Translate this page into:
Harvesting season dependent variation in chemical composition and biological activities of the essential oil obtained from Inula graveolens (L.) grown in Chebba (Tunisia) salt marsh
⁎Corresponding author at: Center of Biotechnology of Sfax, Road of Sidi mansour Km 6, P.O. Box. 1177, 3018 Sfax, Tunisia. lotfi.mallouli@cbs.rnrt.tn (Lotfi Mellouli)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The chemical composition and the biological activities of aromatic plants may be influenced by seasonal changes. Therefore, the essential oil of Inula graveolens (IGEO), collected at Chebba salt marsh, was studied in terms of yields, compositions and biological activities, throughout four different seasons, namely spring, summer, autumn and winter (April, July, October and January, respectively). GC/MS analysis identified 30 compounds. Mostly quantitative rather than qualitative, variation was observed in the oil composition of each sample. It had been revealed a predominant presence of bornyl acetate and borneol, as well as significant differences of several compound amounts in function of the seasonal change. Biological activities were also related to the harvest season; hence, IGEO from spring gave the best antioxidant activity results and IGEO from autumn seemed to be the most potent against pathogenic microorganisms, while oils from summer and winter were the strongest inhibitors of acetylcholinesterase and tyrosinase.
A matrix linking IGEO major compounds to biological was composed to identify relationships between concentrations of the volatile molecules and the biological activities of the samples. Furthermore, the seasonal variation of these main volatile constituents was also investigated through principal compound analysis (PCA). The obtained results revealed that each biological activity depends on the seasonal fluctuation of the amounts of certain chemical compounds.
Keywords
Inula graveolens
Essential oil
Seasonal variations
Chemical composition
Biological activities
PCA
1 Introduction
Inula graveolens (L.) Desf. (synonym: Dittrichia graveolens L. Greuter) is well known and wide spread in many places of the Mediterranean area, such as road sides, waste lands and pastures. This species reaches a height of about 80 cm, has sticky leaves and bright yellow flowers appearing on September / October, and is characterized by a very strong aromatic odor. Chemical and biological investigation of the essential oil of I. graveolens has been a subject of several studies (Abou-Douh, 2008; Braham et al., 2001; Öksüz and Topçu, 1992; Topcu et al., 1992), especially its bactericidal effect (Guinoiseau et al., 2010). Furthermore, these studies displayed the chemical and biological variations yielded by the change of the harvesting geographical zones, as a main factor of diversity.
Many other ecological and biological factors were shown to have significant effect on the quality and quantity variations of the plant natural compounds and their targeted biological activities, especially the harvesting season or period. Hence, the choice of the optimal harvesting time is necessary to obtain plant active products with high value of the sought biological activity.
In as much as the annual growth of I. graveolens could be a source of chemical and biological variations, this present investigation was aimed to obtain a better insight on I. graveolens essential oil (IGEO) composition and its biological activities following the harvesting season. IGEO was extracted from plants collected at 4 seasonal periods, from Chebba salt marsh coast. Our work was divided into two parts. First, in order to carry out a detailed analysis, we investigated the IGEO composition and its biological activity assessment. The second part was focused on the link between IGEO chemical features and the biological power, as well as the impact of the seasons.
2 Material and methods
2.1 Reagents
Dichloromethane (Sigma), anhydrous sodium sulphate (Fluka), Butylated hydroxytoluene (BHT) (Supelco), 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Aldrich), dimethylsulfoxide (DMSO) (Scharlau), 2, 2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) (Fluka), MK2S2O8 (Merck), β-carotene (Sigma), Linoleic acid (Acros Organics), Tween 40 (Scharlau), Peptone (Accumix), Yeast extract (Accumix), NaCl (Suvchem), Agar (Biokar), Glucose (Accumix), Electric eel acetylcholinesterase AChE (Type-VI-S, EC 3.1.1.7, Sigma), Acetylthiocholine iodide (ATCI) (Sigma), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) (Sigma), Tris (Vivantis), Galanthamine (Sigma), Tyrosinase from mushroom TyrE (EC 1.10.3.1) (Sigma-Aldrich), Tyrosine (Fluka), Kojic acid (Sigma).
2.2 Plant material collection and preparation
I. graveolens aerial parts were collected from the marsh area of Chebba (Mahdia, Tunisia, latitude 35.23° and longitude 11.11°), this region is known as strong saline with a semi-arid climate and a scarce rainfall (Mabrouk et al., 2014; Saidi et al., 2009). The harvest had been scheduled in 4 periods: spring (April 2017), summer (July 2017), autumn (October 2017), and winter (January 2018). The identification of the species was performed according to the flora of Tunisia deposited in the Center of Biotechnology of Sfax under the identification number: IGLMB/CBS-17-18
2.3 Isolation of the essential oil
For each season, 6 kg of fresh plant were used for oil extraction by steam distillation during 3 h using a Clevenger-type apparatus (Ben Hsouna et al., 2014). The floral water was extracted with dichloromethane (3 × 50 mL), and then the solvent was evaporated using a rotavapory vacuum evaporator. Each resulted I. graveolens essential oil (IGEO) was dried with anhydrous sodium sulphate, weighted, and stored at 4 °C.
2.4 Physicochemical analysis of IGEOs
2.4.1 Physical properties
The assessed physical properties were mainly the density, using a densitometer preset at 25 °C, and some qualitative features such as the appearance, the colour, and the odor.
2.4.2 Chemical analysis
The chemical analysis of the IGEOs was performed on a GC/MS HP model 6980 inert MSD (Agilent Technologies, J&W Scientific Products, Palo Alto, CA, USA), equipped with an Agilent Technologies capillary HP-5MS column (60 m length; 0.25 mm i.d.; 0.25 mm film thickness), and coupled to a mass selective detector (MSD5973, ionization voltage 70 eV; all Agilent, Santa Clara, CA). The carrier gas was Helium and was used at 1.2 mL min−1 flow rate. The oven temperature program was as follows: 1 min at 100 °C ramped from 100 to 280 °C at 5 °C min−1 and 25 min at 280 °C. Diluted samples (1:100 in dichloromethane (v/v)) of 1.0 mL was injected in the split mode (ratio 1:10). The components were identified based on the comparison of their Kovax index (KI) and mass spectra with those of standards, Wiley 2001 library data (NIST 02 version2.62) of the GC/MS system, and literature data (Adams).
2.5 Biological activities
2.5.1 Antioxidant testing assays
2.5.1.1 DPPH• radical scavenging activity
Radical scavenging activity of extracts was determined using the 2,2-diphenyl-1-picrylhydrazyl (DPPH•) as a reagent (Kirby and Schmidt, 1997). Briefly, IGEOs were dissolved in 10% DMSO and diluted at different concentrations (500, 250, 125, 60.25, and 30.125 µg mL−1). Then, 150 µL of a 4% (w/v) solution of DPPH• radical in methanol were mixed with 150 µL of samples. The mixture was incubated for 30 min in the dark at room temperature. The scavenging capacity was determined by monitoring the decrease in absorbance at 517 nm against a blank. The percentage of antiradical activity (% ArA) has been calculated as follows:
2.5.1.2 ABTS+• radical scavenging activity
The potential of IGEOs to scavenge free radicals was also assessed by their ability to quench the free radical cation ABTS+•, following the method developed by Re and colleagues. Briefly, the pre-formed radical monocation of ABTS+• was generated by reacting ABTS solution (7 mM) with 2.45 mM of MK2S2O8. The mixture was allowed to stand for 15 h in the dark at room temperature, and then diluted with ethanol to obtain the absorbance of 0.7 ± 0.2 units at 734 nm. Samples were diluted to yield the following concentrations: 500, 250, 125, 60.25, 30.125 and 15.625 µg mL−1. Twenty µL of each concentration were added to 180 µL of ABTS+• and vortexed for 30 s, then incubated for 6 min in dark at room temperature (Re et al., 1999). The absorbance was measured at 734 nm, and the percentage of scavenging activity was calculated as follow:
2.5.1.3 β-Carotene bleaching assay
The β-carotene bleaching test was performed according to Pratt’s method (Pratt, 1980), with some optimisations (Sellem et al., 2016): 0,5 mg of β-carotene was dissolved in 1 mL chloroform. Then, 25 µL of linoleic acid and 200 µL of Tween 40 were added. The chloroform was then evaporated under rotary evaporator, and the residue was dissolved in 100 mL of tape H2O.
IGEOs were diluted at different concentrations (500, 250, 125, 60.25, and 30.125 µg mL−1), and for 500 µL of each sample, 2.5 mL of β-carotene- linoleic acid solution were added. The absorbance was read at 470 nm firstly at zero time and after 120 min of incubation in dark.
The antioxidant activity in β-carotene bleaching model in percentage (AA %) was calculated using the following equation:
A0S and A0C: absorbance of the sample and the control, respectively, measured at zero time, A120S and A120C: absorbance of the sample and the control, respectively, measured after 120 min.
2.5.2 Antimicrobial activity
2.5.2.1 Microorganisms and growth conditions
Bacteria and Candida species were obtained from international culture collections (ATCC) and local culture collection of the Laboratory of Microorganisms and Biomolecules of the Centre of Biotechnology of Sfax-Tunisia. They included Gram-positive bacteria: Staphylococcus aureus (S. aureus) ATCC 6538, Micrococcus luteus (M. luteus) LB14110, Listeria monocytogenes (L. monocytogenes) ATCC 19,117 and Gram-negative bacteria: Salmonella typhimurium (S. typhimurium) ATCC 14028, Pseudomonas aeruginosa (P. aeruginosa) ATCC 49189, Enterobacter aerogenes (E. aerogenes) ATCC 13048. Two Candida strains were also tested: Candida tropicalis (C. tropicalis) R2 CIP 203 and Candida albicans (C. albicans) ATCC 10231.
Following a previous work (Sellem et al., 2016), the bacterial cultures were performed in Luria-Bertani (LB) agar medium composed of (g L-1): peptone, 10; yeast extract, 5; NaCl, 5; and agar, 20 at pH 7.2, then the bacterial strains were incubated at 37 °C, except M. luteus and P. aeruginosa which were incubated at 30 °C. Candida species were cultivated on Sabouraud agar medium composed of (g L-1): peptone, 10; glucose, 10; and agar, 20 at pH = 5.6, and incubated at 28 °C.
Working bacterial cultures were prepared by inoculating a loopful of each test bacteria in 3 mL of LB broth. C. tropicalis was cultivated in YP10 medium composed of (g L-1): yeast extract, 10; peptone, 10; glucose, 100; and 15 mL of 2 g L-1 adenine solution. C albicans was cultivated in YEPD medium composed of (g L-1): yeast extract, 10; peptone, 20; and dextrose, 20 at pH 5.6. Each microorganism was incubated for 12 h at its adequate temperature. For the test, the used final inoculum concentration was 106 CFU mL−1.
2.5.2.2 Agar diffusion method
Antimicrobial activity of the IGEOs was evaluated by agar-well diffusion assay. Fifteen milliliters of the molten agar (45 °C) were poured into sterile petri dishes (Ø 90 mm). Working cell suspensions were prepared and 100 μL were evenly spread onto the surface of the agar plates of LB agar medium for bacteria and Sabouraud agar medium for Candida. Once the plates had been aseptically dried, 5 mm wells were punched into the agar with a sterile Pasteur pipette. The oils were dissolved in DMSO/water (1/9; v/v) to a final concentration of 1 mg mL−1 and then filtered through 0.22 μm pore-size black polycarbonate filters (Millipore). Thus, 100 μL were placed into the wells and the plates were incubated at the adequate temperature overnight for bacterial strains and 48 h for Candida strains (Güven et al., 2006).
2.5.2.3 Minimal inhibitory concentration (MIC) and Minimal Bactericidal/Fungicidal concentration (MBC/MFC)
The micro-dilution method with serially dilution (Chandrasekaran and Venkatesalu, 2004) was used to determine MIC or MLC values. The IGEOs were added to growth broth medium to get a final concentration of 1 mg mL−1, and serially diluted to reach 0.0048 mg mL−1. The final volume in each tube was 100 μL. 2.5 μL of the tested microorganism were transferred to each microtube. A positive control (2.5 μL inoculum and 100 μL growth medium) and a negative control (2.5 μL of extract and 100 μL of growth medium) were included in each micro tube. The contents of the tubes were mixed by pipetting and were incubated for 24 h. The MIC was defined as the lowest concentration of an antimicrobial agent that inhibits the visible growth of a microorganism. The lowest concentration of the extract that did not permit any visible bacterial and fungal colony growth on the agar plate recorded as MBC and MFC, respectively. Ampicillin (antibacterial standard) and Fluconazole (antifungal standard) were used according to Chandrasekaran and Venkatesalu, (2004).
2.5.3 Enzymatic inhibitory power
2.5.3.1 Acetylcholinesterase inhibitory potential
The assessment of AChE inhibitory activity was optimized by Sellem et al., (2016) following the method of Ellman et al., (1961). Electric eel AChE (Type-VI-S, EC 3.1.1.7) was used, and acetylthiocholine iodide (ATCI) was employed as substrate of the reaction. 5,5′-dithiobis-(2-nitrobenzoic acid), (DTNB) was the reagent for the measurement of the anti-acetylcholinesterase activity. Briefly, 100 µL of Tris buffer at 50 mM (pH 8.0), 30 µL of sample or standard and 5 µL of AChE enzyme (0,5U mL−1) were added in a 96- well microplate and incubated for 10 min at 25 °C. Then, 142 µL of DTNB (3 mM) and 23 µL of substrate (75 mM) were added. Hydrolysis of ATCI was monitored by the formation of the yellow 5-thio-2-nitrobenzoate anion as a result of the reaction of DTNB with thiocholines, catalyzed by enzymes at 405 nm utilising a 96-well microplate reader. The kinetic reaction has been followed until equilibrium, and then the reaction has been stopped. Percentage of inhibition of AChE was determined by comparison of rates reaction of samples relative to negative control using the following formula:
δA sample: Sample absorbance at zero time – Sample absorbance at the end of reaction.
δA control: Control absorbance at zero time - Control absorbance at the end of reaction.
Galanthamine, the antiacetylcholinesterase alkaloid-type of drug obtained from the bulbs of snowdrop (Galanthus sp.), was used as standard.
2.5.3.2 Antityrosinase activity
The tyrosinase inhibitory activity was determined spectrophotometrically using L-tyrosine as substrate and carried out in a 96-well microplate (Rangkadilok et al., 2007), according to some modifications. Seventy μL of L-tyrosine were added to 100 μL of phosphate buffer (pH 6.5, 50 mM) and 70 μL of sample (or standard) dissolved in 10% of DMSO. The mixture was incubated at 37 °C for 10 min, and then 20 μL of the mushroom tyrosinase (TyrE) were added to 33 U mL−1. The optical density was read at 475 nm in a microplate reader. After 15 min of incubation, absorbance was read again. The data were expressed as percent inhibition of tyrosinase activity (% TyrEI).
δA sample: sample absorbance at zero-time - sample absorbance after 15 min.
δA control: control absorbance at zero-time - control absorbance after 15 min.
Kojic acid was used as standard.
2.6 Statistical analysis
To grantee the accuracy of our results, all tests were assayed in triplicate and expressed as theaverage ± standard deviation of the measurements. EC50 (the maximal concentration that gives 50% of effect) and IC50 (the maximal concentration that inhibits the enzymatic activity by the half) were obtained by non-linear least-squares method. The statistical program SPSS version 21.00 for Windows (SPSS Inc., Chicago, IL) was used to analyse data. Variance was analysed by one-way ANOVA and Duncan’s multiple range tests were calculated for the significant data at p < 0.05. Correlations were analyzed by Pearson correlation coefficient, significant data were at p < 0.05 and very significant ones were at p < 0.01. Principal compound analysis (PCA) was performed within Minitab software version 17 and presented as biplot graphic.
3 Results & discussion
3.1 Yields and physical characteristics
The four samples of essential oil from I. graveolens aerial parts (IGEOs) were weighted to score their yields and studied for their features: appearance, colour, odor, and density at 25 °C. Following the result tabulated in Table 1, IGEOs recorded differences in their abundance and odor; indeed, the oil obtained from July harvested plants was remarkably (p 〈0 0 5) the most abundant (0.678%) and its odor was the strongest (p 〈0 0 5). The remarkably increased oil yield on July could be explained that the essential oil production was enhanced, especially in the sexual organs, the month preceding the flowering period of the species to attract pollinators such as bees and other insects (Castelo et al., 2012). ±: Standard deviation of three replicates; Values with a different letter (a–b) of the same line are significantly different (p < 0.05).
Harvest season, month
April
July
October
January
Yield, % (w/w)
0.308 ± 0.034 a
0.678 ± 0.081b
0.389 ± 0.017 a
0.271 ± 0.009 a
Appearance
mobile liquid, clear
mobile liquid, clear
mobile liquid, clear
mobile liquid, clear
Colour
light yellow
light yellow
light yellow
light yellow
Odor
agreeable
strong
quite strong
agreeable
Density at 25 °C (g mL−1)
0.889 ± 0.067b
0.890 ± 0.021b
0.902 ± 0.011b
0.873 ± 0.034 a
Likewise, as indicated on the Table 1, there were no differences recorded in the appearance and colour, which were mobile liquid, clear and light yellow, respectively. While, the density recorded slight variations across the seasons, with the highest value given with October oil. This could be explained by the variation of the monoterpenes and sesquiterpenes amounts following the synthesis stages and plant needs. As a major class of terpenes, monoterpenes are small volatile molecules of low density, so with high ability of diffusion (de Amaral et al., 2015). These properties due to their role as pollinator attractors during the sexual reproduction stage (Li et al., 2013). Whilst, sesquiterpenes are larger, denser and less volatile molecules than the monoterpenes, which often have protective functions, such as the antimicrobial action (Cysne et al., 2005). The highest essential oil content (0.678%) was obtained in July and the lowest value (0.271%) was obtained in October. This change in the essential oil content values can be explained by the higher average monthly temperatures in July compared to October. As is known, increasing average monthly temperatures create an effect of temperature stress on aromatic plants and cause an increase in essential oil contents (Said-Al Ahl et al., 2018; Katar et al., 2019). Blanc et al., (2004) reported that the essential oil content of I. graveolens from Corsica (France) was between 0.06 and 0.29%. However, the highest essential oil content of I. graveolens from Algeria (Beghidja et al., 2014) and Serbia (Miladinović et al., 2016) was 0.89 and 0.9% respectively.
3.2 Oil chemical compositions
IGEOs GC-MS analysis exhibited 30 different components that were listed according to their kovat index (KI) in Table 2, for each season. The main components were bornyl acetate (40.90–45.34%) and borneol (23.65–37.29%), with remarkable percentage of molecules belonging to several families, namely, monoterpenes (camphene), monoterpene alcohol (terpineol), monoterpene phenol (tymol), sesquiterpene (β-caryophyllene), sesquiterpene alcohol (τ-cadinol and farnesol), and sesquiterpene oxide (caryophyllene oxide). aCompounds listed in order of their KI and identified by GC/MS Wiley 7.0 version library and National Institute of Standards and Technology 05 MS (NIST) library data.
Compounds
RI (KI) b
Sampling periods, % c
April
July
October
January
α-Pinene
932.12
nd†
0.85
trace*
0.22
Camphene
950.04
7.03
3.20
4.57
4.39
β-Pinene
979.25
nd
nd
nd
0.16
α-Phellandrene
1,002.72
0.58
0.53
0.57
0.55
l-Limonene
1,028.16
trace
trace
trace
Trace
1.8-cineole
1,034.09
0.22
trace
0.10
0.19
Linalool
1,094.96
0.30
0.58
0.36
0.18
β-thujone
1,110.86
0.21
0.50
0.54
0.14
Camphor
1,148.77
0.39
0.22
0.21
0.27
Borneol
1,168.59
33.47
37.29
23.65
25.08
α-Terpineol
1,191.59
0.55
1.71
2.74
0.65
Carveol
1,215.41
trace
trace
0.51
0.46
Bornyl Acetate
1,283.69
40.90
45.34
41.88
40.16
Thymol
1,315.72
4.49
4.62
3.15
1.48
β-caryophyllene
1,416.92
1.00
1.22
2.30
0.19
α-humulene
1,453.31
0.17
0.21
0.53
0.45
γ -Muurolene
1,471.67
0.39
0.34
0.52
0.21
β-Selinene
1,487.08
nd
1.20
nd
nd
δ-Cadinene
1,523.58
0.15
0.14
0.27
0.23
Caryophyllene oxide
1,584.61
1.11
1.30
1.35
1.28
τ-cadinol
1,625.02
3.30
6.09
7.12
2.64
neointermedeol
1,658.56
0.19
0.17
0.22
trace
Heptadecane
1,700.00
trace
trace
trace
trace
Pentadecanal
1,712.81
trace
trace
trace
trace
α-Cyperone
1,735.50
trace
0.19
0.16
0.16
(2E,6E)-Farnesol
1,741.63
0.64
1.37
2.65
0.88
3-Eicosyne
1,837.94
trace
trace
trace
trace
4-Epidehydro abietol
2,038.23
nd
nd
trace
nd
Manool
2,057.44
trace
0.11
trace
nd
6,10,14-trimethyl-2-pentadecanone
2,138.59
trace
trace
trace
nd
Total identified, %
95.79
96.57
94.09
93.98
Earlier, another Tunisian investigation undertook the same species and was in accordance with the present study; indeed, the most important compounds obtained from the aerial parts of plants without flowers were: τ-cadinol (9.2%), borneol (21.4%) and bornyl acetate (33.4%). However, thymol wasn’t a major compound in this previews work (Harzallah-Skhiri et al., 2005). World widely, the literature revealed a wide variations in the essential oil composition of I. graveolens collected from different locations (Beghidja et al., 2014; Mirza and Ahmadi, 2000), but all the studies were almost agreed that borneol and bornyl acetate are the major compounds found in I. graveolens essential oil. High concentration of bornyl acetate 69.78% (Beghidja et al., 2014), 63.9% (Lamiri et al., 2001) and 56.8% (Blanc et al., 2004), has characterised certain I. graveolens oils, while borneol dominated in two oils collected in Iran (60.7%) (Mirza and Ahmadi, 2000) and Turkey (64%) (Karamendere and Zeybek, 2000). Moreover, bornyl acetate (43.1 to 73.1%) and borneol (3.7 to 32.2%) were found to be the major compounds in 22 oils extracted from several plants collected during the flowering stage in different localities of Corsica (Lamiri et al., 2001). In contrast, further studies revealed other major compounds; indeed, essential oils isolated from the aerial parts of two different Lebanese wild populations of I. graveolens were mainly rich in bornyl acetate (70.6 to 72.3%) and epi-a-cadinol (1.4– 13.4%). Furthermore, among 22 identified compounds, 1,8-cineole (54.89%) and p-cymene (16.2%) were the majority of an essential oil obtained from the aerial parts of I. graveolens grown in Iran. In the same context, in Greece, the main compounds among the 57 identified in an essential oil of I. graveolens were τ -cadinol (30.2%) and bornyl acetate (25.4%) (Petropoulou et al., 2004). Finally, in the case Algerian I. graveolens, bornyl acetate (69.78%) and caryophyllene oxide (5.7%) were the main compounds of its oil (Beghidja et al., 2014).
Thus the composition of the essential oils of this same species differs widely from one country to another. This chemical variation could be a consequence of the variation in climate, harvest period and soil type (Mitic et al., 2016).
As far as variation in the contents of individual components was concerned, the IGEOs showed considerable variations due to the season of collection (Table 2); indeed, the compounds could increase, decrease, or disappear following the season. For example, bornyl acetate, a major compound, recorded its highest amount on July (45.34%), while it decreased on January (40.16%), β-Selinene was only found on July (1.20%). Whereas, τ-cadinol recorded an amount of 7.12% on October, then dramatically decreased to reach 2.64% on January. Many factors could influence the seasonal variation of the essential oil composition. Thermoregulatory is among the main causes, as the essential oil hydrophobic compounds could increase during the hot periods to protect the plant from desiccation (Kamatou et al., 2008). Moreover, luminosity fluctuation rates are associated with the change in the content of essential oils and may also influence their chemical composition (de Amaral et al., 2015).
3.3 Antioxidant activity
IGEOs antioxidant activity was evaluated by three different methods: DPPH• (1,1-Diphenyl-2-picrylhydrazyl) antiradical assay, ABTS•+ [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)] scavenging assay, and β-carotene bleaching system test, and presented by the EC50 values that exhibited a wide range of variation among the tested oil samples (Table 3). Globally, IGEOs have recorded an important antiradical power through the three tests. IGEO extracted from plants collected on July showed the strongest (p 〈0 0 5) scavenging activity against the radical DPPH• and was better than the synthetic antioxidant BHT. IGEO from plant harvested on April has displayed the strongest (p 〈0 0 5) activity in the free radical ABTS•+ and β-carotene bleaching tests (EC50 were 7.58 ± 0.4 and 79.10 ± 0.59 µg mL−1, respectively) better (p 〈0 0 5) than BHT activity (EC50 were 17.21 ± 1.03 and 89.30 ± 3.67 µg L-1 respectively). It should be noted that essential oils and organic extracts of I. graveolens plant were well known for their important antioxidant activity (Al-Snafi, 2018). Among the identified compounds in IGEOs (Table 2), the two major compounds borneol and bornyl acetate may be considered as main contributors of antioxidant activity (Mahboubi, 2011; Horváthová et al., 2009). As it was mentioned above, IGEOs possess antioxidant activity higher than that of synthetic (standard) antioxidant. ±: Standard deviation of three replicates; Values with a different letter (a–e) of the same antioxidant activity are significantly different (p < 0.05).
EC50, µg mL−1
April
July
October
January
BHT
DPPH
35.45 ± 0.25c
23.12 ± 0.29d
58.66 ± 0.12a
40.20 ± 0.67b
31.2 ± 0.5c
ABTS
7.58 ± 0.4d
16.04 ± 0.82b
19.25 ± 0.5a
11.18 ± 0.33c
17.21 ± 1.03b
β-carotene
79.10 ± 0.59e
118.21 ± 1.48c
192.63 ± 1.21b
345.67 ± 2.54a
89.30 ± 3.67d
3.4 Antimicrobial activity
IGEOs have been also screened for their antimicrobial activity against two Candida species and 6 bacterial strains using agar diffusion method (Fig. 1), and then the MIC and MBC/MFC were determined.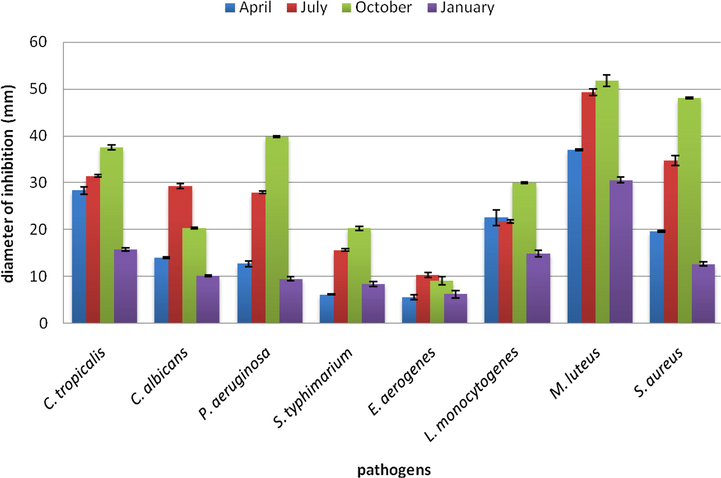
I. graveolens EO antimicrobial activity assessed by agar diffusion test (diameter of inhibition zone in mm includes 3 mm corresponding to the diameter of the hole).
Agar diffusion test outlined variable degrees of antimicrobial activity against the tested pathogen strains; IGEO from plants harvested on October exhibited the most (p 〈0 0 5) promising activity followed by IGEO from July, this late oil was remarkably (p 〈0 0 5) the most efficient against C. albicans. Furthermore, a great potency against Gram-positive bacteria than Gram-negative was reported and could be explained by the fact that Gram-negative bacteria have an outer membrane consisting of lipoprotein and lipopolysaccharide, which is selectively permeable and thus regulates access to the underlying structures (El-Chaghaby et al., 2014).
The results of Table 4 showed IGEOs MIC and MBC/MFC values, assessed by micro-dilution method. The lowest (p 〈0 0 5) MIC value (15.6 µg mL−1) was obtained with IGEO of October against M. luteus and S. aureus. While S. typhimarium showed the highest resistance, IGEO of July exhibited the greatest (p 〈0 0 5) inhibitor potential against this pathogen (500 µg mL−1). This same essential oil inhibited C. albicans at the lowest (p 〈0 0 5) concentration (250 µg mL−1). It has been reported by Al-Snafi, (2018) that the antimicrobial activity of the essential oil of D. graveolens (L.) Greuter, studied against five bacterial and one fungal strain using a disk-diffusion assay, was active only against Gram-positive bacteria.
IGEO
April
July
October
January
Pathogens
MIC*
MBC/MFC§
MIC
MBC/MFC
MIC
MBC/MFC
MIC
MBC/MFC
C. tropicalis
250
500
250
1,000
125
1,000
500
NA†
C. albicans
500
NA
250
1,000
500
1,000
1,000
NA
P. aeruginosa
500
1,000
250
500
125
500
500
NA
S. typhimarium
1,000
NA
500
500
1,000
NA
1,000
NA
E. aerogenes
500
NA
500
1,000
500
1,000
1,000
NA
L. monocytogenes
250
1,000
250
500
125
1,000
250
500
M. luteus
62.5
250
31.2
250
15.6
125
62.5
125
S. aureus
125
500
62.5
1,000
15.6
250
125
500
MBC/MFC were exhibited with various concentrations in all oil samples (125 ∼ 1,000 µgmL−1) (Table 4). These variations were also observed on tested microorganisms. The most susceptible strain was M. luteus, whereas S. typhimurium, E. aerogenes and C. albicans were the most resistant. Only the IGEO of July recorded a bactericidal activity against S. typhimarium (MBC = 500 µg mL−1). Therefore, this oil seemed to be the most promising extract, providing the most microbicidal activity among the 4 samples of IGEOs, while the oil of January is the less efficient.
Previous studies proposed that the essential oil antimicrobial activity may be in part related to its hydrophobicity, responsible for its partition of the cell membrane lipid bilayer, leading to a change in permeability and cell membrane damage resulting in a reduced ability to maintain cellular functions (de Macêdo et al., 2018; Hsu et al., 2013). Other findings supposed that essential oil compounds may alter different metabolic pathways, such as reduction of 3′:5″-cyclic adenosine monophosphate (cAMP) formation and, together with a mitogenic activation protein (MAP) signaling pathway, responsible for playing an important role in the formation of yeast filamentous forms (de Macêdo et al., 2018; Dižová and Bujdáková, 2017). Several studies outlined the antimicrobial properties of IGEOs. Guinoiseau et al., (2010) have mentioned the antimicrobial activity of borneol, a major volatile compound of I. graveolens. Topcu et al., (1992) reported the antimicrobial effect of sesquiterpenes. It should be noted that the process of extraction of I. graveolens products influences their chemical composition and this may also influence their antmicrobial activity (Burt 2004). This could be an explanation for the different activities observed between the different extracts and the essential oil of I. graveolens.
3.5 Enzymatic inhibitory potential
As part of a current study on potential bioactive agents, acetylcholinesterase and tyrosinase inhibitor power of IGEOs have been also evaluated, the results are tabulated in Table 5 and presented by the IC50 of each extract. ±: Standard deviation of three replicates; Values with a different letter (a–e) of anti-acetylcholinesterase (AChEI) and anti-tyrosinase (TyrEI) activity are significantly different (p < 0.05).
IC50, µg mL−1
April
July
October
January
Galanthamine
Kojic acid
AChEI
5.50 ± 0.25d
5.33 ± 0.67c
5.01 ± 0.34b
8.12 ± 0.54e
0.118 ± 0.037a
–
TyrEI
18.34 ± 0.21b
49.25 ± 0.5e
30.97 ± 2.4d
26.34 ± 1.1c
–
4.05 ± 0.25a
As regard the anti-acetylcholinesterase (AChEI) activity, IGEO obtained on October displayed the strongest (p 〈0 0 5) inhibitory potential (5.01 ± 0.34 µg mL−1), while IGEO from January was the weakest (8.12 ± 0.54 µg mL−1). This IGEO activity was previously reported (Dohi et al., 2009), and it may be due to the presence of terpene alcohols, such as α-terpeniol.
Although all samples have shown a less (p 〈0 0 5) activity than kojic acid, the EO from plants collected on April has displayed an important (p 〈0 0 5) inhibitory power (18.34 ± 0.21 µg mL−1). Other Inula species were studied for their antityrosinase activity such as Inula britannica and Inula nervosa. Sesquiterpenes of I. Britannica were reported as inhibitors of tyrosinase in B16 melanoma cells (Choo et al., 2014), and inulavosin of I. nervosa , was also reported to inhibits tyrosinase (Fujita et al., 2009).
3.6 Correlation determination and principal compound analysis (PCA)
A correlation analysis was done among IGEOs major compounds and the different biological activities, according to Pearson correlation coefficient (Table 6).
Camphene
Borneol
α-terpineol
Bornyl acetate
Thymol
Caryophyllene
Caryophyllene oxide
τ-Cardinol
(2E,6E)farnesol
DPPH
0.086
−0.797**
0.122
0.100
−0.394
0.221
0.364
0.286
0.334
ABTS
−0.718**
−0.399
0.254
−0.129
0.009
0.198
0.300
0.245
0.156
β-carotene
−0.324
−0.135
−0.108
0.215
−0.860**
−0.424
0.465
−0.293
0.032
C. tropicalis
−0.020
0.402
−0.730**
−0.101
−0.573**
−0.905**
−0.127
−0.744**
−0.613**
C. albicans
0.137
−0.112
−0.464*
0.441
−0.812**
−0.590**
0.000
−0.618**
−0.269
P. aeruginosa
0.378
0.430
−0.982**
0.102
−0.193
−0.831**
−0.744**
−0.901**
−0.852**
S. typhimarium
0.187
−0.515*
−0.186
0.389
−0.468*
−0.020
−0.256
−0.349
0.012
E. aerogenes
−0.119
0.118
−0.491*
0.117
−0.783**
−0.731**
0.126
−0.603**
−0.344
L.monocytogenes
0.191
0.077
−0.855**
−0.470*
0.134
−0.815**
−0.575
−0.665**
−0.853**
M. luteus
0.280
0.432
−0.979**
0.114
−0.187
−0.830**
−0.744**
−0.897**
−0.846**
S. aureus
0.334
0.192
−0.995**
0.007
−0.162
−0.856**
−0.740**
−0.894**
−0.881**
AChEI
−0.045
0.210
−0.606**
0.051
−0.732**
−0.805**
0.007
−0.683**
−0.462*
TyrEI
−0.814**
0.274
0.286
−0.761**
0.271
0.199
0.316
0.453
0.301
A trend can be found, relating the high contents of borneolto thegood DPPH• antiradical scavenging power (p < 001; R2 = −0.797) and of camphene to ABTS•+ antiradical scavenging power (p < 001; R2 = −0.718), while the β-carotene antioxidant activity is highly correlated to the percentage of tymol (p < 001; R2 = −0.860).
A correlation was likewise found between the major compound percentages and the antimicrobial activity through the obtained MIC values. Among the 9 major compounds, terpeneol, tymol, caryophyllene, τ-cardinol and (2E,6E) farnesol seemed to be the most correlated to the MIC values except that obtained with S. typhimurium, which was moderately related only to the percentage of borneol (p < 005; R2 = −0.515) and tymol (p < 005; R2 = −0.468). The percentage of this late compound was strongly correlated with MIC values obtained against C. albicans (p < 001; R2 = −0.812).
As regard the enzymatic inhibitory potential, AChEI activity displayed a strong relation with the amount of several compounds especially tymol and caryophyllen. While, TyrEI seemed only related to the presence of camphen and bornyl acetate.
In order to assess the influence of the harvesting season on the relationship between IGEO composition and the different biological activity, multivariate analysis by principal components analysis (PCA) was used. For statistical significance, the oil components with concentration higher than 1% (bold in Table 2) of the total oil were selected. PCA procedure reduced the several initial variables and highlighted two principal components explaining most of the variance (74.4%), which were therefore used to prepare the PCA biplot (Fig. 2).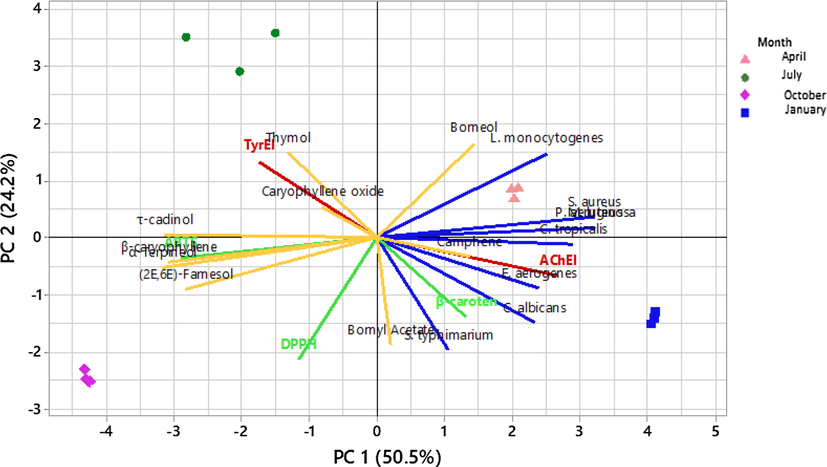
IGEOs ordination biplot according to a principal component analysis (PCA) based on the correlation matrix of IGEO major compounds (>1%) and the biological activities following the harvest period.
The first principal component (PC 1), accounting for 50.5% of total variance allowed distinguishing the oil from the autumn (October) which is characterised by its relatively highest contents of terpene alcohols (α-terpineol, τ-cadinol, and (2E,6E) farnesol). This class of terpenic compounds may have structural and functional similarities because they have the same biosynthetic origin and are formed through the action of enzymes called terpene synthases (Beran et al., 2016; Pazouki and Niinemets, 2016). Taking into account all the assessed biological activities, PC1 highlighted the relationship between terpene alcohols enhancement on October and the antimicrobial activity, which was in accordance with correlation results. Many studies unveiled the antimicrobial capacity of these terpene alcohols. Indeed, Li et al., (2014) reported that α-terpineol had a bactericidal effect against Escherichia coli, Salmonella enteritidis and Staphylococcus aureus. α-terpineol seemed cause a cell structure changes. Likewise, the antimicrobial action of farnesol was recorded against resistant pathogens and their biofilm such as Staphylococcus aureus (Jabra-Rizk et al., 2006) and Candida albicans (Yu et al., 2012).
PC1 also outlined the high concentration of borneol in the oil from April and related it to the excellent antioxidant capacity assessed by the scavenging of the free radicals ABTS+• and especially DPPH•. Several studies previously confirmed the antioxidant property of borneol; indeed, this compound is able to reduce oxidative stress in animal model (Cherneva et al., 2012; Kodikonda and Naik, 2017). However, the capacity of protection of β-carotene against oxidation is only related to the high level of thymol on the oil of July. This oil is distinguished following PC1 and PC2 and showed the highest content in thymol and caryophyllene oxide. The late compound was correlated to the AChEI potential. There is no studies directly implicated the AChE inhibitory power of caryophyllene oxide; however, several works reported this property in essential oils that caryphyllene oxide is among their major compounds (Ayaz et al., 2015; Yu et al., 2011). PCA also distinguished IGEO from Junuary that was related to the highest concentrations on bornyl acetate and camphen. The relationship between camphene content and TyrEI activity, which was revealed by the correlation study, was confirmed by PCA. However, this relationship is unclear and not yet reported in the literature. Indeed, camphen is known for its strong antioxidant and anti-inflammatory power, but not cited as tyrosinase inhibitor (Tiwari and Kakkar, 2009). Likewise, bornyl acetate was reported to be a great anti-inflammatory agent, but a possible enzyme inhibitory features was never reported (Zhong et al., 2011). Further studies are urged to assess the tyrosinase inhibitory power of these two compounds.
4 Conclusion
The essential oil of I. graveolens (IGEO), collected at Chebba-Tunisia salt marsh, was studied in terms of yields, compositions and biological activities, throughout four different periods (April, July, October and January). The highest essential oil content (0.678%) was obtained in July and the lowest value (0.271%) was obtained in October. IGEOs GC-MS analysis exhibited 30 different components and the main products were bornyl acetate (40.90–45.34%) and borneol (23.65–37.29%). The four studied IGEOs have recorded an important antiradical power through the three antioxidant tests. IGEO extracted from plants collected on July showed the strongest scavenging activity against the radical DPPH• and was better than the synthetic antioxidant BHT. IGEO from plant harvested on April has displayed the strongest activity in the free radical ABTS•+ and β-carotene bleaching tests (EC50 7.58 ± 0.4 and 79.10 ± 0.59 µg mL−1, respectively) better than BHT activity (EC50 17.21 ± 1.03 and 89.30 ± 3.67 µg L-1 respectively). The IGEO of July recorded a bactericidal activity against S. typhimarium and it was the most efficient against C. albicans. The IGEO from July was potent as AChE inhibitor and that of January inhibits TyrE. Our investigation approves the traditional use of I. graveolens as medicinal plant and each sought IGEO biological virtue depends on season of the collection. The most interesting biological activity of the essential oil of I. graveolens (IGEO), collected at Chebba-Tunisia salt marsh, is the antioxidant activity and notably for the IGEOs extracted from plants collected on April and on July. Thus we propose these two IGEOs as natural products to protect human from oxidative stress damage.
Acknowledgement
This study was supported by the Tunisian Ministry of Higher Education and Scientific Research (Program Contract 2015-2018 of the Laboratory of Microorganisms and Biomolecules of the Center of Biotechnology of Sfax – Tunisia)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- New eudesmane derivatives and other sesquiterpenes from the epigeal parts of dittrichia graveolens. Chem. Pharm. Bull. (Tokyo). 2008;56:1535-1545.
- [CrossRef] [Google Scholar]
- Seasonal influence on the essential oil production of Nectandra megapotamica (Spreng.) Mez. Brazilian Arch. Biol. Technol.. 2015;58:12-21.
- [CrossRef] [Google Scholar]
- Chemical constituents and pharmacological effect of Inula graveolens (SYN: Dittrichia graveolens). A Review. Indo Am. J. P. Sci. 2018 05, 2183-2190
- [Google Scholar]
- Comparative chemical profiling, cholinesterase inhibitions and anti-radicals properties of essential oils from Polygonum hydropiper L: A Preliminary anti- Alzheimer’s study. Lipids Health Dis.. 2015;14:1-12.
- [CrossRef] [Google Scholar]
- Composition of the essential oil of Inula graveolens Algerian origin species. J. Nat. Prod. Plant Resour. 2014;4:1-3.
- [Google Scholar]
- Myrtus communis essential oil: chemical composition and antimicrobial activities against food spoilage pathogens. Chem. Biodivers.. 2014;11:571-580.
- [CrossRef] [Google Scholar]
- Novel family of terpene synthases evolved from trans -isoprenyl diphosphate synthases in a flea beetle. Proc. Natl. Acad. Sci.. 2016;113:2922-2927.
- [CrossRef] [Google Scholar]
- Chemical composition and variability of the essential oil of Inula graveolens from Corsica. Flavour Fragr. J.. 2004;19:314-319.
- [CrossRef] [Google Scholar]
- Etudes Biologique et Chimique d’Inula graveolens. Structures de Sesquiterpenes, Identification Par CPG/SM de Constituants des Huiles Essentielles. J. la Société Chim. Tunisie. 2001;4:1215-1222.
- [Google Scholar]
- Essential oils: their antibacterial properties and potential applications in foods a review. Int. J. Food Microbiol.. 2004;94:223-253.
- [CrossRef] [Google Scholar]
- Seasonal variation in the yield and the chemical composition of essential oils from two brazilian native arbustive species. J. Appl. Sci.. 2012;12:753-760.
- [CrossRef] [Google Scholar]
- Antibacterial and antifungal activity of Syzygium jambolanum seeds. J. Ethnopharmacol.. 2004;91:105-108.
- [CrossRef] [Google Scholar]
- The effect of camphor and borneol on rat thymocyte viability and oxidative stress. Molecules. 2012;17:10258-10266.
- [CrossRef] [Google Scholar]
- Erratum to: Hypo-pigmenting effect of sesquiterpenes from Inula britannica in B16 melanoma cells (archives of pharmacal research) Arch. Pharm. Res.. 2014;37:687.
- [CrossRef] [Google Scholar]
- Leaf essential oils of four piper species from the State of Ceará - Northeast of Brazil. J. Braz. Chem. Soc.. 2005;16:1378-1381.
- [CrossRef] [Google Scholar]
- Effect of seasonality on chemical profile and antifungal activity of essential oil isolated from leaves Psidium salutare (Kunth) O. Berg. PeerJ. 2018;6:e5476.
- [CrossRef] [Google Scholar]
- Properties and role of the quorum sensing molecule Farnesol in relation to the yeast Candida albicans. Pharmazie. 2017;72:307-312.
- [CrossRef] [Google Scholar]
- Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J. Agric. Food Chem.. 2009;57:4313-4318.
- [CrossRef] [Google Scholar]
- Evaluation of the antioxidant and antibacterial properties of various solvents extracts of Annona squamosa L. leaves. Arab. J. Chem.. 2014;7:227-233.
- [CrossRef] [Google Scholar]
- A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol.. 1961;7:88-95.
- [Google Scholar]
- Inulavosin, a melanogenesis inhibitor, leads to mistargeting of tyrosinase to lysosomes and accelerates its degradation. J. Invest. Dermatol.. 2009;129:1489-1499.
- [CrossRef] [Google Scholar]
- Cellular effects induced by Inula graveolens and Santolina corsica essential oils on Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis.. 2010;29:873-879.
- [CrossRef] [Google Scholar]
- Antimicrobial activities of fruits of Crataegus and Pyrus species. Pharm. Biol.. 2006;44:79-83.
- [CrossRef] [Google Scholar]
- Chemical composition of essential oils from leaves-stems, flowers and roots of Inula graveolens from Tunisia. Pakistan J. Biol. Sci.. 2005;8:249-254.
- [CrossRef] [Google Scholar]
- Effects of borneol on the level of DNA damage induced in primary rat hepatocytes and testicular cells by hydrogen peroxide. Food Chem. Toxicol.. 2009;47:1318-1323.
- [CrossRef] [Google Scholar]
- The inhibitory activity of linalool against the filamentous growth and biofilm formation in Candida albicans. Med. Mycol.. 2013;51:473-482.
- [CrossRef] [Google Scholar]
- Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother.. 2006;50:1463-1469.
- [CrossRef] [Google Scholar]
- Seasonal variation in essential oil composition, oil toxicity and the biological activity of solvent extracts of three South African Salvia species. South African J. Bot.. 2008;74:230-237.
- [CrossRef] [Google Scholar]
- Composition of the Essential Oils of Inula viscosa, I. graveolens and I.helenium subsp. turcoracemosa. J. Fac. Pharm. Istansboul. 2000;33:10-14.
- [Google Scholar]
- Determination of relationship between yield components in rosemary (Rosmarinus officinalis L.) genotypes. J. Agricul. Faculty of Gaziosmanpasa Univ.. 2019;36:177-186.
- [CrossRef] [Google Scholar]
- The antioxidant activity of Chinese herbs for eczema and of placebo herbs - I. J. Ethnopharmacol.. 1997;56:103-108.
- [CrossRef] [Google Scholar]
- Ameliorative effect of borneol, a natural bycyclic monoterpene against hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic Wistar rats. Biomed. Pharmacother.. 2017;96:336-347.
- [CrossRef] [Google Scholar]
- Insecticidal effects of essential oils against Hessian fly, Mayetiola destructor (Say) F. Crop. Res.. 2001;71:9-15.
- [CrossRef] [Google Scholar]
- Antibacterial activity of α-terpineol may induce morphostructural alterations in Escherichia coli. Brazilian J. Microbiol.. 2014;45:1409-1413.
- [CrossRef] [Google Scholar]
- Variations in essential oil yields and compositions of Cinnamomum cassia leaves at different developmental stages. Ind. Crops Prod.. 2013;47:92-101.
- [CrossRef] [Google Scholar]
- Diversity and temporal fluctuations of epiphytes and sessile invertebrates on the rhizomes Posidonia oceanica in a seagrass meadow off Tunisia. Mar. Ecol.. 2014;35:212-220.
- [CrossRef] [Google Scholar]
- Chemical composition, antimicrobial and antioxidant activities of Dittrichia graveolens (L.) Greuter essential oil. Herba Pol.. 2011;57:20-32.
- [Google Scholar]
- In vitro trials of Dittrichia graveolens essential oil combined with antibiotics. Nat. Prod. Commun.. 2016;11:865-868.
- [CrossRef] [Google Scholar]
- Composition of the essential oil of dittrichia graveolens (l.) greuter. J. Essent. Oil Res.. 2000;12:507-508.
- [CrossRef] [Google Scholar]
- Dittrichia graveolens (L.) Greuter Essential Oil: Chemical Composition, Multivariate Analysis, and Antimicrobial Activity. Chem. Biodivers.. 2016;13:85-90.
- [CrossRef] [Google Scholar]
- Öksüz, S., Topçu, G., 1992. and Other Constituents From Galeana. Phytochemistry 31, 195–197.
- Multi-substrate terpene synthases: their occurrence and physiological significance. Front. Plant Sci.. 2016;7:1-16.
- [CrossRef] [Google Scholar]
- Volatile Constituents of Dittrichia graveolens (L.) Greuter from Greece. J. Essent. Oil Res.. 2004;16:400-401.
- [CrossRef] [Google Scholar]
- Natural Antioxidants of Soybeans and Other Oil-Seeds. In: Simic M.G., Marcus K., eds. Autoxidation in Food and Biological Systems. US, Boston, MA: Springer; 1980. p. :283-293. https://doi.org/10.1007/978-1-4757-9351-2_18
- [Google Scholar]
- Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol.. 2007;45:328-336.
- [CrossRef] [Google Scholar]
- Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med.. 1999;26:1231-1237.
- [CrossRef] [Google Scholar]
- Essential oil contentent and concentration of constituents of Lemon balm (Melissa officinalis L.) at different harvest dates. J. Ess. oil bearing Plants.. 2018;21:1410-1417.
- [CrossRef] [Google Scholar]
- A GIS-based susceptibility indexing method for irrigation and drinking water management planning: Application to Chebba-Mellouleche Aquifer. Tunisia. Agric. Water Manag.. 2009;96:1683-1690.
- [CrossRef] [Google Scholar]
- Anti-oxidant, antimicrobial and anti-acetylcholinesterase activities of organic extracts from aerial parts of three tunisian plants and correlation with polyphenols and flavonoids contents. Bangladesh J. Pharmacol.. 2016;11
- [CrossRef] [Google Scholar]
- Plant derived antioxidants - Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. Vitr.. 2009;23:295-301.
- [CrossRef] [Google Scholar]
- Cytotoxic and Antibacterial Sesquiterpenes from Inula graveolens. Phytochemistry. 1992;33:407-410.
- [Google Scholar]
- Possible inhibitory molecular mechanism of farnesol on the development of fluconazole resistance in Candida albicans biofilm. Antimicrob. Agents Chemother.. 2012;56:770-775.
- [CrossRef] [Google Scholar]
- Original article chemical composition and anti-acetylcholinesterase activity of flower essential oils of artemisia annua at different flowering stage. Iran. J. Pharm. Res.. 2011;10:265-271.
- [Google Scholar]
- Quercetin and Bornyl Acetate regulate T-lymphocyte subsets and INF-γ/IL-4 ratio in utero in pregnant mice Evidence-based Complement. Altern. Med.. 2011;2011
- [CrossRef] [Google Scholar]







