Grignard Reaction: An ‘Old-Yet-Gold’ synthetic gadget toward the synthesis of natural Products: A review
⁎Corresponding authors. fawad.zahoor@gcuf.edu.pk (Ameer Fawad Zahoor), mezaki@imamu.edu.sa (Magdi E.A. Zaki)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Natural product synthesis is an important area of chemical study having various prospects for medicinal, industrial, and agricultural industry. Grignard reaction was developed as a flexible and necessary tool for the production of complex organic compounds in synthetic procedures. Additionally, the synthetic flexibility of the Grignard reaction facilitates its usage in the construction of more complicated ring frameworks and stereoselective transformations. It has been substantially applied in natural product synthesis, demonstrating its effectiveness in the generation of crucial C-C, C-X bonds and the development of intricate molecular structures. Use of Grignard reaction has been significantly studied towards the synthesis of biologically active natural products such as terpenoids, polyketides, alkaloids and amino acids. This review outlines the numerous instances where Grignard reagents have been exploited in the synthesis of notable natural products since past two years.
1 Introduction
The Grignard reaction was a powerful invention of the famous French chemist Francois Auguste Victor Grignard (Sakamoto et al., 2001). The significance of this advancement in synthetic chemistry was acknowledged in 1912, when Grignard received the Chemistry Nobel Prize in recognition of his invention. It entails the creation of a C-C bond that links an electrophilic substrate, frequently a carbonyl molecule and an organomagnesium halide (Guggenberger and Rundle, 1964). In industry and academia, this reaction is frequently used to synthesize a wide range of several organic substances i.e., ketones, carboxylic acids and alcohols. In the Grignard reaction, an aryl or alkyl halide reacted with magnesium (Mg) metal in tetrahydrofuran or diethyl ether under anhydrous conditions (Scheme 1) (Seyferth, 2009). In general, Grignard reaction involves the treatment of carbonyl group of aldehyde or ketone with organo-magnesium compound to access corresponding alcohol.

- General schematic layout for Grignard reaction.
Many essential characteristics associated with the Grignard reaction led to its significant synthetic value. It results in the generation of both C-C and C-X bonds. Furthermore, Grignard reagents can be employed as reductants (Clémancey et al., 2017), trans-metalation reagents (Jaumier et al., 1999; Akhtar and Zahoor, 2020), and catalysts for metal-catalyzed cross-coupling processes (Qin et al., 2024). All of these mentioned processes are generally useful in creating complex organic compounds from simple building blocks. In addition to this, the synthetic utility of the Grignard reagent has been enhanced by the development of halogen magnesium exchange (Ziegler et al., 2019). Moderate reaction conditions are required for halogen magnesium exchange (Knochel et al., 2003), which ensures excellent functional group tolerance, thereby broadening way for new alternatives in organic synthesis. The Grignard reagent has also been found to introduce regio- and stereoselectivity in some reactions (Vidal et al., 2014).
During the past ten years, natural products (Singh et al., 2024; Sims & Dai, 2023; Liang et al., 2022; Saridakis et al., 2020) have acquired growing importance in organic synthesis because of their distinct structural designs and variety of pharmacological actions (Jiang et al., 2021; Kinfe, 2019; Abu-Hashem et al., 2023). These naturally occurring products are currently being exploited in research to create novel pharmaceuticals (Blakemore et al., 2018; Bai et al., 2017; Abu-Hashem et al., 2021) and agricultural products (Depres et al., 2016; Ley et al., 2015). Grignard reagent has also been utilized for the synthesis of a large number of natural products such as cylindricine A (Donohoe et al., 2010) 1 (active against brine shrimp larvae), (−)-aurantioclavine (Brak and Ellman, 2010) 2, amphidinolide X (Lepage et al., 2004) 3 (exhibits cytotoxicity against murine lymphoma), hopeahainol A (Nicolaou et al., 2010) 4 (shows anti-inflammatory, cardiovascular and antitumor properties), (±)-crispine A (Knölker and Agarwal, 2005) 5 (adrenoreceptor antagonist) and radicamines A (Yu and Huang, 2006) 6 (Fig. 1).
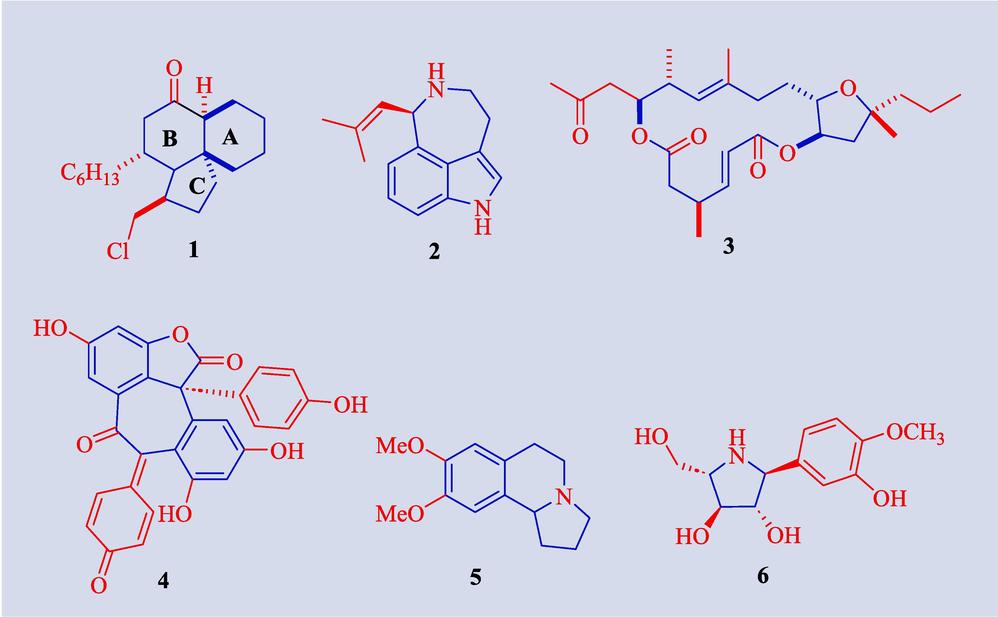
- Structure of natural products 1–6 synthesized from Grignard reagent.
The Grignard reaction's importance and its broad use in the formation of organic compounds have been extensively described in the literature. Several research publications and review articles have been reported in this field (Franzén, 2000; Guérinot & Cossy, 2020) which emphasize the advancement of novel techniques and their applications in organic synthesis (Harutyunyan et al., 2008; Douchez et al., 2018; Tkachenko et al., 2018). However, to the best of our knowledge, despite its continuous utility, any review specifically demonstrating the applications of Grignard reagent towards natural product synthesis has not been published yet, which significantly urged us to work in this domain.
2 Alkaloids based natural products
2.1 Daphgraciline alkaloids
The Daphniphyllum alkaloids are a huge family of natural products that feature complex polycyclic skeletons with different variations (Kobayashi and Kubota, 2009). Synthetic researchers are quite interested in such alkaloids. One of its important subfamilies is yuzurine-type alkaloids, that possess high cytotoxic and pesticidal efficacy against murine lymphoma and brine shrimp respectively (Kang et al., 2014). Li et al. in 2022, proposed the total synthesis of daphgraciline, a yuzurine-type alkaloid by utilizing the Grignard reagent in one of their key steps (Li et al., 2022). The first step of this synthetic scheme involved the formation of compound 8 from 7 which was then made to react with dihydroquinidine at 55 °C to attain single diastereomer 9 with 75 % yield. In the next step, diastereoselective 1,2-addition of Grignard reagent 10 to compound 9 took place in dichloromethane to afford compound 11 in 85 % yield which was then transformed into compound 12 over few steps. In the next step, compound 12 was reacted with EtNH2 and KHDMS in the presence of imidazole tetrahydrofuran followed by Ti-mediated coupling with acrylonitrile 13 to afford spirolactone 14 in 81 % yield. The following step involved the treatment of compound 14 with Grignard reagent and then reacting it with tetraphenyl porphyrin to produce alcohol 15 with an overall of 70 % yield (4-steps). Last but not least, compound 15 was treated with MgSO4 at 140 °C in the presence of DCM to generate dehydrodaphgraciline 16 in 83 % yield. The resulting compound 16 was further made to react with p-TsOH in THF/water mixture to furnish the title compound i.e., daphgraciline 17, having 80 % yield (Scheme 2).
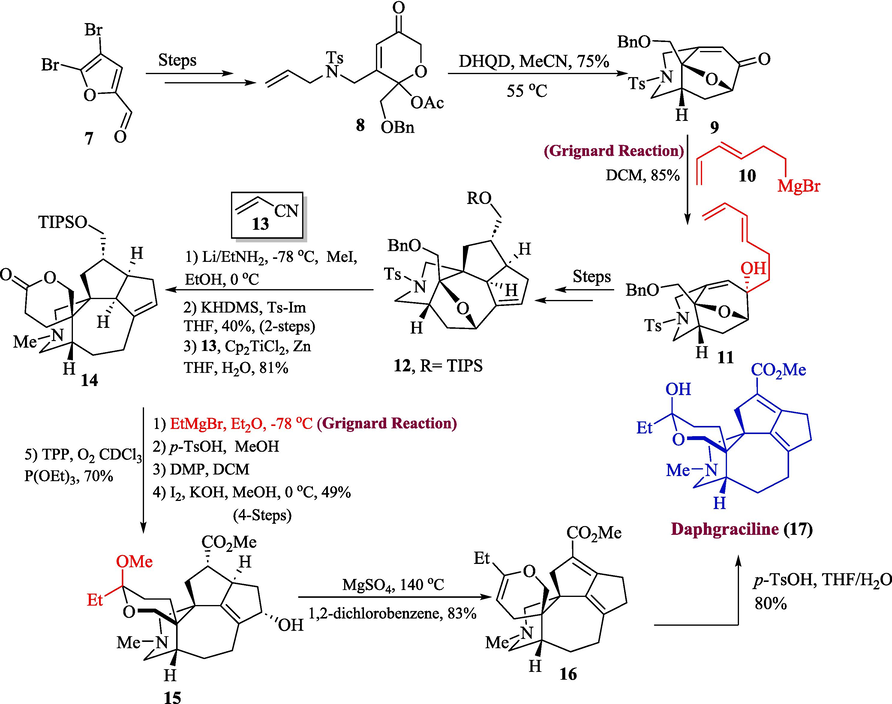
- Synthesis of daphgraciline alkaloid 17.
2.2 Pileamartines alkaloids
The Pilea genus contains a large number of active alkaloids which have an ancient legacy of usage as traditional remedies for a variety of medical conditions (Ren et al., 2012). The pileamartines A-D (Thuy et al., 2019) are very important among them, which have been isolated from Pilea aff. martinii leaves. For instance, pileamartine D exhibited impressive and specific inhibitory activity against HepG-2 and KB cells (Thuy et al., 2018). In addition, pileamartine B demonstrated negligible inhibition of glucosidase enzymes. Considering the wide-ranging significance of pilemartines, Xu et al. in 2022, reported the synthesis of pileamartines A and B by employing Grignard reagent in one of their important steps (Xu et al., 2022). The first step of their efficient synthetic strategy involved the formation of indanone 21 by the treatment of t-butyl (p-tolyl(tosyl)methyl)carbamate 18 with thiazolium salt 19 and acrylate 20. In the next step, compound 21 was treated with trifluoroacetic acid followed by allylation to obtain dihydroindenone 22 with a 79 % yield. Furthermore, the transformation of C10 carbonyl into methylene was achieved by reduction with NaBH4 followed by Grignard addition and LiAlH4 reduction respectively to afford the desired isomer 23 in 32 % yield. After a few steps, pileamartine B 24 was formed which was transformed into pileamartine A 25 in 96 % yield by the methylation of the phenol group (Scheme 3).
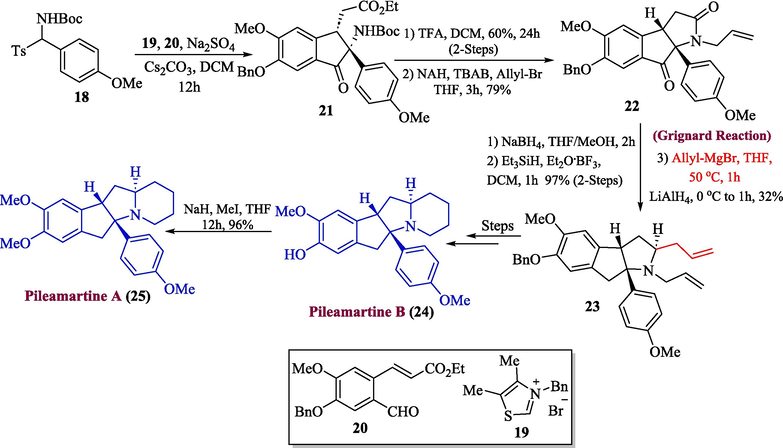
- Synthesis of pileamartine alkaloids 24 and 25 from Grignard reaction.
2.3 Morphinan alkaloids
Dihydroetorphine and buprenorphine are important morphinan alkaloids that were originally synthesized in the 1960 s (Blakemore and White, 2002). In addition to developing appealing synthetic targets, their complex structures serve as the ideal building blocks for the development of novel drugs with increased effectivity (Trost and Tang, 2002). Buprenorphine has demonstrated excellent results in the treatment of opioid misuse due to its competitive antagonist activity at κ-receptor and partial agonist activity at µ-receptors. It is a well-known and effective substitute for methadone (Olson et al., 2018). In 2022, Tang et al. synthesized dihydroetorphine 33 and buprenorphine 31 (Tang et al., 2022). The synthesis of compounds 33 and 31 was initiated by the formation of intermediate 26 which underwent cycloaddition with methyl vinyl ketone 27 to give compound 28 in 92 % yield followed by double bond hydrogenation to afford compound 29 in 92 % yield. Grignard reaction of compound 29 t-BuMgCl was carried out in the presence of toluene for the introduction of the tert-butyl group. This resulted in compound 30 in 55 % yield along with an unexplained by-product (23 % yield) which arose from the stereospecific reduction of the keto group of compound 29 and ultimately gave buprenorphine 31 after a few steps. The steric hindrance originating from ethylene linkage (C6 to C14) was hypothesized to be the cause of the stereoselective addition of Grignard reagent. In this scenario, the t-Bu group was selectively attached to the less-hindered re-face of a carbonyl group. Similarly, the synthesis of dihydroetorphine 33 also commenced from intermediate 26. The stereoselective addition of propylmagnesium chloride to compound 29 gave compound 32 in 66 % yield. The tosyl group in compound 32 was then removed by using LiAlH4 followed by reductive amination in the presence of paraformaldehyde and o-methylation by treating tert-dodecanethiol and EtONa to afford dihydroetorphine 33 in 85 % yield (Scheme 4).
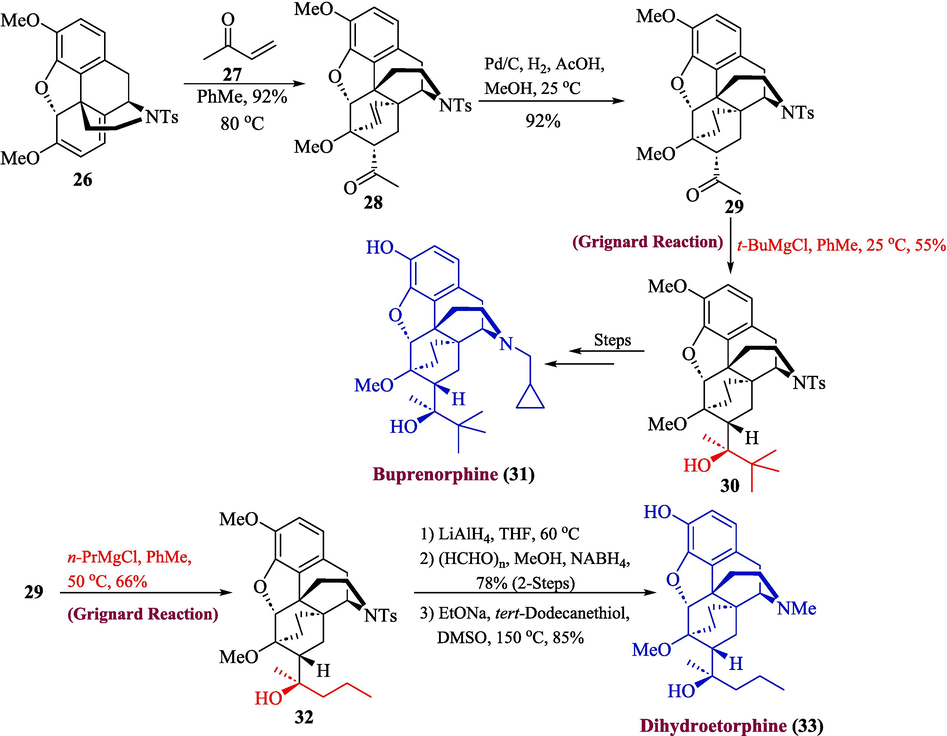
- Synthesis of dihydroetorphine 33 and buprenorphine alkaloids 31.
2.4 Cylindricine alkaloids
The tricyclic alkaloids known as cylindricines were isolated from the ascidian Clavelina cylindrica and are found on the east coast of Tasmania. (Hoye and Sizova, 2009; Wong, 2018). Huang et al. in 2022 proposed an enantioselective total synthesis of cylindricines (Huang et al., 2022). The synthesis of 2-epi-cylindricine D 43 and (+)-cylindricine D 42 was initiated by the reaction of (S)-N-benzylpyroglutaminol methyl ether 35 (synthesized from (S)-N-benzylpyroglutaminol 34) with Grignard reagent 36 followed by deprotection with sodium bis(trimethylsilyl)amide) to furnish derivative prolinol 37 along with their diastereomer with 75 % yield (dr = 7:1). The Liotta’s method was employed to get compound 38 in 96 % yield which underwent catalytic hydrogenolysis in the presence of Boc2O and Pearlman’s catalyst to afford compound 39 with 93 % yield. In the next step, ynone 40 was obtained in 94 % yield by the reaction of alkyny lithium (produced by the reaction of n-BuLi with a terminal alkyne) with Weinreb amide 39. Compound 40 was smoothly treated with a Linder catalyst (under hydrogen atmosphere) followed by the reaction with trifluoroacetic acid (TFA) and potassium carbonate in the presence of methanol, which yielded compound 41 in 88 % yield with diastereoselectivity (1:1). In the last step, compound 41 was reacted with mesityl chloride and potassium tert-butoxide to get 2-epi-cylindricine D 43 and (+)-cylindricine D 42 (40 % yield for each). Similarly, the synthesis of (+)-cylindricine C and (+)-cylindricine E was started from benzyl ether 44 (synthesized from 34) which was reacted with Grignard reagent 36 to afford proline 45 with 80 % yield (dr ≥ 20:1). Further treatment of compound 45 with isopropyl magnesium chloride and tetrahydrofuran gave Weinreb amide 46 in 96 % yield which upon treatment with (Boc)2O furnished carbamate 47 in 96 % yield. The ynone 48 was formed in 88 % yield by the alkynylation of compound 47 which gave compound 49 (94 % yield) over a few steps. The compound 49 underwent O-debenzylation in the presence of Pd/C/H2 and AcOH/MeOH/H2O to furnish 2-epi-cylindricine C 51 in 95 % yield and (+)-cylindricine C 50 in 41 % yield for each. Acetylation of compounds 51 and 50 gave 2-epi-cylindricine E 53 and (+)-cylindricine E 52 in 95 % and 97 % yield respectively (Scheme 5).
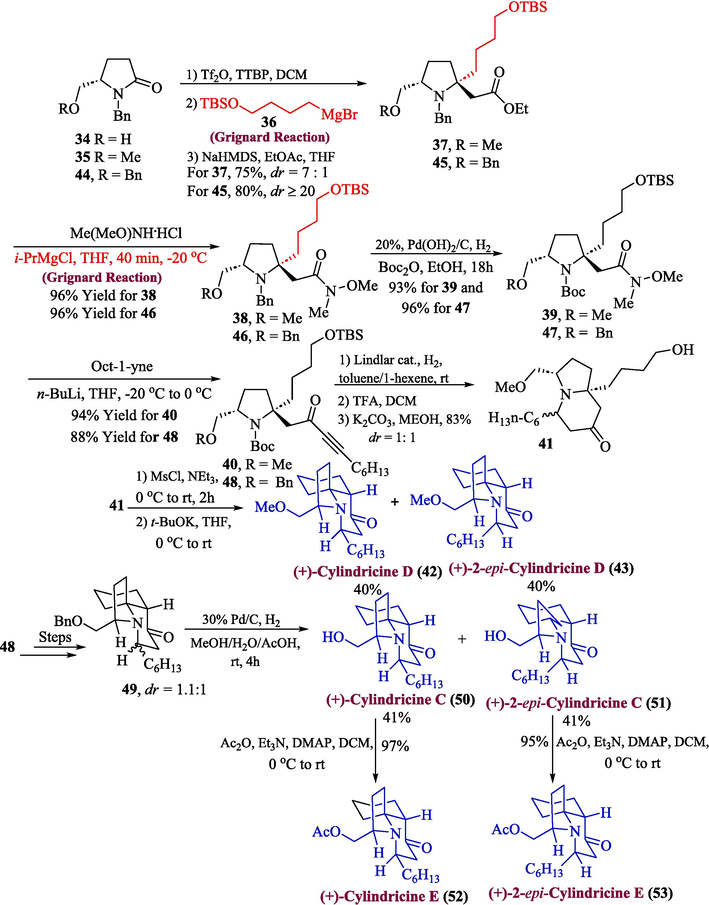
- Synthesis of 2-epi-cylindricine D 43, (+)-cylindricine D 42 and cylindricine 50–53 from Grignard reaction.
2.5 Pyrrolizidine alkaloids
One of the noteworthy six adjacent stereocenters containing pyrrolizidine alkaloid is (+)-casuarine, which was first reported in 1994 by Nash et al. and it was extracted from Casuarina equisetifolia L (Nash et al., 1994). There have been numerous publications that infer the glycosidase inhibitory action potential of natural casuarines (uniflorine A and casuarine). The Casuarine 61 has attracted the attention of synthetic chemists because of its unique structure and biological activity. Myeong et al. in 2022 reported a total synthesis of (+)-casuarine 61 by utilizing the Grignard reagent (Myeong, 2022). To achieve this, the substituted pyrrolidine 54 was reacted with osmium tetrachloride to get products 55 and 56 (87 % yield at 0 °C, 74 % yield at 40 °C respectively). In the next step, the secondary benzoyl group of compound 55 was deprotected to further react with pivaloyl chloride, and secondary diol 57 was obtained with a 93 % yield. The primary alcohol 58 was synthesized in 83 % yield by MOM protection and pivaloyl deprotection of diol 57. Compound 58 then underwent oxidation using Dess-Martin periodinane (DMP) in the subsequent step followed by reaction with vinyl Grignard reagent (chelation controlled) to furnish compound 59 (74 % yield), which produced the sixth stereocenter with the required stereochemistry. A few more steps led to the formation of compound 60, which, upon deprotection, produced (+)-casuarine 61 in 89 % yield (Scheme 6).
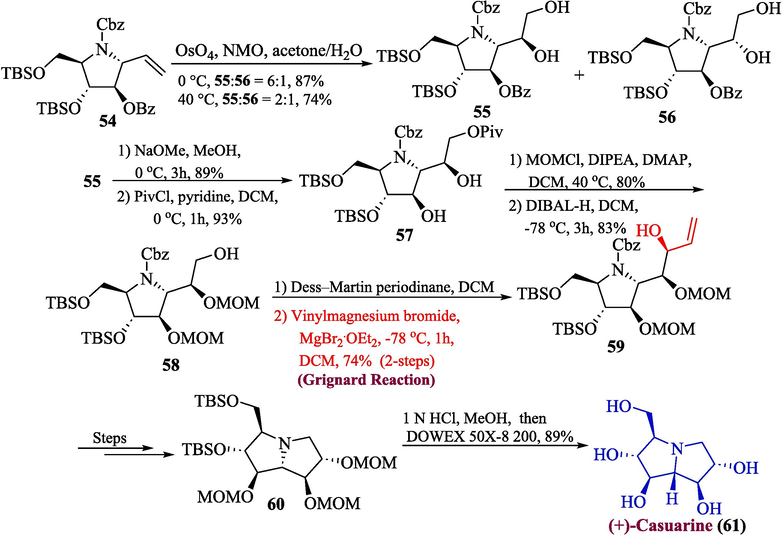
- Synthesis of (+)-casuarine 61 alkaloid.
2.6 Voacafricines A and B alkaloids
One of the important alkaloid-based natural products is voacafricines A and B that has been isolated from the African tree fruit Voacanga Africana and were utilized to cure bacterial infections (Ding et al., 2018). voacafricines A and B exhibit strong inhibitory action against Salmonella typhi and Staphylococcus aureus higher than two antibacterial drugs (fibrauretine and berberine) (Soysal, et al., 2022). Andres et al. in 2023 proposed the asymmetric total synthesis of voacafricines A 71 and B 72 by using the Grignard reagent in one of their key steps (Andres et al., 2023). To achieve this, compound 62 was subjected to react with bicyclic lactone 63 in the presence of Grignard reagent and Me2S.CuBr to get alcohol 64 which was transformed into ether 65 in a 92 % yield without the need for purification. After being transformed into benzoate 66, molecule 64 was discovered to have an enantiomeric excess of 98 %. The following stage produced compound 67 in 92 % yield by hydrolyzing, oxidizing, and esterifying dimethyl acetal 65. Subsequently, compound 67 was deprotected and then reacted with Davis oxaziridine to produce α-hydroxy ester. This ester was then oxidized with DMP to produce α-keto ester 68 in 58 % yield. The compound 68 was then subjected to Pictet-Spengler reaction in the presence of substituted tryptamine 69a/b to obtain diastereomers 70a/b (dr 1:1) in 95 % yield. The resulting diastereomers were then treated over few steps to afford voacafricines A 71 and B 72 in overall good yield respectively (Scheme 7).
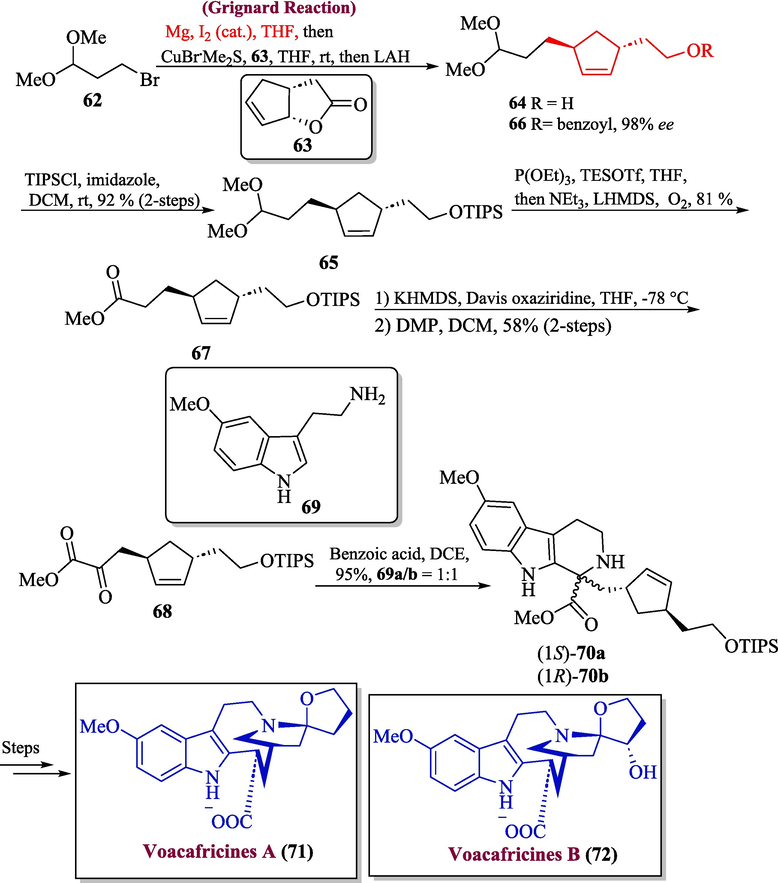
- Synthesis of voacafricines A and B alkaloids via Grignard reaction.
2.7 Zoanthamine alkaloids
A well-known marine natural product is zoanthamine alkaloid, which demonstrates the potential to be used in the development of new drugs (Altmann, 2017). This natural product has garnered a lot of interest as a potential anti-osteoporotic medication candidate. The chemical and biological characteristics of norzoanthamine are still not completely understood because of its limited concentration in aquatic habitats and complicated heptacyclic structure (Guillen et al., 2020). In 2023, Chen et al., proposed the total synthesis of norzoanthamine (Chen et al., 2023). The synthesis commenced from the reaction of chiral ketone 73 with formaldehyde and DBU to afford compound 74 in 62 % yield, which was transformed into aldehyde 75) via a few steps. Aldehyde 75 was further converted into ester 77 (with an overall 65 % yield) via a two-step process that included the acetylation with Ac2O after nucleophilic addition of Grignard reagent 76. Next, compound 78 was formed from ester 77 by treating it via several steps, which upon subsequent treatment with anhydrous sodium sulfate and acetic acid furnished norzoanthamine 79 in 30 % yield (over two steps) (Scheme 8).
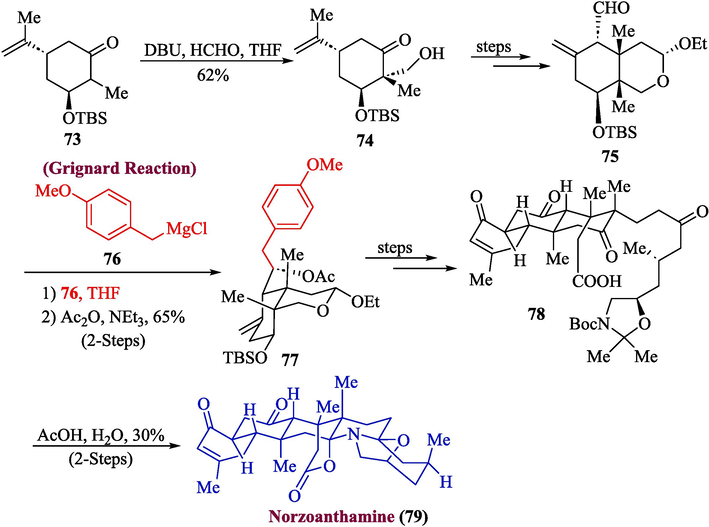
- Synthesis of norzoanthamine 79 from Grignard reaction.
2.8 Lissoclin C, haploscleridamine and villagorgin alkaloids
Villagorgin A and B are alkaloids-based natural products that were extracted from Villagorgia rubra while haploscleridamine was found to be very potent against cathepsin IC50 = 26 µM (Searle and Molinski, 1994). Lissoclin C is also an alkaloid-based natural product that was obtained from Lissoclinum sp. and it has been also found to be very effective against Candida albicans (Patil et al., 2002). In 2023, Roy et al. reported the total synthesis of lissoclin C 89, haploscleridamine 90, and villagorgin, in which Grignard reagent was utilized for the synthesis of 89 and 90 (Roy et al., 2023). The methodology involved the reaction of methyl ester 80 with tosyl chloride followed by the reaction with allyl bromide 81 in the presence of potassium carbonate to give 82 in 92 % yield. In the next step, compound 82 was subjected to a reduction in the presence of DIBAL-H followed by the addition of Grignard reagent 83 to afford allylic alcohol 84 as a single diastereomer in 77 % yield. X-ray crystallography was used to assess the relative stereochemistry of this compound and the obtained results were consistent with a Feldin-Ahn model where the polar group was opposed to the incoming nucleophile, and after a few steps, compound 85 was formed with good yield (85 %). Next, compound 85 was reacted with 86 in the presence of p-toluene sulfonic acid followed by reduction with Mg in methanol to successfully deliver 87 in 52 % yield. Similarly, Compound 85 was also treated with 88 under similar conditions to give a mixture of 89 and 87 with an overall good yield. Next, villagorgin A 90 was also synthesized from compound 87 by using several steps involving synthetic strategy (Scheme 9).
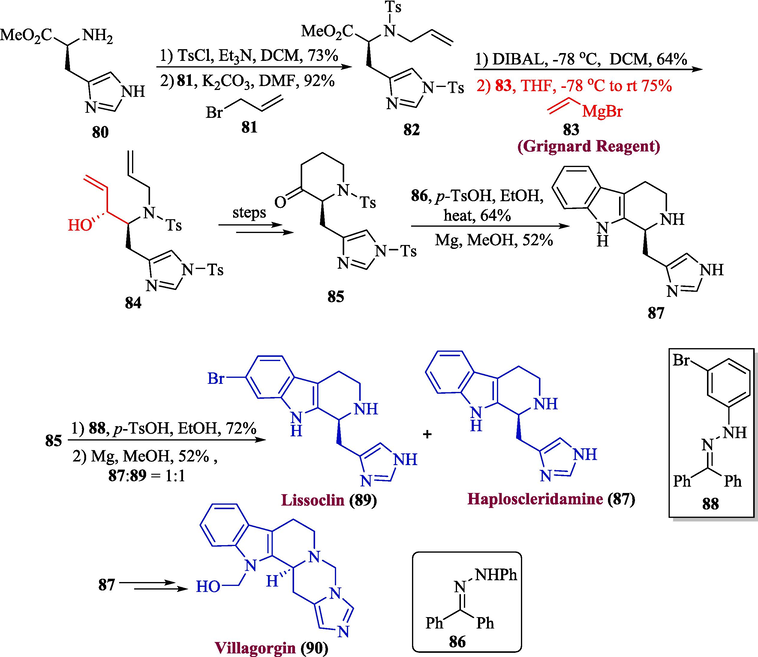
- Synthesis of lissoclin C 89 and haploscleridamine 87 and villagorgin 90 alkaloids.
2.9 (–)-Normacusine B alkaloids
One area of interest in synthetic chemistry is the preparation of monoterpenoid indole alkaloids, which have complex structures and exhibit potent biological activities (Rosales et al., 2020; Lounasmaa et al., 1999). There have been many attempts in the past to synthesize these natural products, including the total synthesis of asparagine and related alkaloids, which are primarily found in plants of the Apocynaceae family and consist of over 100 different members. These alkaloids have demonstrated diverse pharmacological effects such as anti-inflammatory, anti-hypertension, and anti-cancer properties (Namjoshi and Cook, 2016; Shahzadi et al., 2020). In 2022, Zhu et al. proposed an efficient method for the synthesis of (−)-normacusine B by utilizing Grignard reagent as one of the key steps (Zhu et al., 2022). The first step of the synthesis involved the condensation of compound 91 with dimethoxybenzylamine to get amide 92 in 65 % yield followed by zinc-catalyzed reduction and tosyl protection in sequence to furnish sulfonamide 93 with an overall 65 % yield over two steps. The next step involved the irradiation of compound 93 with acrolein 94 in the presence of potassium bicarbonate and Ir(dtbbpy)(ppy)2PF6 followed by Wittig olefination to afford lactam 95 in 81 % yield. Compound 95 then underwent titanium-mediated coupling in the presence of Grignard reagent 96 to generate low-valent titanium species followed by oxidation to furnish compound 97 with a 52 % yield. In the next step, the silyl protection of compound 97 gave compound 98 which underwent Barton deoxygenation to afford compound 99 in 75 % yield. Further, compound 99 was treated with trifluoroacetic acid followed by alkylation with 1-bromo-2-iodobute-2-ene 100 to procure vinyl iodide 101 in 55 % yield (2-steps), which was further transformed into (–)-normacusine B 102 after few steps (Scheme 10).
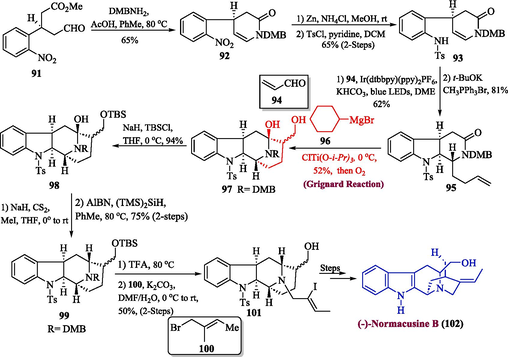
- Synthesis of (−)-normacusine B 102 alkaloids via Grignard reaction.
3 Terpenoids based natural products
3.1 Toxicodenanes terpenoids
A dried resin, Resina Toxicodendri is extracted from a Chinese lacquer tree and is a well-known Chinese traditional medicine, used to cure different diseases such as stomach and gastric cancer. One of the important sesquiterpenoids is toxicodenane, which is also extracted from Resina Toxicodendri. Different studies showed that toxicodenanes B and C greatly reduce the overproduction of fibronectin, brought on by high glucose (He et al., 2013). In this context, one of the crucial steps proposed by Qin et al. in 2022 was the entire synthesis of toxicodenane A and epi-toxicodenane A by utilizing the Grignard reagent (Qin et al., 2022). For this purpose, dimedone 103 was methylated by using MeI followed by its reaction with 1,3-dioxolane 104 to obtain compound 105 in 68 % yield which was further reacted with DIBAL-H to afford substituted cyclohexanones 106. In the next step, compound 106 interacted well with Grignard Reagent 107 to give major anti-product 108, which was correlated with the formation of a small amount of regio-isomer 109 in total 90 % yield (ca 8:1). The stereochemistry of compound 108 was verified by transforming 108 and 109 mixtures into appropriate separable pivaloyl protected derivative 110 as a major product in 73 % yield (2-Steps from 106). The synthesized derivative 110 was further transformed into (±)-toxicodenane 111 in moderate yield (Scheme 11).
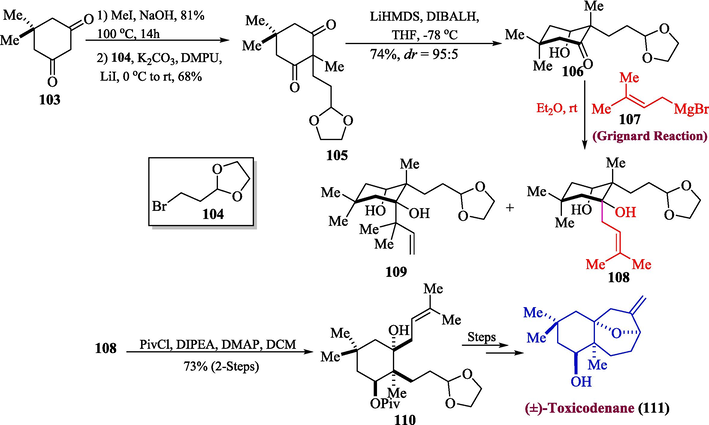
- Synthesis of (±)-toxicodenane 111 via Grignard reaction.
The enantioselective total synthesis of compound 119 was also performed. The synthesis was initiated by treating compound 112 and substituted amine 114 in a reaction mediated by organo-catalyst 113 to obtain compound 115 in 96 % yield with 92 % ee. The compound 115 was further treated with Grignard reagent 116 to obtain product 117 (81 % yield) which was made to react with the pivaloyl group to afford an ester 118 with 99 % enantiomeric excess. The resulting ester was converted into (+)-toxicodenane A 119 after several steps (Scheme 12).
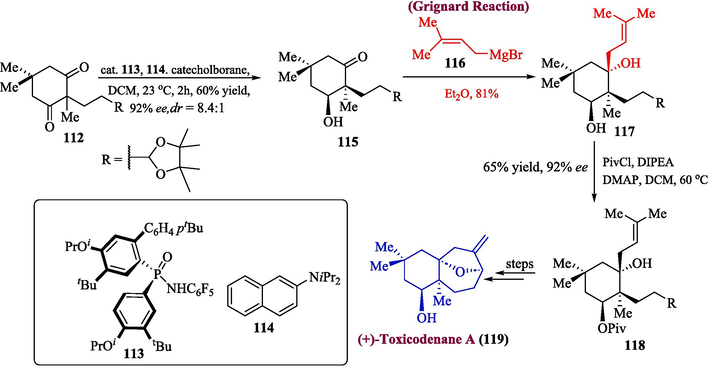
- Synthesis of (+)-toxicodenane A 119.
3.2 Illisimonin A terpenoids
The illicium plants are widely recognized as a significant source of sesquiterpenoids, which are renowned for both their highly oxidized, polycyclic frameworks and their powerful neurotrophic effects (Lane et al., 1952). A note-worthy sesquiterpenoid is illisimonin A, which has been extracted from the fleshy parts of Illicium simonsii and they are found to be potent against neurodegenerative diseases (Ma et al., 2017). In 2023, Etling et al. reported the total synthesis of illisimonin A by utilizing the Grignard reagent in one of their key steps (Etling et al., 2023). Their synthetic scheme involved the reaction of propargylic alcohol 120 with Ni-catalyst by utilizing Liu’s method to afford compound 121 in 61 % yield which (after silylation) was reduced to aldehyde. The aldehyde then underwent treatment with isopropenyl lithium to attain precursor 122 which gave compound 123 in good yield (81 %), via several steps involving a synthetic route. In the next step, oxasilolane 123 was reacted with Grignard reagent to produce TMS-epoxide 24 in 76 % yield followed by TES deprotection and in sequence reduction to get diol 125 in 84 % yield. The diol 125 was then protected with Ph2SiCl2, followed by a reaction with compound 126 under Boran’s conditions to give compound 127 (22 % yield) was then transformed into illisimonin A 128 in poor yield. (Scheme 13).
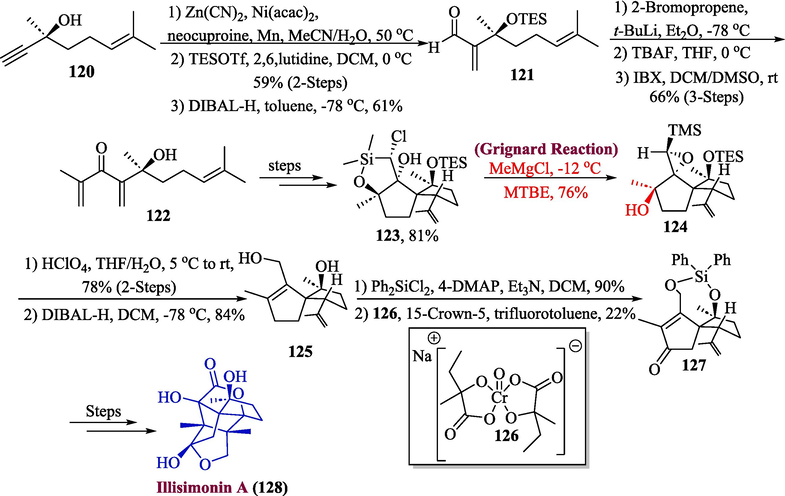
- Synthesis of illisimonin A 128 through Grignard reaction.
3.3 Fornicin D terpenoids
For over a thousand years, East Asian conventional medicine has employed mushrooms from the genus Ganoderma fornicatum in the treatment of a range of maladies and persistent illnesses such as asthma, cancer and hypertension etc. Among these monoterpenoids, an important natural product is fornicin D, which was extracted from Ganoderma cochlear (Niu et al., 2006; Yajima et al., 2014). In 2023, Bunt et al. planned the total synthesis of fornicin D, fornicin A, and danodercin D, in which fornicin D was produced using the Grignard reagent as one of the key steps (Bunt et al., 2023). Using the MeMgBr, compound 129 was reacted to yield 130, which was made to react with another Grignard reagent to furnish compound 131. In the next step, compound 133 (synthesized from 132) underwent an aldol reaction with compound 131 in the presence of LiHMDS to produce 134 in 56 % yield which was later converted to fornicin D 135 via several steps (Scheme 14).
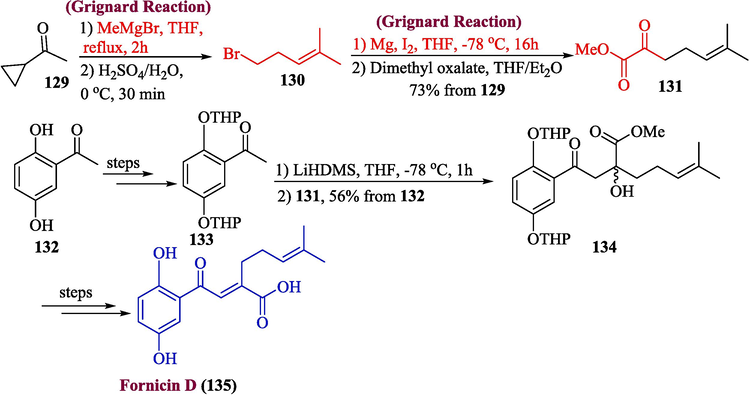
- Synthesis of fornicin D 135.
3.4 Wickerol A and B terpenoids
Omura and coworkers in 2009, described the tetracyclic compound wickerol A, which was extracted from the fungal strain Trichoderma atroviride (Omura et al., 2006; Sun et al., 2011). Chung and coworkers in 2023, developed an effective method for producing biologically active diterpenoids wickerols B and A by using Grignard reagent as one of the significant steps (Chung et al., 2023). The synthesis was initiated by the reaction of compound 136 with catalyst Rh(I)/R-BINAP to obtain 137, which further underwent reaction with TBSCl in the presence of N-methylimidazole to afford 138 in 60 % yield over three steps. Next, Grignard reagent 139 was subjected to conjugate addition with 138, affording product 140 with good diastereoselectivity. Both HMPA (hexamethylphosphoramide) and TMSCl (trimethylsilyl chloride) were required for low-temperature reactivity as well as for high stereochemical control. Hydrindenone 141 was produced in 72 % yield by intramolecular aldol condensation and acid-mediated acetal cleavage over two steps. Compound 141 was further treated with Grignard reagent 142 to get a mixture of 143 and 144, which resulted in the synthesis of compound 145, after a few steps. In the next step, compound 145 was subjected to Gui’s conditions to furnish compound 146 and wickerol B 147 in 45 % yields. In the last step, Barton-McCombie conditions were employed to afford wickerol A 148 in moderate yield (30 %) (Scheme 15).
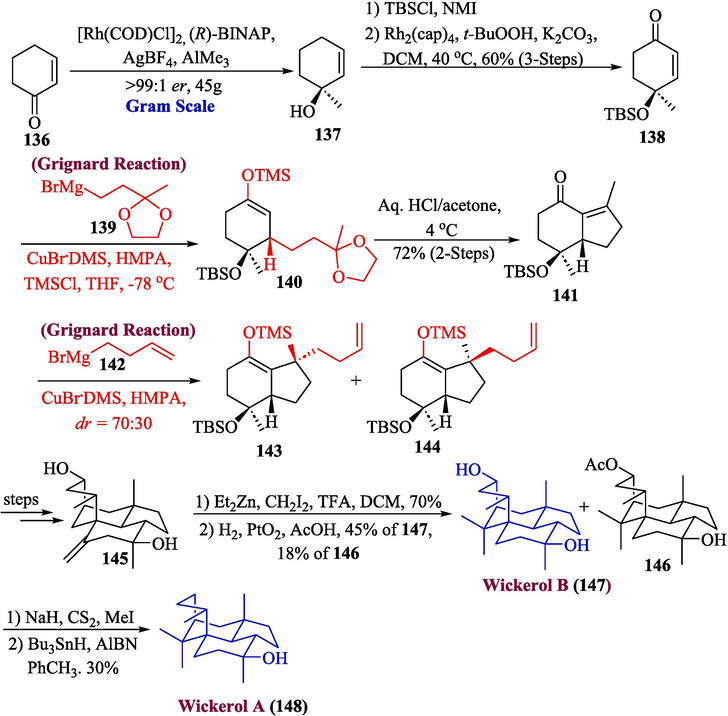
- Synthesis of wickerol B 147 and A 148.
4 Polyketide-Based natural products
4.1 Plakortone Q
The plakortone Q is a polyketide-based natural product that was extracted from the marine sponge Plakortis simplex. This unique polyketide-based natural product owns 4 stereogenic centers sequentially present in tetrahydrofuran moiety (Seibert et al., 2007; Sohn and Oh, 2007). In 2022, Okazaki et al. reported the total synthesis of plakortone Q by utilizing the Grignard reagent as a key step (Okazaki et al., 2022). For this purpose, compound 150 (synthesized from Roche ester 149 was reacted with ethyl magnesium bromide (Grignard Reagent) followed by oxidation, thereby yielding ketone 151 in three steps (57 % yield). The ketone 151 was then subjected to HWE reaction followed by reduction with DIBAL-H to furnish compound 152 in 82 % yield. In the next step, the Katsuki Sharpless epoxidation of compound 152 was performed followed by Parikh Doering oxidation to afford aldehyde 153 in 82 % yield. The aldehyde 153 was again treated with EtMgBr (Grignard reagent) followed by another Parikh-Doering oxidation to acquire compound 154 (48 % yield) and 155 (24 % yield). After a few steps, compounds 154 was observed to generate 156 and 157 in 72 % and 27 % yield respectively. In the next step, the oxidation of compound 156 took place to afford aldehyde followed by Wittig homologation, hydroboration, and oxidation by using NaBO3·4H2O to obtain alcohol 157. In the last step, the compound 157 was transformed into carboxylic acid by using 1-methyl-2-azaadamantane N-oxyl (1-Me-AZADO) in catalytic amounts along with NaClO4/NaOCl as co-oxidants. This reaction was proceeded by an esterification reaction utilizing a catalytic amount of 10-camphorsulfonic acid (CSA) to afford plakortone Q 158 in 82 % yield (Scheme 16).
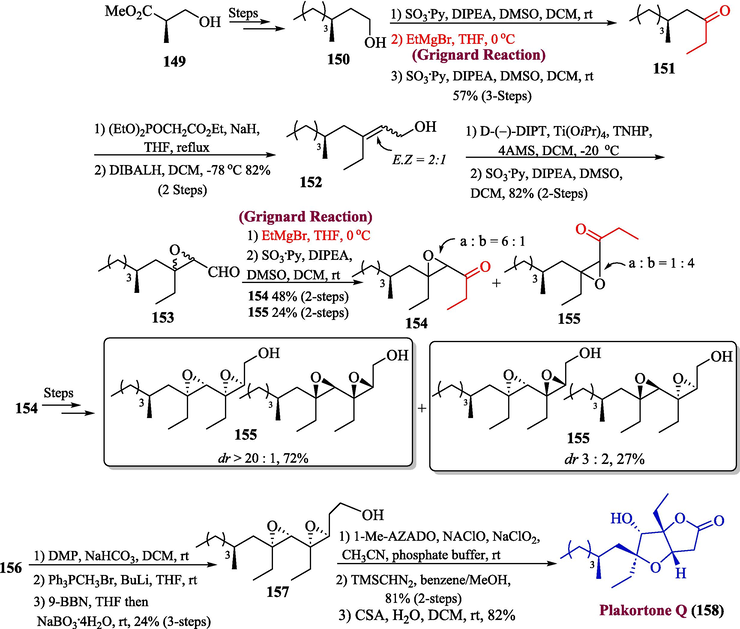
- Synthesis of plakortone Q 158 by virtue of Grignard reaction.
4.2 Prorocentin
Prorocentin is a polyketide-based natural product that was obtained from the P. lima species of the northern Taiwan coast and they are found to be potent against two types of human cancer cells (Domínguez et al., 2014; Anderl et al., 2018). Zachmann and coworkers in 2023, proposed the total synthesis of prorocentin by utilizing the Grignard reagent in one of important steps (Zachmann et al., 2023). The commercially available compound 159 was initially subjected to Krische allylation in the presence of the provided catalyst and ligand 160, resulting in alcohol 161 with 96 % ee (enantiomeric excess). Subsequently, cobalt catalyst 162 was added, yielding a single isomer 163 with a 72 % yield. The primary alcohol 163 was then transformed into Weinreb amide 164 followed by the addition of Grignard reagent to furnish enone 165 in good yield. Next, the compound 165 was reduced by using Luche conditions, followed by reaction with ligand 166 that produced diol 167 in 76 % yield. The next step involved the treatment of diol 167 with NaH and imidazole 168 followed by Grignard 169 addition to yield 170 and after a few steps, compound 171 was formed in good yield. Subsequently, compound 172 (synthesized from D-glucose) underwent Sonogashira coupling in the presence of 171 to create 173. A few steps later, the intended prorocentin 174 was synthesized with a modest yield (50 %) (Scheme 17).
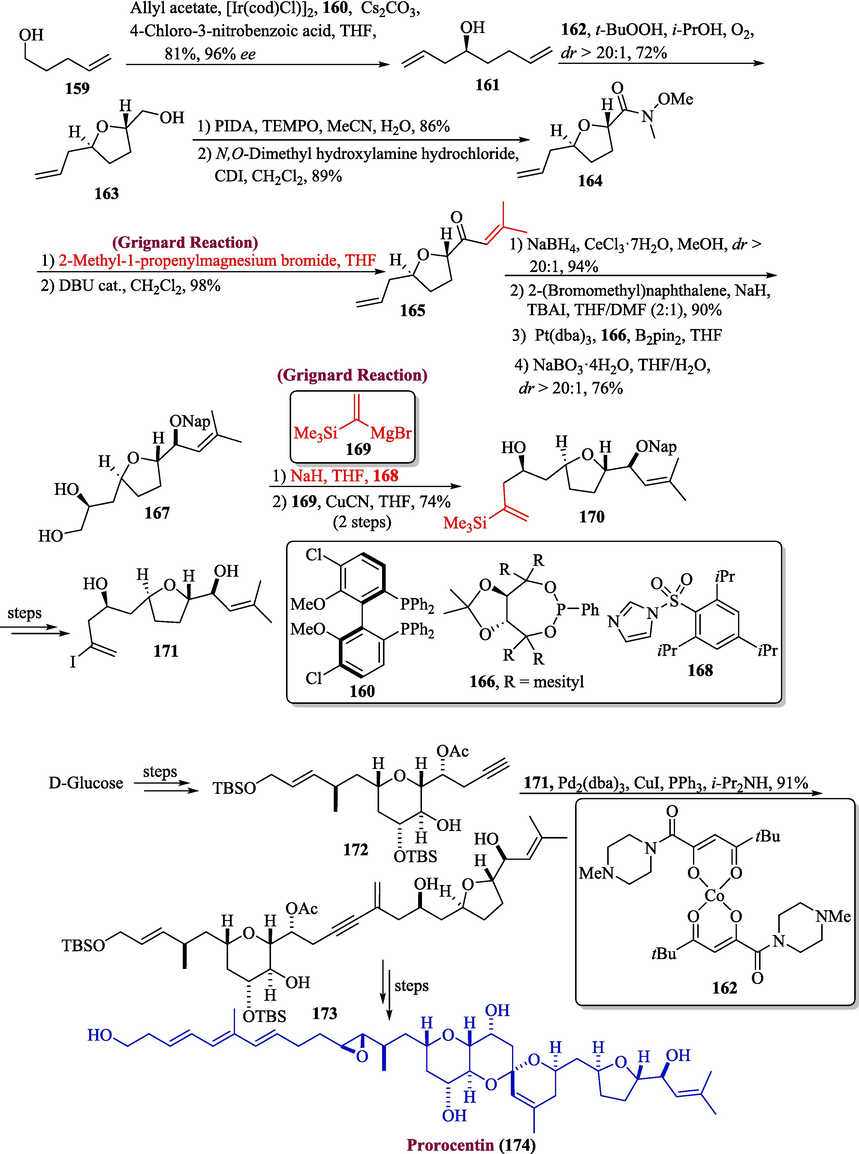
- Synthesis of prorocentin 174 via Grignard reaction.
4.3 Sanctolide A
The sanctolide A is a polyketide-based macrolide that was first isolated from Oscillatoria sancta cyanobacteria. The initial studies revealed that this natural product showed toxicity in brine shrimp (Shou et al., 2016; Kang et al., 2012). Dissanayake and colleagues presented the total synthesis of sanctolide A in 2023 by utilizing the Grignard reagent in one of their key steps (Dissanayake et al., 2023). The synthesis was initiated by the reaction of diepoxide 175 with Grignard reagent 176 to afford 177 in 70 % yield followed by tripodal coupling in the presence of lithium alkoxide and POCl3 to get 178 in 72 % yield, which got transformed in compound 179 after a few steps. In addition to this, compound 179 was reacted with 180 in the presence of DCM to afford 181 (51 % yield), which was reacted with 182 and 183 in sequence to deliver 184 in 67 % yield, which later resulted in the synthesis of sanctolide A 185 over several steps (Scheme 18).

- Synthesis of sanctolide A 201 through Grignard reaction.
4.4 Salimabromide
Salimabromide is an important natural product that was obtained from Enhygromyxa/Plesiocystis and research has shown it to be a powerful inhibitor of Arthrobacter crystallopoietes. However, as a consequence of its exceedingly low natural abundance, further assessment regarding its biological activities was restricted (Herrmann et al., 2017; Fudou et al., 2001; Felder et al., 2013). Lu and coworkers in 2022, proposed a concise method for the synthesis of salimabromide (Lu et al., 2022). For this purpose, the cycloheptadienone 186 was initially reacted with Grignard reagent 187 followed by reaction with compound 188 in the presence of Lewis acid to obtain compound 189 in 60 % yield along with small amount of compound 190 (15 % yield) which was later transformed into 189. The compound 189 was then treated with methyl lithium along with PCC in the presence of Al2O3 to furnish substituted cycloheptenone 191 in 83 % yield. In the next step, compound 191 was reacted with iron catalyst to get product 192 with 28 % yield and after a few steps, compound 193 was formed which underwent dibromination utilizing Bronsted acid as catalyst to afford salimabromide 194 in 61 % yield (Scheme 19).
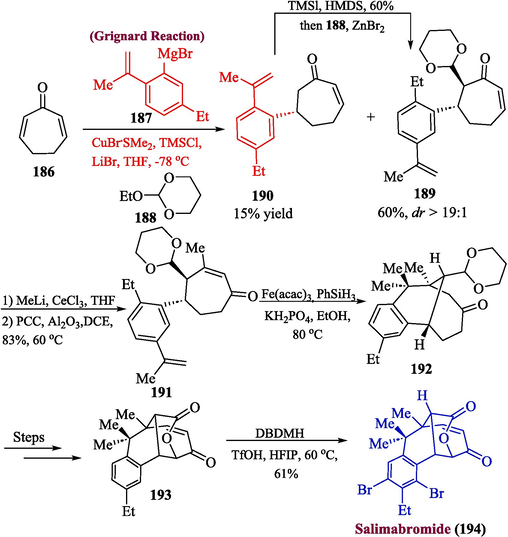
- Synthesis of salimabromide 194 via Grignard reaction.
5 Macrolide-based natural products
5.1 Aspergillide D
The polyhydroxylated macrolides have attracted the attention of many synthetic chemists because of their fascinating structure and biological potential, which includes the ability to inhibit the biosynthesis of cholesterol, and antibacterial and antimalarial activity (Zahoor et al., 2017). One of the important polyhydroxylated macrolides is aspergillide D, which was extracted from the Gorgonian fungal strain (Aspergillus sp.) (Atanasov et al., 2015; Newman and Cragg, 2016). In 2022, Kumari et al. proposed an efficient and facile method for the synthesis of aspergillide D. (Kumari et al., 2022). The synthesis was initiated by reaction of compound 196 (synthesized from 195) with TBS-Cl followed by treatment with Grignard reagent and MPMBr to produce ether 197 in 86 % yield. In the next step, allylic alcohol 198 was formed by ozonolysis, Wittig olefination, and DIBAL-H reduction in 85 % yield. The Sharpless oxidation of compound 198 was achieved by reacting it with cumene hydroperoxide, Ti(OiPr)4, and (+)-DIPT that furnished 199, and after a few steps, compound 200 was synthesized which was desilylated with TBAF to furnish compound 201 in 93 % yield. In the last step, the Yamaguchi conditions were used to achieve compound 202 in 67 % yield followed by deprotection using DDQ to obtain aspergillide D 203 in 86 % yield (Scheme 20).
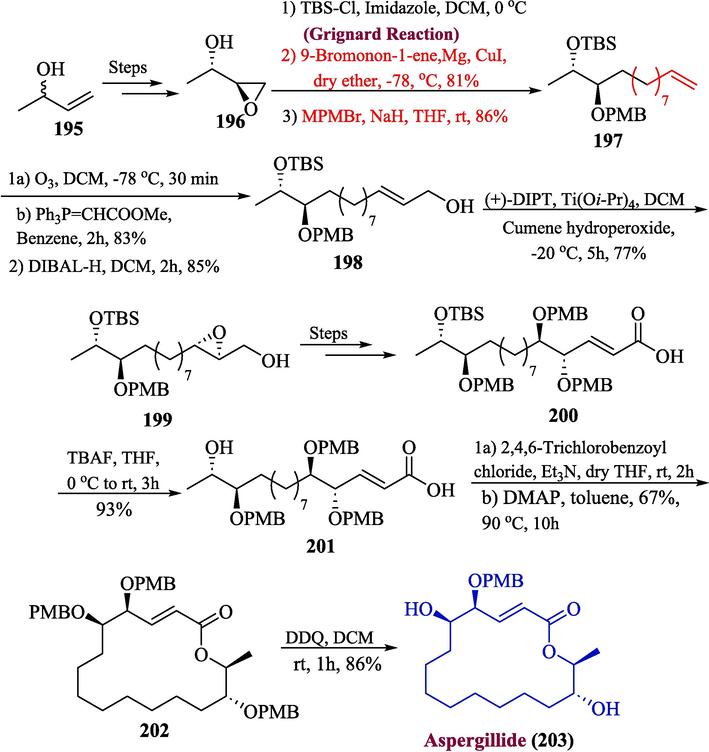
- Synthesis of aspergillide D 203 by Grignard reaction.
5.2 Pandangolide
In recent years, several naturally existing macrolides with different ring diameters have been identified via fungal metabolites. One of the noteworthy naturally occurring macrolides is 12-membered pandangolide, which was obtained from fungi broth Cladosporium sp. Later reports showed pandangolide was also isolated from Lambertella brunneola and Cladosporium oxysporum fungus (Kobayashi and Tsuda, 2004; Gesner et al., 2005). In 2023, Nimmareddy et al. proposed the total synthesis of pandangolide by utilizing the Grignard reagent as a key step (Nimmareddy et al., 2023). For this purpose, aldehyde 204 was reacted with vinyl-magnesium bromide to form allylic alcohol 205 with 84 % yield. In the next step, the compound 205 underwent Swern oxidation followed by a reaction with ceric ammonium nitrate and 1,3-propanedithiol to afford thioacetal 206 in 74 % yield. The compound 206 was subjected to Sharpless asymmetric dihydroxylation by reaction with AD-mix-β to furnish diol 207 which upon tosylation gave 208 in 88 % yield. Next, compound 208 was immediately exposed to potassium carbonate in the presence of methanol to yield epoxide 209 (81 % yield) and after a few steps, carboxylic acid 210 was synthesized. In addition to this, the compound 210 underwent a Yamaguchi reaction followed by a reaction with alcohol 211 to get ester 212 with a 78 % yield. In the last step, the deprotection of ester took place in the presence of I2 and CaCO3 followed by ring-closing reaction using Grubbs catalyst to yield lactone 213, which upon reduction and debenzylation with Pd/C afforded desired compound 214 in 88 % yield (Scheme 21).
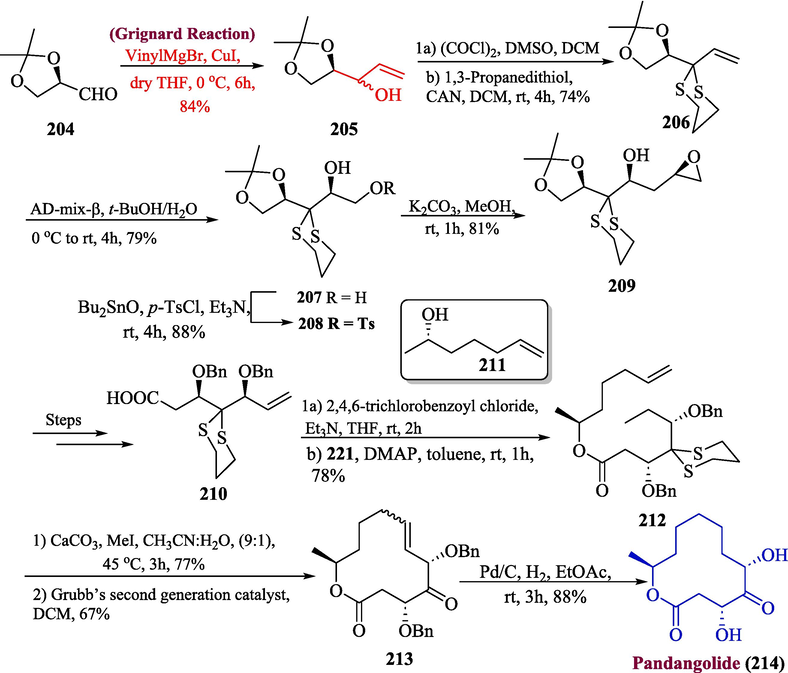
- Synthesis of pandangolide 214 via Grignard reaction.
6 Peptide-based natural products
6.1 (−)-Negamycin
An unusual antibiotic negamycin was extracted from strains of Streptomyces purpeofuscus. The negamycin has potent inhibitory effects against both Gram-negative and Gram-positive bacteria such as Klebsiella pneumonia and Pseudomonas aeruginosa (Hamada et al., 1970; Kondo et al., 1971). Lin and Tseng, proposed the total synthesis of (–)-negamycin (Lin and Tseng, 2023) The first step of this synthesis involved the reaction of epoxide 215 with vinyl magnesium chloride to obtain alcohol 216 in 88 % yield, which was transformed into the compound 217 and 218 over few steps. The compound 218 was further subjected to Mitsunobu reaction followed by oxidative cleavage using OsO4 to furnish diazide acid 219 with 77 % yield, which was treated over few steps to afford (–)-negamycin 220 (Scheme 22).
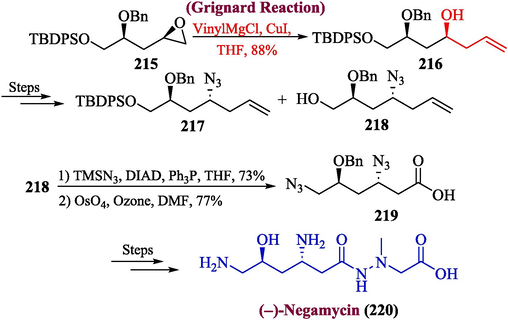
- Synthesis of (−)-negamycin 220.
7 Miscellaneous
7.1 Penicyclone A
Penicyclone A is a spiro lactone-containing naturally occurring substance that has been obtained from the ocean depths. Penicyclone A is a derivative of ambuic acid, that has traditionally been quite a synthetic target alongside structurally similar dimeric torreyanic acid and jesterone. A novel six-membered spiro-lactone and a heavily substituted cyclohexanone backbone are both present in penicyclone A. (Guo et al., 2015; Quintavalla, 2018; Talebi et al., 2019) In 2022, Talajic et al. proposed the total synthesis of penicyclone A by utilizing Girgnard reagent in one of the key steps (Talajic et al., 2022). The synthesis of penicyclone was initiated by a double Grignard reaction of compound 221 with compound 222 and allyl magnesium bromide 223 to afford diastereoselective tertiary alcohol 224, followed by deprotection to obtain compound 225 in 57 % yield. In the next step, compound 225 was treated with PIDA/TEMPO catalyst along with Dess-Martin periodinane to attain compound 226 in 97 % yield, which was then transformed to compound 228 in the presence of compound 227 by using Julia − Kociensky reaction and after a few steps, compound 229 was obtained which was treated with trifluoroacetic acid to afford penicyclone A 230 in excellent yield (81 %) (Scheme 23).
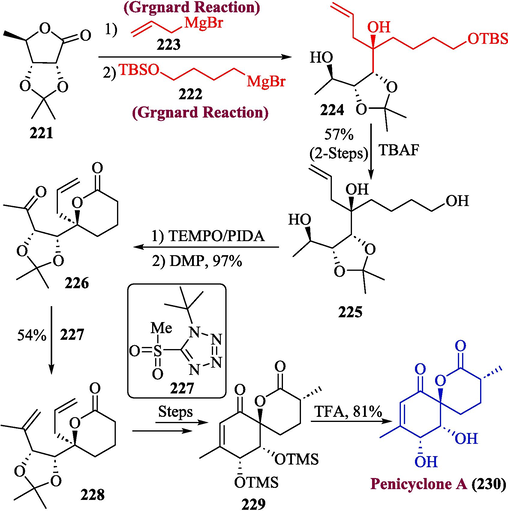
- Synthesis of penicyclone A 230 via Grignard reaction.
7.2 Thelepamide
Thelepamide is a rare amino acid that was obtained from the Thelepus crispus (marine annelid worm). It features an unusual oxazolidinone ring made of valine, glycine, and cysteine. The oxazolidinone ring seems to be a desirable synthetic candidate due to its scarcity in nature and its medicinal potential. (Cai et al., 2017; Shigezane et al., 1971) In 2022, Ashida et al. proposed a new method for the synthesis of hydoxyoxazolidinone 233 by utilizing Grignard reagent in one of the significant steps (Ashida et al., 2022). In the first step, an amide 231 bearing oxalic acid was reacted with an excess amount of paraformaldehyde to afford 232 in 72 % yield, followed by the reaction with Grignard reagent in THF to afford hydoxyoxazolidinone 233 in 84 % yield (Scheme 24).
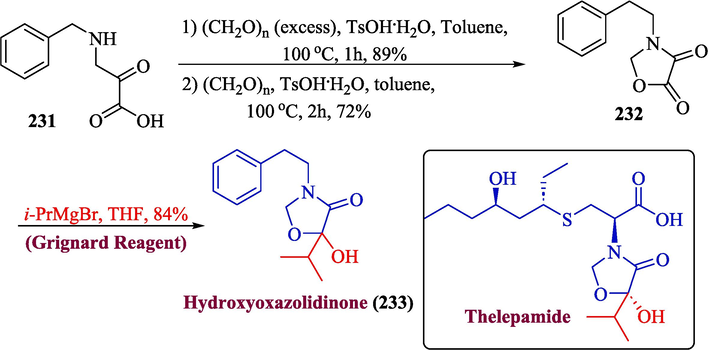
- Synthesis of hydoxyoxazolidinone 233.
7.3 Prostaglandins
One of the best-known prominent natural products that have served humanity for about a century is prostaglandins (PGs). They are common hormone-like lipid molecules comprising 20 carbon atoms that have been found in both animals and humans (Kurzrok and Lieb, 1930). They have distinctive compositions and a variety of biological properties, such as vasodilation, inflammatory responses, blood coagulation, and reproductive processes (Das et al., 2007; Dams et al., 2013; Funk, 2001) In 2022, Yi et al. proposed the total synthesis of TBS-based prostaglandin ether 244 by using Grignard reagent in one of the pivotal steps (Yi et al., 2022). In the first step, the gram scale synthesis compound 234 was carried out to afford alcohol 235 in 95 % (96 % ee) which was transformed into ketone precursor 236 over few steps (87 % yield). In the next step, compound 236 was reacted with Sml2 in the presence of THF to get product 237 (58 % yield) along with compounds 238 (11 % yield) and 239 (<5% yield) as side products. The compound 237 was further transformed into keto-lactone 240 via a few steps, followed by the addition of vinyl magnesium bromide along with dehydration of alcohol to afford diene 241 in 64 % yield. In addition to this, the compound 241 was treated with alkene 242 in the presence of Grubbs II catalyst to yield lactone 243. In the last step, the compound 243 was reduced using DIBAL-H along with the addition of phosphonium salt 244, followed by oxidation using Dess Martin to afford target molecule 245 in 78 % yield (Scheme 25).

- Synthesis of prostaglandin 245 via Grignard reaction.
7.4 Millpuline B
The unsubstituted benzofuran acts as a key component in many biologically active molecules and natural products (Simonetti et al., 2013; Radadiya and Shah, 2015). They show significant biological activities such as anti-microbial (Faiz et al., 2019), antioxidant, and cytotoxicity, etc. (Rindhe et al., 2010; Rida et al., 2006; Bovicelli et al., 2016). One of the important benzofuran-based natural products is millpuline B, which was extracted from Millettia pulchra (Benth) leaves. Recent research on this molecule has revealed that this molecule shows great inhibitory effects (Li et al., 2018). In 2022, Dong et al. proposed the total synthesis of mellipuline B by using Grignard reagent in one of their significant steps (Dong et al., 2022). For this purpose, compound 247 (formed from 246 in multiple steps) was allowed to react with phenethyl Grignard reagent to obtain secondary alcohol 248 in 89 % yield which was treated with CH3I to get a racemic mixture of millpuline B 249 in 86 % yield. The compound 248 was further oxidized with DMP to afford ketone 250 (85 % yield) which was reduced by using the Corey-Bakshi-Shibata (Haroon et al., 2023) (CBS) reaction to yield compounds 251 and 252 in 71 % and 77 % yield respectively. In the last step, the methylation of 251 and 252 isomers was achieved by DMF and CH3I to afford millpuline B 253 and enantiomer 254 (Scheme 26).
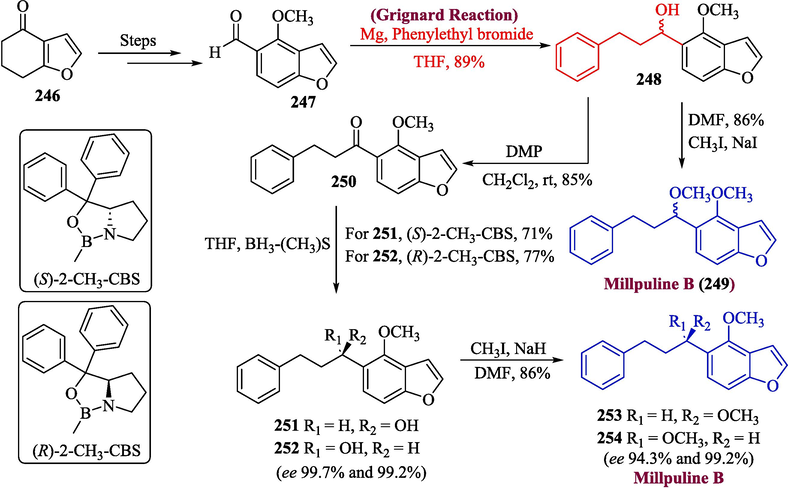
- Synthesis of millpuline B 249 & 253.
7.5 Phomonol synthesis
One of the important tetrahydropyran moieties containing natural products is phomonol, isolated from Phomopsis sp. fungal strain which was procured from Kandelia candel, a mangrove species. Structurally, phomonol contains four stereo-genic centers, for this reason, their synthesis has remained a challenge for the synthetic chemist. (Searle and Molinski, 1995; Li et al., 2010). In 2022, Dada et al. proposed the total synthesis of pheromonal utilizing Grignard reagent as a key step (Dada and Yaragorla, 2022). The synthesis of phomonol 265 was initiated by diazotization of D-Aspartic acid 255 along with bromination to get (R)-bromosuccinic acid 256. After a few steps, epoxide (Ahmad et al., 2018) 257 was formed, which was treated with vinyl magnesium bromide alongside tert-butyldimethylsilyl chloride to obtain silyl ether 258 in 68 % yield. In the next step, syn-diol 259 in 73 % yield was formed by ozonolysis, Wittig olefination, and Sharpless dihydroxylation of compound 258 which after a few steps, got transformed into Weinreb amide 260 in 78 % yield. Further, the allylation of compound 260 with allyl magnesium bromide was performed to furnish allylic ketone 261 in 83 % yield, followed by the deprotection of the silyl group with a trace amount of p-TSA along with reductive etherification to afford syn-pyran 262 in 61 % yield over two steps. In addition to this, acetonide 263 was formed by treating compound 262 with 2,2-DMP in 52 % yield. In the last step, compound 263 was reacted with methyl magnesium bromide alongside Li2CuCl4 followed by an acidic workup to afford phomonol 264 in 82 % yield (Scheme 27).
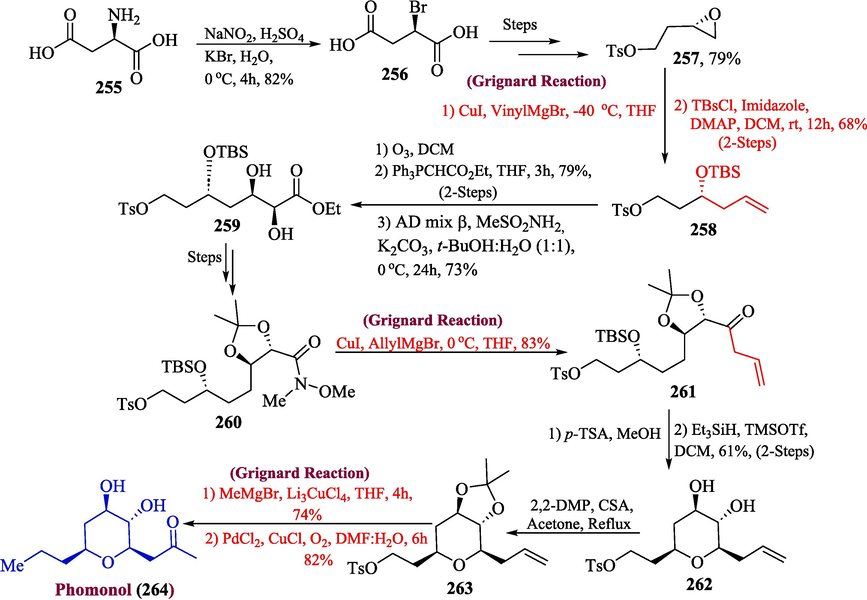
- Synthesis of phomonol Synthesis 264 via Grignard reagent.
7.6 Protulactone A
One of the important naturally existing bicyclic lactones is protulactone A, extracted from the marine fungus Aspergillus sp. This naturally occurring lactone has an intriguing furanofuranone structure, produced by fungi. This secondary metabolite has found ample significance in the medical field. A large number of efforts have been made by synthetic chemists to develop their synthetic routes. (Sohn and Oh, 2010; Blázquez et al., 1999; Fang et al., 1990) Despite previously developed commercial synthetic methodologies, Djokic et al. proposed the total synthesis of protulactone A (Djokić et al., 2022). In the first step, ring-closing reaction of furanofuranone 265 was carried out by using the Koll procedure and Meldrum’s acid which gave two products 266 and 267 while the use of t-BuNH2 yielded only one compound 266 with 54 % yield. Compound 266 was further reacted with 2,2-DMP in the presence of p-TsOH to get compound 268 in 94 % yield followed by the hydroxyl protection in the presence of BnBr and Ag2O to afford products 269 (85 % yield) and 270 (2 % yield) in major and minor amounts respectively. The treatment of compound 269 with Grignard reagent in the presence of toluene gave two products 271 (13 % yield), and 272 (7 % yield), and a trace amount of compound 273 (3 % yield) upon dehydration of the unreacted starting compound. In the last step, compounds 271 272, and 273 were reacted separately with acetyl chloride in the presence of DMAP and acetonitrile to get protulactone A 274, protulactone epimer 275, and protulactone analog 276 respectively with 92, 88 and 89 % yields (Scheme 28).
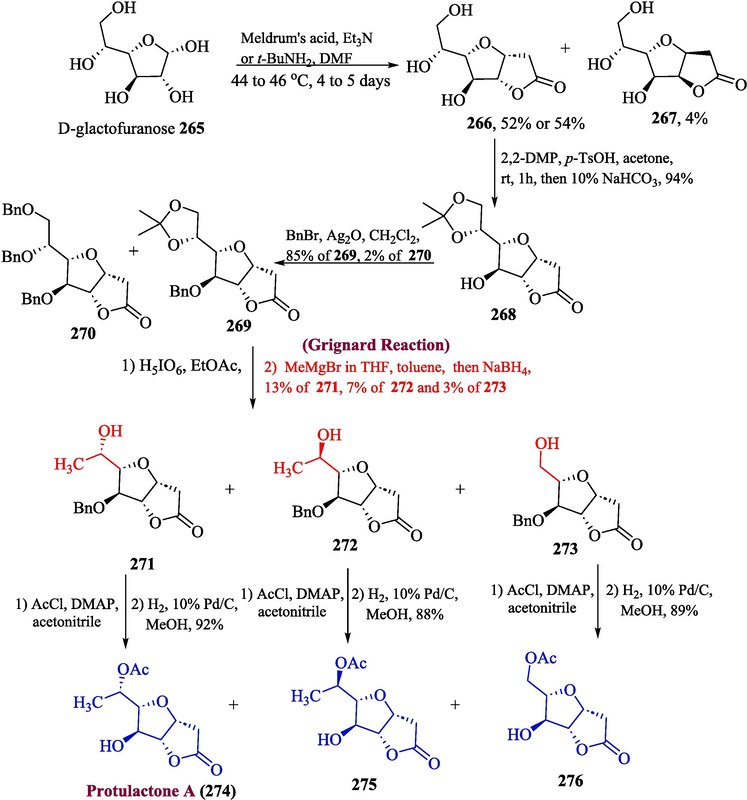
- Synthesis of protulactone A 274 via Grignard reaction.
7.7 Patulone
Patulone was derived from Hypericum sampsonii and Hypericum patulum blocks the release of PGE2 as well as inhibits PAF-induced hypotension in mice (Ishiguro et al., 1997; Nazeer et al., 2023; Drašar and Moravcova, 2004). The distinctive structure of this chemical has also received significant attention from chemotaxonomic and biogenetic disciplines. In 2022, Kobayashi et al. proposed the total synthesis of patulone by using Grignard reagent in one of the key steps (Kobayashi et al., 2023). The synthesis was initiated by reacting substituted benzene derivatives 277 and 278 to get compound 279 via few steps, followed by the addition of Grignard reagent 280 to obtain xanthenol 281 in 99 % yield. The substituted prenylxanthone 282 was formed in 92 % yield by the addition of 18-crown-6 and KN(SiMe3)2. In the next step, compound 282 was treated with Grignard reagent 283 to yield compound 284, which was treated with KN(SiMe3)2 to acquire compound 285 and 286. Next, the treatment of compound 285 with sulfuric acid and ethanol gave tomentanone 287, which was further reacted with NaN(SiMe3) and O2 to afford patulone 288 in 54 % yield (Scheme 29).
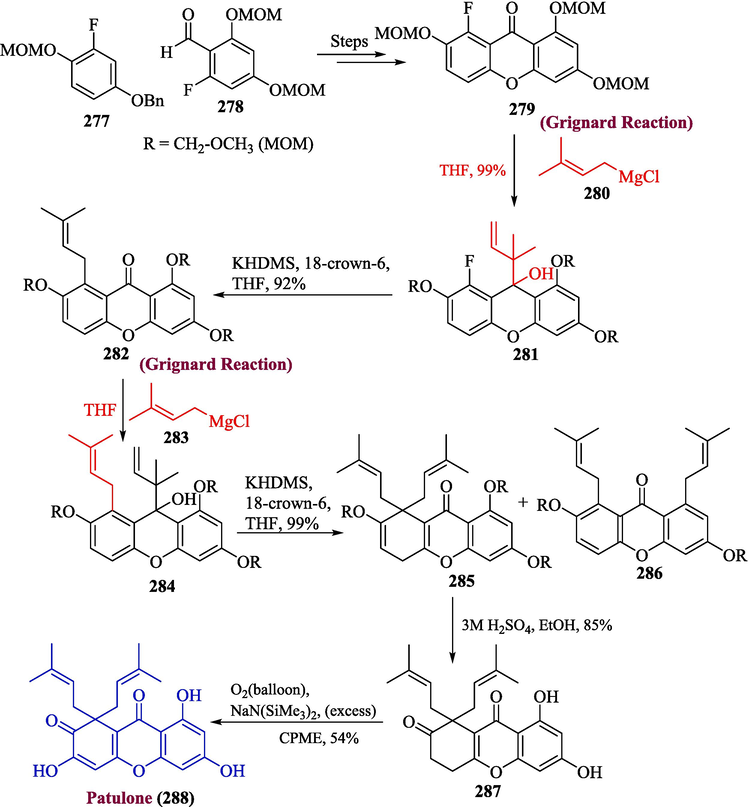
- Synthesis of patulone 288 through Grignard reaction.
7.8 Mollanol A
Grayanoids, which are found in plants, belonging to the Ericaceae family, have significant biological features such as anticancer (Akhtar et al., 2019), insecticidal, analgesic, and antibacterial effects. mollanol A, a significant member of the grayanoid, was discovered in the Rhododendron molle. According to many studies, mollanol had impacts on the Xbp1 upstream promoters in intestinal epithelial cell lines that activated transcription. According to the latest research revealing that the malfunction of transcriptional factors Xbp1 within ICEs might generate bowel inflammation. Such findings imply that mollanol A might also have promising clinical utility (Wang and Qin, 1997; Li et al., 2013; Li et al., 2017; Shahzadi et al., 2021; Li et al., 2019). Wang et al. in 2022 reported an efficient approach for the synthesis of mollanol A by employing Grignard Reagent in one of important steps (Wang et al., 2022). Their synthetic procedure commenced with the synthesis of precursors 290 and 293. For this purpose, the cyclopentanone 288 was treated with Grignard reagent to get compound 389 in 72 % yield (2 steps), which was further converted to precursor 290 over several steps. Similarly, the treatment of carboxylate 291 with 1-bromo-2-butyne in the presence of base followed by the addition of catalyst InCl3 to get compound 292, which was treated over several steps to furnish precursor 293. In the next step, the compounds 290 and 293 were allowed to couple in the presence of a catalyst (Pd(PPh3)4/CuCl) to get an in-separatable mixture of compounds 294 and 295. The mixture of compounds 294 and 295 was separated by using DMP with 64 % yield (2 steps). In the next step, an aldol reaction was performed by using 1,5,7-triazabicyclo-dec-5-ene (TBD) followed by the addition of pyridinium p-toluenesulfonate (PPTS) to furnish compound 296, which led towards the synthesis of mollanol A 297, by undergoing reaction via numerous steps (Scheme 30).
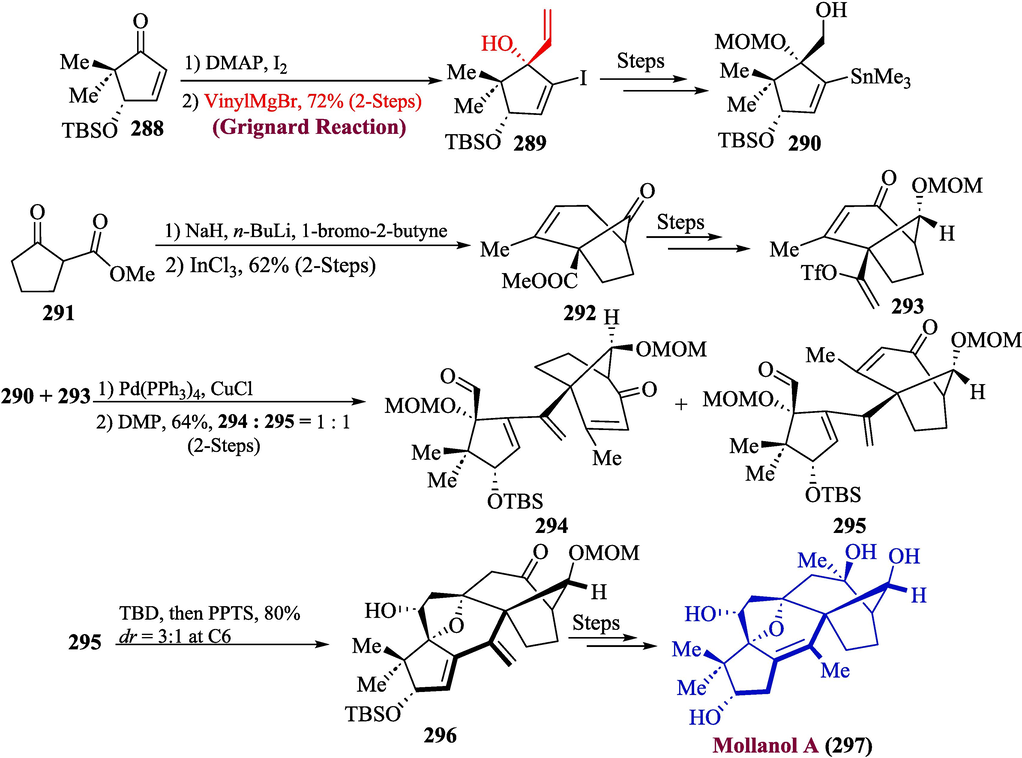
- Synthesis of mollanol A 297 by using Grignard reaction.
7.9 Kibdelomycin
A well-known natural product kibdelomycin was discovered in 2012 by a famous chemist Merk. It was discovered to be a powerful topoisomerase inhibitor and exhibits strong antimicrobial activity against different human diseases such as Acinetobacter baumannii (MIC90 = 0.125 μgmL−1, MIC50 = ≤ 0.015 μgmL−1) (Phillips et al., 2011). He et al. in 2022 proposed the total synthesis of kibdelomycin by using Grignard reagent in one of their key steps (He et al., 2022). In the first step, the Weinreb amide 298 was allowed to react with Grignard reagent 299 followed by CBS reduction and TBS protection to get compound 300 in 93 % yield, which was converted into precursor 301, over a few steps. After the successful synthesis of precursor 301, compound 302 was treated with chiral sulfinimine 303 to provide compound 304. In the next step, the compound 304 underwent silyl deprotection followed by a reaction with singlet oxygen, HCl in methanol and acylation with pyrrole 305 to furnish compound 306 which over few steps, resulted in silyl ether 307. The reaction of compound 307 with 301 in the presence of TfOH gave α 308 and β 309 isomers. In the next step, the deprotection of compound 309 was achieved by using LiHMDS, which was further reacted with S,S-dimethyl dithiocarbonate followed by the addition of silver trifluoroacetate in the presence of compound 311 (synthesized from 310) followed by treatment with MeOH/Et3N TBAF to form β-ketoamide intermediate 312. The resulting intermediate was immediately treated with formic acid (CH3CN/H2O) to afford epi-kibdelomycin 313 in 78 % yield (Scheme 31).
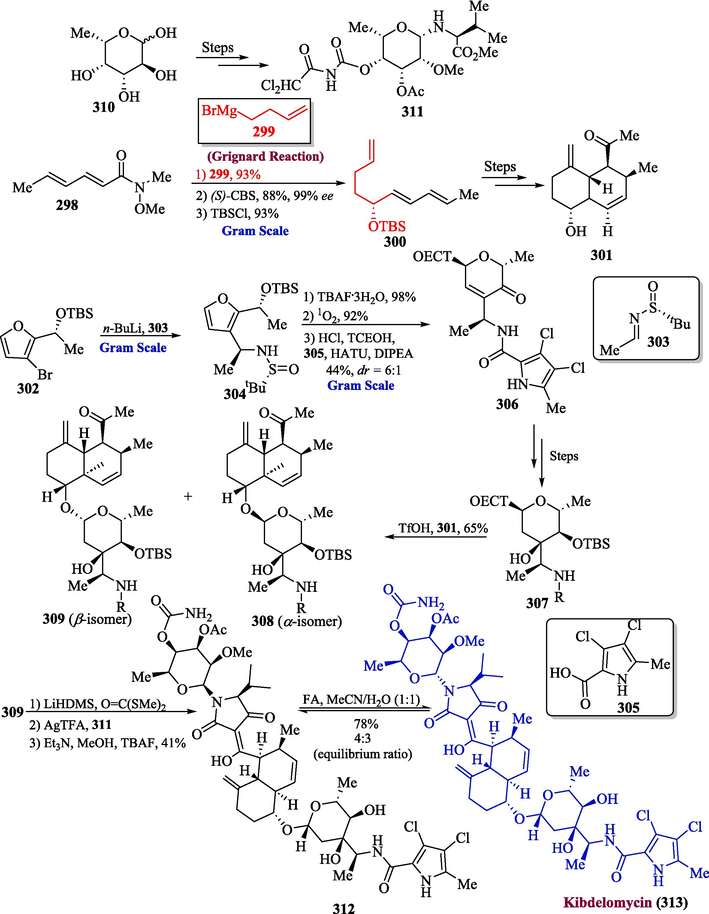
- Synthesis of kibdelomycin 313 from Grignard reaction.
7.10 Diarylheptanoids
The diarylheptanoids, which include yakuchione B, oxyphyllacinol, and yakuchione A, are significant natural products that have been discovered to be essential parts of the zingiberaceae family, which is a significant Chinese traditional medicine with noteworthy biological effects. Several attempts have been made towards the synthesis of yakuchione B, oxyphyllacinol, and yakuchione A but the use of an expensive substance makes this process unsuitable for large-scale synthesis (Park et al., 2021; Hassan et al.,). In 2022, Shi et al. reported an efficient and facile method for the total synthesis of yakuchione B, oxyphyllacinol, and yakuchione A (Shi et al., 2022). In the first step, compounds 316 (derived from vanillin 315) and 318 (synthesized from 317) were allowed to interact with potassium tert-butoxide and tetrahydrofuran to form Weinreb amide 319 in 96 % yield. In the next step, compound 319 underwent hydrogenation in the presence of H2 and Pd/C followed by desilylation in the presence of TBAF to furnish compound 320. The compound 320 was further reacted with compound 322 (synthesized from phenyl-butanol 321) in the presence of Grignard reagent to afford yakuchinone A 323 in 72 % yield, which was proceeded further for a reduction in the presence of NaBH4 to obtain oxyphyllacinol 324 with 87 % yield. In addition to this, compound 319 was also subjected to desilylation followed by a reaction with compound 322 in the presence of Grignard reagent to afford yakuchinone B 325 in 77 % yield (Scheme 32).
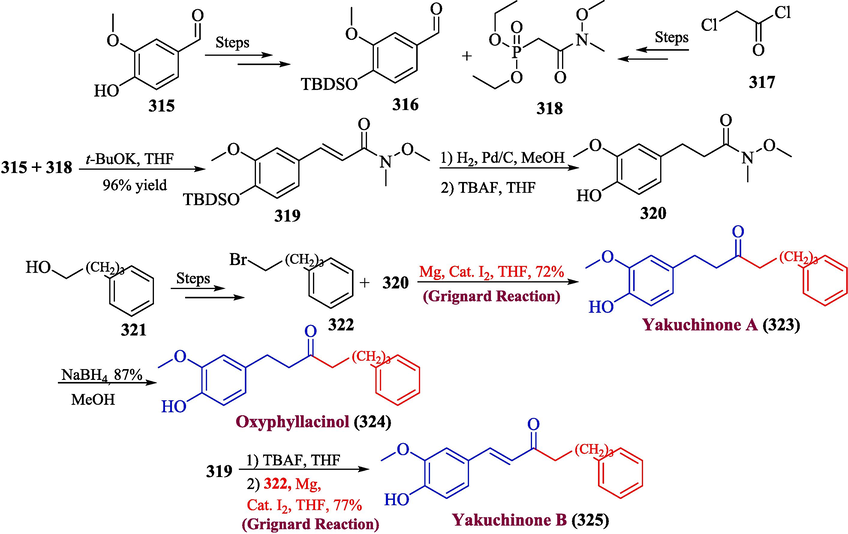
- Synthesis of diarylheptanoids 323, 324 & 325 by Grignard reaction.
7.11 Catellatolactams
The naturally occurring macrolactams known as ansamycins have an aliphatic chain connected to naphthalene or benzene ring by an amide bond. Catellatolactam, a recognizable member of this family, have been a challenge to the synthetic community because of their strong biological activity and distinctive architecture (Floss et al., 2011; Wang et al., 2023; Munir et al., 2023). Yang et al. in 2023, proposed a total synthesis of catellatolactams B and A (Yang et al., 2023). The synthesis of these unique macrolactams was initiated by the treatment of compound 326 with Grignard reagent 327 to get compound 328 in 93 % yield. The compound 328 was then reacted with 2,4-dimethoxybenzaldehyde 329 to provide 330 in 95 % yield, which over a few steps, gave 331. Similarly, catellatolactam B 332 was synthesized on the same pattern from compound 330 (Scheme 33).
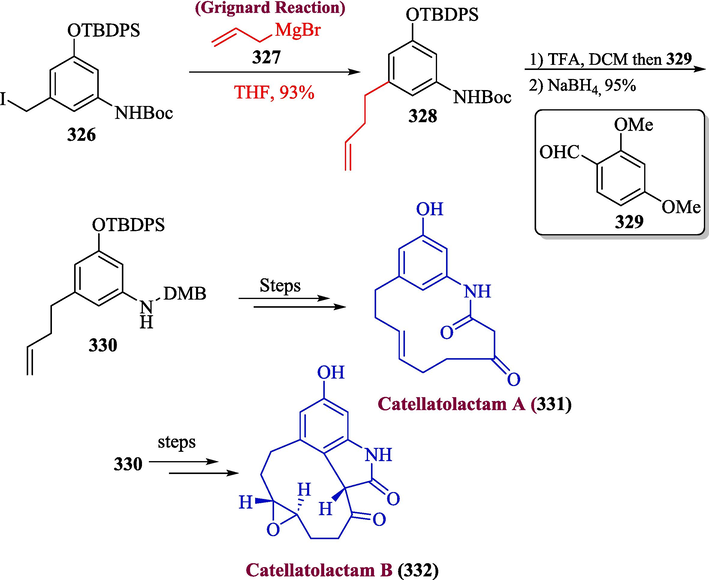
- Synthesis of catellatolactams A and B 331 & 332 via Grignard reaction.
7.12 Karnatakafuran B
Christophersen and colleagues isolated the karnatakafurans A and B coming from the previously unknown species Aspergillus karnatakaensis. They have been scientifically demonstrated to display in vitro mild antimalarial efficacy versus Plasmodium falciparum (Dixit et al., 2007). In 2023, Hieda et al. reported the total synthesis of bioactive karnatakafuran B natural product by using Grignard reagent in one of their important steps (Hieda et al., 2023). The synthesis involved the Stille coupling reaction of benzofuran 334 (synthesized from 2-methoxyacetophenone 333 in the presence of 2-ethoxyvinylstannane and PdCl2(PPh3)2 to provide substituted benzofuran 335 in 87 % yield. Next, the compound 335 was treated with Grignard reagent to yield alcohol 336 in 80 % yield, which was further reacted with t-BuOK along with MOM protection to give compound 337, which provided karnatakafuran B 338 by several steps involving synthetic pathway (Scheme 34).
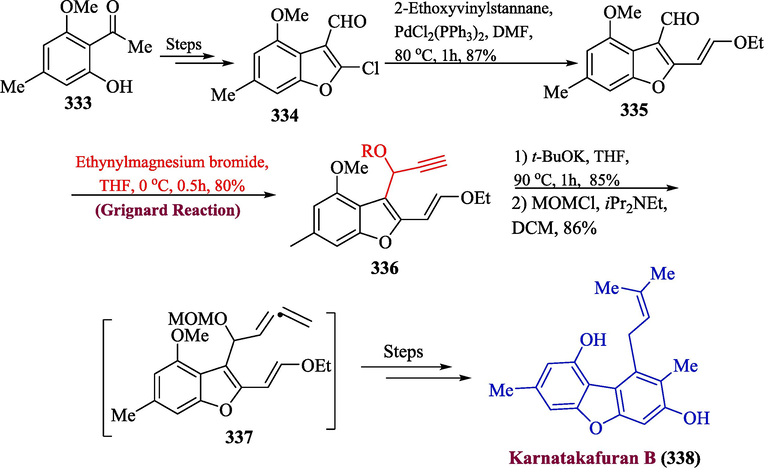
- Synthesis of karnatakafuran B 338 through Grignard reaction.
7.13 Nyasol
Nyasol is one of the noteworthy natural products that was isolated from A. cochinchinensis and Asparagus africanus (Tsui et al., 1996). In 2023, Kobayashi and co-workers proposed the total synthesis of nyasol by utilizing Grignard reagent in one of the key steps (Kobayashi et al., 2023). The methodology involved the reaction of phosphate 340 (synthesized from acid 339) with Grignard reagent 341 to furnish 342 in 70 % yield. In the next step, compound 342 was desilylated using TBAF to get 343 in 63 % yield, which was transformed into nyasol 344 by undergoing reaction over multiple steps (51 % yield, 94.5 % ee, 99 % es, 97 % cis) (Scheme 35).
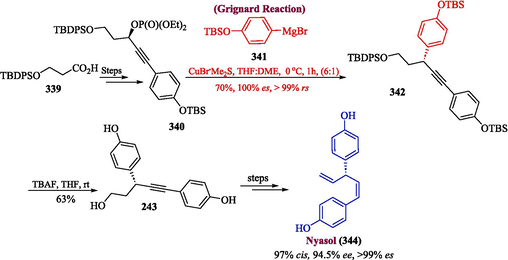
- Synthesis of nyasol 344 by Grignard reaction.
7.14 (+)-Petromyroxol
One of the noteworthy natural products is (+)-petromyroxol, which was extracted from Petromyzon marinus, a sea lamprey (Li et al., 2015). Fernandes and colleagues proposed an efficient and facile synthesis of (+)-petromyroxol in which the Grignard reagent was employed in one the significant steps (Fernandes and Gorve, 2023). The synthesis was initiated by reaction of compound 345 with palladium catalyst to get 346 in 96 % yield which was further protected with TBS to afford 347 in 86 % yield. In the next step, compound 347 was subjected to ozonolysis followed by Grignard addition to furnish diastereomers 348 and 349 in 49 and 16 % yield respectively, which ultimately afforded (+)-petromyroxol 350 in moderate yield, over few steps (Scheme 36).
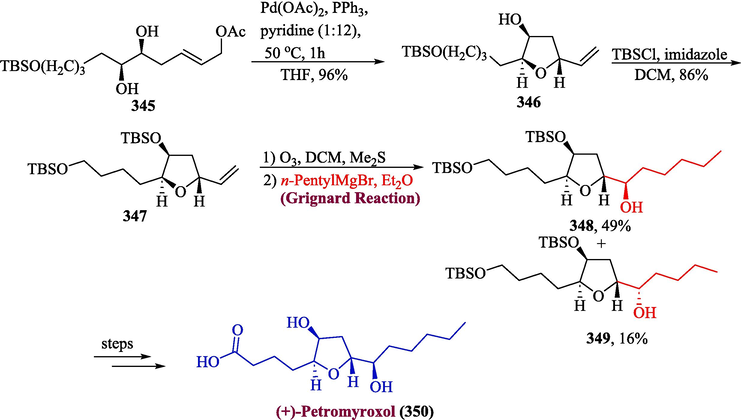
- Synthesis of (+)-petromyroxol 350 by Grignard reaction.
8 Conclusion
In conclusion, Grignard reagents have been established as potent and adaptable tools for natural product synthesis. Their exceptional reactivity, ability to form both C-C and C-X bonds, make them essential candidates for the construction of complex organic compounds. Grignard reagents have been used to successfully synthesize biologically active natural products such as polyketides, terpenoids, and amino acids. Recent advances in Grignard chemistry, such as their usage as reductants, trans-metalation reagents, and catalysts for metal-catalyzed cross-coupling processes, all of which help create complex organic compounds from simple building blocks. These breakthroughs have made possible the synthesis of new bioactive chemicals and broadened the scope of chemical creativity in a variety of scientific fields. The investigation of Grignard chemistry continues to offer significant possibilities for the synthesis of natural products and their analogs with potential medicinal uses. We are strongly hopeful that this review will urge researchers to unleash more innovative environmentally friendly processes and tackle hurdles in the production of complex compounds by further improving and enhancing the synthetic potential of Grignard reagents.
CRediT authorship contribution statement
Muhammad Haroon: Writing – original draft, Writing – review & editing. Sajjad Ahmad: Writing – original draft, Resources, Writing – review & editing. Ameer Fawad Zahoor: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Visualization, Investigation. Sadia Javed: Writing – review & editing, Formal analysis, Data curation. Mirza Nadeem Ahmad: Writing – review & editing, Formal analysis, Resources. Samreen Gul Khan: Data curation, Writing – original draft, Resources. Aamal A.Al-Mutairi: Data curation, Writing – review & editing, Funding acquisition. Ali Irfan: Validation, Formal analysis, Methodology, Resources, Project administration. Sami A.Al-Hussain: Funding acquisition, Data curation, Writing – review & editing. Magdi E.A.Zaki: Supervision, Resources, Funding acquisition, Project administration.
Acknowledgments
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (Grant number IMSIU-RG23013).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Design, synthesis and anticancer activity of new polycyclic: imidazole, thiazine, oxathiine, pyrrolo-quinoxaline and thienotriazolopyrimidine derivatives. Molecules. 2021;26(7):2031.
- [Google Scholar]
- Synthesis and antimicrobial activity of new triazines, tetrazines, thiazinoquinoxalines, thienotriazepine-imidazo [4, 5-b] quinolines from isatin derivatives. Polycycl. Aromat. Compd.. 2023;43(8):7073-7092.
- [Google Scholar]
- Recent trends in the ring opening of epoxides with sulfur nucleophiles. Mol. Divers.. 2018;22:191-205.
- [Google Scholar]
- Design, synthesis, in-silico study and anticancer potential of novel n-4-piperazinyl- ciprofloxacin-aniline hybrids. Pak. J. Pharm. Sci. 2019;32(5):2215-2222.
- [Google Scholar]
- Transition metal catalyzed glaser and glaser-Hay coupling reactions: scope, classical/green methodologies and synthetic applications. Synth. Commun.. 2020;50(22):3337-3368.
- [Google Scholar]
- Drugs from the oceans: marine natural products as leads for drug discovery. Chimia. 2017;71:646.
- [Google Scholar]
- Total synthesis of belizentrin methyl Ester: report on a likely conquest. Angew. Chem. Int. Ed.. 2018;57:10712-10717.
- [Google Scholar]
- Divergent asymmetric Total synthesis of (−)-voacafricines a and B. Angew. Chem.. 2023;135:e202301517.
- [Google Scholar]
- Synthesis of the oxazolidinone fragment of thelepamide. Nat. Prod. Res.. 2022;36:1686-1692.
- [Google Scholar]
- Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv.. 2015;33:1582-1614.
- [Google Scholar]
- Natural product synthesis via palladium-catalyzed carbonylation. J. Org. Chem.. 2017;82(5):2319-2328.
- [Google Scholar]
- Organic synthesis provides opportunities to transform drug discovery. Nat. Chem.. 2018;10(4):383-394.
- [Google Scholar]
- Styryl-lactones from goniothalamus species-a review. Phytochemical Analysis: Phytochem. Anal.. 1999;10:161-170.
- [Google Scholar]
- Simple and efficient synthesis of benzofuran derivatives from tyrosol. Synth. Commun.. 2016;46:242-248.
- [Google Scholar]
- Divergent Total synthesis of fornicin a, fornicin D, and ganodercin D. Meroterpenoids from Ganoderma Mushrooms. Eurjoc.. 2023;26:e202201446.
- [Google Scholar]
- Biosynthesis of the antibiotic nematophin and its elongated derivatives in entomopathogenic bacteria. Org. Lett.. 2017;19:806-809.
- [Google Scholar]
- Asymmetric total synthesis of norzoanthamine and formal synthesis of zoanthenol. Org. Chem. Front.. 2023;10:651-660.
- [Google Scholar]
- Enantioselective syntheses of wickerols a and B. J. Am. Chem. Soc.. 2023;145:6486-6497.
- [Google Scholar]
- Structural insights into the nature of Fe0 and FeI low-valent species obtained upon the reduction of iron salts by aryl grignard reagents. Inorg. Chem.. 2017;56(7):3834-3848.
- [Google Scholar]
- Chiral pool approach to the total synthesis of phomonol. Synth. Commun.. 2022;52:445-450.
- [Google Scholar]
- Therapeutic uses of prostaglandin F2α analogues in ocular disease and novel synthetic strategies. Prostaglandins & Other Lipid Mediators.. 2013;104:109-121.
- [Google Scholar]
- Chem. Rev.. 2007;107:3286.
- Diverse natural products from dichlorocyclobutanones: an evolutionary tale. Acc. Chem. Res.. 2016;49(2):252-261.
- [Google Scholar]
- Antibacterial indole alkaloids with complex heterocycles from voacanga africana. Organic Lett.. 2018;20:2702-2706.
- [Google Scholar]
- Total synthesis of sanctolide a and formal synthesis of (2 S)-sanctolide a. J. Org. Chem.. 2023;88:805-817.
- [Google Scholar]
- One-pot regioselective synthesis of dihydronaphthofurans and dibenzofurans. Tetrahedron. 2007;63:1610-1616.
- [Google Scholar]
- Natural product protulactone a: Total synthesis from D-galactose, X-ray analysis and biological evaluation. Bioorg. Chem.. 2022;127:105980
- [Google Scholar]
- Belizentrin, a highly bioactive macrocycle from the dinoflagellate Prorocentrum belizeanum. Organic Lett.. 2014;16:4546-4549.
- [Google Scholar]
- Synthesis of cylindricine C and a formal synthesis of cylindricine a. Tetrahedron. 2010;66:6411-6420.
- [Google Scholar]
- Applications of γ, δ-unsaturated ketones synthesized by copper-catalyzed Cascade addition of vinyl grignard reagents to esters. Acc. Chem. Res.. 2018;51(10):2574-2588.
- [Google Scholar]
- Recent advances in analysis of chinese medical plants and traditional medicines. J. Chromatogr Sci.. 2004;812:3-21.
- [Google Scholar]
- Asymmetric Total synthesis of illisimonin a. J. Am. Chem. Soc.. 2023;145(12):7021-7029.
- [Google Scholar]
- Design, synthesis, antimicrobial evaluation, and laccase catalysis effect of novel benzofuran–oxadiazole and benzofuran–triazole hybrids. J. Heterocycl. Chem.. 2019;56(10):2839-2852.
- [Google Scholar]
- Novel bioactive styryl-lactones: goniofufurone, goniopypyrone, and 8-acetylgoniotriol from goniothalamus giganteus (annonaceae). X-ray molecular structure of goniofufurone and of goniopypyrone. J. Chem. Soc. Perkin Trans.. 1990;I:1655-1661.
- [Google Scholar]
- Salimabromide: unexpected chemistry from the obligate marine myxobacterium enhygromyxa Salina. Chem. Eur. J.. 2013;19:9319-9324.
- [Google Scholar]
- Jha, A. K. protecting-group-directed stereodivergent tsuji-trost cyclization: total synthesis of oxylipids and (+)-petromyroxol. Chem. Comm.. 2023;59:2007-2010.
- [Google Scholar]
- The biosynthesis of 3-amino-5-hydroxybenzoic acid (AHBA), the precursor of mC7N units in ansamycin and mitomycin antibiotics: a review. J. Antibiot.. 2011;64:35-44.
- [Google Scholar]
- Utilization of grignard reagents in solid-phase synthesis: a review of the literature. Tetrahedron. 2000;56(5):685-691.
- [Google Scholar]
- Novel antifungal metabolite produced by a marine myxobacterium. J. Antibiot.. 2001;54:153-156.
- [Google Scholar]
- Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871-1875.
- [Google Scholar]
- Pandangolide 1a, a metabolite of the sponge-associated fungus cladosporium sp. J. Nat. Prod.. 2005;68:1350-1353.
- [Google Scholar]
- Cobalt-catalyzed cross-couplings between alkyl halides and grignard reagents. Acc. Chem. Res.. 2020;53(7):1351-1363.
- [Google Scholar]
- The structure of ethylmagnesium bromide dietherate. an X-ray diffraction study. J. Am. Chem. Soc.. 1964;86:5344-5345.
- [Google Scholar]
- Marine natural products from zoantharians: bioactivity, biosynthesis, systematics, and ecological roles. Nat. Prod. Rep.. 2020;37:515-540.
- [Google Scholar]
- Penicyclones, A. E. antibacterial polyketides from the Deep-Sea-derived fungus penicillium sp. F23–2. J. Nat. Prod.. 2015;78:2699-2703.
- [Google Scholar]
- The corey-seebach reagent in the 21st century: a review. Molecules. 2023;28(11):4367.
- [Google Scholar]
- Catalytic asymmetric conjugate addition and allylic alkylation with grignard reagents. Chem. Rev.. 2008;108(8):2824-2852.
- [Google Scholar]
- Kaempferol derivatives from the rhizomes of Zingiber montanum (J. koenig) link ex a. Dietr. from Bangladesh Separations. Dietr. from Bangladesh. Separations.. 2019;6:31.
- [Google Scholar]
- Sesquiterpenoids with new carbon skeletons from the resin of Toxicodendron vernicifluum as new types of extracellular matrix inhibitors. Organic Lett.. 2013;15:3602-3605.
- [Google Scholar]
- Natural products from myxobacteria: novel metabolites and bioactivities. Nat. Prod. Rep.. 2017;34:135-160.
- [Google Scholar]
- Total synthesis of the reported structure of bioactive dibenzofuran natural product karnatakafuran B. Tetrahedron. 2023;135:133327
- [Google Scholar]
- Hoye, T. R., Sizova, E., 2009. The evolution of chemistry through synthesis (and of synthesis in chemistry).
- Enantioselective total syntheses of marine natural products (+)-cylindricines C, D, E and their diastereomers. Org. Chem. Front.. 2022;9:58-63.
- [Google Scholar]
- prenylated xanthone from cell suspension cultures of Hypericum patulum. Phytochem.. 1997;44:1065-1066.
- [Google Scholar]
- Transmetalation of tetraalkynyltin compounds with grignard reagents: access to mono-and dialkyltin products. Angew. Chem. Int. Ed.. 1999;38(3):402-404.
- [Google Scholar]
- Advances in polycyclization cascades in natural product synthesis. Chem. Soc. Rev.. 2021;50(1):58-71.
- [Google Scholar]
- Strategies towards the synthesis of calyciphylline A- type daphniphyllum alkaloids. Nat. Prod. Rep.. 2014;31:550-562.
- [Google Scholar]
- Sanctolide a, a 14-membered PK–NRP hybrid macrolide from the cultured cyanobacterium Oscillatoria sancta (SAG 74.79) Tetrahedron Lett.. 2012;53:3563-3567.
- [Google Scholar]
- Versatility of glycals in synthetic organic chemistry: coupling reactions, diversity oriented synthesis and natural product synthesis. Org. Biomol. Chem.. 2019;17(17):4153-4182.
- [Google Scholar]
- Highly functionalized organomagnesium reagents prepared through halogen– metal exchange. Angew. Chem. Int. Ed.. 2003;42(36):4302-4320.
- [Google Scholar]
- Total synthesis of the antitumor active pyrrolo [2, 1-a] isoquinoline alkaloid (±)-crispine a. Tetrahedron Lett.. 2005;46:1173-1175.
- [Google Scholar]
- Synthesis of (S)-nyasol through a copper-catalyzed propargylic substitution. Synlett. 2023;34:159-162.
- [Google Scholar]
- Bioactive products from okinawan marine micro-and microorganisms. Phytochem Rev.. 2004;3:267.
- [Google Scholar]
- Total synthesis of patulone: a natural xanthonoid possessing a geminally diisoprenylated structure. Synlett. 2023;34:953-957.
- [Google Scholar]
- Biochemical studies of human semen. II. the action of semen on the human uterus. Proc. Soc. Exp. Biol. Med.. 1930;28:268-272.
- [Google Scholar]
- On the toxin of illicium anisatum. I. the isolation and characterization of a convulsant principle: anisatin1. J. Am. Chem. Soc.. 1952;74:3211-3215.
- [Google Scholar]
- Organic synthesis: march of the machines. Angew. Chem. Int. Ed.. 2015;54(11):3449-3464.
- [Google Scholar]
- (+)-and (−)-petromyroxols: antipodal tetrahydrofurandiols from larval sea lamprey (Petromyzon marinus L.) that elicit enantioselective olfactory responses. Org. Lett.. 2015;17:286-289.
- [Google Scholar]
- Grayanoids from the ericaceae family: structures, biological activities and mechanism of action. Phytochem. Rev.. 2013;12:305-325.
- [Google Scholar]
- Total synthesis of yuzurine-type alkaloid daphgraciline. J. Am. Chem. Soc.. 2022;144(41):18823-18828.
- [Google Scholar]
- Drug interaction study of flavonoids toward CYP3A4 and their quantitative structure activity relationship (QSAR) analysis for predicting potential effects. Toxicol. Lett.. 2018;294:27-36.
- [Google Scholar]
- Structural diversity and biological activity of the genus pieris terpenoids. J. Agric. Food Chem.. 2017;65:9934-9949.
- [Google Scholar]
- An overview of grayanane diterpenoids and their biological activities from the ericaceae family in the last seven years. Eur. J. Med. Chem.. 2019;166:400-416.
- [Google Scholar]
- Achmatowicz rearrangement-inspired development of green chemistry, organic methodology, and total synthesis of natural products. Acc. Chem. Res.. 2022;55(16):2326-2340.
- [Google Scholar]
- Total synthesis of (–)-negamycin from a chiral advanced epoxide. Synth. Commun.. 2023;53:119-126.
- [Google Scholar]
- The sarpagine group of indole alkaloids. In: The Alkaloids. Academic Press; 1999. p. :103-195.
- [Google Scholar]
- Illisimonin a, a caged sesquiterpenoid with a tricyclo [5.2. 1.01, 6] decane skeleton from the fruits of Illicium simonsii. Organic Lett.. 2017;19:6160-6163.
- [Google Scholar]
- Gilman reagent toward the synthesis of natural products. RSC Adv.. 2023;13(50):35172-35208.
- [Google Scholar]
- Stereoselective Total synthesis of (+)-casuarine via a functionalized pyrrolidine. Synthesis. 2022;54:2473-2479.
- [Google Scholar]
- Casuarine: a very highly oxygenated pyrrolizidine alkaloid. Tetrahedron Lett.. 1994;35:7849-7852.
- [Google Scholar]
- Cloke– Wilson rearrangement: a unique gateway to access five-membered heterocycles. RSC Adv.. 2023;13(50):35695-35732.
- [Google Scholar]
- Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod.. 2016;79:629-661.
- [Google Scholar]
- Total synthesis and biological evaluation of the resveratrol-derived polyphenol natural products hopeanol and hopeahainol a. J. Am. Chem. Soc.. 2010;132:7540-7548.
- [Google Scholar]
- Biomimetic total synthesis of plakortone Q via acid-mediated tandem cyclization. Org. Biomol. Chem.. 2022;20:6771-6775.
- [Google Scholar]
- Chemical interventions for the opioid crisis: key advances and remaining challenges. J. Am. Chem. Soc.. 2018;141:1798-1806.
- [Google Scholar]
- Oh, improvement of obesity and dyslipidemic activity of amomum tsao-ko in C57BL/6 mice fed a high-carbohydrate diet. Molecules. 2021;26:1638.
- [Google Scholar]
- Haploscleridamine, a novel tryptamine-derived alkaloid from a sponge of the order haplosclerida: an inhibitor of cathepsin K. J. Nat. Prod.. 2002;65:628-629.
- [Google Scholar]
- Discovery of kibdelomycin, a potent new class of bacterial type II topoisomerase inhibitor by chemical-genetic profiling in Staphylococcus aureus. Chem. Biol.. 2011;18:955-965.
- [Google Scholar]
- Transition-metal-free cross-coupling of acetals and grignard reagents to form diarylmethyl alkyl ethers and triarylmethanes. Synthesis. 2024;56(04):527-538.
- [Google Scholar]
- Synthetic studies on the synthesis of toxicodenane a and 8, 11-epi-toxicodenane a. JOC. 2022;87(5):3223-3233.
- [Google Scholar]
- Spirolactones: recent advances in natural products, bioactive compounds and synthetic strategies. Curr. Med. Chem.. 2018;25:917-962.
- [Google Scholar]
- Bioactive benzofuran derivatives: an insight on Lead developments, radioligands and advances of the last decade. Eur. J. Med. Chem.. 2015;97:356-376.
- [Google Scholar]
- Chemical constituents of Pilea cavaleriei subsp. cavaleriei. zhongguo zhong Yao za zhi= zhongguo zhongyao zazhi. J. Chin. Mater. Med.. 2012;37:2581-2584.
- [Google Scholar]
- Synthesis of novel benzofuran and related benzimid azole derivatives for evaluation of in vitro anti-HIV-1, anticancer and antimicrobial activities. Arch. Pharm. Res.. 2006;29:826-833.
- [Google Scholar]
- New benzofuran derivatives as an antioxidant agent. Indian J. Pharm. Sci.. 2010;72:231-235.
- [Google Scholar]
- Indole alkaloids: 2012 until now, highlighting the new chemical structures and biological activities. Fitoterapia. 2020;143:104558
- [Google Scholar]
- Total synthesis of haploscleridamine, villagorgin a and an approach towards lissoclin C. Org. Biomol. Chem.. 2023;21:1422-1434.
- [Google Scholar]
- Constitution of grignard reagent RMgCl in tetrahydrofuran. Organic Lett.. 2001;3:1793-1795.
- [Google Scholar]
- Unconventional macrocyclizations in natural product synthesis. ACS Cent. Sci.. 2020;6(11):1869-1889.
- [Google Scholar]
- Five new alkaloids from the tropical ascidian, lissoclinum sp. lissoclinotoxin a is chiral. JOC.. 1994;59:6600-6605.
- [Google Scholar]
- Phorboxazoles a and B: potent cytostatic macrolides from marine sponge phorbas species. J. Am. Chem. Soc.. 1995;117:8126-8131.
- [Google Scholar]
- Ascospiroketals a and B, unprecedented cycloethers from the marine-derived fungus Ascochyta salicorniae. Org. Lett.. 2007;9:239-242.
- [Google Scholar]
- Synthesis, anticancer, and computational studies of 1, 3, 4-oxadiazole-purine derivatives. J. Heterocycl. Chem.. 2020;57(7):2782-2794.
- [Google Scholar]
- Synthesis, hemolytic studies, and in silico modeling of novel Acefylline–1,2,4-triazole hybrids as potential anti-cancer agents against MCF-7 and A549. ACS Omega. 2021;6:11943-11953.
- [Google Scholar]
- Concise and efficient total synthesis of oxyphyllacinol, yakuchione-a and yakuchione-B. Synth. Commun.. 2022;52:513-520.
- [Google Scholar]
- Synthesis of antirenin active peptides II. Yakugaku Zasshi. J. Pharm. Soc. Jpn.. 1971;91:987-996.
- [Google Scholar]
- A hybrid polyketide–nonribosomal peptide in nematodes that promotes larval survival. Nat. Chem. Biol.. 2016;12:770-772.
- [Google Scholar]
- Angular tricyclic benzofurans and related natural products of fungal origin. isolation, biological activity and synthesis. Nat. Prod. Res.. 2013;30:941-969.
- [Google Scholar]
- Palladium-catalyzed carbonylations: application in complex natural product Total synthesis and recent developments. J. Org. Chem.. 2023;88(8):4925-4941.
- [Google Scholar]
- Biocatalyst for the synthesis of natural flavouring compounds as food additives: bridging the gap for a more sustainable industrial future. Food Chem.. 2024;435:137217
- [Google Scholar]
- Protulactones a and B: two new polyketides from the marine- derived fungus aspergillus sp. SF-5044. Bull. Kor. Chem. Soc.. 2010;31:1695-1698.
- [Google Scholar]
- Theoretical investigation of the biogenetic pathway for formation of antibacterial indole alkaloids from voacanga africana. ACS Omega. 2022;7:31591-31596.
- [Google Scholar]
- Trichodermanin a, a novel diterpenoid from endophytic fungus culture. J. Nat. Med.. 2011;65:381-384.
- [Google Scholar]
- Total synthesis of penicyclone a using a double grignard reaction. ]OC.. 2022;87:16054-16062.
- [Google Scholar]
- World Health Organization report: current crisis of antibiotic resistance. J. Bionanosci.. 2019;9:778-788.
- [Google Scholar]
- Asymmetric total synthesis of buprenorphine and dihydroetorphine. Tetrahedron Lett.. 2022;104:154027
- [Google Scholar]
- Pileamartines a and B: alkaloids from Pilea aff. martinii with a new carbon skeleton. Tetrahedron Lett.. 2018;59:1909-1912.
- [Google Scholar]
- Cytotoxic alkaloids from leaves of Pilea aff. martinii. Planta Med.. 2019;85(06):496-502.
- [Google Scholar]
- Grignard reagents and their N-analogues in the synthesis of tricyclic and tetracyclic cage-like lactams. J. Heterocycl. Chem.. 2018;55(10):2381-2391.
- [Google Scholar]
- Enantioselective synthesis of (−)-codeine and (−)-morphine. J. Am. Chem. Soc.. 2002;124:14542-14543.
- [Google Scholar]
- Introducing deep eutectic solvents to polar organometallic chemistry: chemoselective addition of organolithium and grignard reagents to ketones in air. Angew. Chem.. 2014;126:6079-6083.
- [Google Scholar]
- PCR screening reveals considerable unexploited biosynthetic potential of ansamycins and a mysterious family of AHBA-containing natural products in actinomycetes. J. Appl. Microbiol.. 2023;115:77-85.
- [Google Scholar]
- Progress of grayanoids in ericaceae on chemistry and bioactivity. Nat. Prod. Res. Dev.. 1997;9:82-90.
- [Google Scholar]
- Efficiency in natural product total synthesis. John Wiley & Sons; 2018.
- Concise total synthesis of (±)-pileamartines a and B. Org. Chem. Front.. 2022;9:6968-6972.
- [Google Scholar]
- Concise syntheses and biological activities of ganomycin I and fornicin a. Eur. J. Org. Chem.. 2014;2014(4):731-738.
- [Google Scholar]
- Scalable Total syntheses of (±)-catellatolactams a and B. Org. Lett.. 2023;25:1003-1007.
- [Google Scholar]
- Asymmetric total synthesis of prostaglandin C 2 TBS ether. Chem. Commun.. 2022;58:6000-6003.
- [Google Scholar]
- Radicamines a and B: synthesis and revision of the absolute configuration. Organic Lett.. 2006;8:3021-3024.
- [Google Scholar]
- Total syntheses of nominal and actual prorocentin. J. Am. Chem. Soc.. 2023;145:2584-2595.
- [Google Scholar]
- Synthetic strategies toward the synthesis of enoxacin-, levofloxacin-, and gatifloxacin- based compounds: a review. Synth. Commun.. 2017;47(11):1021-1039.
- [Google Scholar]
- Total synthesis of sarpagine alkaloid (−)-normacusine B. Organic Lett.. 2022;24:3515-3520.
- [Google Scholar]
- Improving the halogen-magnesium exchange by using new turbo-grignard reagents. chemistry–a. European Journal. 2019;25(11):2695-2703.
- [Google Scholar]






