Translate this page into:
Green formulation of Ag nanoparticles by Hibiscus rosa-sinensis: Introducing a navel chemotherapeutic drug for the treatment of liver cancer
⁎Corresponding author. hwldr123456@gmail.com (Hongwei Lu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
An eco-friendly biosynthesized Ag NPs immobilized Hibiscus rosa-sinensis extract has been introduced. The as-prepared nanoparticles were characterized using UV–Vis, SEM, and FT-IR analysis. In the FT-IR test, the presence of many antioxidant compounds with related bonds caused the excellent condition for reducing of silver in the silver nanoparticles. In UV–Vis, the clear peak in the wavelength of 428 nm indicated the formation of silver nanoparticles. The synthesized nanoparticles had very low cell viability and high anti-liver cancer activities dose-dependently against pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr) cell lines without any cytotoxicity on the normal cell line (HUVEC). The synthesized nanoparticles inhibited half of the DPPH molecules in the concentration of 78 µg/mL. Perhaps notable anti-liver cancer activities of the synthesized nanoparticles against common liver cancer cell lines are linked to their antioxidant activities.
Keywords
Silver
Hibiscus rosa-sinensis
Plant extract
Liver ccancer
Antioxidant
1 Introduction
Liver is an essential organ accountable for the metabolism, bile secretion, elimination of many substances, blood detoxifications, synthesis and regulation of vital hormones (Abdalla et al., 2001). The main contributory factors for the liver diseases in developed countries are too much alcohol consumption, and viral-induced chronic liver diseases while in the developing countries the most common causes are environmental toxins, (carbon tetrachloride (CCl4) and insecticides), hepatitis B and C viruses, and hepatotoxic drugs (certain antibiotics, chemotherapeutic agents, high doses of paracetamol, etc.) (Abdalla et al., 2001; Abulkhir et al., 2008). Liver diseases have turn out to be a global problem and are associated with substantial morbidity and mortality (Abulkhir et al., 2008). Chronic liver cirrhosis and drug induced liver injury are the ninth leading cause of death in western and developing countries (Altekruse et al., 2009; Altekruse et al., 2012; Bosetti et al., 2008). Among all liver diseases, the liver cancers are the most agent of death. Liver cancer or hepatic cancer in first occurs in the liver organ and after progressing the disease, it transfer to all parts of body by the lymphatic system or bloodstream (Breitenstein et al., 2009; Breous and Thimme, 2011; Bruix et al., 2014). The main types of hepatica cancers are hepatobiliary cancers, hepatocellular carcinoma, cholangiocarcinoma, liver Angiosarcoma, and hepatoblastoma. Among all types of hepatic cancers, the role of hepatobiliary cancers in death of human is significant. For the treatment of hepatobiliary carcinoma, surgery, radiation therapy, chemotherapy, targeted therapy, immunotherapy, and EGFR-targeted therapy are used (Altekruse et al., 2009; Altekruse et al., 2012; Bosetti et al., 2008). The main anti-hepatobiliary carcinoma chemotherapeutic drugs are included cisplatin with lipiodol; sorafenib, and doxorubicin (Abulkhir et al., 2008). According to the high side effects of chemotherapeutic drugs such as mouth sores, weight loss, diarrhea, vomiting, hair loss, fatigue, and nausea, the formulation of modern chemotherapeutic drugs is necessary (Burroughs et al., 2004; Butterfield et al., 2006). Recently, scientists have understood that metallic nanoparticles especially iron nanoparticles have excellent anticancer properties (Breous and Thimme, 2011).
There are three biological, chemical, and physical methods to synthesize the nanoparticles. The synthesis of nanoparticles by similar biological methods results in greater catalytic activity and limits the use of toxic and expensive chemicals (Sierra-Ávila et al., 2014; Kooti et al., 2017; Sujayev et al., 2020). In biological methods, plant extracts, enzymes or proteins carrying natural resources are used to produce or stabilize nanoparticles. The nature of the materials used to make nanoparticles influences the shape, structure and morphology of these nanoparticles (Butterfield et al., 2006; Sierra-Ávila et al., 2014; Kooti et al., 2017; Sujayev et al., 2020; Abdoli et al., 2020). Biological systems involved in the green synthesis of nanoparticles, plants and their derivatives, as well as microorganisms such as algae, fungi, and bacteria (Kooti et al., 2017; Sujayev et al., 2020; Abdoli et al., 2020). Chemical and physical methods are time-consuming and costly. In addition, these methods use some toxic additive chemicals that cause adverse effects on medical applications by adsorption on the surface. Applying the principles of green chemistry has decreased the use of toxic compounds or hazardous solvents, provided optimal regeneration conditions and ameliorated materials for the chemical processes, and raised new sources for green synthesis (Kooti et al., 2017; Sujayev et al., 2020; Abdoli et al., 2020). Therefore, one of the primary goals of green nanotechnology is to produce nanomaterials without harm to human health or environment, and to develop and design nanomaterials and products that are suitable solutions to environmental problems.
Plant parts such as roots, leaves, stems, fruits, and tiny parts such as the kernel and skin of the fruit are suitable to synthesize the nanoparticles because their extracts are rich in phytochemicals that act as stabilizing and reducing substances (Butterfield et al., 2006; Sierra-Ávila et al., 2014; Kooti et al., 2017; Sujayev et al., 2020). The use of natural plant extracts is a cheap and environmentally friendly process and does not require intermediate groups. Short time, no need for expensive equipment, precursors, high purity product and excellent quality without impurities are the features of this method. This is possible very quickly, at room temperature and pressure as well as easily on a large scale. Bio-reduction in the conversion of base metal ions is carried out by various plant metabolites such as alkaloids, phenolic compounds, terpenoids and coenzymes (Abdoli et al., 2020; Shaneza, 2018). Nanoparticles centered on inorganic materials such as magnetic metals, their oxides and alloys, and semiconductors have the most studies and potential in biomedicine from diagnosis to treatment of diseases (Sierra-Ávila et al., 2014; Kooti et al., 2017; Sujayev et al., 2020). Nanoparticles are generally effective in a wide variety of sectors that if their production is based on green chemistry, they have great applications in the fields of food, medicine, cosmetics and health. The effects of nanoparticles should be predictable, controllable and get the desired results with minimal toxicity. Metallic nanoparticles used in treatment and diagnosis, in addition to being non-toxic, must be biocompatible and stable in vivo. Also, by making appropriate changes in the surface of metallic nanoparticles, they will have a wide range of applications by binding to biomolecules and various carriers to cross the cell membrane and target the desired part in the body. One of the important points in the production of nanoparticles is the use of cost-effective and efficient precursors (Kooti et al., 2017; Sujayev et al., 2020; Abdoli et al., 2020; Shaneza, 2018).
For many years, herbal medicines have been used and are still used in developing countries as the primary source of medical treatment. Plants have been used in medicine for their natural antiseptic properties. Thus, research has developed into investigating the potential properties and uses of terrestrial plants extracts for the preparation of potential nanomaterial based drugs for diseases including cancer (Ahmad et al., 2015; Olas et al., 2015; Akbar, 2020; Al-Saleem et al., 2019). Many plant species are already being used to treat or prevent development of cancer. Multiple researchers have identified species of plants that have demonstrated anticancer properties with a lot of focus on those that have been used in herbal medicine in developing countries (Hamed et al., 2012; Al-Snai et al., 2019; Laghari et al., 2011; Awaad Amani et al., 2006; Laghari et al., 2012). Recently, the anticancer effects of Tinospora cordifolia, Sophora subprostrata, Euphoria hirta, Barleria prionitis, Lubinus perennis, Maytenus boaria, Cephaelis acuminate, Phyllanthus niruri, Solanum seaforthianum, Boswellia serrate, Lavendula officinalis, and Cephalotaxus harringtonia drupacea have been proved (Soni and Krishnamurthy, 2013).
In the current research, the properties of silver nanoparticles formulated by Hibiscus rosa-sinensis leaf aqueous extract against common liver cancer cell lines i.e. pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr) were evaluated.
2 Material and methods
2.1 Green synthesis and chemical characterization of Ag nanoparticles
First, the dried leaves of Hibiscus rosa-sinensis were grounded. Then, 75 g of the sample was macerated in 800 mL of boiling water for 5 h. After that, the extract was filtrated and evaporated to concentrate. Finally, the extract was placed in a freeze drier for 72 h. The obtained extract as brown powder was kept in cold place. The green synthesis of Ag nanoparticles was carried out according to a previous study. A 20 mL of Hibiscus rosa-sinensis extract (1 g in 10 mL of deionized water) was added to 50 mL of 15 mM NiSO4·6H2O in a flask. Then, to adjust of the pH, 2 mL of NaOH (1%) was added dropwise and shake for 25 min. Next the flask was put in an ultrasonic bath (75 W) for 30 min. The AgNPs was formed as green participates during the reaction time. The AgNPs was washed with water for three times and then centrifuged at 10,000 rpm for 15 min. Finally, the precipitate was dried in an oven at 45 °C.
UV–Vis. and FT-IR spectroscopy and SEM techniques were used to characterize the biosynthesized AgNPs. Different parameters of the nanoparticles, such as shape, particle size, fractal dimensions, crystallinity, and surface area are obtained by these techniques. The UV–Vis. spectra were obtained by a PhotonixAr 2015 UV–Vis. Spectrophotometer (200–800 nm); The FT-IR spectra were recorded using a Shimadzu FT-IR 8400 in the range of 400–4000 cm−1(KBr disc); MIRA3TESCAN-XMU was used to report the FE-SEM images.
2.2 Antioxidant activities of Ag nanoparticles
DPPH radical can directly react to antioxidants. When this radical is trapped by antioxidants, DPPH is revitalized from an antioxidant by receiving a hydrogen atom. The free radicals of this compound produce a purple color and can absorb at the wavelength of 517 nm. Its absorption intensity decreases by revitalization and it changes to yellow. The antioxidant strength depends on the reduction percentage of initial dark purple color to the yellow color. The greater is the number of hydroxyl groups of antioxidant phenyl loop; the higher is the number of hydrogen atoms for reaction with DPPH and its stabilization (Shaneza, 2018). In our study, to determine the trapping potential of DPPH, different concentrations of the AgNPs were mixed with 2 mL 0.004% DPPH solution. The control solution contained 2 mL DPPH and 2 mL ethanol. The solutions were kept in darkness at room temperature for 30 min. Then, the absorption rate of the samples was measured at 517 nm by the following formula compared to the control sample (Shaneza, 2018):
IC50 factor was used to evaluate better the antioxidant activity, which indicates the concentration of the Ag nanoparticles that can reduce the concentration of free radical DPPH. The initial is 50% of the initial value, and the lower the amount, the greater the antioxidant activity (Shaneza, 2018).
2.3 Anti-human liver cancer potentials of Ag nanoparticles
In this research, we used the following Cell lines to evaluating anti-human liver cancer and cytotoxicity effects of Ag nanoparticles using an MTT method.
-
Human liver cancer cell lines: pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr).
-
Normal cell line: HUVEC.
The cytotoxic assay was performed using MTT reagent (3-(4, 5-dimetylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide) (Sigma, Germany) according to the manufacturer's protocol. Viable cells (1.5 × 104) were seeded in 96-well flat bottom plates. When cells reached more than 80% confluence, the medium was replaced and cells were incubated with AgNPs at several concentrations, at a maximum concentration of dimethyl sulphoxide (DMSO) 0.05% (v/v). After 24 h, the supernatants were removed and cell layers were washed with phosphate buffer saline (PBS, Invitrogen Gibco) and incubated with MTT (50 µL, 2 mg/mL) in RPMI 1640 for 4 h in a humidified atmosphere at 37 °C. The cell cultures were centrifuged at 1000 g for 5 min and the supernatants were discarded. Subsequently, 200 µL of dimethyl sulfoxide (DMSO, Sigma, USA) and 25 µL Sorenson buffer were added to dissolve the formazan crystals formed. The optical density colored solution was quantified at 570 nm wavelengths by an enzyme linked immunoabsorbent assay reader (ELISA Reader, Bio-Rad). The absorbance of untreated cells was considered as 100%. All treatments were assayed in triplicate in three independent experiments. Fifty percent of inhibition concentration (IC50) was calculated by Graph Pad Prim 4 software. Percent growth inhibition of cells exposed to treatments was calculated as follows (Arunachalam, 2003):
Finally, linear regression was done to gain IC50, which indicates the nanoparticles concentration, which causes 50% cancer cell growth inhibition. Using the curve, the line equation for cancer cells was obtained, respectively, then by replacing 50% inhibition in the equation, the IC50 value for cancer cells was obtained (Olas et al., 2015).
2.4 Statistical analysis
SPSS statistical software version 22 was used for data analysis and the findings were determined as the mean standard deviation of 5 replications. Data were analyzed using one-way analysis of variance and Duncan post hoc test and the significance level in the test was considered 0.05.
3 Results and discussion
3.1 Chemical characterization of Ag nanoparticles
3.1.1 UV–Vis. analysis
UV–Vis spectroscopy is an analytical technique that measures the amount of discrete wavelengths of UV or visible light that are absorbed by or transmitted through a sample in comparison to a reference or blank sample. This property is influenced by the sample composition, potentially providing information on what is in the sample and at what concentration. Since this spectroscopy technique relies on the use of light, let’s first consider the properties of light (Katata-Seru et al., 2018; Harshiny et al., 2015).
The UV–Vis. spectra of the green-synthetic nanoparticles of Ag nanoparticles is presented in Fig. 1. The surface plasmon resonance (SPR) of Ag nanoparticles was completed using UV–Vis. spectroscopy. The produce of the biosynthetic Ag nanoparticles was observed. The advanced SPR bands at the wavelength of 428 nm approved the formation of the Ag nanoparticles. The bands are very close to a previously reported on the green synthesized of Ag nanoparticles (Katata-Seru et al., 2018; Harshiny et al., 2015).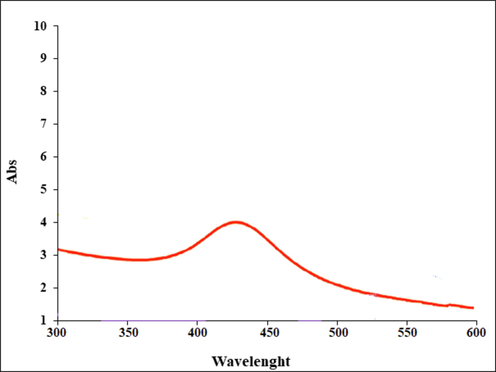
UV–Vis. the spectrum of biosynthesized Ag nanoparticles.
3.1.2 FT-IR analysis
Fourier Transform Infrared Spectroscopy, also known as FTIR Analysis or FTIR Spectroscopy, is an analytical technique used to identify organic, polymeric, and, in some cases, inorganic materials. The FTIR analysis method uses infrared light to scan test samples and observe chemical properties (Mahdavi et al., 2020; Iqbal et al., 2019; Zhang et al., 2021).
The FT-IR spectrum of Ag nanoparticles is shown in Fig. 2. The formation of Ag nanoparticles is approved by the presence of the peak at wavenumber of 552 cm−1. These peaks attribute to bending vibration of Ag-O. Similar peaks with some differences in the wavenumber have been reported for green-synthetic Ag nanoparticles by other research groups (Mahdavi et al., 2020; Iqbal et al., 2019; Zhang et al., 2021). The other peaks in the spectrum are attributed to the functional groups of different organic compounds in Hibiscus rosa-sinensis extract, which are linked to the surface of Ag nanoparticles. The presence of secondary metabolites such as phenolic, flavonoid, triterpenes in Hibiscus rosa-sinensisextract has been reported previously (Breous and Thimme, 2011; Bruix et al., 2014; Burroughs et al., 2004). The peaks in 3432 and 2916 cm−1 are related to O—H and aliphatic C—H stretching; the peaks from 1418 to 1612 cm−1 are corresponded to C⚌C and C⚌O stretching, and the peak at 1102 cm−1 could be ascribed to C—O—C stretching.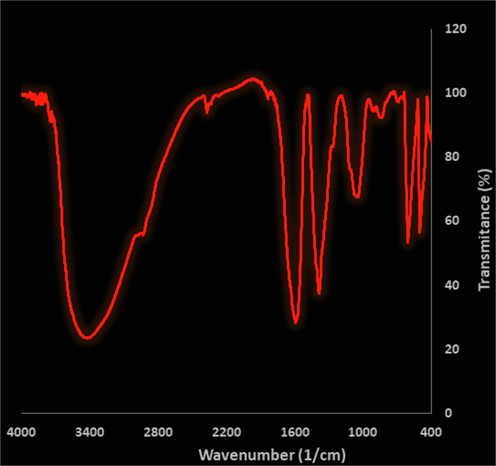
FT-IR spectra of biosynthesized Ag nanoparticles.
3.1.3 SEM analysis
Scanning Electron Microscopy (SEM) is a test process that scans a sample with an electron beam to produce a magnified image for analysis. The method is also known as SEM analysis and SEM microscopy, and is used very effectively in microanalysis and failure analysis of solid inorganic materials. Electron microscopy is performed at high magnifications, generates high-resolution images and precisely measures very small features and objects (Mahdavi et al., 2019; Baghayeri et al., 2018; Nwanya et al., 2020; Baranwal et al., 2018; Rameshthangam and Chitra, 2018; Ibraheem et al., 2019; Chen et al., 2013).
The morphology of Ag nanoparticles was assessed by the SEM technique. Fig. 3 presents the SEM of Ag nanoparticles. The images show the spherical shape for the nanoparticles with average particle size of 48.52 nm. Furthermore, the nanoparticles are aggregated. In our literature review, 10–100 nm was reported for biosynthesized of Ag using plant extracts as the capping agent (Mahdavi et al., 2019; Baghayeri et al., 2018; Nwanya et al., 2020; Baranwal et al., 2018; Rameshthangam and Chitra, 2018; Ibraheem et al., 2019; Chen et al., 2013).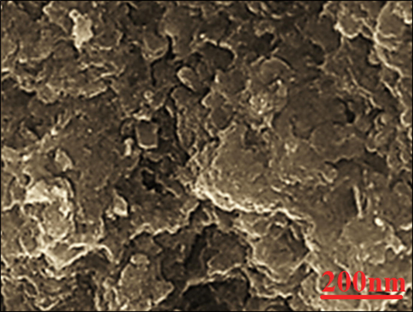
SEM Images of FeSNPs.
3.2 Cytotoxicity, anti-human liver cancer, and antioxidant activities of Ag nanoparticles
Free radicals are destructive compounds that are produced as a by-product by the body's chemical reactions and are destroyed by the body's defense system and enzyme system and antioxidants. However, in cases where the body's metabolic disorders and the production of free radicals are high and they are not destroyed by the neutralizing system, due to their instability, these compounds have a strong tendency to react with a variety of molecules in the body (Kuang et al., 2013; Mao, 2016; You et al., 2012; Katata-Seru et al., 2018). It is estimated that each cell in the human body is exposed to free radicals 10,000 times a day and DNA strands 5000 times a day. Damage to cell components includes proteins (genetic disorder), fats (lipid oxidation), and cell membranes (permeability disorder) that if the damage is not repaired, it leads to disruption of the chemical reaction and normal proteinization of the cell and the formation of harmful compounds and sometimes cancer cells in the body (Sangami and Manu, 2017; Namvar et al., 2014; Radini et al., 2018; Sankar et al., 2014). It is reported that thousands of cancer cells are produced daily in the human body that are killed by the body's defense system. In some cases, due to dysfunction of the above systems, cancer cells proliferate and conditions for cancer development in different tissues (Iqbal et al., 2019; Zhang et al., 2021; Mahdavi et al., 2019; Baghayeri et al., 2018; Nwanya et al., 2020). According to the above, antioxidants play a vital role in preventing disorders caused by the effects of free radicals and thus the prevention and treatment of cancer. Antioxidants are a wide range of molecular compounds with complex properties that combine with and neutralize free radicals. The results show that more than 60,000 types of molecular antioxidants have been identified so far. Antioxidants can be effective in three known ways to prevent and treat cancer; 1. Destruction of free radicals 2. Strengthen the immune system to destroy cancer cells. Prevent the adhesion of cancer cells to other cells and prevent their proliferation (Mao, 2016; You et al., 2012; Katata-Seru et al., 2018; Sangami and Manu, 2017; Namvar et al., 2014).
The treated cells with different concentrations of the present Ag salt, Hibiscus rosa-sinensis leaf aqueous extract, and Ag nanoparticles were assessed by MTT assay for 48 h about the cytotoxicity properties on normal (HUVEC) and liver malignancy cell lines i.e. pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr). The absorbance rate was evaluated at 570 nm, which represented viability on normal cell line (HUVEC) even up to 1000 μg/mL for Ag salt, Hibiscus rosa-sinensisleaf aqueous extract, and Ag nanoparticles (Tables 1 and Fig. 4). The viability of malignant liver cell lines reduced dose-dependently in the presence of Ag salt, Hibiscus rosa-sinensis leaf aqueous extract, and Ag nanoparticles. The IC50 of Ag nanoparticles were 223, 185, 265, and 188 µg/mL against pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr) cell lines, respectively (Tables 1 and Fig. 4).
Silver nanoparticles (µg/mL)
IC50 against HUVEC
–
IC50 against pleomorphic hepatocellular carcinoma (SNU-387)
223
IC50 against hepatic ductal carcinoma (LMH/2A)
185
IC50 against morris hepatoma (McA-RH7777)
265
IC50 against novikoff hepatoma (N1-S1 Fudr)
188
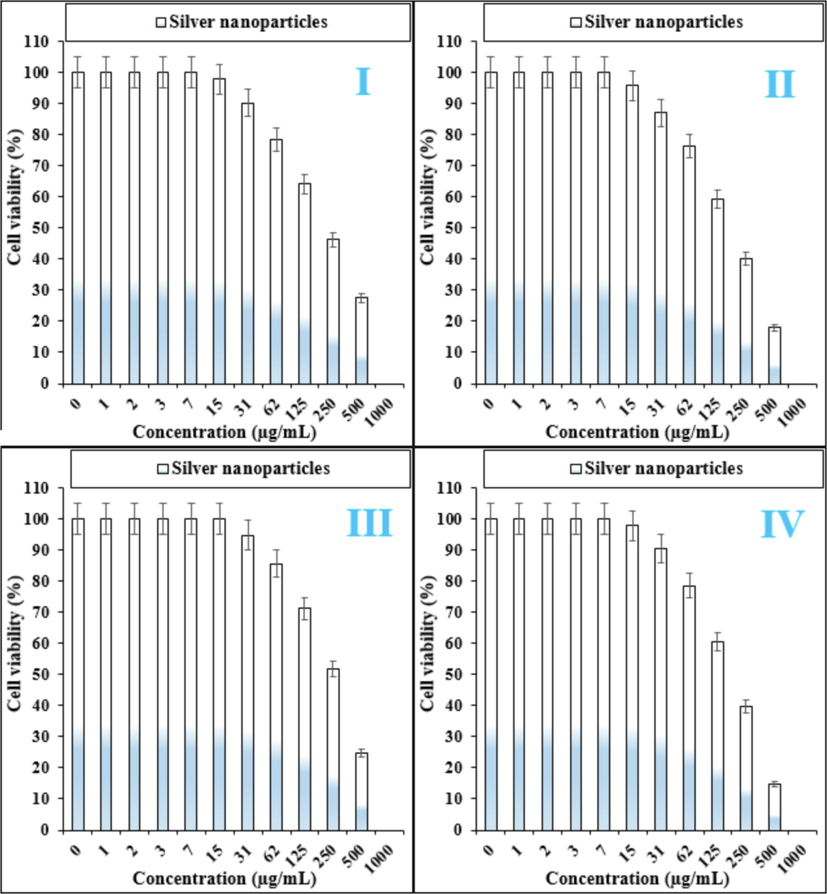
The anti-liver cancer properties of silver nanoparticles against pleomorphic hepatocellular carcinoma (SNU-387 (I)), hepatic ductal carcinoma (LMH/2A (II)), morris hepatoma (McA-RH7777 (III)), and novikoff hepatoma (N1-S1 Fudr (IV))) cell lines.
Many nanoparticles have pharmacological and biochemical properties, including antioxidant and anti-inflammatory properties, which appear to be involved in anticarcinogenic and antimutagenic activities (Radini et al., 2018; Sankar et al., 2014; Beheshtkhoo et al., 2018). It seems that the anti-human liver cancer effect of recent nanoparticles is due to their antioxidant effects. Because tumor progression is so closely linked to inflammation and oxidative stress, a compound with anti-inflammatory or antioxidant properties can be an anticarcinogenic agent (Radini et al., 2018; Sankar et al., 2014).
In this study, we assessed the antioxidant properties of Hibiscus rosa-sinensis leaf aqueous extract green-synthesized Ag nanoparticles by using the DPPH test as a common free radical. Antioxidants produced in the body fight free radicals with two systems: enzymatic defense and non-enzymatic defense. Superoxide dismutase, catalase, and glutathione peroxidase metabolize lipid peroxide, hydrogen peroxide, and superoxide and prevent the production of toxic hydroxyl radicals (Kooti et al., 2017; Shaneza, 2018). In non-enzymatic defense, there are two classes of fat-soluble antioxidants (such as carotenoids and vitamin E) and water-soluble (glutathione and vitamin C) that trap free radicals. These two systems help neutralize oxidants. However, oxidants can escape from antioxidants and damage tissues. In this case, the activated antioxidant repair system (which is the enzymes lipase, protease, transferase and DNA repair enzymes), counteract the oxidant effects. However, due to deficiencies in the production of antioxidants in the body or due to physiopathological factors and situations (such as smoking, air pollution, UV radiation, diets containing high unsaturated fatty acids, inflammation, ischemia, bleeding, etc) that ROS are yielded in large quantities at the wrong place and time, oral antioxidants are needed to counteract the oxidative damage cumulative effects (Al-Snai et al., 2019; Laghari et al., 2011; Soni and Krishnamurthy, 2013).
The scavenging capacity of Hibiscus rosa-sinensis leaf aqueous extract green-synthesized Ag nanoparticles and BHT at different concentrations expressed as percentage inhibition has been indicated in Table 2 and Fig. 5.
Silver nanoparticles (µg/mL)
BHT (µg/mL)
IC50 against DPPH
106
70
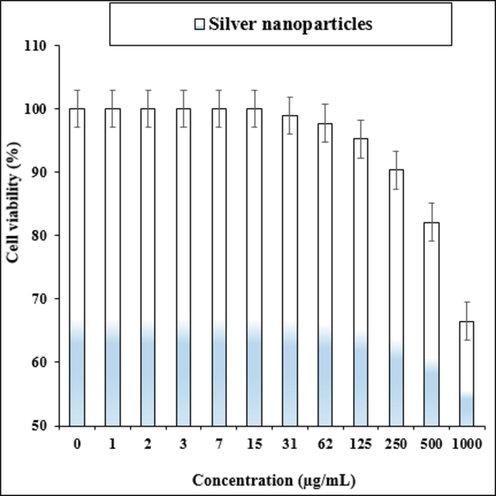
The cytotoxicity effects of silver nanoparticles against normal cell line.
In the antioxidant test, the IC50 of Ag nanoparticles and BHT against DPPH free radicals were 106 and 70 µg/mL, respectively (Fig. 5) (see Fig. 6).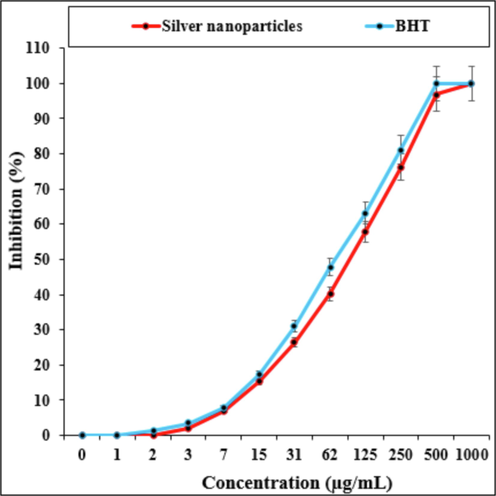
The antioxidant properties of silver nanoparticles and butylated hydroxytoluene (BHT) against DPPH.
4 Conclusion
After clinical study, Ag nanoparticles containing Hibiscus rosa-sinensis leaf aqueous extract can be utilized as an efficient drug in the treatment of liver cancer in humans. In summary, the nanoparticles were characterized using common chemical techniques such as UV–Visible, FT-IR, and SEM. The IC50 of Ag nanoparticles and BHT against DPPH free radicals were 316 and 231 µg/mL, respectively. The SEM images indicated a spherical morphology for Ag nanoparticles with average size of lesser than 50 nm, which is well known as a sufficient size for the synthetic nanoparticles. The viability of malignant liver cell lines reduced dose-dependently in the presence of Ag nanoparticles. The IC50 of Ag nanoparticles were 477, 548, and 605 µg/mL against pleomorphic hepatocellular carcinoma (SNU-387), hepatic ductal carcinoma (LMH/2A), morris hepatoma (McA-RH7777), and novikoff hepatoma (N1-S1 Fudr) cell lines, respectively.
Funding
Research on targeted therapy of liver cancer CTCs with CTLA-4 inhibitor monoclonal antibody nanoparticles, cross cooperation project of basic scientific research business fee of Xi'an Jiaotong University, (1191320139).
Killing effect of CD80 monoclonal antibody and CCL22 on hepatoma TSCs through chemotaxis and function of Tregs, key R & D project of Shaanxi Province, (2020sf-058).
MiRNA through PPAR in liver fibrosis γ Research on the mechanism of affecting hepatic stellate cells, Shaanxi Natural Science Foundation, (2018JM7034).
Author contribution
Le Lu and Ziyun Zhuang contributed equally to this research and should be considered as co-first author.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Portal vein embolization: rationale, technique and future prospects. Br. J. Surg.. 2001;88:165-175.
- [Google Scholar]
- (a) Abdoli, M., Sadrjavadi, K., Arkan, E., Zangeneh, M.M., Moradi, S., Zangeneh, A., Shahlaei, M., Khaledian S., 2020. J. Drug Deliv. Sci. Technol. 60, 102044. (b) Dou, L., Zhang, X., Zangeneh, M.M., Zhang, Y. 2020. Bioorg. Chem. 106: 104468. (c) Han, Y., Gao, Y., Cao, X., Zangeneh, M.M., Liu, S., Li, J., 2020. Int. J. Biol. Macromol. 164, 2974–2986.
- Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann. Surg.. 2008;247:49-57.
- [Google Scholar]
- Phytochemicals, antibacterial and antioxidative investigations of Alhagi maurorum medik. Pak. J. Bot.. 2015;47:121-124.
- [Google Scholar]
- Akbar, S., 2020. Handbook of 200 medicinal plants: A comprehensive review of their traditional medical uses and scientific justifications.
- Antioxidant flavonoids from Alhagi maurorum with hepatoprotective effect. Pharmacogn. Mag.. 2019;15:592.
- [Google Scholar]
- Antifungal effect of Alhagi maurorum phenolic extract. IOSR J. Pharm.. 2019;9:7-14.
- [Google Scholar]
- Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol.. 2009;27:1485-1491.
- [Google Scholar]
- Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992–2008. Hepatology. 2012;55:476-482.
- [Google Scholar]
- One-step green synthesis and characterization of leaf extract-mediated biocompatible silver and gold nanoparticles from Memecylon umbellatum. Int. J. Nanomed.. 2003;8:1307-1315.
- [Google Scholar]
- Green synthesis of silver nanoparticles using water extract of Salvia leriifolia: Antibacterial studies and applications as catalysts in the electrochemical detection of nitrite. Appl. Organomet. Chem.. 2018;32:e4057
- [Google Scholar]
- Guar gum mediated synthesis of NiO nanoparticles: An efficient catalyst for reduction of nitroarenes with sodium borohydride. Int. J. Biol. Macromol.. 2018;120:2431-2441.
- [Google Scholar]
- Green synthesis of iron oxide nanoparticles by aqueous leaf extract of Daphne mezereum as a novel dye removing material. Appl. Phys. A. 2018;124:363-369.
- [Google Scholar]
- Trends in mortality from hepatocellular carcinoma in Europe, 1980–2004. Hepatology. 2008;48:137-145.
- [Google Scholar]
- Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br. J. Surg.. 2009;96:975-981.
- [Google Scholar]
- Potential of immunotherapy for hepatocellular carcinoma. J. Hepatol.. 2011;54:830-834.
- [Google Scholar]
- STORM: A phase III randomized, double-blind, placebo-controlled trial of adjuvant sorafenib after resection or ablation to prevent recurrence of hepatocellular carcinoma (HCC) In: 2014 Annual Meeting of the American Society of Clinical Oncology (ASCO), Abstract 4006. 2014.
- [Google Scholar]
- Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol.. 2004;5:409-418.
- [Google Scholar]
- A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four α-fetoprotein peptides. Clin. Cancer Res.. 2006;12:2817-2825.
- [Google Scholar]
- Evaluation of the antitumor activity by Ag nanoparticles with verbascoside. J. Nanomater.. 2013;2013
- [Google Scholar]
- Oleanane glycosides from the roots of Alhagi maurorum. Phytochem. Lett.. 2012;5:782-787.
- [Google Scholar]
- Biogenic synthesis of iron nanoparticles using Amaranthus dubius leaf extract as a reducing agent. Powder Technol.. 2015;286:744-749.
- [Google Scholar]
- In vitro Cytotoxicity, MMP and ROS activity of green synthesized nickel oxide nanoparticles using extract of Terminalia chebula against MCF-7 cells. Mater. Lett.. 2019;234:129-133.
- [Google Scholar]
- Green synthesis and characterizations of Nickel oxide nanoparticles using leaf extract of Rhamnus virgata and their potential biological applications. Appl. Organomet. Chem.. 2019;33:e4950
- [Google Scholar]
- Green synthesis of iron nanoparticles using Moringa oleifera extracts and their applications: Removal of nitrate from water and antibacterial activity against Escherichia coli. J. Mol. Liq.. 2018;256:296-304.
- [Google Scholar]
- Green synthesis of iron nanoparticles using Moringa oleifera extracts and their applications: removal of nitrate from water and antibacterial activity against Escherichia coli. J. Mol. Liq.. 2018;256:296-304.
- [Google Scholar]
- Effective medicinal plant in cancer treatment, part 2: review study. J. Evid. Based Complementary Altern. Med.. 2017;22(4):982-995.
- [CrossRef] [Google Scholar]
- Heterogeneous Fenton-like oxidation of monochlorobenzene using green synthesis of iron nanoparticles. J. Colloid Interface Sci.. 2013;410:67-73.
- [Google Scholar]
- Alhagi maurorum: a convenient source of lupeol. Ind. Crops Prod.. 2011;34:1141-1145.
- [Google Scholar]
- Determination of free phenolic acids and antioxidant capacity of methanolic extracts obtained from leaves and flowers of camel thorn (Alhagi maurorum) Nat. Prod. Res.. 2012;26:173-176.
- [Google Scholar]
- Ziziphora clinopodioides Lam leaves aqueous extract mediated synthesis of zinc nanoparticles and their antibacterial, antifungal, cytotoxicity, antioxidant, and cutaneous wound healing properties under in vitro and in vivo conditions. Appl. Organomet. Chem.. 2019;33:e5164
- [Google Scholar]
- Assessment of antioxidant, cytotoxicity, antibacterial, antifungal, and cutaneous wound healing activities of green synthesized manganese nanoparticles using Ziziphora clinopodioides Lam leaves under in vitro and in vivo condition. Appl. Organomet. Chem.. 2020;34:e5248
- [Google Scholar]
- Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicol.. 2016;10:1021-1040.
- [Google Scholar]
- Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int. J. Nanomed.. 2014;19:2479-2488.
- [Google Scholar]
- Zea mays lea silk extract mediated synthesis of nickel oxide nanoparticles as positive electrode material for asymmetric supercabattery. J. Alloy. Compd.. 2020;822:153581
- [Google Scholar]
- Comparison of biological activity of phenolic fraction from roots of Alhagi maurorum with properties of commercial phenolic extracts and resveratrol. Platelets. 2015;26:788-794.
- [Google Scholar]
- Biosynthesis of iron nanoparticles using Trigonella foenum-graecum seed extract for photocatalytic methyl orange dye degradation and antibacterial applications. J. Photochem. Photobiol., B. 2018;183:154-163.
- [Google Scholar]
- Synergistic anticancer effect of green synthesized nickel nanoparticles and quercetin extracted from Ocimum sanctum leaf extract. J. Mater. Sci. Technol.. 2018;34:508-522.
- [Google Scholar]
- Synthesis of Green Iron Nanoparticles using Laterite and their application as a Fenton-like catalyst for the degradation of herbicide Ametryn in water. Environ. Technol. Innov.. 2017;8:150-163.
- [Google Scholar]
- Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mat. Sci. Eng. C. 2014;44:234-239.
- [Google Scholar]
- Herbal treatment for the ovarian cancer. SGVU J. Pharm. Res. Educ.. 2018;3(2):325-329.
- [Google Scholar]
- (a) Sierra-Ávila, Rubén, Pérez-Alvarez, Marissa, Cadenas-Pliego, Gregorio, Alberto Ávila-Orta, Carlos, Betancourt-Galindo, Rebeca, Jiménez-Regalado, Enrique, Martha Jiménez-Barrera, Rosa, Guillermo Martínez-Colunga, Juan, 2014. Synthesis of copper nanoparticles coated with nitrogen ligands. J. Nanomater. 2014, Article ID 361791, 8. (b) Pérez-Alvarez, M., Cadenas-Pliego, G., Pérez-Camacho, O., Comparán-Padilla, V.E., Cabello-Alvarado, C.J., Saucedo-Salazar, E., 2021. Green synthesis of copper nanoparticles using cotton. Polymers. 13(12), 1906. 10.3390/polym13121906. (c) Yasar, H., Ho, D.-K., De Rossi, C., Herrmann, J., Gordon, S., Loretz, B., Lehr, C.-M., 2018. Starch-chitosan polyplexes: a versatile carrier system for anti-infectives and gene delivery. Polymers 10, 252. 10.3390/polym10030252.
- Indian J. Plant Sci.. 2013;2:117-125.
- (a) Sujayev, A., Taslimi, P., Garibov, E., Karaman, M., Zangeneh, M.M., 2020. Bioorg. Chem. 104, 104216. (b) Sun, T., Gao, J., Shi, H., Han, D., Zangeneh, M.M., Liu, N., Liu, H., Guo, Y., Liu. X., 2020. Int. J. Biol. Macromol. 165, 787–795.
- The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol. Biol. Rep.. 2012;39:9193-9201.
- [CrossRef] [Google Scholar]
- Green synthesis of NiO nanoparticles using calendula officinalis extract: chemical charactrization, antioxidant, cytotoxicity, and anti-esophageal carcinoma properties. Arabian J. Chem.. 2021;14:103105
- [Google Scholar]







