Translate this page into:
Geraniin ameliorates streptozotocin-induced diabetic retinopathy in rats via modulating retinal inflammation and oxidative stress
⁎Corresponding author. diaolixia_66@hotmail.com (Lixia Diao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Diabetic retinopathy (DR) is the major complication of diabetes, which causes acquired vision loss in the working-age group population.
Objective
Here, we planned to address the therapeutic roles of geraniin against the streptozotocin (STZ)-challenged DR in rats
Methodology
The DR was induced in the animals by 60 mg/kg of STZ, and then treated with 25 mg/kg of geraniin for 60 days. Later, bodyweight, food consumption, and blood glucose levels were investigated. The levels of antioxidants, MMP-9, MCP-1, and VEGF, and inflammatory cytokine status were measured using marker-specific kits. The morphometric study was conducted to assess the retinal thickness. The pancreatic tissues were analyzed microscopically.
Results
Geraniin reduced the blood glucose (270.36 ± 81 mg/dL), hemoglobin, and enhanced bodyweight (261.93 ± 72 g)in the DR rats. The antioxidant levels in the STZ-challenged DR rats were substantially improved by geraniin. Geraniin also decreased inflammatory cytokines, MCP-I, MMP-9, and VEGF levels and enhanced the retinal thickness. A histological study demonstrated that geraniin reduced the pancreatic islet cell damage in STZ-induced DR rats.
Conclusion
Our outcomes witnessed that geraniin reduced retinal inflammation and oxidative stress in the STZ-induced DR rats.
Keywords
Diabetic retinopathy
Geraniin
Oxidative stress
Inflammation
Streptozotocin
1 Introduction
Diabetic retinopathy (DR) is a common problem among diabetic patients and a major cause of acquired vision loss in the working-age population (Yamagishi and Matsui, 2011). The occurrence of DR among diabetic patients was estimated at 35 %, and almost 10 % of patients among them had the risk of vision loss (Sayres et al., 2019; Hu et al., 2012). DR results in the detachment and edema of the retina, which further directs to vision loss. Diabetic patients are more likely to suffer from chronic vascular damage caused by high blood sugar levels, and they are at a higher risk of developing diabetes (Barot et al., 2013). The exact etiology of DR is not yet known, but the causes may comprise hyperglycemia, inflammation, oxidative stress, and depletion of antioxidants that could initiate the DR progression (Abougalambou and Abougalambou, 2015; Zheng et al., 2018). The uncontrolled hyperglycemia due to diabetes leads to oxidative stress via facilitating increased glucose oxidation, protein kinase-C and polyol fluctuations, and elevated end products of glycation (Rains and Jain, 2011; Singh et al., 2011). The eye cells are more vulnerable to oxidative stress than other body tissues because of the high levels of membrane-bound polyunsaturated fatty acids and higher glucose oxidation and oxygen utilization. The depletion of antioxidant defensive mechanisms and improved oxidative stress are the crucial contributors to hyperglycemia-mediated injury (Cao et al., 2014). The increased energy needs during diabetes and retinal exposure to light could elevate the DR risks. Hence, adjusting the oxidative and antioxidant proportions could be a hopeful approach to prevent DR-provoked injuries (Vakifahmetoglu-Norberg et al., 2017).
Chronic inflammation at the retinal blood vessels is caused by arteriosclerotic plate depositions caused by high blood glucose levels, making them vulnerable to microrupture, which results in fluid leakage into the retina. If this condition is left undiagnosed, there could be new vessel growth that alters the retinal microvasculature and this consequently triggers the detachment of retina (Aylward, 2005; Kern, 2007). NF-B activates many genes involved in disease progression during DR progression, enhancing inflammatory regulators such as cytokines and intercellular adhesion molecules-1 (ICAM-1) (Leal et al., 2010). These regulators simultaneously control the BRB permeability combined with leukostasis (Zhong and Kowluru, 2011). Angiogenesis is one of the imperative pathological features of DR progression. The elevated expression of angiogenic proteins like VEGF is tightly connected with diabetes-provoked neurodegeneration and oxidative stress. Calcium dobesilate is a well-known angioprotective and vasoactive drug, which is extensively employed to treat DR at the local ocular and systematic level with hopeful outcomes (Cai et al., 2017). For many years, laser photocoagulation has been considered as the gold standard therapeutic option to treat the DR. However, much recent evidence has shown that the intravitreal administration of anti-VEGF agents has the potential to alleviate the DR and is hence currently being explored broadly (Wells et al., 2016). Nonetheless, not all the DR patients responded well to the anti-VEGF drugs and also experienced several adverse effects. Hence, the discovery of novel therapeutic agents with minimal adverse effects from natural sources to treat the DR were highly demanded.
Geraniin is a crystalline tannin found in several medicinal plant species from the Sapindaceae, Geranium, and Euphobiaceae families, e.g., Nephelium lappaceum. Geraniin is reported to exhibit numerous biological actions like antioxidants (Lin et al., 2008), antihypertensive and antihyperglycaemic properties (Palanisamy et al., 2011). It was also highlighted that the geraniin revealed potent hepatoprotective (Aayadi et al., 2017), anti-inflammatory (Wang et al., 2016; Okabe et al., 2001), anticancer (Wang et al., 2017), antitumor (Guo et al., 2018), insulin sensitizing (Perera et al., 2020), and anti -atherosclerosis (Lin et al., 2022 Jul). In contrast, the therapeutic role of geraniin in the DR has yet to be investigated. As a result, we conducted this study to address the therapeutic role of geraniin against the streptozotocin (STZ)-triggered DR in rats via ameliorating oxidative stress and inflammation.
2 Materials and methods
2.1 Chemicals
Geraniin, STZ, citrate buffer (CB), and other chemicals were purchased from Sigma Aldrich, USA. The marker-specific assay kits were procured from Thermofisher and R&D Systems, USA.
3 Experimental animals
The 6–8 week old Wistar rats (male breed) weighing approximately 230 ± 30 g were chosen for this study and purchased from the institutional animal house. The experimental protocol has been approved by the institutional ethics committee at Shandong First Medical University, Shandong, China. Rats were caged with the utmost care in organized laboratory conditions, which were conserved with a 22–26 °C temperature, 40–70 % air moisture, and a 12-h dark-light sequence. All rats were administered pure water and standard rat chow during the study period. Before the experiments started, all of the rats were in the lab for a week to get used to it.
3.1 Experimental design
The rats were distributed into four groups (n = 6). Group I was control rats received only 0.1 M of CB instead of STZ. Group II rats were administered with 60 mg/kg of STZ (dissolved in 0.1 M of CB) to provoke the DR and were considered as a DR control. After 48 h of STZ administration, the blood glucose of rats was examined, and the animals with 200 mg/dl or above were deliberated as diabetic. Group III were diabetic rats provided orally with 25 mg/kg of geraniin for 60 days. Group IV were diabetic rats provided with 350 mg/kg of metformin for 60 days (standard drug). Finally, the blood was gathered and utilized for the biochemical assessments.
3.2 Measurement of changes in the blood glucose, glycosylated hemoglobin (HbA1c), food intake, and bodyweight
The average food intake levels of all the experimental animals were measured and tabulated carefully. The body weight of animals was detected using a sensitive weighing balance and values were tabulated. The blood glucose in the treated animals was examined with the help of a glucometer. The HbA1c levels in the blood samples of rats were examined using an assay kit according to the protocols described by the manufacturer (Thermofisher, USA).
3.3 Measurement of enzymatic and non-enzymatic antioxidant levels
The activities of superoxide dismutase (SOD) and SOD/catalase (CAT) in the retina of experimental animals were investigated using marker-specific kits using manufacturer’s guidelines (Thermofisher, USA). The levels of glutathione (GSH) and GSH/oxidized glutathione (GSSG) in the retina were investigated using assay kits by the manufacturer’s guidelines (Thermofisher, USA).
3.4 Determination of VEGF, MCP-1, and MMP-9 levels
The angiogenic protein MCP-1, VEGF and MMP-9 status in the experimental rats were investigated using assay kits as per the described protocols by the manufacturer (R&D Systems, USA). Each assay was done in triplicate and the outcomes were depicted as ng/l.
3.5 Assay of pro-inflammatory cytokine levels
The inflammatory cytokines, i.e., TNF-α, IL-6, IL-1β, IFN-γ, IL-8, IL-12, IL-2, IL-3, and IL-10 contents in the serum of experimental animals were assayed using marker specific kits in accordance with the recommended protocols of the manufacturer (Thermofisher and MyBioSource, USA, respectively). Each assay was done in triplicate and the outcomes were depicted as ng/l.
3.6 Morphometric analysis
The morphometric assessment was conducted on the retinal tissues of experimental rats with the aid of a computer-assisted image analysis technique. The thickness of the retina and the cells in the ganglion cell layer (GCL) was detected using a morphometric study using the Image J software.
3.7 Histopathological study
The pancreatic tissues were removed from the experimental animals and processed with 10 % formalin for 24 h and then dehydrated by the addition of isopropyl alcohol/xylene for 12 h. Afterward, pancreatic tissues were paraffin fixed and cut into 5 mm size. The sections were then stained with H&E and the changes in pancreatic islet cells were assayed using an optical microscope at 40 × magnification.
3.8 Statistical analysis
The outcomes were interpreted statistically using GraphPad Prism software (ver.7.0). Outcomes were illustrated as mean ± SD of triplicates, which were investigated using one-way ANOVA and Tukey’s post hoc assay. Significance was fixed at ‘p’ <0.05.
4 Results
4.1 Geraniin treatment modulates the changes in the STZ-provoked DR rats
Fig. 1 demonstrates the impacts of geraniin on bodyweight, food consumption, HbA1c, and blood glucose in the STZ-provoked DR rats. The improved bodyweight, HbA1c, and blood glucose were evidenced in the STZ-triggered DR rats, which is in contrast to the control. STZ-induced rats also had a lower bodyweight than control. Conversely, the 25 mg/kg of geraniin treatment appreciably suppressed the food consumption, HbA1c, and blood glucose in the STZ-challenged DR rats. The bodyweight of the STZ-challenged rats was also improved by the geraniin treatment. These results were found to be consistent with the results of standard drug metformin treatment.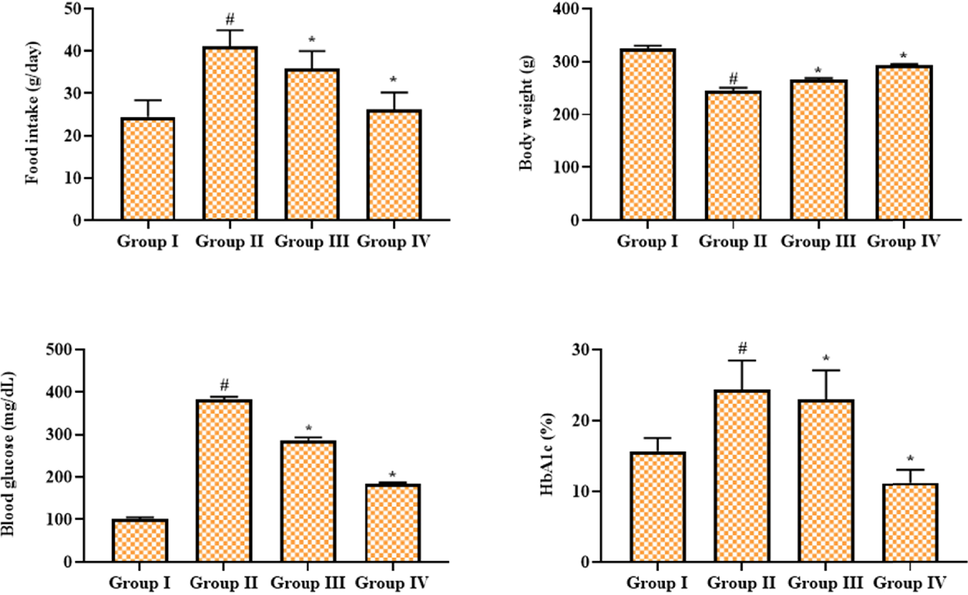
Effect of geraniin on the bodyweight, blood glucose, hemoglobin, and food intake in the STZ-challenged DR rats. Group I: Control; Group II: STZ-induced DR rats; Group III: STZ-induced DR + 25 mg/kg of geraniin treated rats; Group IV: STZ-induced DR + 350 mg/kg of metformin treated rats. Values are represented as the mean ± SD of triplicates assessed statistically by one-way ANOVA and Tukey’s multiple comparison assay. ‘#’ reveals significance at p < 0.05 from control and ‘*’ reveals significance at p < 0.01 from STZ group.
4.2 Geraniin treatment improved the antioxidant levels in the retinal tissues of STZ-challenged DR rats
Fig. 2 shows the effects of geraniin on the antioxidants in the retina of STZ-challenged DR animals. When compared to control, STZ-administered DR rats had lower SOD and SOD/CAT activity as well as lower GSH and GSH/GSSG contents. The 25 mg/kg of geraniin administration remarkably enhanced the SOD and SOD/CAT activities and GSH and GSH/GSSG contents in the STZ-provoked DR rats, which proves the antioxidant action of geraniin. The outcomes of geraniin treatment were slightly similar to those of metformin treatment.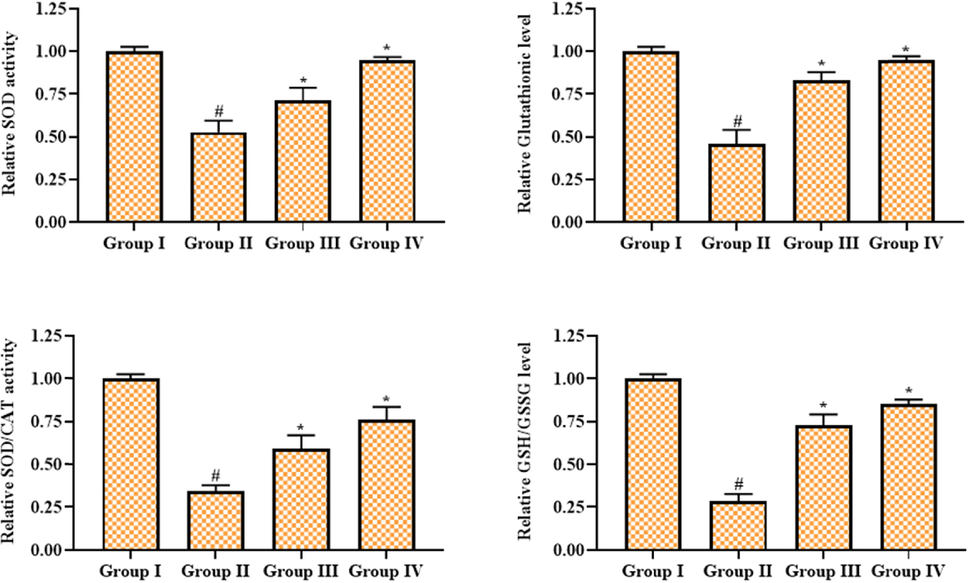
Effect of geraniin on the antioxidant levels in the retinal tissues of STZ-provoked DR rats. Group I: Control; Group II: STZ-induced DR rats; Group III: STZ-induced DR + 25 mg/kg of geraniin treated rats; Group IV: STZ-induced DR + 350 mg/kg of metformin treated rats. Values are represented as the mean ± SD of triplicates assessed statistically by one-way ANOVA and Tukey’s multiple comparison assay. ‘#’ reveals significance at p < 0.05 from control and ‘*’ reveals significance at p < 0.01 from STZ group.
4.3 Geraniin treatment decreased the angiogenic and inflammatory markers in the serum of STZ-challenged DR rats
Fig. 3 exhibits the levels of the angiogenic protein VEGF and the inflammatory proteins MMP-9 and MCP-1 in the experimental rats. The STZ-provoked rats displayed improved levels of VEGF, MCP-I, and MMP-9 in the serum. Nonetheless, these elevations were remarkably decreased by the geraniin treatment. The 25 mg/kg of geraniin appreciably reduced the levels of VEGF, MCP-I, and MMP-9. Metformin also decreased the amount of VEGF, MCP-I, and MMP-9 in the serum of DR rats that had been given STZ.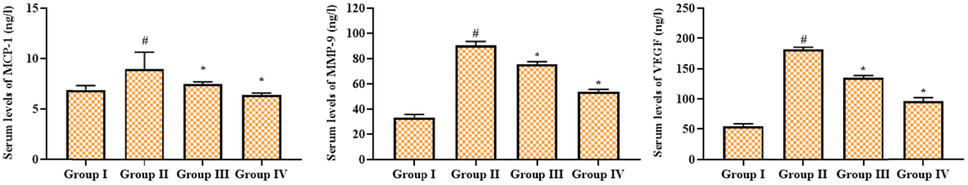
Effect of geraniin on the angiogenic marker levels in the serum of STZ-challenged DR rats. Group I: Control; Group II: STZ-induced DR rats; Group III: STZ-induced DR + 25 mg/kg of geraniin treated rats; Group IV: STZ-induced DR + 350 mg/kg of metformin treated rats. Values are represented as the mean ± SD of triplicates assessed statistically by one-way ANOVA and Tukey’s multiple comparison assay. ‘#’ reveals significance at p < 0.05 from control and ‘*’ reveals significance at p < 0.01 from STZ group.
4.4 Geraniin treatment reduced the inflammatory cytokines level in the serum of STZ-challenged DR rats
Fig. 4 displays the inhibitory actions of geraniin on the inflammatory cytokine content in the serum of STZ-challenged DR animals. The levels of inflammatory cytokines, i.e., TNF-α, IL-6, IL-1β, IFN-γ, IL-8, IL-12, IL-2, IL-3, and IL-10 were found elevated in the serum of DR rats, which is in contrast to the control. Interestingly, the drastic reductions in the contents of TNF-α, IL-6, IL-1β, IFN-γ, IL-8, IL-12, IL-2, IL-3, and IL-10 were witnessed in the 25 mg/kg of geraniin administered to DR animals. Hence, it was clear that the geraniin could decrease the inflammatory cytokine level, which is found to be similar to the outcomes of metformin.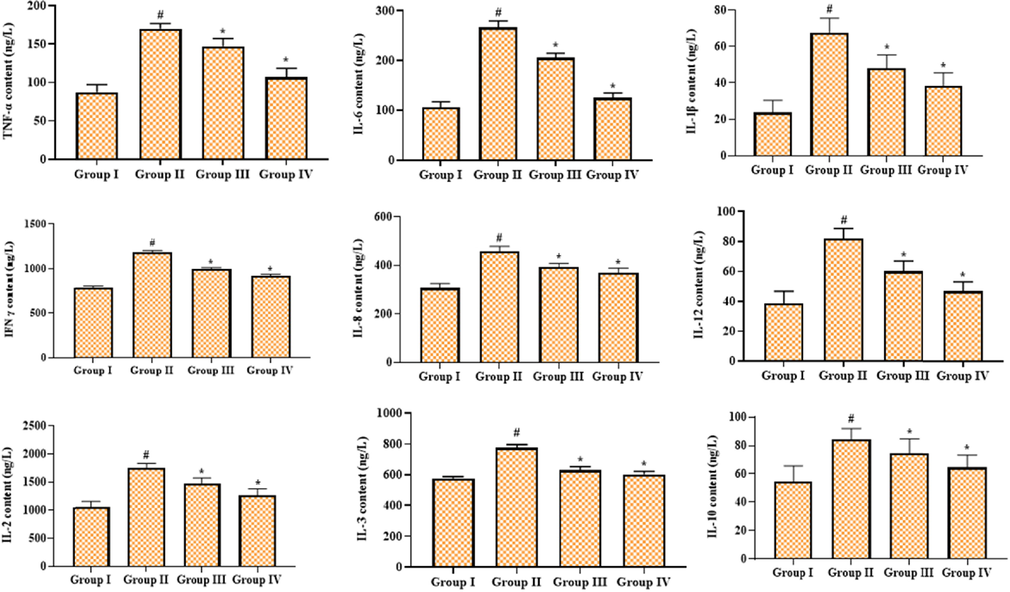
Effect of geraniin on the inflammatory cytokines level in the serum of STZ-challenged DR rats. Group I: Control; Group II: STZ-induced DR rats; Group III: STZ-induced DR + 25 mg/kg of geraniin treated rats; Group IV: STZ-induced DR + 350 mg/kg of metformin treated rats. Values are represented as the mean ± SD of triplicates assessed statistically by one-way ANOVA and Tukey’s multiple comparison assay. ‘#’ reveals significance at p < 0.05 from control and ‘*’ reveals significance at p < 0.01 from STZ group.
4.5 Geraniin enhanced the retinal thickness and cell number in CGL of STZ-challenged DR rats
Fig. 5 demonstrates the findings of morphometric analysis of the retinas of experimental rats. The findings demonstrated that retinal thickness and the number of cells in the CGL of the retina were diminished in the DR rats. Fascinatingly, geraniin effectively modulated these changes in the STZ-challenged animals. The increased retinal thickness and cell numbers in the CGL were witnessed in the retinal tissues of 25 mg/kg of geraniin administered DR rats. The metformin also moderated these alterations in the STZ-challenged DR rats.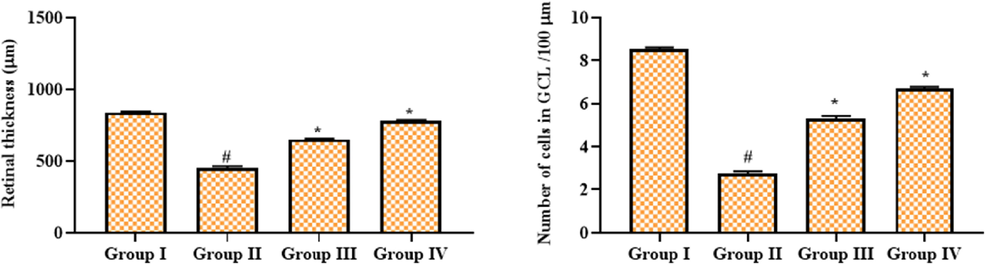
Effect of geraniin on the retinal thickness and cell numbers in CGL of STZ-challenged DR rats. Group I: Control; Group II: STZ-induced DR rats; Group III: STZ-induced DR + 25 mg/kg of geraniin treated rats; Group IV: STZ-induced DR + 350 mg/kg of metformin treated rats. Values are represented as the mean ± SD of triplicates assessed statistically by one-way ANOVA and Tukey’s multiple comparison assay. ‘#’ reveals significance at p < 0.05 from control and ‘*’ reveals significance at p < 0.01 from STZ group.
4.6 Geraniin treatment improved the pancreas histology of the STZ-challenged DR rats
Fig. 6 displays the histological changes of pancreas tissues from control and treated rats. The control rats demonstrated the normal histological appearance without inflammatory signs. Conversely, the inflammatory symptoms and inflammatory cell infiltrations, as well as pancreatic islet contractions were observed in the pancreas of STZ-provoked DR rats. However, these histological alterations were remarkably ameliorated by the geraniin treatment. The 25 mg/kg of geraniin demonstrated reduced pancreatic lesions and improved islet appearances in the STZ-challenged DR rats, which is consistent with the results of metformin treatment.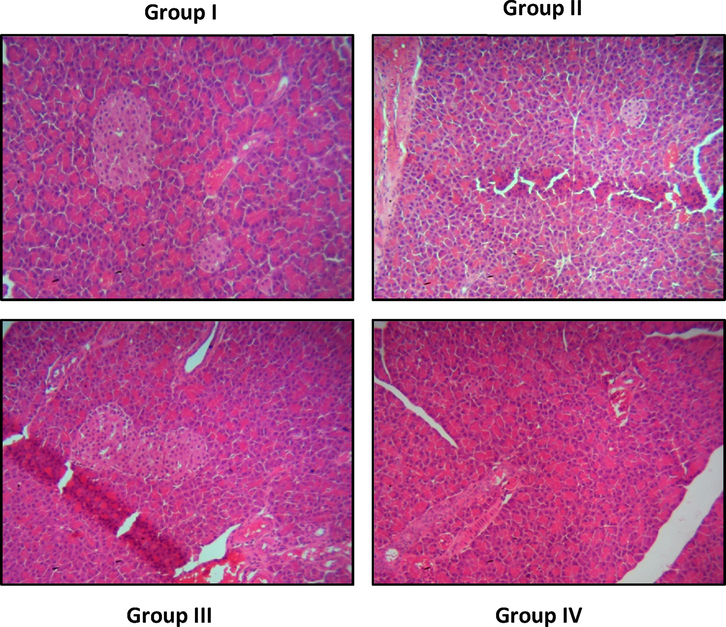
Effect of geraniin on the pancreas histology of the STZ-provoked DR rats. Group I: control animals demonstrated the normal histological appearance. Group II: STZ-challenged DR animals demonstrated the inflammatory signs and pancreatic islets contractions in the pancreas. Group III & IV: The reduced pancreatic lesions and improved islets appearances were noted in the geraniin and metformin treated animals, respectively.
5 Discussion
DR is a major difficulty faced by diabetic patients, which is responsible for their blindness. DR has a complex pathogenesis, but it is widely thought that chronic hyperglycemia could be the initiator of DR (Rui et al., 2017). The changes in the retinal microvascular system are the distinctive feature of DR. The diabetic neurodegeneration of the retina comprises retinal cell apoptosis that further leads to the retinal layer thinning and loss of neuronal activity (Sohn et al., 2016). The pathological features of DR are elevated vascular permeability, neovascularization, and edema. In particular, the main pathological feature is the elevated vascular permeability. This occurrence is intimately related to the expression of inflammatory mediators (Funatsu et al., 2009). The retinal neurovascular system was primarily affected during DR that further provoked inflammation, plasma leakage, neurodegeneration, and BRB integrity loss. DR also develops retinal edema, neovascularization, vision loss, and ultimately blindness (Abcouwer, 2013).
There is strong evidence that oxidative stress and local inflammation are actively involved in the progression of DR and play a critical role in the disorder's development. In vascular inflammation, the participation of several pro-inflammatory factors like TNF-α and IL-1β were identified (Williams et al., 2013). It has already been reported that inflammation is a crucial regulator of DR progression, and DR is primarily recognized as the inflammatory ailment (Semeraro et al., 2015). Increased production of proinflammatory cytokines is a major cause of DR development, and inflammation actively contributes to DR development. Several retinal pathological and biochemical complications are connected with diabetes, which is consistent with inflammation (Joussen et al., 2004). A number of studies have demonstrated that the elevated leukocyte adhesion to the retinal vessels in experimental diabetic models and the status of several proinflammatory regulators were elevated in the retina to activate the proinflammatory response. Such elevated leukostasis and inflammatory mediators may play a role in BRB breakdown, which is an early event in the development of DR (Joussen et al., 2001).
In inflammatory conditions, several regulators like chemokines and cytokines are actively involved. Inflammation is fundamentally involved in the progression of DR. General proinflammatory mediators like TNF-α, IL-6, and IL-1β were primarily augmented in the retinal tissues and serum of diabetic patients as well as animal models. In diabetic animal models of DR, inflammatory cytokines such as IL-2, IL-3, IL-8, and IFN-γ were found to be elevated (Liu et al., 2012; Li et al., 2012). It has already been stated that IL-1 increases retinal capillary cell apoptosis by activating the NF-B cascade. Additionally, IL-1β also linked with angiogenesis and improved vascular permeability, and promotes retinal cell apoptosis, and the mechanism is aggravated in hyperglycemic situations (Santiago et al., 2020). During DR development, IL-1β is activated and further improves the vascular permeability due to leukocyte adhesion and retinal capillary cell apoptosis. TNF-α is an important inflammatory regulator that causes cell differentiation, apoptosis, and inflammation (Wu et al., 2017). TNF-α is required for the breakdown of BRB in DR, and it may improve BRB breakdown and leukostasis (Huang et al., 2011). TNF-α inhibition has been shown in preliminary studies to suppress inflammatory responses. Similarly, the level of IL-1β was also augmented during DR (Mukhopadhyay et al., 2006). Here, our findings showed that geraniin remarkably suppressed the inflammatory cytokines in the serum of DR rats.
Oxidative stress due to the disproportion of free radical development and antioxidant mechanisms is tightly connected with the damage of several biological macromolecules like proteins, carbohydrates, nucleic acids, and lipids and participates in the stimulation of numerous ailments like diabetes, eye diseases, neurodegenerative ailments, glaucoma, and DR (Sies et al., 2017; Masuda et al., 2017). Our outcomes confirmed that the geraniin remarkably enhanced the antioxidants in the DR rats.
Uncontrolled oxidative stress enhances the VEGF expression that eventually translocate to the nucleus to enhance the proinflammatory mediators' expression like ICAM-1 and MCP-1(Behl and Kotwani, 2015). During diabetic conditions, ICAM-1 improves the leukocyte chemoattraction in the vascular membranes of the retina. TNF-α regulates ICAM-1 expression and improves leukostasis and BRB injury in DR (Zhang et al., 2011). ICAM-1 is a vital player that mediates the leukocytes' adhesion to the endothelial cells, and augmented ICAM-1 was mentioned to be connected with DR initiation and development. The well-known pro-inflammatory mediators IL-1β and TNF-α have been linked to the development of DR (Ugurlu et al., 2013; Capitao and Soares, 2016). The adhesion of leukocytes is the major cause of retinal endothelial cell damage and apoptosis. It directs to capillary occlusion, local ischemia, and vessel non-perfusion. Consequently, it elevates the VEGF and ICAM-1 and aggravates vascular leakage, finally resulting in the DR. ICAM-1 actively participates in the migration of inflammatory cells and controls the expression of inflammatory mediators. Hence, the alteration in the ICAM-1 level is closely connected with inflammation (Miyamoto et al., 1999).
VEGF is one of the crucial proteins that improves angiogenesis. It has already been discovered that an increased level of VEGF in the retina is closely linked to the development of DR (Rezzola et al., 2019). In recent decades, several clinical reports have identified that the expressions of inflammatory factors are found augmented in the retina of DR rats. Overexpression of inflammatory markers such as VEGF, TNF-α and IL-1β may disrupt BRB homeostasis and lead to hypoxia and ischemia (Wang et al., 2018). It was identified that the vitreous VEGF expressions were augmented in the DR patients (Abu El-Asrar et al., 2012). Our results have demonstrated that the geraniin treatment efficiently decreased the VEGF, MCP-1, and MMP-2 status in the serum of STZ-challenged DR rats.
6 Conclusion
Our results showed that geraniin inhibited retinal inflammation and oxidative stress in the STZ-triggered DR rats. The findings also proved that geraniin could alleviate the retinal inflammation, lower retinal MCP-1, ICAM-1, and VEGF levels, and ameliorate retinal oxidative stress, in that way, lessening the DR development. As a result, geraniin could be considered as a therapeutic agent to treat DR patients, followed by additional research in the future to elucidate the systematic role of geraniin.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Protective effects of geraniin against carbon tetrachloride induced acute hepatotoxicity in Swiss albino mice. Biochem. Biophys. Res. Commun.. 2017;487:62-67.
- [Google Scholar]
- Angiogenic factors and cytokines in diabetic retinopathy. J. Clin. Cellular Immunol. Suppl.. 2013;1(11):1-12.
- [Google Scholar]
- Risk factors associated with diabetic retinopathy among type 2 diabetes patients at teaching hospital in Malaysia. Diabetes Metab. Syndr. Clin. Res. Rev.. 2015;9:98-103.
- [Google Scholar]
- High-mobility group box-1 and endothelial cell angiogenic markers in the vitreous from patients with proliferative diabetic retinopathy. Mediators Inflamm.. 2012;2012:697489
- [Google Scholar]
- Microvascular complications and diabetic retinopathy: recent advances and future implications. Future Medicinal Chem.. 2013;5(3):301-314.
- [Google Scholar]
- Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol. Res.. 2015;99:137-148.
- [Google Scholar]
- Calcium dobesilate prevents diabetic kidney disease by decreasing bim and inhibiting apoptosis of renal proximal tubular epithelial cells. DNA Cell Biol.. 2017;36:249-255.
- [Google Scholar]
- Effects of L-carnitine on high glucose-induced oxidative stress in retinal ganglion cells. Pharmacology. 2014;94(3–4):123-130.
- [Google Scholar]
- Angiogenesis and inflammation crosstalk in diabetic retinopathy. J. Cell Biochem.. 2016;117(11):2443-2453.
- [Google Scholar]
- Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;1:73-79.
- [Google Scholar]
- Geraniin selectively promotes cytostasis and apoptosis in human colorectal cancer cells by including catastrophic chromosomal instability. Mutagenesis. 2018;33:271-281.
- [Google Scholar]
- Endoplasmic reticulum stress-related factors protect against diabetic retinopathy. Exp. Diabetes Res.. 2012;2012:507986
- [Google Scholar]
- Choroidal microvascular proliferation secondary to diabetes mellitus. Oncotarget. 2017;2:2034-2036.
- [Google Scholar]
- TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Investig. Ophthalmol. Vis. Sci.. 2011;3:1336-1344.
- [Google Scholar]
- Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am. J. Pathol.. 2001;158(1):147-152.
- [Google Scholar]
- A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J.. 2004;18(12):1450-1452.
- [Google Scholar]
- Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp. Diabetes Res.. 2007;2007:95103.
- [Google Scholar]
- Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes. 2010;59(10):2637-2645.
- [Google Scholar]
- Angiogenesis-related cytokines in serum of proliferative diabetic retinopathy patients before and after vitrectomy. Int. J. Ophthalmol.. 2012;5(6):726-730.
- [Google Scholar]
- Antioxidant, anti-semicarbazide-sensitive amine oxidase, and anti-hypertensive activities of geraniin isolated from Phyllanthus urinaria. Food Chem. Toxicol.. 2008;46(7):2485-2492.
- [Google Scholar]
- Anti-atherosclerotic effects of geraniin through the gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway in mice. Phytomedicine. 2022;101:154104
- [Google Scholar]
- Pigment epithelium-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2Akita mice. Mol. Med.. 2012;18(1):1387-1401.
- [Google Scholar]
- Retinal diseases associated with oxidative stress and the effects of a free radical scavenger (Edaravone) Oxid. Med. Cell. Longev.. 2017;2017:1-14.
- [Google Scholar]
- Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl. Acad. Sci. U. S. A.. 1999;19:10836-10841.
- [Google Scholar]
- New TNF-alpha releasing inhibitors, geraniin and corilagin, in leaves of Acer nikoense. Megusurino-ki. Biol. Pharm. Bull.. 2001;24:1145-1148.
- [Google Scholar]
- Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chem.. 2011;127(1):21-27.
- [Google Scholar]
- The insulin-sensitising properties of the ellagitannin geraniin and its metabolites from Nephelium lappaceum rind in 3T3-L1 cells. Int. J. Food Sci. Nutr.. 2020;71(8):940-953.
- [Google Scholar]
- Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med.. 2011;50:567-575.
- [Google Scholar]
- Vascular endothelial growth factor in the vitreous of prolferative diabetic retinopathy patients: chasing a hiding prey? Diabetes Care. 2019;42:e105-e106.
- [Google Scholar]
- Keep an eye on adenosine: Its role in retinal inflammation. Pharmacol. Ther.. 2020;210:107513
- [Google Scholar]
- Using a deep learning algorithm and integrated gradients explanation to assist grading for diabetic retinopathy. Ophthalmology. 2019;126:552-564.
- [Google Scholar]
- Diabetic retinopathy: vascular and inflammatory disease. J. Diabetes Res.. 2015;2015:582060
- [Google Scholar]
- Oxidative stress: annual review of biochemistry. Annu. Rev. Biochem.. 2017;86:715-748.
- [Google Scholar]
- Resveratrol prevents embryonic stress and apoptosis associated with diabetic embryopathy and improves glucose and lipid profile of diabetic dam. Mol. Nutr. Food Res.. 2011;55:1186-1196.
- [Google Scholar]
- Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA. 2016;113:E2655-E2664.
- [Google Scholar]
- The levels of the circulating cellular adhesion molecules ICAM-1, VCAM-1 and endothelin-1 and the flow-mediated vasodilation values in patients with type 1 diabetes mellitus with early-stage diabetic retinopathy. Intern Med.. 2013;52(19):2173-2178.
- [Google Scholar]
- The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun.. 2017;482(3):426-431.
- [Google Scholar]
- Geraniin suppresses ovarian cancer growth through inhibition of NF-jB activation and downregulation of Mcl-1 expression. J. Biochem. Mol. Toxicol.. 2017;31:e21929.
- [Google Scholar]
- Inhibitory effects of geraniin on LPS-induced inflammation via regulating NF-jB and Nrf2 pathways in RAW 264.7 cells. Chem. Biol. Interact. 2016;253:134-142.
- [Google Scholar]
- Catechin weakens diabetic retinopathy by inhibiting the expression of NF-κB signaling pathway-mediated inflammatory factors. Ann. Clin. Lab. Sci.. 2018;5:594-600.
- [Google Scholar]
- Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351-1359.
- [Google Scholar]
- Association between Aqueous cytokines and diabetic retinopathy stage. J. Ophthalmol.. 2017;2017:9402198.
- [Google Scholar]
- Advanced glycation end products (AGEs), oxidative stress and diabetic retinopathy. Curr. Pharm. Biotechnol.. 2011;12:362-368.
- [Google Scholar]
- Inflammation and diabetic retinal microvascular complications. J. Cardiovasc. Dis. Res.. 2011;2(2):96-103.
- [Google Scholar]
- Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol.. 2018;14:88.
- [Google Scholar]
- Epigenetic changes in mitochondrial superoxide dismutase in the retina and the development of diabetic retinopathy. Diabetes. 2011;60(4):1304-1313.
- [Google Scholar]







