Translate this page into:
Green biosynthesis of Pt-nanoparticles from Anbara fruits: Toxic and protective effects on CCl4 induced hepatotoxicity in Wister rats
⁎Corresponding author. nsa@taibahu.edu.sa (Najlaa S. Al-Radadi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Platinum Nanoparticles (PtNPs) are synthesized from the Anbara fruit (Phoenix dactylifera L.) and are characterized using various spectroscopic analytical methods. These PtNPs were used to study the Hepatotoxic and Hepatoprotective effects on acute liver damage caused by CCl4 in Wister rats. Seventy-two rats of both sexes are divided into twelve groups and are treated with PtNPs and aqueous Anbara extract (AAE). Histopathological examinations were performed to identify the toxic effects on the vital organs of the rats. Hepatoprotective activity was monitored by observing the serobiochemical and hematological parameters and the intensity of hepatic marker enzymes alanine transferase (ALT), aspartate amino transferase (AST) and alkaline phosphatase (ALP) in the organs such as liver, intestine and kidney. Considerable experimental results were obtained when compared with the standard drug Silymarine. The PtNPs and AAE were proven to have protective activity of enzymes in the liver of Wister rats.
Keywords
Aqueous Anbara extract
Pt-nanoparticles
Biomimetic synthesis
Hepatotoxicity
Histopathology
Liver enzymes
1 Introduction
Nano-chemistry is attributed as a rapidly growing family of research in the empire of chemistry in these days (Al-Radadi, 2018; Barua and Mitragotri, 2014; Cai et al., 2010; Ferreira et al., 2017; Mahdavi et al., 2013; Reddy, 2017; Sathishkumar et al., 2013; Thakkar et al., 2010). Noble metals such as Au, Pt and Ag contained nanoparticles explored interesting applications in the areas of biomedical devices and in biosensors (Al-Radadi and Al-youbi, 2018a; Aswathy Aromal and Philip, 2012; Deokar and Ingale, 2016; Kumar et al., 2011; Mukherjee et al., 2017; Rajan et al., 2017; Rao et al., 2000; Sanchez-Mendieta and Rafael, 2012; Shah et al., 2015; Shipway et al., 2000; Smitha et al., 2009; Vinod et al., 2011; Yu et al., 2016). These metal nanoparticles are gaining popularity as they are widely used in the manufacturing of cosmetics, soaps, detergents, shoes, toothpastes and shampoos (Al-Radadi and Al-youbi, 2018; Bankar et al., 2010; Bavykin et al., 2006; Meena and Chouhan, 2015; Prokop and Davidson, 2008; Vedelago et al., 2018). In addition, these metal based NPs are used for drug synthesis and as drug delivery systems. In particular, Platinum nanoparticles (PtNPs) in recent years have gained attention for their thriving applications in the treatment of coronary artery disease, brain aneurysms (Chanda et al., 2011; Da Rocha et al., 2014; Dreaden et al., 2011; Lin et al., 2012; Mirza and Siddiqui, 2014; Muniyappan and Nagarajan, 2014; Ramalingam et al., 2016; Yew et al., 2020; Yoon et al., 2016) and in chemotherapy due to their size and shape dependent therapeutic properties, volume to large surface area ratio, and electrocatalytic properties. PtNPs are also finding significant role in energy related industrial fields such as fuel cells, hydrogen storage and electro catalysis (Bendale et al., 2017; Miklášová et al., 2012; Pansare et al., 2016; Vrana et al., 2016). PtNPs are also combined with other metal nanoparticles in the form of bimetallic nanoclusters (Ganaie et al., 2016; Majzik, 2008; Sahu et al., 2015; Zhan et al., 2011), core-shell (Chaudhuri and Paria, 2012; Wang et al., 2013), or as alloys (Malathi et al., 2014; Rashid et al., 2018; Valodkar et al., 2011; Xu et al., 2012) and are extensively used in biomedical applications. Biomimetic synthetic approaches accompanied with plant extracts, live plants, and microorganisms (El-Sherbiny et al., 2016; El Khoury et al., 2015; Ibrahim, 2015; Mavukkandy et al., 2016; Puišo et al., 2014; Rivera-Rangel et al., 2018; Sathishkumar et al., 2009) are considered as a probable non-toxic and eco-friendly techniques which are alternatives to the conventional chemical and physical methods (Abdel-misih and Bloomston, 2010; Cruz-López et al., 2015; David et al., 2014; Dubey et al., 2010; Dzimitrowicz et al., 2016; Ghorbanpour, 2015; Ghoreishi et al., 2011; Karthik et al., 2016; Kumar et al., 2013; Molnár et al., 2018; Muthu and Priya, 2017; Philip, 2009; Wang et al., 2015; Yadav et al., 2017).
Liver is one of the vital organs which regulate the homeostasis inside the body. It works with almost all biochemical pathways associated with nutrient supply, energy stipulation, growth, fight against diseases and reproduction. This detox organ also protects us from harmful chemicals including drugs (Althnaian et al., 2013; Diniz-santos et al., 2004). In recent years, increased deaths are observed due to morbidity and co-morbidities of liver problems including hepatitis and Jaundice. Inadequate pharmacotherapeutic agents to cure the liver diseases scares and increase the demand to propel the requirement of novel and effective drugs for the medication of hepatic disorders (Adam et al., 2011; Czaja, 2014).
Carbon tetrachloride (CCl4) is generally used for liver injury in the research experiments. It decreases antioxidant enzymes activities and generate free radicals leads that to hepatocyte damage in both invitro and invivo conditions (Johnston and Kroening, 1998). CCl4 causes damage associated with free radical and oxidative stress mediate dliver tissue damage was determined in humans and rats (Mohammadinejad et al., 2016). It creates critical effects on membranes by raising the levels of plasma aminotransferases of the hepatocyte, causing leakage of the enzyme present in the cell. It directs the decomposition of fat in the liver due to obstacle of secretion of hepatic triglycerides into plasma. CCl4 toxicity intricate the splitting of C—Cl bond forms (CCl3O2) free radical which occurs in endoplasmic reticulum and mediated by cytochrome p-450 oxidase system. The free radicals are then bound to lipids and hepatic proteins (Das and Brar, 2013).
Green synthetic approach of metal nanoparticles is an intriguing research area showed increased consideration due to its simplicity. It has several advantages over traditional techniques such as it is non-toxic, clean, environmental friendly, cost effective, simple and high productivity. They can be synthesized from extracts of plant materials by several chemical, physical and biological methods and obtained functioning in the prevention of chronic diseases such as cancer due to their unique interactions at the molecular & cellular levels (Abdul Salam et al., 2012; Alshatwi et al., 2015; Banerjee et al., 2014; Jee et al., 2012; Mishra et al., 2014; Nair, 2013; Taylor et al., 2012).
Date palm fruits have commercial importance in countries like Saudi Arabia, Oman, Emirates, Bahrain, Iraq, North Africa and South Africa (Al-farsi et al., 2007; Al-orf et al., 2012; Aron and Hahidi, 2005; Vayalil, 2012). In most of the Arabian countries, dates are fundamental components of diet and clipped food which are assumed to be a good resource of energy. Fruits of date palm composed of proteins (0.2–0.5%), minerals (2.3–5.6%), dietary fiber (6.4–11.5%) in addition to high percentage of carbohydrates (44.8%) and vitamins. These fruits have rich contents made up of antioxidants, amino acids, sugars, macro minerals, trace elements and several fatty acids like linolenic, oleic and palmitoleic acids (Abdul Salam et al., 2012; Alshatwi et al., 2015; Jee et al., 2012; Khan et al., 2016; Kumar and Yadav, 2009; Nair, 2013).
There are several reports on anticancer, antiviral and liver protective activities of dates (Al-qarawi et al., 2004; Al-Radadi, 2019; Al-yahya et al., 2016; El-far, 2014; Nadaroglu et al., 2017; Saafi et al., 2011). The aqueous extract of date fruit was used invivo for hepatoprotective properties and induced hepatic injury in mice, besides some information indicate that this fruit could be helpful to prevent the oxidative stress induced hepatotoxicity (Martins et al., 2012). Liver damage caused by CCl4 were reorganized by the treatment of dates flesh or pits extractin rats and rabbits (Dobrucka, 2015; Nadaroglu et al., 2017; Yew et al., 2020). The extract of date palm Ajwa from almadine-almonawara shows strong hepatoprotective activity against ochratoxin (Al-Radadi, 2019). The effect of particle size of Pt-Anbara NPs and aqueous Anbara extract (AAE) on the hepatic and kidney tissues of rats, identification of potential threats of diagnostic and therapeutic use and hepato-toxicity with the time of exposure is studied in the present work.
2 Experimental
2.1 Materials
The UV–visible absorption spectra were recorded on Agilent, Cary 100 UV–VIS spectrophotometer, IR spectra was performed using Thermo Nicolet 6700 FTIR Spectrometer, powder X-Ray diffraction was recorded with a Shimadzu XRD-6000 with Cu Kα radiation (λ = 1.54056 Å). The size and surface morphology of NPs were identified with Energy dispersive X-ray (EDX) with modelNOVA-450 instrument and Transmission electron microscope (TEM).
2.2 Animals
Seventy-two clinically healthy Wister rats with standard husbandry conditions such as temperature, diet and drinking water having 3 months of age of two sexes with an average body weight of 120–152 g, were housed in the animal house of collage of Science and Technology, Al Neelain University, Khartoum, Sudan. Animals were prepared to the experimental conditions for a period of two weeks prior to the initiation of the experiments. Animal experiments were designed and conducted according to the guidelines of institutional animal ethical committee.
2.3 Synthesis of Pt nanoparticles (Pt NPs)
Anbara (Phoenix dactylifera) date fruits were obtained from a local farm in Madinah Monawara, Saudi Arabia. The fruits were washed with distilled water to remove the impurities, dried at 60 °C and preserved under vacuum. An Anbara stock solution was prepared where Anbara was mixed with 4 ml of 10−3 M H2PtCl6 solution and make up to 10 ml with de-ionized water. The reduction of Pt+4 to Pt0 NPs was confirmed when the solution color changed to yellow to yellowish-brown. The pH of the solution was adjusted by the addition of 0.1 M NaOH or 0.1 M HCl. The solid black colored Pt NPs were obtained when the mixture was boiled for about 7 h. The solvent was reduced to 1/4th of its original volume, centrifuged and washed several times with water and ethanol to get the pure product.
2.4 Preparation of the aqueous Anbara extract (AAE)
Anbara stock solution was prepared by taking 9 g. of Anbara and was boiled in 200 ml water for 15 min. and was reduced to a volume of 100 ml. The solution obtained was filtered, kept in dark at 100 °C and the filtrate was used as a reducing agent and utilized with 24 h.
2.5 Preparation of AAE concentrations
The Anbara dissolved in distilled water at a dose of 0.78 g/kg/day (low dose), equivalent to 7 units Anbara dates ∼47.25 g/200 ml water/60 kg/body weight per person per day (according to the words of our prophet mohammed (peace be upon him) who eats seven Anbara dates in a day which is equal to 1.56 g/kg/body weight (high dose).
2.6 Preparation of Pt NPs dose concentration
The Pt NPs prepared every day at 5 µg (low dose), and 10 µg (high dose) dissolved in phosphate buffer saline (PBS).
2.7 Experimental design
2.7.1 Toxicity assessment
Thirty male adult Wistar rats were divided randomly to 6 rats each in 5 groups. Group-1served as control allowed for feed the normal diet. Groups 2 and 3 were given AAE at 0.78 g/kg/day and 1.56 g/kg/day orally. To the groups 4 and 5 rats, PtNPs were given at 5 µg/g/day and 10 µg/g/day orally by cathedral tube, respectively. The dosage of all rats was given for 7 days according to their designated experimental oral doses. Body weights of rats were measured on day 0 and the 7th day of the treatment. Tissue samples for histopathology were taken at the end of the experiment after scarifying animals under mild chloroform anesthesia.
2.8 Evaluation of hepatoprotective activity
After two weeks of acclimation, forty-two Wistar rats of both sexes were divided into seven groups contain 6 rats in each group. Group 1 represents control in which the rats were fed with normal diet and distilled water for 21 days. Group 2 represents induction control received 3 ml/kg CCl4 subcutaneously and diluted on 7th day in 1:3 with olive oil. Group 3 received 70 mg/kg/day Silymarin for 15 days and CCl4 injected on 7th day. Groups 5 received 10 µg/g/day of Pt NPs orally for 7 days and CCl4 injected on 7th day. Group 6 received 0.78 g/kg (lower dose) of AAE orally for 7 days and CCl4injected on 7th day. Group 7 received 1.56 g/kg (higher dose) of AAE orally for 21 days and CCl4injected on 7th day. Clinical signs were recorded throughout the experimental period where body weights were weekly reported for each group. Animals were killed on 21st day under mild chloroform anesthesia whereas blood samples for serum and hematological analysis were collected immediately. To identify the gross lesion, all rats were examined at necropsy and the small intestine, liver and kidney specimens were quickly recovered after autopsy and fixed in 10% formalin for histopathological study.
2.9 Hematological analysis
Ethylene diamine tetra acetic acid (EDTA) contained dry test tubes were taken and collected the blood samples of rats to determine the red blood cells (RBC) count, differential white blood cell (WBC) count, packed cell volume (PCV), mean corpuscular volume (MCV), hemoglobin concentration (Hb), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). Automated heamatology analyzer was used to perform the measuring techniques.
2.10 Serobiochemical analysis
Blood samples were collected in a plain container and allowed to clot. Serum was separated after centrifugation and stored at −20 °C. This serum was used to analyze the serum activities on alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP) and for total protein concentration, urea, albumin, globulin, bilirubin, and creatinine.
2.11 Histological examination
To identify the gross lesion all rats were examined and Necropsy was conducted. Kidney, small intestine and liver specimens were collected, fixed in 10% formalin immediately, covered with paraffin wax, sectioned at 5 μm thickness and stained with eosin and haematoxylin (H&E) using Harris’s haemalum.
2.12 Statistical analysis
Statistical Package for Social Science (SPSS) used for the data analysis. For the T-test classified data Duncan’s multiple range tests were performed to compare the difference between means at each point after ANOVA. The obtained results were presented as mean ± Standard error (M ± S.E). P < 0.05 was considered as the statistically significant difference.
3 Results and discussion
The characterization of synthesized Pt NPs under different conditions were analyzed using UV–visible absorption spectra, Transmission Electron Microscope (TEM) analysis, Fourier Transform Infra Red (FTIR), Powder XRD and Energy Dispersive X-ray spectroscopy (EDX).
3.1 UV–Visible absorption and Transmission electron microscope (TEM) analysis
The formation of Pt NPs was monitored using UV–visible absorption spectra. The UV–visible absorption spectra were taken for in three batches. For the first batch the UV–visible absorption spectra were measured for the mixture of a constant 4 ml of 10−3 MH2PtCl6 solution and different volumes of aqueous Anbara extract. The UV–Visible spectra showed a prominent peak at a wavelength (λmax) of 380 nm. There is a gradual increase in the absorbance intensity upon increasing the Anbara extract volume from 1 ml to 6 ml, with a slight red shift in the peak position in the UV–visible spectra was observed (Fig. 1).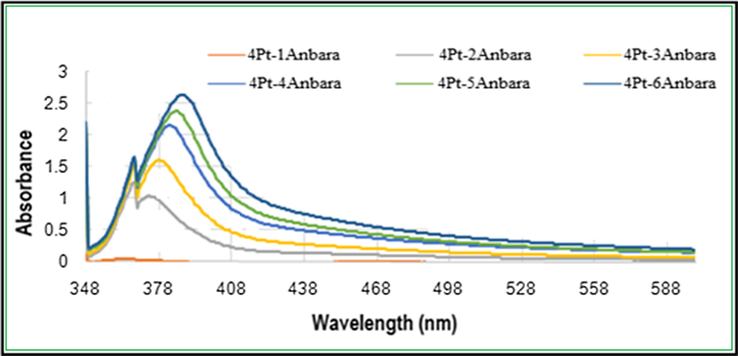
UV–visible absorption spectra of Pt NPs synthesized with different concentrations of Anbara extract and 4 ml H2PtCl6 stock solutions after 7 h of addition at 25 °C.
The peak at 380 nm is an indication for the formation of Pt0 species which is the ideal surface plasmon resonance (SPR) of Pt NP (Huang et al., 2004; Link and El-Sayed, 2005; Rajathi and Nambaru, 2014; Riddin et al., 2010; Song et al., 2010; Soundarrajan et al., 2012). Whereas the red shift in the peak position indicates the completion of reaction and no more Pt0will be formed. Furthermore, the TEM images of synthesized Pt NPs were taken for the mixtures of 4 ml 10−3 MH2PtCl6 with 4,5 and 6 ml solutions of Anbara extract. The images showed the presence of homogeneous, quasi- spherical and smaller size with an average size range of 2.3–3.0 nm nanoparticles (Fig. 2).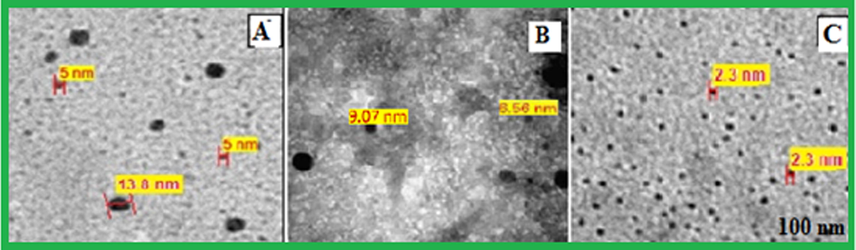
TEM micrograph of Pt NPs prepared with different mixtures of 4 ml of H2PtCl6 and Anbara extract (A) 4 ml (B) 5 ml and (C) 6 ml, after 7 h of addition at 25 °C.
For the second batch the UV–visible absorption spectra were measured for the mixture of a constant 6 ml of Anbara extract and different volumes (0.5–4 ml) of H2PtCl6 solution. A prominent peak at λmax = 380 nm was obtained. Red shift in the peak position was observed with increase in the concentration of H2PtCl6 solution from (0.5 ml) to (4 ml) (Fig. 3). TEM images of the synthesized Pt NPs were taken for the mixtures of 6 ml Anbara extract and 2, 3 and 4 ml H2PtCl6 solutions. The images showed the presence of smaller size homogeneous quasi- spherical nanoparticles with an average size range of 2.3–3.0 nm (Fig. 4). From the TEM images it was observed that the NPs size decreased with the addition of the volume of H2PtCl6 solution from 0.5–4 ml to 6 ml Anbara extract.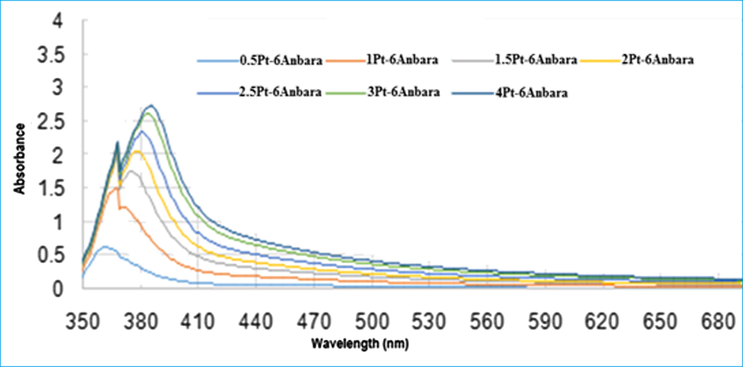
UV–visible absorption spectra of Pt NPs prepared with different volumes of 10−3 M H2PtCl6 stock solutions with 6 ml Anbara extract after 7 h of addition at 25 °C.
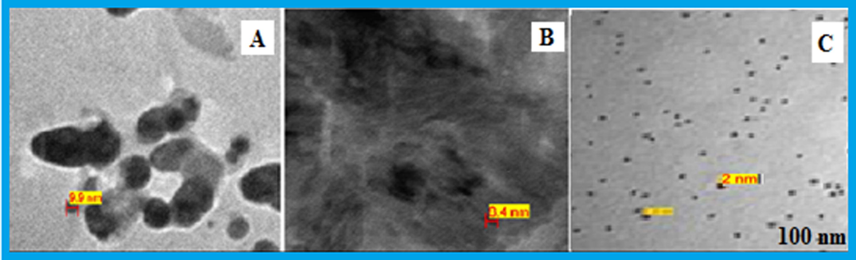
TEM micrograph of Pt NPs formed with different mixtures of 6 ml Anbara extract and 10−3 M H2PtCl6 stock solutions (A) 2 ml (B) 3 ml and (C) 4 ml, after 7 h of addition at 25 °C.
The third batch UV–visible absorption spectra were measured for the Pt NPs synthesized with 4 ml of 10−3 MH2PtCl6 and 6 ml Anbara extracts a function of time at room temperature. Two peaks were obtained at 310 nm and 380 nm up to 1 h. The intensity of the peak at 310 nm increased and the 380 nm peak intensity was decreased initially for 30 min. Further increasing the time the peak at 310 nm decreased, and then vanished, but the peak intensity at 380 nm increased and stabilized at 7 h and no subsequent increase in intensity occurred up to48 hours (Fig. 5). The peak at 310 nm is corresponding to Pt4+ species which increased up to 30 min. and converted to 380 nm peak which corresponding to the formation of Pt0 NPs. The absorbance to time spectrum showed the variation in the absorbance and the time required for maximum stability of Pt0 NPs (Fig. 6).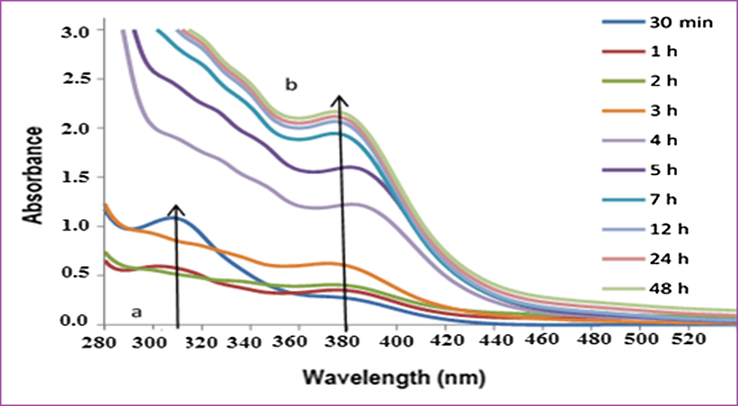
UV–visible absorption spectra of Pt NPs obtained with 4 ml H2PtCl6 solution and 6 ml of Anbara extract as a function of time at 25 °C.
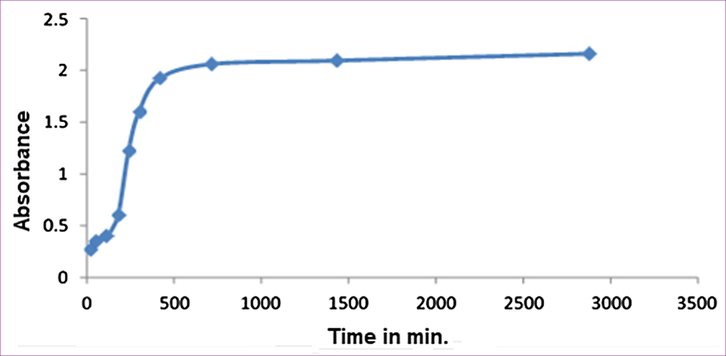
Absorption intensity variation in the UV–visible absorption of Pt NPs obtained with 4 ml H2PtCl6 and 6 ml of Anbara extract as a function of time at 25 °C.
These results suggests that the highest concentration of Pt0 NPs were achieved within first 420 min (7 h) and then stabilized. It optimized the reaction time for the synthesis of Pt0 NPs with 4 ml of 10−3M H2PtCl6 and 6 ml Anbara extract. The color of the solution turns darker with increasing the reaction time. The TEM images of Pt NPs reactions at 4, 7 and 11 h were taken for the third batch compound mixture. The most adequate time at 7 h (Fig. 7) offers the optimum reaction time to obtain Pt NPs which is in good agreement with the TEM images of the proposed reactions conditions. More over the TEM analysis showed that the desired Pt NPs formed acquired nearly spherical shape contained the particle size of 2.3–3.0 nm.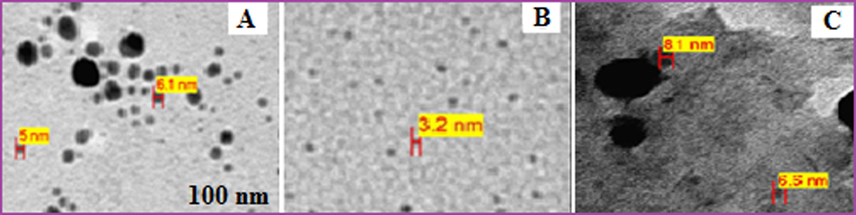
TEM micrograph of Pt NPs (4 ml of H2PtCl6 solution and 6 ml of Anbara extract) at different time (A) 4 h (B) 7 h (C) 11 h at 25 °C.
3.2 Ftir
FTIR was recorded to identify the presence of functional groups in AAE and PtNPs. Similar pattern of IR bands at 3550–3150, 1732, 1626, 1347, 1226 and 1042 cm−1 were obtained in both spectra (Thirumurugan et al., 2016; Venu et al., 2011). A broad band at 3550–3150 cm−1 was obtained in Pt NPs which was obtained at 3400 cm−1 in AAE. There is a slight variation in the position and intensity of the peaks observed due to the complexation of Anbara with Pt to obtain the Pt NPs (Fig. 8).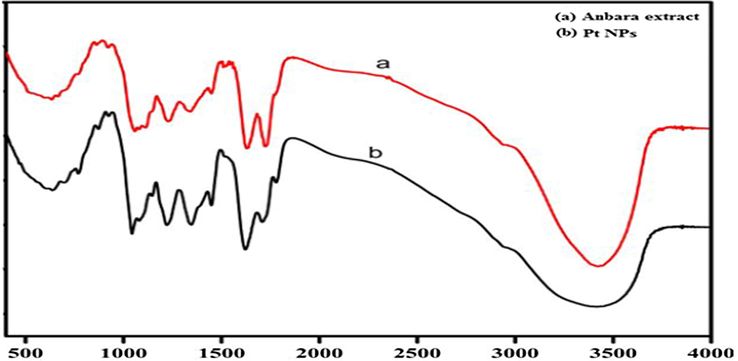
FTIR spectra of AAE and Pt NPs.
3.3 Powder XRD analysis
The Powder pattern of the synthesized Pt NPs was recorded to determine the crystalline nature and the phase purity of the Pt NPs (Onizawa et al., 2009; Yoshida et al., 2008) (Fig. 9).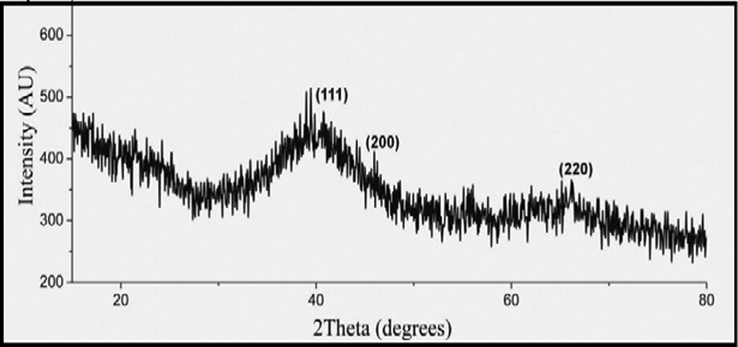
Powder XRD of synthesized Pt NPs.
3.4 Energy dispersive X-ray spectroscopy (EDX)
The EDX spectrum results of the synthesized Pt NPs showed a strong Pt signal that confirmed the presence and purity of Pt metal in the PtNPs. Whereas the presence of other elements were also identified (Gholami-Shabani et al., 2016) (Fig. 10).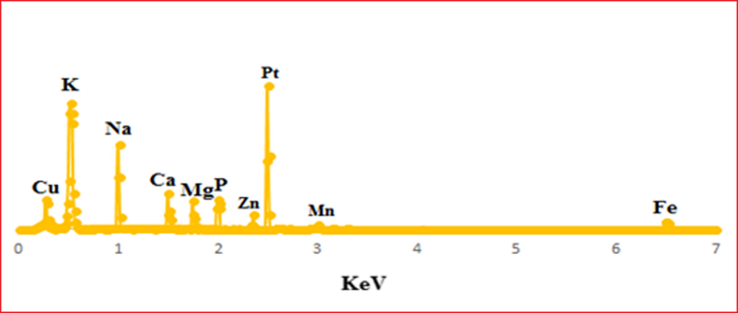
The elemental composition analysis of Pt NPs using EDX spectroscopy.
3.5 Toxicity assessment
3.5.1 Growth changes
Oral doses of Pt NPs and AAE were given to 5 groups contain 6 rats in each group, and the change in body weight was observed after 7 days (Table 1). Body weight of all groups were decreased and group 3 showed significant decreased body weight (P < 0.05) when compared with control (Group 1). All rats were alive even after completion of the experiments.
S.No.
Treatment groups
Experimental period
Body weight (g)
0 dayBody weight gain (g)
After 7th days
1.
Control (normal diet)
122.0 ± 4.7
21.3 ± 6.3
2.
Anbara (0.78 g/kg/day)
124.3 ± 4.9
11.0 ± 5.9
3.
Anbara (1.56 g/kg/day)
123.5 ± 5.7
27.1 ± 4.8
4.
Pt NPs (5 µg/g/day)
122.7 ± 6.3
17.3 ± 6.4
5.
Pt NPs (10 µg/g/day)
123.5 ± 8.7
12.5 ± 7.7
3.6 Histopathological changes
Oral doses of Pt NPs and Anbara aqueous extract at different concentrations were given to 5 groups contain 6 rats in each group as in Table 1. After one week of the treatment, there were no lesions or alterations appeared on all the treated groups and other vital organs of control rats (group 1). The microscopic analysis showed no change in the sectioned small intestine, kidney and liver organs (Fig. 11).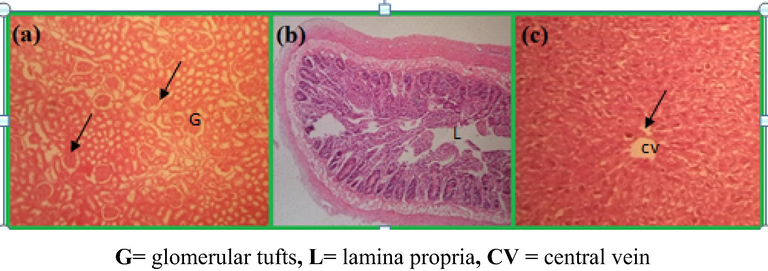
Microscopic images of tissues of rats taken after oral doses of PtNPs at 5 and 10 µg/g/day for one week: Glomerular in kidney (a) no changes observed in the intestine (b) and hepatocytes in liver.
3.7 Hepatoprotective activity
3.7.1 Clinical findings and growth changes
Details of oral doses of Pt NPs and AAE at various concentrations were given to 7 groups contain 6 rats in each group as in Table 2. CCl4 induced in ratson 7th day and the treatment continued for 21 days. Depression, huddling together, reluctance to move, locomotors disturbances, erection of hair, weakness in limbs and emaciation are prominent manifestations which appeared on 7, 8 and 9 days after CCl4 induction. These symptoms were moderate in groups 4 and 6, where severe in group 2. Ingroups 6 and 7 rats which received5 and 10 µg/g/day of Pt NPs and group 5 rats which received 1.56 g/kg of AAE. Mild symptoms aroused and no deaths occurred except in group 2. The kidney and liver postmortem report of the groups 2–7 explained moderate to severe fatty change, congestion and/or necrosis. The groups 2, 4 and 6 rats showed severe necrosis of kidneys and liver whereas group 1 had no clinical signs. The observed changes in the body weight of rats were presented in Table 3 where the groups 4, 5 and 7 showed increased body weight significantly (P < 0.05) and group 6 exhibits significant decrease in body weight when compared to group 1 (control). NS = not significant. *Significant (P < 0.05).
S. No.
Treatment groups
After 7 days
No. of rats/group
No of rats died
Mortality (%)
1
Control (normal diet)
6
0
0
2
Anbara (0.78 g/kg/day)
6
0
0
3
Anbara (1.56 g/kg/day)
6
0
0
4
PtNPs (5 µg/g/day)
6
0
0
5
PtNPs (10 µg/g/day)
6
0
0
After 21 days + CCl4 induction on 7th day
1
Control (normal diet)
6
0
0
2
CCl4 control
6
1
16.6
3
Silymarine control
6
0
0
4
Anbara (0.78 g/kg/day)
6
0
0
5
Anbara (1.56 g/kg/day)
6
0
0
6
PtNPs (5 µg/g/day)
6
0
0
7
PtNPs (10 µg/g/day)
6
0
0
S.No.
Treatment groups
Body weight (g)
0 dayBody weight (g)
7 dayBody weight (g)
14 dayBody weight (g)
21 dayBody weight gain (g)
After 21 days
1
Control (normal diet)
135.0 ± 6.1
143.0 ± 5.9
146.3 ± 5.2
154.3 ± 9.9
19.3 ± 5.1
2
CCl4 control
133.0 ± 8.0
140.0 ± 6.9
144.7 ± 7.3
148.7 ± 13.4
15.7 ± 7.2*
3
Silymarine control
130.2 ± 3.0
141.0 ± 5.8
143.5 ± 5.7
147.53 ± 5.9
17.3 ± 2.8 NS
4
Anbara (0.78 g/kg/day)
134.0 ± 9.4
135.3 ± 8.8
140.7 ± 6.9
149.9 ± 9.8
15.9 ± 8.4 *
5
Anbara (1.56 g/kg/day)
134.7 ± 9.3
144.0 ± 9.3
152.3 ± 8.9
160.75 ± 10.0
26.0 ± 5.2*
6
PtNPs (5 µg/g/day)
130.0 ± 4.0
135.0 ± 5.0
139.5 ± 1.0
142.5 ± 0.0
12.5 ± 3.0*
7
PtNPs (10 µg/g/day)
135.3 ± 10.4
142.0 ± 6.9
145.7 ± 4.8
152.7 ± 4.8
17.4 ± 7.2 NS
3.8 Hematological analysis
The hematological data after 3 weeks of treatment with Pt NPs and AAE after CCl4 induction on 7th day are presented in Table 4. Hematological changes of treated rats with low dose and high dose showed significant decrease in mean concentration of Hb and RBCs and the P-value (P < 0.05) was compared with untreated group. There was a significant increase mean concentration of WBCs and lymphocytes of low and high dose treated rats when compared with untreated group (P < 0.05). Except in group 5, other hematological parameters showed no significant change. The correlated concentrations of HB, RBC and WBC for all the groups after the treatment of rats with PtNPs and AAE for 3 weeks were shown in (Fig. 12). Values are expressed as mean ± S.E; NS = not significant; *Significant = (P < 0.05); n = 6 rats per group.
Parameters
Groups
1. Control (normal diet)
2. CCl4 Control
3. Silymarin Control
4. Anbara (0.78 g/kg/day)
5. Anbara (1.56 g/kg/day)
6. PtNPs (5 µg/g/day) (low dose)
7. PtNPs (10 µg/g/day) (high dose)
Hb (g/dl)
15.3 ± 0.4
13.0 ± 2.6*
15.3 ± 1.4NS
13.8 ± 0.3*
13.0 ± 0.6*
14.4 ± 0.6NS
13.6 ± 0.1*
RBC (×106 mm3)
8.6 ± 0.3
7.3 ± 1.4*
7.8 ± 1.4NS
8.1 ± 0.2NS
7.7 ± 0.7NS
8.1 ± 0.4NS
7.7 ± 0.1*
PCV (%)
48.0 ± 1.3
41.0 ± 3.1NS
49.0 ± 1.6NS
43.9 ± 1.1 NS
41.1 ± 4.5 NS
45.6 ± 1.8NS
43.0 ± 0.5*
MCV (m3)
55.6 ± 0.4
55.6 ± 1.0NS
54.2 ± 0.2NS
54.0 ± 0.9NS
53.5 ± 1.3NS
56.3 ± 0.4NS
55.2 ± 0.9NS
MCH (pg)
17.6 ± 0.3
17.4 ± 0.4 NS
17.2 ± 0.6NS
16.9 ± 0.2NS
16.8 ± 0.6NS
17.7 ± 0.1NS
17.4 ± 0.3 NS
MCHC (%)
31.8 ± 0.3
31.4 ± 0.4 NS
31.7 ± 1.0NS
31.4 ± 0.1NS
31.5 ± 0.4NS
31.5 ± 0.2NS
31.6 ± 0.1NS
WBC (×106 mm3)
11.5 ± 0.8
9.7 ± 2.3*
11.3 ± 1.8NS
11.0 ± 1.1NS
7.8 ± 0.4*
14.8 ± 3.3*
15.3 ± 2.8*
Lymphocytes (%)
53.9 ± 0.7
58.3 ± 0.5*
50.9 ± 0.6 NS
68.9 ± 2.0*
58.8 ± 3.4*
55.1 ± 4.5NS
66.6 ± 0.3*
Granulocytes (%)
46.1 ± 0.7
41.7.7 ± 0.5*
49.1 ± 6.0NS
32.1 ± 2.0*
41.2 ± 0.3NS
44.9 ± 4.5NS
33.4 ± 0.3 *
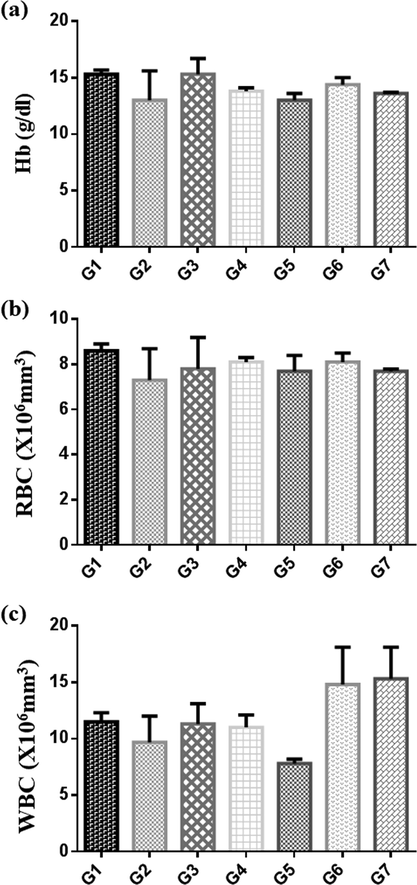
Graphs of concentrations of HB (a), RBC (b) and WBC (c), after treatment of rats with Pt NPs and Anbara aqueous extract for 3 weeks as shown in Table 4. Groups expressed as mean ± SR.
3.9 Serobiochemical analysis
The results of Serobiochemical changes for the given pre and post-treated rats with Pt NPs, and AAE for 21 days with a dose of CCl4 (3 ml/kg body weight) on day 7, are presented in Table 5. After 21 days of treatment, significant decrease in activity of AST and increase in ALP in treated groups than in CCl4 control (group 2). ALT increase in group 4 and decrease in group 7 (Fig. 13). The concentrations of total protein and creatinine in group 6 and 7 were significantly decreased than in CCl4 control. While the concentration of urea showed no significant change when compared with CCl4 control (Fig. 14). Values are expressed as mean ± S.E; NS = not significant; *Significant = (P < 0.05); n = 6 rats per group.
Parameters
Groups
1. Control (normal diet)
2. CCl4 Control
3. Silymarin Control
4. Anbara (0.78 g/kg/day)
5. Anbara (1.56 g/kg/day)
6. Pt NPs (5 µg/g/day) (low dose)
7. Pt NPs (10 µg/g/day) (high dose)
AST (IU)
300.3 ± 18.9
396.3 ± 44.7*
250.7 ± 0.4*
308.7 ± 24.9
273.7 ± 27.3*
286.7 ± 21.9*
276.0 ± 31.0*
ALT (IU)
59.7 ± 7.5
60.7 ± 10.5 NS
60.0 ± 4.5 NS
72.3 ± 5.6*
53.3 ± 5.9*
67.7 ± 12.3*
48.5 ± 2.5*
ALP (IU)
127.7 ± 31.3
216.0 ± 9.1 *
128.0 ± 5.9*
247.7 ± 4.5*
358.8 ± 5.5*
281.0 ± 5.5*
234.3 ± 5.9*
Total protein (g/dl)
8.1 ± 0.3
7.5 ± 0.1NS
8.2 ± 0.4NS
7.5 ± 0.1NS
7.7 ± 0.4NS
6.7 ± 0.3*
7.0 ± 0.1*
Albumin (g/dl)
3.7 ± 0.1
3.3 ± 0.0 NS
3.6 ± 0.2NS
3.3 ± 0.1 NS
3.2 ± 0.2NS
3.2 ± 0.1*
3.1 ± 0.0*
Globulin (g/dl)
4.4 ± 0.3
4.2 ± 0.1NS
4.3 ± 0.3 NS
4.2 ± 0.2 NS
4.5 ± 0.2 NS
3.5 ± 0.2 *
3.9 ± 0.1 NS
Bilirubin (mg/dl)
0.7 ± 0.7
1.9 ± 0.3*
1.5 ± 0.3*
1.7 ± 0.9*
1.4 ± 0.2 *
1.6 ± 0.3*
1.3 ± 0.2*
Creatinine (mg/dl)
0.3 ± 0.1
0.6 ± 0.1 NS
0.6 ± 0.1 NS
0.6 ± 0.0 NS
0.7 ± 0.1 NS
0.3 ± 0.1*
0.3 ± 0.0*
Urea (mg/dl)
29.7 ± 3.0
39.7 ± 2.3*
32.3 ± 4.1 NS
29.0 ± 1.7 NS
36.7 ± 7.0*
29.7 ± 4.4 NS
32.5 ± 1.5 NS
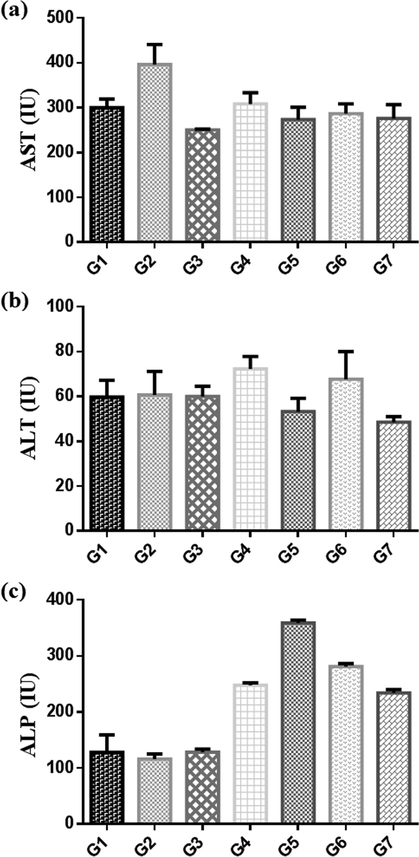
Comparitive activity of enzymes (a) AST, (b) ALT and (c) ALP, after treatment for 3 weeks with Pt NPs and aqueous Anbara extract in control group (G1), CCl4 (G2), Silymarin control (G3), low does Anbara 0.78 g/kg (G4), high dose Anbara 1.56 g /kg (G5), low does Pt NPs 5 µg/g (G6) and high dose Pt NPs 10 µg/g (G7).
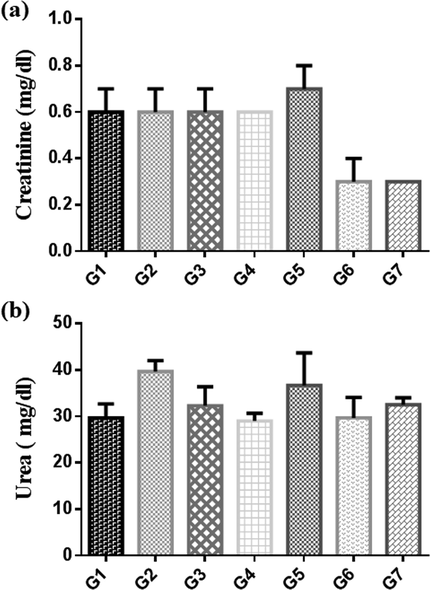
Concentrations of (a) urea and (b) creatinine, after treatment for 3 weeks with Pt NPs and AAE in control (G1), CCl4 (G2), Silymarin control (G3), low does AAE 0.78 g/kg (G4), high dose AAE 1.56 g/kg (G5), low does Pt NPs 5 µg/g (G6) and high dose Pt NPs 10 µg/g (G7). Groups expressed as mean ± SR.
3.10 Histopathological changes
Microscopic examinations after 3 weeks of treatment with Pt NPs and AAE under CCl4 induction on 7th day are summarized in Table 6 and presented in Figs. 11, 15–19. The results from various treatment groups revealed that the hepatocytes and the cells of the renal proximal convoluted tubules contained fatty vacuoles, showed hepatocellular degeneration, cytoplasmic vacuolar diffusion (Fig. 15), vacuolar degeneration of the glomerular tufts and necrosis of CCl4 (Fig. 16), and showed less damage of hepatocytes and low index of changes in treated groups including mild catarrhal enteritis with infiltration of lymphocytes in the lamina propria, fatty cytoplasmic vacuolation of hepatocytes and epithelial cells of the renal tubules with varying degrees of cellular infiltration in groups 4 and 6 (Fig. 17). These changes were less marked in groups 5 and 7 (Figs. 18 and 19). No significant lesions appeared in Control rats (Group1) and no death occurred among the rats recorded along with the treatment. *Cytoplasmic fatty change in low and high dose dates; Semi normal = Semi similar to the control group.
Treatment groups
Histopathological results
Experiment
liver
kidney
intestine
1. Control (normal diet)
Not given any thing (not effected)
Normal
Normal
Normal
2. CCL4 control
Given Carbon tetra chloride (CCl4) after 7th day
Severe damage
Severe damage
Abnormal
3. Silymarine control
Given sylimarine after 7th day
Better than CCl4
Semi normal
Semi normal
4. Anbara 0.78 g/kg/day)
This group given Low dose Anbara from day 1 to day 7 before given CCl4
Abnormal liver better than CCl4
No treated (but better than CCl4)
Better than CCl4
5. PtNPs (5 µg/g/day)
This group given low dose platinum nanoparticles (PtNPs) from day 1 to day 7 before given CCl4
Abnormal
Abnormal
Semi normal
6. Anbara 1.56 g/kg/day)
This group given high dose Anbarabefore and after CCl4
Semi normal
Medium effect
Semi normal
7. PtNPs (10 µg/g/day)
Given platinum nanoparticles before and after CCl4
Semi normal
Semi normal
Abnormal
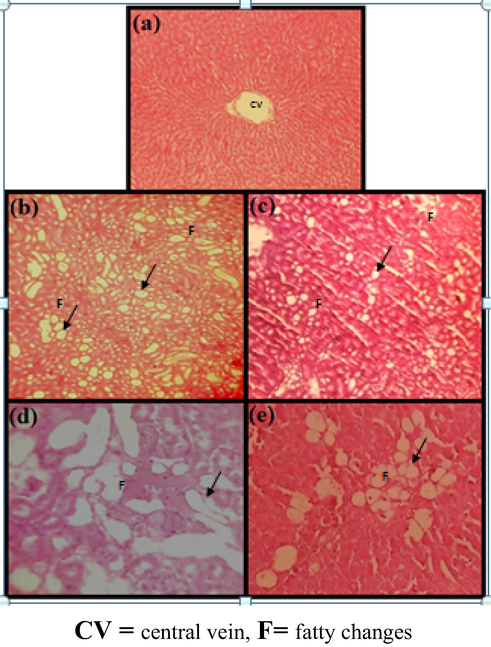
Liver damage comparison in normal control and CCl4 induction single dose 3 ml/kg subcutaneous (s.c), (a) liver of normal rat (b, c) CCl4 control × 100, (d, e) CCl4 control × 200 showing fatty cytoplasmic vacuolation of entrilobularhepatocytes. (H & E) × 100.
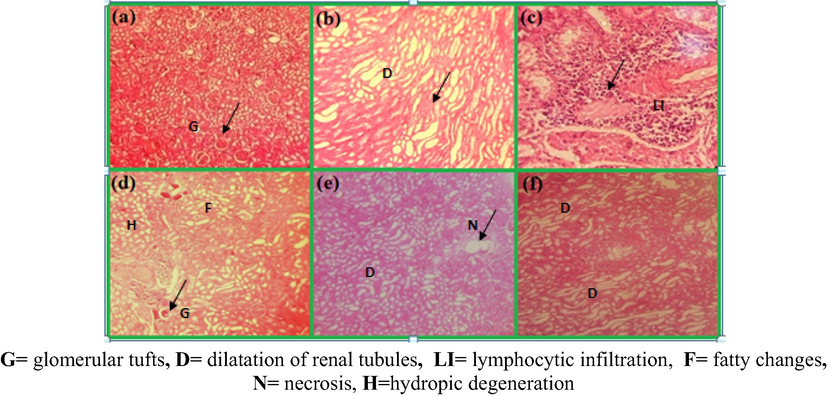
Comparison of kidneys of rats after 3 weeks of oral doses of Pt NPs and AAE, (a) kidney of normal rats (no change), (b) CCl4 control × 100, (Sever cytoplasmic fatty vaculation and dilatation of renal tubules in cortex), (c) represents CCl4 control × 200, (severe lymphacytic infiltration),(d) 0.78 g/kg AAE- (cytoplasmic fatty vacuolation change), (e) 1.56 g/kg AAE, (f) PtNPs (high dose degeneration of glomeruli and dilatation of renal tubules in cortex) (H & E) × 100.
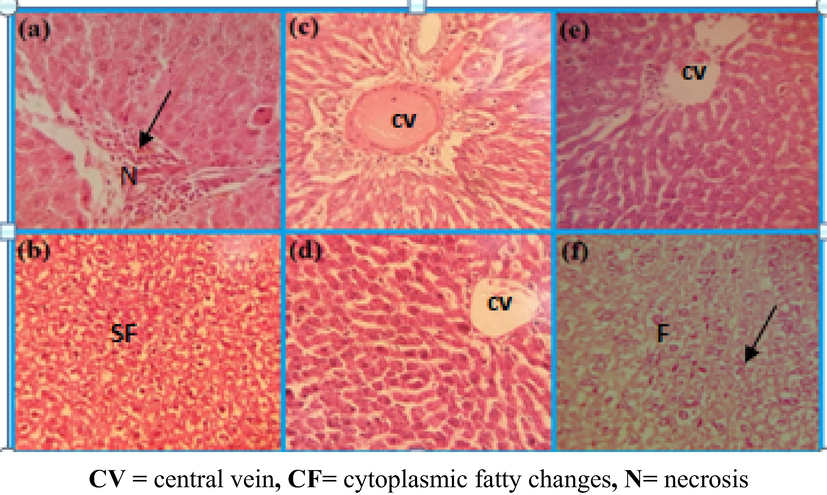
Comparison of liver damage in rats after providing daily high oral dose of PtNPs (10 µg/g) for 3 weeks, (a, b) CCl4 control single dose (shows fatty changes, necrosis and lymphocytic infiltration) (c, d) PtNPs, before and after CCl4induction (shows view cytoplasmic vacuolation of entrilobularhepatocytes and necrosis of the central vein) (e, f) represents PtNPs (shows semi similar to the control group) (H & E) × 100.
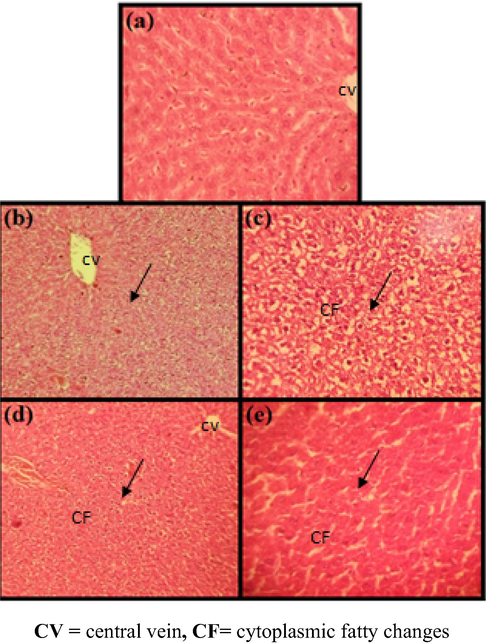
Liver images of rats receiveda daily oral low dose of PtNPs (5 µg/g) for 3 weeks and CCl4induced on 7th day. (a) No lesions were observed in hepatocytes, (b, c) and Cytoplasmic fatty vaculation of the interlobular hepatocytes, (d, e) Semi normal H & E (×100).
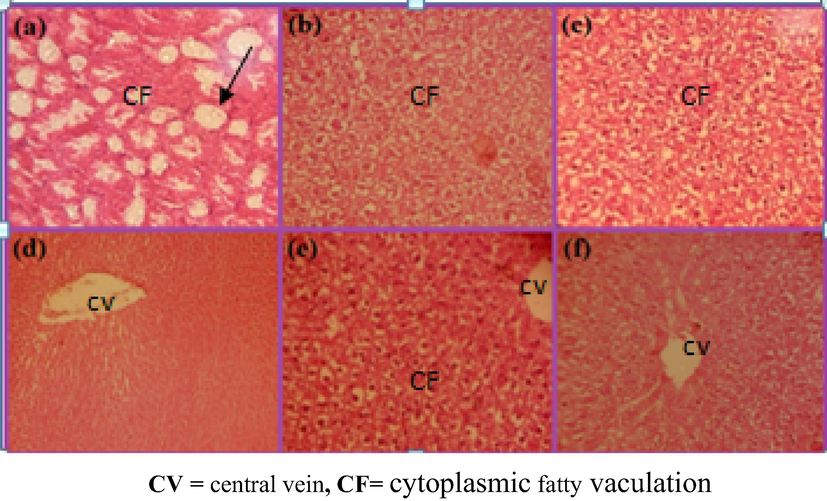
Liver images of rats received a daily oral low dose of AAE (0.78 g/kg) for 7 days and CCl4induced on 7th day showing, Cytoplasmic fatty vaculation(a, b, c) and high dose (1.56 g/kg) for 21 days and CCl4 on 7th day showing less cytoplasmic fatty vaculation(d, e, f) (H & E) × 100.
4 Discussion
Date palm fruits are rich source of different nutritional compounds like proteins, minerals, lipids, carbohydrates and polyphenolic compounds (Behija et al., 2011; El et al., 2014; Park et al., 2010). Consumption of these fruits naturallyare beneficial due to its carbohydrates content and great potential as a medicinal food (Al-orf et al., 2012; Bankar et al., 2010; Meena and Chouhan, 2015) contains antioxidants (El et al., 2014; Taylor et al., 2012), and protective towards human health. The beneficial roles of Pt NPs in green synthesis attributed for its antioxidant, anticancer, antimicrobial activity, and protective effect on reactive oxygen species (Ahmed and Hasona, 2008; Arem et al., 2013; Aswathy Aromal and Philip, 2012; Bouhlali et al., 2017; Fang et al., 2009; Hamad et al., 2015; Kostova, 2006; Shrinath et al., 2011). But lack of toxicity information and no research reports till yet leads us to investigate the safety and possible side effects of Pt NPs. The present work was carried out to investigate the toxic and protective effects and histopathological studies of Pt NPs and aqueous Anbara Fruit extract at different doses on CCl4induced liver damage in Wistar rats.
Hepatic damage due to CCl4 intoxication was assessed by employing hematological and biochemical parameters. In addition, CCl4-induced pathological changes in liver and kidneys were evaluated by histopathological studies. Significant changes in mean body weight of treated groups were observed when compared with control. After 3 weeks of administration of the Pt NPs and aqueous Anbara extract, the body weight significantly decreased (P < 0.05) in groups 4, 6 and 7, when compare to group 1 and 2. Where the higher dosed group 5 gain weight when compared to group 1 (Table 3). The decrease in body weight may be due to decrease of total protein or attributed to gastroenteritis but actual mechanism for the loss of weight is not clear.
The serum alanine transferase (ALT), aspartate amino transferase (AST) and alkaline phosphatase (ALP) enzymes of liver exhibits enhanced activity after exposed with a single dose of CCl4 which indicated the liver toxicity with CCl4 (Kim et al., 2012; Martins et al., 2012; Salim et al., 2018; Xiao et al., 2018). These enzymes ALT, AST and ALP activity in rats significantly decreased (p ≤ 0.05) when pretreated and post-treated with Pt NPs than in CCl4 treated rats. Decrease in the concentration of serum creatinine and bilirubin also observed in rats with pre and post treated Pt NPs than in CCl4 treated rats. This indicates the hepatocytic cell membrane structural integrity protection or damaged liver cells regeneration and restoration of the normal functioning of the poisoned liver, which further protect against CCl4 hepatotoxicity (Nadaroglu et al., 2017). The administration of Pt NPs in all treated groups enhanced the kidney tissue and concentration of creatinine and bilirubin compared to CCl4 group. No significant effect occurred in blood biochemical parameters (Kim et al., 2012). Histopathological examinations showed necrosis of central vein, fatty cytoplasmic vacuolation of entrilobular hepatocytes, hepatocellular degeneration, renal tubular necrosis and fatty degeneration in rats treated with CCl4 (Fig. 15).
The histological examination of rats pretreated with the PtNPs, exhibited good liver protection against the toxicant rats. But with pretreated AAE exhibited no significant liver protection against the CCl4. Pretreated and post-treated with PtNPs and aqueous Anbara extract, were exhibited excellent protective effect in the kidney and liver in all the groups as evidenced by the normal hepatocytes, absence of necrosis and less cytoplasmic fatty vaculation (Figs. 16–19). Our findings demonstrate that PtNPs, has a potent hepato-protective effect on CCl4 induced liver injury by improving the change in histopathological structure and enhancing liver enzyme activity in rats. The mechanism for hepatoprotective activity by PtNPs is not assured. Oral administration of AAE at a dose of 1.56 g/kg/day attenuate the elevated level of enzymes (AST and ALT) produced by CCl4, caused subsequent recovery towards normalization like silymarin. The rats pretreated with AAE (0.78 g/kg/day), showed a good recovery. All the obtained values were evaluated with control animals (Table 5).
The decrease levels of serum marker enzymes and serum bilirubin concentration in pre and post-treated rats with AAE (1.56 g/kg/day) indicates the liver protective effect of Anbara against CCl4 poisoning. The AAE administration reinstate physiological integrity of hepatocytes, thus normalize the values of transaminases in serum. This suggests that the free radical and antioxidant properties of aqueous Anbara extract palliate the liver injuriespossibly by antioxidative effect. Hence the CCl4induced harmful effects of toxic metabolites were eliminated. These findings indicate the protective effect of AAE against CCl4 induced liver damage in rats (Kim et al., 2012; Nadaroglu et al., 2017; Salim et al., 2018).
From the in vivo study the significant decrease in body weight in all the groups when administered the Pt NPs and Anbara extract in various doses (low and high doses) orally for 7 days in Wistar rats indicate the decrease in total protein compared to control (group-1). In group-3, increase in the body weight-gain (Table 5) indicates the interference of Anbara in growth processes. There is no significant change occurred in biological parameters, toxic effects or damage on the vital organs, liver and kidney (Schmid et al., 2007).
It is assumed that the secondary metabolites of Anbara fruits are non toxic or they might be low molecular weight components that can be easy to pass through and excrete by kidneys. Hence Anbara fruits showed non toxic effects on Wistar rats. Even at higher concentrations the Anbara extract doesn't affected the vital organs and there is no significant changes in parameters occurred upon administration.
All the results in the present study explained that the Pt NPs and Anbara fruits extract are non-toxic to rats and protects the liver against CCl4 toxicity.
5 Conclusion
In summary, the best types of Saudi Anbara dates contained higher antioxidants were used for the biosynthesis of Pt NPs and aqueous Anbara extract. The flavonoids, amino acids, phenols, vitamins and minerals present in the aqueous extract act as both reducing and capping/stabilizing agents supports the synthesis of Pt NPs. These synthesized Pt NPs and aqueous Anbara extract were characterized using UV–visible, FTIR, TEM, XRD and EDX spectroscopic analytical techniques. The histopathological and biochemical examinations concluded that the Pt NPs have considerable potential in healing and regeneration of liver cells. Thus, it can be used as not toxic, potent liver tonic and efficient to utilizein the treatment of diseases. The aqueous Anbara extract obtained its protective nature on rat liver from CCl4induced injury. By observing the results, we recommend Pt NPs and aqueous Anbara extractfor potential therapeutic use. Further studies can be used to develop possible mechanisms and the hepatoprotective activity can be used to compose standard therapeutic doses.
Acknowledgment
The authors desired to express gratitude to the Department of Chemistry, Taibah University and are thankful to the King Fahd Medical Research Center, Jeddah. Authors are greatly indebted to Ms. Sara Hassan for the laboratory work and all people who provided the invaluable assistance directly or indirectly, throughout the course of this work. This work was supported by the Bio-technology Research Center, Al Neelain, University, Khartoum, Sudan.
References
- Liver anatomy liver anatomy surgery hepatic vasculature biliary tree. Surg. Clin. NA. 2010;90:643-653.
- [CrossRef] [Google Scholar]
- Plants: green route for nanoparticle synthesis. Int. Res. J. Biol. Sci. Sept. I. Res. J. Biol. Sci. 2012;1:2278-3202.
- [Google Scholar]
- Adam, S.I.Y., Idris, I.A., Abdelgadir, E.H., Ahmed, R.H., Kamal, E.E., 2011. Evaluation of Hepatoprotective Activity of Argemone mexicana Aerial Part Extracts on CCL 4 Induced Liver Damage in Wistar Rats. 2, pp. 251–256.
- Ahmed, M.B., Hasona, N.A., 2008. Protective effects of extract from dates (Phoenix Dactylifera L.) and ascorbic acid on thioacetamide-induced hepatotoxicity in rats. 7, pp. 193–201.
- Al-farsi, M., Alasalvar, C., Al-abid, M., Al-shoaily, K., 2007. Food chemistry compositional and functional characteristics of dates, syrups, and their by-products. 104, pp. 943–947. https://doi.org/10.1016/j.foodchem.2006.12.051.
- Al-orf, S.M., Ahmed, M.H.M., Atwai, N.A., Ali, H., Dehwah, A., Dehwah, S., Arabia, S., 2012. Review: nutritional properties and benefits of the date fruits (Phoenix dactylifera L.). Bull. Natl. Nutr. Inst. Arab Repub. Egypt. 2012, pp. 97–129.
- Al-qarawi, A.A., Eldin, B., Ali, H., Abdel-rahman, H., 2004. Protective effect of extracts from dates (Phoenix dactylifera L.) on carbon tetrachloride – induced hepatotoxicity in rats. 2, pp. 176–180.
- Green synthesis of platinum nanoparticles using Saudi’s Dates extract and their usage on the cancer cell treatment. Arab. J. Chem.. 2019;12:330-349.
- [CrossRef] [Google Scholar]
- Artichoke (Cynara scolymus L.), mediated rapid analysis of silver nanoparticles and their utilisation on the cancer cell treatments. J. Comput. Theor. Nanosci.. 2018;15:1818-1829.
- [CrossRef] [Google Scholar]
- Al-Radadi, N.S., Al-youbi, A.N., 2018a. One-step synthesis of Au nano-assemblies and study of their anticancer activities. 15, pp. 1–10. https://doi.org/10.1166/jctn.2018.7323.
- Environmentally-safe synthesis of gold and silver nano-particles with AL-Madinah Barni fruit and their applications in the cancer cell treatments. 2018;15:2-9.
- [CrossRef]
- Al-yahya, M., Raish, M., Alsaid, M.S., Ahmad, A., Mothana, R.A., Al-sohaibani, M., Al-dosari, M.S., Parvez, M.K., Rafatullah, S., 2016. Phytomedicine ‘Ajwa’ dates (Phoenix dactylifera L .) extract ameliorates isoproterenol-induced cardiomyopathy through downregulation of oxidative, inflammatory and apoptotic molecules in rodent model. 23, pp. 1240–1248. https://doi.org/10.1016/j.phymed.2015.10.019.
- Green synthesis of platinum nanoparticles that induce cell death and G2/M-phase cell cycle arrest in human cervical cancer cells. J. Mater. Sci. Mater. Med.. 2015;26:1-9.
- [CrossRef] [Google Scholar]
- Althnaian, T., Albokhadaim, I., El-bahr, S.M., 2013. Biochemical and histopathological study in rats intoxicated with carbontetrachloride and treated with camel milk pp. 1–7.
- Nephroprotective effect of date fruit extract against Dichloroacetic acid exposure in adult rats. FOOD Chem. Toxicol. 2013
- [CrossRef] [Google Scholar]
- Aron, M.A.R.K.B., Hahidi, F.E.S., 2005. Comparison of Antioxidant Activity, Anthocyanins, Carotenoids, and Phenolics of Three Native Fresh and Sun-Dried Date (Phoenix dactylifera L.) Varieties Grown in Oman pp. 7592–7599. https://doi.org/10.1021/jf050579q.
- Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2012;97:1-5.
- [CrossRef] [Google Scholar]
- Banerjee, P., Satapathy, M., Mukhopahayay, A., Das, P., 2014. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis, pp. 1–10.
- Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp.. 2010;368:58-63.
- [CrossRef] [Google Scholar]
- Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today. 2014;9:223-243.
- [CrossRef] [Google Scholar]
- Deposition of Pt, Pd, Ru and Au on the surfaces of titanate nanotubes. Top. Catal.. 2006;39:151-160.
- [CrossRef] [Google Scholar]
- Experimental and Toxicologic Pathology Protective effect of date palm fruit extract (Phoenix dactylifera L.) on dimethoate induced-oxidative stress in rat liver. Exp. Toxicol. Pathol.. 2011;63:433-441.
- [CrossRef] [Google Scholar]
- Evaluation of cytotoxic activity of platinum nanoparticles against normal and cancer cells and its anticancer potential through induction of apoptosis. Integr. Med. Res.. 2017;6:141-148.
- [CrossRef] [Google Scholar]
- Functional composition and antioxidant activities of eight Moroccan date fruit varieties (Phoenix dactylifera L.) J. Saudi Soc. Agric. Sci.. 2017;16:257-264.
- [CrossRef] [Google Scholar]
- Green synthesis of soya bean sprouts-mediated superparamagnetic Fe 3O4 nanoparticles. J. Magn. Magn. Mater.. 2010;322:2938-2943.
- [CrossRef] [Google Scholar]
- An effective strategy for the synthesis of biocompatible gold nanoparticles using cinnamon phytochemicals for phantom CT imaging and photoacoustic detection of cancerous cells. Pharm. Res.. 2011;28:279-291.
- [CrossRef] [Google Scholar]
- Chaudhuri, R.G., Paria, S., 2012. Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. pp. 2373–2433.
- Synthesis and characterization of gallium nitride nanoparticles by using solvothermal-soft-chemical methodology. Mater. Sci. Semicond. Process.. 2015;30:435-441.
- [CrossRef] [Google Scholar]
- Czaja, A.J., 2014. Hepatic inflammation and progressive liver fibrosis in chronic liver disease 20, 2515–2532. https://doi.org/10.3748/wjg.v20.i10.2515.
- Nanotechnology meets 3D in vitro models: Tissue engineered tumors and cancer therapies. Mater. Sci. Eng. C. 2014;34:270-279.
- [CrossRef] [Google Scholar]
- Plant mediated green synthesis: modified approaches. Nanoscale. 2013;5:10155.
- [CrossRef] [Google Scholar]
- Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surfaces B Biointerfaces. 2014;122:767-777.
- [CrossRef] [Google Scholar]
- Green synthesis of gold nanoparticles (Elixir of Life) from banana fruit waste extract – an efficient multifunctional agent. RSC Adv.. 2016;6:74620-74629.
- [CrossRef] [Google Scholar]
- Diniz-santos, D.R., Clotildes, M., Melo, N. De, 2004. Acute liver failure complicating viral hepatitis A 8, pp. 180–183.
- Biofabrication of platinum nanoparticles using Fumariae herba extract and their catalytic properties. Saudi J. Biol. Sci. 2015
- [CrossRef] [Google Scholar]
- Beating cancer in multiple ways using nanogold. Chem. Soc. Rev.. 2011;40:3391.
- [CrossRef] [Google Scholar]
- Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem.. 2010;45:1065-1071.
- [CrossRef] [Google Scholar]
- Preparation and characterization of gold nanoparticles prepared with aqueous extracts of Lamiaceae plants and the effect of follow-up treatment with atmospheric pressure glow microdischarge. J. Chem Arab 2016
- [CrossRef] [Google Scholar]
- El-far, A., 2014. The Ameliorative Effect of Phoenix Dactylifera Extract on CCl4 Hepatotoxicity in New Zealand Rabbits The Ameliorative Effect of Phoenix Dactylifera Extract on CCl4 Hepatotoxicity in New Zealand Rabbits.
- Photo-induced green synthesis and antimicrobial efficacy of poly (∊-caprolactone)/curcumin/grape leaf extract-silver hybrid nanoparticles. J. Photochem. Photobiol. B Biol.. 2016;160:355-363.
- [CrossRef] [Google Scholar]
- Hepatoprotective activity of date fruit extracts against dichloroacetic acid-induced liver. J. Funct. Foods. 2014;9:119-130.
- [CrossRef] [Google Scholar]
- Green synthesis of curcumin conjugated nanosilver for the applications in nucleic acid sensing and anti-bacterial activity. Colloids Surfaces B Biointerfaces. 2015;127:274-280.
- [CrossRef] [Google Scholar]
- Fang, B., Chaudhari, N.K., Kim, M., Kim, J.H., Yu, J., 2009. Homogeneous deposition of platinum nanoparticles on carbon black for proton exchange membrane fuel cell pp. 15330–15338.
- Sustainable production of high purity curcuminoids from Curcuma longa by magnetic nanoparticles: A case study in Brazil. J. Clean. Prod.. 2017;154:233-241.
- [CrossRef] [Google Scholar]
- Rapid and green synthesis of bimetallic Au–Ag nanoparticles using an otherwise worthless weed Antigonon leptopus. J. Exp. Nanosci.. 2016;11:395-417.
- [CrossRef] [Google Scholar]
- Biogenic approach using sheep milk for the synthesis of platinum nanoparticles: the role of milk protein in platinum reduction and stabilization. Int. J. Nanosci. Nanotechnol.. 2016;12:199-206.
- [Google Scholar]
- Major essential oil constituents, total phenolics and flavonoids content and antioxidant activity of Salvia officinalis plant in response to nano-titanium dioxide. Indian J. Plant Physiol.. 2015;20:249-256.
- [CrossRef] [Google Scholar]
- Green synthesis of silver and gold nanoparticles using Rosa damascena and its primary application in electrochemistry. Phys. E Low-Dimensional Syst. Nanostruct.. 2011;44:97-104.
- [CrossRef] [Google Scholar]
- Metabolic analysis of various date palm fruit (Phoenix dactylifera L.) cultivars from Saudi Arabia to assess their nutritional quality. Molecules. 2015;20:13620-13641.
- [CrossRef] [Google Scholar]
- Huang, J., He, C., Liu, X., Xiao, Y., 2004. Formation and characterization of water-soluble platinum nanoparticles using a unique approach based on the hydrosilylation reaction metal and semiconductor nanoparticles in the colloidal solution have size-dependent optical, optoelectronic, and materia. pp. 5145–5148.
- Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci.. 2015;8:265-275.
- [CrossRef] [Google Scholar]
- Cancer targeting strategies in nanomedicine: Design and application of chitosan nanoparticles. Curr. Opin. Solid State Mater. Sci.. 2012;16:333-342.
- [CrossRef] [Google Scholar]
- Johnston, D.E., Kroening, C., 1998. Mechanism of Early Carbon Tetrachloride Toxicity in Cultured Rat Hepatocytes, pp. 231–239.
- Green synthesized gold nanoparticles decorated graphene oxide for sensitive determination of chloramphenicol in milk, powdered milk, honey and eye drops. J. Colloid Interface Sci.. 2016;475:46-56.
- [CrossRef] [Google Scholar]
- Cinnamon extract exhibits potent anti-proliferative activity by modulating angiogenesis and cyclooxygenase in myeloma cells. J. Herbal Med.. Elsevier GmbH. 2016
- [CrossRef] [Google Scholar]
- Kim, W., Kim, J., Park, H., Sul, O., Lee, M., 2012. Platinum nanoparticles reduce ovariectomy-induced bone loss by decreasing osteoclastogenesis. 44, pp. 432–439.
- Kostova, I., 2006. Platinum Complexes as Anticancer Agents. 2, pp. 1–22.
- Green synthesis of nano platinum using naturally occurring polyphenols. RSC Adv.. 2013;3:4033.
- [CrossRef] [Google Scholar]
- Green synthesis of gold nanoparticles with Zingiber officinale extract: Characterization and blood compatibility. Process Biochem.. 2011;46:2007-2013.
- [CrossRef] [Google Scholar]
- Kumar, V., Yadav, S.K., 2009. Plant-mediated synthesis of silver and gold nanoparticles and their applications. 176061, pp. 151–157. https://doi.org/10.1002/jctb.2023.
- Mesoporous silica nanoparticles for the improved anticancer efficacy of cis-platin. Int. J. Pharm.. 2012;429:138-147.
- [CrossRef] [Google Scholar]
- Simulation of the optical absorption spectra of gold nanorods as a function of their aspect ratio and the effect of the medium dielectric constant. J. Phys. Chem. B. 2005;109:10531-10532.
- [CrossRef] [Google Scholar]
- Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules. 2013;18:5954-5964.
- [CrossRef] [Google Scholar]
- Majzik, Z., 2008. Surface Structure and Composition of Au - Rh Bimetallic Nanoclusters on TiO2 (110): A LEIS and STM Study, 2, pp. 0–5.
- One pot green synthesis of Ag, Au and Au-Ag alloy nanoparticles using isonicotinic acid hydrazide and starch. Carbohydr. Polym.. 2014;111:734-743.
- [CrossRef] [Google Scholar]
- Martins, A.J., Benelmekki, M., Teixeira, V., Coutinho, P.J.G., 2012. Platinum nanoparticles as pH sensor for intelligent packaging, pp. 97–104.
- A Clean-Green Synthesis of Platinum Nanoparticles Utilizing a Pernicious Weed Lantana (Lantana Camara) Am. J. Eng. Appl. Sci.. 2016;9:84-90.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles from plant (Fenugreek Seeds) reducing method and their optical properties. Res. J. Recent Sci. Res. J. Recent. Sci.. 2015;4:47-52.
- [Google Scholar]
- Antiproliferative effect of novel platinum(II) and palladium(II) complexes on hepatic tumor stem cells in vitro. Eur. J. Med. Chem.. 2012;49:41-47.
- [CrossRef] [Google Scholar]
- Nanomedicine and drug delivery: a mini review. Int. Nano Lett.. 2014;4:94.
- [CrossRef] [Google Scholar]
- International journal of green and a review on green synthesis of nanoparticles and evaluation of antimicrobial activity. Int. J. Green Herb. Chem.. 2014;3:81-94.
- [Google Scholar]
- Plant-derived nanostructures: types and applications. Green Chem.. 2016;18:20-52.
- [CrossRef] [Google Scholar]
- Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci. Rep.. 2018;8:1-12.
- [CrossRef] [Google Scholar]
- Mukherjee, S., Nethi, S.K., Patra, C., 2017. Metadata of the chapter that will be visualized in SpringerLink. https://doi.org/10.1007/978-981-10-3647-7.
- Green synthesis of gold nanoparticles using Curcuma pseudomontana essential oil, its biological activity and cytotoxicity against human ductal breast carcinoma cells T47D. J. Environ. Chem. Eng.. 2014;2:2037-2044.
- [CrossRef] [Google Scholar]
- Green synthesis, characterization and catalytic activity of silver nanoparticles using Cassia auriculata flower extract separated fraction. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2017;179:66-72.
- [CrossRef] [Google Scholar]
- Green synthesis and characterisation of platinum nanoparticles using quail egg yolk. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2017;172:43-47.
- [CrossRef] [Google Scholar]
- Nair, M.G., 2013. Antioxidant and Anti-in fl ammatory Assays Con fi rm Bioactive Compounds in Ajwa Date Fruit.
- Pulmonary Pharmacology & Therapeutics Platinum nanoparticle antioxidants inhibit pulmonary inflammation in mice exposed to cigarette smoke. Pulm. Pharmacol. Ther.. 2009;22:340-349.
- [CrossRef] [Google Scholar]
- Green synthesis of anticancerous honeycomb PtNPs clusters: Their alteration effect on BSA and HsDNA using fluorescence probe. J. Photochem. Photobiol. B Biol.. 2016;162:473-485.
- [CrossRef] [Google Scholar]
- Effects of platinum nanoparticles on the postnatal development of mouse pups by maternal. Exposure. 2010;25:279-286.
- [Google Scholar]
- Honey mediated green synthesis of gold nanoparticles. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2009;73:650-653.
- [CrossRef] [Google Scholar]
- Nanovehicular intracellular delivery systems. J. Pharm. Sci.. 2008;97:3518-3590.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles using lingonberry and cranberry juices and their antimicrobial activity. Colloids Surfaces B Biointerfaces. 2014;121:214-221.
- [CrossRef] [Google Scholar]
- Elettaria cardamomum seed mediated rapid synthesis of gold nanoparticles and its biological activities. OpenNano. 2017;2:1-8.
- [CrossRef] [Google Scholar]
- Phytofabrication of nano-crystalline platinum particles by leaves of Cerbera manghas and its antibacterial efficacy. Int. J. Pharma Biol. Sci.. 2014;5
- [Google Scholar]
- Biogenic gold nanoparticles induce cell cycle arrest through oxidative stress and sensitize mitochondrial membranes in A549 lung cancer cells. RSC Adv.. 2016;6:20598-20608.
- [CrossRef] [Google Scholar]
- Rao, C.N.R., Kulkarni, G.U., John, P., Thomas, P.J., Edwards, P.P., 2000. Metal nanoparticles and their assemblies, pp. 27–35.
- Biogenic synthesis of shape-tunable Au-Pd alloy nanoparticles with enhanced catalytic activities. J. Alloys Compd.. 2018;763:399-408.
- [CrossRef] [Google Scholar]
- Green synthesis, morphological and optical studies of CuO nanoparticles. J. Mol. Struct.. 2017;1150:553-557.
- [CrossRef] [Google Scholar]
- Biological synthesis of platinum nanoparticles: Effect of initial metal concentration. Enzyme Microb. Technol.. 2010;46:501-505.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles in oil-in-water microemulsion and nano-emulsion using geranium leaf aqueous extract as a reducing agent. Colloids Surfaces A Physicochem. Eng. Asp.. 2018;536:60-67.
- [CrossRef] [Google Scholar]
- Protective effect of date palm fruit extract (Phoenix dactylifera L.) on dimethoate induced-oxidative stress in rat liver. Exp. Toxicol. Pathol.. 2011;63:433-441.
- [CrossRef] [Google Scholar]
- A Bifunctional nano-electrocatalyst based on a flower-like gold/palladium bimetallic alloy nanostructure and its graphene hybrid. ChemCatChem. 2015;7:4042-4049.
- [CrossRef] [Google Scholar]
- Growth and characterization of spherical cinnamon nanoparticles: Evaluation of antibacterial efficacy. LWT - Food Sci. Technol.. 2018;90:346-353.
- [CrossRef] [Google Scholar]
- Green Synthesis of Noble Metal (Au, Ag, Pt) Nanoparticles, Assisted by Plant-Extracts. Noble Met. 2012
- [CrossRef] [Google Scholar]
- Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surfaces B Biointerfaces. 2009;73:332-338.
- [CrossRef] [Google Scholar]
- Green fabrication of zirconia nano-chains using novel Curcuma longa tuber extract. Mater. Lett.. 2013;98:242-245.
- [CrossRef] [Google Scholar]
- Influence of platinum, palladium and rhodium as compared with cadmium, nickel and chromium on cell viability and oxidative stress in human bronchial epithelial cells. Environ. Int.. 2007;33:385-390.
- [CrossRef] [Google Scholar]
- Green synthesis of metallic nanoparticles via biological entities. Materials 2015
- [CrossRef] [Google Scholar]
- Shipway, A.N., Katz, E., Willner, I., 2000. Nanoparticle arrays on surfaces for electronic, optical, and sensor applications, pp. 18–52.
- A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.) FRIN. 2011;44:1812-1822.
- [CrossRef] [Google Scholar]
- Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2009;74:735-739.
- [CrossRef] [Google Scholar]
- Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst. Eng.. 2010;33:159-164.
- [CrossRef] [Google Scholar]
- Rapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applications. Bioprocess Biosyst. Eng.. 2012;35:827-833.
- [CrossRef] [Google Scholar]
- Taylor, P., Mukunthan, K.S., Balaji, S., 2012. Cashew apple juice (Anacardium occidentale L.) speeds up the synthesis of silver nanoparticles cashew apple juice (Anacardium occidentale L.) speeds up the synthesis of silver nanoparticles, pp. 37–41. https://doi.org/10.1080/19430892.2012.676900.
- Biological synthesis of metallic nanoparticles. Nanomedicine Nanotechnology. Biol. Med.. 2010;6:257-262.
- [CrossRef] [Google Scholar]
- Green synthesis of platinum nanoparticles using Azadirachta indica – An eco-friendly approach. Mater. Lett.. 2016;170:175-178.
- [CrossRef] [Google Scholar]
- Synthesis and anti-bacterial activity of Cu, Ag and Cu-Ag alloy nanoparticles: A green approach. Mater. Res. Bull.. 2011;46:384-389.
- [CrossRef] [Google Scholar]
- Vayalil, P.K., 2012. Date Fruits (Phoenix dactylifera L.): an emerging medicinal food date fruits (Phoenix dactylifera L.), 8398. https://doi.org/10.1080/10408398.2010.499824.
- Green synthesis of silver nanoparticles aimed at improving theranostics. Radiat. Phys. Chem.. 2018;146:55-67.
- [CrossRef] [Google Scholar]
- Bio-directed synthesis of platinum nanoparticles using aqueous honey solutions and their catalytic applications. Colloids Surfaces A Physicochem. Eng. Asp.. 2011;384:733-738.
- [CrossRef] [Google Scholar]
- A facile synthesis and characterization of Ag, Au and Pt nanoparticles using a natural hydrocolloid gum kondagogu (Cochlospermum gossypium) Colloids Surfaces B Biointerfaces. 2011;83:291-298.
- [CrossRef] [Google Scholar]
- Internalization of ineffective platinum complex in nanocapsules renders it cytotoxic. Chem. - A Eur. J.. 2016;22:2728-2735.
- [CrossRef] [Google Scholar]
- Structurally ordered intermetallic platinum-cobalt core-shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater.. 2013;12:81-87.
- [CrossRef] [Google Scholar]
- In situ green synthesis of Ag nanoparticles on tea polyphenols-modified graphene and their catalytic reduction activity of 4-nitrophenol. Colloids Surfaces A Physicochem. Eng. Asp.. 2015;485:102-110.
- [CrossRef] [Google Scholar]
- Cyclooxygenase-1 serves a vital hepato-protective function in chemically induced acute liver. Injury. 2018;143:430-440.
- [CrossRef] [Google Scholar]
- Compositional dependence of the stability of AuCu alloy nanoparticles. Chem. Commun.. 2012;48:5626-5628.
- [CrossRef] [Google Scholar]
- Structural, magnetic, optical, dielectric, electrical and modulus spectroscopic characteristics of ZnFe2O4spinel ferrite nanoparticles synthesized via honey-mediated sol-gel combustion method. J. Phys. Chem. Solids. 2017;110:87-99.
- [CrossRef] [Google Scholar]
- Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: a review. J. Chem. Arab.. 2020;13:2287-2308.
- [CrossRef] [Google Scholar]
- PEGylation density-modulated anticancer drug release on gold nanoparticles in live cells. J. Ind. Eng. Chem.. 2016;33:345-354.
- [CrossRef] [Google Scholar]
- Yoshida, S., Hiyoshi, K., Ichinose, T., Takano, H., Oshio, S., Sugawara, I., Takeda, K., 2008. Effect of nanoparticles on the male reproductive system of mice, pp. 337–342. https://doi.org/10.1111/j.1365-2605.2008.00865.x
- Facile one-step green synthesis of gold nanoparticles using Citrus maxima aqueous extracts and its catalytic activity. Mater. Lett.. 2016;166:110-112.
- [CrossRef] [Google Scholar]
- Green synthesis of Au-Pd bimetallic nanoparticles: Single-step bioreduction method with plant extract. Mater. Lett.. 2011;65:2989-2991.
- [CrossRef] [Google Scholar]







