Translate this page into:
Improved anti-inflammatory and anticancer properties of celecoxib loaded zinc oxide and magnesium oxide nanoclusters: A molecular docking and density functional theory simulation
⁎Corresponding Authors. mj.ansari@psau.edu.sa (Mohammad Javed Ansari), alireza.soltani@goums.ac.ir (Alireza Soltani), aliariannia@gmail.com (Ali Arian Nia), tavassolisam54@gmail.com (Samaneh Tavassoli), lotfor@ums.edu.my (Md Lutfor Rahman), chsu@mail.mcut.edu.tw (Chia-Hung Su)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Present study offers great prospects for the adsorption of anti-inflammatory celecoxib molecule (CXB) over the surface of zinc oxide (Zn12O12) and magnesium oxide (Mg12O12) nanoclusters in several environments by performing robust theoretical calculations. Density functional theory (DFT), time-dependent density functional theory (TDDFT) and molecular docking calculations have been extensively carried out to predict the foremost optimum site of CXB adsorption. It has been observed that the CXB molecule prefers to be adsorbed by its SO2 site on the Zn-O and Mg-O bonds of the Zn12O12 and Mg12O12 nanoclusters instead of NH2 and NH sites, where electrostatic interactions dominate over the bonding characteristics of the conjugate complexes. Furthermore, the presence of interactions between the CXB molecule and nanoclusters has also been evidenced by the UV–Vis absorption spectra and IR spectra. Molecular docking analysis has revealed that both adsorption states including CXB/Zn12O12 and CXB/Mg12O12 have good inhibitory potential against protein tumor necrosis factor alpha (TNF-α) and Interleukin-1 (IL-1), and human epidermal growth factor receptor 2 (HER2). Hence they might be explored as efficient TNF-α, IL-1, and HER2 inhibitors. Hence from the study, it can be anticipated that these nanoclusters can behave as an appropriate biomedical carrier for the CXB drug delivery.
Keywords
Zn12O12
Mg12O12
Celecoxib
Adsorption mechanism
Optoelectronic properties
1 Introduction
Nanocarrier emergence in drug delivery systems has significantly improved the solubility, stability and chemotherapeutic effects of drug by reducing its toxicity (Fakhar et al., 2017; Cao et al., 2021a; Cao et al., 2021b). Among a wide variety of nanocarriers in the field of nanomaterials, magnetic and nanoelectronic, metal oxides have been extensively used in the area of biomedicine including gene delivery, cancer therapy, bio-imaging and drug delivery (Hassanian et al.; Javan; Zhang et al., 2016; Zhang et al., 2021; Yang et al., 2021; Zhao and Guo, 2021; Deng et al., 2021; Sharma et al., 2020; Chen et al., 2020). Good biocompatibility, non-toxicity, high stability and selectivity of ZnO and MgO make them potential candidates for bio-imaging and drug delivery (Li et al., 2018; Cai et al., 2016). Moreover, anti-microbial and anti-inflammatory properties of these n-type semiconductors, i.e. ZnO and MgO nanoparticles broaden their biomedical application related to skin inflammation (Lin et al., 2019; Alalaiwe et al., 2019; Ansari, 2017).
In recent studies, various nanoclusters of ZnO and MgO have been explored as efficient drug carrier via adsorption of different biomolecules and drug molecules (Krishnamoorthy et al., 2012; Malarkodi et al., 2014). For instance, the encapsulation of 2-methoxyestradiol into MgO nanoparticles modified with the polymer polyethylene glycol were used for anticancer prostate therapy (Alfaro et al., 2019). Furthermore, photophysical and electronic properties of DNA nucleotide conjugated (ZnO)3 cluster had also been studied by Kumari et al. in aqueous phase using density functional theory (DFT) and time dependent-density functional theory (TDDFT) method (Kumari et al., 2019). Moreover, Tayebee and co-workers investigated the adsorption of some aliphatic aldehydes on the Zn12O12 nanocage and revealed the significant reduction in the adsorption energy and changes in the electronic properties which indicated Zn12O12 nanocage as an efficient sensor for toxic formaldehyde (Tayebee et al., 2015).
Fallahi et al. (2018) have explored a variety of nanocages including Al12N12, Al12P12, B12N12, Be12O12, C12Si12, Mg12O12 and C24 towards the detection as well as adsorption of tabun molecule using DFT studies and observed that Mg12O12 nanocluster was the best candidate for tabun adsorption without any chemical change in its structure whereas Al12N12 behaves as a good sensor for it. In addition to drug carrier, ZnO nanoparticles are efficient and fast in drug release as well. Farmanzadeh and Keyhanian have theoretically observed an increase in the release of amantadine molecule from Zn12O12 in comparison with B12N12 nanocage because of low binding energy in aqueous phase (Farmanzadeh and Keyhanian, 2019). Zhao et al. reported the role of ZnO nanoparticle capsules for loading isotretinoin with a capacity as high as 34.6 wt% and showed at low pH (below 6), ZnO-Isotretinoin decomposed quickly and more than 90% of the drug was released within 8 h (Zhao et al., 2017). Ravaei et al. showed the strong interaction of isoniazid (INH) drug via its nitrogen and oxygen atoms to the Mg atoms of the Mg12O12, while the electronic properties of the nanocage was not significantly changed (Ravaei et al., 2019).
Celecoxib (CXB) is a well-known nonsteroidal anti-inflammatory drug which is commonly prescribed to relieve pain and inflammation. CXB inhibits the transformation of arachidonic acid to prostaglandin precursors by blocking the enzyme cyclooxygenase, which result in decreasing the prostaglandins and hence acts as antipyretic, analgesic and anti-inflammatory (Mandracchia et al., 2016). In addition, CXB has also been considered as an effective drug to prevent the development of sporadic adenomatous polyps, precursors of colorectal cancer (Alamdarsaravi et al., 2017). Cao et al. have shown the naproxen loaded with OH-B12N12 was able to inhibit both IL-1 receptor and TNF-α receptor targets in comparison to B12N12 and B12P12 systems (Cao et al., 2021). To the best knowledge of the authors, the adsorption of CXB drug molecule on Zn12O12 and Mg12O12 metal clusters was not explored for biomedical applications. Thus, in this study, metal-oxide nanoclusters i.e. Zn12O12 and Mg12O12 have been theoretically explored for the adsorption of CXB drug molecule. By using DFT, TDDFT and molecular docking calculations, the attempt to design an effective drug carrier for CXB was made through the utilization of the unique properties of such metal oxide nanoclusters.
2 Computational details
All theoretical calculations including geometry optimization, interaction energies, frontier molecular orbitals (FMO) and transition state (TDDFT) studies of pristine and CXB adsorbed metal oxide nanoclusters (Zn12O12 and Mg12O12) were performed using B3LYP and B3PW91 functionals with Gaussian 09 suite of program (Gaussian et al., 2009). In all cases, 6-311 g(d,p) basis set was chosen for H, C, N, O, F and S atoms while LANL2DZ effective core potential (ECP) was chosen for Zn atoms. In order to evaluate the total density of state (TDOS), the projected density of state (PDOS) and shaded surface map with projection of electron density plots Multiwfn 3.3.9 were done (Lu and Chen, 2012). Furthermore, the effect of solvent i.e. water, was also examined by implementing polarizable continuum model (PCM) as the solvation model to mimic the biological environment. To compute total energy and electron density, the SCF (self-consistent field) convergence limit was set to 1.0 × 10−6 a.u. All computations were fully relaxed with the relaxation criteria (RMS force = 0.0003, Max. force = 0.00045, RMS displacement = 0.0012, and max. displacement = 0.0018). The adsorption energies (
) of CXB on metal oxide nanoclusters were calculated using the formula:
where
is the total energy of the adsorption complex,
is the total energy of the perfect metal-oxide nanocluster and
is the total energy of CXB molecule. The adsorption energies were corrected by adding basis set superposition error (BSSE) which accounts for the incompleteness of basis set in the calculation of weak intermolecular interactions by implementing the counterpoise method. Quantum molecular descriptors of CXB, nanoclusters and adsorption complexes were calculated to predict their respective physicochemical properties (Soltani et al., 2018; Soltani et al., 2014):
where ionization potential, electron affinity, electronegativity, global hardness, global softness, chemical potential and electrophilicity index are , , , , , and respectively. The values of and were respectively defined as negative orbital energies of the HOMO (highest occupied molecular orbital), , and the LUMO (lowest unoccupied molecular orbital), , based on Koopmans’ approximation.
Molecular docking simulations were calculated by the Auto Dock software (4.2) (Morris et al., 2009). Three-dimensional structures of TNF-alpha (PDB ID: 2AZ5), HER2 kinase (PDB ID: 3RCD), and IL1A-S100A13 complexes (PDB ID: 2L5X) were retrieved from Protein Data Bank. Protein files for docking were created via cognate ligand removal; non-polar hydrogen atoms, polar hydrogen atoms and the Kollman atom partial charges were added by the Auto Dock Tools (ADT). Lamarckian genetic algorithm was used for local search method. A grid box of 60x60x60 with a spacing of 0.375 Å was created for preparation of autogrid (Mirzaei et al., 2017a; Mirzaei et al., 2017b). A total of 100 GA runs were performed for docking. Maestro 11.0 Schrodinger program was used for preparation of 2D and 3D presentation.
3 Result and discussion
3.1 Structural analysis of CXB loaded Zn12O12/Mg12O12
The relaxed structural parameters of Celecoxib drug on Zn12O12 and Mg12O12 nanoclusters were calculated by the B3LYP and B3PW91 methods for both vacuum and aqueous environments and the optimized structures were shown in Figs. 1, 2, 3 and 4. The lengths of Zn-O, O1-S, N3-S, N1-C3, N1-N2, and C4-F bonds for the Zn12O12 and CXB were computed to be 1.983, 1.461, 1.694, 1.393, 1.421 and 1.346 Å by the B3LYP functional and 1.983, 1.463, 1.670, 1.384, 1.408 and 1.356 Å by the B3PW91 functional and the studies represent good agreement with the experimental and theoretical results (Pulay et al., 1983; Vijayakumar et al., 2016). Farmanzadeh and Keyhanian have shown the interaction of amantadine drug with Zn12O12 nanocluster and reported two types of Zn–O bonds with the values of 1.89 and 1.98 Å by the PBE functional (Li et al., 2018). The Zn-O-Zn angles increase from 87.22˚ in pure Zn12O12 to 88.30˚ and 87.63˚ in State I and II within the water phase also there have been not many changes in other scenarios. O-S-O angles for the State I (121.72°) and II (119.09°) in vacuum phase observed more changes than any other scenario while in the aqueous phase are 115.39° and 113.08°. The geometry of Zn12O12 nanocluster is made of six 4-MRs (four-membered rings) with the symmetry of Th (Baei et al., 2013). Fig. 1 represents HOMO orbital of CXB drug which is more distributed on the C-C and C-N bonds in aromatic and pyrazole rings and LUMO orbital which is more localized on the C-C bonds in aromatic ring.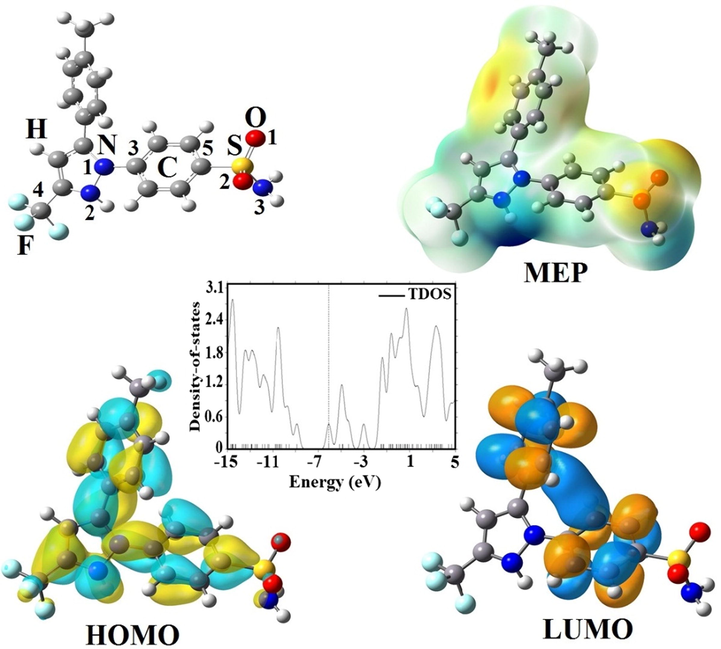
Optimized structures, FMO and TDOS plots of CXB.
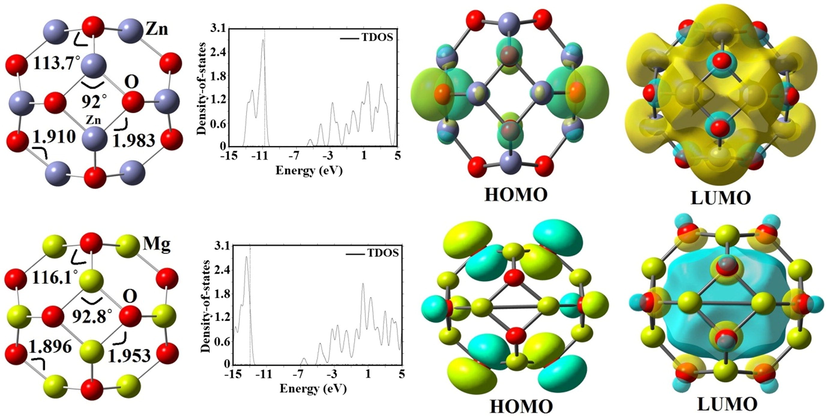
Optimized structures, FMO and TDOS plots of Zn12O12 and Mg12O12 nanoclusters.
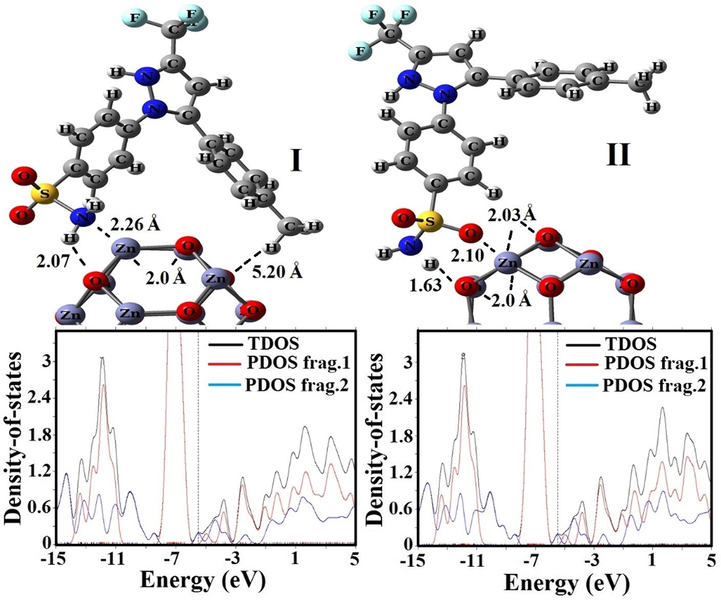
Optimized structures and PDOS plots of CXB on the surface of Zn12O12 fullerene.
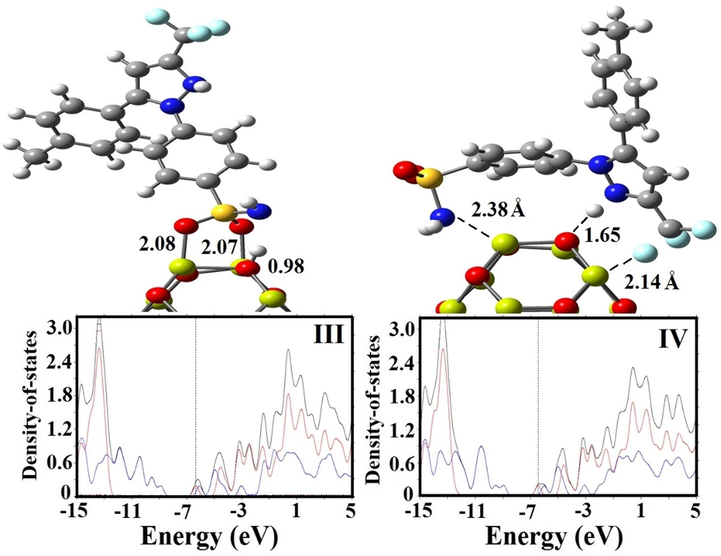
Optimized structures and PDOS plots of CXB on the surface of Mg12O12 fullerene.
To better perceive the adsorption behavior of CXB with Zn12O12 and Mg12O12 nanoclusters, we evaluated various orientations of the CXB through –NH2 and –SO2 groups by changing their separation distances on Zn12O12 and Mg12O12 surfaces. As depicted in Figs. 3 and 4, CXB drug in relaxed structures indicate the best trend for the interaction with the electrophilic Zn and Mg atoms of nanoclusters as compared with other positions through its O atom as the nucleophilic agent; this result is in agreement with MEP plots. In comparison between Tables 1 and 2, the
values and separation distance (BD) of CXB in the most stable state (State II) on the Zn12O12 surface were −1.18 eV (2.10 Å) in vacuum environment and −1.38 eV (2.02 Å) in solvent environment by the B3PW91 functional, while for the B3LYP functional were −1.20 eV (2.10 Å) in vacuum environment and −1.26 eV (2.10 Å) in solvent environment, respectively. In contrast with Zn12O12, the adsorption process of CXB through the sulphonamide (III: SO2NH2) group demonstrates a strong hybridization in vacuum environment due to a strong covalent interaction between the CXB and the adsorbent in comparison to amino (IV: NH2) group of the drug.
and BD values related to B3LYP functional in the most stable state (State III) indicate strong binding state for the CXB on the Mg atom rather than O atom of the Mg12O12 with the amount of −1.87 eV and 2.07 Å respectively.
Property
Vacuum
Aqueous
Zn12O12
I
II
Zn12O12
I
II
EHOMO (eV)
−6.95
−4.35
−4.24
−5.96
−3.92
−3.92
ELUMO (eV)
−2.80
−2.69
−2.70
−1.58
−1.70
−1.63
Eg (eV)
4.15
1.66
1.54
4.38
2.22
2.29
ΔEg (%)
–
60.0
62.9
–
49.3
47.7
Eads (eV)
–
−0.98
−1.20
–
−1.03
−1.26
BD (Ǻ)
–
2.26
2.10
–
2.08
2.02
DM (Debye)
0.00
3.696
2.694
0.00
19.38
14.01
EF (eV)
−4.88
−3.52
−3.47
−3.77
−2.81
−2.77
I (eV)
6.95
4.35
4.24
5.96
3.92
3.92
A (eV)
2.80
2.69
2.70
1.58
1.70
1.63
(eV)
−4.88
−3.52
−3.47
−3.77
−2.81
−2.78
(eV)
4.88
3.52
3.47
3.77
2.81
2.78
(eV)
2.08
0.83
0.77
2.19
1.11
1.15
(eV−1)
0.24
0.60
0.65
0.23
0.45
0.44
(eV)
5.73
7.46
7.82
3.24
3.56
3.36
Property
Vacuum
Aqueous
Zn12O12
I
II
Zn12O12
I
II
EHOMO (eV)
7.00-
−4.36
−4.25
−6.00
−3.94
−3.97
ELUMO (eV)
−2.61
−2.52
−2.53
−1.41
−1.47
−1.47
Eg (eV)
4.39
1.82
1.72
4.59
2.47
2.5
ΔEg (%)
–
58.5
60.8
–
46.2
45.5
Eads (eV)
–
−0.94
−1.18
–
−1.21
−1.38
BD (Ǻ)
–
2.26
2.10
–
2.08
2.02
DM (Debye)
0.0
3.761
2.851
0.00
16.53
14.66
EF (eV)
4.805-
3.44-
3.39-
−3.705
−2.705
−2.72
I (eV)
7.00
4.36
4.25
6.00
3.94
3.97
A (eV)
2.61
2.52
2.53
1.41
1.47
1.47
(eV)
−4.81
−3.44
−3.39
−3.71
−2.71
−2.72
(eV)
4.81
3.44
3.39
3.71
2.71
2.72
(eV)
2.20
0.92
0.86
2.30
1.24
1.25
(eV−1)
0.23
0.54
0.58
0.22
0.40
0.40
(eV)
5.26
6.43
6.68
2.99
2.96
2.96
The stability of CXB through the amino (NH2) group (State IV) in solvent environment is spontaneous and it could be increased due to the negative values of solvation energy (
) as the
and BD values were calculated to be −2.08 eV and 2.25 Å, respectively. On the other hand, BD value from CXB to the nanoclusters seems to remarkably depend on the orientation of drug and curvature of the clusters. Lower
values represent that the CXB adsorption on the Zn12O12 in comparison with Mg12O12 becomes weak; therefore, drug with a non-covalent interaction is often separated from the adsorbent and can be delivered into target site (Chen et al., 2020; Abd El-Mageed, 2020). The adsorption behavior of isoniazid drug over the surface of Mg12O12 nanocluster has been reported by Lin et al. (2019) and feasible adsorption between the N and O atom of drug with Mg atom of complex has been demonstrated on the basis of negative value of adsorption energy i.e. −0.96 to −2.58 eV based on the dispersion corrected M062X level of theory. As shown in Fig. 5, shaded surface map with projection of electron density (SPE) plot demonstrates that the celecoxib could adsorbed on the surface of Zn12O12 fullerene through electrostatic interaction in nature (Ansari et al., 2014a; Ansari et al., 2014b). Table 1 and 2 demonstrate that the CXB through NH2 group (I: 19.38 Debye at B3LYP and 16.53 Debye at B3PW91) in solvent environment has a higher value of dipole moment (DM) in comparison with the SO2NH2 group (II: 14.01 Debye at B3LYP and 14.66 Debye at B3PW91). The higher DM value demonstrates the higher complex reactivity. In a physiological medium, significant increment in DM value indicates enhanced solubility which is an acceptable characteristic for drug delivery systems (Dastani et al., 2021).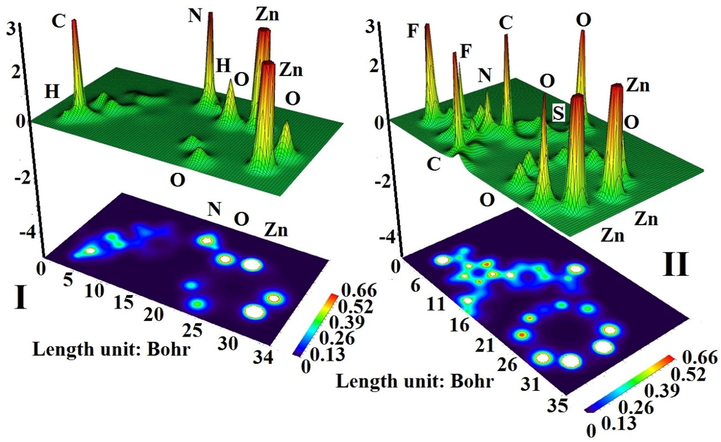
SPE plots of the CXB adsorbed on the of Zn12O12 surface in State I and II.
Infrared (IR) spectroscopy has also been employed to study the possibility of molecular interactions of CXB with Zn12O12 and Mg12O12 nanoclusters by the B3LYP functional. Theoretical IR spectrum of Mg12O12 demonstrates the band at 656 cm−1 strongly corresponded to Mg-O stretching vibration (Moorthy et al., 2015). Theoretical IR spectrum of Zn12O12 exhibits bands at 465, 552, 584, and 644 cm−1 corresponded to Zn–O stretching vibration (Aggarwal, 2006; Wahab et al., 2009). The IR spectrum of State I demonstrated characteristic bands at 1038 and 3338 cm−1 that are attributed to N–H stretching vibration of SO2NH2 group and 1293 and 1527 cm−1 for S = O asymmetric and symmetric stretching, and 1202 cm−1 for C–F stretching. In contrast, IR spectrum of State II exhibited characteristic bands at 1066 and 3667 cm−1 that are assigned to N–H stretching vibration of SO2NH2 group and 1254 cm−1 for C–F stretching, and S = O asymmetric and symmetric stretching are distributed to 1271 and 1482 cm−1. These results were in good agreement with the experimental data obtained by He and co-authors (He et al., 2017).
3.2 Electronic properties of CXB-Zn12O12 and CXB-Mg12O12
The values of energy gap (Eg), Fermi level (EF), and change of energy gap (ΔEg) for CXB, Zn12O12 and Mg12O12, and considered complexes are summarized in Tables 1, 2 and 3. The Eg values for the Zn12O12 nanocluster in both vacuum and solvent phases were 4.15 and 4.38 eV by B3LYP functional and 4.39 and 4.59 eV by B3PW91 functional, whereas they reduce to 1.66 and 1.54 eV in vacuum phase and 2.22 and 2.29 eV in solvent phase for the State I and II respectively after CXB adsorption on Zn12O12 surface, which is close to the theoretical results by Ahmadi Peyghan et al. (2013), Ghenaatian et al. (2013). This represents that interaction of CXB with Zn12O12 induces some shifts in the electronic features and therefore Eg values were reduced when compared with both pure CXB and Zn12O12. The change in energy gap for the CXB-Zn12O12 complex in solvent phase is more than the vacuum phase. Similar Eg reduction trend was also observed in CXB-Mg12O12 complexes however the reduction effect was lesser as compared with the former. For CXB adsorbed on the Zn12O12 and Mg12O12 nanoclusters, PDOS of a complex appeared with small Eg in which each fragment (where the nanoclusters and CXB respectively were determined as frags1 and 2) has the electronic total density of state (TDOS) near the states of HOMO and LUMO (Figs. 3 and 4).
Property
Vacuum
Aqueous
Mg12O12
III
IV
Mg12O12
III
IV
EH/eV
−6.58
−4.13
−4.02
−5.99
−4.0
−3.22
EL/eV
−1.74
−1.66
−1.78
−1.03
−1.06
−1.11
Eg/eV
4.84
2.47
2.24
4.96
2.94
2.11
ΔEg (%)
–
48.97
53.72
–
40.72
57.46
Eads/eV
–
−1.87
−1.17
–
−1.90
−2.08
D/Ǻ
–
2.07
2.38
–
2.14
2.25
DM/Debye
0.0
2.05
3.75
0.0
3.78
10.88
EF/eV
−4.16
−2.89
−2.90
−3.51
−253
−2.17
I (eV)
6.58
4.13
4.02
5.99
4.00
3.22
A (eV)
1.74
1.66
1.78
1.03
1.06
1.11
(eV)
−4.16
−2.90
−2.90
−3.51
−2.53
−2.17
(eV)
4.16
2.90
2.90
3.51
2.53
2.17
(eV)
2.42
1.24
1.12
2.48
1.47
1.06
(eV−1)
0.21
0.40
0.45
0.20
0.34
0.47
(eV)
3.58
3.39
3.75
2.48
2.18
2.22
Tables 1, 2, and 3 represent the quantum molecular descriptors (QMDs) of CXB, Zn12O12, Mg12O12 and adsorption complexes. The interaction of Zn12O12 and Mg12O12 nanoclusters with CXB, a significant decrement in the Eg value has enabled a more facile electron transition thus increasing its reactivity for CXB interaction (Abdolahi et al., 2020; Soltani et al., 2022). QMDs of adsorption complexes also indicated that they are less electronegative and chemically softer. In terms of electrophilicity, CXB-Zn12O12 becomes more electrophilic while CXB-Mg12O12 becomes less electrophilic. These consequences demonstrated a notable change in the electronic properties of adsorption complexes as comparing to the pure Zn12O12 and Mg12O12 nanoclusters, making them suitable for applications involving molecular sensor of high sensitivity.
3.3 Analysis of UV–visible spectra
We have calculated the UV–visible absorption spectra of CXB interaction with Zn12O12 and Mg12O12 nanoclusters in vacuum and aqueous phases using TDDFT/B3PW91 level of theory, as displayed in Tables 4 and 5. HOMOs and LUMOs that were involved in the electronic transitions were illustrated in Fig. 6. Table 4 represents the utmost values of E (vertical excitation energies), λmax (excitation wavelength), and f (oscillator strength). The UV–vis spectra of pure Zn12O12 and Mg12O12 nanoclusters reveal existence of one main maximum band with λmax at 251.06 and 252.78 nm and therefore the E values of 4.938 and 4.909 eV in the aqueous environment respectively. Sharma et al. reported the absorption band of the synthesized ZnO nanoparticles with a λmax at 270.5 nm (Sharma et al., 2018); which is close to our theoretical calculations. Moazzen and co-workers experimentally have shown the UV–visible absorption spectra of the ZnO nanoparticles with a band at 317 nm (3.30 eV) by dispersing the powder in deionized water (Moghri Moazzen et al., 2012).
E (eV)
λmax (nm)
f
Assignment
Vacuum
I
2.258
549.05
0.0849
H → L + 9(A) (89%)
2.381
520.53
0.0335
H → L + 6 (48%), H → L + 10 (-29%)
2.477
500.53
0.0296
H → L + 6 (-21%), H → L + 12 (60%)
II
2.251
550.56
0.0825
H → L + 8 (-14%), H → L + 9 (74%)
2.44
508.03
0.0296
H → L + 12(A) (70%)
2.396
517.46
0.0265
H → L + 5(39%), H → L + 6(-17%), H → L + 11
Aqueous
I
3.582
346.11
0.2049
H → L + 15(10%), H → L + 1(71%)
4.262
290.88
0.1826
H → L + 19 (25%), H-1 → L + 1(35%)
2.655
466.96
0.0943
H → L + 4 (–22%), H → L + 6(25%), H → L + 7 (20%), H → L + 8 (11%)
II
3.212
385.91
0.1969
H → L + 1(78%)
2.192
565.56
0.1231
H → L + 3 (16%), H → L + 4(71%)
2.414
513.41
0.0479
H → L + 6(82%)
E (eV)
λmax (nm)
f
Assignment
Mg12O12
4.994
248.24
0.0889
H-17 → L (86%)
4.909
252.78
0.2599
H-14 → L (88%)
4.231
292.99
0.0057
H-1 → L (93%)
III
3.889
317.94
0.0727
H → L + 16(58%), H-5 → L + 1(2%)
3.178
390.14
0.1991
H → L + 5(13%), H-1 → L (68%)
2.255
549.86
0.1098
H → L + 1(76%), H → L + 3(9%)
IV
3.196
387.88
0.0421
H → L + 17(79%), H → L + 1(6%)
2.281
543.62
0.0125
H → L + 7(65%), H → L + 8(8%)
1.322
937.70
0.0510
H → L + 1(62%), H → L + 3(27%)
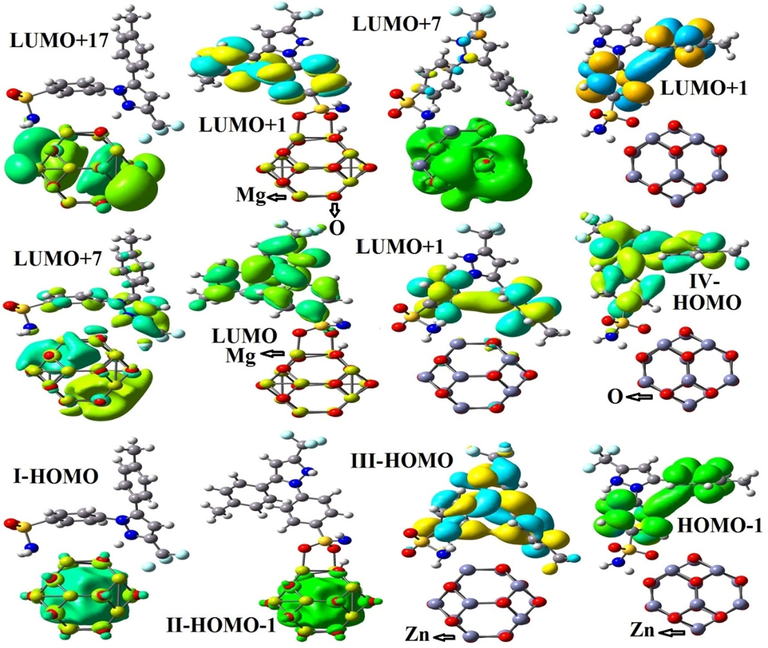
FMO plots of CXB adsorbed on Zn12O12 and Mg12O12 nanoclusters in the most stable states.
UV–visible absorption spectra for the pure CXB represents four maximum absorption bands with the λmax value of 260 (4.768 eV), 294 (4.214 eV), 354 (3.498 eV) and 470 nm (2.635 eV) which are in agreement with theoretical (Abdolahi et al., 2018) and experimental (Revathi et al., 2011) reports. The UV–visible absorption spectra demonstrate that the strong interaction of CXB through its –NH2 group with Zn12O12 and Mg12O12 nanoclusters leading to the optical changes compared to -SO2 group of the drug because of the electronic transitions between the non-bonding p-orbital of nitrogen and the unoccupied sp-hybrid orbitals of zinc and magnesium atoms (Nagare et al., 2017). The maxima absorbance band in the State I and III were observed at 346.11 nm (f: 0.2049) and 390.14 nm (f: 0.1991) which were superimposed on the absorption band of CXB. The maxima absorbance bands in the State I and III occurred at longer wavelengths with the main electron transitions in solvent phase as H → L + 1 (71%) and H-1 → L (68%) respectively.
3.4 Molecular docking studies of CXB loaded with Zn12O12 and Mg12O12
The binding affinities for the investigated compounds toward TNF-α receptor and IL-1 receptor targets were evaluated via molecular docking. To determine the inhibition mechanism of TNF-α receptor and IL-1 receptor, CXB-Zn12O12 and CXB-Mg12O12 complexes were docked in the binding pocket of the targets. The docking results reveal that the CXB-Zn12O12 complex (State I) is a potent inhibitor of TNF-α and IL-1 as compared to other compounds. As displayed in Table 6, CXB through NH2 group interacts with the Zn12O12 nanocluster and showed high binding energy in comparison with the CXB-Mg12O12 complex as the value of the binding energy of the TNF-α and IL-1 receptors in order were −10.7 and −9.3 kcal/mol. CXB-Zn12O12 compound (State I) had a good binding affinity with protein TNF-α through amino acid residues (Tyr119, Leu120, Tyr59, Tyr151, Leu57, and Ile155 and Gly121) from the C chain and also amino acid residues (Tyr151, Leu120, Tyr59, Tyr119, and Gly121) from the D chain by hydrophobic interactions. Hence, the State I was also bounded with other amino acid residues like Ser60, Gln61 and His15 from the C chain and Ser60 from the D chain through polar interactions. Besides, State I bound to amino-acid residue of Tyr59 from the C chain by π-π stacking. Previous study proved that the corresponding amino acid residues (Tyr59, Tyr151, Leu120, Ser60, and Gln61) were important residues in the active site of the target protein (Xu et al., 2018) that exert anti-inflammatory effects through TNF-α inhibition (Fig. 7). Olbert et al. experimentally demonstrated zinc oxide nanoparticles at a dose of 14 mg/kg ip decreased the carrageenan-induced paw edema and improved the anti-inflammatory activity of ketoprofen (Olbert et al., 2017). Agarwal et al. experimentally showed anti-inflammatory effect of zinc oxide nanoparticles at the concentration of 1 mg/mL by blocking the production and release of inflammatory mediators such as IL-1β, IL-6, TNF-α, and COX-2 (Agarwal and Kumar Shanmugam, 2019). The sulfonamide group of State I was stabilized in the binding pocket of IL-1 receptor (PDB ID: 2L5X) in two hydrogen bonds of amino acid residues Thr77 and Arg34. The length of the hydrogen bond distance was 2.39 Å. Hence, the complex was established in the binding pocket of the target by the hydrophobic behavior of amino acid residues (Ala51, Val52, Ile74 and Leu79). In addition, amino acid residues like Asp49, Glu50, Arg34, Lys53, Glu94 and Lys76 bound to the target by electrostatic interactions. The calculations showed that State I bound to the active site consists of amino acid residues like Arg34, Asp49 and Glu50 and lead to the inhibition of the IL-1 receptor (Shokri et al., 2018; Emami et al., 2018). Fig. 7 demonstrates that State I was placed in the active site pocket of the IL-1 receptor. Molecular docking calculations exhibited that State I had a strong binding affinity to TNF-α and IL-1 receptors and observed that the complex inhibits more influentially the active site of the protein than states II, III, and IV. Therefore, the hydrophobic and hydrogen bond interactions play a crucial role in occupation of the binding pocket. The results demonstrated that CXB/Zn12O12 complex (State I) could be considered as promising inhibitors against the TNF-α and IL-1 receptors (see Fig. 8).
Compound
PDB ID: 2AZ5
PDB ID:2L5X
PDB ID:3RCD
BE (kcal/mol)
Ki (µM)
BE (kcal/mol)
Ki (µM)
BE (kcal/mol)
Ki (µM)
ZnO
−6.0
8.6
−4.2
11.1
−6.1
10.3
CXB
−6.3
8.2
−6.1
9.7
−6.3
9.5
I
−10.7
2.4
−9.3
3.7
−9.1
7.5
II
−9.7
3.7
−8.8
4.2
−8.5
8.7
Mg12O12
−6.1
8.4
−4.2
11.3
−5.9
10.8
III
−8.5
5.3
−7.6
6.3
−8.8
8.2
IV
−9.8
3.3
−8.1
5.5
−9.7
7.1
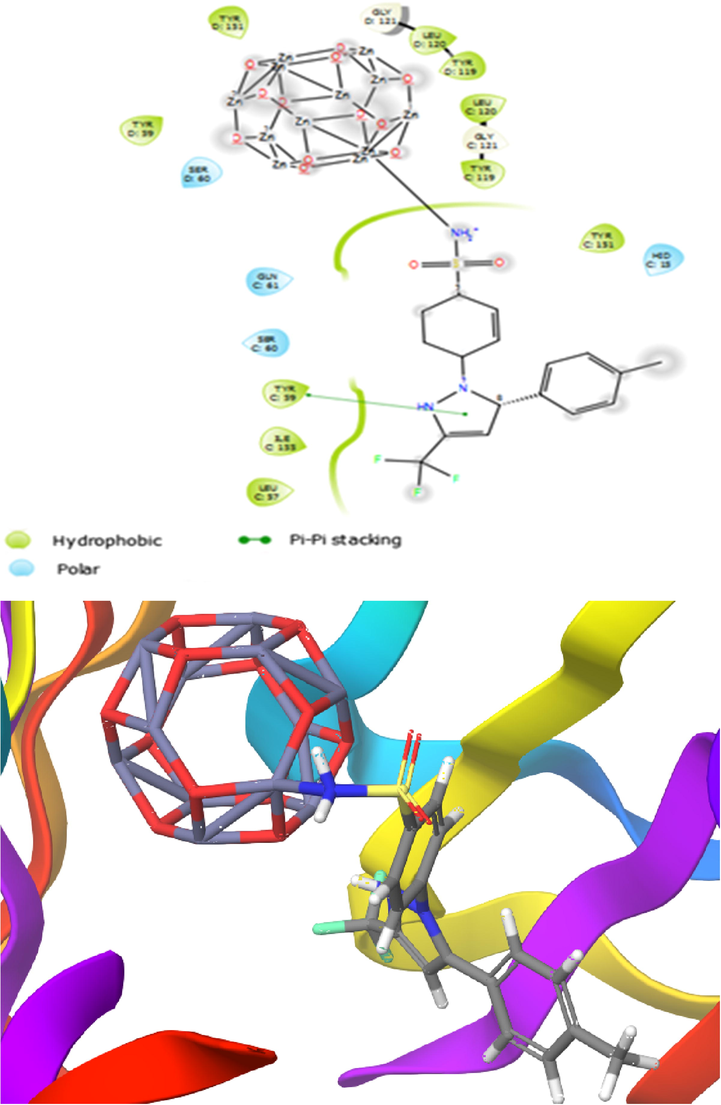
Presentation of 2D and 3D models of interactions between CXB-Zn12O12 complex (State I) and IL-1 receptor (PDB ID: 2LX5).
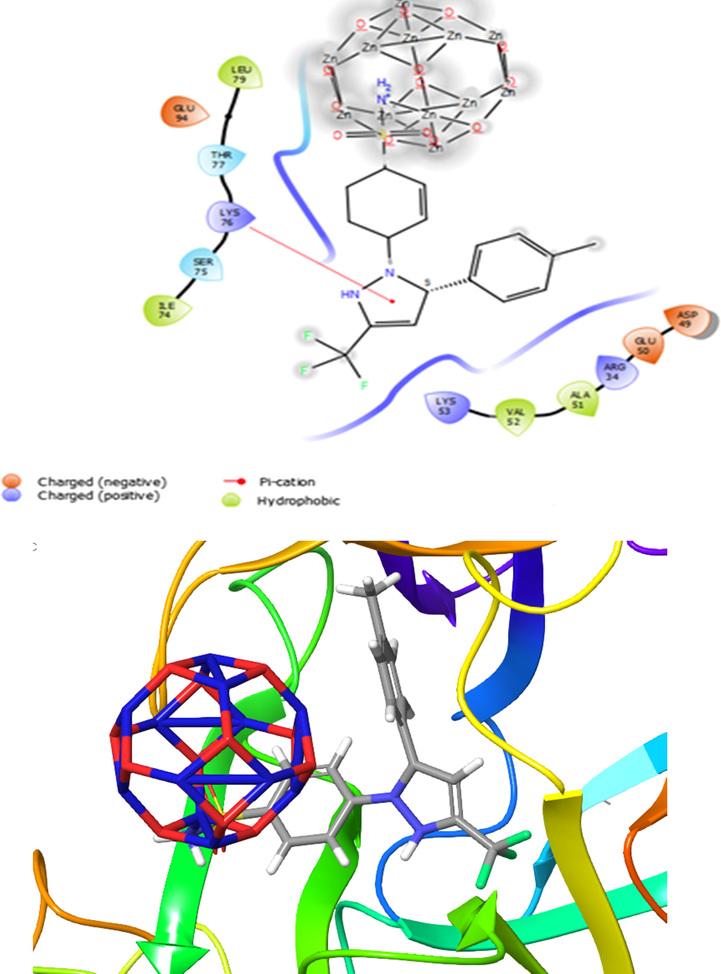
Presentation of 2D and 3D models of interactions between CXB-Zn12O12 complex (State II) and IL-1 receptor (PDB ID: 2LX5).
Furthermore, to evaluate binding affinity of selected complexes toward human epidermal growth factor receptor 2 (HER2) kinase, molecular docking was done. The aforementioned results revealed that State IV has the lowest binding free energy (−9.7 kcal/mol) compared to other complexes. This binding affinity is well associated with the anticancer activity, where CXB/Mg12O12 complex inhibits the HER2 more potently than CXB/Zn12O12 complex (Xiang et al., 2017; Mozdoori et al., 2017). Moreover, amino acids residues involved in the active site of the receptor were like Pro945, Cys947, Thr917, Val956, Cys958, Met916, Val912, Arg940, Glu914, Lys887, Ser907, Trp888 and also amino acid residue of Tyr923 interacted with the receptor via hydrogen bond interactions. Therefore, molecular docking simulation exhibited that COX-2 inhibitors could be associated with anticancer properties. Hence, finding of the study illustrated importance of CXB loaded on Zn12O12 and Mg12O12 nanoclusters for the interaction with the amino acids involved in the binding pocket of HER2 receptor. Accordingly, this compound (State IV) could be developed as potential anticancer compound (El-Azab et al., 2018; Mokhtary et al., 2018).
4 Conclusion
We devised the interaction of CXB through their –NH2 and –SO2 groups on the surface of Zn12O12 and Mg12O12 nanoclusters in the vacuum and aqueous phases using DFT calculations and molecular docking. The Zn and Mg atoms of Zn12O12 and Mg12O12 nanoclusters were shown to attract CXB molecules via its positive charge. Our calculation represents that the Eads of CXB is negative in the exothermic process (State II), with values of −1.18 eV (B3PW91) and −1.20 eV (B3LYP) in vacuum phase and −1.38 eV (B3PW91) and −1.26 eV (B3LYP) in aqueous phase. Our calculations demonstrated that CXB from its –NH2 group leads to more changes in the optical and electronic features of the Zn12O12 and Mg12O12 nanoclusters compared to the –SO2 group. The evaluation of molecular docking represents that State I contain a good binding affinity with these main cytokines (TNF-α and IL-1) as compared with State II, III, and IV.
Acknowledgements
We thank Golestan University of Medical Sciences for providing partial support of the instrumental analysis facilities. The authors want to thank the clinical Research Development Unit (CRDU), Sayad Shirazi Hospital, Golestan University of Medical Sciences, Gorgan, Iran. The authors want to thank the Zhejiang Shuren University Basic Scientific Research Special Funds (2020XZ011).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Zinc oxide nanoclusters and nanoparticles as a drug carrier for cisplatin and nedaplatin anti-cancer drugs, insights from DFT methods and MC simulation. Mol. Phys.. 2020;119:e1842533
- [Google Scholar]
- Adsorption of Celecoxib on B12N12 fullerene: Spectroscopic and DFT/TD-DFT study. Spectrochim. Acta, Part A. 2018;204:348-353.
- [Google Scholar]
- Gold decorated B12N12 nanocluster as an effective sulfasalazine drug carrier: A theoretical investigation. Physica E. 2020;124:114296
- [Google Scholar]
- Synthesis and optimization of zinc oxide nanoparticles using Kalanchoe pinnata towards the evaluation of its anti-inflammatory activity. J. Drug Delivery Sci. Technol.. 2019;54:101291
- [Google Scholar]
- SHI induced modification of ZnO thin film: Optical and structural studies. Nucl. Instrum. Methods Phys. Res. B. 2006;244:136-140.
- [Google Scholar]
- Ahmadi Peyghan, A., Baei, M.T., Hashemian, S., 2013. ZnO Nanocluster as a Potential Catalyst for Dissociation of H2S Molecule. J. Clust. Sci., 24, 341-347.
- Influence of chitosan coating on the oral bioavailability of gold nanoparticles in rats. Saudi Pharmaceut. J.. 2019;27(2):171-175.
- [Google Scholar]
- Efficacy and Safety of Celecoxib Monotherapy for Mild to Moderate Depression in Patients with Colorectal Cancer: A Randomized Double-Blind, Placebo Controlled Trial. Psychiatry Res.. 2017;255:59-65.
- [Google Scholar]
- MgO nanoparticles coated with polyethylene glycol as carrier for 2-Methoxyestradiol anticancer drug. PLOS ONE E. 2019;14(8):e0214900
- [Google Scholar]
- Factors Affecting Preparation and Properties of Nanoparticles by Nanoprecipitation Method. Indo Am. J. Pharmaceut. Sci.. 2017;4(12):4854-4858.
- [Google Scholar]
- solubility and stability enhancement of curcumin through cyclodextrin complexation. Int. J. Biol., Pharm. Allied Sci.. 2014;3(11):2668-2675.
- [Google Scholar]
- Influence of hydrophilic polymers on complexation and solubilizing efficiencies of beta cyclodextrin over silymarin. Int. J. Biol., Pharm. Allied Sci.. 2014;3(10):237-242.
- [Google Scholar]
- Quantum chemical analysis on hydrogenated Zn12O12 nanoclusters. C. R. Chim.. 2013;16:122-128.
- [Google Scholar]
- pH-Sensitive ZnO Quantum Dots-Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl. Mater. Interfaces. 2016;8:22442-22450.
- [Google Scholar]
- Cao, Y., Khan, A., Balakheyli, H., Ng Kay Lup, A., Taghartapeh, M.R., Mirzaei, H., Khandoozi, S.R., Soltani, A., Aghaei, M., Heidari, F., Sarkar, S.M., Albadarin, A.B., 2021a. Penicillamine functionalized B12N12 and B12CaN12 nanocages act as potential inhibitors of proinflammatory cytokines: A combined DFT analysis, ADMET and molecular docking study. Arab. J. Chem., 14, 103200.
- Cao, Y., Khan, A., Mirzaei, H., Khandoozi, S.R., Javan, M., Ng Kay Lup, A., Norouzi, A., Tazikeh Lemeski, E., Pishnamazi, M., Soltani, A., Albadarin, A.B., 2021b. Investigations of adsorption behavior and anti-cancer activity of curcumin on pure and platinum-functionalized B12N12 nanocages. J. Mol. Liquids, 334, 116516.
- Predicting adsorption behavior and anti-inflammatory activity of naproxen interacting with pure boron nitride and boron phosphide fullerene-like cages. J. Mol. Liq.. 2021;339:116678
- [Google Scholar]
- Effect of metal atoms on the electronic properties of metal oxide nanoclusters for use in drug delivery applications: a density functional theory study. Mol. Phys.. 2020;118:e1692150
- [Google Scholar]
- DFT study of Ni-doped graphene nanosheet as a drug carrier for multiple sclerosis drugs. Comput. Theor. Chem.. 2021;1196:113114
- [Google Scholar]
- Inter-hours rolling scheduling of behind-the-meter storage operating systems using electricity price forecasting based on deep convolutional neural network. Int. J. Electr. Power Energy Syst.. 2021;125:106499
- [Google Scholar]
- El-Azab, A.S., Abdel-Aziz, A.A.-M., Abou-Zeid, L.A., El-Husseiny, W.M., El_Morsy, A.M., El-Gendy, M.A., El-Sayedd, M.A.-A., 2018. Synthesis, antitumour activities and molecular docking of thiocarboxylic acid ester-based NSAID scaffolds: COX-2 inhibition and mechanistic studies, J. Enzyme Inhib. Med. Chem., 33, 989–998.
- Acetophenone benzoylhydrazones as antioxidant agents: synthesis, in vitro evaluation and structure-activity relationship studies. Food Chem.. 2018;268:292-299.
- [Google Scholar]
- Fakhar ud Din, Aman, W., Ullah, I., Qureshi, O.S., Mustapha, O., Shafique, S., Zeb, A., 2017. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 12, 7291-7309.
- Theoretical studies on the potentials of some nanocages (Al12N12, Al12P12, B12N12, Be12O12, C12Si12, Mg12O12 and C24) on the detection and adsorption of Tabun molecule: DFT and TD-DFT study. J. Mol. Liq.. 2018;260:138-148.
- [Google Scholar]
- Computational assessment on the interaction of amantadine drug with B12N12 and Zn12O12 nanocages and improvement in adsorption behaviors by impurity Al doping. Theor. Chem. Acc.. 2019;138:11.
- [Google Scholar]
- Gaussian 09, Revision D.01, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, €O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian, Inc., Wallingford CT, 2009.
- Zn12O12 nano-cage as a promising adsorbent for CS2 capture. Superlattices Microstruct.. 2013;58:198-204.
- [Google Scholar]
- Hassanian, M., Aryapour, H., Goudarzi, A., Javan, M.B. Are zinc oxide nanoparticles safe? A structural study on human serum albumin using in vitro and in silico methods. J. Biomol. Struct. Dynam. 39, (1), 330-335
- Preparation and evaluation of celecoxib nanosuspensions for bioavailability enhancement. RSC Adv.. 2017;7:13053-13064.
- [Google Scholar]
- Javan, M.B. Magnetic properties of Mg12O12 nanocage doped with transition metal atoms (Mn, Fe, Co and Ni): DFT study. J. Magn. Magn. Mater., 385, 138-144.
- Mechanistic investigation on the toxicity of MgO nanoparticles toward cancer cells. J. Mater. Chem.. 2012;22:24610-24617.
- [Google Scholar]
- Nucleotide conjugated (ZnO)3 cluster: Interaction and optical characteristics using TDDFT. J. Mol. Graph. Model.. 2019;87:211-219.
- [Google Scholar]
- Keratin-Templated Synthesis of Metallic Oxide Nanoparticles as MRI Contrast Agents and Drug Carriers. ACS Appl. Mater. Interfaces. 2018;10:26039-26045.
- [Google Scholar]
- Quantitative Proteomic Analysis to Understand the Mechanisms of Zinc Oxide Nanoparticle Toxicity to Daphnia pulex (Crustacea: Daphniidae): Comparing with Bulk Zinc Oxide and Zinc Salt. Environ. Sci. Technol.. 2019;53:5436-5444.
- [Google Scholar]
- Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem.. 2012;33:580-592.
- [Google Scholar]
- Biosynthesis and Antimicrobial Activity of Semiconductor Nanoparticles against Oral Pathogens. Bioinorg. Chem. Appl.. 2014;2014:1-10.
- [Google Scholar]
- Inulin based micelles loaded with curcumin or celecoxib with effective anti-angiogenic activity. Eur. J. Pharm. Sci.. 2016;93:141-146.
- [Google Scholar]
- An overview of anticancer chalcones with apoptosis inducing activity. J. Mazandaran Univ. Med. Sci.. 2017;26(146):254-268.
- [Google Scholar]
- Synthesis, cytotoxic activity and docking study of two indole-chalcone derivatives. J. Mazandaran Univ. Med. Sci.. 2017;27(154):12-25.
- [Google Scholar]
- Synthesis and Characterization of Nano-Sized Hexagonal and Spherical Nanoparticles of Zinc Oxide. J. Nanostruct.. 2012;2:295-300.
- [Google Scholar]
- Cationic vesicles for efficient shRNA transfection in the MCF-7 breast cancer cell line. Int. J. Nanomed.. 2018;13:7107-7121.
- [Google Scholar]
- Synthesis and Characterization of Mgo Nanoparticles by Neem Leaves through Green Method. Mater. Today. 2015;2:4360-4368.
- [Google Scholar]
- AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem.. 2009;30(16):2785-2791.
- [Google Scholar]
- Augmentation of the cytotoxic effects of zinc oxide nanoparticles byMTCP conjugation: Non-canonical apoptosis and autophagy induction inhuman adenocarcinoma breast cancer cell lines. Mater. Sci. Eng., C. 2017;78:949-959.
- [Google Scholar]
- Study of electronic and optical properties of ZnO clusters using TDDFT method. Mater. Res. Express. 2017;4:106304
- [Google Scholar]
- Beneficial effect of nanoparticles over standard form of zinc oxide in enhancing the anti-inflammatory activity of ketoprofen in rats. Pharmacol. Rep.. 2017;69:679-682.
- [Google Scholar]
- J. Am. Chem. Soc.. 1983;105:7037-7047.
- A DFT, AIM and NBO study of isoniazid drug delivery by MgO nanocage. Appl. Surf. Sci.. 2019;469:103-112.
- [Google Scholar]
- Simple UV Spectrophotometric Determination of Celecoxib in Pure Form and in Pharmaceutical Formulations. Int. J. Pharmaceut. Sci. Lett.. 2011;1(2):49-55.
- [Google Scholar]
- Dietary Inclusions and Exclusions: Preparation Against Cancer. Oncologie. 2020;22(4):213-224.
- [Google Scholar]
- Green synthesis, characterization and electrochemical sensing of silymarin by ZnO nanoparticles: Experimental and DFT studies. J. Electroanal. Chem.. 2018;808:160-172.
- [Google Scholar]
- “Promising antileishmanial activity of novel imidazole antifungal drug Luliconazole against Leishmania major: in vitro and in silico studies. J. Global Antimicrob. Resistance. 2018;14:260-265.
- [Google Scholar]
- The study of SCN- adsorption on B12N12 and B16N16 nano-cages. Superlattices Microstruct.. 2014;75:716-724.
- [Google Scholar]
- Serine adsorption through different functionalities on the B12N12 and Pt-B12N12 nanocages. Mater. Sci. Eng. C. 2018;92:216-227.
- [Google Scholar]
- Improvement of anti-inflammatory and anticancer activities of poly(lactic-co-glycolic acid)-sulfasalazine microparticle via density functional theory, molecular docking and ADMET analysis. Arabian J. Chem.. 2022;15:103464
- [Google Scholar]
- Density functional study on the adsorption of some aliphatic aldehydes on (ZnO)12 and M-doped (ZnO)12 nanocages. Polyhedron. 2015;102:503-513.
- [Google Scholar]
- J. Mol. Struct.. 2016;1121:16-25.
- A non-aqueous synthesis, characterization of zinc oxide nanoparticles and their interaction with DNA. Synth. Met.. 2009;159:2443-2452.
- [Google Scholar]
- Tumor detection using magnetosome nanoparticles functionalizedwith a newly screened EGFR/HER2 targeting peptide. Biomaterials. 2017;115:53-64.
- [Google Scholar]
- Inhibition of TNF-α and IL-1 by compounds from selected plants for rheumatoid arthritis therapy: In vivo and in silico studies. Trop. J. Pharm. Res.. 2018;17(2):277-285.
- [Google Scholar]
- A CRISPR-based and post-amplification coupled SARS-CoV-2 detection with a portable evanescent wave biosensor. Biosens. Bioelectron.. 2021;190:113418
- [Google Scholar]
- Zhang, L., Xu, Y., Liu, H., Li, Y., You, S., Zhao, J.,... Zhang, J., 2021. Effects of coexisting Na+, Mg2+ and Fe3+on nitrogen and phosphorus removal and sludge properties using A2O process. J. Water Process Eng., (44).
- A Novel Aluminum-Graphite Dual-Ion Battery. Adv. Energy Mater.. 2016;6(11):1502588.
- [Google Scholar]
- June). MusiCoder: A Universal Music-Acoustic Encoder Based on Transformer. In: International Conference on Multimedia Modeling. 2021. p. :417-429.
- [Google Scholar]
- Self-Assembled ZnO Nanoparticle Capsules for Carrying and Delivering Isotretinoin to Cancer Cells. ACS Appl. Mater. Interfaces. 2017;9(22):18474-18481.
- [Google Scholar]







