Translate this page into:
Formulation of a novel anti-leukemia drug and evaluation of its therapeutic effects in comparison with Cytarabine
⁎Corresponding authors. anchangliu03@163.com (Anchang Liu), jichunyan@sdu.edu.cn (Chunyan Ji)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the recent research, we investigated the application of gold nanoparticles green-synthesized by Alhagi maurorum aqueous extract in the treatment of several types of leukemia, i.e. acute T cell leukemia, acute lymphoblastic leukemia, and acute myeloid leukemia. Different techniques such as transmission electron microscopy (TEM), fourier-transform infrared spectroscopy (FTIR), and ultraviolet–visible spectroscopy analysis were used to characterize AuNPs. The TEM images show a spherical morphology for AuNPs with the range size of 21 to 59 for the synthetic nanoparticles. In the antioxidant test, the IC50 of AuNPs and butylated hydroxytoluene (BHT) against DPPH free radicals were 117 and 87 µg/mL, respectively. In the oncological part of the recent study, the treated cells with AuNPs and Cytarabine were assessed by MTT assay for 48 h about the cytotoxicity and anti-leukemia properties on normal (HUVEC) and leukemia cell lines i.e., acute myeloid leukemia (Human HL-60/vcr and 32D-FLT3-ITD), acute lymphoblastic leukemia (MOLT-3 and TALL-104), and acute T cell leukemia (J.RT3-T3.5 and Jurkat, Clone E6-1). The IC50 of AuNPs were 242, 297, 383, 207, 234, and 218 µg/mL against acute myeloid leukemia (Human HL-60/vcr and 32D-FLT3-ITD), acute lymphoblastic leukemia (MOLT-3 and TALL-104), and acute T cell leukemia (J.RT3-T3.5 and Jurkat, Clone E6-1) cell lines, respectively. In addition, the IC50 of Cytarabine were 117, 113, 145, 119, 131, and 135 µg/mL against acute myeloid leukemia (Human HL-60/vcr and 32D-FLT3-ITD), acute lymphoblastic leukemia (MOLT-3 and TALL-104), and acute T cell leukemia (J.RT3-T3.5 and Jurkat, Clone E6-1) cell lines, respectively. The viability of malignant leukemia cell line reduced dose-dependently in the presence of AuNPs and Cytarabine.
Keywords
Anti-leukemia
Cytarabine
Alhagi maurorum
Gold nanoparticles
Green formulation
Chemotherapeutic drug
1 Introduction
Traditional medicine has a unique role in producing herbal therapeutic supplement and drug (Shaker et al., 2010). Alhagi maurorum is an herb with many pharmaceutical properties. It belongs to the Plantae kingdom, Fabales order, Fabaceae family, and Alhagi genus. The several parts of Alhagi maurorum and its products have been used in different world regions for the treatment of diseases such as several cancers, dropsy, rheumatism, asthma, bronchitis, loss of appetite, amenorrhoea, dysmenorrhoea, diarrhea, indigestion, skin eruptions, and worms (Atta and Mouneira, 2004; Laghari et al., 2011; Laghari et al., 2010). Since ancient times people have used Alhagi maurorum to cure many diseases related to the respiratory, liver function, cardiovascular, gastrointestinal, immune, and urinary and genital systems (Laghari et al., 2011; Laghari et al., 2010; Almeida et al., 2001). The chemical antioxidant composition isolated from Alhagi maurorum are as fallow: 2-nonadecanone, Nonacosane, actinidiolide, 6,10,14-trimethyl-2-pentadecanone, trans-β-ionone, pentacosane, furanacetic acid, 9-octylheptadecane, drimenol, squalene, tetracosane, docosane, eicosane, octadecane, and drimenol (Atta and Mouneira, 2004; Laghari et al., 2011; Laghari et al., 2010; Almeida et al., 2001; Abu-Dahab and Afifi, 2007). The therapeutic features of this plant due to its chemical antioxidant composition were mentioned above.
Nowadays, nanotechnology has been developed in several ways due to its wide range of applications. The applications in medical diagnosis, food, medicine, biotechnology, environment, energy, chemistry, physics, etc. have been considered, which introduces this technology as an interdisciplinary and cross-sectorial field (Zangeneh, 2019; Ahmeda et al., 2020). One of the fields of nanotechnology is the production of nanoparticles to prevent and treat various diseases (Zangeneh, 2020). Metallic nanoparticles have received a great attention due to their applications in different branches of technology including pharmaceuticals, electronics, photonics, sensing technologies, therapeutics and antimicrobial products (Zangeneh, 2019). Moreover, nanoparticles interact with plants causing different morphological and physiological modifications. Their effects are reported to be positive or negative depending on the physico-chemical, size, concentration, and composition characteristics of nanoparticles as well as plant species (Zangeneh, 2019; Ahmeda et al., 2020). The current control measures are focused mainly on inhibiting diffusion of disease to uninfected plants. Recent works are concentrated on the application of nanoparticles for controlling plant diseases (Zangeneh, 2019; Ahmeda et al., 2020; Zangeneh, 2020). Due to the recent advances in research on metallic nanoparticles, the gold nanoparticles are effective antifungal agents due to their powerful cytotoxic activity toward a broad range of microorganisms compared to other metals (Ahmeda et al., 2020; Zangeneh, 2020; Zangeneh et al., 2019a). Metallic nanoparticles made fastidious attention because of its wide applications in chemical, electrical, optical, bioremediation, sensor, and biological fields (Zangeneh, 2020; Zangeneh et al., 2019a; Zangeneh et al., 2019b; Zangeneh et al., 2019c). Several methods for the synthesis of metallic nanoparticles are under consideration. These include chemical and physical methods. However, they are expensive, time-consuming, and environmentally toxic. A green synthesis is proposed as a simple, cost-efficient and environment friendly method. The biological synthesis of nanoparticles uses different biomaterials such as bacteria, yeast, algae, fungi, and plants (Zangeneh et al., 2019a; Zangeneh et al., 2019b; Zangeneh et al., 2019c; Mahdavi et al., 2019). The use of plants attracts the attention of researchers as a simple biosynthesis process compared to other conventional methods. In addition, due to the high availability and low cost of the medicinal plants, the metallic nanoparticles green-synthesized using natural compounds significantly have increased (Zangeneh et al., 2019a; Zangeneh et al., 2019b; Zangeneh et al., 2019c). Metallic nanoparticles green-synthesized using medicinal plants have been abundantly used in the biomedical sciences for the treatment of many diseases. Every year, the notable applications of metallic nanoparticles green-synthesized are being gained and this trend is continuing (Mahdavi et al., 2019; Zangeneh et al., 2019d). The results of many studies have indicated the significant antifungal effects of metallic nanoparticles green-synthesized by plants in the cure of candida diseases and their antibacterial properties in the treatment of Streptococcus; Staphylococcus, Pseudomonas, Salmonella, and Bacillus infectious. Also, metallic nanoparticles synthesized by plants have been formulated due to the antiviral, antibacterial, antioxidant, anti-parasitic, anti-inflammatory, antifungal, wound healing, and anti-cancer properties (Mahdavi et al., 2019). The main therapeutic properties of metallic nanoparticles green synthesized by plants are anticancer effects (Zangeneh et al., 2019c; Mahdavi et al., 2019; Zangeneh et al., 2019d).
In the present study, we decided to investigate the anti-acute leukemia potentials of gold nanoparticles formulated by Alhagi maurorum against the leukemia cell lines i.e., acute myeloid leukemia (Human HL-60/vcr and 32D-FLT3-ITD), acute lymphoblastic leukemia (MOLT-3 and TALL-104), and acute T cell leukemia (J.RT3-T3.5 and Jurkat, Clone E6-1).
2 Materials and methods
2.1 Material
Dimethyl sulfoxide (DMSO), Antimycotic antibiotic solution, hydrolysate, decamplmaneh fetal bovine serum, Ehrlich solution, 4-(Dimethylamino)benzaldehyde, 2,2-diphenyl-1-picrylhydrazyl (DPPH), carbazole reagent, borax-sulphuric acid mixture, Dulbecco's Modified Eagle Medium (DMED), and phosphate buffer solution (PBS) all were achieved from Sigma-Aldrich company of USA.
2.2 Preparation and extraction of aqueous extract
First, the dried leaves of A. maurorum were grounded. Then, 100 g of the sample was macerated in 1000 mL of boiling water for 6 h. After that, the extract was filtrated and evaporated to concentrate. Finally, the extract was placed in a freeze drier for 72 h. The obtained extract as brown powder was kept in cold place.
2.3 Green synthesis of AuNPs
The green synthesis of the AuNPs was initiated with a reaction mixture of 100 mL of HAuCl4·H2O in the concentration of 1 × 10−3 M and 200 mL of aqueous extract solution of A. maurorum leaf (20 µg/mL) in the proportion 1:10 in a conical flask. The reaction mixture was kept under magnetic stirring for 12 h at room temperature. At the end of the reaction time, the dark red colored colloidal solution of Au was formed. The mixture was centrifuged at 10000 rpm for 15 min. The precipitate was triplet washed with water and centrifuged subsequently.
2.4 Determination of the antioxidant property of gold nanoparticles
At the beginning of the study, 100 mL of methanol (50 %) was added to the 39.4 g of DPPH. Also, several concentrations of AuNPs i.e., 0–1000 µg/mL were considered. The above DPPH was added to the various concentrations of AuNPs and all samples were transfer to an incubator at the temperature of 37 °C. After 30 min incubating, the absorbances were measured at 517 nm. In this study, methanol (50 %) and butylated hydroxytoluene (BHT) were negative and positive controls, respectively. Acceding to the following formula, the antioxidant properties of AuNPs were determined in detail (Ahmeda et al., 2020):
2.5 Determination of anti-acute leukemia effects of gold nanoparticles
In this research, the following cell lines have been used for investing the cytotoxicity and anti-lung carcinoma effects of the AuNPs using the common cytotoxicity test i.e., MTT assay:
-
Leukemia cell lines: Acute myeloid leukemia (Human HL-60/vcr and 32D-FLT3-ITD), acute lymphoblastic leukemia (MOLT-3 and TALL-104), and acute T cell leukemia (J.RT3-T3.5 and Jurkat, Clone E6-1)
-
Normal cell line: HUVEC.
The cells were in RPMI1640 liquid culture medium containing 2 mM glutamine, 10% inactivated bovine fetal serum (FBS), solution of streptomycin (100 μg/ml) and penicillin (100 units per ml) at 37 °C and 5% CO2 and 95% moisture was cultured and passaged so that the cells reached the desired number in terms of morphology and number (after 3–4 passages). After separating the cells from the flask surface, trypsin-EDTA (Gibco BRL, Scotland) counted and evaluated cell viability, and 3000 cells were cultured in 96-well wells without or with AuNPs. Morphological changes and general characteristics of the cell were assessed 24 h using an inverted microscope (Motic, AE31 model, China) (Ahmeda et al., 2020).
The effect of cytotoxicity of the AuNPs was evaluated by MTT Methy Thiazol Tetrazolium colorimetric test. MTT (Sigma-Aldrich, USA) is a tetrazolium salt based on the function of living cell mitochondrial dehydrogenase succinate enzyme, which converts the yellow solution of MTT to insoluble purple crystals of formazan that is soluble in dimethyl sulfoxide (Ahmeda et al., 2020).
The cells began to grow in 75 cm square T-flasks in 15 mL of medium with 106 cells initial number. After 72 h and covering the bottom of the flask with cell, the cell layer adhering to the bottom of the flask was separated enzymatically using trypsin-Versen and transferred to a sterile test tube was centrifuged at 1200 rpm for 10 min (Germany). Sigma, 3–30 k model). The cells were then resuspended in new culture medium with a pasteurizer pipette and 100 μl of cell suspension without or with AuNPs was added to each well of the 96-well plate so that there were 3000 cells in each well. The plates were incubated for 24 h in an incubator (Germany, Memmert) to return the cells to normal from the stress of trypsinization. Then, suitable dilutions of the desired AuNPs were prepared and 100 μl of each dilution was added columnar to the plate wells and the cells were incubated for 37 h at 37 °C and 5% CO2. After 72 h of adding the AuNPs to the cells, 20 μl of 5 mg/ml MTT solution was added to each well. The plates were incubated for 4 h after the required time, the culture medium containing MTT was carefully removed and 100 μl of DMSO was added to each well to dissolve the resulting formazan. After 10 min and shaking the plates using the plate shaker, the light absorption at 570 nm was read by ELISA Reader (Awareness Technology Inc, Stat Fax 2100, USA). Cells containing no AuNPs were considered as controlled light density and wells without cells and only RPMI1640 medium with bovine fetal serum were considered as blank (Ahmeda et al., 2020).
Finally, linear regression was performed to obtain the IC50 level, which represents the concentration of the extract, which causes 50% inhibition of cancer cell growth (Ahmeda et al., 2020):
2.6 Statistical analysis
The obtained results were fed into SPSS-22 software and analyzed by one-way ANOVA, followed by Duncan post-hoc test (p ≤ 0.01).
3 Results and discussion
3.1 Chemical characterization of AuNPs
UV–Vis spectroscopic analysis: It showed the presence of an absorption peak at 533 nm which confirmed the formation of the gold nanoparticles (Fig. 1).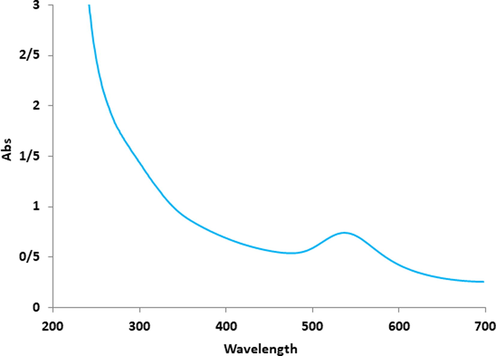
UV–Vis analysis of AuNPs.
In agreement with our study, Shahriari et al. (Shahriari et al., 2019) reported Allium noeanum Reut. ex Regel aqueous extract synthesized gold nanoparticles with a peak at 542 nm in the UV–Visible spectrum (Shahriari et al., 2019). Zhaleh et al. (Zhaleh et al., 2019) reported the absorbance at 528 nm for gold nanoparticles synthesized by Gundelia tournefortii L. (Zhaleh et al., 2019). Zangneh et al. (2019) studied Falcaria vulgaris aqueous extracts mediated synthesis of gold nanoparticles. Absorption in the spectrum was noted in the wavelength of 535 nm (Zangeneh and Zangeneh, 2019). Hemmati et al. (Hemmati et al., 2019) reported Thymus vulgaris leaf aqueous extract mediated gold nanoparticles and absorption peak was observed at 532 nm (Hemmati et al., 2019). These reports support the results of the current work.
TEM analysis: TEM is the other test for determining the morphology and size of metallic nanoparticles. In our study, the range size of the nanoparticles (21–59 nm) calculated through TEM images (Fig. 2).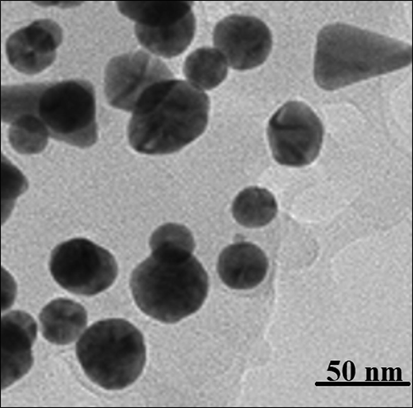
TEM image of AuNPs.
Furthermore, the histogram plot from the TEM image showed the particle size distribution of biosynthesized gold nanoparticles ranges of 18 to 62 nm. In the previous studies, the size of gold nanoparticles formulated by aqueous extract of medicinal plants had been calculated in the ranges of 10–100 nm with the shape of spherical (Shahriari et al., 2019; Zhaleh et al., 2019; Zangeneh and Zangeneh, 2019; Hemmati et al., 2019). These reports support the results of the current work.
FT-IR analysis: Fig. 3 exhibits the spectra of AuNPs. The peaks at 478 and 529 cm−1 belong to the bending vibration of Au-O. These peaks for Au nanoparticles have been reported previously with a small difference in the wavenumber (Shahriari et al., 2019; Zhaleh et al., 2019; Zangeneh and Zangeneh, 2019; Hemmati et al., 2019). FT-IR technique is an acceptable method to evaluate the secondary plant metabolites as the capping and reducing agents of gold chloride precursor to AuNPs. FT-IR spectrum of AuNPs shows the presence of different-IR bands that correlate to the presence of various functional groups in A. maurorum extract that covers the AuNPs. A broad peak around 3349 relates to O-H, peak at 2955 cm-1shows an aliphatic C-H stretching band. These peaks can be considered for some phytochemical compounds found in A. maurorum extract such as phenolic, flavonoid, triterpenes (Katata-Seru et al., 2018).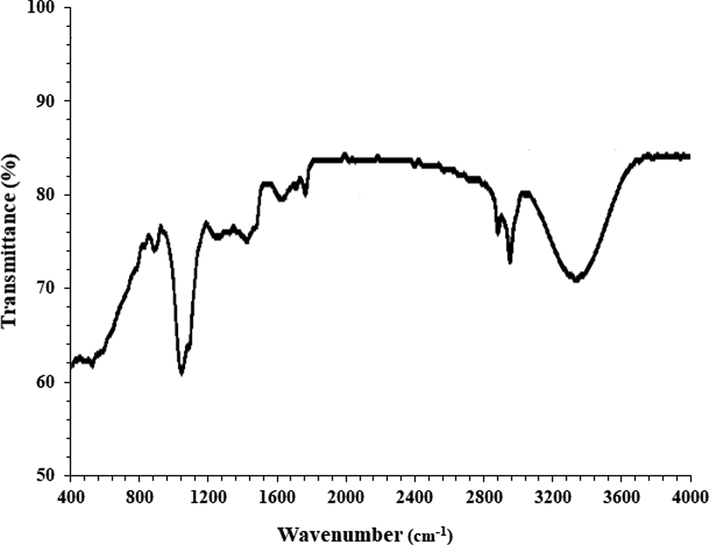
FT-IR analysis of AuNPs.
3.2 Antioxidant properties of gold nanoparticles synthesized using A. maurorum leaf aqueous extract
In recent years, researchers evaluated plants and bio mediated synthesized nanoparticles for antioxidant activity. The green-synthesized gold nanoparticles exhibit higher antioxidant activity for the formation of free radicals into the living system (Cheirmadurai et al., 2014; Gultekin et al., 2016; Rehana et al., 2017). The gold nanoparticles have redox properties and play a significant role in deactivating free radicals in the living system (Rehana et al., 2017).
For determining of antioxidant properties of several materials such as medicinal plants and metallic nanoparticles green-synthesized by medicinal plants the free radicals are used that the most main of them is DPPH. In the high antioxidant capacities of several materials, the color of the DPPH molecules changes from violet to the pale yellow or colorless (Zangeneh et al., 2019d). In our study, the antioxidant effects of the gold nanoparticles synthesized using plant leaf aqueous extract were evaluated by DPPH assay revealed concentration-dependent effects i.e., an increase in the concentration of the gold nanoparticles leads to an increase in antioxidant activities. In the concentrations of studied, the best result was seen in the high concentration or 1000 µg/mL (Fig. 4).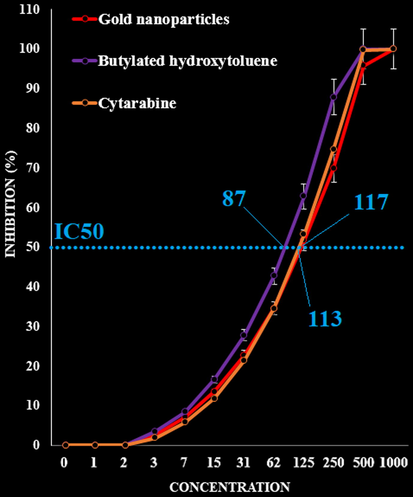
The antioxidant activities of Cytarabine, gold nanoparticles and BHT against DPPH.
Comparative analysis of the individual antioxidant assays showed significant variations in the exertion of radical scavenging effects. Standard (butylated hydroxytoluene) demonstrated similar antioxidant effects compared to the gold nanoparticles. The exact IC50 of Cytarabine, gold nanoparticles and BHT were 113, 117, and 87 µg/mL, respectively (Fig. 4).
The reason behind the antioxidant activity of green or biosynthesized nanoparticles could be due to the presence of metabolites compounds (Gultekin et al., 2016; Rehana et al., 2017; Del Mar Delgado-Povedano et al., 2016; Jeong et al., 2012; Sankar et al., 2014). Also, many researchers reported phenolic and flavonoids attached to the nanoparticles exhibited the antioxidant activity. Previously it has been indicated that A. maurorum leaf is rich in antioxidant compounds such as 2-nonadecanone, Nonacosane, actinidiolide, 6,10,14-trimethyl-2-pentadecanone, trans-β-ionone, pentacosane, furanacetic acid, 9-octylheptadecane, drimenol, squalene, tetracosane, docosane, eicosane, octadecane, and drimenol (Shaker et al., 2785). Several studies were carried out in the nanotechnology field using various medicinal plants, but still, no report is available on gold nanoparticles synthesized using A. maurorum leaf aqueous extract.
3.3 Cytotoxicity and anti-human lung cancer potentials of gold nanoparticles synthesized using A. maurorum leaf aqueous extract
The morphological parameters of gold nanoparticles which affect anticancer properties of these nanoparticles against several cancer cell lines are size, form, and surface coating. Among the above parameters, the role of size of gold nanoparticles is the most (Sankar et al., 2014; Suman et al., 2013; Jacob et al., 2012; Vivek et al., 2012; Namvar et al., 2014). Previously, it was showed whatever the size of gold nanoparticles reduced, the ability of these nanoparticles for transferring to the cancer cell lines and killing them increased (Namvar et al., 2014).
As can be observed in the TEM image of our study, the sizes of gold nanoparticles synthesized by A. maurorum leaf aqueous extract are at the ranges of 21 to 59 nm. In the similar study, it has been indicated that gold nanoparticles at the size of 100 nm and lower have significant roles in the removing tumor cell lines (Gultekin et al., 2016; Rehana et al., 2017; Del Mar Delgado-Povedano et al., 2016; Jeong et al., 2012; Sankar et al., 2014; Suman et al., 2013; Jacob et al., 2012).
The anticancer effects of gold nanoparticles green-synthesized by medicinal plants have been confirmed in the previous studies (Suman et al., 2013; Jacob et al., 2012; Vivek et al., 2012). In the study of Suman et al. (Suman et al., 2013) was clarified the anti-cervix cancer effects of metallic nanoparticles containing natural compound (Morinda citrifolia) against HeLa cell line. In the previous study, the metallic nanoparticles killed all HeLa cells in high doses (Suman et al., 2013). In another study, the anti-liver cancer properties of metallic nanoparticles containing Piper longum leaf against Hep-2 cell lines were proved (Jacob et al., 2012). In the previous study has been indicated that metallic nanoparticles green-synthesized by Annona quamosal leaf have excellent anti-breast cancer potentials against MCF-7 cell line (Vivek et al., 2012).
In the recent research, the treated cells with several concentrations of the present Cytarabine, A. maurorum leaf aqueous extract, and gold nanoparticles were examined by MTT test for 48 h regarding the cytotoxicity properties on normal (HUVEC) and human acute leukemia cell lines. The absorbance rate was determined at 570 nm, which indicated extraordinary viability on normal cell line (HUVEC) even up to 1000 μg/mL for Cytarabine, A. maurorum leaf aqueous extract, and gold nanoparticles.
The IC50 of AuNPs were 242, 297, 383, 207, 234, and 218 µg/mL against acute myeloid leukemia (Human HL-60/vcr and 32D-FLT3-ITD), acute lymphoblastic leukemia (MOLT-3 and TALL-104), and acute T cell leukemia (J.RT3-T3.5 and Jurkat, Clone E6-1) cell lines, respectively. In addition, the IC50 of Cytarabine were 117, 113, 145, 119, 131, and 135 µg/mL against acute myeloid leukemia (Human HL-60/vcr and 32D-FLT3-ITD), acute lymphoblastic leukemia (MOLT-3 and TALL-104), and acute T cell leukemia (J.RT3-T3.5 and Jurkat, Clone E6-1) cell lines, respectively (Figs. 5–8).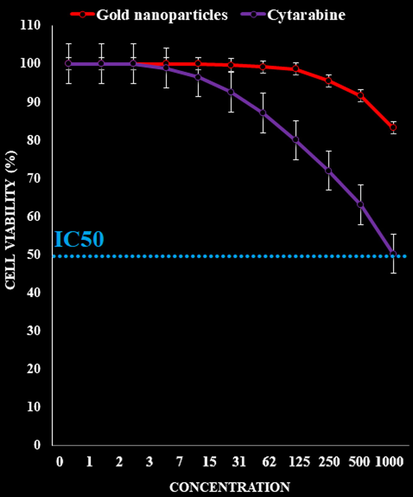
The cytotoxicity effects of Cytarabine and gold nanoparticles against normal (HUVEC) cell line.
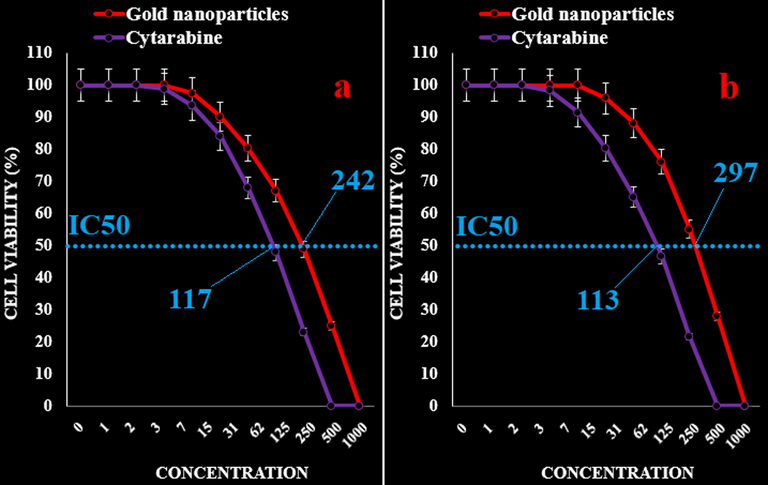
The anti-acute leukemia activities of Cytarabine and gold nanoparticles against acute myeloid leukemia (Human HL-60/vcr (a) and 32D-FLT3-ITD (b)) cell lines.
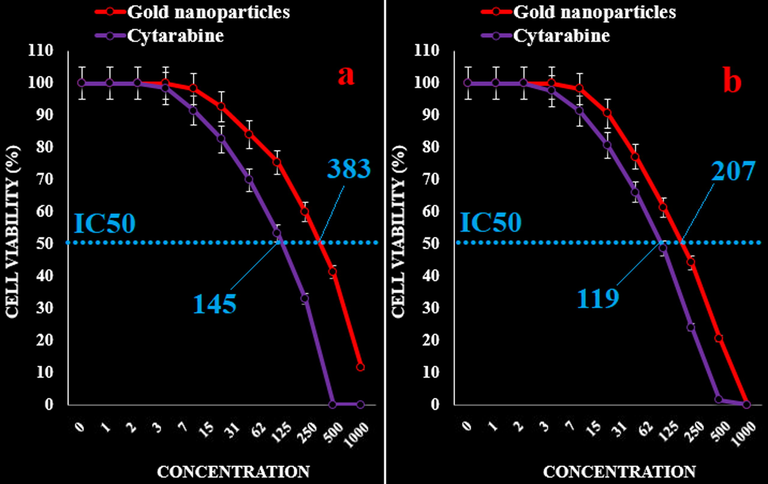
The anti-acute leukemia activities of Cytarabine and gold nanoparticles against acute lymphoblastic leukemia (MOLT-3 (a) and TALL-104 (b)) cell lines.
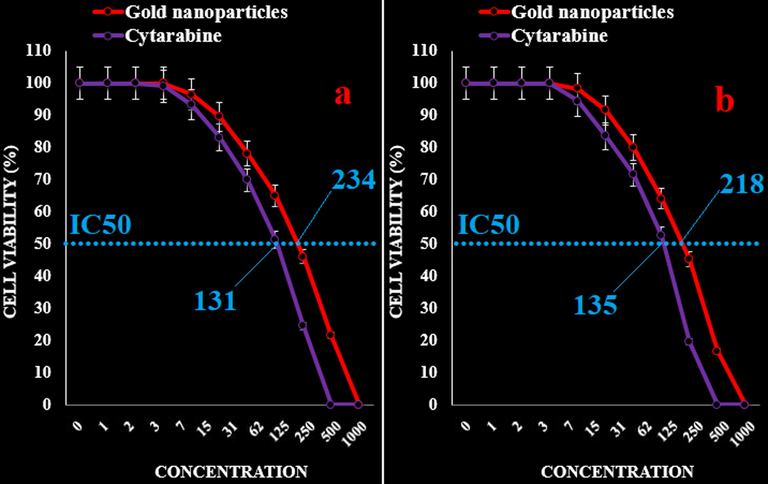
The anti-acute leukemia activities of Cytarabine and gold nanoparticles against acute T cell leukemia (J.RT3-T3.5 (a) and Jurkat, Clone E6-1 (b)) cell lines.
Likely the significant anti-acute leukemia potentials of gold nanoparticles synthesized by A. maurorum leaf aqueous extract are linked to their antioxidant activities. The similar researches have revealed the antioxidant materials such as metallic nanoparticles especially gold nanoparticles and ethno medicinal plants reduce the volume of tumors by removing free radicals (Katata-Seru et al., 2018).
The high presence of free radicals in the normal cells make many mutation in their DNA and RNA, destroy their gene expression and then accelerate the proliferation and growth of abnormal cells or cancerous cells (Sangami and Manu, 2017; Beheshtkhoo et al., 2018). The free radicals high presences in all cancers such as breast, gallbladder, stomach, rectal, liver, gastrointestinal stromal, esophageal, bile duct, small intestine, pancreatic, colon, parathyroid, thyroid, bladder, prostate, testicular, fallopian tube, vaginal, ovarian, hypopharyngeal, throat, lung, and skin cancers indicate significant role of these molecules in making angiogenesis and tumorigenesis (Beheshtkhoo et al., 2018; Radini et al., 2018). Many researchers reported that gold nanoparticles synthesized by ethno medicinal plants have remarkable role in the removing free radicals and growth inhibition of all cancerous cells (Radini et al., 2018; Oganesvan et al., 1991).
4 Conclusion
In summarize the gold nanoparticles were green-synthesized using an aqueous extract of Alhagi maurorum. The AuNPs was characterized using common chemical techniques such as UV–Visible, FT-IR, and TEM. The results approved the synthesis of nanoparticles in form of Au nanoparticles with a spherical morphology. The size of AuNPs was in the range of 21 to 59 nm which is well known as an efficient size for the synthetic nanoparticles. The IC50 of AuNPs were 242, 297, 383, 207, 234, and 218 µg/mL against acute myeloid leukemia (Human HL-60/vcr and 32D-FLT3-ITD), acute lymphoblastic leukemia (MOLT-3 and TALL-104), and acute T cell leukemia (J.RT3-T3.5 and Jurkat, Clone E6-1) cell lines, respectively. In addition, the IC50 of Cytarabine were 117, 113, 145, 119, 131, and 135 µg/mL against acute myeloid leukemia (Human HL-60/vcr and 32D-FLT3-ITD), acute lymphoblastic leukemia (MOLT-3 and TALL-104), and acute T cell leukemia (J.RT3-T3.5 and Jurkat, Clone E6-1) cell lines, respectively. It appears these nanoparticles may be administrated as a chemotherapeutic drug.
Funding
The National Natural Science Foundation of China (grant no. 81803635).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abu-Dahab, R., Afifi, F., 2007. Sci. Pharm., 75, 121.
- Appl. Organometal. Chem.. 2020;34:e5378.
- Almeida, R.N., Navarro, D.S., Barbosa-Filho, J.M., 2001. Phytomedicine, 8, 310.
- Atta, A.H., Mouneira, S.M., 2004. J. Ethnopharmacol., 92, 303.
- Appl. Phys. A. 2018;124:363-369.
- Green synthesis of copper nanoparticles and conducting nanobiocomposites using plant and animal sources. RSC Adv.. 2014;4:19507-19511.
- [Google Scholar]
- Del Mar Delgado-Povedano, M., De Medina, V.S., Bautista, J., Priego-Capote, F., De Castro, M.D., 2016. J. Function Foods. 24, 403-19.
- J. Turk. Chem. Soc. A: Chem.. 2016;3:623-636.
- Appl. Organometal. Chem.. 2019;33:e5267.
- [CrossRef]
- Colloids Surf. B Biointerfaces. 2012;91:212-214.
- J. Med. Food. 2012;1:58-65.
- J. Mol. Liq.. 2018;256:296-304.
- Laghari, A.H., Memon, S., Nelofar, A., Khan, K.M., Yasmin, A., Syed, M.N., Aman, A., 2010. J. Mol. Struct., 965, 65.
- Laghari, A.H., Memon, S., Nelofar, A., Khan, K.M., Yasmin, A., 2011. Ind. Crops. Prod., 34, 1141.
- Appl. Organometal. Chem.. 2019;33:e5248.
- [CrossRef]
- Int. J. Nanomed.. 2014;19:2479-2488.
- Chem. Nat.. 1991;27:247.
- J. Photochem. Photobiol., B. 2018;183:154-163.
- Biomed. Pharmacother.. 2017;89:1067-1077.
- Environ. Technol. Innov.. 2017;8:150-163.
- Mater. Sci. Eng. C. 2014;44:234-239.
- Appl. Organometal. Chem.. 2019;33:e5189.
- [CrossRef]
- E. Shaker, H. Mahmoud, and S. Mnaa, Food Chem. Toxicol., 48, 2785 (2010).
- Colloids Surf. B Biointerfaces. 2013;106:74-78.
- Process Biochem.. 2012;47:2405-2410.
- Appl. Organometal. Chem.. 2019;33:e4963.
- Appl. Organometal. Chem.. 2020;34:e5295.
- Appl. Organometal. Chem.. 2019;33:e4961.
- Appl. Organometal. Chem.. 2019;33:e5216.
- [CrossRef]
- Appl. Organometal. Chem.. 2019;33:e5290.
- [CrossRef]
- Appl. Organometal. Chem.. 2019;33:e5247.
- [CrossRef]
- Appl. Organometal. Chem.. 2019;33:e5246.
- [CrossRef]
- Appl. Organometal. Chem.. 2019;33:e5015.







