Translate this page into:
From nature to protection: Unleashing the protective potential of Hedera helix leaves against corrosion in harsh acidic environments using experimental and theoretical insights
⁎Corresponding authors. maryam.chafiq@yu.ac.kr (Maryam Chafiq), abdelkarim.chaouiki@yu.ac.kr (Abdelkarim Chaouiki), younggun@ynu.ac.kr (Young Gun Ko)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The corrosion of metals is a major problem in many industrial sectors. To develop sustainable and naturally friendly alternatives to classical corrosion inhibitors, the use of plant extracts has attracted increasing interest. Plant extracts contain a variety of bioactive substances, such as polyphenols, which have corrosion-inhibitory properties. Plant extracts are biodegradable and pose minimal risk to human health and ecosystems. Consistent with the prevailing research trends in this field, in this study, the ability of Hedera helix leaf extract (HHLE) to protect XC18 steel from corrosion in 1 M HCl was evaluated. The polyphenols present in HHLE were identified using HPLC-DAD analysis. The weight loss, potentiodynamic polarization (PDP), and electrochemical impedance spectroscopy (EIS) measurements were examined. Six phenolic compounds were detected in HHLE. The results showed that the inhibitory efficiency of the leaf extract increased with increasing concentration. At 298 K, weight loss corrosion and electrochemical tests demonstrated that the greatest protection efficiency of 92.67 % for the steel was achieved at a concentration of 1000 mg/L of HHLE. This was attributed to a reduction in the cathodic currents and the obstruction of the active corroding sites. Thermodynamic analysis indicated an endothermic process governed by physisorption, and the Langmuir adsorption isotherm model highlighted the influence of both physical and chemical mechanisms on the adsorption process. Scanning electron microscopy (SEM) was used to confirm the reduction in XC18 steel corrosion in the presence of HHLE. Density functional theory (DFT) was employed to carry out quantum chemistry studies on the two primary components of the extract: kaempferol (KMP) and trans-chalcone (CHL). The quantum parameters obtained from the calculations were used to explore the active sites and interactions between donor and acceptor molecules, as well as the interfacial mechanism behavior of HHLE molecules on the metal surface.
Keywords
Hedera helix
XC18 steel
Adsorption
Electrochemical behavior
Green corrosion inhibitor
DFT
Interfacial mechanism
1 Introduction
Corrosion represents a significant cause of damage in both industrial and social domains. The National Association of Corrosion Engineers (NACE) reports that approximately 2.5 trillion US$ in economic damage occurs annually due to the corrosion of metal components (Hossain et al., 2023). This corresponds to roughly 3–4 percent of the annual Gross World Product (GWP) (Shang and Zhu, 2021). Metal corrosion is influenced by several factors, including the reactivity of the metals, the presence of contaminants, the pH and temperature of the solution, as well as industrial processes such as acidizing, acid cleaning, pickling, descaling, and so forth (Hossain et al., 2023). Carbon steel finds widespread use in industries such as petrochemicals, marine ships, machinery manufacturing, power equipment, and others, due to its exceptional mechanical strength, favorable thermal conductivity, ease of processing, and affordability (Wang et al., 2022). XC18, a type of carbon steel, is widely employed. However, the corrosion of XC18 presents a significant concern due to its low resistance to corrosion and its broad range of applications (Salim et al., 2019). In various industrial applications, acid solutions find extensive use, including important processes such as acid pickling of iron and steel, chemical cleaning and processing, ore production, and acidification of oil wells (Soltani and Khayatkashani, 2015). Furthermore, HCl is commonly employed in diverse industrial processes. There are several alternative methods used to reduce the dissolution of metals, including coating, cathodic protection, and the addition of acid corrosion inhibitors (Kalkhambkar et al., 2022). Among these methods, the use of corrosion inhibitors (CIs), which, when added to the acid medium in small quantities, are employed to minimize metal decomposition, continues to be one of the most practical approaches due to its exceptional effectiveness and wide-ranging suitability across various industries (Bobby Kannan et al., 2022). Although inorganic CIs like tungstate, molybdate, and chromate exhibit high efficiency, their utilization is accompanied by significant disadvantages such as high cost, adverse environmental impact, and the need for large quantities of the inhibitor (Lin et al., 2023). Various synthetic organic inhibitors are very efficient in preventing corrosion (Stanly Jacob and Parameswaran, 2010). Within industries, CIs prove more effective compared to alternative methods like corrosion protection, protective coatings, cathodic protection, and anodic protection. The use of CIs is straightforward, offering the advantage of being applied “in situ” without disrupting ongoing processes (Khayatkashani et al., 2022). Organic CIs containing heteroatoms (S, P, O, and N) possess lone pairs, facilitating the interaction with molecules featuring empty d orbitals to adhere to the base metal more easily (Assad et al., 2023). Numerous synthetic organic compounds have been examined for their corrosion inhibition properties, and ongoing research continues to focus on their synthesis and evaluation (Ahchouch et al., 2023; Chaouiki et al., 2020; Elmacı et al., 2019, 2018; Goyal et al., 2018; Singh et al., 2022). While synthetic organic compounds demonstrate effective anti-corrosion properties, they still expensive and they also pose a significant risk of high toxicity to both the environment and human life because they contain toxic components (Assad et al., 2023; Méndez-Figueroa et al., 2022). Consequently, the central goal has been to formulate CIs that possess efficiency, affordability, and eco-friendly.
The continuous quest for sustainable and eco-friendly methods in corrosion control has stimulated the exploration of natural resources, including the utilization of plant extracts. Plant extracts have recently become an alternative to traditional corrosion remedies because of environmental concerns (Zehra et al., 2022). The availability, varied chemical compositions, and inherent corrosion inhibitory potential of plant extracts make them an attractive option. These extracts do really offer a number of benefits, including the highest level of inhibitory efficiency, availability, biodegradability and non-toxicity (Mwakalesi, 2022). Plant extracts are composed of a variety of organic compounds, typically including oxygen (O), nitrogen (N), sulfur (S), as well as double bonds, triple bonds, or conjugate systems. These characteristics resemble those of conventional organic inhibitors, rendering them a promising choice for high-efficiency inhibition (Wang et al., 2022).
In recent years, researchers in the field of corrosion inhibitors derived from plant extracts have invested significant efforts. They have reported on the use of natural substances taken from leaves, fruit, or seeds to prevent corrosion in metallic materials (Dehghani et al., 2020; Orubite and Oforka, 2004). In fact, they include organic substances (tannins, flavonoids, polyphenols and carbohydrates) that have good chelating capabilities toward cations (Fe3+ and Fe2+) produced by steel oxidation (Wamba-Tchio et al., 2022). Recently, notable inhibitory effects on reducing the corrosion rate of carbon steel in acid environments have been reported for various plant extracts, including Lantana camara (Shrestha et al., 2019), Syzygium cumini (Silva et al., 2021), Datura stramonium (Hjouji et al., 2023), Urtica dioica L. (Maizia et al., 2023), Kleinhovia hospita (Gapsari et al., 2023), Aconitum carmichaelii Debx (Xu et al., 2024), Almond waste (Batah et al., 2022), Barringtonia acutangula (Tran et al., 2024), Pelargonium gravelons (Boussalem et al., 2023), and acacia melanoxylon (Minagalavar et al., 2024). Wang et al. studied he inhibitive effect of Fatsia japonica (Belongs to the same family, Araliaceae, as Hedera helix) extract on the corrosion of carbon steel, and the experimental findings indicated that the corrosion inhibition achieved a level of 89.6 % at a concentration of 1000 mg/L (Wang et al., 2023).
Hedera helix L. is a species of evergreen climbing vines, belonging to the Araliaceae family. Hedera helix L. also known as ivy plant, and is primarily utilized for decorative purposes as ornamental grass. Many in vitro and in vivo studies have previously shown its pharmacological efficacy. H. helix exhibits potent spasmolytic and bactericidal properties (Bezruk et al., 2022). As a result, in several countries, the use of ivy leaf extracts has been approved as a medicinal product for the treatment of acute bronchitis (Kim et al., 2017). In addition to phenolic acids and flavonoids, several triterpenoid saponins were previously identified from H. helix leaf extracts (Shokry, 2022). These organic substances have adsorption centers, which are polar functional groups containing N, S, and O atoms or aromatic rings. This indicates that leaf extracts may operate as corrosion inhibitors (Feng et al., 2022). In addition to that, the practical scalability of implementing Hedera helix extract is supported by several key factors. Firstly, Hedera helix is widely distributed globally, ensuring a readily available and abundant source. Secondly, as an ornamental plant cultivated in urban areas, Hedera helix generates significant waste material during routine pruning, facilitating easy accessibility. Thirdly, the leaves are easily processed through grinding, and transportation logistics are uncomplicated. Additionally, the extraction methods outlined in this article, or through simple maceration, offer versatility for large-scale applications. Despite these favorable attributes, it is noteworthy that there is currently a gap in the literature concerning the corrosion inhibition properties of H. helix leaf extracts (HHLE). For this purpose, the present work aims to address this gap by investigating and elucidating the corrosion inhibitory potential and interfacial mechanism behavior of HHLE. The intention is to propose the development of green corrosion inhibitors that exhibit both high effectiveness and minimal toxicity effects. Therefore, HHLE was examined as a sustainable corrosion inhibitor for XC18 steel in a 1 M HCl solution. Weight loss and electrochemical measurements were employed to investigate the impact of HHLE concentration and temperature on the experimental outcomes. The surface morphology and chemical composition of the metal were assessed through SEM-EDS analysis. The inhibition and adsorption phenomena were thoroughly examined and discussed in detail. To the best of our knowledge, there is a lack of comprehensive efforts aimed at elucidating (i) the interfacial interaction mechanism of plant extarcts, (ii) the impact of inter- and intra-molecular interactions on structure stabilization, and (iii) the driving forces underlying the specific rearrangement of spatial architectures proposed to establish a new, durable, and stable anti-corrosion system. Following the identification of multiple organic molecules present in HHLE, two representative molecules were selected and simulated through modern concepts in theoretical chemistry. The remarkable inhibitory effectiveness of HHLE has inspired us to focus on comprehending the adsorption behavior and interfacial mechanism of these two prominent polyphenolic molecules, KMP and CHL. Quantum chemistry calculations based on density functional theory (DFT) and density functional tight binding (DFTB) simulations were then employed to evaluate and estimate their respective abilities to interact with the surface of XC18 steel. The findings reveal that two key molecules in HHLE, KMP and CHL, exhibit near-parallel adsorption onto the XC18 surface. This adsorption is facilitated by both oxygen groups and non-covalent interactions (NCIs). Such arrangement maximizes the contact area between these molecules and the iron surface, thereby effectively preventing steel corrosion. These insights can be extended and applied in the field of environmental chemical engineering for various purposes.
2 Experimental methodology
2.1 Extraction protocol and corrosion inhibitor elaboration
The Hedera helix leaves were collected from the Agadir region in Morocco. After being washed, the samples are left to dry in the dark at room temperature for 72 h, before being ground. The resulting powder is stored in glass bottles in the darkness and away from humidity, to prepare for the extraction process. In this study, the hydro-alcoholic extract of Hedera helix leaves was utilized as a corrosion inhibitor. To prepare it, 20 g of Hedera helix leaves powder was packed into a cellulose extraction thimble and placed to a Soxhlet instrument. Around 500 mL of a melange of ethanol and water (70/30, v/v) is poured into the apparatus, and the solution is refluxed at 56 °C for 5 h until the solvents become colorless. After filtration through a 10 µm filter paper, the extract is evaporated using a rotary evaporator (Buchi Rotavapor R-200 coupled with Buchi Vac V-500 pump, Switzerland) at 50 °C. The resulting extract is then stored in darkness at a temperature of 4 °C (Das et al., 2019). The formula utilized to determine the extraction yield is (Ngamkhae et al., 2022): yield (%) = (Weight of extracted material / Weight of the original sample) × 100 %
The dried extract's yield was 1.4 g (7 % w/w). Various HHLE concentrations were prepared as test solutions for the corrosion tests. The HHLE was added to 1 M HCl to produce corrosive solutions with HHLE concentrations of 125 mg/L, 250 mg/L, 500 mg/L, and 1000 mg/L.
2.2 High-performance liquid chromatography with diode-array detection (HPLC-DAD) analysis
In the normal phase, HPLC-DAD is employed to analyze and measure the phenolic compounds present in the HHLE. The phenolic compounds were separated using a column (Agilent 1200, Agilent Technologies, USA) in conjunction with a Bruker diode array UV detector (Germany). The mobile phase consists of solvent A (H2O/0.5 % of H3PO4 acid) and methanol (CH3OH) as a solvent B, flowing at a constant rate of 0.5 mL/min for a duration of 35 min. A 20 μL volume of HHLE was introduced into a Zorbax XDB-C18 column (5 µm pore size, 250 × 4.6 mm; Agilent Technologies Serie 1100 system) provided with a 4 × 3 mm C18 cartridge pre-column (Agilent Technologies). The gradient elution method employed the following conditions: 0–25 min with 20 % of methanol, 25–30 min with 100 % of methanol, and 30–35 min with 20 % of methanol, as specified in Table 1. The separation took place under a temperature of 40 °C. Spectrophotometric detection was conducted using wavelengths of 254 nm, 280 nm, 320 nm, and 350 nm, while identification specifically occurred at 280 nm. The retention times of peaks are compared with those of the reference standards to determine their identification. Quantification is achieved by utilizing calibration curves, which allow the expression of results as milligrams per 100 g of dry matter. The linearity of all standard curves was excellent, with R2 values exceeding 0.99. The phenolic compounds used as standards include Syringic acid, vanillic acid, catechin, p-hydroxybenzoic acid, caffeic acid, rutin, vanillin, quercetin-3-glucoside, naringenin, p-coumaric acid, quercetin, salicylic acid, ferulic acid, kaempferol, trans-chalcone, gallic acid, and sinapic acid. Solvent A: (H2O/0.5 % H3PO4 acid); Solvent B: CH3OH.
Solvent gradient
Time (minutes)
0
25
30
35
A (%)
80
0
0
80
B (%)
20
100
100
20
2.3 Material and test solution
For electrochemical/gravimetric tests, XC18 steel plates were utilized. Table 2 indicates the chemical constitution of the plates. The plates underwent a polishing process with abrasive paper with different granulations (600, 1000, 1200, and 1800) before each usage. They were then cleaned with distilled water, followed by ethanol, and dried with airflow. A hydrochloric acid (HCl) solution with a concentration of 1 M is prepared using a 37 % HCl (PanReac®) as the corrosive medium.
Elements
C
S
P
Mn
Si
Fe
Weight %
0.18
≤ 0.035
0.06
0.05
0.16
≈ 99.52
2.4 loss measurements
A fundamental technique for determining how corrosion is prevented in an electrolytic solution for a certain metal is the measurement of mass loss. For 24 h, 100 mL of a 1 M HCl solution is used to immerse rectangular-shaped XC18 steel samples (1.5 cm × 1.5 cm × 0.1 cm) that were previously prepared. The trials were conducted at a range of temperatures between 298 K and 328 K to investigate the impact of temperature on thermodynamic activation parameters. After each experiment, steel specimens were removed from the test solution, corrosion products were removed, cleaned with distilled water, followed by ethanol, dried, and accurately reweighed. The tests were conducted in triplicate, and the standard deviation for the three measurements was calculated.
2.5 Electrochemical measurement
A silicone rubber was used to encapsulate the cylindrical XC18 electrode, with a 0.14 cm2 working area. To complete electrochemical testing, a three-electrode cell was used, which included a platinum coil as a counter electrode, in addition to the XC18 working electrode and SCE as a reference electrode. All measurements were performed using Corrtest potentiostat (Corrtest Instruments Corp. Ltd., Hubei, China) with a water-bath thermostat set at 298
0.5 K. EIS measurements were initiated following the immersion of the electrode in the electrolyte for an appropriate duration (30 min) until the open circuit potential (OCP) was reached. And then the electrochemical impedance spectroscopy measurements were performed with the frequency ranging from 10 kHz to 10 mHz with a sinusoidal fluctuation of 10 mV. After then, potentiodynamic polarization (PDP) was carried out at a rate of 1 mV/s between –700 and –300 mV vs EOCP. The tests were carried out at a stable OCP value (EOCP). The inhibition percentage can be determined using the charge transfer resistance, as follows (Xu et al., 2023):
and are the polarization resistance with and without HHLE, respectively.
The diameter of the half-circle represents the value of the charge transfer resistance (
) in the Nyquist plot. Using PDP data, the corrosion inhibition efficiency was assessed using the following equation:
Where and represent respectively the corrosion current density without and with the addition of HHLE.
2.6 Surface morphology observation
The surface morphologies of XC18 specimens that had been submerged in 1 M of HCl for 48 h with and without 1000 mg/L of HHLE were investigated using scanning electron microscopy (SEM, HITACHI) equipped with energy dispersive spectroscopy (EDS, HORIBA EMAX).
2.7 Computational details
The adsorption behavior of inhibitor molecules on the metal surfaces is significantly influenced by their molecular structure and electronic parameters. To delve into the optimized structure and electronic characteristics of the inhibitors, DFT has emerged as a powerful technique in the field of computational chemistry. The molecules under investigation in the aqueous phase was modeled, optimized, and their electron donor–acceptor character further computed using the Gaussian 16 W software (Frisch, 2016). In order to obtain the entry record, the ground state computation and the B3LYP/6-311G++ (d,p) functional were incorporated alongside the DFT parameter (Cossi et al., 1998, 1996). In addition, first-principles DFTB simulations were carried out using the DFTB+ code (Aradi et al., 2007; Spiegelman et al., 2020), to investigate the adsorption behavior and interfacial mechanism of the organic layer on the XC18 surface. To achieve this, the trans3d Slater-Koster library was employed in conjunction with A self-consistent charge (SCC) formalism (Perdew et al., 1998). The modern dispersion-corrected method (DFT-D2) is used as recommended by Grimme to explore long-range interactions (Elstner et al., 1998). The optimization of bulk lattice parameters utilized an initial (8 × 8 × 8) k-point grid, subsequently refined to a (2 × 2 × 1) k-point grid for the adsorption models. Construction of inhibitor-metal adsorption models involved building a Fe(1 1 0) surface with a (3 × 3) supercell and a 30 Å vacuum spacing along the z-direction, establishing separation between periodic images in each direction. In this setup, HHLE molecules were initially placed on the top side of the slab, and all atoms were allowed to relax. During the optimization of each model, the following factors were taken into account: a maximum displacement of 0.001 Å, 0.5 kcal mol−1 Å−1 for force, and an energy tolerance SCC of 0.05 kcal mol−1. More details about the computational models used in this study were constructed, building upon our previous works (Chafiq et al., 2023a; Chaouiki et al., 2023).
3 Results and discussions
3.1 HPLC-DAD analysis
Polyphenols are a naturally occurring group of organic compounds present in plants (Abbas et al., 2017). Several studies have shown that polyphenols exhibit very interesting corrosion- inhibitory features. This can primarily be attributed to the existence of aromatic OH groups and the ability of heteroatoms to form coordinate with the metal's vacant d-orbitals through electron donation. Likewise, the presence of rings containing conjugated bonds and their π-electrons enables the adsorption of molecules onto the metallic surface through interactions (Marzorati et al., 2019). Hence, the primary polyphenolic constituents in HHLE were identified and quantified using HPLC-DAD by comparing their Rt and UV spectra with those of the established standards (Kadda et al., 2022; Loukili et al., 2022, 2021). The chromatogram of HPLC for the identified phytoconstituents in HHLE (Fig. 1) indicated the presence of six compounds, namely p-hydroxybenzoic acid, naringenin, rutin, quercetin, kaempferol, and trans-chalcone with retention times of 5.83, 9.25, 9.72, 12.31, 13.95, and 17.29 min, respectively. However, other compounds such as catechin, salicylic acid, p-coumaric acid, ferulic acid, vanillic acid, quercetin-3-glucoside, syringic acid, sinapic acid, vanillin, caffeic acid, and gallic acid are not detected in the studied extract. Table 3 provides the extract's quantitative content of phenolic components. The mean content of abundant phenolic compounds is ordered as kaempferol > trans chalcone > naringenin > quercetin > p-hydroxybenzoic acid > rutin, with concentrations of 61230.32, 30972.29, 15259.05, 13754.30, 15259.05, and 589.76 mg/100 g of dry extract, respectively. Pop et al, (Pop et al., 2017) have identified rutin and quercetin in the hydro-ethanolic extract (70 %) of Hedera helix leaves using the cold repercolation extraction method. However, p-Coumaric acid and Kaempferol are not identified. These differences might be associated with many factors, such as geographical conditions, genetic factors, cultivation conditions, season of cultivation, the extraction method, and the type of extraction solvent.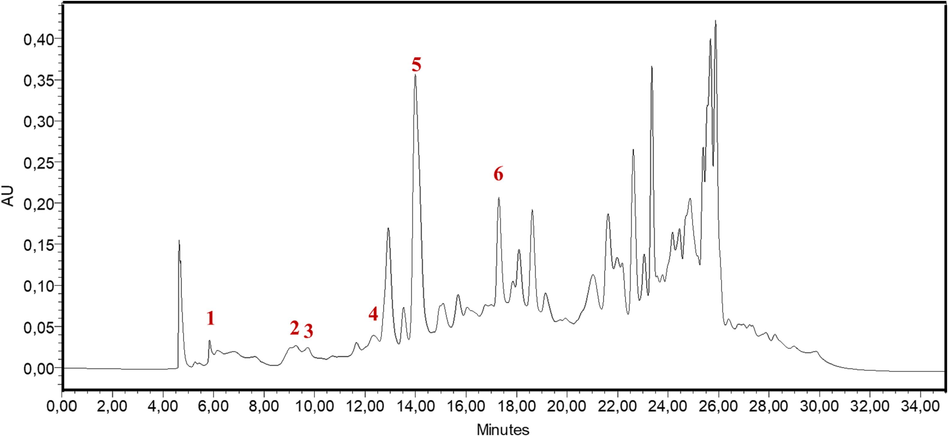
HPLC-DAD chromatogram patterns of HHLE: (1) p-hydroxybenzoic acid, (2) Naringenin, (3) Rutin, (4) Quercetin, (5) Kaempferol, and (6) Trans chalcone.
Peak
Compound name
Rt
(min)
Area
% Area
Concentration (mg/100 g)
Linear regression
1
p-hydroxybenzoic acid
5.83
309,734
0.53
10803.36
y = 3406.7x + 235.1
2
Naringenin
9.25
676,495
1.15
15259.05
y = 443.3x + 15.3
3
Rutin
9.72
258,750
0.44
589.76
y = 578.4x + 8.3
4
Quercetin
12.31
471,316
0.80
13754.30
y = 7989.6x + 116.2
5
Kaempferol
13.95
6,128,556
10.45
61230.32
y = 1000.9x + 12.5
6
Trans-chalcone
17.29
2,281,268
3.89
30972.29
y = 2784x + 133.2
3.2 Mass loss measurements: Effect of the inhibitor concentration and temperature
The effectiveness of corrosion inhibition is assessed using the mass loss approach. Following a 24-hour period of submersion in a 1 M HCl solution at a constant temperature range, from 298 to 328 K, both before and after the introduction of different doses of inhibitor, the mass variation of the XC18 samples is determined. Calculations of the corrosion rate
, inhibition efficiency
and surface coverage (θ) were performed using equations (3), (4) and (5) (Fan et al., 2022).
where and represent the specimen's mass before and after being submerged in different corrosion inhibitor concentrations. S is the XC18 sample's surface area (in cm2), and t is the interval time of the measurement (in hours). and are corrosion rates of XC18 with and without inhibitor, respectively.
Table 4 displays the variation of
,
and θ in response to increasing concentrations of the extract of HHLE. The decrease in
demonstrates the good inhibitory activity of HHLE, and the maximum degree of inhibition efficiency was therefore reached at 1000 mg/L of HHLE. The adsorption of HHLE molecules at the XC18 surface can be used to explain the inhibitory mechanism of the components of this HHLE. It may be attributed to the creation of a protective coating by substituting H2O molecules on the exposed surface (Berrissoul et al., 2022).
Conc. (mg/L)
Temperature (K)
νcorr (mg cm−2 h−1)
ϴ
ηML
(%)
Blank
298
0.724
–
–
308
1.428
–
–
318
2.457
–
–
328
3.190
–
–
125
298
0.378
0.479
47.89
308
0.765
0.464
46.41
318
1.408
0.427
42.69
328
1.947
0.390
38.97
250
298
0.227
0.687
68.73
308
0.478
0.665
66.50
318
0.896
0.635
63.53
328
1.282
0.598
59.81
500
298
0.151
0.792
79.16
308
0.340
0.762
76.18
318
0.640
0.739
73.95
328
0.926
0.710
70.97
1000
298
0.076
0.896
89.57
308
0.191
0.866
86.61
318
0.366
0.851
85.11
328
0.570
0.821
82.14
In order to estimate various thermodynamic parameters ( , and ) and fully comprehend the energy exchange present at the Fe/solution interface, it is crucial to study the effect of temperature on the corrosion mechanism of XC18 in HCl medium without and in the presence of different concentrations of HHLE. Corrosion occurs faster as the temperature rises. In fact, at 1000 mg/L concentration, the corrosion rate at 298 K was 0.076 mg cm–2 h−1 and 0.570 mg cm–2 h−1 at 328 K (Table 4). At 328 K, the inhibition effectiveness for 1000 mg/L dropped from 89.57 % at 298 K to 82.14 %. The observed change indicates the presence of an adsorption/desorption equilibrium of the inhibiting molecules on the XC18 surface as the temperature is raised. The equilibrium in question favors desorption (Zehra et al., 2022). This implies that the adsorption mechanism of HHLE molecules onto the XC18 surface might be predominantly physical and that the interaction between HHLE molecules and XC18 steel is affected by changes in temperature.
3.3 Activation and thermodynamic parameters
The corrosion inhibition mechanism is strongly influenced by thermodynamic and activation parameters. In our present study, we observed a correlation between corrosion rate and temperature that follows an Arrhenius-type equation. This correlation enabled us to determine the activation energies associated with the corrosion phenomena in 1 M HCl and various concentrations of HHLE at varying temperatures. Obtaining the corrosion rate from the mass loss measurements enables the acquisition of kinetic parameters such as activation energy (
), activation enthalpy (
), and activation entropy (
), which can be measured with or without an inhibitor. The
of corrosion process can be determined both with and without HHLE by the Arrhenius Equation (Zehra et al., 2022):
Where, is the dissolution rate, R the gas constant, T absolute temperature and is the frequency factor.
By utilizing transition state theory (Assad et al., 2023), we derived the thermodynamic parameters, namely enthalpy and entropy, associated with metal degradation. These factors are represented by Eq.
Fig. 2(a) shows that the Arrhenius curve, which plots
against 1000/T, is utilized to compute the
in both in the presence and absence of HHLE. Fig. 2(b) demonstrates that by plotting
as a function of
for the dissolution of XC18, straight lines can be obtained both in the presence and absence of HHLE. The slope of these lines is -
, and the intercept is
. Therefore, using the slope and intercept,
and
can be respectively calculated. Table 5 presents the values of
,
, and
for XC18 in 1 M HCl with and without HHLE. The obtained data shows that the
measured under the influence of HHLE is greater than that observed in 1 M HCl, indicating a strong inhibitive action of HHLE by raising the energy barrier for the corrosion process (Noor and Al-Moubaraki, 2008). The observed increase in
as the HHLE extract concentration increased is explained by electrostatic interactions between the HHLE entities and the XC18 surface. In both the absence and presence of HHLE, the activation entropy
has negative values. This shows that the activated complex in the rate-determining step represents an association rather than a dissociation, which means that as one moves from reactants to the activated complex, disordering decreases (Noor and Al-Moubaraki, 2008). The presence of positive values for
indicates that the adsorption process is driven by an endothermic reaction (Quraishi et al., 2020). This result demonstrates that HHLE acts as a protective layer, inhibiting the corrosion of XC18 steel.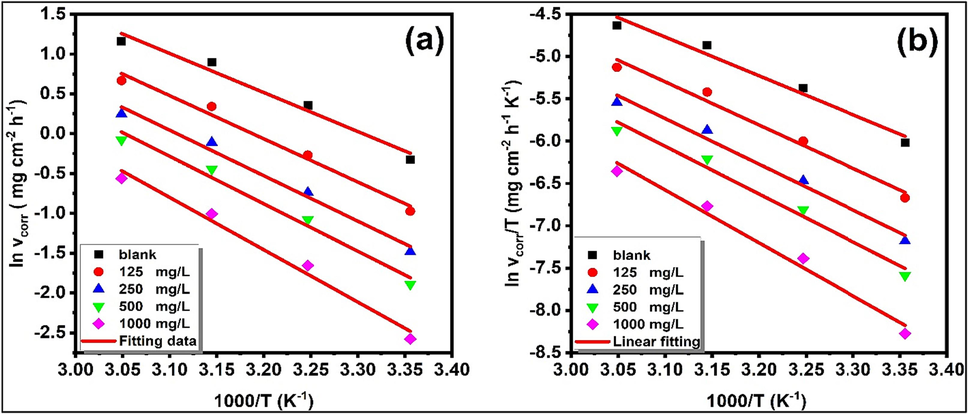
(a) Arrhenius and (b) transition state plots of XC18 steel in 1 M HCl solution with and without various concentrations of HHLE at different temperatures.
Parameters
Concentration (mg/L)
Blank
125
250
500
1000
(kJ mol−1)
40.71
45.04
47.45
49.52
54.59
(kJ mol−1)
38.14
42.46
44.87
46.95
52.02
(J mol−1 K−1)
−118.85
−109.87
−106.05
−102.31
−90.93
3.4 Adsorption isotherm analysis
The active compounds present in the extract can effectively adhere to the metal surface through both chemical and physical mechanisms. Chemisorption is associated with the creation of chemical bonds between the inhibitor's heteroatom lone electron pair and the transition metal's vacant orbital, while physisorption is ascribed to the electrostatic effect. In this adsorption process, the inhibitor molecules replace the H2O molecules on the metal surface. The process can be summarized as follows (Wang et al., 2022):
Once the inhibitor reaches adsorption equilibrium, the adsorptive properties on the metal surface can be quantitatively characterized using an adsorption isotherm. One can use adsorption isotherms to examine how HHLE adheres to the surface of XC18. Fig. 3(a) demonstrates that the adsorption of the HHLE molecules on the surface follows the Langmuir isotherm model. Moreover, various other models, such as Temkin, El-Awady, and Freundlich, were employed to fit the experimental outcomes obtained from mass loss measurements in Fig. 3 (b, c, and d). The following are the expressions used for the adsorption isotherm models (Lima et al., 2020; Wang et al., 2022):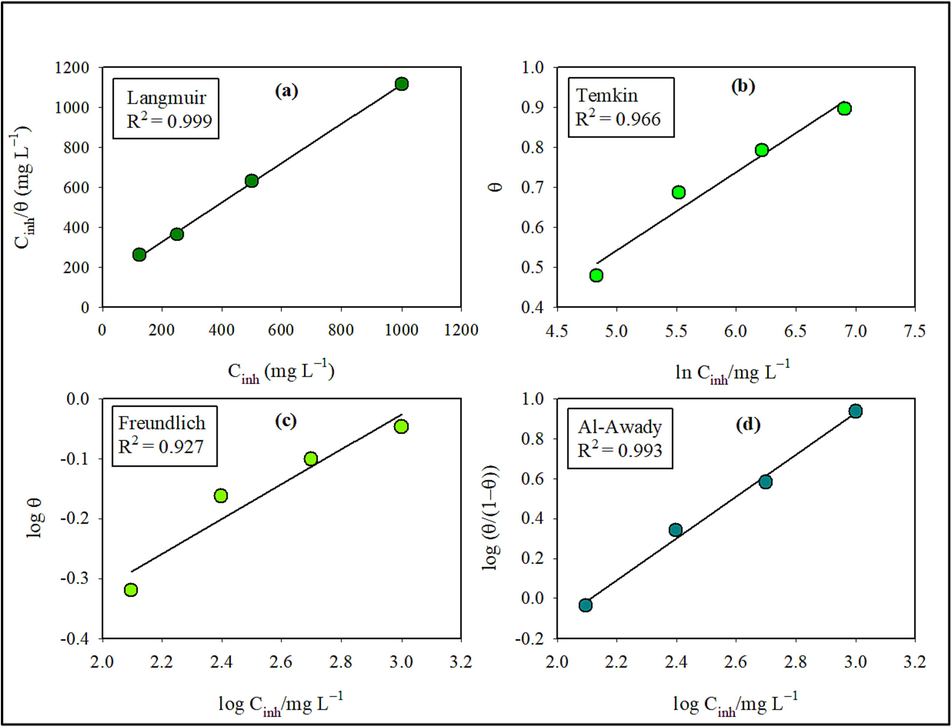
Fitting plots of different isothermal adsorption curves: (a) Langmuir, (b) Temkin, (c) Freundlich and (d) EI-Awady.
To fit the mass loss data, the adsorption isotherm models mentioned above were employed, and the results are presented in Fig. 3. Estimating correlation coefficients by fitting the different results of the above isotherm models is depicted in Table 6. The high linear correlation coefficient (R2 > 0.99) presented in Table 6 provides strong evidence that the Langmuir isotherm effectively describes the experimental data. This confirmation supports the conclusion that HHLE molecules were adsorbed onto the XC18 surface in a single layer, aligning with the assumptions of the Langmuir adsorption isotherm.
Isotherm model
parameters
Values at (298 K)
Langmuir
0.999
slope
0.987
(L mg−1)
7.67 × 10−3
(kJ mol−1)
–22.16
Temkin
0.966
0.196
–2.551
(L mg−1)
0.108
(kJ mol−1)
–28.71
Freundlich
0.927
n
0.292
(L mg−1)
0.126
(kJ mol−1)
–29.09
Al-Awady
0.993
0.954
1.048
(L mg−1)
7.70 × 10−3
(kJ mol−1)
–22.17
Gibbs free energy can be used to determine the adsorption nature of the inhibitor. Typically, the adsorption type (physisorption or chemisorption) of inhibitors can be determined by the value of
. Specifically, if
is less than –40 kJ/mol, it suggests chemisorption type, while a value greater than –20 kJ/mol can be regarded as physisorption. Furthermore,
between –40 kJ/mol to –20 kJ/mol are classified as mixed adsorption (Chaouiki et al., 2020). The calculation of the Gibbs free energy can be expressed using equation (Liu, 2009).
Where 106 (mg/L) denotes the concentration of H2O at the metal/solution interface and is expressed in the same unit as that of the inhibitor, the gas constant (R = 8.314 J mol−1 K−1), absolute temperature (T = 298, 308, 318, and 328 K) and is calculated from Langmuir equation.
A noticeable trend is the decrease in (from 7.67 × 10−3 to 5.64 × 10−3 ) with increasing temperature (298 K to 328 K). This decrease suggests that the strength of the adsorptive binding between the HHLE molecules and XC18 surface diminishes as the temperature rises, potentially due to the molecules desorbing from the metal surface (Zhang et al., 2021). At 298 K, 308 K, 318 K and 328 K, the calculated values –22.16 kJ/mol, –22.82 kJ/mol, –23.18 kJ/mol, and –23.55 kJ/mol, respectively. This indicates that the adsorption of HHLE components on the XC18 steel surface involved both physisorption and chemisorption processes, with the physisorption type predominant.
3.5 Electrochemical behavior by HHLE inhibitor
3.5.1 Polarization behavior
The impact of HHLE on the anodic dissolution of XC18 steel and the reduction of cathodic H+ in a 1 M HCl solution was evaluated using the potentiodynamic polarization technique (Thakur et al., 2022). Fig. 4 displays the polarization plots obtained from the experiment. By analyzing these curves, various potential kinetic parameters, such as corrosion current densities (
), corrosion potential (
), inhibition effectiveness (
), anode Tafel slope (
), and cathode Tafel slope (
) were determined and are listed in Table 7. By comparing the polarization curves in the presence and absence of the HHLE extract, it is evident from Table 7 that the addition of the HHLE inhibitor leads to a significant reduction in corrosion current density. The decreasing trend observed in Fig. 4 provides evidence that the inhibitor effectively inhibited the corrosion of XC18. This was achieved by reducing the process of H2 evolution at the cathode and anodic dissolution rate, along with the formation of a protective film on the surface of XC18. As the concentration of the HHLE increased, the estimated value of
derived from the extrapolation of the Tafel traces decreased gradually (Table 7). Meanwhile, the values of
increased (Table 7), reaching a peak value of 92.67 % at a concentration of 1000 mg/L. This suggests that the extract can effectively reduce the process of metal dissolution by adsorbing its molecules onto the specific sites that play a role in the process of dissolution (Chaouiki et al., 2022b). Moreover, the presence of HHLE inhibitors results in alterations to the Tafel coefficients (βc and βa). Indeed, various factors can influence the polarization of the electrode. With the addition of corrosion inhibitors, molecules of this inhibitor have the capacity to adsorb onto the surface of the XC18, creating a protective film. The presence of adsorbed molecules induced a modification in the properties of the interface between the solution and the XC18 steel. These modifications directly influenced the polarization of the electrode by affecting both the electrode reaction and the diffusion properties of the medium. From the data presented in Table 6, it is apparent that both the cathode slope and the anode slope exhibit substantial changes when compared to the blank sample. This observation suggests that the synthesized extract functions as a mixed-type corrosion inhibitor (Chafiq et al., 2021). The complex organic compounds present in the composition of HHLE are typically responsible for anti-corrosion activity. The molecular structures of these organic substances typically include polar functional groups with oxygen atoms and possess either conjugated double bonds or aromatic rings within their molecular structures., which act as the primary sites of adsorption. The inhibition effect on corrosion can also attributed to the existence of heterocyclic components, including flavonols, phenolic acid, flavones, and flavanols, which are capable of forming an adsorbed film on the metal surface (Zehra et al., 2022). The inhibitor under investigation is identified as a mixed-type inhibitor for the following reasons: the first is the observed shift of both anodic and cathodic Tafel traces towards lower current density in Fig. 4, which suggests that the tested compounds are regulating both cathodic and anodic reactions. The second reason is that the variation in
(in mV) between the blank solution without inhibitor and solutions containing different inhibitor concentrations is within 85 mV (Elaryian et al., 2022; Sun et al., 2022). Moreover, as the concentration of our extract increases, the inhibitory effectiveness also improves, due to a large fraction of the electrode surface being covered by adsorption (Altunbaş Şahin, 2022).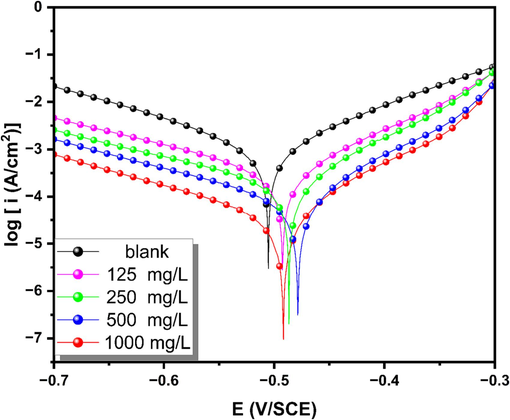
Potentiodynamic polarization curves for XC18 steel in 1 M HCl at 298 K with and without different concentrations of HHLE.
Conc. (mg/L)
(mV/ECS)
(μA.cm−2)
(mV/dec)
(mV/dec)
η (%)
Blank
−505.1
671.83
−138.31
106.04
–
125
−492.0
218.77
−165.01
91.65
67.43
250
−497.8
135.89
−165.13
81.43
79.77
500
−494.8
99.18
−158.98
81.69
85.23
1000
−493.7
49.19
−146.84
81.03
92.67
3.5.2 Impedance spectra (EIS)
3.5.2.1 Concentration effect
Changes occurring at the electrode/solution interface, which are induced by substances like corrosion inhibitors, can be explored using the EIS technique. The Nyquist and Bode graphs for XC18 steel are depicted in Fig. 5(a) and Fig. 5(b), respectively. These plots illustrate the electrode's behavior in the absence of any treatment (blank) and at varying concentrations of HHLE. A circuit consisting of electrolyte resistance (
), and constant phase element
) placed in parallel with a charge transfer resistance (
) (Fig. 6) was employed to fit the results. At various concentrations, the Nyquist and Bode plots exhibit similar shapes with a singular capacitive loop for all concentrations, but with differing intensity or height, indicating that the primary mode of control for the decomposition of XC18 in the HCl medium appears to be the charge transfer process, while the corrosion mechanism remains unaffected (Silva et al., 2021). Increasing the concentration results in a higher peak and diameter, indicating greater resistance in the solution. This may be attributed to the HHLE inhibitor forming a shielding layer on the XC18 steel surface, thereby reducing the corrosion rate in the corrosive medium. It is worth noting that these capacitive loops are not perfectly semicircular, which is likely due to the frequency dispersion effect (Elaryian et al., 2022). By measuring the diameter of the half-circle in the Nyquist plot,
was determined, and
was calculated through fitting, and subsequently converted into the capacitance of the double layer (
). The equations used to determine the impedance of the double-layer
an double layer capacitance (
in circuits that include
are as follows (Kalkhambkar et al., 2022; Mobin and Rizvi, 2017):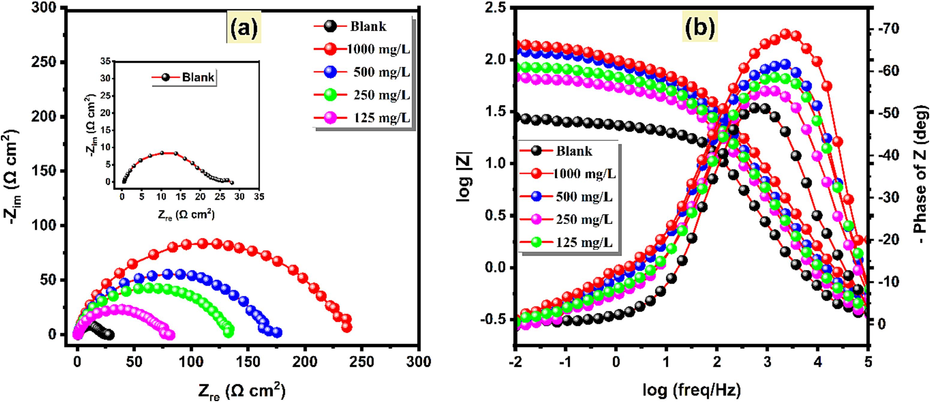
EIS plots; (a) Nyquist plot, and (b) Bode plot for the corrosion of XC18 in the absence and presence of diverse concentrations of HHLE at 298 K.
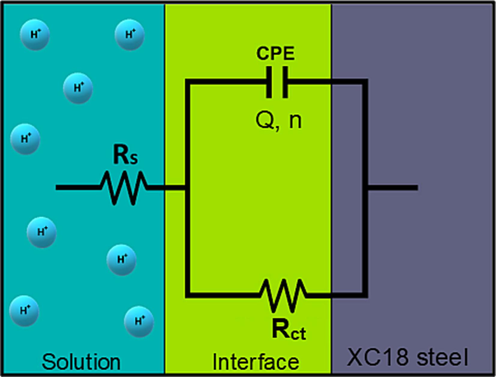
The proposed circuit model for impedance results modeling.
The value is denoted by , the heterogeneous constant is represented by , where –1 < < 1. In cases of semicircles, the value of falls between 0.5 and 1, is the imaginary unit. In the given formula, , where refers to the frequency at which the highest value of the imaginary component is observed.
With the intention of confirming the impact of HHLE on the resistive-capacitive properties of the protective layer, Bode diagrams were employed to analyze the impedance modulus |
|. Fig. 5(b) demonstrates that HHLE effectively enhanced |
| at a low frequency of 0.01 Hz. Furthermore, the inhibitor's concentration-dependent effect on the phase angle was observed, as it resulted in a higher phase angle than that of the untreated sample and increased in magnitude with increasing HHLE concentration. This indicates that the HHLE molecules were adsorbed onto the metal surface, impeding access to reaction sites. The non-ideal capacitor behavior of the XC18-steel/electrolyte interface is indicated by the phase angle deviation from 90°. The impedance response for both treated and untreated samples were quantitatively interpreted through the simulation of electronic circuit components. This generated a set of parameter values that are summarized in Table 8 such as
,
,
, n (phase shift),
(double layer capacitance), χ2 (Goodness of fit), and
. Reportedly, values of χ2 ranging from 10−3 to 10−5 are considered indicative of optimal fitting, signifying that the equivalent circuit diagram is stable and dependable (Khayatkashani et al., 2022).
Conc. (mg/L)
)
n
(μF cm−2)
η
(%)
Blank
0.40
22.18
3.60
0.80
71.23
1.0
–
125
0.40
72.76
2.95
0.77
36.65
0.3
69.51
250
0.45
129.30
2.51
0.77
31.18
0.9
82.84
500
0.45
170.40
1.92
0.78
23.36
0.1
86.98
1000
0.40
245.60
1.25
0.79
16.73
1.1
90.96
Table 8 shows that the charge transfer resistance ( ) and corrosion inhibition efficiency augmented with the rising concentration of HHLE while the and values decreased, this suggests effective substitution of water molecules on the surface with HHLE molecules, leading to a well-coated surface (Nid-Bella et al., 2023). The presence of HHLE molecules on the metal surface causes a decrease in , which in turn leads to a decrease in the dielectric constant or an increase in the thickness of the double layer (Chaouiki et al., 2022a). At 1000 mg/L, the maximum corrosion inhibition efficiency of 90.96 % was achieved, which is consistent with the results of weight loss and potentiodynamic polarization investigations. After the introduction of the inhibitor, the value did not exhibit significant changes. Moreover, the phase shift values ( ) did not exhibit significant alterations. This suggests that the XC18 dissolution in a 1 M HCl media was governed by charge transfer (Thakur et al., 2022). During EIS experiments, measurements are typically obtained at or near the OCP, which is typically within a range of ± 10 mV. As a consequence, the results from PDP and EIS may exhibit slight difference (Silva et al., 2021). Nevertheless, the trend observed for the inhibition efficiency values remains consistent between PDP and EIS.
3.5.2.2 Effect of long-term immersion
Following the examination of the effect of concentration, the primary focus of this part is the exploration of immersion time effect on the corrosion performance of synthesized extract. We anticipate that this variable plays a crucial role in influencing the inhibition performance. The study aims to evaluate the stability of HHLE in the presence of a corrosive environment. The study monitors its performance throughout a timeframe ranging from 30 min to 48 h of immersion, maintaining consistent conditions of inhibitor concentration (1000 mg/L) and a temperature of 298 K. Fig. 7 depicts a noticeable trend in which the diameters of the capacitive semicircles steadily diminish with an increase in the immersion time in the corrosive solution. The figure distinctly shows a substantial decrease in
of XC18 with the prolonged immersion time, both in the absence and presence of 1000 mg/L of HHLE. After 48 h of immersion, the
values are approximately 9.07 Ω cm2 without HHLE and around 58.37 Ω cm2 with the addition of HHLE. This is primarily attributed to the development of a protective layer on the electrode surface, which occurs upon contact with the inhibited corrosive solution. The formation of a protective film, composed of organic compounds, on the surface of XC18 is influenced not only by the molecular structure of the studied inhibitors but also by experimental conditions, such as immersion time. These findings suggest the continuous deposition of the inhibitor film on the XC18 surface even during extended immersion times.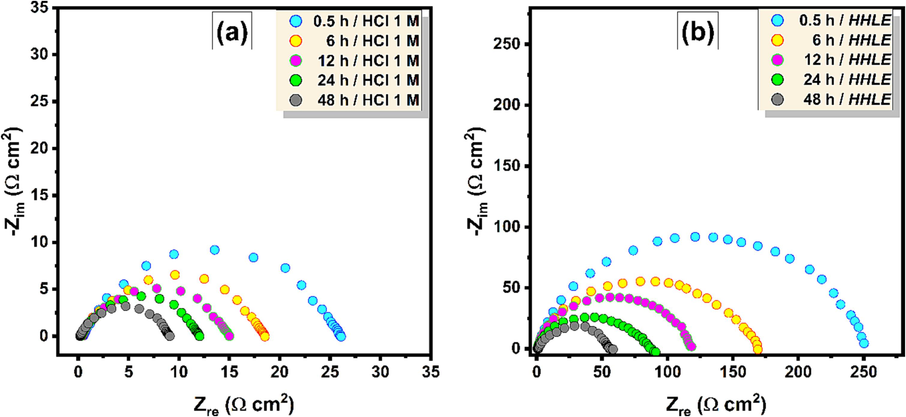
Time-dependent impedance responses of the XC18 steel corrosion, presented in the Nyquist plots of (a) uninhibited and (b) inhibited (1000 mg/L of HHLE) for prolonged immersion in 1 M HCl solution at 298 K.
3.6 Formation of protective film by HHLE inhibitor
The microscopic surface morphologies and chemical compositions of the samples were analyzed using SEM equipped with EDS. Fig. 8 illustrates the microstructures of XC18 steel after immersion in inhibited and uninhibited 1 M HCl solutions for 48 h. It was observed that the steel plate without treatment had sustained significant damage in the form of holes and crevices on its surface, as shown in Fig. 8(b), which verify the extensive deposition of corrosion byproducts on the surface of the XC18 steel, implying that the XC18 sample is highly susceptible to attack in HCl media. The image presented in Fig. 8(d) provided visual evidence that the introduction of 1000 mg/L of HHLE led to the development of a shielding layer that impedes the corrosion of XC18 steel in the 1 M HCl medium (Dehghani et al., 2023). A relatively smooth surface is well protected against corrodent attack, with the polishing scratches still obvious on the metal surface. The EDS spectra of XC18 steel immersed in 1 M HCl and with 1000 mg/L of the HHLE are shown in Fig. 8(a,b) and (c,d), respectively. Fig. 8(a) displays the presence of Fe, Cl, and O on the surface. These elements are present on the surface due to the presence of corrosion products as a result of the oxidation of iron during the corrosion process. In contrast, Fig. 8(c) shows that the presence of HHLE inhibitor reduces the O and Cl content and increases the Fe peak intensity compared to 1 M HCl. This suggests that the iron surface is protected from the corrosive chloride ion. The HHLE shields the metal surface by forming a protective film on the metal surface. These findings validate the significant values of inhibition efficiency obtained through mass loss and electrochemical methods, which can be linked to the successful shielding film formed on the XC18 surface by the HHLE inhibitor.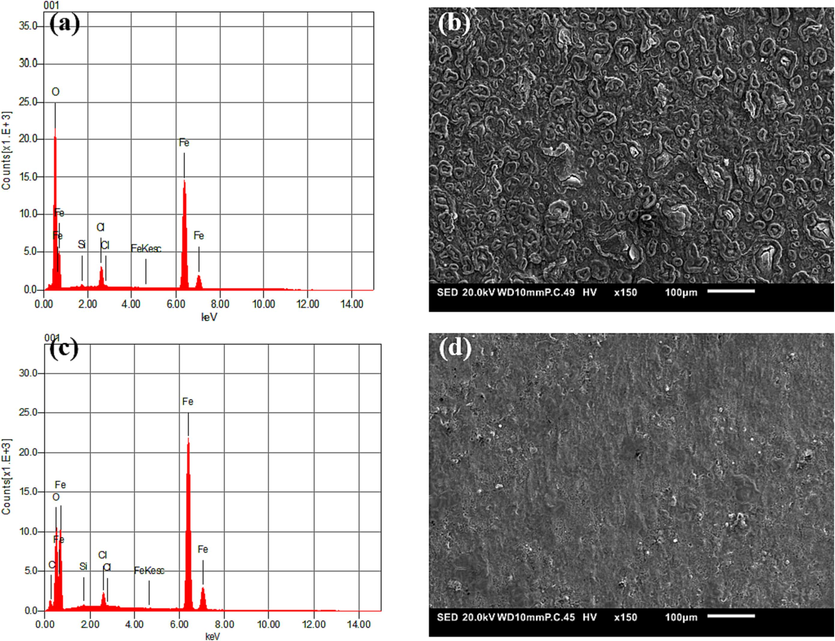
Surface morphology and corresponding EDS spectra of XC18 steel after prolonged corrosion test in (a, b) uninhibited and (c, d) inhibited HCl solution containing 1000 mg/L of HHLE for 48 h.
3.7 Theoretical approach
In order to enhance our comprehension of the interaction between inhibitor molecules and the XC18 surface and to provide an explanation for these experimental findings, density functional theory (DFT) computations were performed. The calculations specifically targeted the two predominant molecules within HHLE, namely Kaempferol (KMP) and trans-Chalcone (CHL), which constitute higher percentages of the plant's chemical composition, as indicated in Table 3.
3.7.1 Role of electron donor–acceptor character of HHLE via DFT calculations
The evaluation of charge transfer (CT) effects on the quantum calculation (QC) parameters resulting from complex formation necessitates the utilization of suitable DFT-based computational methods. The frontier orbitals consist of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), and the energy gap between them is referred to as the energy gap (
). Typically, the HOMO energy characterizes the ionization potential, while the LUMO energy reflects the electron affinity (Elmacı et al., 2015). Initially, we assessed the physicochemical characteristics of the separated molecules using the frontier molecular orbital (FMO) theory. Fig. 9 depicts the molecular orbital (MO) spreads of KMP and CHL. The figure shows the optimized structures of KMP and CHL molecules and FMO distribution, respectively. The relative positions of the HOMO and LUMO energy levels, as well as the energy gap (
) of the molecules under investigation, can be utilized to anticipate the interaction between each molecule with Fe2+. Typically, a molecule that exhibits a higher HOMO energy and a lower LUMO energy (smaller
) is a potent adsorption inhibitor due to its ability to donate electrons to the empty orbitals of Fe (Shahini et al., 2021). The higher reactivity of the KMP compound compared to the CHL compound is evident through its significantly lower gap energy (
) value, making it a prominent candidate for corrosion inhibition.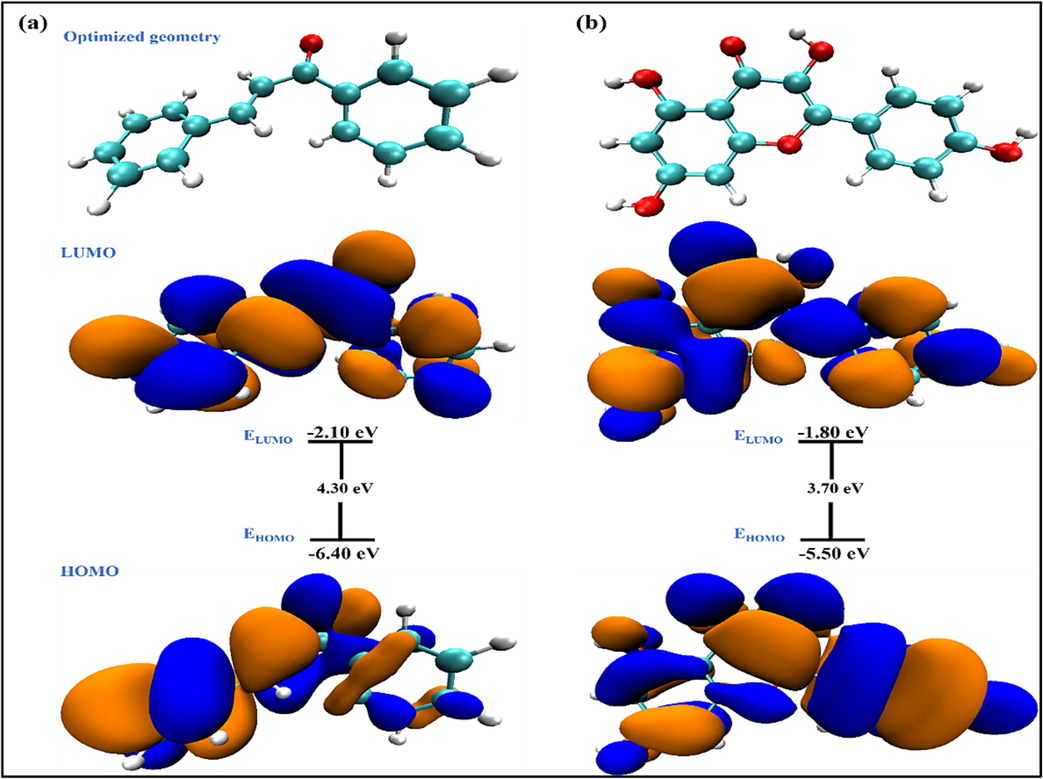
Geometry-optimized structure and HOMO/LUMO orbital distribution of (a) CHL and (b) KMP.
3.7.2 Computational insights into the significant interactions between Fe2+ and HHLE molecules
To gain a deeper understanding of how molecule-Fe2+ interactions influence the overall electronic properties of complex systems, an investigation was conducted on the capacities of KMP and CHL to create complexes with Fe2+. Firstly, an analysis was performed to evaluate the physical or chemical coordination of KMP and CHL molecules in forming complexes with Fe2+ the intermolecular interactions between KMP and CHL molecules using a combination of techniques including the distribution of MO energy levels, total density of states (TDOS), and a noncovalent interaction (NCI) method called reduced density gradient (RDG). Fig. 10 displays the acquired results. Fig. 10(a) shows the optimized KMP/Fe/CHL structure. In the process of forming the KMP/Fe/CHL complex, the LUMO spans across both molecules, encompassing their entire structures, while the HOMO is confined to the KMP molecule alone (Fig. 10(b,c)). Fig. 10(d) illustrates the energy gap and DOS spectra of the KMP/Fe/CHL system and also provides partial densities of state (PDOS) for KMP and CHL, which can aid in comprehending their impact on the TDOS. The PDOS curves reveal that KMP and CHL had comparable contributions to the TDOS. It is noteworthy that the interaction between KMP, CHL, and Fe2+ leads to a small energy gap of 0.96 eV. This reduction indicates that changes in charge density occur during the interaction, resulting in a straightforward transfer of charge (Chaouiki et al., 2022a). Establishing coordination bonds and enabling charge transfer (CT) are greatly aided by the electron-donor group, thus explaining the observed reduction in the energy gap. These findings validate the proposed calculation model, which demonstrates a consistent correlation between molecular reactivity during molecule/Fe interactions and the experimental outcomes.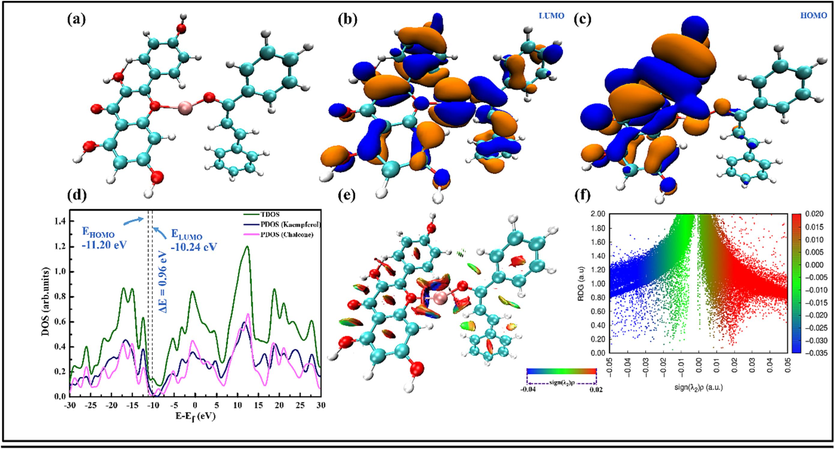
(a) Optimized KMP/Fe/CHL complex, (b) LUMO, (c) HOMO, (d) DOS spectra, (e) RDG isosurfaces and (f) scatter plot.
To explore the potential impact of intermolecular interactions on the adsorption power of the inhibitor molecules, an RDG analysis was conducted to uncover the chemical and weak interaction regions within the molecule-Fe2+ complex. Despite being weaker than covalent bonds, NCI still has a significant impact on the inhibitory effect that cannot be disregarded. The RDG method plays a crucial role as a valuable and intuitive tool in the study and analysis of intermolecular interactions (Chaouiki et al., 2022b). It provides insights into the fundamental bonding mechanism between Fe ions and inhibitor molecules, enhancing our understanding of these processes. In order to comprehend these interactions, RDG analyses were conducted on the KMP/Fe/CHL complex. The RDG isosurfaces and scatter plot (Fig. 10(e,f)) utilize various colors to represent the values of the sign( )ρ function, which effectively characterizes the types and strengths of noncovalent interactions. The strength of interactions can be determined by the electron density (ρ), while the sign of can be used to differentiate between bonded ( < 0) and non-bonded ( > 0) interactions. The occurrence of hydrogen bond interactions, van der Waals (vdW) effects, and the creation of bonding interactions are denoted by a larger negative sign( )ρ for H-bonding (blue), a smaller sign ( )ρ (green) for vdW interaction, and a more positive sign( )ρ for the steric effect (red), respectively (Chafiq et al., 2023b). As depicted in Fig. 10(e), the KMP and CHL establish van der Waals (vdW) interactions and hydrogen bonds with Fe2+ through the involvement of OH groups. Furthermore, the system demonstrates attractive intermolecular interactions, and steric effects present within the aromatic rings. In addition, the scatter plot provides a measure of both the intensity and nature of intermolecular interactions. The presence of spikes in the negative and highly negative region of sign( )ρ, as well as the observed steric effect at positive values in Fig. 10(f), demonstrate the robust interactions between the inhibitor molecules and the Fe surface.
3.7.3 Adsorption behavior and interfacial mechanism of KMP and CHL on the Fe (1 1 0) surface by DFTB calculations
To obtain a comprehensive understanding of the inhibition mechanism and verify the structural configurations of the KMP and CHL on the Fe surface, we conducted DFTB simulations. These simulations provide valuable insights into the actual inhibition mechanism and allow us to examine how the adsorption mechanism may be influenced by the presence of these molecules. To model the intermolecular interactions of KMP and CHL, various initial distances were chosen between the reactive sites and the surface. This allowed us to examine and analyze the range of relationship/interactions between the molecules and the surface. Fig. 11(a) and 11(b) depict the interactions between the inhibitor molecules in parallel geometries with the Fe surface, both before and after adsorption optimization. The process of adsorption leads to a notable alteration in the geometry, resulting in a significant decrease in the optimized bond lengths (dFe-O) when compared to their initial values (Fig. 11(b)). In its parallel state, KMP forms bonds with Fe atoms through its three oxygen atoms, with bond lengths measuring 1.43 Å, 1.95 Å, and 2.29 Å (Fig. 11(b)). Similarly, CHL binds to Fe atoms through its oxygen atom, with a bond length of 2.00 Å. According to Cordero et al., the combined covalent radii of Fe-O (rO + rFe) were reported to be approximately 1.98 Å (Cordero et al., 2008). This value represents the combined size of the covalent radii for oxygen (rO) and iron (rFe) atoms when they form a covalent bond. The chemical adsorption of KMP and CHL on the Fe (1 1 0) surface can be considered plausible, as the bonds formed between the active sites of the molecules and the iron atoms fall within the range of the sum of the covalent radii. The involvement of oxygen atoms in the adsorption-induced interaction mechanism indicates their role in facilitating the formation of chemical bonds with the iron surface. To evaluate the adsorption strength, the system's adsorption energy (
) was computed. This energy corresponds to the release of energy when one mole of inhibitor species is adsorbed onto the metal surface (Quraishi et al., 2020):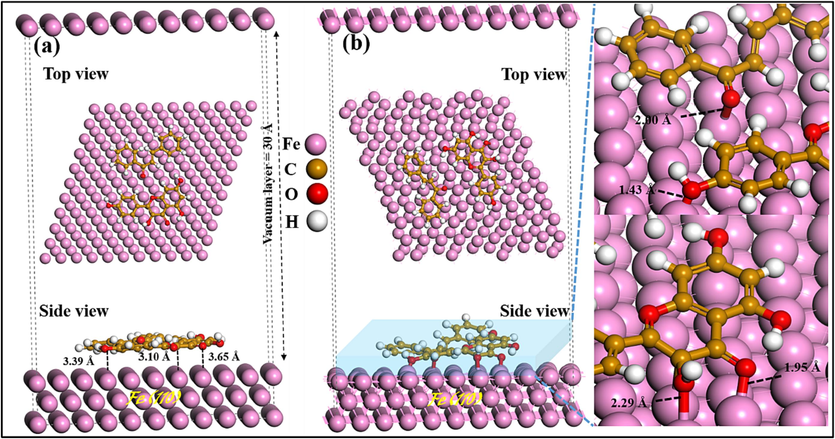
Inhibitor molecules adsorbed on Fe (1 1 0) surface: Side and top views with initial (a) and final (b) adsorption profiles.
represents the overall energy of the entire system. indicates the combined energy of the metal surface and solution in the absence of the inhibitor. corresponds to the total energy associated with the inhibitor.
It is recognized that a more negative signifies a more robust interaction between the inhibitor and the metal (Singh et al., 2021). The obtained value is −5.83 eV (−134.44 kcal/mol). The presence of negative values in the interaction energy of the system indicates the spontaneous adsorption of these molecules onto the atoms of the Fe (1 1 0) surface. Hence, these outcomes align with the experimentally derived results, confirming their agreement.
3.8 Possible adsorption mechanism
To emphasize the interactions between KMP, CHL, and the surface, as described in the MD simulations and DFT calculations, we suggest an interfacial process, illustrated in Fig. 12. Hence, based on the molecular configuration, KMP and CHL molecules are adsorbed onto the XC18 surface through two fundamental modes of adsorption. During the initial stage, the inhibitor molecules undergo chemisorption, leading to the replacement of H2O molecules at the surface and the exchange of electrons between oxygen and the surface. The parallel adsorption form not only offers optimal stability but also promotes the self-organization of the molecules. In corrosive aqueous media, the surface tends to accumulate negative charges (Cl −) and H2O molecules. Thus, after this stage, KMP and CHL molecules can readily interact with the metal surface through electrostatic interactions via Cl− and H2O. Furthermore, as time progresses, the HHLE molecules adsorbed on the surface have the potential to spontaneously bond together and form a complete and stable tissue structure through hydrogen bonding. As a result of these interactions, the HHLE molecules effectively span a significant portion of the metal surface, providing extensive coverage.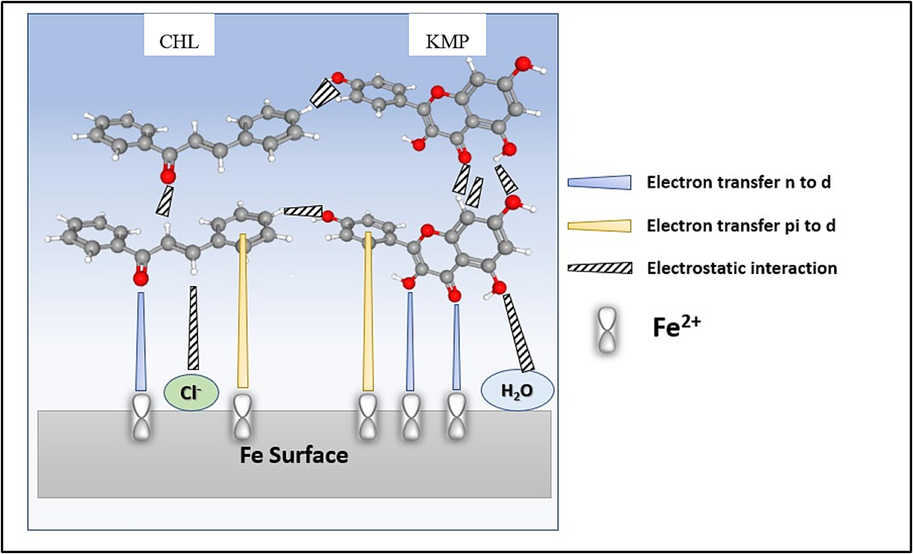
Schematic depiction of the interaction mechanism between HHLE molecules and XC18 surface for extraordinary corrosion performance.
4 Conclusion
HHLE was obtained via a decoction process. To evaluate its performance as an environmentally friendly corrosion inhibitor for XC18 steel in acidic solutions, a comprehensive analysis was conducted using electrochemical/surface techniques and theoretical calculations. Based on these investigations, the following conclusions were drawn.
-
The main phenolic constituents of HHLE were kaempferol, trans-chalcone, naringenin, quercetin, p-hydroxybenzoic acid, and rutin. HPLC-DAD analysis was employed to confirm the presence of these chemical components. These components exhibit a significant potential as corrosion inhibitors.
-
As a mixed-type inhibitor, HHLE exhibited remarkable effectiveness in inhibiting XC18 steel corrosion in an HCl acid solution. With a concentration of 1000 mg/L, the inhibition efficiency (η) can reach up to 92.67 %.
-
As the concentration of HHLE increases, the inhibition efficiency (η%) increases at a constant temperature. Conversely, when the temperature rises, there is a modest decrease in η(%) for the same concentration.
-
The adsorption of HHLE onto the XC18 steel surface conforms to the Langmuir adsorption model, as confirmed by fitting the weight loss data. This adsorption process is spontaneous and involves a combination of chemisorption and physisorption.
-
SEM and EDS analyses were conducted, revealing a substantial reduction in the corrosion proportion of the XC18 steel surface. Additionally, the formation of a protective film on the XC18 steel surface was observed, confirming the outstanding inhibitory capability of HHLE, as determined through the electrochemical and weight loss methods.
-
The anticorrosion mechanism of HHLE is further elucidated through quantum chemistry calculations and DFTB simulations. According to the results, two of the active molecules of HHLE were adsorbed onto the XC18 steel surface in a near-parallel orientation, suggests that the covalent and non-covalent interactions including π-π interaction, vdW forces, hydrogen and chemical bonding play a key role in the adsorption process and are important for the effective electron transfer between the molecules and metal surface. This arrangement optimizes the contact area between those molecules and the iron surface, effectively inhibiting steel corrosion.
CRediT authorship contribution statement
Hamid Ahchouch: Conceptualization, Methodology, Investigation, Writing – original draft. Mohamed El house: . Aisha H. Al-Moubaraki: Visualization, Formal analysis. Ehteram A. Noor: Visualization, Formal analysis. Abdallah Hadfi: . Ali Driouiche: . Lahcen Bammou: Visualization, Formal analysis, Supervision. M'hamed Belkhaouda: Visualization, Formal analysis, Supervision. Rachid Salghi: Visualization, Formal analysis, Supervision. Maryam Chafiq: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Abdelkarim Chaouiki: Conceptualization, Investigation, Writing – original draft, Writing – review & editing, Supervision. Young Gun Ko: Funding acquisition.
Acknowledgements
This work was supported by the Fundamental-Core National Project of the National Research Foundation (NRF) funded by the Ministry of Science and ICT, Republic of Korea (2022R1F1A1072739).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Natural polyphenols: An overview. Int. J. Food Prop.. 2017;20:1689-1699.
- [CrossRef] [Google Scholar]
- Inter- and intra-molecular synergism in designing MgO-MCC composite-based coating: An efficient inhibitor for excellent anticorrosion performance. Process Saf. Environ. Prot.. 2023;177:1461-1476.
- [CrossRef] [Google Scholar]
- Experimental and theoretical studies of acridine orange as corrosion inhibitor for copper protection in acidic media. J. Indian Chem. Soc.. 2022;99:100358
- [CrossRef] [Google Scholar]
- DFTB+, a Sparse Matrix-Based Implementation of the DFTB Method. Chem. A Eur. J.. 2007;111:5678-5684.
- [CrossRef] [Google Scholar]
- Evaluating the adsorption and corrosion inhibition capabilities of Pyridinium - P - Toluene Sulphonate on MS in 1 M HCl medium: An experimental and theoretical study. Inorg. Chem. Commun.. 2023;153:110817
- [CrossRef] [Google Scholar]
- Almond waste extract as an efficient organic compound for corrosion inhibition of carbon steel (C38) in HCl solution. Sustain. Chem. Pharm.. 2022;27:100677
- [CrossRef] [Google Scholar]
- Exploitation of a new green inhibitor against mild steel corrosion in HCl: Experimental, DFT and MD simulation approach. J. Mol. Liq.. 2022;349:118102
- [CrossRef] [Google Scholar]
- Estimation of the influence of the environmental factors on the accumulation of phytochemicals and antioxidant capacity in the ivy leaves (Hedera helix L.) Nat. Prod. Res.. 2022;36:1014-1019.
- [CrossRef] [Google Scholar]
- Antipsychotic drug waste: A potential corrosion inhibitor for mild steel in the oil and gas industry. Waste Manag.. 2022;145:38-47.
- [CrossRef] [Google Scholar]
- The study of the methanolic extract of “Pelargonium gravelons” as a green corrosion inhibitor of mild steel in H2SO4 0.5 M. Chemical Data. Collections. 2023;48:101084
- [CrossRef] [Google Scholar]
- Adsorption mechanism of 3-(1,4-disubstituted-1,2,3-triazolyl) uridine nucleosides against the corrosion of mild steel in HCl. Mater. Chem. Phys.. 2021;268:124742
- [CrossRef] [Google Scholar]
- Interface engineering of LDH-based material as efficient anti-corrosive system via synergetic performance of host, interlayers, and morphological features of nature-mimic architectures. Chem. Eng. J. 2023
- [Google Scholar]
- Interface engineering of LDH-based material as efficient anti-corrosive system via synergetic performance of host, interlayers, and morphological features of nature-mimic architectures. Chem. Eng. J.. 2023;462:142239
- [CrossRef] [Google Scholar]
- Comprehensive assessment of corrosion inhibition mechanisms of novel benzimidazole compounds for mild steel in HCl: An experimental and theoretical investigation. J. Mol. Liq.. 2020;320:114383
- [CrossRef] [Google Scholar]
- Advanced prediction of organic–metal interactions through DFT study and electrochemical displacement approach. J. Magnesium Alloys 2022 S2213956722001062
- [CrossRef] [Google Scholar]
- Computational molecular-level prediction of heterocyclic compound–metal surface interfacial behavior. J. Colloid Interface Sci.. 2022;622:452-468.
- [CrossRef] [Google Scholar]
- Advanced prediction of organic–metal interactions through DFT study and electrochemical displacement approach. J. Magnesium Alloys. 2023;11:301-316.
- [Google Scholar]
- Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem. Phys. Lett.. 1996;255:327-335.
- [Google Scholar]
- Ab initio study of ionic solutions by a polarizable continuum dielectric model. Chem. Phys. Lett.. 1998;286:253-260.
- [Google Scholar]
- Das, S., Ray, A., Nasim, N., Nayak, S., Mohanty, S., 2019. Effect of different extraction techniques on total phenolic and flavonoid contents, and antioxidant activity of betelvine and quantification of its phenolic constituents by validated HPTLC method. 3 Biotech 9, 37. 10.1007/s13205-018-1565-8.
- Potential role of a novel green eco-friendly inhibitor in corrosion inhibition of mild steel in HCl solution: Detailed macro/micro-scale experimental and computational explorations. Constr. Build. Mater.. 2020;245:118464
- [CrossRef] [Google Scholar]
- Synergistic anticorrosion effect of Brassica Hirta phytoconstituents and cerium ions on mild steel in saline media: Surface and electrochemical evaluations. Colloids Surf. A Physicochem. Eng. Asp.. 2023;656:130503
- [CrossRef] [Google Scholar]
- Synthesis, characterization of novel coumarin dyes as corrosion inhibitors for mild steel in acidic environment: Experimental, theoretical, and biological studies. J. Mol. Liq.. 2022;346:118310
- [CrossRef] [Google Scholar]
- Synthesis, molecular structure and computational study of (Z)-2-((E)-4-nitrobenzylidene)hydrazone)-1,2-diphenylethan-1-one. J. Mol. Struct.. 2015;1099:83-91.
- [CrossRef] [Google Scholar]
- The syntheses, molecular structure analyses and DFT studies on new benzil monohydrazone based Schiff bases. J. Mol. Struct.. 2018;1162:37-44.
- [CrossRef] [Google Scholar]
- Novel benzildihydrazone based Schiff bases: Syntheses, characterization, thermal properties, theoretical DFT calculations and biological activity studies. J. Mol. Struct.. 2019;1184:271-280.
- [CrossRef] [Google Scholar]
- Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B. 1998;58:7260-7268.
- [CrossRef] [Google Scholar]
- Inter-component synergetic corrosion inhibition mechanism of Passiflora edulia Sims shell extract for mild steel in pickling solution: Experimental, DFT and reactive dynamics investigations. Sustain. Chem. Pharm.. 2022;29:100821
- [CrossRef] [Google Scholar]
- Cucumber (Cucumis sativus L.) Leaf Extract as a Green Corrosion Inhibitor for Carbon Steel in Acidic Solution: Electrochemical, Functional and Molecular Analysis. Molecules. 2022;27:3826.
- [CrossRef] [Google Scholar]
- Frisch, M. ea, Trucks, G., Schlegel, H., Scuseria, G., Robb, M., Cheeseman, J., Scalmani, G., Barone, V., Petersson, G., Nakatsuji, H., 2016. Gaussian 16.
- Analysis of corrosion inhibition of Kleinhovia hospita plant extract aided by quantification of hydrogen evolution using a GLCM/SVM method. Int. J. Hydrogen Energy. 2023;48:15392-15405.
- [CrossRef] [Google Scholar]
- Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq.. 2018;256:565-573.
- [CrossRef] [Google Scholar]
- Datura stramonium plant seed extracts as a new green corrosion inhibitor for mild steel in 1M HCl solution: Experimental and surface characterization studies. Sustain. Chem. Pharm.. 2023;34:101170
- [CrossRef] [Google Scholar]
- Advances of plant-extracted inhibitors in metal corrosion reduction – Future prospects and challenges. Results Chem.. 2023;5:100883
- [CrossRef] [Google Scholar]
- Temperature and extraction methods effects on yields, fatty acids, and tocopherols of prickly pear (Opuntia ficus-indica L.) seed oil of eastern region of Morocco. Environ. Sci. Pollut. Res. 2022:1-9.
- [CrossRef] [Google Scholar]
- Kalkhambkar, A.G., S K, R., Manjanna, J., Malimath, G.H., 2022. Effect of expired doxofylline drug on corrosion protection of soft steel in 1 M HCl: Electrochemical, quantum chemical and synergistic effect studies. Journal of the Indian Chemical Society 99, 100639. 10.1016/j.jics.2022.100639.
- Insight into the corrosion inhibition of Biebersteinia multifida root extract for carbon steel in acidic medium. Sci. Total Environ.. 2022;836:155527
- [CrossRef] [Google Scholar]
- Simultaneous Determination of Six Compounds in Hedera helix L. Using UPLC-ESI–MS/MS. Chromatographia. 2017;80:1025-1033.
- [CrossRef] [Google Scholar]
- Glycine max meal extracts as corrosion inhibitor for mild steel in sulphuric acid solution. J. Mater. Res. Technol.. 2020;9:12756-12772.
- [CrossRef] [Google Scholar]
- Passiflora edulis Sims peel extract as a renewable corrosion inhibitor for mild steel in phosphoric acid solution. J. Mol. Liq.. 2023;375:121296
- [CrossRef] [Google Scholar]
- Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data. 2009;54:1981-1985.
- [CrossRef] [Google Scholar]
- Chemical Composition, Antibacterial, Antifungal and Antidiabetic Activities of Ethanolic Extracts of Opuntia dillenii Fruits Collected from Morocco. J. Food Qual.. 2022;2022
- [CrossRef] [Google Scholar]
- Loukili, E.L.H., Abrigach, F., Bouhrim, M., Bnouham, M., Fauconnier, M., Ramdani, M., 2021. Chemical Composition and Physicochemical Analysis of Opuntia dillenii Extracts Grown in Morocco Chemical Composition and Physicochemical Analysis of Opuntia dillenii Extracts Grown in Morocco.
- Experimental assessment and molecular-level exploration of the mechanism of action of Nettle (Urtica dioica L.) plant extract as an eco-friendly corrosion inhibitor for X38 mild steel in sulfuric acidic medium. Arab. J. Chem.. 2023;16:104988
- [CrossRef] [Google Scholar]
- Green corrosion inhibitors from natural sources and biomass wastes. Molecules. 2019;24
- [CrossRef] [Google Scholar]
- Electrochemical evaluation of an Acanthocereus tetragonus aqueous extract on aluminum in NaCl (0.6 M) and HCl (1 M) and its modelling using forward and inverse artificial neural networks. J. Electroanal. Chem.. 2022;918:116444
- [CrossRef] [Google Scholar]
- Ferrous metal corrosion studies in presence of eco-friendly acacia melanoxylon leaves extract in 1M HCl condition. Inorg. Chem. Commun.. 2024;160:111900
- [CrossRef] [Google Scholar]
- Adsorption and corrosion inhibition behavior of hydroxyethyl cellulose and synergistic surfactants additives for carbon steel in 1 M HCl. Carbohydr. Polym.. 2017;156:202-214.
- [CrossRef] [Google Scholar]
- Mwakalesi, A.J., 2022. Corrosion Inhibition of Mild Steel in Sulphuric Acid Solution with Tetradenia riparia Leaves Aqueous Extract: Kinetics and Thermodynamics. Biointerface Res Appl Chem 13, 32. 10.33263/BRIAC131.032.
- Optimization of extraction method for Kleeb Bua Daeng formula and comparison between ultrasound-assisted and microwave-assisted extraction. J. Appl. Res. Med. Aromat. Plants. 2022;28:100369
- [CrossRef] [Google Scholar]
- The performance of acetaldehyde ammonia trimer in corrosion inhibition of 3003 aluminum alloy in Na2CO3/NaCl mixture solution: Experimental and computational study. J. Mol. Struct.. 2023;1274:134453
- [CrossRef] [Google Scholar]
- Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4[4′(-X)-styryl pyridinium iodides/hydrochloric acid systems. Mater. Chem. Phys.. 2008;110:145-154.
- [CrossRef] [Google Scholar]
- Inhibition of the corrosion of mild steel in hydrochloric acid solutions by the extracts of leaves of Nypa fruticans Wurmb. Mater. Lett.. 2004;58:1768-1772.
- [CrossRef] [Google Scholar]
- Investigation of antioxidant and antimicrobial potential of some extracts from hedera helix L. Farmacia. 2017;65:624-629.
- [Google Scholar]
- Quraishi, M.A., Chauhan, D.S., Saji, V.S., 2020. Heterocyclic organic corrosion inhibitors: principles and applications. Elsevier, Amsterdam, Netherlands ; Cambridge, MA.
- A Review on the Assessment of Imidazo[1,2-a]pyridines As Corrosion Inhibitor of Metals. J. Bio Tribo Corros.. 2019;5:14.
- [CrossRef] [Google Scholar]
- Recent advances in biopolymers/carbohydrate polymers as effective corrosion inhibitive macro-molecules: A review study from experimental and theoretical views. J. Mol. Liq.. 2021;325:115110
- [CrossRef] [Google Scholar]
- Overview on plant extracts as green corrosion inhibitors in the oil and gas fields. J. Mater. Res. Technol.. 2021;15:5078-5094.
- [CrossRef] [Google Scholar]
- Shokry, A.A., 2022. Bioactive phenolics fraction of Hedera helix L. (Common Ivy Leaf) standardized extract ameliorates LPS-induced acute lung injury in the mouse model through the inhibition of proinflammatory cytokines and oxidative stress.
- Bark Extract of Lantana camara in 1M HCl as Green Corrosion Inhibitor for Mild Steel. EJ. 2019;23:205-211.
- [CrossRef] [Google Scholar]
- Syzygium cumini leaf extract as an eco-friendly corrosion inhibitor for carbon steel in acidic medium. J. Taiwan Inst. Chem. Eng.. 2021;129:342-349.
- [CrossRef] [Google Scholar]
- Corrosion inhibition behavior of piperidinium based ionic liquids on Q235 steel in hydrochloric acid solution: Experimental, density functional theory and molecular dynamics study. Colloids Surf. A Physicochem. Eng. Asp.. 2021;623:126708
- [CrossRef] [Google Scholar]
- Adsorption study of N (-benzo[d]thiazol-2-yl)-1-(thiophene-2-yl) methanimine at mild steel/aqueous H2SO4 interface. Surf. Interfaces. 2022;33:102169
- [CrossRef] [Google Scholar]
- Gundelia tournefortii as a Green Corrosion Inhibitor for Mild Steel in HCl and H2SO4 Solutions. Int. J. Electrochem. Sci.. 2015;10:46-62.
- [CrossRef] [Google Scholar]
- Density-functional tight-binding: basic concepts and applications to molecules and clusters. Adv. Phys.: X. 2020;5:1710252.
- [CrossRef] [Google Scholar]
- Corrosion inhibition of mild steel in hydrochloric acid solution by Schiff base furoin thiosemicarbazone. Corros. Sci.. 2010;52:224-228.
- [CrossRef] [Google Scholar]
- Cabbage extract as an eco-friendly corrosion inhibitor for X70 steel in hydrochloric acid medium. J. Mol. Liq.. 2022;362:119733
- [CrossRef] [Google Scholar]
- Computational and experimental studies on the corrosion inhibition performance of an aerial extract of Cnicus Benedictus weed on the acidic corrosion of mild steel. Process Saf. Environ. Prot.. 2022;161:801-818.
- [CrossRef] [Google Scholar]
- Precise major compounds in Barringtonia acutangula flower – water extract for mitigating carbon steel corrosion. J. Taiwan Inst. Chem. Eng.. 2024;155:105251
- [CrossRef] [Google Scholar]
- Electrochemical study and experimental simulation of the synergistic effect of a formulation based on Ficus pumila Linn. Leaves extract and zinc sulfate on the XC38 steel corrosion inhibition in NaCl solution. J. Electroanal. Chem.. 2022;919:116553
- [CrossRef] [Google Scholar]
- Wang, Q., Zhang, Q., Liu, L., Zheng, H., Wu, X., Li, Z., Gao, P., Sun, Y., Yan, Z., Li, X., 2022. Experimental, DFT and MD evaluation of Nandina domestica Thunb. extract as green inhibitor for carbon steel corrosion in acidic medium. Journal of Molecular Structure 1265, 133367. 10.1016/j.molstruc.2022.133367.
- Evaluation for Fatsia japonica leaves extract (FJLE) as green corrosion inhibitor for carbon steel in simulated concrete pore solutions. J. Build. Eng.. 2023;63:105568
- [CrossRef] [Google Scholar]
- Exploring of an ecological corrosion inhibitor of wood hibiscus leaf extract for the Cu/H2SO4 system based on experimental study and theoretical calculations. J. Taiwan Inst. Chem. Eng. 2023
- [CrossRef] [Google Scholar]
- Insight into interfacial adsorption and inhibition mechanism of Aconitum carmichaelii Debx extract as high-efficient corrosion inhibitor for carbon steel in acidic solution. J. Mol. Liq.. 2024;393:123602
- [CrossRef] [Google Scholar]
- Crataegus oxyacantha leaves extract for carbon steel protection against corrosion in 1M HCl: Characterization, electrochemical, theoretical research, and surface analysis. J. Mol. Struct.. 2022;1259:132737
- [CrossRef] [Google Scholar]
- Chemically modified resveratrol as green corrosion inhibitor for Q235 steel: Electrochemical, SEM, UV and DFT studies. J. Mol. Liq.. 2021;343:117672
- [CrossRef] [Google Scholar]







