Translate this page into:
Homopolymerization of benzylmethacrylate and methylvinylketone using Ni(acac)2–methylaluminoxane catalyst system
*Corresponding author (taouak@yahoo.fr)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The homopolymerization of benzylmethacrylate and methylvinylketone was carried out with a Ni(acethylacetonate)2–MAO catalytic system. The catalytic efficiency of this complex was almost identical towards ester methacrylic monomers. The yield of polybenzylmethacrylate (84–86%) was not influenced by the bulkiness of the substituent directly attached to the sp3 oxygen, and in the meantime, it gave a small amount of oligomers (3–16%). The molecular weights of polybenzylmethacrylate obtained by this catalytic system were relatively high ( ) and were not practically influenced by the temperature. The polymerization of methylvinylketone (MVK) gave a low polymer yield (8–25%), with low molecular weights ( ) and a higher quantity of oligomers (53–67%). This result indicated that the ketone-enol equilibrium of the momoner perturbed highly the polymerization process, which was due to the presence of a methyl group in α position of the carbonyl. The polymerization of vinylacetate using the same catalytic system was also investigated in this work. However, the polymerization process was unsuccessful. Ni(acac)2–MAO catalyst system was able to initiate the polymerization of various monomers, such as those containing conjugated double bonds C=C–C=C or C=C–C=O, but those containing a donor group in α-position, such as methylvinylketone appeared to give essentially the oligomers. 13C and 1H NMR analyses of the polymer revealed that the catalytic system gave a mixture of mr (27.4–46%) and rr (43.8–55.5%) triads in the main chain preferentially. The fraction of triad rr increased when the temperature of polymerization decreased (40% at 80 °C and 70% at 0 °C).
Keywords
Benzylmethacrylate
Methylvinylketone
Nickel (II) acetylacetonate
Methylaluminoxane
Polymerization
1 Introduction
The polymerization of benzylmethacrylate has been investigated by different methods. Demirelli et al. (2004) have polymerized this monomer by atom transfer radical polymerization method (ATRP) at different temperatures.
It was observed that the number-average molecular weight and polydispersities decreased when the temperature decreased. Polybenzylmethacrylate (PBMA) has also been synthesized previously from the monomer by free radical polymerization (FRP) (Caron et al., 1991) and group transfer polymerization (GTP) (Mykytiuk et al., 1992). It was found that palladium based supported catalysts are more efficient dibenzylation agents for the polymeric benzyl ester (Dyakonov et al., 2000).
On the other hand, Ni-based catalysts are more versatile, as they are able to polymerize various monomers such as cyclic olefins (Endo, 1999; Breunig and Risse, 1992; Safir and Novak, 1995; Liaw et al., 1996), non-cyclic olefins such as ethylene, 1-olefins (Pellecchia and Zambelli, 1996; Pellecchia et al., 1996; Mohring et al., 1985; Johnson et al., 1995), styrene and its derivatives (Endo et al., 1994, 1995; Crossetti et al., 1997; Longo et al., 1990; Johson et al., 1995), alkylmethacrylate monomers (Endo et al., 1994; Coutinho et al., 1997; Lecomte et al., 1996) and conjugated dienes, such as isoprene and butadiene (Deming et al., 1994; Taube et al., 1995; Endo et al., 1996, 1997). Some nickel (II) catalysts require a multistep synthesis and are sensitive to oxygen, but Ni(acac)2 is very stable at ambient atmosphere. The polymerization of vinyl monomers, such as styrene (Endo et al., 1994), methylmethacrylate (MMA) (Endo and Inukai, 2000), ter-butylmethacrylate (t-BMA) (Endo, 1999) using Ni(acac)2–MAO catalytic system were also investigated by Kioschi et al.
Ni(acac)2 appears to be a versatile catalytic system for these polymerizations, with a high catalytic activity. It has also been observed that the results are not influenced by the bulkiness of the substituent directly attached to the sp3 oxygen. The polymerization of methylvinylketone was investigated by Fujio et al. (1963) using zinc ethyl or calcium zinc tetraethyl as a catalyst. The product obtained was a crystalline polymer, whereas amorphous polymers were obtained with radical catalysts.
Thomas (1963), Fujio et al. (1963) and Wasai et al. (1963) have reported that highly crystalline polymers of various vinylketones were obtained in the polymerization with organometallic catalysts, and they investigated the structures by X-ray diffractometry and IR spectrometry. Fujio et al. (1963) and Hay (1963) also reported on the cycliced structure of polymethylvinylketone. The stereoregularity of polymethylketone was investigated by Merle-Aubry et al. (1975) using 13C NMR spectroscopy and Matzuzaki et al. (1981) by 1H NMR. It was found that radically obtained polymethylvinylketone has an atactic-rich structure.
In the present investigation, a study of benzylmethacrylate (BMA) polymerization was carried out using the Ni(acac)2–MAO catalytic system. According to previous studies, on the polymerization of MMA (Endo and Inukai, 2000), our reactions followed the same conditions leading to a high activity with smaller amounts of Ni(acac)2 and a ratios of MAO:Ni = (1:10). Because of its weak conversion, polymerization study of MVK was limited in the present work, to its conversion and structure characterization. In our results we have made a comparison with those obtained by the authors quoted above, concerning the polymerization of other vinylic monomers using the same catalytic system.
2 Experimental
2.1 Chemicals
Toluene, the polymerization solvent (Aldrich, 99% purity) was dried over calcium hydride followed by distillation under nitrogen and collected in a flask which was previously degassed, flushed with purified nitrogen and fitted with “Subaseal” type stopper.
Methylvinylketone, styrene and methylmethacrylate (Aldrich, 99% purity), benzylmethacrylate (Aldrich, 96% purity), t-butylmethacrylate (Aldrich, 98% purity), and vinylacetate (Aldrich, +99% purity) were purified by distillation under vacuum, dried and collected similar to toluene. All these monomers were kept at 5 °C.
Methylaluminoxane 10% in toluene (Aldrich, 98%) was used without further purification and diluted with anhydrous toluene. Acetylacetone was used as purchased from Aldrich (+99% purity). Nickel acetylacetonate was prepared according to Pham et al. (1991). Nickel chloride (Aldrich, 98% purity) was used.
2.2 Polymerization
The polymerization was carried out in a Pyrex reactor (volume: 100 ml) fitted with devices designed for dispensing liquids under inert atmosphere. The reactor was initially charged with a known quantity of nickel acetylacetonate then connected to a vacuum pump. After degassing the setup to 10−4 mm Hg, toluene, and methylaluminoxane solution were carefully injected to the reactor through a rubber septum cap with a hypodermic syringe, then the system was finally left under a nitrogen atmosphere.
The catalytic system was allowed to age for 15 min at 50 °C with stirring. At the desired polymerization temperature, the monomer was added to the reactor and left for a desired time. After the required time elapsed, the polymerization was quenched by the addition of a small quantity of methanol containing 5% by weight of hydrochloric acid.
The polymer was isolated by a precipitation with acidified methanol then dried in a vacuum oven. The oligomers were isolated by evaporation of the mixture residue (solvent and precipitant).
2.3 Characterization
13C and 1H NMR spectra of polybenzylmethacrylate and polymethylvinylketone in deuterated chloroform were obtained using a JEOL FX 90 Q NMR spectrometer at room temperature. Tetramethylsilane (TMS) was used as an internal reference and the chemical shifts were expressed in ppm.
Infrared spectra of a polymer thin films spread on KBr pellets were analyzed with a Thermo Nicolet model Nexus 470 FT-IR Fourier Transform spectrometer. UV/Visible spectra of polymer solution in acetonitrile were run on a double-beam, UV–Vis spectrophotometer Lambda 5 Perkin–Elmer.
The molecular weights of the polymers were estimated in THF at 30 °C by size exclusion chromatography (SEC) on a Varian apparatus equipped with a JASCO HPLC-pump type 880-PU refractive index/UV detectors and TSK Gel columns calibrated with polystyrene standards.
DSC thermograms were performed on Shimadzu DSC 60 device. The heating rate was 20 °C/min, and the data were collected from the second and third scans. Glass transition temperatures (Tgs) were taken as the mean value between the onset and the end point temperatures. Nitrogen atmosphere was used to minimize thermal degradation of the polymers.
3 Results and discussion
The polymerization of benzylmethacrylate (BMA) and some vinylic monomers were carried out under the same conditions using Ni(acac)2/MAO catalytic system in toluene, at 50 °C. The results are shown in Table 1.All the isolated polybenzylmethacrylate and polymethylvinylketone were solid powder, and highly soluble in many solvents, such as chlorinated, aromatic, and oxygenated solvents. The polymerization of both BMA and MVK was neither initiated by the catalyst nor the co-catalyst alone, since binary compounds were necessary to initiate this type of polymerization. In the present work, it was also observed that the catalyst system did not succeed to polymerize vinylacetate monomer. FT-IR spectrum of PBMA (Fig. 1) shows a strong band at 1729 cm−1, this was attributed to the stretching vibration of the ester carbonyl group. Both bands at 1143 and 1238 cm−1 were assigned to symmetric and asymmetric stretching of the –C(=CO)–O–C group, respectively.
| Polymer | Conversion (wt%) | Polymer (wt%) | Oligomer (wt%) | ||
|---|---|---|---|---|---|
| PBMA | 90 | 84 | 06 | 4.0 | 2.63 |
| PMVK | 78 | 25 | 53 | 0.4 | 4.82 |
| PS | 87 | 84 | 03 | 1.8 | 2.42 |
| PMMA | 91 | 88 | 03 | 2.5 | 1.78 |
| Pt-BMA | 98 | 86 | 12 | 1.8 | 1.87 |
Polymerization conditions: ageing of the initiating system: 15 min at 50 °C; reaction time: 5 h; reaction temperature: 50 °C; [Ni] = 4 mmol l−1; [Monomer] = 2.0 mol l−1; [MAO]/[Ni] = 100.
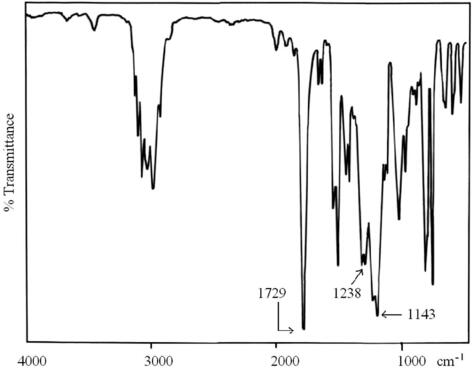
- IR spectra of PBMA prepared in the presence of Ni(acac)2–MAO catalyst system.
FT-IR spectrum of PMVK (Fig. 2) shows the presence of a carbonyl group by the strong signal at 1709 cm−1, this is in full agreement with a diketone structure (1710 cm−1).
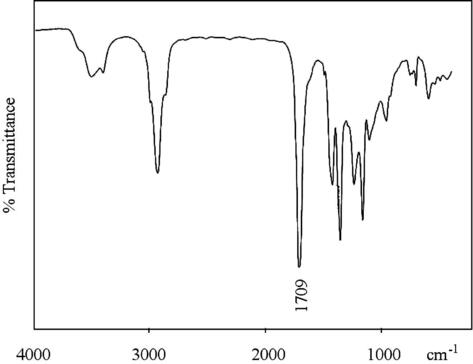
- IR spectra of PMVK prepared in the presence of Ni(acac)2–MAO catalyst system.
1H NMR spectrum of PBMA illustrated in Fig. 3 shows signals at 7.71–6.92 ppm due to the five phenyl ring protons, while the signal at 5.05 ppm was assigned to the methylene adjacent to oxygen in the BMA unit. The peaks at 1.11–1.35 ppm were assigned to α-CH3 protons and that at 2.55–1.80 ppm was assigned to β-CH2 protons.
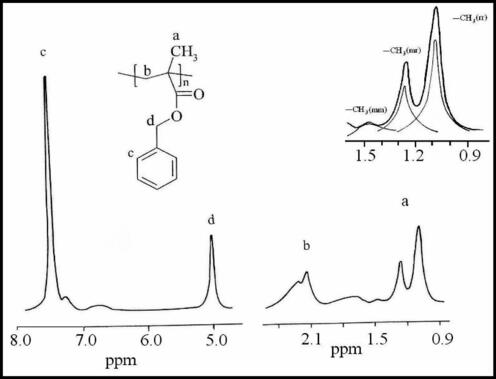
- 400 MHz 1H NMR of PBMA prepared in the presence of Ni(acac)2–MAO catalyst system.
13CNMR spectrum of PBMA is shown in Fig. 4. The peak at 185.0 ppm corresponded to the carbonyl group and those at 139.0 and 130.0 were assigned to carbons (b) and (c) of the phenyl ring, respectively. The signals at 66.0 and 50.0 ppm corresponded to –OCH2– carbon and β-CH2– carbon, respectively. The peaks observed at 32.0–44.0 ppm for PBMA were attributed to Cα existing in (rr), (mr) and (mm) triads Al-Saigh and Munk, 1984. The peaks at 7.0–20.0 ppm corresponded to the resonances of α-CH3 carbon atoms.1H NMR spectrum of PVMK illustrated in Fig. 5 shows the peaks that were attributed to the protons of α-CH3 at 2.0, 2.11 and 2.16 ppm in (rr), (mr) and (mm) triads; respectively Matzuzaki et al., 1981. The signal at 2.41 ppm was attributed to the proton (b), 1.20 ppm to the proton (c) and 1.80 ppm to the proton (d). The peak at 1.54 was attributed to –CH2– syndio. This spectrum shows also two short peaks at 4.0–3.70 and 4.50 ppm corresponding probably to enolic form protons of some monomeric units in this polymer.
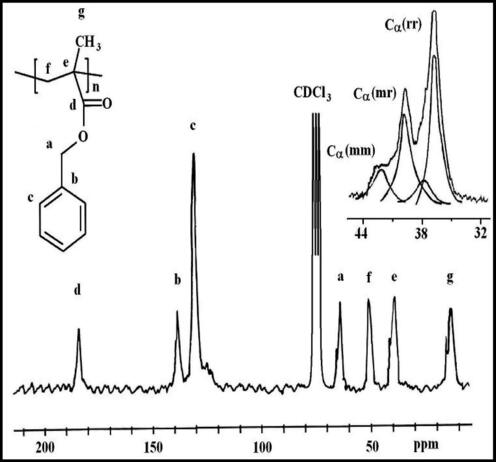
- 100 MHz 13C NMR of PBMA prepared in the presence of Ni(acac)2–MAO catalyst system.
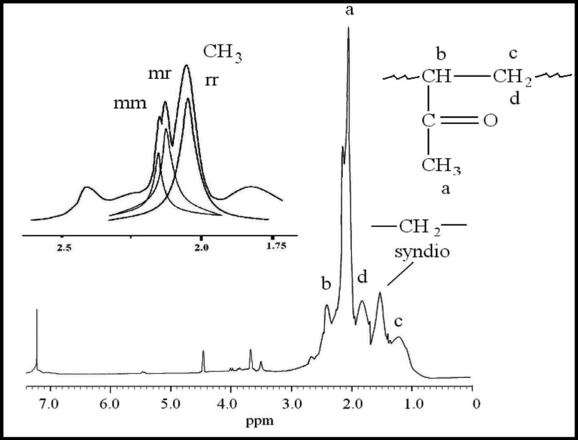
- 400 MHz 1H NMR of PMVK prepared in the presence of Ni(acac)2–MAO catalyst system.
13CNMR spectrum of PMVK is shown in Fig. 6. The peaks at 208.5, 209.8 and 210.6 ppm corresponded to the carbonyl group in (mm), (mr) and (rr) triads; respectively (Merle-Aubry et al., 1975). The signals at 29.9 and 32.6, 47.5 ppm were assigned to carbons (d), (a) and (b); respectively. The spectrum confirmed the presence of an enolic form in some monomeric units by apparition of a short peak at 148.6 ppm.
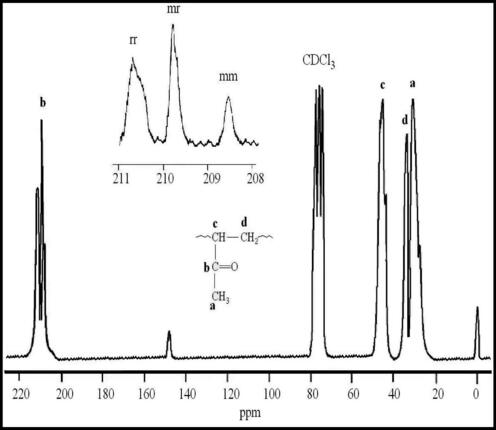
- 100 MHz 13C NMR of PMVK prepared in the presence of Ni(acac)2–MAO catalyst system.
In this investigation, the stereoregularities of polybenzylmethacrylate and polymethylvinylketone were determined by 1H and 13C NMR after performing a deconvolution of the multiplets α-CH3 (1H) and Cα (13C) of the carbonyl group to Lorentzian singlets as shown in Figs. 3–6. It should be noted that in case of PMVK, the deconvolution of 13C NMR spectrum was not necessary because the signals attributed to the α-CH3 corresponding to the different triads were very distinct. Tables 2 and 3 show the results of average tacticities of PBMA and PMVK determined by both methods.
| Temperature (°C) | Tacticity (%) | |||||
|---|---|---|---|---|---|---|
| PBMAa | PBMAb | |||||
| mm | mr | rr | mm | mr | rr | |
| 0 | 10.2 | 46.0 | 43.8 | – | – | – |
| 25 | 8.7 | 42.2 | 49.1 | – | – | – |
| 50 | 6.6 | 40.3 | 53.1 | – | – | – |
| 80 | 6.0 | 38.5 | 55.5 | – | – | – |
| 90 | – | – | – | 7.3 | 37.0 | 55.7 |
| 100 | – | – | – | 7.1 | 37.0 | 55.9 |
| Temperature (°C) | Tacticity (%) | ||
|---|---|---|---|
| PMVK | |||
| mm | rr | mr | |
| 0 | 21.0 | 46.0 | 33.0 |
| 25 | 23.2 | 46.6 | 29.2 |
| 50 | 23.1 | 48.5 | 27.4 |
| 80 | 16.0 | 55.0 | 29.0 |
Polymerization conditions: Ni(acac)2–MAO catalysts system; ageing of the initiating system: 15 min at 50 °C; time of polymerization: 5 h; [Ni] = 4.0 mmol l−1; [monomer] = 2.0 mol l−1; [MAO]/[Ni] = 100; solvent: toluene.
It was found that for PBMA, the Ni(acac)2–MAO catalytic system gave essentially a mixture of triads mr (38.5–46.0%), rr (43.8–55.5%) and a small quantity of triad mm (6.0–10.2%).
On the other hand, the same catalytic system gave for PMVK, a mixture of triads rr (46–55%), mr (27.4–33%) and mm (16–23.2%). In case of PBMA, these percentages of tacticity appeared to be similar to those obtained in this work using the free radical initiator (benzyl peroxide) under the same conditions (Table 2). The percentage of triad (rr) increased when the temperature decreased. In this way, it was found that these tacticity results confirmed those obtained by Endo and Inukai (2000) for the polymerization of methylmethacrylate and tert-butylmethacrylate synthesized, using the same initiator. Table 3 shows the tacticity of PMVK and indicated that the triads rr in PMVK increased slightly with the temperature.
The UV spectrum illustrated in Fig. 7 shows the spectra of PMBA and PMVK. The first spectrum revealed two peaks at 259 and 280 nm assigned to two π → π* transition (K-bands), while the second spectrum revealed only one peak at 280 nm assigned to the same transition.
![Polymerization of BMA with the Ni(acac)2–MAO catalyst system in toluene at different temperatures; ageing of the initiating system: 15 min at 50 °C; reaction time: 5 h; [Ni] = 4.0 mmol l−1; [BMA] = 2.0 mol l−1; [MAO]/[Ni] = 100.](/content/184/2009/2/2/img/10.1016_jarabjc200910003-fig7.png)
- Polymerization of BMA with the Ni(acac)2–MAO catalyst system in toluene at different temperatures; ageing of the initiating system: 15 min at 50 °C; reaction time: 5 h; [Ni] = 4.0 mmol l−1; [BMA] = 2.0 mol l−1; [MAO]/[Ni] = 100.
Differential scanning calorimetry studies characterized further these PBMA and PMVK materials. The DSC thermograms show the appearance of glass-transition temperature (Tg) for PBMA and PMVK. The first spectrum shows a Tg at 58 °C and the second shows a Tg at 34 °C. These values were similar to those reported in Demirelli et al. (2004) and Al-Saigh and Munk (1984).
The effect of temperature on the polymerization of BMA and MVK was investigated and the results are illustrated, for BMA in Fig. 10 and in Table 2. It was observed that, as the temperature changed from 25 to 80 °C, the yields and the tacticity of the obtained PBMA remained practically unchanged. Measurements of polymer molecular weights showed that the molecular weight of the produced PBMA increased by raising the polymerization temperature. It was revealed that a small quantity of oligomers (3–16%) was also obtained by increasing the temperature. In the polymerization of MVK, Table 4 shows that the polymer yields increased slightly in the temperature range from 0 to 50 °C and decreased beyond that. We have also noticed that the molecular weights increased weakly by increasing the polymerization temperature, while the polydispersity index (I) reached a minimum at 50 °C. It was also revealed that high fractions of oligomers (53–67%) were obtained by the catalytic system, and remained practically unchanged when the temperature increased.
| Temperature (°C) | Conversion (wt%) | Polymer (wt%) | Oligomer (wt%) | ||
|---|---|---|---|---|---|
| 0 | 75 | 8 | 67 | 2.1 | 5.5 |
| 25 | 75 | 21 | 54 | 3.0 | 3.6 |
| 50 | 78 | 25 | 53 | 4.2 | 3.3 |
| 80 | 85 | 21 | 64 | 4.4 | 4.7 |
Polymerization conditions: Ni(acac)2–MAO catalysts system; ageing of the initiating system: 15 min at 50 °C; time of polymerization: 5 h; [Ni] = 4.0 mmol l−1; [monomer] = 2.0 mol l−1; [MAO]/[Ni] = 100; solvent: toluene.
As reported by Kioshi et al., in the polymerization of methylmethacrylate (Endo and Inukai, 2000) and tert-butylmethacrylate (Endo, 1999), the Ni(acac)2–MAO catalytic system has the advantage of requiring a low molar ratio of MAO ([MAO]/[Ni] = 10–100) to reach high activity. In this work and those reported by Kioshi before, we approved the high polymerization activity of various monomers, such as the ones containing conjugated double bonds C=C–C=C or C=C–C=O, while those containing a donor group in α-position, such as methylvinylketone gave essential oligomers.
The kinetic study of BMA polymerization with Ni(acac)2–MAO catalytic system was carried out, and the results are shown in Fig. 8a and b. As for the polymerization of MMA initiated with the same catalytic system (Endo and Inukai, 2000), the maximum yield and molecular weight of PBMA was reached at 3 h and 50 °C. The equation of the polymerization rate of BMA using this catalytic system can be expressed as: where [C*] is the active center concentration, kp is the polymerization rate constant. For an almost stationary regime, this stipulates that the appearance of active centers is equal to their disappearance: where kp′ is the apparent polymerization rate constant, is:
![Kinetic curve for the polymerization of BMA with Ni(acac)2–MAO catalyst system in toluene; ageing time of initiating system: 15 min at 50 °C; [BMA] = 2.0 mol l−1; [Ni] = 4.0 mmol l−1; [MAO]/[Ni] = 100.](/content/184/2009/2/2/img/10.1016_jarabjc200910003-fig8.png)
- Kinetic curve for the polymerization of BMA with Ni(acac)2–MAO catalyst system in toluene; ageing time of initiating system: 15 min at 50 °C; [BMA] = 2.0 mol l−1; [Ni] = 4.0 mmol l−1; [MAO]/[Ni] = 100.
The relationship between the logarithm of Rp and the logarithm of [MBA] is a linear relationship as shown in Fig. 9. The kinetic order of the polymerization reaction was obtained from the slope of the line and estimated to be 1.68, which is almost similar to the result obtained by Endo and Inukai (2000) for other methacrylate esters. Therefore, the equation of the polymerization rate will be as follows:
![Effect of BMA concentration on the polymerization of BMA with Ni(acac)2–MAO catalyst system in toluene; ageing of initiating system: 15 min at 50 °C; polymerization time: 5 h; [Ni] = 4.0 mmol l−1; [MBA] = 2.0 mol l−1; [MAO]/[Ni] = 100.](/content/184/2009/2/2/img/10.1016_jarabjc200910003-fig9.png)
- Effect of BMA concentration on the polymerization of BMA with Ni(acac)2–MAO catalyst system in toluene; ageing of initiating system: 15 min at 50 °C; polymerization time: 5 h; [Ni] = 4.0 mmol l−1; [MBA] = 2.0 mol l−1; [MAO]/[Ni] = 100.
As shown in Fig. 9, the polymerization rate Rp increased as a function of the reaction time. The plot of ln Rp versus ln[MBA] was almost linear, indicating that the concentration of propagation centers was constant during the polymerization procedure; this confirmed the hypothesis of almost stationary regime.
Arrhenius plots were obtained from the initial rates of the polymerization of BMA as in Fig. 10. From the slope of the straight line, the overall activation energy Ea for the polymerization of BMA with Ni(acac)2 and MAO associated, was estimated to be 16.76 kJ/mol. This value looks to be reasonable and in accordance with those obtained from the polymerizations of methylmethacrylate (15.0 kJ/mol) (Endo and Inukai, 2000), styrene (22.0 kJ/mol) (Endo et al., 1994) and butadiene (18.0 kJ/mol) (Endo et al., 1996), using the same catalytic system.
![Arrhenius plot for the polymerization of BMA with Ni(acac)2–MAO catalyst system in toluene; ageing of initiating system: 15 min at 50 °C; polymerization time: 5 h; [Ni] = 4.0 mmol l−1; [MBA] = 2.0 mol l−1; [MAO]/[Ni] = 100.](/content/184/2009/2/2/img/10.1016_jarabjc200910003-fig10.png)
- Arrhenius plot for the polymerization of BMA with Ni(acac)2–MAO catalyst system in toluene; ageing of initiating system: 15 min at 50 °C; polymerization time: 5 h; [Ni] = 4.0 mmol l−1; [MBA] = 2.0 mol l−1; [MAO]/[Ni] = 100.
The polymerization of methacrylate esters, conjugated diene and styrene proceeded through the coordination of the monomer to the active site, followed by an insertion reaction. The unsuccessful result of vinylacetate polymerization using the same catalytic system shows that conjugated bonds are required for the mechanism, as proposed by Endo and Inukai (2000). In the polymerization of methylvinylketone which has conjugated bonds, the low conversion and molecular weights obtained in this work indicated, that the methyl group in the α position of the carbonyl in the monomer favored the ketone-enol equilibrium and affected highly the polymerization by the coordination process.
According to the results concerning the polymerization yield of BMA and those obtained from the polymerization of methylmethacrylate and tert-butylmethacrylate using Ni(acac)2/MAO, as cited by different authors, it was found also that the yield was not practically influenced by the steric hindrance of the substituent.
4 Conclusion
From the data obtained by the polymerization of benzylmethacrylate, methylvinylketone and vinylacetate in this work, and their comparison with those obtained by different authors using other vinylic monomers initialized with Ni(acac)2/MAO catalytic system, the following conclusions could be thoroughly drawn.
-
The above catalytic system is very efficient in the polymerization of alkylmethacrylate monomers.
-
The result shows that the polymerization is not influenced by the bulkiness of the substituent directly attached to the sp3 oxygen.
-
The polymer yields and the molecular weights are more influenced by the ketone-enol equilibrium of methylvinylketone due to the presence of methyl group directly attached to the carbonyl.
-
The polymerization of vinylacetate using this catalysts system has been unsuccessful, the presence of conjugated bonds C=C–C=C or C=C–C=O in the monomers is necessary.
-
Both the polymer yield and molecular weight of PBMA increased as a function of the reaction time.
-
The kinetic order of the polymerization reaction was estimated to be 1.68.
-
The tacticity of PBMA obtained is a mixture composed essentially from mr and rr triads and practically not affected by the temperature.







