Translate this page into:
[Hmim]PF6 enhanced the extraction of polycyclic aromatic hydrocarbons from soil with the QuEChERS method
⁎Corresponding author at: Guangdong University of Technology, 100 Wai Huan Xi Road, Guangzhou Higher Education Mega Center, Guangzhou, Guangdong 510006, PR China. lqjzhch@gdut.edu.cn (Qianjun Liu),
⁎⁎Corresponding author at: Foshan University, Jiangwan 1 st Road, Chancheng, Foshan, Guangdong 528000, PR China. zhangmin@gic.ac.cn (Min Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
To detect, identify, and quantify the polycyclic aromatic hydrocarbons (PAHs) released into the environment, the PAHs need to be isolated from the soil matrix. In this work, a modified quick, easy, cheap, efficient, rugged and safe (QuEChERS) method with ionic liquid was combined with liquid chromatography to identify 16 selected PAHs in soil. Ionic liquid 1-hexyl-3-methylimidazolium hexafluorophosphate ([Hmim]PF6) was applied as an extractant component to enhance the process. The [Hmim]PF6 content in acetonitrile (ACN) was optimized. The [Hmim]PF6 modified QuEChERS method has the advantages defined by its name and a similar recovery to other extraction methods reported in the literature. Adding [Hmim]PF6 may eliminate the co-extract proportion and achieve a more effective extraction. Compared with ACN alone, the matrix effect (ME) of ACN containing 5% [Hmim]PF6 was reduced by approximately 35%. Additionally, the ME of using ACN containing [Hmim]PF6 without a clean-up procedure was similar to that of using ACN followed by a clean-up procedure. The recoveries of the QuEChERS method implemented with [Hmim]PF6 ranged from 75.19% to 100.98%. The limits of detection (LOD) and limits of quantification (LOQ) ranged from 0.86 to 4.51 µg/kg and from 2.87 to 15.13 µg/kg, respectively.
Keywords
[Hmim]PF6
QuEChERS
Polycyclic aromatic hydrocarbons
Soil
1 Introduction
Polycyclic aromatic hydrocarbons (PAHs) are organic pollutants mostly derived from the processing and combustion of fossil fuels and anthropogenic activities. Due to their recalcitrance and bioaccumulation potential, PAHs do not degrade easily under natural conditions and their persistence increases with increasing molecular weight. Therefore, PAHs have received significant environmental concern (Fernández-Luqueño et al., 2016). Upon exposure to high levels of pollutant mixtures containing PAHs, occurring short-term effects include eye irritation, nausea, vomiting, diarrhoea, etc. Mixtures of PAHs are also known to cause skin irritation and inflammation. The carcinogenic and mutagenic potencies of PAHs are also an important concern (Soukarieh et al., 2018). Sixteen basic PAHs are included in the United States Environmental Protection Agency (16 US EPA PAHs) priority pollutant list (Kosnar et al., 2018). Because of the hydrophobic character of PAHs, they tend to accumulate in soils, sediments, and sewage sludge. Soil is considered to be a major reservoir of PAHs. PAHs may further accumulate in vegetables and other biota after deposition on surface soils, and then be transferred to humans via the food chain. Some PAHs may strongly sorb on soil, where they persist for a long period of time (Soukarieh et al., 2018).
To detect, identify, and quantify the PAHs released into the environment, the PAHs need to be isolated from the soil matrix. Several extraction methods have been developed (Wang et al., 2007; Camel, 2001; Page et al., 2004). In 2003, Anastassiades and Lehotay (2003) described the “quick, easy, cheap, effective, rugged and safe” (QuEChERS) method for the multiclass, multiresidue analysis of pesticides in fruits and vegetables. The original QuEChERS method (Anastassiades and Lehotay, 2003) has the following steps: (a) use acetonitrile (ACN) as the extractant; (b) add magnesium sulfate (MgSO4) and sodium chloride (NaCl) to induce phase separation; and (c) perform clean-up and drying of the ACN phase simultaneously during dispersive solid phase extraction (d-SPE) with anhydrous MgSO4 and the primary secondary amine (PSA). The steps of the modified method AOAC 2007.01 (Lehotay, 2007) include: (a) use ACN containing 1% (v/v) acetic acid (AcOH) as an extractant; (b) separate the phase by MgSO4/sodium acetate (NaAc); and (c) for substrates with a fat content greater than 1%, add 50 mg of C18 solid phase adsorbent per mL of extract (if analysing compounds without planar structure, add 50 mg of graphitized carbon black (GCB) per mL of extract agent for solid phase adsorption). The ruggedness characteristics of the QuEChERS approach have been thoroughly evaluated in the original (Anastassiades and Lehotay, 2003) and subsequent publications by the original authors (Mastovska et al., 2010; Koesukwiwat et al., 2010). Compared with other extraction method (Ma et al., 2010, 2011; Song et al., 2012), the QuEChERS method has a low cost per sample, short elapse time and all the other advantages defined by its name.
The QuEChERS method is very flexible and the original method has evolved into a template for modification depending on the analyte properties, matrix composition, equipment and analytical techniques available in the lab. The template also can be achieved for many pesticides in many matrices, even if different ratios and types of sample sizes, solvents, salts and sorbents are use (Lehotay et al., 2010). Some examples were showed in Table 1. EtOAc: ethyl acetate; GPC: Gel Permeation Chromatography; DCM: dichloromethane; Na2EDTA: Ethylenediaminetetraacetic acid disodium salt.
No
Target
Matrix
Extraction and Separation
Recovery
1
PAHs
Animal tissues
Extraction: ACN
84–107%
Kiełbasa and Buszewski (2017))
Clean-up: PSA, C18, MgSO4
2
PCBs, PAHs, PBDES, PCDD/Fs
Biological samples
Extraction: EtOAc
∼100%
Cloutier et al. (2017)
Clean-up: GPC, n- hexanes, DCM
3
Drugs
Food
Extraction: ACN
90%
Schmidt and Snow (2016)
Clean-up: PSA, MgSO4,
4
Pesticides, PAHs, PCBs
Honey
Extraction: ACN
60–103%
Al-Alam et al. (2017)
Clean-up: PSA
5
PAHs
Food
Extraction: ACN
72–112%
Petrarca and Godoy (2018)
Clean-up: PSA, C18, MgSO4
6
PAHs
Cachaça
Extraction: DCM
84.8–118.2%
da Silva et al. (2019)
Elution: EtOAc
7
Veterinary drugs
Milk
Extraction: ACN, AcOH, Na2EDTA
70–110%
Aguilera-Luiz et al. (2008)
8
PAHs
Soil
Extraction: hexane, acetone
70–110%
Nikolić et al. (2018)
Clean-up: MgSO4, PSA, C18, clinoptilolite, florisil, diatomaceous earth
9
PAHs
Fish
Extraction: ACN, AcOH
63.5–110%
Ramalhosa et al. (2009)
Clean-up: MgSO4, PSA, C18
10
PAEs
Soil
Extraction: ACN, acetone, n-hexane, DCM
70–117.9%
Liu et al. (2018)
Clean-up: MgSO4, PSA, C18
11
Neonicotinoid insecticide residues
Soil
Extraction: ACN, AcOH
72–104.8%
Dankyi et al. (2014)
Clean-up: GCB, PSA, C18
12
Herbicides
Soil
Extraction: ACN, AcOH
80–110%
Pang et al. (2016)
Clean-up: GCB
13
Chlorinated compounds
Soil
Extraction: EtOAc
62–93%
Pinto et al. (2010)
14
Pyraclostrobin
Soil
Extraction: ACN
80.3–109.4%
Zhang et al. (2012)
Clean-up: MgSO4
15
Aromatic organochlorines
Soil
Extraction: ACN
60–100%
Rouviere et al. (2012)
Clean-up: MgSO4, PSA
The matrix consists of one or more undetected components from the sample, which often significantly affects the accuracy of analytical results. Such disturbances from matrixes are called matrix effect (ME) which are likely caused by different compounds in soils such as salts, ion-pairing agents, endogenous compounds, metabolites and proteins given that soil is an extremely variable mixture of minerals, organic particles and diverse microbial communities. Furthermore, the extraction efficiency of target analytes can be affected by the sorption and limitation in analytical procedures for examining soils with high organic matter (Varona-Torres et al., 2018).
Ionic liquids (ILs) are organic salts that exist as liquids below a threshold temperature. ILs offer a highly solvating and non-coordinating medium in which a number of organic and inorganic solutes may be dissolved (Blanchard and Brennecke, 2001). ILs are considered as green solvents that are non-volatile, non-flammable, highly thermostable, relatively undemanding and inexpensive to manufacture (Ngo et al., 2000). The usable liquid range may include that used for conventional synthetic chemistry, and separation ionic liquids are considered to be ideal substitutes for traditional volatile organic solvents (Liu et al., 2005). Han et al. (2012) provided an overview of the applications of ILs in liquid phase microextraction technology. Aqueous solutions containing aggregates of the ionic liquid (IL) 1-hexadecyl-3-methylimidazolium bromide (HDMIm-Br) could be an extracting medium to extract PAHs from sediments (Pino et al., 2008).
1-hexyl-3-methylimidazolium hexafluorophosphate ([Hmim]PF6) is one of the most stable and hydrophobic ionic liquids in ambient conditions (Martínez-Romero et al., 2017). Additionally, [Hmim]PF6 has been applied in different separation methods, such as single-drop microextraction (SDME) (Chisvert et al., 2009), liquid-phase microextraction (LPME) (Liu et al., 2004) and ultrasound-assisted microextraction (UAME) (Zhou et al., 2009). The extracted target substance in liquid–liquid separation processes with [Hmim]PF6 can be heavy metal ions and organic compounds (common pollutant aromatic compounds and acetone) (Vidal et al., 2010; Hirayama et al., 2005; Saien et al., 2015).
The aim of this study was to develop and validate a [Hmim]PF6evolved QuEChERS method for extracting 16 PAHs from soil. By comparing the recoveries and the ME of the 16 PAHs, the role of [Hmim]PF6 in the QuEChERS extraction process was explored.
2 Materials and methods
2.1 Chemicals and reagents
The standard mixture of the sixteen PAHs (200 mg/L), dissolved in 1 mL of ACN and containing naphthalene (NAP), acenaphthylene (ANY), acenaphthene (ANA), fluorine (FLU), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLT), pyrene (PYR), benzo[a]anthracene (BaA), chrysene (CHR), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), dibenzo[a,h]anthracene (DBA), benzo[g,h,i]perylene (BPE), and indeno[1,2,3-cd]pyrene (IPY), was purchased from ANPEL Scientific Instrument (Shanghai, China). The sixteen standard stock solutions of the PAHs (2 mg/L) were obtained by 1:100 dilution with ACN.
The ionic liquid [Hmim]PF6 (99%, 5 g) was purchased from ANPEL Scientific Instrument (Shanghai, China). HPLC-grade n-hexane and acetone were purchased from Merck (Darmstadt, Germany). HPLC-grade ACN, analytical-grade anhydrous MgSO4 and NaCl were obtained from Aladdin Industrial Corporation (Shanghai, China). PSA (40–63 µm) and LC-C18 (40–63 µm), which were used as the clean-up materials, were purchased from ANPEL Scientific Instrument (Shanghai, China). Ultrapure water was produced in the laboratory with a Milli-Q gradient system from Millipore (Vienna, Austria).
2.2 Apparatus and conditions
All the PAHs samples were quantified using a Shimadzu LC-20AD high performance liquid chromatography (HPLC) coupled to a Shimadzu SPD-M20A diode array detector (DAD) (Shimadzu corporation, Kyoto, Japan) with a 4.6 mm × 250 mm × 5 µm C18 column (Shimadzu corporation, Kyoto, Japan). The mobile phase, which was degassed and filtered before analysis, consisted of ACN (A) and ultrapure water (B). Flow rate of eluent was 1.0 mL/min with the mobile phase initially consisting of 65% A and 35% B, with a linear increase to 80% A from 30.0 min to 40.0 min and to 100% A from 50.0 min to 51.0 min, finally decreased to 40% A from 54.5 min to 56 min and held for 1.0 min. The temperature of the column oven was maintained at 30 °C and the injection volume was 20 µL. Fig. 1 shows the chromatogram of 16 PAHs at the conditions above which registered in 254 nm.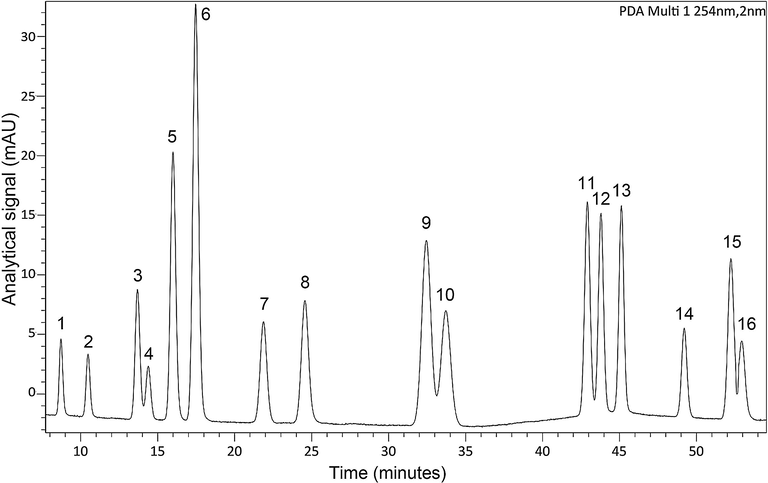
The chromatogram of 16 PAHs in 254 nm. Compounds: (1) NAP; (2) ANT; (3) ANA; (4) FLU; (5) PHE; (6) ANT; (7) PLT; (8) PYR; (9) BaA; (10) CHR; (11) BbF; (12) BkF; (13) BaP; (14) DBA; (15) BPE; (16) IPY.
The identification of sample peaks was based on the retention time of the standard peaks. The detection wavelengths were based on the maximum UV absorption wavelength of each PAHs, which were according to the Soil and sediment—Determination of polycyclic aromatic hydrocarbons—High performance liquid chromatography (HJ784-2016, China).
2.3 Sample preparation
The soil samples were collected from middle soil layers (20–60 cm depth) in an area in Guangzhou China, as far away from PAHs pollution sources as possible. Soil samples were transported and stored at 4 °C in precleaned glass containers. After removing the plant tissues and silt, soil samples were dried at room temperature before being ground in a mortar and sieved. The soil pH was 4.42 in the soil/water suspension (1:2.5, v/v), and the organic matter content was 1.017%. Then, the soil samples were spiked with 200 µg/kg of the PAHs standards and stirred with a mechanical stirring apparatus to obtain a homogeneous sample. Samples were then left in a ventilated location to allow all the solvent to evaporate. The standards are thought to bind to the soil samples in a manner similar to natural processes.
2.4 The QuEChERS method
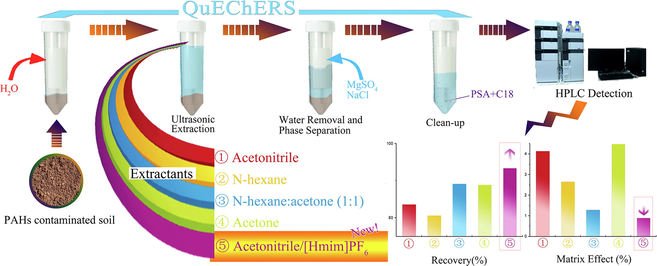
The modified QuEChERS procedure in this paper consisted of the following steps, which were based on the literature (Liu et al., 2018; Schenck and Hobbs, 2004). (a) Sample (5.0 g) was weighed into a 50 mL disposable polypropylene centrifuge tube, and 5 mL of ultrapure water was added. The sample was stood for 10 min. (b) Twenty millilitres of extraction solvent (ACN, n-hexane, acetone, n-hexane/acetone (1:1, v/v) or ACN containing[Hmim]PF6 (5%,10%,15%)) was added, and the tube containing samples was ultrasonicated for 20 min. (c) NaCl (2.0 g) and MgSO4 (2.0 g) were added to the centrifuge tube, and the tube was shaken vigorously by hand for 1 min and centrifuged at 4000 rpm. (d) A 10 mL aliquot of the supernatant was transferred into a 15 mL disposable polypropylene centrifuge tube. PSA (500 mg) and C18 (500 mg) were added to the tube, which was ultrasonicated for 5 min. (e) Finally, 5 mL of the supernatant was filtered with 0.22 µm organic phase membrane. The filtrate was transferred and concentrated by rotary evaporation to less than 1 mL. Three millilitres ACN was added before rotary evaporation and the solution was concentrated to less than 1 mL again. (f) The extract was transferred to an autosampler vial (CNW, Germany) and then diluted with ACN to 1.5 mL for analysis by HPLC.
2.5 Calibration curve
The matrix standard solutions (ACN, n-hexane, acetone, n-hexane/acetone (1:1, v/v) and ACN/5% [Hmim]PF6) were prepared following the steps in Section 2.4. The concentrations of the working standards were 20.0, 50.0, 100.0, 200.0, 500.0, and 1000.0 µg/L, which were diluted from the PAHs standard stock solutions (2 mg/L) with matrix standard solutions.
2.6 Method validation
The performance of the method was validated by evaluating the parameters including recovery, relative standard deviation (RSD), the limits of detection (LOD) and the limits of quantification (LOQ). The LOD and LOQ were calculated based on the official method of the Official Analytical Chemists Association, that is, derived from the blank and quantified as the mean plus three times and ten times the field blank standard deviation (SD), respectively.
2.7 Matrix effect
To compare the ME, solutions of 1000 µg/L PAHs were prepared using five matrix standard solutions (ACN, n-hexane, acetone, n-hexane/acetone (1:1, v/v) and ACN/5% [Hmim]PF6) and ACN pure solvent. The ME is calculated by the following formula (Matuszewskiet al., 2003):
In the formula, ME refers to the matrix effect, A1 refers to the peak area of PAHs in the pure solvent sample at a certain concentration, and A2 refers to the peak area of PAHs in the matrix standard solution at the same concentration. If the average ME exceed 20%, the ME can significantly affect the detection results, and vice versa, the influence of the ME on the analysis results can be neglected (Matuszewski et al., 2003).
3 Results and discussion
3.1 Effect of [Hmim]PF6 on the extraction of PAHs
The extraction efficiency strongly depends on the extraction solvent, the nature of the sample, and the chemical properties of the pollutants. In this study, an extraction solvent of ACN/5% [Hmim]PF6 was used to investigate the effects of ionic liquids on the extraction of PAHs from soil. The extraction results were compared those obtained with ACN, n-hexane, acetone and n-hexane/acetone (1:1, v/v). ACN, n-hexane and acetone are typically used for the extraction of PAHs (Liu et al., 2018; Socas-Rodríguez et al., 2017). A mixed solvent of n-hexane/acetone (1:1, v/v) was recommended for the extraction of semivolatile organics from soil by the EPA Method 3540C and 3550C. DCM can also be used as an extractant for PAHs (Albinet et al., 2013; Pena et al., 2009). However, DCM is denser than water, and after solvent delamination, the DCM was below the aqueous phase, making its removal difficult. Therefore, DCM was not suitable for the QuEChERS extraction process (Liu et al., 2018).
Table 2 shows the recoveries and the RSD of the sixteen PAHs. Using n-hexane/acetone (1:1, v/v) as an extractant has advantages in terms of both the recovery and the RSD. The extraction effect of ACN is slightly worse than that of n-hexane/acetone (1:1, v/v), but when ACN containing 5% [Hmim]PF6 is used, the recoveries and the RSD increased to 75.19–100.98% and 0.58–6.44%, respectively. The recovery of most PAH components was increased. Eleven PAH components have higher recovery when using ACN containing 5% [Hmim]PF6 than when using n-hexane/acetone (1:1, v/v). It appears that [Hmim]PF6 can enhance the extraction of PAHs from soil with the QuEChERS method.
ACN
N-hexane
Acetone
N-hexane/acetone (1:1, v/v)
ACN/5% [Hmim]PF6
PAHs
R (%)
RSD (%)
R (%)
RSD (%)
R (%)
PAHs
R (%)
RSD (%)
R (%)
RSD (%)
NAP
91.47
4.41
61.39
2.75
86.87
8.43
90.27
3.29
89.64
3.42
ANY
86.41
0.98
77.55
0.64
80.24
4.58
81.84
0.21
100.09
4.32
ANA
72.48
1.51
89.71
2.31
86.01
0.34
91.18
2.30
93.06
3.47
FLU
74.12
3.26
76.42
2.60
72.34
1.59
88.51
2.35
75.19
0.73
PHE
87.99
1.19
82.87
5.47
66.92
4.58
82.82
3.16
90.35
6.44
ANT
78.32
1.38
70.71
4.21
91.12
1.31
80.95
0.68
87.59
3.22
FLT
112.69
0.28
85.16
1.62
110.58
4.69
97.02
5.22
100.97
5.05
PYR
110.29
1.10
86.13
0.69
101.38
6.04
79.45
2.64
100.98
6.35
BaA
76.10
7.54
66.17
3.18
107.54
1.83
105.39
1.07
94.96
2.62
CHR
70.26
7.85
81.67
0.65
106.05
3.34
84.37
0.73
100.94
6.15
BbF
85.89
2.30
83.63
6.45
73.72
2.98
95.51
2.61
78.75
5.77
BkF
81.08
8.00
89.31
0.21
88.89
2.09
96.45
2.42
100.11
5.25
BaP
80.52
4.80
79.51
6.59
86.10
8.22
77.38
3.01
100.68
0.58
DBA
71.84
6.87
77.23
3.95
77.72
4.25
90.33
2.80
75.79
3.94
BPE
88.92
1.66
91.73
0.65
92.05
0.55
95.76
1.09
100.22
1.63
IPY
70.00
5.81
88.30
1.68
92.88
2.93
90.48
0.90
79.81
6.40
ACN containing [Hmim]PF6 (5%, 10%, or 15%) was used as the extractant to investigate the effect of [Hmim]PF6 content on the extraction process. The recoveries and the RSD of the sixteen PAHs are listed in Table 3. Compared with ACN, ACN containing [Hmim]PF6 shows higher recovery and RSD. It shows that the content of [Hmim]PF6 in the extractant has a certain influence on the extraction efficiency of a variety of PAH compounds, which may be related to the structure difference. A [Hmim]PF6 concentration of 5% was selected considering the extraction efficiency and convenient operation.
ACN
ACN/5% [Hmim]PF6
ACN/10% [Hmim]PF6
ACN/15% [Hmim]PF6
PAHs
R (%)
RSD (%)
R (%)
RSD (%)
R (%)
RSD (%)
R (%)
RSD (%)
NAP
91.47
4.41
89.64
3.42
85.30
5.67
77.84
0.41
ANY
86.41
0.98
100.09
4.32
95.60
0.88
94.76
5.17
ANA
72.48
1.51
93.06
3.47
93.11
4.87
89.47
0.31
FLU
74.12
3.26
75.19
0.73
75.91
1.96
74.68
1.32
PHE
87.99
1.19
90.35
6.44
92.25
4.21
80.06
2.88
ANT
78.32
1.38
87.59
3.22
92.36
5.08
96.43
1.13
FLT
112.69
0.28
100.97
5.05
100.29
1.73
97.98
0.52
PYR
110.29
1.10
100.98
6.35
98.36
4.81
101.16
4.96
BaA
76.10
7.54
94.96
2.62
98.98
0.42
99.38
0.81
CHR
70.26
7.85
100.94
6.15
97.58
5.87
95.15
4.10
BbF
85.89
2.30
78.75
5.77
85.79
0.12
90.88
5.28
BkF
81.08
8.00
100.11
5.25
91.17
1.68
100.17
3.59
BaP
80.52
4.80
100.68
0.58
95.08
1.75
89.94
3.05
DBA
71.84
6.87
75.79
3.94
75.51
1.92
86.81
0.56
BPE
88.92
1.66
100.22
1.63
91.17
4.18
92.39
0.61
IPY
70.00
5.81
79.81
6.40
93.94
2.24
93.90
1.40
3.2 Matrix effect
ME was originally discussed by Liang and Kebarle (1993). Because of the existence of interferences which would co-elute along with the analyte of interest in a sample compared to a pure standard solution, leading to a significant increase or decrease in the detector response (Liang and Kebarle, 1993). Hence, the evaluation of ME is required to obtain more accurate and reliable results (Annesley, 2003). The ME observed with five extract solvents were shown in Fig. 2.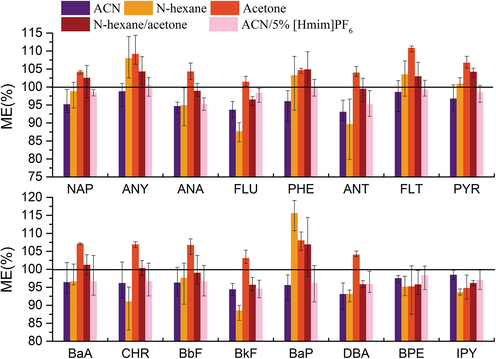
ME with five solvents.
The ME of ACN, n-hexane, acetone, n-hexane/acetone (1:1, v/v) and ACN/5% [Hmim]PF6 were 93.08–98.79%, 87.68–115.60%, 94.78–110.87%, 95.68–106.88% and 94.61–100.57%, respectively. Among these effects, the maximum ME of n-hexane and acetone reached 110% or more, and the minimum ME of n-hexane was lower than 90%.ACN, hexane/acetone (1:1, v/v) and ACN/5% [Hmim]PF6 had ME within 90–110%. The ME of ACN/5% [Hmim]PF6 were close to 100%. Compared with ACN, ME of ACN containing 5% [Hmim]PF6 was reduced by approximately 35%. The results show that the ME was within the range of 85–115% for both the QuEChERS method and the [Hmim]PF6 modified QuEChERS method for PAHs. Compared with that extracted by ACN, the chromatogram of the analyte extracted by ACN/5% [Hmim]PF6 showed a clean baseline and narrower peaks, in consistence with references (Bi et al., 2011; Fernandez-Navarro et al., 2012; Ubeda-Torres et al., 2015), with a more inconspicuous impurity peak. All the results demonstrate the effectiveness of [Hmim]PF6 in the extraction selectivity of PAHs in soil.
3.3 Clean-up procedure
Clean-up procedure in QuEChERs method can recede ME, remove the co-extractants and eliminate the interference peaks which present in the chromatograms. Some sorbents have the ability to attenuate ME, such as PSA and NH2 have the ability to remove fatty acids, sugars, and other matrix co-extractives, GCB can remove planar molecules such as natural pigments (e.g., chlorophyll, hemoglobin, and carotenoids), C18 has extreme retentive nature for non-polar compound like fat, and strong anion exchange material (SAX) is suited for the extraction of compounds like carboxylic acids (Molina et al., 2014; Yang et al., 2015; Hercegova et al., 2007; Wen et al., 2014). The combined effects of ACN/5% [Hmim]PF6 and clean-up procedure on ME were studied. Four matrix standard solutions (ACN, ACN without clean-up, ACN/5% [Hmim]PF6 and ACN/5% [Hmim]PF6 without clean-up) were prepared following the steps in Section 2.4. Among these solutions, the matrix standard solutions of ACN without clean-up and ACN/5% [Hmim]PF6 without clean-up bypassed this step (d). Then formula (1) was used to calculate the ME. The ME of four matrix solutions were compared and are shown in Fig. 3.![The ME of four process matrix solutions. A: ACN extraction followed by clean-up. B: ACN extraction without clean-up. C: ACN/5% [Hmim]PF6 extraction followed by clean-up. D: ACN/5% [Hmim]PF6extraction without clean-up.](/content/184/2020/13/2/img/10.1016_j.arabjc.2019.06.009-fig4.png)
The ME of four process matrix solutions. A: ACN extraction followed by clean-up. B: ACN extraction without clean-up. C: ACN/5% [Hmim]PF6 extraction followed by clean-up. D: ACN/5% [Hmim]PF6extraction without clean-up.
Fig. 3 showed that the ME of ACN followed by clean-up, ACN without clean-up, ACN/5% [Hmim]PF6 followed by clean-up and ACN/5% [Hmim]PF6 without clean-up were 93.08–98.79%, 87.61%-106.41%, 94.61–100.57%, and 92.67–103.43%, respectively. It was evident that the most severe ME was found for ACN without clean-up. The ME of ACN/5% [Hmim]PF6 followed by clean-up were relatively weaker than ACN followed by clean-up, while the ME of adding [Hmim]PF6 without clean-up were similar to that of ACN followed by clean-up. These results proved that [Hmim]PF6 has a purifying effect due to the high selectivity of [Hmim]PF6 (Chisvert et al., 2009; Liu et al., 2004; Zhou et al., 2009; Vidal et al., 2010; Hirayama et al., 2005; Saien et al., 2015). Since the MEs were similar, it was feasible to extract PAHs from soil by using an ACN solution with the addition of [Hmim]PF6, thereby bypassing the clean-up step to achieve a rapid analysis.
3.4 Method validation
When the linear concentration ranges from 20 to 1000 µg/L, the ranges of correlation coefficients (R2) and LOD for the calibration curves of the 16 PAHs extracted by the five extractants are listed in Table 4. The good linearity was found for ACN/5% [Hmim]PF6 with R2 ranging from 0.999 to 0.9999. The LOD and LQD ranged from 0.86 to 4.51 µg/kg and 2.87 to 15.13 µg/kg, respectively. Compared with other four extractions, the minimum LOD and minimum LQD of ACN/5% [Hmim]PF6 are lower by 30%. This finding means that using ACN/5% [Hmim]PF6 as the extractant might effectively increase sensitivity. Some proposed methods reported in the literature are shown in Table 5. The 5% [Hmim]PF6 modified QuEChERS method has a similar recovery to other extraction methods. However, the QuEChERS method requires less solvent and less extraction time. The No. 7 QuEChERS method with ACN/5% [Hmim]PF6 as an extractant has a higher recovery and RSD than using ACN/water as an extractant. The lower LOD of observed with the No. 7 method might be related to the detection method. UAE: ultrasonic assisted extraction; SPE: solid phase extraction; FLD/FD: fluorescence detector; MAE: microwave-assisted extraction; FI: flow injection; UV: UV–vis detector; MSPD: matrix solid-phase dispersion; SLE: soil-liquid extraction; MS: mass spectrometry.
Extractant
Line range (µg/L)
Recovery (%)
LOD (µg/kg)
LQD (µg/kg)
ACN
20–1000
70.00–112.69
1.64–4.51
5.47–15.03
n-hexane
20–1000
61.39–91.73
1.37–4.45
4.57–14.83
acetone
20–1000
66.92–110.58
1.61–5.45
5.37–18.17
n-hexane/acetone
20–1000
77.38–105.39
1.42–4.55
4.73–15.17
ACN/5% [Hmim]PF6
20–1000
75.19–100.98
0.86–4.51
2.87–15.03
No.
Extractant
Pretreatment Method
Instrument detection
Recovery (%)
RSD (%)
LOD (µg/kg)
Source
1
ACN/5% [Hmim]PF6
QuEChERS
HPLC-DAD
75.19–100.98
0.58–6.44
0.86–4.51
This work
2
DCM
UAE + SPE
HPLC-FLD
70.32–115.51
3.80–13.56
0.0015–0.2
Pan et al. (2013)
3
ACN
MAE + SPE
FI-LC- UV
98.00–99.00
2.40–4.80
2.00–40.00
Criado et al. (2004)
4
ACN
MAE + SPE
FI-LC- FD
98.00–99.00
2.50–5.40
0.30–2.00
Criado et al. (2004)
5
Hexane/acetone(1:1)
MSPD + SPE
HPLC-DAD
94.30–103.90
0.2–1.9
0.004–0.20
Pena et al. (2007)
6
Cyclohexane
UAE + SLE
HPLC-FLD
70.00–98.00
2.00–15.00
0.138–2.688
Kayali-Sayadi et al. (2000)
7
ACN/water
QuEChERS
GC-MS
80.59–109.82
0.97–18.95
0.39–1.53
Cvetkovic et al. (2016)
4 Conclusion
A QuEChERS method that uses less solvent and less time than traditional methods was applied to extract 16 PAHs from soil. Based on the high selectivity of ILs, [Hmim]PF6 was added to ACN to explore the effects of ILs on the extraction efficiency and the ME. The recoveries and the RSD of the sixteen PAHs in the soil were 75.19–100.98% and 0.58–6.44%, respectively. The extraction results were better than using n-hexane/acetone (1:1, v/v) which was recommended by the EPA Method 3540C and 3550C. The content of [Hmim]PF6 in the extractant has a certain influence on the extraction efficiency of a variety of PAH compounds. The addition of [Hmim]PF6 can effectively reduce the ME. Compared with ACN, the ME of ACN containing [Hmim]PF6were reduced by approximately 35%. Meanwhile, the ME of four procedures (ACN followed by clean-up, ACN without clean-up, ACN/5% [Hmim]PF6 followed by clean-up and ACN/5% [Hmim]PF6 without clean-up) were compared. The ME of the ACN/5% [Hmim]PF6 without clean-up procedure were similar to that of the ACN followed by clean-up procedure, which proved that [Hmim]PF6 has a purifying effect due to the high selectivity of [Hmim]PF6. The QuEChERS method has the advantages of fastness, high efficiency, and high selectivity. When this method is optimized by adding [Hmim]PF6, the extraction efficiency can be increased and the ME can be reduced significantly. [Hmim]PF6 modified QuEChERS method may be applied for the rapid analysis of PAHs in soil samples, and field application of this method may be broadened accordingly.
Acknowledgment
This work was supported by the National Key Research and Development Program (Project Nos. 2018YFC1802803 and 2017YFD0801302), the National Natural Science Foundation of China (Nos. 21677052 and 21677041), the Science and Technology Planning Project of Guangdong (Nos. 2016A040403111 and 2015A030401106). The authors also thank the chemical pollution control and ecological restoration teams for their support.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- Multi-residue determination of veterinary drugs in milk by ultra-high-pressure liquid chromatography-tandem mass spectrometry. J. Chrom. A. 2008;1205:10-16.
- [Google Scholar]
- A multiresidue method for the analysis of 90 pesticides, 16 PAHs, and 22 PCBs in honey using QuEChERS-SPME. Anal. Bioanal. Chem.. 2017;409:5157-5169.
- [Google Scholar]
- A really quick easy cheap effective rugged and safe (QuEChERS) extraction procedure for the analysis of particle-bound PAHs in ambient air and emission samples. Sci. Total. Environ.. 2013;450–451:31-38.
- [Google Scholar]
- Fast and easy multiresidue method employing acetonitrile extraction partitioning and dispersive solidphase extraction for the determination of pesticide residues in produce. J. AOAC Int.. 2003;86:412-431.
- [Google Scholar]
- Chiral separation and determination of ofloxacin enantiomers by ionic liquid-assisted ligand-exchange chromatography. Analyst. 2011;136:379-387.
- [Google Scholar]
- Recovery of organic products from ionic liquids using supercritical carbon dioxidebiodiesel. Ind. Eng. Chem. Res.. 2001;40:287-292.
- [Google Scholar]
- Recent extraction techniques for solid matrices—supercritical fluid extraction, pressurized fluid extraction and microwave-assisted extraction: their potential and pitfalls. Analyst. 2001;126:1182-1193.
- [Google Scholar]
- Simple and commercial readily-available approach for the direct use of ionic liquid-based single-drop microextraction prior to gas chromatography determination of chlorobenzenes in real water samples as model analytical application. J. Chrom. A. 2009;1216:1290-1295.
- [Google Scholar]
- QuEChERS extraction for multi-residue analysis of PCBs, PAHs, PBDEs and PCDD/Fs in biological samples. Talanta. 2017;165:332-338.
- [Google Scholar]
- Direct automatic screening of soils for polycyclic aromatic hydrocarbons based on microwave-assisted extraction/fluorescence detection and on-line liquid chromatographic confirmation. J. Chrom. A. 2004;1050:111-118.
- [Google Scholar]
- Optimization of the QuEChERS extraction procedure for the determination of polycyclic aromatic hydrocarbons in soil by gas chromatography-mass spectrometry. Anal. Meth.. 2016;8:1711-1720.
- [Google Scholar]
- Simultaneous extraction of pesticides and polycyclic aromatic hydrocarbons in Brazilian Cachaca using a modified QuEChERS method followed by gas chromatography coupled to tandem mass spectrometry quantification. J. Agric. Food Chem.. 2019;67:399-405.
- [Google Scholar]
- Quantification of neonicotinoid insecticide residues in soils from cocoa plantations using a QuEChERS extraction procedure and LC-MS/MS. Sci. Total Environ.. 2014;499:276-283.
- [Google Scholar]
- Why wastewater sludge stimulates and accelerates removal of PAHs in polluted soils? Appl. Soil Ecol.. 2016;101:1-4.
- [Google Scholar]
- 1-Hexyl-3-methyl imidazolium tetrafluoroborate: an efficient column enhancer for the separation of basic drugs by reversed-phase liquid chromatography. J. Chrom. A. 2012;1258:168-174.
- [Google Scholar]
- Application of ionic liquid in liquid phase microextraction technology. J. Sep. Sci.. 2012;35:2949-2961.
- [Google Scholar]
- Sample preparation methods in the analysis of pesticide residues in baby food with subsequent chromatographic determination. J. Chrom. A. 2007;1153:54-73.
- [Google Scholar]
- Use of 1-alkyl-3-methylimidazolium hexafluorophosphate room temperature ionic liquids as chelate extraction solvent with 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione. Talanta. 2005;65:255-260.
- [Google Scholar]
- Rapid determination of PAHs in soil samples by HPLC with fluorimetric detection following sonication extraction. Fresen. J. Anal. Chem.. 2000;368:697-701.
- [Google Scholar]
- PAHs in animal tissues – the analytics of PAHs in new reference materials and their homogeneity. Anal. Meth.-UK. 2017;9:76-83.
- [Google Scholar]
- Extension of the QuEChERS method for pesticide residues in cereals to flaxseeds, peanuts, and doughs. J. Agric. Food Chem.. 2010;58:5950-5958.
- [Google Scholar]
- Ability of natural attenuation and phytoremediation using maize (Zea mays L.) to decrease soil contents of polycyclic aromatic hydrocarbons (PAHs) derived from biomass fly ash in comparison with PAHs-spiked soil. Ecotoxicol. Environ. Saf.. 2018;153:16-22.
- [Google Scholar]
- Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chrom. A. 2010;1217:2548-2560.
- [Google Scholar]
- Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate collaborative study. J. AOAC Int.. 2007;90:485-520.
- [Google Scholar]
- Ionic liquid-based liquid-phase microextraction, a new sample enrichment procedure for liquid chromatography. J. Chrom. A. 2004;1026:143-147.
- [Google Scholar]
- Application of ionic liquids in analytical chemistry. TrAC-Trends Anal. Chem.. 2005;24:20-27.
- [Google Scholar]
- Determination of phthalate esters in soil using a quick, easy, cheap, effective, rugged, and safe method followed by GC–MS. J. Sep. Sci.. 2018;41:1711-1896.
- [Google Scholar]
- Bamboo charcoal as adsorbent for SPE coupled with monolithic column-HPLC for rapid determination of 16 polycyclic aromatic hydrocarbons in water samples. J. Chrom. Sci.. 2011;49:683-688.
- [Google Scholar]
- Determination of 16 polycyclic aromatic hydrocarbons in environmental water samples by solid-phase extraction using multi-walled carbon nanotubes as adsorbent coupled with gas chromatography-mass spectrometry. J. Chrom. A. 2010;1217:5462-5469.
- [Google Scholar]
- Electrochemical stability and electron transfer across 4-methyl-40-(nmercaptoalkyl) biphenyl monolayers on Au(100)-(1X1) electrodes in 1-hexyl-3-methylimidazolium hexafluorophosphate ionic liquid. Electrochim. Acta. 2017;231:44-52.
- [Google Scholar]
- Pesticide multiresidue analysis in cereal grains using modified QuEChERS method combined with automated direct sample introduction GC-TOFMS and UPLC-MS/MS techniques. J. Agric. Food Chem.. 2010;58:5959-5972.
- [Google Scholar]
- Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MSMS. Anal. Chem.. 2003;75:3019-3030.
- [Google Scholar]
- Optimization of the QuEChERS method for determination of pesticide residues in chicken liver samples by gas chromatography-mass spectrometry. Food Anal. Meth.. 2014;8:898-906.
- [Google Scholar]
- Novel sorbent and solvent combination for QuEChERS soil sample preparation for the determination of polycyclic aromatic hydrocarbons by gas chromatography-mass spectrometry. Anal. Lett.. 2018;51:1087-1107.
- [Google Scholar]
- Polycyclic aromatic hydrocarbon sources related to biomarker levels in fish from Prince William Sound and the Gulf of Alaska. Environ. Sci. Technol.. 2004;38:4928-4936.
- [Google Scholar]
- Ultrasonic assisted extraction combined with titanium-plate based solid phase extraction for the analysis of PAHs in soil samples by HPLC-FLD. Talanta. 2013;108:117-122.
- [Google Scholar]
- Method validation and dissipation kinetics of four herbicides in maize and soil using QuEChERS sample preparation and liquid chromatography tandem mass spectrometry. Food Chem.. 2016;190:793-800.
- [Google Scholar]
- Optimization of the matrix solid-phase dispersion sample preparation procedure for analysis of polycyclic aromatic hydrocarbons in soils: comparison with microwave-assisted extraction. J. Chrom. A. 2007;1165:32-38.
- [Google Scholar]
- Development of an ionic liquid based dispersive liquid-liquid microextraction method for the analysis of polycyclic aromatic hydrocarbons in water samples. J. Chrom. A. 2009;1216:6356-6364.
- [Google Scholar]
- Gas chromatography-mass spectrometry determination of polycyclic aromatic hydrocarbons in baby food using QuEChERS combined with low-density solvent dispersive liquid-liquid microextraction. Food Chem.. 2018;257:44-52.
- [Google Scholar]
- The ionic liquid 1-hexadecyl-3-methylimidazolium bromide as novel extracting system for polycyclic aromatic hydrocarbons contained in sediments using focused microwave-assisted extraction. J. Chrom. A. 2008;1182:145-152.
- [Google Scholar]
- Simplified QuEChERS approach for the extraction of chlorinated compounds from soil samples. Talanta. 2010;81:385-391.
- [Google Scholar]
- Analysis of polycyclic aromatic hydrocarbons in fish: evaluation of a quick, easy, cheap, effective, rugged, and safe extraction method. J. Sep. Sci.. 2009;32:3529-3538.
- [Google Scholar]
- Multiresidue analysis of aromatic organochlorines in soil by gas chromatography-mass spectrometry and QuEChERS extraction based on water/dichloromethane partitioning. Comparison with accelerated solvent extraction. Talanta. 2012;93:336-344.
- [Google Scholar]
- Ionic liquid 1-hexyl-3-methylimidazolium hexafluorophosphate, an efficient solvent for extraction of acetone from aqueous solutions. J. Chem. Thermodyn.. 2015;91:404-413.
- [Google Scholar]
- Evaluation of the quick, easy, cheap, effective, rugged, and safe (QuEChERS) approach to pesticide residue analysis. Bull Environ. Contam. Toxicol.. 2004;73:24-30.
- [Google Scholar]
- Making the case for QuEChERS-gas chromatography of drugs. TrAC Trends Anal. Chem.. 2016;75:49-56.
- [Google Scholar]
- Recent advances and developments in the QuEChERS method. Compreh. Anal. Chem.. 2017;76:319-374.
- [Google Scholar]
- Determination of 16 polycyclic aromatic hydrocarbons in seawater using molecularly imprinted solid-phase extraction coupled with gas chromatography-mass spectrometry. Talanta. 2012;99:75-82.
- [Google Scholar]
- Impact of Lebanese practices in industry, agriculture and urbanization on soil toxicity. Evaluation of the Polycyclic Aromatic Hydrocarbons (PAHs) levels in soil. Chemosphere. 2018;210:85-92.
- [Google Scholar]
- Dependence of ion intensity in electrospray mass spectrometry on the concentration of the analytes in the electrosprayed solution. Anal. Chem.. 1993;65:3654-3668.
- [Google Scholar]
- Gaining insight in the behaviour of imidazolium-based ionic liquids as additives in reversed-phase liquid chromatography for the analysis of basic compounds. J. Chrom. A. 2015;1380:96-103.
- [Google Scholar]
- Matrix-effect-free determination of BTEX in variable soil compositions using room temperature ionic liquid co-solvents in static headspace gas chromatography mass spectrometry. Anal. Chim. Acta. 2018;1021:41-50.
- [Google Scholar]
- Studies on the use of ionic liquids as potential extractants of phenolic compounds and metal ions. Sep. Sci. Technol.. 2010;39:2155-2169.
- [Google Scholar]
- Extraction of polycyclic aromatic hydrocarbons and organochlorine pesticides from soils: a comparison between Soxhlet extraction, microwave-assisted extraction and accelerated solvent extraction techniques. Anal. Chim. Acta. 2007;602:211-222.
- [Google Scholar]
- Recent advances in solid-phase sorbents for sample preparation prior to chromatographic analysis' TrAC-Trends Anal. Chem.. 2014;59:26-41.
- [Google Scholar]
- A multiresidue method for simultaneous determination of 44 organophosphorous pesticides in Pogostemon cablin and related products using modified QuEChERS sample preparation procedure and GC-FPD. J. Chrom B Anal. Technol. Biomed. Life Sci.. 2015;974:118-125.
- [Google Scholar]
- Residue dynamics of pyraclostrobin in peanut and field soil by QuEChERS and LC-MS/MS. Ecotoxicol. Environ. Saf.. 2012;78:116-122.
- [Google Scholar]
- Ultrasound-assisted ionic liquid dispersive liquid-phase micro-extraction: a novel approach for the sensitive determination of aromatic amines in water samples. J. Chrom. A. 2009;1216:4361-4365.
- [Google Scholar]







