Translate this page into:
Gamma globulin binding of hinokiflavone with anti-osteoporosis effects: A mechanistic study
⁎Corresponding author at: Department of Spine Surgery, Xi’an Honghui Hospital, School of Medicine, Xi’an Jiaotong University, 555 Youyidonglu, Xi’an 710000, Shaanxi Province, China. hhdingjun@163.com (Dingjun Hao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Oxidative stress can trigger apoptosis and associated reduction of osteoblast activity. Hinokiflavone (HNK) with a biflavonoid-based structure might show potent antioxidant activity. However, before the application of this compound as a promising therapeutic bioactive material, its interaction with blood proteins should be investigated to further reveal its pharmacokinetic and pharmacodynamic properties. Therefore, in this paper, the interaction of HNK with γ-globulin, one of the main blood proteins, was assessed. Then, the antioxidant properties of HNK against H2O2-induced osteoblast cytotoxicity in MC3T3-E1 cells were assessed. It was shown that HNK can potentially bind to γ-globulin mostly with the aid of hydrophilic forces, with a slight effect on the protein structure. It was then determined that HNK significantly recovered cell survival and alkaline phosphatase (ALP) activity and collagen-I content in MC3T3-E1 cells exposed to H2O2. In addition, HNK decreased the production of intracellular reactive oxygen species (ROS) and malondialdehyde (MDA) in MC3T3-E1 cells triggered by H2O2. Finally, HNK was shown to control the apoptosis induction in MC3T3-E1 cells triggered by H2O2 mediated by regulation of Bax, Bcl-2 and caspase-3. Taken together, these data demonstrated that HNK with promising blood protein binding properties can show significant protective effects in MC3T3-E1 cells mediated by inhibition of osteoblast dysfunction, oxidative stress, and apoptosis.
Keywords
Hinokiflavone
γ-Globulin
Interaction
Osteoblast
Oxidative stress
Apoptosis
1 Introduction
Dimeric flavonoids, biflavonoids, found in a wide range of plants are “composed of two phenyl-chromenone units which linked via a C–C or C–O–C bond” (Gontijo et al., 2017; Goossens et al., 2021). The dimerization between two apigenin units through an ether linkage occurs for compounds like hinokiflavone (HNK), ochnaflavone and several other C–O–C-type biflavonoids (Goossens et al., 2021).
HNK, as a C–O–C-type biflavonoid (Schematic 1) has shown different pharmacological activities.
Schematic structure of hinokiflavone (HNK) as a biflavonoid.
For example, HNK showed potential hepatoprotective (Abdel-Kader et al., 2018), anticancer (Goossens et al., 2021), antibacterial (Kong et al., 2022), and antioxidant effects (Setyawan, 2011). However, the main antioxidant mechanism of HNK as a potential bioflavonoid is not well-understood.
As oxidative stress which is deduced from the excessive formation of reactive oxygen species (ROS) can lead to serious side effects against all components of the cell, the evaluation of antioxidant properties of HNK can provide useful information for the modulation of a wide range of diseases. For example, reduced bone mineral density is linked with high oxidative stress index markers in osteoporotic patients (Zhao et al., 2021).
As a result, it is tempting to recommend that HNK might play a key role in regulating the overproduction of ROS and acting as an effective candidate for the treatment of osteoporosis.
However, before assessing the pharmacologic activity of a bioactive material, its pharmacokinetic properties should be investigated in detail. The interaction of therapeutic biflavonoids with main plasma proteins (albumin, γ-globulin, fibrinogen) could provide us with useful information about their bioavailability and subsequent therapeutic applications (Koly et al., 2015). γ-Globulin as one of the main components of blood proteins has been widely used as a model to study the interaction of antioxidant flavonoids with proteins (Yang et al., 2011; Li et al., 2021). However, the interaction of HNK with γ-globulin has not been investigated to understand the binding affinity and probable structural changes of γ-globulin.
As a consequence, the first objective of this study was to determine the binding mechanism of HNK and γ-globulin under physiological conditions by spectroscopic and theoretical approaches. Secondly, as it is unclear whether HNK can modulate the oxidative stress in osteoblasts, another aim of the current study was to investigate the protective effects of HNK on H2O2-induced oxidative stress in MC3T3-E1 cells, which was addressed by the determination of different markers such as osteoblast dysfunction, oxidative stress, and apoptosis.
2 Materials and methods
2.1 Materials
Hinokiflavone (98 %, HPLC) was purchased from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, China). γ-Globulin, Fetal bovine serum (FBS), α-modified minimal essential medium (α-MEM), dichlorodihydrofluorescein diacetate (DCFH-DA), and N-acetyl-l-cysteine (NAC) were purchased from Sigma Chemical (St. Louis, MO, USA). The osteoblastic MC3T3-E1 cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). Other materials were of high grade and purchased from Sigma Chemical (St. Louis, MO, USA). The HNK stock solution (1 mM) was dissolved in dimethyl sulphoxide (DMSO) and was further diluted with sodium phosphate buffer (50 mM, pH 7.5). The γ-globulin solution was prepared in sodium phosphate buffer (50 mM, pH 7.5).
2.2 Spectroscopy measurements
The intrinsic fluorescence measurements were done on an F-2500 spectrophotometer (Hitachi, Japan), where the excitation wavelength was set at 280 nm and the emission wavelengths were set between the ranges of 300–420 nm. To explore the interaction of HNK and γ-globulin, 2.0 mL of γ-globulin solution (5 µM) was titrated with consecutive addition of HNK (1–30 µM). The experiments were performed at the four different temperatures of 298, 305, 310, and 315 K. All fluorescence data were corrected against HNK intrinsic fluorescence and inner filter effects (Zia et al., 2024). Synchronize fluorescence spectroscopy (SFS) study for determination of the microenvironmental changes of Tyr and Trp residues of γ-globulin was done at Δλ between excitation and emission wavelength at 15 nm and 60 nm, respectively.
2.3 Molecular docking study
To understand the molecular interaction of HNK with γ-globulin at the atomic level, a molecular docking study was done using AutoDock Vina package. The 3D molecular building of HNK was downloaded from PubChem (PubChem CID: 528444) (https://pubchem.ncbi.nlm.nih.gov/). The γ-globulin crystal structure (PDB code: 1AJ7) was obtained from Protein Data Bank (https://www.rcsb.org/). AutoDock Vina Tool was used to control the hydrogen atoms and charges as well as the elimination of water molecules. A blind docking study was performed at a box of 28 × 28 × 28 Å, and then the lowest interaction score was selected for further analysis.
2.4 Cell culture
The osteoblastic MC3T3-E1 cells were cultured in a-MEM supplemented with 10 % heat-inactivated FBS, 100 U/ml penicillin and 100 μg/ml streptomycin with 5 % CO2 at 37 °C.
2.5 Cell viability assay
The cells were incubated with H2O2 with a concentration of 100 µM for different times of 6 h, 12 h, 24 h, 48 h, and 72 h to induce oxidative stress. To measure the cytotoxicity of HNK, the cells were treated with different concentrations of HNK (1–50 µM) for 72 h. To investigate the protective effects of HNK on H2O2-induced cytotoxicity in MC3T3-E1 cells, the cells were incubated with H2O2 (100 µM) and H2O2 + HNK (20 μM) for 72 h. NAC with a concentration of 1 mM was also used as the positive control for all assays. Then the cell viability assay was done using the MTT assay as described previously at 570 nm absorbance using an ELISA reader (Thermo Scientific, MA, USA) (Park et al., 2020).
2.6 Alkaline phosphatase (ALP) activity
Alkaline phosphatase (ALP) activity was done based on the previous study (Zhang, Yang et al., 2012). Briefly, following treating the cells in an osteogenic medium for 6 days, the cells were cultured with serum-free medium containing H2O2 (100 µM), HNK (20 μM), H2O2 (100 µM) + HNK (20 μM), or H2O2 + NAC (1 mM) for 72 h. Then, the cells were harvested, washed, lysed, and centrifuged at 10,000g for 5 min. The supernatant was then used to assess the ALP activity using the relevant kit (MAK447, Sigma) according to the manufacturer's protocol.
2.7 Quantitative real-time PCR (qPCR)
The cells cultured in an osteogenic medium for 6 days were exposed to H2O2 (100 µM), HNK (20 μM), H2O2 (100 µM) + HNK (20 μM), or H2O2 + NAC (1 mM) for 72 h. Total cellular RNA was then extracted using the Trizol reagent according to the manufacturer's instructions (TaKara, Dalian, China). Single-strand cDNA synthesis was synthesized by the PrimeScript RT reagent kit (TaKaRa, Dalian, China). Real-time PCR was performed using a SYBR® Premix Ex Taq™ (Takara, Dalian, China). qPCR was performed on an ABI PRISM 7700 sequence detection system (Applied Biosystems, Grand Island, NY, USA).
The primer sequences and experiment procedure for ALP, collagen-I, Bax, Bcl-2, Caspase-3, and β-actin were developed based on the previous studies (Zhang et al., 2012; Guo et al., 2014).
2.8 ELISA assay
ELISA kits for the quantitative determination of Bax, Bcl-2, caspase-3, and collagen-I were purchased from Cusabio Biotechnology (Wuhan, China). The cells were cultured in an osteogenic medium for 6 days. Then, the cells cultured with serum-free medium containing H2O2 (100 µM), HNK (20 μM), H2O2 (100 µM) + HNK (20 μM), or H2O2 + NAC (1 mM) for 72 h were harvested, washed, lysed, and centrifuged at 10,000g for 5 min. The supernatant was then used to perform ELISA assay according to the manufacturer's protocol.
2.9 Measurement of intracellular ROS
The hydrolysis of the non-fluorescent DCFH-DA probe can be catalyzed by intracellular esterase and intracellular ROS to produce fluorescent dichlorofluorescein (DCF). The measurement of intracellular ROS was done based on a previous study (Yu et al., 2013). In brief, the cells were The cells incubated with H2O2 (100 µM), HNK (20 μM), H2O2 (100 µM) + HNK (20 μM), or H2O2 + NAC (1 mM) for 72 h were rinsed with PBS, incubated with 10 μM DCFH-DA for 30 min at 37 °C, harvested, and washed. Then, ROS production was assessed by reading the fluorescence intensity of cells at an excitation wavelength of 488 nm and an emission wavelength of 525 nm on a fluorescence microplate reader (Victor® X3, Perkin Elmer, Waltham, MA, USA).
2.10 Measurement of membrane lipid peroxidation
The extent of lipid peroxidation was determined using the MDA assay Kit (Beyotime Institute of Biotechnology (Shanghai, China) according to the manufacturer's protocol. Briefly, the cells were treated with H2O2 (100 µM), HNK (20 μM), H2O2 (100 µM) + HNK (20 μM), or H2O2 + NAC (1 mM). After 72 h, the cell samples were mixed with a working solution (200 μl MDA assay), heated at 100 °C for 15 min, cooled, and centrifuged at 1000 × g for 10 min. The optical density of supernatants was read at 532 nm using an ELISA reader (Thermo Scientific, MA, USA).
2.11 Statistical analysis
The data in cell culture assays were from three replicates and the data were expressed as the mean ± standard deviation (S.D.). The data were analyzed using one-way ANOVA using SPSS 22.0 software (Chicago, IL, USA). A statistically significant difference was considered at p < 0.05.
3 Results and discussion
The interaction between γ-globulin and HNK at the molecular level was investigated by adopting spectroscopic as well as theoretical studies. Therefore, this report might decipher the interaction of antioxidants with serum proteins at the molecular level in the process of ligand–protein complexation.
3.1 Spectroscopic studies
Quenching of the aromatic residue fluorescence intensity of γ-globulin was detected following consecutive addition of HNK at different temperatures (Fig. 1A). The fluorescence spectrum of HNK at an excitation at 280 nm was subtracted from protein fluorescence signal to ensure that the fluorescence intensity of HNK does not play a significant role in the quenching experiment. The addition of HNK to γ-globulin was observed to not only induce a fluorescence quenching of protein but also change the λmax of aromatic residue (redshift). This phenomenon can be associated with the changes in the surrounding polarity of aromatic residues in γ-globulin upon interaction with HNK.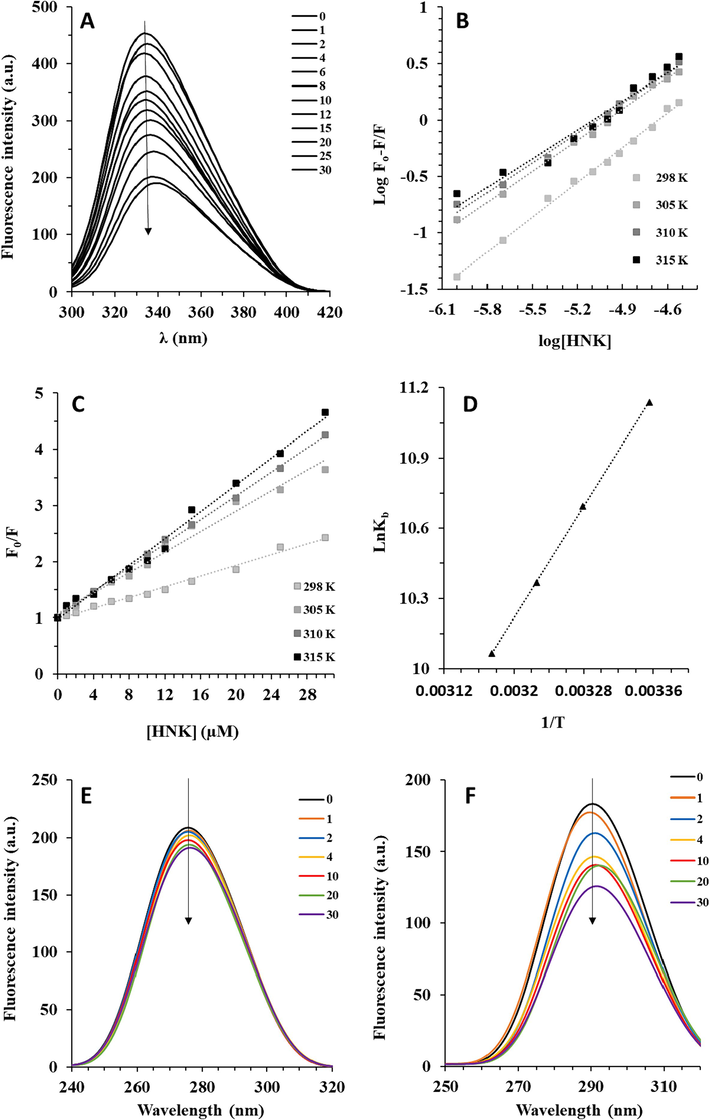
(A) The interaction of γ-globulin with consecutive addition of HNK measured by fluorescence quenching study at 298 K. (B) The modified Stern-Volmer plots of γ-globulin-HNK system at four different temperatures. (C) The Stern-Volmer plots of γ-globulin-HNK system at four different temperatures. (D) The van’t Hoff plots of γ-globulin-HNK system at four different temperatures. SFS measurement of γ-globulin-HNK system at (E) Δλ = 60 nm and (F) Δλ = 15 nm.
It can be indicated that the surrounding polarity of the aromatic residue in γ-globulin was changed by HNK, which has already been reported for the interaction of some other polyphenols with this protein (Li et al., 2021).
Binding parameters for interaction between HNK and γ-globulin were determined using a double logarithmic equation as follows (Sharma et al., 2022):
The resultant plots derived from Eq. (1) are shown in Fig. 1B. Kb is indicative of the stability of the formed protein–ligand complex. The in vitro Kb and n values between γ-globulin and HNK were found in the order 104 M−1 and 1, respectively, suggesting a moderate probable binding with one binding site between HNK and γ-globulin in vivo (Li et al., 2023). In line with this study, Li et al. reported that the formation of baicalin–γ-globulin, quercetin–γ-globulin, myricetin–γ-globulin, rutine–γ-globulin, diadzein–γ-globulin, hesperidin–γ-globulin, and isoliquiritin–γ-globulin complexes occurs with Kb values of around 104 (Li et al., 2021).
Also, it was detected that the Kb values of HNK–γ-globulin declined with increasing temperature (Fig. 1B, Table 1), implying that the covalent forces are not predominated in the HNK–γ-globulin complex formation, while noncovalent forces are mainly involved and these interactions become stronger at lower temperature compared to higher temperatures.
Temp. (K)
logKb
n
R2
298
4.78
1.02
0.9957
305
4.59
0.91
0.9848
310
4.45
0.87
0.9928
315
4.32
0.84
0.9634
A similar temperature effect was also observed for the Kb values of baicalin–γ-globulin, quercetin–γ-globulin, rutin–γ-globulin, daidzein–γ-globulin, and hesperidin–γ-globulin complexes, while myricetin–γ-globulin and isoliquiritin–γ-globulin complexes behaved differently (Li et al., 2021). As a result, affinities of flavonoids for protein binding were heavily affected by their structural differences (Yang et al., 2011). Bioconjugate between γ-globulin and HNK as a C–O–C-type biflavonoids was found to have similar stability with the complex between γ-globulin and several classic flavonoids. This indicates a potential complexation between γ-globulin and HNK. Also, it was detected upon an increase in temperature, the n values decreased slightly because of subtle structural changes at the binding site of the γ-globulin.
Quenching constant (KSV) was computed from the Stern–Volmer (SV) Eq. as follows (Sharma et al., 2022):
SV plots for the HNK–γ-globulin complex at three different temperatures are exhibited in Fig. 1C and the calculated KSV values are listed in Table 2.
Temp. (K)
KSV (105 M−1)
kq (1013 M−1 s−1)
R2
298
0.47
1.42
0.9906
305
0.91
2.76
0.9853
310
1.06
3.22
0.9988
315
1.19
3.61
0.9915
The KSV values give information regarding the fluorescence quenching efficiency of protein by ligand and the variation of these values with temperature indicates the static or dynamic quenching mechanisms.
The higher value of KSV for HNK (105 M−1) compared to baicalin, quercetin, myricetin, rutin, diadzein, hesperidin, and isoliquiritin (104 M−1) and puerarin (103 M−1) (Li et al., 2021) indicated that this C-O-C-type biflavonoid quenched the aromatic residue fluorescence of γ-globulin more efficiently than the classical flavonoids (Table 1). A rise in temperature increased the quenching process of γ-globulin by HNK. A similar phenomenon was already reported during the fluorescence quenching of γ-globulin by most classical flavonoids, which suggests a dynamic quenching mechanism (Yang, Zhao et al., 2011; Li, Wang et al., 2021). Also, the determination of the quenching process was done using the kq values as calculated from Eq. (2).
The calculated values of kq were determined as ∼1013 M−1 s−1, which was significantly higher than the maximum reported value of kq (1010 M−1 s−1) for dynamic quenching (Sharma et al., 2022). Accordingly, it can be implied that both static and dynamic mechanisms of quenching are involved in this system, which was similar to the quenching by other classical flavonoids (Li et al., 2021). Li et al. suggested that the possible quenching mechanism may be initiated by static quenching and is further followed by dynamic one (Li et al., 2021).
Different intermolecular forces typically contribute to the protein–ligand complexation process. The sign and magnitude of the enthalpy change (ΔH) and entropy change (ΔS) are usually used to evaluate the nature of the binding forces (Abarova et al., 2024; Chaves et al., 2024). ΔS and ΔH values along with Gibbs free energy (ΔG) parameter were then determined by the van’t Hoff Eq. (3) and Gibbs Eq. (4) as follows (Chaves et al., 2024):
Fig. 1D shows the van’t Hoff plot for the interaction of HNK and γ-globulin at four different temperatures. The thermodynamic parameters were then listed in Table 3.
Temp. (K)
ΔH (kJ/mol)
ΔS (J/K.mol)
ΔG (kJ/mol)
298
−49.48
−73.12
−27.69
305
−27.17
310
−26.81
315
−26.44
A negative ΔG value emphasizes that the HNK–γ-globulin complexation process occurred spontaneously. The values of ΔH and ΔS for the interaction of HNK and γ-globulin revealed that this process was based on an enthalpy driven system, indicating that hydrogen bond and van der Waals forces are predominant in the formation of such complex (Abarova et al., 2024; Chaves et al., 2024). An earlier report has shown that classical flavonoids such as baicalin, quercetin, myricetin, rutin, diadzein, hesperidin, isoliquiritin, and puerarin interact with γ-globulin through hydrophobic or electrostatic forces, while hydrogen bonds and van der Waals forces have played a minimum role (Li et al., 2021), revealing that HNK as a C–O–C-type biflavonoid interacts with γ-globulin differently from other classical flavonoids. Changes in the flavonoid structure from classical geometry to biflavonoid altered the nature of binding forces between the γ-globulin and HNK.
In this study, the conformation changes of γ-globulin induced by HNK were studied using SFS spectroscopy. The SFS technique is known as a very serviceable approach to obtaining information about the polarity changes in the vicinity of the Trp and Tyr residues. When Δλ values are fixed at 60 nm and 15 nm, this technique can give some details about the changes in the polarity of Trp and Tyr residues, respectively (He et al., 2015; Samandar et al., 2023). Fig. 1, exhibits the SFS analysis of γ-globulin in the presence of consecutive addition of HNK. It can be observed that when Δλ = 60 nm (Fig. 1E), a gradual quenching occurs in the fluorescence intensity accompanied by no shift, while when Δλ = 15 nm (Fig. 1F), an obvious red shift was observed. This implies that the polarity around Trp remains unchanged in the presence of HNK, while the red shift in the emission maxima of Try designates a reduction in the hydrophobicity surrounding the Tyr residues (Li et al., 2021; Sun et al., 2021).
Hence, these findings suggested that the interaction of γ-globulin with HNK does not alter substantially the polypeptide backbone and associated β-sheet structure of γ-globulin.
3.2 Molecular docking study
One of the most widely utilized approaches for investigating the binding affinity of ligands with protein is the analysis of molecular interaction using molecular docking study (Azimirad et al., 2023). In the present study, the interaction of the NHK (Fig. 2A) and γ-globulin was explored by using the AutoDock Vina package. The tertiary structure of γ-globulin is characterized by β-sheet structure (Fig. 2B). The major NHK-γ-globulin location is depicted in Fig. 2B and C, which exhibited that HNK as a ligand potentially binds to an interface of light and heavy chains. The docking energies for 10 runs were −44.768 to −30.543 kJ/mol, indicating a strong binding affinity between HNK and γ-globulin. The lowest binding energy conformation (−44.768 kJ/mol) was applied and the results showed that NHK could interact with several amino acid residues (Fig. 2D and E) mediated by different binding forces. It was shown that hydrogen bond: Ala 84 and Gln 39, weak hydrogen bond: Gly 106, and hydrophobic forces: Asp 85 and Phe 83 contributed to the formation of HNK-γ-globulin complex. This finding is in line with experimental results, which indicated that hydrogen bonding and van der Waals forces might play a role in the interaction of HNK and γ-globulin.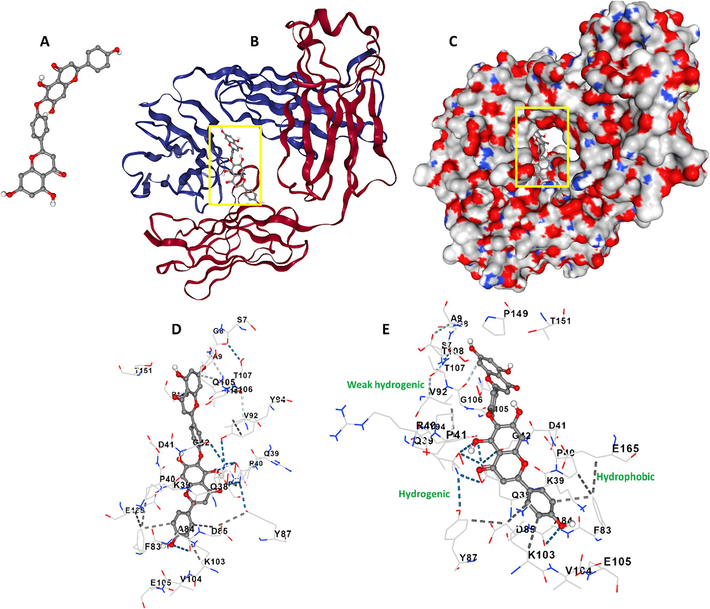
Molecular docking study of γ-globulin-HNK system. (A) HNK structure, (B) γ-globulin-HNK complex, (C) γ-globulin-HNK complex, (D) amino acids in the binding site, (E) amino acids in the binding site.
Also, experimental assays showed that the interaction of HNK with γ-globulin leads to the fluorescence quenching of protein and slight conformational changes in the vicinity of Tyr residues. A molecular docking study showed that Tyr 87 (light chain) and Tyr 94 (heavy chain) amino acid residues are in the vicinity of the binding pocket of HNK on γ-globulin.
Both light and heavy chains are important binding sites on γ-globulin and typically a minimum number of small molecules show the possibility to bind there due to the extended structure (Wang et al., 2015; Krzyżak et al., 2023). The importance of these chains lies in their capability to interact with a number of small molecules, which makes them a notable candidate for drug delivery (Wang et al., 2015; Wang et al., 2016; Krzyżak et al., 2023). Also, the high affinity of γ-globulin to HNK not only causes their sequestration and reduced adverse effects but also changes the pharmacokinetics and pharmacodynamics of the HNK.
3.3 Protective effect of HNK on H2O2 stimulated cytotoxicity in MC3T3-E1 cells
Cell viability was done to assess the protective effect of HNK on the behavior of MC3T3-E1 cells against oxidative stress triggered by H2O2. First, we detected that H2O2 treatment at a concentration of 100 µM for 72 h induced the most significant cell death among other studied time intervals (Fig. 3A). We also found that the incubation of MC3T3-E1 with HNK at concentrations above 20 µM for 72 h induced significant cytotoxicity (Fig. 3B). Therefore, we treated the cells with H2O2 (100 µM) or H2O2 (100 µM) + HK (20 µM) for 72 h to assess the following experiments. As shown in Fig. 3C, when the cells were treated with HNK in the presence of H2O2, HNK could remarkably regulate the cell viability comparable to NAC as a potential antioxidant, suggesting that HNK might be able to suppress H2O2 triggered cytotoxicity.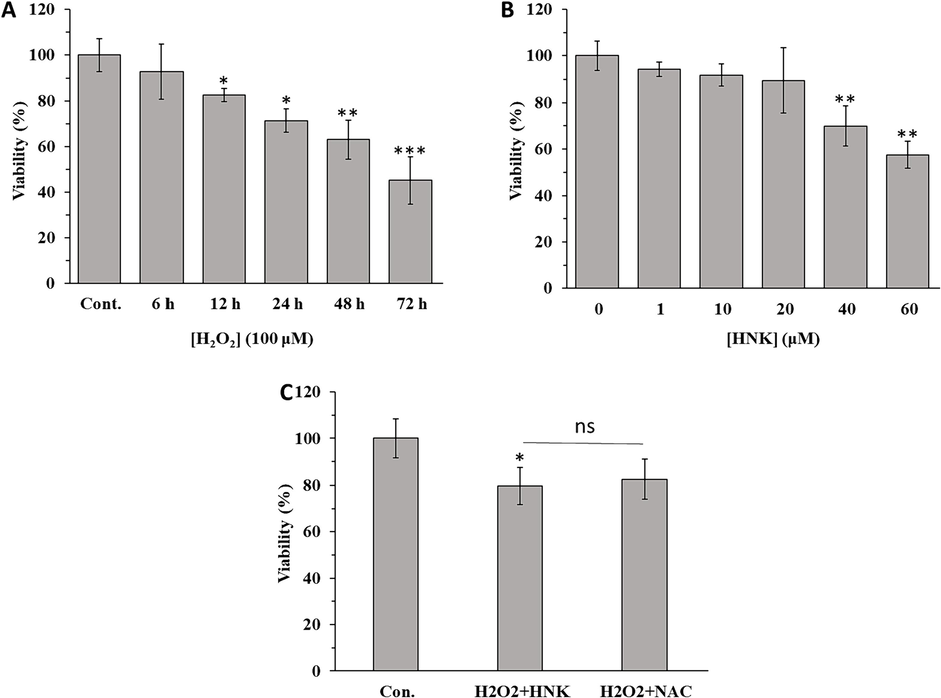
(A) Effect of H2O2 on the viability of MC3T3-E1 cells at different time intervals. (B) Effect of HNK on viability of MC3T3-E1 cells at different concentrations after 72 h. (C) Effect of H2O2 (100 µM) + HK (20 µM) and H2O2 (100 µM) + NAC (1 mM) on viability of MC3T3-E1 cells after 72 h. The data were determined by MTT assay (SD, n = 3). *p < 0 0.05, **p < 0 0.01, ***p < 0 0.001, relative to control group.
3.4 Protective effect of HNK on osteoblast dysfunction triggered by H2O2
In order to determine the protective effects of HNK on osteoblast dysfunction stimulated by H2O2, ALP activity was assessed. Compared with control cells, the addition of H2O2 significantly reduced ALP activity (Fig. 4A) and ALP mRNA expression (Fig. 4B) in MC3T3-E1 cells However, when osteoblasts were treated with HNK (20 µM) in the presence of H2O2 (100 μM), HNK significantly elevated ALP activity and ALP mRNA expression (Fig. 4A and B), which is known as one of the most important osteoblast differentiation markers (Khotib et al., 2023).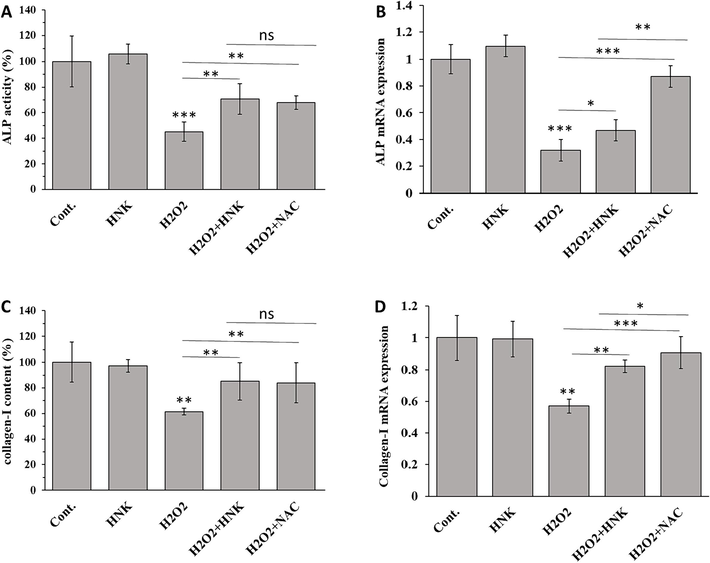
Effect of HNK (20 µM), H2O2 (100 µM), H2O2 (100 µM) + HK (20 µM) and H2O2 (100 µM) + NAC (1 mM) on (A) ALP activity (B) ALP mRNA expression, (C) collagen-I content, and (D) collagen-I mRNA of MC3T3-E1 cells after 72 h. *p < 0.05, **p < 0.01, ***p < 0.001, relative to control group.
The evaluation of collagen-I contents at both protein (Fig. 4C) and mRNA (Fig. 4D) levels in MC3T3-E1 cells also indicated that when the cells were incubated with H2O2, collagen-I markers were reduced. However, when osteoblasts were treated with HNK (20 µM) in the presence of H2O2 (100 μM), HNK significantly elevated collagen-I protein and mRNA levels relative to H2O2-treated groups. Based on this data, in agreement with previous studies, we can discuss that biflavonoids similar to classical flavonoids could stimulate osteoblast differentiation through the regulation of ALP and collagen-I (Lee et al., 2006; Suh et al., 2013; Sekaran et al., 2022).
The increased collagen content and ALP activity are indicative of the osteo-stimulatory properties of HNK. It has been shown that apigenin as a building block of NHK can elevate MC3T3-E1 cell growth, collagen content and ALP activity at very low concentrations (Choi, 2007), which was comparable to the luteolin and hesperetin properties by restoration of collagen and ALP levels (Kim et al., 2011; Lin et al., 2020; Sekaran et al., 2022).
3.5 Protective effect of HNK on apoptosis induction triggered by H2O2
To determine the features of H2O2-induced death in MC3T3-E1 cells, ELISA and qPCR assays were carried out to evaluate the expression of Bax, Bcl-2, and caspase-3 as the typical markers of apoptosis. After treatment with H2O2for 72 h, the Bax protein and mRNA expression levels (Fig. 5A and B) were upregulated. However, when osteoblasts were treated with HNK (20 µM) in the presence of H2O2 (100 μM) for 72 h, HNK significantly decreased Bax protein and mRNA levels relative to H2O2-treated groups. Furthermore, it was observed that although the expression levels of Bcl-2 protein and mRNA were mitigated in the presence of H2O2, HNK remarkably upregulated the expression of Bcl-2 protein and mRNA levels relative to H2O2-treated groups (Fig. 5C and D).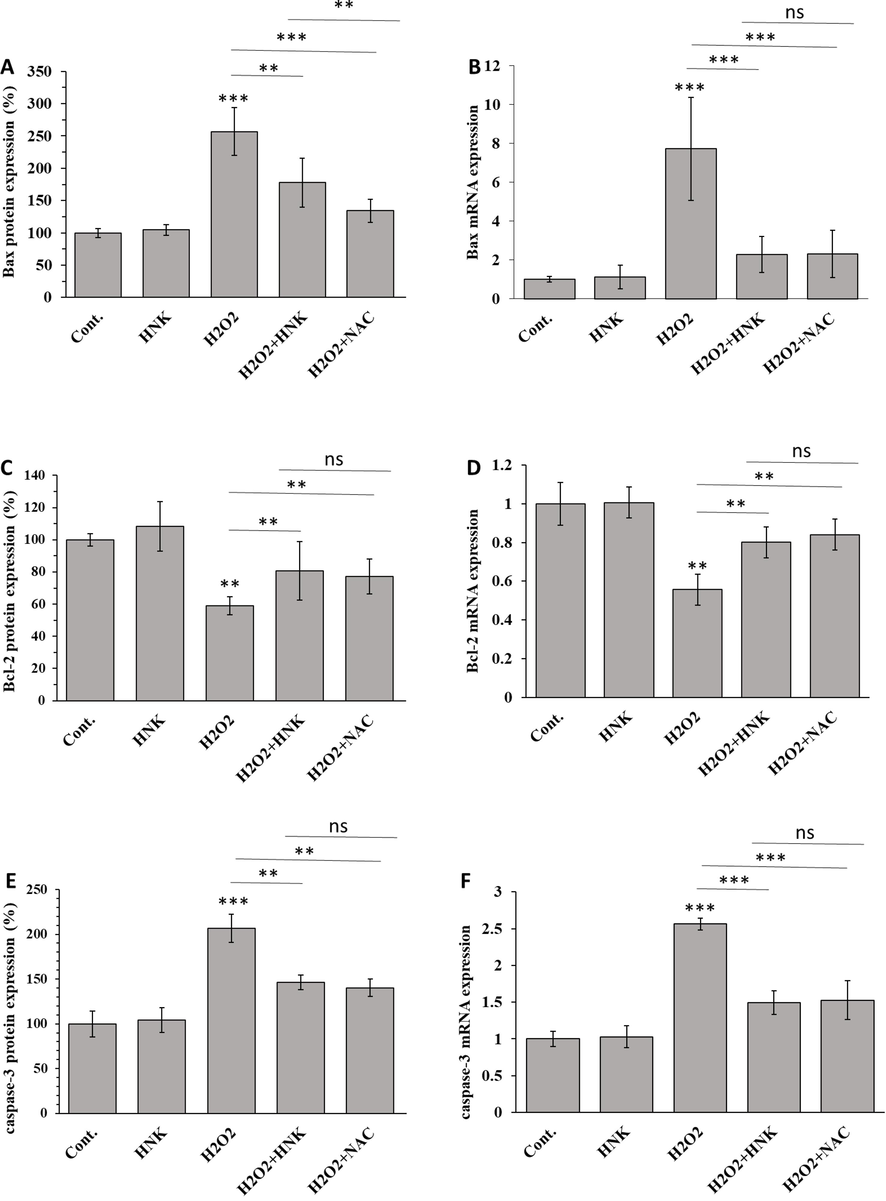
Effect of HNK (20 µM), H2O2 (100 µM), H2O2 (100 µM) + HK (20 µM) and H2O2 (100 µM) + NAC (1 mM) on (A) Bax protein expression, (B) Bax mRNA expression, (C) Bcl-2 protein expression, (D) Bcl-2 mRNA expression, (E) caspase-3 protein expression, and (F) caspase-3 mRNA expression of MC3T3-E1 cells after 72 h. *p < 0.05, **p < 0.01, ***p < 0.001, relative to control group.
We then assessed the antiapoptotic effects of HNK by determination of the caspase-3 content in MC3T3-E1 cells. Results demonstrated that H2O2 upregulated the expression of caspase-3 protein and mRNA (Fig. 5E and F). However, HNK was shown to reverse the H2O2-triggered apoptotic effect indicated through the downregulation of caspase-3 factor.
These results implied that HNK was capable of mitigating apoptosis induced by H2O2 in MC3T3-E1 cells. The HNK was able to inhibit H2O2-stimulated osteoblastic MC3T3-E1 cell apoptosis via ameliorating mitochondrial dysfunction evidenced by regulation of Bax, Bcl-2, and finally caspase-3. Bax and Bcl-2 play a key role in the mitochondrial integrity and deregulation of these factors can result in apoptosis induction through upregulation of caspase-3 (Adams and Cory, 2007; Asadi et al., 2022).
Osteoblast as the main bone cell type is the main driver of bone formation. A previous study has shown that exposure of MC3T3-E1 cells to 200 µM H2O2 can induce apoptosis and necrosis after 1 h (Fatokun et al., 2006). In this study, we found that 100 µM H2O2 can substantially reduce cell viability after 72 h, but the cytotoxicity effect was reduced significantly upon being treated with HNK. HNK has a polyphenolic structure similar to that of apigenin and these protective effects might be associated with the antioxidant characteristics of this compound.
3.6 Protective effect of HNK on oxidative stress induction triggered by H2O2
To investigate the antioxidant effects of HNK, the effects of HNK on H2O2-triggered ROS and MDA formation were examined. As expected, incubation with H2O2 for 72 h significantly increased ROS production (Fig. 6A) and MDA level (Fig. 6B). Co-treatment of H2O2 and NHK for 72 h significantly attenuated the effect of H2O2.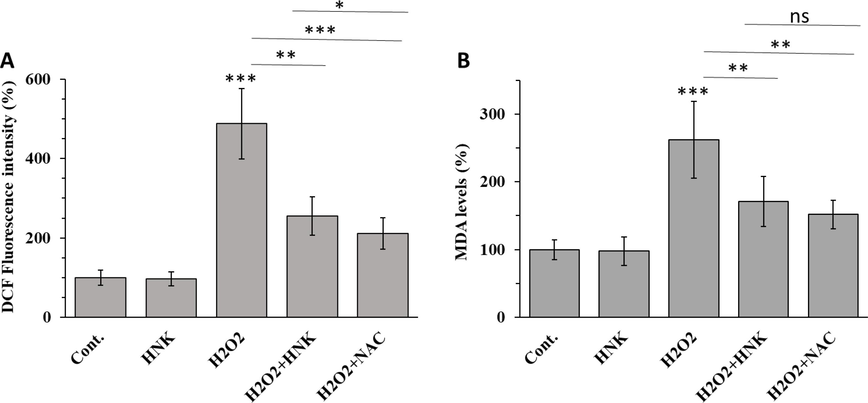
Effect of HNK (20 µM), H2O2 (100 µM), H2O2 (100 µM) + HK (20 µM) and H2O2 (100 µM) + NAC (1 mM) on (A) ROS production and (B) lipid peroxidation of MC3T3-E1 cells after 72 h. *p < 0.05, **p < 0.01, ***p < 0.001, relative to control group.
Oxidative stress could stem from deregulation of the intracellular oxidation–antioxidation balance and is linked with a significant increase in intracellular ROS levels. Upregulation of ROS could lead to biomolecule oxidation such as lipids, proteins, and DNA, which would result in tissue damage (Juan et al., 2021). Lipid peroxides (MDA) can lead to elevated cell and mitochondrial membrane damage and associated apoptosis (Su et al., 2019). It has been documented that the overproduction of ROS contributes to the deregulation of mineral tissue homeostasis and causes bone remodeling mediated by decreasing bone formation and upgrading bone resorption (Marcucci et al., 2023). In the present study, although H2O2 increased the production of ROS and MDA, after co-treatment with HNK for 72 h, these effects were reversed to a significant extent.
4 Conclusion
In conclusion, HNK moderately interacted with γ-globulin mainly via hydrogen bonds and van der Waals forces, without any significant changes in the protein structure. The results of the present study also showed that HNK could serve as a potential antioxidant and protect osteoblasts from H2O2-induced cytotoxicity, dysfunction, apoptosis, and oxidative stress. Accordingly, we determined that the protective effect of HNK on osteoblastic MC3T3-E1 cells could be associated, at least in part, through its antioxidant, antiapoptotic and modulatory abilities. However, the interaction with other blood proteins and protection mechanism of HNK against H2O2-induced apoptosis of osteoblastic MC3T3-E1 cells and regulation of osteogenic differentiation remain to be explored in future studies.
CRediT authorship contribution statement
Zhen Zhang: Conceptualization, Investigation, Methodology, Formal analysis, Writing – review & editing, Writing – original draft. Jialang Zhang: Conceptualization, Investigation, Methodology, Formal analysis, Writing – review & editing, Writing – original draft. Baorong He: Investigation, Methodology, Formal analysis, Writing – review & editing, Writing – original draft. Dingjun Hao: Formal analysis, Writing – review & editing, Writing – original draft, Conceptualization, Supervision, Project administration, Investigation, Data curation, Validation, Resources, Visualization, Funding acquisition.
Acknowledgement
This study was supported by the Basic Scientific Research Project of Xi’an Jiaotong University (No. xzy012022128)
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Spectroscopic and thermodynamic characterization of the interaction of a new synthesized antitumor drug candidate 2H4MBBH with human serum albumin. Pharmacia. 2024;71:1-5.
- [Google Scholar]
- Evaluation of the hepatoprotective effect of combination between hinokiflavone and Glycyrrhizin against CCl4 induced toxicity in rats. Saudi Pharm. J.. 2018;26(4):496-503.
- [Google Scholar]
- Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr. Opin. Immunol.. 2007;19(5):488-496.
- [Google Scholar]
- Caspase-3: structure, function, and biotechnological aspects. Biotechnol. Appl. Biochem.. 2022;69(4):1633-1645.
- [Google Scholar]
- Mechanistic and kinetic aspects of Natamycin interaction with serum albumin using spectroscopic and molecular docking methods. Arab. J. Chem. 2023105043
- [Google Scholar]
- Spectroscopic and in silico evaluation on the interactive behavior between substituted β-2, 3-dihydrofuran naphthoquinones and human serum albumin. Chem. Phys. Impact 2024100465
- [Google Scholar]
- Apigenin increases osteoblastic differentiation and inhibits tumor necrosis factor-α-induced production of interleukin-6 and nitric oxide in osteoblastic MC3T3-E1 cells. Die Pharmazie-an Int. J. Pharm. Sci.. 2007;62(3):216-220.
- [Google Scholar]
- Hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells: the effects of glutamate and protection by purines. Bone. 2006;39(3):542-551.
- [Google Scholar]
- Biological and chemical aspects of natural biflavonoids from plants: a brief review. Mini Rev. Med. Chem.. 2017;17(10):834-862.
- [Google Scholar]
- Hinokiflavone and related C-O–C-type biflavonoids as anti-cancer compounds: properties and mechanism of action. Nat. Prod. Bioprospecting. 2021;11:365-377.
- [Google Scholar]
- Lipopolysaccharide (LPS) induces the apoptosis and inhibits osteoblast differentiation through JNK pathway in MC3T3-E1 cells. Inflammation. 2014;37:621-631.
- [Google Scholar]
- Characterization of the binding of shikonin to human immunoglobulin using scanning electron microscope, molecular modeling and multi-spectroscopic methods. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2015;150:514-522.
- [Google Scholar]
- The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci.. 2021;22(9):4642.
- [Google Scholar]
- Differentiation of osteoblasts: the links between essential transcription factors. J. Biomol. Struct. Dyn.. 2023;41(19):10257-10276.
- [Google Scholar]
- The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. Nutr. Biochem.. 2011;22(1):8-15.
- [Google Scholar]
- Analysis of aceclofenac and bovine serum albumin interaction using fluorescence quenching method for predictive, preventive, and personalized medicine. EPMA J.. 2015;6:1-6.
- [Google Scholar]
- Hinokiflavone attenuates the virulence of methicillin-resistant Staphylococcus aureus by targeting caseinolytic protease P. Antimicrob. Agents Chemother.. 2022;66(8):e00240-00222
- [Google Scholar]
- Spectroscopic and theoretical analysis of the interaction between plasma proteins and phthalimide analogs with potential medical application. Life. 2023;13(3):760.
- [Google Scholar]
- Osteoblast differentiation stimulating activity of biflavonoids from Cephalotaxus koreana. Bioorg. Med. Chem. Lett.. 2006;16(11):2850-2854.
- [Google Scholar]
- Mechanism evaluation of the interactions between eight flavonoids and γ-globulin based on multi-spectroscopy. J. Mol. Struct.. 2021;1225:129291
- [Google Scholar]
- Effects of B-ring structures on binding behavior of flavonols with proteins: experimental and molecular docking approaches. J. Mol. Struct.. 2023;1287:135614
- [Google Scholar]
- The protective effect of hesperetin in osteoarthritis: an in vitro and in vivo study. Food Funct.. 2020;11(3):2654-2666.
- [Google Scholar]
- Oxidative stress and natural antioxidants in osteoporosis: novel preventive and therapeutic approaches. Antioxidants. 2023;12(2):373.
- [Google Scholar]
- Cytoprotective effects of fermented oyster extracts against oxidative stress-induced DNA damage and apoptosis through activation of the Nrf2/HO-1 signaling pathway in MC3T3-E1 osteoblasts. EXCLI J.. 2020;19:1102.
- [Google Scholar]
- New perspective on the interaction behavior between riboflavin and β lactoglobulin-β casein complex by biophysical techniques. Cell Biochem. Biophys. 2023:1-17.
- [Google Scholar]
- Re-appraising the role of flavonols, flavones and flavonones on osteoblasts and osteoclasts – A review on its molecular mode of action. Chem. Biol. Interact.. 2022;355:109831
- [Google Scholar]
- Natural products from genus Selaginella (Selaginellaceae) Nusantara Biosci.. 2011;3(1)
- [Google Scholar]
- Multispectroscopic studies on the molecular interactions between bovine γ-globulin and borohydride-capped silver nanoparticles. Luminescence. 2022;37(7):1200-1207.
- [Google Scholar]
- Su, L.-J., Zhang, J.-H., Gomez, H., Murugan, R., Hong, X., Xu, D., Jiang, F., Peng, Z.-Y., 2019. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longevity.
- Sciadopitysin protects osteoblast function via its antioxidant activity in MC3T3-E1 cells. Food Chem. Toxicol.. 2013;58:220-227.
- [Google Scholar]
- Elucidating the interaction of propofol as an intravenous anesthetic drug with blood components: IgG and peripheral blood mononuclear cell as targets. Arab. J. Chem.. 2021;14(2):102965
- [Google Scholar]
- Mechanistic and conformational studies on the interaction of sulfamethazine with human immunoglobulin G by molecular modeling and multi-spectroscopic approach in vitro. Luminescence. 2015;30(6):798-804.
- [Google Scholar]
- Molecular modeling and multi-spectroscopic approaches to study the interaction between antibacterial drug and human immunoglobulin G. Luminescence. 2016;31(3):704-711.
- [Google Scholar]
- Interaction of dietary flavonoids with gamma-globulin: molecular property-binding affinity relationship aspect. Food Funct.. 2011;2(2):137-141.
- [Google Scholar]
- Effect of danofloxacin on reactive oxygen species production, lipid peroxidation and antioxidant enzyme activities in kidney Tubular epithelial cell line, LLC-PK 1. Basic Clin. Paharmacol. Toxicol.. 2013;113(6):377-384.
- [Google Scholar]
- Protective effect of tetrahydroxystilbene glucoside against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. Eur. J. Pharmacol.. 2012;689(1–3):31-37.
- [Google Scholar]
- Correlation of oxidative stress-related biomarkers with postmenopausal osteoporosis: a systematic review and meta-analysis. Arch. Osteoporos.. 2021;16:1-10.
- [Google Scholar]
- Insight into the molecular interaction between the anticancer drug, enzalutamide and human alpha-2-macroglobulin: biochemical and biophysical approach. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2024;123957
- [Google Scholar]







