Translate this page into:
Feeding deterrence towards Helicoverpa armigera by Tithonia diversifolia tagitinin C-enriched extract
⁎Corresponding authors. alessandra.vacari@unifran.edu.br (Alessandra Marieli Vacari), rodrigo.veneziani@unifran.edu.br (Rodrigo Cassio Sola Veneziani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The use of botanical insecticides is a tactic that can be highly useful for the management of Helicoverpa armigera populations in the field. However, most botanical insecticides are produced using primarily macerated leaves. The problem with this process is that all components of the leaves are present in the extract, and not just the plant defence chemicals. Therefore, the objective of this research was to evaluate the feeding deterrence of a tagitinin C-rich leaf rinse extract (TagCE) obtained from Tithonia diversifolia (which concentrates mainly the plant defence substances) towards H. armigera larvae. The RP-HPLC-PDA analytical method allowed the identification and quantification of the major constituent TagCE, showing that this compound is suitable to be considered as its chemical marker. Regarding biological activity, we observed high insect mortality, with up to 80%, and high antifeedant activity for H. armigera larvae for all host plant species treated with T. diversifolia extract. These results open up the possibility to use T. diversifolia extract as a potential botanical insecticide, mainly against plant-defoliating Lepidoptera.
Keywords
Botanical insecticide
Chemical ecology
IPM
1 Introduction
On a global level, the species Helicoverpa armigera (Hübner, 1805) (Lepidoptera: Noctuidae), with an extensive geographical distribution and polyphagous habits, is among the main pests that attack agricultural crops, causing significant damage to the economy (Jones et al., 2019; Gonçalves et al., 2019). The first record of this species in the Brazilian territory was in 2013 in the state of Goiás (Czepak et al., 2013). In its larval stage, H. armigera feeds on more than 100 plant species of native and cultivars of approximately 47 families, including Solanaceae, Poaceae, Malvaceae, Fabaceae and Asteraceae. It generally feeds on the leaves and stems of these plants and may also reach inflorescences and fruits of some species (Jallow et al., 2004). In Brazil, the larvae mainly affect cotton, crotalaria, beans, guandu, millet, corn, soybean, sorghum, tomato and wheat crops, including some weeds such as Conyza bonariensis (Bueno and Sosa-Gómez, 2014).
The control tactics available for H. armigera are chemical control using synthetic chemical insecticides and biological control using Bacillus thuringiensis bioinsecticides. However, there are records of H. armigera populations resistant to both chemical insecticides (Yang et al., 2013; Bird, 2015; Bird, 2018) as well as to bioinsecticides (Estela et al., 2004; Avilla et al., 2005). Studies have shown that there may be future risks in the use of Bt crops mainly due to increased resistance of H. armigera populations (Ahmad et al., 2019; Leite et al., 2018; Dandan et al., 2019).
In this context, new plant-derived natural insecticides, known as “botanical insecticides”, can help to avoid these problems because instead of being lethal to several non-target arthropods, they act specifically as impediments to oviposition and feeding of insects (Cameron et al., 2014). Deterrent activity against Chlosyne lacinia (Lepidoptera: Nymphalidae) larvae was observed using leaves of T. diversifolia (Ambrosio et al., 2008). Thus, the use of botanical insecticides is a tactic that can be highly useful for the management of H. armigera populations in the field (Mabou Tagne et al., 2018). However, most botanical insecticides are produced using primarily macerated leaves (Arivoli and Tennyson, 2012; Amoabeng et al., 2019; Cerda et al., 2019), and the issue with this process is that all the components of the leaves are present in the extract and not just the plant defence chemicals. Therefore, in this study, we evaluated the feeding deterrence of a T. diversifolia leaf rinse extract obtained from leaf trichomes (TagCE, which concentrates only the plant defence substances) in H. armigera larvae.
2 Material and methods
2.1 General experimental procedures and reagents
The Liquid Chromatography system consisted of a three-pump Shimadzu chromatograph model LC-20AR Prominence equipped with a SIL-10AF auto sampler, a CTO- 20A column oven, a CBM-20A communications bus module, a DGU-20A3R in-line degasser, and an SPD-M20A photodiode array detector. The Lab solution® software was used to process the data; the analyses were conducted in a Shim-pack VP-ODS column (250 × 4.6 mm i.d., 5 μm; Shimadzu). Acetonitrile (chromatographic grade) was supplied by Mallinkrodt Baker Inc. (Phillipsburg, NJ, USA), and water was purified with a Milli-Q-plus filter system (Millipore, Bedford, MA, USA). Classic and vacuum liquid chromatography (respectively CC and VLC; glass columns of 450 X 25 mm and 50–100 mm i.d.) were used to purify tagitinin C (Fig. 1A) by using silica gel 60 (Merck, art. 9385) and silica gel 60H (Merck, art. 7736). Commercial hexanes and ethyl acetate (EtOAc) were purified by distillation in our facilities. The NMR spectra were run on a Bruker DPX 400 spectrometer (400 MHz for 1H and 100 MHz for 13C). The samples were dissolved in CDCl3, and the spectra were calibrated with the solvent signals at 7.26 (1H) and 77.0 (13C). We used HPLC grade p-coumaric acid (Fig. 1B, CA), a natural phenylpropanoid that is not found in T. diversifolia leaves, as Internal Standard, acquired from Sigma-Aldrich (≥98% purity, Darmstadt, Germany).
Molecular structures of tagitinin C (A) and p-cumaric acid (B).
2.2 Plant material
Tithonia diversifolia leaves were collected in Patrocínio (Minas Gerais state, Brazil; S18° 56′ 38″ W46° 59′ 34″) by Gabriel da Costa Inacio. Plant identification was carried out by Rosângela de Oliveira Araújo from the University Center of the Cerrado-Patrocínio (UNICERP), Patrocínio, Minas Gerais, Brazil, and the voucher specimen was deposited in the Herbarium Uberlandense (HUFU) under the number HUFU 73.901.
2.3 Obtaining of tagitinin C-rich leaf rinse extract (TagCE) and isolation of tagitinin C
Initially, 8.0 kg of T. diversifolia leaves were rinsed with dichloromethane (6 L) for 3 min to obtain 23,290 g of TagCE after filtering and solvent evaporation (Ambrosio et al., 2008). About 9.0 g of the extract were then chromatographed over 600.0 g of silica gel 60H (Merck, art. 7736), using vacuum liquid chromatography (VLC) (Pelletier et al., 1986) with increasing amounts of ethyl acetate in n-hexane as eluent (2 L for each fraction). This procedure delivered seven fractions that were analysed through TLC, using silica gel PF254 (Merck art. 9385; 1 mm thickness) and isocratic n-hexane: ethyl acetate 1:1 as mobile phase after solvent evaporation. The second and third fractions (0.511 and 2.309 g, respectively) were combined and then submitted to another fractionation using VLC (150.0 g of silica gel 60H; increasing amounts of n-hexane: ethyl acetate – 1 L for each fraction). After TLC analysis using the conditions previously described, the tenth fraction showed a single spot and was identified as tagitinin C after 1H and 13C NMR analyses and comparison with literature data (Baruah et al. 1979). The NMR data also allowed us to conclude that the purity of the isolated compound was above 99%.
2.4 Development and validation of the analytical method
Chromatographic analyses of TagCE were performed through RP-HPLC-PDA using a Shim-pack VP-ODS column (250 mm × 4.6 mm i.d., 5 μm; Shimadzu) and an isocratic system consisting of acetonitrile and water with 0.1% acetic acid (45:55 v/v) as mobile phase, which was established through initial experiments using the gradient scouting run evaluation as previously described (Snyder and Dolan, 1996). The temperature of the column was set at 40 °C, the flow rate was 1.0 mL.min−1, the injected volume was 20.0 µL and the detection wavelength was set at 254 nm. The developed and validated analytical method complies with the requirements established by the National Agency for Sanitary Vigilance in Brazil (ANVISA, 2003).
2.5 Preparation of sample solutions, construction of linear analytical curves, determination of limits of detection (LOD) and quantification (LOQ)
The analytical solutions were prepared by dissolving 1.0 mg of the sample plus 0.5 mg of p-cumaric acid (CA), used as internal standard in 5 mL of acetonitrile, followed by filtration through a UNIFLO 25/0.2 PTFE syringe filter (Whatman/Schleicher & Schuell, Maidstone, UK).
A dilution series of metabolite tagitinin (400.0, 300.0, 200.0, 100.0, 80.0, 40.0, and 20.0 µg·mL−1) with internal standard (CA; 100.0 µg·mL−1) was prepared in acetonitrile. Then, 20.0 µL aliquots of these solutions were injected into the HPLC equipment in triplicate. Linear analytical curves were obtained by plotting the area ratio of the individual chromatographic standards to the internal standard CA. The correlation coefficient was calculated using Microsoft Excel®.
The LOD and LOQ values were determined on the basis of the standard deviation of the response (σ) and of the slope of the analytical curve (S), using the expressions LOD = 3.3σ/S and LOQ = 10σ/S.
2.6 Selectivity
The selectivity of the method was evaluated by comparing DAD spectral data in the ascending, upper and descending regions of the respective peaks of tagitinin C in the linear analytical curve and in the sample (Souza et al., 2012). All the spectra matched, confirming that no other metabolites co-eluted with the target compound.
2.7 Precision and accuracy
Intra-day precision was evaluated by analysing six samples, and inter-day precision was assayed on two consecutive days. The concentration and the relative standard deviation (RSD) of the analyte were determined in TagCE. For the inter-day precision, the concentration values of tagitinin C were compared by applying the t test (p < 0.05), using GraphPad Prism®.
The accuracy of the method was determined by spiking known contents of tagitinin C into TagCE at low, medium and high levels (respectively 25, 50 and 100% of the compound content determined in the precision experiment) in triplicate. The spiked samples were analysed, and the recoveries were calculated by comparing the measured to the spiked concentrations (Moreira et al., 2013, 2016).
2.8 Robustness
The robustness of the chromatographic method was assessed by following the experimental design proposed by Plackett-Burman for seven factors and eight experiments, as previously described (Vander Heyden et al., 2001; Moreira et al., 2016). Briefly, the selected operational factors related to the chromatographic method were as follows: mobile phase flow rate (Flow), column oven temperature (Temp.), percentage of organic solvent in the mobile phase (%B), detection wavelength (λ) and volume of injected samples (Inj.). Two dummy factors had to be included to reach a saturated design and variation levels for each of these operational factors (Table 1). Robustness values were expressed as RSD (%) of the responses.
Factors
Unit
Limit (±)
Level (−1)
Level (+1)
Nominal
Flow
mL·min−1
0.1
0.9
1.1
1.0
Temp.
° C
5
35.0
45.0
40.0
%B
%
1
40
50
45.0
λ
nm
5
259
249
254
Inj.
µL
1
19.0
21.0
20.0
2.9 Feeding deterrence on artificial diet
The artificial diet used in the bioassay was prepared according to Greene et al. (1976). The extract was sprayed when the diet temperature decreased and reached 25 °C, to avoid the potential degradation of compounds present in the extract. Dichloromethane extract was tested at concentrations of 100, 500 and 1000 mg mL−1. In the control treatment, only dimethyl sulfoxide (DMSO) solvent was used. The extract was dissolved in DMSO at a ratio of 1% (w/w) extract on the dry weight of the artificial diet. A 0.1-mL aliquot of the diluted extract was applied to the diet with a micropipette, and the diet remained at room temperature for 30 min for drying. The procedure was repeated for each tested extract concentration, for the control (DMSO) and for the positive control (flubendiamide insecticide used at the manufacturer's recommended dose of 0.35 µL mL−1). After diet drying, 10-day-old third instar larvae were added; each repetition consisted of a Petri dish (90 mm diameter) containing an artificial diet and one larva. Evaluations were performed every 24 h until pupae formation and subsequent emergence of adults. The pupae were transferred to 1000-mL plastic cups where they remained until the emergence of the adults. The experiment was conducted in a BOD chamber adjusted to a temperature of 25 ± 2 °C, a relative humidity of 70 ± 10% and a photoperiod of 14 h light and 10 h dark. The experimental design was completely randomised, with 10 replications for each treatment. The mortality of the larval and pupal insects was evaluated.
2.10 Feeding deterrence on plants
The effect of feeding deterrence, besides being tested on the artificial diet, was also confirmed in host plants. The plant species used in the experiments (cotton, beans, corn, soybeans, sorghum and tomato) were selected due to the frequent occurrence of H. armigera in these crops in Brazil (Czepak et al., 2013; Specht et al., 2013; Pratissoli et al., 2015). The experiment was conducted in a BOD chamber adjusted to a temperature of 25 ± 2 °C, a relative humidity of 70 ± 10% and a photoperiod of 14 h light and 10 h dark. The arena was made up of a 10-cm diameter acrylic Petri dish. Each experimental unit consisted of an arena containing six discs of 1-cm diameter leaf, interspersed with discs treated and untreated with the extract. In each arena, there were discs of only one host plant. Prior to the experiment, the 10-day-old third instar larvae were kept 24 h without food. One larva was inserted into the centre of each arena, and after 3 h, the leaf discs were removed, and the leaf area was measured to record the consumption in a Lee Tools device, with an accuracy of 0.01 cm2. After measuring the leaf area, the discs were oven-dried at 60 °C for 48 h and weighed on a precision scale.
The procedure was repeated for each tested extract concentration (100, 500 and 1000 mg mL−1) and each host plant (cotton, beans, corn, soybeans, sorghum and tomato). The control used was DMSO, and for the positive control, we used flubendiamide insecticide at the manufacturer’s recommended dose of 0.35 µL mL−1. The experimental design was completely randomised, with 10 replications for each treatment.
The feeding deterrence index was calculated using the following equation (Simmonds et al., 1989):
Ac = control disc consumed area (mm2)
At = treated disc consumed area (mm2).
Feeding deterrence values above 0.50 and 0.90 are considered significant and highly significant, respectively (Simmonds et al., 1989).
2.11 Data analysis
Data on the feeding deterrence on artificial diets and on plants for H. armigera were submitted to the Kolmogorov and Bartlett tests to verify the normality of their residuals and the homogeneity of their variances, respectively. The data met the assumptions and were then submitted to analysis of variance (ANOVA). Treatment means were compared using Tukeýs test (P < 0.05). All analyses were conducted using the SAS software (SAS Institute, 2015).
3 Results
3.1 Chemical analysis
After obtaining TagCE through the leaf rinse process, its tagitinin C content was determined using the developed and validated reverse-phase HPLC analytical method, whose validation parameters are presented herein. In this sense, the linear calibration curve obtained by plotting the area ratio of tagitinin C to that of the internal standard (CA) against the standard concentrations, and a correlation coefficient of 0.9998 was achieved. The linear regression equation (y = 0.0070x + 0.0029) and the values of LOD (2.8670 µg·mL−1) and LOQ (8.6879 µg·mL−1) indicate that the developed method is highly sensitive and adequate to determine the tagitinin C content in TagCE. Fig. 2 displays a comparison between typical HPLC-DAD chromatograms obtained for the sample (TagCE plus CA) and for the analytical standard (tagitinin C plus CA).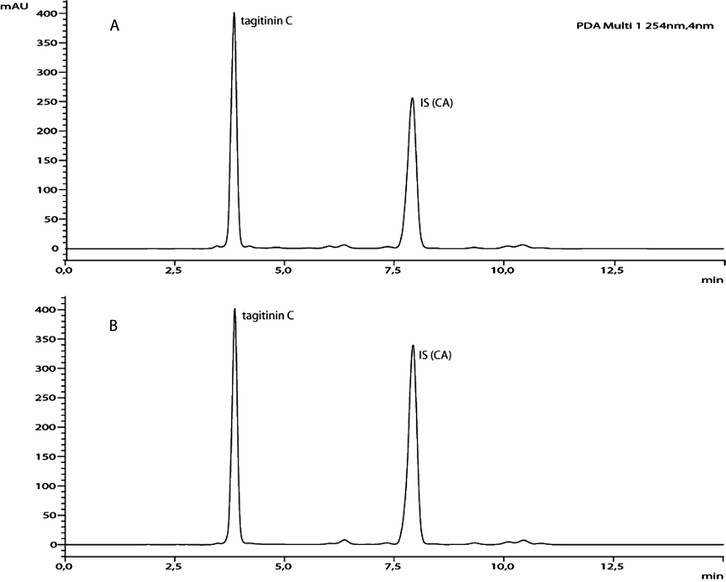
HPLC-DAD chromatograms showing (A) TagCE and p-coumaric acid (CA; internal standard) and (B) the analytical standard (tagitinin C) and p-coumaric acid (CA; internal standard) peaks, obtained in the established analytical conditions (isocratic mobile phase system of 45% acetonitrile in Milli Q water containing 0.1% acetic acid, mobile phase flow rate of 1 mL·min−1, column temperature of 40 °C, injected sample volume of 20 μL and detection at 254 nm).
Intra-day precision was determined by analysis of tagitinin C in six samples of TagCE, and inter-day precision was assayed on two consecutive days. The obtained concentrations were respectively 98.724 µg·mL−1 and 101.763 µg·mL−1, which corresponded to about 11.36% of tagitinin C content in TagCE, thus confirming the previously reported high contents of this compound in T. diversifolia and leading us to propose this compound as the chemical marker of TagCE (Ambrosio et al., 2008; Baruah et al. 1979; Orsomando et al., 2016; Sut et al., 2018). The RSD values for tagitinin C concentrations were bellow 5%, and the means obtained for different days did not differ statistically (p < 0.05). To determine the accuracy of the method and after the addition of known contents of tagitinin C (25, 50 and 100%), the RSD values for all concentrations obtained, in triplicate, were also below 5%, indicating that the results were adequate for the analytical purpose of the developed method. Moreover, the robustness of the method was assessed as previously described by Moreira et al. (2013) and satisfactorily met the requirements of the main Brazilian regulatory agency (Anvisa, 2003).
3.2 Feeding deterrence
In the feeding deterrence bioassay, spraying the extract on the artificial diet caused larval mortality of 50.0% and pupal mortality of 70.0 and 80.0% at concentrations of 500 and 1000 mg mL−1, respectively (Table 2). Positive control using flubendiamide insecticide showed 100.0% mortality of individuals tested within 24 h.
Concentration (mg mL−1)
Mortality (%)
Larval
Pupal
Control
0.0 ± 0.00 b1
0.0 ± 0.00 b
100
30.0 ± 0.42 a
50.0 ± 0.50 a
500
50.0 ± 0.50 a
70.0 ± 0.42 a
1000
50.0 ± 0.50 a
80.0 ± 0.32 a
The T. diversifolia extract showed feeding deterrence activity for H. armigera larvae in the six host plants studied. The concentrations of 500 and 1000 mg/mL presented higher levels of feeding deterrence, ranging from 0.5 to 1.0, and higher leaf dry mass due to the lower leaf consumption at these concentrations (Table 3).
Plant species
Common name
Concentration (mg mL−1)
Feeding deterrence index2
Leaf dry mass (mg)
Glycine max
Soybean
Control DMSO
0.0
0.040 b1
100
0.0
0.089 b
500
1.0
0.115 a
1000
1.0
0.119 a
Gossypium herbaceum
Cotton
Control DMSO
0.0
0.187 b
100
0.0
0.193 b
500
0.5
0.210 a
1000
1.0
0.215 a
Phaseolus vulgaris
Bean
Control DMSO
0.0
0.091 b
100
1.0
0.147 a
500
1.0
0.155 a
1000
1.0
0.152 a
Solanum lycopersicum
Tomato
Control DMSO
0.0
0.093 b
100
1.0
0.143 a
500
1.0
0.173 a
1000
1.0
0.190 a
Sorghum bicolor
Sorghum
Control DMSO
0.0
0.141 b
100
0.5
0.164 a
500
0.5
0.170 a
1000
1.0
0.193 a
Zea mays
Corn
Control DMSO
0.0
0.174 b
100
0.0
0.154 b
500
0.6
0.222 a
1000
1.0
0.210 a
4 Discussion
This is the first study that evaluated the feeding deterrence towards H. armigera by T. diversifolia extract. Dichloromethane rinse extracts of the leaves and inflorescences were chemically investigated, and 16 compounds were isolated and identified: 14 sesquiterpene lactones, one flavonoid and one diterpenoid (Ambrosio et al., 2008). We showed that tagitinin C is a main metabolite of the T. diversifolia extract obtained from foliar glandular trichomes. To determine if this compound is responsible for the observed antifeedant activity, HPLC qualitative analysis of the leaf trichomes extract and quantitative analysis of tagitinin C, the main metabolite, were performed.
The extract used in this study was obtained by leaf washing. We dipped the fresh, intact leaf into the organic solvent so that only the glandular trichomes were ruptured. Since these structures concentrate the defence substances (in this case of T. diversifolia, the sesquiterpene lactones), we actually lent the sesquiterpene lactones to the studied crop plants (cotton, beans, corn, soybeans, sorghum and tomato), which have no inherent defence at all.
The reported RP-HPLC-PDA analytical method allowed the identification and quantification of the major constituent of T. diversifolia in the rinse leaf extracts, showing that this compound is suitable to be considered as the chemical marker of TagCE. Also, the developed analytical method described herein is simple, reliable and should be used routinely in analyses of T. diversifolia rinse leaf extracts as well as in other studies, since it met the requirements of the Brazilian Regulatory Agency.
The results show high mortality, with up to 80% of larvae, and antifeedant activity for all plant species tested using T. diversifolia extract for H. armigera larvae. This suggests that H. armigera larvae avoid feeding when T. diversifolia extract is present in the host plants. Previous studies have shown that T. diversifoilia dichloromethane leaf rinse extract presented an antifeedant activity for other pest insects such as Chlosyne lacinia (Lepidoptera: Noctuidae) (Ambrosio et al., 2008). In addition, T. diversifolia leaf extract also showed high bioactivity on mortality of Callosobruchus maculatus (Coleoptera: Bruchidae) (Adedire and Akinneye, 2004).
Our results not only help in understanding that tagitinin C is a main compound present in the glandular trichomes from T. diversifolia leaves, but also clarify the mode of action of T. diversifolia extract. A previous study characterising the mode of action of sesquiterpene lactones on Trypanossoma cruzi showed that psilostachyin accomplished its antiparasitic effect by interacting with hemin, while psilostachyin C interfered with sterol synthesis, and both sesquiterpene lactones induced parasite death by apoptosis (Sülsen et al., 2016).
This opens up the possibility to use T. diversifolia extract as a potential botanical insecticide, mainly for plant-defoliating Lepidoptera. In Brazil, there are only five botanical pesticides available to use in crop protection. Most insecticides of botanical origin are extracts containing a group of active ingredients of diverse chemical synthetics and could be used as a good strategy in insect resistance management. Plant origin insecticides also have a great advantage by being compatible with other low-risk options which are acceptable for insect management, such as entomopathogenic fungi, predators and parasitoids, which greatly increase the probabilities of being integrated in IPM programs. Further studies should be performed to understand the relationships between the isolated compounds from T. diversifolia extract and the antifeedant activity for Lepidoptera pests.
Acknowledgements
The authors are grateful to the State of São Paulo Research Foundation (FAPESP) for financial support, grant number 18/25010-2, as well as to CAPES and CNPq for fellowships.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biological activity of tree marigold, Tithonia diversifolia, on cowpea seed bruchid, Callosobruchus maculatus (Coleoptera: Bruchidae) Ann. Appl. Biol.. 2004;144:185-189.
- [Google Scholar]
- Resistance status of Helicoverpa armigera Bt cotton in Pakistan. Transgen. Res.. 2019;28(2):119-212.
- [Google Scholar]
- Constituents of glandular trichomes of Tithonia diversifolia: relationships to herbivory and antifeedant activity. Phytochemistry. 2008;69:2052-2060.
- [Google Scholar]
- Natural enemy enhancement and botanical insecticide source a review of dual use companion plants. Appl. Entomol. Zool.. 2019;54(1):1-19.
- [Google Scholar]
- ANVISA, 2003. Guia para validação de métodos analíticos e bioanalíticos. In: ANVISA (Ed.). Diário Oficial da União, Brasília.
- Antifeedant activity of plant extracts against Spodoptera litura (Fabr.) (Lepidoptera: Noctuidae) Am. Eurasian J. Agric. Environ. Sci.. 2012;12(6):764-768.
- [Google Scholar]
- Toxicity of several δ-endotoxins of Bacillus thuringiensis against Helicoverpa armigera (Lepidoptera: Noctuidae) from Spain. J. Invertebr. Pathol.. 2005;90(1):51-54.
- [Google Scholar]
- Sesquiterpene lactones of Tithonia diversifolia. Stereochemistry of the tagitinins and related compounds. J. Org. Chem.. 1979;44(11):1831-1835.
- [Google Scholar]
- Baseline susceptibility of Helicoverpa armigera (Lepidoptera: Noctuidae) to indoxacarb, emamectin benzoate, and chlorantraniliprole in Australia. J. Econ. Entomol.. 2015;108(1):294-300.
- [Google Scholar]
- Pyrethroid and carbamate resistance in Australian Helicoverpa armigera (Lepidoptera: Noctuidae) from 2008 to 2015: what has changed since the introduction of Bt cotton? Bull. Entomol. Res.. 2018;108:781-791.
- [Google Scholar]
- The old world bollworm in the neotropical region: the experience of Brazilian growers with Helicoverpa armigera. Outlooks Pest Manage.. 2014;25(4):261-264.
- [Google Scholar]
- Effects of aqueous extracts from Amazon plants on Plutella xylostella (Lepidoptera: Plutellidae) and Brevicoryne brassicae (Homoptera: Aphididae) in laboratory, semifield, and field trials. J. Insect Sci.. 2019;9(5):1-9.
- [Google Scholar]
- First reported occurrence of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) in Brazil. Pesq. Agropec. Trop.. 2013;43(1):110-113.
- [Google Scholar]
- Feeding deterrence of cabbage looper (Lepidoptera: Noctuidae) by 1-allyloxy-4-propoxybenzene, alone and blended with neem extract. J. Econ. Entomol.. 2014;107(6):2119-2129.
- [Google Scholar]
- Field monitoring of Helicoverpa armigera (Lepidoptera: Noctuidae) Cry1Ac insecticidal protein resistance in China (2005–2017) Pest Manage. Sci.. 2019;75:753-759.
- [Google Scholar]
- Interaction of Bacillus thuringiensis toxins with larval midgut binding sites of Helicoverpa armigera (Lepidoptera: Noctuidae) Appl. Environ. Microbiol.. 2004;70(3):1378-1384.
- [Google Scholar]
- Invasion origin, rapid population expansion, and the lack of genetic structure of cotton bollworm (Helicoverpa armigera) in the Americas. Ecol. Evol.. 2019;9:7378-7401.
- [Google Scholar]
- Velvetbean cater- pillar: A rearing procedure and artificial medium. J. Econ. Entomol.. 1976;69:487-488.
- [Google Scholar]
- Intra-specific variation for host plant use in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae): implications for management. Crop Prot.. 2004;23:955-964.
- [Google Scholar]
- Movement ecology of pest Helicoverpa: implications for ongoing spread. Annu. Rev. Entomol.. 2019;64:277-295.
- [Google Scholar]
- Susceptibility of Brazilian populations of Helicoverpa armigera and Helicoverpa zea (Lepidoptera: Noctuidae) to Vip3Aa20. J. Econ. Entomol.. 2018;111(1):399-404.
- [Google Scholar]
- Tithonia diversifolia (Hemsl.) A. Gray as a medicinal plant: a comprehensive review of its ethnopharmacology, phytochemistry, pharmacotoxicology and clinical relevance. J. Ethnopharmacol.. 2018;28(220):94-116.
- [Google Scholar]
- RP-HPLC analysis of manool-rich Salvia officinalis extract and its antimicrobial activity against bacteria associated with dental caries. Braz. J. Pharmacog.. 2013;23:870-876.
- [Google Scholar]
- Ent-Kaurenoic acid-rich extract from Mikania glomerata: In vitro activity against bacteria responsible for dental caries. Fitoterapia. 2016;112:211-216.
- [Google Scholar]
- Mexican sunflower (tithonia diversifolia, Asteraceae) volatile oil as a selective inhibitor of Staphylococcus aureus nicotinate mononucleotide adenylyltrasferase (NadD) Ind. Crops Prod.. 2016;85(7):181-189.
- [Google Scholar]
- Separation of diterpenoid alkaloid mixtures using vacuum liquid chromatography. J. Nat. Prod.. 1986;49:892-900.
- [Google Scholar]
- Occurrence of Helicoverpa armigera (Lepidoptera: Noctuidae) on tomato in the Espírito Santo state. Hort. Bras.. 2015;33(1):101-105.
- [Google Scholar]
- SAS Institute Inc., 2015. SAS® University Edition: Installation Guide for Windows. Cary, NC: SAS Institute Inc.
- The antifeedant activity of clerodane diterpenoids from Teucrium. Phytochemistry. 1989;28:1069-1071.
- [Google Scholar]
- Initial experiments in high-performance liquid chromatographic method development I. Use of a starting gradient run. J. Chromatogr. A. 1996;721(1):3-14.
- [Google Scholar]
- Development and validation of a rapid RP-HPLC method for analysis of (-)-copalic acid in Copaiba oleoresin. Planta Med.. 2012;78(11):1255.
- [Google Scholar]
- Morphological and molecular identification of Helicoverpa armigera (Lepidoptera: Noctuidae) and expansion of its occurrence record in Brazil. Pesqu. Agropec. Bras.. 2013;48(6):689-692.
- [Google Scholar]
- Mode of action of the sesquiterpene lactones psolostachyin and psilostachuin C on Trypanossoma cryzi. PLoS ONE. 2016;11(3):e0150526.
- [Google Scholar]
- Identification of tagitinin C from Tithonia diversifolia as antitrypanosomal compound using bioactivity-guided fractionation. Fitoterapia. 2018;124:145-151.
- [Google Scholar]
- Guidance for robustness/ruggedness tests in method validation. J. Pharm. Biomed. Anal.. 2001;24(5–6):723-753.
- [Google Scholar]
- Current status of insecticide resistance in Helicoverpa armigera after 15 years of Bt cotton planting in China. J. Econ. Entomol.. 2013;106(1):375-381.
- [Google Scholar]







