Translate this page into:
High performance of PES-GNs MMMs for gas separation and selectivity
⁎Corresponding authors at: Department of Production Engineering, Faculty of Mechanical Engineering and Design, Kaunas University of Technology, LT-51424 Kaunas, Lithuania alakha@kth.se (Alaa Mohamed), ahmed.saed@ktu.lt (Alaa Mohamed), ahmed.saed@ktu.lt (Samy Yousef)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, graphene nanosheets (GNs) were incorporated into polyethersulfone (PES) by phase inversion approach for preparing PES-GNs mixed matrix membranes (MMMs). To investigate the impact of filler content on membrane surface morphology, thermal stability, chemical composition, porosity and mechanical properties, MMMs were constructed with various GNs loadings (0.01, 0.02, 0.03, and 0.04 wt%). The performance of prepared MMMs was tested for separation and selectivity of CO2, N2, H2 and CH4 gases at various pressures from 1 to 6 bar and temperature varying from 20 to 60 °C. It was observed that, compared to the pristine PES membrane, the prepared MMMs significantly improved the gas separation and selectivity performance with adequate mechanical stability. The permeability of CO2, N2, H2 and CH4 for the PES + 0.04 wt% GNs increases from 9 to 2246, 11 to 2235, 9 to 7151, and 3 to 4176 Barrer respectively, as compared with pure PES membrane at 1 bar and 20 °C due to improving the membrane absorption and porosity. In addition, by increasing the pressure, the permeability and selectivity of CO2, N2, H2 and CH4 are increased due to the increased driving force for the transport of gas via membranes. Furthermore, the permeability of CO2, N2, H2 and CH4 increased by increasing the temperature from 20 to 60 °C due to the plasticization in the membranes and the improvement in polymer chain movement. This result proved that the prepared membranes can be used for gas separation applications.
Keywords
Polyethersulfone (PES)
Graphene nanosheets (GNs)
Mixed matrix membranes (MMMs)
Gas separation membrane
CO2/N2, CO2/H2 and CO2/CH4 selectivity
1 Introduction
Worldwide energy demand is predicted to rise because of continued economic progress and a growing global population. As a result, greenhouse gas emissions such as carbon dioxide, methane, and nitrous oxide will be increased (Martin-Gil et al., 2019; Juber et al., 2021). According to the World Meteorological Organization (WMO), carbon dioxide levels in the atmosphere reached 393 ppm in 2012, an average increase of 2 ppm per year over the previous ten years (Wong et al., 2021; Yousef et al., 2021). In addition, CO2, which is a pollutant in natural gas wells, lowers the energy content of the gas and becomes acidic and caustic in the presence of water, carbon dioxide/methane separation is critical for natural gas processing. Traditional CO2 capture methods (such as cryogenic distillation and amine-based absorption) consume a lot of energy (Castro-Muñoz et al., 2020; Asif et al., 2021). Therefore, a cost-effective, efficient, and environmentally friendly capture process is required.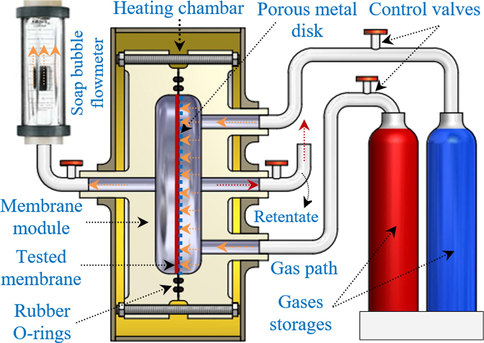
(a) Schematic of the gas permeation set-up.
Among numerous technologies, membrane technology has attracted great attention in water and gas separation due to its highly competitive energy prices and environmental issues (Khalil and Schäfer, 2021; Mohamed et al., 2020). Currently, the removal of CO2 and acidic gases from natural gas is carried out on a large-scale using the membrane separation process. Polyethersulfone (PES) is the most widely used material for the preparation of ultrafiltration (UF) and nanofiltration (NF) membranes for the gas separation process due to its strong mechanical, thermal, and chemical stability, as well as the fact that the inclusion of an ether unit in the polymer provides an alternative binding mechanism for CO2 molecules (Abdel-Karim et al., 2018; Alenazi et al., 2017). The phase inversion process developed for the manufacture of polymeric membranes that are commercially viable and reproducible (Castro-Muñoz et al., 2018). These membranes possess have a skin layer on the top, followed by a porous sublayer, which achieved high permeability and selectivity (Sazali et al., 2019; Yousef et al., 2022). The pore size of the polymeric membranes is greatly affected by external forces due to the deformation of the polymer material under high pressure and elevated temperature (Tan and Rodrigue, 2019; Mohamed et al., 2016). Mechanical properties of the membrane depend on the polymer properties and the pore topology (Karim et al., 2018; Salama et al., 2018). Hence, to increase the mechanical strength and enhance membrane performance, polymeric membranes have been incorporated into a variety of nanomaterials with sufficient properties (physical, morphological and permeation characteristics) to improve their efficiency for gas applications, such as TiO2 (Tran et al., 2020; Yu et al., 2020), MOF (Daglar et al., 2021; Yang et al., 2020), SiO2 (Kang, 2020; Arjunan et al., 2020), GO (Shukla et al., 2017; Ouyang et al., 2015), and CNTs (Cao et al., 2020; Mohamed et al., 2017).
Graphene nanosheets (GNs) have attractive great properties such as high surface area and high hydrophilic behavior in gas separation and water treatment in the fabrication of mixed matrix membranes (MMMs) (Fatemi et al., 2020; Gontarek-Castro et al., 2021). The main important feature of the GNs membrane is the interlayer spacing between the GNs sheets that can be tailored for efficient transport of the desired molecules through the membrane (Abdel-Mottaleb et al., 2019; Castro-Muñoz et al., 2019). In addition, GNs based membrane materials have high mechanical strength and flexibility, as well as high stability in aqueous suspension owing to the presence of the functional groups such as carboxyl, carbonyl, hydroxyl, and epoxy groups (Luque-Alled et al., 2020; Abdel-Mottaleb et al., 2019). These behaviours are very important in the preparation of homogeneous membranes for gas separation (Nair et al., 2012; Li et al., 2013). For instance, Gotthold et al. (Shin et al., 2019) incorporated GO into PES membrane for the selectivity of water–ethanol separation. The results showed excellent H2O/EtOH selectivity and the membrane can effectively separate water-contaminated biofuels and the same for Geim (Nair et al., 2012) used for water-helium separation. Roberto et al. (Castro-Muñoz et al., 2019) combining graphene oxide into cross-linked PVA membranes to achieve better performance for ethanol dehydration. Moreover, the effect of filler dispersion in the polymer matrix to reach a synergistic effect has been addressed by Roberto et al. (Castro-Muñoz and Fíla, 2019). The preparation of Matrimid®-PEG 200 and ZIF-8 nanoparticles have been demonstrated. The result shows that the incorporation of 30 wt% ZIF-8 nanoparticles allowed to increase the CO2 permeability in MMMs. Srabani et al. showed that the incorporation of Mg-MOF-74 into PVAc enhanced the CO2 separation (Majumdar et al., 2020). Furthermore, the addition of PEG and ZIF-8 enhanced the CO2 permeability (Castro-Muñoz et al., 2019).
Nanostructured fillers help to reduce the shortcomings of polymer-based membranes such as aging, plasticization, and stability (e.g., physical, chemical, and thermal). However, improper merging, such as poor interface compatibility and membrane preparation process, causes particular flaws in the filler–polymer membranes, such as new non-selective gas transport channels, which enhance permeability but impair selectivity (Castro-Muñoz et al., 2017; Ahmad et al., 2020). Ultrathin inorganic membranes prepared from the nanosheets have been identified as good candidates for producing higher performance membranes while using less inorganic nanomaterials, which is one of the key advantages of this type of membrane (Mohamed et al., 2021). There are a few other advantages to using ultrathin membranes: selective-layer materials in tiny quantities, resulting in significant material cost reductions; an optimized membrane material and morphology in each layer; minimal constraints on membrane materials' mechanical qualities and processability as long as they can be produced or deposited as a thin layer on top. Membrane cost and permeance are both favored when membrane thickness decreases (Castro-Muñoz et al., 2020; Ahmad et al., 2021).
Biogas applications are typically composed of numerous gases mixed with flammable gases such as CO2, N2, and CH4. Which negatively affects the performance and economic value of the energy product and the separation process. The primary contamination sources in biogas are agglomerates, which tend to cluster together because of the force of attraction between particles gases, resulting in the formation of numerous agglomerate particles and significant pipe issues, such as corrosion. In this regard, the MMMs were developed to overcome these issues. In the literature, different studies show that the percentage of loading of NPs into the polymer are in the range of 0.5–15 wt% (Ismail et al., 2011; Yousef et al., 2021). At higher loading, NPs started to agglomerate and block the membrane pores as well as inhibit gas transportation across the produced membranes, resulting in decreased membrane lifespan and high operating costs (Parsamanesh et al., 2021). But in our work, we used only 0.04 wt% with a high gas permeability. The main objective of this paper is to prepare a pristine PES membrane as well as mixed matrix membranes incorporated with different loading of GNs by a phase inversion approach. The performance of the prepared membranes is tested for separation and selectivity performance of CO2/CH4, CO2/N2 and CO2/H2 under high pressure and elevated temperature. The physical and chemical properties of the developed membranes are characterized. The influence of the membrane deformation was also studied.
2 Experimental
2.1 Materials
Polyethersulfone (PES, Ultrason E 6020P) was purchased from BASF Company (Germany). Polyvinylpyrrolidone (PVP, Mw = 40,000 g mol−1) and N,N-Dimethylformamide (DMF, 99.8% purity) was supplied from Sigma Aldrich. GNs (95% purity, average thickness 15 nm, and average length 2 µm) were fabricated as described elsewhere (Yousef et al., 2018). CO2, N2, H2, and CH4 gases with purity ≥ 99.99% were supplied by the Lithuanian Energy Institute, Lithuania.
2.2 Membrane preparation
The pristine PES membrane and four mixed matrix membranes incorporated with different loadings of GNs (0.01, 0.02, 0.03, and 0.04 wt%) were fabricated using the phase inversion technique. The homogeneous solutions were prepared by dissolving 14 wt% PES and 1 wt% PVP in DMF at 50 °C for 6 h. After degassing, the solutions were cast on a glass substrate using a casting blade (ZAA 2300, Germany) with a 100 µm gap height and 20 mm/s shear rate (Yousef et al., 2021; Gouveia et al., 2021). The membranes were immediately immersed into a coagulant bath containing deionized water at 70 °C for 10 min and subsequently, the membranes were detached from the glass surface and transferred to a fresh deionized water for 24 h at room temperature to remove the excess solvent (Mohamed et al., 2021). After that, the membranes were dried at room temperature. For the MMM, the GNs was added to the homogeneous solutions and then followed the same procedure.
2.3 Gas permeation test
In order to determine the separation and selectivity of CO2/CH4, CO2/N2 and CO2/H2, the permeability test was applied under various pressure and temperature. The experiments were performed in a gas permeation system using a circular membrane with an effective diameter of 60 mm (see Scheme 1). The membrane was supported into the cell and gently tightly and evacuated using a vacuum pump to remove all remaining gases from the cell. Before each gas experiment, the same procedures were done. The feed flow of each gas was passing through the membrane by adjusting the pressure from 1 bar to 8 bar at different temperatures in the range of 20–60 °C. All measurements have been made triple and the average values are recorded. The gas permeability (Pi,j) and membrane selectivity (α) were calculated according to Eqs. (1) and (2)
where i refers to CO2 and j represents N2 or H2 or CH4. Q is the gas flow rate (cm3/s), A is the membrane area (cm2), t is the membrane thickness (cm), and ΔP is the transmembrane pressure difference (bar).
2.4 Characterization of the fabricated membranes
A field emission scanning electron microscope (FESEM, FEI Philips XL 30) was used to analyze the membrane's surface and the cross-section morphology. A thin coating of gold film (3–5 nm) was applied to all samples. The tests were made using a 20 keV acceleration voltage in a high vacuum (1.5 Torr). The thermal stability of the fabricated membranes was done using thermogravimetric analysis (TGA, Mettler Toledo TGA2 Star system) from 30 to 900 °C at a rate of 10 °C min−1 under a nitrogen environment. The chemical structures and composition of membranes were examined using the Fourier-Transform Infrared spectroscopy from 4000 to 400 cm−1, 64 scans, and resolution 4 cm−1 (FTIR, Vertex70 spectrometer) and X-ray crystallography of 2θ from 3° to 60° with a 0.018–0.025° 2θ step width and 384 s per step for all samples (XRD, D8-Advance, Bruker, USA). Membrane porosity and pore size were measured by using mercury intrusion porosimetry (MIP, Pascal 440 Evo, Thermo Scientific). Tensile tests of the membranes were determined using Lloyd Universal Testing Machine (model LR10K) under ambient conditions and 5 mm/min loading rate. Three specimens 100 mm × 10 mm from each membrane were cut off before the test.
3 Results and discussion
Fig. 1 demonstrates the surface morphology, porous structure and the cross section of the synthesized MMMs. Fig. 1a-e shows that the synthesized MMMs were uniform, smooth and homogeneous without obvious defects such as pores or cracks. All the cross section membranes were prepared by being broken into liquid nitrogen. The cross-sectional images showed an asymmetrical structure consisting of a thin dense top layer and a porous sublayer with a macropores finger and mesoporous sponge structure underneath. The thin dense layer shows an infinite number of pores created during the preparation process, responsible for the permeation and retention of the solution. While the porous bulk acts as mechanical support. For the pristine PES membrane, there are smaller porous fingers and a decrease in the number of voids in comparison with the PES-GNs matrix membrane with a thickness of about 100 µm. This behaviour is attributed to the hydrophilic of GNs which improve the replacement of the water in the phase inversion process. In addition, the pore size distribution of the prepared MMMs (Fig. 1k) decreased from 100 to 65 nm with increasing the GNs content. While the porosity increased with the incorporation of GNs from 82.4% for the PES membrane to 86.5% for PES + 4 wt% GNs which is in line with the analysis of the membrane morphology.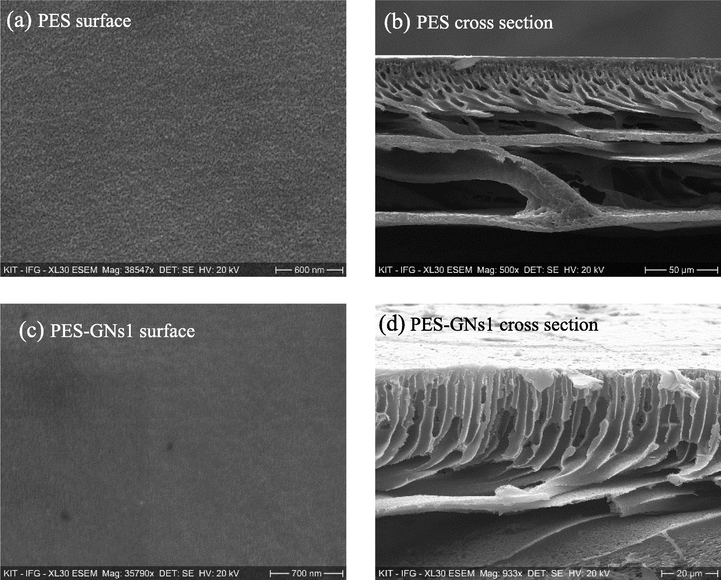
Surface morphology and cross-sectional of a, b) PES, c, d) PES-GNs1, e, f) PES-GNs2, g, h) PES-GNs3 and I, j) PES-GNs4. K) pore size and porosity of membranes.
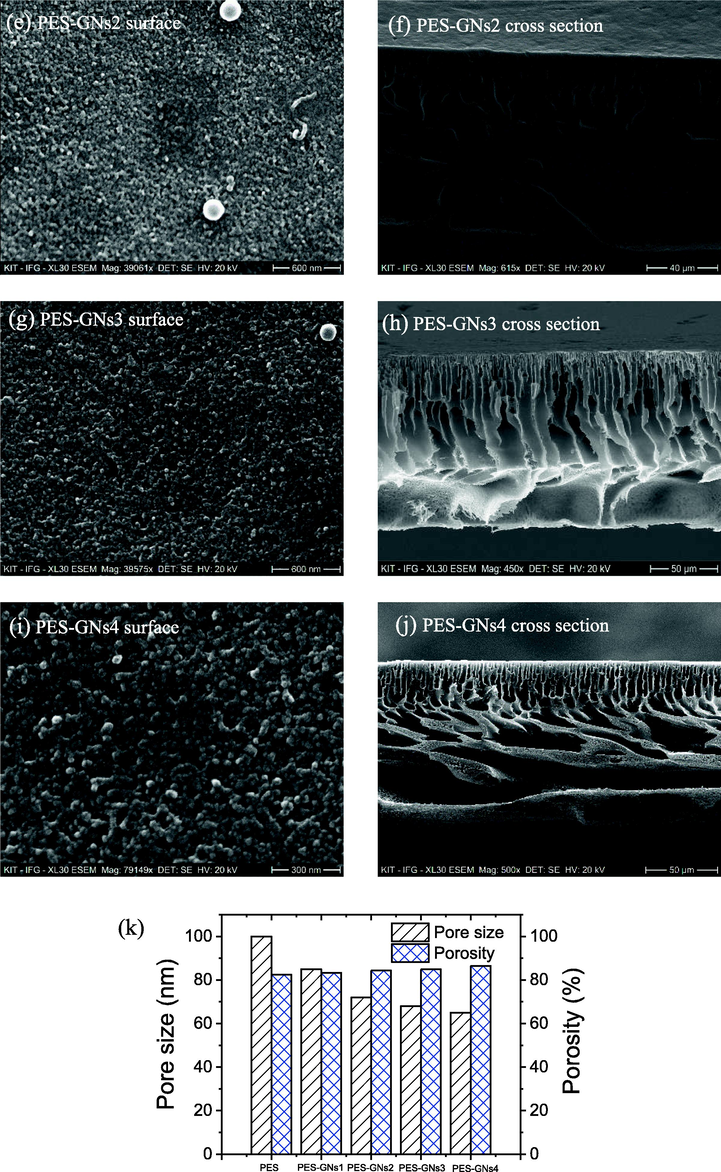
Surface morphology and cross-sectional of a, b) PES, c, d) PES-GNs1, e, f) PES-GNs2, g, h) PES-GNs3 and I, j) PES-GNs4. K) pore size and porosity of membranes.
Fig. 2a shows the FTIR spectra of pristine PES membrane and PES with different loading of GNs. The result demonstrated that the FTIR of MMMs is largely indistinguishable from pristine PES, due to the limited amount of GNs. The result shows several broad absorbance peaks at 557, 1149 and 1296 cm−1 corresponding to the sulfone groups and at 1579 and 1485 cm−1 attributed to C = C skeleton in the aromatic rings (Shin et al., 2019; Abdel-Karim et al., 2017). The peaks at 1725 and 1668 cm−1 were assigned to the carbonyl group (C = O) and the two peaks at 1105 and 1238 cm−1 were attributed to C-O stretching vibration (Yousef et al., 2021; Angione et al., 2015). In addition, Fig. 2b shows the XRD spectra of pristine PES membrane and PES with different loading of GNs. The two peaks of the PES membrane are obvious at 2θ of 13.6 and 30°, indicating the amorphous nature of PES (Guan et al., 2005; Laghaei et al., 2016). In addition, for the MMMs another sharp peak is seen around 26.3° which is corresponding to GNs into the membranes (Mohamed et al., 2020).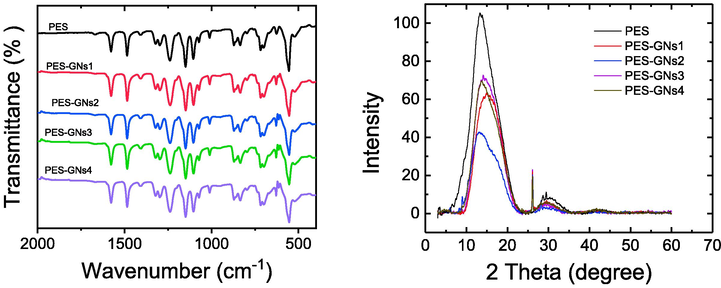
a) FTIR and b) XRD spectra of the prepared MMMs.
The main criteria for membranes used in gas separation processes are strong mechanical properties to withstand high pressures in the gas permeation system (Aboamera et al., 2019). Therefore, to determine their mechanical properties, tensile strength experiments were conducted on the membranes. Experiments were performed by stretching the specimen and measuring the instantaneous tension before the tearing at various strain rates. Fig. 3a shows the tensile stress and strain for the prepared MMMs samples. The result shows that incorporating of GNs improved the tensile strength of the MMMs in comparison to the pristine PES membrane. Furthermore, the tensile strength of MMMs is shown to be highly dependent on the loading of GNs. The pristine PES membrane showed 2.8 MPa tensile strength and by increasing the loading of GNs from 0 to 0.04 wt%, the tensile strength increased from 2.8 to 3.7 MPa with an increase of 32.14%. At the same time, the strain declined with increasing the amount of GNs, reaching a decrease of 17.46%. In addition, Young’s modulus increased from 44.44 to 71.15 MPa with an increase of 60.1%. This behaviour may be attributed to the high strength properties of the GNs, good dispersion of GNs in the PES, and the improved interfacial interaction between the two phases (Abdel-Mottaleb et al., 2020; Li et al., 2019). Furthermore, the thermal stability of the prepared membranes was conducted by TGA analysis as shown in Fig. 3b. It can be observed that the thermal decomposition curve of all samples begins from 375 to 580 °C with a weight loss of 65 wt% due to the composition of the polymer (Yousef et al., 2021). Three is no weight loss up to 100° C for all the preparations membranes; confirm the evaporation of the remaining solvent in the membranes. According to the results, adding GNs to the PES matrix did not show significant improvement in the membrane thermal stability. The beginning degradation temperatures of pristine PES and MMMs were similar and the degradation temperature of MMMs was not considerably changed when GNs was added (2 wt%) (Farnam et al., 2016).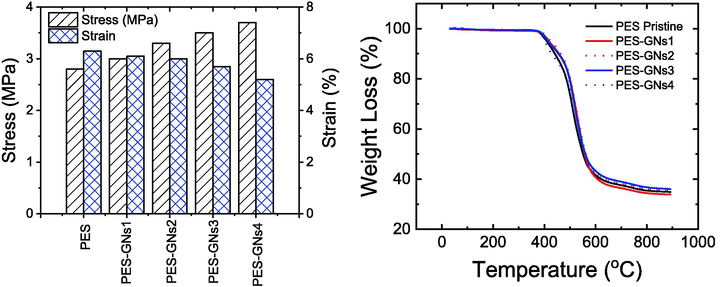
a) Mechanical properties and b) TGA analysis of the prepared MMMs.
3.1 Evaluation of gas permeability
Pure gas permeation experiments were carried out using pure CO2, N2, H2 and CH4 gases at various pressures of 1, 2, 3, 4, 5 and 6 bar to investigate the efficiency of membrane gas separation. From the preliminary results, it appears that the maximum membrane capacity to withstand the transmembrane pressure and temperature are 8 bar and 70 °C without deteriorating membrane properties. Based on that, the main experiments were performed up to 6 bar and 60 °C. Fig. 4 shows the CO2, N2, H2 and CH4 permeability of all the prepared mixed matrix membranes at 20 °C. It was observed that by increasing the pressure from 1 to 6 bar, the permeability of CO2, N2, H2 and CH4 are increased. The permeability of CO2, N2, H2 and CH4 for the PES-GNs4 increases from 2246 to 21739, 2235 to 21535, 7151 to 53589, and 4176 to 37,159 Barrer, respectively with increasing the pressure. In addition, the CO2, N2, H2 and CH4 permeability of the PES increases from 9 to 13, 11–14, 9–12, and 3–5 Barrer by increasing the pressure. This behaviour attributed to the increased driving force for the transport of gas via membranes. As the pressure increases, the macromolecular segments are displaced close to each other. Therefore, the inter‐segmental void space decreases, the selective layer area increases and the density of the polymer increases, which leads to the increase of permeability (Farnam et al., 2016; Azizi et al., 2019; Alsari et al., 2007).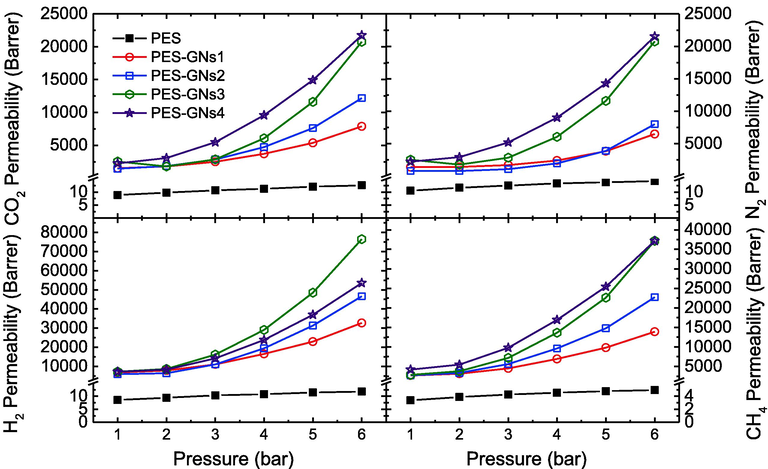
Effect of feed pressure on CO2, N2, H2 and CH4 permeability values of the prepared membranes at 20 °C.
Moreover, H2 and CH4 have the maximum permeability more than CO2 and N2. This is due to the affinity and the larger diffusivity of H2 and CH4 molecules to the MMMs are greater than that of CO2 and N2 (Alkhouzaam et al., 2016; Wang et al., 2015). In addition, it was noticed that by increasing the loading of GNs from 0.01 and 0.04 wt%, the gas permeability was further enhanced. The permeability of CO2, N2, H2 and CH4 for the PES-GNs4 increases from 9 to 2246, 11 to 2235, 9 to 7151, and 3 to 4176 Barrer, respectively as compared with pure PES membrane at 1 bar. This is due to the presence of GNs assisting the absorption of gasses in the polymer and the increases of the porosity that offers more channels for the gas, resulting in higher permeation through the membrane (Luque-Alled et al., 2020; Kamble et al., 2020). Therefore, the combination of PES and GNs into the polymer matrix enhanced the membrane permeability and separation performance. PES-GNs4 showed the highest permeability for all the gasses among the synthesized MMMs at all the feed pressure.
The effects of different temperatures on the permeability of CO2, N2, H2 and CH4 gases for the prepared membranes at various pressures have been shown in Fig. 5a, b, c, d and e. The result shows that by increasing the temperature from 20 to 60 °C, the permeability of CO2, N2, H2 and CH4 gases through prepared membranes improves due to the enhancement of polymer chain movements and the presence of plasticization in the membranes (Zia-ul-Mustafa et al., 2019; Lasseuguette et al., 2018). For pristine PES membrane, the permeability of CO2, N2, H2 and CH4 increases from 12.5 to 14.2, 14.2 to 16.1, 11.8 to 12.1, and 5 to 5.4 Barrer respectively at 6 bar. Whereas, at PES-GNs4, the permeability of CO2, N2, H2 and CH4 increases from 21,740 to 24171.5, 21534.7 to 26425.3, 53589.1 to 103951.6, 37159.6 to 54678.2 Barrer respectively at 6 bar. This means that the permeability of CO2, N2, H2 and CH4 for PES and PES-GNs4 increased about 13.6, 13.4, 2.6, 8 % and 11.2, 22.7, 94, 47.1% respectively when the temperature raised from 20 to 60 °C.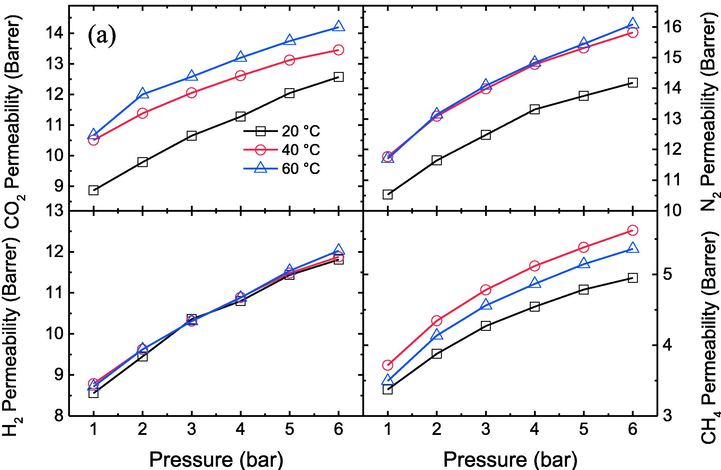
Effect of different temperatures on CO2, N2, H2 and CH4 permeability values of the prepared membranes a) PES, b) PES-GNs1, c) PES-GNs2, d) PES-GNs3 and e) PES-GNs4.
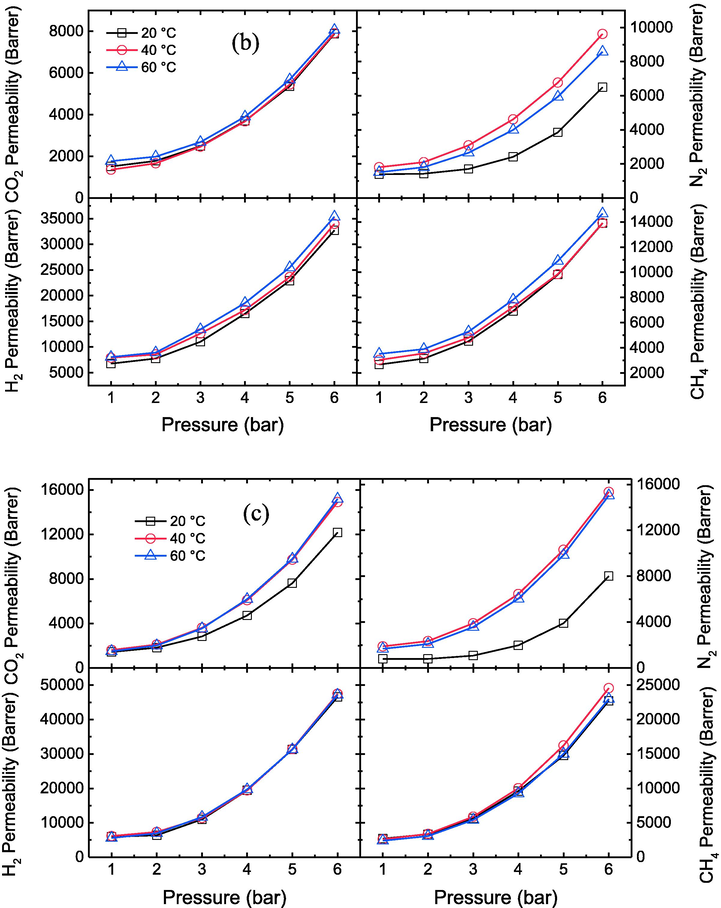
Effect of different temperatures on CO2, N2, H2 and CH4 permeability values of the prepared membranes a) PES, b) PES-GNs1, c) PES-GNs2, d) PES-GNs3 and e) PES-GNs4.
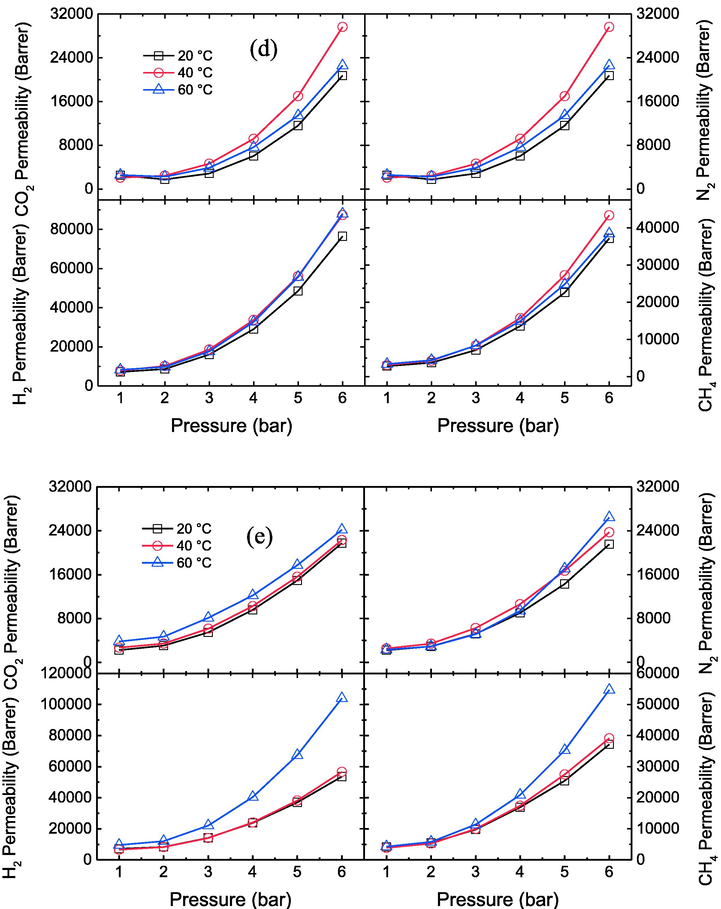
Effect of different temperatures on CO2, N2, H2 and CH4 permeability values of the prepared membranes a) PES, b) PES-GNs1, c) PES-GNs2, d) PES-GNs3 and e) PES-GNs4.
3.2 Evaluation of gas selectivity
Selectivity illustrates how well a polymeric membrane can separate the two gases from each other and is known as the permeability ratio of pure gas components (PA/PB) (Alkhouzaam et al., 2016). Fig. 6 shows the CO2/N2, CO2/H2 and CO2/CH4 selectivity at different pressure (1–6 bar) and 20 °C. The result shows that by increasing the pressure, the selectivity decreased slightly. For PES membrane, the H2/CO2, H2/N2 and H2/CH4 selectivity decreased from 0.97 to 0.94, 0.81 to 0.83 and 2.5 to 2.4, respectively, with the decreasing rates of 3, 2 and 4 %. In addition, the H2/CO2, H2/N2 and H2/CH4 selectivity for the PES-GNs4 membrane is decreased by increasing the pressure from 1 to 6 bar about 22, 22 and 18 % respectively at 20 °C. Meanwhile, the selectivity of H2/N2 for the PES-GNs2 membrane is decreased by increasing the pressure by about 23 %. It can be observed that for all the prepared membranes, H2/N2 exhibited the highest gas selectivity and H2/CH4 has the lowest selectivity due to the gas diffusion and solubility mechanism (Lasseuguette et al., 2018). The maximum selectivity of the H2/N2 gas obtained via the PES-GNs2 membrane is 7.5 without a decrease in the permeability. This behaviour is due to the properties of PES, which is considered a strong H2 adsorbent that facilitated the H2 permeability over N2 gas through the membrane (Yousef et al., 2021; Akhmetshina et al., 2019).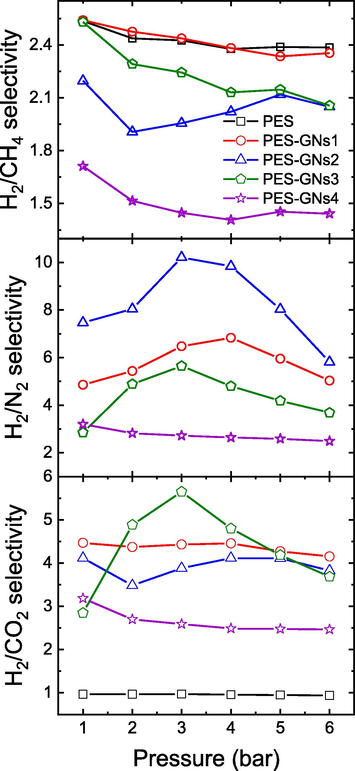
H2/CO2, H2/N2 and H2/CH4 Selectivity behavior with different pressure at 20 °C.
MMMs are a viable alternative to regular polymeric membranes because they can eliminate the “trade-off” impact that plagues commercial membranes. Several aspects influence the effectiveness of MMMs in CO2 separation procedures, including filler type and physicochemical qualities, nature of a polymer matrix, filler-matrix interaction, processing techniques, and so on. One of the most important aspects of developing effective MMMs is the choice of filler material. The selection of an adequate filler is difficult because filler materials may have poor adherence in the polymer matrix, resulting in poor CO2 separation performance (Rodenas et al., 2015). Table 1 displays recent developments in the fabrication of MMMs using different filler exhibiting unique textural and surface properties such as MWCNTs, GO, GNs, and MIL-53.
Materials
Parameter
Permeability (Barrer)
Selectivity
Ref
Pressure(Bar)
Temperature(°C)
CO2
N2
H2
CH4
PES
2
25
3
0.24
12.83
(Zia-ul-Mustafa et al., 2019)
PES
22
3675.4 GPU
3118.1 GPU
1.2
(Wang et al., 2015)
PDMS/seed/PES
22
810.1 GPU
52.4 GPU
15.5
(Wang et al., 2015)
PES
2
25
55 GPU
11 GPU
5
(Farnam et al., 2016)
PES + 20wt.%PVA
2
25
119 GPU
7
17
(Farnam et al., 2016)
PES/MWCNTs
4
2.99 GPU
1.5 GPU
0.75 GPU
2, 4
(Ismail et al., 2011)
GO/PES
1
30
662 GPU
18 GPU
36,
(Wang et al., 2016)
GO/PES
1
25
8500
425
20
(Kim et al., 2013)
PES/A-MIL-53 (Al)
5
4.5 GPU
0.08 GPU
54
(Ahmadijokani et al., 2019)
PES
2
20
10
12
10
4
1, 0.8, 2.5
Present study
PES-GNs1
2
20
1780
1432
7780
3143
4.4, 5.4, 2.5
Present study
PES-GNs2
2
20
1828
792
6367
3339
3.5, 8, 1.9
Present study
PES-GNs3
2
20
1775
1775
8667
3782
4.9, 4.9, 2.3
Present study
PES-GNs4
2
20
3067
2934
8275
5462
2.7, 2.8, 1.5
Present study
4 Conclusions
In this work, GNs with different loading were successfully embedded into PES for preparing mixed matrix membranes by a phase inversion process that has excellent separation and selectivity for gases. The prepared MMMs exhibited high mechanical stability, hydrophilicity and permeability. SEM results showed that the MMMs are smooth and homogeneous surfaces with a uniform structure consisting of a thin dense and porous layer. The TGA results showed that the thermal stability of the MMMs didn’t change significantly in comparison to the PES membrane. The prepared MMMs have the capacity to operate without thermal degeneration and plasticization under high pressure of up to 6 bar and high temperature of up to 60 °C. The gas separation and selectivity have been increased with increasing the GNs loading, resulting in the formation of nanofiltration membrane. By increasing the GNs loading from 0 to 0.04 wt%, the permeability of CO2, N2, H2, and CH4 increased about 24856, 20218, 79,356 and 139,100 % respectively at 1 bar and 20 °C due to improving the membrane absorption and porosity. The result demonstrated that by increasing the pressure, the permeability of CO2, N2, H2, and CH4 have also increased. In addition, the selectivity of CO2/N2, CO2/H2, and CO2/CH4 increased by increasing the pressure and the GNs loading. CO2/N2 exhibited the highest gas selectivity and CO2/H2 has the lowest selectivity for all the MMMs. Consequently, the prepared membranes exhibited higher gas separation and selectivity as well as better mechanical properties compared to the pure PES membrane. Finally, the synthesized membrane achieved improved gas separation performance, which significantly contribute to post-combustion carbon capture and storage in the industry, lowering CO2 emissions and reducing climate change risk.
Acknowledgements
This project has received funding from the Research Council of Lithuania (LMTLT), agreement No. S-MIP-20-27.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fabrication of modified polyethersulfone membranes for wastewater treatment by submerged membrane bioreactor. Separation and Purification Technology. 2017;175:36-46.
- [Google Scholar]
- High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. Chemical Engineering Journal. 2018;334:789-799.
- [Google Scholar]
- Removal of hexavalent chromium by electrospun PAN/GO decorated ZnO. Journal of the Mechanical Behavior of Biomedical Materials. 2019;98:205-212.
- [Google Scholar]
- High performance of PAN/GO-ZnO composite nanofibers for photocatalytic degradation under visible irradiation. Journal of the Mechanical Behavior of Biomedical Materials. 2019;96:118-124.
- [Google Scholar]
- Preparation, characterization, and mechanical properties of polyacrylonitrile (PAN)/graphene oxide (GO) nanofibers. Mechanics of Advanced Materials and Structures. 2020;27:346-351.
- [Google Scholar]
- Characterization and mechanical properties of electrospun cellulose acetate/graphene oxide composite nanofibers. Mechanics of Advanced Materials and Structures. 2019;26:765-769.
- [Google Scholar]
- Boosting gas separation performance and suppressing the physical aging of polymers of intrinsic microporosity (PIM-1) by nanomaterial blending. Nanoscale. 2020;12:23333-23370.
- [Google Scholar]
- Novel MMM using CO2 selective SSZ-16 and high-performance 6FDA-polyimide for CO2/CH4 separation. Separation and Purification Technology. 2021;254:117582
- [Google Scholar]
- Amino-silane-grafted NH2-MIL-53(Al)/polyethersulfone mixed matrix membranes for CO2/CH4 separation. Dalton Transactions. 2019;48:13555-13566.
- [Google Scholar]
- Acidic Gases Separation from Gas Mixtures on the Supported Ionic Liquid Membranes Providing the Facilitated and Solution-Diffusion Transport Mechanisms. Membranes (Basel). 2019;9
- [Google Scholar]
- Modified polyether-sulfone membrane: a mini review. Designed Monomers and Polymers. 2017;20:532-546.
- [Google Scholar]
- High-pressure CO2/N2 and CO2/CH4 separation using dense polysulfone-supported ionic liquid membranes. Journal of Natural Gas Science and Engineering. 2016;36:472-485.
- [Google Scholar]
- Effect of Pressure and Membrane Thickness on the Permeability of Gases in Dense Polyphenylene Oxide (PPO) Membranes: Thermodynamic Interpretation. Separation Science and Technology. 2007;42:2143-2155.
- [Google Scholar]
- Enhanced Antifouling Properties of Carbohydrate Coated Poly(ether sulfone) Membranes. ACS Applied Materials & Interfaces. 2015;7:17238-17246.
- [Google Scholar]
- Superior Ionic Transferring Polymer with Silicon Dioxide Composite Membrane via Phase Inversion Method Designed for High Performance Sodium-Ion Battery. Polymers (Basel). 2020;12
- [Google Scholar]
- A Molecular Simulation Study of Silica/Polysulfone Mixed Matrix Membrane for Mixed Gas Separation. Polymers (Basel). 2021;13
- [Google Scholar]
- Experimental Study of CO2 and CH4 Permeability Values Through Pebax®-1074/Silica Mixed Matrix Membranes. Silicon. 2019;11:2045-2057.
- [Google Scholar]
- The preparation of a modified PVDF hollow fiber membrane by coating with multiwalled carbon nanotubes for high antifouling performance. RSC Advances. 2020;10:1848-1857.
- [Google Scholar]
- Ultrathin permselective membranes: the latent way for efficient gas separation. RSC Advances. 2020;10:12653-12670.
- [Google Scholar]
- Tuning of Nano-Based Materials for Embedding Into Low-Permeability Polyimides for a Featured Gas Separation. Frontiers in Chemistry. 2020;7
- [Google Scholar]
- R. Castro-Muñoz, J. Buera-González, Ó.d.l. Iglesia, F. Galiano, V. Fíla, M. Malankowska, C. Rubio, A. Figoli, C. Téllez, J. Coronas, Towards the dehydration of ethanol using pervaporation cross-linked poly(vinyl alcohol)/graphene oxide membranes, Journal of Membrane Science, 582 (2019) 423-434.
- Mixed Matrix Membranes Based on PIMs for Gas Permeation: Principles, Synthesis, and Current Status. Chemical Engineering Communications. 2017;204:295-309.
- [Google Scholar]
- Effect of the ZIF-8 Distribution in Mixed-Matrix Membranes Based on Matrimid® 5218-PEG on CO2 Separation. Chemical Engineering & Technology. 2019;42:744-752.
- [Google Scholar]
- Matrimid® 5218 in preparation of membranes for gas separation: Current state-of-the-art. Chemical Engineering Communications. 2018;205:161-196.
- [Google Scholar]
- Graphene oxide – Filled polyimide membranes in pervaporative separation of azeotropic methanol–MTBE mixtures. Separation and Purification Technology. 2019;224:265-272.
- [Google Scholar]
- Enhanced CO2 permeability in Matrimid® 5218 mixed matrix membranes for separating binary CO2/CH4 mixtures. Separation and Purification Technology. 2019;210:553-562.
- [Google Scholar]
- Exploring the performance limits of MOF/polymer MMMs for O2/N2 separation using computational screening. Journal of Membrane Science. 2021;618:118555
- [Google Scholar]
- An investigation of blended polymeric membranes and their gas separation performance. RSC Advances. 2016;6:102671-102679.
- [Google Scholar]
- Gas separation using graphene nanosheet: insights from theory and simulation. Journal of Molecular Modeling. 2020;26:322.
- [Google Scholar]
- Characterization of PVDF/Graphene Nanocomposite Membranes for Water Desalination with Enhanced Antifungal Activity. Water. 2021;13
- [Google Scholar]
- CO2/H2 separation through poly(ionic liquid)–ionic liquid membranes: The effect of multicomponent gas mixtures, temperature and gas feed pressure. Separation and Purification Technology. 2021;259:118113
- [Google Scholar]
- Polyethersulfone sulfonated by chlorosulfonic acid and its membrane characteristics. European Polymer Journal. 2005;41:1554-1560.
- [Google Scholar]
- Gas separation performance of polyethersulfone/multi-walled carbon nanotubes mixed matrix membranes. Separation and Purification Technology. 2011;80:20-31.
- [Google Scholar]
- Development of novel blend poly (Ethylene Glycol) / Poly(Ethersulfone) polymeric membrane using N-Methyl-2-Pyrollidone and dimethylformamide solvents for facilitating CO2/N2 gas separation. Materials Today: Proceedings. 2021;46:1963-1970.
- [Google Scholar]
- Polyethersulfone based MMMs with 2D materials and ionic liquid for CO2, N2 and CH4 separation. Journal of Environmental Management. 2020;262:110256
- [Google Scholar]
- Enhanced Olefin Transport by SiO2 Particles for Polymer/Ag Metal/Electron Acceptor Composite Membranes. Polymers (Basel). 2020;12
- [Google Scholar]
- Mechanical Properties and the Characterization of Polyacrylonitrile/Carbon Nanotube Composite Nanofiber. Arabian Journal for Science and Engineering. 2018;43:4697-4702.
- [Google Scholar]
- Cross-linked β-cyclodextrin nanofiber composite membrane for steroid hormone micropollutant removal from water. Journal of Membrane Science. 2021;618:118228
- [Google Scholar]
- Selective Gas Transport Through Few-Layered Graphene and Graphene Oxide Membranes. Science. 2013;342:91-95.
- [Google Scholar]
- The role of compatibility between polymeric matrix and silane coupling agents on the performance of mixed matrix membranes: Polyethersulfone/MCM-41. Journal of Membrane Science. 2016;513:20-32.
- [Google Scholar]
- Temperature and Pressure Dependence of Gas Permeation in a Microporous Tröger's Base Polymer. Membranes (Basel). 2018;8:132.
- [Google Scholar]
- Ultrathin, Molecular-Sieving Graphene Oxide Membranes for Selective Hydrogen Separation. Science. 2013;342:95.
- [Google Scholar]
- A review on enhancement of mechanical and tribological properties of polymer composites reinforced by carbon nanotubes and graphene sheet: Molecular dynamics simulations. Composites Part B: Engineering. 2019;160:348-361.
- [Google Scholar]
- Polyethersulfone membranes: From ultrafiltration to nanofiltration via the incorporation of APTS functionalized-graphene oxide. Separation and Purification Technology. 2020;230:115836
- [Google Scholar]
- Mg-MOF-74/Polyvinyl acetate (PVAc) mixed matrix membranes for CO2 separation. Separation and Purification Technology. 2020;238:116411
- [Google Scholar]
- Economic Framework of Membrane Technologies for Natural Gas Applications. Separation & Purification Reviews. 2019;48:298-324.
- [Google Scholar]
- Composite nanofibers for highly efficient photocatalytic degradation of organic dyes from contaminated water. Environmental Research. 2016;145:18-25.
- [Google Scholar]
- Photocatalytic degradation of organic dyes and enhanced mechanical properties of PAN/CNTs composite nanofibers. Separation and Purification Technology. 2017;182:219-223.
- [Google Scholar]
- Tribological characterization and rheology of hybrid calcium grease with graphene nanosheets and multi-walled carbon nanotubes as additives. Journal of Materials Research and Technology. 2020;9:6178-6185.
- [Google Scholar]
- Rapid photocatalytic degradation of phenol from water using composite nanofibers under UV. Environmental Sciences Europe. 2020;32:160.
- [Google Scholar]
- Microstructure and modeling of uniaxial mechanical properties of Polyethersulfone nanocomposite ultrafiltration membranes. International Journal of Mechanical Sciences. 2021;204:106568
- [Google Scholar]
- Highly Efficient Visible Light Photodegradation of Cr(VI) Using Electrospun MWCNTs-Fe3O4@PES Nanofibers. Catalysts. 2021;11
- [Google Scholar]
- Unimpeded Permeation of Water Through Helium-Leak–Tight Graphene-Based Membranes. Science. 2012;335:442.
- [Google Scholar]
- Remarkable permeability enhancement of polyethersulfone (PES) ultrafiltration membrane by blending cobalt oxide/graphene oxide nanocomposites. RSC Advances. 2015;5:70448-70460.
- [Google Scholar]
- Improving the efficacy of PES-based mixed matrix membranes incorporated with citric acid–amylose-modified MWCNTs for HA removal from water. Polymer Bulletin. 2021;78:1293-1311.
- [Google Scholar]
- T. Rodenas, I. Luz, G. Prieto, B. Seoane, H. Miro, A. Corma, F. Kapteijn, F.X. Llabrés i Xamena, J. Gascon, Metal–organic framework nanosheets in polymer composite materials for gas separation, Nature Materials, 14 (2015) 48-55.
- Characterization and mechanical properties of cellulose acetate/carbon nanotube composite nanofibers. Advances in Polymer Technology. 2018;37:2446-2451.
- [Google Scholar]
- A brief review on carbon selective membranes from polymer blends for gas separation performance. Reviews in Chemical Engineering 2019:20180086.
- [Google Scholar]
- Highly Selective Supported Graphene Oxide Membranes for Water-Ethanol Separation. Scientific Reports. 2019;9:2251.
- [Google Scholar]
- Development of a nanocomposite ultrafiltration membrane based on polyphenylsulfone blended with graphene oxide. Scientific Reports. 2017;7:41976.
- [Google Scholar]
- A Review on Porous Polymeric Membrane Preparation. Part I: Production Techniques with Polysulfone and Poly (Vinylidene Fluoride) Polymers (Basel). 2019;11:1160.
- [Google Scholar]
- Immobilization of TiO2 and TiO2-GO hybrids onto the surface of acrylic acid-grafted polymeric membranes for pollutant removal: Analysis of photocatalytic activity. Journal of Environmental Chemical Engineering. 2020;8:104422
- [Google Scholar]
- Rapid synthesis of faujasite/polyethersulfone composite membrane and application for CO2/N2 separation. Microporous and Mesoporous Materials. 2015;208:72-82.
- [Google Scholar]
- A highly permeable graphene oxide membrane with fast and selective transport nanochannels for efficient carbon capture. Energy & Environmental Science. 2016;9:3107-3112.
- [Google Scholar]
- A polyethylene glycol (PEG) – polyethersulfone (PES)/multi-walled carbon nanotubes (MWCNTs) polymer blend mixed matrix membrane for CO2/N2 separation. Journal of Polymer Research. 2021;28:6.
- [Google Scholar]
- Energy-efficient separation alternatives: metal–organic frameworks and membranes for hydrocarbon separation. Chemical Society Reviews. 2020;49:5359-5406.
- [Google Scholar]
- S. Yousef, J. Eimontas, N. Striūgas, A. Mohamed, M. Ali Abdelnaby, Pyrolysis kinetic behavior and TG-FTIR-GC–MS analysis of end-life ultrafiltration polymer nanocomposite membranes, Chemical Engineering Journal, 428 (2022) 131181.
- Mass production of graphene nanosheets by multi-roll milling technique. Tribology International. 2018;121:54-63.
- [Google Scholar]
- CO2/CH4, CO2/N2 and CO2/H2 selectivity performance of PES membranes under high pressure and temperature for biogas upgrading systems. Environmental Technology & Innovation. 2021;21:101339
- [Google Scholar]
- Morphology, compositions, thermal behavior and kinetics of pyrolysis of lint-microfibers generated from clothes dryer. Journal of Analytical and Applied Pyrolysis. 2021;155:105037
- [Google Scholar]
- Ultra-permeable CNTs/PES membranes with a very low CNTs content and high H2/N2 and CH4/N2 selectivity for clean energy extraction applications. Journal of Materials Research and Technology 2021
- [Google Scholar]
- High-performance composite photocatalytic membrane based on titanium dioxide nanowire/graphene oxide for water treatment. Journal of Applied Polymer Science. 2020;137:48488.
- [Google Scholar]
- Effect of imidazolium based ionic liquids on PES membrane for CO2/CH4 separation. Materials Today: Proceedings. 2019;16:1976-1982.
- [Google Scholar]







