Translate this page into:
Green chemistry-based synthesis of gold nanoparticles using Nicotiana plumbaginifolia and their anticancer properties against cervical cancer
⁎Corresponding author. Jungao22@outlook.com (Jun Gao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Although various strategies have been reported for the synthesis of gold nanoparticles (AuNPs) for possible anticancer effects, green synthesis can be considered an efficient strategy for the production of efficient NPs with minimal toxic effects. In this study, we investigate the green synthesis of gold nanoparticles (AuNPs) using an aqueous leaf extract of Nicotiana plumbaginifolia and evaluated their protein/DNA binding, hemolysis characteristics, and anticancer properties against HeLa cervical cancer cells, while normal HUVECs were used as the control sample. Different techniques were utilized to characterize the development of AuNPs. Then, the interaction properties of biosynthesized AuNPs with HeLa cancer cells and HUVECs were assessed by cellular assays. The results showed that the Au nanosphere had an average particle size of 18 nm with four distinctive X-ray diffraction (XRD) peaks at 38.00°, 44.10°, 63.75° and 77.10°. Moreover, the UV–Vis spectrum showed a maximum absorption peak at about 538 nm, corresponding to the surface Plasmon resonance (SPR) characteristics of the AuNPs. Furthermore, an observed Fourier-transform infrared spectroscopy (FTIR) peak at about 769 cm−1 was attributed to the Au—O stretching vibration. Dynamic light scattering (DLS) analysis also showed that the maximum size and zeta potential values for colloid AuNPs were 68.69 nm and −47.92 mV, respectively. Then, it was detected that after incubation of maximum % solution of AuNPs, 0.1, % BSA and %DNA binding values were 58.76 % and 32.15 %, respectively. It was also determined that following incubation of red blood cells (RBC) with maximum concentrations of AuNPs, 20 μM, the hemolysis value was 2.29 %. Cellular assays showed that the IC50 value of AuNPs was 1.53 μM in HeLa cells, whereas this value for HUVEC normal cells was 30.55 μM. It was also shown that incubation of cells with AuNPs for 24 h led to a higher upregulation in the levels of released Cytc. C, caspase-9, and caspase-3 in HeLa cancer cells compared with HUVECs. Then, it was determined that stimulation of ROS generation and subsequent apoptosis, upregulation of Bax/Bcl-2, caspase-9, and caspase-3 mRNA, induced by AuNPs can be reversed by pretreatment of cells with NAC, a potential antioxidant. Finally, it was demonstrated that although the exposure of HeLa cells to AuNPs after 24 h caused a significant reduction in cell viability mediated by apoptosis, pre-treatment of cells with caspase-9 and caspase-3 inhibitors, significantly mitigated apoptosis induction and recovered cell viability. In conclusion, this paper might provide useful information about the medicinal purposes enabled by AuNPs.
Keywords
Gold nanoparticles
Green synthesis
Protein binding
Hemolysis
Anticancer
Cervical
Data availability
Data will be made available on reasonable request.
1 Introduction
Nano-based structures, owing to their very small dimension present unique physicochemical features in comparison with bulk materials, and find different implementations in the field of biomedicine. Artificial engineering of nanoparticles (NPs) is known as one of the fastest-growing subfields of nanomedicine (Zhang, 2015, Ko et al., 2024). NPs have been potentially used in cancer diagnosis and treatment (Baranwal et al., 2023, Ko et al., 2024). The interaction of NPs with the surrounding biological microenvironment plays a key role in their biological responses and thorough investigation of the nature of the contributed forces, we can design properly the nanoscale materials for diagnostic and therapeutic perspectives (Falahati et al., 2023). Based on the increased surface area to volume, nanostructures have potential wanted or unwanted interaction with biological systems and as a result of this, making the right material selections is essential for developing therapeutics based on NPs (Baranwal et al., 2023; Ubanako et al., 2024). Depending on the type of cell and the different uptake pathways or organelles they target, NPs can interact with biological systems in a variety of manners which is associated with their surface modifications, sizes, shapes, and aggregation states. Therefore, the physicochemical properties of NPs are important variables for their utilization in biomedical fields, especially in interaction with protein and cells, which may be responsible for the more substantial pharmacokinetic, pharmacodynamics, and anticancer effects (Kang et al., 2015).
Researchers are currently interested in the green synthesis of metal oxide NPs because this biosynthetic method increases the NPs' biocompatibility and there is no need for costly, hazardous, or carcinogenic chemicals (Chandran et al., 2006; Kumar et al., 2011; Dhas et al., 2012; Sharifi-Rad et al., 2024; Youssef et al., 2024). Biomaterials derived from plants in the formation of NPs serve as capping, stabilizing, and reducing agents. Even tough plant extracts could act as promising stabilizing and reducing agents for the biosynthesis of NPs, detailed research is still required to fully explore how reaction parameters influence synthesis pathways and address challenges facing “green” synthesis (Duan et al., 2024). Indeed, the biosynthesis of NPs could be affected by several factors such as temperature, incubation time, material content, pH, and, most importantly, the synthesis process protocols (Teimouri et al., 2018). The small size and surface charge may be crucial factors that determine the type of interaction of NPs with proteins and cells.
Cervical cancer as a common disease could be controlled and prevented with early detection and treatment. To eradicate cervical cancer, the World Health Organization has determined three essential key actions on populations, targets, and measurements. The best course of action and timing for eliminating cervical cancer have been determined through model predictions conducted by the WHO and a number of other nations. Nonetheless, particular implementation plans must be created based on the regional circumstances. China with a low population coverage and vaccination for cervical cancer shows a high disease burden of cervical cancer (Ji et al., 2023). Therefore, developing potential studies for cervical cancer modulation can be one of the aims of this paper. Also as bloodstream is known as the first entry route of NPs in vivo, investigating the interaction of NPs with the blood proteins, including albumin, hemoglobin, fibrinogen, gamma globulin, and transferrin is important so that the pharmacokinetic properties of NPs could be assessed (Zeinabad et al., 2016). Moreover, the synthesis route, the protocols, and the type of plants may have a crucial effect on the interaction of NPs with DNA (Khan et al., 2023).
Recently, gold NPs (AuNPs) have been widely used in the development of biochemical and therapeutic platforms (Liu et al., 2021). Depending on their physicochemical properties derived from the synthesis route, they might have unique interactions with blood proteins and DNA. Furthermore, induced apoptotic effects were reported upon the interaction of cervical cancer cells (HeLa) with green synthesized AuNPs by Alternanthera sessilis extract (Qian et al., 2019). Also, activation of caspase-mediated signaling pathways in human cervical cancer cells was reported following the interaction with greenly synthesized AuNPs (Baharara et al., 2016). Therefore, the green synthesis of AuNPs can be considered an efficient strategy for cancer treatment (Abed et al., 2023).
Therefore, we have synthesized AuNPs using Nicotiana plumbaginifolia leaf extract, as a member of Solanaceae family, which is also known as tobacco or wild tobacco. Nicotine and nornicotine are highly detected alkaloids found in this species (Duan et al., 2024). Then, biosynthesized AuNPs were analyzed for protein/DNA binding, hemolysis and anticancer activity (HeLa cervical cancer cell line). This study tried to characterize the physicochemical properties of AuNPs synthesized based on green synthesis and further evaluate their interaction with transferrin and cervical cancer cells.
2 Materials and methods
2.1 Materials
Gold chloride trihydrate (III) (HAuCl4·3H2O, CAS No.:16961-25-4), bovine serum albumin (BSA CAS No.: 9048-46-8), deoxyribonucleic acid sodium salt from calf thymus (DNA, CAS No.: 73049-39-5), 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA, CAS No.: 4091-99-0), Z-DEVD-FMK (caspase-3 inhibitor), Z-LEHD-FMK (caspase-9 inhibitor), and MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, CAS No.: 298-93-1] were purchased from Sigma Co. (MO, USA). For DNA and BSA binding assays, 0.01 M tris-HCl buffer solution was used for the preparation of the working solutions. The primers for the quantitative real-time PCR (qPCR) study were obtained from Genscript (China). Dulbecco’s modified Eagle’s medium (DMEM), N-Acetyl-L-cystein (NAC), and fetal bovine serum (FBS) were purchased from Gibco (USA). The medium 199 (M199) was purchased form from HyClone (Thermo Fisher Scientific, Waltham, MA, USA). All other materials were of analytical grade and aqueous solutions were prepared using double-distilled water. The number of AuNPs per liter, the molar absorbance ɛ, and the concentration of AuNPs were determined in terms of the equations previously reported by Tournebize et al. (2011). The working solution of the AuNPs (1 mM) was prepared by dissolving in methanol. The final concentration of methanol in different assays did not exceed 1 %.
2.2 The extraction process of Nicotiana plumbaginifolia leaf
Nicotiana plumbaginifolia leaves taken from the native habitats were washed properly with distilled water and chopped. For the preparation of the aqueous extract of the plant leaves, 10 g of leaves were crushed using a mortar pestle and added with 100 mL distilled water, heated at 70 °C for 10 min, cooled, and filtered using Whatman No. 1 filter paper. Finally, the sample was stored in the refrigerator.
2.3 Green synthesis of AuNPs
10 ml of plant extract was added to 1 mM solution of HAuCl4·3H2O with a final volume of 100 ml. The sample was then stirred for 50 min (100 RPM) at room temperature and centrifuged at 8000 rpm for 20 min. Afterward, the pellet was collected, washed with ethanol and distilled water and dried at 60 °C
2.4 Characterization of greenly synthesized AuNPs
The synthesis of AuNPs was assessed by analyzing the UV absorption range using a UV–vis spectrophotometer (UV–Vis, PerkinElmer – Lambda 750) at the wavelength of 300–800 nm. FTIR analysis was also performed to evaluate the chemical bonds and functional groups. The dried powder of the AuNPs synthesized was mixed with KBr and analyzed by FTIR (Spectrum 65, Perkin Elmer). Transmission electron microscopy (TEM) was used to analyze the morphology and size of the AuNPs. After dissolving the NPs in ethanol, 10 µl of the AuNPs was dropped on the copper-coated carbon grades and left to dry at room temperature. Then, the TEM images were taken on a TEM machine (TECNAI-G-20). The X-ray diffraction (XRD) method was employed to reveal the metal nature of the synthesized AuNPs on an XRD apparatus (Panalytical, X’ pert PRO-MPD, Netherlands) using CuKα radiation (λ = 0.15405 nm). Dynamic light scattering (DLS) study was done to measure the hydrodynamic diameter and zeta-potential of AuNPs using a Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK), equipped with a 633 nm He/Ne laser, scattered data angle of 173°. The AuNPs were filtered (0.2 µm, Milli-Q water) and diluted (1:10 for DLS and 1:1000 for zeta potential).
2.5 Albumin and DNA binding measurements
BSA and DNA binding of greenly synthesized AuNPs were evaluated based on the method reported previously (Duan et al., 2024). Briefly, macromolecule solutions were mixed with different dispread solutions of AuNPs ranging from 0.01 to 0.1 % with a 1:1 ratio. After 30 min, the samples were centrifuged, and the concentration of protein and DNA were determined spectroscopically at 280 and 260 nm, respectively.
2.6 Hemolysis assay
The hemolysis assay for greenly synthesized AuNPs was done based on the method reported previously (Behera and Awasthi, 2021). Blood samples taken from the blood bank were drawn into K2-EDTA-coated Vacutainer tubes. Briefly, isolated red blood cells (RBCs) were treated with different concentrations of AuNPs ranging from 0.01 to 20 µM at 37 °C for 1 h followed by centrifugation at 300 rpm for 5 min. Then, 100 µl of supernatant was removed and the optical density of samples was read at 590 nm using a microplate reader (Epoch, BioTek Instruments, USA). Positive control and negative control were considered as 100 μl of 1 % SDS and 100 μl of PBS, respectively, +50 μl of cells. The hemolysis (%) was calculated based on the equation reported previously (Singh et al., 2020).
2.7 Cell culture
The human cervical cancer HeLa cells and Human umbilical vein endothelial cells (HUVECs) were obtained from the Tumor Hospital of the Chinese Academy of Medical Sciences. HeLa cells were cultured in Dulbecco’s modified Eagle’s medium containing 10 % (v/v) fetal calf serum, 1 % penicillin–streptomycin-glutamine (Invitrogen) in a humidified incubator containing 5 % CO2 at 37 °C. HUVECs as the normal cells were cultured in M199 containing 10 % FBS, 1 ng/ml basic fibroblast growth factor (bFGF; Invitrogen), and 1 % penicillin–streptomycin-glutamine (Invitrogen) at 37 °C with a humidified atmosphere of 5 % CO2 in air.
2.8 MTT assay
The viability of the cells was assessed by MTT assay. The cells (5x103 cells/well) seeded in 96-well plates were treated with AuNPs at different final concentrations of 0.01–20 μM for 24 h. The maximal concentrations of the methanol used in all cellular experiments were 3.0 % (v/v). After incubation, the cells were prepared for MTT assay. 20 μL of MTT solution with a concentration of 5 mg/mL was added to each well and incubated at 37 °C for 4 h. The supernatant was then removed and formazan precipitates were added by 150 μL of dimethyl sulfoxide (DMSO). After 15 min, the absorbance of samples was read at 490 nm on a microplate reader (Epoch, BioTek Instruments, USA).
2.9 ELISA assay
The quantitative determination of cytochrome C (Cyt. C), caspase-3, and caspase-9 in cell lysate of cells treated with 1.5 µM greenly synthesized AuNPs for 24 h was done by ELISA assay using relevant ELISA Kit (Cusabio Biotech Co., LTD, Wuhan, China), according to the instructions.
2.10 Detection of mitochondrial membrane potential (MMP)
For the detection of MMP collapse, cells treated with 1.5 µM greenly synthesized AuNPs for 24 h were incubated with Rh123, 5 μg/ml, for 45 min in the dark at 37 °C. Then, the cells were harvested, suspended in PBS, and the fluorescence intensity changes were determined using a fluorescent microplate reader (XFLUOR4, GENios) with an excitation peak at 508 nm and an emission peak at 528 nm.
2.11 Reactive oxygen species (ROS)-generation assay
Intracellular ROS generation was determined by the DCFH-DA probe. Cells plated in 6-well plates and attached overnight were treated with 1.5 µM greenly synthesized AuNPs for 24 h. The cells were then harvested, washed twice with PBS, resuspended, and incubated with 10 μM DCFH-DA for 15 min at 37 °C. The cells were then washed with PBS, resuspended, and cell fluorescence was read on a fluorescent microplate reader (XFLUOR4, GENios) at excitation and emission wavelengths of 480 nm and 530 nm, respectively. For ROS-related studies, the cells were pretreated with NAC (10 μM) for 2 h.
2.12 RNA extraction and RT-qPCR
After treating the cells with 1.5 µM greenly synthesized AuNPs for 24 h, total RNA was extracted by the standard protocol using TRIzol Reagent (TaKaRa, China) followed by synthesis of the first-strand cDNA using PrimeScript™ RT reagent Kit (TaKaRa, China) based on the manufacturer's protocols. The qPCR reaction was done on a Real-Time machine (Roche, Basel, Switzerland) with a SYBR® Green Premier Pro Taq HS qPCR Kit. All primers used were designed based on the previous study (Xu et al., 2021). The expression of the genes of interest was calculated using the 2−ΔΔCT method.
2.13 Caspase-3 and caspase-9 activity assays
The cells treated with 1.5 µM greenly synthesized AuNPs for 24 h, were harvested for caspase analysis using Caspase-3 and Caspase-9 Colorimetric Assay Kits (R&D System Inc.) in accordance with the manufacturer's instruction. Totally, after cell lysis and centrifugation, the samples were incubated with specific caspase-3 substrate (DEVD-pNA) or caspase-9 substrate (LEHD-pNA) for 1 h at 37 °C and the optical densities of samples were read at 405 nm.
2.14 Determination of effect of caspase inhibitors by MTT and flow cytometry assays
To determine the apoptosis signaling pathway induced by AuNPs, the cells were pretreated with 20 µM each of Z-DEVD-FMK (caspase-3 inhibitor) and Z-LEHD-FMK (caspase-9 inhibitor) for 2 h. Then, cells were incubated with 1.5 µM greenly synthesized AuNPs for 24 h and cell survival was assessed using MTT assay as described above. Also, flow cytometry analysis for the detection of apoptosis was done using the staining of cells with Annexin-V/FITC. For this reason, the cells were washed, suspended in the binding buffer as instructed by the manufacturer's protocol (Sigma, USA), incubated with 1.5 μl of Annexin-V/FITC for 15 min in dark, diluted with PBS, and finally analyzed using FACScan cytometer (FACSCalibur; BectonDickinson, San Jose, CA).
2.15 Statistical analysis
Statistical analysis data were expressed as the means ± standard deviation of three experiments. The one-way analysis of variance assay was done using SPSS software. Values of p < 0.05 were reported statistically significant.
3 Results and discussion
3.1 Characterization of greenly synthesized AuNPs
Fig. 1 shows the characterization of greenly synthesized AuNPs using Nicotiana plumbaginifolia leaf extract by different techniques. The different phytochemicals present in the leaf extract such as alkaloids, polyphenols, and flavonoids serve as reducing as well as capping agents during the synthesis of NPs (Duan et al., 2024, Yadav et al., 2024). The interaction of phytochemicals with metal ions resulted in a color change of the mixture, revealing the fabrication of the NPs (Yadav et al., 2024). Following the combination of gold salt and leaf extract under different reaction conditions, the salt ions not only are reduced upon the interaction with phytochemicals but also covered with different functional groups (Yadav et al., 2024).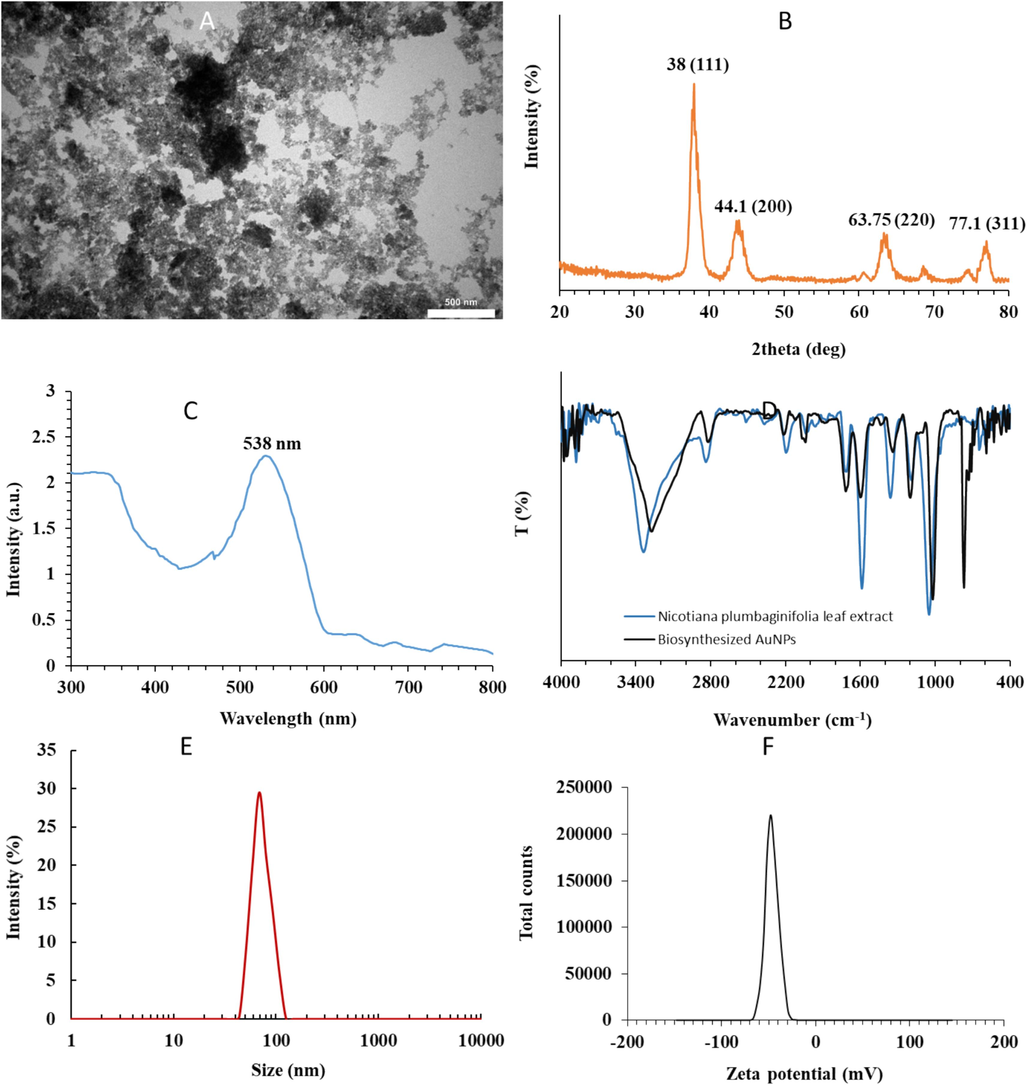
Cauterization of synthesized AuNPs through green chemistry method. (A) TEM, (B) XRD, (C) UV–Vis, (D) FTIR, (E) DLS, (F) Zeta potential measurements.
Fig. 1A exhibits the morphology and size of synthesized AuNPs determined by TEM imaging. It reveals that AuNPs mostly possess spherical-shaped morphology with subtle aggregation. The nanosphere had an average particle size of 18 nm (Fig. 1A). Also, it was seen that there is almost a homogenous distribution of AuNPs with similar geometrical shapes and sizes. Overall, the outcome of TEM of AuNP samples confirmed the successful reduction of Au salt and subsequent NP development. Duan et al. showed that few spherical iron oxide NPs were synthesized using Nicotiana plumbaginifolia leaf extract and the formed NPs had a size of around 15 nm (Duan et al., 2024).
It is also important to investigate the crystal structure of the AuNPs developed and this can be addressed by analyzing the XRD pattern of the NPs. The XRD peaks at 38.00°, 44.10°, 63.75° and 77.10° are indexed to the “(1 1 1), (2 0 0), (2 2 0) and (3 1 1) reflections of FCC (face-centered cubic) structure of metallic gold (JCPDS no. 04-0784)” (Suman et al., 2014), indicating that the biosynthesized AuNPs are developed based on pure crystalline Au (Fig. 1B). Also, it was observed that the peak at (1 1 1) is the most intense peak among other planes, revealing that this plane is the dominant orientation and the biosynthesized AuNPs are crystalline (Suman et al., 2014; Patil et al., 2023).
UV–Vis spectroscopy is an analytical technique that is typically used to characterize the surface Plasmon resonance (SPR) properties of NPs because of the surface plasmon resonance (SPR) phenomenon (Abdelhalim et al., 2012). SPR characteristics known as a collective excitation of the electrons are heavily associated with the size and shape of NPs. Therefore, metallic NPs could display different optical absorption signals in the UV–Vis region (Abdelhalim et al., 2012; Parthiban et al., 2023). The UV–Vis spectrum detected from NP solution after the completion of the biosynthesis process showed a maximum absorption peak at about 538 nm (Fig. 1C), attributing to the SPR features of the AuNPs (Parthiban et al., 2023).
The narrow bandwidth of SPR also indicates the biosynthesized AuNPs might possess a homogenous size distribution. Actinidia deliciosa (Naraginti and Li 2017), Fagopyrum esculentum (Babu et al., 2011), Gloriosa superba (Gopinath et al., 2016) extracts have all been employed to fabricate AuNPs with similar outcomes.
Following the combination of Au salt and leaf extract under different reaction conditions, the salt ions not only reduced following the interaction with phytochemicals but also covered with different functional groups (Yadav et al., 2024) which can be determined by FTIR study.
The FTIR spectra of Nicotiana plumbaginifolia leaf extract and biosynthesized AuNPs measured in the range of 4000–400 cm−1 are shown in Fig. 1D. The Nicotiana plumbaginifolia leaf extract sample shows FTIR bands at 3340, 1713, 1581, 1362, 1189 and 1048 cm−1. For plant extract sample the FTIR band at 1713 cm−1 could be attributed to carbonyl peak, which might be responsible for the reduction of Au ions to AuNPs (Dubey et al., 2010). FTIR bands stemming from the —OH group at ∼3340 and 1048 cm−1, and 1581 cm−1 are indicative of the presence of NH2 groups (Dubey et al., 2010). Comparison between FTIR spectra of Nicotiana plumbaginifolia leaf extract and biosynthesized AuNPs displays some alterations in the wavenumber and the magnitude of the bands. For biosynthesized AuNPs a strong and wide FTIR band was detected at about 3270 cm−1, attributing to the O—H stretching vibration which can be derived from plant extract or absorption of water molecules by NP samples during analysis (Barnawi et al., 2022). Moreover, the typical band at about 1720 cm−1 could be assigned to C⚌ O stretching of a carbonyl group found in plant extract (Rao et al., 2019; Döll et al., 2023). It should be noted that C⚌O band position was shifted to higher wavenumbers relative to plant extract, revealing the interaction and coordination of Au with an oxygen atom in C⚌O (Barnawi et al., 2022). Also, the appeared band at 1020 cm−1 is derived from C—C or C—O stretching vibration (Jayaseelan et al., 2013). An observed peak at about 769 cm−1 is attributed to the Au—O stretching vibration (Suriyakala et al., 2022).
The FTIR analysis verified the capping behavior of Nicotiana plumbaginifolia leaf extract which might impart high stability to the biosynthesized AuNPs.
The DLS instrument can be used to analyze the size of NPs along with the shell thickness of biochemical agents and water molecules enveloping their core surface (Sujitha and Kannan 2013). The average size of the biosynthesized AuNPs was then detected by DLS measurement. The size distribution vs. intensity graph exhibited in Fig. 1E revealed that the maximum size for colloid AuNPs was 68.69 nm. Upon comparison of particle sizes determined by TEM (Fig. 1A) and DLS (Fig. 1E) analyses, we found that the detected particle size of AuNPs by DLS was larger than that of the TEM result. This difference is derived from the inclusion of the bioorganic agents enveloping the surface of the AuNPs in DLS measurement (Sujitha and Kannan 2013). Also, van der Waals forces between NPs appearing in the solution could affect the agglomeration of NPs and their sizes accordingly (Sujitha and Kannan 2013). Also, different sample preparations for microscopic and DLS techniques could result in different size results (Sujitha and Kannan 2013).
Zeta potential value could provide us with some useful information about the surface charge, interaction forces, and stability of the synthesized NPs (Pochapski et al., 2021). The result of the zeta potential value for biosynthesized AuNPs was −47.92 mV (Fig. 1F), indicating the strong stability of the biosynthesized NPs.
3.2 Protein and DNA binding as well as hemolysis assays
Binding assays between BSA and DNA with greenly synthesized AuNPs were evaluated by UV–Vis study. Proteins and DNA exhibit distinctive absorption at 280 nm and 260 nm owing to the presence of aromatic amino acid residues and bases, respectively. Fig. 2A shows the percentage binding of BSA and DNA with AuNPs. It was detected that after incubation of different % solution of AuNPs, 0, 0.01, 0.02, 0.04, 0.06, 0.08, and 0.1 the % BSA binding and %DNA binding were 0.00 %, 6.22 %, 10.19 %, 20.51 %, 32.82 %, 50.69 %, and 58.76 % and 0.09 %, 3.11 %, 13.56 %, 19.34 %,29.14 %, 30.24 %, and 32.15 %, respectively.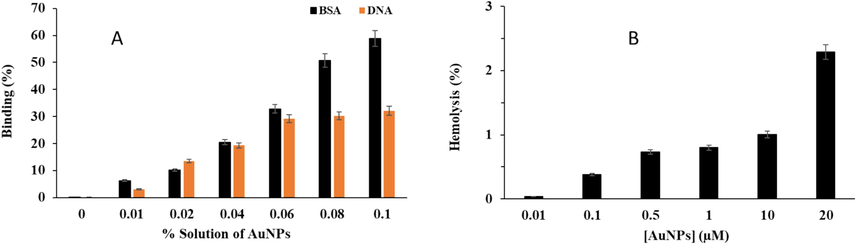
Cauterization of binding of synthesized AuNPs through green chemistry method with DNA and protein as well as hemocompatibility assay. (A) DNA and BSA binding, (B) hemolysis assay.
In general, it was detected that the interaction of AuNPs with biomolecules depends on the concentration of NPs and types of biomolecules. As expected, BSA has a more substantial binding affinity with AuNPs compared with DNA, which might be due to the presence of 20 different amino acid residues in BSA which can be involved in the establishment of different forces with AuNPs, while DNA with four types of purine and pyrimidine bases could contribute in the formation of hydrogen bonding interactions (Duan et al., 2024).
Also, the hemolysis assay was done to analyze the hemocompatibility of biosynthesized AuNPs. It was shown that after incubation of RBCs with different concentrations of AuNPs, 0.01, 0.1, 0.5, 1, 10, and 20 μM, the hemolysis values were 0.03 %, 0.38 %, 0.72 %, 0.79 %, 1.00 %, and 2.29 %, respectively (Fig. 2B). Overall, it was detected that greenly synthesized AuNPs do not significantly trigger hemolytic activity. At the highest concentration of AuNPs, 20 μM, the hemolysis percentage of 2.29 % is detected as shown in Fig. 2B, which is whitin the safe limit and in agreement with the previous studies (Dey et al., 2016; Kumar et al., 2020). The results confirmed that greenly synthesized AuNPs might possess safe therapeutic potency since it inserts minimum hemolytic activity on human RBCs.
3.3 Growth inhibitory effects of greenly synthesized AuNPs
First, the effects of AuNPs on the growth of the human cervical cancer HeLa cells and human umbilical vein endothelial cells (HUVECs) were compared. After 24 h incubation with greenly synthesized AuNPs at concentrations ranging from 0.01 to 20 μM, the MTT assay was carried out. As depicted in Fig. 3, AuNPs were much more efficient as cell proliferation inhibitors in HeLa cells compared to normal HUVEC: the IC50 value of AuNPs was 1.53 μM in HeLa cells, whereas this value for HUVEC normal cells was higher than 20 μM (30.55 μM). Indeed, after incubation of HeLa cells with different concentrations of AuNPs, 0, 0.01, 0.1, 0.5, 1, 10, and 20 μM, the cell viability was reported to be 100.05 %, 91.18 %, 82.11 %, 72.88 %, 64.93 %, 53.05 %, and 45.76 %, while these amounts for normal HUVEC were 100.05 %, 98.37 %, 91.74 %, 89.31 %, 87.33 %, 74.72 %, and 63.27 %, respectively. These data indicate that AuNPs synthesized through green chemistry route from Nicotiana plumbaginifolia leaf extract are likely to be more potent antiproliferative NPs against HeLa calls than normal HUVEC (Fig. 3). These outcomes suggest that the green synthesis of AuNPs can be a potentially important factor in determining the selective antiproliferative efficacy of AuNPs. We can also conclude that the IC50 values of AuNPs depended on the cell type under investigation. Collectively, these results revealed that the synthesis of AuNPs through green chemistry played a key role in mediating the selective proliferation inhibitory effect of AuNPs in cervical HeLa cancer cells. In other words, AuNPs synthesized from Nicotiana plumbaginifolia leaf extract are likely to be more potent antiproliferative nanostructures in HeLa cancer cells than normal HUVEC.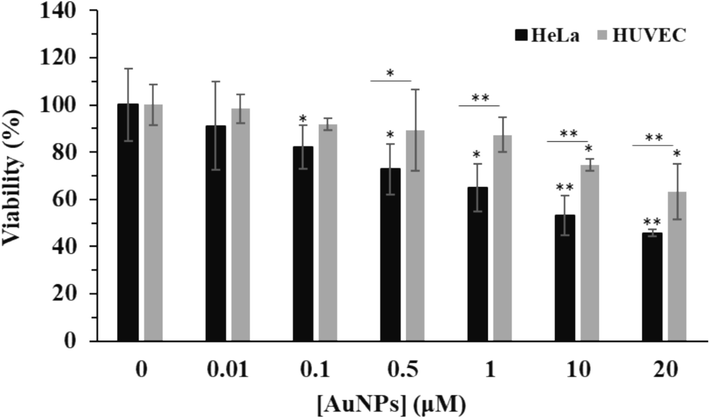
MTT assay for determination of the effects of greenly synthesized AuNPs on the growth of the human cervical cancer HeLa cells and human umbilical vein endothelial cells (HUVECs) after 24 h. *P < 0.05, **P < 0.01, ***P < 0.001 compared to control.
3.4 Induction of apoptosis by AuNPs
Earlier reports have shown that eco-friendly AuNPs mitigated cell growth by triggering apoptosis in different types of cancer cells including cervical cancer cells (Geetha et al., 2013; Qian et al., 2019; Dickson et al., 2023). To further investigate that weather induced growth inhibition by AuNPs synthesized through green chemistry route from Nicotiana plumbaginifolia leaf extract was linked with a capability to stimulate apoptosis, an ELISA test was employed to measure the content of released Cytc. C from mitochondria to cytosol as well as the content of caspase-9 and caspase-3. Cytc. C release and caspase-9 and caspase-3 cleavage are general features of mitochondrial-associated apoptosis. In this study, then the contents of Cytc. C, caspase-9, and caspase-3 in both Hela cancer cells and normal HUVEC were assessed by ELISA assay. Therefore, the cells were exposed to 1.5 µM AuNPs for 24 h and an ELISA assay was performed. It was shown that at 24 h incubation with AuNPs a significant increase in the levels of released Cytc. C (Fig. 4A), caspase-9 (Fig. 4B) and caspase-3 (Fig. 4C) in HeLa cancer cells were observed.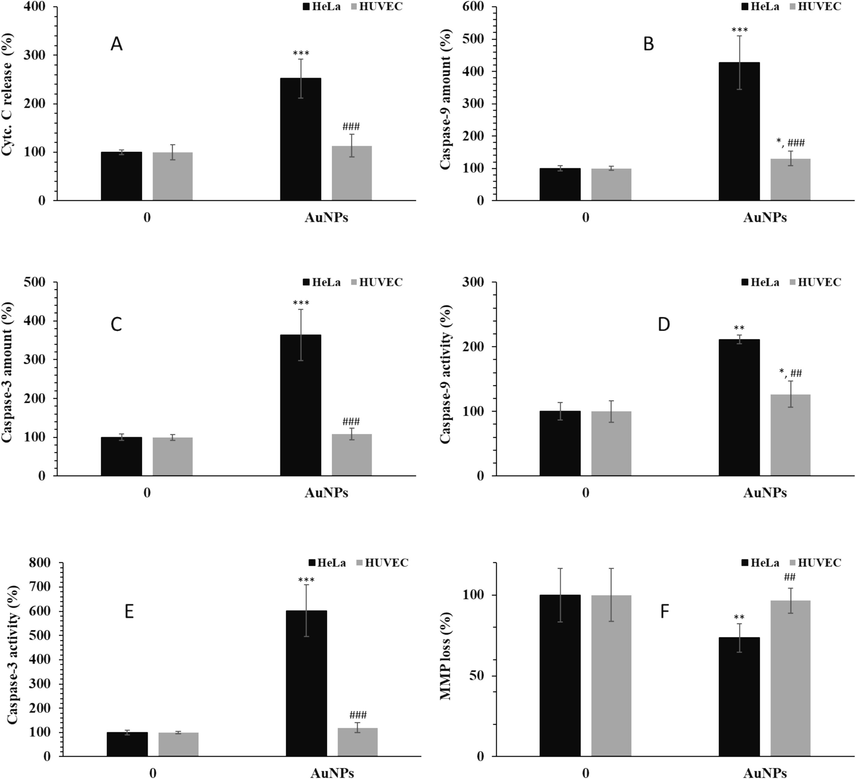
The effects of 1.5 µM greenly synthesized AuNPs on the (A) Cyt. C release, (B) caspase-9 amount, (C) caspase-3 amount, (D) caspase-9 activity, (E) caspase-3 activity, (F) MMP loss in human cervical cancer HeLa cells and human umbilical vein endothelial cells (HUVECs) after 24 h. *P < 0.05, **P < 0.01, ***P < 0.001 compared to control. ##P < 0.01, ###P < 0.001 compared to HeLa cells.
Also, it was found that the elevation of these markers was significantly high in HeLa cancer cells, but not in AuNP-treated HUVEC, compared with control cells. The same trend was also observed for caspase-9 (Fig. 4D) and caspase-3 activity (Fig. 4E) assays. Moreover, the extent of MMP loss, another indicator of mitochondria-mediated apoptosis, induced by AuNPs was further decreased in HeLa cancer cells compared with normal HUVEC (Fig. 4F). Indeed, MMP analysis done after 24 h of incubation of cells with 1.5 μM AuNPs indicated that synthesized AuNPs could induce MMP collapse in HeLa cancer cells, whereas exposure to AuNPs afforded only a subtle MMP loss in normal HUVECs. These data indicate that proliferation inhibition induced by greenly synthesized AuNPs after 24 h was preceded by mitochondria-mediated apoptosis.
3.5 Stimulation of ROS generation and subsequent apoptosis induced by AuNPs
Oxidative stress mediated by NPs has been reported to be associated with apoptosis in various cell lines (Enea et al., 2020; Ozcicek et al., 2021). Also, it has been shown that AuNPs-induced apoptosis is upregulated by stimulation of ROS generation in several cancer cells (Liu et al., 2013; Kondath et al., 2020). To explore whether apoptosis-inducing abilities induced by AuNPs were afforded by changes in ROS-generating possibility, DCF fluorescence intensity as a marker of intracellular ROS level was detected after treatment of cells with 1.5 µM AuNPs for 34 h. AuNPs significantly elevated DCF fluorescence intensity compared to that of negative control HeLa cancer cells, whereas AuNPs showed no such effect in normal HUVECs, thus indicating that AuNPs are more proficient ROS inducers in HeLa cells than normal cells (Fig. 5A). Moreover, pretreatment with NAC (10 µM), a well-known ROS scavenger, remarkably decreased AuNPs-induced ROS generation in HeLa cells (Fig. 5A). These data indicate that greenly synthesized AuNPs are potent ROS inducers in HeLa cancer cells. It was then sought to determine whether AuNPs-induced ROS generation contributed to the induction of apoptosis. For this reason, the expression of Bax, caspase-9, and caspase-3 mRNA (apoptotic markers) and Bcl-2 mRNA (antiapoptotic marker) in AuNPs-treated cells were explored. In fact, it has been reported that inorganic NPs-induced apoptosis is regulated by Bax/Bcl-2, caspase-9, and caspase-3 mRNA expression (Katifelis et al., 2018; Ji et al., 2019), which is indicative of mitochondrial intrinsic apoptotic pathway.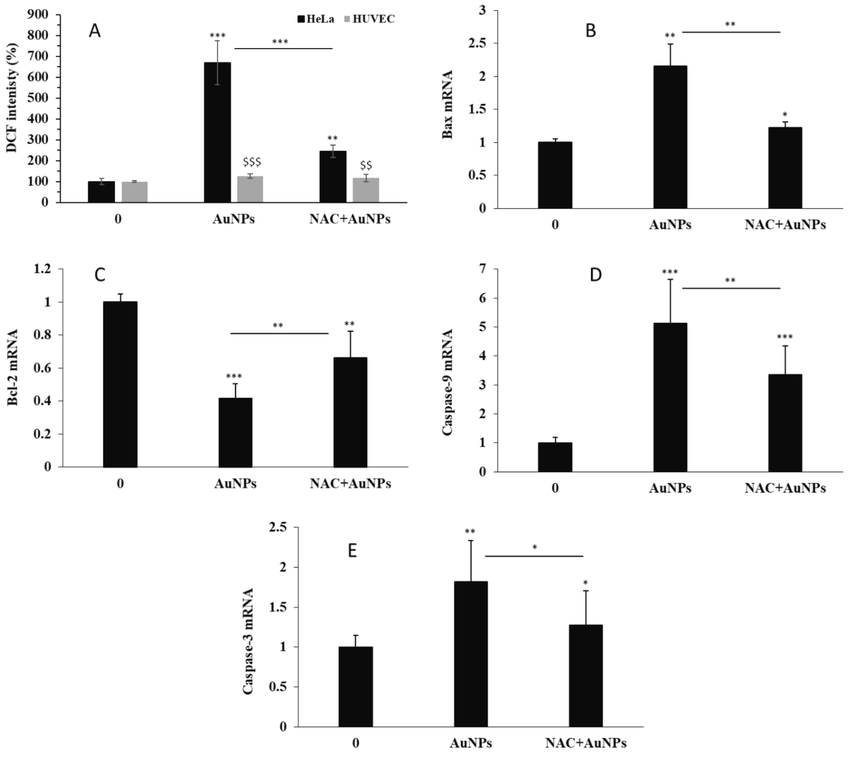
(A) The effects of 1.5 µM greenly synthesized AuNPs alone or with NAC on the ROS generation in human cervical cancer HeLa cells and human umbilical vein endothelial cells (HUVECs) after 24 h. The effects of 1.5 µM greenly synthesized AuNPs alone or with NAC on (B) Bax mRNA, (C) Bcl-2 mRNA, (D) caspase-9 mRNA, (E) caspase-3 mRNA in human cervical cancer HeLa cells after 24 h. *P < 0.05, **P < 0.01, ***P < 0.001 compared to control. $XXX$P < 0.01, $XXX$XXX$P < 0.001 compared to HeLa cells.
As shown in Fig. 5, such upregulation of Bax mRNA (Fig. 5B) and downregulation of Bcl-2 mRNA (Fig. 5C) induced by AuNPs were recovered by pretreatment of HeLa cells with NAC. Also, the caspase-9 (Fig. 5D) and caspase-3 mRNA expression (Fig. 5E) stimulated by exposure of cells to AuNPs were reduced before exposure of HeLa cells to NAC. These data suggested that AuNPs-triggered ROS generation played a key role in initiating apoptotic cell death. In agreement with these outcomes, Kowsalya et al. also reported that greenly synthesized AuNPs from leaf aqueous extract of 10 different plants with particle dimensions ranging between 2 and 26 nm stimulated mitochondria-mediated apoptosis through oxidative stress in breast cancer cells (Kowsalya et al., 2021). Furthermore, Ramalingam et al. revealed that biogenic AuNPs from an aqueous extract of Sesuvium portulacastrum L. stimulated oxidative stress and mitochondrial membrane disruption in A549 lung cancer cells (Ramalingam et al., 2016). Prasad et al. also showed that a low concentration of biosynthesized AuNPs (25 μg mL−1) in combination with glutamine deprivation promoted the growth inhibition if HeLa cancer cells through oxidative stress-mediated mitochondrial damage (Prasad et al., 2022).
3.6 Apoptosis mechanism
To further explore the probable apoptosis mechanism regulated by greenly synthesized AuNPs, we used caspase-9 and caspase-3 inhibitors and then evaluated the apoptosis and cell viability rates in the HeLa cancer cell line. Indeed, to clarify the role of caspases in the AuNPs-induced apoptosis, specific caspase-9 and caspase-3 inhibitors were utilized. It was shown that the percentage of Annexin-V FITC + in AuNPs-treated cells is 4.33-fold higher than that of control cells. However, pre-treatment of cells with specific caspase-3 inhibitor Z-DEVD-FMK and specific caspase-9 inhibitor Z-LEHD-FMK could mitigate the percentage of Annexin-V FITC+ (%) to 2.26- and 2.53-folds compared to the control cells (Fig. 6A). Therefore, it was found that specific caspase-9 inhibitor Z-LEHD-FMK and specific caspase-3 inhibitor Z-DEVD-FMK could block the apoptosis induced by AuNPs.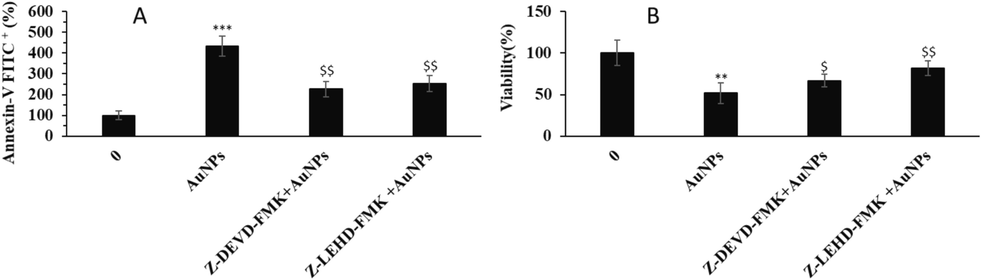
The effects of 1.5 µM greenly synthesized AuNPs alone or in combination with specific caspase-9 inhibitor Z-LEHD-FMK or specific caspase-3 inhibitor Z-DEVD-FMK on (A) apoptosis, (viability) of human cervical cancer HeLa cells after 24 h. **P < 0.01, ***P < 0.001 compared to control. $P < 0.05, $XXX$P < 0.01 compared to HeLa cells.
As intrinsic apoptosis induction and relevant cell death are mediated by the upregulation of caspase-9 and caspase-3, we also evaluated the rate of viable cells by MTT assay. Fig. 6B shows that although the incubation of HeLa cells with 1.5 µM AuNPs resulted in a significant reduction in cell viability after 24 h, pre-treatment of cells with caspase-9 and caspase-3 inhibitors, significantly recovered cell viability. Taken together, these data indicated that greenly synthesized AuNPs induced caspase-mediated apoptosis through the mitochondrial pathway in HeLa cervical cancer cells.
4 Conclusion
This study has investigated the green synthesis of AuNPs using Nicotiana plumbaginifolia leaf extract. Besides, different properties of AuNPs, including protein and DNA binding, hemolysis, and cancer cells and normal cell interactions were assessed. Different characterization techniques showed that the synthesized AuNPs had spherical morphology with an average size of 18 nm with good colloidal stability. Also, it was shown that biosynthesized AuNPs had a potential BSA and DNA binding ability with negligible hemolysis activity. Cellular assays also depicted that ROS generation induced by AuNPs triggers apoptosis through a caspase-mediated pathway in HeLa cervical cancer cells. These findings suggest that AuNPs could be used to treat cervical cancer, but future research should include the use of positive control samples, the study of synergistic effects, and in vivo studies.
CRediT authorship contribution statement
Xiaorong Yang: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Yin Bao: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Xia Zhou: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Hong Zhu: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Jun Gao: Funding acquisition, Formal analysis, Data curation, Conceptualization.
Funding
This project is supported by the National Natural Science Foundation of China (82060477) and the Talent Training Support Plan of Jiangxi Cancer Hospital (the Second Affiliated Hospital of Nanchang Medical College), “Five-level Progressive” talent cultivation project of Jiangxi Cancer Hospital & Institute (WCDJ2024XK01).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Physical properties of different gold nanoparticles: ultraviolet-visible and fluorescence measurements. J. Nanomed. Nanotechol.. 2012;3(3):178-194.
- [Google Scholar]
- Green synthesis of gold nanoparticles as an effective opportunity for cancer treatment. Results Chem.. 2023;5:100848
- [Google Scholar]
- Green synthesis of biocompatible gold nanoparticles using Fagopyrum esculentum leaf extract. Front. Mater. Sci.. 2011;5:379-387.
- [Google Scholar]
- Induction of apoptosis by green synthesized gold nanoparticles through activation of caspase-3 and 9 in human cervical cancer cells. Avicenna J. Med. Biotechnol.. 2016;8(2):75.
- [Google Scholar]
- Biosynthesis and characterization of gold nanoparticles and its application in eliminating nickel from water. J. Mater. Res. Technol.. 2022;17:537-545.
- [Google Scholar]
- Anticancer, antimicrobial and hemolytic assessment of zinc oxide nanoparticles synthesized from Lagerstroemia indica. BioNanoScience. 2021;11(4):1030-1048.
- [Google Scholar]
- Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol. Prog.. 2006;22(2):577-583.
- [Google Scholar]
- Alginate stabilized gold nanoparticle as multidrug carrier: evaluation of cellular interactions and hemolytic potential. Carbohydr. Polym.. 2016;136:71-80.
- [Google Scholar]
- Sargassum myriocystum mediated biosynthesis of gold nanoparticles. Spectrochim. Acta A: Mol. Biomol. Spectrosc.. 2012;99:97-101.
- [Google Scholar]
- Anti-neoplastic effects of gold nanoparticles synthesized using green sources on cervical and melanoma cancer cell lines. BioNanoScience. 2023;13(1):194-202.
- [Google Scholar]
- Green synthesis of gold nanoparticles using peach extract incorporated in graphene for the electrochemical determination of antioxidant butylated hydroxyanisole in food matrices. Biosensors. 2023;13(12):1037
- [Google Scholar]
- Green synthesis of Iron Oxide Nanoparticles using Nicotiana plumbaginifolia and their biological evaluation. J. Mol. Liq. 2024123985
- [Google Scholar]
- Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf. A: Physicochem. Eng. Aspects. 2010;364(1–3):34-41.
- [Google Scholar]
- Gold nanoparticles induce oxidative stress and apoptosis in human kidney cells. Nanomaterials. 2020;10(5):995
- [Google Scholar]
- Engineering of pulmonary surfactant corona on inhaled nanoparticles to operate in the lung system. Nano Today. 2023;52:101998
- [Google Scholar]
- Green synthesis of gold nanoparticles and their anticancer activity. Cancer Nanotechnol.. 2013;4:91-98.
- [Google Scholar]
- Green synthesis of silver, gold and silver/gold bimetallic nanoparticles using the Gloriosa superba leaf extract and their antibacterial and antibiofilm activities. Microb. Pathog.. 2016;101:1-11.
- [Google Scholar]
- Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind. Crop. Prod.. 2013;45:423-429.
- [Google Scholar]
- Green synthesis of gold nanoparticles using a Cordyceps militaris extract and their antiproliferative effect in liver cancer cells (HepG2) Artif. Cells Nanomed. Biotechnol.. 2019;47(1):2737-2745.
- [Google Scholar]
- Pharmacokinetics, pharmacodynamics and toxicology of theranostic nanoparticles. Nanoscale. 2015;7(45):18848-18862.
- [Google Scholar]
- Ag/Au bimetallic nanoparticles induce apoptosis in human cancer cell lines via P53, CASPASE-3 and BAX/BCL-2 pathways. Artif. Cells Nanomed. Biotechnol.. 2018;46(sup3):389-398.
- [Google Scholar]
- Nano-bio interaction: An overview on the biochemical binding of DNA to inorganic nanoparticles for the development of anticancer and antibacterial nano-platforms. Int. J. Biol. Macromol.. 2023;225:544-556.
- [Google Scholar]
- Magnetic nanoparticles for ferroptosis cancer therapy with diagnostic imaging. Bioact. Mater.. 2024;32:66-97.
- [Google Scholar]
- Curcumin reduced gold nanoparticles synergistically induces ROS mediated apoptosis in MCF-7 cancer cells. Inorg. Nano-Metal Chem.. 2020;51(5):601-613.
- [Google Scholar]
- Gold nanoparticles induced apoptosis via oxidative stress and mitochondrial dysfunctions in MCF-7 breast cancer cells. Appl. Organomet. Chem.. 2021;35(1):e6071
- [Google Scholar]
- Facile green synthesis of gold nanoparticles using leaf extract of antidiabetic potent Cassia auriculata. Colloids Surf. B Biointerfaces. 2011;87(1):159-163.
- [Google Scholar]
- Biocompatibility of surface-modified gold nanoparticles towards red blood cells and haemoglobin. Appl. Surf. Sci.. 2020;512:145573
- [Google Scholar]
- Gold nanoparticles trigger apoptosis and necrosis in lung cancer cells with low intracellular glutathione. J. Nanopart. Res.. 2013;15:1-14.
- [Google Scholar]
- Gold nanoparticles: synthesis, physiochemical properties and therapeutic applications in cancer. Drug Discov. Today. 2021;26(5):1284-1292.
- [Google Scholar]
- Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J. Photochem. Photobiol. B Biol.. 2017;170:225-234.
- [Google Scholar]
- The effects of surface functionality and size of gold nanoparticles on neuronal toxicity, apoptosis, ROS production and cellular/suborgan biodistribution. Mater. Sci. Eng. C. 2021;128:112308
- [Google Scholar]
- Green synthesis of gold nanoparticles using quercetin biomolecule from mangrove plant, Ceriops tagal: assessment of antiproliferative properties, cellular uptake and DFT studies. J. Mol. Struct.. 2023;1272:134167
- [Google Scholar]
- Green synthesis of gold nanoparticles via Capsicum annum fruit extract: characterization, antiangiogenic, antioxidant and anti-inflammatory activities. Appl. Surf. Sci. Adv.. 2023;13:100372
- [Google Scholar]
- Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: effects of experimental conditions and electrokinetic models on the interpretation of results. Langmuir. 2021;37(45):13379-13389.
- [Google Scholar]
- Low-dose exposure to phytosynthesized gold nanoparticles combined with glutamine deprivation enhances cell death in the cancer cell line HeLa via oxidative stress-mediated mitochondrial dysfunction and G0/G1 cell cycle arrest. Nanoscale. 2022;14(29):10399-10417.
- [Google Scholar]
- Synthesis and characterization of gold nanoparticles from aqueous leaf extract of Alternanthera sessilis and its anticancer activity on cervical cancer cells (HeLa) Artif. Cells Nanomed. Biotechnol.. 2019;47(1):1173-1180.
- [Google Scholar]
- Biogenic gold nanoparticles induce cell cycle arrest through oxidative stress and sensitize mitochondrial membranes in A549 lung cancer cells. RSC Adv.. 2016;6(25):20598-20608.
- [Google Scholar]
- Green synthesis and structural classification of Acacia nilotica mediated-silver doped titanium oxide (Ag/TiO2) spherical nanoparticles: assessment of its antimicrobial and anticancer activity. Saudi J. Biol. Sci.. 2019;26(7):1385-1391.
- [Google Scholar]
- Green synthesis of silver nanoparticles (AgNPs) by Lallemantia royleana leaf extract: their bio-pharmaceutical and catalytic properties. J. Photochem. Photobiol. A: Chem.. 2024;448:115318
- [Google Scholar]
- Hemolysis tendency of anticancer nanoparticles changes with type of blood group antigen: an insight into blood nanoparticle interactions. Mater. Sci. Eng. C. 2020;109:110645
- [Google Scholar]
- Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochim. Acta A: Mol. Biomol. Spectrosc.. 2013;102:15-23.
- [Google Scholar]
- The Green synthesis of gold nanoparticles using an aqueous root extract of Morinda citrifolia L. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc.. 2014;118:11-16.
- [Google Scholar]
- Green synthesis of gold nanoparticles using Jatropha integerrima Jacq. flower extract and their antibacterial activity. J. King Saud Univ.-Sci.. 2022;34(3):101830
- [Google Scholar]
- Gold nanoparticles fabrication by plant extracts: synthesis, characterization, degradation of 4-nitrophenol from industrial wastewater, and insecticidal activity–a review. J. Clean. Prod.. 2018;184:740-753.
- [Google Scholar]
- Simple spectrophotocolorimetric method for quantitative determination of gold in nanoparticles. Talanta. 2011;83(5):1780-1783.
- [Google Scholar]
- Nanoparticles and the tumor microenvironment: challenges and opportunities. In: Site-specific Cancer Nanotheranostics. 2024. p. :1-20.
- [Google Scholar]
- Cordyceps cicadae polysaccharides inhibit human cervical cancer hela cells proliferation via apoptosis and cell cycle arrest. Food Chem. Toxicol.. 2021;148:111971
- [Google Scholar]
- Biogenic synthesis of nanomaterials: bioactive compounds as reducing, and capping agents. In: Biogenic Nanomaterials for Environmental Sustainability: Principles, Practices, and Opportunities. Springer; 2024. p. :147-188.
- [Google Scholar]
- Green synthesis and biomedical applications of zinc oxide nanoparticles. Review. Egypt. J. Vet. Sci.. 2024;55(1):287-311.
- [Google Scholar]
- Thermodynamic and conformational changes of protein toward interaction with nanoparticles: a spectroscopic overview. RSC Adv.. 2016;6(107):105903-105919.
- [Google Scholar]
- Gold nanoparticles: recent advances in the biomedical applications. Cell Biochem. Biophys.. 2015;72:771-775.
- [Google Scholar]







