Translate this page into:
Holistic quality evaluation of Saposhnikoviae Radix (Saposhnikovia divaricata) by reversed-phase ultra-high performance liquid chromatography and hydrophilic interaction chromatography coupled with ion mobility quadrupole time-of-flight mass spectrometry-based untargeted metabolomics
⁎Corresponding authors at: State Key Laboratory of Component-based Chinese Medicine, Tianjin University of Traditional Chinese Medicine, 10 Poyanghu Road, Jinghai, Tianjin 301617, China (W. Yang). zyyjy@163.com (Weiming Wang), wzyang0504@tjutcm.edu.cn (Wenzhi Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Untargeted metabolomics more suits the quality evaluation of TCM because of its holistic property. To assess the holistic quality difference of Saposhnikoviae Radix (the roots of Saposhnikovia divaricata), we integrate ultra-high-performance liquid chromatography coupled with ion mobility/quadrupole time-of-flight mass spectrometry (UHPLC/IM-QTOF-MS)-based untargeted metabolomics and quantitative assay. A BEH C18 column in the reversed-phase mode and a BEH Amide column in Hydrophilic Interaction Chromatography (HILIC) mode were utilized for metabolites profiling, which enabled high coverage of the non-polar to polar components in Saposhnikoviae Radix. Moreover, the application of major components knockout strategy enlarged the exposure of those minor components. Integrated use of high-definition MSE (HDMSE) and data-dependent acquisition (DDA) could enhance the metabolites characterization by providing reliable fragmentation information and collision cross section values. Computational in-house library-driven automated peak annotation of the HDMSE and DDA data assisted to characterize 104 components from Saposhnikoviae Radix. Chemometric analyses of the commercial Saposhnikoviae Radix samples (64 batches collected from 11 cultivars aging from 1 to 4 years), based on the positive MSE data, in general could indicate large discrimination between Guan-Fang-Feng (from Heilongjiang) and the others, but negligible difference among Saposhnikoviae Radix from the other ten provinces of China and with different ages. Quantitative assays of prim-O-glucosylcimifugin and 4′-O-β-D-glucosyl-5-O-methylvisamminol, by a rapid and fully validated UHPLC-UV method, could primarily deduce that Guan-Fang-Feng aging 2 and 3 years exhibited better quality. The methods established can holistically assess the quality of TCM with wide spans of plant metabolites covered.
Keywords
Saposhnikovia divaricata
Untargeted metabolomics
IM-QTOF-MS
Quality evaluation
1 Introduction
Different from the chemical and biological medicine, herbal medicine (or traditional Chinese medicine, TCM) is well known as a complex chemical system, which is typically featured by the co-existing of multiple classes of the primary and secondary metabolites possessing wide spans of molecular weight, acid-base property, polarity, and contents, etc. (Lu et al., 2019; Yan et al., 2017). Moreover, the quality of TCM can be affected by a series of factors, such as the producing region (cultivar), growing age, collection time, processing technology, and even the condition and period of storage (Li et al., 2017; Luo et al., 2019; Kim et al., 2012; Nie et al., 2019; Xu et al., 2018). These characteristics render a systematic work to develop powerful analytical methods (e.g. fingerprinting and multicomponent quantitative assay) capable of assessing the holistic quality of TCM, which is not only crucial to ensuring the efficacy and safety supporting the clinical use, but also an imperative segment to promote the modernization and internationalization of TCM (Le et al., 2017; Yang et al., 2017).
Chemical basis elucidation, authentication, and content determination, are three of the most important segments involved in the quality research of TCM. The utilization of multiple mechanism of chromatography is able to capture the information of almost all the plant metabolites. Aside from the most commonly used reversed-phase liquid chromatography (RPLC) that enables the separation of a majority of the plant metabolites, gas chromatography (GC) and supercritical fluid chromatography (SFC) are advantageous in resolving non-polar components (such as the volatile oil and lipids), while Hydrophilic Interaction Chromatography (HILIC) offers possibilities to separate those polar ingredients, such as the saccharides, amino acids, and nucleic acids (Khan et al., 2020; Ganzera and Sturm, 2018; Shi et al., 2018; West, 2018; Zhang et al., 2016). Particularly, multi-dimensional liquid chromatography coupled with high-resolution mass spectrometry (HRMS) provides a practical solution to the in-depth profiling and characterization of herbal multicomponents, enabling the identification or tentative characterization of up to hundreds of compounds (Cao et al., 2017; Qiu et al., 2015). Notably, the introduction of ion mobility high-resolution mass spectrometry can provide an additional dimension of separation for ions and the determined collision cross section (CCS) can support more reliable identification of metabolites and even isomers discrimination (Clément et al., 2018; Christian et al., 2017). A distinct trend in authentication of TCM in recent years is the application of untargeted metabolomics, which has been proven as practical and powerful in discriminating the easily confusing species, different parts, producing regions, ages, and processing technologies, etc. (Jia et al., 2019; Li et al., 2020; Sun et al., 2012; Tajidin et al., 2019; Wang et al., 2019; Zhang et al., 2019b). In contrast to conventional approaches (e.g. microscopic features examination, physiochemical identification, thin layer chromatography, and HPLC fingerprint), untargeted metabolomics can capture the complete metabolome information and visualize the holistic metabolome differences beneficial to the discovery of marker compounds. LC-MS approaches by multiple reaction monitoring (MRM), on a triple quadrupole or a hybrid triple quadrupole/linear ion-trap (QTrap) mass spectrometer, is preferably selected in multicomponent content determination for evaluating TCM quality because of its high selectivity, ultra-high sensitivity, and wide dynamic linearity range (Song et al., 2018).
Saposhnikoviae Radix, derived from the roots of Saposhnikovia divaricata (Turcz.) Schischk., is a reputable TCM used for the treatment of headache, vertigo, generalized aching and arthralgia (Okuyama et al., 2001). Saposhnikoviae Radix is widely distributed in China, including the provinces of Heilongjiang, Liaoning, Inner Mongolia, Hebei, Ningxia, Gansu, Shanxi (陕西), Shanxi (山西), and Shandong, etc. (Maruyama et al., 2018). Phytochemical researches of Saposhnikoviae Radix have isolated multiple subtypes of plant metabolites, such as the chromones, coumarins, volatile oils, polysaccharides, polyacetylenes, and organic acids. A reversed-phase liquid chromatography/quadrupole time-of-flight mass spectrometry (RPLC/QTOF-MS) approach has been established, and 45 components (including 13 chromones, 28 coumarins, and four others) were identified or tentatively characterized by analyzing the fragmentation pathways of six reference compounds (Chen et al., 2018). Moreover, ionic liquid-based ultrasonic-assisted extraction could enhance the extraction efficiency of chromones, compared with the conventional ultrasonic extraction method (Han et al., 2017). HPLC-DAD and LC/MRM were utilized in the simultaneous content determination of five chromones and six coumarins from Saposhnikoviae Radix (Kim et al., 2011). Ultra-high-performance liquid chromatography quadrupole time-of-flight mass spectrometry (UHPLC/QTOF-MS) coupled with principal component analysis (PCA) and partial least squared discriminant analysis (PLS-DA) was used to rapidly discover and identify the metabolites of Saposhnikovia divaricate decoction (Li et al., 2020). However, currently we know little about the impacts of cultivar and age on the quality of Saposhnikoviae Radix. To fully address this issue, powerful analytical approaches are in need to achieve the holistic evaluation.
In this work, we aimed to evaluate the quality difference of Saposhnikoviae Radix (64 batches in total; Table S1 and Fig. S1), resulting from different cultivars (eleven provinces of China: Heilongjiang, Inner Mongolia, Hebei, Henan, Qinghai, Shanxi (山西), Shanxi (陕西), Anhui, Shandong, Gansu, and Ningxia) and different ages (from one to four years) by both untargeted metabolomics and qualitative assay. Both RPLC and HILIC coupled with a VionTM ion mobility/quadrupole time-of-flight (IM-QTOF) mass spectrometer were utilized for metabolites profiling from Saposhnikoviae Radix. Moreover, a “Major Components Knockout” strategy was utilized to expand the exposure of those minor ingredients. Two data acquisition approaches (data independent high-definition MSE, HDMSE; data-dependent acquisition, DDA) and an in-house library (containing 172 known compounds) incorporated into UNIFI were employed to boost the performance, efficiency, and reproducibility in the multicomponent characterization (24 reference compounds were used with their structures and information given in Fig. 1 and Table S2). Quantitative assays of two index compounds (prim-O-glucosylcimifugin and 4'-O-β-D-glucosyl-5-O-methylvisamminol) collected by China Pharmacopoeia (Committee of National Pharmacopoeia, 2020) were performed by a validated UHPLC-UV approach to offer more data supporting the quality assessment of Saposhnikoviae Radix. To our knowledge, it is the first report that systematically evaluates the quality differentiation of Saposhnikoviae Radix due to the different cultivars and ages.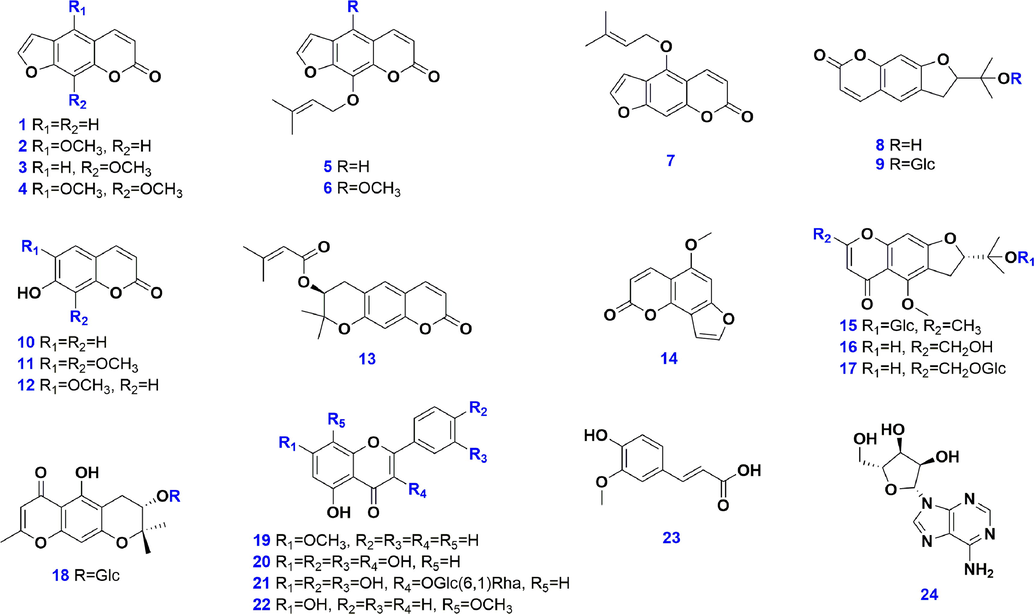
Chemical structures of 24 reference compounds used in the current work.
2 Experimental section
2.1 Materials and reagents
Twenty-four compounds (Fig. 1 and Table S2), involving psoralen (1), 5-methoxypsoralen (2), methoxsalen (3), isopimpinellin (4), imperatorin (5), phellopterin (6), isoimperatorin (7), (+)-marmesin (8), nodakenin (9), umbelliferone (10), 7-hydroxy-6,8-dimethoxychromen-2-one (11), scopoletin (12), decursin (13), 5-methoxyfuro[2,3-h]chromen-2-one (14), 4′-O-β-D-glucosyl-5-O-methylvisamminol (15; GMV), cimifugin (16), prim-O-glucosylcimifugin (17; PGC), sec-O-glucosylhamaudol (18), tectochrysin (19), quercetin (20), rutin (21), wogonin (22), trans-ferulic acid (23), and adenosine (24), purchased from Chengdu Desite Biotechnology Co., Ltd. (Chengdu, China) or Shanghai Standard Biotech. Co., Ltd. (Shanghai, China), were used as the reference compounds in this work. HPLC-grade acetonitrile, methanol (Fisher, Fair Lawn, NJ, USA), formic acid (Sigma-Aldrich, MO, Switzerland), and ultra-pure water in-house prepared using a Milli-Q Integral 5 water purification system (Millipore, Bedford, MA, USA), were used. Information with respect to 64 batches of Saposhnikoviae Radix samples, analyzed in this work, is detailed in Table S1. Specimens were deposited at the authors' lab in Tianjin University of Traditional Chinese Medicine (Tianjin, China).
2.2 Sample preparation
An easy-to-implement ultrasonic extraction method was utilized for sample preparation. In brief, 50 mg of accurately weighed powder of Saposhnikoviae Radix was soaked in a 15-mL centrifuge tube containing 4 mL methanol. After being vortexed for 2 min, the sample was extracted on a water bath at 25 °C with ultrasound assistance (power, 1130 W; frequency, 40 kHz) for 40 min. The extract was centrifuged for 10 min at a rotate speed of 4000 rpm, with the supernatant further transferred into a 10-mL volumetric flask. The extraction process was repeated by using 3 mL of methanol. The pooled supernatants were diluted to a constant volume (10 mL), and then mixed well. The solution was centrifuged at 14,000 rpm for 10 min, and the resulting supernatant was stored at 4 °C prior to LC-MS analysis (reaching a final concentration of the drug material: 5 mg/mL). A quality control sample (QC1) by pooling the equal volume of the test solutions of five batches of Saposhnikoviae Radix samples (No. 1, 7, 29, 48, and 55, Table S1) was prepared for method establishment, while another QC sample (QC2) prepared from all the tested solutions (64 batches in total) was used in the untargeted metabolomics experiments. In the quantitative assay experiment, 250 mg powder of Saposhnikoviae Radix was extracted by the same manner. A quality control sample (QC3) by pooling the equal volume of the test solutions of four batches of Saposhnikoviae Radix samples (No. 35, 37, 26, and 57, Table S1) was prepared for monitoring the system stability all through the analysis batch.
2.3 Metabolites profiling and untargeted metabolomics analysis of Saposhnikoviae Radix by ultra-high-performance RPLC and HILIC coupled with IM-QTOF-MS
Two different mechanisms of chromatographic separations, ultra-high-performance RPLC and HILIC, were utilized to extend the coverage in profiling the multicomponents of Saposhnikoviae Radix on an ACQUITY UPLC I-Class/Vion™ IM-QTOF system (Waters Corporation, Milford, MA, USA). A BEH C18 column (2.1 × 100 mm, 1.7 μm) hyphenated with a VanGuard Pre-column (2.1 × 50 mm, 1.7 μm) maintained at 35 °C was used for the UHP-RP separation. A binary mobile phase, composed by 0.1% formic acid in water (water phase: A) and 0.1% formic acid in acetonitrile (organic phase: B), ran at a flow rate of 0.3 mL/min in consistency with an optimal gradient program: 0–4 min, 5–32% (B); 4–7 min, 32–50% (B); 7–10 min, 50–70% (B); 10–12 min, 70–75% (B); 12–14 min, 75–100% (B); and 14–17 min, 100% (B). A BEH Amide column (2.1 × 100 mm, 1.7 μm) hyphenated with a VanGuard Pre-column (2.1 × 50 mm, 1.7 μm) maintained at 35 °C was used for the UHP-HILIC separation. The same binary mobile phase at the same flow rate as those in RP separation was employed, but followed a different gradient program: 0–1 min, 95–94% (B); 1–6 min, 94–93% (B); 6–9 min, 93–92% (B); 9–10 min, 92–50% (B); and 10–12 min, 50% (B). In both two separation modes, 3 μL of the test solution was injected onto the column.
High-accuracy MS data for both the multicomponent characterization and multivariate statistical analysis experiments were acquired on a Vion™ IM-TOF mass spectrometer in the positive ESI mode (Waters). The LockSpray ion source was equipped adopting the following parameters: capillary voltage, 1.0 kV; cone voltage, 20 V; source offset, 80 V; source temperature, 120 °C; desolvation gas temperature, 500 °C; desolvation gas flow (N2), 800 L/h; and cone gas flow (N2), 50 L/h. Default parameters were defined for the travelling wave IM separation (Zhang et al., 2019a; Zuo et al., 2019). The QTOF analyzer scanned over a mass range of m/z 80–1500 at a low collision energy of 6 eV for HDMSE at 0.3 s per scan (MS1). Multicomponent characterization was conducted in both RP and HILIC separations by using HDMSE and DDA (including a precursor ion list consisting of 103 molecular formulae), while the MSE data were recorded for 64 batches of Saposhnikoviae Radix samples. Ramp collision energy (RCE) of 30–50 eV was set in the high-energy channel for HDMSE. When TIC (total ion chromatogram) intensity exceeded 1000 detector counts, MS/MS fragmentation of three most intense precursors was automatedly triggered in DDA mode under mass-dependent ramp collision energy (MDRCE) of a constant 30 eV in low mass ramp and 50 eV in high mass ramp (scan range m/z 80–1500 for MS2). The MS/MS acquisition stopped if either TIC dropped below 100 detector counts or time exceeded 0.3 s. MS data calibration was conducted by constantly infusing the leucine enkephalin solution (LE; Sigma-Aldrich; 200 ng/mL) at a flow rate of 10 µL/min. CCS calibration was conducted according to the manufacture’s guidelines by using a mixture of calibrants (Giuseppe et al., 2015).
Uncorrected HDMSE, DDA, and MSE data in Continuum format, were processed using the UNIFI™ 1.9.3.0 software (Waters). Impressively, the UNIFI software was able to efficiently perform data correction, peak picking, and peak annotation (Zhang et al., 2019a; Zuo et al., 2019). Key parameters of UNIFI were as follows. Find 4D Peaks (only set in HDMSE): High-energy intensity threshold, 100.0 counts; low-energy intensity threshold, 50.0 counts. Target by mass: Target match tolerance: 10.0 ppm; screen on all isotopes in a candidate, generate predicted fragments from structure, and look for in-source fragments; fragment match tolerance, 10.0 ppm. Adducts: Positive adduct was +H. Lock Mass: Combine width, 3 scans; mass window, 0.5 m/z; reference mass, 556.2766; reference charge, +1. The corrected HDMSE data were further processed by Progenesis QI 2.1 software (Waters). Isotope and adduct fusion were applied to reduce the number of detected metabolic features. Menu-guided processing of multi-batch MSE data (peak alignment and peak picking) generated a data matrix, involving the information of tR, m/z, and normalized peak area. The components, after “30% variation” filtering, were the variables for multivariate statistical analysis using SIMCA-P 14.1 (Umetrics, Umea, Sweden) by PCA and OPLS-DA (orthogonal partial least squares discriminant analysis) with the data Pareto scaled. Those variables showing VIP > 3.0 were considered as the potential markers diagnostic for comparing Saposhnikoviae Radix samples, in the current work.
2.4 Quantitative determination of prim-O-glucosylcimifugin and 4′-O-β-D-glucosyl-5-O-methyl-visamminol by UHPLC-UV
Quantitative assays of prim-O-glucosylcimifugin (PGC) and 4′-O-β-D-glucosyl-5-O-methyl-visamminol (GMV) were performed by a rapid UHPLC-UV method, established on an Agilent 1290 Infinity Ⅱ UHPLC system. Chromatographic separation was conducted on a Kinetex® Biphenyl column (2.1 × 100 mm, 1.7 µm) using a binary mobile phase consisting of 0.1% formic acid in water (water phase: A) and methanol (organic phase: B) which ran at 0.3 mL/min following an optimized gradient program: 0–2 min, 25–40% (B); 2–10 min, 40–50% (B); 10–12 min, 50–98% (B); and 12–14 min, 98% (B). The injection volume was 2 µL, and the DAD detector was set at 254 nm. Detailed information regarding the preparation of standard solutions and method validation items are provided as Supplementary Materials.
3 Results and discussion
3.1 An in-house library of Saposhnikoviae Radix incorporated into UNIFI™
The bioinformatics platform UNIFI™ can standardize peak annotation of the acquired HDMSE and DDA spectra by matching with the incorporated or commercial database (Jia et al., 2019; Zhang et al., 2019a; Zuo et al., 2019). Considering the restricted coverage of the commercial library on TCM components, we have recourse to the in-house library by giving a systematic summary on the phytochemical reports of Saposhnikoviae Radix retrieved from multiple available database (e.g. Web of Science, SciFinder, and China National Knowledge Infrastructure-CNKI, etc.). A chemical library of Saposhnikoviae Radix was thus established which recorded the trivial name, molecular formula, and chemical structure for each of the 172 known compounds (involving 51 coumarins, 25 chromones, 8 flavonoids, 10 volatile oils, 13 fatty acids, 11 aromatic hydrocarbons, 6 polyacetylenes, 13 alcohols, 14 esters, 7 aldehydes, 3 ketones, and 11 others; Table S3). On one hand, the collected structure information was input into an EXCEL file (.xls) in a format compatible with the UNIFI software. On the other hand, each structure was drawn using ChemDraw Professional, which was subsequently saved as a .mol file with the file name in consistency with the trivial name. The resulting EXCEL file and all structure files were incorporated into the UNIFI software, which could be utilized to characterize the multicomponents from Saposhnikoviae Radix.
3.2 Establishment and optimization of UHPLC/IM-QTOF-MS and HILIC/IM-QTOF-MS for the metabolites profiling and characterization of Saposhnikoviae Radix by the positive-mode HDMSE and DDA data
Aimed to extend the coverage and enhance the monitoring of various metabolites from Saposhnikoviae Radix, in the current work, we developed two chromatographic separation approaches (UHP-RP and UHP-HILIC) by coupling to a Vion IM-QTOF hybrid high-resolution mass spectrometer (Waters Corporation, Milford, MA, USA). The factors influencing the chromatography performance (e.g. stationary phase, column temperature, and gradient elution program) and MS detection (involving the ESI mode, source parameters, and collision energy) were optimized in sequence by single-factor experiments. We firstly compared the negative and positive ESI modes in characterizing Saposhnikoviae Radix components using a solution of mixed standards containing 24 reference compounds. Evidently, the positive mode had remarkable advantages, by which 22 compounds displayed high intensity, compared to only 13 detectable in the negative mode with much weaker abundance (Fig. 2).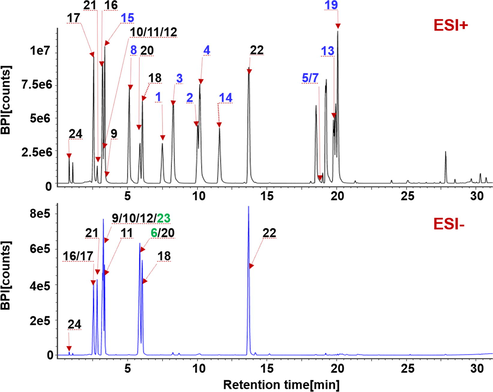
Comparison of the full-scan spectra of a mixed reference compounds solution containing 24 compounds (the numbering is consistent with that in Table S1) obtained in the positive (ESI+) and negative (ESI−) modes.
In establishing the UHP-RP chromatography condition, the stationary phase was screened, in the first step. On consideration of the differential factors involving silica gel spheres (fully porous or core–shell) and the bonding techniques/groups, ten chromatographic columns from Waters and Agilent (HSS SB C18, CSH C18, BEH Shield RP18, CORTECS UPLC C18, HSS T3, BEH C18, ZORBAX SB C18, ZORBAX Extend C18, ZORBAX SB-Aq, and ZORBAX Eclipse Plus C18) were compared under the unified gradient elution condition (column temperature at 30 °C). It was clearly exhibited in Fig. 3 that the BEH C18 column could be the best choice, as the largest number of peaks got resolved (5386 in total) showing more symmetric peak shape. Column temperature by using the BEH C18 column was further evaluated (Fig. S2), and we considered the best resolution was achieved when 35 °C was set. Minor modifications were made on the gradient elution program, which finally enabled satisfactory separation of Saposhnikoviae Radix components in the RP mode. The HILIC condition was established in a similar manner. Six commercial HILIC columns, involving XBridge Amide, CORTECS UPLC HILIC, BEH HILIC, ZORBAX HILIC Plus, Exsil Pure ZIK HILIC, and BEH Amide, were examined (Fig. S3). Comparatively, the BEH Amide column gave satisfactory resolution, which was thus selected.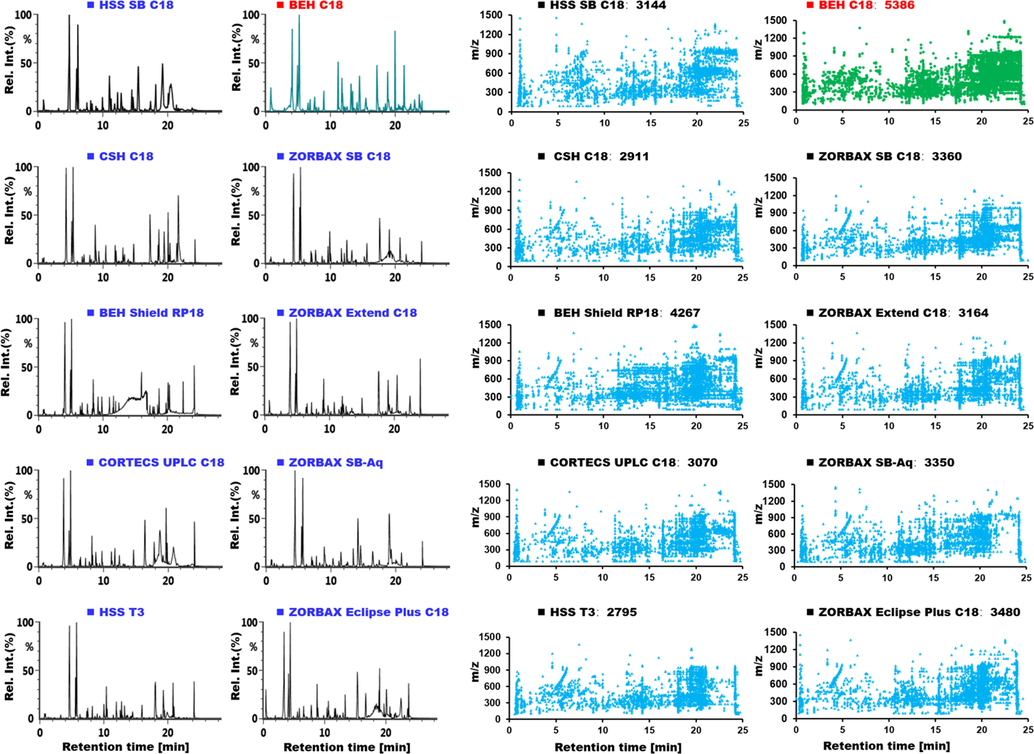
Selection of the stationary phase for reversed-phase UHPLC separation of the multicomponents from Saposhnikoviae Radix. The left shows the base peak chromatograms of a mixed sample obtained on ten candidate sub-2 µm particles packed columns; the right represents the numbers and scatter plots for the resolved peaks by different columns.
Detection of Saposhnikoviae Radix components was performed on the Vion IM-QTOF instrument, and two key ion source parameters, capillary voltage (0.5–3.0 kV) and cone voltage (20–100 V), were optimized to increase the ion response. Ten compounds representative of four common subclasses of natural products (nucleotide: adenosine; chromones: PGC, GMV, and sec-O-glucosylhamaudol; coumarins: methoxsalen, psoralen, and isopimpinellin; flavonoids: quercetin, wogonin, and tectochrysin) were used as the index components. As shown in Fig. 4, by increasing the capillary voltage from 0.5 to 3.0 kV, the ion response of all the nucleotide, coumarins, and flavonoids, exhibited remarkable decreasing trend, and the same circumstances were witnessed for one chromone, PGC. However, the other two chromone compounds gave the highest intensity at 1.0 kV. We selected to set capillary voltage at 1.0 kV in all subsequent experiments. In addition, most of these index components gave decreasing intensity when cone voltage increased from 20 to 100 V, and thereby we considered cone voltage at 20 V enabled high ion response of Saposhnikoviae Radix components.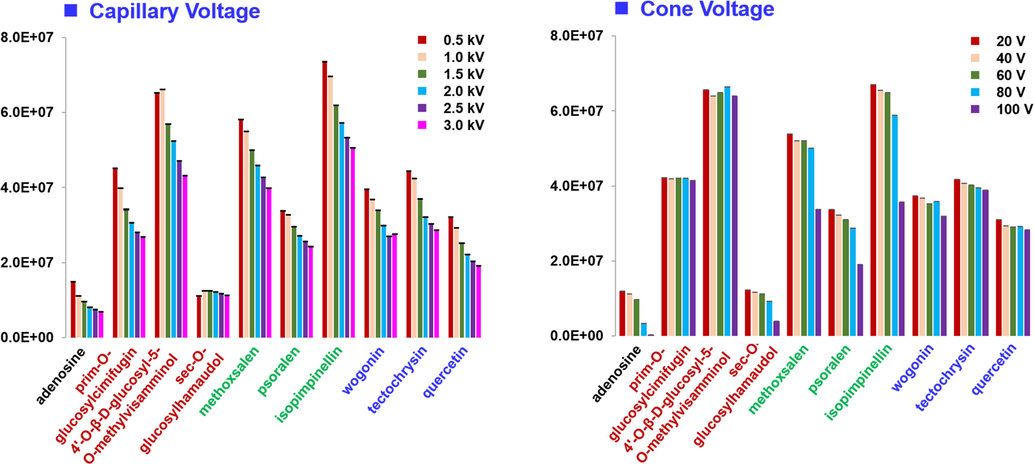
Optimization of two key ion source parameters (capillary voltage and cone voltage) of the Vion IM-QTOF hybrid high-resolution mass spectrometer using ten representative compounds.
DDA and DIA have their individual merits and demerits in untargeted metabolites characterization. We were aimed to expand the coverage in metabolites profiling and characterization from Saposhnikoviae Radix by integrating both DDA and HDMSE. Collision energy for these two data acquisition modes was optimized using the QC1 sample. For both two modes, we primarily set four fixed levels of collision energy (including 20, 40, 60, and 80 eV), based on which another three levels of ramp collision energy (RCE; 30–50, 40–60, and 50–70 eV) were further compared, as RCE could assist to acquire more balanced MS2 spectra for a complex sample (Zhang et al., 2019a; Zuo et al., 2019). Using two chromones (PGC and GMV) and two coumarins (decursin and (+)-marmesin) (Fig. S4), higher collision energy could enable more thorough fragmentation, and RCE of 30–50 eV was suitable for collision-induced dissociation (CID) of both the chromones and coumarins. To this end, the approaches utilizing two orthogonal chromatography coupled to enhanced MS detection by combining the positive-mode DDA and HDMSE were established, which conduced to characterizing more components from Saposhnikoviae Radix and assessing the factors that could affect its quality.
In addition, a “Major Components Knockout” strategy, which was similar to our previous report (Zuo et al., 2019), was utilized by feat of the automatic elute switching function of the Vion IM-QTOF instrument. As exhibited in Fig. S5, a total of four major peaks were knocked out in both the HDMSE and DDA modes for the acquisition of CID-MS2 data. In the knockout mode, the concentration of Saposhnikoviae Radix extract increased from 1 mg/mL to 10 mg/mL. The results benefitting from the use of knockout strategy were the increasing on the numbers of the components that could be characterized. UNIFI by searching the in-house Saposhnikoviae Radix library was able to annotate 100 peaks in the normal analysis and 124 in the knockout mode based on the DDA data, and 183 in the normal analysis and 292 in the knockout mode based on the HDMSE data, which testified the beneficial effect of the knockout strategy useful to identify more components.
3.3 Computational database-driven automated annotation of the DDA and HDMSE data for the multicomponent characterization of Saposhnikoviae Radix
The multicomponent characterization of Saposhnikoviae Radix was based on the positive-mode DDA and HDMSE data obtained by the established UHPLC/IM-QTOF-MS approaches applying both UHP-RP and HILIC separations. Efficient structural elucidation was achieved by the computational in-house library (Table S3)-driven automated annotation of the obtained HRMS data using UNIFI. By further confirming the characterization results the software gave, as a consequence, a total of 104 compounds were identified or tentatively characterized from Saposhnikoviae Radix, which included 27 coumarins, 45 chromones, 7 flavonoids, and 25 other components, with their information detailed in Table S4). Notably, the CCS information of the precursor ions were offered due to the application of IM separation.
Coumarins refer to a common class of natural compounds with a phenylpyrene α-pyrone core. Coumarins from Saposhnikoviae Radix have been reported with various pharmacological properties (Kreiner et al., 2017), and by the integral strategy we characterized 27 coumarin compounds belonging to the simple coumarin, furancoumarin, and pyrancoumarin, etc. The positive-mode CID-MS2 fragmentation pathways of coumarins were featured by the neutral elimination of CO and H2O (Ren et al., 2019), which could be evidenced by compounds 47# and 15# as the typical examples (Fig. 5). Compound 47# (tR 6.38 min), giving a predominant protonated ([M+H]+) ion at m/z 217.0502 suggestive of the molecular formula C12H8O4 (calculated at m/z 217.0495, 3.0 ppm), was identified as methoxsalen due to the aid of reference compound comparison. As displayed in the MS2 spectrum, a weak fragment by free radical cleavage was observed at m/z 202.0273 ([M+H–CH3]+), which could be easily further fragmented with CO eliminated forming the base peak ion of m/z 174.0320 ([M+H–CH3–CO]+). In addition, a fragment of m/z 175.0364 was observed as well, which might further lose CH2O to produce a medium-intensity fragment at m/z 145.0289. The product ion of m/z 118.0422 should result from the successive neutral loss of 3 × CO ([M+H–CH3–3CO]+) from the fragment m/z 202.0273. Compound 15# (tR 3.84 min) was an unknown compound giving a [M+H]+ precursor ion at m/z 247.0971, and accordingly the molecular formula was characterized to be C14H14O4 (calculated at m/z 247.0964, 2.7 ppm). The MS/MS spectrum showed fragmentations similar to the furan-type coumarins (Yang et al., 2015). From the transition m/z 247.0964 → 213.0554 consistent with neutral elimination of H2O + CH4 (34 Da), we speculated a hydroxyl-substituted pyrancoumarin. Moreover, the product ions of m/z 175.0404, 147.0449, 119.0499, and 91.0548, could match the product ions of ostenol (C14H14O3) (Ren et al., 2019). The element composition of compound 15# differing from that of ostenol was an additional oxgen, and taking together, all these fragments assisted to tentatively characterize compound 15# as decursinol, a known pyrancoumarin compound ever-isolated from Saposhnikoviae Radix (Yang et al., 2015).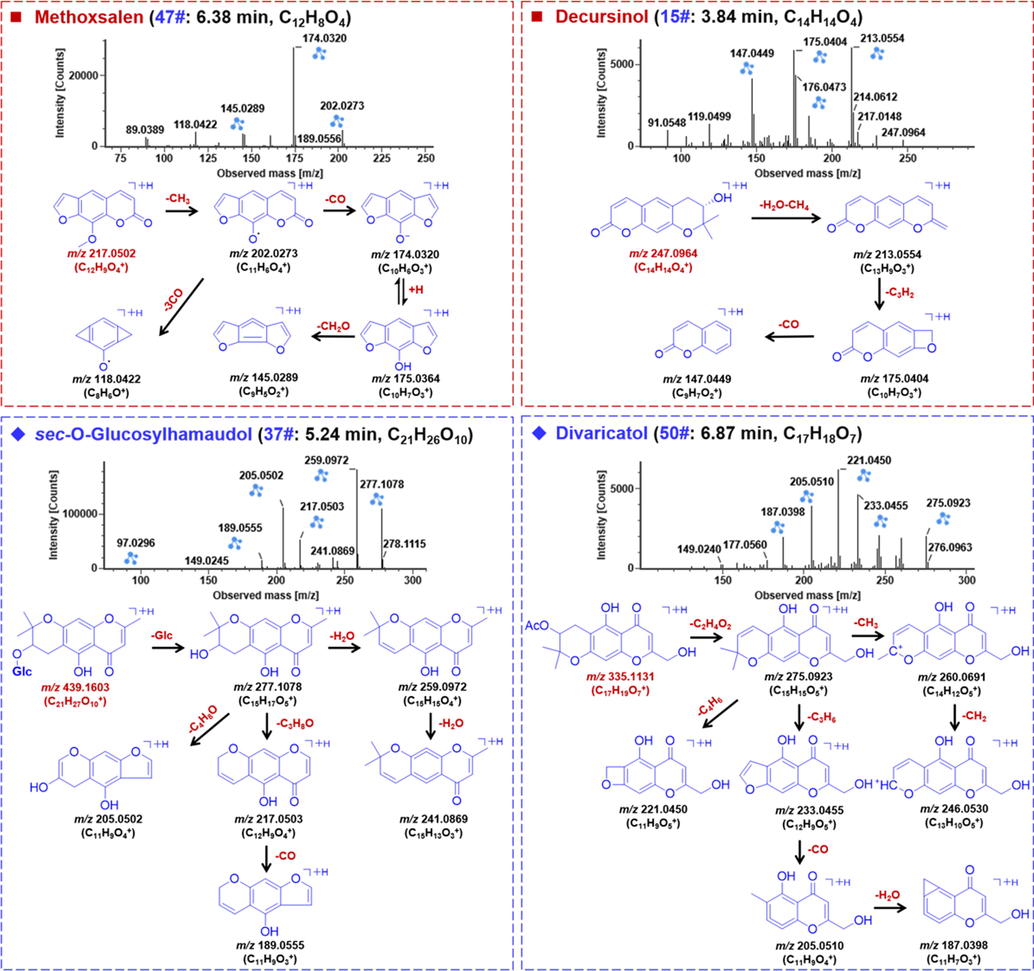
The positive CID-MS2 spectra and proposed fragmentation pathways for representative compounds of coumarins and chromones from Saposhnikoviae Radix.
Chromones are known as an important class of bioactive ingredients for SR (Kreiner et al., 2017). We were able to characterize 45 chromones from Saposhnikoviae Radix in the current work, and the fragmentation pathways and characterization of compounds 37# (tR 5.24 min) and 50# (tR 6.87 min) were illustrated (Fig. 5). Because of the availability of reference compound comparison, compound 37# that showed a rich [M+H]+ precursor ion at m/z 439.1603, was identified as sec-O-glucosylhamaudol with the molecular formula C21H26O10 (calculated at m/z 439.1598, 1.0 ppm). In its CID-MS2 spectrum, a fragment, due to the neutral loss of the Glc moiety, was observed at m/z 277.1078 ([M+H–Glc]+). Further cleavage on the obtained chromone aglycone included the eliminations of H2O producing product ions of m/z 259.0972 ([M+H–Glc–H2O]+) and 241.0869 ([M+H–Glc–2H2O]+). The other fragments of m/z 217.0503 and 205.0502 were due to the additional eliminations of C3H8O and C4H8O, corresponding to 60 Da and 72 Da, respectively, from the fragment m/z 277. What’s more, a fragment of m/z 189.0555 was considered resulting from the ion m/z 217.0503 by further elimination of CO. Compound 50# gave the predominant protonated precursor at m/z 335.1131, indicative of the molecular formula C17H18O7 (calculated at m/z 335.1125, 1.7 ppm). By searching against the in-house library of Saposhnikoviae Radix, a hit of divaricatol was observed, and the MS/MS spectrum exhibited typical fragmentation pathways consistent with the characterization result. The fragment at m/z 275.0923 resulted from the neutral loss of acetic acid (C2H4O2) from the precursor ion. In addition, typical cleavages on the pyran moiety occurred, such as the eliminations of C3H6, C4H6, and C4H6O, generating the product ions of m/z 233.0455, 221.0450, and 205.0510, respectively. Accordingly, we tentatively characterized compound 50# as divaricatol (Chen et al., 2018).
Giving a summary on the components characterized from Saposhnikoviae Radix, we can demonstrate the complementarity by applying two different mechanism of chromatography, RPLC and HILIC. Among the 104 components, 84 and 57 ones were characterized from the RPLC and HILIC modes, respectively. In particular, seven compounds (saccharides and aldehydes) were newly identified by the use of HILIC separation. Moreover, the application of two MS2 data acquisition approaches, HDMSE and DDA, was able to boost the coverage, to some extent (Zhang et al., 2019a). In this work, structural characterization of multicomponents from Saposhnikoviae Radix was mainly based on the DDA data, while the HDMSE data could supplement the CCS value and additional identification results due to its high coverage on the profiled precursor ions.
3.4 Evaluation of the impacts of cultivars and ages on the holistic quality of Saposhnikoviae Radix by untargeted metabolomics and quantitative assay
Untargeted metabolomics has been demonstrated as a powerful tool in quality control of TCM achieving the holistic evaluation (Jia et al., 2018). Both the UHP-RPLC and HILIC were utilized in metabolomics analysis of the quality difference of Saposhnikoviae Radix by monitoring the positive MSE data. The multi-batch positive MSE data were primarily corrected by UNIFI, and the corrected data were input into the Progenesis QI software for data processing (peak alignment and peak picking). In the PR mode, a table consisting of the tR, m/z, and normalized abundance of 2086 ions was generated, and 1649 thereof in the QC data showed variation no more than 30%. In the case of HILIC mode, a total of 1730 metabolic features were recorded, and 1308 retained after 30% variation filtering. These metabolic features were used as the variables for pattern-recognition chemometrics by PCA and OPLS-DA.
By unsupervised PCA, we were able to evaluate the major factors that affected the quality of Saposhnikoviae Radix. As exhibited in Fig. 6, in both the RP and HILIC modes, no clear segregation trend was observed for all the tested samples of Saposhnikoviae Radix. No matter the samples were divided by ages or cultivars, in general, the quality difference of Saposhnikoviae Radix was not significant. In contrast, nine batches of Saposhnikoviae Radix collected from Heilongjiang (namely Guan-Fang-Feng) showed remarkable differentiation from the other commercial 55 batches of Saposhnikoviae Radix samples (collected from 10 different cultivars aging from 1 to 4 years), despite several samples were the outliers. It could indicate certain quality difference between Guan-Fang-Feng and the other Saposhnikoviae Radix samples. OPLS-DA was utilized to further probe into the difference between Guan-Fang-Feng and the other 55 batches of commercial Saposhnikoviae Radix samples (Fig. 7). It was evident that, in both the PR and HILIC modes, two groups between Guan-Fang-Feng and the other commercial Saposhnikoviae Radix samples could be clearly separated, despite the fitness and predictability were both low for the established OPLS-DA model (section [1] of Fig. 7). The major variables contributing to the clustering were further discerned by VIP ranking. In the PR mode, 22 ions showing VIP > 3.0 were selected as the potential differential ions, while 24 ones discovered in the HILIC mode (section [2] of Fig. 7). Heat map was used to visualize these differential ions among all the determined 64 batches of Saposhnikoviae Radix samples (section [3] of Fig. 7).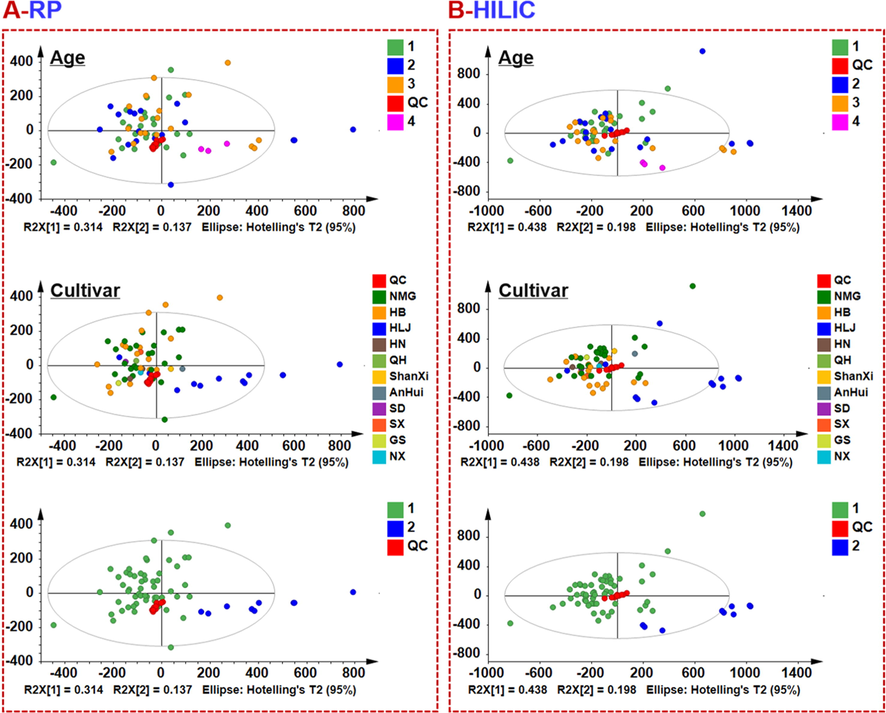
PCA score plots of 64 batches of Saposhnikoviae Radix samples based on the positive-mode MSE data acquired by applying both RPLC and HILIC, visualized in terms of the cultivars, ages, and between Guan-Fang-Feng and others.
![Multivariate statistical analysis between Guan-Fang-Feng and the other Saposhnikoviae Radix samples based on the positive MSE data acquired by RPLC and HILIC. [1] Score plot of OPLS-DA; [2] VIP plot with the cutoff set at 3.0; [3] heat map visualizing the differentiated ions (VIP > 3.0).](/content/184/2020/13/12/img/10.1016_j.arabjc.2020.10.013-fig7.png)
Multivariate statistical analysis between Guan-Fang-Feng and the other Saposhnikoviae Radix samples based on the positive MSE data acquired by RPLC and HILIC. [1] Score plot of OPLS-DA; [2] VIP plot with the cutoff set at 3.0; [3] heat map visualizing the differentiated ions (VIP > 3.0).
Quantitative assays can absolutely determine the content difference of index components, and thus offer reliable information to the metabolomics experiments (Wu et al., 2018). A rapid UHPLC-UV method was successfully established and fully validated for quantitative assays of prim-O-glucosylcimifugin (PGC) and 4′-O-β-D-glucosyl-5-O-methylvisamminol (GMV) in 64 batches of Saposhnikoviae Radix samples (Fig. 8). The LOD, LOQ, and linearity of two analytes are presented in Table S5. Both the calibration curves showed good linearity (r2 > 0.999). The inter- and intra-day precision (RSD, %) were in the range of 0.12–1.47% and 0.05–1.44% (Table S6), respectively. RSDs of the repeatability (Table S7) and stability (Table S8) tests were no more than 1.60% for both two analytes. The recovery was in the range of 98–104.30% with RSD less than 2% for three different concentration levels (Table S9). All these data could testify a reliable quantitative determination method applicable to quantify two major bioactive components from Saposhnikoviae Radix, and the results of quantitative assays are exhibited in Table S10. Aside from the Batch 3 sample (Table S1), the contents of PGC and GMV among the tested Saposhnikoviae Radix samples (63 batches) varied in the ranges of 0.54–8.98 mg/g and 0.35–8.57 mg/g, respectively. Notably, Batch 41 sample (3-year old from Hebei) possessed the highest content of PGC. Generally, the 2-year and 3-year Guan-Fang-Feng have better quality than the others, and the total trend is consistent with the PCA results of untargeted metabolomics (Fig. 6). According to Chinese Pharmacopoeia (2020 version), the contents of PGC and GMV should sum up not less than 2.4 mg/g (0.24%) (Committee of National Pharmacopoeia, 2020), and based on this content standard, six batches of Saposhnikoviae Radix samples (No. 3, 15, 28, 33, 35, and 45) were considered with inferior quality.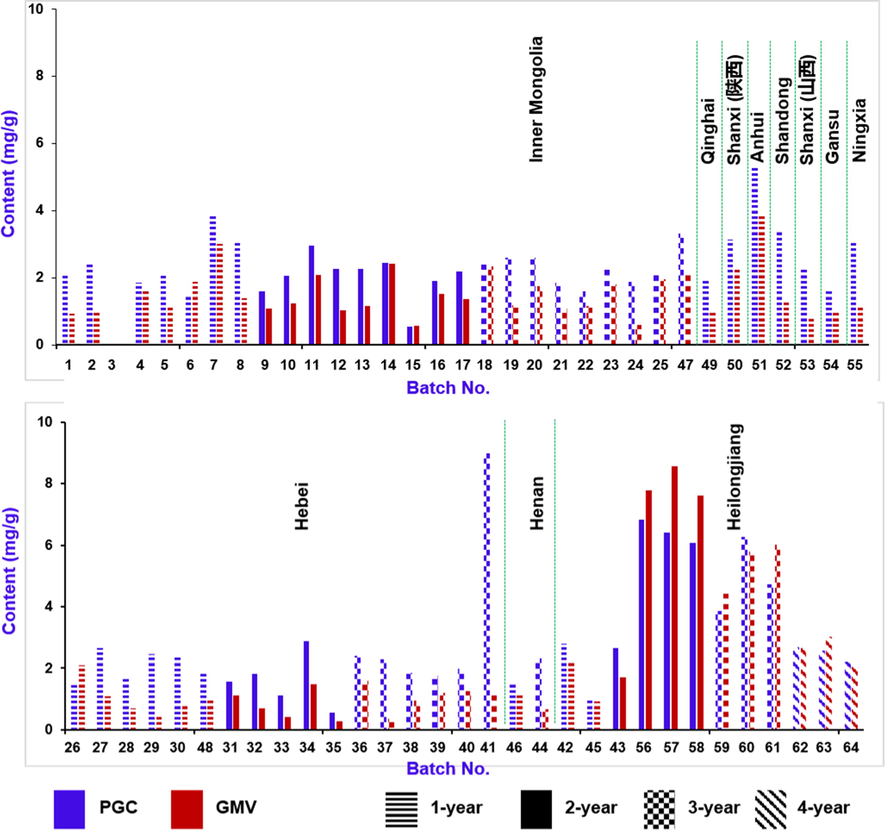
Bar charts showing the contents of prim-O-glucosylcimifugin (PGC) and 4′-O-β-D-glucosyl-5-O-methylvisamminol (GMV) in 64 batches of Saposhnikoviae Radix samples determined by a rapid UHPLC-UV approach.
4 Conclusion
With the view of evaluating the factors that can influence the quality of Saposhnikoviae Radix, in the current work, untargeted metabolomics and quantitative assay approaches were successfully established. In particular, the integration of UHP-RPLC and HILIC was able to enhance the metabolites profiling by expanding the coverage of polarity range, while the combination of HDMSE and DDA was beneficial to generate complementary MS information. In addition, the application of a major components knockout strategy could improve the exposure of minor ingredients from Saposhnikoviae Radix. By these efforts, a total of 104 components were identified or tentatively characterized from Saposhnikoviae Radix, displaying remarkable superiority over the currently available approaches. By integrative analyses of the untargeted metabolomics and quantitative assay results, we could primarily deduce that Guan-Fang-Feng showed large difference from the other Saposhnikoviae Radix samples, and Guan-Fang-Feng aging 2 and 3 years had good quality. Conclusively, the methods established enable the comprehensive chemical basis elucidation and holistic quality assessment of TCM.
Acknowledgments
Funding: This work was financially supported by National Natural Science Foundation of China (Grant No. 81872996), State Key Project for the Creation of Major New Drugs (Grant No. 2018ZX09711001-009-010 and 2018ZX09735-002), and National Key R&D Program of China (Grant No. 2018YFC1707904, 2018YFC1707905, and 2017YFC1702104).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Online comprehensive two-dimensional hydrophilic interaction chromatography × reversed-phase liquid chromatography coupled with hybrid linear ion trap Orbitrap mass spectrometry for the analysis of phenolic acids in Salvia miltiorrhiza. J. Chromatogr. A. 2017;1536:216-227.
- [CrossRef] [Google Scholar]
- Rapid characterisation and identification of compounds in Saposhnikoviae Radix by high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry. Nat. Prod. Res.. 2018;32(8):898-901.
- [CrossRef] [Google Scholar]
- A four dimensional separation method based on continuous heart-cutting gas chromatography with ion mobility and high resolution mass spectrometry. J. Chromatogr. A. 2017;1536:50-57.
- [CrossRef] [Google Scholar]
- Identification of phase-II metabolites of flavonoids by liquid chromatography–ion-mobility spectrometry–mass spectrometry. Anal. Bioanal. Chem.. 2018;410(2):471-482.
- [CrossRef] [Google Scholar]
- China Pharmacopoeia (Part 1). Beijing: Chemical Industry Press; 2020.
- Recent advanced on the HPLC/MS in medicinal plant analyses-An update covering 2011–2016. J. Pharm. Biomed. Anal.. 2018;147:211-233.
- [CrossRef] [Google Scholar]
- Giuseppe, P., Peggi, A., Jonathan, P.W., Keith, R., Hernando, J.O., J, W.T., Lochana, M., Steven, L., Callee, W., Arthur, M., Robert, S.P., David, F.G., Bernhard, O.P., James, L., Scott, G., Giuseppe, A., 2015. Ion mobility-derived collision cross section as an additional measure for lipid fingerprinting and identification. Anal. Chem. 87 (2), 1137−1144. https://doi.org/10.1021/ac503715v.
- Application of ionic liquid-based ultrasonic-assisted extraction followed by rapid resolution LC–ESI/MS to chromone analysis from Radix Saposhnikoviae. Acta Chromatogr.. 2017;29(3):359-374.
- [CrossRef] [Google Scholar]
- Simultaneous profiling and holistic comparison of the metabolomes among the flower buds of Panax ginseng, Panax quinquefolius, and Panax notoginseng by UHPLC/IM-QTOF-HDMSE-based metabolomics analysis. Molecules. 2019;24(11):2188.
- [CrossRef] [Google Scholar]
- Characterization of secondary metabolites of leaf and stem essential oils of Achillea fragrantissima from central region of Saudi Arabia. Arab. J. Chem.. 2020;13:5254-5261.
- [CrossRef] [Google Scholar]
- Simultaneous determination of chromones and coumarins in Radix Saposhnikoviae by high performance liquid chromatography with diode array and tandem mass detectors. J. Chromatogr. A. 2011;1218:6319-6330.
- [CrossRef] [Google Scholar]
- Nontargeted metabolomics approach for age differentiation and structure interpretation of age-dependent key constituents in hairy roots of Panax ginseng. J. Nat. Prod.. 2012;75(10):1777-1784.
- [CrossRef] [Google Scholar]
- Saposhnikoviae divaricata: a phytochemical, pharmacological and pharmacokinetic review. Chin. J. Nat. Med.. 2017;15(4):255-264.
- [CrossRef] [Google Scholar]
- High-performance liquid chromatography method development for the quality control of Ginkgonis Semen. Arab. J. Chem.. 2017;10:792-800.
- [CrossRef] [Google Scholar]
- Integration of multicomponent characterization, untargeted metabolomics and mass spectrometry imaging to unveil the holistic chemical transformations and key markers associated with wine steaming of Ligustri Lucidi Fructus. J. Chromatogr. A. 2020;1624:461228
- [CrossRef] [Google Scholar]
- Strategy for comparative untargeted metabolomics reveals honey markers of different floral and geographic origins using ultrahigh-performance liquid chromatography-hybrid quadrupole-orbitrap mass spectrometry. J. Chromatogr. A. 2017;1409:78-89.
- [CrossRef] [Google Scholar]
- Rapid analysis of Saposhnikovia divaricate decoction metabolism in rats by UHPLC–Q-TOFMS and multivariate statistical analysis. Biomed. Chromatogr.. 2020;34(3):e4778
- [CrossRef] [Google Scholar]
- Application of multiple chemical and biological approaches for quality assessment of Carthamus tinctorius L. (safflower) by determining both the primary and secondary metabolites. Phytomedicine. 2019;58:152826
- [CrossRef] [Google Scholar]
- Discrimination of Citrus reticulata Blanco and Citrus reticulata ‘Chachi’ as well as the Citrus reticulata ‘Chachi’ within different storage years using ultra high performance liquid chromatography quadrupole/time-of-flight mass spectrometry based metabolomics approach. J. Pharm. Biomed. Anal.. 2019;171:218-231.
- [CrossRef] [Google Scholar]
- Botanical origin and chemical constituents of commercial Saposhnikoviae radix and its related crude drugs available in Shaanxi and the surrounding regions. J. Nat. Med. 2018;72(1):267-273.
- [CrossRef] [Google Scholar]
- An integration of UPLC-DAD/ESI-Q-TOF MS, GC–MS, and PCA analysis for quality evaluation and identification of cultivars of cultivars of Chrysanthemi Flos (Juhua) Phytomedicine. 2019;59:152803
- [CrossRef] [Google Scholar]
- Analgesic components of saposhnikovia root (Saposhnikovia divaricata) Chem. Pharm. Bull.. 2001;49(2):154-160.
- [CrossRef] [Google Scholar]
- A green protocol for efficient discovery of novel natural compounds: Characterization of new ginsenosides from the stems and leaves of Panax ginseng as a case study. Anal. Chim. Acta. 2015;893:65-76.
- [CrossRef] [Google Scholar]
- Analysis of chemical components as coumarin in Saposhnikovia divaricata by UPLC-Q-TOF-MS. China Pharm.. 2019;30(3):349-354.
- [Google Scholar]
- Systematic profiling and comparison of the lipidomes from Panax ginseng, P. quinquefolius, and P. notoginseng by ultrahigh performance supercritical fluid chromatography/high-resolution mass spectrometry and ion mobility-derived collision cross section measurement. J. Chromatogr. A. 2018;1548:64-75.
- [CrossRef] [Google Scholar]
- Serially coupled reversed phase-hydrophilic interaction liquid chromatography tailored multiple reaction monitoring, a fit-for-purpose tool for large-scale targeted metabolomics of medicinal bile. Anal. Chim. Acta. 2018;1037:119-129.
- [CrossRef] [Google Scholar]
- Classification of cultivation locations of Panax quinquefolius L samples using high performance liquid chromatography−electrospray ionization mass spectrometry and chemometric analysis. Anal. Chem.. 2012;84(8):3628-3634.
- [CrossRef] [Google Scholar]
- Metabolite profiling of Andrographis paniculata (Burm. f.) Nees. Young and mature leaves at different harvest ages using 1H NMR-based metabolomics approach. Sci. Rep.. 2019;9(1):16766.
- [CrossRef] [Google Scholar]
- In-depth profiling, characterization, and comparison of the ginsenosides among three different parts (the root, stem leaf, and flower bud) of Panax quinquefolius L. by ultra-high performance liquid chromatography/quadrupole-Orbitrap mass spectrometry. Anal. Bioanal. Chem.. 2019;411(29):7817-7829.
- [CrossRef] [Google Scholar]
- Current trends in supercritical fluid chromatography. Anal. Bioanal. Chem.. 2018;410(25):6441-6457.
- [CrossRef] [Google Scholar]
- Geographic impact evaluation of the quality of Alismatis Rhizoma by untargeted metabolomics and quantitative assay. J. Sep. Sci.. 2018;41:839-846.
- [CrossRef] [Google Scholar]
- Remarkable impact of steam temperature on ginsenosides transformation from fresh ginseng to red ginseng. J. Ginseng. Res.. 2018;42(3):277-287.
- [CrossRef] [Google Scholar]
- Simultaneous determination of components with wide polarity and content ranges in Cistanche tubulosa using serially coupled reverse phase-hydrophilic interaction chromatography-tandem mass spectrometry. J. Chromatogr. A. 2017;1501:39-50.
- [CrossRef] [Google Scholar]
- Three new coumarins from Saposhnikovia divaricata and their porcine epidemic diarrhea virus (PEDV) inhibitory activity. Tetrahedron. 2015;71:4651-4658.
- [CrossRef] [Google Scholar]
- Approaches to establish Q-markers for the quality standards of traditional Chinese medicines. Acta Pharm. Sin. B. 2017;7(4):439-446.
- [CrossRef] [Google Scholar]
- Integration of data-dependent acquisition (DDA) and data-independent high-definition MSE (HDMSE) for the comprehensive profiling and characterization of multicomponents from Panax japonicus by UHPLC/IM-QTOF-MS. Molecules. 2019;24(15):2708.
- [CrossRef] [Google Scholar]
- Comparison of triterpene compounds of four botanical parts from Poria cocos (Schw.) wolf using simultaneous qualitative and quantitative method and metabolomics approach. Food Res. Int.. 2019;121:666-677.
- [CrossRef] [Google Scholar]
- An intelligentized strategy for endogenous small molecules characterization and quality evaluation of earthworm from two geographic origins by ultra-high performance HILIC/QTOF MSE and Progenesis QI. Anal. Bioanal. Chem.. 2016;408(14):3881-3890.
- [CrossRef] [Google Scholar]
- Data-dependent acquisition and database-driven efficient peak annotation for the comprehensive profiling and characterization of the multicomponents from Compound Xueshuantong Capsule by UHPLC/IM-QTOF-MS. Molecules. 2019;24(19):3431.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.10.013.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supplementary data 2
Supplementary data 2







