Translate this page into:
Green synthesis of platinum nanoparticles using Atriplex halimus leaves for potential antimicrobial, antioxidant, and catalytic applications
⁎Corresponding authors. abdelazeemeltaweil@alexu.edu.eg (Abdelazeem S. Eltaweil), MohamedHosny@alexu.edu.eg (Mohamed Hosny)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
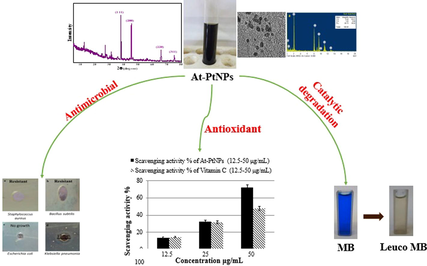
Herein, green platinum nanoparticles (PtNPs) were synthesized using an aqueous extract of Atriplex halimus leaves as a reductant. Atriplex platinum nanoparticles (At-PtNPs) were stable for up to three months. At-PtNPs were characterized by several techniques including UV–Visible spectroscopy, Fourier Transform Infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), Energy Dispersive X-ray spectroscopy (EDX), EDX elemental mapping, High-resolution Transmission electron microscope (HRTEM), Selected Area Electron Diffraction (SAED), and X-ray Photoelectron Spectroscopy (XPS) and Zeta measurements. At-PtNPs were black-colored and mainly spherical with a plasmon peak at 295 nm with ultra-small particle size (1–3 nm) and high surface charge (−25.4 mV). At-PtNPs were verified as a superb catalyst as they were able to catalytically degrade MB dye. At-PtNPs exhibited a high antibacterial efficiency against gram-negative bacteria. At-PtNPs were proved as a highly efficient antioxidant agent. Thus, the attained results offer a promising route of the green synthesis of PtNPs using the aqueous extract of Atriplex halimus.
Keywords
Platinum
Green synthesis
Atriplex halimus
Antibacterial
Antioxidant
Methylene blue
1 Introduction
In today’s world, inorganic nanoparticles are a critical cornerstone that represents significant scientific and technological achievements (Abdelfatah, 2021; Mallikarjuna, 2017; Bathula, 2020; Eltaweil, 2020). Noble metal nanoparticles, including gold, silver, palladium, and platinum, have been proved to be applied in lots of different applications in material science, chemistry, and medicine (Jameel, 2020; Şahin, 2018; Mallikarjuna, 2019). Among these nanoparticles, platinum nanoparticles (PtNPs) have sparked special interest owing to their unique structural, optical, catalytic properties, high surface area, and good resistance to corrosion making them a potential candidate for catalysis and biomedical applications (Dong, 2021; Fan, 2021). PtNPs were reckoned to be efficient and can serve as drug carriers (Barua and Mitragotri, 2014; Al-Radadi, 2019). Also, PtNPs have a diversity of medicinal uses, including anti-cancer, anti-diabetic, antibacterial, and antifungal applications (Naseer, 2020).
Recently, green chemistry is urgently needed to synthesize environmentally sustainable products (Duan et al., 2015). Green synthesis of NPs has gained a lot of interest as the demand for non-toxic, safe, environmentally sustainable, and green procedures grow (Muthu and Priya, 2017; El-Borady et al., 2021). Plants are favored over other biological synthetic methods because they obviate the need for long times of bacterial and fungal culturing and preservation. Furthermore, extracellular plant extracts were proved to be more selective and powerful in regulating nanoparticle size, shape, and dispersity (Muthu and Priya, 2017). Therefore, employing plant extracts in the synthesis of nanoparticles advocates the principles of green chemistry including less hazardous chemical syntheses, safer solvents and auxiliaries, design for energy efficiency, and use of renewable feedstocks.
Plant sections such as roots, leaves, stems, and fruits have also been used to make NPs since their extract contains phytochemicals like flavonoids, tannins, and phenolic compounds that serve as both a reducing and stabilizing agent simultaneously (Rajeshkumar, 2016; Maisa'a and Awwad, 2021). Plants are employed in PtNPs synthesis because they are simpler, smoother, eco-friendly, durable, and cost-effective, and they produce more stable synthesized particles than other, more orthodox approaches (Aygun, 2020). Nymphaea alba; Tragia involucrate, and Crocus sativus are just some examples of plant extracts that were recently utilized in PtNPs synthesis.
A substantial environmental concern comes from the textile industry's release of harmful dyes, especially into aquatic areas (El-Monaem, 2021; Eltaweil, 2021; Chandrasekaran et al., 2020; Arul et al., 2020; Arul, 2021; Eltaweil, 2021). Methylene blue (MB), which is a common harmful dye, inhibits sunlight from reaching a water body so it has a long-term negative impact on the aquatic ecosystem (Eltaweil, 2020; Eltaweil, 2020). Therefore, this deleterious dye was chosen to test the catalytic degradation potency of the phytosynthesized At-PtNPs in this work.
Generally, bacterial pathogens are suspected of causing a wide range of life-threatening illnesses in people and animals (Selvi, 2020; Omer, 2021). Bacterial strains whether gram-negative or gram-positive bacteria such as Escherichia coli and Staphylococcus aureus that are commonly existing in the environment, food, and the intestines of people and animals (Iyer, 2018), can result in severe dermal infections, problems with urinary and respiratory tract systems, and intravenous catheters especially in immune-compromised patients (Carezzano, 2017). Various antibiotics were employed in the treatment of these pathogenic bacteria yet continuous application or consumption of these antibiotics resulted in the development of drug-resistant bacteria (Allen, 2010). Thus, novel materials such as PtNPs have to be synthesized and employed in such an onerous task. Additionally, the antifungal activities of biogenic synthesized PtNPs against various fungal species were previously reported (Wang and Lippard, 2005).
Methods for estimating the efficacy of nanoparticles such as antioxidants are becoming more common. In addition, the use of the stable free radical 1,1-diphenyl-2-picrylhydrazyl (DPPH) is one such process that is currently common for the assessment of nanoparticles as antioxidants (Chen, 2021).
Herein, the aqueous extract of A. halimus leaves was utilized for the first time in PtNPs synthesis and stabilization as a green and efficient route for the synthesis of PtNPs with excellent catalytic and biological activities as well as very high stability. Therefore, the aim of the current work is twofold, firstly to investigate the potentiality of A. halimus leaves aqueous extract in the synthesis of highly stable PtNPs and secondly to test the applicability of At-PtNPs in medical applications including antibacterial and antioxidant test and also in the catalytic degradation of MB.
2 Materials and methods
2.1 Chemicals and reagents
All reagents used in this study were of analytical grade and used without purification including hexachloroplatinic acid (H2PtCl6·6H2O), sodium hydroxide (NaOH), sodium borohydride (NaBH4), and methylene blue (MB) were purchased from Merck, USA.
2.2 Collection of plant specimens and preparation of extracts
Representative samples of A. halimus leaves were collected from their natural habitats on the Mediterranean coastal land of Egypt and the Nile Delta. Leaves were rinsed with deionized water (D.W.) numerous times to remove any impurities and debris. Then leaves were shredded and allowed to dry in the open air followed by overnight drying at 60 °C till reaching constant weight. Afterward, dry leaves were ground in a stainless steel mixer to have a fine powder. Five grams from the resultant powder were mixed with 100 mL of D.W, then the mixture was heated at 80 °C and stirred at 500 rpm using a magnetic stirrer for approximately 20 min. Eventually, the hot solution was filtered and the filtrate was stored in a glass beaker at 4.0 °C for further use.
2.3 Green synthesis of PtNPs
Briefly, 10 mL of A. halimus leaves extract was added to 1 mL of platinum chloride solution (H2PtCl6·6H2O) with a molarity of 0.0019 M. Afterwards, to achieve fast synthesis process, the pH of the mixture solution was adjusted at 9.6 (based on preliminary experiments) using NaOH (0.1 M) then the mixture solution was subjected to stirring and heating at 95 °C for almost one hour till a color change was observed from dark yellow to black. The purification step was carried out by centrifugation of A. halimus-PtNPs (At-PtNPs) colloidal solution then collecting the precipitated pellets and washing them using repeatedly with D.W. Then the formed At-PtNPs solution was preserved at 4.0 °C.
2.4 Characterization techniques
The UV–Visible spectroscopy measurements (200–800 nm) using a double-beam spectrophotometer (T70/T80 series UV/Vis Spectrophotometer, PG instruments Ltd, UK) to ensure the green reduction of the Pt4+ into PtNPs. HRTEM measurements were done on a JOEL, JEM-2100F, Japan, accelerating voltage of 200 kV FT-IR spectra were conducted using a JASCO spectrometer to assess the possible involvement of functional groups in A. halimus extract that were responsible for the reduction and stabilization process. Zeta potential and hydrodynamic size of fabricated At-PtNPs was examined in a zeta potential analyzer (Zetasizer Nano ZS Malvern) to determine the surface charge of At-PtNPs. The XRD measurements of powdered At-PtNPs were done on an X-ray diffractometer (X'PERT PRO. Netherland) operated at a voltage of (45KV) and current of (40 mA) with CuKa1 radiation ( 1.54056Ao) in the 2θ range from 20° to 80° to make sure of the crystalline nature of At-PtNPs. The crystallite size was calculated from the width of the XRD peaks using the Scherrer formula as given by:
Where D is average crystallite size, indicates the line broadening the value of the full width at half maximum (FWHM) of a peak, is the wavelength of irradiated X-rays, and θ is the maximum peak position value. Energy-dispersive X-ray spectroscopy (EDX), performed using JEOL model JSM-IT100 to investigate the elemental composition of At-PtNPs. XPS was collected on K-ALPHA (Thermo Fisher Scientific, USA) with monochromatic X-ray Al K-alpha radiation −10 to 1350 eV spot size 400 micro m at pressure 10-9 mbar with full-spectrum and narrow-spectrum 50 eV to confirm the reduction of Pt4+ into Pt0.
2.5 Catalytic degradation of MB
100 μL of freshly prepared aqueous NaBH4 solution (0.058 M) were added to 10 mL of MB (20–100 ppm). Subsequently, 100 μL of At-PtNPs with a concentration of 50 μg/mL were added to the mixture at 25° C with continuous stirring. The reaction was monitored by recording the time-dependent UV–Vis absorption spectra of these mixtures at 664 nm. Control experiments were conducted using the same experimental conditions yet without At-PtNPs and/or NaBH4. The degradation efficacy was measured by the following equation:
Where (A0) represents the initial absorbance of MB at time 0, while (A) refers to the absorbance of MB at time t.
2.6 Antimicrobial test
Inoculum preparation: The stock culture of reference strain (in glycerol broth) was subcultured onto tryptic soy agar plates. The tested samples are Escherichia coli (ATCC 8739), Klebsiella pneumonia (ATCC 1388), Bacillus subtilis (ATCC 6633), and Staphyllococcus aureus (MRSA)(ATCC 25923). Turbidity of the suspended colonies was compared with the 0.5 McFarland turbidity standard equivalent to 2x108 CFU/mL.
Preparation of seeded agar: Muller Hinton agar is weighed and dissolved in distilled water then sterilized by autoclaving after being divided into 25 mL portions into 6 separate flasks. Flasks were shaken and poured onto sterile petri dishes and left to solidify.
Placing of tested materials (At-PtNPs): At-PtNPs were placed directly in wells after sterilization by filtration; the plates were put in the refrigerator overnight to allow diffusion of At-PtNPs material.
Incubation: Plates were incubated at 35 ± 2 °C for 24 h.
Reading results: All measurements were made with the unaided eye while viewing the back of the Petri dish a few inches above a non-reflecting background and illuminated with reflected light.
2.7 Antioxidant activity of PtNPs (DPPH assay)
The free radical scavenging activity was examined via DPPH (2, 2-diphenyl-1-picrylhydrazyl) assay to determine the antioxidant efficiency of At-PtNPs samples. The assay was conducted in triplicates. In the process, 1 mL of each PtNPs sample was mixed with 1 mL of DPPH (0.2 mM) along with control DPPH which does not contain any nanoparticles. This mixture was blended for 3 min in dark conditions at ambient temperature. Then, after 20 min the concentration of radical is examined by the reduction in absorbance percentage of the mixture at 517 nm wavelength. The control was set up as over. Change in absorbance was estimated at 517 nm. Vitamin C (ascorbic acid) was used as a reference (positive control). The activity was measured by the following equation (El-Borady, 2020).
Where control absorbance is the absorbance in the absence of antioxidants and sample absorbance is the absorbance in the presence of antioxidants (At-PtNPs or Vitamin C) at 517 nm.
2.8 Statistical analysis
All experiments were conducted in triplicate (n = 3), while the gained data were presented as a mean value corrected by the standard deviation (±SD).
3 Results and discussion
3.1 Characterization of At-PtNPs
A.halimus aqueous extract was previously confirmed to contain a diversity of phytoconstituents, including flavonoids, glycosides, and alkaloids (Benhammou et al., 2009; Walker, 2014). As a result, Fig. 1 was used to represent a simplified mechanism for the phytosynthesis of At-PtNPs using A.halimus aqueous extract. Consequently, there was no need for any chemicals. These phytoconstituents are postulated to play a dual role as a reducing and stabilizing agent at the same time since they were proven to be capable of donating electrons or hydrogen atoms to platinum ions to get these ions reduced (Brewer, 2011). Firstly, A.halimus extract reduced platinum ions into a metallic or zero-valent form of platinum (PtNPs). Secondly, they contributed to the stability of the resultant At-PtNPs as they remained stable for more than three months as displayed in Fig. 2a and b. Thus, confirming the great potentiality of A.halimus aqueous extract in the phytosynthesis of several metallic and non-metallic nanoparticles in the near future and denoting its cost-effectiveness. Especially, A.halimus strains that are affected by environmental pressures including droughts and high levels of salinity as it was proven that the concentration of the phytoconstituents increases in most living plant species when the severity of these environmental pressures increases (Oh et al., 2009).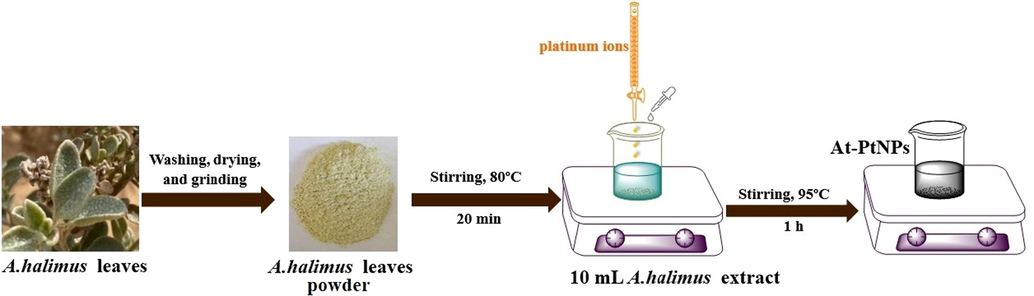
Schematic representation for the green synthesis of PtNPs by A. halimus aqueous extract.
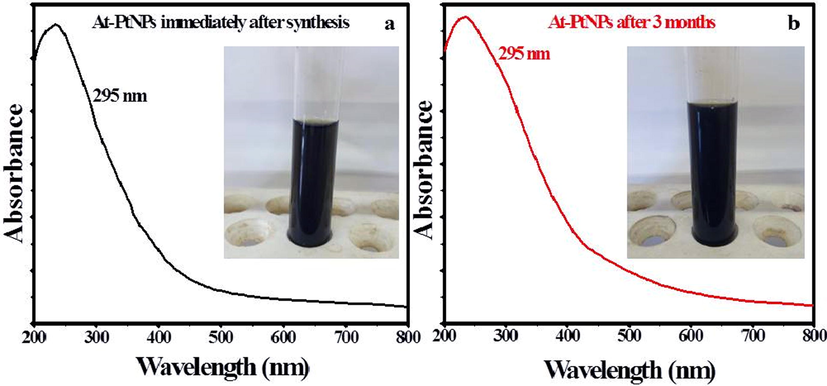
UV–Vis spectrum and images (inset) of At-PtNPs (a) Immediately after synthesis and (b) After 3 months.
Moreover, the plausible mechanism for the reduction of Pt4+ by polyphenols in A.halimus aqueous extract is elucidated in Scheme 1.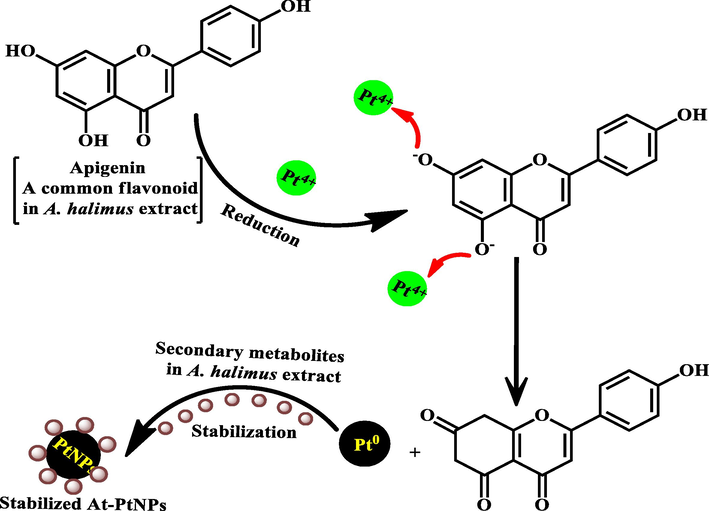
A plausible mechanism for the reduction of Pt4+ by polyphenols in A.halimus aqueous extract.
3.2 UV–Visible spectroscopy
The excitation produced by a source of light at a certain wavelength has a distinct peak at that wavelength known as surface plasmonic resonance which is determined by UV–visible spectroscopy (Zada, 2018). Most of the metallic nanoparticles have unique surface plasmon resonance (SPR) bands (Noruzi et al., 2012). Moreover, the NPs shape and size usually define the form and location of the SPR peak. In the current study, a black-colored colloidal solution (Inset Fig. 2a) and an SPR peak that appeared at 295 nm as shown in Fig. 2a which is characteristic to PtNPs confirmed the successful phytosynthesis of At-PtNPs using the aqueous extract of A. halimus. Furthermore, At-PtNPs were stable for up to three months as indicated by the unaffected black color (Inset Fig. 2b) and UV–Vis surface plasmon resonance (Fig. 2b). Concomitantly with the current study, other researchers reported similar results for the characteristic peak of green synthesized PtNPs (Al-Radadi, 2019; Thirumurugan, 2016).
3.3 XRD analysis
The face-centered cubic crystalline structure of At-PtNPs was confirmed by the XRD pattern. Distinct diffraction peaks of PtNPs at 38.1°, 44.6°, 64.7°, and 78.3° corresponding to (1 1 1), (2 0 0), (2 2 0), and (3 1 1) planes were observed (Fig. 3). Furthermore, the green synthesized At-PtNPs' preferred growth orientation was the (1 1 1) plane. The obtained results matched the stated requirements for crystalline platinum; JCPDS file no. 04–0802. The Scherrer formula was used to determine the average crystallite size of At-PtNPs, which was found to be about 1.5 nm, which was close to the size range (1–2 nm) observed by HRTEM spectroscopy. The undefined sharp peaks are attributed to the plant residues (Hosny, 2021). In addition, the smaller crystallite size reported in this study suggesting the higher proficiency for the synthesized At-PtNPs.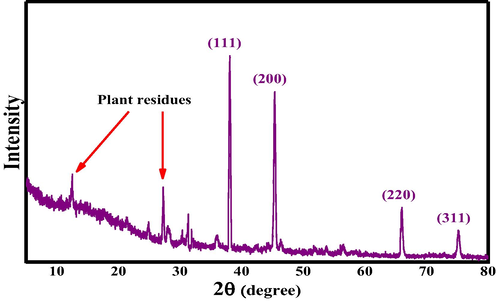
XRD pattern of At-PtNPs.
3.4 FT-IR analysis
FTIR spectrum of A. halimus showed several peaks at different wavenumbers including O-H group at 3360 cm−1, C = C group at 1625 cm−1, C–N group at 1397 cm−1, and C-O stretching group at 1080 cm−1 (Fig. 4a). However, in the spectrum of At-PtNPs (Fig. 4b), some of these peaks appeared but at a lower wavenumber including the C = C group at 1619 cm−1, C–N group at 1317 cm−1, and C-O group at 1019 cm−1. Also, the O-H group of A. halimus disappeared confirming the active role of O-H functional group-containing phytoconstituents in the reduction and stabilization of At-PtNPs. In addition, a new C–H stretch appeared at 2920 cm−1 and 2850 cm−1. Consequently, it was concluded that phytoconstituents of A. halimus extract including glycosides, terpenoids, flavonoids, and alkaloids (Rahman, 2011) were responsible for the reduction and capping of At-PtNPs. The current results are concomitant with other previously published works that were concerned with the green synthesis of PtNPs (Thirumurugan, 2016; Yang, 2017). The obtained results indicated that the dielectric constant of the medium may have been changed because of the involvement of a variety of different phytochemicals from A. halimus extract such as glycosides, flavonoids, phenolic acids, and alkaloids as capping agents which are supposed to cause the deviation or the shift of the peak positions in the At-PtNPs spectrum as previously elaborated (Mahmoud, 2012; Pandey and Mishra, 2014).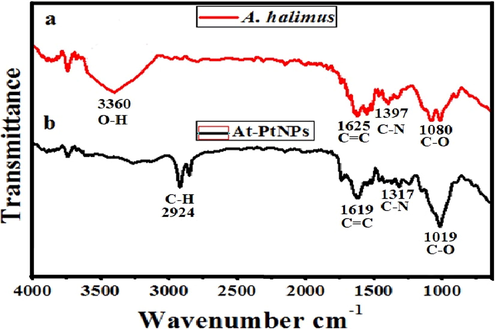
FT-IR spectra of A. halimus (a) and At-PtNPs (b).
3.5 High resolution transmission electron microscopy (HRTEM)
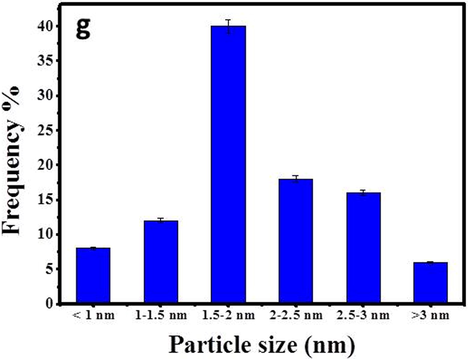
Providing essential information of nanoparticles such as shape and size is usually achieved by using High Resolution Transmission electron microscopy (HRTEM) (Hosny, 2021). It was found that the average particle size of At-PtNPs approximately ranged between 1 and 3 nm as shown in Fig. 5 (a-e), similar to other previous works (Jameel, 2020; Tahir, 2017), which is a very small particle size that could result in outstanding efficiency when these nanoparticles used in various applications. When the hydrodynamic size was measured via zetasizer as previously done by (Cui, 2021), it was found to be 9.4 nm. It has to be noticed that the hydrodynamic size is larger than the HRTEM particle size as it includes the size of biomolecules that are capping the nanoparticles (Dong, 2021; Ullah, 2017). The predominantly observed shape was spherical. The crystalline structure of At-PtNPs was confirmed by utilizing the Selected Area Electron Diffraction (SAED) technique demonstrated in Fig. 5f. SAED results were concomitant with the results obtained from XRD analysis indicating the (1 1 1), (2 0 0), (2 2 0), and (3 1 1) planes of crystalline At-PtNPs. In addition, crystalline At-PtNPs consisted of several crystalline lattices with well-defined inter-planer spacing d = 0.21 nm that is shown in (inset) Fig. 5e. Furthermore, a histogram size distribution of At-PtNPs that is presented in Fig. 5g indicated that approximately 40 % of these particles lie in the size range of 1.5–2 nm. The obtained results were in line with other previous studies (Jameel et al., 2020; Nishanthi et al., 2019). Furthermore, a comparison between the physical properties of At-PtNPs and other green synthesized PtNPs is presented in Table 1.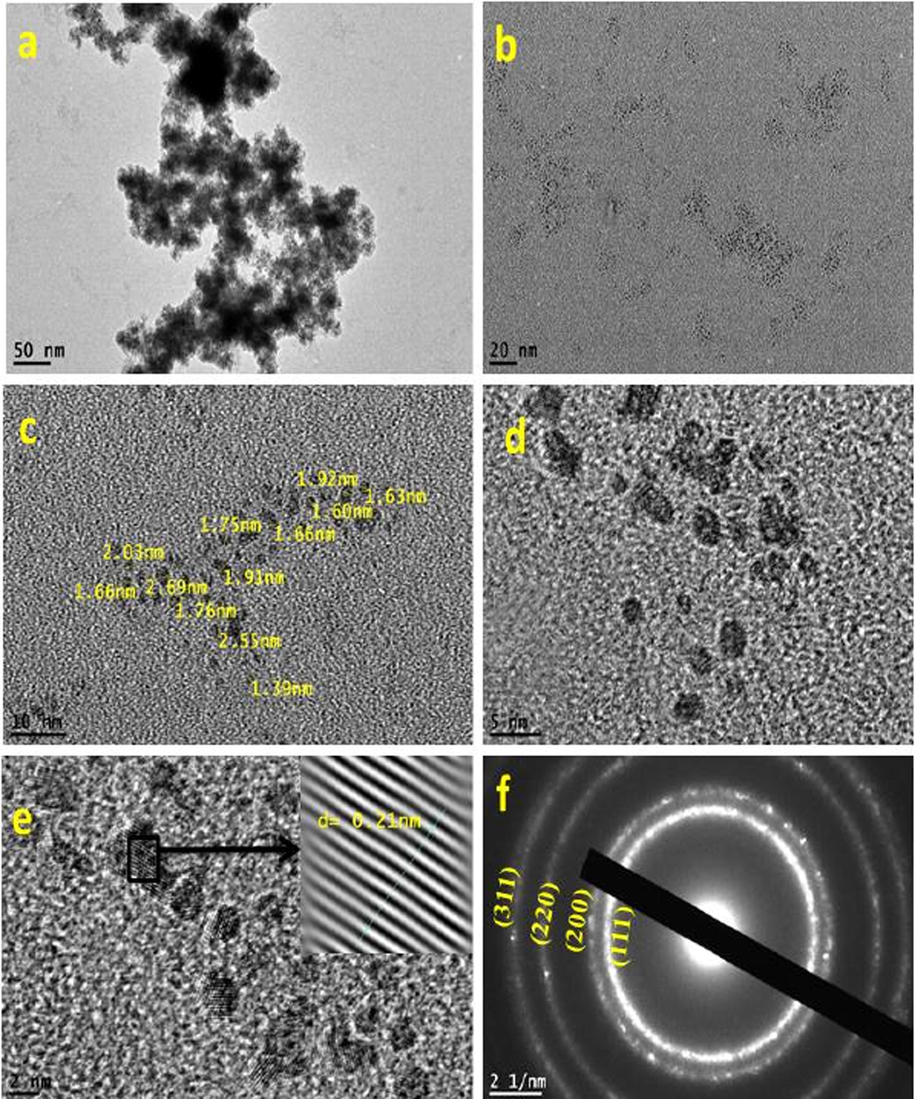
(a-e) HRTEM images of At-PtNPs (f) SAED image of At-PtNPs (g) Size distribution of At-PtNPs.
(a-e) HRTEM images of At-PtNPs (f) SAED image of At-PtNPs (g) Size distribution of At-PtNPs.
Plant
Size (nm)
Shape
Zeta potential (mV)
Ref.
Maytenus royleanus
5
Spherical
−41
(Ullah, 2017)
Taraxacum laevigatum
2–7
Spherical
−29
(Tahir, 2017)
Garcinia mangostana L.
∼2
Spherical
−13
(Nishanthi et al., 2019)
Prosopis farcta
10–40
Spherical
−15.6
(Jameel et al., 2020)
Atriplex halimus
1–3
Spherical
−25.4
Current study
3.6 EDX analysis and mapping
Energy-dispersive X-ray spectroscopy (EDX) was used to confirm the phytoreduction of H2PtCl6·6H2O into At-PtNPs. This was confirmed by strong signals of elemental platinum at energy levels of 2.2, 8.2, 9.5, 10, 11.2, and 13 keV in At-PtNPs as shown in Fig. 6a which are concomitant with the results of other workers (Konishi, 2007). It has to be pointed that the percentage of elemental platinum in At-PtNPs was approximately 90 % indicating the outstanding efficiency of A. halimus leaves extract in the phytosynthesis of At-PtNPs. Carbon and oxygen were derived from A. halimus extract. Also, carbon was derived from the carbon-coated copper grid. In the current analysis, the atoms on the At-PtNPs were excited by a specific wavelength electron beam. These, in turn, emit X-rays at an energy level that is element-specific as previously indicated (Bharati and M., C. Byram, and V.R. Soma, , 2018). As revealed in Fig. 6c, the yellow EDX mapping revealed that the nanoparticles presented in Fig. 6b are composed of platinum because the yellow color is centered over At-PtNPs. Similar results were obtained by Yang et al. (Yang, 2018). Furthermore, the red EDX mapping, as illustrated in Fig. 6d, indicated that the oxygen was equally distributed all over the map. Thus, confirming that the At-PtNPs are of zero-valent state of platinum (Pt0), not platinum oxide (PtO2) nanoparticles. Moreover, the white color in Fig. 6e represented the carbon that was equally distributed all over the map. Therefore, the obtained results designated that the oxygen and carbon were resulting from the phytoconstituents of A. halimus extract. In other words, they were of plant origin.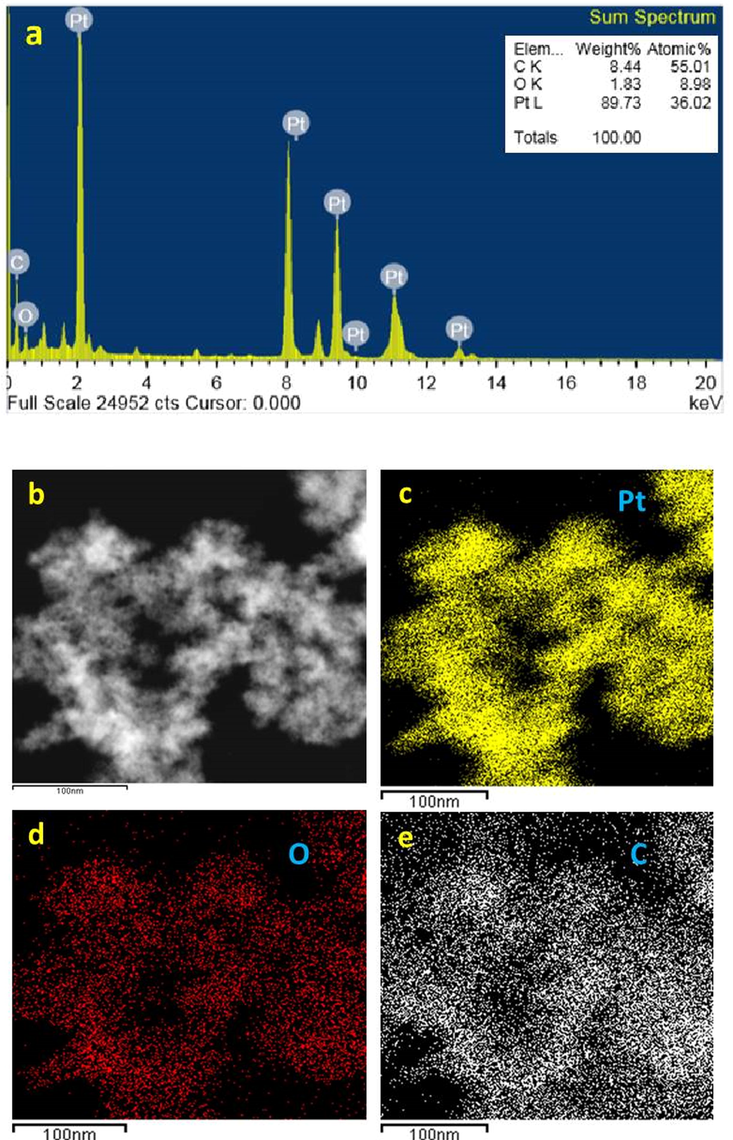
(a) EDX of At-PtNPs (b-e) elemental mapping images of At-PtNPs.
3.7 XPS analysis
In the current investigation, XPS analysis was used to determine the oxidation state of At-PtNPs as it was previously utilized (Aygun, 2020; Zou, 2018). Pt, C, O, and N were found to be the most prevalent elements in our findings as shown in the survey in Fig. 7a. High resolution spectrum of C1s (Fig. 7b) showed three main peaks with binding energies of 284.28, 286.08, and 287.98 eV resulting from hydrocarbon chains, α-carbon, and single bond COOH groups present in the A. halimus's phytoconstituents as it was elaborated by Syed and Ahmad (Syed and Ahmad, 2012). The peaks in Fig. 7c correspond to chemically distinct O1s and with binding energies at 531.38 eV and 532.48 eV that are attributed to the carbonyl group and molecular water (Hussain, 2019). While Fig. 7d demonstrated a peak at 399.78 eV which is related to N1s core levels. The obtained high-resolution spectrum of Pt4f (Fig. 7e) was characteristic for metallic Pt with Pt04f7/2 and Pt04f5/2 at binding energies of 72.48 and 75.58 eV, respectively. The obtained results were concomitant with other published ones (Celebioglu, 2017; Benaissi, 2010). As a result, At-PtNPs were concluded to be in the metallic Pt0 form (only the zero-valent state is produced), rather than Pt2+ or Pt4+. The obtained result may be explained by the straightforward reduction of an oxidized platinum species to its zero-valent form (the stable state of Pt) by the action of phytoconstituents found in A. halimus extract.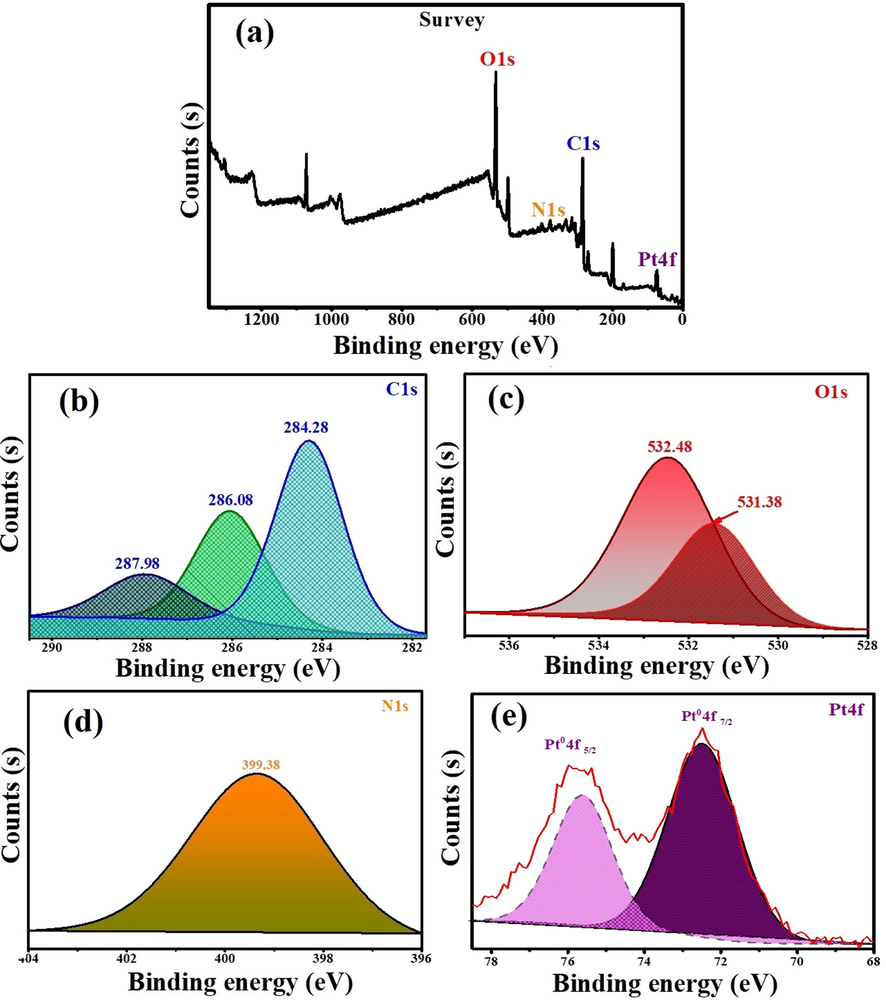
XPS spectrum of At-PtNPs (a) survey (b) C1s (c) O1s (d) N1s (e) Pt4f.
3.8 Zeta potential
The stability and surface charge of green synthesized At-PtNPs were investigated using a zeta analyzer. This approach is commonly utilized to measure nanoparticles dispersion stability (Tahir, 2017). At-PtNPs had a zeta potential value of − 25.4 mV (Fig. 8), confirming the high stability of At-PtNPs. As the zeta potential value becomes more negative, nanoparticles become more stable (Shah, 2014). At-PtNPs have a negative zeta potential, denoting that they are capped with negatively charged phytoconstituents that cause repulsion (dispersion) among them and increase their stability. The obtained results in the current study were lower than (Ullah, 2017) who mentioned that the zeta potential of PtNPs was − 41 mV and higher than that of (Dong, 2021) who reported a zeta value of − 7.33 mV.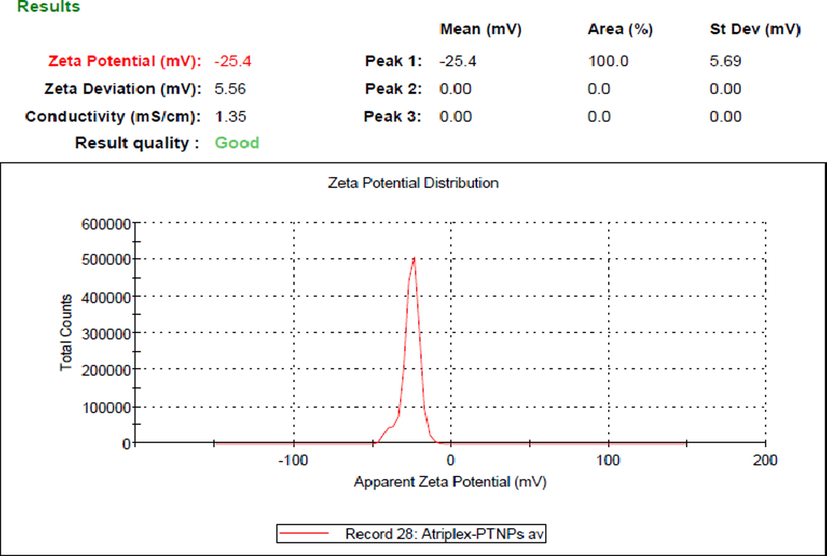
Zeta potential of At-PtNPs.
3.9 Applications of green synthesized At-PtNPs
3.9.1 Catalytic degradation of MB
In the current study, the catalytic degradation of different concentrations (20, 40, 60 and, 100 ppm) MB was conducted using 100 μL of At-PtNPs in the presence of 100 μL of 0.058 M NaBH4. The obtained results indicated that the characteristic blue color of the three concentrations (20, 40, and 60 ppm) of MB instantly disappeared after the addition of At-PtNPs to confirm the complete degradation of MB as shown in Fig. 9 (a-c). However, in the case of the highest concentration (100 ppm), it took approximately five min for complete MB degradation and color removal as shown in Fig. 9d and inset of Fig. 9d, respectively. When control experiments were carried out either without NaBH4 or At-PtNPs there was no color change observed. Moreover, when the catalytic effect of A. halimus extract was tested, it was figured out that the removal efficiency was only 4% as shown in Fig. 9e indicating the essential role of At-PtNPs in the catalytic degradation of MB. It has to be noticed that the obtained results in this study were reckoned to be superior to those reported by Dobrucka (Dobrucka, 2019) who reached a catalytic efficiency of approximately 37.5 % for MB solution with a concentration of just 40 ppm using PtNPs phytosynthesized by the extract of Fumariae herba after 15 min. As well as, our results are far away better than the results attained by Jameel et al. (Jameel, 2020) who achieved complete degradation of MB with a concentration of only 10 ppm in 15 min using sonochemical synthesized PtNPs. Therefore, it was concluded that the green synthesized At-PtNPs in the current study unequivocally have a better performance than other phyto or chemically synthesized PtNPs, which could be accredited to the phytoconstituents of A. halimus extract that resulted in the formation of highly stable and efficient PtNPs. Therefore, it is considered to be a highly potent and prominent catalyst that could be harnessed in the catalytic degradation of other toxic dyes from wastewater. In addition, a detailed comparison between At-PtNPs and other nanocatalysts including different variables such as the dye concentration, the degradation efficiency, and time is provided in Table 2. From Table 2, it can be concluded that the synthesized At-PtNPs are highly efficient for the catalytic degradation of MB.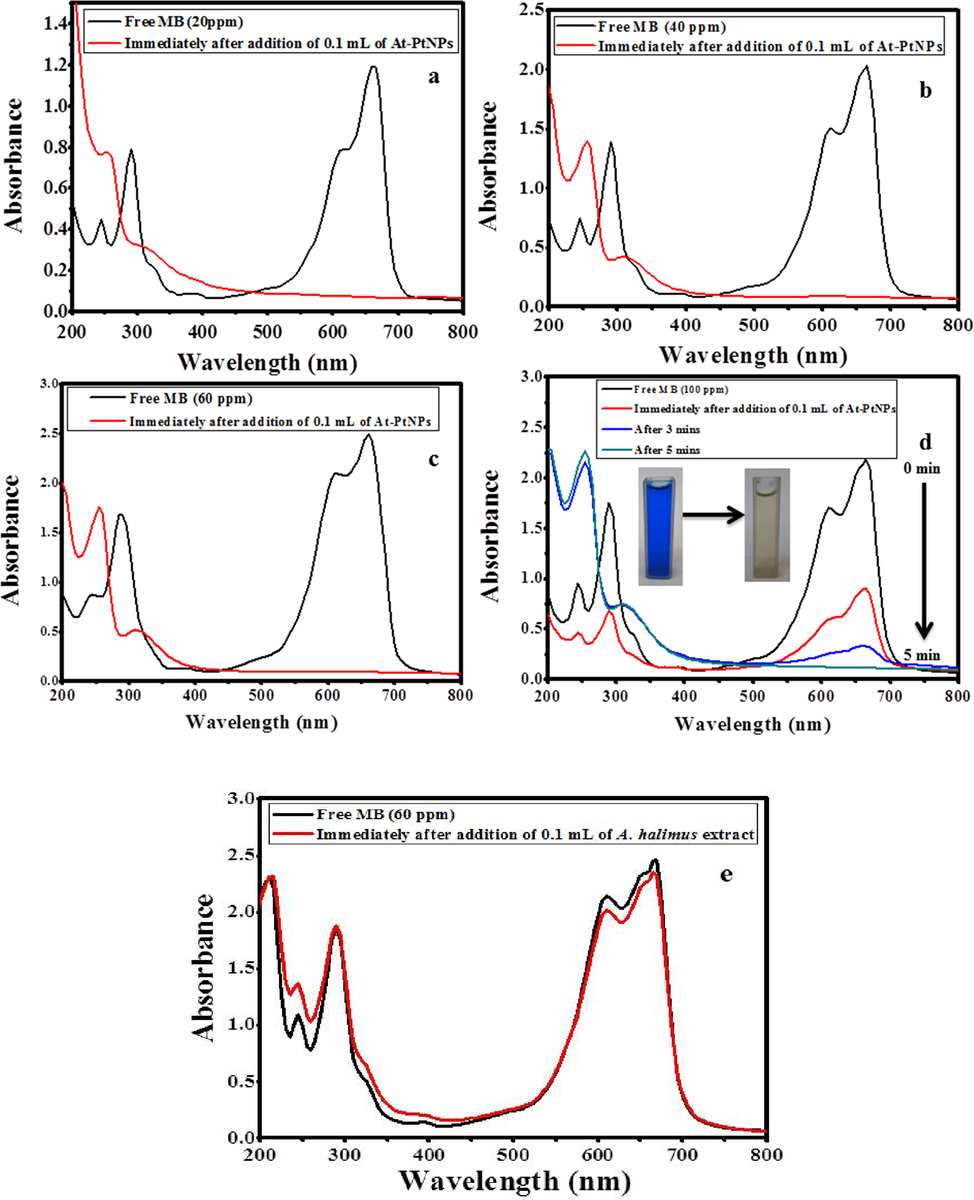
Catalytic degradation of MB (a) 20 ppm (b) 40 ppm (c) 60 ppm (d) 100 ppm using100 μL of At-PtNPs (e) Catalytic degradation of MB (60 ppm) using100 μL of A. halimus extract.
Catalyst
MB concentration (ppm)
Degradation efficiency (%)
Time (min)
Ref.
AuNPs
–
100
12
(Bogireddy et al., 2015)
AuNPs
50
100
10
(Hosny, 2021)
AuNPs
320
100
9
(Ganapuram, 2015)
AgNPs
16
100
30
(Edison, 2016)
AgNPs
320
100
20
(Bonnia, 2016)
PdNPs
240
100
2
(Kora and Rastogi, 2016)
PdNPs
25
100
5
(Subhan, 2020)
PdNPs
3
70
75
(Anand, 2016)
PtNPs
10
100
15
(Jameel, 2020)
At-PtNPs
25–75
100
Immediate
This study
At-PtNPs
100
100
5
This study
Five cycles of At-PtNPs utilization in the removal of MB with a concentration of 60 ppm were carried out to test their reusability. The obtained results indicated that the removal efficiency remained 100% after three cycles of regeneration (Fig. 10a, b, c). However, the efficiency started to diminish slightly to 93% in the fourth cycle (Fig. 10d) and eventually it reached 91.1% (Fig. 10e) after five cycles of reuse confirming the great potentiality of At-PtNPs as a catalyst as shown in Fig. 10f.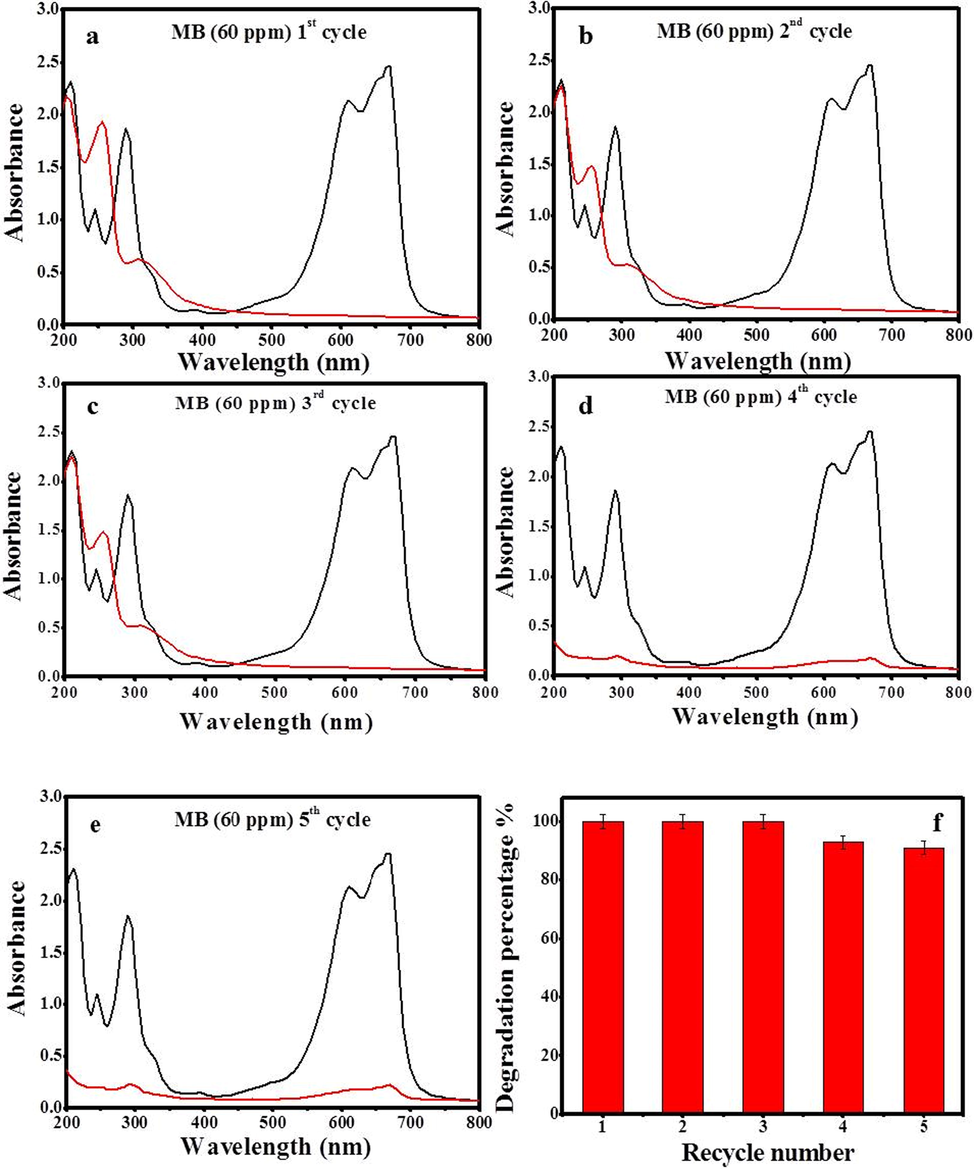
Recycling of At-PtNPs against MB (60 ppm) (a) 1st cycle (b) 2nd cycle (c) 3rd cycle (d) 4th cycle (e) 5th cycle (f) degradation percentage with recycling number.
A suggested mechanism that is concomitant with previous ones using other types of nanoparticles including AgNPs and AuNPs (Atta, 2020) for the catalytic degradation of MB into leuco MB is illustrated in Fig. 11. The reduction process occurs as both MB and BH4- ions are adsorbed on the surface of At-PtNPs and an electron relaying from the BH4- ions to the MB molecules resulting in the catalytic degradation of MB into leuco (El-Subruiti et al., 2019; Sallam et al., 2018). It has to be noticed that the degradation of dimethylamino groups in MB molecule by strong catalysts such as At-PtNPs facilitates the removal of MB. In addition, when Liu et al. (Liu, 2014) investigated the final products of MB molecules after its catalytic degradation using Ion Chromatography (IC), they observed that MB molecules were totally degraded into inorganic compounds such as NH4+, S2-, SO42-, NO3–, CO2, and H2O.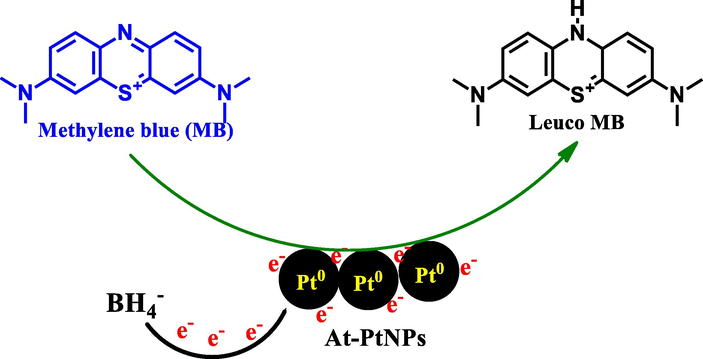
A proposed mechanism for the catalytic degradation of methylene blue (MB) into leuco MB onto At-PtNPs.
3.9.2 Antimicrobial study of At-PtNPs
The efficacy of green produced At-PtNPs at a concentration of 1 mg.L-1 as an antibacterial agent against gram-negative and gram-positive bacteria was determined using the zone of inhibition as shown in Fig. 12. The bacterial strains which were used in the current study are Escherichia coli, and Klebsiella pneumonia (gram-negative bacteria), and also gram-positive bacteria such as Bacillus subtilis and Staphyllococcus aureus (Mrsa). Our results showed that the inhibition zone was 17 mm for Klebsiella pneumonia and no growth was detected at all in the case of Escherichia coli indicating that At-PtNPs were highly efficient against Escherichia coli as it prevented the growth of the bacteria. However, At-PtNPs did not show any activity against gram-positive bacteria. As a result of the current findings, At-PtNPs may have antibacterial characteristics by changing the shape of the cell membrane and inhibiting normal budding due to a loss of membrane integrity, as has been demonstrated in earlier studies (Aygun, 2020; Pedone, 2017). At-PtNPs can result in increasing the formation of reactive oxygen species (ROS), resulting in down-regulation of DNA, oxidative stress, and finally apoptosis of bacterial cells (Pedone, 2017). At-PtNPs exhibited considerable antibacterial activity owing to their small size, uniform dispersion, and spherical shape. Generally, nanoparticles with small particle sizes often have a higher surface area and so are more effective than those with larger particle sizes. The antibacterial activity is usually related to the local activity of the material that eliminates or reduces bacteria's growth, without general tissue toxicity (Hajipour, 2012). Various studies have shown that both the physical and chemical properties including size, shape, and surface-volume relation of nanoparticles are associated with their antibacterial activities not only the surface charge, as well as their method of synthesis (Allaker, 2010). Moreover, it was previously revealed that negatively charged AgNPs can attack the gram-negative bacteria by metal depletion as proposed by many workers (Fayaz, 2010; Amro, 2000). As a consequence, the acquired data revealed that the newly synthesized At-PtNPs constitute a potential antibacterial that has high efficacy against gram-negative bacteria with a high concentration (2x108 CFU/mL). A comparison between the antibacterial potency of At-PtNPs and other different nanoparticles is provided in Table 3.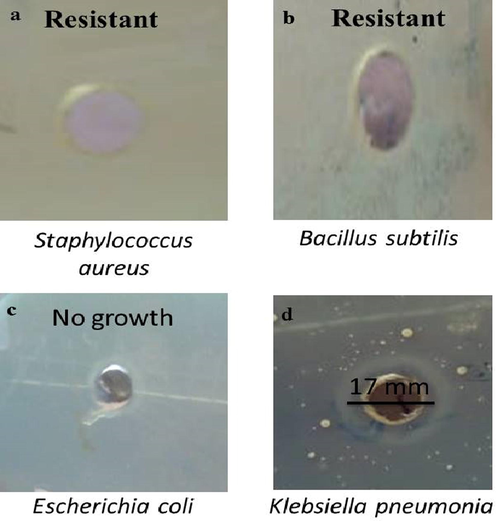
Antibacterial effect of At-PtNPs against (a) Staphyllococus aureus (b) Bacillus subtilis (c) Escherichia coli (d) Klebsiella pneumonia.
Sample
Concentration (mg.mL−1)
Bacterial strain
Zone of inhibition (mm)
Ref.
AgNPs
0.0075
Escherichia coli
18.5
(Hernández-Morales, 2019)
Staphylococcus aureus
14.9
AgNPs
–
Escherichia coli
14
(Naaz, 2021)
Staphylococcus aureus
13
Pseudomonas aeruginosa
16
AuNPs
10
Staphylococcus aureus
24
(Bindhu and Umadevi, 2014)
Pseudomonas aeruginosa
27.5
AuNPs
100
Escherichia coli
17.6
(Bakur, 2019)
Staphylococcus aureus
17
AuNPs
–Bacillus subtilis
9
(Poojary et al., 2016)
Staphylococcus aureus
12
Escherichia coli
7
Pseudomonas aeruginosa
5
AgNPs
Bacillus subtilis
10
Staphylococcus aureus
14
Escherichia coli
8
Pseudomonas aeruginosa
6
PtNPs
0.5
Escherichia coli
2
(Aygun, 2020)
Staphylococcus aureus
25
Pseudomonas aeruginosa
25
PtNPs
–Pseudomonas aeruginosa
15
(Tahir, 2017)
Bacillus subtilis
18
PtNPs
–Staphylococcus aureus
10
(Nishanthi et al., 2019)
Pseudomonas aeruginosa
11
Bacillus subtilis
Resistant
Klebsiella pneumonia
12
At-PtNPs
1
Escherichia coli
No growth
(sensitive)
This study
Klebsiella pneumonia
17
Bacillus subtilis
Resistant
Staphylococcus aureus
Resistant
3.9.3 Antioxidant study
The production of by-products such as perilous and noxious free radicals including reactive oxygen species (ROS) is generally related to common metabolic processes, which are deemed to be quintessential for the survival and welfare of biotic entities including humans (Sen, 2010; Mittler, 2017). These free radicals commonly result in oxidative stress and other physiological problems (Hosny and Fawzy, 2021). DPPH has been thought of as one of the most important and common free radicals that can negatively affect human cells (Patil, 2019). DPPH is identified as a stable free radical by virtue of the delocalization of the free electron over the molecule as a whole, so that it is presumed to be not easily degradable, like the preponderance of other free radicals (Kedare and Singh, 2011; Thaipong, 2006). DPPH has long been used to test the free radical scavenging capacity of antioxidants as it is an uncharged free radical that can accept hydrogen or free electrons and lead to the production of a stable diamagnetic molecule (Nakkala et al., 2016; Mensor, 2001). A substantial and efficient role against harmful free radicals including DPPH is usually played by antioxidants such as nanomaterials including metallic nanoparticles, metal oxides, graphene, and other nanostructures (Bhakya, 2016; Shah, 2017). On mixing the DPPH solution with a substance that can donate a hydrogen atom, in other words, it is working as an antioxidant, it causes the formation of the reduced form confirmed by the loss of the characteristic violet color (Sharma and Bhat, 2009) such as gold, palladium, silver, platinum, and other metallic nanoparticles which commonly play an essential role in eliminating DPPH (Zayed, 2020; Lee, 2014; Singh and Dhaliwal, 2019). In the current study, the scavenging % of DPPH increased dramatically from 13.77% to approximately 72% when the concentration of At-PtNPs increased from 12.5 to 50 µg/mL as shown in Fig. 13 which is reckoned to be a promising and a high percentage compared to most of the scavenging percentages mentioned by other studies (Selvi, 2020; Ramachandiran et al., 2021) that could be attributed to the biomolecules of A. halimus extract that led to the formation of highly stable and efficient PtNPs. In addition, the IC50 was figured out to be 36 µg/mL confirming the high antioxidant efficiency of At-PtNPs. Concerning the efficacy of A. halimus extract, it improved from 9.83% to 48.35% when the concentration of this extract increased in the same manner as At-PtNPs. Vitamin C, which was used as a positive control, achieved 14.45%, 31.8%, and 47.75% of DPPH scavenging at the concentrations of 12.5, 25, and 50 µg/mL (Fig. 13), in a respective manner, which is lower than At-PtNPs. Therefore, the obtained results confirmed the high antioxidant capacity of both A. halimus extract and At-PtNPs against DPPH and their promising application in the scavenging of other free radicals in further work. A comparison among At-PtNPs and other metallic nanoparticles in the scavenging efficiency of DPPH is presented in Table 4.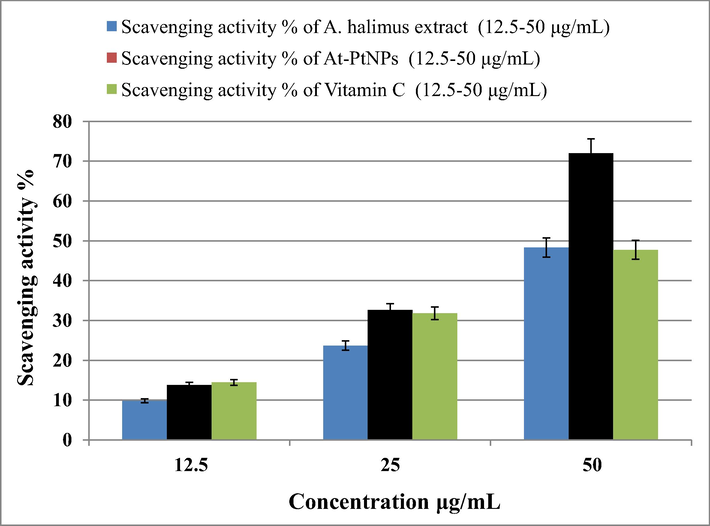
Antioxidant efficiency of A. halimus extract, At-PtNPs, and Vitamin C (positive control) against DPPH.
Antioxidant
Concentration µg/mL
Scavenging activity
Ref.
PdNPs
25
82.27
(Anju et al., 2020)
PtNPs
25
78.14
PtNPs
1000
59.37
(Selvi, 2020)
PtNPs
100
70
(Ramachandiran et al., 2021)
AgNPs
50
89.5
(Sivasankar, 2018)
AgNPs
4
80
(Sreelekha, 2021)
AgNPs
250
85.9
(Kharat and Mendhulkar, 2016)
AgNPs
500
62
(Niraimathi, 2013)
AuNPs
300
57.70
(Hosny and Fawzy, 2021)
AuNPs
120
84.64
(Veena, 2019)
AuNPs
300
70
(Manivasagan, 2015)
AuNPs
125
50
(Bakur, 2019)
At-PtNPs
50
72
This study
4 Conclusion
The current work is the first to demonstrate a rapid, one-step, cost-effective, and environmentally friendly production of platinum nanoparticles (At-PtNPs) utilizing the aqueous extract of A. halimus leaves. The phytosynthesis of At-PtNPs using the investigated species' aqueous extract was deemed to be successful and efficient, as they were found stable for more than three months and evidenced by the formation of dark brown and spherical At-PtNPs with a zeta potential of – 25.4 mV, very fine microscopic diameter ranging between 1 and 3 nm, and a surface plasmon peak at 295 nm. At-PtNPs were confirmed to be an excellent catalyst since they were able to catalytically degrade MB at three different concentrations in no time, and at 100 ppm it took only 5 min. Against gram-negative bacteria, At-PtNPs had strong antibacterial effect as they were able to stop Klebsiella pneumonia from growing with an inhibition zone of 17 mm and prevent the growth of Escherichia coli at all. Moreover, they have been confirmed to be a highly effective antioxidant agent with an IC50 value of 36 µg/mL. So, the current findings show that using an aqueous extract of A. halimus to synthesize PtNPs is a straightforward and effective way for producing a powerful nanomaterial that could be efficiently employed in a variety of different applications. Additionally, it can be concluded that At-PtNPs synthesis in the current study upholds many of the principles of green chemistry such as less hazardous chemical syntheses, safer solvents and auxiliaries, design for energy efficiency, and use of renewable feedstocks.
Acknowledgement
This work was financially supported by SMARTWATIR, ERANETMED-3-227 project, Academy of Scientific Research and Technology & Young Scientist Research Grant 2020, MAB Committee - Egyptian National Commission to UNESCO.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Green Synthesis of Nano-Zero-Valent Iron Using Ricinus Communis Seeds Extract: Characterization and Application in the Treatment of Methylene Blue-Polluted Water. ACS. Omega 2021
- [Google Scholar]
- The use of nanoparticles to control oral biofilm formation. J. Dent. Res.. 2010;89(11):1175-1186.
- [Google Scholar]
- Call of the wild: antibiotic resistance genes in natural environments. Nat. Rev. Microbiol.. 2010;8(4):251-259.
- [Google Scholar]
- Green synthesis of platinum nanoparticles using Saudi’s Dates extract and their usage on the cancer cell treatment. Arabian J. Chem.. 2019;12(3):330-349.
- [Google Scholar]
- High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: structural basis for permeability. Langmuir. 2000;16(6):2789-2796.
- [Google Scholar]
- Biosynthesis of palladium nanoparticles by using Moringa oleifera flower extract and their catalytic and biological properties. J. Photochem. Photobiol., B. 2016;165:87-95.
- [Google Scholar]
- In Vitro Antimicrobial and Antioxidant Activity of Biogenically Synthesized Palladium and Platinum Nanoparticles Using Botryococcus braunii. Turkish Journal of Pharmaceutical Sciences. 2020;17(3):299.
- [Google Scholar]
- Efficient green synthesis of N, B co-doped bright fluorescent carbon nanodots and their electrocatalytic and bio-imaging applications. Diam. Relat. Mater.. 2021;116:108437
- [Google Scholar]
- Reduction of Congo red using nitrogen doped fluorescent carbon nanodots obtained from sprout extract of Borassus flabellifer. Chem. Phys. Lett.. 2020;754:137646
- [Google Scholar]
- Methylene blue catalytic degradation using silver and magnetite nanoparticles functionalized with a poly (ionic liquid) based on quaternized dialkylethanolamine with 2-acrylamido-2-methylpropane sulfonate-co-vinylpyrrolidone. ACS Omega. 2020;5(6):2829-2842.
- [Google Scholar]
- Biogenic platinum nanoparticles using black cumin seed and their potential usage as antimicrobial and anticancer agent. J. Pharm. Biomed. Anal.. 2020;179:112961
- [Google Scholar]
- Synthesis of gold nanoparticles derived from mannosylerythritol lipid and evaluation of their bioactivities. Amb Express. 2019;9(1):62.
- [Google Scholar]
- Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today. 2014;9(2):223-243.
- [Google Scholar]
- Ultrasonically driven green synthesis of palladium nanoparticles by Coleus amboinicus for catalytic reduction and Suzuki-Miyaura reaction. Colloids Surf., B. 2020;192:111026
- [Google Scholar]
- Synthesis of platinum nanoparticles using cellulosic reducing agents. Green Chem.. 2010;12(2):220-222.
- [Google Scholar]
- Antioxidant activity of methanolic extracts and some bioactive compounds of Atriplex halimus. C. R. Chim.. 2009;12(12):1259-1266.
- [Google Scholar]
- Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Applied Nanoscience. 2016;6(5):755-766.
- [Google Scholar]
- Femtosecond laser fabricated Ag@ Au and Cu@ Au alloy nanoparticles for surface enhanced Raman spectroscopy based trace explosives detection. Front. Physics. 2018;6:28.
- [Google Scholar]
- Antibacterial activities of green synthesized gold nanoparticles. Mater. Lett.. 2014;120:122-125.
- [Google Scholar]
- Gold nanoparticles—synthesis by Sterculia acuminata extract and its catalytic efficiency in alleviating different organic dyes. J. Mol. Liq.. 2015;211:868-875.
- [Google Scholar]
- Green biosynthesis of silver nanoparticles using ‘Polygonum Hydropiper’and study its catalytic degradation of methylene blue. Procedia Chem.. 2016;19:594-602.
- [Google Scholar]
- Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf.. 2011;10(4):221-247.
- [Google Scholar]
- Inhibitory effect of Thymus vulgaris and Origanum vulgare essential oils on virulence factors of phytopathogenic Pseudomonas syringae strains. Plant Biology. 2017;19(4):599-607.
- [Google Scholar]
- Surface Decoration of Pt Nanoparticles via ALD with TiO 2 Protective Layer on Polymeric Nanofibers as Flexible and Reusable Heterogeneous Nanocatalysts. Sci. Rep.. 2017;7(1):1-10.
- [Google Scholar]
- Ecofriendly synthesis of fluorescent nitrogen-doped carbon dots from coccinia grandis and its efficient catalytic application in the reduction of methyl orange. Journal of fluorescence. 2020;30(1):103-112.
- [Google Scholar]
- Green synthesis, characterization, cytotoxicity, antioxidant, and anti-human ovarian cancer activities of Curcumae kwangsiensis leaf aqueous extract green-synthesized gold nanoparticles. Arabian J. Chem.. 2021;14(3):103000
- [Google Scholar]
- “Stealth” dendrimers with encapsulation of indocyanine green for photothermal and photodynamic therapy of cancer. Int. J. Pharm.. 2021;600:120502
- [Google Scholar]
- Biofabrication of platinum nanoparticles using Fumariae herba extract and their catalytic properties. Saudi Journal of Biological Sciences. 2019;26(1):31-37.
- [Google Scholar]
- Green synthesis of platinum nanoclusters using lentinan for sensitively colorimetric detection of glucose. Int. J. Biol. Macromol.. 2021;172:289-298.
- [Google Scholar]
- Green chemistry for nanoparticle synthesis. Chem. Soc. Rev.. 2015;44(16):5778-5792.
- [Google Scholar]
- Caulerpa racemosa: a marine green alga for eco-friendly synthesis of silver nanoparticles and its catalytic degradation of methylene blue. Bioprocess Biosyst. Eng.. 2016;39(9):1401-1408.
- [Google Scholar]
- Green synthesis of gold nanoparticles using Parsley leaves extract and their applications as an alternative catalytic, antioxidant, anticancer, and antibacterial agents. Adv. Powder Technol.. 2020;31(10):4390-4400.
- [Google Scholar]
- Antioxidant, anticancer and enhanced photocatalytic potentials of gold nanoparticles biosynthesized by common reed leaf extract. Applied Nanoscience 2021:1-12.
- [Google Scholar]
- Cobalt Nanoparticles Supported on Reduced Amine-Functionalized Graphene Oxide for Catalytic Reduction of Nitroanilines and Organic Dyes. NANO. 2021;16(04):2150039.
- [Google Scholar]
- Synthesis of active MFe2O4/γ-Fe2O3 nanocomposites (metal= Ni or Co) for reduction of nitro-containing pollutants and methyl orange degradation. NANO. 2019;14(10):1950125.
- [Google Scholar]
- Carboxymethyl cellulose/carboxylated graphene oxide composite microbeads for efficient adsorption of cationic methylene blue dye. Int. J. Biol. Macromol.. 2020;154:307-318.
- [Google Scholar]
- Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: Characterization, adsorption kinetics, thermodynamics and isotherms. Adv. Powder Technol.. 2020;31(3):1253-1263.
- [Google Scholar]
- Efficient removal of toxic methylene blue (MB) dye from aqueous solution using a metal-organic framework (MOF) MIL-101 (Fe): isotherms, kinetics, and thermodynamic studies. Desalin. Water Treat. 2020;189:395-407.
- [Google Scholar]
- Zero valent iron nanoparticle-loaded nanobentonite intercalated carboxymethyl chitosan for efficient removal of both anionic and cationic dyes. ACS Omega. 2021;6(9):6348-6360.
- [Google Scholar]
- Highly efficient removal for methylene blue and Cu2+ onto UiO-66 metal–organic framework/carboxylated graphene oxide-incorporated sodium alginate beads. ACS Omega 2021
- [Google Scholar]
- Green synthesis of stable platinum nanoclusters with enhanced peroxidase-like activity for sensitive detection of glucose and glutathione. Microchem. J.. 2021;166:106202
- [Google Scholar]
- Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med.. 2010;6(1):103-109.
- [Google Scholar]
- Catalytic reduction of methylene blue and Congo red dyes using green synthesized gold nanoparticles capped by salmalia malabarica gum. International Nano Letters. 2015;5(4):215-222.
- [Google Scholar]
- Antibacterial properties of nanoparticles. Trends Biotechnol.. 2012;30(10):499-511.
- [Google Scholar]
- Study of the green synthesis of silver nanoparticles using a natural extract of dark or white Salvia hispanica L. seeds and their antibacterial application. Appl. Surf. Sci.. 2019;489:952-961.
- [Google Scholar]
- Comparative study on the potentialities of two halophytic species in the green synthesis of gold nanoparticles and their anticancer, antioxidant and catalytic efficiencies. Adv. Powder Technol.. 2021;32(9):3220-3233.
- [Google Scholar]
- Comparative study between Phragmites australis root and rhizome extracts for mediating gold nanoparticles synthesis and their medical and environmental applications. Adv. Powder Technol. 2021
- [Google Scholar]
- Instantaneous phytosynthesis of gold nanoparticles via Persicaria salicifolia leaf extract, and their medical applications. Adv. Powder Technol. 2021
- [Google Scholar]
- Platinum nanoparticles photo-deposited on SnO2-C composites: An active and durable electrocatalyst for the oxygen reduction reaction. Electrochim. Acta. 2019;316:162-172.
- [Google Scholar]
- Nanodiamonds facilitate killing of intracellular uropathogenic e. Coli in an in vitro model of urinary tract infection pathogenesis. PLoS ONE. 2018;13(1):e0191020
- [Google Scholar]
- Rapid sonochemically-assisted green synthesis of highly stable and biocompatible platinum nanoparticles. Surf. Interfaces. 2020;20:100635
- [Google Scholar]
- Rapid methanol-assisted amalgamation of high purity platinum nanoparticles utilizing sonochemical strategy and investigation on its catalytic activity. Surf. Interfaces. 2020;21:100785
- [Google Scholar]
- Comparative analysis of platinum nanoparticles synthesized using sonochemical-assisted and conventional green methods. Nano-Structures & Nano-Objects. 2020;23:100484
- [Google Scholar]
- Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol.. 2011;48(4):412-422.
- [Google Scholar]
- Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng., C. 2016;62:719-724.
- [Google Scholar]
- Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J. Biotechnol.. 2007;128(3):648-653.
- [Google Scholar]
- Catalytic degradation of anthropogenic dye pollutants using palladium nanoparticles synthesized by gum olibanum, a glucuronoarabinogalactan biopolymer. Ind. Crops Prod.. 2016;81:1-10.
- [Google Scholar]
- Characterization of the antioxidant activity of gold@ platinum nanoparticles. RSC Adv.. 2014;4(38):19824-19830.
- [Google Scholar]
- Microwave-enhanced catalytic degradation of methylene blue by porous MFe2O4 (M= Mn, Co) nanocomposites: Pathways and mechanisms. Sep. Purif. Technol.. 2014;135:35-41.
- [Google Scholar]
- Effect of the dielectric constant of the surrounding medium and the substrate on the surface plasmon resonance spectrum and sensitivity factors of highly symmetric systems: silver nanocubes. J. Am. Chem. Soc.. 2012;134(14):6434-6442.
- [Google Scholar]
- A novel route for the synthesis of copper oxide nanoparticles using Bougainvillea plant flowers extract and antifungal activity evaluation. Chem. Int.. 2021;7(1):71-78.
- [Google Scholar]
- Green synthesis of palladium nanoparticles using fenugreek tea and their catalytic applications in organic reactions. Mater. Lett.. 2017;205:138-141.
- [Google Scholar]
- Au-Pd bimetallic nanoparticles embedded highly porous Fenugreek polysaccharide based micro networks for catalytic applications. Int. J. Biol. Macromol.. 2019;126:352-358.
- [Google Scholar]
- Extracellular synthesis of gold bionanoparticles by Nocardiopsis sp. and evaluation of its antimicrobial, antioxidant and cytotoxic activities. Bioprocess Biosyst. Eng.. 2015;38(6):1167-1177.
- [Google Scholar]
- Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res.. 2001;15(2):127-130.
- [Google Scholar]
- Green synthesis, characterization and catalytic activity of silver nanoparticles using Cassia auriculata flower extract separated fraction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2017;179:66-72.
- [Google Scholar]
- Green synthesis of silver nanoparticles using Syngonium podophyllum leaf extract and its antibacterial activity. Mater. Today:. Proc. 2021
- [Google Scholar]
- The antioxidant and catalytic activities of green synthesized gold nanoparticles from Piper longum fruit extract. Process Saf. Environ. Prot.. 2016;100:288-294.
- [Google Scholar]
- Biogenic and eco-benign synthesis of platinum nanoparticles (Pt NPs) using plants aqueous extracts and biological derivatives: environmental, biological and catalytic applications. J. Mater. Res. Technol.. 2020;9(4):9093-9107.
- [Google Scholar]
- Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Colloids Surf., B. 2013;102:288-291.
- [Google Scholar]
- Green synthesis and characterization of bioinspired silver, gold and platinum nanoparticles and evaluation of their synergistic antibacterial activity after combining with different classes of antibiotics. Mater. Sci. Eng., C. 2019;96:693-707.
- [Google Scholar]
- A rapid biosynthesis route for the preparation of gold nanoparticles by aqueous extract of cypress leaves at room temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2012;94:84-88.
- [Google Scholar]
- Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem.. 2009;47(7):578-583.
- [Google Scholar]
- Formulation and Antibacterial Activity Evaluation of Quaternized Aminochitosan Membrane for Wound Dressing Applications. Polymers. 2021;13(15):2428.
- [Google Scholar]
- Catalytic reduction of p-nitrophenol by using platinum nanoparticles stabilised by guar gum. Carbohydr. Polym.. 2014;113:525-531.
- [Google Scholar]
- Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surf., B. 2019;183:110455
- [Google Scholar]
- Green synthesis of silver and gold nanoparticles using root bark extract of Mammea suriga: characterization, process optimization, and their antibacterial activity. BioNanoScience. 2016;6(2):110-120.
- [Google Scholar]
- Antibacterial activity of some wild medicinal plants collected from western Mediterranean coast, Egypt: Natural alternatives for infectious disease treatment. Afr. J. Biotechnol.. 2011;10(52):10733-10743.
- [Google Scholar]
- Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J. Genet. Eng. Biotechnol.. 2016;14(1):195-202.
- [Google Scholar]
- Structural, optical, biological and photocatalytic activities of platinum nanoparticles using salixtetraspeama leaf extract via hydrothermal and ultrasonic methods. Optik 2021167494
- [Google Scholar]
- Cytotoxic effects of platinum nanoparticles obtained from pomegranate extract by the green synthesis method on the MCF-7 cell line. Colloids Surf., B. 2018;163:119-124.
- [Google Scholar]
- Facile synthesis of Ag–γ-Fe 2 O 3 superior nanocomposite for catalytic reduction of nitroaromatic compounds and catalytic degradation of methyl orange. Catal. Lett.. 2018;148(12):3701-3714.
- [Google Scholar]
- Synthesis of Tragia involucrata mediated platinum nanoparticles for comprehensive therapeutic applications: Antioxidant, antibacterial and mitochondria-associated apoptosis in HeLa cells. Process Biochem.. 2020;98:21-33.
- [Google Scholar]
- Free radicals, antioxidants, diseases and phytomedicines: current status and future prospect. International journal of pharmaceutical sciences review and research. 2010;3(1):91-100.
- [Google Scholar]
- Optimisation and stability assessment of solid lipid nanoparticles using particle size and zeta potential. Journal of Physical. Science. 2014;25(1)
- [Google Scholar]
- Surface functionalization of iron oxide nanoparticles with gallic acid as potential antioxidant and antimicrobial agents. Nanomaterials. 2017;7(10):306.
- [Google Scholar]
- Novel green synthesis and characterization of the antioxidant activity of silver nanoparticles prepared from Nepeta leucophylla root extract. Anal. Lett.. 2019;52(2):213-230.
- [Google Scholar]
- Characterization, antimicrobial and antioxidant property of exopolysaccharide mediated silver nanoparticles synthesized by Streptomyces violaceus MM72. Carbohydr. Polym.. 2018;181:752-759.
- [Google Scholar]
- A comparative study on the synthesis, characterization, and antioxidant activity of green and chemically synthesized silver nanoparticles. BioNanoScience. 2021;11(2):489-496.
- [Google Scholar]
- Unusual Pd nanoparticle dispersion in microenvironment for p-nitrophenol and methylene blue catalytic reduction. J. Colloid Interface Sci.. 2020;578:37-46.
- [Google Scholar]
- Extracellular biosynthesis of platinum nanoparticles using the fungus Fusarium oxysporum. Colloids Surf., B. 2012;97:27-31.
- [Google Scholar]
- Facile and green synthesis of phytochemicals capped platinum nanoparticles and in vitro their superior antibacterial activity. J. Photochem. Photobiol., B. 2017;166:246-251.
- [Google Scholar]
- Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal.. 2006;19(6–7):669-675.
- [Google Scholar]
- Green synthesis of platinum nanoparticles using Azadirachta indica – An eco-friendly approach. Mater. Lett.. 2016;170:175-178.
- [Google Scholar]
- Green synthesis of platinum nanoparticles using Azadirachta indica–An eco-friendly approach. Mater. Lett.. 2016;170:175-178.
- [Google Scholar]
- Bio-fabrication of catalytic platinum nanoparticles and their in vitro efficacy against lungs cancer cells line (A549) J. Photochem. Photobiol., B. 2017;173:368-375.
- [Google Scholar]
- Green synthesis of gold nanoparticles from Vitex negundo leaf extract: characterization and in vitro evaluation of antioxidant–antibacterial activity. J. Cluster Sci.. 2019;30(6):1591-1597.
- [Google Scholar]
- Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discovery. 2005;4(4):307-320.
- [Google Scholar]
- Bio-synthesis of peppermint leaf extract polyphenols capped nano-platinum and their in-vitro cytotoxicity towards colon cancer cell lines (HCT 116) Mater. Sci. Eng., C. 2017;77:1012-1016.
- [Google Scholar]
- Graphene supported platinum nanoparticles modified electrode and its enzymatic biosensing for lactic acid. J. Electrochem. Soc.. 2018;165(14):B665.
- [Google Scholar]
- Biofabrication of gold nanoparticles by Lyptolyngbya JSC-1 extract as super reducing and stabilizing agents: Synthesis, characterization and antibacterial activity. Microb. Pathog.. 2018;114:116-123.
- [Google Scholar]
- In-vitro antioxidant and antimicrobial activities of metal nanoparticles biosynthesized using optimized Pimpinella anisum extract. Colloids Surf., A. 2020;585:124167
- [Google Scholar]
- Functionalization of silk with in-situ synthesized platinum nanoparticles. Materials. 2018;11(10):1929.
- [Google Scholar]







