Translate this page into:
Hemicellulose structural changes during steam pretreatment and biogradation of Lentinus edodes
⁎Corresponding authors at: School of Materials Science and Engineering, Central South University of Forestry and Technology, Changsha 410004, China (W. Peng). pengwanxi@163.com (Wanxi Peng), 641038556@qq.com (Zhongfeng Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
To disclosed the internal factors for the growth of mycelium and Lentinus edodes, Quercus Linn wood, which was biotransformed during the artificial cultivation of Lentinus edodes, were synergistically characterized by TGA/DTG, FT-IR and NMR. The results showed that the different ingredients of hemicellulose decreased during steam explosion and biodegradation of Lentinus edodes, however hemicellulose content continued to increased. FT-IR showed that the transmittance of the characteristic peaks in hemicellose gradually increased after decreased after steam explosion and biodegradation of Lentinus edodes. TGA/DTG curves that thermal stability and maximum thermal degradation rates of hemicelloses were contiguous after steam explosion and biodegradation of Lentinus edodes. Structural determination based on FT-IR and 1H, and 2D-HSQC NMR analyses showed that the alkali-extractable hemicelluloses shared the structure composed of (1 → 4)-linked β-d-xylopyranosyl backbone with 4-O-methyl-R-d-glucuronic acid attached to O-2 of the xylose residues and l-arabinose attached to O-3 of the xylose residues. And it revealed that the extractable hemicelluloses retained original structure without cleaving chemical linkages. Furthermore, a small amount of other minor hemicelluloses (β-glucans) including xylans in the extractable hemicelluloses could be identified by NMR and other approaches.
Keywords
Hemicellulose of Quercus Linn wood
Steam explosion
Biodegradation of Lentinus edodes
TGA/DTG
FT-IR
NMR
HSQC
1 Introduction
With the increase of global energy requirements and greater environmental awareness, alternatives to fossil fuels as energy sources have been widely followed on. Photosynthesis conversion solar energy into the storage of energy in the form of plant cell-wall polymers. The energy stored in these polymers could be accessed in many ways, ranging from simple burning to complex bioconversion processes. Currently plant cell walls were considerable biomass resources of biofuels and other chemicals. What’s more, lignocellulosic biomass, which contained the agricultural residues, forestry waste and municipal solid waste, presented a sustainable and renewable source for the production of liquid biofuels such as bioethanol (Taherzadeh and Karimi, 2008; Razali and Said, 2017). That was, lignocellulose feedstock was considered to be an attractive raw material for liquid fuel and the production of chemicals and materials. As most often being a by-product from food and feed production, lignocellulosic biomass did not compete with the production of edible crops (Chen and Qiu, 2010; Petersson et al., 2007) and had the potential to be the feedstock for the production of a considerable proportion of transport fuels if cost effective conversion processes were available (Kristensen et al., 2008; Halim and Phang, 2017). The major components in lignocellulosic biomass were cellulose, hemicellulose and lignin. Hemicellulose sugars are the second most abundant carbohydrates in nature and its conversion to ethanol could provide an alternative liquid fuel source for the future (Jeffries, 2006; Shamsudin et al., 2017). However, hemicelluloses, which were restricted to poales and a few other groups, composed of different fiveand six-carbon monosaccharide units (Rubin, 2008; Ghafar et al., 2017). The detailed structure of the hemicelluloses and their abundance varied widely between different species and cell types. Especially, the biosynthesis of xylans and beta-(1 → 3, 1 → 4)-glucans remains very elusive, and recent studies have led to more questions than answers. Hence, the effects of hemicelluloses on biomass digestibility need to be revealed.
In the plant cell wall, hemicelluloses were bound to lignin and cellulose, and detailed isolation procedures were required to separate these components from plant raw material (Hansen and Plackett, 2008; Khan et al., 2017). Removal of hemicelluloses in a pure form from plant cell wall involved hydrolysis of ester and ether bonds, which linked the hemicelluloses to lignin (Aziz and Hanafiah, 2017). A number of methods were used to isolate and richen hemicelluloses from plant sources, including extraction with alkali, dimethyl sulfoxide, water, methanol, steam or microwave treatment (Hansen and Plackett, 2008; Wen et al., 2010, 2011). These methods were developed for characterization purposes, but it could also be used as a preparative method. Besides, hot water could be used as a tool to separate water-soluble hemicelluloses from plant cell wall (Sun et al., 2004, 1998). Unfortunately, only a few papers about the comparison of hemicellulosic fractions obtained by hot water and biopretreatments had been published (Xu et al., 2007; Ismail and Hanafiah, 2017). With the depletion of fossil fuel and the concern of environmental protection, the utilization of biomass resources has attracted increasing worldwide interest.
Steam explosion was the most commonly used method for pretreatment of lignocellulosic materials (Fernandez-Bolanos et al., 2001). Steam explosion, which was invented in 1925, was introduced to treat aspen wood in early 1980s. In the method, the factors of steam explosion pretreatment are residence time, temperature, chip size and moisture content (Duff and Murray, 1996). Optimal hemicellulose solubilization and hydrolysis could be achieved by different temperature and residence time (Duff and Murray, 1996; Lee et al., 2009). Steam explosion, whose advantages included the low energy requirement and no recycling or environmental costs, was recognized as one of the most cost-effective pretreatment processes for hardwoods and agricultural residues, but it is less effective for softwoods (Clark and Mackie, 1987; Basheer et al., 2017). However, Hemicellulose recovery in the range of 55–84%, together with low levels of inhibitory by-products, has been obtained through autohydrolysis (Ulbricht et al., 1984; Mes-Hartree et al., 1988).
The most promising method for enzymatic hydrolysis of the lignocellulosic biomass was preceeded by a pretreatment process in which the lignin component was separated from the cellulose and hemicellulose to make it amenable to the enzymatic hydrolysis. In biopretreatment processes, microorganisms such as white-, brown-, and soft-rot fungi are used to degrade lignin and hydrolyse hemicellulose in waste materials (Njoku et al., 2013; Sassner et al., 2008). Brown rots mainly attack cellulose, while white and soft rots attack both cellulose and lignin. White-rot fungi are the most effective Basidiomycetes for biological pretreatment of lignocellulosic materials (Sukor et al., 2017; Hassan et al., 2017). Using varieties of white-rot fungi, 35% of the straw was converted to reducing sugars by Pleurotus ostreatus in five weeks (Hatakka, 1983). In order to prevent the loss of cellulose, a cellulase-less mutant of Sporotrichum pulverulentum was developed for the degradation of lignin in wood chips (Akin et al., 1995), also reported the delignification of Bermuda grass by white-rot fungi. The white-rot fungus P. chrysosporium produces lignin-degrading enzymes, lignin peroxidases and manganese-dependent peroxidases, during secondary metabolism in response to carbon or nitrogen limitation (Boominathan and Reddy, 1992; Halim et al., 2017). Many white-rot fungi could degraded wood cell walls, and other enzymes including laccases, polyphenol oxidases, H2O2 producing enzymes and quinone-reducing enzymes can also degrade lignin (Blanchette, 1991). The advantages of biopretreatment include low energy requirement and mild environmental conditions. However, the rate of hydrolysis in most biopretreatment processes was very low (Jeffries, 2006; Njoku et al., 2013; Zhu et al., 2009; Singh et al., 2011).
Lentinula edodes was first typically cultivated in the dead logs of hardwood. Before 1982, the Japanese variety of Lentinula edodes could only be grown in traditional locations using ancient methods. And a 1982 report on the budding and growth of the Japan Islands variety revealed opportunities for commercial cultivation in the United States (Leatham, 1982). Lentinula edodes has been now widely cultivated all over the world (Chang and Hayes, 2013). Commercially, Lentinula edodes was typically grown in conditions similar to their natural environment on either artificial substrate or hardwood logs, such as oak. Now, Lentinus edodes was commercially cultivated on oak wood particles. Although Lentinula edodes had the wide growth, researches mainly concentrated in the pharmacodynamics, cultivation conditions and culinary uses (Ramasamy and Kandaswamy, 1976; Dhillon and Chahal, 1979; Miller and Jong, 1987; Bhatti et al., 1987; Royse et al., 1990; Palomo et al., 1998; Chang, 1999; Zhang et al., 2002; Obodai et al., 2003; Permana et al., 2009; Jiang et al., 2013; Kim et al., 2014), but little attention to wood biodegradation and reuse. Profiling of wood chips may help growers optimize their production media and reduce production costs (Royse et al., 1990). The extractives of Lentinula edodes contained the antibacterial substances (Njoku et al., 2013; Yamamoto et al., 1997; Hirasawa et al., 1999). However, oak wood extractives could be inhibitory to growth of Lentinus edodes (Leatham, 1985), and oak wood need be pretreated before cultivation of Lentinula edodes. Lentinus edodes had their ability to produce lignocellulolytic enzyme in solid-state and submerged fermentation of various plant raw material (Elisashvili et al., 2008; Rahman et al., 2017). They cellulolytic system enzymes, hemicellulases, the ligninolytic system, (gluco-)amylase, pectinase, acid protease, cell wall lytic enzymes (laminarinase, 1,4-β-d-glucosidase, β-N-acetyl-d-glucosaminidase, α-d-galactosidase, β-d-mannosidase), acid phosphatase, and laccase (Leatham, 1985). Lentinus edodes was the important wood lignin-degrading fungi (Leatham, 1986). Degradation of the lignin fraction of the wood cell wall by compounds of lower molecular weight than enzymes is implicated (Goodell et al., 1998). The overall effect of L. edodes on oak is similar to that of many white-rot fungi, which simultaneously degrade all cell wall components (Vane, 2003; Vane et al., 2003). Unfortunately, shiitake dermatitis were recorded by French Poison Control Centers – New case series with clinical observations (Hérault et al., 2010; Boels et al., 2014). The inexplicable cases become more and more from the vegetable growers in China. Merely, it was easily found that the biodegradation of wood by L. edodes was not deeply understand to reuse. To date, the physicochemical features and structural characteristic of hemicelluloses from wood biodegraded by L. edodes had been poorly characterized (Aoyama et al., 1999). Therefore, the aim of this study was to firstly prepare hemicellulosic polymers isolated from Quercus Linn wood after steam pretreatment and biodegradation of L. edodes, and their chemical structure would be examined and analyzed by ourier-Transform Infrared Spectroscopy (FT-IR), thermogravimetric analysis (TGA), nuclear magnetic resonance (NMR) in order to recognize hemicelluses richen. And it was particularly important to elucidate the physicochemical features and structural characterization in this study.
2 Material and methods
The scheme of structural analysis on hemicellulosic polymers isolated from Quercus Linn wood during the artificial cultivation of Lentinus edodes were illustrated in Fig. 1.
Scheme of hemicelluloses isolated from natural, steam pretreatment and biodegraded wood.
2.1 Materials
The Quercus Linn was collected from Tongbaishan Forest Region China. Biomass was crushed into particles (GM1). The mycelium of Lentinus edodes were clonal and Industrial grade. Ethanol, benzene, acetic acid, and KOH were all analytic reagent for the following tests.
2.2 Experiment methods
2.2.1 Biotorrefaction process
The above 1.0 t particles were steam pretreated for 60 h in order to ensure further decomposition, and then each 2 kg were packed in plastic bag tied by rope. These bags were drilled and put into by the Lentinus edodes mycelium. Then further storage were done under the confined space, high humidity for 140 days. Lentinus edodes mycelium could live in some samples (GM3), and died in others (GM2). After small plant of Lentinus edodes, grown, GM3 samples were placed in plastic shed, ventilation and sunlight during the daytime, airproof at night. The small plants could live in some samples (GM5), and died in others (GM4). After GM5 samples raised Lentinus edodes five times, these samples were the waste wood (GM6). The samples were crushed into 40–60 mu powder, dried to moisture content of 0%, and extracted in ethanol-benzene solution (Vethanol:Vbenzene = 2:1) at 85–90 °C for 6 h. After ethanol-benzene extraction, the extracted flour was dried to moisture content of 0%.
2.2.2 Isolation and purification of hemicelluloses
The above extracted flour was treated in 10% KOH solution at 10 °C for 24 h (Vextracted flour:VKOH solution = 1:5). The KOH extracted flour was filtrated, washed five times with 0.5% acetic acid solution, dried to a moisture content of 0%, then weighed. The filtrate was neutralized with 6 M hydrochloric acid or acetic acid solution to pH 5.5 ∼ 6.0 and then concentrated under reduced pressure to about 50 mL. Three volumes of ethanol were added to each concentrated solution with continuous stirring, and then the flocculent precipitate appeared. After filtration with filter paper on a Buchner funnel, the isolated hemicelluloses were purified by thorough washing with 70% ethanol and then freeze-dried (Wen et al., 2010, 2011).
2.2.3 Determination of chemical composition
To determine the composition of isolated hemicelluloses, the neutral sugars and uronic acids in isolated hemicellulosic fractions were determined by HPAEC (high-performance anion exchange chromatography). The neutral sugars and uronic acids in the hemicellulosic preparations were obtained by hydrolyzing 5 mg of sample with 10% H2SO4 for 2.5 h at 105 °C. After hydrolysis, the hydrolysate was diluted 50-fold, filtered, and injected into the HPAEC system (Dionex ICS3000) with pulsed amperometric detector and an ion exchange Carbopac PA-1 column (4 × 250 mm). The neutral sugars were separated in 18 mMNaOH(carbonate free and purged with nitrogen) with post-column addition of 0.3 M NaOH at a rate of 0.5 mL/min. Run time was 45 min, followed by a 10 min elution with 0.2 M NaOH to wash the column and then a 15 min elution with 18 mMNaOH to reequilibrate the column. Calibration was performed with a standard solution of l-rhamnose, l-arabinose, l-glucose, l-galactose, d-mannose, d-xylose, glucuronic acid, and galacturonic acids. Measurements were conducted with two parallels, and reproducibility of the values was found within the range of 5% (Wen et al., 2010, 2011).
2.2.4 TG analysis
Each sample was analyzed using less than 10 mg of hemicellulosic polymers. TG spectra were measured from room temperature to 805 °C on a TG20 thermogravimetric analyzer (209-F1 TG, Netzsch, Germany) using a carrier gas (N2) velocity of 40 mL/min and a heating rate of 20 °C/min.
2.2.5 FT-IR analysis
The FT-IR spectra of the samples were obtained on a FT-IR spectrophotometer (Bruker Tensor 27) using KBr discs containing 1% finely ground sample. Thirty-two scans were taken for each sample recorded from 4000 to 400 cm−1 at a resolution of 2 cm−1 in the transmission mode.
2.2.6 NMR analysis
The solution-state 1H NMR spectra were recorded on a Bruker AV III NMR spectrometer at 400.13 MHz using 15 mg of hemicelluloses in 1.0 mL of D2O. In addition, to increase solubility of alkali-soluble hemicelluloses in D2O, a few drops of sodium deuteroxide (7.5 M NaOD) were added. The chemical shifts were calibrated relative to the signals from D2O, used as an internal standard, at 4.70 ppm for the 1H NMR spectra. A semiquantitative analysis of the HSQC cross-signal intensities was performed. The spectral widths for the HSQC were 5000 and 20,000 Hz for the 1H and 13C dimensions, respectively. The number of collected complex points was 1024 for the 1H dimension with a recycle delay of 5 s. The number of transients for the HSQC spectra was 128, and 256 time increments were always recorded in the 13C dimension. The 1JC-H used was 146 Hz. Prior to Fourier transformation, the data matrices were zero filled to 1024 points in the 13C dimension. Data processing was performed using standard Bruker Topspin-NMR software. Because the cross-signal intensity depended on the particular 1JC-H value, as well as on the relaxation time, a direct analysis of the intensities was indeed impossible. Therefore, the integration of the cross-signals was performed separately for the different regions of the HSQC spectra, which contain signals that correspond to chemically analogous carbon-proton pairs (in similar samples). For these signals, the 1JC-H coupling value was relatively similar and was used semiquantitatively to estimate the relative abundance of the different constituents (Wen et al., 2010, 2011).
3 Results and discussion
3.1 Yield and chemical composition
Hemicelluloses, which were the second most abundant natural polymers and the main ingredient of plant cell wall, played a vital role in the lifetime of wood cell. It composed of different fiveand six-carbon monosaccharide units (Rubin, 2008), and included xyloglucans, xylans, mannans and glucomannans, and beta-(1 → 3, 1 → 4)-glucans. The hemicellulose content varied roughly from 20 to 35% in the wood. Although the test results were different by the different methods, they could assess the chemical changes of Quercus Linn wood during the growth of Lentinus edodes. The extractives, cellulose, hemicellulose and lignin content of Quercus Linn wood were 3.09%, 20.40%, 22.86%, 53.64%, respectively. After steam explosion and biodegradation of Lentinus edodes, the test results of hemicellulose content were 23.80%, 25.30%, 47.50%, 26.20%, and 56.10% in GM2, GM3, GM4, GM5, and GM6, respectively. And the measurement yield and relative content of sugars and uronic acids of hemicellulose isolated from natural, steamed and biodegraded Quercus Linn wood. Table 1 showed that hemicellulose of Quercus Linn wood regularly changed during steam explosion and biodegradation of Lentinus edodes. During steam explosion in 100 °C water vapor for 60 h, xylose of hemicellulose was hydrolyzed and degraded, and decreased from 70.37% to 48.70% or 40.47%, resulting that other ingredients increased relatively (Table 1). Though xylose decreased after steam explosion, hemicellulose content increased because cellulose hydrolyzed and extractives were volatilized. Moreover, if xylose of hemicellulose was deficiently degradation during steam explosion, Lentinus edodes mycelium would not live until die in Quercus Linn wood. During the mycelium growth, arabinose and glucose of hemicellulose biodegraded, and other ingredients increased relatively. Furtherly, if arabinose and glucose of hemicellulose was over degradation during the mycelium growth, Lentinus edodes would not live until die in Quercus Linn wood. During Lentinus edodes growth, arabinose, galactose and glucose of hemicellulose biodegraded and continued to decresed, and other ingredients increased relatively. The above results suggested that the different ingredients of hemicellulose decreased during steam explosion and biodegradation of Lentinus edodes, however hemicellulose content continued to increased because cellulose, lignin and extractives produced the great changes.
Samples
Arabinose
Galactose
Glucose
Xylose
Glucose acid
GM1
2.37
2.21
15.14
70.37
9.92
GM2
10.37
3.66
32.63
48.70
4.64
GM3
8.84
2.36
43.99
40.47
4.34
GM4
6.31
2.79
26.58
58.14
6.18
GM5
7.99
2.86
37.65
47.09
4.40
GM6
7.02
2.08
26.08
56.87
7.95
3.2 FT-IR analysis
Infrared spectroscopy (FT-IR) had been proved to be useful for analysis and identification on functional groups of polysaccharides in plant materials. In addition, it could be applied to explore structural features when combined with chemicalmethods as well as other spectrum approaches, such as NMR spectroscopy. After steam explosion and biodegradation of Lentinus edodes, hemicellulose of Quercus Linn wood had the larger transmittance in the peaks. FT-IR spectra could be used to investigate the structural goups of Quercus Linn wood and biodegraded. For comparison, the spectra of the above six samples were demonstrated Fig. 2. For wood, the peaks at 3388 cm−1, 2924 cm−1, 1596 cm−1, 1408 cm−1, and 1042 cm−1 were O—H stretch, —C—H stretch stretch, C—C stretch, CH2 bend, and C—O stretch respectively (Wen et al., 2010, 2011; Kwon et al., 2013; Aggarwal et al., 2003). After steam explosion, the transmittance of the characteristic peaks in hemicellose increased, and hemicellulose of Quercus Linn wood had the larger transmittance and were fit to grow for Lentinus edodes mycelium. After the mycelium growth, the transmittance of the characteristic peaks in hemicellose continued to increase, and hemicellulose of Quercus Linn wood had the larger transmittance and were fit to grow for Lentinus edodes. After Lentinus edodes growth, the transmittance of the characteristic peaks in hemicellose continued to increase. Morever, the different ingredients of hemicellulose decreased during steam explosion and biodegradation of Lentinus edodes, it was resulted that the transmittance of the characteristic peaks in hemicellose gradually increased after decreased after steam explosion and biodegradation of Lentinus edodes.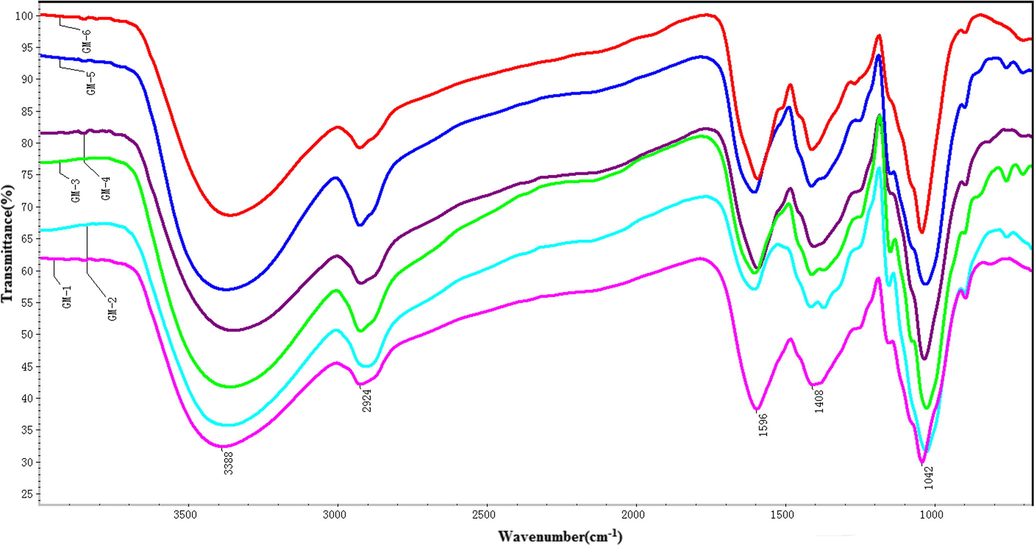
FT-IR spectra of hemicelluloses isolated natural, steamed and biodegraded wood.
3.3 TG analysis
In the present study, the pyrolysis of cellulose, hemicellulose, and lignin in TGA and packed bed, together with the energy consumption and gas product releasing behaviors were investigated in detail. The objective of this study is to gain a comprehensive understanding to hemicelluloses pyrolysis, thus facilitate to establish an universe model to simulate hemicelluloses pyrolysis. It was favorable for the development of advanced bioethanol feedstocks pyrolysis process. Morever, the regular pattern of hemicelluloses changes was revealed. During steam explosion and biodegradation of Lentinus edodes, hemicelluloses of Quercus Linn wood was degraded by steam and mycelium. And their thermal stability was a kind of effective method of characterization by TG. The thermogravimetric analyzer (TGA) was an essential laboratory tool used for material characterization. TGA was used as a technique to characterize materials used in various environmental. That was to say, TGA could measure weight changes in a controlled atmosphere with the temperature variation. In the controlled hot N2, hemicelluloses of Quercus Linn wood could react oxidation, reduction, hydration, dehydration, decomposition, and so on to cause weight loss. The GM1, GM2, GM3, GM4, GM5, and GM6 samples were contrastively investigated by TGA between room temperature and 804 °C. The TGA and DTG curves were illustrated in Fig. 4. As could be seen from Fig. 3, the thermal degradation of three samples proceeded over the wide temperature range (100–805 °C). At 10%, 30%, and 50% weight loss, the decomposition temperature were determined to be 118.9 °C, 272 °C, and 647.5 °C for GM1 sample; 114.1 °C, 276.8 °C, and 805.5 °C for GM2 sample; 116.1 °C, 269 °C, and 508 °C for GM3 sample; 130.7 °C, 275.8 °C, and 666.91 °C for GM4 sample; 118.3 °C, 275.5 °C, and 727 °C for GM5 sample; 168.4 °C, 285.62 °C, and 694.8 °C for GM6 sample; respectively. It was suggested that their thermal stability of hemicelloses were adjacent at 10% and 30% weight loss, whereas these were variant at 50% weight loss. At 100 °C, the weight loss of GM1, GM2, GM3, GM4, GM5, and GM6 samples were 8.79%, 8.26%, 8.83%, 8.20%, 7.70%, and 6.06%, respectively. At 200 °C, the weight loss of GM1, GM2, GM3, GM4, GM5, and GM6 samples were 17.06%, 18.43%, 16.16%, 15.86%, 16.66%, and 13.11, respectively. At 300 °C, the weight loss of GM1, GM2, GM3, GM4, GM5, and GM6 samples were 37.16%, 33.44%, 37.52%, 34.81%, 34.39%, and 32.63%, respectively. At 803.3 °C, the weight loss of GM1, GM2, GM3, GM4, GM5, and GM6 samples were 58.97 49.29%, 63.28%, 56.71%, 52.97%, and 58.21%, respectively. It was resulted that their thermal stability of hemicelloses had tiny difference at 100 °C, 200 °C, 300 °C and 803.3 °C.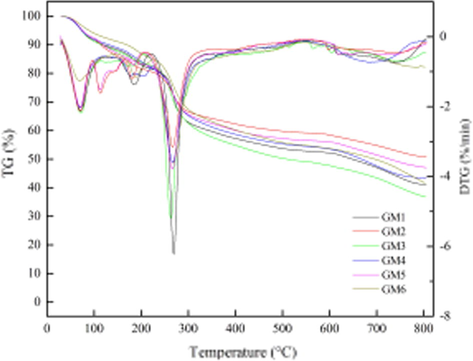
TGA/DTG Curves of hemicelluloses isolated from natural, steamed and biodegraded wood.
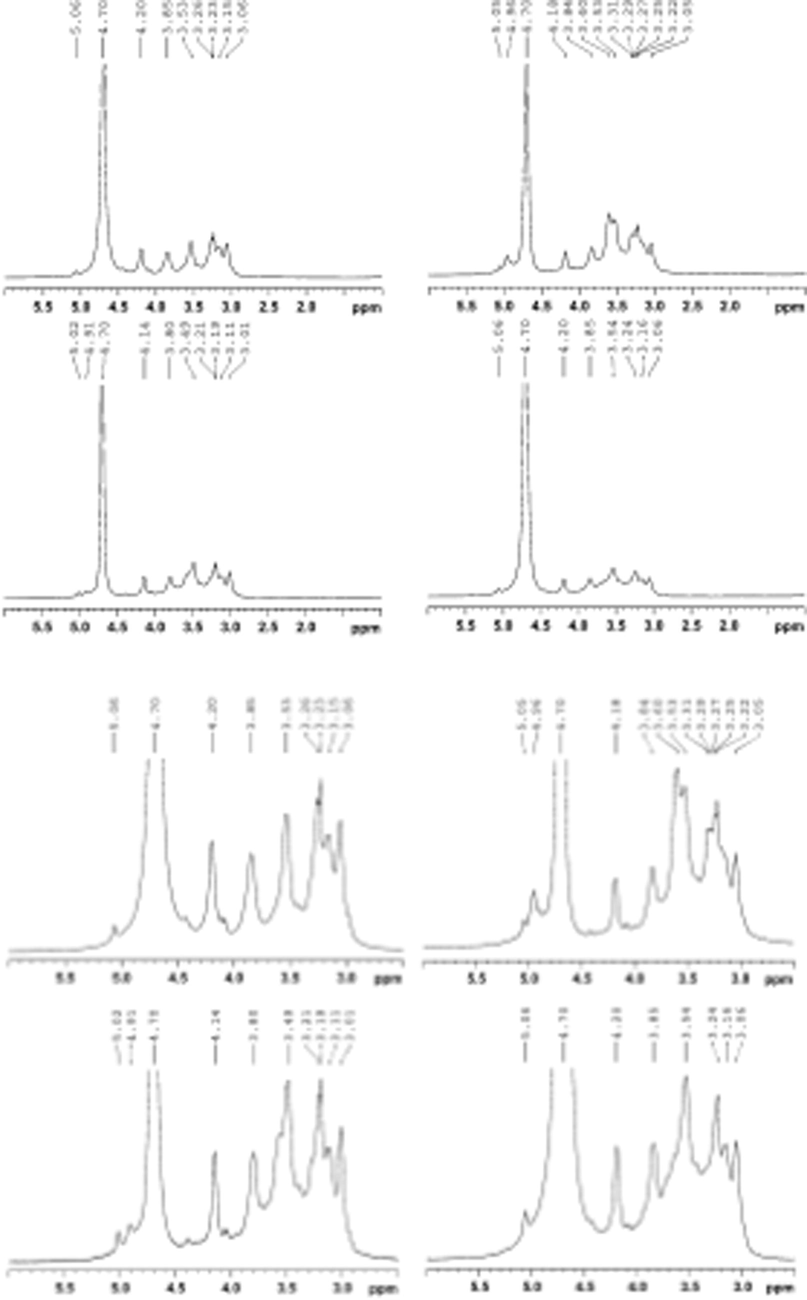
1H NMR spectra of hemicellulosic fractions GM1, GM3, GM5 and GM6.
Furtherly, the DTG curves presented the weight loss rates, and DTGmax was the maximum thermal degradation rate, which could estimate degree of thermal degradation (Gedemer, 1974). The DTG curves of six samples had three turning points with the increase of temperature. The first DTGmax values, which ethanol evaporated, were found to be 70.67 °C, 68.16 °C, 73.21 °C, 69.52 °C, 70.53 °C and 69.24 °C for GM1, GM2, GM3, GM4, GM5 and GM6 sample, respectively. The second DTGmax values, which hemicelloses produced the thermal cracking reaction, were found to be 184 °C, 182 °C, 182 °C, 178 °C, 185 °C and 184 °C for GM1, GM2, GM3, GM4, GM5 and GM6 sample, respectively. The third DTGmax values, which hemicelloses produced the carbonation reaction, were found to be 269.90 °C, 264.85 °C, 262.97 °C, 266.61 °C, 267.16 °C and 265.93 °C for GM1, GM2, GM3, GM4, GM5 and GM6 sample, respectively. Whereas the other turning point of DTGmax values of GM2 and GM5 were 113.58 °C and 112.93 °C. Ir induced that the maximum thermal degradation rates of six amples were contiguous after steam explosion and biodegradation of Lentinus edodes.
3.4 NMR spectra
The most powerful tool for biopolymers analysis was NMR spectroscopy. For the majority of these biopolymers, well-resolved 1D and 2D NMR Spectra (13C, 1H, and HSQC spectra) could be acquired from the intact polymers in D2O, DMSO‑d6, or other solvents. To further investigate the structural characteristics of polymers extracted with different saples, the hemicellulosic preparations of GM1, GM3, GM5 and GM6 were studied using 1D and 2D NMR spectroscopy. NMR spectras were supposed to identify and assay the type of sidechain branching along the backbone and the polymer backbone. Hemicelluloses fractions GM1, GM3, GM5 and GM6 were isolated from wood samples during the growth of Lentinus edodes. The 1H and HSQC spectra of hemicelluloses fractions GM1, GM3, GM5 and GM6 were shown in Figs. 4 and 5, respectively. The signals for 1H NMR were assigned on the basis of the HSQC spectra and previous literature (Wen et al., 2010, 2011; Chaikumpollert et al., 2004; Vignon et al., 1998; Xiao et al., 2013).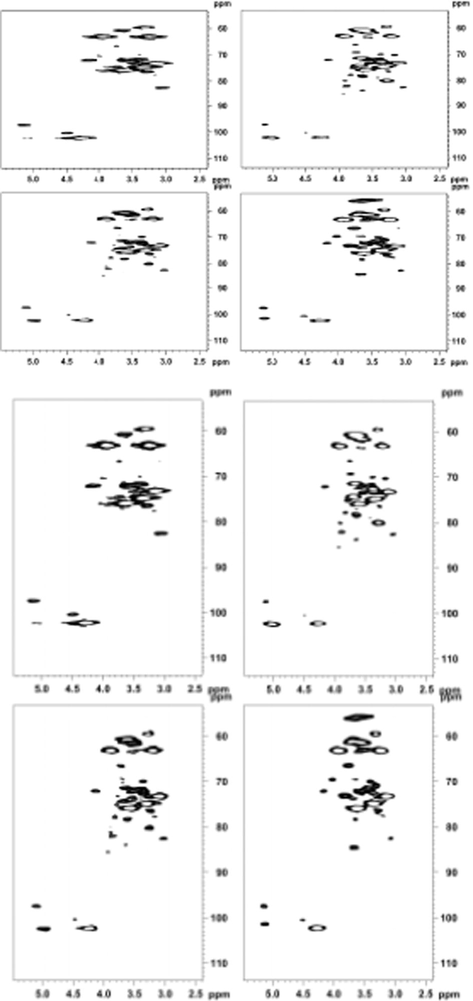
HSQC spectra of hemicellulosic fractions GM1, GM3, GM5 and GM6.
As could be seen from Fig. 4, the two hemicellulosic preparations showed that analogous 1H NMR spectra indicated a similar structure of hemicelluloses. The anomeric signals in the 1H NMR spectra of GM1, GM3, GM5 and GM6 were assigned according to sugar analysis and the literature data, and listed in the spectral region of 4.3–5.6 ppm. The relevant signals distinguished at two regions, namely, the ring proton region (δ4.5–3.0) and the anomeric region (δ5.6–4.9 for α-anomers and δ4.9–4.3 for β-anomers). The anomeric protons were well occurred in δ 4.28 and δ4.30, which were assigned as (1 → 4)-β-d-Xylp of GM1, GM3, GM5 and GM6, respectively. It confirmed that (1 → 4)-β-d-Xylp was linked β-glycosidically, which was consistent with the presence of the small sharp peak at about 890 cm−1 in the IR spectra of GM1, GM3, GM5 and GM6.
Fig. 5 showed the HSQC spectras of GM1, GM3, GM5 and GM6. And the dominant four cross-peaks could be expressly identified at 63.3/3.94, 102.7/4.30, 76.0/3.63, 75.2/3.36, 73.3/3.15 ppm, which were assigned to C5-H5, C4-H4, C3-H3, C2-H2, and C1-H1 of the (1 → 4)-linked-β-d-Xylp units, respectively. And the presence of the methyl group (OCH3) of 4-O-methyl-d-glucuronic acid was found by a corresponding small but distinguishable cross-peak at 60.7/3.33 ppm. What’s more, some weak cross-peaks, which represented 4-O-methyl-d-glucuronic acid and l-arabinose, were also found. But the weak signals were probably due to the low abundance of these components. HSQC contour volume integral was a promising method to estimate the relative quantity of components in hemicelluloses. Based on HSQC, the relative ratios of compositions in hemicelluloses could be estimated by integrating contour volumes corresponding to carbohydrates’ anomeric C1-H1 correlations. The relative ratio of xylose/arabinose in GM1, GM3, GM5 and GM6 was determined based on the integration of corresponding anomeric cross-peaks, and the ratio of xylose/arabinose was subsequently calculated. However, the relatively lower xylose/arabinose ratio obtained by HSQC integration was probably because the low content of hemicelluloses could not be detected in HSQC in Fig. 5. Compared with GM1, GM3, GM5 and GM6 fraction presented a similar molar ratio of xylose/arabinose estimated by contour volume integral. The discrepancy of ratios xylose/arabinose obtained from sugar analysis and NMR contour volume integral was similarly attributed to the above-mentioned reason. However, In comparison with the relative sugar molar ratio obtained by sugar analysis, the HSQC contour volume integral could be still considered as a methodology of semiquantitative estimates. And then, as a novel and speedy approach to achieve both structural information and quantitative information, 2D-HSQC-NMR spectroscopy demonstrated the powerful functionalities in quantitative and qualitative estimation of polysaccharides.
Simultaneously, hemicellulosic structural analysis using 1H NMR spectroscopy was limited because of the structural diversity of the different protons along the chain and their coupling patterns. HSQC together with the 1H NMR spectrum clearly showed the typical signal patterns allowed accurate quantitative analysis and expected for a hemicellulosic moiety. The direct correlations between the hydrogen and carbon atom of the glycosyl residues of GM1, GM3, GM5 and GM6 could also be observed through the crosspeaks in the HSQC spectrum. The signals at δ3.1–5.4 were caused by the protons of the arabinose and xylose residues except for the strong signal at δ4.7. The resonance at δ1.29 was the methyl protons of ethanol retained from the ethanol precipitation of the hemicelluloses. Another resonance at δ1.82 was to the methyl protons of a few acetyl groups on the hemicelluloses. One point should be explained, which was probably due to degradation of ester bonds in the acetyl groups. While the 1H NMR spectrum was conducted the sample was dissolved in D2O, therefore, the occurrence of a signal at δ1.82 represented the protons of the CH3 from the acetyl groups. The chemical shifts of δ3.1–4.3 were assigned to the protons of the anhydroxylose units of the hemicelluloses. The signal at δ8.42 was assigned to the proton of the carboxy group from 4-O-Me-a-d-GlcpA. The signals at δ5.25 in the 1H NMR spectrum have been assigned to protons at C-1 of 4-O-methyl-d-glucuronic acid residues. The relevant signals occurred in two regions, namely, the anomeric region (δ4.9–4.3 for the b anomers and δ5.6–4.9 for the a anomers) and the ring proton region (δ4.5–3.0). Another signal at δ5.36 in the 1H NMR spectrum was attributed to protons at C-1 of the a-LAraf residues. It confirmed that 4-O-methyl-d-glucuronic acid and l-arabinose were of the a-configuration, which was in agreement with the presence of a small peak at about 830 cm−1 in the FTIR spectrum of GM1, GM3, GM5 and GM6.
Based on the 2D-HSQC and 1H NMR spectra, it was deduced that the anhydroxylose units of H3 were indicated by signals at δC/δH 63.6/4.06 (H-5 eq), 102.3/4.45 (H-1), and 63.6/3.34 (H-5ax), 74.7/3.54 (H-3), 76.4/ 3.75 (H-4), and 73.4/3.28 (H-2), in which chemical shifts of δ3.34 and δ4.06 were assigned to the equatorial and axial protons linked at C-5, respectively. A very weak signal atδ6.36 and δ6.32 corresponds to the C-2 of ferulic acid and residual p-coumaric acid (Hallac et al., 2009). In addition, some very weak signals at 6.87, 6.89, 7.29, 7.33, 7.48, 7.50 ppm were assigned to the fractions of the associated lignin. The relative molar ratio of xylan and 4-O-Me-a-d-GlcA in the hemicelluloses GM1, GM3, GM5 and GM6 could be obtained by the integrated values for the H-1 in (1 → 4)-b-d-Xylp-2-O-(4-O-Me-a-d-GlcpA) and (1 → 4)-b-d-Xylp to H-1 in 4-O-Me-a-d-GlcpA (anomeric protons) in the 1H NMR spectrum. It indicated that GM1, GM3, GM5 and GM6 contained 15 xylose units for every one 4-O-Me-a-d-GlcpA unit in the hemicelluloses extracted from GM1, GM3, GM5 and GM6. The discrepancies could be easily explained by the fact that aldouronic acids were difficult to hydrolyze under acidic conditions. Thus the 4-OMe-a-d-GlcpA was obtained in relatively low proportions in hemicelluloses. And one detail that should not be ignored was that the peak was more intense at δ5.36 than at δ5.25. GM1, GM3, GM5 and GM6 contained more arabinose than glucuronic acid, which was confirmed by the sugar analysis. To be more precise, it was obtained the relative molecular ratio of arabinose and 4-O-Me-a-d-GlcpA for the H-1 in l-Araf and 4-O-Me-a-d-GlcpA (anomeric protons) in the 1H NMR spectrum. It further quantitatively described the side-chain structure of hemicelluloses extracted by aqueous alkali. Besides some weak signals at 59.8/3.43, 97.9/5.25 ppm, corresponding to OCH3, and H-6 of the 4-O-methyl-d-glucuronic acid residues were also found.
Based on NMR spectra and FT-IR spectra concerning the linkages between the monosaccharides of GM1, GM3, GM5 and GM6, it could be concluded that hemicelluloses (GM1, GM3, GM5 and GM6) were formed by a linear backbone of (β-1 → 4)-Xylp residues and the xylose residues was substituted at C-3 by arabinose, whereas the xylose residue was substituted at C-2 by 4-O-Me-GlcA. Furthermore, the (β-1 → 4)-Xylp residues in the backbone might contain substituted phenolic acids (p-coumaric acid and ferulic acid).
The NMR spectra of GM1, GM3, GM5 and GM6 presented the straightforward structure as revealed by four major signals corresponding to (1 → 4)-linked b-DXyl residues and other less intense signals characteristic of a 4-Omethyl-a-d-glucuronic acid residue as well as an l-Araf residue and b-d-Xyl units substituted with 4-O-methyl-a-d-GlcpA. However, the signals of the glucans couldn’t be found in the NMR spectrum. And various analytical techniques, such as FTIR, sugar analysis, and NMR data, a possible chemical structure of hemicelluloses extracted with alkalis from GM1, GM3, GM5 and GM6 was l-arabino-4-O-methyl-d-glucurono-d-xylan.
4 Conclusions
The different ingredients of hemicellulose decreased during steam explosion and biodegradation of Lentinus edodes, however hemicellulose content continued to increased. During steam explosion in 100 °C water vapor for 60 h, xylose of hemicellulose was decreased from 70.37% to 40.47%, and other ingredients increased relatively. During the mycelium growth, arabinose and glucose of hemicellulose biodegraded, and other ingredients increased relatively. During Lentinus edodes growth, arabinose, galactose and glucose of hemicellulose biodegraded and continued to decresed, and other ingredients increased relatively.
The FTIR spectroscopy shows that hemicellulose of Quercus Linn wood groups firstly began to fracture and degrade after wood was steam explosion and biodegradation of Lentinus edodes. The six hemicellose samples of natural, steamed and biograded wood had the similar spectral patterns. And the transmittance of the characteristic peaks in hemicellose gradually increased after decreased after steam explosion and biodegradation of Lentinus edodes. TGA and DTG curves that thermal stability and maximum thermal degradation rates of six amples were contiguous after steam explosion and biodegradation of Lentinus edodes. According to the weight loss, the thermal stability of hemicelloses had tiny difference at 100 °C, 200 °C, 300 °C and 803.3 °C. According to the DTGmax values, the maximum thermal degradation rates of six amples were contiguous after steam explosion and biodegradation of Lentinus edodes.
By combining NMR with FTIR spectroscopy, The NMR spectra of GM1, GM3, GM5 and GM6 presented the straightforward structure as revealed by four major signals corresponding to (1 → 4)-linked b-d-Xyl residues and other less intense signals characteristic of a 4-Omethyl-a-d-glucuronic acid residue as well as an l-Araf residue and b-d-Xyl units substituted with 4-O-methyl-a-d-GlcpA. Furthermore, a small amount of other minor hemicelluloses (β-glucans) including xylans in the extractable hemicelluloses could be identified by NMR and other approaches.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31170532), and Invitation Fellowship Programs for Research in Japan of Japan Society for the Promotion of Science (ID No. S14748). Thank Jialong Wen and Runcang Sun (Beijing Forestry University) for the help provided in the experiment.
References
- Water repellent treatments for catamaran grade Bombax ceiba Linn. (Spermatophyta/Dicotyledoneae) wood. Indian J. Mar. Sci.. 2003;32:340-343.
- [Google Scholar]
- Alterations in structure, chemistry, and biodegradability of grass lignocellulose treated with the white rot fungi Ceriporiopsis subvermispora and Cyathus stercoreus. Appl. Environ. Microbiol.. 1995;61:1591-1598.
- [Google Scholar]
- Acid catalysed steaming for solubilization of bamboo grass xylan. Bioresour. Technol.. 1999;69:91-94.
- [Google Scholar]
- The potential of palm oil mill effluent (POME) as a renewable energy source. Acta Sci. Malaysia. 2017;1(2):09-11.
- [Google Scholar]
- A study on water quality from Langat River, Selangor. Acta Sci. Malaysia. 2017;1(2):01-04.
- [Google Scholar]
- Effect of different bedding materials on relative yield of oyster mushroom in the successive flushes. Pakistan J. Agr. Res.. 1987;8:256-259.
- [Google Scholar]
- Shiitake dermatitis recorded by French Poison Control Centers–new case series with clinical observations. Clin. Toxicol.. 2014;52:625-628.
- [Google Scholar]
- cAMP-mediated differential regulation of lignin peroxidase and manganese-dependent peroxidase production in the white-rot basidiomycete Phanerochaete chrysosporium. Proc. Natl. Acad. Sci. USA. 1992;89:5586-5590.
- [Google Scholar]
- Structural elucidation of hemicelluloses from Vetiver grass. Carbohydr. Polym.. 2004;57:191-196.
- [Google Scholar]
- World production of cultivated edible and medicinal mushrooms in 1997 with emphasis on Lentinus edodes (Berk.) Sing, in China. Int. J. Med. Mushrooms.. 1999;1:291-300.
- [Google Scholar]
- Chang, S.T., Hayes, W.A., 2013. Volvariella volvacea - The Biology and Cultivation of Edible Mushrooms - 27. The Biology and Cultivation of Edible Mushrooms, pp. 573–603.
- Key technologies for bioethanol production from lignocellulose. Biotechnol. Adv.. 2010;28:556-562.
- [Google Scholar]
- Steam explosion of the softwood Pinus radiata with sulphur dioxide addition. I. Process optimisation. J. Wood Chem. Technol.. 1987;9:135-166.
- [Google Scholar]
- Synthesis of Single Cell Protein (SCP) from wheat straw and its fractions. Ind. J. Microbiol.. 1979;25:793-797.
- [Google Scholar]
- Bioconversion of forest products industry waste cellulosics to fuel ethanol: a review. Bioresour. Technol.. 1996;55:1-33.
- [Google Scholar]
- Lentinus edodes and Pleurotus species lignocellulolytic enzymes activity in submerged and solid-state fermentation of lignocellulosic wastes of different composition. Bioresour. Technol.. 2008;99:457-462.
- [Google Scholar]
- Steam-explosion of olive stones: hemicellulose solubilization and enhancement of enzymatic hydrolysis of cellulose. Bioresour. Technol.. 2001;79:53-61.
- [Google Scholar]
- The use of derivative thermogravimetry to estimate degree of thermal degradation. J. Macromol. Sci. Chem.. 1974;8:95-104.
- [Google Scholar]
- Total phenolic content and total flavonoid content in moringa oleifera seed. Sci. Heritage J.. 2017;1(1):23-25.
- [Google Scholar]
- Laccase immunolabelling and microanalytical analysis of wood degraded by Lentinus edodes. Holzforschung. 1998;52:345-350.
- [Google Scholar]
- Biomass characterization of Buddleja davidii: a potential feedstock for biofuel production. J. Agric. Food Chem.. 2009;57(4):1275-1281.
- [Google Scholar]
- Salicylic acid mitigates pb stress in Nicotiana tabacum. Sci. Heritage J.. 2017;1(1):16-19.
- [Google Scholar]
- Comparison between measured traffic noise in Klang Valley, Malaysia and existing prediction models. Eng. Heritage J.. 2017;1(2):10-14.
- [Google Scholar]
- Sustainable films and coatings from hemicelluloses: a review. Biomacromolecules. 2008;9:1493-1505.
- [Google Scholar]
- Influence of seed loads on start up of modified anaerobic hybrid baffled (MAHB) reactor treating recycled paper wastewater. Eng. Heritage J.. 2017;1(2):05-09.
- [Google Scholar]
- Pretreatment of wheat straw by white-rot fungi for enzymic saccharification of cellulose. Eur. J. Appl. Microbiol. Biotechnol.. 1983;18:350-357.
- [Google Scholar]
- La shiitake dermatitis (dermatose toxique au lentin) est arrivée en France. Ann. Dermatol. Venereol.. 2010;137:290-293.
- [Google Scholar]
- Three kinds of antibacterial substances from Lentinus edodes (Berk.) Sing. (Shiitake, an edible mushroom) Int. J. Antimicrob. Agents. 1999;11:151-157.
- [Google Scholar]
- Management of end-of-life electrical and electronic products: the challenges and the potential solutions for management enhancement in developing countries context. Acta Sci. Malaysia. 2017;1(2):05-08.
- [Google Scholar]
- Physicochemical responses and microbial characteristics of shiitake mushroom (Lentinus edodes) to gum arabic coating enriched with natamycin during storage. Food Chem.. 2013;138:1992-1997.
- [Google Scholar]
- Engineering yeasts for xylose metabolism. Curr. Opin. Biotechnol.. 2006;17:320-326.
- [Google Scholar]
- Khan, I.U., Sajid, S., Javed, A., Sajid, S., Shah, S.U., Khan, S.N., Ullah, K., 2017. Comparative diagnosis of typhoid fever by polymerase chain reaction and Widal test in Southern Districts (Bannu, Lakki Marwat And D.I.Khan) Of Khyber Pakhtunkhwa, Pakistan. Acta Sci. Malaysia 1(2), 12–15.
- A polysaccharide isolated from the liquid culture of Lentinus edodes (Shiitake) mushroom mycelia containing black rice bran protects mice against a Salmonella lipopolysaccharide-induced endotoxemia. Food Chem.. 2014;61:10987-10994.
- [Google Scholar]
- Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol. Biofuels. 2008;1:1-9.
- [Google Scholar]
- Change of heating value, pH and FT-IR spectra of charcoal at different carbonization temperatures. J. Korean Wood Sci. & Tech.. 2013;41:440-446.
- [Google Scholar]
- Cultivation of shiitake, the Japanese forest mushroom, on logs: a potential industry for the United States. Forest. Prod. J.. 1982;32:29-35.
- [Google Scholar]
- Extracellular enzymes produced by the cultivated mushroom Lentinus edodes during degradation of a lignocellulosic medium. Appl. Environ. Microbiol.. 1985;50:859-867.
- [Google Scholar]
- The ligninolytic activities of Lentinus edodes and Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol.. 1986;24:51-58.
- [Google Scholar]
- Autohydrolysis pretreatment of Coastal Bermuda grass for increased enzyme hydrolysis. Bioresour. Technol.. 2009;100:6434-6441.
- [Google Scholar]
- Comparison of steam and ammonia pretreatment for enzymatic hydrolysis of cellulose. Appl. Microbiol. Biotechnol.. 1988;29:462-468.
- [Google Scholar]
- Commercial cultivation of shiitake in sawdust filled plastic bags. Develop. Crop Sci.. 1987;10:421-426.
- [Google Scholar]
- Production of ethanol from hemicellulose fraction of cocksfoot grass using pichia stipitis. Sustain. Chem. Process.. 2013;1:1-7.
- [Google Scholar]
- Comparative study on the growth and yield of Pleurotus ostreatus mushroom on different lignocellulosic by-products. J. Ind. Microbiol. Biotechnol.. 2003;30:146-149.
- [Google Scholar]
- Comparative study of different substrates for the growth and production of Lentinus edodes Berk (“ Shiitake”) Fitopatologia. 1998;8:566-569.
- [Google Scholar]
- Permana, I.G., Meulen, U.T., Zadrazil, F., 2009. Cultivation of Pleurotus ostreatus and Lentinus edodes on Linocellulosic Substrates Tes for human food and animal feed production. J. Agric and Rural Dev. Tropics Subtropics.
- Potential bioethanol and biogas production using lignocellulosic biomass from winter rye, oilseed rape and faba bean. Biomass Bioenergy. 2007;31:812-819.
- [Google Scholar]
- Effect of certain amendments on cellulases and yield of straw mushroom. Indian J. Mushroom. 1976:360-374.
- [Google Scholar]
- Validation of microscopic dynamics of grouping pedestrians behavior: from observation to modeling and simulation. Eng. Heritage J.. 2017;1(2):15-18.
- [Google Scholar]
- Red pigment production by monascus purpureus in stirred-drum bioreactor. Sci. Heritage J.. 2017;1(1):13-15.
- [Google Scholar]
- Enhanced yield of shiitake by saccharide amendment of the synthetic substrate. Appl. Environ. Microbiol.. 1990;56:479-482.
- [Google Scholar]
- Steam pretreatment of H2SO 4-impregnated Salix for the production of bioethanol. Bioresour. Technol.. 2008;99:137-145.
- [Google Scholar]
- Tight repression of elastase strain K overexpression by Pt7 (A1/O4/O3) shuttle expression system. Sci. Heritage J.. 2017;1(1):20-22.
- [Google Scholar]
- Utilization of hemicellulosic fraction of lignocellulosic biomaterial for bioethanol production. Adv. Appl. Sci. Res.. 2011;2:508-521.
- [Google Scholar]
- Analysis of passengers’ access and egress characteristics to the train station. Eng. Heritage J.. 2017;1(2):01-04.
- [Google Scholar]
- Fractional extraction and structural characterization of sugarcane bagasse hemicelluloses. Carbohydr. Polym.. 2004;56:195-204.
- [Google Scholar]
- Extraction and characterization of xylose-rich pectic polysaccharide from wheat straw. Int. J. Polym. Anal. Charact.. 1998;4:345-356.
- [Google Scholar]
- Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int. J. Mol. Sci.. 2008;9:1621-1651.
- [Google Scholar]
- A review of 5-hydroxymethylfurfural (HMF) in parenteral solutions. Toxicol. Sci.. 1984;4:843-853.
- [Google Scholar]
- Monitoring decay of black gum wood (Nyssa sylvatica) during growth of the shiitake mushroom (Lentinula edodes) using diffuse reflectance infrared spectroscopy. Appl. Spectrosc.. 2003;57:514-517.
- [Google Scholar]
- Biodegradation of oak (Quercus alba) wood during growth of the shiitake mushroom (Lentinula edodes): a molecular approach. J. Agric. Food Chem.. 2003;51:947-956.
- [Google Scholar]
- Isolation 1H and 13 C NMR studies of (4-O-methyl-D-glucurono)-D-xylans from luffa fruit fibres, jute bast fibres and mucilage of quince tree seeds. Carbohydr. Res.. 1998;307:107-111.
- [Google Scholar]
- Fractional isolation and chemical structure of hemicellulosic polymers obtained from Bambusa rigida species. J. Agric. Food Chem.. 2010;58:11372-11383.
- [Google Scholar]
- Comparative study of alkali-soluble hemicelluloses isolated from bamboo (Bambusa rigida) Carbohydr. Res.. 2011;346:111-120.
- [Google Scholar]
- Biodegradation of lignocellulose by white-rot fungi: structural characterization of water-soluble hemicelluloses. BioEnergy Res.. 2013;6:1154-1164.
- [Google Scholar]
- Comparative study of water-soluble and alkali-soluble hemicelluloses from perennial ryegrass leaves (Lolium peree) Carbohydr. Polym.. 2007;67:56-65.
- [Google Scholar]
- Immunopotentiating activity of the water-soluble lignin rich fraction prepared from LEM—the extract of the solid culture medium of Lentinus edodes Mycelia. Biosci. Biotechnol. Biochem.. 1997;61:1909-1912.
- [Google Scholar]
- Adaptation fermentation of Pichia stipitis and combination detoxification on steam exploded lignocellulosic prehydrolyzate. Nat. Sci.. 2009;01:47-54.
- [Google Scholar]
- Oyster mushroom cultivation with rice and wheat straw. Bioresour. Technol.. 2002;82:277-284.
- [Google Scholar]







