Green manure (Raphanus stativus L.) alters soil microbial structure and promotes acetochlor degradation
⁎Corresponding authors. plu@gzu.edu.cn (Ping Lu), gzu_dyhu@126.com (Deyu Hu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Microorganisms play important roles in the remediation of contaminated soil. However, the relationship between the soil microbial communities and herbicide degradation remains unclear after applying green manure. The aim of this study was to investigate the effect of green manure on soil physicochemical properties, between acetochlor degradation and microbial communities. Adoption of pot experiments, the effects and mechanism of four fertilization treatments (CK: without green manure; GMV: Vicia villosa Roth; GML: Lolium perenne L.; GMR: Raphanus stativus L.) on the degradation of acetochlor were evaluated. Appling to Raphanus stativus L., acetochlor degraded in 5.82 days, which was 74.81 % higher than that of CK. Community differences in soil microbes were analysed using high-throughput sequencing. The predominant phyla of fungi were Ascomyocta, Mortierellomycota, Basidiomycota, unclassified_k_Fungi, and Chytridiomycota, which accounted for 85.01 %–116.46 % of the fungal OTUs. The green manure treatment showed higher abundance of Mortierella (9.54 %–13.05 %), diversity of saprotrophic fungi and symbiotic fungi and faster rate of degradation of acetochlor as compared to CK. In addition, soil properties such as soil total phosphorus (TP) and pH have significant effects on microorganisms. Fungal functional analysis revealed that the microorganisms associated with acetochlor biodegradation mainly belonged to Ascomycota and Glomeromycota. This study offers new insights into the relationship between soil properties and the environmental risks associated with the presence of pesticides in soil and the related mechanisms.
Keywords
Acetochlor
Biodegradation
Soil
Green manure
1 Introduction
Pesticides are used worldwide, and play a vital role in protecting crop growth and enhancing the security of agricultural products (Jolodar et al., 2021). The widespread use of pesticides in agriculture poses, a significant risk to ecosystems in terms of water, air, soil, crops, and human health (Achour et al., 2017). The adverse effects of pesticide residues on the environment and their potential threat to food safety are of great concern to the general public (Wang et al., 2022b).
Acetochlor (C14H20ClNO2; ACE) is a chloroacetamide herbicide that is used to control weeds in crops such as corn, soybeans, and sunflowers (Bedmar et al., 2017). Owing to its low sorption coefficient, ACE is highly mobile and may cause environmental risks including contamination of arable land, surface water, and groundwater, which is frequently detected in water and soil due to its wide application and persistence. The research results of Wang et al. (2023) showed that the detection rate of ACE in surface water and soil are 17.5 % and 49.6 %. The long-term application of ACE has adverse effects on plants. Studies have demonstrated that the use of ACE impacts the growth characteristics of wheat, including plant height, spike length, tiller number, and dry weight (Gao et al., 2021), and that the addition of ACE-containing herbicides causes 15 % damage to maize (Sarangi and Jhala, 2018). ACE can interfere with soil microorganisms’ respiration, reduce soil bacterial populations, and alter the composition of microbial communities, thus, it affects the nutrient cycling process in the soil (Barriuso et al., 2010). Excessive use of ACE over a long period of time can lead to the continuous accumulation of ACE in the soil beyond the self-purification capacity of the soil (Feng et al., 2023). Therefore, it is crucial to study the green, environmentally friendly and economical degradation of ACE after it has been applied to the soil, as to reduce the hazards it poses to the environment, crops, and human health.
Green manure consists of a combination of plant material (freshly cut plants or pieces of rotational crops) that are added to the soil while they are recently harvested. Employing green manure can ameliorate the soil characteristics, and increase the capacity of soil nutrient, and advance soil biological activities, leading to higher yields (Meena et al., 2018). It also replenishes carbon sources, increase soil organic matter (SOM), and promotes soil aggregation to prevent the movement of organic pesticides (Kareem et al., 2022). Studies have shown that microorganisms are the main factors affecting pesticides degradation, green manure can increase soil microbial activity, and pesticide In agriculture, Raphanus stativus L is often turned into the soil as green manure. Radish roots are capable of metabolizing a large amount of sucrose (Kang et al., 2021), which means it can contribute a significant amount of organic matter to the soil when incorporated and help improve microbiological activity biodegradation in the soil is mainly caused by microbial activity (Yang and Ji, 2015). Teófilo et al. (2020) investigated the remediation of soil herbicides using green manure (Raphanus stativus L.) on soil herbicides and found that the application of green manure significantly increased soil root microbial activity. Microorganisms typically utilize these chemicals like herbicide as a source of energy, Xu et al. (2008) desired to utilize biodegradation by microorganisms to promote the degradation of pesticides in the soil. Thus, the environmental behavior of ACE can be assessed by studying how microbial communities, diversity, and composition are affected by green manure. Although herbicide phytoremediation has attracted interest in recent years, few studies have linked this technique to the introduction of herbicide-degrading green manure species into agricultural fields.
In this study, an integrated approach was undertaken to understand the interrelationships among soil physical and chemical properties, ACE degradation, and microbial communities after applying different green manures. We hypothesize that herbicide could have different effects on the diversity, structure, and function of the soil microbial community in the presence of green manures. Hence, the objectives of this study were to: (1) explore the effect of different green manure conditions on the degradation rate of ACE, including the kinetics of ACE degradation, (2) evaluate the effects of ACE on soil bacterial and fungal microbial communities, and (3) determine the optimal green manure for ACE degradation. This study aims to provide a comprehensive view of microbiological technology for removing herbicides from the environment.
2 Materials and methods
2.1 Reagents
ACE standard (purity 99.40 %) was purchased from Beijing Manhag Biotechnology Co., Ltd (Beijing, China). Emulsifiable ACE concentrates (81.5 %) were obtained from Nantong Nanshen Plant protection Technology Development Co., Ltd. (Jiangsu, China). Acetonitrile (ACN), methanol (MeOH), and sodium chloride (NaCl) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Primary secondary amines (PSA) were obtained from Shanghai McLean Biochemical Technology Co., Ltd. (Shanghai, China). Syringe filters (0.22 μm, nylon) were from Tianjin Jinteng Experimental Equipment Co., Ltd. (Tianjin, China).
2.2 Materials
The soil used was a local yellow soil in Guizhou. The physical and chemical properties of the soil include soil pH, soil organic matter, total nitrogen, total phosphorus, total potassium, alkali-hydrolyzed nitrogen, soil-available phosphorus, and soil-available potassium, which are 7.08, 17.39 g·kg−1, 1.26 g·kg−1, 0.4 g·kg−1, 15.52 g·kg−1, 146.79 mg·kg−1, 24.41 mg·kg−1 and 222.62 mg·kg−1 respectively.
The seeds of green manure were obtained from Guizhou Academy of Agricultural Sciences, and were sown in October 2022 in each plot of the experimental field (26°29′56″N, 106°39′34″E). Management of green manure cultivation was carried out, and the details of the nutrient composition of green manure are shown in Table S1.
2.3 Experimental design
The pot incubation test was conducted by selecting pots (25 × 45 cm) with 8 kg soils (Erenoğlu et al., 2023). During the experiment, the pesticide was applied according to the recommended dosage (110–155 mL·ha−1), and evenly sprayed into the soil by spraying method. Except for the control (CK) treatment, the green manures were cut into small sections of 10–15 cm, wiped off the surface water and then pressed into the soil. The uniform overturning capacity of the three green manures was 22500 kg·ha−1 (Wang et al., 2022a). In this experiment, four kinds of treatment containing different green manure were selected (Table S1): CK (without adding green manure), GMV (with Vicia villosa Roth as green manure), GML (with Lolium perenne L. as green manure) and GMR (with Raphanus stativus L.as green manure). The experiment was designed with three parallel treatments, totaling 12 treatments, conducted in a shaded area. In the pre-test, the amount of water applied was 200 mL to ensure that the water could moisten the soil and the pesticides would not be lost with the water, and the amount of water added to each treatment was consistent to avoid human error.
Different soil depths (0–20 cm; mixed) were obtained according to the time period of 0, 1, 3, 7, 14, 30, and 60 days. The mixed soil samples were separated into two parts after removing impurities such as stones and twigs and passing through a sieve (per size, 2 mm). Some of the soil samples were air-dried in a cool, well-ventilated room to test for pesticide residues and to assess soil properties, while another set of samples was stored at −80 °C for future microbiological analysis (with sterilized tools used for sampling).
2.4 Test method
As for physicochemical properties of the soil, the soil samples were first dried at 35℃ for 4 h and then pulverized using a pulverizer (Feng et al., 2023). Detailed steps for the determination of soil physical and chemical properties, including pH, soil organic matter (SOM), total nitrogen (TN), total phosphorus (TP), total potassium (TK), alkali-hydrolyzed nitrogen (AHN), soil-available phosphorus (AP), soil-available potassium (AK), and soil organic matter (SOM) refer to Wang et al. (2022a).
The QuEChERS (Anastassiades et al., 2003) technique was used to extract ACE from soil using ACN as the extracting agent and PSA as the purifying agent. The degradation of ACE was monitored by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Detailed information on sample preparation and determination of ACE can be found in Supplemental material Text S1, while specifics on the elution process are outlined in Table S2 and optimized MS/MS parameters can be found in Table S3. The method was validated, with matrix effect, limit of detection (LOD) and limit of quantitation (LOQ) among the specific validation parameters presented in Table S4. The recoveries of ACE in soil samples, together with the corresponding relative standard deviations, are documented in Table S5.
2.5 Microbial community detection
DNA was extracted from 0.5 g soil using the PowerSoil® DNA Isolation Kit from Mo Bio Laboratories. High-throughput sequencing was used to study microbial communities. Mi Seq amplicons derived from the V3-V4 region of the bacterial 16S rRNA gene were sequenced using specific barcode-labeled primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTA-CHVGGGTATCAAT-3′). Similarly, Mi Seq amplicons from the fungal ITS2 region were sequenced with primers specifically designed for the barcode markers ITS1-F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2-R (5′-GCTGCGTTCTTCATCGATGC-3′). Sequencing and subsequent bioinformatic analysis were performed at Shanghai Majorbio Bio-pharm Technology Co. Ltd (Yan et al., 2021).
2.6 Data analysis
The degradation of ACE was determined using the first-order kinetic equation Ct = C0 e-kt as described by Yu et al. (2019). In this equation, Ct represents the concentration of ACE on day t (mg·kg−1), C0 is the initial concentration of ACE (mg·kg−1), k (d-1) is the rate constant of ACE degradation, and t1/2 is the half-life of ACE. Data processing was performed in Microsoft Excel 2016, and analysis of Duncan's multiple range test data was performed using IBM SPSS Statistics version 24.0. Bioinformatic statistical analysis of operational taxonomic units (OTUs) at the 97 % similarity level was performed using Uparse (version 7.0). Taxonomic analysis of fungal and bacterial communities was performed using the fungal database Unite (release 8.0 at https://unite.ut.ee/index.php) and the bacterial 16S rRNA database Silva (release 138 at https://www.arb-silva.de). Statistical analyses and graphs were performed using the R language (version 3.3.1) and Circos-0.67–7 (https://circos.ca/). The significance of differences between two groups was tested using Welch's t-test, with p-values corrected for multiple testing using Benjamini-Hochberg and a two-tailed test with a confidence interval of 0.95. Tools for taxonomic analysis of fungal communities were used through the software FUNGuild (http://www.funguild.org/).
3 Results and Discussion
3.1 Effect of different green manures on the degradation of ACE
The degradation of ACE in the CK with different green manure treatments were shown in Table 1 and Fig. S1. The half-life of ACE was 23.10 days in the blank treatment, and the half-life of several other treatments was significantly lower than that of CK. Among them, GMR (Raphanus stativus L.) showed the best effect as green manure, and the degradation time of the soil was shortened to 5.82 days, which improved the degradation efficiency by 74.81 %. This indicated that the ACE degradation was positively affected by the different green manure treatments, and the presence of fertilizer sources may improve the existing environmental system, leading to differences in tpesticide degradation (Zhang et al., 2022). Studies have demonstrated that green manure treatment can increase the adsorption of pesticides in soil, promote the degradation of pesticides and reduce soil residues (Ferreira et al., 2021). The ACE degradation rate of green manure treatment was higher than that of the CK treatment, indicating that green manure was conducive to the ACE degradation in soil (Cai et al., 2007).
| Pesticide | Treatment | Linear equation | K | R2 | t1/2(d) |
|---|---|---|---|---|---|
| ACE | CK | Ct = 1.2848e-0.03x | 0.0300 | 0.9320 | 23.10 |
| GMV | Ct = 1.479e-0.079x | 0.0790 | 0.9445 | 8.77 | |
| GML | Ct = 1.2841e-0.104x | 0.1040 | 0.9173 | 6.66 | |
| GMR | Ct = 1.4837e-0.119x | 0.1190 | 0.9114 | 5.82 |
3.2 Soil microbial diversity under different green manures
A thorough analysis of different types of green manures was conducted to evaluate their influence on pesticide by examining the microbial community structure and diversity. Venn diagram illustrates the distribution of soil OTUs across various treatment conditions (Fig. 1). A total of 583 bacterial OTUs were found to be common to all four soil types (Fig. 1A), suggesting that these OTUs were not significantly affected by ACE. A total of 419 fungal OTUs were identified in all (Fig. 1B). Among these, CK, GMV, GML, and GMR contained 160, 187, 241, and 230 OTUs, respectively, indicating that these endemic fungi effected ACE degradation.
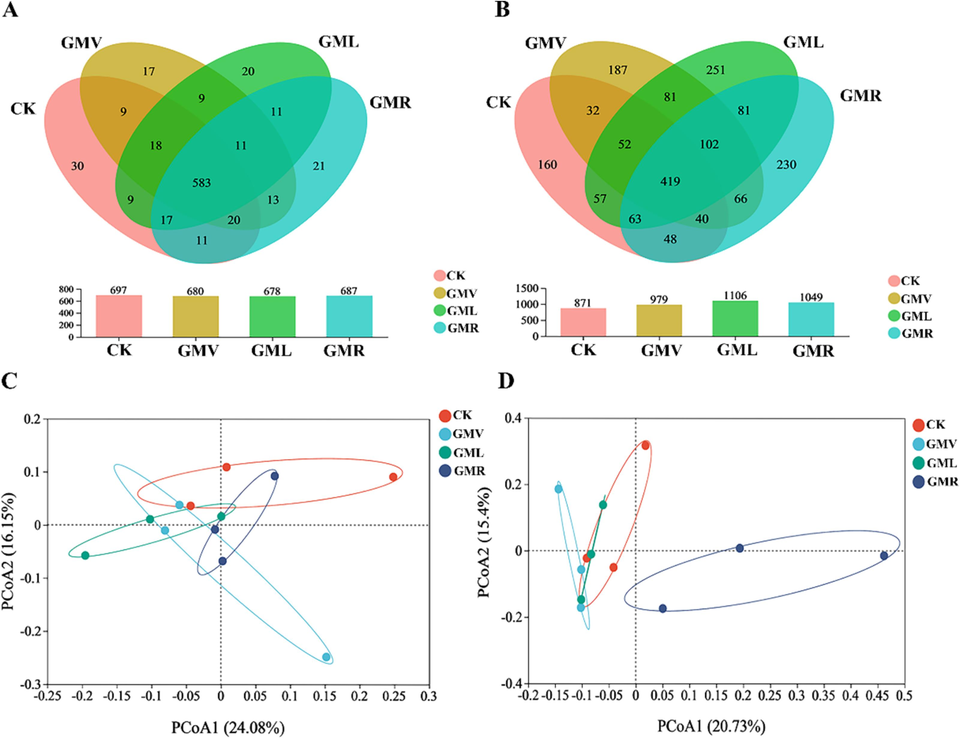
- Venn diagram showing the number of unique and shared OTUs of microbial communities in different green manure soils exposed to ACE. (A) bacteria;(B) fungi. Principal coordinates analysis (PCoA) based on the relative abundance of microbial OTUs showing different microbial community structure: (C) bacteria; (D) fungi. CK: without green manure; GMV: Vicia villosa Roth; GML: Lolium perenne L.; GMR: Raphanus stativus L.
Chao, Shannon, and Simpson indices represent the abundance and diversity of microbial species (Wang et al., 2022b). In our study, Chao and OTUs varied consistently, with CK having the lowest value (Table 2). The Shannon index indicated that soil microbial community diversity was higher and more evenly distributed in the green manure treatment group. The diversity of fungal communities under the four treatments was ranked in the following order: GML > GMR > GMV > CK, and the richness was ranked in the order GMV > GML > GMR > CK (Table 3). Furthermore, we found that application of GMV, GML, and GMR increased the abundance and diversity of bacterial and fungal microbial communities in the soil compared with CK. Xu et al. (2023) showed that green manure ryegrass can alter the microbial taxa involved in soil carbon, nitrogen, and sulfur cycling, thereby increasing the α-diversity of soil bacteria.
| Samples | Sequencing results | Diversity estimates | |||
|---|---|---|---|---|---|
| (Bacterial) | OTUs | Ace | Chao | Shannon | Simpson |
| CK | 2341 ± 64a | 2807.38 ± 10.54a | 2778.30 ± 25.58a | 6.51 ± 0.01a | 0.0043 ± 0.0008a |
| GMV | 2363 ± 123a | 2770.58 ± 130.35a | 2737.79 ± 121.17a | 6.54 ± 0.17a | 0.0047 ± 0.0022a |
| GML | 2458 ± 72a | 2890.99 ± 79.94a | 2868.90 ± 69.18a | 6.55 ± 0.04a | 0.0056 ± 0.0011a |
| GMR | 2406 ± 99a | 2889.89 ± 59.74a | 2862.97 ± 84.31a | 6.53 ± 0.11a | 0.0042 ± 0.0002a |
ACE and Chao indices present the species richness, while the Shannon and Simpson indices estimate the microbial community diversity. CK: without green manure; GMV: Vicia villosa Roth; GML: Lolium perenne L.; GMR: Raphanus stativus L. Statistical signature (a, b) was obtained by comparison between groups (CK vs GMV vs GML vs GMR).
| Samples | Sequencing results | Diversity estimates | |||
|---|---|---|---|---|---|
| (Fungal) | OTUs | Ace | Chao | Shannon | Simpson |
| CK | 477 ± 59b | 494.06 ± 69.11b | 497.22 ± 70.27b | 4.24 ± 0.27a | 0.0388 ± 0.0147a |
| GMV | 634 ± 35a | 664.09 ± 51.83a | 664.32 ± 52.07a | 4.20 ± 0.16a | 0.0437 ± 0.0061a |
| GML | 604 ± 89a | 649.31 ± 105.35a | 654.05 ± 103.15a | 3.84 ± 0.36a | 0.0694 ± 0.0301a |
| GMR | 550 ± 25ab | 572.02 ± 38.20ab | 570.67 ± 32.06ab | 3.99 ± 0.38a | 0.0677 ± 0.0339a |
ACE and Chao indices present the species richness, while the Shannon and Simpson indices estimate the microbial community diversity. CK: without green manure; GMV: Vicia villosa Roth; GML: Lolium perenne L.; GMR: Raphanus stativus L. Statistical signature (a, b, ab) was obtained by comparison between groups (CK vs GMV vs GML vs GMR).
Principal coordinate analysis (PCoA) was performed evaluate the modifications in the composition of microbial communities ACE after exposure. Fig. 1D illustrates that approximately 36.13 % of the variability in the microbial community was accounted for by 20.73 % in the first dimension and 15.4 % in the second dimension. The fungal community composition significantly differed among the four treatment groups, with GMR showing a distinct composition compared to GMV and GML which exhibited a bacterial community composition more similar to that of CK soil as per PCoA. Moreover, bacterial PCoA indicated that 24.08 % and 16.15 % of the variation could explained by PCoA1 and PCoA2, respectively (Fig. 1C). The sample sites were tightly clustered, so there were no significant differences in bacterial community composition between treatments.
3.3 Structure of soil microbial communities under different green manures
3.3.1 Variations in bacterial community composition
The top five dominant bacterial communities at the phylum level were Proteobacteria (20.69 %-28.53 %), Actinobacteriota (22.24 %-25.42 %), Chloroflexi (17.20 %-19.62 %), Acidobacteriota (9.11 %-15.41 %), and Bacteroidata (3.50 %-3.95 %), accounting for more than 72.74 %-92.93 % of the bacterial sequences (Fig. 2A). The prevalence of Proteobacteria, Actinobacteria, Chlorobacteria, and Acidobacteria in this study was consistent with previous findings (Gao et al., 2023; Zhang et al., 2023). The proportion of both Actinobacteriota and Acidobacteriota were higher in the green manure treatment than in the CK. Acidobacteria isis widespread in highly acidic fibrous tissue soils rich in soil organic matter and effective phosphorus and can survive in extreme environments and nutrient-poor soils (Geng et al., 2020). Similarly, Actinobacteriota is widely present in soil and can be cleaned up by bioremediation, making them a good choice for contaminated soil (Alvarez et al., 2017).
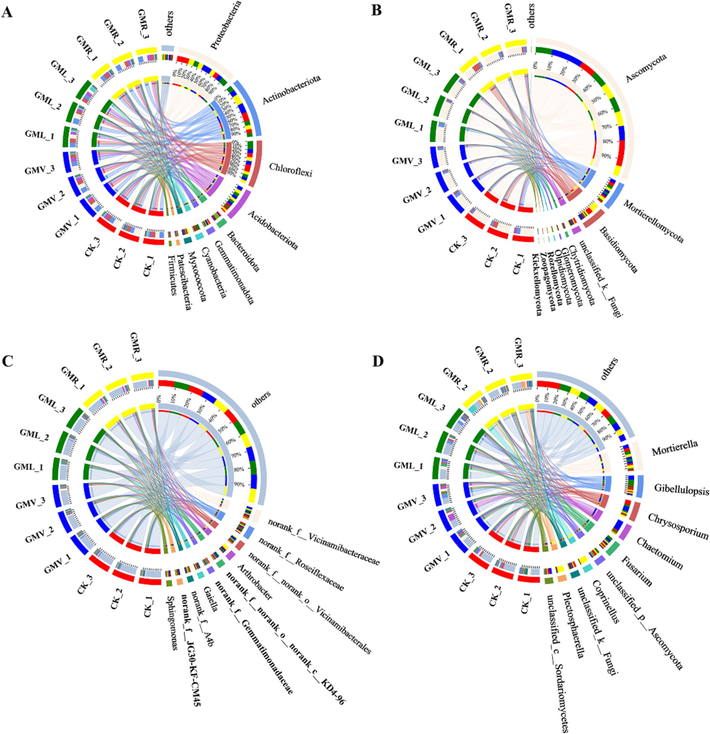
- Distribution of microbial community for each sample at phylum and genus level. The data were visualized by Circos. The width of the bars from each phylum and genus indicates the relative abundance of that phylum and genus in the sample. (A), (B) phylum level; (C), (D) genus level. CK: without green manure; GMV: Vicia villosa Roth; GML: Lolium perenne L.; GMR: Raphanus stativus L.
Microbial communities were further tested for differentiation at the genus level. Fifteen genera were detected in bacterial communities affected by GMV, GML, and GMR (Fig. 3A). The five genera with the highest relative abundance were norank_f__Vicinamibacteraceae, norank_f__Roseiflexaceae, norank_f__norank_o__Vicinamibacterales, Arthrobacter, and norank_f__norank_o__norank_c__KD4-96, which accounted for 13.22 %-24.49 % of the bacterial community (Fig. 2C). Among the four treatments, the green manure treatment had higher norank__f__Vicinamibacteraceae, norank__f__norank__o___Vicinamibacterales, and norank__f__norank__o__norank__c__KD4-96 than the control. Ellin6067 was less abundant in all treatments, whereas Arthrobacter was more abundant in the GMR treatment (Fig. 3A). Aerobic bacteria (Arthrobacter) can establish populations in the rhizosphere that facilitate the rapid degradation of pesticides in soil when they are present, and are a promising bioremediation agent for contaminated soils (Bazhanov et al., 2017). The varying effects of different fertilization conditions on Arthrobacte could be attributed to the distinct soil properties present in the four treatments (Li et al., 2022). Studies have shown that bacteria of the genus Arthrobacter can degradate environmental organic pollutants such as benzene derivatives, polycyclic aromatic hydrocarbons (PAHs), N-heterocyclic compounds, and other environmental organic pollutants. And they have an abundance of selective factors and resilience such as oxidative stress, which is important for the degradation of pollutants in complex and variable environments (Hasan et al., 2011; Vandera et al., 2015). Therefore, considering the pesticide remediation properties of Arthrobacter spp. (Actinobacteriota) in soils, it was speculated that they might be involved in the degradation of ACE.
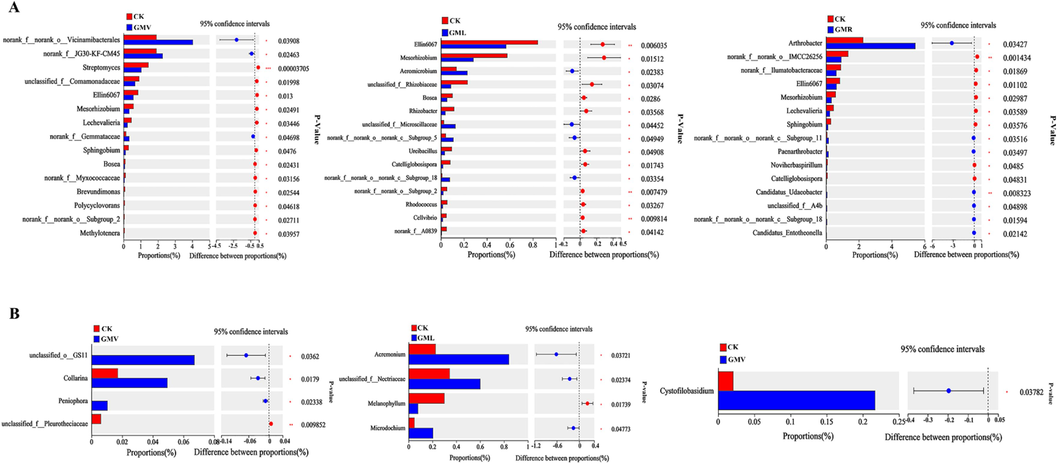
- Plots showing the genera with differential abundance in soils with GMV, GML and GMR comparing the CK. (A) bacteria;(B) fungal. P-values were calculated based on Welch’s t-test with Benjamini-Hochberg correction (p < 0.05). CK: without green manure; GMV: Vicia villosa Roth; GML: Lolium perenne L.; GMR: Raphanus stativus L.
3.3.2 Variations in fungal community composition
Ten phyla were identified based on the classification of the fungal communities at the phylum level (Fig. 2B). The top five dominant fungi were Ascomyocta (68.46 %-79.64 %), Mortierellomycota (9.78 %-13.06 %), Basidiomycota (3.87 %-17.49 %), unclassified_k__Fungi (2.36 %-4.88 %), and Chytridiomycota (0.54 %-1.39 %), accounting for more than 85.01 %-116.46 % of fungal sequences (Fig. 2B). As shown in Fig. 2B, Ascomyocta and Mortierellomycota were the most prominent phyla in the fungal community, although other phyla constituted a smaller percentage of the fungal community. Ascomyocta abundance was higher in the green manure treatments than in CK, except for GMR. In most cases, Ascomyocta has a greater ability to withstand environmental stressors and use more resources, which may contribute to their increased dominance in the soil (Egidi et al., 2019).
Mortierella, Gibellulopsis, Chrysosporium, Chaetomium, and Fusarium are the genera with a high relative abundance, comprising 25.09 %-58.33 % of the fungal community (Fig. 2D). Previous research has indicated that Mortierella (a genus from the Mortierellomycota phylum) is the dominant fungal community for herbicides, such as isopropylmethachlor, with Mortierella and Chaetomium being the primary fungi responsible for degrading amide herbicides (Li et al., 2019). The relative abundance of Chaetomium (Ascomycetes spp.) and Mortierella varied between treatments, and the GMV treatment group had a significantly higher relative abundance than the other treatment groups, which has been found to be similar in other studies (Zhao et al., 2021) (Fig. 2D, p < 0.05). The abundance of differential fungal genera was elevated in all treatments compared to CK. Among them, the difference was more significant for Cystofilobasidium, and GMR had a much higher relative abundance compared to CK (Fig. 3B). Cystofilobasidium belongs to the fungi under the phylum Ascomycota, which are widely distributed in environments such as soils and plant surfaces, and utilizes the ability of d-glucuronide and myo-inositol to serve as the sole source of carbon as well as nitrate as the sole source of nitrogen for its assimilation (Pontes et al., 2016). The study also showed that Cystofilobasidium is resistant to pesticides and there are some differences in pesticide inhibition (Sláviková and Vadkertiová, 2003). Nevertheless, variations in soil microbial communities may also arise owing to differences in soil nutrient levels and other environmental factors (Han et al., 2022). Feng et al. (2023) in their report elaborated that fungi have the potential to degrade herbicides, but that this activity may be direct or indirect, and speculated that fungi may play an important role in the degradation of ACE. Therefore, Mortierella (Mortierellomycota spp.) and Chaetomium (Ascomycetes spp.) may be important for the addition of green manure to promote ACE degradation.
3.4 Analysis of microbial communities in relation to soil properties
Earlier findings have indicated that soil physicochemical properties significantly influence both compositional and functional changes in soil microbial communities (Li et al., 2021a). The thermograms showed that soil pH, soil total phosphorus (TP) and soil total potassium (TK) had a significant effect on bacterial microbial communities, and soil pH and soil total phosphorus (TP) had a significant effect on the microbial communities of fungi (Fig. 4). Therefore, the present study focused on the effect of common influencing factors pH and TP on soil microbial communities. This finding is in agreement with that of other studies (Li et al., 2021b). Among the most well-known environmental factors influencing how the organization of soil microbial communities varies is pH (Shen et al., 2013). It has recently been shown that soil phosphorus addition mainly influences microorganisms by affecting soil carbon cycling and chemical properties (e.g., pH) (Xiao et al., 2022). Correlation analysis showed that pH was mainly positively correlated with Myxococcota (Fig. 4A, p < 0.05), which was similar to previous research results (Chen et al., 2021). In addition, several other studies have shown positive associations between TP and certain bacterial phyla, including Protcobacteria and Myxococcota (Dao et al., 2023). Protcobacteria is the more abundant phylum in the bacterial microbial community (Fig. 2A), and previous studies have shown that Actinobacteriota and Proteobacteria, among others, are the dominant bacteria in soils where amide herbicides have been applied, and that the duration of herbicide exposure is a major determinant of bacterial community variability (Wang et al., 2022b). In addition, bacteria belonging to α-, β-, and γ-Proteobacteria can utilize hydrocarbons as carbon sources and degrade pesticide contaminants into simpler forms (Klase et al., 2019). This suggests that Proteobacteria may contribute to the degradation of ACE. Among the fungal communities, only Basidiomycota and Kickxellomycota were positively associated with pH and TP, whereas the other phyla were not significantly correlated with pH and TP (Fig. 4B) (Shen et al., 2013). Overall, there was a correlation between soil physico-chemical properties and changes in soil bacterial and fungal community structure under different green manure treatments. However, soil is a complex environment that influenced by many factors, making it difficult to determine correlations between soil, pesticides, and microbial communities (Han et al., 2022).
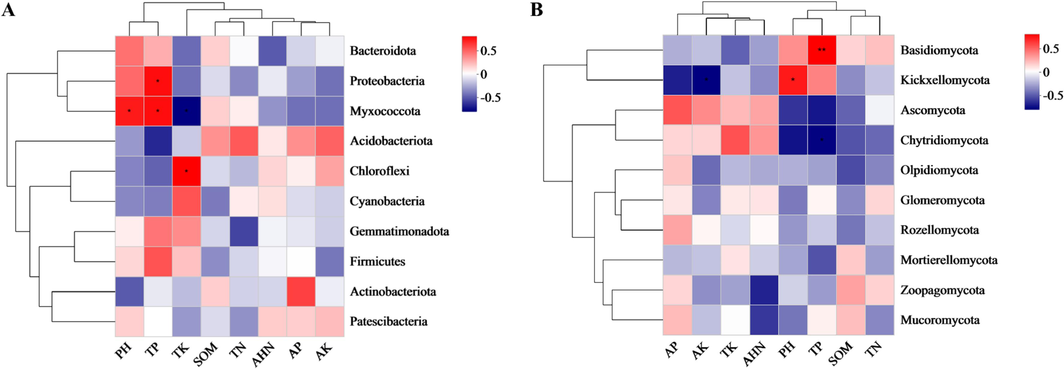
- The correlation heatmap analysis between environmental factors and species of bacterial by Bray-Curtis distances. (A) bacteria; (B) fungi. TP: total phosphorus.
3.5 Prediction of soil microbial communities for degradation of ACE
Microbial biodegradation is an important process in the removal of organic pollutants from soil and that fungi are prominent in the degradation of metolachlor in soil (Sun et al., 2020). FUNGuild was used to categorize the fungi based on their functions to study the impact of microorganisms on soil ACE degradation in the four different treatments. A total of nine trophic modes were identified based on Fig. 5A, of which the three main trophic modes were pathotrophic, saprotrophic, and symbiotrophic. Pathotrophic fungi had the highest relative abundance in GMR, while the relative abundance of saprotrophic fungi and symbiotic fungi were higher in CK treatment than green manure treatment group (Table S6). The application of green manure altered the relative abundance of saprotroph, pathotroph, and symbiotroph. The presence of highly pesticide-sensitive fungal genera in the saprophytic fungal phylum is associated with the residues deethylatrazine, pyrimethamine, and S-isopropylmethachlor (Walder et al., 2022). The decrease in saprophytic fungi under green manure treatments in this study may be linked to the degradation of ACE in the soil by these fungi (Table S6). The three main nutrient modes were further analyzed by guilds, and 11 major dominant taxa were found, of which the top five guilds in abundance were undefined saprotrophs, plant pathogens, dung saprotrophs, animal pathogens, and arbuscular mycorrhizalss (Fig. 5B). (According to the above diversity analysis, there was no significant difference in bacteria, therefore, it was not be repeated). According to Fig. 5C, the test of fungi related to the undefined saprotrophic fungi and arbuscular mycorrhizal fungi (AMF) showed that the dominant fungal groups were Ascomycota and Glomeromycota. According to Fig. 2B and 3B, application of green manure can change the abundance of fungal microbial communities. Among them, the abundance of Ascomycota increased under green manure treatment, the difference of Cystofilobasidium was more significant, and the relative abundance of GMR was much higher than that of CK. Egidi et al. (2019) showed that Ascomycota has a strong ability to withstand environmental pressure and utilize more resources, which is more conducive to its survival in soil. Sláviková and Vadkertiová (2003) found that Cystofilobasidium was resistant to pesticides, and there were some differences in pesticide inhibition. As soil saprotrophic fungus, Ascomycota can degrade lignified plant residues in the soil and promote rapid growth and reproduction of the fungal community (Boer et al., 2005). Whiteway et al. (2015) in their study found that the phylum Ascomycetes and Ascomycetes can degrade harmful aromatic pollutants in soil. In the CK treatment, the mycorrhizal fungus Glomeromycota was most abundant (Fig. 5D). The presence of arbuscular mycorrhizal fungi (AMF) enhanced the plant root response to atrazine by upregulating relevant enzymes, alleviating toxic effects, and promoting soil atrazine degradation (Huang et al., 2009). Based on the results of bubble abundance maps and previous studies, we concluded that Ascomycota and Glomeromycota may be involved in ACE degradation. However, because of the numerous unknown factors in the soil environment, further analysis is required to fully understand the degradation pathways (Chen et al., 2023).
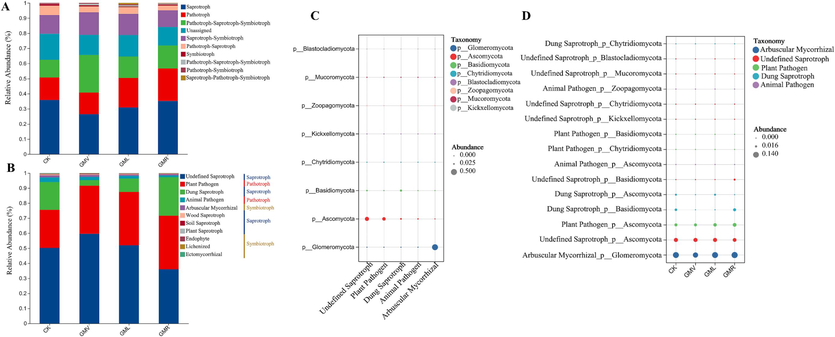
- Variations in fungal function (A), composition of fungal functional groups (guilds) inferred by FUNGuild (B), and comparison of fungal distribution in fungal function groups and different samples (C-D). CK: without green manure; GMV: Vicia villosa Roth; GML: Lolium perenne L.; GMR: Rhaphanus stativus L.
4 Conclusion
In this study investigated ACE microbial degradation under various green manure conditions. The application of green manure promoted the degradation of ACE, in which the degradation time of Raphanus stativus L. was shortened to 5.82 days, and the degradation rate was increased by 74.81 % compared with CK. Correlational analysis reveal that TP and pH significantly affected the microbial community. PCoA showed some differences in the fungal community composition and non-significant differences in the bacterial community composition between treatments. FUNGuild inferred that the three main trophic modes were pathotrophic, saprotrophic, and symbiotic. The application of green manure altered the relative abundance of saprotrophic, pathotrophic, and symbiotic fungi. The relative abundance of saprotrophic fungi and symbiotic were lower in the green manure treatment than in the CK treatment, and the rate of ACE degradation was higher. Fungal functional analysis revealed that the microorganisms associated with ACE biodegradation mainly belonged to Ascomycota and Glomeromycota. In summary, the application of green manure can affect the soil microbial community and promote the degradation of ACE. It is recommended to choose Raphanus stativus L. as green manure to more effectively promote the degradation of ACE in soil. This study provides a valuable theoretical basis for the isolation of microbial degradation of ACE, this study provides a valuable theoretical basis.
CRediT authorship contribution statement
Qian Liu: Writing – original draft, Visualization, Investigation, Data curation. Wanqiu Jing: Investigation. Wansheng Yang: Investigation, Data curation. Min Huang: Investigation. Ping Lu: Project administration, Methodology, Investigation, Funding acquisition. Deyu Hu: Supervision, Project administration.
Acknowledgements
This work was supported by Guizhou Provincial Science and Technology Project (grant number ZK [2022] ZD-013), National Natural Science Foundation of China (grant number 32260692).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue from northern Tunisia: Current extent of contamination and contributions of socio-demographic characteristics and dietary habits. Environ. Res.. 2017;156:635-643.
- [CrossRef] [Google Scholar]
- Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere. 2017;166:41-62.
- [CrossRef] [Google Scholar]
- Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int. 2003;86:412-431.
- [Google Scholar]
- Effect of the herbicide glyphosate on glyphosate-tolerant maize rhizobacterial communities: a comparison with pre-emergency applied herbicide consisting of a combination of acetochlor and terbuthylazine. Environ. Microbiol.. 2010;12:1021-1030.
- [CrossRef] [Google Scholar]
- Colonization of plant roots and enhanced atrazine degradation by a strain of Arthrobacter ureafaciens. Appl. Microbiol. Biotechnol.. 2017;101:6809-6820.
- [CrossRef] [Google Scholar]
- Persistence of acetochlor, atrazine, and S-metolachlor in surface and subsurface horizons of 2 typic argiudolls under no-tillage. Environ. Toxicol. Chem.. 2017;36:3065-3073.
- [CrossRef] [Google Scholar]
- Boer, W., de, Folman, L.B., Summerbell, R.C., Boddy, L., 2005. Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiology Reviews. 29, 795–811. 10.1016/j.femsre.2004.11.00.
- Degradation and detoxification of acetochlor in soils treated by organic and thiosulfate amendments. Chemosphere. 2007;66:286-292.
- [CrossRef] [Google Scholar]
- High-throughput sequencing analysis of the composition and diversity of the bacterial community in cinnamomum camphora soil. Microorganisms.. 2021;10:72.
- [CrossRef] [Google Scholar]
- Insights into the metabolic pathways and biodegradation mechanisms of chloroacetamide herbicides. Environ. Res.. 2023;229:115918
- [CrossRef] [Google Scholar]
- Diversity of soil bacteria in alpine coal slag mountain grassland in different vegetation restoration years. Ann. Microbiol.. 2023;73:12.
- [CrossRef] [Google Scholar]
- A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun.. 2019;10:2369.
- [CrossRef] [Google Scholar]
- The effect of organomineral fertilization phosphorus on the availability of phosphorus in a calcareous soil Appl. Ecol. Environ. Res.. 2023;21:4545-4562.
- [CrossRef] [Google Scholar]
- Degradation of acetochlor in soil by adding organic fertilizers with different conditioners. Soil Tillage Res.. 2023;228:105651
- [CrossRef] [Google Scholar]
- Green manure species for phytoremediation of soil with tebuthiuron and vinasse. Front. Bioeng. Biotechnol.. 2021;8
- [CrossRef] [Google Scholar]
- The effect of long-term controlled-release urea application on the relative abundances of plant growth-promoting microorganisms. Eur. J. Agron.. 2023;151:126971
- [CrossRef] [Google Scholar]
- Effects of Acetochlor on Wheat Growth Characteristics and Soil Residue in Dryland. Gesunde Pflanzen. 2021;73:307-315.
- [CrossRef] [Google Scholar]
- Bacterial community structure and diversity in the soil of three different land use types in a coastal wetland. Appl. Ecol. Env. Res.. 2020;18:8131-8144.
- [CrossRef] [Google Scholar]
- Two new sexual talaromyces species discovered in estuary soil in China. J. Fungi. 2022;8:36.
- [CrossRef] [Google Scholar]
- Complete biodegradation of 4-Fluorocinnamic acid by a consortium comprising Arthrobacter sp. Strain G1 and Ralstonia sp. Strain H1. Appl. Environ. Microbiol.. 2011;77:572-579.
- [CrossRef] [Google Scholar]
- Influence of glomus etunicatum/Zea mays mycorrhiza on atrazine degradation, soil phosphatase and dehydrogenase activities, and soil microbial community structure. Soil Biol. Biochem.. 2009;41:726-734.
- [CrossRef] [Google Scholar]
- Human health and ecological risk assessment of pesticides from rice production in the Babol Roud River in Northern Iran. Sci. Total Environ.. 2021;772:144729
- [CrossRef] [Google Scholar]
- Analysis of phenotypic characteristics and sucrose metabolism in the roots of raphanus sativus L. Front. Plant Sci.. 2021;12:716782
- [CrossRef] [Google Scholar]
- The microbiome and antibiotic resistance in integrated fishfarm water: Implications of environmental public health. Science of Total Environ.. 2019;649:1491-1501.
- [CrossRef] [Google Scholar]
- Nutrients available in the soil regulate the changes of soil microbial community alongside degradation of alpine meadows in the northeast of the Qinghai-Tibet Plateau. Sci. Total Environ.. 2021;792:148363
- [CrossRef] [Google Scholar]
- Structure and driving factors of the soil microbial community associated with Alhagi sparsifolia in an arid desert. PLoS One. 2021;16:e0254065
- [CrossRef] [Google Scholar]
- Shifts in rhizosphere microbial communities in Oplopanax elatus Nakai are related to soil chemical properties under different growth conditions. Sci. Rep.. 2022;12:11485.
- [CrossRef] [Google Scholar]
- Restructured fungal community diversity and biological interactions promote metolachlor biodegradation in soil microbial fuel cells. Chemosphere. 2019;221:735-749.
- [CrossRef] [Google Scholar]
- Legume green manuring: an option for soil sustainability. In: Meena R.S., Das A., Yadav G.S., Lal R., eds. Legumes for Soil Health and Sustainable Management. Singapore: Springer; 2018. p. :387-408.
- [CrossRef] [Google Scholar]
- Cystofilobasidium intermedium sp. nov. and Cystofilobasidium alribaticum f.a. sp. nov., isolated from Mediterranean forest soils. Int. J. Syst. Evol. Microbiol.. 2016;66:1058-1062.
- [CrossRef] [Google Scholar]
- Comparison of a premix of atrazine, bicyclopyrone, mesotrione, and S-metolachlor with other preemergence herbicides for weed control and corn yield in no-tillage and reduced-tillage production systems in Nebraska, USA. Soil and Tillage Research. 2018;178:82-91.
- [CrossRef] [Google Scholar]
- Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem.. 2013;57:204-211.
- [CrossRef] [Google Scholar]
- Effects of pesticides on yeasts isolated from agricultural soil. Z. Naturforsch. c: J. Biosci.. 2003;58:855-859.
- [CrossRef] [Google Scholar]
- Response of soil bacterial and fungal community structure succession to earthworm addition for bioremediation of metolachlor. Ecotoxicol. Environ. Saf.. 2020;189:109926
- [CrossRef] [Google Scholar]
- Phytoextraction of diuron, hexazinone, and sulfometuron-methyl from the soil by green manure species. Chemosphere. 2020;256:127059
- [CrossRef] [Google Scholar]
- Comparative proteomic analysis of Arthrobacter phenanthrenivorans Sphe3 on phenanthrene, phthalate and glucose. J. Proteomics. 2015;113:73-89.
- [CrossRef] [Google Scholar]
- Soil microbiome signatures are associated with pesticide residues in arable landscapes. Soil Biol. Biochem.. 2022;174:108830
- [CrossRef] [Google Scholar]
- The green manure (Astragalus sinicus L.) improved rice yield and quality and changed soil microbial communities of rice in the karst mountains area. Agronomy. 2022;12:1851.
- [CrossRef] [Google Scholar]
- Insights of microbial community evolution under benzisothiazolinone exposure in different soil environments. Chemosphere. 2022;307:135868
- [CrossRef] [Google Scholar]
- Occurrence and risk assessment of three chloroamide herbicides in water and soil environment in northeastern, eastern and southern China. Environ. Res.. 2023;219:115104
- [CrossRef] [Google Scholar]
- Metabolic regulation in model ascomycetes – adjusting similar genomes to different lifestyles. Trends Genet.. 2015;31:445-453.
- [CrossRef] [Google Scholar]
- Characteristics of endophytic bacterial community structure in roots of sugarcane under different fertilizer applications. Acta. Agronom. Sin.. 2022;48:1222-1234.
- [CrossRef] [Google Scholar]
- Various green manure-fertilizer combinations affect the soil microbial community and function in immature red soil. Front. Microbiol.. 2023;14:1255056.
- [CrossRef] [Google Scholar]
- Degradation of acetochlor by four microbial communities. Bioresour. Technol.. 2008;99:7797-7802.
- [CrossRef] [Google Scholar]
- Enantioselective degradation mechanism of beta-cypermethrin in soil from the perspective of functional genes. Chirality. 2015;27:929-935.
- [CrossRef] [Google Scholar]
- Bacterial community compositions and nitrogen metabolism function in a cattle farm wastewater treatment plant revealed by Illumina high-throughput sequencing. Environ. Sci. Pollut. Res.. 2021;28:40895-40907.
- [CrossRef] [Google Scholar]
- Dissipation dynamics and residue of four herbicides in paddy fields using HPLC-MS/MS and GC-MS. Int. J. Environ. Res. Public Health. 2019;16:236.
- [CrossRef] [Google Scholar]
- Bacterial community structure in rhizosphere of barley at maturity stage. Agronomy. 2023;13:2825.
- [CrossRef] [Google Scholar]
- Effects of fertilizers and soil amendments on the degradation rate of allyl isothiocyanate in two typical soils of China. Pest Manage. Sci.. 2022;78:5191-5202.
- [CrossRef] [Google Scholar]
- Variation of rhizosphere microbial community in continuous mono-maize seed production. Sci. Rep.. 2021;11:1544.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.106017.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







