Translate this page into:
Green synthesis of magnetic Fe3O4/Ag nanocomposite using Pomegranate peel extract for the treatment of ovarian cancer
⁎Corresponding author. liujuncs@med.uestc.edu.cn (Jun Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
In this work, we have reported the biogenic supported silver nanoparticles over surface of magnetic Fe3O4 nanoparticles by using Pomegranate peel extract. The extract was used as a green reducing agent and an excellent stabilizer of the synthesized Ag NPs. Physicochemical characterization of the as-synthesized Fe3O4/Ag NPs was carried out through electron microscopy (SEM and TEM), energy dispersive X-ray spectroscopy (EDX), elemental mapping (WDX), vibrating sample magnetometer (VSM), X-ray diffraction (XRD) and inductively coupled plasma-optical emission spectroscopy (ICP-OES). The anti-ovarian cancer effects of biologically synthesized Fe3O4/Ag NPs against ovarian cancer cell lines were assessed. The anti-ovarian cancer properties of the Fe3O4/Ag NPs could significantly remove NIH: OVCAR-3, ES-2, TOV-21G cancer cell lines in a time and concentration-dependent manner by MTT assay. The antioxidant activity of Fe3O4/Ag NPs was determined by DPPH method. The Fe3O4/Ag NPs showed the high antioxidant activity according to the IC50 value. It seems that the anti-human ovarian cancer effect of recent nanoparticles is due to their antioxidant effects. After clinical study, Fe3O4/Ag NPs bio-composite can be utilized as an efficient drug in the treatment of human ovarian cancer in humans.
Keywords
Silver
Magnetic
Pomegranate peel extract
Human ovarian cancer
1 Introduction
Nanotechnology is defined in different ways in several countries, which affects the nanodrugs clinical validation. However, what these different definitions have in common is the use of nanoscale structures (Hilger and Kaiser, 2012; Orel et al., 2015; Van Landeghem et al., 2009; Silva et al., 2011). There are several distinct benefits to using nanotechnology in the diseases treatment. Nanoparticles, especially metal nanoparticles and metal oxides, have been widely used by medical consumers and manufacturers. The mechanism of nanoparticle-induced toxicity against cancer cells is the production of reactive oxygen species (ROS) (Johannsen et al., 2010; Bañobre-López et al., 2013). Excessive production of reactive oxygen species can lead to oxidative stress, disruption of normal physiological maintenance, and oxidation regulation. These effects in turn lead to DNA damage, unregulated cell signaling pathways, changes in cell evolution, cytotoxicity, apoptotic death, and the onset of cell death (Orel et al., 2015; Van Landeghem et al., 2009; Silva et al., 2011; Johannsen et al., 2010). Critical-deterministic factors can affect the production of reactive oxygen species. These critical-deterministic factors include shape, size, nanoparticle surface area, particle surface baroelectricity, surface-forming groups, Particle solubility, metal ion emission from nanomaterials and nanoparticles, optical activation, model of cell reactions, inflammatory effects and ambient pH (Johannsen et al., 2010; Bañobre-López et al., 2013; Klein et al., 2014; Chatterjee et al., 2008; Zhang and Sun, 2004). Metal nanoparticles and oxides of metal nanoparticles due to their optical properties due to the large active area and high atomic number, amplify the photoelectric and Compton effects of both X-ray and gamma-ray interactions with the adsorbent in the diagnostic and therapeutic range (Thevenot et al., 2008; Colon et al., 2010; Wason et al., 2013; Veisi et al., 2021; Hamelian et al., 2022; Alikhani et al., 2022; Shahriari et al., 2021; Tarnuzzer et al., 2005; Gürbüz et al., 2021; Ertürk et al., 2021; Gürbüz et al., 2021). Finally, they can lead to the development of methods for the destruction of tumor cells and reduce their survival with minimal side effects in radiation therapy. As a result, increasing industrial knowledge in the field of scalable nanoparticle synthesis, along with the design of multifunctional nanoparticles, will dramatically change the strategies of microenvironmental preparation and therapeutic-diagnostic nanoparticles for cancer treatment (Ali et al., 2014; Neri and Supuran, 2011; Seo et al., 2007; Hou et al., 2015; Cui et al., 2013; Lucky et al., 2015; Idris et al., 2014).
The reasons for the clinical trials' failed to achieve the desired multilayer results are complex and intertwined. Unfortunately, therapeutic agents (chemotherapy, biology, and nanotechnology) are so selective and effective in targeting in vitro cancer cells, and even in proportionate animal specimens (Stewart et al., 2014; Rasmussen et al., 2010; Felice et al., 2014; Fernandes et al., 2015). However, failing in clinical trials is not a rule but a rule. This is because the biological distribution of therapeutic agents can be a fundamental factor in these fractures. Inadequate concentration at unwanted concentration and target sites elsewhere, leading to dose-limiting poisoning (Rasmussen et al., 2010; Felice et al., 2014). The biological distribution of drug agents is controlled largely by the drugs ability to penetrate biological barriers. Strategy for Adding Targeting Sections to Therapeutic Nanoparticles to Improve Location Specification to date, despite 30 years of effort in pharmaceutical companies and many laboratories, it has not yet been able to produce clinically approved drugs (Felice et al., 2014; Fernandes et al., 2015). This failure is because the addition of molecular agents increases the targeting of cognitive characteristics. However, it does so in the face of much greater difficulty in managing biological barriers (Caputo et al., 2014; Danhier et al., 2010; Laurent et al., 2011; Maier-Hauff et al., 2011; Kolosnjaj-Tabi et al., 2014; Bhattacharyya et al., 2011).
In recent times, it has been a continuous trend to develop the functionalized nanomaterials in advanced level to improve their physicochemical, catalytic and biological properties. In this particular study, we report the biogenic synthesis of Ag NPs immobilized on magnetite nanoparticles using Pomegranate peel extract as a green reducing and stabilizing agent (Scheme 1). The as-synthesized Fe3O4/Ag NPs exhibited excellent potential for the treatment of ovarian cancer.
Schematic green synthesis of magnetic Fe3O4/Ag NPs mediated by Pomegranate peel extract.
2 Experimental
2.1 Preparation of Pomegranate peel extract
Pomegranate peel were washed with deionized water and dried at room temperature. 2.0 g of dried Pomegranate peel was added to 50 ml deionized water and heated to 80 °C for 30 min. It was then filtered through Whatman-1 filter paper and maintained at 4 °C for further study.
2.2 Synthesis of the Fe3O4/Ag magnetite nanoparticles
Magnetite nanoparticles (Fe3O4) were synthesized by co-precipitation of its precursors, namely, FeSO4⋅7H2O (4.2 g) and FeCl3⋅6H2O (6.1 g) into deionized water (100 ml). Initially, the two salts were dissolved in water and stirred at 80 °C for 30 min which was followed by the dropwise addition of 10 ml anhydrous ammonia. The mixture was stirred at 80 °C for 30 min again. The brown colored Fe3O4 NPs were retrieved using a strong magnet, washed with deionized water to neutrality and dried at 80 °C. Then 0.5 g Fe3O4 NPs were sonicated in deionized water (100 ml) for 20 min to complete dispersion. The precursor, a solution of AgNO3 (30 mg) in 20 ml H2O was then added dropwise and stirred for 20 min to afford the Fe3O4/Ag(I). The prepared Pomegranate peel extract was subsequently added and stirred for 1 h at room temperature. It was isolated by magnetic decantation, rinsed with DI-H2O and treated in vacuum at 40 °C. Finally, ICP-OES analysis was performed to assess the Ag content, as being 0.12 mmol/g.
2.3 DPPH assay protocol
1 ml of DPPH methanol solution was added to 1 ml of concentrate to 3 ml of nanoparticles and the resulting mixture was stirred vigorously. The test tubes were placed in a dark place for 30 min. After this period, the absorbance at the wavelength of 517 nm was read. It should be noted that in the control sample, the nanoparticles was replaced with 3 ml of methanol. Finally, the DPPH radical's inhibition percentage was calculated with this formula (Chen et al., 2006; Das et al., 2007; Korsvik et al., 2007; Hong et al., 2006; Zangeneh et al., 2019):
DPPH is a free radical that changes color in the presence of substances with antioxidant properties and captures electrons. Yellow to purple color change is the basis of antioxidant properties. Solutions with different concentrations (10 to 1000 μg/ml) were prepared from phenolic powder and BHT synthetic antioxidant in methanol solvent (Chen et al., 2006; Das et al., 2007; Korsvik et al., 2007; Hong et al., 2006; Zangeneh et al., 2019).
2.4 MTT assay protocol
In this study, the anticancer effects of Fe3O4/Ag magnetite nanoparticles samples against the ovarian cancer cells (NIH: OVCAR-3, ES-2, TOV-21G) were investigated. These cells in DMEM culture medium (Gibco, USA) with 10 % FBS (Gibco, USA) and penicillin/streptomycin (100 μl/100 μg/ml) in an incubator containing 5 % Carbon dioxide with 90 % humidity was stored at 37 °C. Then, when about 80 % of the flask was filled, cell passage was performed and about 5 × 104 cells (per square centimeter) were placed in 24 house bacterial petri dishes in the usual environment. The cells were treated with different concentrations of nanoparticles 24 h later and kept in this condition for 3 days. The survival rate of cultured cells was prepared with different concentrations. In this experiment, cells were cultured at 3 × 104 cells/well in 24-well plates and kept in an incubator at 37 °C for 24 h. Then the old culture medium was taken out of the wells and the cells were treated with different concentrations of nanoxidro. This test was performed on the first, second and third days after exposing the cells to the compounds; thus, at the appropriate time after culturing the cells in plates of 24 cells, the culture medium was removed and about 300 μl of fresh medium containing 30 μl of MTT solution was added to each cell. After 3–4 h of incubation at 37 °C, MTT solution is removed and 200 μl (Dimethyl Sulfoxide, Merck, USA, 100 %) DMSO is added to each house. Then the sample absorption was read at 570 wavelengths using ELISA rider (Expert 96, Asys Hitch, Ec Austria). This experiment was repeated 3 times and each time, four wells were considered for each nano oxide concentration. Cell survival percentage was evaluated by the following formula (Jalalvand et al., 2019):
The results were evaluated as Mean ± SE using SPSS software version 12 and statistical tests of variance of completely randomized block design. Drawing graphs in Excel software was performed and the significance level of the differences was considered p < 0.01.
3 Results and discussion
3.1 Characterization of Fe3O4/Ag NPs
This study describes an eco-friendly method for supporting of Ag NPs on the surface of magnetic Fe3O4 NPs using Pomegranate peel extract as reducing and stabilizing agent without using of any toxic reagents. The Fe3O4/Ag NPs were synthesized following two steps, the plant modified Fe3O4 MNPs adsorbs Ag+ ions on its surface and then in situ reduction and stabilization of the adsorbed ions by the extract (Scheme 1). The structural, morphological and physicochemical properties of the Fe3O4/Ag NPs were determined by various analytical techniques including SEM, EDX, TEM, ICP-OES, VSM and XRD study.
In order to have an idea of structural morphology, shape and size of Fe3O4/Ag NPs, the electronic microscopic analysis (TEM and SEM) were performed (Figs. 1–4). The particles are of globular shape. Ag NPs are larger in size than the Fe3O4 NPs. The homogeneous growth in the form of a thin layer of Pomegranate peel extract can be detected by close observation. The nanocomposite looks aggregated, possibly due to manual sample preparation (Fig. 1).
SEM image of Fe3O4/Ag NPs.
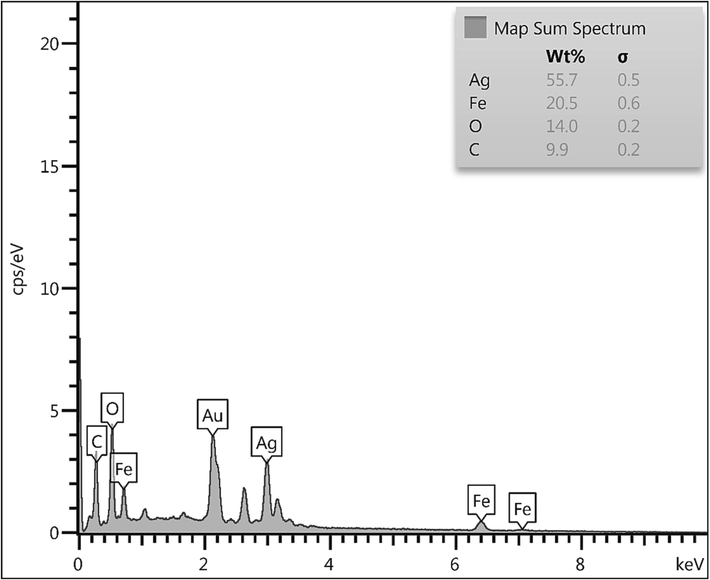
EDX spectrum of Fe3O4/Ag NPs.
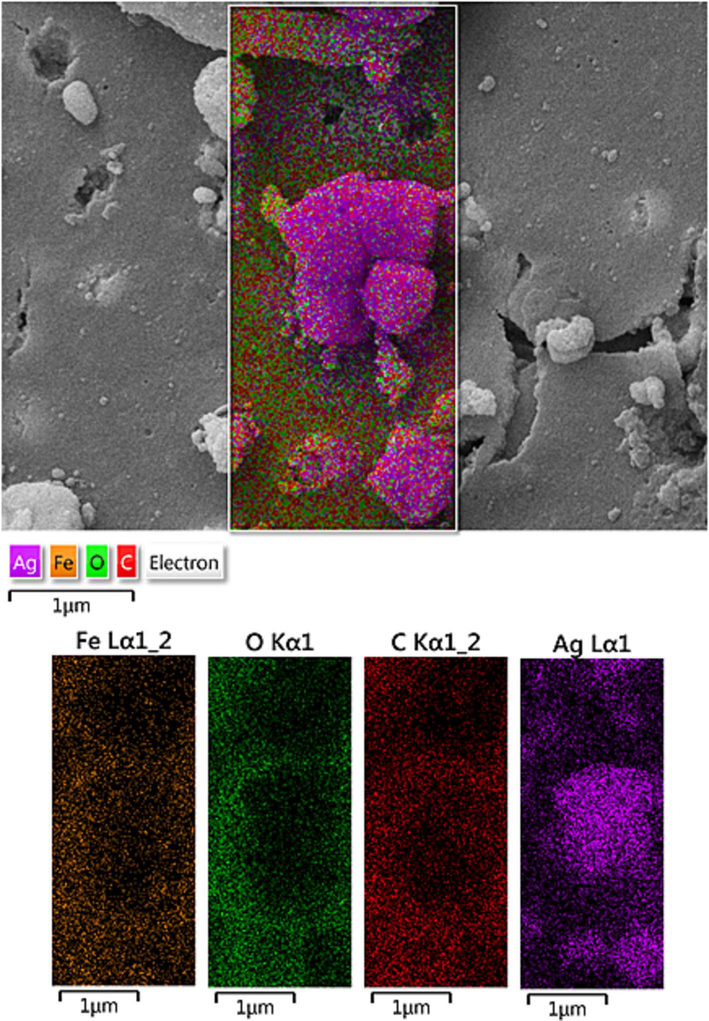
SEM image of Fe3O4/Ag NPs with its elemental mapping.
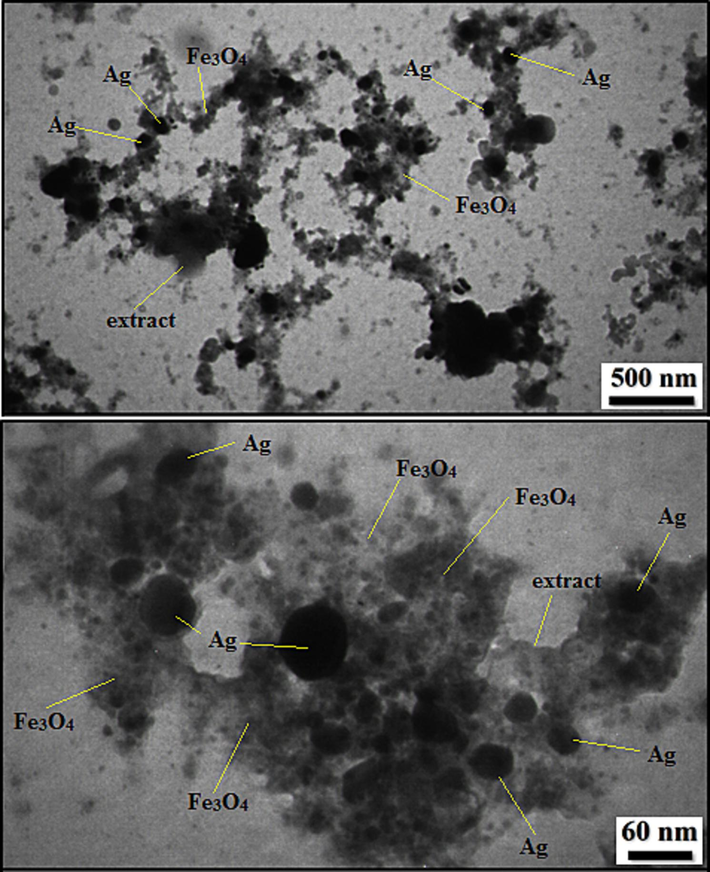
TEM images of Fe3O4/Ag NPs.
Chemical composition of the Fe3O4/Ag NPs was assessed from EDX analysis and the profile is shown in Fig. 2. It represents Fe and Ag as metallic components. The occurrence of Ag confirmed the successful fabrication of Ag NP over the composite surface. Presence of C and O are the evidence of phytomolecular attachments. The results were further justified by SEM elemental mapping analysis (Fig. 3). The compositional map reveals the Fe, C, and Ag species to exist with excellent dispersion throughout the matrix surface.
The FE-SEM data were further justified and more intrinsically studied by TEM analysis. Fig. 4 displays the resultant images of Fe3O4/Ag NPs. The particles are invariably globular shaped. A thin layer of Pomegranate peel extract over the particle surface can be noticed. The grey and black particles represent the Fe3O4 and Ag NPs The dark colored Ag NPs are also round shaped and are almost bigger than Fe3O4 NPs. Size of the particles of Fe3O4 and Ag NPs are 10–20 and 20–30 nm respectively.
Crystalline phases and the diffraction planes of the Fe3O4/Ag NPs were ascertained by XRD study, that shown in Fig. 5. It represents a single phase profile indicating a united entity of the assembled counterparts. The typical diffraction peaks due to Fe3O4 are observed at 2θ = 30.2, 35.4, 43.3, 53.6, 57.2, 62.7° corresponding to (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1) and (4 4 0) Bragg reflection planes respectively (JCPDS file, PDF No. 65–3107). The extra peaks appeared at 2θ = 39.1°, 44.4°, 64.5° and 77.4° can be allocated to the (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes of Ag fcc crystalline phases.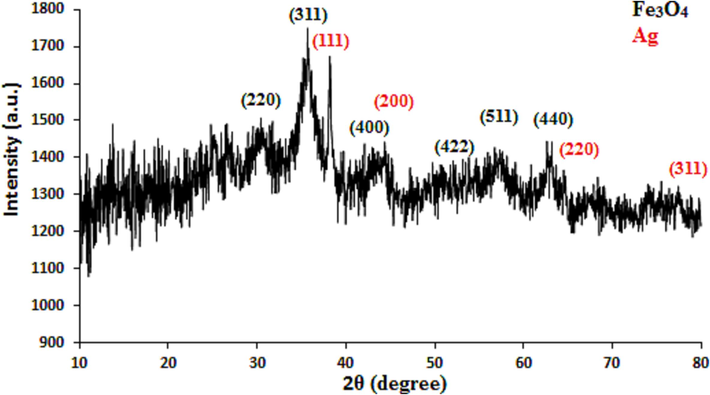
XRD pattern of Fe3O4/Ag NPs.
VSM study was carried out to determine the magnetic properties of the Fe3O4/Ag NPs. On passing an external magnetic field of −20kOe to + 20kOe, a magnetic hysteresis curve is obtained and the corresponding saturation magnetization (Ms) value of Fe3O4/Ag NPs was 31.4 emu/g (Fig. 6). It clearly reveals the material to be superparamagnetic in nature.
VSM analysis of Fe3O4/Ag NPs.
Regarding cancer, efforts have been made to use smart nanomaterials (nanoparticles, nanostructures), which have a greater ability to target cancer cells, to treat such patients. That is, they kill malignant cells by irradiating them, providing a microscopic therapeutic effect within electrons (Stewart et al., 2014; Rasmussen et al., 2010; Felice et al., 2014; Fernandes et al., 2015; Caputo et al., 2014). Nanoparticles are programmed to achieve optimal therapeutic efficacy, delivering therapeutic loads to target cells. Studies have also been performed on several nanocarriers based on lipids, polymers, and peptides for delivery to the respiratory system (Danhier et al., 2010; Laurent et al., 2011; Maier-Hauff et al., 2011; Kolosnjaj-Tabi et al., 2014). Properties of nanoparticles for targeted delivery of nanoparticles to tumors is the motivation for targeted drug delivery in cancer treatment to kill cancer cells. In a way, that has the least damage to healthy cells (Idris et al., 2014; Stewart et al., 2014; Rasmussen et al., 2010; Felice et al., 2014; Fernandes et al., 2015). One of the nanotechnology goals is to mount drugs on carriers, send them and release them into the target cell, which is called targeted drug delivery. Using nanoparticles, the drug can be intelligently delivered to the desired tissue, and improve the tissue without damaging other tissues (Caputo et al., 2014; Danhier et al., 2010; Laurent et al., 2011; Maier-Hauff et al., 2011; Kolosnjaj-Tabi et al., 2014; Bhattacharyya et al., 2011).
The scavenging capacity of Fe3O4/Ag NPs and BHT at different concentrations expressed as percentage inhibition has been indicated in Table 1 and Fig. 7.
Nanocomposite
BHT
IC50 (µg/mL)
131 ± 5a
109 ± 3a
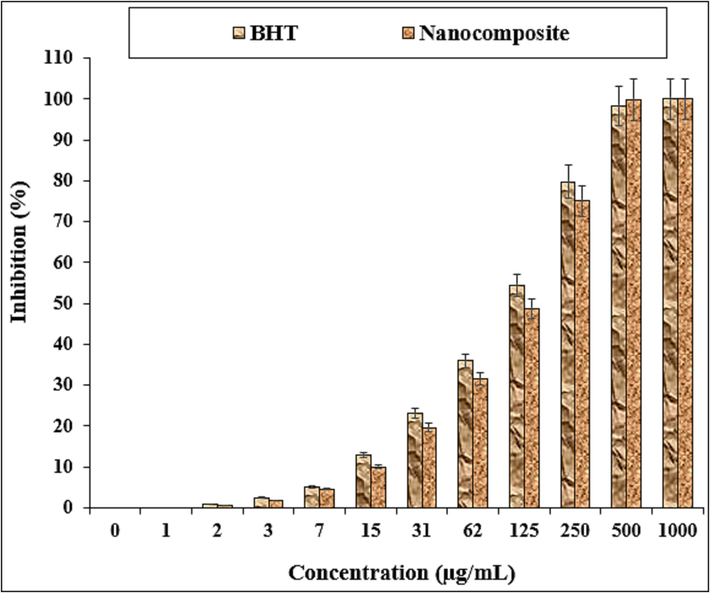
The antioxidant properties of nanocomposite and BHT against DPPH.
In this study, the treated cells with different concentrations of the present Fe3O4/Ag NPs were assessed by MTT assay for 48 h about the cytotoxicity properties on normal (HUVEC) and ovarian malignancy cell lines i.e. NIH: OVCAR-3, ES-2, TOV-21G. The absorbance rate was evaluated at 570 nm, which represented viability on normal cell line (HUVEC) even up to 1000 μg/mL for Fe3O4/Ag NPs (Table 2 and Fig. 8).
HUVEC
NIH: OVCAR-3
ES-2
TOV-21G
IC50 (µg/mL)
–
432 ± 6b
451 ± 8b
334 ± 5a
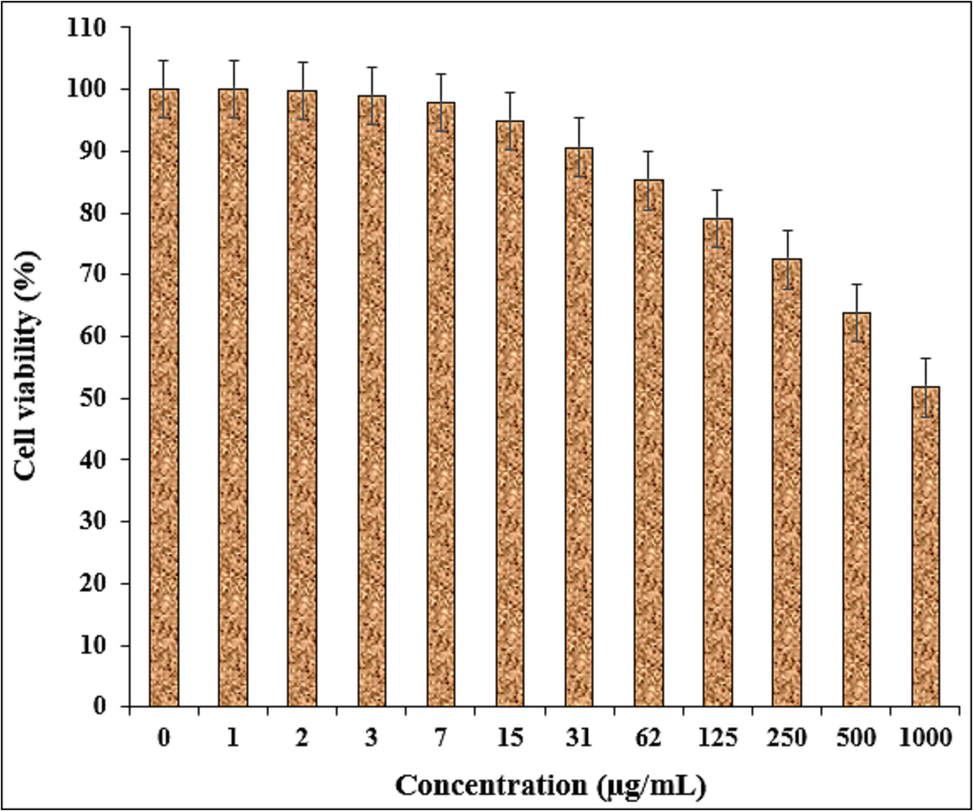
The cytotoxicity potentials of nanocomposite against HUVEC cell line.
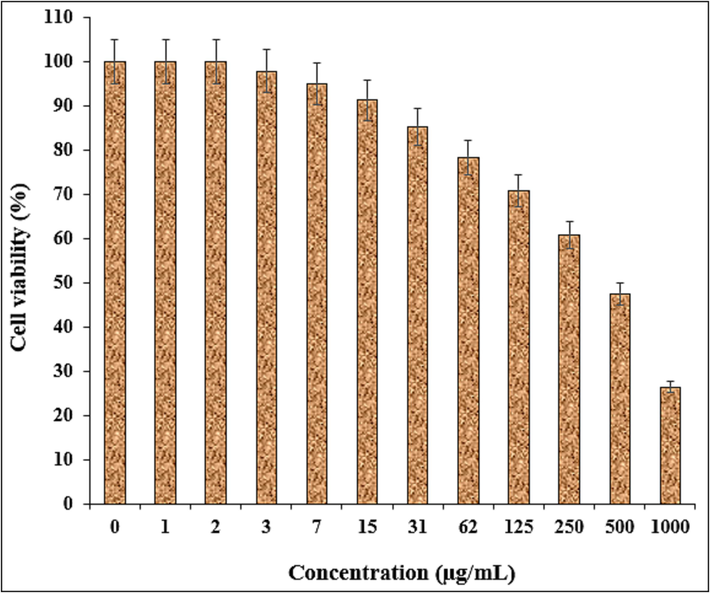
The viability of malignant ovarian cell line reduced dose-dependently in the presence of Fe3O4/Ag NPs. The IC50 of Fe3O4/Ag NPs were 432, 451, and 334 µg/mL against NIH: OVCAR-3, ES-2, TOV-21G cell lines, respectively (Table 2 and Figs. 9–11).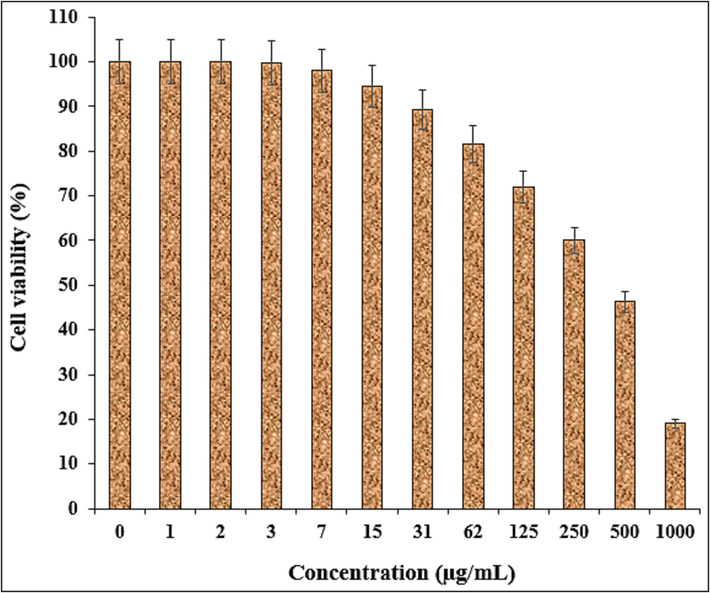
The anti-ovarian cancer potentials of nanocomposite against NIH: OVCAR-3 cell line.
The anti-ovarian cancer potentials of nanocomposite against ES-2 cell line.
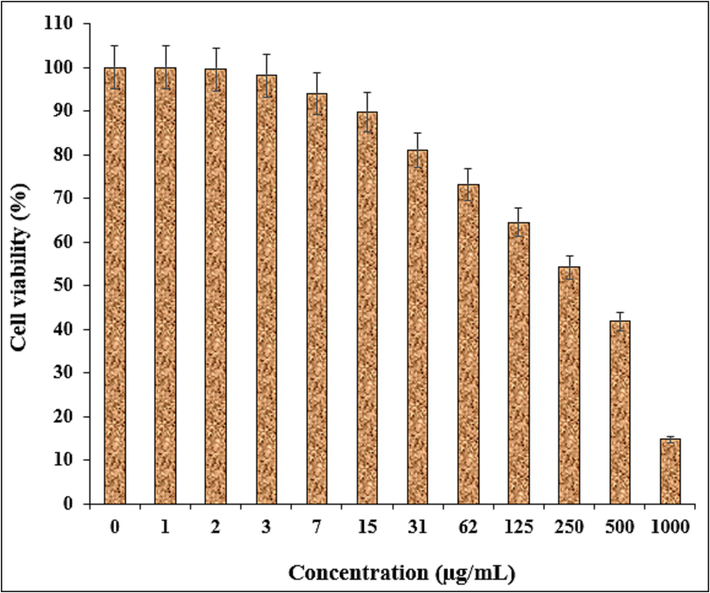
The anti-ovarian cancer potentials of nanocomposite against TOV-21G cell line.
4 Conclusion
In this work, an eco-friendly approach for in situ supported of Ag nanoparticles on the surface of Fe3O4 nanoparticles mediated by Pomegranate peel extract, without using any toxic reducing and capping agents. The structure, morphology, and physicochemical properties were characterized. The viability of malignant cancer cell lines reduced dose-dependently in the presence of Fe3O4/Ag NPs. The Fe3O4/Ag NPs showed the best antioxidant activities against DPPH. So, the findings of the recent research show that biologically synthesized Fe3O4/Ag NPs might be used to cure ovarian cancer. In addition, the current study offer that Fe3O4/Ag NPs could be a new potential adjuvant chemopreventive and chemotherapeutic agent against cytotoxic cells.
Funding
Title: Ultrasound-guided transplantation of neonatal porcine islet tissue via hepatic portal vein in diabetic rhesus macaques for the treatment of type 1 diabetic rhesus macaques' liver tissue: a study on the key technology (Natural Science Foundation of Sichuan Province 2022NSFSC0836).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cerium oxide nanoparticles induce oxidative stress and genotoxicity in human skin melanoma cells. Cell Biochem. Biophys.. 2014;71:1643-1651.
- [Google Scholar]
- Inorg. Chem. Commun.. 2022;139:109351
- Magnetic nanoparticle-based hyper-thermia for cancer treatment. Rep. Pract. Oncol. Radiother.. 2013;18:397-400.
- [Google Scholar]
- Pharmacological potential of bioactive engineered nanomaterials. Biochem. Pharmacol.. 2014;92:112-130.
- [Google Scholar]
- Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev.. 2008;60:1627-1637.
- [Google Scholar]
- Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol.. 2006;1:142-150.
- [Google Scholar]
- Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine. 2010;6:698-705.
- [Google Scholar]
- In vivo targeted deep-tissue photodynamic therapy based on near-infrared light triggered upconversion nanoconstruct. ACS Nano. 2013;7:676-688.
- [Google Scholar]
- To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release. 2010;148:135-146.
- [Google Scholar]
- Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28:1918-1925.
- [Google Scholar]
- Turk. J. Chem.. 2021;45:1968-1979.
- Drug delivery vehicles on a nano-engineering perspective. Mater. Sci. Eng. C. 2014;41:178-195.
- [Google Scholar]
- New trends in guided nanotherapies for digestive cancers: A systemic review. J. Control. Release. 2015;209:288-307.
- [Google Scholar]
- Appl. Organomet. Chem.. 2021;35:e6284
- Appl. Organomet. Chem.. 2021;35:e6230
- Catal. Lett. 2022:1-11.
- [CrossRef]
- Iron oxide-based nanostructures for MRI and magnetic hyperthermia. Nanomedicine. 2012;7:1443-1459.
- [Google Scholar]
- Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc.. 2006;128:1078-1079.
- [Google Scholar]
- UV-emitting upconversion-based TiO2 photosensitizing nanoplatform: Near-infrared light mediated in vivo photodynamic therapy via mitochondria-involved apoptosis pathway. ACS Nano. 2015;9:2584-2599.
- [Google Scholar]
- Photoactivation of core-shell titania coated upconversion nanoparticles and their effect on cell death. J. Mater. Chem. B. 2014;2:7017-7026.
- [Google Scholar]
- J. Photochem. Photobiol. B Biol.. 2019;192:103-112.
- Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperth.. 2010;26:790-795.
- [Google Scholar]
- Superparamagnetic iron oxide nanoparticles as novel X-ray enhancer for low-dose radiation therapy. J. Phys. Chem. B. 2014;118:6159-6166.
- [Google Scholar]
- Heat-generating iron oxide nanocubes: Subtle “destructurators” of the tumoral microenvironment. ACS Nano. 2014;8:4268-4283.
- [Google Scholar]
- Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007:1056-1058.
- [Google Scholar]
- Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci.. 2011;166:8-23.
- [Google Scholar]
- Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy. ACS Nano. 2015;9:191-205.
- [Google Scholar]
- Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011;103:317-324.
- [Google Scholar]
- Interfering with pH regulation in tumors as a therapeutic strategy. Nat. Rev. Drug Discov.. 2011;10:767-777.
- [Google Scholar]
- Magnetic properties and antitumor effect of anocomplexes of iron oxide and doxorubicin. Nanomedicine. 2015;11:47-55.
- [Google Scholar]
- Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Exp. Opin. Drug Deliv.. 2010;7:1063-1077.
- [Google Scholar]
- Development of watersoluble single-crystalline TiO2 nanoparticles for photocatalytic cancer-cell treatment. Small. 2007;3:850-853.
- [Google Scholar]
- Int. J. Biol. Macromol.. 2021;172:55-61.
- Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int. J. Nanomed.. 2011;6:591-603.
- [Google Scholar]
- Stewart, B., Wild, C.P. World Cancer Report 2014. International Agency for Research on Cancer World Health Organization; Lyon, France: 2014. [(accessed on 24 March 2015)]. Available online: http://www.iarc.fr/en/publications/books/wcr/wcr-order.php.
- Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett.. 2005;5:2573-2577.
- [Google Scholar]
- Surface chemistry influences cancer killing effect of TiO2 nanoparticles. Nanomedicine. 2008;4:226-236.
- [Google Scholar]
- Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30:52-57.
- [Google Scholar]
- Ultrasound assisted synthesis of Pd NPs decorated chitosan-starch functionalized Fe3O4 nanocomposite catalyst towards Suzuki-Miyaura coupling and reduction of 4-nitrophenol. Int. J. Biol. Macromol.. 2021;172:104-113.
- [Google Scholar]
- Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS production. Nanomedicine. 2013;9:558-569.
- [Google Scholar]
- Appl. Organometal. Chem.. 2019;33:e5246.
- Photocatalytic killing effect of TiO2 nanoparticles on Ls- 174-t human colon carcinoma cells. World J. Gastroenterol.. 2004;10:3191-3193.
- [Google Scholar]







