Translate this page into:
Fractionation and purification of antioxidant peptides from Chinese sturgeon (Acipenser sinensis) protein hydrolysates prepared using papain and alcalase 2.4L
⁎Corresponding authors. anwarnoman15@yahoo.com (Anwar Noman), wangyx5166@163.com (Yuxia Wang), zhangch8619@163.com (Chao Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Protein hydrolysates have the potential to be natural and safer sources of bioactive peptides. In this study, two proteases were used to hydrolyze Chinese sturgeon (Acipenser sinensis) protein, and the hydrolysates were then purified to yield antioxidant peptides. The degree of hydrolysis of 23.56 % and 18.14 % was obtained using papain and alcalase 2.4L, respectivly, and hydrolysates had 96.80 % and 87.24 % total amino acid content, respectivly. The papain hydrolysate (PH) and alcalase 2.4L hydrolysate (AH) showed good antioxidant activity against DPPH• (IC50 of 3.64 and 3.15 mg/mL) and ABTS•+ (IC50 of 1.92 and 1.58 mg/mL), respectively. The low-molecular-weight (<1000 Da) fraction of both hydrolysates demonstrated the highest antiradical activity (IC50 of 2.59 and 2.31 mg/mL, DPPH) and (IC50 of 1.54 and 1.36 mg/mL, ABTS), respectively. Nine peptides were separated from both hydrolysates using reverse phase high performance liquid chromatography (RP-HPLC). The IC50 for ABTS•+ scavenging activity of peptide P5 with valine, glycine and asparagine (MW of 282.13 Da) from PH, and peptide P3 with histidine, glycine and alanine (MW of 302.74 Da) from AH was 0.89 and 0.72 mg/mL, respectively. The fractions and purified peptides obtained from Chinese sturgeon hydrolysates could be utilized as natural antioxidant substitutes in pharmaceuticals and food products.
Keywords
Acipenser sinensis
Hydrolysate
Ultrafiltration
Peptides purification
Antiradical activity
- FPH
-
fish protein hydrolysate
- RP-HPLC
-
reverse phase high performance liquid chromatography
- DPPH
-
1,1-diphenyl-2-picrylhydrazyl
- ABTS
-
2,2-azino-bis(3-ehtylbenzothiazoline-6-sulfonic acid)
- PH
-
papain hydrolysate
- AH
-
alcalase 2.4L hydrolysate
- DH
-
degree of hydrolysis
- AN
-
α-amino nitrogen
- TN
-
total nitrogen
- TAA
-
total amino acids
- BHT
-
butylated hydroxytoluene
- MWCO
-
molecular weight cut-off
- MW
-
molecular weight
- Da
-
dalton
- TFA
-
trifluoroacetic acid
- P
-
peptide
Abbreviations
1 Introduction
Humans generate extremely reactive free radicals and oxygen species from a variety of sources, especially oxidative metabolic pathways involving many enzymes and hormones. These naturally occurring oxidants have the ability to oxidize DNA, polyunsaturated fatty acids, proteins, and nucleic acids. The human body is subjected to oxidative stress due to elevated levels of free radicals, which damages the internal redox balance and contributes to many chronic disorders such as atherosclerosis, cardiovascular disorders, neurological diseases, cancer, and aging-related illnesses (Cai et al., 2015; Zhang et al., 2021). Furthermore, free radicals in foods can cause lipid oxidation, as well as the formation of toxic substances and unpleasant flavors and odors (Jang et al., 2016). Recently, there has been a surge in interest in discovering natural and safe antioxidant sources such as fish protein hydrolysates (FPH), which have remarkable potential because of the presence of unique antioxidant peptides (Pan et al., 2020).
The primary method for obtaining protein hydrolysate is enzymatic hydrolysis, which employs a variety of enzymes (Chalamaiah et al., 2015; Noman et al., 2018). Peptides found in protein hydrolysates produced via the enzymatic hydrolysis are a heterogeneous group that affects the biological properties of peptides based on their molecular weights (Chalamaiah et al., 2013), with the majority of these peptides containing between 3 and 20 amino acid residues (Chalamaiah et al., 2019). Peptides derived from hydrolysates of various fish parts have recently been shown in numerous studies to have high antioxidant properties (Cai et al., 2015; Zhang et al., 2017; Wang et al., 2021). Peptides obtained from enzymatically hydrolyzed protein have properties that make them beneficial in the treatment and prevention of certain diseases caused by the negative effects of free radicals, as well as inhibiting or preventing the oxidation process of food, thereby extending its shelf life (Noman et al., 2022).
One of the world's biggest freshwater fish is Chinese sturgeon (Acipeneser sinensis) (Yi et al., 2016). China has been known for massive commercial aquaculture of various sturgeon species over the last two decades (Abraha et al., 2018), with an annual production of these fish of about 77 % according to global update 2017 (Bronzi et al., 2019). Sturgeons have a high protein content of up to 21 % (wet weight basis) (Abraha et al., 2018). In the study of Noman et al. (2020), they demonstrated that protein hydrolysates produced via the enzymatic hydrolysis procedure from Chinese sturgeon have high antioxidant properties.
In several investigations, antioxidant peptides were fractionated and purified from fish and its byproduct hydrolysates using ultrafiltration and chromatography techniques (Fan et al., 2012; Chi et al., 2015; Wang et al., 2019). Due to its excellent sensitivity, speed, and reproducibility, the reverse phase high performance liquid chromatography (RP-HPLC) technique is commonly used to isolate and purify bioactive peptides of various sizes (Zhang et al., 2017).
To that end, the goals of this study were to isolate a series of antioxidant peptides from the Chinese sturgeon (Acipenser sinensis) protein hydrolysates prepared using enzymatic hydrolysis and investigate their antioxidant capacities using in vitro assays. These peptides were further purified using ultrafiltration membranes and RP-HPLC.
2 Material and methods
2.1 Materials
Hua Da Aquatic Products Science and Technology Industry Co., ltd. supplied raw sturgeon samples (Acipenser sinensis) (10 Kg). Papain (EA/1000 casein, pH 6.0 and 40 °C: ≥6000/(USP-U/mg)), and alcalase 2.4L (2.4 AU-A/g) were provided by Ourchem Co., ltd, Guangdong, China, and Nanjing Chengna Chemical Co., ltd. China, respectively. The enzymes were kept in the fridge at 4 °C. Bomei Biotechnology Co., ltd (Hefei, China) provided 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical. Sigma Chemical Co. (Shanghai, China) provided 2,2-azino-bis (3-ehtylbenzothiazoline-6-sulfonic acid) (ABTS). The standard amino acids were obtained from Sigma Chemical Company (Shanghai, China). In this study, chemicals and solvents of high purity and analytical quality were used.
2.2 Sturgeon protein hydrolysates preparation
To obtain enzymatically hydrolyzed protein from A. sinensis, the optimal conditions of the enzymolysis process using papain (solid/liquid (S/L) ratio of 1:1(w/v), enzyme/substrate (E/S) ratio of 3 % (w/w), temperature of 70 °C and pH 6)), were chosen according to findings of Noman et al. (2018), while the optimal conditions for the alcalase 2.4L (S/L of 1:1(w/v), E/S of 3.5 %, %, temperature of 55 °C, and pH of 8.5)) were selected in accordance with Noman et al. (2019). The prepared sturgeon muscle samples were enzymatically hydrolyzed for 6 h in 200 mL jacketed glass vials using a magnetic stirrer that was continuously attached to a water bath (Shanghai Blue pard Yiheng Technical Co., ltd, Shanghai, China) by tubes to maintain the optimum temperature during the hydrolysis period. Enzyme activity was suppressed after the indicated incubation time by indirect heating for 20 min at 90 °C, followed by cooling to 25 ± 2 °C. The supernatant was separated by centrifugation at 13,440xg and 4 °C for 20 min, followed by freeze-drying at − 50 °C (SCIENTZ-10ND, Ningbo SCIENTZ Biotechnology Co., ltd., Ningbo, China) with a vacuum of 0.3 mbar to obtain the papain hydrolysate (PH) and alcalase 2.4L hydrolysate (AH), which were stored in airtight bags at − 20 °C.
2.3 Degree of hydrolysis (DH) measurement
The α-amino nitrogen (AN) content under enzymolysis conditions applied in this study was identified using a modified version of Taylor's (1957) formal titration procedure. Using deionized water, 1.5 g of the homogeneous hydrolyzed mixture was weighted up to 50 g. The pH of the mixture was raised to 7.0 by adding 0.1 N solution of sodium hydroxide (NaOH). 10 mL of formaldehyde solution (38 %, v/v) was neatly added and kept at 25 ± 2 °C for 5 min before continuing the titration with the same standard NaOH solution to pH 8.5. In accordance with AOAC standards (AOAC, 1998), the Kjeldahl procedure was used to determine total nitrogen (TN) in samples of Chinese sturgeon muscle; the following formulas were applied to determine AN and DH:
2.4 Composition analysis of amino acids
To determine the amino acid profile, the method described by Noman et al. (2019) was used. Carefully, 100 mg of the freeze-dried hydrolysate or 0.5 mg of the most active pure peptides were separately digested in HCl solution (6 M) at 120 °C for 22 h. After allowing the digested samples to cool to room temperature, 4.8 mL of NaOH (10 N) was added. Deionized water was used to dilute the new combination to a volume of 25 mL. Before centrifugation at 7280xg for 10 min, the mixture of digested sample and water was distilled using two layers of filter paper (No. 40). For amino acid analysis, 1 µL of the pre-prepared sample was placed on Zorbax, 80 A/C8 Column (250 × 4.0 mm, 5 μm particle size; Agilent, USA) using RP-HPLC (Agilent 1100; Agilent ltd., Palo Alto, CA, USA) with detection at 338 nm and 40 °C. The mobile phase A in this procedure was prepared using a mixing ratio of 500:0.12:2.5 (v/v/v) of sodium acetate (7.35 mM)/triethylamine/tetrahydrofuran, respectively, and its pH was set to 7.5 by acetic acid. Whereas the mobile phase B (pH 7.2) was prepared with a mixing ratio of 1:2:2, v/v/v of sodium acetate (7.35 mM)/methanol/acetonitrile, respectively. The amino acid composition of PH, AH, and their purified peptides was identified using standard amino acid retention time, and the results were reported as g per 100 g of protein or a percentage of the total amino acids (TAA), respectively.
2.5 Antioxidant activities estimation
2.5.1 DPPH• scavenging activity
The DPPH• scavenging activity of lyophilized PH and AH and their fractions was estimated using the method described by Jemil et al. (2014) with minor modifications. Protein hydrolysate solution (2 mL; 1, 2, 3, 4, and 5 mg/mL) and ultrafiltration fractions (1 mL; 1, 2, 3, 4, and 5 mg/mL) were added separately to 95 % ethanol-based DPPH• solution (3 mL, 0.1 mM).These combinations were shaken and maintained for 30 min in the dark at ambient temperature. The decrease in DPPH• was detected at 517 nm using UV-spectrophotometer (Mapada Instruments Co., ltd., Shanghai, China). As a control sample, deionized water was utilized in place of the sturgeon hydrolysate samples. According to hydrolysate concentration (mg/mL) that achieves 50 % inhibition of DPPH, IC50 was calculated according to the antioxidant activity of samples. Butylated hydroxytoluene (BHT) was utilized as a positive control. To identify the DPPH• scavenging activity, the following equation was used:
2.5.2 ABTS•+ scavenging activity
The ABTS•+ scavenging activity of lyophilized PH and AH, their fractions and purified peptides was evaluated using a modified procedure reported by Cai et al. (2015). The ABTS•+ working solution (7.4 mM) and potassium persulfate (K2S2O8) solution (2.6 mM) were prepared, and equal volumes were mixed to prepare the working solution, which was then allowed to react in a dark place at 25 ± 2 °C for nearly 16 h. Before measuring the activity of the samples, the absorbance of this solution was adjusted to 0.70 ± 0.02 at 734 nm by diluting 1 mL in 50 mL ethanol (95 %). To estimate the antiradical activity, 2 mL of hydrolysates solution, 1 mL of peptide factions or 200 µL purified peptides solution (1–5 mg/mL) were combined with 3.5 mL of ABTS•+ working solution and kept in the absence of light at ambient temperature for 10 min. The control sample was prepared using the same procedure, but instead of protein hydrolysate, deionized water was used. As a positive control, BHT was used. UV- spectrophotometer (Mapada Instruments Co., ltd., Shanghai, China) was utilized to detect the samples absorbance at 734 nm and determine the ABTS•+ scavenging activity using the following formula:
2.6 Isolation of antioxidant sturgeon peptides
2.6.1 Ultrafiltration of protein hydrolysate
Accurately 50 mg/mL of enzymatically hydrolyzed protein (PH and AH) were subjected to ultrafiltration (Millipore Minitan unit, Millipore, Bedford, MA) by three different molecular weight cut-off (MWCO, 3000, 2000, and 1000 Da) membranes. To dissolve each hydrolysate, 60 mg/mL deionized water was used, and four fractions were isolated and collected (fraction, >3000 Da), (fraction, 2000–3000 Da), (fraction, 1000–2000 Da), and (fraction, <1000 Da). The resultant peptide fractions were freeze-dried using the same procedure as described above, and their antioxidant capacity was identified using DPPH and ABTS assays.
2.6.2 Purification of peptides using RP-HPLC
Using ultrafiltration membranes, the most active fraction (fraction, <1000 Da) derived from PH and AH was purified using an RP-HPLC unit (Agilent 1260, Santa Clara, CA, USA). In brief, these fractions were diluted (50 mg/mL) in 0.1 % trifluoroacetic acid (TFA), and 20 μL of each sample was loaded into an Agilent Zorbax, SB C-18 column (4.6 mm 250 mm, 5 µm particle size). TFA (0.1 %, v/v) and acetonitrile (30 %, v/v) with 0.1 % (v/v) TFA were the mobile phases A and B, respectively. With a flow rate of 0.8 mL/min, the gradient program of 0 % B, 0–9 min; 10 % B, 10–20 min; 20 % B, 20.5–31.5 min; 40 % B, 32–41 min; and 50 % B, 41.5–43 min was used. The eluate was measured at 280 nm, and nine peptides (P1-P9) were separated from each sample, gathered, and lyophilized. The antioxidant capability of these peptides was estimated in order to verify the most active peptide.
2.7 Determination of the molecular weights of peptides
Gel permeation chromatography with an HPLC (Waters 1525, USA) was applied to determine the molecular weight (MW) of purified peptides (P5 of pH and P3 of AH). In the presence of 0.1 % TFA (v/v), the TSK gel 2000 SWXL (300 × 7.8 mm) column (Tosoh, Tokyo, Japan) was equilibrated with a mixing ratio of 40 acetonitrile: 60 water (v/v). The purified peptide samples were injected and eluted at a flow rate of 0.5 mL/min. Finally, the peptide samples were monitored at 220 nm with the column temperature set to 30 °C.
2.8 Statistical analysis
The experiments were performed in triplicate (n = 3), and values were reported as average ± standard deviation (SD). The CoStat 9.4 for Windows software program was used for statistical analysis. One-way analysis of variance (ANOVA) was applied to define significant differences between averages, and Duncan's test was used for multiple comparisons among averages at a p < 0.05. The differences between PH and AH in terms of different concentration and fractions was statistically analyzed using a t-test (p < 0.05).
3 Results and discussion
3.1 Degree of hydrolysis
Enzymatic hydrolysis is a process for generating food-derived peptidic antioxidants by degrading proteins with endogenous or exogenous proteases. Protein hydrolysis includes the cleavage of peptide linkage to produce peptides with varying MW and amino acids. According to this concept, the DH is determined as the ratio of enzyme-cleaved peptide bonds to the total number of these bonds per unit weight in the substrate sample used in the hydrolysis experiment (García-Moreno et al., 2014). As a result, it is primarily utilized to measure reaction kinetics and determine the extent to which proteins are broken down or hydrolyzed (Kristinsson and Rasco, 2000). The DH under optimal conditions using papain and alcalase 2.4L was 23.56 ± 0.76 % and 18.14 ± 0.81 %, respectively. This value of DH using papain was higher than previous results obtained for hydrolysis of monkfish muscle sample (19.83 %) using trypsin (Chi et al., 2014), while DH using alcalase 2.4L was close to this result. Nevertheless, the DH in our study was greater than that reported for tilapia frame samples hydrolysis, which ranged from 5.30 % to 15.10 % using various enzymes (Fan et al., 2012). This variation could be explained by a number of factors influencing the enzymatic hydrolysis process, including experimental conditions and enzyme specificity (Ktari et al., 2013; Noman et al., 2018). Villamil et al. (2017) reported that enzyme concentration, temperature, and pH all have an effect on the rate of enzymatic hydrolysis. In another context, the alcalase enzyme is an endopeptidase with a wide range of peptide bond selectivity for hydrolysis. Alternatively, papain is a combination of endopeptidase and exopeptidase, providing it a broader range of activity and consequently a higher DH when compared to alcalase 2.4L hydrolysis. These results are expected when the specificity of each enzyme is considered (Pedroche et al., 2002). Consequently, increased papain enzyme effectiveness towards sturgeon protein may refer to exopeptidase activity. In this study, the optimum pH values of two proteases were used, which were 6 for papain (Noman et al., 2018) and 8.5 for alcalase 2.4L (Noman et al., 2019). According to Do Evangelho et al. (2017), because asparagine and glutamine are deaminated to glutamic acid and aspartic acid via acidolysis rather than enzymolysis, variations in DH may be caused by the pH value for the enzyme activity used in the enzymolysis process. Thus, they noticed that the DH was higher in the acidic medium.
3.2 Amino acid composition of hydrolysates
Hydrolyzed proteins provide polypeptide, oligopeptide, and amino acid mixtures. The nutritional value of protein hydrolysate is most strongly influenced by its amino acid content, particularly essential amino acids required for proper physiological activities (Islam et al., 2021). As indicated in Table 1, the essential amino acid composition of pH and AH was 48.33 and 42.45 g/100 g protein, respectively. Asparagine and glutamine were the most abundant amino acids, accounting for 26.79 % and 27.77 % of the total amino acids in both hydrolysates, respectively. The essential amino acids leucine, isoleucine, arginine, valine, and lysine were abundant in both PH and AH, representing approximately 35.86 % and 33.86 % of the total amino acids, sequentially. Therefore, these enzymatically hydrolyzed proteins have potential nutritional value and could be added as dietary supplements or included into health claim products. The differences in content and amino acid profile of pH and AH could be attributed to the specificity of the enzymes used to cleave the peptide bonds in the substrate.
Amino Acids
Papain hydrolysate
Alcalase 2.4L hydrolysate
Histidine*
2.01 ± 0.07a
1.97 ± 0.11a
Threonine*
3.08 ± 0.08b
3.38 ± 0.14a
Arginine*
9.41 ± 0.09a
5.61 ± 0.13b
Tyrosine*
2.52 ± 0.08a
2.05 ± 0.09b
Valine*
4.26 ± 0.14b
4.39 ± 0.07a
Methionine*
2.97 ± 0.07a
2.22 ± 0.08b
Phenylalanine*
3.04 ± 0.17b
3.29 ± 0.17a
Isoleucine*
3.85 ± 0.16b
4.11 ± 0.14a
Leucine*
7.33 ± 0.17a
6.86 ± 0.09b
Lysine*
9.86 ± 0.21a
8.57 ± 0.12b
Asparagine
9.16 ± 0.17a
8.54 ± 0.21b
Glutamine
16.77 ± 0.21a
15.69 ± 0.19b
Serine
3.67 ± 0.11a
3.22 ± 0.11b
Glycine
6.40 ± 0.13a
5.47 ± 0.16b
Alanine
4.11 ± 0.12b
6.44 ± 0.08a
Cystine
1.77 ± 0.06a
0.14 ± 0.02b
Proline
6.59 ± 0.15a
5.29 ± 0.17b
Total amino acid
96.80 ± 1.07a
87.24 ± 0.92b
Total essential amino acids
48.33 ± 0.62a
42.45 ± 0.54b
Total non-essential amino acids
48.47 ± 0.43a
44.79 ± 0.48b
When the amino acid profile in the current findings was compared with protein hydrolysates from other sources, it was discovered that the total amino acid of pH was greater (96.80 g/100 g protein) than the protein hydrolysate of the Persian sturgeon (Ovissipour et al., 2009), which was 93.52 g/100 g protein. However, the amino acid content of AH was lower (87.24 g/100 g protein). In our study, the total amino acids content of both hydrolysates was greater than that of tuna protein hydrolysate (Saidi et al., 2014), which was 60.74 g/100 g protein. Amino acid content differences in different fish protein hydrolysates could be related to one or more of the following factors: fish species, organs, age, living location, feeding, and fishing season (Hamzeh et al., 2015; Noman et al., 2018).
3.3 Antioxidant activities
3.3.1 Antioxidant activity of protein hydrolysates
The scavenging activity of pH and AH was identified against DPPH• at 517 nm. There are two mechanisms of the antiradical action of antioxidants include single electron transfer and hydrogen atom transfer; hence, these two mechanisms produce the same end products (Osipova et al., 2021). The compounds that contribute to hydrogen (H+) act as radical scavengers, causing the purple sample mixture to turn yellow, resulting in reduced absorption (Ktari et al., 2012). As illustrated in Fig. 1A, the DPPH• scavenging activity of pH and AH obtained from Chinese sturgeon was demonstrated at concentrations ranging from 1 to 5 mg/mL. The radical scavenging activity of both hydrolysates significantly increased (p < 0.05) as their concentration raised from 1 to 5 mg/mL, which is consistent with previous reports showing that protein hydrolysates activity against radicals increased as their concentration increased (Bougatef et al., 2009; Ktari et al., 2012). At a concentration of 5 mg/mL, the highest capacity was found to be 62.45 % for PH and 67.42 % for AH. Bahari et al. (2020) reported that leucine, valine, methionine, and alanine play a fundamental role in peptide activity to scavenge DPPH•. The amino acid profiles of pH and AH indicated the existence of these amino acids with appropriate levels; however, the content of alanine and valine was higher in AH, as shown in Table 1. According to the current results, AH was more active (IC50 of 3.15 mg/mL) than PH (IC50 of 3.64 mg/mL). Both results were within the IC50 range (1.63–4.95 mg/mL) of salmon byproduct protein hydrolysate obtained by (Ahn et al., 2014) using different enzymes. However, it was less than IC50 of standard BHT (IC50 = 0.24 ± 0.01 mg/mL). The difference between synthetic scavengers and protein hydrolysate can be attributed to the fact that synthetic scavengers are pure compounds, whereas hydrolysate mostly is a mixture of protein/peptide and non-protein ingredients. This type of protein activity is generally associated with the composition and amino acid sequence of peptides, as well as their MW and the hydrophobic property caused by various DH (Bougatef et al., 2010). According to Phongthai et al. (2016), the amino acid profile released in the hydrolysis process could affect the antioxidant activities, especially phenylalanine tyrosine, histidine, and methionine, which were shown to be higher proton donors compared to other amino acids.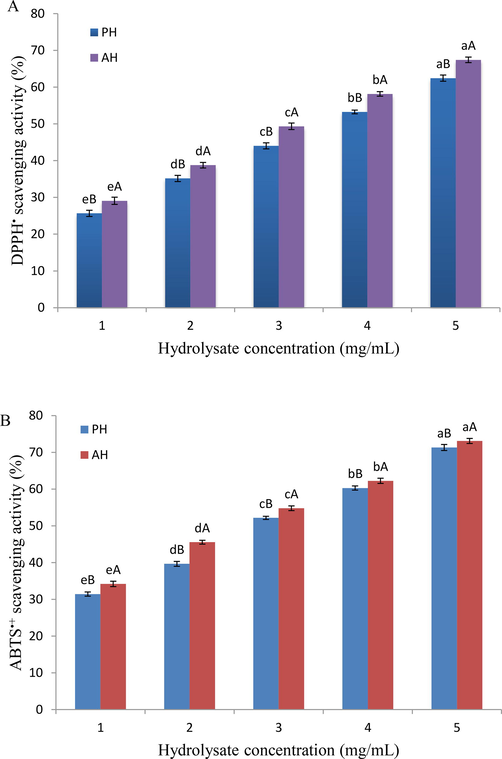
Antioxidant activities of protein hydrolysates powder obtained by using two different enzymes (PH; papain hydrolysate and AH; alcalase 2.4L hydrolysate), (A) DPPH• scavenging activity; (B) ABTS•+ scavenging activity. Data are expressed as mean ± standard deviation. Different lower-case letters (a and b) indicate significant differences (p < 0.05) between the different concentrations in each hydrolysate, and upper-case letters (A and B) indicate significant differences at the same concentration with the different hydrolysates.
When an antioxidant substance or active sample is included in the mixture, ABTS•+ is scavenged, which is proportional to the sample's antioxidant activity. This assay depends on the formation of a blue/green ABTS radical, which can be limited by antioxidants capable of donating hydrogen atoms to scavenge aqueous phase radicals or lipid peroxyl radicals (Wang et al., 2021), and the color changes can be detected by reading the absorbance at 734 nm. Fig. 1B depicts the PH and AH ABTS•+ scavenging activity. When the concentration of pH was raised from 1 mg/mL to 5 mg/mL, the radical scavenging capacity ranged from 31.43 % to 71.32 % and from 34.19 % to 73.07 % for AH. According to the findings in Fig. 1B, both hydrolysates contained peptides that may differ in amino acid content and sequence, and thus their antioxidant activity differed. The AH activity was higher (IC50 of 1.58 mg/mL) than the PH activity (IC50 of 1.92 mg/mL). Because the size of the peptides released during the hydrolysis process is determined by enzyme specificity, the differences in DH could explain the difference in antioxidant activity between PH and AH (Noman et al., 2022). Both hydrolysates had lower activity compared to standard BHT (IC50 = 0.19 ± 0.02 mg/mL). According to Sarmadi and Ismail (2010), peptides with MW in the range from 500 to 1500 Da had higher antioxidant activity than peptides with MW > 1500 Da and <500 Da. Noman et al. (2018), found that the MW of pH peptides (<500 Da) obtained with papain enzyme was >87 %, while it was significantly lower for AH peptides (Noman et al., 2019). Chalamaiah et al. (2015), on the other hand, mentioned that the histidine, phenylalanine, tyrosine, and methionine, may lead to increased ABTS•+ scavenging activities.
3.3.2 Antioxidant activity of hydrolysate fractions
The ultrafiltration procedure is frequently applied to separate fractions of protein hydrolysate with specific biological properties based on their MW (Chi et al., 2014). The Chinese sturgeon hydrolysates were separated by ultrafiltration membranes, and four fractions (>3000, 2000–3000, 1000–2000, and <1000 Da) were collected from PH and AH (Fig. 2). Fig. 2A depicts the PH and AH peptide fractions activities against DPPH•. The findings show that the peptide fractions exhibited varying levels of antioxidant activity, but the peptide fractions of AH were more active than PH peptide fractions, which could be related to the distribution of amino acids in each fraction's peptides. The fractions of pH and AH with MW < 1000 Da displayed the highest antioxidant activity compared to other fractions, which was 80.30 and 84.81 % with IC50 of 2.59 and 2.31 mg/mL, respectively. According to these findings, it can be concluded that this fraction (<1000 Da) from both hydrolysates may have contained more active antiradical peptides, which could efficiently transform DPPH• into more stable compounds, preventing the free radical chain reaction. The IC50 values of pH and AH fractions to scavenge DPPH• decreased with decreasing MW compared to pre-fractionation values. Previous studies found that protein hydrolysates or low MW peptide molecules interact more effectively with radicals and exhibit higher antioxidant capability (Wang et al., 2012; He et al., 2013; Onuh et al., 2014; Cai et al., 2015). Aromatic amino acids including tyrosine, histidine, and phenylalanine, as well as the hydrophobic acids, such as valine, methionine leucine and alanine are abundant in the PH and AH and play a remarkable role in bioactive peptide antioxidant activities (Bahari et al., 2020). However, because DH affects the amino acid profile, as well as the sizes and structures of the bioactive peptides released during the enzymolysis process, hence, the antioxidant activity of these fractions is linked to DH (Jang et al., 2016).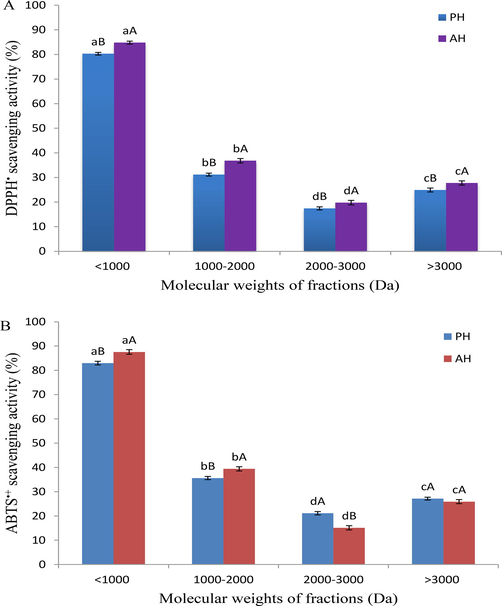
Radical-scavenging activity of ultrafiltration fractions of protein hydrolysates obtained using two different enzymes (PH; papain hydrolysate and AH; alcalase 2.4L hydrolysate), (A) DPPH; (B) ABTS•+. Data are expressed as mean ± standard deviation. Different lower-case letters (a and b) indicate significant differences (p < 0.05) between the different fractions in each hydrolysate, and upper-case letters (A and B) indicate significant differences at the same fraction with the different hydrolysates.
The ABTS•+ scavenging activity assay is a common method for determining the antioxidant capacity of hydrophilic and lipophilic compounds (You et al., 2009). In the presence of proton-donating compounds, such as antioxidants, the ABTS•+ are scavenged and the absorption is reduced by changing the solution color. As shown in Fig. 2B, based on MW of both protein hydrolysates, peptidic fractions exhibited significantly different antioxidant activities at p < 0.05, whereas peptide fractions with a MW of <1000 Da demonstrated significantly stronger activity compared to the other fractions in both hydrolysates. Most AH fractions showed higher antioxidant activities against ABTS•+ than those of PH, particularly the fraction with MW of <1000 Da, which were 82.87 % and 87.46 % (IC50 of 1.54 and 1.36 mg/mL), respectively. Reduced scavenging activity in papain peptide fractions samples may be attributed to an increased DH, as Intarasirisawat et al. (2012) found that protein hydrolysates with DH > 20 % have a lower ability to scavenge ABTS•+. Moreover, the distribution of MW of peptides within the fraction is critical in determining the antioxidant activity (Sarmadi and Ismail, 2010).
3.3.3 Antioxidant activity of purified peptides
The <1000 Da fraction of pH and AH was the most active in scavenging free radicals, particularly the ABTS•+. Therefore, this fraction was separated further by RP-HPLC, and chromatogram shows nine peaks corresponding to the P1-P9 peptides as shown in Figs. 3 and 4. Among these nine fractions, the peak corresponding to P5 peptide from PH and P3 peptide from AH demonstrated higher (p < 0.05) than the other isolated peptides, with 87.78 and 93.34 %, respectively. According to this result, the purification process increased the activity of the purified peptides compared to the previous stages, where the IC50 values were 0.89 and 0.72 mg/mL, sequentially. Although these values were higher than the IC50 of standard BHT (0.19 ± 0.02 mg/mL), they were remarkably close to BHT value compared to hydrolysates and their fractions, enhancing their potential as natural substitutes for synthetic antioxidant compounds. On the other hand, the result in Table 2 revealed that the purified peptide (P5) of pH contained valine and glycine, whereas the purified peptide (P3) of AH contained glycine and alanine. These acids are classified as hydrophobic amino acids that exhibited strong antioxidant capacity (Cai et al., 2015). Furthermore, the peptide isolated from AH (P3) contains histidine (aromatic acid) residue, which has the potential to donate electrons to radicals and convert them to stable molecules. The difference in activity between the two peptides can thus be traced to the peptides' varied distribution of these amino acids.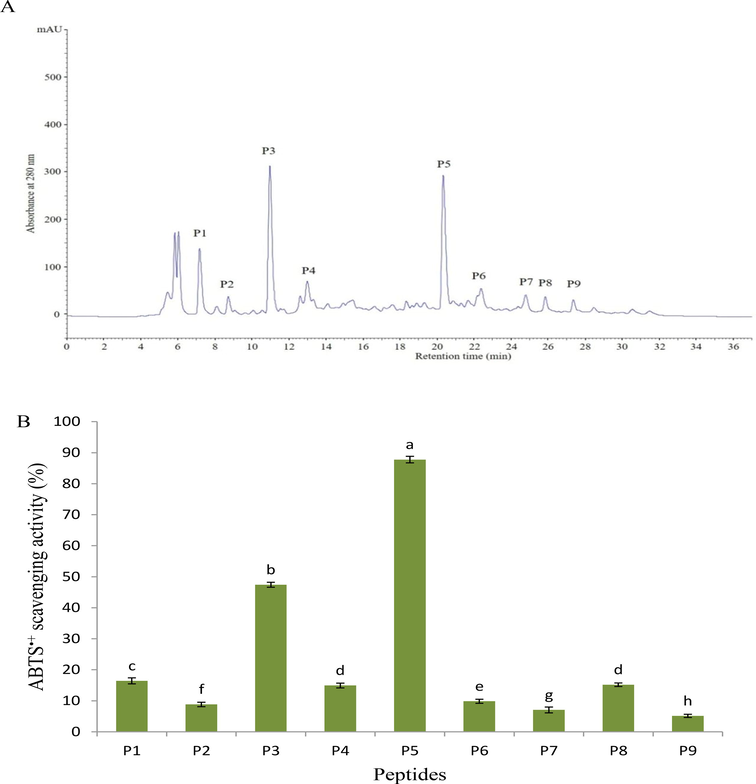
RP-HLPC chromatogram on a Zorbax, SB C-18 column of the papain hydrolysate fraction < 1000 Da obtained using ultrafiltration process (A), and ABTS•+ scavenging activity of the separated peptides (B). Data are expressed as mean ± standard deviation. Different letters above bars indicate significant differences at p < 0.05.
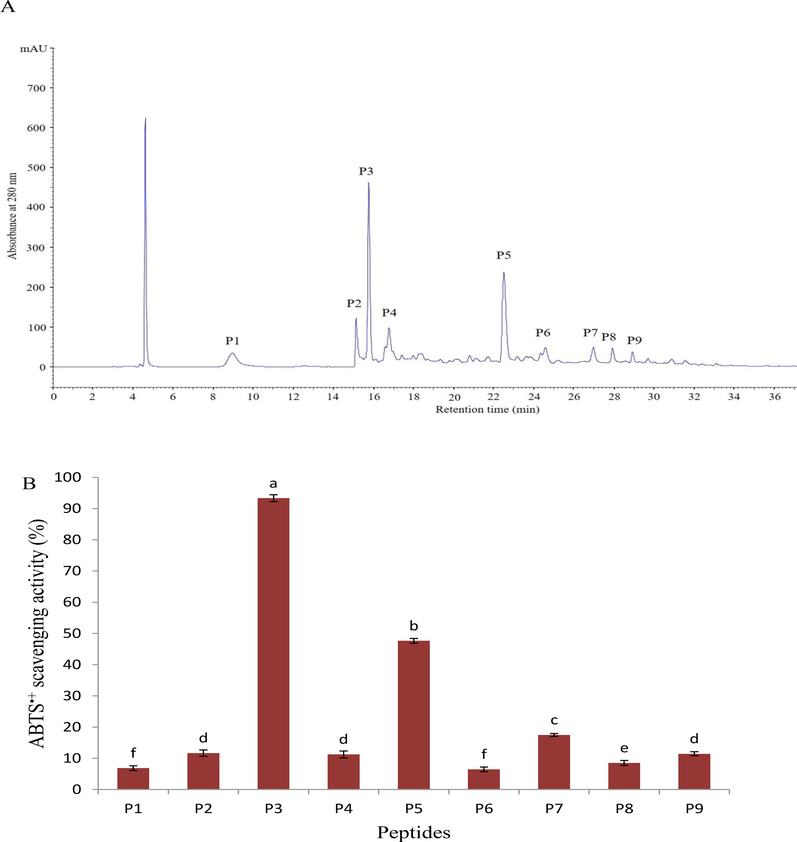
RP-HLPC chromatogram on a Zorbax, SB C-18 column of the alcalase 2.4L hydrolysate fraction < 1000 Da obtained using ultrafiltration (A), and ABTS•+ scavenging activity of the separated peptides (B). Data are expressed as mean ± standard deviation. Different letters above bars indicate significant differences at p < 0.05.
Purified peptide from papain hydrolysate (P5)
Purified peptide from alcalase hydrolysate (P3)
MW (Da)
Amino acids
Composition (%)
MW (Da)
Amino acids
Content (%)
282.13
Valine
25.49 ± 1.48
302.74
Histidine
31.86 ± 1.87
Glycine
33.85 ± 2.01
Glycine
48.0.37 ± 1.35
Asparagine
40.66 ± 1.79
Alanine
19.78 ± 1.56
Total
100
Total
100
Protein structural properties such as amino acid profile, sequences, hydrophobicities, and MW highly affect the antioxidant activity of peptides purified prepared from various protein sources (Zou et al., 2016). Furthermore, hydrophobic amino acids can increase peptide solubility in lipids, improving interaction with free radicals, which in turn might improve the antioxidant properties of peptides (Sarbon et al., 2018). In contrast, the purified peptides (P3 from PH and P5 from AH) obtained in this study have low MW as shown in Table 2. Although amino acids content in the peptide structure are responsible for antioxidant activity, the MW of peptides was found to be critical in peptide efficiency, where Kumar et al. (2012) documented that peptides with smaller MW can pass the intestinal barrier and perform a biological activity.
4 Conclusions
Antioxidant peptides were successfully produced by hydrolyzing Chinese sturgeon (Acipenser sinensis) proteins with papain and alcalase 2.4L. The PH and AH with DH of 23.56 % and 18.14 % contained 96.80 % and 87.24 % total amino acids, respectively. Ultrafiltration membranes were used to separate four fractions from PH and AH, and the highest antioxidant activities were found in fraction <1000 Da for both hydrolysates. Among the nine peptides separated using RP-HPLC the P3 peptide (MW of 302.74 Da) of the AH was the most active (IC50 of 0.72 mg/mL) against ABTS•+ contained histidine, glycine and alanine, while the peptide P5 (MW of 282.13 Da) of PH (IC50 of 0.89 mg/mL) contained valine, glycine and asparagine. The fractionation and purification process significantly increased the activity of the peptides, indicating that the purified peptides of Chinese sturgeon hydrolysates possess excellent antioxidant activities to scavenge radicals.
Author contributions
Anwar Noman: Investigation, Data curation, Formal analysis, Writing – original draft. Yuxia Wang: Conceptualization, Methodology, Funding acquisition, Supervision. Chao Zhang: Conceptualization, Project administration, Methodology, Supervision. Yin Liguo: Methodology, Data curation. Sherif M. Abed: Methodology, Writing – review & editing. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This work sponsored by Model in curriculum’s ideology and politics of Sichuan province “Principle of microbiological engineering and process” (SKCSZKC202001), Agricultural research and practice project of Sichuan province “Reformation and practice on teaching model targeted fusing agriculture-industry and linking tertiary industries” (SXNK20201), and First-class offline curriculum of Sichuan province “Principle of microbiological engineering and process”.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Production of biscuit from chinese sturgeon fish fillet powder (Acipeneser sinensis): A snack food for children. J. Aquat. Food Prod. Technol.. 2018;27:1048-1062.
- [CrossRef] [Google Scholar]
- Purification and antioxidant properties of octapeptide from salmon byproduct protein hydrolysate by gastrointestinal digestion. Food Chem.. 2014;147:78-83.
- [CrossRef] [Google Scholar]
- Official Methods of Analysis of AOAC International (16th ed. & 4th rev ed.). Rockville, MD: AOAC International; 1998.
- Response factorial design analysis on papain-generated hydrolysates from actinopyga lecanora for determination of antioxidant and antityrosinase activities. Molecules. 2020;25:2663.
- [CrossRef] [Google Scholar]
- Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem.. 2009;114:1198-1205.
- [CrossRef] [Google Scholar]
- Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinellaaurita) by-products proteins. Food Chem.. 2010;118:559-565.
- [CrossRef] [Google Scholar]
- Sturgeon meat and caviar production: Global update 2017. J. Appl. Ichthyol.. 2019;35:257-266.
- [CrossRef] [Google Scholar]
- Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Foods. 2015;16:234-242.
- [CrossRef] [Google Scholar]
- Chemical composition, molecular mass distribution and antioxidant capacity of rohu (Labeo rohita) roe (egg) protein hydrolysates prepared by gastrointestinal proteases. Food Res. Int.. 2013;52:221-229.
- [CrossRef] [Google Scholar]
- Antioxidant activity and functional properties of enzymatic protein hydrolysates from common carp (Cyprinus carpio) roe (egg) J. Food Sci. Technol.. 2015;52:5817-5825.
- [CrossRef] [Google Scholar]
- Regulatory requirements of bioactive peptides (protein hydrolysates) from food proteins. J. Funct. Foods. 2019;58:123-129.
- [CrossRef] [Google Scholar]
- Isolation and characterization of three antioxidant pentapeptides from protein hydrolysate of monkfish (Lophius litulon) muscle. Food Res. Int.. 2014;55:222-228.
- [CrossRef] [Google Scholar]
- Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J. Funct. Foods. 2015;12:1-10.
- [CrossRef] [Google Scholar]
- Black bean (Phaseolus vulgaris L.) protein hydrolysates: Physicochemical and functional properties. Food Chem.. 2017;214:460-467.
- [CrossRef] [Google Scholar]
- Purification and identification of antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) frame protein. Molecules. 2012;17:12836-12850.
- [CrossRef] [Google Scholar]
- Antioxidant activity of protein hydrolysates obtained from discarded mediterranean fish species. Food Res. Int.. 2014;65:469-476.
- [CrossRef] [Google Scholar]
- Amino acid composition of roe from wild and farmed beluga sturgeon (Huso huso) J. Agr. Sci. Tech.. 2015;17:357-364.
- [Google Scholar]
- Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods. 2013;5:219-227.
- [CrossRef] [Google Scholar]
- Antioxidative and functional properties of protein hydrolysate from defatted skipjack (Katsuwonous pelamis) roe. Food Chem.. 2012;135:3039-3048.
- [CrossRef] [Google Scholar]
- Degree of hydrolysis, functional and antioxidant properties of protein hydrolysates from grass turtle (Chinemys reevesii) as influenced by enzymatic hydrolysis conditions. Food Sci. Nutr.. 2021;9:4031-4047.
- [CrossRef] [Google Scholar]
- Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. J. Funct. Foods. 2016;20:433-442.
- [CrossRef] [Google Scholar]
- Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented by bacillus subtilis a26. Process Biochem.. 2014;49:963-972.
- [CrossRef] [Google Scholar]
- Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr.. 2000;40:43-81.
- [CrossRef] [Google Scholar]
- Functionalities and antioxidant properties of protein hydrolysates from muscle of zebra blenny (Salaria basilisca) obtained with different crude protease extracts. Food Res. Int.. 2012;49:747-756.
- [CrossRef] [Google Scholar]
- Effect of degree of hydrolysis and protease type on the antioxidant activity of protein hydrolysates from cuttlefish (Sepia officinalis) by-products. J. Aquat. Food Prod. Technol.. 2013;22:436-448.
- [CrossRef] [Google Scholar]
- Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber) Amino Acids. 2012;42:1641-1649.
- [CrossRef] [Google Scholar]
- Influence of enzymatic hydrolysis conditions on the degree of hydrolysis and functional properties of protein hydrolysate obtained from Chinese sturgeon (Acipenser sinensis) by using papain enzyme. Process Biochem.. 2018;67:19-28.
- [CrossRef] [Google Scholar]
- Effects of ultrasonic, microwave, and combined ultrasonic-microwave pretreatments on the enzymatic hydrolysis process and protein hydrolysate properties obtained from Chinese sturgeon (Acipenser sinensis) J. Food Biochem.. 2020;44
- [Google Scholar]
- Influence of degree of hydrolysis on chemical composition, functional properties, and antioxidant activities of Chinese sturgeon (Acipenser sinensis) hydrolysates obtained by using alcalase 2.4 L. J. Aquat. Food Prod. Technol.. 2019;28:583-597.
- [CrossRef] [Google Scholar]
- Antioxidant activity of hybrid sturgeon (Huso dauricus× Acipenser schrenckii) protein hydrolysate prepared using bromelain, its fractions and purified peptides. Pol. J. Food Nutr. Sci.. 2022;72:79-89.
- [CrossRef] [Google Scholar]
- In vitro antioxidant properties of chicken skin enzymatic protein hydrolysates and membrane fractions. Food Chem.. 2014;150:366-373.
- [CrossRef] [Google Scholar]
- Antioxidant activity of some organosulfur compounds in vitro. Arabian J. Chem.. 2021;14:103068.
- [CrossRef] [Google Scholar]
- The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (Acipenser persicus) viscera. Food Chem.. 2009;115(1):238-242.
- [CrossRef] [Google Scholar]
- Advances on food-derived peptidic antioxidants—a review. Antioxidants. 2020;9:799.
- [CrossRef] [Google Scholar]
- Utilisation of chickpea protein isolates for production of peptides with angiotensin I-converting enzyme (ACE)-inhibitory activity. J. Sci. Food Agric.. 2002;82:960-965.
- [CrossRef] [Google Scholar]
- Optimization of microwave-assisted extraction of rice bran protein and its hydrolysates properties. J. Cereal Sci.. 2016;70:146-154.
- [CrossRef] [Google Scholar]
- Production and fractionation of tuna by-product protein hydrolysate by ultrafiltration and nanofiltration: Impact on interesting peptides fractions and nutritional properties. Food Res. Int.. 2014;65:453-461.
- [CrossRef] [Google Scholar]
- Purification and characterization of antioxidative peptides derived from chicken skin gelatin hydrolysate. Food Hydrocoll.. 2018;85:311-320.
- [CrossRef] [Google Scholar]
- Antioxidative peptides from food proteins: A review. Peptides. 2010;31:1949-1956.
- [CrossRef] [Google Scholar]
- Formol titration: An evaluation of its various modifications. Analyst. 1957;82:488-498.
- [CrossRef] [Google Scholar]
- Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem.. 2017;224:160-171.
- [CrossRef] [Google Scholar]
- Preparation and evaluation of antioxidant peptides from ethanol-soluble proteins hydrolysate of sphyrna lewini muscle. Peptides. 2012;36:240-250.
- [CrossRef] [Google Scholar]
- Antioxidant activity and functional properties of alcalase-hydrolyzed scallop protein hydrolysate and its role in the inhibition of cytotoxicity in vitro. Food Chem.. 2021;344:e128566.
- [Google Scholar]
- Preparation and identification of antioxidative peptides from pacific herring (Clupea pallasii) protein. Molecules. 2019;24:1946.
- [CrossRef] [Google Scholar]
- Assessment of Chinese sturgeon habitat suitability in the Yangtze River (China): Comparison of generalized additive model, data-driven fuzzy logic model, and preference curve model. J. Hydrol.. 2016;536:447-456.
- [CrossRef] [Google Scholar]
- Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Innov. Food Sci. Emerg. Technol.. 2009;10:235-240.
- [CrossRef] [Google Scholar]
- Identification and characterization of two novel antioxidant peptides from silkworm pupae protein hydrolysates. Eur. Food Res. Technol.. 2021;247:343-352.
- [CrossRef] [Google Scholar]
- Purification and characterization of antioxidant peptides of pseudosciaena crocea protein hydrolysates. Molecules. 2017;22:57.
- [CrossRef] [Google Scholar]
- The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules. 2016;21:72.
- [CrossRef] [Google Scholar]







