Translate this page into:
Highly efficient removal of diazinon pesticide from aqueous solutions by using coconut shell-modified biochar
⁎Corresponding author: Department of Environment, Faculty of Forestry and Environment, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia Telephone: +603 9769 7455 Fax: +603 9769 8019 zaharin@upm.edu.my (Ahmad Zaharin Aris)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study evaluates the adsorption of diazinon from aqueous solutions onto coconut shell-modified biochar using a batch system. The amount of dosage and initial pH are the main parameters being studied to obtain maximum adsorption capacity of the probe molecules. The carbonized coconut shell biochar (BC1), activated coconut shell biochar (BC2), chemically modified phosphoric acid (BC3) and sodium hydroxide coconut shell biochar (BC4) were prepared and tested as variables in the adsorption experiment. The characteristic of biochar via SEM, EDX and BET analysis revealed the large porous of surface morphology and slight changes in the composition with high surface area (405.97 – 508.07 m2/g) by following the sequence of BC3 > BC2 > BC4. Diazinon removal percentage as high as 98.96% was achieved at pH 7 with BC3 as adsorbent dosing at 5.0 g/L. The high coefficient of determination, R2 with a small value of ERRSQ and χ2 error analysis present the BC1 (0.9971) and BC2 (0.9999) are best fitted with Freundlich isotherm indicates multilayer sorption onto heterogeneous surface whereby the Langmuir isotherm model is the best fitting is described of monolayer adsorption process onto the homogenous surface of BC3 and BC4. The results indicated the maximum adsorption capacity (qm) was achieved by BC3 with 10.33 mg/g, followed by BC2 (9.65 mg/g) in accordance to the Langmuir isotherm while Freundlich isotherm showed the highest adsorption capacity (kF) with 1.73 mg/g (L/mg)1/n followed by BC4 with 0.63 mg/g (L/mg)1/n at favorable adsorption isotherm (1 ≤ n ≤ 10). Thus, the results obtained depicted that BC2 and BC3 are highly efficient adsorbents and both exhibit great potential in removing diazinon from aqueous solutions.
Keywords
Adsorption
Biochar
Organophosphorus pesticides
Diazinon
Treatment
1 Introduction
Despite the fact that pesticides are known to be harmful, their use is prevalent since the end of World War II due to the many benefits they bring to the agricultural sector. The pesticide are relatively low in price and highly effective for controlling weeds, insects and other pests while minimizing harm to the agricultural products (Leong et al., 2007; Mansouriieh et al., 2016). It can be grouped according to their functions and structures. Structurally, the pesticides can be categorized into three major synthetic organic groups, namely the organochlorine, organophosphorus and organonitrogen pesticide. The United Nations Environmental Program (UNEP) has included nine organochlorine pesticides (OCPs) such as DDT, aldrin and chlordane which are chlorine-containing organic compounds in the list of 12 chemicals as the persistent organic contaminants (POPs). POPs are persistent substances, easily transported and distributed over long distances, and often bioaccumulates via food chain. Therefore, they pose the threat of causing detrimental effects to the environment and human health (Mansouriieh et al., 2016). Due to these adverse consequences, the agricultural industry has shifted usage of pesticides towards organophosphorus pesticides (OPPs) in their field instead.
The main sources of OPPs in the environment found in agricultural runoff and drainage, wastewater treatment plants and other industrial water resources (Moussavi et al., 2013). The exposure of pesticides, particularly the OPPs, commonly occur via leaching, surface runoff, deposition, atmospheric transport and spray drift pathways. Despite contamination risks, the use of pesticide continues to gain popularity, thus endangering aquatic life and affecting water quality (Sanagi et al., 2011). The OPPs residues are traceable and detectable in water, soil, sediment and biota and the bioaccumulation of OPPs may occurred at any point along the food chain (Gobas et al., 2018; Navarrete et al., 2018; Omar et al., 2018). Even though rapidly degrade in the environment, they are normally toxic to vertebrates as compared to the other classes of insecticides (Leong et al., 2007) including humans as the top hierarchy of the food chain (Wee et al., 2016). As a consequence, human health is seriously at risk of OPPs contamination as current studies have confirmed the mutagenicity and cytotoxicity of numerous OPPs (Kamboh et al., 2016). Due to the public health concern through the residues of OPPs presence in water bodies, the appropriate technologies in OPPs removal is required (Abdelhameed et al., 2018a).
Diazinon is an example of a widely utilized OPPs. World Health Organization (WHO) has described diazinon which is one of the broad spectrum of OPPs as “moderately hazardous” Class II. The hazard lies in its acute toxicity which is inhibition of acetylcholinesterase (AChE) enzyme. OPPs acts by suppressing the action of enzymes in the nervous system which can result in several neurobehavioral side effects (Esfandian et al., 2016). Apart from that, the trace level of OPPs in drinking water even at very low concentration (as low as ng/L or lower) is a major concern (Kamboh et al., 2016). As established by the European Union (EU/1998), the maximum allowable concentrations in drinking water guidelines is 0.1 µg/L for single compound of pesticide and 0.5 µg/L for total pesticides respectively. Thus, the removal or treatment of this pesticides (OPPs) from contaminated water is pertinent.
Several methods have been introduced and employed to remove pesticides which include biological treatment, membrane filtration, advanced oxidation treatment, photocatalyst, and ion exchange treatment. Every method has its own benefits and drawbacks. However, the inadequacies of most of these treatment methods are high maintenance with high operational costs and may generate by-products after the operation of the treatment (De Gisi et al., 2016). Thus, the adsorption method is preferred due to the development of a robust process, simplicity of design and operation towards the environmentally sustainable of removing pesticides (De Gisi et al., 2016; Esfandian et al., 2016).
The vast majority of researchers used various types of adsorbents such as biochar, activated carbon, minerals, clays and the hybrid materials through metal organic frameworks (MOFs) as solutions in organic pollutants especially pesticides elimination from water bodies (Abdelhameed et al., 2019; Emam and Shaheen, 2019a; Durán et al., 2019; Emam et al., 2019b; Derylo-Marczewska et al., 2017; Abdelhameed et al., 2017). Due to high surface area, the amount of adsorption capacity in biochar increases the removal efficiency and therefore becoming one of the chosen adsorbents in pesticide removal (Ponnam et al., 2020; Okoya et al., 2020; Yang et al., 2017). Besides, biochar has the capability to eliminate organic pollutants such as dyes and pharmaceuticals compounds from water (Tran et al., 2020; Wu et al., 2020). Biochar is a by-product material of the pyrolysis process during biomass burning, under low oxygen supply in a closed system and the characteristics of the material varies depending on the pyrolysis conditions such as heating temperature and exposure time (Taha et al., 2014). The raw organic parent materials are significant factors in fabricating of adsorbent through pyrolysis. Several studies have investigated organic materials in pesticides removal by using the (i) agricultural wastes; corn stover (Taha et al., 2014), rice stalk (Liu et al., 2015), corn straw (Suo et al., 2019), orange peel and apricot kernels (Abdelhameed et al., 2020), (ii) wood derivatives; birch wood and Norway spruce wood (Cederlund et al., 2016), bamboo chips (Mandal et al., 2017) and (iii) manures; poultry manure, cattle manure, pig manure (Liu et al., 2015). However, there is no study which has focused on the pesticide (diazinon) removal from water by using the simple modification of low-cost coconut sell biomass with the comparison of less environmental toxicological acid (H3PO4) and low-cost alkaline (NaOH) as an activating agents until now. This study focusing on providing the environmentally safe sounds materials from the biomass waste that potentially can be used to remediate the contaminants of emerging concern at high efficiency and produce no environmental side effects.
The current study aims to determine the removal percentage and the maximum adsorption capacity of diazinon adsorbed per unit mass of different types of biochar prepared from coconut shell biomass. Diazinon is preferred as a model for OPPs because of its extensive application and the prevalence with which it has been traced in many water resources. The conversion of biomass from coconut shell to biochar were achieved through carbonization and pyrolysis with chemical activation method. The biomass of coconut shell was selected because of its abundant availability whereas the activating agent (H3PO4 and NaOH) was chosen due to its non-polluting, non-corrosive nature and ease of elimination by extraction with water which is also the most important factor as it may increase material porosity. The characteristics of produced carbonized coconut shell biochar (BCs) were investigated by various techniques, such as scanning electron microscope (SEM), energy dispersive X-ray (EDX) and BET surface area analysis. The targeted diazinon adsorption process was examined in a batch technique at various operations conditions such as adsorbent dosage and pH of adsorbate solution. Furthermore, factors such as removal efficiency, adsorption capacity of diazinon and adsorption mechanism were herein discussed, makes it appropriate to be considered as a green process.
2 Materials and methods
2.1 Chemicals, glassware and standards
Diazinon stock standard (0.25 g of 1000 mg/L) was supplied by Dr. Ehrenstrorfer GmbH (Augsburg, Germany) with structure, identify and physicochemical properties as illustrated in Table 1. Deionized water was supplied from a Milli-Q water purification system manufactured by Millipore (USA) throughout the analysis. HPLC-grade methanol (MeOH) and acetonitrile (ACN) were purchased from Fischer Scientific (UK). The stock standards (1000 mg/L) was prepared by weighting and dissolving 100 mg of diazinon analyte with 100% HPLC-grade ACN in 100 mL volumetric flask from the stock standard. Afterwards, working standard of 100 mg/L was prepared by dilution of stock standard. The calibration solutions which were freshly prepared include 0.25, 0.5, 1.0, 2.0, 5.0 and 10.0 mg/L. It was then stored in amber glass-stoppered volumetric flasks at ±4 °C before analysis.
Name
Diazinon
Structure
Molecular structure
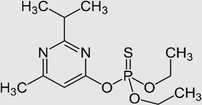
Identity
IUPAC name
O,O-diethyl O-2-isopropyl-6-methyl-4-pyrimidinyl phosphorothioate
Chemical formula
C12H21N2O3PS
CAS number
333–41-5
Molecular weight (g/mol)
304.3
Physicochemical properties
Octanol–water partition coefficient, log Kow
3.81b,c
Solubility in water
40 mg/L at 25 °C
Soil sorption coefficient, Koc
40–854a,b
2.2 Adsorbent preparation and production
The carbonized coconut shell biochar (BC) was collected from Biorefinery Complex of Environmental Biotechnology, UPM Serdang, Selangor. The carbonized BC (BC1) was activated and chemically modified while the pyrolysis temperature and retention time was chosen in accordance to the previous studies (Taha et al., 2014; Lin et al., 2012). In brief, the activated BC (BC2) was produced through slow pyrolysis by using electrical muffle furnace (Carbolite). The pyrolysis temperature started from ±35 °C and slowly increased up to 700 °C with rate of 7 °C/min. The process was maintained at this temperature for 2 h. The BC2 was chemically modified with acid and alkaline activating agents via 1.0 M phosphoric acid (BC3) and 1.0 M sodium hydroxide (BC4). The adsorbents amounted to 5.0 g was heated with 200 mL of respective chemicals at 80 °C for 1 h. Next, the BCs was cooled down and left overnight at room temperature. Subsequently, the treated adsorbents (BC3 and BC4) were filtered, rinsed with distilled water and dried at 80 °C for 24 h (Taha et al., 2014; Lin et al., 2012). Afterwards, the BCs collected were crushed and sieved through 1 mm size according to U.S. Department of Agriculture (USDA) to obtain a size that is considered as very coarse.
2.3 Adsorbent characterization
The elemental composition, characteristic images and morphology of BCs were scrutinized using a couple of instrument of scanning electron microscope (SEM) and energy dispersive X-ray (EDX) (Hitachi S-3400N SEM). Generally, the SEM analysis is an efficient means to explain the features of adsorbent structure morphology changes after the adsorption towards obtaining quantitative knowledge for general use application. Other than that, the characterization of adsorbent is based on the elemental composition of materials via EDX (Hitachi S-3400N SEM) analysis for changes of composition before and after adsorption of diazinon. The Brunauer, Emmett and Teller (BET) surface area via Micromeritics ASAP 2020 (USA) automated system was used to measure the surface area (m2/g), pore volume (cm3/g) and pore diameter (nm) of selected biochar. The parameters were obtained through nitrogen adsorption physisorption at 77 K that was degassed at approximately 115 °C. The samples of BC before and after adsorption treatment was collected and dried before processed on copper support and coated with gold prior to analysis.
2.4 Batch adsorption experiments
A known concentration of diazinon aqueous solution containing 100 mL of 1 mg/L was transferred into a 250 mL conical flask. The working solution were thoroughly stirred, mixed and shaken at 150 rpm for 2 h at room temperature until equilibrium was achieved. Subsequently, the samples were pre-filtered through filter paper to separate the solid and aqueous solution before proceed to solid-phase extraction (SPE) and samples clean-up. The samples of conditioning, loading, washing, drying and elution steps were used Strata X33u polymeric reversed-phase of 200 mg/6 mL (Strata, Phenomenex, CA, USA) in a vacuum manifold system (Phenomenex, CA, USA). The optimized SPE method was performed before and after treatment involving batch adsorption experiments (Wee et al., 2016). Afterwards, the initial and residual quantification of diazinon was determined using HPLC (Agilent, USA). These adsorption procedures were repeated for the following pH (3, 5, 7 and 9) and adsorbent dosages (0.2, 0.5, 1.0, 2.0, 5.0 and 10.0 g/L) respectively. The adsorption of diazinon onto BC3 and BC4 are a function of adsorbent dosage and initial solution pH. The effect of sorbent dose on the equilibrium uptake of diazinon was investigated with adsorbent masses of 0.2 to 10.0 g. Diazinon aqueous solutions were adjusted to different pH values with either 0.1 M NaOH or 0.1 M HNO3 using a pH meter. All adsorption experiments were conducted in duplicate.
2.5 HPLC-DAD analysis
Agilent Series 1200 High-Performance Liquid Chromatography (HPLC), equipped with a diode array detector (DAD) was used to perform this analysis. A standard solution of diazinon compound in a chromatogram was analyzed by identifying its peak concentrations. The retention time of the standard was identified and the peak area was then quantified. A Poroshell 120 EC-C18 3.0 × 100 mm analytical column (Agilent, USA) was picked. The limit of detection (LOD) of this instrument was recorded at 0.25 mg/L. The optimized chromatographic conditions as illustrated in Table 2 were modified based on Wee et al. (2016). All details of experimental was illustrated in Fig. 1.
Instrument
High-performance liquid chromatography agilent series 1200
Column
Poroshell 120 EC-C18 3.0 × 100 mm
Injection volume
10 µL
Mobile phase
40% H2O (A) and 60% ACN (B)
Flow rate
0.6 mL/min
Wavelength
210 nm
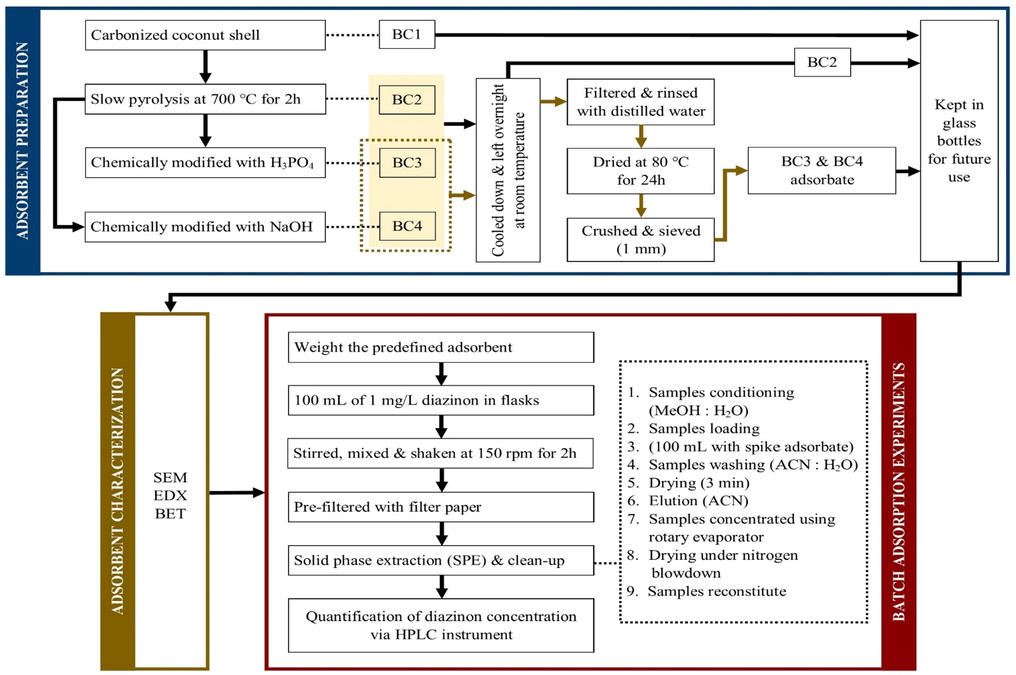
Schematic flow diagram of experiments.
2.6 Quality assurance and quality control
For every batch adsorption experiment of pH and adsorbent dosage, a recovery of spiked diazinon synthetic aqueous solution was carried out. Method recovery was conducted to determine the loss of diazinon from early sample preparation process, the extraction and enrichment of diazinon in SPE method and matrix interference. Each sample was analyzed in duplicate to inspect the reproducibility and the mean value used for each set of values. Additionally, blank samples without adsorbent were also tested. Accuracy, precision, recovery, linearity and limits of detection were evaluated from SPE method until development and optimization of HPLC analysis (Wee et al., 2016).
2.7 Statistical analysis
2.7.1 Removal percentage and adsorption capacity
The removal percentage of diazinon by BCs was calculated at equilibrium as written in Eq. (1) where, Co (mg/L) represents the initial diazinon concentration before adsorption, Ce (mg/L) represent the final concentration at equilibrium after adsorption. Under optimized conditions, the amount of diazinon adsorbed per unit mass of adsorbent, BC at equilibrium was calculated as written in Eq. (2)
2.7.2 Adsorption isotherm models
Langmuir isotherms and Freundlich isotherms were applied and tested for diazinon adsorption mechanism. The linear Langmuir isotherm was expressed as follow (Eq. (3))
The result obtained from the RL values illustrates the fit of BC as an adsorbent in adsorption study in Langmuir model. The value of RL = 1 shows a linear relationship between adsorbent and adsorbate whereas RL > 1 implies the unfavorable relationship meanwhile 0 < RL < 1 indicates the favorable isotherm and RL = 0 points out an irreversible relationship.
The Freundlich isotherm applied describes the mechanism occurred in heterogeneous surface of the BC for the adsorption of diazinon compound. The Freundlich isotherm, is expressed as mentioned below (Eq. (5))
The errors analysis were used in order to overcome the shortfall in the isotherm modelling due to the created inherent bias from the linearization. The error value in small number represents the data from the isotherm model is close with the experimental data whereas on the contrary to the large error value. This study extensively used the nonlinear isotherm models to calculate the error analysis through the sum of the square error (ERRSQ) and chi-square test (χ2) as in Eqs. (6) and (7), respectively to evaluate the fit of experimental equilibrium data.
The subscript of ‘exp’ and ‘cal’ indicates the obtained equilibrium data through the experimental and calculated data from the isotherm modelling, respectively.
3 Results and discussion
3.1 Characterization of adsorbents
3.1.1 Scanning electron microscopy (SEM)
The characteristic image and morphology of BC2 was analyzed using scanning electron microscope (SEM). The surface area, pore volume and pore diameter for BCs (Table 4) shown the change for each BCs after modification steps. Based on Fig. 2a and b, it was found that BC2 has a regular structure and highly porous surface with internal and external pores. The images from Fig. 2c and 1d featured the surface of BC2 before and after the adsorption treatment process, respectively. Based on the SEM images, Fig. 2d displayed a slight change of appearance with the presence of white clumps adsorbed onto the surface after the treatment process. The white clumps represent the compounds, presumably, diazinon adsorbed onto the surface due to the comparison of elemental composition in EDX analysis which showed the phosphorus element is present by weight (0.22%), atom (0.09%) and compounds (0.22%) after the diazinon (C12H21N2O3PS) adsorption. This clearly showed the fabricated biochar materials in Fig. 2b is pristine from the targeted pollutant. In comparison to Fig. 2c, the effect of after adsorption treatment did not show clear observable difference. It is suggested that, regardless of its low concentration, the adsorption of diazinon onto the BC surface still occurred. This implies the adsorption of diazinon has occurred successfully using the fabricated biochar derived from coconut shell. In addition, most diazinon compounds were very likely has been adsorbed into the internal pores instead of external pores of the BC2 surface.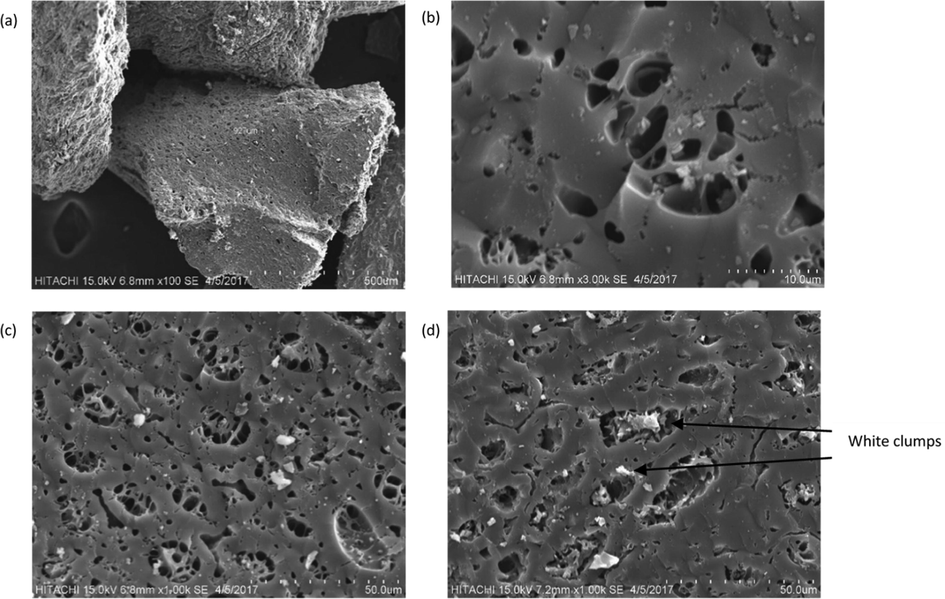
SEM images of BC2 (a) at 1000x magnification before adsorption treatment, (b) at 3000x magnification before adsorption treatment, (c) before adsorption treatment and (d) after adsorption treatment.
3.1.2 Energy dispersive X-ray spectroscopy (EDX)
The EDX analysis was employed to determine the purity and elemental composition of BC2. The elemental analyses of BC2 demonstrated that the composition of these elements changed after adsorption treatment (Table 3). Before adsorption process, the BC2 was primarily composed of carbon with 98.28% whereas the other compounds were oxygen, sodium, magnesium and potassium with a very low percentage. The greater content of carbon found before treatment could be due to the increased carbonization degree in which the pyrolysis temperature at 700 °C plays the critical role. Additionally, the content of alkaline elements (Na, Mg, K) with inorganic basic minerals present may attributed to the main component of ash derived from the pyrolysis process of BC (Taha et al., 2014). The resultant BC2 after adsorption treatment process showed the reduction of carbon and significant increase in oxygen and potassium whereby the presence of phosphorus compound was observed. The presence of the phosphorus thereby confirming the adsorption of diazinon onto the adsorbent occurred due to the diazinon structure of having phosphorus element in it (Mezenner et al., 2017; Moussavi et al., 2013). Thus, EDX analysis can be considered as a quantitative evidence of the diazinon adsorption onto the BC2 surface.
Element line
Formula
Weight (%)
Atom (%)
Compound (%)
Before
After
Before
After
Before
After
Carbon
C
98.28
89.91
99.27
95.12
98.28
89.91
Oxygen
O
0.36
2.59
0.27
2.06
–
–
Sodium
Na2O
0.06
–
0.03
–
0.08
–
Magnesium
MgO
0.15
1.61
0.08
0.84
0.25
2.67
Potassium
K2O
1.16
5.27
0.36
1.71
1.39
6.35
Phosphorus
P
–
0.22
–
0.09
–
0.22
Adsorbent
Surface area (m2/g)
Pore volume (cm3/g)
Total pore volume (cm3/g)
BC2
434.833
0.124
0.174
BC3
508.072
0.146
0.203
BC4
405.976
0.126
0.162
3.1.3 Brunauer-Emmett-Teller (BET) analysis
The obtained surface area of coconut shell biochar showed that BCs have a high surface area which is more than 400 m2/g as presented in Table 4. The pyrolysis biochar (BC2) showed higher surface area compared to BC4 which NaOH activated with following the sequence, BC3 > BC2 > BC4. As reported in previous studies, the H3PO4 activation lead to the improvement of porosity (Lin et al., 2012; Jagtoyen and Derbyshire, 1993) and fabricate the greatest textural characteristics (Yang et al., 2011). The average pore diameter distribution for all BCs adsorbent was in the mesoporous structure ranging from 4.454 to 6.961 nm. Fig. 3 presented the pore size distribution for each adsorbent which strongly indicates a mesoporous structure as reported in IUPAC classification. The presence of mesoporous is crucial in order to enhance the diazinon adsorption percentage from aqueous solutions.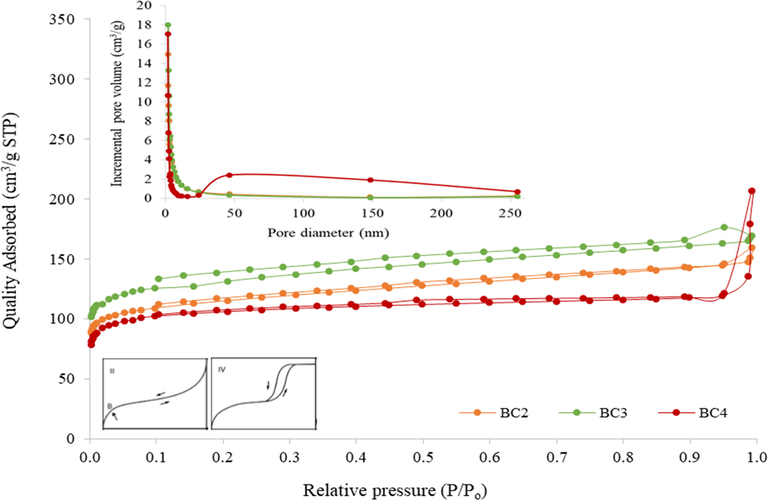
N2 adsorption–desorption isotherm models and pore size distribution for BCs.
The pore volume showed that BC3 (0.146 cm3/g) have higher value followed by BC4 and BC2 which acquired a quite similar pore volume of 0.126 cm3/g and 0.124 cm3/g, respectively. The large opening pore volume indicates rapid access of the adsorbate adsorption into BC3 adsorbent. It also supports the removal percentage in BC3 with 35.3–88.4% ±1.2 – 5.2 which showed low removal at acidic and alkaline condition due to the diazinon becoming unstable in those conditions. The same results were found in BC3 where adsorbent in different dosage showed the removal percentage is between 40 and 99.7% ±1.0 – 1.7. The present study is in agreement with previous studies which showed high surface area and large pore volume with tunable pore size (Wang et al., 2020; Xia et al., 2020; Abdelhameed et al., 2018b). Hence, the high surface area with a large pore volume presents in the BCs have potential for diazinon removal application.
The N2 adsorption–desorption isotherm models of prepared BCs (Fig. 3) showed the isotherms belongs to a combination of type II and type IV (IUPAC classification) of the Brunauer classification. The characteristic types approached monolayer at a lower region which is before 0.1 (P/Po) is an agreement with Type II adsorption pattern and followed by multilayers ascended at last stage >0.9 of P/Po that represent Type IV pattern. The isotherm of all BCs assumed as capillary condensation step and presented a slight hysteresis loop about 0.45 of relative pressure (P/Po) that specify of mesoporous (2–50 nm of pore diameter) materials (Lufrano and Staiti, 2010). These mesoporous materials give comparatively weak adsorbent-adsorbate interaction in the selected type isotherms. On the other hand, the same fabrication product in mesoporus structure were obtained through biochar of coconut shell (0.2619 nm), cotton stalk (0.376 nm), corn straw (8.36 nm), corncob (7.07 nm) and corn starch (7.47 nm) as illustrated in Table 5 with reported characteristics (Suo et al., 2019; Hao et al., 2018; Chen et al., 2012). Thus, this study revealed the highest surface area (434.8–508.1 m2/g) were obtained in the characterization of fabricated biochar which is a great accomplishment of mesoporous materials compared with previous studies.
Biomass
Pollutant types
Pyrolysis temperature (℃)
Surface area (m2/g)
Pore diameter (nm)
References
Coconut shell biochar
Pesticide
700
434.8–508.1
2.227–2.439
This study
Coconut shell
NA
700
341.0
NA
Wang et al. (2013)
Coconut shell
Herbicide
400
90.9
NA
Kearns et al. (2014)
Coconut shell activated carbon
phenol
NA
416.7
0.2619
Hao et al. (2018)
Cotton-stalk
Gas and liquid oil
650
750224.1
94.90.376 ± 0.025
Chen et al. (2012)
Neem tree bark
Pesticide
300
30.4
NA
Ponnam et al. (2020)
Bamboo char
Gas and oil
650
750266.7
228.7NA
Yang et al. (2016)
Oak wood biochar
Herbicides
550
226.0
210.9NA
Gámiz et al. (2019)
Soybean and rice straws
Organochlorine pesticides
500
2.63
2.21NA
Ali et al., (2019)
Corn straw CSW
Corncob CC
corn starch CSHerbicide
300
6.7
3.9
2.58.36
7.07
7.47Suo et al. (2019)
Groundnut shell char
Pigeon plant charPesticide
650
128.0
106.0NA
Gokhale et al. (2020)
Chinese herbs
Herbicide
750
85.3
3.47
Wei et al. (2020)
3.2 Adsorption of diazinon onto BCs
3.2.1 Effect of pH solution
The pH of a solution which contains adsorbate is an imperative factor for adsorption process (Hassan et al., 2017; Ouznadji et al., 2016). The effects of pH solution on the removal of diazinon utilizing the BC as an adsorbent were investigated at different pH ranged from 3 to 9 due to the half-life of diazinon as mentioned in literature. The weak acidic condition of pH 5 showed diazinon half-life is 14 days while at pH 7 is 70 days and 29 days at pH 9 (Deer and Beard, 2001). According to Peoples, (2019), most of pesticides compounds are quite stable in acidified condition except for diazinon. This strongly proved the diazinon compounds is highly unstable which rapidly degrades at low pH in water. Hence, this study proved the diazinon compounds potential to be removed in acidic condition in agreement with Ouznadji et al. (2016). Fig. 4 manifested not less than 75% reduction of diazinon compounds in each pH solution by all BCs adsorbent excluding BC1 where the highest removal of 41.84% at pH 3 due to unmodified the other activating agent such as phosphoric acid and sodium hydroxide. The modified BCs (BC and BC4) showed that high surface area contributes to the porous structure and enhances the removal efficiency of diazinon. The removal percentage of BC2 is 92.16% for diazinon achieved at pH 3. This is presumably due to acidic solution where hydrolysis of diazion was accelerated whereby the pKa of diazinon is 2.6 (Hassan et al., 2017; Moussavi et al., 2013). This study follows Ku et al. (1998) which showed that high removal efficiency of diazinon was achieved at the acidic condition specific at pH 4 (Liu et al., 2018; Kazemizad et al., 2016) and pH 3 (Pirsaheb et al., 2014; Fadaei et al., 2012). Besides, Dehghani et al. (2017) strongly agreed with previously reported results where the high removal percentage of diazinon found in sequence condition as follows neutral < alkaline < acidic. Generally, functionalized BC consists of –OH, –COOH and C = C groups on the surface.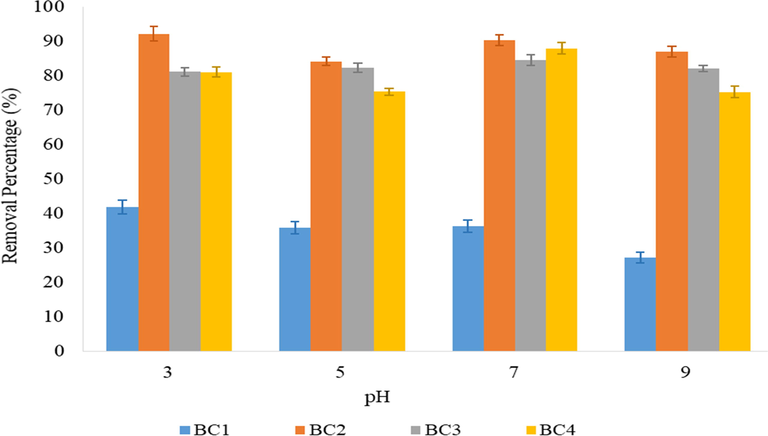
Effect of pH solution on diazinon removal percentage onto BCs. Note: diazinon concentration: 1 mg/L; solution pH: 3–9; BC adsorbent dosage: 1.0 g/L, contact time: 2 h.
At different pH, the change in charge of the BC surface is possibly due to protonation or deprotonation of oxygen-containing functional groups (Ahmed et al., 2017; Wang et al., 2015). The removal efficiency of diazinon increased in the acidic pH due to the protonation of hydroxyl group on the BCs adsorbent and the nitrogen atoms protonation is occurred on the primidine groups of diazinon (Amani et al., 2018). The surface of BC is positively charged when the pH of aqueous solution is above the diazinon pKa value of 2.6. Subsequently, the diazinon molecules begin to dissociate into anionic species with electrostatic forces of attraction to the positively charged surface of the BC. Moreover, the degree of diazinon dissociation is greater when pH of the solution is exceeding pKa of diazinon whereby the deprotonated form of amino acid side chain is predominates. As a result, the solution becomes more negatively charged. Apart from that, at a higher pH in alkaline condition, the decline in diazinon removal can be explained by the negatively charged surface of BC which cause the electrostatic repulsion of the diazinon anionic molecules leading to a decrease in the adsorption as the condition becomes more alkaline (Hassan et al., 2017; Hassani et al., 2015). The pattern of these results were similar to Kamboh et al. (2016) where the removal efficiency at pH 3 was slightly higher than pH 5. The removal percentage increased by raising the pH to 7, nevertheless, started to decline when the pH level was over 7.
The chemically modified BC3 and BC4 were reported as being able to remove diazinon maximally at its natural state, pH 7 with 84.55% and 87.93%, respectively. The diazinon adsorption onto the BC is highly dependent on the pH solution as it affects the surface charge and ionization of the BC and the degree of ionization and speciation of the diazinon (Rashidi Nodeh et al., 2017; Farmany et al., 2016; Ouznadji et al., 2016; Regmi et al., 2012). Thus, the optimum pH of adsorbate occurred at pH 3 which is consistent with reported studies and the maximum removal percentage of diazinon at pH 7 due to hydrolysis process.
3.2.2 Effect of adsorbent dosage
The effect of BC dosage was assessed by diversifying the dosage of adsorbent from 0.2 g/L to 10.0 g/L on the diazinon adsorption at pH 7. The removal percentage of diazinon increased when the amount of BC increasing, while the adsorption capacity reduced (Fig. 5). At equilibrium phase, the BC dosage of 2.0 g/L of all adsorbents except unmodified BC (BC1) exhibited the capability of removing diazinon for more than 90%. The highest BC dosage, which is 10.0 g/L illustrated the highest quantitative removal of OPPs by the respective adsorbent, BC1, BC2 and BC4 with 82.12%, 98.28% and 97.95%, respectively. At a definite time, the increased of adsorbent dosage did not result in higher removal percentage of diazinon compound and the removal percentage remained nearly constant. This indicated that the highest and maximum removal percentage of diazinon was reached, therefore no further removal was likely to occur (Lim and Aris, 2013). Hence, the optimum dosage of 2.0 g/L was selected for all BCs. Even though the lower dosage was able to uptake the diazinon compound, the removal efficiency was lesser compared to the efficiency of optimum dosage due to inadequate binding surface of adsorbents. In addition, the increment of adsorbent dosage has been proven to enhance the diazinon removal efficiency as more and suffice active adsorption sites are present in the adsorption process. The quantity of BC dosage regulates the removal percentage of diazinon and also the availability of active sites (Lim and Aris, 2013).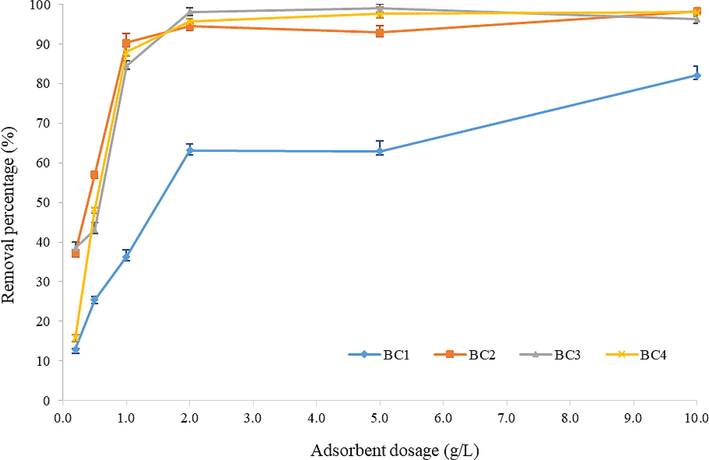
Effect of adsorbent dosage on diazinon removal percentage.
A consistent initial concentration of diazinon at 1 mg/L was utilized for adsorption batch experiments. The enhancement of diazinon removal with higher dosage of BC can be attributed to high availability of active binding sites for a specific quantity of OPPs molecules in the solution and a decrement of intra particle diffusion (Akl et al., 2015; Leyßner, 2011). In other words, the greater BC adsorbent dosage, the higher the quantity of active sites available, hence more OPPs molecules are able to adsorb with the available active sites leading to a prominent removal efficiency (Moussavi et al., 2013). The results are in parallel with the obtained surface area by the following sequence: BC3 > BC2 > BC4 > BC1 (from the highest to lowest) and with the contrary results of pore diameter of BCs adsorbent. Therefore, based on the aforementioned results, it can be concluded that BCs was an effective adsorbent for the removal of OPPs from aqueous solutions.
3.2.3 Adsorption capacity (qe)
The equilibrium adsorption of diazinon on different pH (3–9) and adsorbent dosage (0.2–10.0 g/L) of BCs within 2 h contact time were measured. The adsorption capacity was determined under optimum conditions of pH and adsorbent dosage and calculated using Eq. (2). The results showed significant difference (p < 0.05) in adsorption capacity of BCs at different pH in removing diazinon compounds from the aqueous solutions. For all the BCs, ones with pH 7 solution depicted the greatest adsorption capacity in comparison to BC with pH 3, 5 and 9. The removal percentage of diazinon and the highest adsorption capacity achieved at pH 7 by respective BCs are shown in Fig. 6. The highest adsorption capacity amongst all BCs was achieved primarily by using BC2 with 5.85 mg/g, followed by BC4 and BC3 with 5.69 mg/g and 5.47 mg/g respectively.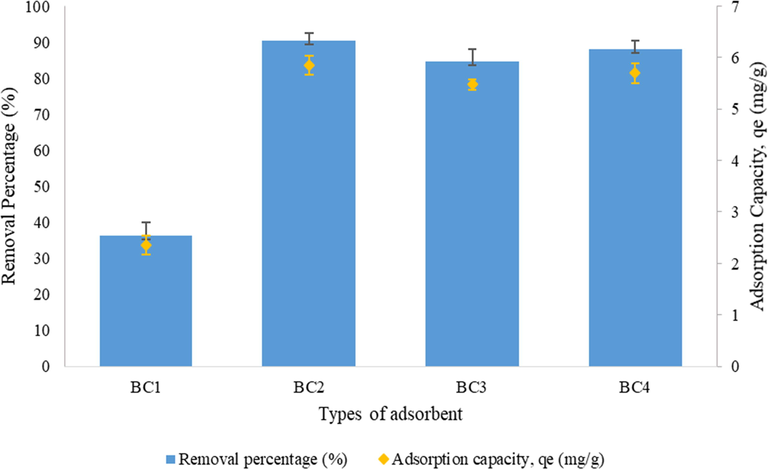
Effect of pH 7 on diazinon removal percentage and adsorption capacity of BCs.
Nonetheless, in term of adsorbent dosage, the result of adsorption capacity of BC3 and BC2 were determined to be the best compared to the other two BCs. Based on Fig. 7, generally the adsorption capacity (qe) observed was reduced with increasingly of adsorbent dosage. The removal percentage of diazinon by BC3 at equilibrium conditions initially raised from 38.48% to 96.18% in correspondence to the qe of 13.26 mg/g and reduced to 0.58 mg/g when adsorbent dosage of 0.2 g/L was increased to 10.0 g/L. The diazinon adsorption capacity at equilibrium conditions by BC2 decreased from 12.87 mg/g to 0.53 mg/g at adsorbent dosage of 0.2 g/L when the dosage was increased to 10.0 g/L. This demonstrated that the vacant and available active sites were not entirely used up or acted upon. Hence, the adsorption process still occurs despite of low adsorption capacity. Subsequently, Fig. 7 revealed that the adsorption capacity would further decline as the adsorbent dosage raised (p < 0.05) (Lim and Aris, 2013). Afterwards, when the adsorbent sites became completely saturated, an increase in adsorbent dosage does not remarkably enhance the adsorption capacity (qe) further (Kamboh et al., 2016).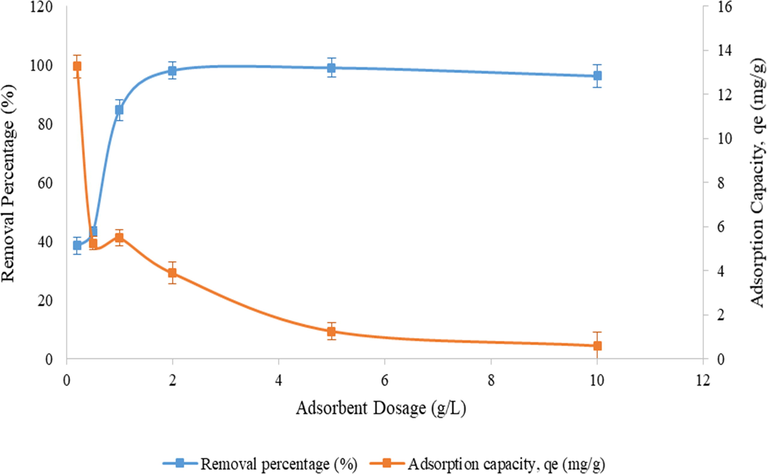
The removal percentage and adsorption capacity of diazinon onto BC3 under equilibrium conditions. Notes: diazinon concentration: 1 mg/L; pH: 7; BC3 dosage: 0.2–10.0 g/L.
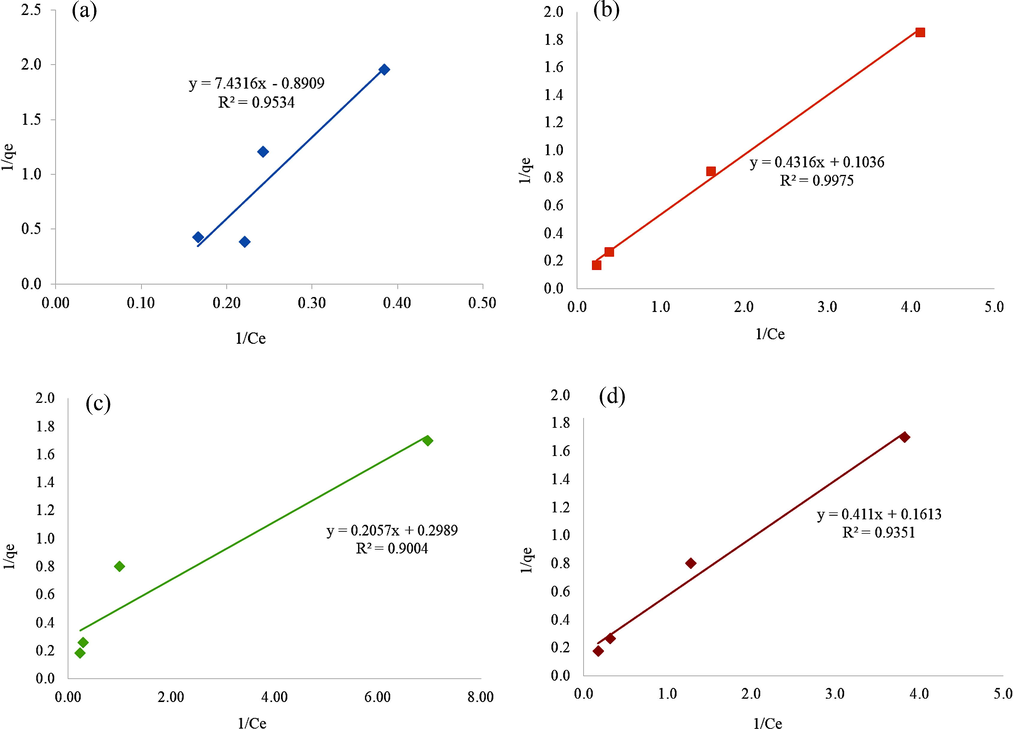
Linearized form of Langmuir isotherm model for diazinon adsorption onto (a) BC1 (b) BC2 (c) BC3 and (d) BC4.
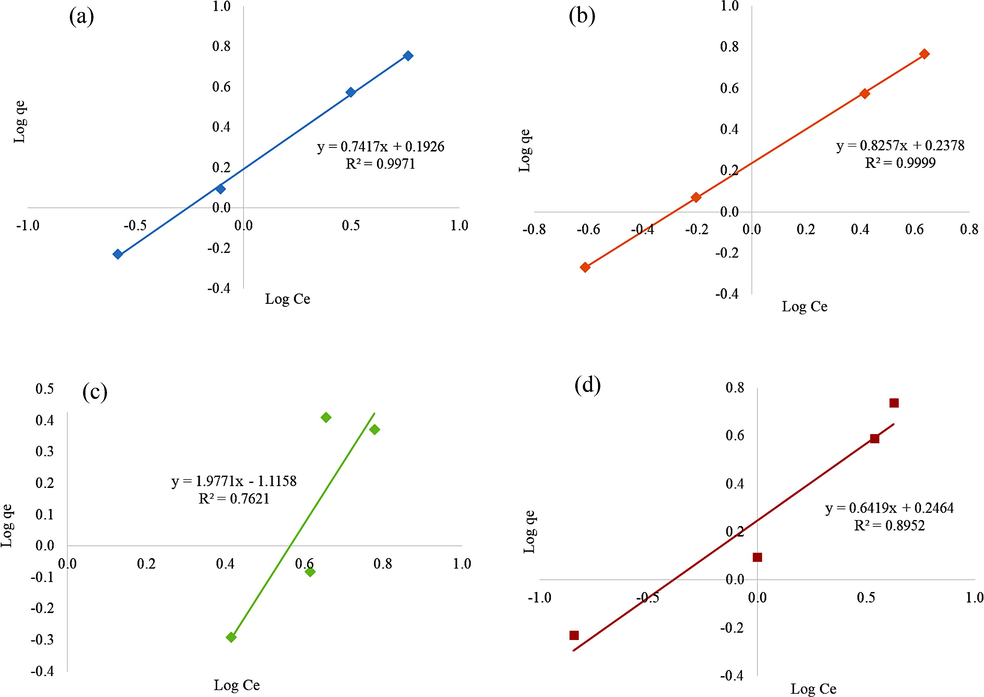
Linearized form of Freundlich isotherm model for diazinon adsorption onto (a) BC1 (b) BC2 (c) BC3 and (d) BC4.
In consideration of consistent diazinon concentration in the solutions, the increased efficiency of the removal percentage is due to the effect of BC dosage. It can be associated to the reduction of diazinon to BC dosage mass ratio (Zuo and Balasubramanian, 2013; Raposo et al., 2009). As a matter of fact, available adsorption sites increase when the BC dosage increased. Overall, the results indicates that the qe of the adsorbent decreases as the adsorbent dosage increases. Next, the adsorption data at equilibrium was exploited for isotherm model analysis.
3.3 Adsorption isotherm models
An isotherm model depicts the interaction between adsorbent and adsorbate for optimizing an adsorption process (Moussavi et al., 2013) by interpreting the occurrence of governing retention (or release) or kinetics of a substance from the aqueous to a solid-phase at optimized conditions. The adsorption isotherm models namely Langmuir and Freundlich were employed to scrutinize the adsorption process of diazinon onto BCs. The structure of the adsorbed layer, interaction between adsorbate and adsorbent and adsorption capacity of the BCs towards diazinon at optimized pH and adsorbent dosage were investigated. The Langmuir isotherm basically involved in determining the monolayer adsorption mechanism whereas Freundlich isotherm corresponded to the heterogeneous layer (Lim and Aris, 2013). These isotherm models were tested in equations as previously mentioned.
The linearized forms of these models and the data acquired from fitting the experimental equilibrium adsorption data are presented in Table 6. The values of coefficient of determination, R2 of BC3 and BC4 are higher (R2 = 0.9004 and R2 = 0.9351) in the Langmuir model whereby the values of R2 of BC1 and BC2 (R2 = 0.9971 and R2 = 0.9999) were better in Freundlich model. The nearer the value of R2 to one, the stronger the correlation, the better equation fits to the experimental data respectively. To determine the best-fitted isotherm, the sum of square error (ERRSQ) and chi-square test (χ2) as illustrated in Eq. (6) and Eq. (7) was used for both isotherm models. The evaluated error analysis through ERRSQ and χ2 is totally in agreement with the mentioned results of determination coefficient which presented the BC1 and BC2 as best fitted with the Langmuir isotherm model. The ERRSQ and χ2 (Emam et al., 2019c; Abdelhameed et al., 2018c; Abdel-Gawad et al., 2011) values showed that Freundlich isotherm model was the best fits model in the elucidation of diazinon removal onto BC3 and BC4 due to the small values of both sums of square error (ERRSQ) and chi-square test (Table 6). The large value of error analysis obtained on BC1 and BC2 for Freundlich model as well as BC3 and BC4 for the Langmuir model explained the obtained value in isotherm models is different from the data of experiments.
BCs
Langmuir
Freundlich
Langmuir
Freundlich
R2
qm (mg/g)
KL (L/mg)
RL
R2
kF(mg/g (L/mg)1/n)
n
ERRSQ
χ2
ERRSQ
χ2
BC1
0.9534
NA
NA
NA
0.9971
0.0013
0.2357
25.64
18.12
6.26
4.47
BC2
0.9975
9.653
0.2400
0.2941
0.9999
1.7290
1.2110
2.71
1.68
1.27
0.71
BC3
0.9004
10.331
0.0598
0.6257
0.7621
0.5488
0.9430
0.559
0.24
1.25
1.20
BC4
0.9351
4.228
0.2031
0.3299
0.8952
0.6548
1.1655
1.488
0.65
2.37
1.44
3.3.1 Langmuir isotherm model
The R2 value achieved from the Langmuir equation in respect to the BCs were 0.9534, 0.9975, 0.9004 and 0.9351, respectively (Figure 8). The R2 of BC3 and BC4 were best fits to the Langmuir compared to Freundlich isotherm model. This model emphasized the adsorption of diazinon which took place over a monolayer coverage rather than heterogeneous layers onto the homogenous surface of BC3 and BC4. As this model deduces a monolayer adsorption throughout the process, it can be indicated the active binding sites have equally scattered on the surface of BC with no interactions between the adsorbed molecules (Tan et al., 2016; Moussavi et al., 2013).
This study depicted the positive maximum adsorption capacity (qm) for all BCs with the exception of BC1 where the value was negative. The maximum adsorption capacity from Langmuir model calculated is 10.331 mg/g signifying a high capacity for BC3 for adsorption of diazinon followed by BC2 with 9.653 mg/g indicating BC3 as more promising adsorbent rather than BC2. The highest value of affinity binding site, KL acquired from the Langmuir isotherms were BC2 (0.2400 L/mg) and BC4 (0.2031 L/mg). The greater value of KL suggested functional group that exists in BC2 and BC4 has stronger chemical bonding with the adsorbate (diazinon) as compared to other BCs (Jusoh et al., 2011). On top of that, the Langmuir dimensionless separation factor, RL of BC2, BC3 and BC4 demonstrated the value between 0 and 1 inferred adsorption of diazinon onto respected BCs is a favorable process in contrast of BC1 where the qm resulted in a negative value that assumed the RL and KL also are negative values. Thus, the type of favorable isotherm was not able to be determined. The negative value of qm, RL and KL present the experimental data is not fitted or follow the assumption of Langmuir isotherm possibly due to the low diazinon concentration. Thus, the process of desorption takes place instead of adsorption of diazinon and the other isotherm model is highly recommended for further analysis.
3.3.2 Freundlich isotherm model
The R2 acquired from Freundlich isotherm in regards to the BCs were 0.9971, 0.9999, 0.7621 and 0.8952 respectively (Figure 9). The comparative analysis of R2 indicates that adsorption of diazinon onto the BC1 and BC2 follows Freundlich adsorption models better than the Langmuir isotherms. This model described the adsorption of diazinon initial interaction occurred over heterogeneous layers onto the surface of BC1 and BC2 and subsequently interact with each other (Kim et al., 2016). On top of that, the adsorption capacity, Kf acquired from this model with the highest KF value was BC2 with 1.7270 mg/g (L/mg)1/n followed by BC4 with 0.6548 mg/g (L/mg)1/n. On the other hand, the Freundlich constant, 1/n is related to energy adsorption and measures the intensity adsorption. Usually, the adsorbate was adsorbed onto the adsorbent favorably when the n value is in a range of 1–10 whereby n < 1 demonstrates the adsorbate was deficiently adsorbed onto the adsorbent (Gupta et al., 2007). In addition, the n value suggests the existence of a broad range of surface functional group since it is inclined to have the strong chemical bonding between the carboxylic group and adsorbate (diazinon) which then affects the adsorption process (Jusoh et al., 2011). The n values were 1.2110 and 1.1655 which indicates the favorable adsorption of diazinon onto the BC2 and BC4 respectively. In fact, the n value reconfirming BC2 and BC4 as a suitable adsorbent for adsorption of diazinon.
Several studies (Table 7) have reported on the adsorption of OPPs on solid adsorbents. A study performed by Lemić et al. (2006) which analyzed the removal of diazinon reported a maximum adsorption capacity of 1.35 mg/g by organo-zeolites while Moussavi et al. (2013) reported prominent maximum adsorption of diazinon onto NH4Cl- induced activated carbon with 250.0 mg/g and Jusoh et al. (2011) revealed maximum adsorption capacity with 909.1 mg/g in removing malathion using commercial coconut shell activated carbon. The maximum adsorption indicated a high capacity for both adsorbents in the adsorption of OPPs. Meanwhile, the maximum adsorption capacity acquired in this study is greater than Lemić et al. (2006) and Ouznadji et al. (2016) but lesser than Moussavi et al. (2013) and Jusoh et al. (2011) studies. The results of this study found that modified coconut shell biochar including BC2 and BC3 has the potential to remove OPPs from aqueous solutions. However, since the maximum adsorption capacity of these BCs were lower compared to the previous studies, the mass ratio of modified BCs needed must be higher in order to successfully remove OPPs from aqueous solutions efficiently.
Adsorbent
Analyte (OPP)
qm (mg/g)
Reference
NH4Cl- induced activated carbon
Diazinon
250.0000
Moussavi et al. (2013)
Organo-zeolite
Diazinon
1.35
Lemic et al. (2006)
Bimetallic Fe/Ni nanoparticles
Profenofos
0.2020
Mansouriieh et al. (2016)
Acid-activated bentonite
Diazinon
5.5630
Ouznadji et al., 2016
Commercial coconut shell activate carbon
Malathion
909.1000
Jusoh et al. (2011)
MOF- Cotton fabric composite
Ethion
182.00
Abdelhameed et al. (2016)
PEI-modified natural cotton fabrics
PEI-modified natural wood fabricsPirimiphos-methyl
monocrotophos pesticides
pirimiphos-methyl
monocrotophos pesticides454.6
333.3
625.0
500.0Abdelhameed et al. (2018)
Phosphoric acid coconut shell biochar
Diazinon
10.3306
This study
4 Adsorption mechanism
The mechanism of adsorption process is partinent to evaluate the removal efficiency of diazinon by using the BCs adsorbent. The interaction of adsorbent-adsorbate in diazinon removal may be owing to the occurrence of electrostatic attraction. The attraction appears when the adsorbent particle and organic contaminant as adsorbate obtained in opposite charges. This is associated with the sorption of ionic and ionizable organic contaminants. For as much as ionic adsorbate are attracted to adsorbents in opposite charges, the anionic of diazinon with the positive charge of BCs surface tends to adsorb through biochar surface as a negative charge. Subsequently, the bonding of ionic between the attractions formed in the primary active mechanism in the diazinon removal. Consequently, the obtained adsorbent-adsorbate interaction completely depends on pH values. The vast majority of researcher portrayed the electrostatic interaction through diazinon removal using the carbon-based (Sean et al., 2019; Nodeh et al., 2019; Moussavi et al., 2013; Zheng et al., 2010).
5 Conclusion
This study showed that activated and chemically modified coconut shell biochar has successfully been applied as promising adsorbent for the removal of selected organophosphorus pesticide, specifically diazinon from aqueous solutions. The optimum conditions of adsorption process for maximum adsorption capacity are at pH 7 and adsorbent dosage 2.0 g/L. The results of isotherm adsorption models were well described by the theoretical Langmuir and Freundlich models. The Langmuir model was best fit with small value of error analysis (ERRSQ and χ2) for BC3 and BC4 whereby the Freundlich data was best fit for BC1 and BC2 with the equilibrium adsorption data. Diazinon was able to be removed and adsorbed efficiently from the synthetic aqueous solutions based on the outstanding removal percentage of more than 97% using activated and chemically modified BCs at the highest adsorbent dosage (10 mg/L). The characteristic of biochar exhibited large porous surface morphology with the changes of chemical composition of high surface area within 405.97 m2/g to 508.07 m2/g by the following sequence of BC3 > BC2 > BC4 in high removal efficiency of diazinon. The study also showed better results than some studies in which the maximum adsorption capacity achieved by the modified BC2 and BC3 were 9.65 mg/g and 10.33 mg/g, respectively. This results proposed that at optimized parameter the modified BCs are highly potential and efficient for adsorption of diazinon from the contaminated surface water. The attained maximum adsorption at neutral pH is a significant advantage for practical full-scale application due to the unnecessity of pH changing which accomplished cost-effective and environmental friendly through H3PO4 activating agent (Heidarinejad et al., 2020; Li et al., 2015; Chowdhury, 2013). Thus, it can be concluded that modified coconut shell BC has a high adsorption affinity towards the selected OPPs making the BC adsorption process a feasible and effective for water treatment containing such pollutants.
Acknowledgements
The authors would like to thank the Ministry of Higher Education, Malaysia through the Fundamental Research Grant Scheme (FRGS), project code 07-01-15-1656FR for providing financial assistance for this research project. This work was also partially supported by GIST Research Institute (GRI) grant funded by the Gwangju Institute of Science and Technology (GIST) 2020. Special thanks to postgraduate students and laboratory staff of Faculty of Forestry and Environment, UPM for their support and assistance.
References
- Distribution and elimination of 14C-ethion insecticide in chamomile flowers and oil. Phosphorus, Sulfur, and Silicon and the Related Elements. 2011;186(10):2122-2134.
- [Google Scholar]
- Effective adsorption of prothiofos (O-2, 4-dichlorophenyl O-ethyl S-propyl phosphorodithioate) from water using activated agricultural waste microstructure. J. Environ. Chem. Eng.. 2020;8(3) 103768
- [Google Scholar]
- Cu–BTC@ cotton composite: design and removal of ethion insecticide from water. RSC Adv.. 2016;6(48):42324-42333.
- [Google Scholar]
- Kinetic and equilibrium studies on the removal of 14 C-ethion residues from wastewater by copper-based metal–organic framework. Int. J. Environ. Sci. Technol.. 2018;15(11):2283-2294.
- [Google Scholar]
- Separation of bioactive chamazulene from chamomile extract using metal-organic framework. J. Pharm. Biomed. Anal.. 2017;146:126-134.
- [Google Scholar]
- Applicable strategy for removing liquid fuel nitrogenated contaminants using MIL-53-NH2@ natural fabric composites. Ind. Eng. Chem. Res.. 2018;57(44):15054-15065.
- [Google Scholar]
- Efficient removal of organophosphorus pesticides from wastewater using polyethylenimine-modified fabrics. Polymer. 2018;155:225-234.
- [Google Scholar]
- Zeolitic imidazolate frameworks: Experimental and molecular simulation studies for efficient capture of pesticides from wastewater. J. Environ. Chem. Eng.. 2019;7(6) 103499
- [Google Scholar]
- Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J.. 2017;311:348-358.
- [Google Scholar]
- Comparative adsorption studies of Cd (II) on EDTA and acid treated activated carbons from aqueous solutions. J. Anal. Bioanal. Tech.. 2015;6(288):2.
- [Google Scholar]
- Influence of biochars on the accessibility of organochlorine pesticides and microbial community in contaminated soils. Sci. Total Environ.. 2019;647:551-560.
- [Google Scholar]
- Removal of diazinon pesticide from aqueous solutions using MCM-41 type materials: isotherms, kinetics and thermodynamics. Int. J. Environ. Sci. Technol.. 2018;15(6):1301-1312.
- [Google Scholar]
- Adsorption of pesticides with different chemical properties to a wood biochar treated with heat and iron. Water Air Soil Pollut.. 2016;227(6):203.
- [Google Scholar]
- Biomass-based pyrolytic polygeneration system on cotton stalk pyrolysis: influence of temperature. Bioresour. Technol.. 2012;107:411-418.
- [Google Scholar]
- Preparation, characterization and adsorption studies of heavy metals onto activated adsorbent materials derived from agricultural residues (Doctoral dissertation. University of Malaya); 2013.
- Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustainable Mater. Technol.. 2016;9:10-40.
- [Google Scholar]
- Effect of water pH on the chemical stability of pesticides. AG/Pesticides. 2001;14:1.
- [Google Scholar]
- Optimizing the removal of organophosphorus pesticide Malathion from water using multi-walled carbon nanotubes. Chem. Eng. J.. 2017;310:22-32.
- [Google Scholar]
- Adsorption of chlorophenoxy pesticides on activated carbon with gradually removed external particle layers. Chem. Eng. J.. 2017;308:408-418.
- [Google Scholar]
- Optimizing a low added value bentonite as adsorbent material to remove pesticides from water. Sci. Total Environ.. 2019;672:743-751.
- [Google Scholar]
- Investigation into the role of surface modification of cellulose nanocrystals with succinic anhydride in dye removal. J. Polym. Environ.. 2019;27(11):2419-2427.
- [Google Scholar]
- Refining of liquid fuel from N-Containing compounds via using designed Polysulfone@ Metal organic framework composite film. J. Cleaner Prod.. 2019;218:347-356.
- [Google Scholar]
- Non-invasive route for desulfurization of fuel using infrared-assisted MIL-53 (Al)-NH2 containing fabric. J. Colloid Interface Sci.. 2019;556:193-205.
- [Google Scholar]
- Development of a novel method for the removal of diazinon pesticide from aqueous solution and modeling by artificial neural networks (ANN) J. Ind. Eng. Chem.. 2016;35:295-308.
- [Google Scholar]
- European Union (Drinking Water) Regulations 2014 (S.I No. 122 of 2104), Dublin.
- Degradation of organophosphorus pesticides in water during UV/H 2 O 2 treatment: role of sulphate and bicarbonate ions. J. Chem.. 2012;9(4):2015-2022.
- [Google Scholar]
- Ultrasond-assisted synthesis of Fe3O4/SiO2 core/shell with enhanced adsorption capacity for diazinon removal. J. Magn. Magn. Mater.. 2016;416:75-80.
- [Google Scholar]
- Changes in sorption and bioavailability of herbicides in soil amended with fresh and aged biochar. Geoderma.. 2019;33:341-349.
- [Google Scholar]
- AGRO-2014: A time dependent model for assessing the fate and food-web bioaccumulation of organic pesticides in farm ponds: Model testing and performance analysis. Sci. Total Environ.. 2018;639:1324-1333.
- [Google Scholar]
- Biomass ashes as potent adsorbent for pesticide: prediction of adsorption capacity by artificial neural network. Environ. Sci. Technol. 2020:1-8.
- [Google Scholar]
- Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. J. Colloid Interface Sci.. 2007;315(1):87-93.
- [Google Scholar]
- Magnetic particles modification of coconut shell-derived activated carbon and biochar for effective removal of phenol from water. Chemosphere. 2018;211:962-969.
- [Google Scholar]
- Adsorption and photocatalytic detoxification of diazinon using iron and nanotitania modified activated carbons. J. Taiwan Inst. Chem. Eng.. 2017;75:299-306.
- [Google Scholar]
- Surfactant-modified montmorillonite as a nanosized adsorbent for removal of an insecticide: Kinetic and isotherm studies. Environ. Technol.. 2015;36(24):3125-3135.
- [Google Scholar]
- Methods for preparation and activation of activated carbon: a review. Environ. Chem. Lett. 2020:1-23.
- [Google Scholar]
- Some considerations of the origins of porosity in carbons from chemically activated wood. Carbon. 1993;31(7):1185-1192.
- [Google Scholar]
- Study on the removal of pesticide in agricultural run off by granular activated carbon. Bioresour. Technol.. 2011;102(9):5312-5318.
- [Google Scholar]
- The removal of organophosphorus pesticides from water using a new amino-substituted calixarene-based magnetic sporopollenin. New J. Chem.. 2016;40(4):3130-3138.
- [Google Scholar]
- Investigation of photo–fenton–like process efficiency in diazinon pesticide removal from aqueous solutions. J. Saf., Environ. Health Res.. 2016;1(1):17-22.
- [Google Scholar]
- 2, 4-D adsorption to biochars: Effect of preparation conditions on equilibrium adsorption capacity and comparison with commercial activated carbon literature data. Water Res.. 2014;62:20-28.
- [Google Scholar]
- Sorptive removal of selected emerging contaminants using biochar in aqueous solution. J. Ind. Eng. Chem.. 2016;36:364-371.
- [Google Scholar]
- Effect of solution pH on the hydrolysis and photolysis of diazinon in aqueous solution. Water Air Soil Pollut.. 1998;108(3–4):445-456.
- [Google Scholar]
- Removal of atrazine, lindane and diazinone from water by organo-zeolites. Water Res.. 2006;40(5):1079-1085.
- [Google Scholar]
- Contamination levels of selected organochlorine and organophosphate pesticides in the Selangor River, Malaysia between 2002 and 2003. Chemosphere. 2007;66(6):1153-1159.
- [Google Scholar]
- Analysis of Functional Organic Molecules at Noble Metal Surfaces by Means of Vibrational Spectroscopies (Doctoral dissertation. Freie Universität Berlin); 2011.
- The role of H3PO4 in the preparation of activated carbon from NaOH-treated rice husk residue. RSC Adv.. 2015;5:32626-32636.
- [Google Scholar]
- A novel approach for the adsorption of cadmium ions in aqueous solution by dead calcareous skeletons. Desalin. Water Treat.. 2013;52(16–18):3169-3177.
- [Google Scholar]
- Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere. 2012;87(2):151-157.
- [Google Scholar]
- Adsorption and removal of organophosphorus pesticides from environmental water and soil samples by using magnetic multi-walled carbon nanotubes@ organic framework ZIF-8. J. Mater. Sci.. 2018;53(15):10772-10783.
- [Google Scholar]
- Characterization of biochars derived from agriculture wastes and their adsorptive removal of atrazine from aqueous solution: A comparative study. Bioresour. Technol.. 2015;198:55-62.
- [Google Scholar]
- Mesoporous carbon materials as electrodes for electrochemical supercapacitors. Int. J. Electrochem. Sci.. 2010;5:903-916.
- [Google Scholar]
- Characterization of pesticide sorption behaviour of slow pyrolysis biochars as low cost adsorbent for atrazine and imidacloprid removal. Sci. Total Environ.. 2017;577:376-385.
- [Google Scholar]
- Adsorption kinetics and thermodynamics of organophosphorus profenofos pesticide onto Fe/Ni bimetallic nanoparticles. Int. J. Environ. Sci. Technol.. 2016;13(5):1393-1404.
- [Google Scholar]
- Biosorption of diazinon by a pre-treated alimentary industrial waste: equilibrium and kinetic modeling. Appl. Water Sci.. 2017;7(7):4067-4076.
- [Google Scholar]
- The investigation of diazinon pesticide removal from contaminated water by adsorption onto NH4Cl-induced activated carbon. Chem. Eng. J.. 2013;214:172-179.
- [Google Scholar]
- Organochlorine pesticide residues in surface water and groundwater along Pampanga River, Philippines. Environ. Monitoring Assessment. 2018;190(5):289.
- [Google Scholar]
- Equilibrium, kinetic and thermodynamic study of pesticides removal from water using novel glucamine-calix [4] arene functionalized magnetic graphene oxide. Environ. Sci. Processes Impacts. 2019;21(4):714-726.
- [Google Scholar]
- Comparative assessment of the efficiency of rice husk biochar and conventional water treatment method to remove chlorpyrifos from pesticide polluted water. Curr. J. Appl. Sci. Technol. 2020:1-11.
- [Google Scholar]
- Occurrence, distribution, and sources of emerging organic contaminants in tropical coastal sediments of anthropogenically impacted Klang River estuary, Malaysia. Mar. Pollut. Bull.. 2018;131:284-293.
- [Google Scholar]
- Adsorptive removal of diazinon: kinetic and equilibrium study. Desalin. Water Treat.. 2016;57(4):1880-1889.
- [Google Scholar]
- Pesticide storage dissipation in surface water samples. In: Pesticides in Surface Water: Monitoring, Modeling, Risk Assessment, and Management. American Chemical Society; 2019. p. :89-100.
- [Google Scholar]
- Removal of diazinon and 2, 4-dichlorophenoxyacetic acid (2, 4-D) from aqueous solutions by granular-activated carbon. Desalin. Water Treat.. 2014;52(22–24):4350-4355.
- [Google Scholar]
- Efficacy of biochar in removal of organic pesticide, Bentazone from watershed systems. J. Environ. Sci. Health, Part B 2020:1-10.
- [Google Scholar]
- Methylene blue number as useful indicator to evaluate the adsorptive capacity of granular activated carbon in batch mode: Influence of adsorbate/adsorbent mass ratio and particle size. J. Hazard. Mater.. 2009;165(1–3):291-299.
- [Google Scholar]
- New magnetic graphene-based inorganic–organic sol-gel hybrid nanocomposite for simultaneous analysis of polar and non-polar organophosphorus pesticides from water samples using solid-phase extraction. Chemosphere. 2017;166:21-30.
- [Google Scholar]
- Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J. Environ. Manage.. 2012;109:61-69.
- [Google Scholar]
- Determination of organophosphorus pesticides using molecularly imprinted polymer solid phase extraction. Malaysian J. Anal. Sci.. 2011;15(2):175-183.
- [Google Scholar]
- Sean, S., Binh, Q.A., Tungtakanpoung, D., Kajityichyanukul, P., (2019, September). Potential adsorption mechanisms of different bio-wastes to remove diazinon from aqueous solution. In IOP Conference Series: Materials Science and Engineering (Vol. 617, No. 1, p. 012012). IOP Publishing.
- Rapid removal of triazine pesticides by P doped biochar and the adsorption mechanism. Chemosphere. 2019;235:918-925.
- [Google Scholar]
- Adsorption of 15 different pesticides on untreated and phosphoric acid treated biochar and charcoal from water. J. Environ. Chem. Eng.. 2014;2(4):2013-2025.
- [Google Scholar]
- Sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresour. Technol.. 2016;211:727-735.
- [Google Scholar]
- Innovative spherical biochar for pharmaceutical removal from water: Insight into adsorption mechanism. J. Hazard. Mater. 2020 122255
- [Google Scholar]
- Optimization of mesoporous activated carbon from coconut shells by chemical activation with phosphoric acid. BioResources.. 2013;8(4):6184-6195.
- [Google Scholar]
- A novel mesoporous hydrogen-bonded organic framework with high porosity and stability. Chem. Commun.. 2020;56(1):66-69.
- [Google Scholar]
- Removal of chlorpyrifos from waste water by wheat straw-derived biochar synthesized through oxygen-limited method. RSC Adv.. 2015;5(89):72572-72578.
- [Google Scholar]
- Surface water organophosphorus pesticides concentration and distribution in the Langat River, Selangor. Malaysia. Exposure and Health 2016:1-15.
- [Google Scholar]
- The ratio of H/C is a useful parameter to predict adsorption of the herbicide metolachlor to biochars. Environ. Res.. 2020;109324
- [Google Scholar]
- High-efficiency removal of dyes from wastewater by fully recycling litchi peel biochar. Chemosphere. 2020;246 125734
- [Google Scholar]
- Simple synthesis of Ni/high porosity biomass carbon composites with enhanced electrochemical performance of lithium–sulfur battery. J. Alloys Compd. 2020153692
- [Google Scholar]
- Biomass-based pyrolytic polygeneration system for bamboo industry waste: evolution of the char structure and the pyrolysis mechanism. Energ Fuel. 2016;30(8):6430-6439.
- [Google Scholar]
- Potential benefits of biochar in agricultural soils: A review. Pedosphere. 2017;27(4):645-661.
- [Google Scholar]
- Yang, R., Liu, G., Xu, X., Li, M., Zhang, J., Hao, X., 2011. Surface texture, chemistry and adsorption properties of acid blue 9 of hemp (Cannabis sativa L.) bast-based activated carbon fibers prepared by phosphoric acid activation. Biomass Bioenergy, 35(1), 437–445.
- Sorption properties of greenwaste biochar for two triazine pesticides. J. Hazard. Mater.. 2010;181(1–3):121-126.
- [Google Scholar]
- Evaluation of a novel chitosan polymer-based adsorbent for the removal of chromium (III) in aqueous solutions. Carbohydrate Polym. 2013:92.
- [Google Scholar]







