Translate this page into:
Facile synthesis of ZnO and Co3O4 nanoparticles by thermal decomposition of novel Schiff base complexes: Studying biological and catalytic properties
⁎Corresponding author. asalwasidi@pnu.edu.sa (Asma S. Al-Wasidi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this paper, a novel Zn(II) and Co(II) Schiff base complexes were synthesized by template method via refluxing 2,3-Naphthalenedicarboxaldehyde, Metal(II) chloride (Metal = Zn or Co), and L-phenylalanine. ZnO and Co3O4 nanoparticles were synthesized by thermal decomposition of Zn(II) and Co(II) complexes, respectively. The products were characterized using different instruments such as CHN, Conductivity, FT-IR, XRD, HR-TEM, and UV–Vis spectrophotometer. The experimental results of elemental analysis for Zn(II) and Co(II) complexes, agree with the calculated results, indicating that the Zn(II) and Co(II) complexes have 1:1 ligand/metal ratios. The molar conductance of the Zn(II) and Co(II) complexes, is less than 5 Ω−1cm−1mol−1, confirming the non-electrolytic nature of the synthesized complexes. The average crystallite diameter of the ZnO and Co3O4 samples is 39.64 and 30.38 nm, respectively. The optical energy gap of the ZnO and Co3O4 samples are 2.75 and 3.25 eV, respectively. Methylene blue dye was utilized to examine the photocatalytic properties of the synthesized nanoparticles using UV irradiations in the absence and presence of hydrogen peroxide. The % degradation of the methylene blue dye in the presence of hydrogen peroxide using ZnO and Co3O4 samples after 40 min is 94.55 and 98.98, respectively. Six pathogenic microbes were utilized to examine the antimicrobial properties of the synthesized Schiff base complexes and their nanoparticles: Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Streptococcus species, Aspergillus species, and Candida species. Zn(II) and Co(II) complexes display inhibition towards all the studied microbes. Besides, ZnO and Co3O4 nanoparticles exhibit less inhibition towards Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Streptococcus species. Moreover, ZnO and Co3O4 nanoparticles have no activity towards Aspergillus and Candida species.
Keywords
Schiff base complexes
Nanoparticles
Photocatalytic degradation
Methylene blue dye
Biological activity
1 Introduction
Metal complexes of Schiff base are a field of increasing importance. These metal complexes were used for various applications such as treatment of cancer, antivirus agents, fungicide agents, and antibactericide agents (Kaczmarek et al., 2018; Liang et al., 2021; Al-Resayes et al., 2016; Alaghaz et al., 2015). Also, metal complexes of Schiff base, which were gotten from amino acids, are discovering applications in understanding several in vivo biochemical reactions (Saikumari, 2021; Otani et al., 2021). Besides, the poisoning task of specific metal ions in living organisms is determined by exploiting these complexes via verifying the activity of the drug. Donmez et al. prepared a novel biosensor that is sensitive to glucose using the microspheres modified with (4-formyl-3-methoxyphenoxymethyl)polystyrene (FMPS) with l-glycine. Polymeric microspheres having Schiff bases were prepared from FMPS using the glycine condensation method. Glucose oxidase enzyme was immobilized onto modified carbon paste electrode by cross-linking with glutaraldehyde. Oxidation of enzymatically produced H2 O2 (+0.5 V vs. Ag/AgCl) was used for determination of glucose. Optimal temperature and pH were found as 50 °C and 8.0, respectively. The glucose biosensor showed a linear working range from 5.0 × 10−4 to 1.0 × 10−2 M, R2 = 0.999. Storage and operational stability of the biosensor were also investigated. The biosensor gave perfect reproducible results after 20 measurements with 3.3% relative standard deviation. It also had good storage stability (Donmez et al., 2017). Amino acids form the basic structural units of proteins and are chemical kinds required for performing an enormous number of biological purposes, as illustrated via the task of enzymes. Ortho-phthalaldehyde acts the main role in the assay of amino acids. The residues of amino acids in several biological fluids and enzymes are determined by exploiting ortho-phthalaldehyde. It achieves requests in the clinical area as a high-level antiseptic (Moitessier et al., 2020; Gyimesi-Forrás et al., 2005). Recently, great interest has been established in copper Schiff base complexes as fundamental models for the active position of the copper proteins. In the copper proteins, a distorted environment of mixed donor groups and low symmetry is present (Dinesh Karthik et al., 2020; Srivastava et al., 2021). Also, nickel complexes are existing in the active positions of urease (Thalamuthu and Neelakantan, 2021; Dong et al., 2012). In the presence of oxidizing agents, square planar nickel-salen was used in the cleavage of plasmid DNA as reported by Morrow and Kolasa (Morrow and Kolasa, 1992). Zinc- including bridged-carboxylate complexes have differed structural styles in hydrolytic metalloenzymes, for example, aminopeptidases and phosphatases (Baltulionis et al., 2021; Karmakar and Chattopadhyay, 2020). Latif et al synthesize a Schiff base via the condensation of 4-(dimethylamino)benzaldehyde and S-benzyldithiocarbazate. Also, Ni(II), Cu(II), and Zn(II) complexes were synthesized. The results of the biological activity of the tested compounds displayed that the complexes were more effective antibiotics than the free Schiff base. The Ni(II) and Cu(II) complexes exhibited high antibacterial effectiveness whereas Zn(II) complex was moderately active against bacteria (Latif et al., 2019). Recently, the complexes have been exploited in the facile and low-cost synthesis of many nanomaterials of small crystallite size by thermal decomposition. Abdelghany et al synthesize Ag and CuO nanoparticles via thermal decomposition of Ag(I) and Cu(II) Schiff base complexes, respectively. The Schiff base was synthesized by the condensation reaction between 2-hydroxy-1-naphthaldehyde and 3-methyl-4-amino-5-mercapto-1,2,4 triazole (Abdelghany et al., 2021). Katouah synthesizes ZnO and Co3O4 nanoparticles by thermal decomposition of Zn(II) and Co(II) Schiff base complexes, respectively. The Schiff base was synthesized via condensation of 4-(dimethylamino)benzaldehyde with 4-amino-5-ethyl-4H-1,2,4-triazole-3-thiol (Katouah, 2021). Nanomaterials are used in many applications, most notably the treatment of water pollutants by adsorption and photocatalytic degradation techniques (Abdelrahman, 2018; Hegazey et al., 2020; Abdelrahman and Hegazey, 2019; Abdelrahman et al., 2021). The photocatalytic degradation method is an effective and simple way to get rid of many dyes because it depends on the use of light and a catalyst to produce free radicals that can degrade these pollutants (Abdelrahman and Hegazey, 2019; Nguyen et al., 2021b; Nasri et al., 2021; Nguyen et al., 2021a; Nguyen et al., 2021c; Shokouhimehr et al., 2019; Nguyen et al., 2020; Kiani et al., 2020; Nayebi et al., 2020; Zhang et al., 2019). So, in this paper, Zn(II) and Co(II) complexes were synthesized by template method via refluxing 2,3-Naphthalenedicarboxaldehyde, cobalt(II) chloride hexahydrate or zinc(II) chloride, and L-phenylalanine. ZnO and Co3O4 nanoparticles were synthesized by thermal decomposition of the Zn(II) and Co(II) complexes, respectively. The biological and catalytic properties were studied.
2 Experimental
2.1 Chemicals
Methylene blue dye (C16H18ClN3S), cobalt(II) chloride hexahydrate (CoCl2·6H2O), zinc(II) chloride (ZnCl2), and L-phenylalanine (C9H11NO2), ethanol (C2H5OH), sodium hydroxide (NaOH), and 2,3-Naphthalenedicarboxaldehyde (C12H8O2) were of analytical grade (Purity = 99.99 %) and obtained from Sigma Aldrich Company and utilized without further purification.
2.2 Synthesis of Zn(II) and Co(II) Schiff base complexes
The template method is operated for the synthesis of Zn(II) and Co(II) Schiff base complexes. About 6 mmol (1.11 g) of 2,3-Naphthalenedicarboxaldehyde in 25 mL ethanol was added to an ethanolic solution of proper cobalt(II) chloride hexahydrate or zinc(II) chloride (6 mmol/25 mL) and stirred at room temperature for one hour. To this stirred solution, 12 mmol of L-phenylalanine in 25 mL of ethanol including 12 mmol sodium hydroxide was added then refluxed for six hours. After that, obtained solid product was filtered using filter paper, washed various times using ether, and then recrystallized from ethanol.
2.3 Synthesis of ZnO and Co3O4 nanoparticles
The Zn(II) and Co(II) Schiff base complexes were thermally decomposed at 550 °C for 5 h to produce ZnO and Co3O4 nanoparticles, respectively.
2.4 Characterization
Elemental analysis of Co(II) and Zn(II) Schiff base complexes was gotten using an Elementar elemental analyzer (Vario EL III). The molar conductance of the Co(II) and Zn(II) complexes in ethanol was assessed utilizing a conductivity bridge (Systronics 304). The Fourier transform (FT-IR) spectra of the complexes, ZnO, and Co3O4 samples, on KBr pellets, were gotten at room temperature using a Shimadzu spectrometer (IRTracer 100) in the range from 4000 to 400 cm−1. Thermal analysis of the Co(II) and Zn(II) complexes was performed using TG/DTG instrument (Perkin-Elmer Diamond) through an oxygen atmosphere at a heating rate of 10 °C/min. The X-ray diffraction (XRD) patterns of the ZnO and Co3O4 samples were conducted utilizing a Bruker diffractometer (D8 Advance) with Cu-Kα irradiation (λ = 0.15 nm) at 40 mA and 40 kV. The high-resolution transmission electron microscopy (HR-TEM) images of the ZnO and Co3O4 samples were taken using JEOL model 2100. Optical energy gaps of ZnO and Co3O4 samples were obtained using a UV–Vis spectrophotometer (Cintra 101).
2.5 Photocatalytic degradation of methylene blue dye
Degradation experiments of the methylene blue dye were achieved in a photochemical batch reactor containing a magnetic stirrer. 15 W four UV lamps (λ = 365 nm) were operated as a UV radiation source. All the experiments of adsorption and photocatalytic degradation started with a 60 mL aqueous solution of 60 mg/L of methylene blue dye and 0.15 g of ZnO or Co3O4. The adsorption experiments were achieved in the dark. The degradation of methylene blue dye in the absence and presence of hydrogen peroxide under the effect of UV light and catalyst (ZnO or Co3O4) was observed using a UV–Vis spectrophotometer at different periods. The concentration of methylene blue dye was determined from a preset calibration curve at 663 nm (maximum absorption wavelength of the methylene blue dye).
% Adsorption (% A) was calculated using Eq. (1) whereas % degradation (% D) was calculated using Eq. (2).
2.6 Biological activity
Six pathogenic microbes were utilized to examine the antimicrobial activity of the synthesized Schiff base complexes and their nanoparticles: Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Streptococcus species, Aspergillus species, and Candida species. The antimicrobial activity of the extracts was qualitatively estimated via a modified disc diffusion approach (Almehizia et al., 2021). A lawn of microorganisms was synthesized via pipetting and equally spreading inoculums (10−4 cm3, adjusted turbidometrically to 10−5 cfu cm3) onto agar group in petri dishes, utilizing potato dextrose agar in the case of fungi and Muller Hinton nutrient agar in the case of bacteria. The liquid media including the bacterial divisions was autoclaved for twenty minutes at 15 lb pressure and 120 °C before inoculation. Then, the bacteria were cultured at 37 °C in an incubator for one day. The samples (50 μg) to be examined were dissolved in dimethyl sulfoxide then dropwise added to a Whatman No. 1 filter paper disc (10 mm diameter) placed at the center of each agar plate. These filter paper discs were then kept at 5 °C for one hour then transported to an incubator maintained at 37 °C. After four days, the inhibition zone, which formed around the discs in each plate, was determined. Commercially available nystatin was utilized as antifungal control whereas ampicillin was utilized for antibacterial control.
3 Results and discussion
3.1 Characterization of Zn(II) and Co(II) complexes
The experimental results of elemental analysis for the Zn(II) and Co(II) complexes agree with the calculated results indicating that the Zn(II) and Co(II) complexes have 1:1 ligand/metal ratios as clarified in Scheme 1 and Table 1. The molar conductance of the Zn(II) and Co(II) complexes in ∼0.001 M ethanol at room temperature is 3.87 and 4.12 Ω−1cm−1mol−1, respectively. These values confirming the non-electrolytic nature of the synthesized complexes because of the absence of counterions in the suggested structure of the complexes as shown in Table 1 (Abdelghany et al., 2021; Katouah, 2021). Fig. 1 represents the FT-IR spectra of the Zn(II) and Co(II) complexes, respectively. The bands, which were observed in the Zn(II) and Co(II) complexes at 480 and 430 cm−1, are due to the stretching vibration of Zn-O and Co-O, respectively (Abdelghany et al., 2021; Katouah, 2021; Nakamoto, 2008). The bands, which were observed in the Zn(II) and Co(II) complexes at 582 and 570 cm−1, are due to the stretching vibration of Zn-N and Co-N, respectively. The bands, which were observed in the Zn(II) and Co(II) complexes in the range 690–960 cm−1 and 680–950 cm−1, are due to CH out of plane bending vibration of aromatic rings, respectively. The bands, which were observed in the Zn(II) and Co(II) complexes at 1390 and 1385 cm−1, are due to the symmetric stretching vibration of COO, respectively. The bands, which were observed in the Zn(II) and Co(II) complexes in the range 1450–1530 cm−1 and 1460–1540 cm−1, are due to the stretching vibration of C = C aromatic, respectively. The bands, which were observed in the Zn(II) and Co(II) complexes at 1601 and 1610 cm−1, are due to the asymmetric stretching vibration of COO, respectively. The bands, which were observed in the Zn(II) and Co(II) complexes at 1640 and 1645 cm−1, are due to the stretching vibration of C = N, respectively. The bands, which were observed in the Zn(II) and Co(II) complexes at 2900 and 2950 cm−1, are due to the stretching vibration of CH aliphatic, respectively. The bands, which were observed in the Zn(II) and Co(II) complexes at 3200 and 3300 cm−1, are due to the stretching vibration of CH aromatic, respectively. The bands, which were observed in the Zn(II) and Co(II) complexes at 3450 and 3500 cm−1, are due to the stretching vibration of H-O-H, respectively (Abdelghany et al., 2021; Katouah, 2021; Nakamoto, 2008). Table 2 represents the data of TG and DTA analyses for Zn(II) and Co(II) complexes. There are three decomposition steps. The first exothermic step, which was observed in the temperature range of 30–300 °C, is due to the loss of two coordinated water molecules. The second exothermic step, which was observed in the temperature range of 300–550 °C, is because of the decomposition of the organic moiety. The third step, which was observed in the temperature range of 550–1000 °C, is because of oxidation of metal (Zn or Co) i.e., formation of ZnO or Co3O4.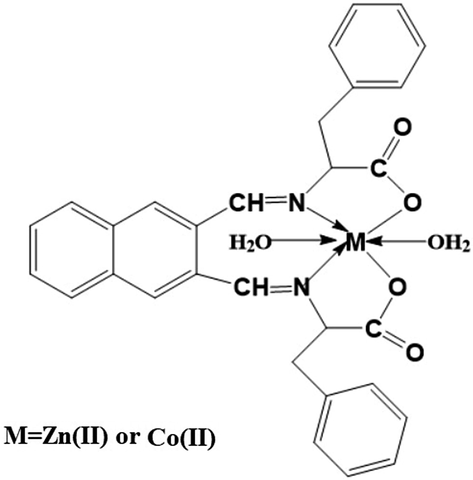
The Structure of the synthesized complexes.
% Element
Zn(II) complex
Formula: C30H28N2O6Zn
Molecular weight: 576.12
Co(II) complex
Formula: C30H28N2O6Co
Molecular weight: 571.13
Calculated
Found
Calculated
Found
%C
62.34
62.12
63.05
63.15
%H
4.88
4.67
4.94
4.90
%N
4.85
4.80
4.90
4.92
%O
16.61
16.45
16.80
16.88
%Zn
11.32
11.96
–
–
%Co
–
–
10.31
10.15
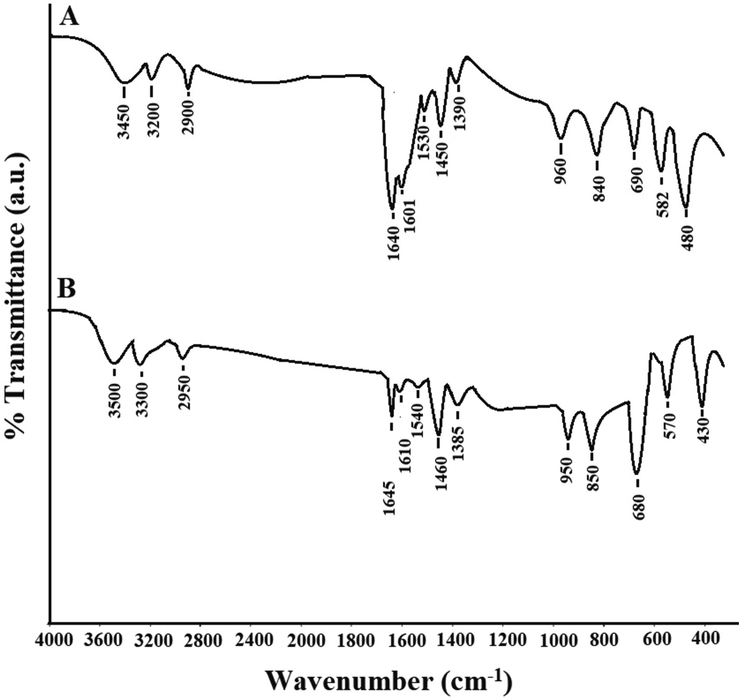
The FT-IR spectra of the Zn(II) (A) and Co(II) (B) complexes.
Steps
Zn(II) complex (C30H28N2O6Zn)
Co(II) complex (C30H28N2O6Co)
Chemical equations
Weight loss%
Chemical equations
Weight loss%
Calculated
Found
Calculated
Found
First step
C30H28N2O6Zn → C30H24N2O4Zn + 2H2O↑
6.25
6.38
C30H28N2O6Co → C30H24N2O4Co + 2H2O↑
6.30
6.15
Second step
C30H24N2O4Zn → Zn + C30H24N2O4↑
82.65
82.26
C30H24N2O4Co → Co + C30H24N2O4 ↑
83.37
83.49
Third step
Zn + 1/2O2 → ZnO
–
–
3Co + 2O2 → Co3O4
–
–
3.2 Characterization of ZnO and Co3O4 nanoparticles
The ZnO and Co3O4 samples were analyzed using X-ray diffraction (XRD) to check their crystalline phases and structures. The XRD patterns of the ZnO and Co3O4 samples are presented in Fig. 2A-B, respectively. In the case of ZnO, the principal reflections observed at the 2θ values of 31.80°, 34.40°, 36.30°, 47.50°, 56.60°, 62.90°, 67.90°, 68.80°, 69.90°, 72.50°, and 78.60° assigned (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2), (2 0 1), (0 0 4), and (2 0 2) plains, respectively as clarified from the JCPDS No. 36–1451 (Katouah, 2021). In the case of Co3O4, the principal reflections observed at the 2θ values of 36.80°, 38.50°, and 44.80° assigned (3 1 1), (2 2 2), and (4 0 0) plains, respectively as clarified from the JCPDS No. 78–1970 (Katouah, 2021). The average crystallite diameter of the ZnO and Co3O4 samples is 39.64 and 30.38 nm, respectively. The FT-IR spectra of the ZnO and Co3O4 samples are presented in Fig. 3A-B, respectively. The band, which was appeared in the case of ZnO at 485 cm−1, is because of the stretching vibration of Zn-O. The bands, which were appeared in the case of ZnO at 1615 and 3445 cm−1, are because of the bending and stretching vibration of adsorbed water, respectively (Katouah, 2021). The bands, which were appeared in the case of Co3O4 at 530 and 630 cm−1, are because of the stretching vibration of Co(II)-O and Co(III)-O, respectively. The bands, which were appeared in the case of Co3O4 at 1620 and 3450 cm−1, are because of the bending and stretching vibration of adsorbed water, respectively (Katouah, 2021). The morphology of the ZnO and Co3O4 samples was examined via HR-TEM as shown in Fig. 4A-B, respectively. The ZnO and Co3O4 samples consist of irregular and spherical shapes with an average diameter of 35.04 and 26.48 nm, respectively. UV–Vis spectrophotometer was utilized for determining the bandgap via the Tauc plot method (Eq. (3)) for the ZnO and Co3O4 samples as shown in Fig. 5A-B, respectively (Katouah, 2021).
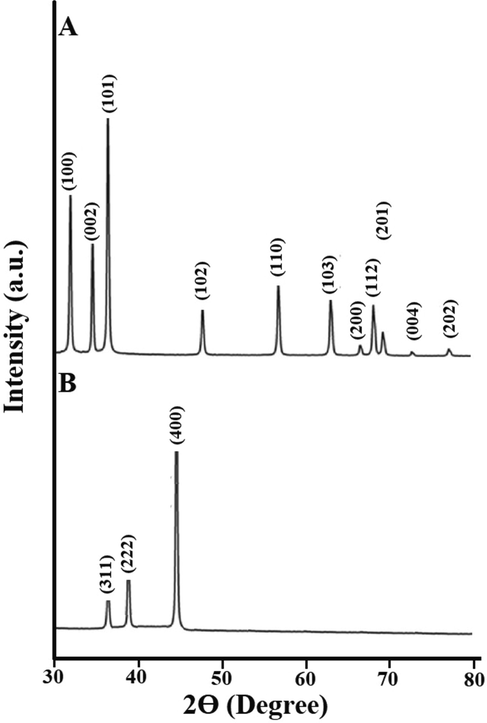
The XRD patterns of the ZnO (A) and Co3O4 (B) samples.
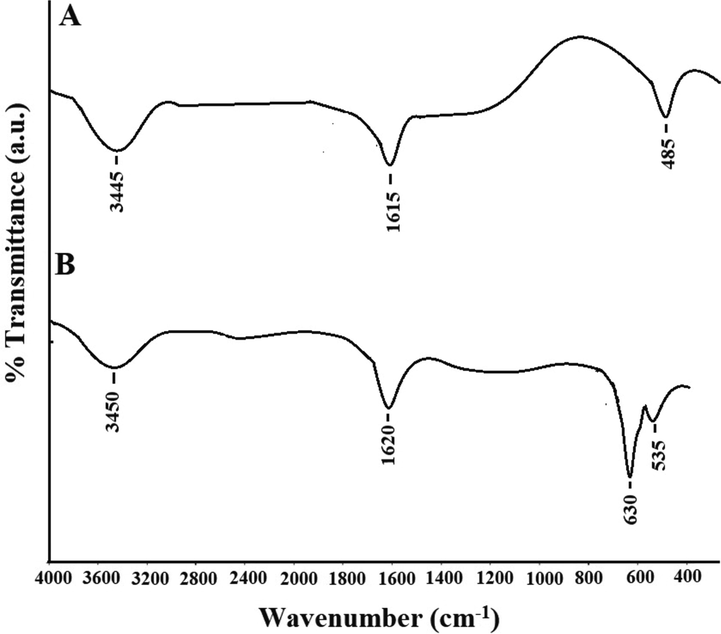
The FT-IR spectra of the ZnO (A) and Co3O4 (B) samples.
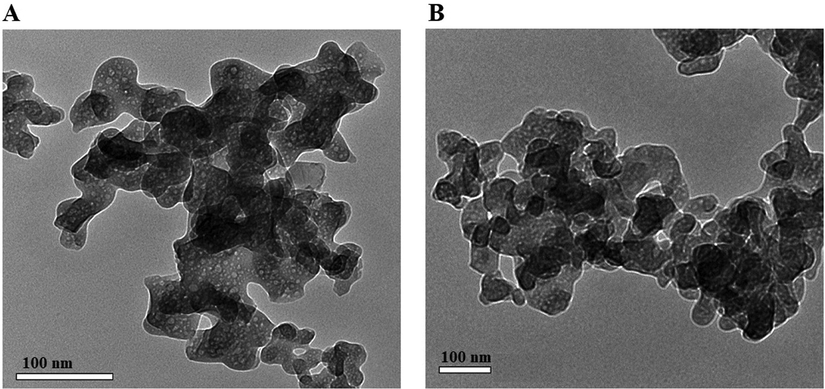
The HR-TEM images of the ZnO (A) and Co3O4 (B) samples.
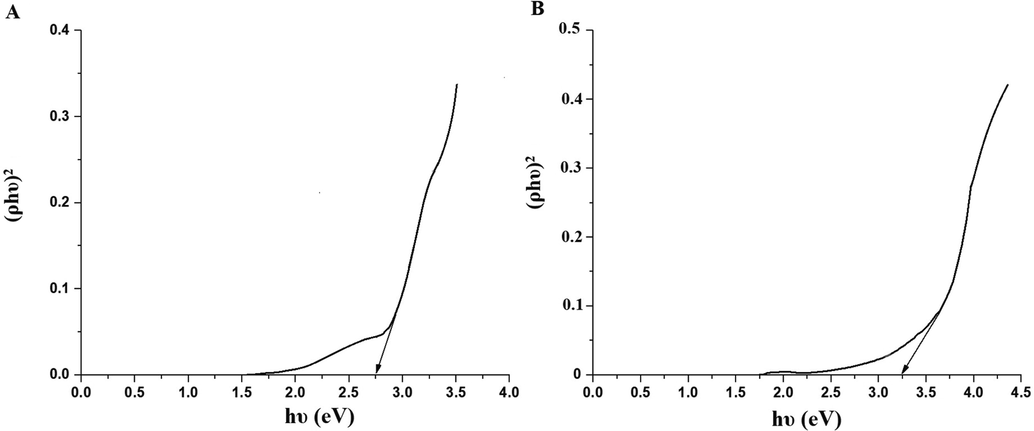
The optical energy gap of the ZnO (A) and Co3O4 (B) samples.
3.3 Photocatalytic degradation of methylene blue dye
% A of the methylene blue dye in the dark place using ZnO and Co3O4 samples is 8.33 and 18.33 %, respectively. To determine the influence of UV irradiation time on % D of methylene blue dye using ZnO and Co3O4 samples in the absence and presence of H2O2, experiments were performed by the analysis of irradiation time variations versus % D as shown in Fig. 6A-B, respectively. In the absence of hydrogen peroxide, % D increased with increasing UV irradiation time from 20 to 80 min. After that, % D almost remains stable when the UV irradiation time increased from 80 to 120 min owing to the saturation of the active centers. Consequently, the optimum UV irradiation time is 80 min. The % D of the methylene blue dye using ZnO and Co3O4 samples after 80 min is 67.27 and 75.51, respectively. In the presence of hydrogen peroxide, % D increased with increasing UV irradiation time from 10 to 40 min. After that, % D almost remains stable when the UV irradiation time increased from 40 to 60 min owing to the saturation of the active centers. Consequently, the optimum UV irradiation time is 40 min. The % D of the methylene blue dye using ZnO and Co3O4 samples after 40 min is 94.55 and 98.98, respectively. Photocatalytic degradation of methylene blue dye fitted well with the first-order kinetic model (Eq. (4)) (Katouah, 2021).
E = Excitation source, V = Volume, C = Concentration, and W = Weight.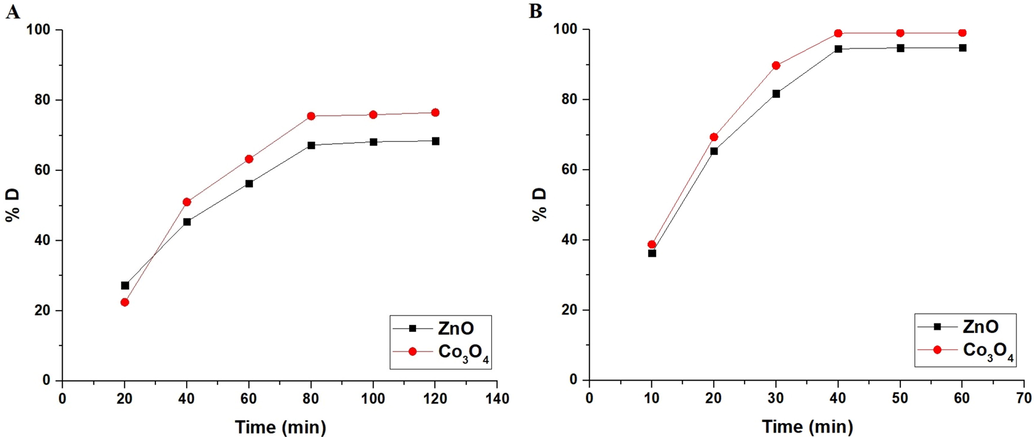
The plot of % D versus time.
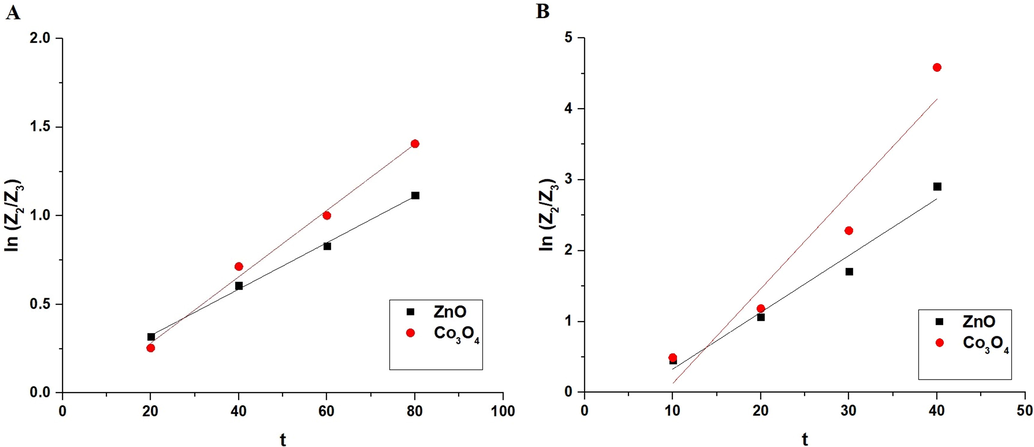
The plot of ln (Z2/Z3) versus time.
Factors
Parameters
R2
K (1/min)
ZnO + UV irradiation
0.986
0.0131
ZnO + UV irradiation + H2O2
0.998
0.0801
Co3O4 + UV irradiation
0.926
0.0187
Co3O4 + UV irradiation + H2O2
0.995
0.1338
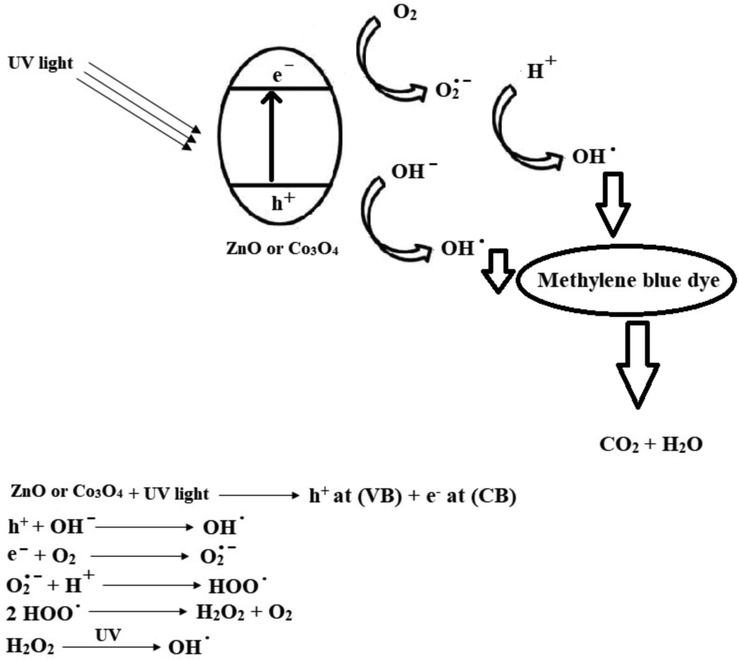
The mechanism of the photocatalytic degradation of methylene blue dye.
Photocatalyst
Dye
E
% Degradation
Ref
Name
W (g)
C (mg/L)
V (mL)
Value
Time (min)
Co alloyed CdZnS
0.03
25
25
UV light
83
100
(Geetha et al., 2021)
ZnWO4
0.1
10
200
UV light
87
60
(de Moura et al., 2021)
TiO2 supported on magnetic core shell
0.03
10
200
UV light
96
120
(Sridevi et al., 2020)
ZnSe
0.05
10
50
UV light
78
150
(Ivanets et al., 2021)
CdSe
0.05
10
50
UV light
56
150
(Ivanets et al., 2021)
ZnO
0.15
60
60
UV light
94.55
40
This study
Co3O4
0.15
60
60
UV light
98.98
40
This study
3.4 Biological activity
In vitro antimicrobial activities of the metal complexes and their nanoparticles were examined against six microbes using the modified disc diffusion procedure (Katouah, 2021). The zone of inhibition versus the growth of microbes for the metal complexes and their nanoparticles is given in Table 5. The complexes and their nanoparticles diffused into microbes through the lipid layer of spore membranes to the site of action eventually killing them via combining with –OH groups of specific cell enzymes (Katouah, 2021). The diversity in the efficiency of different biocidal agents against different organisms relys on the impermeability of the cell. It is clear that Zn(II) and Co(II) complexes display inhibition towards all the studied microbes. Besides, ZnO and Co3O4 nanoparticles exhibit less inhibition towards Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Streptococcus species. Moreover, ZnO and Co3O4 nanoparticles have no activity towards Aspergillus and Candida species.
Sample
Inhibition zone diameter (mm/mg sample)
Staphylococcus aureus
Escherichia coli
Pseudomonas aeruginosa
Streptococcus species
Aspergillus species
Candida species
Control
15
13
12
9
12
11
Zn(II) complex
22
21
17
19
17
14
Co(II) complex
19
15
15
16
14
12
ZnO
12
8
8
11
0
0
Co3O4
11
10
9
10
0
0
4 Conclusions
ZnO and Co3O4 nanoparticles were facilely synthesized using the thermal decomposition of novel Zn(II) and Co(II) Schiff base complexes. The average crystallite diameter of the ZnO and Co3O4 samples is 39.64 and 30.38 nm, respectively. The % degradation of the methylene blue dye in the presence of hydrogen peroxide using ZnO and Co3O4 samples after 40 min is 94.55 and 98.98, respectively. Compared to Schiff base complexes, ZnO and Co3O4 nanoparticles exhibit less inhibition towards Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Streptococcus species. Moreover, ZnO and Co3O4 nanoparticles have no activity towards Aspergillus and Candida species.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Princess Nourah Bint Abdulrahman University, through the Research Groups Program Grant No. (RGP-1440-0003) (3).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Facile Synthesis of CuO and Ag Nanoparticles by Thermal Decomposition of Novel Schiff Base Complexes. J. Inorg. Organomet. Polym. Mater. 2021
- [CrossRef] [Google Scholar]
- Synthesis of zeolite nanostructures from waste aluminum cans for efficient removal of malachite green dye from aqueous media. J. Mol. Liq.. 2018;253:72-82.
- [Google Scholar]
- Utilization of rice husk and waste aluminum cans for the synthesis of some nanosized zeolite, zeolite/zeolite, and geopolymer/zeolite products for the efficient removal of Co(II), Cu(II), and Zn(II) ions from aqueous media. J. Hazard. Mater.. 2021;401:123813
- [Google Scholar]
- Facile Synthesis of HgO Nanoparticles Using Hydrothermal Method for Efficient Photocatalytic Degradation of Crystal Violet Dye Under UV and Sunlight Irradiation. J. Inorg. Organomet. Polym. Mater.. 2019;29:346-358.
- [Google Scholar]
- Utilization of waste aluminum cans in the fabrication of hydroxysodalite nanoparticles and their chitosan biopolymer composites for the removal of Ni(II) and Pb(II) ions from aqueous solutions: Kinetic, equilibrium, and reusability studies. Microchem. J.. 2019;145:18-25.
- [Google Scholar]
- Synthesis, spectral characterization, molecular modeling and antimicrobial studies of tridentate azo-dye Schiff base metal complexes. J. Mol. Struct.. 2015;1084:36-45.
- [Google Scholar]
- Facile synthesis and characterization of ZnO nanoparticles for studying their biological activities and photocatalytic degradation properties toward methylene blue dye. Alexandria Eng. J. 2021
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopic characterization and in vitro antimicrobial studies of Schiff base ligand, H2L derived from glyoxalic acid and 1,8-diaminonaphthalene and its Co(II), Ni(II), Cu(II) and Zn(II) complexes. Arab. J. Chem.. 2016;9:335-343.
- [Google Scholar]
- The role of propeptide-mediated autoinhibition and intermolecular chaperone in the maturation of cognate catalytic domain in leucine aminopeptidase. J. Struct. Biol.. 2021;213:107741
- [Google Scholar]
- Photocatalytic degradation of methylene blue dye by TiO2 supported on magnetic core shell (Si@Fe) surface. J. Iran. Chem. Soc. 2021
- [CrossRef] [Google Scholar]
- Green approach to synthesize, spectral investigation and biological applications of potentially active ternary Schiff base copper(II) complexes. Mater. Today Proc.. 2020;43:2389-2396.
- [Google Scholar]
- Synthesis, structures and urease inhibition studies of copper(II) and nickel(II) complexes with bidentate N, O-donor Schiff base ligands. J. Inorg. Biochem.. 2012;108:22-29.
- [Google Scholar]
- Glucose biosensor based on immobilization of glucose oxidase on a carbon paste electrode modified with microsphere-attached l-glycine. Appl. Biochem.. 2017;64:745-753.
- [Google Scholar]
- Photocatalytic degradation of methylene blue dye using ZnWO4 catalyst prepared by a simple co-precipitation technique. J. Sol-Gel Sci. Technol.. 2021;97:572-580.
- [Google Scholar]
- Comparative study on the use of ortho-phthalaldehyde, naphthalene-2,3- dicarboxaldehyde and anthracene-2,3-dicarboxaldehyde reagents for α-amino acids followed by the enantiomer separation of the formed isoindolin-1-one derivatives using quinine-type chiral stationary phases. J. Chromatogr. A.. 2005;1083:80-88.
- [Google Scholar]
- Facile fabrication of hematite nanoparticles from Egyptian insecticide cans for efficient photocatalytic degradation of rhodamine B dye. J. Mater. Res. Technol.. 2020;9:1652-1661.
- [Google Scholar]
- Effect of metal ions adsorption on the efficiency of methylene blue degradation onto MgFe 2 O 4 as Fenton-like catalysts. Colloids Surfaces A Physicochem. Eng. Asp.. 2019;571:17-26.
- [Google Scholar]
- Effect of magnesium ferrite doping with lanthanide ions on dark-, visible- and UV-driven methylene blue degradation on heterogeneous Fenton-like catalysts. Ceram. Int.. 2021;47:29786-29794.
- [Google Scholar]
- A comparative study on the synthesis of magnesium ferrite for the adsorption of metal ions: Insights into the essential role of crystallite size and surface hydroxyl groups. Chem. Eng. J.. 2021;411:128523
- [CrossRef] [Google Scholar]
- Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord. Chem. Rev.. 2018;370:42-54.
- [Google Scholar]
- Visible light driven photodegradation of methylene blue with two reduced Schiff base complexes of zinc(II): Exploration of their phosphatase mimicking ability. Polyhedron.. 2020;184:114527
- [Google Scholar]
- Facile synthesis of Co3O4 and ZnO nanoparticles by thermal decomposition of novel Co(II) and Zn(II) Schiff base complexes for studying their biological properties and photocatalytic degradation of crystal violet dye. J. Mol. Struct.. 2021;1241:130676
- [Google Scholar]
- Novel Pt-Ag3PO4/CdS/chitosan nanocomposite with enhanced photocatalytic and biological activities. Nanomaterials.. 2020;10:1-21.
- [Google Scholar]
- Synthesis, Characterization, and Biological Activity of the Schiff Base and Its Ni(II), Cu(II), and Zn(II) Complexes Derived from 4-(Dimethylamino)benzaldehyde and S-Benzyldithiocarbazate. Russ. J. Gen. Chem.. 2019;89:1197-1201.
- [Google Scholar]
- Discovery of metal-based complexes as promising antimicrobial agents. Eur. J. Med. Chem.. 2021;224:113696
- [Google Scholar]
- 4-Methoxy-ortho-phthalaldehyde: a promising derivatizing agent for the fluorimetric evaluation of histamine in seafood. Talanta Open.. 2020;2:100014
- [Google Scholar]
- Nakamoto, K., 2008, Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry, sixth ed. http://dx.doi.10.1002/9780470405840.
- Fabrication of g-C3N4/Au nanocomposite using laser ablation and its application as an effective catalyst in the reduction of organic pollutants in water. Ceram. Int.. 2021;47:3565-3572.
- [Google Scholar]
- Boron nitride-palladium nanostructured catalyst: efficient reduction of nitrobenzene derivatives in water. Nano Express.. 2020;1:030012
- [Google Scholar]
- Novel architecture titanium carbide (Ti3C2Tx) mxene cocatalysts toward photocatalytic hydrogen production: A mini-review. Nanomaterials.. 2020;10:602-612.
- [Google Scholar]
- g-C3N4 nanosheet adorned with Ag3BiO3 as a perovskite: An effective photocatalyst for efficient visible-light photocatalytic processes. Mater. Sci. Semicond. Process.. 2021;125:105651.
- [Google Scholar]
- Insitu preparation of g-C3N4 nanosheet/FeOCl: Achievement and promoted photocatalytic nitrogen fixation activity. J. Colloid Interface Sci.. 2021;587:538-549.
- [Google Scholar]
- High-impressive separation of photoinduced charge carriers on step-scheme ZnO/ZnSnO3/Carbon dots heterojunction with efficient activity in photocatalytic NH3 production. J. Taiwan Inst. Chem. Eng.. 2021;118:140-151.
- [Google Scholar]
- Synthesis of amino acid derivative Schiff base copper(II) complexes by microwave and wet mechanochemical methods. J. Indian Chem. Soc.. 2021;98:100004
- [Google Scholar]
- Synthesis and characterization of amino acid Schiff base and its copper (II) complex and its antimicrobial studies. Mater. Today Proc. 2021
- [CrossRef] [Google Scholar]
- Palladium nanocatalysts on hydroxyapatite: Green oxidation of alcohols and reduction of nitroarenes in water. Appl. Sci.. 2019;9:4183-4193.
- [Google Scholar]
- Ultraviolet light induced dye degradation of methylene blue in the presence of photocatalytic CdSe and ZnSe nanoparticles. Mater. Today Proc.. 2020;42:1244-1250.
- [Google Scholar]
- Synthesis, characterization, biological and electrochemical investigation of copper (II) complexes containing 4-chloro-2-[2, 6-diisopropylphenylimino) methyl] phenol Schiff base ligand and aromatic diinines. Chem. Data Collect.. 2021;32:100659
- [Google Scholar]
- Trinuclear nickel(II) amino acid Schiff base complex containing phenolato and acetato bridges: Structural and functional resemblance of urease. Inorganica Chim. Acta.. 2021;516:120109
- [Google Scholar]
- Facile synthesis of monodispersed Pd nanocatalysts decorated on graphene oxide for reduction of nitroaromatics in aqueous solution. Res. Chem. Intermed.. 2019;45:599-611.
- [CrossRef] [Google Scholar]







