Translate this page into:
Green and simple approach for flotation, preconcentration and enhanced spectrophotometric assessment of Ni(II) in aqueous solution by complexation with 1-(3,5-dihydroxybenzylidene)thiosemicarbazone
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the current work, 1-(3,5-dihydroxybenzylidene)thiosemicarbazone (H3L) was produced under mechanochemical ball milling conditions using para-toluenesulfonic acid (p-TSA) as a catalyst in a solvent-free reaction. The generated material was characterized using a number of physical and spectroscopic methods. The synthetic material was also tested as an effective organic chelating agent in the flotation, preconcentration, and spectrophotometric measurement of Ni(II) in aqueous solutions, where H3L reacted with Ni(II) to produce a coffee colored complex. Oleic acid (HOL) was used as a foaming agent to help the complex that was generated float to the scum layer. The concentration of Ni(II) was assessed using a spectrophotometer at 370 nm. The several factors that impact the flotation-separation process were evaluated and adjusted, including pH, the concentration of (Ni(II), H3L, and HOL), surfactant type, sample volume, temperature, shaking duration, and ionic strength. Nearly 100% of Ni(II)-H3L were successfully separated at 25 °C and pH 5 after 5 min of shaking. Utilizing the molar ratio approach, the stoichiometric ratio of the produced complex was determined to be (1:2) of M:L. The formation constant (Kf) was determined as 2.25 × 104. The analytical characteristics of the procedure (limit of detection, limit of quantification, and range of linearity) were evaluated and found to be (0.22 ng/mL, 0.74 ng/mL, and 5–400 ng/mL), respectively. The suggested method was employed to separate Ni(II) that had been spiked into some real water samples. Ni(II) was successfully preconcentrated from different aqueous volumes with a preconcentration factor of 200. Finally, the mechanism for the flotation-separation process was suggested.
Keywords
Ball milling
Flotation
Nickel
Preconcentration
Spectrophotometry
1 Introduction
Metal-containing effluent is released into the environment either directly or indirectly as a consequence of heavy industries like mining, mineral processing, metal smelting, and casting processing. The majority of metals are not biodegradable, in contrast to organic pollutants, and can build up in the body due to their resilience and endurance. Numerous metal ions have been shown to be poisonous or even cancer-causing (Fu and Wang 2011; Genç-Fuhrman et al. 2016; Karnib et al. 2014). Copper, lead, zinc, nickel, mercury, cadmium, and chromium are toxic or hazardous elements that should be of particular worry when it comes to industrial wastewaters (Carolin et al. 2017; Imyim et al. 2016). Nickel, a dangerous and poisonous metal, is one of these metals and may be found in polluted natural water. Nickel is currently utilized in the production of nickel steel, non-ferrous metals, super-alloys, alnico magnets, coins, microphone capsules, rechargeable batteries, plating on pipe fixtures, etc. When ingested through vapors, nickel can cause lung cancer, bronchitis, asthma, and other respiratory system diseases. Nickel is a hazardous metal that may cause skin dermatitis when subjected to it (Cempel and Nikel 2006; Das et al. 2008; Trombetta et al. 2005). The highest concentration of nickel allowed in water by the World Health Organization (WHO) is 70 g/L (Matsumoto et al. 2005; Organization 2008). It has been demonstrated that even low amounts of nickel discharge cause irreversible damage to aquatic ecosystems; therefore, it is critical to reduce their negative effects prior to release into water sources. Furthermore, from an environmental standpoint, it is critical for the public health to identify the existence of nickel at trace amounts in water and other environmental supplies. Inductively coupled plasma optical emission spectrometry (ICP-OES) (Silva et al. 2009), graphite furnace atomic absorption spectrometry (GFAAS) (Bidabadi et al. 2009; Jiang et al. 2008; Matsumiya et al. 2004), flame atomic absorption spectrometry (FAAS) (Ghaedi et al. 2010; Karimi et al. 2008; Lemos et al. 2008; Manzoori and Bavili-Tabrizi 2003; Xie et al. 2008), electrothermal atomic absorption spectrometry (ETAAS) (Gil et al. 2008; Zendelovska et al. 2001), inductively coupled plasma mass spectrometry (ICP-MS) (Hu et al. 2006), and other atomic and molecular methods have been used for the determination of the trace level of metals (Ojeda and Rojas 2009). However, the majority of these processes are time-consuming and costly, and they all need specialized equipment, which limits their applicability. The spectrophotometric method is the most widely used among them due to its superior precision and sensitivity as well as its ease of use, affordability, and operation. Due to increased knowledge of ambient nickel pollution, the measurement of nickel(II) in water samples requires a distinct, more targeted, accurate, and efficient analytical method. Trace metals in water samples can be difficult to detect in a variety of circumstances. This is as a result of the low amounts of these metals and their extremely complex material matrices (Rezende et al. 2011). Therefore, separation and preconcentration methods are required to overcome the matrix effects and concomitants. For the separation and preconcentration of metals from real or simulated wastewater, numerous methods have been explored (Ahmad et al. 2009; Al-Qodah and Al-Shannag 2017; Fu and Wang 2011; Han et al. 2006; Hoseinian et al. 2015; Rubio et al. 2002). The literature clarified that one of the most popular methods for removing heavy metals from effluent is chemical deposition (El-Hendawy 2009; Esalah et al. 2000; Gutiérrez-Segura et al. 2012; Luo et al. 1992; Mansour et al. 2011). This method has a number of drawbacks, including slow solid–liquid separation, low particles density, poor settling, and issues with disposing of sediment, which frequently contains a lot of water (Kurniawan et al. 2006). Precipitate flotation, a technique that combines flotation and precipitation, is suggested due to difficulties in removing the metal precipitate from the suspension and to simplify the separation process. Due to its simplicity, speed, high separation yields (R > 95%) for low concentrations (10-6-10-2 mol/L), suitability for compounds with different physicochemical properties, flexibility of the equipment, and adaptability for recovery purposes, flotation technique is regarded as a good separation-preconcentration method (Ghazy et al. 2003; Shakir et al. 2007; Stoica et al. 1998). Precipitate flotation techniques come in two different kinds (Techniques and Lemlich 1972). Precipitate flotation of the first type (PFFT), which is the first kind, involves initially precipitating the desired ionic species by adding a non-surface active agent, followed by their elimination by adding a surface-active agent and gas bubbles. Precipitate flotation of the second type (PFST), which is the second kind, allows for the floatation of hydrophobic precipitates produced by the precipitation of ionic species without the use of a skimmer by merely introducing gas bubbles to the suspension (Grieves 1975). Based on all of the above, the current study aimed to the flotation, preconcentration and enhanced spectrophotometric assessment of Ni(II) in aqueous solution by complexation with a newely synthesized chelating agent. Due to their importance as sensitive chromogenic reagents and interesting complex-forming agents, many organic compounds have attracted a lot of attention lately. Thiosemicarbazone derivatives are regarded as significant thio-ligands with a high capacity to coordinate with a variety of metal ions and serve as an outstanding example of these organic compounds (Matesanz et al. 2018). The thiosemicarbazone motif converts into a potent electron-pair donor and may readily coordinate with a variety of metal ions when coupled to phenolic compounds, in addition to serving as tridentate chelators (S-N-N donor system) (Udhayakumari et al. 2013; Udhayakumari et al. 2014). Thiosemicarbazone may interact with metal ions with ease because of its elastic, planar, and aliphatic structure, which lowers steric hindrance (Meng et al. 2017). In addition to all of the above, the floatability of Ni(II) from aqueous solution by flotation-preconcentration technique using 1-(3,5-dihydroxybenzylidene)thiosemicarbazone as a complexing agent and oleic acid as a surfactant has not yet been reported in the literature, despite the fact that a vast array of reagents are available for the flotation-separation of Ni(II). As a result, the current study introduced the ball milling technique as a green mechanochemical methodology to synthesize 1-(3,5-dihydroxybenzylidene)thiosemicarbazone for the first time in a solid–solid manner. Ball milling technology was used to achieve a number of green chemistry principles, including a high yield, high purity, fast reaction time, cost-effectiveness, and solvent-free reaction (Fekri and Zaky 2014; Fekri and Zaky 2016; Khaligh et al. 2017; Sharghi et al. 2018). The prepared compound was used as a novel chelating reagent for the separation, preconcentration, and spectrophotometric evaluation of Ni(II) in aqueous solution using the PFFT procedure under the suggested conditions. It has a variety of donor atoms (rich in binding sites) that allow it to selectively bind to trace levels of Ni(II). The variables that influence the efficacy of flotation, such as solution pH, concentrations of (metal ion, chelating agent, and surfactant), flotation duration, sample volume, surfactant nature, and ionic strength, were examined. The developed technique for identifying Ni(II) ions in real water samples was also effectively used and demonstrated good precision, accuracy, and sensitivity. The chemical structure of 1-(3,5-dihydroxybenzylidene)thiosemicarbazone is shown in Fig. 1.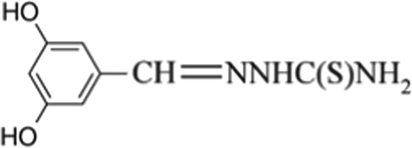
Chemical structure of 1-(3,5-dihydroxybenzylidene)thiosemicarbazone (H3L).
2 Experimental
2.1 Apparatus
The ball mill (MIXER MILL MM 200, Retsch GmbH, Haan, Germany) with a vibrational frequency of 25 Hz (1500 min−1), was used for the synthesis of the chelating agent. In the current investigation, two different types of flotation cells were used. Type 1 was a 2.9 cm long tube with an interior diameter of 1.2 cm. An investigation of the factors affecting the flotation-separation process used such a cell. Type 2 was a cylindrical tube with a quick-fit stopper at the top with an internal diameter of 6.0 cm and a length of 4.5 cm. Such a cell was used to float-separate Ni(II) from rather large quantities during the flotation-separation process. In order to determine the amount of Ni(II), absorbance measurements were carried out and recorded using a double beam UV–VIS spectrophotometer (UV–VIS DOUBLE BEAM PC UVD-3200, Labomed, Inc., Los Angeles, California, U.S.A) for the produced complex at λmax = 370 nm. An elemental analyzer (Vario MACRO cube, Elementar, Langenselbold, Hesse, Germany) was used to do the elemental analysis. Using the KBr disc approach, FTIR spectra in the region of (400–4000 cm−1) were captured using FTIR spectrophotometer (Mattson 5000, Mattson Instruments, Inc., Madison, Wisconsin, U.S.A). Thermal gravimetric analysis (TGA) was achieved by a Thermo-analyzer (TG 209 F1 Libra, NETZSCH, NEDGEX GmbH, SELB, Germany), with a heating temperature range of (20–800 °C), a flow rate of 15 mL/min of N2 atmosphere, and rate of heating 10 °C/min. The conductivity measurements were made using a conductivity meter (Rex DDS-307A, INESA Scientific Instrument Co., Ltd., Shangai, China) at 25 °C. The melting points of the synthesized ligand and the produced complex have been determined using a digital melting point apparatus (Electrothermal IA 9100, Calibre Scientific, Reagecon Diagnostics Ltd., London, United Kingdom). The 1H NMR spectra of the synthesized ligand was done in d6-DMSO using a 400 MHz NMR-spectrometer (Avance DRX, Bruker, Billerica, Massachusetts, United States). Using a digital pH meter equipped with a glass electrode (Hanna 8519, Hanna Instruments, Inc., Leighton Buzzard, Bedfordshire, United Kingdom), the pH values of all working solutions were calculated. Using MST Magnetic Stirrer (VELP Scientifica Srl, Usmate Velate (MB), Italy), all working solutions were stirred. Shaking was done using a flask shaker (BALANCE LOAD, 5 speeds, Gallenkamp & Co. Ltd., London, England). The heating was done using a Thermolyne Type 1900 Hot Plate, Model HPA1915B (Thermolyne Corporation, Dubuque, Iowa, United States). Weighing was done with an analytical balance (Mettler AJ150, Mettler Toledo, Greifensee, Switzerland).
2.2 Materials and solutions
2.2.1 Materials
All the chemicals and reagents used in the current investigation were of analytical grade and supplied from Sigma-Aldrich (Darmstadt, Germany). They were not further purified before use. Deionized water of high purity (Milli-Q) was used for all experimental preparations. Chemicals used involved nickel acetate tetrahydrate Ni(OCOCH3)2·4H2O, 3,5-dihydroxybenzaldehyde, thiosemicarbazide, para-toluenesulfonic acid, oleic acid, Tween 60 (TW 60), cetyltrimethylammonium bromide (CTAB), sodium stearate (SS), sodium lauryl sulfate (SLS), HCl and NaOH.
2.2.2 Preparation of solutions
2.2.2.1 Stock solution of nickel(II)
0.2488 g of Ni(OCOCH3)2·4H2O were dissolved in 1000 mL of deionized water to create a stock solution with a concentration of 1 × 10-3 mol/L.
2.2.2.2 Stock solution of oleic acid
By mixing 20 mL of HOL with 1 L of kerosene, a solution containing 6.36 × 10-2 mol/L of HOL was created.
2.2.2.3 Other ions stock solution
By dissolving the appropriate quantity of salt in deionized water, a stock solution containing 1000 mg/L of each interfering ion was created.
2.2.2.4 Synthesis of the chelating agent using ball milling technique
By combining equal amounts of 3,5-dihydroxybenzaldehyde (1382 mg, 10 mmol) with thiosemicarbazide (920 mg, 10 mmol), the chelating agent 1-(3,5-dihydroxybenzylidene)thiosemicarbazone was created. The mixture was then subjected to a 10-min ball milling process utilizing five 10 mm balls with a 1500 min−1 rotational speed in the presence of 10% para-toluenesulfonic acid as a catalyst. When the mixture cooled, a light red precipitate developed, which was then removed, washed with absolute ethanol, recrystallized from absolute ethanol, and dried (see Scheme 1). The produced chelating agent is denoted by the notation (H3L).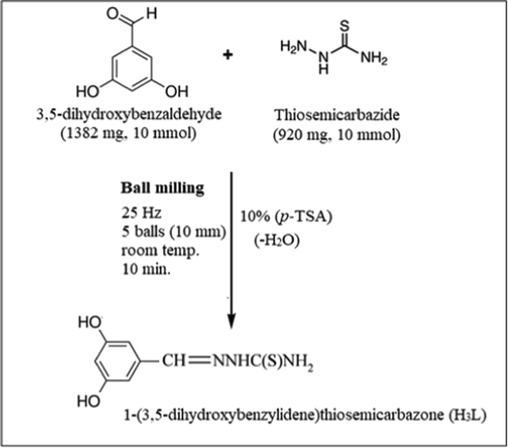
Synthesis of 1-(3,5-dihydroxybenzylidene)thiosemicarbazone.
2.3 Analytical procedure
2.3.1 Flotation-separation protocol
A suitable known amount of Ni(II) solution (5 × 10-5 mol/L) was mixed with 2 mL of H3L solution (1 × 10-4 mol/L) and all were added in an Erlenmeyer flask. The pH of the solution was adjusted by adding drops of 0.1 mol/L of (HCl or NaOH). The mixture was shaken well for few seconds to allow the complexation between H3L and Ni(II) ions. A coffee color complex developed instantaneously. All contents were quantitatively transferred into flotation cell of type (1) and total volume was adjusted to 10 mL with deionized water. Then, 3 mL of HOL were added, with a concentration that is still below the critical micelle concentration (CMC). The cell was then shaken upside down by hand. Vigorous shaking of the flotation cell in the presence of the surfactant creates bubbles in the solution which enhance the floatability of Ni(II)-H3L complex. At equilibrium, a foamy layer was obtained and the aqueous solution in the cell became completely cleared of the colored complex. The aqueous phase was run off through the bottom of the cell. The scum layer, in which Ni(II) was concentrated, was taken into a small vial to determine Ni(II) concentration spectrophotometrically.
2.3.2 Spectrophotometric determination protocol
After 5 min, to ascertain that flotation is completed, a suitable volume of the scum layer was transferred to a 1-cm quartz cell and the absorbance was measured at λmax = 370 nm against a reagent blank to determine the concentration of Ni(II).
The floatability % (F %) of Ni(II) was calculated as follows:
3 Results and discussion
3.1 Characterization of the prepared chelating agent and its Ni-complex
Table 1 provides the analytical data and physical properties of the prepared chelating agent and its Ni(II)-complex. The chelating agent, 1-(3,5-dihydroxybenzylidene)thiosemicarbazone, has been prepared under mechanochemical ball milling conditions in a solvent-free reaction of 3,5-dihydroxybenzaldehyde with thiosemicarbazide. Yield: 88.2%; color: light red; m.p.: >285 °C. The suggested formula of H3L was correlated by its elemental analysis results. Analytical calculations for C8H9N3O2S (F.wt. = 211): C, 45.49; H, 4.26; N, 19.90; S, 15.16. Found: C, 44.98; H, 3.96; N, 19.70; S, 14.91. The suggested formula of Ni(II)-complex was [Ni(H2L)2].2H2O: Yield: 86.5 %; color: coffee; m.p.: >285 °C. The suggested formula was correlated by its elemental analysis results. Analytical calculations for NiC16H20N6O6S2 (F.wt. = 514.69): C, 37.30; H, 3.88; N, 16.32; S, 12.43; Ni,11.40. Found: C, 36.99; H, 3.49; N, 16.01; S, 12.10; Ni, 11.03. The molar conductivity is acceptable for both the prepared chelating agent and its Ni(II)-complex as non-electrolytic activity and falling between 5 and 11 S.cm2/mol (Geary 1971). F.wt: formula weight, m.p: melting point.
Compound
Λ
(S.cm2/mol)
Color
m.p*
(oC)
Calculated (Found) %
Yield (%)
Molecular formula
F.wt*
C
H
N
S
Ni
H3L
211
10.08
light red
>285
45.49
(44.98)4.26
(3.96)19.90
(19.70)15.16
(14.91)---
88.2
C8H9N3O2S
[Ni(H2L)2].2H2O
514.69
6.06
coffee
>285
37.30
(36.99)3.88
(3.49)16.32
(16.01)12.43
(12.10)11.40
(11.03)86.5
NiC16H20N6O6S2
3.1.1 FTIR spectra
There are a number of significant distinctive absorption frequencies shared by the produced chelating agent and its Ni(II)-complex as shown in Fig. 2. Both compounds have a broad peak in the range of 3350–3490 cm−1, revealing the possibility of a dimeric form of the uncoordinated chelating agent, i.e., two molecules of the thiosemicarbazide moiety's OH groups are connected by intermolecular hydrogen bonds. Three bands, weak ν(OH—O), medium ν(—OH) and ν(C—OH) were observed at 1700, 1350 and 1260 cm−1, respectively; suggesting that the phenolic hydroxyls are not coordinated to Ni(II) (Parikh and Shah 1985; Verma et al. 1983). The peak shift form 1622 cm−1 to 1641 cm−1 for ν(CH⚌N) of the Ni(II)-complex indicating coordination of the imino nitrogen to Ni(II). A characteristic peak at 846 cm−1 was observed in the FTIR spectrum of chelating agent, which was absent in Ni(II)-complex. However, a new band at 621 cm−1 was noticed, which was predicted for the produced Ni(II)-complex, due to ν(C—S). Thus, it is suggested that C⚌S in the free chelating agent is converted into C-S in the formed Ni(II)-complex. Metal-nitrogen and metal-sulfur bonds were verified by the appearance of new bands in the region 350–450 cm−1.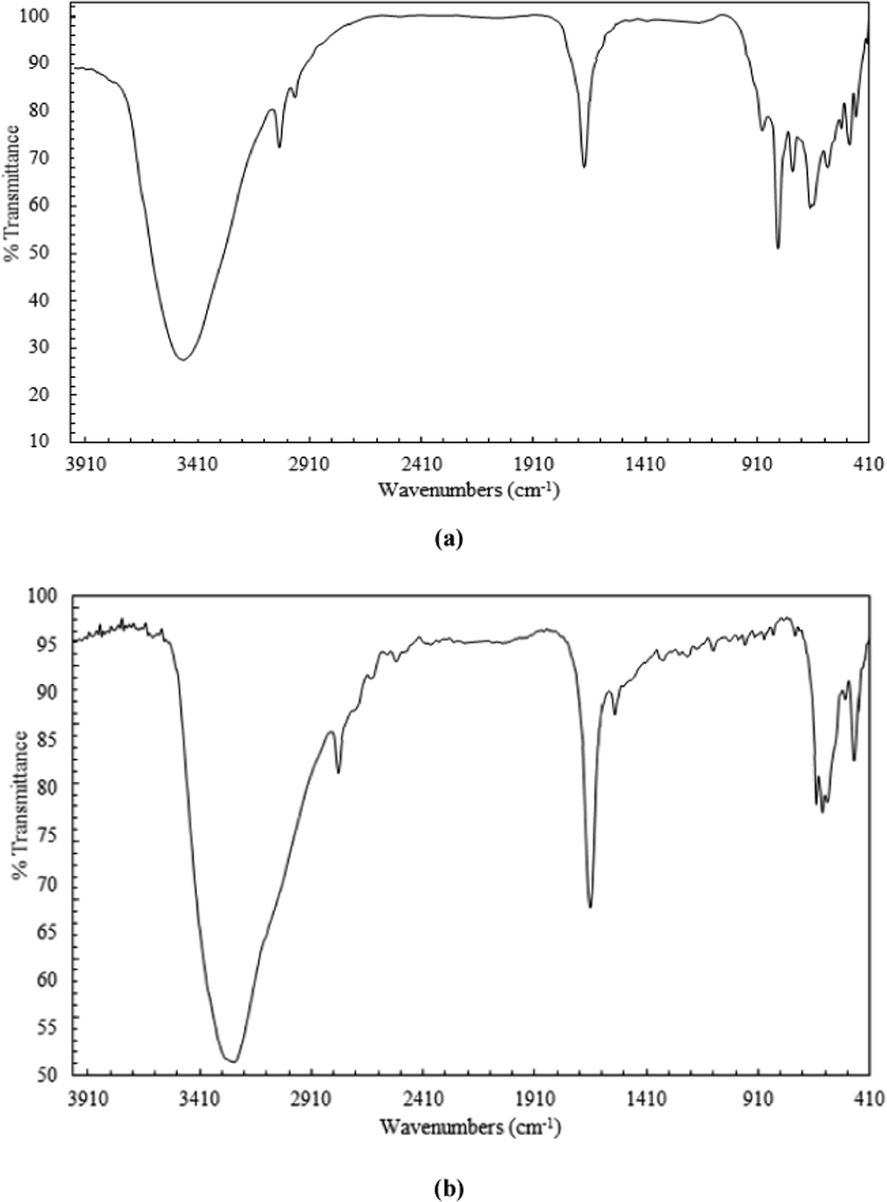
FTIR spectra of: (a) H3L and (b) Ni(II)‐H3L.
3.1.2 UV/VIS spectra
Fig. 3 shows the absorption spectrum curve for the chelating agent H3L and its complex Ni(II)-H3L. It was observed that Ni(II)-H3L has a maximum absorption at λmax = 370 nm which higher than that of H3L which has a maximum absorption at λmax = 300 nm. Accordingly, subsequent analysis of the Ni(II)-H3L complex was carried out at 370 nm. Also, compared to the free chelating agent, the π-π* transition of imino group for the Ni(II)-H3L complex was slightly altered, most likely as a result of imino-nitrogen coordination to Ni(II) by the chelating agent.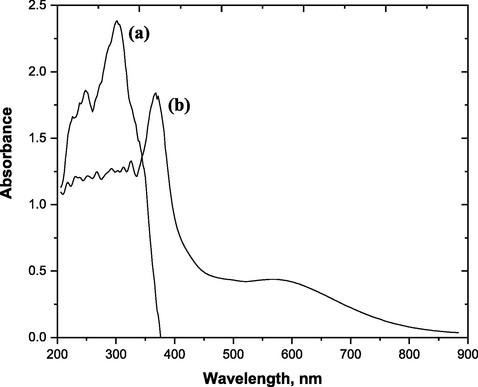
Absorption spectra curve of: (a) H3L and (b) Ni(II)‐H3L (5 × 10-5 mol/L of Ni(II) and 1 × 10-4 mol/L of H3L).
3.1.3 1H NMR spectra
The 1H NMR spectrum of H3L is given in Fig. 4. The signal at δ = 9.587 ppm is due to (2H of 2OH), 8.213 ppm is due to (1H of azomethine CH=N), 7.795 ppm is due to (1H of NH), 7.258 ppm is due to (2H of terminal NH2) and 6.713 ppm is due to (3H of ring). The peaks can be well assigned and further confirm the composition of the free ligand.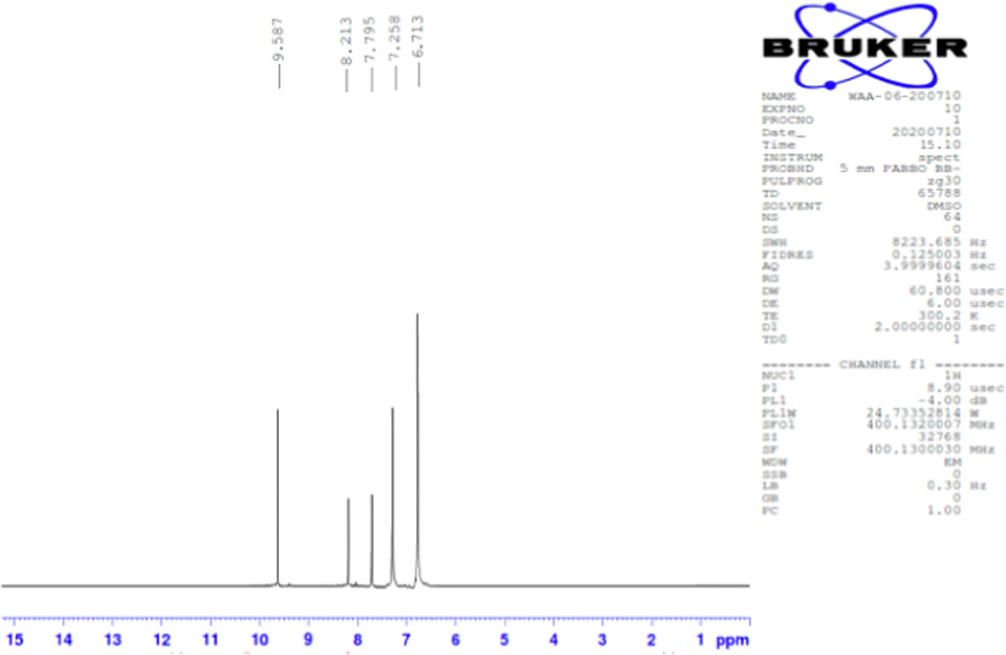
1H NMR spectra of H3L.
3.1.4 TG/DTG analysis
The produced Ni(II)-complex was subjected to the thermogravimetric (TG) and the differential thermogravimetry (DTG) analysis under continuous nitrogen flow in the temperature range of 30–800 °C to insight about its thermal stability and a general scheme for its thermal decomposition. Thermal data showed that the crystallized water molecules are volatilized within the temperature range 30–120 °C. This evidence supports the conclusions of elemental analysis and FTIR spectra indicating the produced Ni(II)-complex included crystallized water. The TG analysis for the Ni(II)-complex is represented in Fig. 5. It is clear that, the TG thermogram for the investigated complex displayed high residual part indicating high stability of the formed chelate.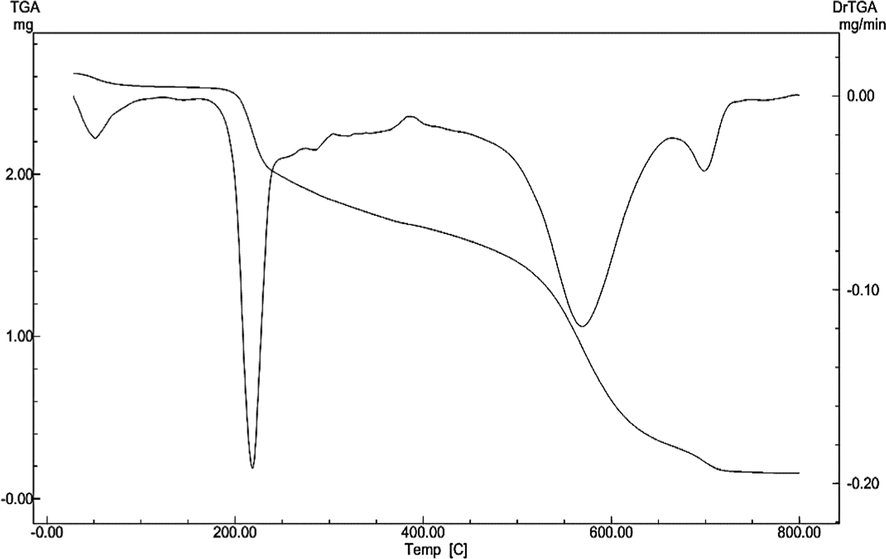
TG/DTG curve of Ni(II)‐H3L.
3.2 Optimization of experimental variables
3.2.1 Effect of pH
In order to determine the effect of pH (1–8) on the floatability percentage of 5 × 10-5 mol/L of Ni(II) using 1 × 10-3 mol/L of HOL in the absence and presence of 1 × 10-4 mol/L of H3L, a series of tests were conducted (see Fig. 6). As indicated by curve (a), the greatest floatability was ≈ 47%. While curve (b) demonstrated that the pH range (5–7) where the greatest percentage of Ni(II) (≈100%) could be removed. According to graphs (a and b), H3L combines with Ni(II) to create a complex that makes them more hydrophobic. HOL then removes them from the bulk of solution. As can be observed, floatability rises progressively as pH rises reaching maximum (≈ 100%) at pH range of (5–7) then decreases. At pH range of (5–7), [Ni(II)-H3L] being combined with un-dissociated oleic acid, which starts to dissociate around pH ≥ 5.2, to make it hydrophobic (Ghazy et al. 2006), through hydrogen bonding and/or chemically with oleate anions. With the help of air bubbles, these hydrophobic aggregates are floated to the solution surface. The floatability decreases above pH 7 due to the solubility of the Ni(II)-H3L species or because the reaction is halted by the formation of an excessive amount of sodium oleate foams and a white emulsion. Consequently, pH 5 has been used in all subsequent tests.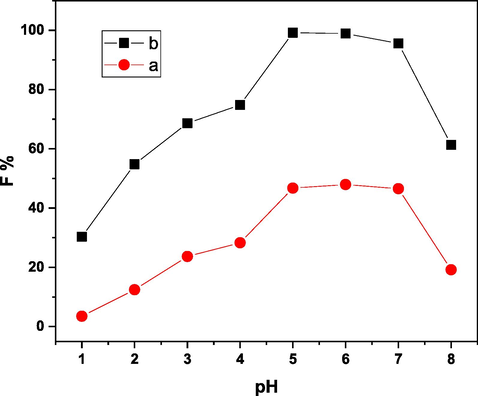
Effect of pH on the floatability % of 5 × 10-5 mol/L of Ni(II) using 1 × 10-3 mol/L of HOL (a) in absence, and (b) in presence of 1 × 10-4 mol/L of H3L.
3.2.2 Effect of H3L and Ni(II) concentration
Using 1 × 10-3 mol/L of HOL, it was determined how the concentration of H3L affected the floatability percentage of 5 × 10-5 mol/L of Ni(II) at pH 5. According to the findings in Fig. 7, the floatability percentage of Ni(II) rose when H3L concentration increased up to 1 × 10-4 mol/L, which is twice the concentration of Ni (II). Additionally, any further excess of H3L has little effect on the floatability percentage. Therefore, 1 × 10-4 mol/L of H3L was used for the investigations that followed.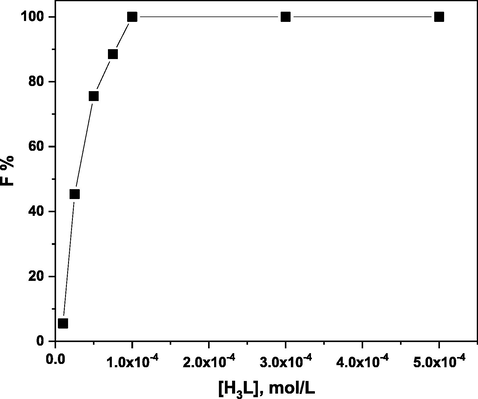
Effect of H3L concentration on the floatability % of 5 × 10-5 mol/L of Ni(II) at pH 5 using 1 × 10-3 mol/L of HOL.
Another set of tests were conducted to examine the floatability percentage of various concentrations of Ni(II) using 1 × 10-4 mol/L of H3L and 1 × 10-3 mol/L of HOL at pH 5. The floatability percentage of Ni(II) in the absence of H3L is shown in Fig. 8 (curve a). The greatest floatability percentage was 43.8%, and as the concentration of Ni(II) increased, the floatability percentage declined because there weren't enough oleate ions present to completely float all of the Ni(II). The floatability percentage of Ni(II) in the presence of 1 × 10-4 mol/L of H3L is shown in Fig. 8 (curve b). The gathered information demonstrated that total flotation happens at a M:L ratio (1:2). The floatability percentage begins to decrease at a high metal ion concentration greater than the ratio of (1:2), which can be attributed to the absence of inadequate H3L, which is required to bind all Ni(II). So, extra H3L should be employed while analyzing Ni(II) in its unidentified samples. Therefore, the optimal concentration of Ni(II) for all tests was determined to be 5 × 10-5 mol/L.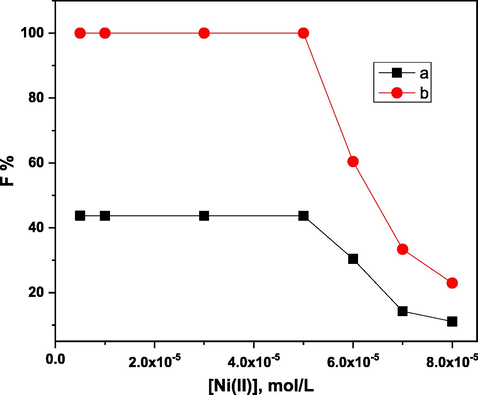
Floatability % of different Ni(II) concentrations at pH 5 (a) in absence, and (b) in presence of 1 × 10-4 mol/L of H3L using 1 × 10-3 mol/L of HOL.
3.2.3 Effect of type and concentration of surfactant
Table 2 displayed how different surfactants affected the amount of 5 × 10-5 mol/L of Ni(II) that was removed while using 1 × 10-4 mol/L of H3L at pH 5. The production of a foam layer and a thick layer of white scum at the top of the aqueous phase was discovered to make non-ionic surfactant (TW-60) and cationic surfactant (CTAB) ineffective for flotation of the generated complex, as a result, Ni(II) remained in the aqueous solution and could not be separated by flotation. However, the anionic surfactants (SS, SLS, and HOL) were very successful in causing the generated species, Ni(II)-H3L, to float. HOL was chosen as an appropriate surfactant for all investigations as a result. HOL: oleic acid, SS: sodium stearate, SLS: sodium lauryl sulfate, CTAB: cetyltrimethylammonium bromide, TW-60: Tween 60.
Surfactant
Floatability %
Anionic
HOL
98.8
SS
93.7
SLS
86.3
Cationic
CTAB
Foam, no flotation
Non-ionic
TW-60
Foam, no flotation
Another set of experiments was conducted to determine the floatability percentage of 5 × 10-5 mol/L of Ni(II) at various HOL concentrations (0.001–0.07 mol/L) in the absence and presence of 1 × 10-4 mol/L of H3L at pH 5 (see Fig. 9). The floatability percentage was shown by curve (a) to be under 50%. The optimal floatability of Ni(II) is 100%, according to curve (b), when HOL concentrations are between 0.001 and 0.007 mol/L. The following explanation fits the results: Higher concentrations of HOL resulted in a decrease in floatability efficiency because the micelles generated by surfactant molecules competing with the created complex, Ni(II)-H3L, caused the floatability efficiency to drop. Additionally, when the concentration of surfactant rises, the size of the bubbles decreases, which ultimately leads to the creation of a creamier foam. As a result, 1 × 10-3 mol/L of HOL was employed as a suitable concentration for the duration of the present study.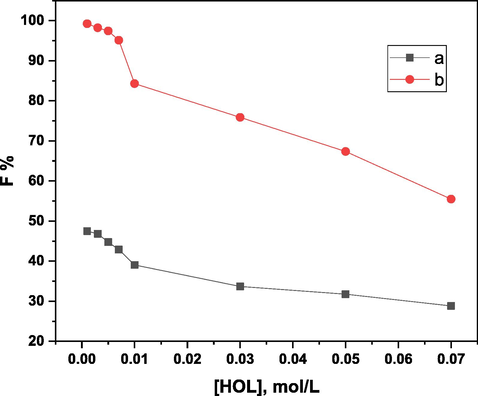
Effect of HOL concentration on the floatability % of 5 × 10-5 mol/L of Ni(II) (a) in absence, and (b) in presence of 1 × 10-4 mol/L of H3L at pH 5.
3.2.4 Effect of shaking and flotation time
Using 1 × 10-4 mol/L of H3L and 1 × 10-3 mol/L of HOL at pH 5, the effect of shaking duration on the floatability percentage of 5 × 10-5 mol/L of Ni(II) was examined throughout a range of (1–30 min). It was discovered that the maximum floatability percentage could be attained in 5 min, as illustrated in Fig. 10. The outcome demonstrates that the flotation-separation process is a quick method. For the next trials, a shaking time of 5 min was used.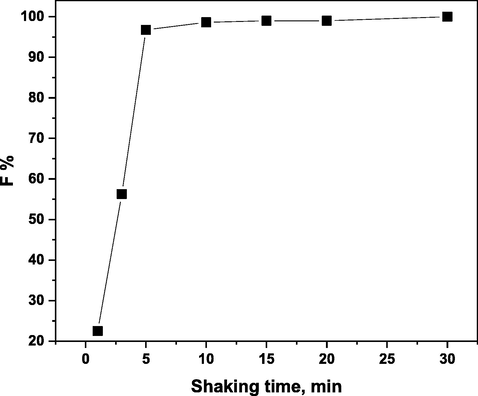
Effect of shaking time on the floatability % of 5 × 10-5 mol/L of Ni(II) at pH 5 in presence of 1 × 10-4 mol/L of H3L and 1 × 10-3 mol/L of HOL.
Investigations were done into how flotation time affected the stability of the produced complex inside the scum layer. The period between the addition of all reagents (Ni(II), H3L, and HOL) to the flotation cell and the beginning of flotation is known as flotation time. Additional experiments from 5 min to 48 h have been run to verify the stability of the Ni(II)-H3L complex. It had been shown that the floatability percentage and the absorbance of the floating [Ni(II)-H3L] complex in the scum layer remained stable for around 36 h.
3.2.5 Effect of sample volume
A series of tests were conducted using the recommended large flotation cells to successfully float a 5 × 10-5 mol/L of Ni(II) from different volumes (10–2500 mL). According to the data obtained and displayed in Fig. 11, 5 × 10-5 mol/L of Ni(II) may be entirely removed from different quantities up to two liters, at which point the floatability percentage reduced to 65.89%. So, using 15 mL of 1 × 10-3 mol/L of HOL and a pre-concentration factor of 200, 5 × 10-5 mol/L of Ni(II) could be pre-concentrated, separated, and measured from various aqueous volumes ranging from 10 mL to 2 L.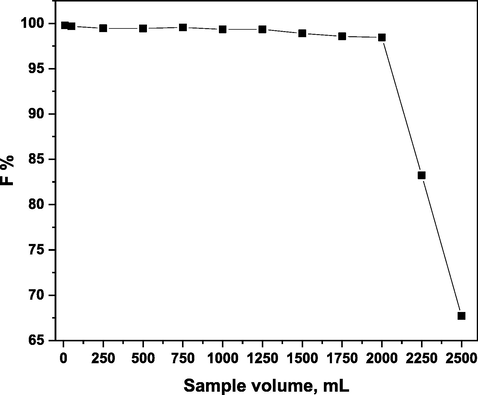
Effect of sample volume on the floatability % of 5 × 10-5 mol/L of Ni(II) in presence of 1 × 10-4 mol/L of H3L and 1 × 10-3 mol/L of HOL at pH 5.
3.2.6 Effect of temperature
For testing the optimum temperature needed for complete floatability of 5 × 10-5 mol/L of Ni(II), a run of trials was done at a range of (5–80 °C) using 1 × 10-4 mol/L of H3L and 1 × 10-3 mol/L of HOL at pH 5. This was accomplished by heating or cooling two solutions (one containing HOL and another containing Ni(II)) to the same temperature in a water bath. The HOL solution was quickly added to the Ni(II) solution. The flotation technique was then carried out after the mixture was added to the flotation cell. The results showed that the flotation-separation process is unaffected by temperatures up to 80 °C (see Table 3). Accordingly, consequent trials have proceeded at room temperature (25 ± 2 °C).
Temperature, oC
Floatability %
5
96.7
10
97.8
15
98.2
25
100
35
100
45
98.7
60
98.3
70
98.2
80
97.8
3.3 Stoichiometric ratio and the probable structure
Utilizing the molar ratio approach, the stoichiometry of the produced compound was demonstrated under the ideal conditions (Beltran-Porter et al. 1983; Momoki et al. 1969). Plot in Fig. 12 of absorbance against [H3L]/[Ni(II)] molar ratio revealed an inflection at a molar ratio of 2.0, indicating the existence of two H3L molecules in the complex. As a consequence, the findings indicated that the stoichiometric ratio was (1:2) for Ni(II):H3L. Also, the complex-formation constant (Kf) was evaluated (De Beer et al. 1994) and was found to be 2.25 × 104. As a result, the complex's structure may possibly be described as follows:
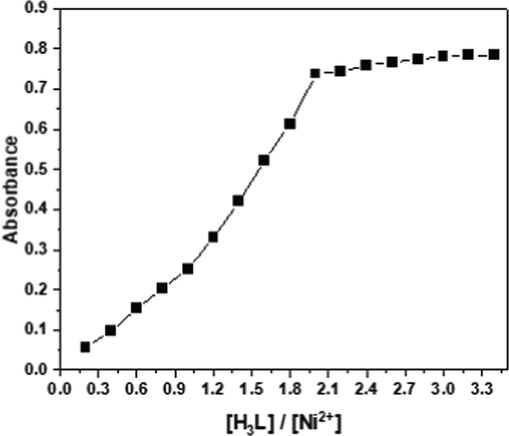
Molar ratio method for the complexation of H3L with Ni(III) at pH 5.
3.4 Effect of ionic strength
Table 4 summarizes the effect of varying the ionic strength of different salts on the floatability % of 5 × 10-5 mol/L of Ni(II) using 1 × 10-4 mol/L of H3L and 1 × 10-3 mol/L of HOL at pH 5. The salts used are generally similar to those present in natural water samples. As seen, it is clear that all added salts to the medium have not markedly affected the flotation-separation process. Only the chloride salts of calcium and magnesium have a little drop in floatability percentage as a result of the production of calcium and magnesium oleate, which lowers the concentration of HOL required for flotation. By adding much more HOL, the antagonistic effects of CaCl2 and MgCl2 can be mitigated.
Salt
Concentration, mol/L
Floatability %
NaCl
0.1
0.5100.0
98.7
KCl
0.1
0.5100.0
97.9
Na2SO4
0.1
0.599.6
97.8
MgCl2
0.1
0.597.3
92.1
CaCl2
0.1
0.596.9
91.8
3.5 Interference study
1 × 10-4 mol/L of H3L, 5 × 10-5 mol/L of Ni(II) and various quantities of each interfering ion (individually or in combination) were introduced to a flotation cell of type 1. Prior protocols were followed for the flotation-separation and spectrophotometric determination of Ni(II). The findings, which are shown in Table 5, revealed that none of the tested foreign ions, even those with large concentrations (in contrast to Ni(II)), significantly influenced the floatability percentage of Ni(II). Excessive H3L was added to the mixture to counteract the effect of interfering ions, which reduce the floatability percentage of Ni(II). Consequently, the suggested technique might be used for removing Ni(II) from water samples with complicated nature.
Foreign ion(s)
Concentration, mg/L
Floatability %
Cations
Individually
Ag(I)
350
97.9
Li(I)
300
98.3
Cu(II)
900
97.4
Pb(II)
850
98.7
Hg(II)
900
98.8
Ca(II)
4000
96.9
Cd(II)
500
97.2
Mg(II)
2400
97.3
Te(II)
300
98.2
Mn(II)
350
98.1
Sr(II)
100
96.8
Co(II)
200
97.9
Zn(II)
50
98.6
Cr(III)
100
96.9
Mo(III)
100
98.1
La(III)
450
97.7
W(III)
100
98.6
As(III)
100
98.2
In(III)
50
97.9
Sn(IV)
100
97.9
Zr(IV)
100
98.8
V(IV)
50
98.2
Th(IV)
150
97.8
Binary
Cu(II) + Mg(II)
450
97.2
Cu(II) + Ca(II)
500
96.8
La(III) + Ti(IV)
400
97.7
Cu(II) + Sr(II)
200
96.8
Co(II) + Sr(II)
100
97.4
Cu(II) + W(III)
50
98.8
Tertiary
Cu(II) + Ca(II) + Mg(II)
350
98.2
Cu(II) + Ca(II) + Hg(II)
450
98.1
Cd(II) + Ca(II) + La(III)
500
98.6
Te(II) + Mg(II) + La(III)
400
97.8
Cu(II) + Cr(III) + Zn(II)
50
97.9
Anions
Individually
Nitrate
1000
98.2
Bicarbonate
900
98.8
Citrate
1000
99.2
Sulphate
190
97.1
Phosphate
800
95.4
Chloride
150
96.4
Oxalate
190
95.6
3.6 Analytical application
Many water samples from different sites in Egypt (Mansoura, Talkha, Damietta, Gamasah, Ras El-Bar, Alexandria, and Sharm El-Sheikh) were collected at a depth of 50 cm below the top level. All of the samples were then filtered using G4 sintered glass. After that, 500 mL of each filtered sample was placed into a beaker, to which 15 mL of concentrated nitric acid and 10 mL of 30 percent (v/v) H2O2 were added to remove and decompose organic compounds. All filtered samples were then heated for 30 min at less than 90 °C, allowed to cool, and then pre-treated for the measurement of Ni(II) content as follows. After that, each sample of the cleaned water was carefully kept for future use in a dark plastic container. A 100 mL aliquot of treated water sample was introduced to a flotation cell of type 2, and then Ni(II) was added with an excess of H3L. The earlier processes for flotation separation and spectrophotometric determination of Ni(II) have been carried out. A series of experiments were conducted to recover spiked quantities of Ni(II) applied to certain real water samples in order to assess the applicability of the suggested approach. The findings obtained, which are shown in Table 6, demonstrated that the recovery was thorough and satisfactory. ND*: not detected.
Sample
Location
Found Ni2+ concentration before addition, ppb
Added concentration of
[Ni2+] × 10-5 mol/L
Recovery %
Distilled water
Our lab.
ND*
1.0
2.0100
100
Domestic water
Talkha
0.38
1.0
2.099.5
98.6
Mansoura
0.26
1.0
2.0100
99.7
Seawater
Sharm El-Shiekh
0.55
1.0
2.098.9
97.5
Gamasa
0.75
1.0
2.099.2
98.8
Ras El-Barr
0.80
1.0
2.099.2
98.5
Alexandria
0.85
1.0
2.099.1
98.2
Nile water
Talkha
0.30
1.0
2.098.2
97.3
Damietta
0.35
1.0
2.098.3
97.5
Mansoura
0.33
1.0
2.097.2
96.8
Wastewater
Talkha
0.44
1.0
2.098.8
97.2
Mansoura
0.65
1.0
2.098.3
97.5
3.7 Analytical characteristics
Under optimum parameters for the proposed flotation-separation procedure of Ni(II), the following characteristics were obtained and presented in Table 7. Beer’s law was obeyed over 5–400 ng/mL concentration range, the correlation coefficient (R2) was calculated and found to be 0.9998. According to IUPAC recommendations, the limit of detection (LOD) and limit of quantification (LOQ) were calculated to be 0.22 and 0.74 µg/L, respectively. The molar absorptivity (Ɛ) was 6.08 × 104 L/mol.cm with RSD of 1.7 % for n = 5.
Parameter
Value
λmax, nm
370
Linear range, ng/mL
5–400
Linearity correlation coefficient (R2)
0.9998
Molar absorptivity coefficient (Ɛ), L/mol.cm
6.08 × 104
Sandell’s sensitivity, µg/cm2
0.0085
Limit of detection (LOD), ng/mL
0.22
Limit of quantification (LOQ), ng/mL
0.74
Relative standard deviation (RSD), %
1.7
3.8 The suggested flotation-separation mechanism and chemistry behind complexation
To investigate the flotation-separation mechanism and chemistry behind complexation, several experimental studies were conducted, and based on experimental data and observations, the suggested flotation-separation mechanism and chemistry behind complexation may be summed up as follows:
-
The floated species, Ni(II)-H3L-HOL, have the same coffee color as that isolated in the aqueous solution, Ni(II)-H3L.
-
The period when the color of the sublate begins to fade after 36 h following the flotation process; such a time is longer than that necessary for our investigation; did not have an impact on the intensity of color for the scum layer.
-
Temperature increases up to 80 °C had little effect on the flotation process, showing that the floated complex is not readily dissociated by heat.
-
The results of the current work has shown that complexation of 1-(3,5-dihydroxybenzylidene)thiosemicarbazone with Ni(OCOCH3)2 produced a coffee color tetradentate (bis-bidentate) nickel complex. The results suggested that the thiosemicarbazone ligand behaved as bidentate ligand coordinated to the Ni(II) ion via its N and S atoms (see Fig. 13).
-
The isolated complex's elemental analysis revealed [C: 37.30 (Found 36.99), H: 3.88 (Found 3.49), N: 16.32 (Found 16.01), and Ni: 11.40 (Found 11.03), showing the complex possesses the molar ratio of M:L (1:2).
-
As a result, Ni2+ ions might combine with H3L in a M:L ratio of (1:2) to produce a coffee color complex according to the following equation:
-
The pH of the solution affects the chemical species of HOL (Ramachandra 1982), where dissociation starts at pH>5.2 (Ghazy et al. 2006) and IR analysis was used to calculate the proportion of its forms, and the findings were published in the literature (Pol’kin et al. 1968). The (–COOH) and (–COO-Na+) groups' distinctive bands at 1300–1800 cm−1 are connected to the IR spectra of HOL (Pol’kin et al. 1968). Moreover, as stated (Klassen and Mokrousov 1963), when HOL is ionized, the C⚌O stretching band at 1705 cm−1 shifts to bands in the 1520–1540 cm−1 region, which are typical of sodium oleate. Therefore, the suggested mechanism could occur either through a coordinate bond between HOL and the formed complex in solution, which results in the formation of a self-floatable species, Ni(II)-H3L-HOL, or through physical interaction (for example, Van Der Waals forces or hydrogen bond formation) between the hydrophilic part of oleic acid and the active sites of H3L in the formed complex.
-
As a result, depending on the pH of the solution, oleic acid might interact with the generated complex either in its undissociated (RCOOH) or dissociated (RCOO-) form as follows:
-
This hypothesis was validated by subsequent IR analyses, where the absence of bands for HOL in the IR spectrum of the complex that had been removed from the float layers (after thorough washing) indicates that HOL can combine with Ni(II)-H3L chelates through weak bonds, depending on the solution pH (Ghazy et al. 2004). Combining HOL with the generated chelates results in hydrophobic aggregates that float on the solution surface with the aid of air bubbles produced within the flotation cell by shaking (Ghazy et al. 2004).

- The suggested chemical reaction of Ni(II) with H3L at pH 5.
Finally, it can be concluded based on the facts presented above that the flotation-separation process took place via physisorption with the aid of air bubbles. Fig. 14 shows the potential separation method.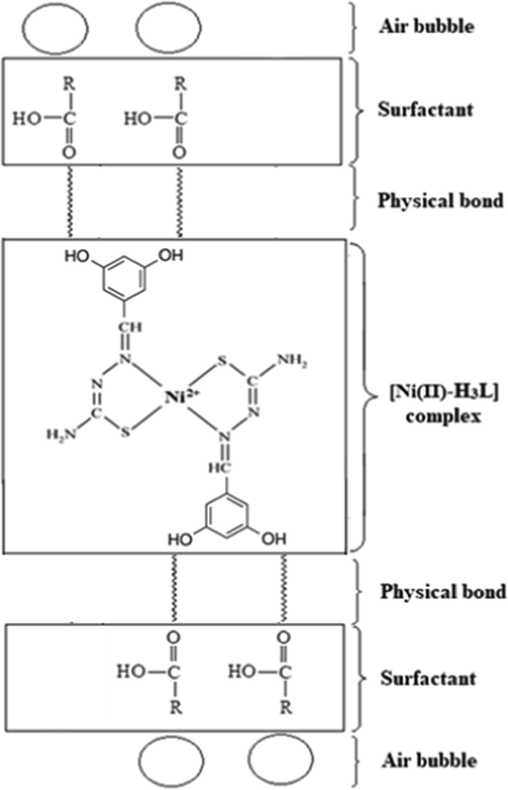
The suggested flotation-separation mechanism of Ni(II) using H3L at pH 5.
3.9 Comparison with other reported methods
The suggested combined process of flotation-separation with spectrophotometric determination employing H3L as a chelating agent and HOL as a surfactant was compared to other published methods for determining Ni(II) in different water samples (see Table 8). As can be observed, the suggested approach and the methods that have been previously published seem to be comparable. CPE: cloud point extraction; SPE: solid phase extraction; CME: capillary micro-extraction; DLPME: dispersive liquid phase micro-extraction; SFODME: solidified floating organic drop micro-extraction; FAAS: flame atomic absorption spectrometry; GFAAS: graphite furnace atomic absorption spectrometry; ETAAS: electro-thermal atomic absorption spectrometry; ICP-MS: inductively coupled plasma mass spectrometry; ICP-OES: inductively coupled plasma optical emission spectrometry; PF: pre-concentration factor; DL: detection limit; %RSD: percentage relative standard deviation.
Separation/preconcentration technique
Determination method
DL (μg/L)
PF
%RSD
References
CPE
FAAS
1.22
29
2.89
(Manzoori and Bavili-Tabrizi 2003)
CPE
ICP-OES
6.3
9.79
2.6
(Silva et al. 2009)
CPE
ETAAS
–
15
–
(Gil et al. 2008)
SPE
FAAS
0.80
43
–
(Lemos et al. 2008)
SPE
FAAS
0.92
200
–
(Xie et al. 2008)
SPE
FAAS
0.75
330
0.9
(Ghaedi et al. 2010)
CME
ICP-MS
0.0015
10
4.1
(Hu et al. 2006)
DLPME
GFAAS
0.033
200
8.2
(Jiang et al. 2008)
SFODME
GFAAS
0.0004
497
3.6
(Bidabadi et al. 2009)
Flotation
GFAAS
9.0
–
–
(Karimi et al. 2008)
Flotation
ETAAS
0.7
93
<1
(Lemos et al. 2008)
Flotation
FAAS
0.0025
100
<1.2
(Akl and Ahmad 2019)
Flotation
Spectrophotometry
1.1
200
1.7
This work
4 Conclusions
Herein, 1-(3,5-dihydroxybenzylidene)thiosemicarbazone (H3L) was prepared via facile solid state ball milling technique as a green and solvent-free mechanochemical approach and introduced as an effective organic chelate for the separation and preconcentration of Ni(II) from aqueous solutions utilizing a straightforward, quick, and affordable flotation method with oleic acid (HOL) as a surfactant. The characterization of the prepared chelating agent was investigated by various methods. The obtained results displayed that H3L acted as a neutral bidentate (N, and S) ligand. The effective elements were selected taking into account several trials in order to fulfill the goal of the current study. The obtained findings demonstrated that 5 min of shaking at 1 × 10-3 mol/L of HOL in the presence of 1 × 10-4 mol/L of H3L produced the maximum floatability percentage of 5 × 10-5 mol/L of Ni(II). Also, it was shown that the floatability % of Ni(II) was ≈ 100% at the Ni(II)‐H3L ratio of (1:2) and pH 5. Large flotation cells have made it possible to identify Ni(II) in water samples with large volumes and significant preconcentration factors. The recommended method could be applied to treat wastewater without cooling, was interference-free, and was unaffected by temperature increases up to 80 °C. This made the method cost-effective. Additionally, the proposed method was effectively used to recover Ni(II) ions supplied to several real water samples. The creation of weak physical interactions between the HOL surfactant and the generated complex, Ni(II)-H3L, was discovered to be the basis of the flotation-separation process. In addition, the suggested ion-flotation methodology provides a non-polluting method for the preconcentration and separation of trace metals. It has been demonstrated to be a green technology for the treatment of water and wastewater.
Funding
This project was supported by the Deanship of Scientific Research at Prince Sattam Bin Abdulaziz University under the research project (PSAU-2022/01/20367).
Declaration of Competing Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Removal of Cu (II) and Pb (II) ions from aqueous solutions by adsorption on sawdust of Meranti wood. Desalination. 2009;247(1–3):636-646.
- [Google Scholar]
- Ion flotation and flame atomic absorption spectrophotometric determination of nickel and cobalt in environmental and pharmaceutical samples using a thiosemicarbazone derivative. Egypt. J. Chem.. 2019;62(10):1917-1931.
- [Google Scholar]
- Heavy metal ions removal from wastewater using electrocoagulation processes: a comprehensive review. Sep. Sci. Technol.. 2017;52(17):2649-2676.
- [Google Scholar]
- Simultaneous determination of stoichiometry, degree of condensation and stability constant: a generalization of the molar-ratio method. Talanta. 1983;30(2):124-126.
- [Google Scholar]
- Solidified floating organic drop microextraction (SFODME) for simultaneous separation/preconcentration and determination of cobalt and nickel by graphite furnace atomic absorption spectrometry (GFAAS) J. Hazard. Mater.. 2009;166(1):291-296.
- [Google Scholar]
- Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J. Environ. Chem. Eng.. 2017;5(3):2782-2799.
- [Google Scholar]
- Nickel: A review of its sources and environmental toxicology. Pol. J. Environ. Stud.. 2006;15(3)
- [Google Scholar]
- Nickel, its adverse health effects & oxidative stress. Indian J. Med. Res.. 2008;128(4):412.
- [Google Scholar]
- Experimental design for the rapid selection of separation conditions for methyl and propyl parahydroxybenzoate, phenylephrine hydrochloride and chlorphenamine maleate by ion-pair liquid chromatography. J. Pharm. Biomed. Anal.. 1994;12(11):1379-1396.
- [Google Scholar]
- The role of surface chemistry and solution pH on the removal of Pb2+ and Cd2+ ions via effective adsorbents from low-cost biomass. J. Hazard. Mater.. 2009;167(1–3):260-267.
- [Google Scholar]
- Removal of lead, cadmium and zinc from aqueous solutions by precipitation with sodium Di-(n-octyl) phosphinate. Can. J. Chem. Eng.. 2000;78(5):948-954.
- [Google Scholar]
- Facile solid state ball milling as a green strategy to prepare 2-(2, 4-dichlorophenoxy)-N′-(2-hydroxybenzylidene) acetohydrazide complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2014;132:846-853.
- [Google Scholar]
- Solvent-free synthesis and computational studies of transition metal complexes of the aceto-and thioaceto-acetanilide derivatives. J. Organomet. Chem.. 2016;818:15-27.
- [Google Scholar]
- Removal of heavy metal ions from wastewaters: a review. J. Environ. Manage.. 2011;92(3):407-418.
- [Google Scholar]
- The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev.. 1971;7(1):81-122.
- [Google Scholar]
- Simultaneous removal of As, Cd, Cr, Cu, Ni and Zn from stormwater using high-efficiency industrial sorbents: Effect of pH, contact time and humic acid. Sci. Total Environ.. 2016;566:76-85.
- [Google Scholar]
- Flame atomic absorption spectrometric determination of copper, zinc and manganese after solid-phase extraction using 2, 6-dichlorophenyl-3, 3-bis (indolyl) methane loaded on Amberlite XAD-16. Food Chem. Toxicol.. 2010;48(3):891-897.
- [Google Scholar]
- Ghazy S, Mostafa H, El Farra S, and Fouda A. 2004. Flotation-separation of nickel from aqueous media using some hydrazone derivatives as organic collectors and oleic acid as surfactant.
- Flotation-separation of aluminum from some water samples using powdered marble waste and oleic acid. Anal. Sci.. 2003;19(10):1401-1406.
- [Google Scholar]
- Removal of aluminum from some water samples by sorptive-flotation using powdered modified activated carbon as a sorbent and oleic acid as a surfactant. Anal. Sci.. 2006;22(3):377-382.
- [Google Scholar]
- Cloud point extraction for cobalt preconcentration with on-line phase separation in a knotted reactor followed by ETAAS determination in drinking waters. Talanta. 2008;76(3):669-673.
- [Google Scholar]
- Adsorption of cadmium by Na and Fe modified zeolitic tuffs and carbonaceous material from pyrolyzed sewage sludge. J. Environ. Manage.. 2012;97:6-13.
- [Google Scholar]
- Effects of surface charge, micro-bubble size and particle size on removal efficiency of electro-flotation. Water Sci. Technol.. 2006;53(7):127-132.
- [Google Scholar]
- Ion flotation for removal of Ni (II) and Zn (II) ions from wastewaters. Int. J. Miner. Process.. 2015;143:131-137.
- [Google Scholar]
- On-line preconcentration and separation of Co, Ni and Cd via capillary microextraction on ordered mesoporous alumina coating and determination by inductively plasma mass spectrometry (ICP-MS) Anal. Chim. Acta. 2006;572(1):55-62.
- [Google Scholar]
- Simultaneous removal of Ag (I), Cd (II), Cr (III), Ni (II), Pb (II), and Zn (II) from wastewater using humic acid-coated aminopropyl silica gel. Desalin. Water Treat.. 2016;57(37):17411-17420.
- [Google Scholar]
- Dispersive liquid phase microextraction (DLPME) combined with graphite furnace atomic absorption spectrometry (GFAAS) for determination of trace Co and Ni in environmental water and rice samples. Talanta. 2008;74(5):1160-1165.
- [Google Scholar]
- Development of a selective and sensitive flotation method for determination of trace amounts of cobalt, nickel, copper and iron in environmental samples. J. Hazard. Mater.. 2008;151(1):26-32.
- [Google Scholar]
- Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia. 2014;50:113-120.
- [Google Scholar]
- Synthesis of N-methyl imines in the presence of poly (N-vinylpyridine) as a reusable solid base catalyst by a mechanochemical process. Res. Chem. Intermed.. 2017;43(2):901-910.
- [Google Scholar]
- An introduction to the theory of flotation”. Butterworths; 1963.
- Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J.. 2006;118(1–2):83-98.
- [Google Scholar]
- Flow injection preconcentration system using a new functionalized resin for determination of cadmium and nickel in tobacco samples. J. Hazard. Mater.. 2008;155(1–2):128-134.
- [Google Scholar]
- Removal of Cd (II) ion from waste water by adsorption onto polyaniline coated on sawdust. Desalination. 2011;272(1–3):301-305.
- [Google Scholar]
- Cloud point preconcentration and flame atomic absorption spectrometric determination of cobalt and nickel in water samples. Microchim. Acta. 2003;141(3):201-207.
- [Google Scholar]
- Mononuclear Pd (ii) and Pt (ii) complexes with an α-N-heterocyclic thiosemicarbazone: cytotoxicity, solution behaviour and interaction versus proven models from biological media. Inorg. Chem. Front.. 2018;5(1):73-83.
- [Google Scholar]
- Multielement preconcentration of trace heavy metals in seawater with an emulsion containing 8-quinolinol for graphite-furnace atomic absorption spectrometry. Anal. Chim. Acta. 2004;507(2):205-209.
- [Google Scholar]
- Interactions among functional groups in the cycling of, carbon, nitrogen and phosphorus in the rhizosphere of three successional species of tropical woody trees. Appl. Soil Ecol.. 2005;28(1):57-65.
- [Google Scholar]
- Inhibition of mild steel corrosion in hydrochloric acid using two novel pyridine Schiff base derivatives: a comparative study of experimental and theoretical results. RSC Adv.. 2017;7(68):43014-43029.
- [Google Scholar]
- Theory of curved molar ratio plots and a new linear plotting method. Anal. Chem.. 1969;41(10):1286-1299.
- [Google Scholar]
- Separation and preconcentration by a cloud point extraction procedure for determination of metals: an overview. Anal. Bioanal. Chem.. 2009;394(3):759-782.
- [Google Scholar]
- Organization WH. 2008. Guidelines for drinking-water quality: second addendum. Vol. 1, Recommendations: World Health Organization.
- Studies on six-coordinate octahedral Chromium (III) chelates with schiff bases derived from 4-Acetyl-3-methyl-1-(3′-chloro Phenyl)-2-pyrazolin-5-one. Synth. React. Inorg. Met.-Org. Chem.. 1985;15(6):769-778.
- [Google Scholar]
- Phase diagram and collector properties of oleic acid with changing pH. Izv Vyssh Ucheb Zaved Tsvet Met. 1968;11:6-11.
- [Google Scholar]
- Ramachandra RS. 1982. Surface Chemistry of Froth Flotation, Vol. 2 Reagents and Mechanisms, Kluer Academic. Plenum Puplishers, New York.
- Cloud point extraction for determination of cadmium in soft drinks by thermospray flame furnace atomic absorption spectrometry. Microchem. J.. 2011;97(2):118-121.
- [Google Scholar]
- Overview of flotation as a wastewater treatment technique. Miner. Eng.. 2002;15(3):139-155.
- [Google Scholar]
- Flotation of cesium coprecipitated with nickel hexacyanoferrate (II) from aqueous solutions and radioactive waste simulants. Sep. Sci. Technol.. 2007;42(6):1341-1365.
- [Google Scholar]
- Supported benzimidazole-salen Cu (II) complex: An efficient, versatile and highly reusable nanocatalyst for one-pot synthesis of hybrid molecules. Appl. Organomet. Chem.. 2018;32(10):e4446.
- [Google Scholar]
- Simultaneous preconcentration of copper, zinc, cadmium, and nickel in water samples by cloud point extraction using 4-(2-pyridylazo)-resorcinol and their determination by inductively coupled plasma optic emission spectrometry. J. Hazard. Mater.. 2009;171(1–3):1133-1138.
- [Google Scholar]
- Mn (II) recovery from aqueous systems by flotation. Water Res.. 1998;32(10):3021-3030.
- [Google Scholar]
- Techniques ABS, and Lemlich R. 1972. Academic Press: New York. London.
- Toxic effect of nickel in an in vitro model of human oral epithelium. Toxicol. Lett.. 2005;159(3):219-225.
- [Google Scholar]
- Thiosemicabazone based fluorescent chemosensor for transition metal ions in aqueous medium. J. Lumin.. 2013;141:48-52.
- [Google Scholar]
- A dual-mode chemosensor: highly selective colorimetric fluorescent probe for Cu2+ and F− ions. Sens. Actuators B. 2014;204:375-381.
- [Google Scholar]
- Reactions of Hafnium Isopropoxide Isopropanolate with Thio-Schiff Bases Derived from S-methyldithiocarbazate. Synth. React. Inorg. Met.-Org. Chem.. 1983;13(7):943-952.
- [Google Scholar]
- Solid phase extraction of lead (II), copper (II), cadmium (II) and nickel (II) using gallic acid-modified silica gel prior to determination by flame atomic absorption spectrometry. Talanta. 2008;74(4):836-843.
- [Google Scholar]
- Electrothermal atomic absorption spectrometric determination of cobalt, copper, lead and nickel traces in aragonite following flotation and extraction separation. Talanta. 2001;54(1):139-146.
- [Google Scholar]







