Translate this page into:
Improved skin delivery and validation of novel stability-indicating HPLC method for ketoprofen nanoemulsion
⁎Corresponding author at: Avenida Ipiranga, 2752/606, 90610-000 Porto Alegre, RS, Brazil. leticia.koester@ufrgs.br (Letícia Scherer Koester)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Ketoprofen is a non-steroidal anti-inflammatory drug (NSAID) widely used to treat rheumatoid arthritis and other inflammatory diseases. Normally used by oral route, this drug presents numerous side effects related to this administration route, such as nausea, dyspepsia, diarrhea, constipation and even renal complications. To avoid that, topical administration of ketoprofen represents a good alternative, since this drug has both partition coefficient and aqueous solubility suitable for skin application, compared to other NSAIDs. In this study, we describe the production of a nanoemulsion containing ketoprofen, its skin permeation and in vitro release study and a novel validation method to analyze this drug in the permeation samples and a forced degradation study using skin and nanoemulsion samples. The new HPLC method was validated, with all specifications in accordance with validation parameters and with an easy chromatographic condition. Forced degradation study revealed that ketoprofen is sensitive to acid and basic hydrolysis, developing degradation peaks after exposure to these factors. Concerning in vitro release from the nanoemulsion, release curves presented first order profile and were not similar to each other. After 8 h, 85% of ketoprofen was release from the nanoemulsion matrix while 49% was release from control group. In skin permeation study, nanoemulsion enabled ketoprofen to pass through the skin and enhanced retention in the epidermis and stratum corneum, layer on which the formulation presented statistically different values compared to the control group.
Keywords
Validation
Forced degradation
Nanoemulsion
1 Introduction
Ketoprofen is a non-steroidal anti-inflammatory drug (NSAID) widely used to treat rheumatoid arthritis and other inflammatory diseases (Jamali and Brocks, 1990; Kantor, 1986). Normally used by oral route, this drug presents numerous side effects related to this administration route, such as nausea, dyspepsia, diarrhea, constipation and even renal complications (De Jalón et al., 2000; Rang et al., 2008). To avoid that, topical administration of ketoprofen represents a good alternative, since this drug has both partition coefficient and aqueous solubility suitable for skin application, compared to other NSAIDs (Rhee et al., 2001).
A nanoemulsion consist in a dispersion of oil in an aqueous media stabilized by surfactants, with submicron droplet size (Roberts et al., 2017). Recently, this carrier appeared as an alternative for topical delivery of lipophilic drugs, since it presents several advantages such as increase intrinsic solubility, rate of dissolution and permeation in biological membranes (Fathi et al., 2012). In addition, nanoemulsions can facilitate skin penetration possibly due to solubilization or extraction of stratum corneum lipids (Roberts et al., 2017). Regarding ketoprofen, different submicrometer systems have been described for topical application of this drug, such as liposome, nanoemulsion, polymeric nanoparticle and nanostructured lipid carrier (Arora et al., 2014; Cirri et al., 2012; Kheradmandnia et al., 2010; Shah et al., 2012; Uchino et al., 2014).
In this paper, we describe the formulation of a nanoemulsion containing ketoprofen, the validation of a method to detect this drug in skin samples, a forced degradation study with ketoprofen in skin and nanoemulsion matrix, in vitro release studies in synthetic membrane and skin permeation studies in porcine ear skin.
2 Materials and methods
2.1 Chemicals and reagents
Ketoprofen standard was purchased from Sigma-Aldrich (Saint Louis, Missouri, United States of America). Medium Chain Triglycerides (MCT) was kindly donated by Lipoid GmbH (Ludwigshaten, Germany). HPLC gradient grade methanol was purchased from Merck (Darmstadt, Germany). HPLC gradient grade trifluoroacetic acid was purchased from Sigma-Aldrich (Saint Louis, Missouri, United States of America). Ultrapure water was obtained from a Milli-Q® apparatus (Millipore, Billerica, United States of America). All other chemicals or reagents were of analytical grade.
2.2 HPLC method validation
2.2.1 Instrumentation and chromatographic conditions
All samples were analyzed by a high performance liquid chromatograph (HPLC, Shimadzu, Japan). Chromatographic conditions consisted in a Perkin C18 column (150 × 4.6 mm, 5 µm), a mobile phase of methanol and trifluoroacetic acid 0.1% (60:40) eluted at 1 mL/min, an oven set at 30 °C and a UV detector set at 254 nm. The mobile phase was filtered under vacuum in a 0.45 µm filter and degassed under sonication for 30 min. The injection volume was 20 µL. Retention time for ketoprofen was 8.2 min.
2.2.2 Specificity
Specificity was evaluated by comparing the chromatograph runs of a ketoprofen standard with blank samples from skin extract, tapes from the tape stripping method after 30 min sonication with methanol, receptor fluid and blank nanoemulsion.
2.2.3 Linearity and limits of quantification and detection
Linearity was assessed by analyzing seven ketoprofen concentrations between 0.5 and 10 µg/mL with or without the skin extract presence. Limits of quantification (LOQ) and detection (LOD) were calculated by the standard deviation divided by the inclination of the curves obtained in the linearity test.
2.2.4 Precision
Precision was assessed by analyzing a medium ketoprofen concentration six times in 3 different days with or without the skin extract presence according to its relative standard deviation (RSD) intra-day and inter-day.
2.2.5 Accuracy
Accuracy was assessed by analyzing three different ketoprofen concentration (low, medium and high) six times with or without the skin extract presence according to its relative standard error (RSE) and recuperation percentage.
2.2.6 Temperature stability
Stability was assessed by analyzing two ketoprofen concentrations (low and high) containing the skin extract under ambient temperature and under three freezing cycles within 24 h according to its recuperation percentage.
2.3 Forced degradation
Forced degradation was assessed by analyzing ketoprofen concentration under: (a) UV radiation, (b) oxidation, (c) acid hydrolysis and (d) basic hydrolysis. Ketoprofen stock solution was analyzed with the presence of blank nanoemulsion and skin extract.
2.3.1 UV radiation
Ketoprofen stock solution (50 µg/mL) was placed in a UVC chamber at 254 nm during 24 h. After 24 h, the solution was diluted to 5 µg/mL and analyzed by the previously described method.
2.3.2 Heat exposure
Ketoprofen stock solution (50 µg/mL) in ambar glass was submitted to a 90 ± 1 °C temperature during 24 h. After 24 h, the solution was diluted to 5 µg/mL and analyzed by the previously described method.
2.3.3 Oxidation
Ketoprofen stock solution (50 µg/mL) was mixed with hydrogen peroxide (H2O2) in two concentrations (29.0% and 2.9%) at a 1:1proportion. After 2 and 24 h, samples were collected and diluted to 5 µg/mL and analyzed by the previously described method.
2.3.4 Acid hydrolysis
Ketoprofen stock solution (50 µg/mL) was mixed with hydrochloric acid (HCl) 2 M at a 1:1 proportion. Samples were analyzed after 2 and 24 h.
2.3.5 Basic hydrolysis
Ketoprofen stock solution (50 µg/mL) was mixed with sodium hydroxide (NaOH) 2 M at a 1:1 proportion. Samples were analyzed after 2 and 24 h.
2.4 Ketoprofen nanoemulsion production and characterization
The oily phase was prepared with ketoprofen (1 mg/mL), medium chain triglycerides (MCT, 10% w/w) and Span 80® (3% w/w). The aqueous phase was prepared with Tween 20® (1% w/w) and water (adjusted to 10 mL). Ketoprofen was solubilized in MCT and Span 80® under magnetic stirring for 24 h at ambient temperature. After, the aqueous phase was added to the oily phase under magnetic stirring to form a coarse emulsion. In order to gradually decrease the droplet size, this coarse emulsion was subjected to high-pressure homogenization (EmulsiFlex-C3®, Avestin, Canada) at 750 bar for 6 cycles, producing the nanoemulsion.
After preparation, the nanoemulsion was characterized for droplet size, polydispersity index, zeta potential and ketoprofen content. Droplet size and polydispersity index were measured in triplicate by dynamic light scattering after dilution of 10 μL of the nanoemulsion in 10 mL of purified water (Zetasizer Nanoseries ZN90, Malvern Instruments, Worcestershire, UK). Zeta potential was measured in triplicate by laser Doppler velocimetry using the same instrument, after dilution of 10 μL of the nanoemulsion in 10 mL in NaCl (1 mM) ultra-filtered in 0.45 μM filter. Ketoprofen content was determined in HPLC as described in Section 2.2.1 diluting the nanoemulsion in methanol to form a 10 µg/mL solution.
2.5 Skin permeation/retention studies
Permeation/retention studies were performed using freshly excised porcine ear skin (n = 6) in Franz-type diffusion cell equipment. Porcine ear was obtained from a local slaughterhouse. Skin was excised from the outer part of the pig’s ear using a scalpel. Hair and fat excess from the extracted skin were removed with scissors and thickness was measured with a thickness gauge (Mitutoyo Corporation, Japan). Only skin cuts in the range of 0.90 and 1.10 mm were used in the experiments (OCDE 428, 2004).
Receptor fluid was a mixture of ethanol and phosphate buffered saline (PBS) pH 7.4 (50:50). A volume of 500 µL of the nanoemulsion was placed on top of the skin. Control group consisted only in ketoprofen solubilized in MCT (500 µL) in the same concentration as the nanoemulsion.
After 8 h, an aliquot of receptor fluid was collected and the skin was cleaned with Milli-Q water. Stratum corneum was collected with the tape stripping method. Epidermis and dermis were separated using a scalpel. Skin layers were placed in a test-tube with 2 mL of methanol and sonicated for 30 min in ambient temperature. After, all samples were filtered in a 0.45 filter to a HPLC vial and analyzed as described in Section 2.2.1.
2.6 In vitro release study
Release study (n = 6) was performed with a cellulose membrane (Merck Millipore, Germany) with 25 mm diameter and 0.05 µm pore size in a Franz diffusion cell apparatus, with temperature set at 32 °C. Receptor fluid consisted in a mixture of ethanol and phosphate buffered saline (PBS) pH 7.4 (50:50). A volume of 500 µL of nanoemulsion was placed on top of the membrane. During 8 h an aliquot of 1 mL was taken from the receptor fluid each hour, filtered in a 0.45 µm filter and was analyzed in HPLC as described in item 2.2.1. Control group consisted only in ketoprofen solubilized in MCT (500 µL) in the same concentration as the nanoemulsion. Release profile from both curves were evaluated by mathematical models (zero order, first order and Higuchi) and compared by values of factors of difference (ƒ1) and similarity (ƒ2).
2.7 Statistical analysis
Statistics were carried out with ANOVA test to verify the difference between the samples in the permeation/retention and liberation studies. P values under 0.05 (*p < 0.05) were considered statistically significant.
3 Results
3.1 Method validation
Method validation consisted in tests of specificity, linearity, precision, accuracy and temperature stability. Specificity compared chromatograms from blank samples of skin extract, tapes from tap-stripping method, receptor fluid and nanoemulsion to a ketoprofen solution. As can be seen in Fig. 1, blank samples did not present interferers in the same retention time as ketoprofen (8.2 min), demonstrating that the method is specific for ketoprofen samples from permeation study and from a nanoemulsion matrix.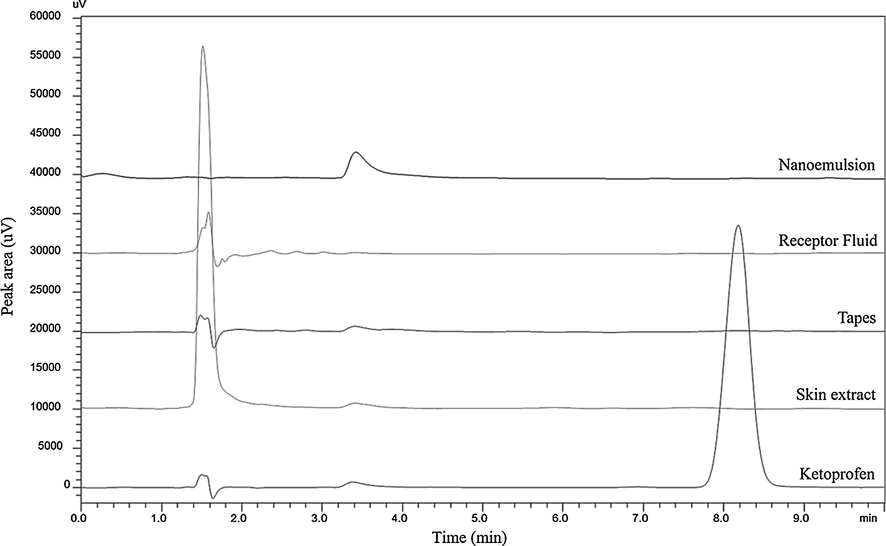
Chromatograms comparing blank samples from skin extract, tapes from tap-stripping method, receptor fluid and nanoemulsion to a ketoprofen solution.
Linearity was evaluated with and without skin permeation samples containing ketoprofen solutions in the range of 0.5–10 µg/mL. All solutions contained also samples from a blank nanoemulsion. Linearity curves showed good correlation coefficient for both curves (r = 0.999), highly significant to the method (p < 0.05). Limits of quantification and detection for the curve with skin extract were 0.34 and 0.10 µg/mL respectively. For the curve without skin extract, limits of quantification and detection were 0.14 and 0.04 µg/mL, respectively. Low values for LOQ and LOD indicate that the method is sensitive for its purpose.
Precision was assessed by analyzing the same ketoprofen concentration (5 µg/mL) six times in three different days and with or without skin extract. Values of relative standard deviation (RSD) were under 15%, which is considered satisfactory for bioanalytical methods (FDA, 1997; Tiwari and Tiwari, 2010). Accuracy was assessed by analyzing a high, a low and an intermediate ketoprofen concentration (4, 5 and 6 µg/mL). Results are show in Table 1. The method presented good values of RSE and high recovery percentage, indicating that it was accurate.
4 µg/mL
5 µg/mL
6 µg/mL
Without skin extract
RSE (%)
11.99
10.98
7.011
Recovery (%)
111.99 ± 0.05
110.98 ± 0.04
107.01 ± 0.20
With skin extract
RSE (%)
0.89
4.28
1.28
Recovery (%)
99.12 ± 0.30
104.28 ± 0.31
101.28 ± 0.11
Temperature stability was tested with 0.5 and 10 µg/mL ketoprofen concentrations. Solutions stayed in ambient temperature and under three freezing and thawing cycles during 24 h. Table 2 shows the results as ketoprofen recovery from these solutions. All recovery values are adequate to a bioanalytical method (90–110%) and showed that ketoprofen samples can stay under similar conditions to sample that stays in the HPLC equipment or that is frozen to be analyzed later.
Ketoprofen solution concentration
0.5 µg/mL
10 µg/mL
Ambient temperature
103.86 ± 0.01
97.13 ± 0.21
Freezing and thawing cycles
107.76 ± 0.05
98.73 ± 0.01
3.2 Forced degradation
Forced degradation studies are important in a new stability-indicating method since they can evidence problems in the method and the formulation development (Blessy et al., 2014). In this paper, we describe ketoprofen degradation under five conditions: UV radiation, heat exposure, oxidation, acid hydrolysis and basic hydrolysis. Table 3 describes the percentage of recovered ketoprofen from samples after degradation step. Samples from stock solution of ketoprofen were mixed with a sample of blank nanoemulsion and skin extract, in order to simulate conditions from the validation method. SS: stock solution; NE: nanoemulsion; SE: skin extract; ND: not determined; TD: total degradation; aOne peak from degradation product; bKetoprofen peak displacement.
% Recovered Ketoprofen
2 h
24 h
SS
SS + NE
SS + NE + SE
SS
SS + NE
SS + NE + SE
UV Radiation
94.59 ± 0.24b
94.08 ± 0.29b
77.71 ± 0.34b
83.46 ± 0.09b
44.51 ± 0.68ab
49.64 ± 0.11ab
Heat Exposure
ND
ND
ND
98.00 ± 0.29b
96.87 ± 0.04b
89.14 ± 0.07b
Oxidation 2.9%
104.31 ± 0.35
98.94 ± 0.05
93.48 ± 0.07
118.69 ± 0.35
100.95 ± 0.27
94.76 ± 0.12
Oxidation 29.0%
99.76 ± 0.14
100.95 ± 0.27
94.76 ± 0.12
105.75 ± 0.01
102.78 ± 0.18
94.34 ± 0.00
Acid Hydrolysis
101.42 ± 0.23
98.99 ± 0.12
95.02 ± 0.13
TD
TD
TD
Basic Hydrolysis
68.29 ± 0.10ab
67.72 ± 0.10ab
65.08 ± 0.06ab
89.77 ± 0.10b
87.46 ± 0.11b
84.65 ± 0.08b
3.3 Skin permeation study
Fig. 2 presents skin permeation results for ketoprofen nanoemulsion and ketoprofen control (ketoprofen dispersed in MCT). A can be seen, there is no statistical difference between formulation concerning dermis retention. However, in the epidermis layer, ketoprofen nanoemulsion presented a significant increase in the retention profile (p < 0.05). Concerning the stratum corneum retention, obtained by the tape-stripping method, the nanoemulsion presented significant increase (p < 0.05) in the ketoprofen retention compared to the control (2.81 ± 0.74 µg/mL and 1.50 ± 0.66 µg/mL, respectively). Both formulations presented similar permeation to the receptor fluid (0.58 ± 0.28 µg/ml for ketoprofen nanoemulsion and 0.79 ± 0.65 µg/mL for ketoprofen control).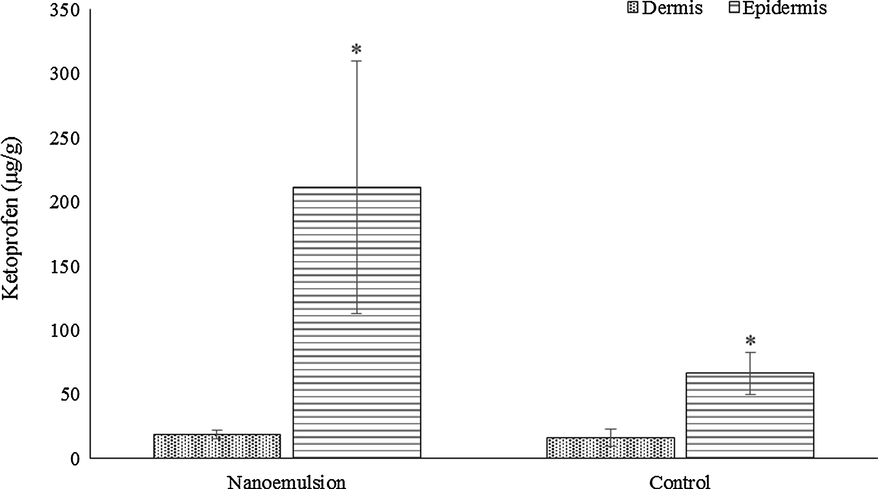
Permeation study results. * Statistically different (p < 0.05).
3.4 In vitro release study
After 8 h, 85% of ketoprofen was release from the nanoemulsion matrix, statistically different from the control group (containing only the nanoemulsion oil core, MCT), which released only 49% of ketoprofen (Fig. 3).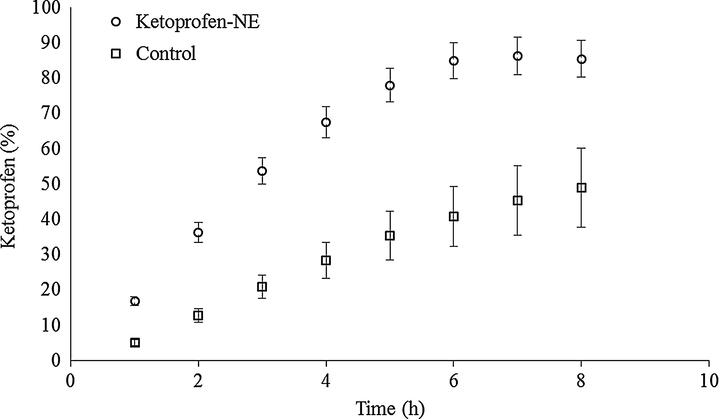
In vitro release of ketoprofen from nanoemulsion formulation and control containing only MTC.
4 Discussion
In this study, a new HPLC method was validated to quantify ketoprofen. Results show that the method was specific, linear, precise, accurate and able to detect the drug after temperature exposure.
Concerning forced degradation, as can be seen in Table 3, in 2 h only under basic hydrolysis ketoprofen presented a decrease in recovery. In addition, in this condition the chromatogram showed the presence of another peak (for conditions with nanoemulsion and skin extract) and displacement for ketoprofen peak (Fig. 4b). Concerning acid hydrolysis, after 24 h there is a total degradation in ketoprofen peak (Table 3) and the appearance of new peaks in the chromatogram (Fig. 4a). At 4.1 min there is a high intensity degradation peak, with can represent the ketoprofen degradation product to 3-(2-carboxyphenyl) propionic acid (Fig. 5a). The second and smaller degradation peak can represent the degradation product 3-acetylbenzophenone (Fig. 5b), which is also a impurity from ketoprofen (Dvořák et al., 2004).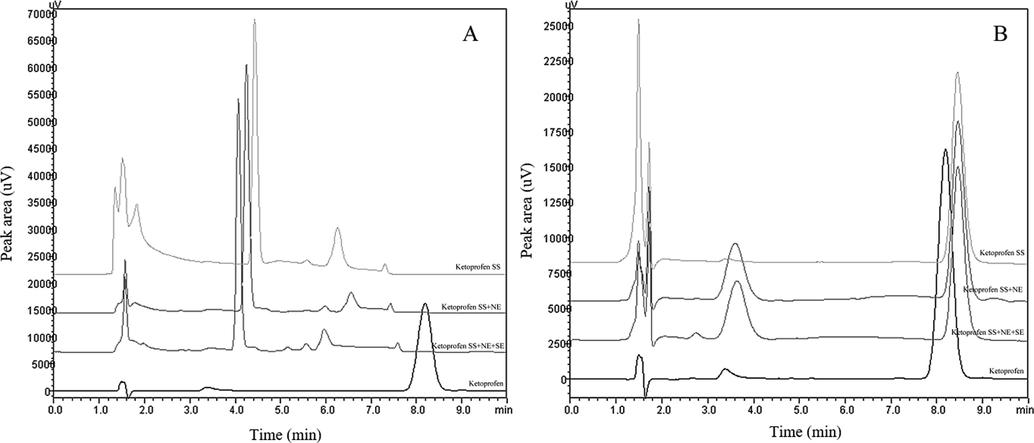
Chromatograms from (A) Acid hydrolysis from ketoprofen samples and degradation peaks after 24 h and (B) Basic hydrolysis from ketoprofen samples and degradation peaks after 2 h. SS: stock solution; NE: nanoemulsion; SE: skin extract.
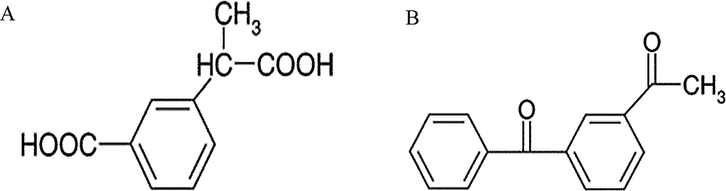
Ketoprofen degradation products (A) 3-(2-carboxyphenyl) propionic acid and (B) 3-acetylbenzophenone.
It is well known that lipophilic drugs, such as ketoprofen, have trouble passing through the skin lipid layer (specially the stratum corneum), and, as shown by skin permeation results, it is possible to see that the nanoemulsion facilitates this passage process, accumulating the drug in the epidermis (Haroutiunian et al., 2010). According to Uchino et al., surfactant-based nanoparticles can enhance the passage through the skin by partitioning the vesicle in the stratum corneum and, by consequence, releasing the drug to the epidermis layer, which can also explain the high amount of ketoprofen in the epidermis when nanoemulsion was applied to the skin (Uchino et al., 2014). The relative error obtained in this assay was a bit large due to inter-animal variability, since in the conduct of the study samples of skins from different animals were used. The other variables in relation to the biological matrix were controlled, such as thickness and use of freshly excised skin.
Therefore, to verify the drug release kinetics and the improvement in the release when it is associated with nanoemulsion, in vitro release studies were performed. Release kinetics, from in vitro release study, were calculated using three mathematical models: zero order, first order and Higuchi. Release curves were also compared by means of factors of difference (ƒ1) and similarity (ƒ2). In our findings, the model that best fitted the release profile is the first order model with correlation coefficients of 0.9636 for the nanoemulsion and 0.9961 for the control. First order kinetics is concentration dependent, which means that the concentration released from the matrix is proportional to the concentration that remains in it (Costa and Sousa Lobo, 2001). Factors ƒ1 and ƒ2 presented values of 24.93 and 18.72 respectively, which indicate that curves are not equivalent to each other.
The data of in vitro skin permeation and release studies should be analyzed together. In our study it was found that both ketoprofen nanoemulsion and the control group showed release model that is best described by a first order kinetics. However, the release curves were not equivalent to each other, and at the last analyzed time (8 h) it was found that ketoprofen nanoemulsion presented a release of approximately 85% and the control group of 49% (Fig. 3). Therefore, it is suggested that the developed nanoemulsioned system has improved permeation due to the reduced droplet size associated with effective drug release.
5 Conclusion
Therefore, in this study a new HPLC method was validated, with all specifications in accordance with validation parameters and with an easy chromatographic condition. Forced degradation study revealed that ketoprofen is sensitive to acid and basic hydrolysis, developing degradation peaks after exposure to these factors. Concerning in vitro release from the nanoemulsion, release curves presented first order profile and were not similar to each other. In skin permeation study, nanoemulsion enabled ketoprofen to pass through the skin and enhanced retention in the epidermis and stratum corneum, presenting a good alternative to topical formulation to this drug.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil).
Acknowledgements
The authors thank the scholarships from CAPES and CNPq/Brasil.
Compliance with Ethical standards
Conflict of interest: The authors declare that they have no conflict interest.
References
- Nanoemulsion based hydrogel for enhanced transdermal delivery of ketoprofen. Adv. Pharm.. 2014;2014:1-12.
- [CrossRef] [Google Scholar]
- Development of forced degradation and stability indicating studies of drugs – a review. J. Pharm. Anal.. 2014;4:159-165.
- [CrossRef] [Google Scholar]
- Development of a new delivery system consisting in “drug – in cyclodextrin – in nanostructured lipid carriers” for ketoprofen topical delivery. Eur. J. Pharm. Biopharm.. 2012;80:46-53.
- [CrossRef] [Google Scholar]
- Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci.. 2001;13:123-133.
- [CrossRef] [Google Scholar]
- Determination by high-performance liquid chromatography of ketoprofen in vitro in rat skin permeation samples. J. Chromatogr. A. 2000;870:143-149.
- [CrossRef] [Google Scholar]
- Simultaneous HPLC determination of ketoprofen and its degradation products in the presence of preservatives in pharmaceuticals. J. Pharm. Biomed. Anal.. 2004;36:625-629.
- [CrossRef] [Google Scholar]
- Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol.. 2012;23:13-27.
- [CrossRef] [Google Scholar]
- FDA, 1997. Guidance for Industry: nonsterile semisolid dosage forms.
- Topical NSAID therapy for musculoskeletal pain. Pain Med. (United States). 2010;11:535-549.
- [Google Scholar]
- Clinical pharmacokinetics of ketoprofen and its enantiomers. Clin. Pharmacokinet.. 1990;19:197-217.
- [CrossRef] [Google Scholar]
- Ketoprofen: a review of its pharmacologic and clinical properties. Pharmacother. J. Hum. Pharmacol. Drug Ther.. 1986;6:93-102.
- [CrossRef] [Google Scholar]
- Preparation and characterization of ketoprofen-loaded solid lipid nanoparticles made from beeswax and carnauba wax. Nanomed. Nanotechnol., Biol. Med.. 2010;6:753-759.
- [CrossRef] [Google Scholar]
- OCDE 428, 2004. OECD - Guideline For The Testing of Chemicals: Skin Absorption: in vitro Method. Test 1–8. doi: 10.1787/9789264071087-en.
- Rang and Dale’s Pharmacology (sixth ed.). London: Elsevier; 2008.
- Transdermal delivery of ketoprofen using microemulsions. Int. J. Pharm.. 2001;228:161-170.
- [CrossRef] [Google Scholar]
- Topical and cutaneous delivery using nanosystems. J. Control. Release. 2017;247:86-105.
- [CrossRef] [Google Scholar]
- Effect of oleic acid modified polymeric bilayered nanoparticles on percutaneous delivery of spantide II and ketoprofen. J. Control. Release. 2012;158:336-345.
- [CrossRef] [Google Scholar]
- Bioanalytical method validation: an updated review. Pharm. Methods. 2010;1:25.
- [CrossRef] [Google Scholar]
- Characterization and skin permeation of ketoprofen-loaded vesicular systems. Eur. J. Pharm. Biopharm.. 2014;86:156-166.
- [CrossRef] [Google Scholar]







