Translate this page into:
Green synthesis, characterization and anti-cancer capability of Co0.5Ni0.5Nd0.02Fe1.98O4 nanocomposites
⁎Corresponding author. fakhan@iau.edu.sa (F.A. Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, Co0.5Ni0.5Nd0.02Fe1.98O4 nanoparticles CoNiNd (NPs) were synthesized by combustion method linked with biosynthesis with and without different plant extracts such as Lavender, Ginger, Flax-Seed, Lemon Juice, Tragacanth Gum, and Dates Fruit. Co0.5Ni0.5Nd0.02Fe1.98O4 nanoparticles (NPs) with plant extracts (CoNiNd plant extracts) were analyzed by XRD, TEM and SEM methods. The structure of Co-Ni spinel ferrite was confirmed by XRD and the shape and the size of nanoparticles were examined via SEM and TEM and the size was found between 17 and 25 nm. The anti-cancer activity of NPs on cancer cells such as human colorectal carcinoma (HCT-116) and human cervical cells (Hela) were investigated. The cytotoxicity of was examined by MTT assay and followed by measuring the inhibitory concentration (IC50) values after 48 h treatments. The cell viability assay confirmed a decrease in the cancer cell viability post NPs treatments and showed dose-dependent inhibitory action. The treatments of CoNiNd (NPs) and CoNiNd plant extracts via Lavender plant extract showed most profound inhibitory action on both cancer cells than extracts other plant extracts. The IC50 values were for HCT-116 cells were found to be in range of 15.75–42.55 µg/mL and 13.44 to 35.65 µg/mL for HeLa cells. In contrast, the treatment of CoNiNd (NPs) and CoNiNd plant extracts showed inhibitory action but the percentage of inhibition was higher in HEK-293 cells. Our results showed that CoNiNd (NPs) and CoNiNd plant extracts possess potential application in the colon and cervical cancer treatments and we recommend molecular analysis of NPs mediated cancer cell death for future applications.
Keywords
Green synthesis
Plant extract
Spinel ferrite nanomaterials
Anti-cancer agent
Colon cancer cells
Cervical cancer cells
1 Introduction
Spinel ferrites are fundamental complex oxide of iron, typically emerged as the most favorable magnetic material in many applications in bioscience (Dönmez et al., 2019), water purification (You et al., 2020), catalysis (Maleki et al., 2020), optics, Ferro fluids (Wu et al., 2020), biosensors (Rozhina et al., 2021), cellular signaling, and biomedicines such as drug delivery (Nogueira et al., 2020) and hyperthermia (Amiri et al., 2019). As described by Matsumura and Maeda in the late 1980 s, nanoscale substances readily diffuse and accumulates into tumor cells (Matsumura and Maeda, 1986), making spinel ferrites nanoparticles (SPN) are suitable for anticancer drug delivery. Unlike other magnetic ferrites that display remanent magnetization, SPN, in contrast, exhibits super-paramagnetic properties at room temperature. This is very essential in biomedical applications, because, in the absence of an external magnet, the material displays no magnetization, no agglomeration, or accumulation around a biological environment. Further, super-paramagnetic is also influenced by the size of spinel ferrite (Galvão et al., 2016). One of the critical challenges is, the systematic synthesis of nano spinel materials, while precisely maintaining the particle size and morphology.
The cobalt iron oxide (CoFe2O4) NPs and its metal substituted derivatives are characterized with unique properties such as high magneto-crystalline anisotropy coupled with adequate saturation magnetization and high coercivity. Furthermore, the tendency of CoFe2O4 NPs to form nanosphere, excellent coupling efficiency as well as high magnetostriction makes them preferred candidate for diverse technological industries and medical applications (Tomitaka et al., 2009; Tran and Webster, 2010; El-Sayed et al., 2020). Fan et al. have reported CoFe2O4 NPs encapsulated with silica shell which resulted in fibrous morphology. The composite was described to have good biocompatibility and can effectively load and release a doxorubicin hydrochloride anticancer drug via pH stimuli (Fan et al., 2018). Cheraghi et al. employed lemon juice to prepare CoFe2O4 NPs via a modified Pechini route. Concentrated fruit extract was found to enhance the saturation magnetization of the product, i.e when 10 ml and 50 ml lemon juice was used, a CoFe2O4 with 16.6 emu/g and 75.7 emu/g were produced respectively. The sample coated with polyethylene glycol showed promising anticancer drug loading and release capacity (Cheraghi et al., 2021).
Several synthetic techniques were used to crystallize an ultra-small sized spinel ferrite of various morphology have been established, usually, a wet-chemical synthesis is employed due to the atomic level building of the crystals. The common preparation methods such as solvothermal decomposition, sol–gel, co-precipitation, and thermal decomposition. All requires an organic/inorganic solvent as a medium of the reaction, surfactant, alkaline solution (concentrated NaOH or NH3) etc. (Srivastava et al., 2018; De Berti et al., 2013; Salunkhe et al., 2015). These chemicals are hazardous and toxic. However, the green chemical processes stressed that chemical hazards should be avoided or minimized during synthesis and offered an environmentally benign process and the products are less harmful to human health (Anastas and Eghbali, 2010) .
Green synthesis methodology using plants extraction was the primary fabrication method that applied for synthesizing nanoparticle. It is easy, economically, facile manufacturing process, less waste production, nontoxic nature, and fast (Liaskovska et al., 2019). In this background, the application of green synthesis provide a better and effective method to minimize the use of hazardous chemicals during nanomaterial preparation.
There are many reports available for green synthesis of ferrites using Moringa plant extract. Domestic fruits such as Lavender, Ginger, Flax-Seeds, Lemon Juice, Tragacanth Gum, and Dates Fruit are known for their medicinal and biomedical applications. These plants have vital advantage such as easy availability, low cost of production, and facile processing, and most important they are eco-friendly. Although significant reports are available for green synthesis of ferrites using Moringa plant extract (Matinise et al., 2018; Aisida et al., 2020; Aisida et al., 2019). K. Kombaiah et al. used Okra extraction for synthesis of CoFe2O4 NPs by microwave approaches (Kombaiah, 2018). They found that the sample showed good magnetic behavior and exceptional antimicrobial activity. Moreover, Dana Gingasu et al. utilized cardamom seeds and ginger root to prepare CoFe2O4NPs with self-combustion technique (Gingasu, 2017). Karunakaran et al. used the Hydrangea paniculata flower extraction to make NiFe2O4 (Karunakaran, 2018). There results approved that the green synthesis is an excellent method to produce NiFe2O4 NPs. M.M. Naik et al. fabricated ZnFe2O4NPs were prepared with Limonia acidissima extraction. They obtained an efficacious photodegradation and antibacterial activity (Naik et al., 2019).
Accordingly, we decided to perform a green synthesis of CoNiNd (NPs) using six different plant extracts viz: Lavender, Ginger, Flax-Seed, and Lemon Juice, Tragacanth Gum, and Date fruits. After detailed spectroscopic and microscopic examination, CoNiNd (NPs) alone and CoNiNd (NPs) plant extracts were used for the first time to screen the in-vitro cytotoxicity against human colorectal carcinoma (HCT-116) and human cervical cells (Hela).
2 Materials & methods
2.1 Preparation plants extraction
The Lavender seeds, Ginger, Flax Seeds, Lemon Juice, Tragacanth Gum and Date fruit (Fig. 1) were collected from local markets. To prepare a plant extract, all plants firstly cleaned and washed with DI water to remove any dust and dried them. Next, the extractions were synthesized by using 3 g of Lavender, 10 g Ginger, 2.5 g of Flax Seeds, 50 ml Lemon Juice, 10 g of Tragacanth Gum and 5 pieces of Date Fruit with 100 ml DI water and heated them at 70 °C for 1 h with stirring, while 50 ml from Lemon juice was used without any further processing. Finally, the solutions contained extraction was filtered to be ready to use for preparing the NPs.
Plants that used for the green synthesis of CoNiNd (NPs).
2.2 Synthesis of CoNiNd (NPs) with and without plant extracts
Initially, CoNiNd (NPs) were prepared via normal sol–gel combustion method. A specific weight of Co(NO3)2·6H2O, Ni(NO3)2·6H2O, Nd(NO3)3 and Fe(NO3)3·9H2O were dissolved with 70 ml of DI water under stirring at 80 °C for 40 min. The solution pH was adjusted to 7 using NH3 solution and heated at 150 °C for 35 min. then increased up to 350 °C to get a gel that burned and turned into a black powder. The Co0.5Ni0.5Nd0.02Fe1.98O4 NPs was sintered at 800 °C for 5 h as explained in Fig. 2(a).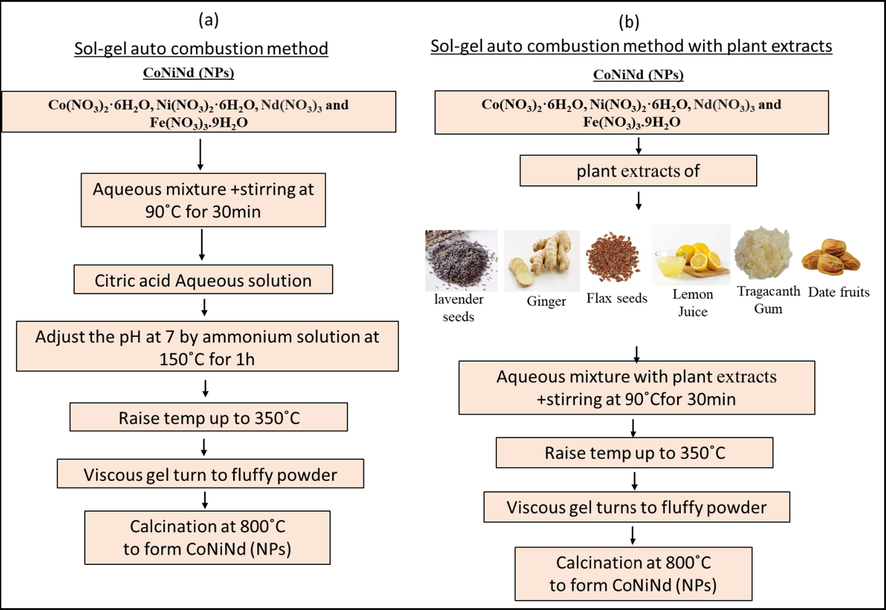
The scheme of preparation CoNiNd (NPs) with (a) Sol-gel auto combustion method and (b) with plant extracts (CoNiNd (NPs) plant extracts).
For the synthesis of CoNiNd (NPs) with plants extraction, a stoichiometric amount of Co(NO3)2·6H2O, Ni(NO3)2·6H2O, Nd(NO3)3 and Fe(NO3)3·9H2O were mixed with 60 ml of each plant extract (Lavender seeds, Ginger, Flax Seeds, Lemon Juice, Tragacanth Gum and Date Fruit) by stirring at 80 °C for 40 min. after that the magnetic stirring was stopped, the temperature was increased to 200 °C and heated until the whole solution was evaporated and turned to a black powder (almost 2 h). The resulted powder was calcinated at 800 °C for 5 h as mentioned in Fig. 2(b). The all products were labeled and listed in Table 1.
2.3 Instrumentation
The structure of CoNiNd (NPs) and CoNiNd (NPs) plant extracts were recorded via Benchtop Rigaku Miniflex X-ray diffractometer (Cu Kα line). The nano particles morphology was gotten by FEI Titan ST SEM and TEM linked with EDX spectroscopy for elemental analysis of the study samples.
2.4 Treatment of CoNiNd (NPs) and CoNiNd (NPs) plant extracts on cancerous cells
We have taken two cancer cell lines, human colorectal carcinoma (HCT-116) and human cervical cells (Hela), to study the impact of CoNiNd (NPs) and CoNiNd (NPs) plant extracts on their viability and proliferation. The cells were cultured and maintained in the DMEM media, L-glutamine (5%), penicillin (1%), streptomycin (1%), FBS (10%), and selenium chloride (1%) as per previously described method (1). The cells were grown in 96 well plates in a 5% CO2 incubator (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37 °C, and 75–80% confluence cells and cell processed for MTT assay (Khan et al., 2018). The MTT assay was done as per the previous study (Rehman et al., 2021). The cells were treated with extracts of CoNiNd (NPs) and CoNiNd (NPs) plant extracts with dosages ranging from 5.0 µg to 40 µg/ml. The cells were treated for 48 h and processed to examine the cell viability using MTT assay. In the control group, we did not add extracts of CoNiNd (NPs) and CoNiNd (NPs) plant extracts. We have also included the non-cancer cells (human embryonic kidney cells (HEK-293) to consider as control cells. Both the control and CoNiNd (NPs) and CoNiNd (NPs) plant extracts -treated groups were treated with 10 µl of MTT (5 mg/ml), and cells were then further incubated in a CO2 incubator for 4 h. After that, cell culture media was replaced with DMSO (100%), and the 96-well plate was then examined under an ELISA plate reader (Biotek Instruments, USA) at a wavelength of 570 nm. The percentage of cell viability was calculated for the statistical analysis.
2.5 Apoptotic DAPI staining:
The morphology changes of cancer nuclear structure due to treatments of CoNiNd (NPs) and CoNiNd (NPs) plant extracts were examined by DAPI staining assay. Cells were divided into two groups, the control group in which no CoNiNd (NPs) and CoNiNd (NPs) plant extracts were added, whereas, in the experimental group, CoNiNd (NPs) and CoNiNd (NPs) plant extracts (25 µg/ml) was added. Post 48 h treatment, both groups were exposed to ice-cold (4%) paraformaldehyde and then with Triton X-100 in phosphate-buffered saline (PBS). After that, cells were stained with DAPI (1.0 μg/mL) for 5 min under a dark environment, and cells were finally washed with PBS and cover slipped. The DNA staining was examined by using Confocal Scanning Microscope (Zeiss, Germany). The data presented as mean (±) standard deviation (SD) obtained from triplicates and one way ANOVA followed by Dennett’s post hoc test with GraphPad Prism Software (GraphPad Software, USA) for final statistical analysis.
3 Results & discussion
3.1 Structure and morphology of CoNiNd (NPs) and CoNiNd (NPs) plant extracts
Fig. 3 exhibited XRD powder patterns of CoNiNd (NPs) with and without plants extract. All samples showed the indexed peaks of Co-Ni spinel ferrite without any presence of impurities which evidenced on the efficiency of Green synthesis as a preparation method. The lattice parameters and crystal size were calculated by full proof program. It was found that the lattice parameters un-systematic variation (Pristine = 8.36 Å , Lavender seeds = 8.31 Å, Ginger = 8.35 Å, Flax Seeds = 8.29 Å , Lemon Juice = 8.30 Å, Tragacanth Gum = 8.33 Å and Date fruit = 8.34 Å) corresponding to the type of plant extraction. The crystal sizes were calculated via Debye–Scherrer equation taking account the most intense peak (3 1 1) and also found that they varied as 44.7,14.3,26.1,14.6,13.2,21.8 and 15.1 nm respectively with changing the extract type.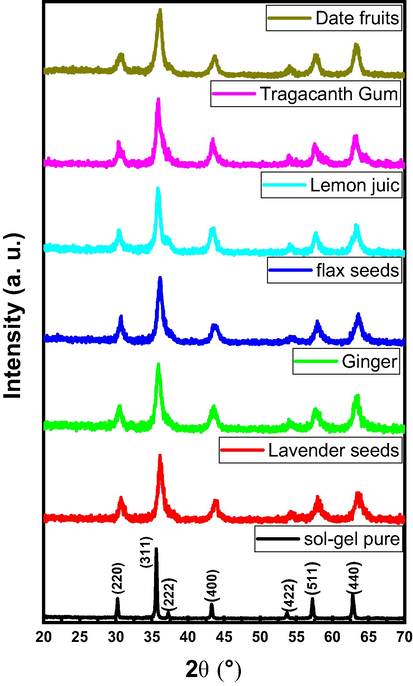
XRD powder patterns of CoNiNd (NPs) and CoNiNd (NPs) plant extracts.
The surface imaging by SEM of CoNiNd (NPs) and CoNiNd (NPs) plant extracts were displayed in Fig. 4. The images revealed aggregation of small grains with various morphology and size distribution according to the type of extraction. Fig. 5 presents the EDX spectrum of CoNiNd (NPs) with Ginger extraction, The EDX spectrum approved the presence of Co, Ni, Nd, Fe and O that showing that the green synthesis is effectively for fabrication nanoparticles.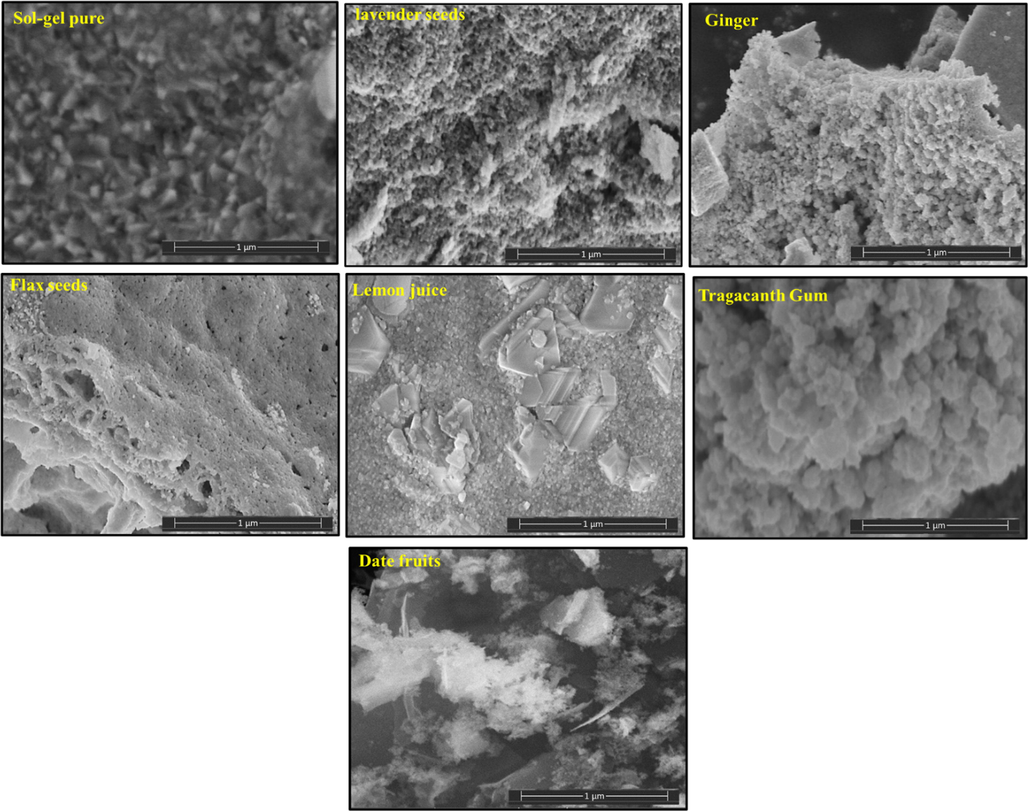
The SEM images of CoNiNd (NPs) plant extracts.
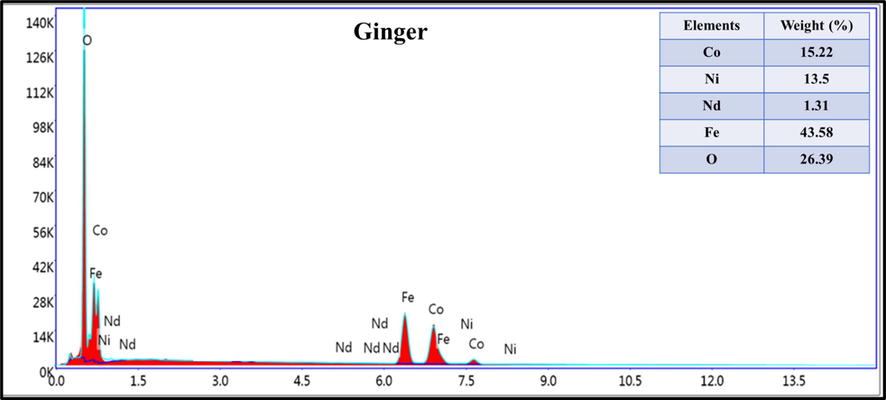
EDX spectrum of CoNiNd (NPs) with Ginger extract.
To confirm the morphology and particles size,TEM analysis was applied on CoNiNd (NPs) with different plant extracts as clear from Fig. 6. The images showed assembling of fine spherical and cubic particles which in turn concurrent with the observations from SEM analysis. The particles size distribution was achieved by treating TEM mages with ImageJ software it found that the size distribution in the range of 17 to 25 nm.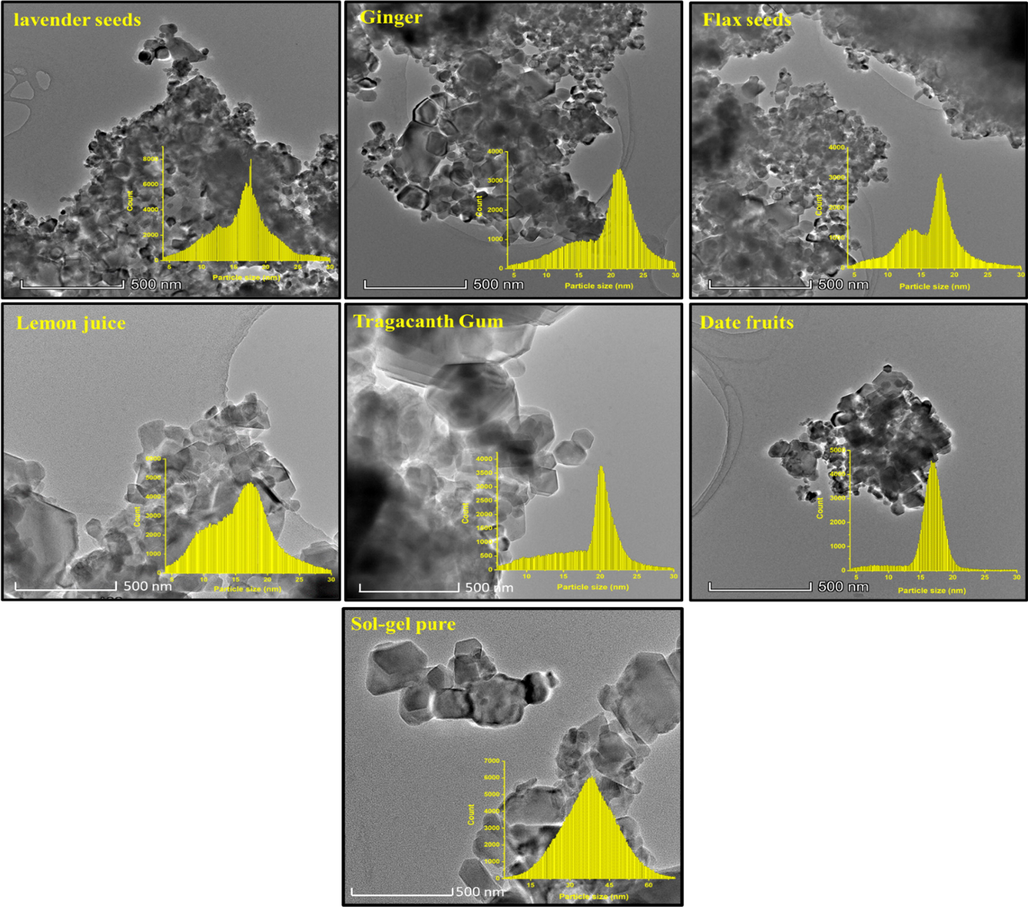
TEM images with histogram of particles size distribution of CoNiNd (NPs) plant extracts.
3.2 Impact of extracts of on cancer cells viability
The impact of CoNiNd (NPs) and CoNiNd (NPs) plant extracts on both colon cancer and cervical cancer cells was examined. The cell viability assay confirmed a decrease in the cell viability after the treatments of CoNiNd (NPs) and CoNiNd (NPs) plant extracts (Fig. 7). The treatments of extracts of CoNiNd (NPs) and CoNiNd (NPs) plant extracts showed dose-dependent inhibitory action on cancer cell growth and proliferation (Fig. 7). We have also tested the impact of extracts of CoNiNd (NPs) and CoNiNd (NPs) plant extracts on non-cancerous cells (HEK-293) to know its impact on normal cells. Data showed that there was decrease in the cell viability due to extracts of CoNiNd (NPs) and CoNiNd (NPs) plant extracts treatments, but the percentage of cell viability was higher than cancer cells (HCT-116, and HeLa). It suggests that treatment of CoNiNd (NPs) and CoNiNd (NPs) plant extracts are more toxic to cancerous cells (HCT-116 and HeLa) cells than normal cells (HEK-293).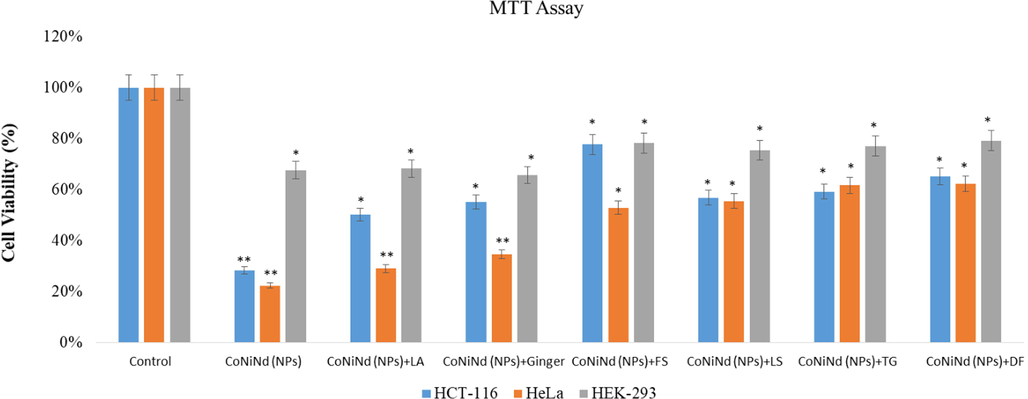
Cell viability by using MTT Assay: It shows the impact of treatment (25 µg/mL) of extracts of CoNiNd (NPs) and CoNiNd (NPs) plant extracts on HCT-116, HeLa and HEK-293 cells post 48 h treatment. * p < 0.05; ** p < 0.01.
The inhibitory concentration (IC50) of extracts of CoNiNd (NPs) and CoNiNd (NPs) plant extracts on colon cancer (HCT-116) cells was calculated, and we have found that IC50 for CoNiNd (NPs) was lowest 15.75 µg/ml and whereas CoNiNd (NPs) + LJ showed highest IC50 44.20 µg/ml for colon cancer cells (Table 2). For the cervical (HeLa) cancer cells, IC50 for extracts of CoNiNd (NPs) was lowest 16.44 µg/ml and whereas CoNiNd (NPs) + TG showed highest IC50 38.15 µg/ml for colon cancer cells.
NPs
HCT-116 (IC50)
(µg/ml)Hela (IC50)
(µg/ml)
CoNiNd (NPs)
15.75 µg/ml
16.44 µg/ml
CoNiNd (NPs) + LS
24.58 µg/ml
26.71 µg/ml
CoNiNd (NPs) + Ginger
28.33 µg/ml
21.55 µg/ml
CoNiNd (NPs) + FS
35.55 µg/ml
32.56 µg/ml
CoNiNd (NPs) + LJ
44.2 µg/ml
33.74 µg/ml
CoNiNd (NPs) + TG
42.83 µg/ml
38.65 µg/ml
CoNiNd (NPs) + DF
35.25 µg/ml
36.15 µg/ml
Based on these observations, we may suggest that synthesized extracts of extracts of CoNiNd (NPs) and CoNiNd (NPs) plant extracts possess better inhibitory effect on HCT-116 and Hela cells than HEK-293 cells. This is the first study demonstrating the cell viability of extracts of extracts of CoNiNd (NPs) and CoNiNd (NPs) plant extracts against HCT-116 and Hela cells. Previously many studies have shown nanoparticles and extracts of Cardamom and Moringa showed cytotoxicity against different cancerous cells (Fagundes et al., 2020 Dec; Tombuloglu et al., 2021 Apr; Rehman et al., 2019; Soshnikova et al., 2018; Jafarain et al., 2014; Ezhilarasi et al., 2016; Aldakheel, 2020; Abou-Hashem et al., 2019; Elsayed et al., 2015).
3.3 Apoptotic cancer cell death due to treatment of CoNiNd (NPs) and CoNiNd (NPs) plant extracts
The treatment of CoNiNd (NPs) and CoNiNd (NPs) plant extracts caused significant decreased in the number of colon cancer cells, as the number of DAPI stained cells were found to be less in the extracts of CoNiNd (NPs) and CoNiNd (NPs) plant extracts -treated cells (Fig. 8 B-C) as compared to control cells (Fig. 2 A). The decrease in the cancer cells is due to cell death which are due to programmed cell death or apoptosis. In contrast the treatments of extracts of CoNiNd (NPs) and CoNiNd (NPs) plant extracts did not show any inhibitory action on the colon cancer cells (Fig. 8A).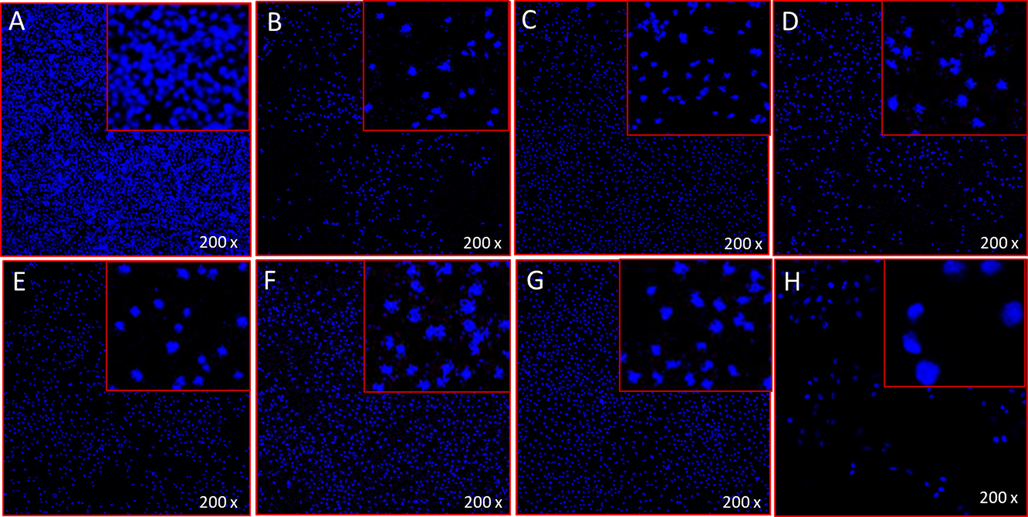
A-H. Apoptotic cells morphology by DAPI staining: It shows the impact of NPs treatments on colon cancer cells (HCT-116) post 48 h treatment. Figure A Control; Figure B CoNiNd (NPs); Figure C CoNiNd (NPs) + LA; Figure D CoNiNd (NPs) + Ginger; Figure E CoNiNd (NPs) + FS; Figure F CoNiNd (NPs) + LS; Figure G CoNiNd (NPs) + TG; and Figure H, CoNiNd (NPs) + DF.
4 Conclusion
The aim of this study was to synthesis CoNiNd (NPs) using six different plant extracts viz: Lavender, Ginger, Flaxseed, Lemon Juice, Tragacanth Gum and Dates Fruit as a biosynthesis method. In this procedure, metals nitrite was mixed with specific amount of plant extracts solution. The resulted nanocomposites were analyzed with XRD, TEM and SEM methods. The XRD confirmed the Co-Ni spinel ferrite structure. Morphology and partials size were estimated through SEM and TEM. The biological activity of all the samples produced by green synthesis CoNiNd (NPs) using six different plant extracts viz: Lavender, Ginger, Flax-Seed, Lemon Juice, Tragacanth Gum, and Dates were done against cancer cells such as human colorectal carcinoma (HCT-116) and human cervical cells (Hela). The cell viability assay confirmed a decrease in the cancer cell viability and showed dose-dependent inhibitory action. The treatments of CoNiNd (NPs) with Lavander plant extract showed most profound inhibitory action on both cancer cells than extracts other plant extracts. The IC50 values were for HCT-116 cells were found to be in range of 15.75–42.55 µg/mL and 13.44 to 35.65 µg/mL for HeLa cells. We may suggest that synthesized plant extracts CoNiNd (NPs) and CoNiNd (NPs) plant extracts possess better inhibitory effect on HCT-116 and Hela cells than HEK-293 cells. Our results showed that CoNiNd (NPs) and CoNiNd (NPs) plant extracts possess potential application in the colon and cervical cancer treatments and would recommend in vivo study experiments to validate their applications.
References
- Comparative heating efficiency of cobalt-, manganese-, and nickel-ferrite nanoparticles for a hyperthermia agent in biomedicines. ACS Appl. Mater. & Interfaces. 2019;11:6858-6866.
- [Google Scholar]
- A review of amino-functionalized magnetic nanoparticles for water treatment: Features and prospects. J. Clean. Prod.. 2020;124668
- [Google Scholar]
- Palladium-decorated o-phenylenediamine-functionalized Fe3O4/SiO2 magnetic nanoparticles: A promising solid-state catalytic system used for Suzuki-Miyaura coupling reactions. J. Phys. Chem. Solids. 2020;136:109200
- [Google Scholar]
- Magnetic particle spectroscopy: a short review of applications using magnetic nanoparticles. ACS Appl. Nano Mater.. 2020;3:4972-4989.
- [Google Scholar]
- Biocompatibility of magnetic nanoparticles coating with polycations using A549 cells. J. Biotechnol.. 2021;325:25-34.
- [Google Scholar]
- Magnetic driven nanocarriers for pH-responsive doxorubicin release in cancer therapy. Molecules. 2020;25:333.
- [Google Scholar]
- Magnetic nanocarriers: evolution of spinel ferrites for medical applications. Adv. Colloid Interf. Sci.. 2019;265:29-44.
- [Google Scholar]
- A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res.. 1986;46:6387-6392.
- [Google Scholar]
- Super-paramagnetic nanoparticles with spinel structure: a review of synthesis and biomedical applications, solid state phenomena. Trans. Tech. Publ. 2016:139-176.
- [Google Scholar]
- Biocompatibility of various ferrite nanoparticles evaluated by in vitro cytotoxicity assays using HeLa cells. J. Magn. Magn. Mater.. 2009;321:1482-1484.
- [Google Scholar]
- Magnetic nanoparticles: biomedical applications and challenges. J. Mater. Chem.. 2010;20:8760-8767.
- [Google Scholar]
- Extracellular biosynthesis of cobalt ferrite nanoparticles by Monascus purpureus and their antioxidant, anticancer and antimicrobial activities: Yield enhancement by gamma irradiation. Mater. Sci. Eng. C. 2020;107:110318
- [Google Scholar]
- A fibrous morphology silica-CoFe2O4 nanocarrier for anti-cancer drug delivery. Ceram. Int.. 2018;44:2345-2350.
- [Google Scholar]
- Effect of lemon juice on microstructure, phase changes, and magnetic performance of CoFe2O4 nanoparticles and their use on release of anti-cancer drugs. Int: Ceram; 2021.
- ZnxFe3− xO4 (0.01≤ x≤ 0.8) nanoparticles for controlled magnetic hyperthermia application. New J. Chem.. 2018;42:7144-7153.
- [Google Scholar]
- Alternative low-cost approach to the synthesis of magnetic iron oxide nanoparticles by thermal decomposition of organic precursors. Nanotechnology. 2013;24:175601
- [Google Scholar]
- Synthesis and magnetostructural studies of amine functionalized superparamagnetic iron oxide nanoparticles. RSC advances. 2015;5:18420-18428.
- [Google Scholar]
- Green Synthesis of Magnetic Spinel Nanoparticles in Nanophotonics, Nanooptics, Nanobiotechnology, and Their Applications. Springer Proceedings in Physics book series (SPPHY). 2019;222:389-398.
- [Google Scholar]
- Green synthesis of novel zinc iron oxide (ZnFe2O4) nanocomposite via Moringa Oleifera natural extract for electrochemical applications. Appl. Surf. Sci.. 2018;446:66-73.
- [Google Scholar]
- Biogenic synthesis of iron oxide nanorods using Moringa oleifera leaf extract for antibacterial applications. Appl. Nanoscience. 2020;10:305-315.
- [Google Scholar]
- Incubation period induced biogenic synthesis of PEG enhanced Moringa oleifera silver nanocapsules and its antibacterial activity. J Polym. Res.. 2019;26:1-11.
- [Google Scholar]
- Okra extract-assisted green synthesis of CoFe2O4 nanoparticles and their optical, magnetic, and antimicrobial properties. Mater. Chem. Phys.. 2018;204:410-419.
- [Google Scholar]
- Green synthesis of cobalt ferrite nanoparticles using plant extracts. Rev Roum Chim.. 2017;62:645-653.
- [Google Scholar]
- Green synthesis of NiFe2O4 spinel-structured nanoparticles using Hydrangea paniculata flower extract with excellent magnetic property, Miner. Met. Mater. Soc.. 2018;70:1337-1343.
- [Google Scholar]
- Green synthesis of zinc ferrite nanoparticles in Limonia acidissima juice: Characterization and their application as photocatalytic and antibacterial activities. Microchemical Journal. 2019;146:1227-1235.
- [Google Scholar]
- Fluorescent magnetic submicronic polymer (FMSP) nanoparticles induce cell death in human colorectal carcinoma cells, Artif. Cells Nanomed. Biotechnol.. 2018;46:S247-S253.
- [Google Scholar]
- Rehman S, Almessiere MA, Al-Jameel SS, Ali U, Slimani Y, Tashkandi N, Al-Saleh NS, Manikandan A, Khan FA, Al-Suhaimi EA, Baykal A. Designing of Co0.5Ni0.5GaxFe2-xO4 (0.0 ≤ x ≤ 1.0) Microspheres via Hydrothermal Approach and Their Selective Inhibition on the Growth of Cancerous and Fungal Cells. Pharmaceutics. 2021 Jun 26;13(7):962. doi: 10.3390/pharmaceutics13070962. PMID: 34206751; PMCID: PMC8309058
- Radiosensitizing effects of citrate-coated cobalt and nickel ferrite nanoparticles on breast cancer cells. Nanomedicine (Lond).. 2020 Dec;15(29):2823-2836. Epub 2020 Nov 26 PMID: 33241971
- [CrossRef] [Google Scholar]
- Synthesis of niobium substituted cobalt-nickel nano-ferrite (Co0.5Ni0.5NbxFe2-xO4 (x ≤ 0.1) by hydrothermal approach show strong anti-colon cancer activities. J Biomol Struct Dyn.. 2021 Apr;39(6):2257-2265. Epub 2020 Apr 7. PMID: 32241211
- [CrossRef] [Google Scholar]
- Sultan, Reem Al Jindan, Khalid Mohammed Khan, Ahsanulhaq Qurashi. Biocompatible Tin Oxide Nanoparticles: Synthesis, Antibacterial, Anticandidal and Cytotoxic Activities, Chemistry Select. 2019;4:4013-4017.
- [Google Scholar]
- Cardamom fruits as a green resource for facile synthesis of gold and silver nanoparticles and their biological applications, Artif. Cells Nanomed. Biotechnol.. 2018;46:108-117.
- [Google Scholar]
- Evaluation of cytotoxicity of Moringa oleifera Lam. callus and leaf extracts on Hela cells. Adv. Biomed. Res.. 2014;3:194.
- [Google Scholar]
- Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: Cytotoxicity effect of nanoparticles against HT-29 cancer cells. J. Photochem. Photobiol. B. 2016;164:352-360.
- [Google Scholar]
- Rehman S, Almessiere MA, Khan FA, Gondal MA, Mostafa A, Baykal, Bactericidal and In Vitro Cytotoxicity of Moringa oleifera Seed Extract and Its Elemental Analysis Using Laser-Induced Breakdown Spectroscopy. Pharmaceuticals (Basel). 2020;13:193.
- [Google Scholar]
- Induction of sub-G0 arrest and apoptosis by seed extract of Moringa peregrina (Forssk.) Fiori in cervical and prostate cancer cell lines. J Integr. Med.. 2019;17:410-422.
- [Google Scholar]
- E.A. Elsayed, M.A. Sharaf-Eldin, M. Wadaan In vitro Evaluation of Cytotoxic Activities of Essential Oil from Moringa oleifera Seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 Cell Lines, Asian Pac. J. Cancer. Prev. 16 (2015) 4671-5.







