Translate this page into:
Genus Chloranthus: A comprehensive review of its phytochemistry, pharmacology, and uses
⁎Corresponding author at: School of Pharmacy, Shaanxi University of Chinese Medicine, No.1, Middle Section of Century Avenue, Qindu District, Xianyang, Shaanxi Province 712046, PR China (D.D. Zhang). School of Pharmacy, Shaanxi University of Chinese Medicine, No.1, Middle Section of Century Avenue, Qindu District, Xianyang, Shaanxi Province 712046, PR China (W. Wang). zhangnatprod@163.com (Dong-dong Zhang), 2051003@sntcm.edu.cn (Wei Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This paper is intended to review advances in the botanical, traditional uses, phytochemical, pharmacological and development and utilization studies of the genus Chloranthus. Chloranthus, a genus of the family Chloranthaceae, which is mainly distributed in the temperate and tropical regions of Asia, has been used as a folk remedy for swollen boils, snake bites and bruises. Up to now, 418 compounds have been reported from the genus Chloranthus, including 383 terpenoids, 4 coumarins, 6 lignans, 2 simple phenylpropanoids, 4 flavonoids, 6 amides, 5 organic acids and some other types of compounds. Among them, the main chemical constituents are sesquiterpenes and their diterpenoids. Modern pharmacological studies have shown that most of the Chloranthus plants possessed anti-cancer, anti-inflammatory, antibacterial, antiviral, and antimalarial activities. As one of the most important genera in China, Chloranthus should be paid further attention to gathering information about the pharmacological mechanism and value active compounds. This paper summarized the phytochemistry, pharmacology, and uses of genus Chloranthus in order to lay a foundation and provide reference for the follow-up research and wide application of the genus.
Keywords
Chloranthus
Traditional uses
Chemical constituents
Pharmacological
Development and utilization
Review
1 Introduction
The genus Chloranthus belongs to the family Chloranthaceae, and consists of 14 species in the world (Chloranthus Swartz in Flora of China @ efloras.org“ eFlora). They are mainly distributed in temperate and tropical Asia (Lu et al., 2020). Among them, 13 species are reported in southwestern, southern, eastern and central China (Chloranthus Swartz in Flora of China @ efloras.org eFlora). As the country with the abundant resources of the genus Chloranthus, China has a long history of application of the genus Chloranthus plants. In traditional Chinese medicine (TCM) theory, their effects are defined as dispersing cold, dispelling wind and relieving pain, removing blood stasis and detoxifying (Zhang, 2016). According to the Dictionary of Traditional Chinese Medicine, Fujian Folk Herbal Medicine, Jiangxi Herbal Medicine and other local herbal standards, most of this genus plants can be used as folk herbal medicine to treat wind-cold cough, bruises and injuries, rheumatism and lumbago.
Due to the novelty of the chemical structure and the richness of biological activities, a large number of scholars at home and abroad have conducted in-depth studies on genus Chloranthus. Modern pharmacology has shown that this genus has excellent pharmacological activities in anticancer, antibacterial, anti-inflammatory and neuroprotective effect (Chen et al., 2021c; Huang et al., 2020). Phytochemical studies discovered the presence of sesquiterpenes, coumarins, lignans, simple phenylpropanoids, flavonoids and amides from this genus. Especially sesquiterpenes dimer macrocyclic compounds possess significant antitumor activity. Such as the types of chloranthalactone and shizukanolide, studies have shown that this class of compounds showed significant activity against A549 cells, human glioma U87 cells, and hepatocellular carcinoma SMMC-7721 cells (Zhang et al., 2021). It has a broad prospect in developing drugs against breast cancer and liver cancer. Eudesmane sesquiterpenes isolated from the C. fortunei, such as fortunilide A (96), sarglabolide J (100), chlorahololide D (103), most of which exhibited antimalarial activity, which was comparable to the potency and selectivity index values of artemisinin (Zhou et al., 2017a).
This review summarized the research advancement of this genus in botanical, traditional uses, phytochemical, pharmacological and development and utilization studies at the past 30 years, in order to provide reference for further applications and research of the genus Chloranthus.
2 Search strategy
Comprehensive research and analysis of previously published literature were conducted for studies on the traditional use, distribution, chemistry, and pharmacological properties of the genus Chloranthus. The search was conducted using databases such as Sciencedirect, SciFinder, Medline PubMed, Google Scholar, Baidu Scholar, and CNKI by using the keywords such as Chloranthus, Chloranthus. japonicus, Chloranthus. henryi and Chloranthus multistachys. Part of the analyzed studies was got by a manual search of articles in the reference lists of the included studies. The chemical structures were drawn using ChemDraw Professional 20.0 software.
3 Botany, description and distribution
To date, about 13 species of the genus Chloranthus have been reported in China, inculding Chloranthus elatior Link, Chloranthus spicatus (Thunb.) Makino, Chloranthus angustifolius Oliv, Chloranthus japonicus Sieb, Chloranthus fortunei (A. Gray) Solms-Laub, Chloranthus holostegius (Hand.-Mazz.) Pei et Shan, Chloranthus anhuiensis K. F. Wu, Chloranthus tianmushanensis K. F. Wu, Chloranthus serratus (Thunb.) Roem. et Schult, Chloranthus multistachys Pei, Chloranthus henryi Hemsl, Chloranthus sessilifolius K. F. Wu and Chloranthus oldhamii Solms Laubach. Among them, about eight species of the genus are available for medicinal use in China. Among which C. japonicus, C. serratus, C. multistachys and C. henryi are extensively studied (Chloranthus in Flora of China @ efloras.org, 2020). Most of the plants in the genus commonly grow on mountain slopes in the forest understory and gully side grasses. They are subshrubs or perennial herbs. Leaves opposite or whorled, serrate; stipules tiny; petioles connected by a transverse ridge on stem. Inflorescences in spikes or branched, arranged in panicles, terminal or axillary. Flowers small, bisexual; perianth absent. Stamens usually 3, rarely 1, on 1 side of apical part of ovary; basal part of connective confluent, or free and connected or overlapped at base, ovoid or lanceolate, sometimes elongated to linear; anthers 1-or 2-loculed; if stamens 3, central anther 2-loculed or occasionally absent, lateral anthers 1-loculed, if stamen 1, anther 2-loculed. Ovary 1-loculed; ovule 1, pendulous, orthotropous; style usually absent, rarely present; stigma truncate or parted. Drupes globose, obovoid, or pyriform (Chloranthus in Flora of China @ efloras.org, 2020).
4 Traditional uses
Most of plants in the genus Chloranthus are used as folk herbal medicine and have a long history of medicinal use. It has the medicinal potencies of dispelling wind and cold, strengthening bones and tendons, activating blood circulation and dispersing blood stasis, removing swelling and relieving pain, and are commonly used to treat bruises, swelling and pain, rheumatic arthritis, boils, sores and swellings in the folk. Moreover, the resources of this genus are rich in species and reserves, and have a high value for development and utilization. In this paper, we have collected proprietary Chinese patent medicines or preparations on the genus Chloranthus, which include empirical prescriptions for folk use, in-hospital preparations and marketed drugs. (Table 3).
5 Phytochemistry and Pharmacology
Literature investigation revealed that Chloranthus include terpenoids, coumarins, amides and phenylpropanoids, among which sesquiterpenoids and diterpenoids are predominant structural types and active components. Up to now, 418 compounds have been reported from the genus Chloranthus, including 383 terpenoids, 4 coumarins, 6 lignans, 2 simple phenylpropanoids, 4 flavonoids, 5 organic acids, 6 amides, and 8 other compounds. Their specific compound names, structures and references are shown in Table 1 and Figs. 1–19. Note: B: Chloranthus elatior Link. C: Chloranthus spicatus (Thunb.) Makino. D: Chloranthus angustifolius Oliv. E: Chloranthus japonicus Sieb. F: Chloranthus fortunei (A. Gray) Solms-Laub. G: Chloranthus holostegius (Hand. -Mazz.) Pei et Shan. H: Chloranthus anhuiensis K. F. Wu. I: Chloranthus tianmushanensis K. F. Wu. J: Chloranthus serratus (Thunb.) Roem. et Schult. K: Chloranthus multistachys Pei. L: Chloranthus henryi Hemsl. M: Chloranthus sessilifolius K. F. Wu. N: Chloranthus oldhamii Solms Laubach.
No.
Name
Plant
Bioactivity
Part
References
Lindenane Sesquiterpenes and Their Polymers
1
shizukanolide
E
Antitumor activity
Aerial
(Kawabata et al., 1981)
2
chloranthalactone A
E
Antitumor activity
Aerial
(Uchida et al., 1980)
(Gong et al., 2021)
3
yinxiancaoside A
E
Antitumor activity
Whole
(Kuang et al., 2008)
4
chloranoside A
E
Whole
(Kuang et al., 2008)
5
chloranthalactone B
E
Antitumor activity
Whole
(Uchida et al., 1980)
(Gong et al., 2021)
6
chloranthalactone C
EF
Whole
(Uchida et al., 1980)
7
chloranthalactone D
E
Whole
(Uchida et al., 1980)
8
chloranthalactone E
E
Whole
(Uchida et al., 1980)
9
9-hydroxy heterogorgiolide
E
Aerial
(Uchida et al., 1980)
10
chlojaponilactone B
E
Whole
(Yan et al., 2013)
11
chlojaponilactone C
E
Whole
(Yan et al., 2013)
12
chlojaponilactone D
E
Whole
(Yan et al., 2013)
13
chlorajapolide C
E
Whole
(Yan et al., 2013)
14
chlojaponilactones E
E
Whole
(Yan et al., 2013)
15
chlorajapolides F
E
Aerial
(Zhang et al., 2012a)
16
chlorajapolides G
E
Aerial
(Zhang et al., 2012a)
17
chlorajapolides H
E
Aerial
(Zhang et al., 2012a)
18
chlojaponilactones F
E
Whole
(Li et al., 2016)
19
chlojaponilactones H
E
Whole
(Li et al., 2016)
20
chlojaponilactones G
E
Whole
(Li et al., 2016)
21
chlojaponilactones I
E
Whole
(Li et al., 2016)
22
chlorajapolides A
E
Antitumor activity
Whole
(Wang et al., 2011)
23
chlorajapolides B
E
Antitumor activity
Whole
(Wang et al., 2011)
24
chlorajapolides C
E
Antitumor activity
Whole
(Wang et al., 2011)
25
chlorajapolides D
E
Antitumor activity
Whole
(Wang et al., 2011)
26
chlorajapolides E
E
Antitumor activity
Whole
(Wang et al., 2011)
27
chlorajaposide
E
Whole
(Wang et al., 2011)
28
chloranthalactone A
E
Roots
(Uchida et al., 1980)
29
shizukanolide C
F
Aerial
(Fang, 2011)
30
shizukanolide H
EFH
Neuroprotective activity
Whole
(Fang, 2011)
31
shizukanolide G
F
Anti-inflammatory activity
Aerial
(Wang et al., 2009)
(Gong et al., 2021)
32
shizukanolide F
F
Aerial
(Wang et al., 2009)
33
lindenanolide H
G
Whole
(Kim et al., 2011)
34
(1R,3S,5S,8S,10R)-14-Acetylshizukanolide
H
Whole
(Xu et al., 2018)
35
isoshizukanolide
F
Whole
(Zhou et al., 2017b)
36
spicachlorantins G
C
Antiinflammatory activity
Roots
(Kim et al., 2011)
37
spicachlorantins H
C
Roots
(Kim et al., 2011)
38
spicachlorantins I
C
Roots
(Kim et al., 2011)
39
spicachlorantins A
C
Roots
(Kim et al., 2011)
40
spicachlorantins B
C
Antiinflammatory activity
Roots
(Kim et al., 2011)
41
spicachlorantins C
C
Roots
(Kim et al., 2011)
42
spicachlorantins D
C
Roots
(Kim et al., 2011)
43
chloramultilide A
CDF
Antineuroinflammatory activity
Antimicrobial activityRoots
(Kim et al., 2011)
(Yang et al., 2014)
44
spicachlorantins E
C
Roots
(Kim et al., 2011)
45
spicachlorantins F
C
Roots
(Kim et al., 2011)
46
chloramultilide B
C
Antimicrobial activity
Whole
(Xu et al., 2007)
47
chloramultilide C
C
Antimicrobial activity
Whole
(Xu et al., 2007)
48
chloramultilide D
C
Whole
(Xu et al., 2007)
49
trichloranoids A
C
Antimalarial activity
Whole
(Zhou et al., 2021)
50
trichloranoids B
C
Whole
(Zhou et al., 2021)
51
trichloranoids C
C
Whole
(Zhou et al., 2021)
52
trichloranoids D
C
Antimalarial activity
Whole
(Zhou et al., 2021)
53
analogue
C
Antimalarial activity
Whole
(Zhou et al., 2021)
54
chlojapolides A
DE
Antiinflammatory activity
Whole
(Guo et al., 2016)
55
chlojapolides B
DE
Whole
(Guo et al., 2016)
56
chlojapolides C
DE
Whole
(Guo et al., 2016)
57
chlojapolides D
DE
Whole
(Guo et al., 2016)
58
chlojapolides E
DE
Whole
(Guo et al., 2016)
59
chlojapolides F
DE
Whole
(Guo et al., 2016)
60
shizukaol A
DEF
Antiinflammatory activity
Whole
(Guo et al., 2016)
(Gong et al., 2021)
61
shizukaol A acetate
E
Roots
(Kawabata et al., 1990)
62
chlojapolides G
DE
Aerial
(Guo et al., 2016)
63
chlojapolides H
DE
Aerial
(Guo et al., 2016)
64
spicachlorantin H
DE
Aerial
(Guo et al., 2016)
65
shizukaol B
CDEFJ
Antitumor activity
Antinflammatory activity
Anti-viral ActivityWhole
(Zhang et al., 2012a)
(Fang et al., 2011)
66
shizukaol F
DEFGJ
Antitumor activity
HIV-1 RNase H inhibitor
Anti-viral ActivityWhole
(Guo et al., 2016)
(Xu, 2016)
(Fang et al., 2011)
67
shizukaol G
DEF
Anti-inflammatory
Anti-tumor activityAerial
(Guo et al., 2016)
68
shizukaol C
CDEFG
Anti-inflammatory activity
Insecticidal activity
Anti-tumor activity
Anti-viral ActivityAerial
(Zhang et al., 2012a)
(Guo et al., 2016)
(Gong et al., 2021)
(Shi et al., 2015)
(Fang, 2011)
69
shizukaol D
DEFJ
Anti-inflammatory activity
Anti-tumor activity
Hypoglycemic Activity
Aerial
(Guo et al., 2016)
(Shi et al., 2015)
(Zhang et al., 2012)
(Hu et al., 2017)
70
shizukaol H
CE
Anti-viral Activity
Aerial
(Fang, 2011)
(Fang et al., 2011)
71
chloramultilide B
DEF GJ
Anti-bacterial activity
Aerial
(Fang, 2011)
72
spicachlorantin B
DEF
Anti-neuroinflammatory activity
Aerial
(Zhou et al., 2017b)
73
chlorahololide C
DEF
Inhibiting K+ channels
Aerial
(Guo et al., 2016)
(Yang et al., 2008)
74
spicachlorantins J
C
Roots
(Guo et al., 2016)
75
henriol A
D
Antimicrobial activity
Aerial
(Xu, 2016)
(Yang et al., 2014)
76
spicachlorantin A
DJ
Antimicrobial activity
Roots
(Yang et al., 2014)
77
tianmushanol
DJI
Inhibiting TYR activity
Antimicrobial activityRoots
(Yang et al., 2014)
78
8-O-methyltianmushanol
DIJ
Inhibiting TYR activity
Antimicrobial activityRoots
(Yang et al., 2014)
(Wu et al., 2008)
79
chlojapolactone A
E
Anti-inflamma tory activity
Whole
(Guo et al., 2015)
80
multistalide C
E
Insecticidal activity
Whole
(Shi et al., 2015)
81
chlorajaponilide I.
E
Whole
(Zhuo et al., 2017)
82
spicachlorantin D
EF
Whole
(Zhuo et al., 2017)
83
chlorajaponilide C
EF
Antimalarial activity
Whole
(Zhuo et al., 2017)
(Zhou et al., 2017b)
84
japonicones A
E
Whole
(Yan et al., 2019)
85
japonicones B
E
Whole
(Yan et al., 2019)
86
japonicones C
E
Whole
(Yan et al., 2019)
87
chlorajaponol
E
Whole
(Wang et al., 2011)
88
chloranthadimeric acid acetate
E
Roots
(Uchida et al., 1980)
89
chlorajaponilides A
E
Whole
(Fang, 2011)
90
chlorajaponilides B
E
Whole
(Fang, 2011)
91
chlorajaponilides D
E
Whole
(Fang, 2011)
92
chlorajaponilides E
E
Whole
(Fang, 2011)
93
cloramultilide C
E
Whole
(Fang, 2011)
94
yinxiancaol
EFG
Whole
(Fang, 2011)
95
chlorafortulide
F
Whole
(Zhang et al., 2012a)
96
fortunilide A
F
Antimalarial activity
Whole
(Zhou et al., 2017b)
97
fortunilide B
F
Whole
(Zhou et al., 2017b)
98
fortunilide C
F
Whole
(Zhou et al., 2017b)
99
sarglabolide I
F
Whole
(Zhou et al., 2017b)
100
sarglabolide J
F
Antimalarial activity
Whole
(Zhou et al., 2017b)
101
shizukaol K
F
Whole
(Zhou et al., 2017b)
102
shizukaol M
F
Whole
(Zhou et al., 2017b)
103
chlorahololide D
FGJ
Inhibiting K+ channels
Whole
(Zhou et al., 2017b)
(Yang et al., 2008)
104
shizukaol N
F
Whole
(Zhou et al., 2017b)
105
sarcandrolide B
F
Whole
(Zhou et al., 2017b)
106
sarcandrolide A
F
Whole
(Zhou et al., 2017b)
107
sarcandrolide J
F
Whole
(Zhou et al., 2017b)
108
sarcandrolide E
F
Whole
(Zhou et al., 2017b)
109
fortunilides D
F
Whole
(Zhou et al., 2017b)
110
fortunilides E
F
Whole
(Zhou et al., 2017b)
111
fortunilides F
F
Whole
(Zhou et al., 2017b)
112
fortunilides G
F
Whole
(Zhou et al., 2017b)
113
fortunilides H
F
Whole
(Zhou et al., 2017b)
114
fortunilides I
F
Anti-inflammatory activity
Whole
(Zhou et al., 2017b)
115
fortunilides J
F
Whole
(Zhou et al., 2017b)
116
fortunilides K
F
Whole
(Zhou et al., 2017b)
117
fortunilides L
F
Whole
(Zhou et al., 2017b)
118
fortunoid A
F
Antimalarial activities
Whole
(Zhou et al., 2017b)
119
fortunoid B
F
Antimalarial activities
Whole
(Zhou et al., 2017b)
120
fortunoid C
F
Aerial
(Zhou et al., 2017b)
121
shizukaol P
F
Aerial
(Zhou et al., 2017b)
122
9-O-β-glucopyranosylcycloshizukaol A
F
Aerial
(Wang et al., 2009)
123
cycloshizulkaol A
F
Anti-tumor activity
Aerial
(Wang et al., 2009)
124
shizukaol L
F
Roots
(Gong et al., 2021)
125
shizukaol O
F
Anti-inflammatory activity
Anti-tumor activityRoots
(Gong et al., 2021)
(Zhang et al., 2012a)
126
cihoranhtaol A
F
Whole
(Luo et al., 2009)
127
chioranthaol B
F
Whole
(Luo et al., 2009)
128
chioranthaol C
F
Whole
(Luo et al., 2009)
129
chlorahololide G
G
Whole
(Xu, 2016)
130
chlorahololide B
G
Inhibiting K+ channels
Whole
(Xu, 2016)
131
chloramultiol D
G
Whole
(Xu, 2016)
132
chlorahololide F
G
Inhibiting K+ channels
Whole
(Xu, 2016)
(Yang et al., 2008)
133
sarcandrolide D
G
Whole
(Xu, 2016)
134
henriol C
GJ
Roots
(Xu, 2016)
135
chlotrichenes A
G
Roots
(Chi et al., 2019)
136
chlotrichenes B
G
Anti-tumor activity
Roots
(Chi et al., 2019)
137
chololactone A
G
Anti-inflammatory activity
Roots
(Shen et al., 2017)
138
chololactone B
G
Anti-inflammatory activity
Roots
(Shen et al., 2017)
139
chololactone C
G
Anti-inflammatory activity
Roots
(Shen et al., 2017)
140
chololactone D
G
Anti-inflammatory activity
Roots
(Shen et al., 2017)
141
chololactone E
G
Anti-inflammatory activity
Roots
(Shen et al., 2017)
142
chololactone F
G
Anti-inflammatory activity
Roots
(Shen et al., 2017)
143
chololactone G
G
Anti-inflammatory activity
Roots
(Shen et al., 2017)
144
chololactone H
G
Anti-inflammatory activity
Roots
(Shen et al., 2017)
145
multistalides A
G
Whole
(Zhang et al., 2010)
146
multistalides B
G
Whole
(Zhang et al., 2010)
147
chloraserrtone A
J
Roots
(Bai et al., 2019)
148
chlorahololide A
F
Inhibiting K+ channels
Whole
(Zhou et al., 2017b)
149
chlorahololide E
F
Inhibiting K+ channels
Whole
(Zhou et al., 2017b)
(Yang et al., 2008)
150
shizukaol
F
Whole
(Wang et al., 2009)
151
13′-acetylshizukaol C
F
Whole
(Gong et al., 2021)
152
chloramuhilide B
F
Whole
(Gong et al., 2021)
153
chlorahupetone A
L
Antitumor activity
Whole
(Zhang et al., 2021)
154
chlorahupetone B
L
Whole
(Zhang et al., 2021)
155
chlorahupetone C
L
Whole
(Zhang et al., 2021)
156
chlorahupetone D
L
Whole
(Zhang et al., 2021)
157
chlorahupetone E
L
Whole
(Zhang et al., 2021)
158
chlorahupetone F
L
Whole
(Zhang et al., 2021)
159
chlorahupetone G
L
Antitumor activity
Whole
(Zhang et al., 2021)
160
chlorahupetone H
L
Antitumor activity
Whole
(Zhang et al., 2021)
161
chlorahupetone I
L
Antitumor activity
Whole
(Zhang et al., 2021)
Eudesmane Sesquiterpenes
162
serralactones A
J
Whole
(Teng et al., 2010)
163
serralactones B
J
Whole
(Teng et al., 2010)
164
serralactones C
J
Whole
(Teng et al., 2010)
165
serralactones D
J
Whole
(Teng et al., 2010)
166
neolitacumone B
J
Whole
(Teng et al., 2010)
167
1β,4β-dihydroxy-5α,8β(H)-eudesm-7(11)Z-en-8,12-olide
C
Aerial
(Yang et al., 2007a)
168
1β,4α-dihydroxy-5α,8β(H)-eudesm-7(11)Z-en-8,12-olide
C
Aerial
(Yang et al., 2007a)
169
homalomenol A
C
Aerial
(Yang et al., 2007a)
170
oplodiol
C
Aerial
(Yang et al., 2007a)
171
chlospicates A
C
Whole
(Yang et al., 2007a)
172
chlospicates B
C
Whole
(Yang et al., 2007a)
173
codonolactone
L
Whole
(Wang et al., 2014a)
174
5-eudesmene-1β,4α-diol
C
Whole
(Yang et al., 2007a)
175
4α,8β-dihydroxyeudesm-7(11)-en-8,12-olide
D
Antimicrobial activity
Roots
(Wang et al., 2014a)
176
4β,7β,11-enantioeudesmantriol
D
Roots
(Xu, 2016)
177
9α-hydroxycurcolonol
D
Antimicrobial activity
Roots
(Yang et al., 2014)
(Wang et al., 2014a)
178
3α-hydroxy-4-deoxy-5-dehydrocurcolonol
D
Antimicrobial activity
Roots
(Yang et al., 2014)
(Wang et al., 2014a)
179
9α-curcolonol
DFJ
Antimicrobial activity
Roots
(Yang et al., 2014)
(Wang et al., 2014a)
180
4α-hydroxy5α,8β(H)-eudesm-7(11)-en-8,12-olide monohydrate
E
Whole
(Lu et al., 2015)
181
shizukafuranol
E
Whole
(Kawabata et al., 1984)
182
shizukolidol
E
Whole
(Kawabata et al., 1984)
183
1β,10β-dihydroxy-eremophil-7(11),8-dien-12,8-olide
E
Whole
(Lu et al., 2016)
184
8,12-epoxy-1β-hydroxyeudesm-3,7,11-trien-9-one
E
Whole
(Lu et al., 2016)
185
4α-hydroxy-5α(H)-8β-methoxy-eudesm-7(11)-en-12,8-olide
E
Whole
(Lu et al., 2016)
186
CJ-01
E
Antimicrobial activity
Whole
(Yim et al., 2008)
187
chlojaponilactone A
E
Whole
(Fang, 2011)
188
tsoongianolide D
E
Whole
(Yan et al., 2013)
189
tsoongianolide E
E
Whole
(Yan et al., 2013)
190
(10α)-10-hydroxy-1-oxoeremophila-7(11),8-dien-12,8-olide
E
Whole
(Yan et al., 2013)
191
chlorajapolides I
E
Aerial
(Zhang et al., 2012a)
192
chlojaponols A
E
Whole
(Li et al., 2016)
193
chlojaponols B
E
Antimicrobial activity
Whole
(Li et al., 2016)
194
chlorajapotriol
E
Whole
(Zhuo et al., 2017)
195
chloraeudolide
E
Antitumor activity
Whole
(Wang et al., 2011)
196
chlorantene B
E J
Whole
(Yuan et al., 2008)
197
chlorantene C
EJ
Neuroprotective activity
Whole
(Yuan et al., 2008)
(Chen et al., 2021a)
198
chlorantene D
EJ
Antibacterial activity
Whole
(Yuan et al., 2008)
199
chlorantene G
E
Antibacterial activity
Whole
(Yuan et al., 2008)
200
atractylenolactam
F
Whole
(Wang et al., 2008)
201
curcodione
F
Neuroprotective activity
Whole
(Chen et al., 2021a)
202
1β,8β-dihydroxyeudesman −3,7(11)-dien-8α,12-olide
G
Whole
(Xu, 2016)
203
4(15)-eudesmene-1β,7α,11-triol
G
Whole
(Xu, 2016)
204
3,4,8α-trimethyl-4α,7,8,8α-tetrahydro-4α-naphto[2,3-b]furan-9-one
G
Whole
(Zhan et al., 2021)
205
(5S,10S)-9-Oxo-atractylon
H
Whole
(Xu et al., 2018)
206
chlorantene J
H
Whole
(Xu et al., 2018)
207
(7R,10S)-7-hydroxyeudesm-4-en-3,6-dione
H
Whole
(Xu et al., 2018)
208
1α-hydroxy-4αH,5αH-eudesma-7,11-diene-6,9-dione
H
Whole
(Xu et al., 2018)
209
4α-hydroxy-8,12-epoxyeudesma-7,11-diene-1,6-dione
H
Whole
(Xu et al., 2018)
210
(3R)-3-hydroxyatractylenolide III
H
Roots
(Xu et al., 2010)
211
8β-hydroxy-1-oxoeudesma-3,7(11)-dien-12,8α-olide
H
Roots
(Xu et al., 2010)
212
chlorantene M
J
Whole
(Huang et al., 2021)
213
5α,7α(H)-6,8-cycloeudesma-1β,4β-diol
C
Aerial
(Yang et al., 2007a)
214
5α-(cinnamoyloxy)-8,12-epoxy-3-methoxy-7βH,8αH-eudesma-3,11-dien-6-one
E
Aerial
(Fang, 2011)
215
8β-(cinnamoyloxy)eudesma-4(14),7(11)-dien-12,8-olide
E
Aerial
(Fang, 2011)
216
8,12-epoxy-1α-hydroxy-4αH,5αH-eudesma-7,11-diene-6,9-dione
E
Aerial
(Fang, 2011)
217
8,12-epoxy-1α-methoxy-4αH,5αH-eudesma-7,11-diene-6,9-dione
E
Aerial
(Fang, 2011)
218
sarcaglaboside A
E
Hepatoprotective activity
Aerial
(Fang, 2011)
(Li et al., 2006)
219
chlorajapodiolide
E
Whole
(Fang, 2011)
220
chloranholide A
G
Whole
(Zhan et al., 2021)
221
1α-methoxy-8,12-epoxyeudesma-4,7,11-trien-6-one
L
Steem
(Wu et al., 2008)
222
11,12,13-trihydroxyeudesma-4(15),8-dien-9-one
L
Steem
(Wu et al., 2008)
223
1α-hydroxy-8,12-epoxyeudesma-4,7,11-triene-3,6-dione
L
Roots
(Gan et al., 2009)
224
curcolone
L
Roots
(Gan et al., 2009)
225
endesm-4(15)-en-7α,11-diol
L
Roots
(Gan et al., 2009)
226
1α-hydroxy-8,12-epoxyeudesma-4,7,11-triene-6,9-dione
L
Antitumor activity
Whole
(Wu et al., 2006)
Germacrane Sesquiterpenes
227
germacra-5E,10(14)-dien-1β,4β-diol
C
Whole
(Yang et al., 2012)
228
4α,5α-epoxy1(10),7(11)-dienegermacr-8α,12-olide
C
Whole
(Yang et al., 2012)
229
furanodienone
DE
Roots
(Yang et al., 2014)
230
glechomanolid
E
Aerial
(Kawabata et al., 1981)
231
isofuranodiene
E
Aerial
(Kawabata et al., 1981)
232
chlorantene E
EJ
Anti-bacterial activity
Whole
(Yuan et al., 2008)
233
chloranthatone
F
Roots
(Wang et al., 2008)
234
zederone
F
Neuroprotective activity
Whole
(Chen et al., 2021a)
235
(1E,4Z)-8-hydroxy-6-oxogermacra-1(10),4,7(11)-trieno-12,8-lactone
F
Neuroprotective activity
Whole
(Wu et al., 2008)
(Chen et al., 2021a)
236
8-methoxy-6-oxogermacra-1(10),4,7(11)-trieno-12,8-lactone
L
Steem
(Wu et al., 2008)
237
zederone epoxide
F
Antitumor activity
Anti-neuroinflammatory activity Neuroprotective activityWhole
(Wang et al., 2014a)
(Chen et al., 2021a)
238
4β,5α-dihydroxy-10(β)H-8,12-epoxygermacra-7,11-diene-9-one
G
Whole
(Xu, 2016)
239
curcuzederone
H
Whole
(Xu et al., 2018)
240
15-hydroxy-11βH-8-oxogermacra-1(10),4-dieno-12,6α-lactone
L
Steem
(Wu et al., 2008)
241
(1S,4S,5S,10S)-1,10:4,5-diepoxygermacrone
L
Whole
(Wang et al., 2014a)
242
chlogermacrone A
L
Roots
(Chen et al., 2020)
243
chlogermacrone C
L
Neuroprotective effects activity
Roots
(Chen et al., 2020)
Cadinane Sesquiterpenes
244
(7R,9S,10R)-3,9-di-hidroxicalameneno
G
Whole
(Xu, 2016)
245
chloranholide B
G
Whole
(Zhan et al., 2021)
246
chloranholide C
G
Whole
(Zhan et al., 2021)
247
chloranholide D
G
Anti-inflammatory activity
Whole
(Zhan et al., 2021)
248
phacadinane E
H
Whole
(Xu et al., 2018)
249
chlomultin C
H
Whole
(Xu et al., 2018)
250
chlorantene N
K
Whole
(Huang et al., 2021)
251
(4α)-8-hydroxy-12-norcardina-6,8,10-trien-11-one
L
Whole
(Wang et al., 2014a)
252
(4α,11β)-8,11-dihydroxycadina-6,8,10-trien-12-oic acid g-lactone
L
Whole
(Wang et al., 2014a)
253
4-epimer
L
Whole
(Wang et al., 2014b)
254
(8α)-6,8-dihydroxycadina-7(11),10(15)-dien-12-oic acid g-lactone1)
L
Anti-tumor activity
Steem
(Wu et al., 2007)
255
tanapraetenolide
L
Steem
(Wu et al., 2007)
256
dayejijiol
L
Anti-tumor activitity
Whole
(Wu et al., 2006)
Guaiane Sesquiterpenes
257
(1R,4S,5R,8S,10S)-Zedoalactone A
K
Whole
(Liu et al., 2013)
258
multistalactone D
K
Whole
(Liu et al., 2013)
259
multistalactone E
K
Whole
(Liu et al., 2013)
260
multistalactone F
K
Whole
(Liu et al., 2013)
261
chlospicate D
C
Whole
(Yang et al., 2012)
262
chloraniolide A
H
Whole
(Xu et al., 2010)
263
chlospicates C
C
Whole
(Yang et al., 2012)
264
chlohenriol A
L
Neuroprotective activitity
Roots
(Chen et al., 2021c)
265
chlohenriol B
L
Neuroprotective activitity
Roots
(Chen et al., 2021c)
266
chlohenriol C
L
Neuroprotective activitity
Roots
(Chen et al., 2021c)
Acorane Sesquiterpenes
267
shizuka-acoradienol
EF
Roots
(Kawabata et al., 1984)
268
spiro[4.5]dec-6-ene-8α,9β,15α-triol,4β-methyl-1α-isopropyl
G
Whole
(Xu, 2016)
269
Spiro[4.5]dec-6-ene-8β,9β,15α-triol,4β-methyl-1α-isopropyl
G
Whole
(Xu, 2016)
270
8-desmethylacor-6,9-dien-8-one-3α-ol
G
Whole
(Xu, 2016)
Eremophilane Sesquiterpenes
271
(3R,4S,5R,10S,11S)-3-hydroxy-8-oxo-6-eremophilen-12-oic acid
H
Leaves
(Wu et al., 2010)
272
Anhuienol
H
Leaves
(Wu et al., 2010)
273
(3R,4S,5R,6R,8R,10S)-3,6,8-trihydroxy-7(11)-eremophilen-12,8-olide
H
Leaves
(Wu et al., 2010)
274
3R,6R-dihydroxy-8αH-7(11)-eremophilen-12,8-olide
H
Leaves
(Wu et al., 2010)
275
anhuienoside A
H
Leaves
(Wu et al., 2010)
276
6αH,8αH-7(11)-eremophilen-12,8:15,6-diolide
H
Leaves
(Wu et al., 2010)
277
(7α)-8-oxoeudesm-4(14)-en-12-oic acid
L
Leaves
(Wu et al., 2010)
Oplopanone Sesquiterpenes
278
oplopanone
C
Aerial
(Yang et al., 2007a)
Drimane Sesquiterpene
279
11- hydroxydrim-8,12-en-14-oic acid
L
Whole
(Gan et al., 2009)
Elemene Sesquiterpene
280
curzerenone
F
Neuroprotective activity
Whole
(Chen et al., 2021a)
281
isogermafurenolide
H
Whole
(Xu et al., 2018)
Brasilane Sesquiterpene
282
chlospicates E
C
Whole
(Yang et al., 2012)
Others Sesquiterpene
283
chloranholides E
G
Whole
(Zhan et al., 2021)
284
chlorantolide A
H
Whole
(Xu et al., 2018)
285
(7S,1(10)Z)-4,5-secoguaia-1(10),11-diene-4,5-dione
L
Whole
(Wang et al., 2014b)
286
chlogermacrone B
L
Roots
(Chen et al., 2020)
Monoterpenes
287
pressafonin
D
Roots
(Wang et al., 2014a)
288
(3R,4S,6R)- p-menth-1-en-3,6-diol
E
Whole
(Lu et al., 2016)
289
(R)-p-menth-1-en-4,7-diol
E
Whole
(Lu et al., 2016)
290
(–) loliolide
F
Whole
(Chen et al., 2021a)
Diterpenoids
291
13-epitorulosol
FJ
Whole
(Chen et al., 2019)
292
(12R,13E)-15-(acetoxy)-12-hydroxylabda-8(20),13-dien-19-oic acid
H
Roots
(Xu et al., 2010)
293
(12S,13E)-15-(acetoxy)-12-dihydroxylabda-8(20),13-dien-19-oic acid
H
Roots
(Xu et al., 2010)
294
12R,13S-dihydroxylabda-8(17),14-dien-19-oic acid
J
Roots
(Chen et al., 2019)
295
henrilabdane A
J
Roots
(Chen et al., 2019)
296
henrilabdane C
J
Roots
(Chen et al., 2019)
297
12S,15-dihydroxylabda-8(17),13E-dien-19-oic acid
J
Roots
(Chen et al., 2019)
298
henrilabdane B
J
Roots
(Chen et al., 2019)
299
12,15-expoxylabda-8(17),13-dien-19-oic acid
J
Roots
(Chen et al., 2019)
300
serralabdanes A
J
Anti-inflammatory activity
Whole
(Zhang et al., 2013)
301
serralabdanes B
J
Anti-inflammatory activity
Whole
(Zhang et al., 2013)
302
serralabdanes C
J
Anti-inflammatory activity
Whole
(Zhang et al., 2013)
303
serralabdanes D
J
Anti-inflammatory activity
Whole
(Zhang et al., 2013)
304
serralabdanes E
J
Anti-inflammatory activity
Whole
(Zhang et al., 2013)
305
ent-17-hydroxyl-16-methoxyl-kauran-3-one
K
Whole
(Luo et al., 2014)
306
ent-17-acetoxyl-16-methoxyl-kauran-3-one
K
Whole
(Luo et al., 2014)
307
ent-17-hydroxylkaur-15-en-3-one
K
Whole
(Luo et al., 2014)
308
ent-3-acetoxyl-kaur-15-en-16, 17-diol
K
Whole
(Luo et al., 2014)
309
ent-kauran-3, 16, 17-triol
K
Whole
(Luo et al., 2014)
310
ent-3-acetoxyl-kauran-16, 17-diol
K
Whole
(Luo et al., 2014)
311
ent-kauran-16, 17-diol
K
Whole
(Luo et al., 2014)
312
abbeokutone
K
Whole
(Luo et al., 2014)
313
ent-17α-acetyl-16β-hydroxyl- kauran-3-one
K
Anti-tumor activity
Whole
(Luo et al., 2014)
314
15-norlabda-8(20),12E-diene-14-carboxalde-19-oic acid
C
Whole
(Yang et al., 2012)
315
12R,15-dihydroxy-8(17),13E-labdadien-19-oic acid
D
Roots
(Wang et al., 2014a)
316
chloranhenryin A
L
Whole
(Xie et al., 2015)
317
oryzalexin A
L
Whole
(Xie et al., 2015)
318
15-hydroxysessilifol F
L
Whole
(Xie et al., 2015)
319
decandrin B
L
Whole
(Xie et al., 2015)
320
sessilifol F
L
Anti-inflammatory activity
Whole
(Xie et al., 2015)
321
13-O-methylsessilifol D
L
Whole
(Xie et al., 2015)
322
sessilifol D
L
Whole
(Xie et al., 2015)
323
chloranhenryin B
L
Antibacterial activity
Whole
(Xie et al., 2015)
324
chloranhenryin C
L
Whole
(Xie et al., 2015)
325
15-O-methylsessilifol J
L
Whole
(Xie et al., 2015)
326
chloranhenryin D
L
Whole
(Xie et al., 2015)
327
chloranhenryin E
L
Whole
(Xie et al., 2015)
328
chloranhenryin F
L
Whole
(Xie et al., 2015)
329
15-ene-3α,8α-diol
L
Whole
(Xie et al., 2015)
330
ent-pimara-8(14),15-diene-3α,7β-diol
L
Antibacterial activity
Whole
(Xie et al., 2015)
331
3β-hydroxyabieta-8,11,13-trien-7-one
L
Antibacterial activity
Whole
(Xie et al., 2015)
332
3β,7α-dihydroxyabieta-8,11,13-triene
L
Antibacterial activity
Whole
(Xie et al., 2015)
333
sessilifol O
L
Whole
(Xie et al., 2015)
334
henrilabdanes A
L
Hepatoprotective activity
Roots
(Li et al., 2008)
335
henrilabdanes C
L
Hepatoprotective activity
Roots
(Li et al., 2008)
336
henrilabdanes B
L
Hepatoprotective activity
Roots
(Li et al., 2008)
337
(13S)-13-hydroxy-19-methoxy-5αH-8(17),14-labdadien
L
Whole
(Wu et al., 2006)
338
7β,12α-Dihydroxy-13-epi-manoyl oxide
L
Roots
(Gan et al., 2009)
339
7β,12α-Dihydroxymanoyl oxide
L
Roots
(Gan et al., 2009)
340
(12R)-Labda-8(17),13E-dien-12,15,19-triol
L
Roots
(Gan et al., 2009)
341
15-Nor-14-oxolabda-8(17),12E-dien-19-ol
L
Roots
(Gan et al., 2009)
342
12(R)-12,15-dihydroxylabda-8(17),13E-dien-19-oic acid
L
Roots
(Gan et al., 2009)
343
15-hydroxy-12-oxolabda-8(17),13E-dien-19-oic acid
L
Roots
(Gan et al., 2009)
344
15-nor-14-oxolabda-8(17),12E-dien-19-oic acid
L
Roots
(Gan et al., 2009)
345
(12R),(13S)-12,13-dihydroxylabda-8(17),14-dien-19-oic acid
L
Roots
(Gan et al., 2009)
346
(12S)-12,15-dihydroxylabda-8(17),13E-dien-19-oic acid
L
Roots
(Gan et al., 2009)
347
12,15-Epoxy-5αH,9βH-labda-8(17),13-dien-19-oic acid
L
Whole
(Wu et al., 2006)
348
14-methoxy-15,16-dinor-5αH,9αH-labda-13(E),8(17)-dien-12-one
L
Antitumor activity
Whole
(Wu et al., 2006)
349
(3R,5S,9R,10S)-3-hydroxy-ent-podocarpa-8(14)-ene-13-one
M
Whole
(Wang et al., 2015b)
350
3α-hydroxy-ent-torara-8-en-7,13-dione
M
Whole
(Wang et al., 2015b)
351
decandrin G
M
Whole
(Wang et al., 2015b)
352
3α,7β-dihydroxyabieta-8,11,13-triene
M
Anti-inflammatory activity
Whole
(Wang et al., 2015b)
353
decandrin B
M
Whole
(Wang et al., 2015b)
354
sessilifol A
M
Whole
(Wang et al., 2015b)
355
sessilifol B
M
Whole
(Wang et al., 2015b)
356
sessilifol C
M
Whole
(Wang et al., 2015b)
357
sessilifol G
M
Whole
(Wang et al., 2015b)
358
sessilifol H
M
Whole
(Wang et al., 2015b)
359
sessilifol I
M
Anti-inflammatory activity
Whole
(Wang et al., 2015b)
360
sessilifol J
M
Whole
(Wang et al., 2015b)
361
sessilifol K
M
Whole
(Wang et al., 2015b)
362
sessilifol M
M
Whole
(Wang et al., 2015b)
363
sessilifol N
M
Whole
(Wang et al., 2015b)
364
sessilifol P
M
Whole
(Wang et al., 2015b)
365
sessilifol Q
M
Whole
(Wang et al., 2015b)
366
chlorabietol A
N
Inhibition of PTP1B activity
Hypoglycemic ActivityRoots
(Xiong et al., 2015)
(Xiong et al., 2015)
367
chlorabietol B
N
Inhibition of PTP1B activity
Hypoglycemic ActivityRoots
(Xiong et al., 2015)
(Xiong et al., 2016)
368
chlorabietol C
N
Inhibition of PTP1B activity
Hypoglycemic ActivityRoots
(Xiong et al., 2015)
(Xiong et al., 2016)
369
19-Hydroxy-ent-abieta-7,13-diene
N
Roots
(Xiong et al., 2015)
370
chlorabietin A
N
Roots
(Xiong et al., 2016)
371
chlorabietin B
N
Anti-inflammatory activity
Roots
(Xiong et al., 2016)
372
chlorabietin C
N
Anti-inflammatory activity
Roots
(Xiong et al., 2016)
373
chlorabietin D
N
Roots
(Xiong et al., 2016)
374
chlorabietin E
N
Roots
(Xiong et al., 2016)
375
chlorabietin F
N
Anti-inflammatory activity
Roots
(Xiong et al., 2016)
376
chlorabietin G
N
Anti-inflammatory activity
Roots
(Xiong et al., 2016)
377
chlorabietin H
N
Roots
(Xiong et al., 2016)
378
chlorabietin I
N
Roots
(Xiong et al., 2016)
379
chlorabietin K
N
Roots
(Xiong et al., 2016)
Triterpenoids
380
2β,9α-dihydroxy-5α-methoxyergosta-7,22-diene
JK
Whole
(Shen et al., 2016)
381
2β,6β-dihydroxy-5α-methoxyergosta-7,22-diene
JK
Whole
(Shen et al., 2016)
C25 Terpenoids
382
hitorins A
E
Aerial
(Kim et al., 2016)
383
hitorins B
E
Aerial
(Kim et al., 2016)
Coumarins
384
isofraxidin
DEH
choleretic activity
Whole
(Zhu et al., 2018)
385
scopoletin
E
Whole
(Kawabata et al., 1984) (Kawabata et al., 1984)
386
isoscopoletin
E
Whole
(Kawabata et al., 1984)
387
isofraxidin-7-O-β-d-glucopyranoside
E
Whole
(Heo et al., 2005)
Lignans
388
(7S,8R)-dihydrodehydrodiconiferyl alcohol
E
Roots
(Kuang et al., 2009)
389
(7S, 8R)-urolignoside
E
Roots
(Kuang et al., 2009)
390
(7S,8R)-dihydrodehydrodiconiferyl alcohol-9-β-d-glucopyranoside
E
Roots
(Kuang et al., 2009)
391
(7S,8R)-dihydrodehydrodiconiferyl alcohol-9′- O-β-d-glucopyranoside
E
Roots
(Kuang et al., 2009)
392
(7S,8R)-5-methoxydihydrodehydrodiconiferyl alcohol-4-O-β-d-glucopyranoside
E
Roots
(Kuang et al., 2009)
393
(±)-erythro-guaiacyl-glycerol-β-O-4′-dihydroconiferylether
D
Aerial
(Du et al., 2017)
Simple phenylpropanoids
394
(E)-cinnamic acid
D
Roots
(Wang et al., 2014a)
395
p-coumaric acid
F
Whole
(Chen et al., 2021a)
Flavonoids
396
7,4′-dimethylnaringenin
F
Whole
(Chen et al., 2021a)
397
quercetin-3-O-α-l-rhamnopyranoside
F
Whole
(Chen et al., 2021a)
398
quercetin-3-O-β-d-glucopyranoside
F
Whole
(Chen et al., 2021a)
399
catechin
F
Whole
(Chen et al., 2021a)
Organic acids
400
stearic acid
D
Roots
(Wang et al., 2014a)
401
vanillic acid
D
Roots
(Wang et al., 2014a)
402
4-Hydroxybenzoic acid
G
Whole
(Xu, 2016)
403
trans-4-Hydroxy-2-nonenoic acid
G
Whole
(Xu, 2016)
404
3,4,5-trimethoxybenzaldehyde
H
Leaves
(Wu et al., 2010)
Amide
405
N-p-trans-coumaroyltyramine
D
Aerial
(Xu, 2016)
406
N-p-trans-feruloyltyramine
D
Aerial
(Xu, 2016)
407
cannabisin G
D
Aerial
(Xu, 2016)
408
thoreliamide A
D
Aerial
(Xu, 2016)
409
cannabisin F
D
Aerial
(Xu, 2016)
410
aurantiamide acetate
D
Aerial
(Xu, 2016)
Others compounds
411
(E)-5-(4-methoxyphenyl)-4-ene-1,2,3-trihydroxyamyl
D
Aerial
(Du et al., 2017)
412
1-acetoxy-2,3,4,5-tetrahydroxy-5-p-metoxyphenylpentane
D
Aerial
(Du et al., 2017)
413
(−)-rosiridol
D
Aerial
(Du et al., 2017)
414
(4S)-p-menth-1-ene-4,7-diol
D
Aerial
(Du et al., 2017)
415
pisumionoside
E
Whole
(Kuang et al., 2008)
416
yinxiancaoside B
E
Antitumor activity
Whole
(Kuang et al., 2008)
417
yinxiancaoside C
E
Antitumor activity
Roots
(Kuang et al., 2008)
418
vomifoliol
F
Whole
(Chen et al., 2021a)
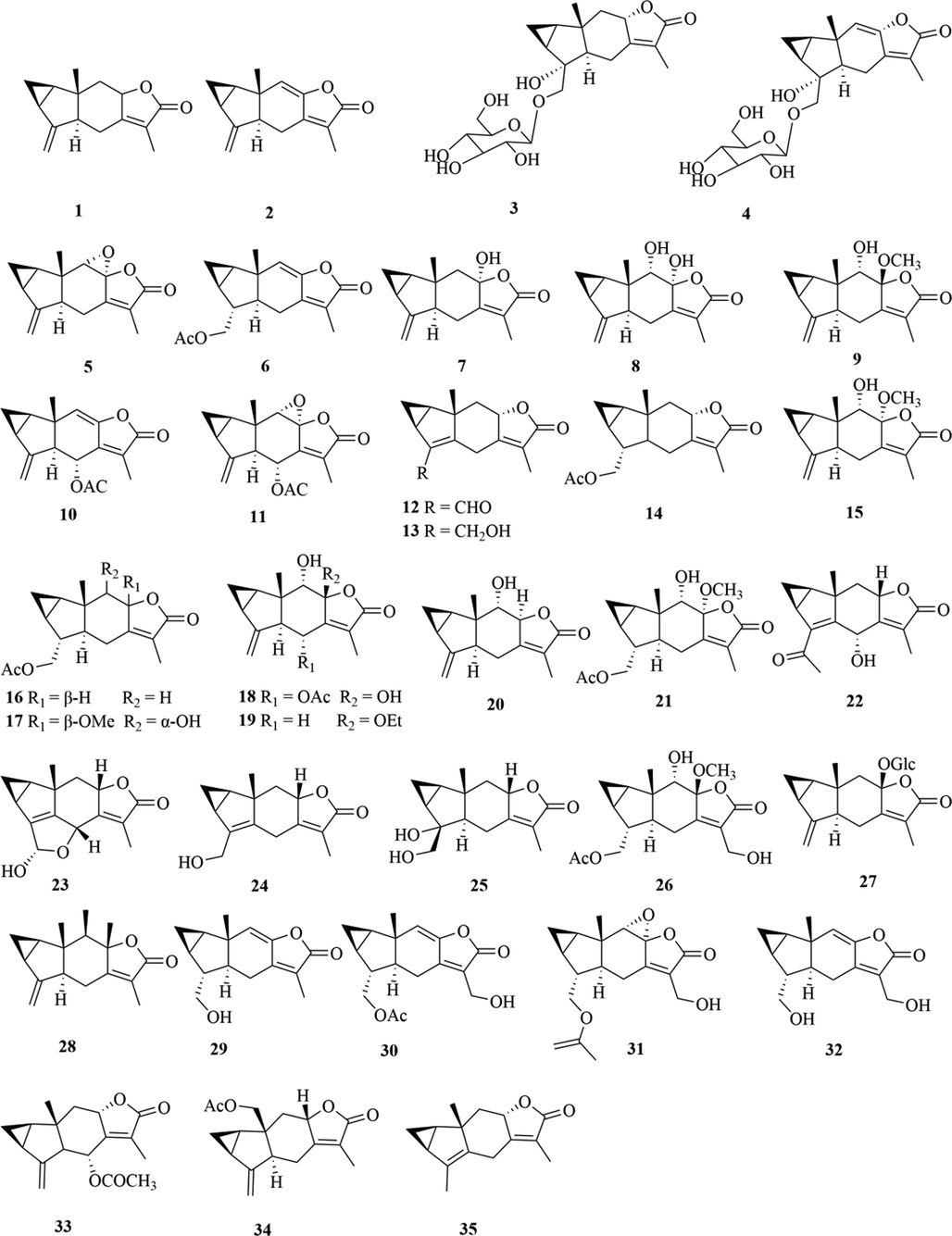
Structures of lindenane sesquiterpenes and their polymers in genus Chloranthus.
Structures of lindenane sesquiterpenes and their polymers in genus Chloranthus.
Structures of lindenane sesquiterpenes and their polymers in genus Chloranthus.
Structures of lindenane sesquiterpenes and their polymers in genus Chloranthus.
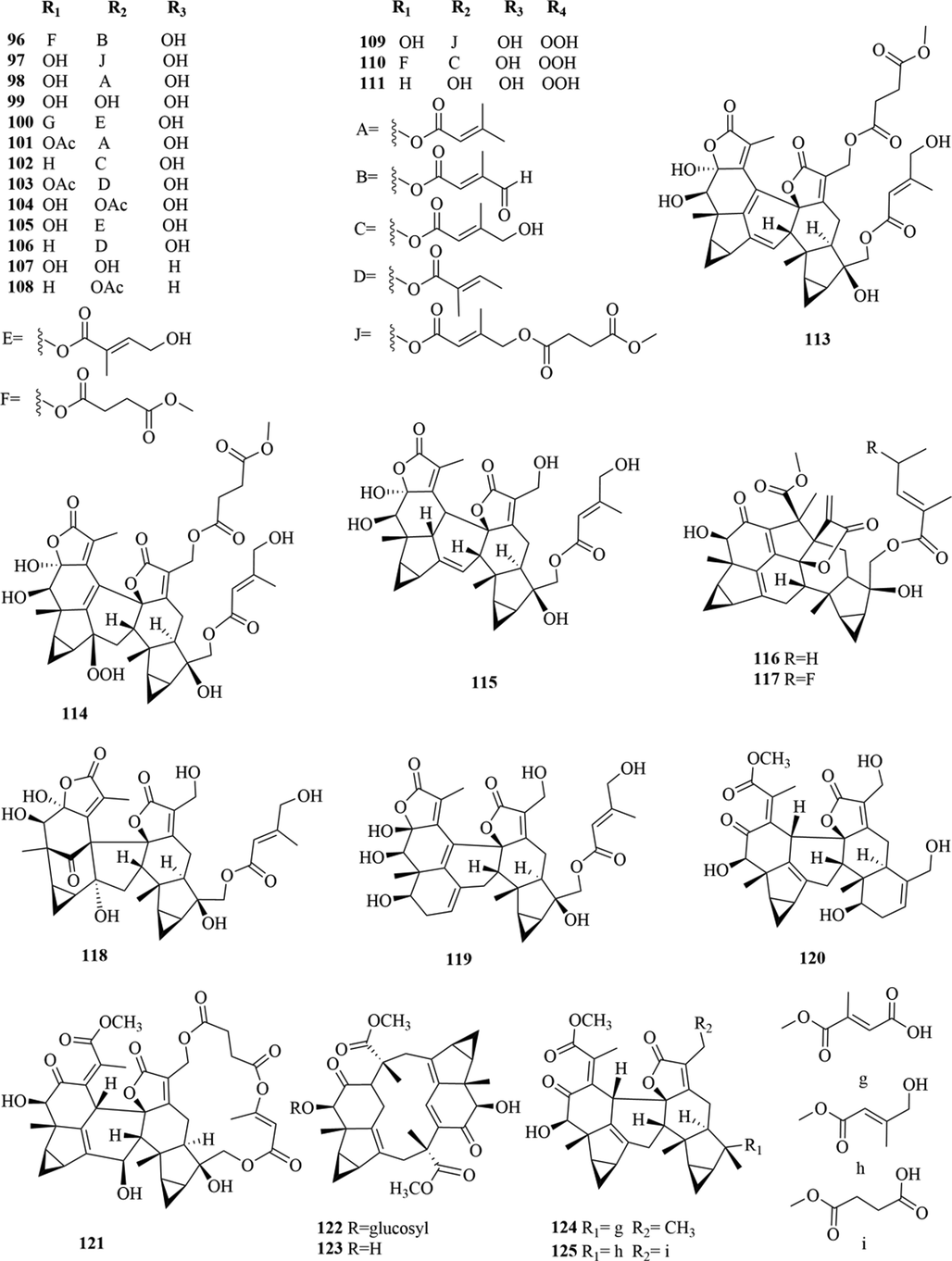
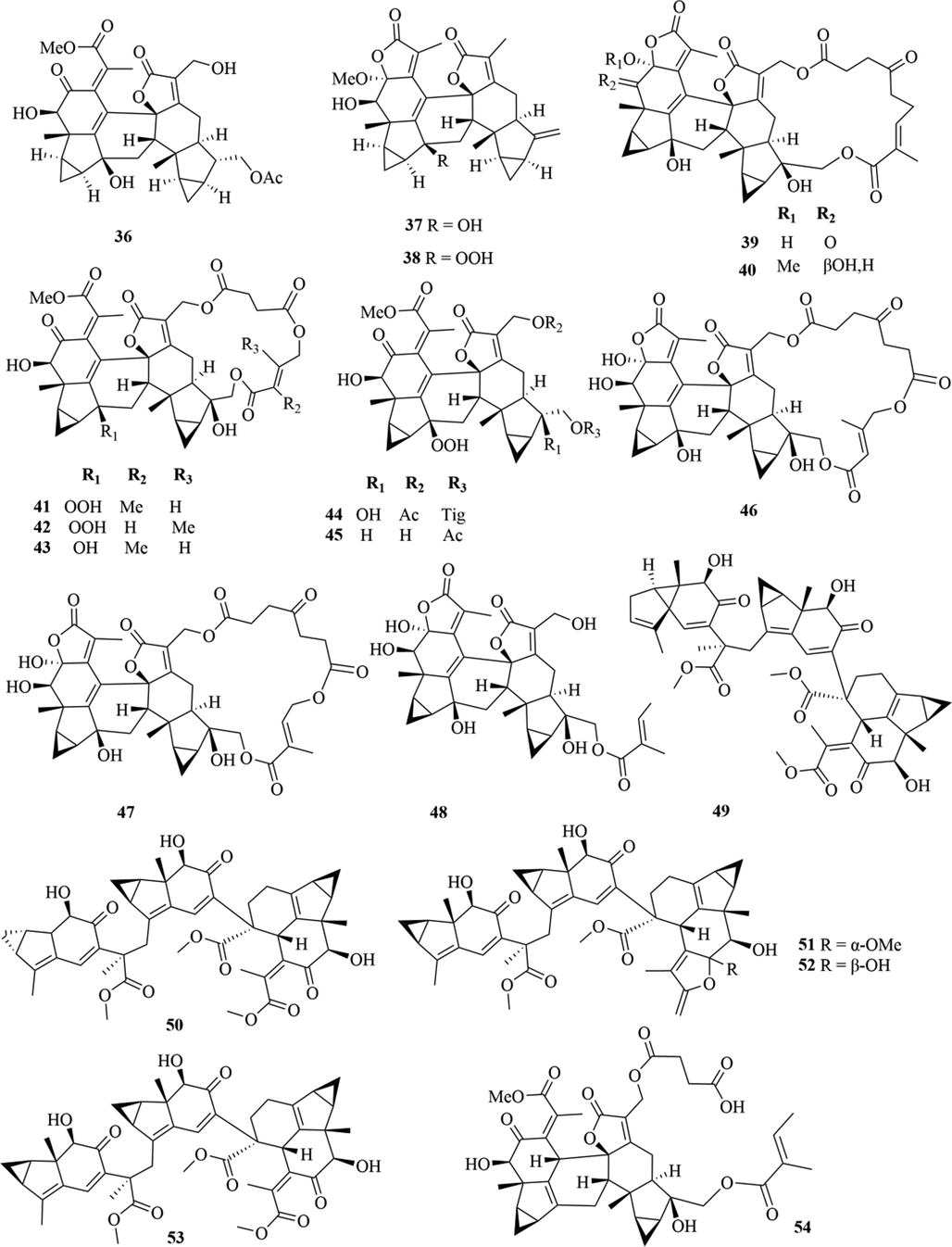
Structures of lindenane sesquiterpenes and their polymers in genus Chloranthus.
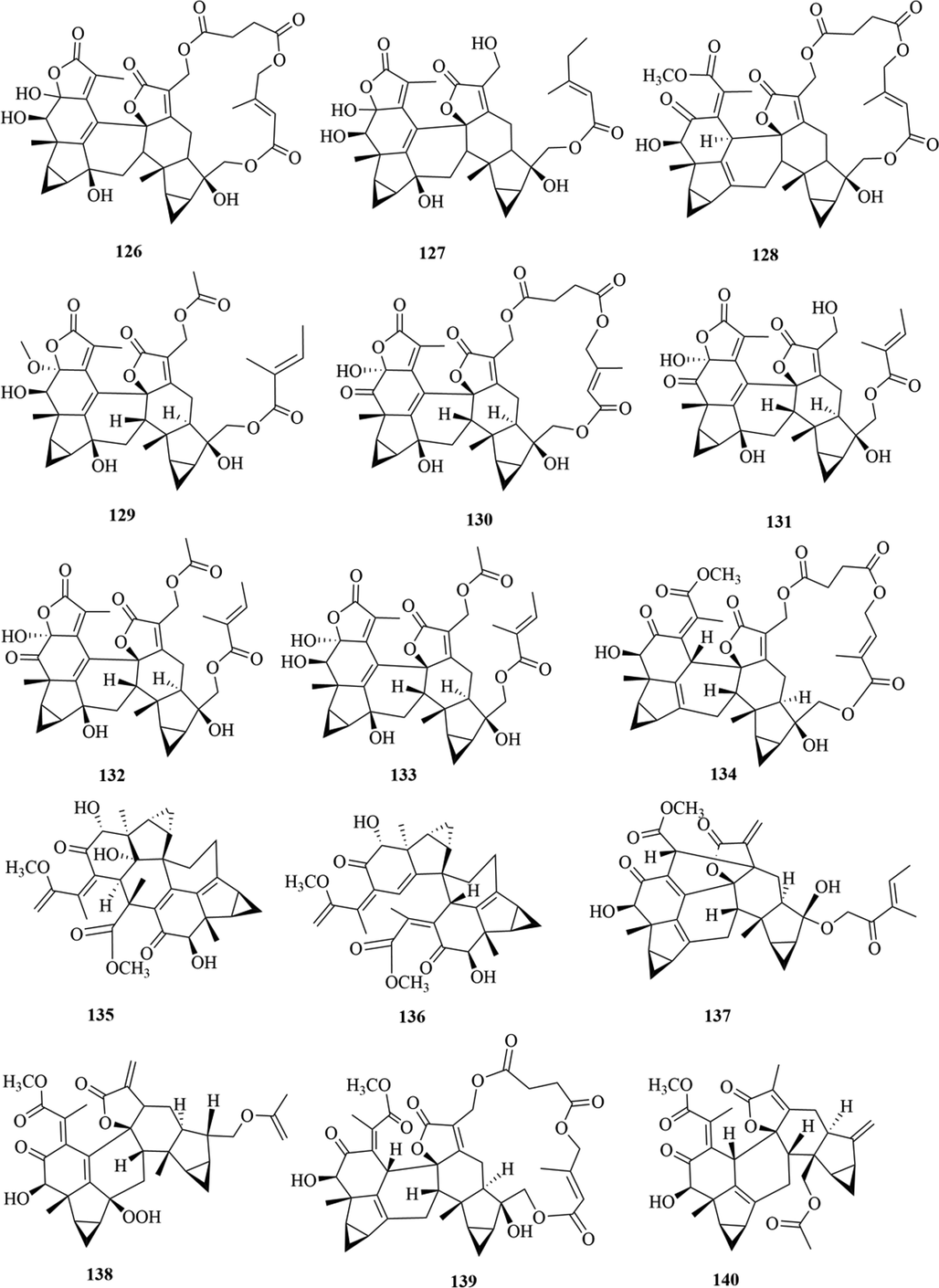
Structures of lindenane sesquiterpenes and their polymers in genus Chloranthus.
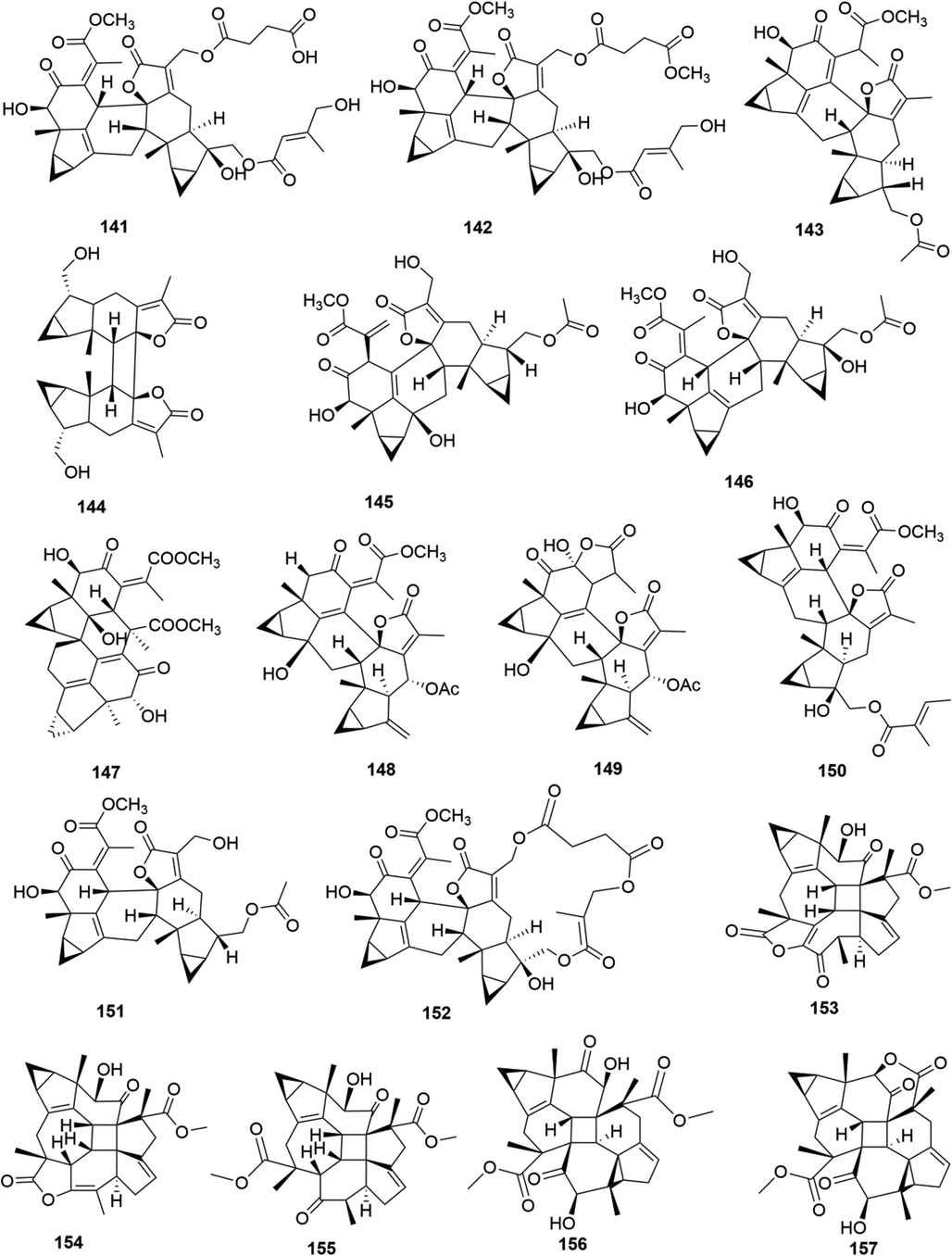
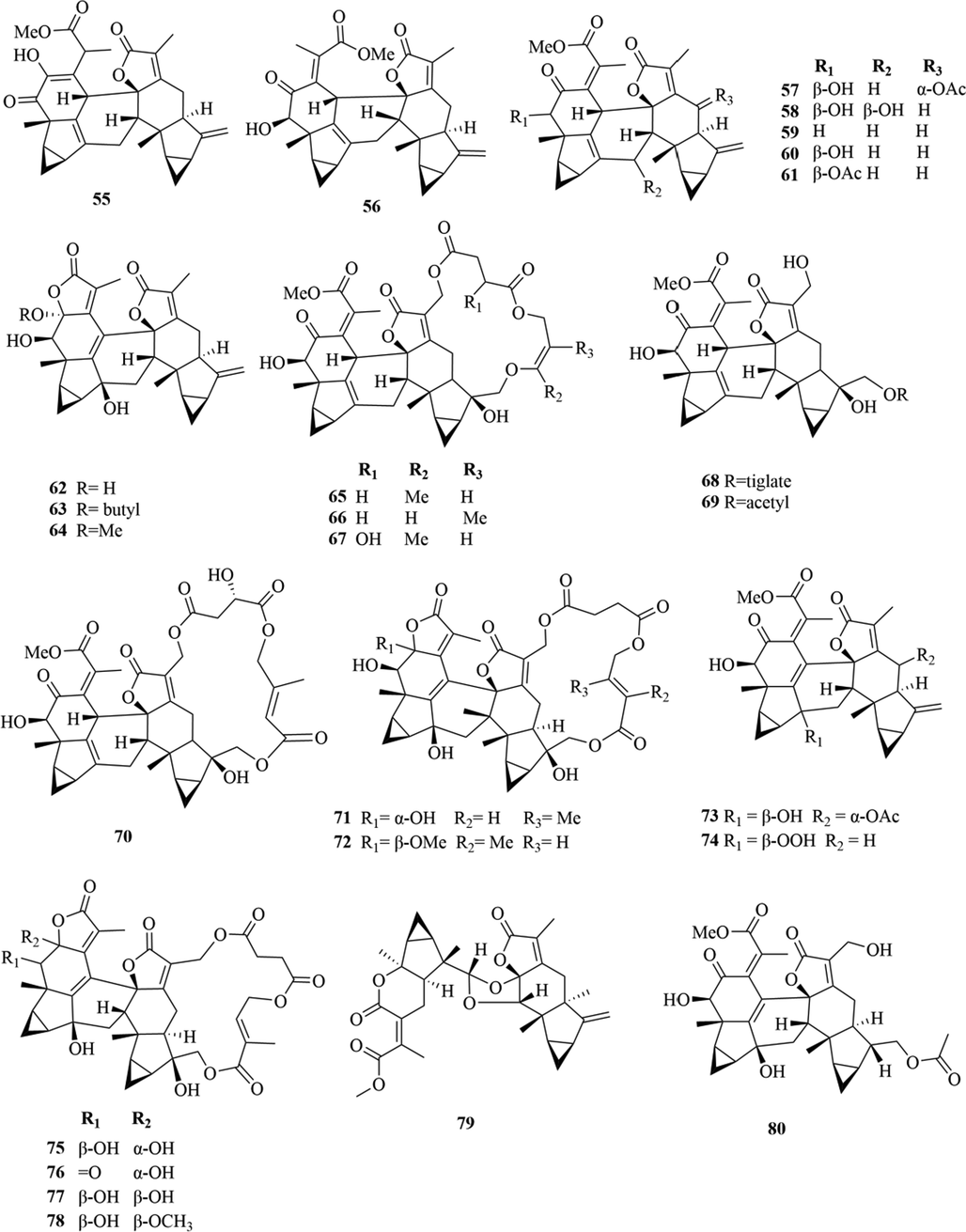
Structures of lindenane sesquiterpenes and their polymers in genus Chloranthus.
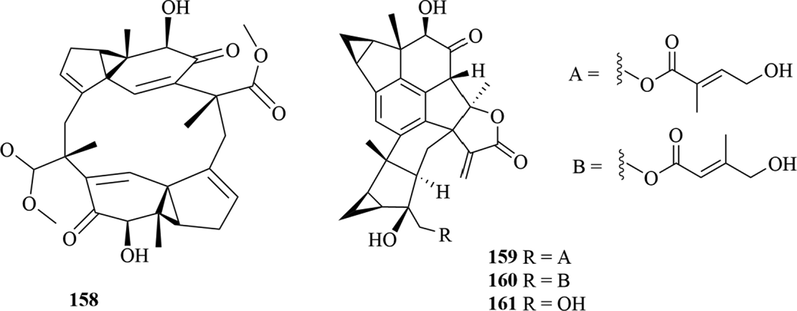
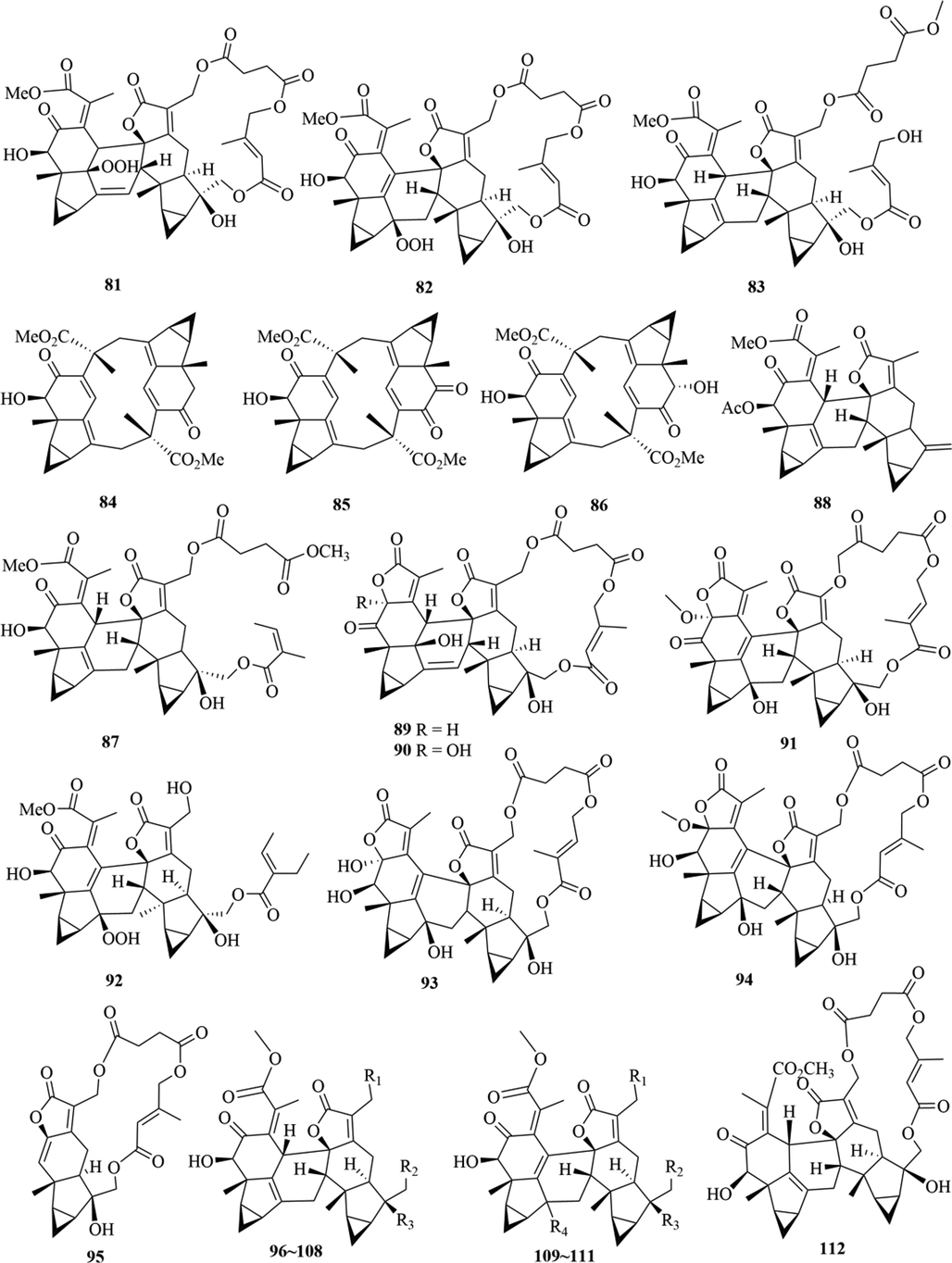
Structures of lindenane sesquiterpenes and their polymers in genus Chloranthus.
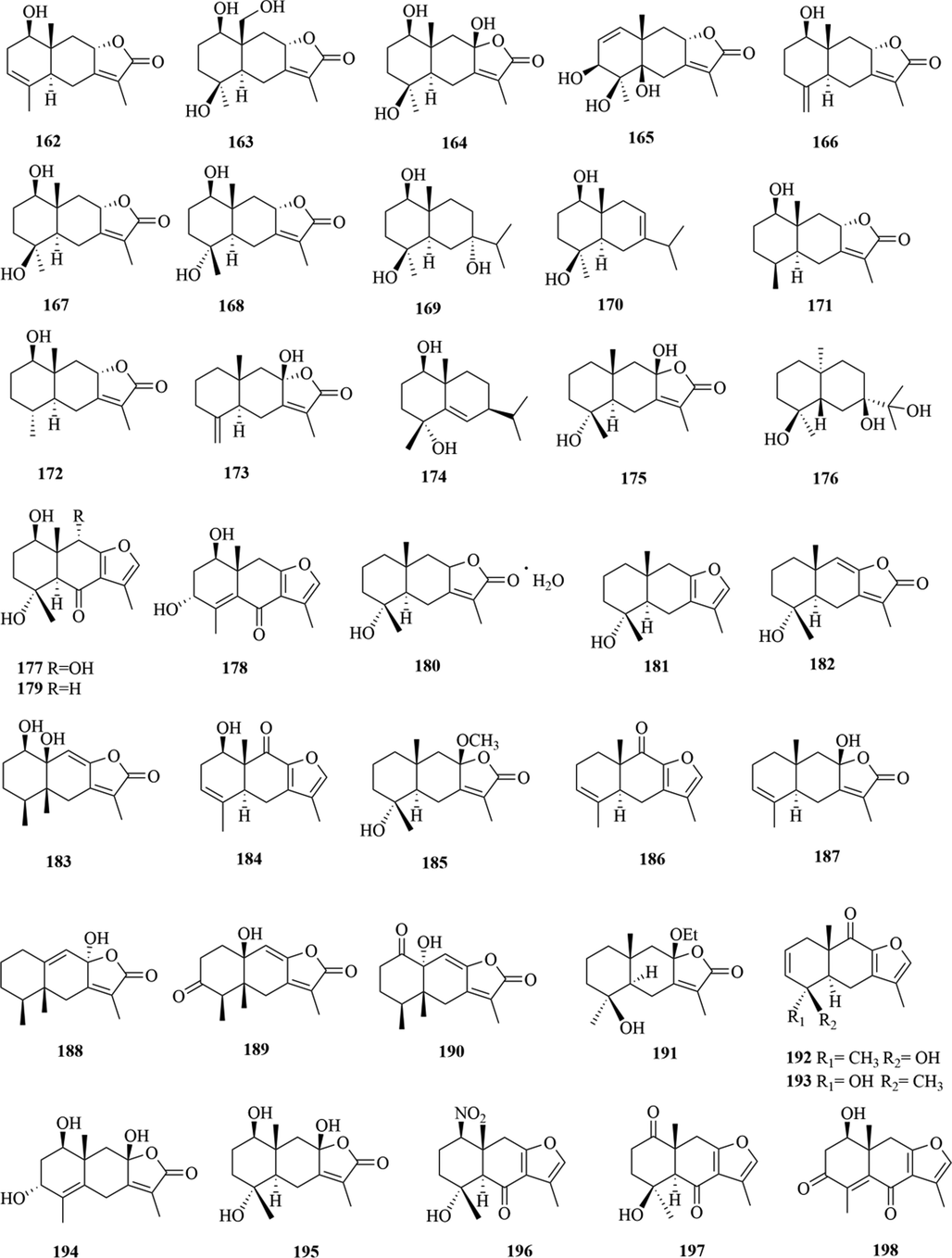
Structures of eudesmane sesquiterpenes in genus Chloranthus.
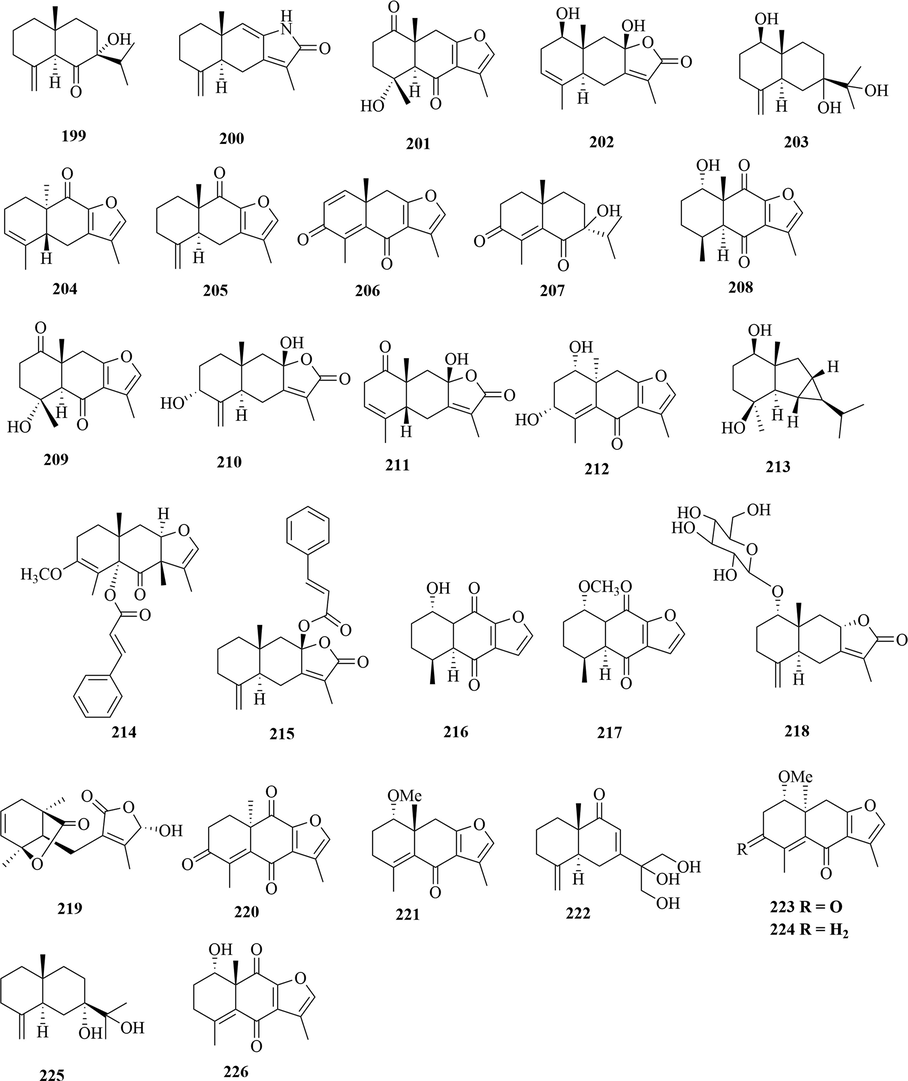
Structures of eudesmane sesquiterpenes in genus Chloranthus.
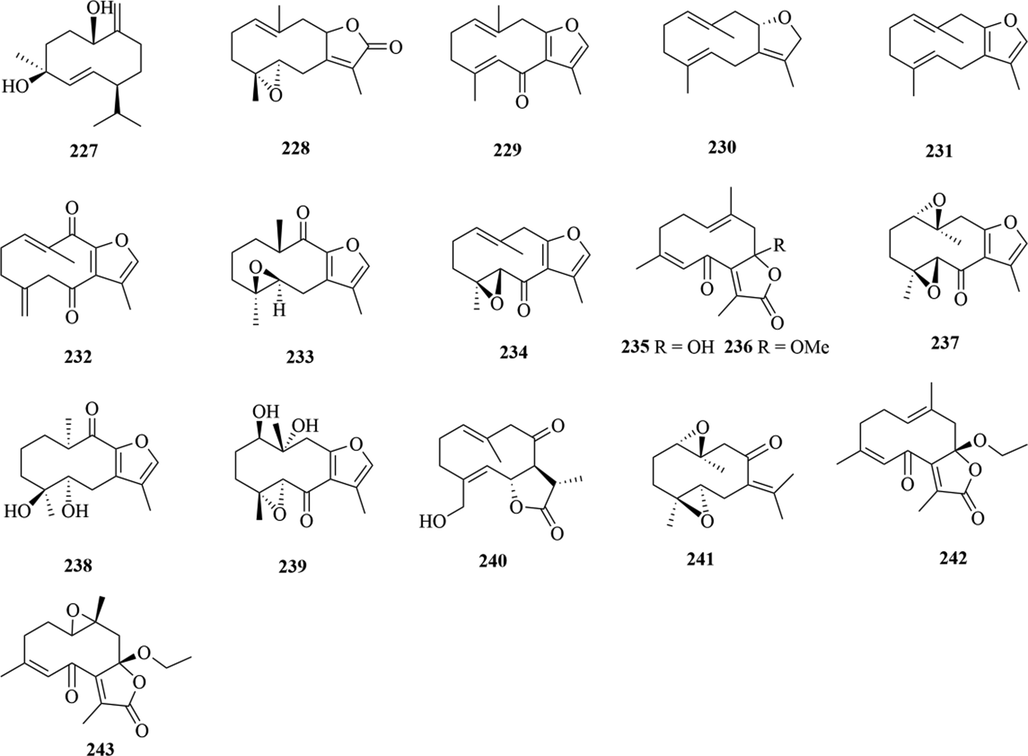
Structures of germacrane sesquiterpenes in genus Chloranthus.
Structures of cadinane sesquiterpenes in genus Chloranthus.
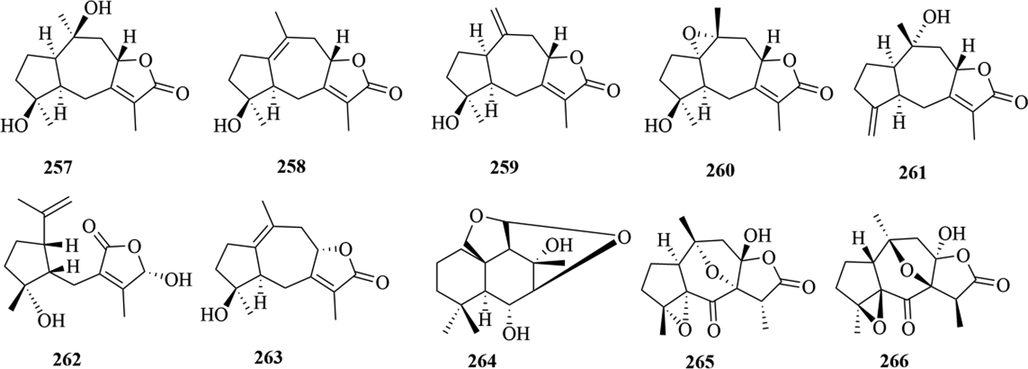
Structures of guaiane sesquiterpenes in genus Chloranthus.
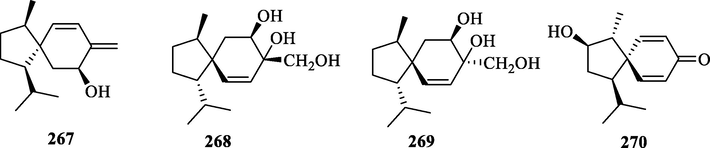
Structures of acorane sesquiterpenes in genus Chloranthus.
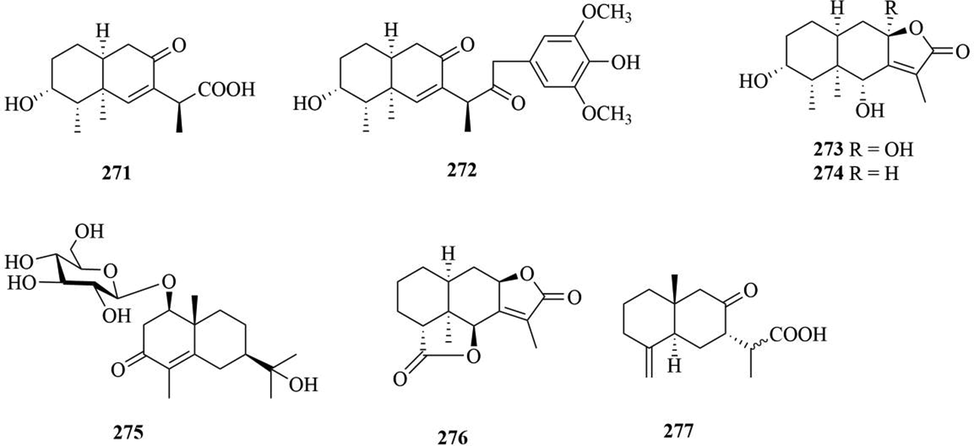
Structures of eremophilane sesquiterpenes in genus Chloranthus.
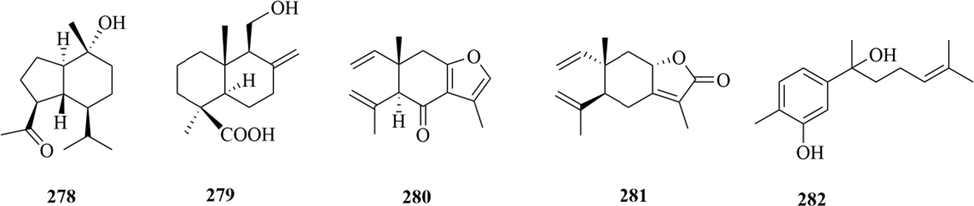
Structures of oplopanone sesquiterpenes, drimane sesquiterpene, elemene sesquiterpene and brasilane sesquiterpene in genus Chloranthus.
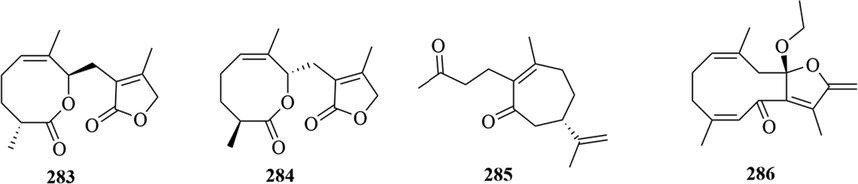
Structures of others sesquiterpene in genus Chloranthus.
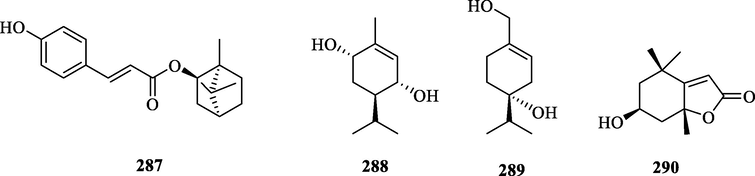
Structures of monoterpenes in genus Chloranthus.
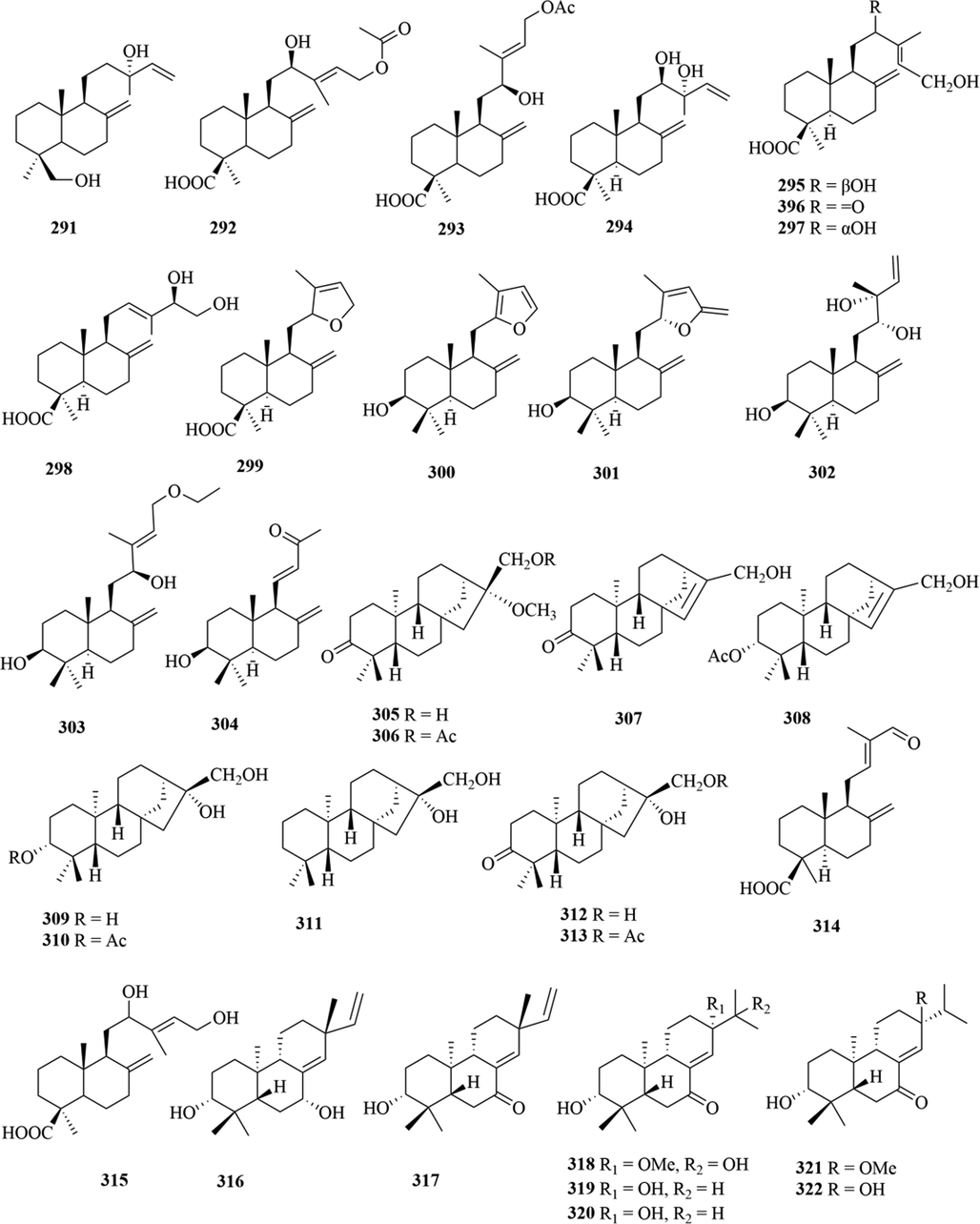
Structures of diterpenoids in genus Chloranthus.
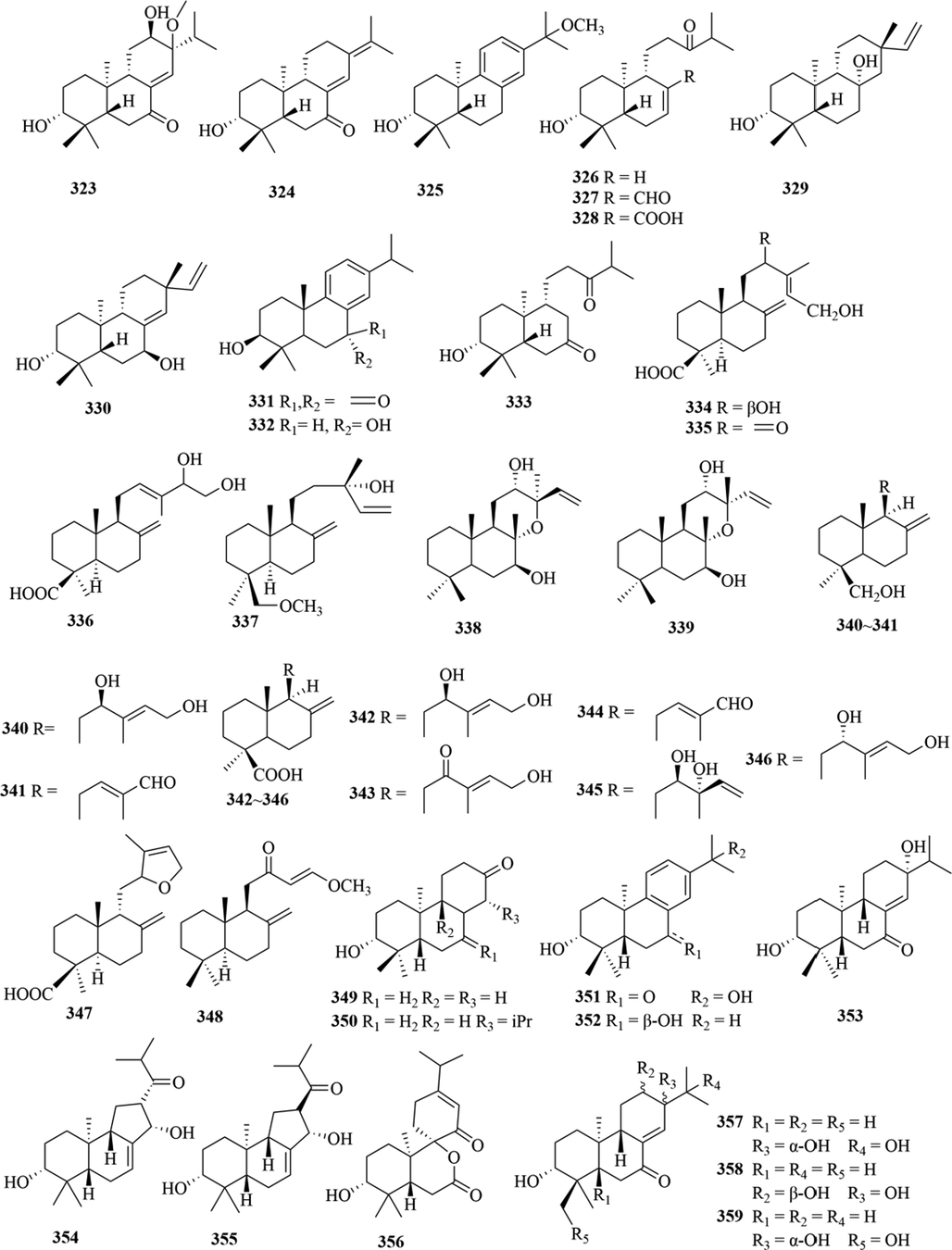
Structures of diterpenoids in genus Chloranthus.
Structures of diterpenoids in genus Chloranthus.
Structures of triterpenoids in genus Chloranthus.
Structures of C25 terpenoids in genus Chloranthus.
Structures of coumarins in genus Chloranthus.
Structures of lignans and simple phenylpropanoids in genus Chloranthu.
Structures of flavonoids in genus Chloranthus.
Structures of Organic acids in genus Chloranthus.
Structures of amides in genus Chloranthus.
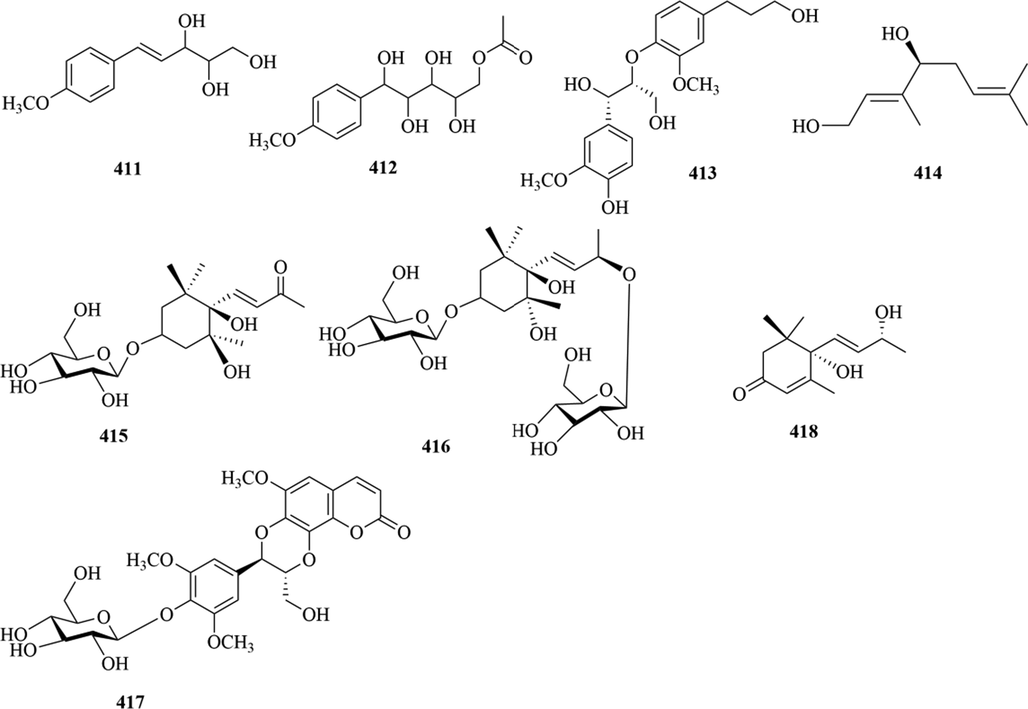
Structures of others compounds in genus Chloranthus.
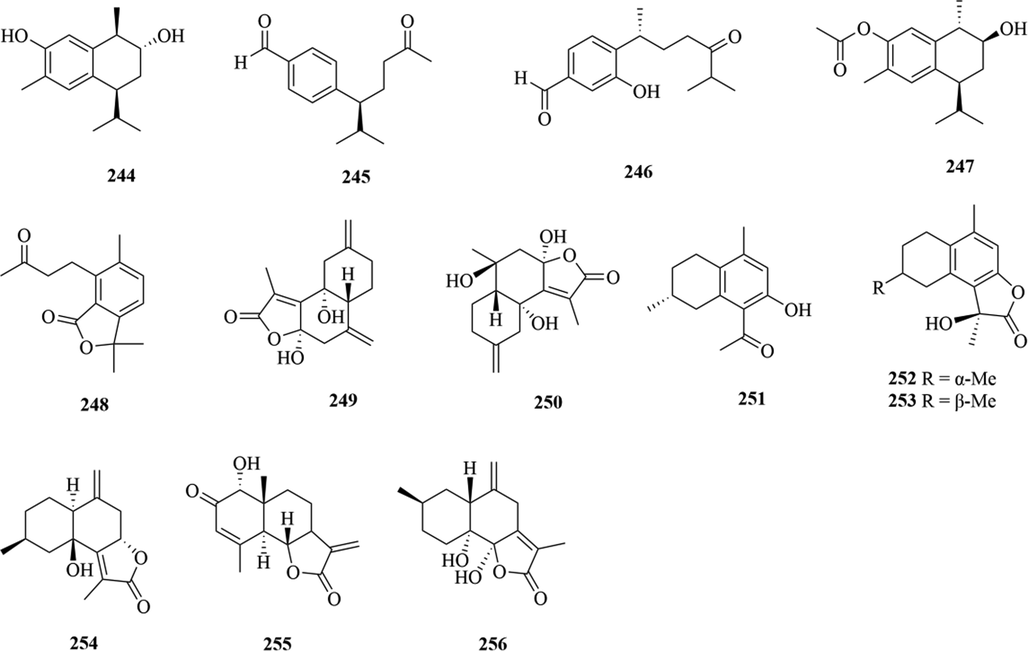
5.1 Sesquiterpenes
Sesquiterpenoids are the main types of chemical constituents in the genus Chloranthus, as well as its main active ingredients. At present, 286 sesquiterpenes were isolated from this genus, mainly distributed in C. japonicus, C. fortunei, C. holostegius and C. spicatus plants, and the structural types include lindenane sesquiterpenes and their polymers (1–161), eudesmane sesquiterpenes (162–226), germacrane sesquiterpenes (227–243), cadinane sesquiterpenes (244–256), guaiane sesquiterpenes (257–266), acorane sesquiterpenes (267–270), eremophilane sesquiterpenes (271–277), oplopanone sesquiterpenes (278), drimane sesquiterpene (279), elemene sesquiterpene (280–281), brasilane sesquiterpene (282) and others sesquiterpene (Wang et al., 2015a; Xu, 2013).
Among them, lindenane sesquiterpenes, sesquiterpenes dimers and eudesmane sesquiterpenes are the most abundant. In particular, sesquiterpene dimers, as indicator components of this genus, which have diverse structural types and significant pharmacological activities (Ma et al., 2020). The specific compound names and structures are shown in Table 1 and Figs. 1–9.
5.2 Monoterpenes
Monoterpenes are less abundant in the genus Chloranthus, and four compounds (287–290) were reported. Wang et al. isolated a monoterpene lactone (287) pressafonin from C. angustifoliu (Wang et al., 2014a). Lu et al. obtained two monoterpenes (3R,4S,6R)-p-menth-1-en-3,6-diol and (R)-p-menth-1-en-4,7-diol from C. japonicus (Lu et al., 2016). (–) loliolide was first isolated from C. fortunei (Chen et al., 2021a). The specific compound names and structures are shown in Table 1 and Fig. 10.
5.3 Diterpenoids
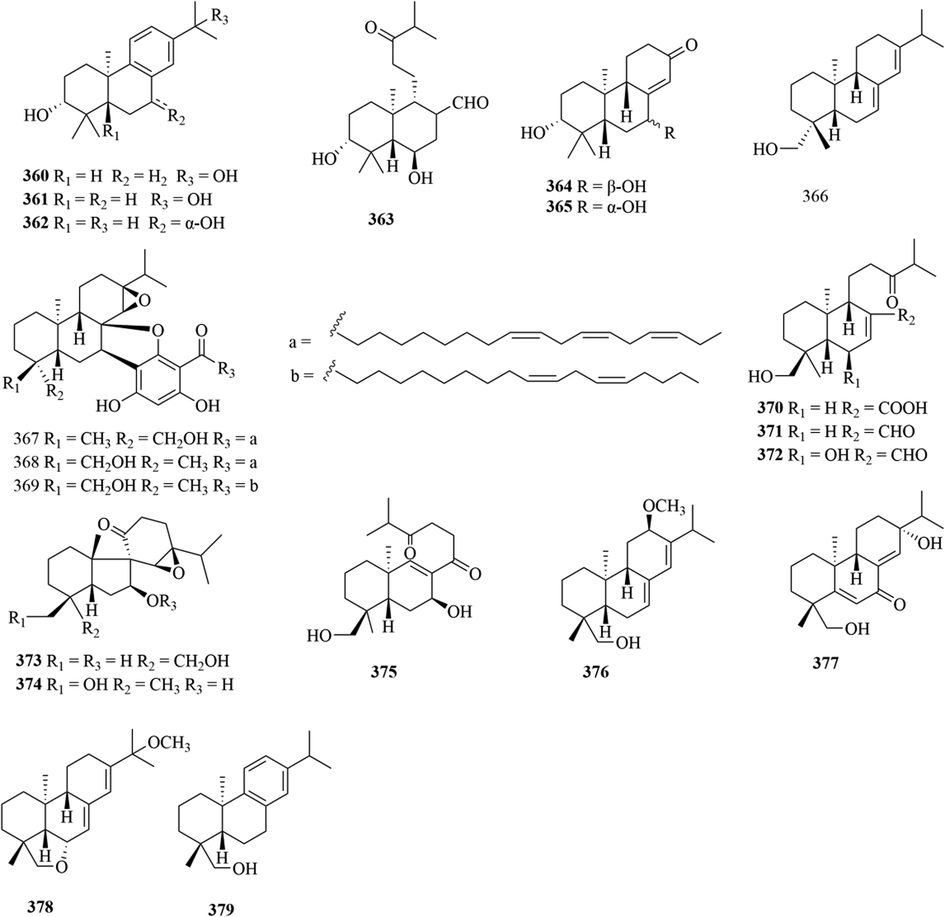
Diterpenoids are abundant in the genus Chloranthus and they are an important source of activity in this genus. So far, a total of 88 compounds (291–379) were isolated from this genus, and the main structural types include abietane diterpenes, pimarane diterpenes, totarane diterpenes, labdane diterpenes and ring-opened chinane diterpenes (Chen et al., 2021b). Among them, the norditerpenoids are new diterpenoid structure types in this genus, and their carbon skeletons are mostly C18 and C19. Xie et al. (2015). reported that chloranhenryin D (326) from C. henryi was a abietane-type diterpenoid at the absence of C-14 position. Wang et al. (2015c). isolated a new ent-podocarpane-type C17 norditerpenoid compound (3R,5S,9R,10S)-3-hydroxy-ent-podocarpa-8(14)-ene-13-one from C. sessilifolius. Furthermore, three new norditerpenoid compounds sessilifol O (333), sessilifol P (364)and sessilifol Q (365), were also isolated from C. serratus (Wang et al., 2015b). The specific compound names and structures are shown in Table 1 and Fig. 11.
5.4 Triterpenoids
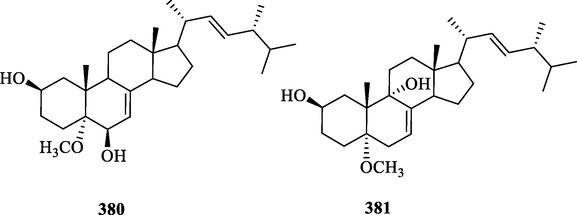
Shen et al. identified two new triterpenoids, 2β, 9α-dihydroxy-5α-me-thoxyergosta-7,22-diene (380) and 2β, 6β-dihydroxy-5α-methoxyergosta-7, 22-diene (381)from the leaves of C. multistachys (Shen et al., 2016). The specific compound names and structures are shown in Table 1 and Fig. 12.
5.5 C25 terpenoids
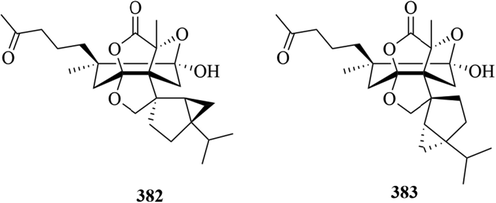
Two new C25 Terpenoids, hitorins A (3 8 2) and hitorins B (3 8 3), were identified from C. japonicus, which has a 6/5/5/5/5/3 hexacyclic skeleton including one γ-lactone ring and two tetrahydrofuran rings (Kim et al., 2016). The specific compound names and structures are shown in Table 1 and Fig. 13.
5.6 Coumarins
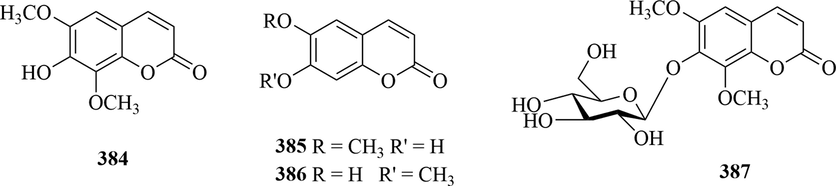
A total of four coumarins isofraxidin (384), scopoletin (385), isoscopoletin (386) and isofraxidin-7-O-β-d-glucopyranoside (387) were isolated from the genus Chloranthus, and most of them were distributed in C. japonicus (Zhu et al., 2018; Kawabata et al., 1984; Heo et al., 2005). The specific compound names and structures are shown in Table 1 and Fig. 14.
5.7 Lignans
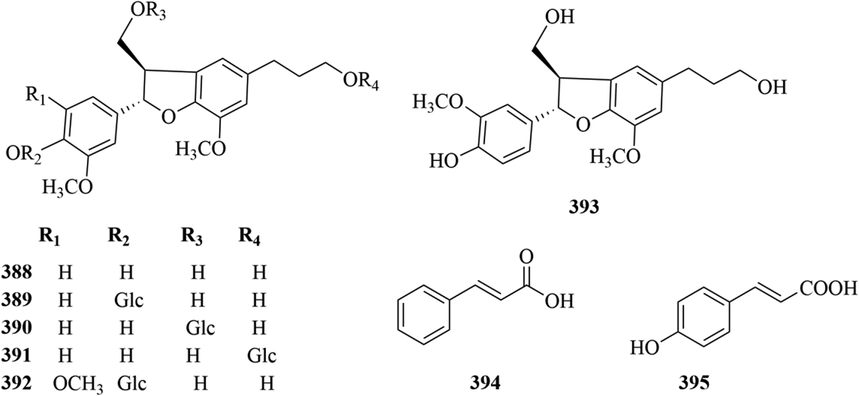
Kuang et al. and Du et al. isolated six new lignans (388–393) from the root parts of C. japonicus and aboveground parts of C. angustifolius, respectively (Kuang et al., 2009; Du et al., 2017). The specific compound names and structures are shown in Table 1 and Fig. 15.
5.8 Simple phenylpropanoids
At present, only two simple phenylpropanes, (E)-cinnamic acid (394) and p-coumaric acid (395), have been isolated from C. angustifolius and C. fortunei (Wang, 2014; Chen et al., 2021a). The specific compound names and structures are shown in Table 1 and Fig. 15.
5.9 Flavonoids
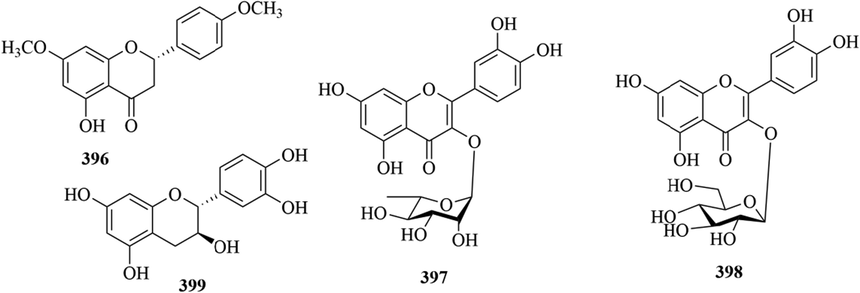
In recent years, there are relatively few reports on flavonoids in the genus Chloranthus. Chen et al. isolated four flavonoids (396–399) from C. fortunei for the first time (Chen et al., 2021a). The specific compound names and structures are shown in Table 1 and Fig. 16.
5.10 Organic acids
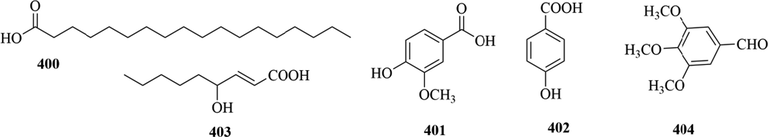
The content of organic acids in the genus Chloranthus is low, and five organic acids compounds (400–404) were found in C. angustifolius and C. Holostegius (Wang et al., 2014a; Xu, 2016; Wu et al., 2010). The specific compound names and structures are shown in Table 1 and Fig. 17.
5.11 Amides
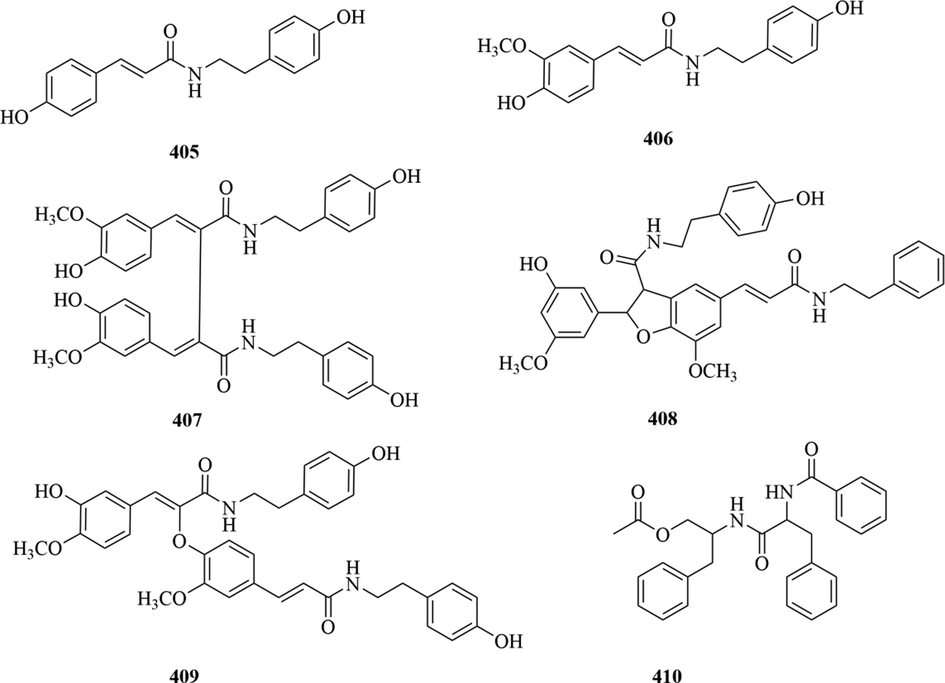
Liu et al. isolated six amides (405–410) from C. angustifolius, all of which were found and reported for the first time in the genus Chloranthus (Liu et al., 2015). The specific compound names and structures are shown in Table 1 and Fig. 18.
5.12 Others compounds
Besides, eight other types of compounds were discovered in this genus. The specific compound names and structures are shown in Table 1 and Fig. 18.
5.13 Pharmacological activity
Modern pharmacological experiments indicated that most species of the genus Chloranthus have anti-cancer, antibacterial, antiviral, hypoglycemic anti-inflammatory, and antimalarial activities (Cao et al., 2008). The bioactivities of monomeric compounds in Chloranthus are listed in Table 2.
Pharmacological
Action
Effective Fraction/ Compounds
Model
Responses along with Critical Assessment
Target or Possible
Mechanism
References
Anti-inflammatory activity
shizukaol B
In vitro / BV-2 microglial cells
At the concentrations ≥ 25 μM
Excellent activityTNF-a and IL-1b
(Pan et al., 2017)
chlorabietin B
BV-2 microglial cells
IC50 = 16.4 ∼ 33.8 μM
Excellent activityInhibiting NO
(Xiong et al., 2016)
chlorabietin C
chlorabietin F
chlorabietin G
sessilifol F
BV-2 microglial cells
IC50 = 8.3, 7.4 μM, respectively
Moderate activity
Inhibiting NO
(Wang et al., 2015b)
sessilifol I
3α,7β-dihydroxyabieta-8,11,13-triene
In vitro /BV-2 microglial cells
IC50 = 4.3 μM
Significant activityCell viability (%)
: 94.6 ± 7.9Inhibiting NO
(Wang et al., 2015c)
shizukaolA
RAW 264.7 cells
IC50 = 7.22 μM, 3.68 μM, 0.15 μM, respectively
Overall good activityInhibiting NO
(Gong et al., 2021)
fortunilide I
shizukanolide G
chloramultilide A
BV-2 microglial cells
IC50 = 31.1 ∼ 79.4 μΜ
Significant activityInhibiting NO
(Wang et al., 2014b)
spicachlorantin G
shizukaol B
spicachlorantin B
chlorajaponol B
RAW 264.7 cells
IC50 = 9.56 ± 0.71 μMpositive control amino guanidine
(IC50 = 8.50 ± 0.35 μM)
Good activityInhibiting NO
(Zhuo et al., 2017)
fortunilide K
RAW 264.7 cells
Not mentioned
Inhibiting NO
(Huang et al., 2020)
chlojaponilactone B
(TPA)-stimulated mice
Not mentioned
iNOS, TNF-α, IL-6, NF-κB
(Sun et al., 2020)
shizukaol D
RAW 264.7 cells
IC50 = 3.7 μM (cell activity (%) at an initial concentration of 50 μM)
positive control l-NIL (IC50 = 7.0 μM)
Good activityInhibiting NO
(Bai et al., 2019)
henriol D
RAW 264.7 cells
IC50 = 1.90, 3.68, 1.95, 7.01, 1.95 μM, respectively
Significant activityInhibiting NO
(Zhang et al., 2012c)
shizukaol E
shizukaol G
shizukaol M
shizukaol O
chololactone A
RAW 264.7 cells
IC50 = 4.4 ∼ 35.4 μM
dexamethasone as a positive control (IC50 = 0.45 ± 0.5 μM)
Moderate activityInhibiting NO
(Shen et al., 2017)
chololactone B
chololactone E
chololactone F
chololactone G
chololactone H
serralabdanes A
RAW 264.7 cells
IC50 = 38.45 ± 1.02, 29.78 ± 0.92, 44.37 ± 0.58, 53.68 ± 1.52, 47.31 ± 1.26 μM, respectivelypositive control dexamethasone
(IC50 = 1.08 ± 0.15 μM)
Overall good activityInhibiting NO
(Zhang et al., 2013)
serralabdanes B
serralabdanes C
serralabdanes D
serralabdanes E
Anti-tumor activity
shizukaol D
liver cancer cells
At the concentrations ≥ 6.25 μM
Excellent activityWnt, β-catenin
(Tang et al., 2016)
serralactone A
breast cancer cells
Against MDA-MB-23, MDA-MB-468 cells
IC50 = 3.14 μM, 4.64 μM
Excellent activityLIM kinase 1
(Fu et al., 2018)
codonolactone
breast cancer cells
Not mentioned
Runx2
(Wang et al., 2014b)
yinxiancaoside A
Hepg-2, OV420 and MCF-7 cells
Against Hepg-2, OV420, MCF-7 cell lines
Marginal activity
Not mentioned
(Kuang et al., 2009) (Kuang et al., 2008)
yinxiancaoside B
yinxiancaoside C
chloranoside A
pisumionoside
sarcaglaboside A
chlotrichenes B
U2 OS
Synergetic cytotoxicity with DOX on U2 OS cells
(CI: 0.94 ± 0.03)Not mentioned
(Chi et al., 2019)
henriols C
BEL7402, BGC-823, HCT-8 cells
Against BEL-7402, BGC823 cells
IC50 = 1.4, 3.2 μM
Good activity
Not mentioned
(Li et al., 2008)
henrilabdanes A
Against BEL-7402, HCT-8, BGC-823 cells
IC50 = 1.7, 0.54, 5.76 μM
Moderate activity
chlorahupetones A
A549, U87, SMMC-7721 cells
Against A549 cells, U87 cells, SMMC-7721 cells
IC50 = 9.82 ± 1.21, 0.43 ± 0.12, 0.94 ± 0.28, 3.15 ± 1.25 μMPaclitaxel
(IC50 = 1.62 ± 0.13 μM)
Excellent activityNot mentioned
(Zhang et al., 2021)
chlorahupetones G
chlorahupetones H
chlorahupetones I
1α-hydroxy-8,12-epoxyeudesma-4,7,11-triene-6,9-dioneHela, K562 human tumor cells
Against Hela, K562 human cells
IC50 = 22.2, 21.8 μM
Moderate activity
Not mentioned
(Wu et al., 2006)
14-methoxy-15,16-dinor-5αH,9αH-labda-13(E),8(17)-dien-12-one
Against Hela, K562 human cells
IC50 = 5.6, 5.9 μM
Strong activity
shizukaol B
C8166 cells
Against C8166 cells
IC50 = 0.020, 0.089, 0.047, 0.022 μM, respectively
Significant activity
Not mentioned
(Fang et al., 2011)
shizukaol C
shizukaol F
shizukaol H
chloranthalactone A
MDA- MB-231, MDA-MB- 468 cells
ID50 = 2.5 μM, 1 ∼ 2.5 μM, respectively
Moderate activitySnail、Slug and p53 protein
(Gong et al., 2021)
chloranthalactone B
Neuroprotective activitychlohenriol A
PC12 cells
Increased cell viability from
55.4 ± 3.1 % to 66.2 ± 9.8, 58.2 ± 2.8, 78.5 ± 4.8 % at 10 μM, respectively,
Moderate activityNot mentioned
(Chen et al., 2021c)
chlohenriol B
chlohenriol C
shizukanolide H
PC12 cells
EC50 = 3.3 ± 0.9 μM
Strong activitycaspase-3, Akt
(Xu et al., 2018)
chlogermacrone C
PC12 cells
Increased cell viability from
43.4 ± 1.3 % to 99.6 ± 8.7, 68.1 ± 4.8 at 10 μM, respectively
Excellent activityNot mentioned
(Chen et al., 2020)
zederone epoxide
curzerenone
PC12 cells
Increased cell viability from
43.41 % ± 1.59 % to 62.61 % ± 5.23 %, 64.87 % ± 8.42 %, 56.06 % ± 6.65 %, 65.87 % ± 5.34 %, 60.54 % ± 3.32 %, 68.11 % ± 4.76 % at 10 μM, respectively
Moderate activityNot mentioned
(Chen et al., 2021a)
zederone
curcodione
chlorantene C
(1E,4Z)-8-hydroxy-6-oxogermacra-1(10),4,7(11)-trieno-12,8-lactone
zederone epoxide
Regulation of glucose metabolism
shizukaol D
C3H10T1/2 cells
Not mentioned
Wnt3a, β-catenin, AMP-activated protein kinase
(Hu et al., 2017)
(Yun et al., 2021)
Antimalarial activity
fortunoid A
P. falciparum strain Dd2
IC50 = 10.2 ± 0.37 μM, 0.5 ± 0.01 μM, respectively
Moderate activityNot mentioned
(Zhou et al., 2017b)
fortunoid B
fortunilide A
P. falciparum strain Dd2
IC50 = 5.2 ± 0.6, 7.2 ± 1.3, 1.1 ± 0.2 μM, respectively
Excellent activityNot mentioned
(Zhou et al., 2017b)
sarglabolide J
chlorajaponilide C
trichloranoids A
P. falciparum strain Dd2
IC50 = 2.50 ∼ 5.00, 10.0 ∼ 15.0, 1.25 ∼ 2.50 μM, respectively
Moderate activityNot mentioned
(Zhou et al., 2021)
trichloranoids D
analogue
Potassium channel blocker
chlorahololides A
rat dissociated hippocampal neurons
IC50 = 10.9, 18.6 μM, respectively
tetraethylammonium chloride as the positive control
Strong activityPotassium (K + ) channels
(Yang et al., 2007b)
chlorahololides B
chlorahololide C
rat hippocampal neurons
IC50 = 3.6 ± 10.1, 2.7 ± 0.3, 27.5 ± 5.1,57.5 ± 6.1 μM, respectively
tetraethylammonium chloride as the positive control
Strong activitydelayed rectifier Kþ current (IK)
(Yang et al., 2008)
chlorahololide D
chlorahololide E
chlorahololide F
Hepatoprotective activity
henriols A
WB-F344 rat cells
IC50 = 0.19, 0.66, 0.09, 0.18 μM, respectively
Moderate activityNot mentioned
(Li et al., 2008)
henrilabdanes A
henrilabdanes B
henrilabdanes C
sarcaglaboside A
WB-F344 rat hepatic epithelial stem-like cells
Against d-galactosamine-induced toxicity
Cell survival rate = 47.5 ± 5.4, 74.9 ± 9.8, 53.0 ± 7.3, 46.3 ± 4.1, 45.5 ± 1.6, 42.4 ± 4.2, 54.5 ± 3.4 μM, respectively
bicyclol = 46.6 (Positive control substance)
Pronounced activity
d-Galactosamine
(Li et al., 2006)
sarcaglaboside B
sarcaglaboside C
sarcaglaboside D
sarcaglaboside E
Cell adhesion inhibitors
shizukaol B
THP-1 cells
MIC = 34.1 nM, 0.9 nM, 27.3 nM, respectively
IC50 = 54.6, 1.2, 34.1 μM
Overall good activityTNF-alpha
(Kwon et al., 2006)
cycloshizukaol A
shizukaol F
Antiviral activity
shizukaol B
HIVwt, HIVRT-K103N, HIVRT-K103N
Against HIVwt, HIVRT-K103N, HIVRT-K103N
EC50 = 0.22, 0.47, 0.50 μM
EC50 = 0.98, 1.36, 1.00 μM
EC50 = 0.11, 3.39, 4.05 μM
EC50 = 0.83, 2.35, 0.86 μM
respectively, Best activityNot mentioned
(Fang et al., 2011)
shizukaol C
shizukaol F
shizukaol H
Species
Local name
Parts
Distribution
Dosage forms
Traditioanal uses
Chloranthus multistachys
Siyexixin
Dasiyedui
Dasikuaiwa
Sidatianwang
Sidajingang
SiyexixinWhole herb
Roots
China (Shaanxi, Jiangxi, Chongqing, Hubei, Hunan, Guangdong, Guangxi and Guizhou)
Decoction, vinum, pill, powder (taken orally);
External application
Itchy skin, bruises and injuries, whole body pain, snakebite, nameless swelling poison and fracture
Chloranthus serratus
Siyedui
Sidatianwang
Sikuaiwa
Zhangerxixin
SiyexixinWhole herb
Roots
Stems and LeavesChina (Jiangsu, Hubei, Hunan, Guangdong, Guangxi, Anhui, Zhejiang, Jiangxi, Sichuan and Fujian), Japan
Liniment;
External applicationBruises and injuries, rheumatism, back and leg pain, furuncle and swelling poison, poisonous snake bite, dysmenorrhea, head sores and white baldness
Chloranthus spicatus
Zhulan
Yuzilan
Chalan
Zhenzhulan
Jizhualan
MizilanWhole herb
Roots
LeavesChina (Yunnan, Sichuan, Guizhou, Fujian and Guangdong), Japan
Decoction, vinum(orally)
;
External applicationRheumatic pain, bruises and injuries, dermatitis and moss, strain and back pain epilepsy, insecticide, indigestion
Chloranthus henryi
Dayejiji
Sidatianwang
Siyedui
Siyexixin
SidatianwangWhole herb
Roots
China (Zhangjiang, Jiangxi, Guangdong, Chongqing, Sichuan, Guizhou, Fujian, Hubei and Shaanxi)
Decoction, vinum(orally)
;
External applicationSnakebite, boils and sores, psoriasis
wind-cold cough and asthma bone fracture, bruises and injuries
Chloranthus holostegius
Sikuaiwa
Siyejin
Heixixin
TuxixinWhole herb
Roots
China (Sichuan, Guangxi, Guizhou and Yunnan)
Decoction, vinum, pill(orally)
;
External application
Paralysis, bone bruises and injuries, functional uterine bleeding, moss, rubella, furuncle, poisonous snake bite, liver wind headache, toothache
Chloranthus fortunei
Shuijinghua
Foshijiinsulan
Yinxianjinsulan
Sizilian
SidajingangWhole herb
China (Guangxi, Shandong, Jiangsu, Anhui, Zhejiang, Taiwan, Jiangxi, Hubei, Hunan, Guangdong and Sichuan)
Decoction (orally);
External application
Rheumatism and cold paralysis, rheumatism and numbness, menstrual disorders, urticaria, bruises and injuries, wind-cold cough, canker sore and swelling poison
Chloranthus sessilifolius
Sikuaiwa
Hongmaoqi
SidatianwangWhole herb
RootsChina (Guangxi, Guizhou, Sichuan and Hunan)
External application
Dispersing cold and relieving cough, promoting blood circulation and relieving pain
Chloranthus oldhamii
Dongnanjinsulan
LuanbaojinsulanWhole herb
China (Fujian, Taiwan and Guangdong)
Decoction, vinum(orally)
;
External application
Stomach pain, poisonous snake bite, painful traumatic bruising to the chest, oral ulceration, dysmenorrhea, bruises and injuries
Chloranthus angustifolius
Siyexixin
Whole herb
China (Hubei, Chonhqing and Sichuan)
Decoction (taken orally)
Dispel wind-dampness, promoting menstruation
Chloranthus japonicus
Siyecao
Sikuaiwa
Siyeqi
Sidatianwang
Baimaoqi
Jingangqi
Maweiqi
Guiduyou
DuyaocaoWhole herb
Roots
Stems
LeavesChina (Jilin, Liaoning, Hebei, Shaanxi, Shanxi, Gansu, Shandong and Hunan), Korea and Japan
Decoction, vinum(orally)
;
External application
Traumatic bruises, sores and boils, breast Knot, itchy skin, menorrhagia, snake bite
Chloranthus elatior
Zhulan
Yezhilan
Xiaogeda
Jiujiefeng
Jiejiecha
ShijiefengWhole herb
Leaves
Branches
FlowersChina (Yunnan, Guangxi, Sichuan and Guizhou), Malaysia, Indonesia, Philippines and India
Decoction, pill, powder(orally)
;
External applicationCold and flu, epilepsy, rheumatic soup bucket, bruises and injuries, postpartum bleeding
5.14 Antitumor activity
More and more research revealed that the genus Chloranthus exhibited strong toxicity against cancer cells. Tang et al. (2016). reported that shizukaol D (69) isolated from C. serratus could inhibit the growth of hepatocellular carcinoma cells by regulating the Wnt pathway. Many studies demonstrated that serralactones A (162) in C. serratus showed significant inhibition activity on LIM domain kinase 1 (LIMK1) by regulating the structure of the actin cytoskeleton of tumor cells in the invasion and metastasis, which may be a potential value in preventing the distant spread of cancer cells. Additionly, its IC50 values on MDA-MB-231 and MDA-MB-468 cells were 3.14 μM and 4.64 μM, respectively (Fu et al., 2018). Wang et al. (2014b) found that codonolactone (1 7 3) obtained from C. henryi exhibited potential antimetastatic properties against breast cancer cells using bioactivity-guided fractionation. Its mechanism may be associated with inhibiting the binding of Runx2 to the mmp-13 promoter through downregulation of invasion and migration of MDA-MB-231 and MDA-MB-157 cells. Zhu Huilin (Zhu et al., 2018) discovered that compound 313 in C. anhuiensis exhibited moderate cytotoxicity on MDA-MB-231, 4 T1, and HepG2 cells with an IC50 value of 39.7 μM. Besides, the compounds yinxiancaoside A (3), yinxiancaoside B (416), chloranoside A (4), pisumionoside (4 1 5) and sarcaglaboside A (2 1 8) separated from C. japonicus exhibited antagonistic effects on HepG-2, OV420 and MCF-7 cells (Kuang et al., 2008).
5.15 Antiinflammatory activity
The genus Chloranthus showed strong effect in anti-inflammatory activity, which are used to treat arthritis and bruises. Pan et al. demonstrated that the sesquiterpene dimer shizukaol B (65) exerted stronger anti-inflammatory activity in LPS-induced BV2 microglia model by modulating the JNK-AP-1 signaling pathway (Pan et al., 2017). Similarly, Wang lijun's group (Wang et al., 2014b) found that the compounds zederone epoxide (2 3 7), chloramultilide A (43), shizukaol B (65) and spicachlorantin B (72) isolated from C. henryi also showed significant anti-inflammatory effects through inhibiting the release of NO. Zhuo et al. (2017) reported that chlorajaponol B (10) identified from C. japonicus significantly inhibited lipopolysaccharide-induced NO release by RAW 264.7 cells. Furthermore, fortunilides K (1 1 6) isolated from C. multistachys whole herb showed the most significant anti-inflammatory activity in LPS-induced RAW 264.7 cell model. By comparison, the sesquiterpene lactones were significantly more active than the other sesquiterpenes (Huang et al., 2020). Besides, chlojaponilactones B (10) from C. japonicus exerted anti-inflammatory activity by inhibiting inflammatory mediators such as iNOS, TNF-α and IL-6, whose mechanism is related to the inhibition of NF-κB signaling pathway (Zhao et al., 2016). Zhang et al. (2012b) found active components shizukaol B (65) and D (69) isolated from C. serratus exhibited significant anti-inflammatory activity in LPS-induced RAW 264.7 inflammation model with IC50 values of 0.22 and 0.15 μM, respectively. Similarly, shizukaol G (67), M (1 0 2), and O (1 2 5) isolated from C. fortunei also showed strong anti-inflammatory activity with IC50 values of 1.90, 3.68, 1.95, 7.01 and 1.95 μM, respectively (Zhang et al., 2012c). Moreover, the compounds chololactones A-H (137–144) from C. holostegius roots showed moderate anti-inflammatory activity by inhibiting NO production against LPS-induced RAW 264.7 (Shen et al., 2017). Sun et al. found that the ethanolic extract of the roots of C. serratus showed the strongest anti-arthritic activity (Sun et al., 2020). In addition, TNF-α and PDE4 were also important signaling molecules involved in the inflammatory response. It was reported that sesquiterpene dimer chlojapolactone B (10) identifed from C. japonicus could exert anti-inflammatory effects by inhibiting the release of TNF-α (IC50 of 76.16 μM) (Li et al., 2019).
5.16 Antibacterial activity
In recent years, it has been confirmed that Chloranthus has antibacterial effects. Li (2011). found that ethyl acetate extracts of C. japonicus and C. multistachys showed a better antibacterial activity against Garcinia octococci. Furthermore, Xu et al. (2007) reported that chloramultilide B (71) isolated from C. spicatus showed inhibitory activity against both Candida albicans and Clostridium parvum with an MIC value of 0.068 µM through antifungal assays. Additionally, the monomeric shizukaol C (68) and F (66) obtained from C. japonicus showed more than 85 % inhibitory activity against Puccinia recondita (wheat leaf rust) and Phytophthora infestans (tomato late blight) (Kang et al., 2017). At the same time, the sesquiterpene dimers shizukaol C and F reported from C. japonicus whole herb showed good inhibitory activity against phytopathogenic fungi (MICs of 4 to 16 μM).
5.17 Neuroprotective activity
Alzheimer's disease (AD) is one of the most common chronic diseases in old age, which has become a major threat to human life and health. The search for natural active drugs from Chinese medicine to treat AD has attracted a lot of attention from researchers. Chen et al (Chen et al., 2021c). demonstrated that chlohenriol A-C (264–266) isolated from C. henryi showed significant neuroprotective activity against H2O2-induced PC12 cell injury model. Furthermore, shizukanolide H (30) isolated from C. anhuiensis exhibited significant neuroprotective activity against glutamate-induced apoptosis in PC12 cells. The active ingredients could reduce PC12 apoptosis by suppressing caspase-3 activity (Xu et al., 2018).
5.18 Antimalarial activity
In recent years, the antimalarial activity of the gens Chloranthus has also been widely studied. Zhou et al. (2017b) demonstrated that 16 lindenane-type sesquiterpenoids dimers isolated from C. fortunei showed antimalarial activity. Among which, fortunilide A (96), sarglabolide J (1 0 0) and chlorahololide D (1 0 3) showed the strongest antimalarial activity, which was comparable to the potency and selectivity index values of artemisinin. Meanwhile, fortunoid A (1 1 8) and B (1 1 9) isolated from C. fortunei also showed moderate antimalarial activity (Zhou et al., 2017a).
5.19 Anti-viral activity
It was reported that shizukaol B (65), shizukaol C (68), shizukaol F (66) and shizukaol H (70) isolated from C. japonilides exhibited anti-HIV activity. However, Fang et al. (2011). found that the compound shizukaol B showed more stronger inhibition.
5.20 Hypoglycemic activity
Few studies have been reported on the hypoglycemic activity of the genus Chloranthus. Hu et al. (2017) discovered that shizukaol D (69) isolated from C. japonicus could activate AMP-activated protein kinase and regulate glucose metabolism. In addition, chlorabietols A-C (366–368) isolated from the roots of C.oldhamii plants exhibited some complexinase inhibitory effects (Xiong et al., 2015).
5.21 Other activities
In addition, the genus Chloranthus also exert other pharmacological effects. Li et al. (2008) found that henriol A (75) and henrilabdanes A-C (334–336) isolated from C. henryi exhibited moderate hepatoprotective activity with IC50 values of 0.19, 0.66, 0.09 and 0.18 μM. Besides, the researchers reported that hexane extract of C. japonicus play a significant role in promoting adipogenesis. The extract activated the Wnt/β-catenin signaling pathway by promoting adipocyte differentiation (Yun et al., 2021). Moreover, three sesquiterpenoids shizukaol B (65), cycloshizukaol A (1 2 3) and shizukaol F (66), isolated from C. japonicus whole herb, also prevented monocyte adhesion to HUVEC by inhibiting TNF-α-stimulated cell adhesion molecule expression (Kwon et al., 2006). And it was reported that chlorahololide A (1 4 8) and B (1 3 0) identified from C. holostegius were two stronger potassium channel blockers (Yang et al., 2007b). In addition, Sun et al. conducted toxicity experiments on rat hearts by taking alcoholic extracts of C. serratus roots, stems and leaves, the results showed that the extracts of the alcoholic parts of C. serratus stems were the most cardiotoxic, followed by the alcoholic extracts of the leaves (Sun et al., 2019).
6 Development and utilization
6.1 Indoleamine 2, 3-dioxygenase 1(IDO1)
IDO1 inhibitors, as drugs with new targets and mechanisms, can be applied to the treatment of tumors, Alzheimer's disease, depression and other diseases, and are potential targets for tumor immunotherapy. It has been found that chloranthalactone A (2), chloranthalactones C-E (6–8) can be effective inhibitors of IDO1, and their inhibition rate has reached about 80 % (5 μM), so inhibition of IDO1 is expected to be a novel tumor treatment strategy (Tan, 2018a; Tan, 2018b; Tan, 2018c; Tan, 2018d; Tan, 2018e).
6.2 Antitumor drugs
In recent years, several components with antitumor activity have been reported from the genus Chloranthus. Researchers found that the application of shizukaol D (69) in the preparation of anti-liver cancer drugs, the addition of shizukaol D to cultures of liver cancer cells can significantly slow down the scratch healing and migration of liver cancer cells (Yu and Tang, 2016), and the component also has the effect of increasing the sensitivity of tumor multidrug-resistant cells to anti-tumor drugs, which can be used as a chemotherapy sensitizer (Yu and Jie, 2017). In addition, chloranthalactone C (6) can significantly inhibit the proliferation of tumor cells, such as blood, cervical, breast or pancreatic cancers. It can be developed as a new anti-tumor drug or its adjuvant component with significant tumor suppression effect (Yu et al., 2013). Yinxiancaoside A (3), yinxiancaoside B (4 1 6), and yinxiancaoside C (4 1 7) were shown to have significant anti-tumor activity in vitro, which can be used to develop into new, low-toxicity antitumor drugs from natural Chinese medicine (Kuang et al., 2010).
6.3 Others
The whole herb of C. fortunei and Chinese patent medicine snakebite detoxification tablet powder or Liushenwan can be mixed to make a kind of detoxification powder which has the function of local detoxification, dispersing blood stasis and reducing swelling. This product provides an effective treatment medicine for people working in the field after preventing poisonous insect bites, and several inventions have disclosed its preparation method (Zhang et al., 2018a; Zhang et al., 2018b). C. spicatus combined with other herbs can be made into a medicinal wine with health effects and treatment of migraine (Cheng & Cheng, 2015; Liang, 2015). In addition, C. japonicus herb of the genus Chloranthus has a very broad development prospect in the treatment of psoriasis (Mao, 2017).
7 Conclusions and discussion
Many species of the genus Chloranthus have been used in TCM or folk medicines to treat various diseases. Among which the most widely studies are C. japonicus, C. serratus, C. multistachys and C. henryi (Fig. 20.). This article updates the references of this genus for the last three decades and summarized all the compounds of genus Chloranthus. To date, 418 compounds have been reported from the genus Chloranthus, which include 383 terpenoids, 4 coumarins, 6 lignans, 2 simple phenylpropanoids, 4 flavonoids, 5 organic acids, 6 amides, and 8 other compounds. Among them, sesquiterpenes were generally considered as major bioactive ingredients in Chloranthus which exhibited various qualities. Furthermore, pharmacological studies showed that Chloranthus plants possessed a wide range of pharmacological activities, such as anti-cancer, antibacterial, antiviral, hypoglycemic anti-inflammatory and antimalarial. Regardless, there are still several aspects that need to be concerned about the further development of genus Chloranthus.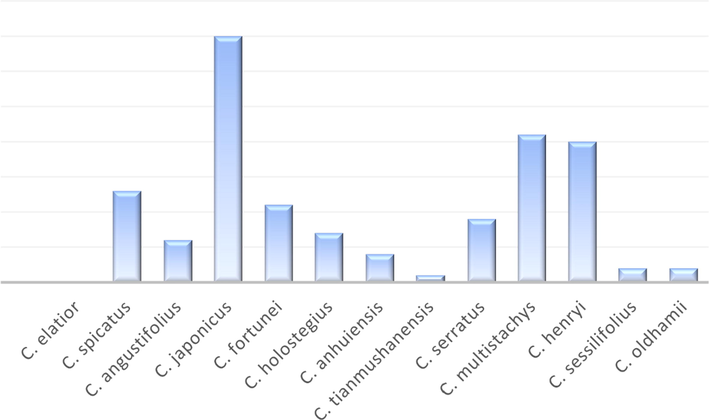
The relative percentage of all published chemical and biological reports regarding Genus Chloranthus species.
In terms of chemical composition, sesquiterpenes are the most important active components of the genus Chloranthus (Fig. 21), which mainly distributed in C. japonicus and C. fortunei (Fig. 22). For further in-depth phytochemical scanning, Fig. 23 is performed to illustrate the type and the relative percentage of each chemical class isolated from Chloranthus species. These notifications are as the following: (1) Terpenoids are its main chemical components, mostly in the form of rings, with great structural variation. Some compounds open part of the ring structure on the original basis or form new rings on the original basis to form new compounds. In addition, the current research hot spot is the large ring structure of sesquiterpene dimer class, especially compounds shizukaol B (65), shizukaol F (66), shizukaol C (68) and chloramultilide B (71) were the most widely distributed and most frequently reported in the literature. (2): Diterpenes are the second major active constituents of the genus, in which more new bioactive monomers are continuously found. And they are mainly discovered in the C. henryi, C. oldhamii, C. sessilifolius and C. serratus, which are also the focus studying of the genus Chloranthus. (3): Fig. 23 is performed to illustrate that the chemical composition of C. japonicus is the most abundant, such as sesquiterpenes, monoterpenes, diterpenoids, C25 terpenoids, coumarins and lignans.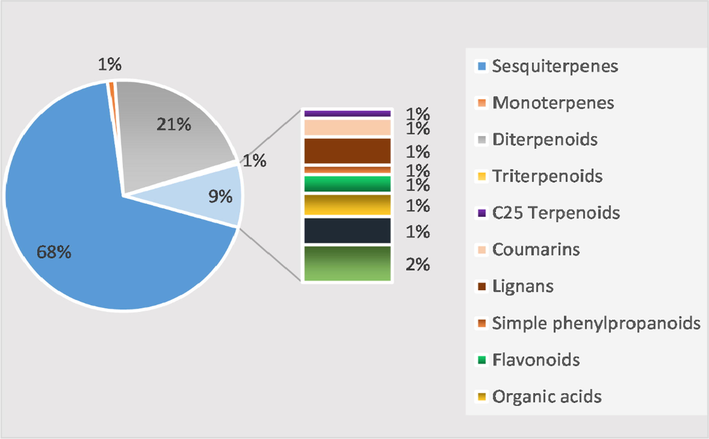
The distribution of the secondary metabolites among Genus Chloranthus species.
The relative percentage of secondary metabolites isolated from Genus Chloranthus species.
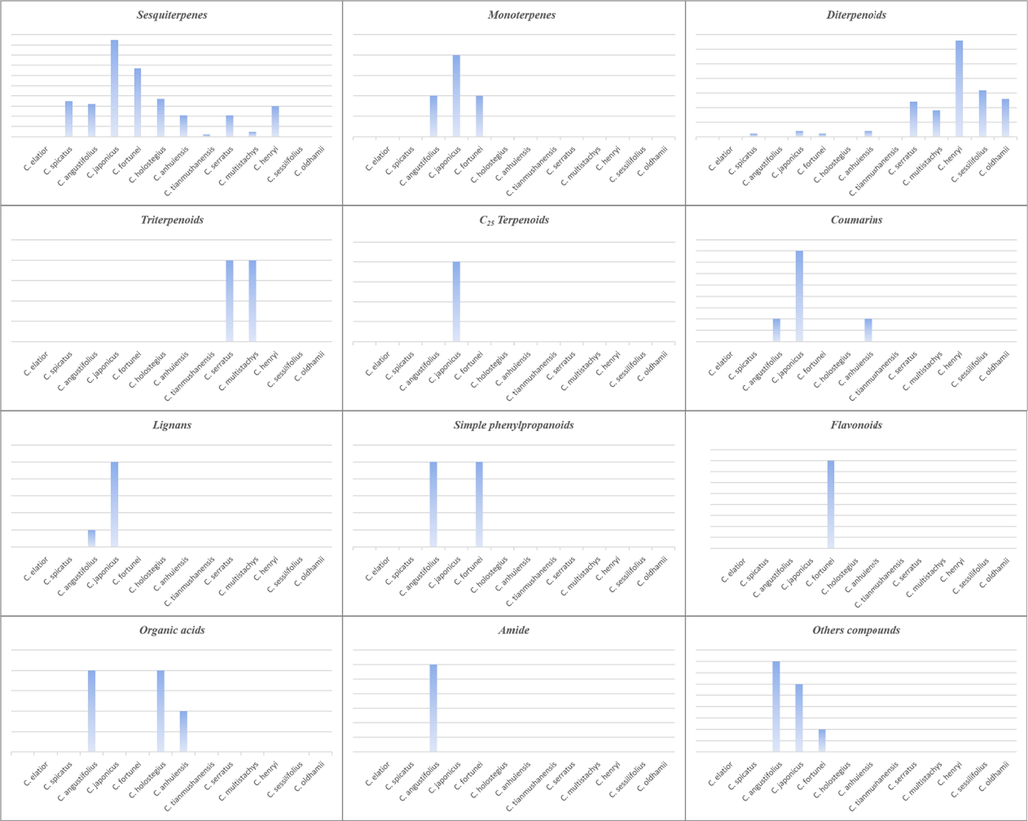
The relative percentage of each chemical classes among different Genus Chloranthus.
As a group of plants possess multiple biological activities, the genus Chloranthus is particularly well studied in terms of pharmacological mechanisms of action (Fig. 24), which include antitumor, anti-inflammatory, hypoglycemic and antimalarial. Among them, shizukanolide C (29) and chloranthalactone A-E (2, 5, 6–8) are the main active compounds, which have varieties of pharmacology activities. Meanwhile, chlorahupetones G, isolated from C. henryi, exhibited the most potent cytotoxicity against A549 cells, even about 4 times than paclitaxel (Zhang et al., 2021). Of course, it cannot be ignored that monomeric compounds with outstanding pharmacological activities can be considered the source of new drugs with excellent therapeutic effects. Furthermore, the characteristic components of aconite-type sesquiterpenes and their dimers in the genus Chloranthus are novel, complex and variable in structure and rich in pharmacological activities, which deserve attention in the subsequent research and development.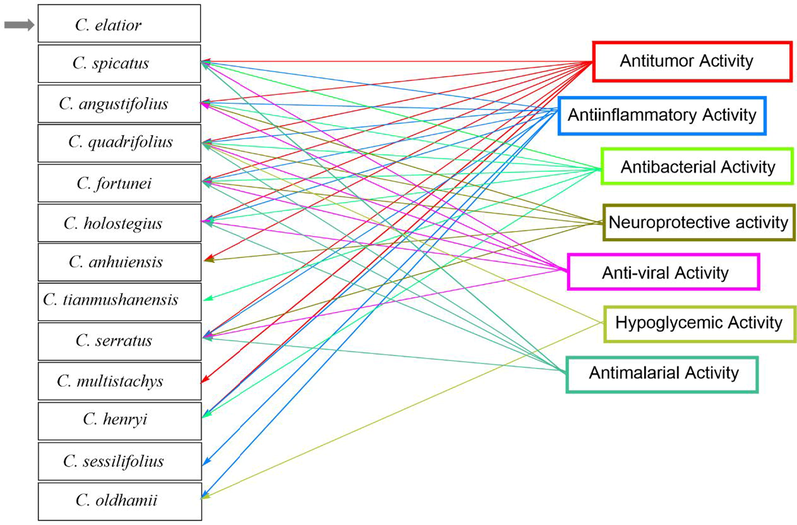
The pharmacological activities of different Genus Chloranthus species.
As a genus with a complete distribution and the presence of endemic species in China, the research and development were still incomplete. Only a bit species has been studied so far, therefore, a systematic and in-depth study and development of the genus Chloranthus is critical.
Author contributions
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Funding
his work is supported by program project for Shaanxi Province (grant No 2019ZDLSF04-03-02); Subject Innovation Team of Shaanxi University of Chinese Medicine (grant number 2019-YL12) and the Natural Science Basic Research Project of Department of science and technology of Shaanxi Province (grant number: 2021JQ-744).
References
- Chloraserrtone A, a sesquiterpenoid dimer from Chloranthus serratus. J. Nat. Prod.. 2019;82:407-411.
- [Google Scholar]
- Advances in study on chemical constituents and pharmacological effects of Chloranthus plants. China J. Chin. Materia Medica. 2008;13:1509-1515.
- [Google Scholar]
- Characteristic lindenane sesquiterpenoid dimers and labdane diterpenoids from Chloranthus serratus. J. Biochem. Syst. Ecol.. 2019;86:103394
- [Google Scholar]
- Neuroprotective racemic germacranolides from the roots of Chloranthus henryi. J. Fitoterapia. 2020;141:104472
- [Google Scholar]
- Chemical constituents from Chloranthus fortunei and their neuroprotective effects. Natl. Prod. Res. Develop.. 2021;33(8):1333-1338.
- [Google Scholar]
- Research progress on chemical constituents from Chloranthus plants and their biological activities. China J. Chin. Materia Medica. 2021;46(15):3789-3796.
- [Google Scholar]
- Sesquiterpenoids with neuroprotective activities from the Chloranthaceae plant Chloranthus henryi. J. Fitoterapia. 2021;151 (prepublish)
- [Google Scholar]
- Cheng, X.G., Cheng, J.Q., 2015. A kind of external multifunctional health drink. CN104983963A.
- Chlotrichenes A and B, Two lindenane sesquiterpene dimers with highly fused carbon skeletons from Chloranthus holostegius. Org. Lett.. 2019;21(3):789-792.
- [Google Scholar]
- Chloranthaceae plant in Flora of China @ efloras.org., 2020. Chloranthaceae plant in Flora of Chloranthus 2020.
- Chloranthus Swartz in Flora of China @ efloras.org” eFlora. Missouri Botanical Garden, St. Louis, MO & Harvard University Herbaria, Cambridge, MA., 2002. Web. Accessed February 2018.
- Study on the chemical composition of the above-ground parts of Chloranthus angustifolius Oliv. Chin. Tradit. Herbal Drugs. 2017;48:42-46.
- [Google Scholar]
- Studies on chemical constituents and biological activities of Chloranthaceae. Qingdao: Qingdao University of Science and Technology; 2011.
- Lindenane disesquiterpenoids with anti-HIV-1 activity from Chloranthus japonicus. J. Nat. Prod.. 2011;74:1408-1413.
- [Google Scholar]
- In vitro inhibitory properties of sesquiterpenes from Chloranthus serratus on cell motility via down-regulation of LIMK1 activation in human breast cancer. Phytomedicine. 2018;49:23-31.
- [Google Scholar]
- Research progress of yao medicine Chloranthus fortunei. Chin. Ethnic Folk Med.. 2021;30
- [Google Scholar]
- Chlojapolactone A, an unprecedented 1,3-dioxolane linked-lindenane sesquiterpenoid dimer from Chloranthus japonicus. RSC Adv.. 2015;5(125):103047-103051.
- [Google Scholar]
- Natural nitric oxide (NO) inhibitors from Chloranthus japonicus. Bioorg. Med. Chem. Lett.. 2016;26(13):3163-3166.
- [Google Scholar]
- Chemical constituents of the aerial parts of Chloranthus japonicus Sieb. Nat. Prod. Sci.. 2005;11:41-44.
- [Google Scholar]
- Modulation of glucose metabolism by a natural compound from Chloranthus japonicus via activation of AMP-activated protein kinase. Sci. Rep.. 2017;7:778.
- [Google Scholar]
- Three pairs of sesquiterpene enantiomers from Chloranthus multistachys Pei. Nat. Prod. Res.. 2021;27:1-8.
- [Google Scholar]
- Huang, W.M., Chen, F.Y., Bian, Y.T., 2020. Study on the chemical composition and anti-inflammatory activity of Chloranthus multistachys Pei., vol. 32, pp. 1688–1697.
- Antifungal activities of dimeric sesquiterpenes, shizukaols C and F, isolated from Chloranthus japonicus Sieb. J. Microbiol. Biotechn.. 2017;27:1272-1275.
- [Google Scholar]
- Isolation and structural elucidation of four sesquiterpenes from Chloranthus japonicus (Chloranthaceae) Agr. Biol. Chem.. 1981;45(6):1447-1453.
- [Google Scholar]
- Structures of novel sesquiterpene alcohols from Chloranthus japonicus. Agric. Biol. Chem.. 1984;48:713-717.
- [Google Scholar]
- a sesquiterpene dimer from Chloranthus japonicus. Phytochemistry. 1990;29(7):2332-2334.
- [Google Scholar]
- Spicachlorantins G-J, new lindenane sesquiterpenoid dimers from the roots of Chloranthus spicatus. Chem. Pharm. Bull.. 2011;59(10):1281-1284.
- [Google Scholar]
- Hitorins A and B, hexacyclic C25 terpenoids from Chloranthus japonicus. Org. Lett... 2016;18(20):5420-5423.
- [Google Scholar]
- Kuang, H.X., Yang, B.Y., Xia, Y.G., Wang, Q.H., Lü, S.W., Sun, G.R., Gu, W.Q., Compounds with antitumor activity isolated from Chloranthus and their uses. CN101824014A. 2010.09.08.
- Sesquiterpene glucosides from Chloranthus japonicus Sieb. Chem. Biodivers.. 2008;5(9):1736-1742.
- [Google Scholar]
- Lignan constituents from Chloranthus japonicus. Sieb. Arch. Pharm. Res.. 2009;32(3):329-334.
- [Google Scholar]
- Dimeric sesquiterpenoids isolated from Chloranthus japonicus inhibited the expression of cell adhesion molecules. J. Ethnopharmacol.. 2006;104:270-277.
- [Google Scholar]
- A new dimeric sesquiterpenoid from Chloranthus japonicus Sieb. Rec. Nat. Prod.. 2019;13:483-490.
- [Google Scholar]
- Antifungal sesquiterpenoids from Chloranthus japonicus. Phytochem. Lett.. 2016;15:199-203.
- [Google Scholar]
- Hepatoprotective sesquiterpene glycosides from sarcandra glabra. J. Nat. Prod.. 2006;69:616-620.
- [Google Scholar]
- Bis-sesquiterpenes and diterpenes from Chloranthus henryi. Phytochemistry. 2008;69:2867-2874.
- [Google Scholar]
- Li, L.X., 2011. Analysis of the antibacterial activity of the extracts of Chloranthus japonicus Sieb and Chloranthus multistachys Pei. Shaanxi University of Normal Studies.
- Liang, D., A kind of medicinal wine for migraine. CN104758643A. 2015.07.08.
- Chemotaxonomic significance of sesquiterpenes and amide derivatives from Chloranthus angustzfolius oliv. Biochem. Syst. Ecol... 2015;58:30-33.
- [Google Scholar]
- Morphological diversity and geographical distribution of the genus Chloranthus in China. Guihaia. 2020;40(1):31-43.
- [Google Scholar]
- Crystal structure of 4α-hydroxy-5α,8β(H)-eudesm-7(11)-en-8,1-2-olide monohydrate. Acta. Crystallogr. Sect. E.. 2015;71:0518.
- [Google Scholar]
- Two new sesquiterpenes from Chloranthus japonicus Sieb. Nat. Prod. Res.. 2016;30(21):2476-2482.
- [Google Scholar]
- Study on the chemical composition of the family of Chloranthaceae. In: Proceedings of the 2009 National Symposium on Traditional Chinese Medicine. 2009. p. :262-265.
- [Google Scholar]
- Mao, Y., A kind of Chinese medicine for the treatment of parapsoriasis. CN106692625A. 2017.05.24.
- Shizukaol B, an active sesquiterpene from Chloranthus henryi, attenuates LPS-induced inflammatory responses in BV2 microglial cells. Biomed. Pharmacother.. 2017;88:878-884.
- [Google Scholar]
- Two new polyhydroxysterols produced by fusarium solani, an endophytic fungus from Chloranthus multistachys. Nat. Prod. Res.. 2016;30(19):2173-2182.
- [Google Scholar]
- Sesquiterpene dimers from the roots of Chloranthus holostegius with moderate anti-inflammatory activity. Phytochemistry. 2017;137:117-122.
- [Google Scholar]
- Three sesquiterpenoid dimers from Chloranthus japonicus: Absolute configuration of chlorahololide A and related compounds. J. Chirality. 2015;28(2):158-163.
- [Google Scholar]
- Mechanisms of Toxicity and Cardiotoxicity of Alcohol Extract from root, stem and leaf of Chloranthus Serratus. J. Forensic Med.. 2019;35:224-229.
- [Google Scholar]
- Anti-inflammatory effects of the root, stem and leaf extracts of Chloranthus serratus on adjuvant-induced arthritis in rats. Pharm. Biol.. 2020;58:528-537.
- [Google Scholar]
- Tan, M.J., 2018. Biomedical use of chloranthalactone A as an indoleamine 2,3-dioxygenase 1 inhibitor. CN108379250A. 2018a.08.10.
- Tan, M.J., 2018. Biomedical use of chloranthalactone D as an indoleamine 2,3-dioxygenase 1 inhibitor. CN108558903A. 2018b.09.21.
- Tan, M.J., 2018. Biomedical use of chloranthalactone F as an indoleamine 2,3-dioxygenase 1 inhibitor. CN108578401A. 2018c.09.28.
- Tan, MJ., 2018. Biomedical use of chloranthalactone C as an indoleamine 2,3-dioxygenase 1 inhibitor. CN108478565A. 2018d.09.04.
- Tan, MJ., 2018. Biomedical use of chloranthalactone E as an indoleamine 2,3-dioxygenase 1 inhibitor. CN108392477A. 2018e.08.14.
- Shizukaol D, a dimeric sesquiterpene isolated from Chloranthus serratus, represses the growth of human liver cancer cells by modulating wnt signalling pathway. PLoS ONE. 2016;11:0152012.
- [Google Scholar]
- Four new eudesmane sesquiterpenoid lactones from Chloranthus serratus. Helv. Chim. Acta. 2010;92:1298-1303.
- [Google Scholar]
- Studies on the constituents of Chloranthus spp. III. Six sesquiterpenes from Chloranthus japonicus. Chem. Pharm. Bull.. 1980;28(1):92-102.
- [Google Scholar]
- Study on the Chemical Constituents of Four Medicinal Plants. Wuhan: South-Central Minzu University; 2014.
- Codonolactone, a sesquiterpene lactone isolated from Chloranthus henryi hemsl, inhibits breast cancer cell invasion, migration and metastasis by downregulating the transcriptional activity of Runx2. Int. J. Oncol.. 2014;45(5):1891-1900.
- [Google Scholar]
- Secondary metabolites of plants from the genus Chloranthus: chemistry and biological activities. Chem. Biodivers.. 2015;12(4):451-473.
- [Google Scholar]
- A New Sesquiterpenoid from the roots of Chloranthus fortunei. Chin. J. Nat. Med.. 2008;6(6):404-407.
- [Google Scholar]
- Sesquiterpenes and dimers thereof from Chloranthus fortunei. Helv. Chim. Acta.. 2009;92(2):313-320.
- [Google Scholar]
- Sesquiterpenoids from Chloranthus henryi and their anti-neuroinflammatory activities. Chem. Biodivers.. 2014;11:919-928.
- [Google Scholar]
- Sesquiterpenoids and further diterpenoids from the rare Chloranthaceae plant Chloranthus sessilifolius. J. Asian. Nat. Prod. Res.. 2015;17(12):1220-1230.
- [Google Scholar]
- Ent-abietane-type and related Seco-/Nor-diterpenoids from the rare Chloranthaceae plant Chloranthus sessilifolius and their antineuroinflammatory activities. J. Nat. Prod... 2015;78(7):1635-1646.
- [Google Scholar]
- Bioactive terpenes from the roots of Chloranthus henryi. Planta Med.. 2006;72(14):1334-1338.
- [Google Scholar]
- Cadinane and eudesmane sesquiterpenoids from Chloranthas henryi. Helv. Chim. Acta. 2007;90:1586-1592.
- [Google Scholar]
- New tyrosinase inhibitory sesquiterpenes from Chloranthus henryi. Chem. Biodivers... 2008;5(7):1298-1303.
- [Google Scholar]
- An unusual stress metabolite induced by CuCl(2) and other constituents from the leaves of Chloranthus anhuiensis. J. Nat. Prod.. 2010;73(6):1069-1074.
- [Google Scholar]
- Bioactive ent-pimarane and ent-abietane diterpenoids from the whole plants of Chloranthus henryi. J. Nat. Prod.. 2015;78(11):2800-2807.
- [Google Scholar]
- Chlorabietols A-C, phloroglucinol-diterpene adducts from the Chloranthaceae plant Chloranthus oldhamii. J. Org. Chem.. 2015;80:11080-11085.
- [Google Scholar]
- Ent-Abietane diterpenoids with anti-neuroinflammatory activity from the rare Chloranthaceae plant Chloranthus oldhamii. Org. Biomol. Chem.. 2016;14:1-15.
- [Google Scholar]
- Phytochemical and biological studies of Chloranthus medicinal plants. Chem. Biodivers.. 2013;10(10):1754-1773.
- [Google Scholar]
- Chemical Composition Study of Chloranthus Holostegius and Sarcandra glabra. Kunming: Yunnan University of Traditional Chinese Medicine; 2016.
- Mono- and di-sesquiterpenoids from Chloranthus spicatus. J. Nat. Prod.. 2007;70(12):1987-1990.
- [Google Scholar]
- Sesquiterpenoids and diterpenoids from Chloranthus anhuiensis. Chem. Biodivers.. 2010;7:151-157.
- [Google Scholar]
- Sesquiterpenoids from Chloranthus anhuiensis with neuroprotective effects in PC12 Cells. J. Nat. Prod.. 2018;81(6):1391-1398.
- [Google Scholar]
- Chlojaponilactones B - E, four new lindenane sesquiterpenoid lactones from Chloranthus japonicus. Helv. Chim. Acta.. 2013;96(7):1386-1391.
- [Google Scholar]
- Japonicones A-C: Three lindenane sesquiterpenoid dimers with a 12-membered ring core from Chloranthus japonicus. Tetrahedron Lett. 2019
- [CrossRef] [Google Scholar]
- Sesquiterpenoids from Chloranthus spicatus (Thunb.) Makino. Chin. J. Chem.. 2007;25(12):1892-1895.
- [Google Scholar]
- Chlorahololides A and B, two potent and selective blockers of the potassium channel isolated from Chloranthus holostegius. Org. Lett.. 2007;9:903-906.
- [Google Scholar]
- Chlorahololides C-F: a new class of potent and selective potassium channel blockers from Chloranthus holostegius. Tetrahedron. 2008;64(9):2027-2034.
- [Google Scholar]
- Antimicrobial sesquiterpenes from the Chinese medicinal plant, Chloranthus angustifolius. Tetrahedron Lett.. 2014;55:5632-5634.
- [Google Scholar]
- Sesquiterpene furan compound CJ-01, a novel chitin synthase 2 inhibitor from Chloranthus japonicus sibe. Biol. Pharm. Bull.. 2008;31(5):1041-1044.
- [Google Scholar]
- Yu, L., Jie, F., 2017. Application of shizukaol D in the preparation of antitumor drug sensitizer. CN106902109A. 2017.06.30.
- Yu, L., Tang, L.S., 2016. Application of shizukaol D in preparation of drugs for hepatocellular carcinoma. CN105213369A. 2016.01.06.
- Yu, L., Tang, L.S., Liu, Z.L., 2013. Application of chloranthalactone C in the preparation of antitumor drugs. CN103054851A. 2013.
- Sesquiterpenoids and phenylpropanoids from Chloranthus serratus. J. Nat. Prod.. 2008;71:2021-2025.
- [Google Scholar]
- Hexane Extract of Chloranthus japonicus increases adipocyte differentiation by acting on Wnt/β-Catenin signaling pathway. Life-Basel.. 2021;11:241.
- [Google Scholar]
- Sesquiterpenoids from the whole plants of Chloranthus holostegius and their anti-inflammatory activities. Chin. J. Chem.. 2021;39
- [CrossRef] [Google Scholar]
- Progress in the study of medicinal plants of the genus Chloranthus. Inner Mongolia Tradit. Chin. Med.. 2016;35(2):155-156.
- [Google Scholar]
- Zhang, K.C., Zhang, Y., Zhang, L., 2018. The invention relates to a preparation method of compound Chloranthus fortunei. CN108904680A 2018a.11.30.
- Zhang, K.C., Zhang, Y., Zhang, L., 2018. An antidote to the poison of Chloranthus fortunei. CN109010393A 2018b.12.18.
- Sesquiterpenes from the aerial part of Chloranthus japonicus and their cytotoxicities. Fitoterapia. 2012;83(8):1604-1609.
- [Google Scholar]
- Terpenoids from Chloranthus serratus and their anti-inflammatory activities. J. Nat. Prod.. 2012;75(4):694-698.
- [Google Scholar]
- Anti-inflammatory sesquiterpenes and sesquiterpene dimers from Chloranthus fortunei. J. Asian Nat. Prod. Res.. 2012;14:708-712.
- [Google Scholar]
- Nine sesquiterpenoid dimers with four unprecedented types of carbon skeleton from Chloranthus henryi var. hupehensis. J. Org. Chem. Front.. 2021;8
- [Google Scholar]
- Multistalides A and B, two novel sesquiterpenoid dimers from Chloranthus multistachys. Tetrahedron Lett.. 2010;51:764-766.
- [Google Scholar]
- Chlojaponilactone B from Chloranthus japonicus: Suppression of inflammatory responses via inhibition of the NF-κB Signaling Pathway. J. Nat. Prod.. 2016;79:2257-2263.
- [Google Scholar]
- Fortunoids A-C, three sesquiterpenoid dimers with different carbon skeletons from Chloranthus fortunei. Org. Lett.. 2017;19:734-737.
- [Google Scholar]
- Trichloranoids A-D, antimalarial sesquiterpenoid trimers from Chloranthus spicatus. Org. Chem. Front.. 2021;8(8):1795-1801.
- [Google Scholar]
- Nanomolar antimalarial agents against chloroquine-resistant plasmodium falciparum from medicinal plants and their structure-activity relationships. J. Nat. Prod.. 2017;80(1):96-107.
- [Google Scholar]
- Chemical constituents from Chloranthus anhuiensis and their cytotoxic activities. Chem. Biodivers.. 2018;15(10):e1800249
- [Google Scholar]
- Chlorajaponols A-F, sesquiterpenoids from Chloranthus japonicus and their in vitro anti-inflammatory and anti-tumor activities. Fitoterapia. 2017;119:90-99.
- [Google Scholar]







