Translate this page into:
In silico and in vivo study of adulticidal activity from Ayapana triplinervis essential oils nano-emulsion against Aedes aegypti
⁎Corresponding author. alexrodrigues.quim@gmail.com (Alex Bruno Lobato Rodrigues) alex.rodrigues@unifap.br (Alex Bruno Lobato Rodrigues)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Nanoinsecticides of plant origin have advantages in the resistance of Aedes aegypti, vectors of infectious diseases. The objective of this study was to evaluate the insecticidal potential of Ayapana triplinervis essential oil nano-emulsions using in silico and in vivo assays in an Aedes aegypti model. Molecular docking showed that minority compounds present in the morphotype A essential oil have a more significant binding affinity to inhibit acetylcholinesterase and juvenile hormone receptors. Aedes aegypti adults were susceptible to A. triplinervis at 150 µg.mL-1 in a diagnostic time of 15 min for morphotype A essential oil nano-emulsion and 45 min for morphotype B essential oil nano-emulsion. The evaluation of toxicity in Swiss albino mice indicated that the nano-emulsions had low acute dermal toxicity and presented LD50 greater than 2000 mg.Kg−1. Thus, it is possible to conclude that nano-emulsions have the potential to be used in the chemical control of A. aegypti.
Keywords
Nanoinsecticide
Molecular docking
Natural products
Nanobiotechnology
1 Introduction
The mosquito Aedes aegypti is the vector of arboviruses such as dengue (DENGV), zika (ZICV), and chikungunya (CHIKV) and represents a major public health problem. The culicide can infect with a single virus type or with double and even triple co-infections of DENGV, ZICV, and CHIKV. There are no vaccines capable of controlling A. aegypti transmitted diseases, so vector control with synthetic insecticides is a key strategy for the management of related epidemics (Rückert et al., 2017).
However, a genetic mutation was detected in the region that decodes the sodium and potassium channel in resistant A. aegypti strains from Brazil. This is done by replacing a valine with leucine and phenylalanine with a cysteine, reducing the mosquitoes' susceptibility to pyrethroid, an important class of insecticides recommended by the World Health Organization (WHO) for chemical vector control (Haddi et al., 2017). In this context, plants have become an alternative in the search for bioactive substances with diverse chemical structures and new mechanisms of action for the control of A. aegypti (Brandão et al., 2021; Chaves et al., 2020).
The Asteraceae family has drawn attention to their ethnobotanical and ethnopharmacological employment in traditional communities. However, the phylogenetic, taxonomic, and chemosystematic information is incipient, reflecting the presence of the little-known genus (Gustafsson and Bremer, 1995).
Among the species of medicinal interest in the Asteraceae family, the Ayapana triplinervis (VAHL) R. M. King & H. Rob can be highlighted. This species is found in Brazil, Ecuador, Peru, Puerto Rico, and Guianas, besides having adapted in other countries such as India and Vietnam (Gauvin-Bialecki and Marodon, 2008). A. triplinervis can be found in two types: morphotype A, popularly called “japana-branca”, and morphotype B, which is known as “japana-roxa”(Nery et al., 2014).
Phytochemically, the methanolic extract of A. triplinervis leaves has a predominance of hexadecanoic acid, tetradecacetic acid, and octadecanoic acid, with proven hypocholestatic, antioxidant, antiproliferative, and anticancer bioactivity (Selvamangai and Bhaskar, 2012). In another study, the methanolic extract showed the inhibition of 59% of melanomas using low cytotoxicity (Arung et al., 2012). The hydroalcoholic extract confirmed the anxiolytic, sedative, and behavioral antidepressant effects in the central nervous system in several animal models (Melo et al., 2013).
The crude ethanolic, methanolic, and petroleum ether extract had antimicrobial activity against different bacteria such as S. epidermis, E. coli, S. typhi, V. parahaemolytcus, E. faecalis, S. aureus, S. epidermidis, M. leuteus, P. aeruginosa, V. cholera, and against fungi A. niger, A. flavus, A. solani and F. solani in different concentrations.
Despite their significant potential for chemical control of vectors of infectious diseases, the essential oils of A. triplinervis morphotypes are susceptible to volatility and oxidation using oxygen and light, affecting the biological activity of their constituents. One way to enable protection against degradation and oxidation of essential oils is through the development of a technological nanoemulsification system, which offers advantages such as ease of handling, stability, protection against oxidation, better distribution, and controlled release (Wu et al., 2015).
Some studies using nano-emulsions as biocides have received prominence in the scientific literature, either by decreasing the lethal concentration of the product or by enabling a delivery and bioavailability mechanism in a polar environment. Nano-emulsions containing essential oils of Ageratum conyzoides, Achillea fragantissima, and Tagetes minuta and had a particle size less than or equal to 136 nm with high insecticidal activity against Callosobruchus maculatus with LC50 < 40.5 µl.L-1 (Nenaah et al., 2015). Nano-emulsions of Cymbopogon citratus essential oil with a hydrodynamic diameter equal to 11 nm and high biocide activity in E. coli (Guerra-Rosas et al., 2016). Thyme nano-emulsion with a particle diameter of 150 nm showed MIC 200 µg.mL−1 in the control of the yeast Zygosaccharomyces bailii, demonstrating the biocide potential of nanostructured systems (Chang et al., 2015).
The potential for inhibition of secondary metabolites of A. triplinervis in receptors of the enzyme acetylcholinesterase and receptors of the juvenile hormone was evaluated. Acetylcholinesterase is an essential enzyme in the insect's nervous system responsible for the hydrolysis of the neurotransmitter acetylcholine, and the juvenile hormone plays a crucial role in the development and reproduction of insects. AChE is the main compound in which nerve impulses are transmitted between cells through cholinergic synapses and its inhibition by essential oils causes super stimulation of neurons, muscle spasms, convulsion, paralysis, and death in the insects (López and Pascual-Villalobos, 2010). Secondary metabolites can influence the hormonal regulation of A. aegypti and cause sublethal effects at the physiological, morphological, and behavioural levels that compromise the insect's development cycle, such as interruption of reproduction and a decrease in longevity (Alomar et al., 2021).
In our previous studies, we reported the larvicidal activity of nanoformulations containing the essential oil of A. triplinervis morphotypes in A. aegypti, which showed low toxicity in non-target mammals (Rodrigues et al., 2021). In this study, we hypothesized that A. triplinervis essential oil nano-emulsion can be used to control A. aegypti in the adult stage. This study aims to evaluate the potential adulticide in A. aegypti through molecular docking of essential oils and biological tests of the nano-emulsions from A. triplinervis morphotypes, and acute dermal toxicity in Swiss albino mice (Mus musculus) of the nanoformulations.
2 Materials and methods
2.1 Molecular docking simulations of essential oils from A. triplinervis morphotypes
2.1.1 Selection of enzymes and inhibiting structures
The crystallographic structure of Drosophila Melanogaster acetylcholinesterase (AChE) complexed with 9- (3-iodobenzylamino) −1,2,3,4-tetrahydroacridine (I40) and Aedes aegypti juvenile hormone complexed with methyl (2E, 6E) − 9 – [(2R) −3,3-dimethyloxiran-2-yl] −3,7-dimethylnona-2,6-dienoate (JH3) were downloaded from the Protein Data Bank (PDB) with the respective codes PDB 1QON (resolution 2.7 Å) and PDB 5 V13 (1.87 Å resolution). I40, JHIII, and pyriproxyfen were used as positive control ligands in molecular docking studies based on the standard protocol established by Ramos and co-workers (Ramos et al., 2020).
There is no crystallographic structure of A. aegypti AChE deposited in the Protein Data Bank. In this study, D. melanogaster AChE was used, which has a 39% similarity with A. aegypti AChE (Ramos et al., 2020).
2.1.2 Docking study with AutoDock 4.2/Vina 1.1.2 via graphical interface PyRx (Version 0.8.30)
The ligands and protein structures were prepared using the Discovery Studio 5.0 software. In the docking study of AChE (D. melanogaster) and Juvenile Hormone, the complexed ligands were used in the AutoDock 4.2/Vina 1.1.2 software, and then in PyRx 0.8.30 (https://pyrx.sourceforge.io). The validation of the molecular docking of the ligand was performed by comparison between the crystallographic ligand and the best conformation obtained with molecular docking (structure of the PDB ID: 1QON), based on the RMSD value. The ×, y, and z coordinates of the receivers are determined according to the average region of the active site, and the coordinates used for the center of the grid can be seen in Table 1.
Enzyme
Inhibitor
Coordinates of the Grid Center
Grid Size
(Points)
AChE (PDB code: 1QON)
9-(3-Iodobenzylamino)-1,2,3,4-Tetrahydroacridine
X = 34.279
Y = 67.55
Z = 9.2842 x
26 y
16 z
Juvenile hormone (PDB code: 5 V13)
methyl (2E,6E)-9-[(2R)-3,3-dimethyloxiran-2-yl]-3,7-dimethylnona-2,6-dienoate
X = 213.483
Y = 2.850
Z = 353.22242 x
40 y
24 z
2.2 Evaluation of adulticidal activity of A. triplinervis nano-emulsions against A. aegypti
The susceptibility of A. aegypti adults was assessed using the Centers for Disease Control and Prevention (CDC) bottle bioassay (Brogdon and Chan, 2013) to determine the dose of insecticide that kills 100% of susceptible mosquitoes (diagnostic dose) within a specific time (diagnostic time).
Nano-emulsions were diluted in acetones in concentrations of 150, 100, and 50 µg.mL−1, and 1 mL of each solution was applied inside Wheaton bottles (250 mL). Nano-emulsion produced only with the blend of surfactants was used as a negative control and malathion at 50 μg.mL−1 was used as a positive control. Solutions were distributed in all internal areas of the bottles through rotation movements in different directions. The bottles were opened at room temperature to evaporate the acetone.
Twenty-five adult females of A. aegypti were gently inserted into each Wheaton with an aspirator, and mortality was assessed every 15 min for a period of 180 min. Mosquitoes that did not stand or slide along the curvature of the bottle were considered dead.
The bioassay was performed in quadruplicate and the data was validated according to the mortality percentage of the negative control. When the mortality of the negative control was greater than 10% the tests were discarded. In the case of the tests in which the mortality of the control was between 3% and 10%, the mortality was corrected by Abbott’s formula (Consoli and Oliveira, 1994).
2.3 Evaluation of acute toxicity of A. triplinervis essential oils Nano-emulsions
This study was approved by the Research Ethics Committee of Universidade Federal do Amapá (CEP – Unifap – 004/2019). All procedures were performed by international animal care protocols and national animal testing regulations. The experiments used male and female, 12-week-old Swiss albino mice (Mus musculus), provided by the Multidisciplinary Center for Biological Research in the Area of Science in Laboratory Animals (CEMIB).
The animals were kept in polyethylene cages at a controlled temperature (22 °C) over a 12 h of light or dark cycle and had free access to food and water, on experiment day when they had private access to food 3 h before, with restitution 1 h after nano-emulsion or negative control treatment.
Acute oral toxicity of the nano-emulsions was performed with 3 experimental groups (morphotype A essential oil nano-emulsion (MAEON), morphotype B essential oil nano-emulsion (MBEON), and negative control), each group with 6 animals (3 males and 3 females).
The test was performed according to Guideline 402 of the Organization for Economic Co-operation and Development (OECD, 2017) for acute toxic dose class testing. On the day before administration of the nano-emulsion, all the fur was removed from the dorsal (at least 10% of the total body surface area). Nanoformulations or negative control were diluted in water at 2,000 mg.kg−1 (limit of 1 mL of the solution) and applied uniformly over the exposed area of the dorsal.
Swiss albino mice (Mus musculus) were observed at 30, 60, 120, 240, and 360 min after application of the nano-emulsion and daily for 14 days. Behavioural changes were assessed and recorded: in skin and fur, eyes and mucous membranes, general activity, vocal frenzy, irritability, touch response, response to tail stimulus, contortion, posterior train position, straightening reflex, body tone, grip strength, ataxia, auricular reflex, corneal reflex, tremors, convulsions, anaesthesia, lacrimation, ptosis, urination, defecation, piloerection, hypothermia, breathing, cyanosis, hyperaemia, and death. Water consumption (mL) and food intake (g) were evaluated daily, and body weight every 3 days.
Animals were sacrificed on the fourteenth day in a CO2 chamber, obeying the ethical principles of animal experimentation. Skin, liver, lung, and kidney were removed for macroscopic and histopathological analysis.
2.4 Histopathological analysis
Organs were fixed in a 10% formalin buffer solution, routinely processed, and embedded in paraffin. Paraffin sections (5 µm) were stained with haematoxylin and eosin. The slides were examined under a light microscope and the magnified images of the tissue structure were captured for further study (Calleja et al., 2013).
2.5 In silico pharmacokinetic and toxicological properties of essential oils from A. triplinervis morphotypes
The molecules of A. triplinervis essential oils were submitted to the QikProp software to obtain pharmacokinetic properties. The parameters generated by the software allowed us to select the candidate having a parameter of 95% of approximation with pharmacokinetic characteristics of drugs already described in the literature, giving reliability to the generated data. The toxicity profile of the molecules was performed using DEREK software. DEREK is in silico toxicological prediction software used for drug design purposes (Ramos et al., 2020).
2.6 Toxicity risk assessment
ProTox, a virtual lab for the prediction of toxicities of small molecules as well as a useful tool to identify any undesirable toxic properties of our molecules, was used (Costa et al., 2019) (see https://tox.charite.de/protox_II/). The prediction was based on functional group similarity for the query molecules with the in vitro and in vivo contained in the database. Toxic properties such as LD50 values in mg/kg and toxicity class were determined.
2.7 Prediction of metabolites in metatox
In MetaTox application, the prediction of substrate modification is based on the rules of transformation. The predictions of metabolite formation were integrated into the MetaTox web service to evaluate the generation and modification of xenobiotic metabolism pathways (https://www.way2drug.com/mg). The LD50 values of acute rat toxicity are calculated for a parent compound and each of the generated metabolites, considering Pa > Pi as an exclusion criterion (Rudik et al., 2017).
2.8 Statistical analysis
The data were organized into mean and standard deviations and analysed using a one‐way Analysis of Variance followed by Bonferroni's multiple comparison test in GraphPad Prism Software v.7 (San Diego, California, USA) with a 95% confidence limit.
3 Results
The docking validation was done through the conformational similarity between the inhibitors used as positive control I40 and JHIII complexed with AChE or juvenile hormone and their crystallographic information. The root-mean-squared distance (RMSD) values were 0.79 Å for I40 and 1.72 Å for JHIII and indicate that the docking protocol is optimized and satisfactory, as the RMSD was lower than 2 Å (Santos et al., 2018). The best results can be seen in Fig. 1.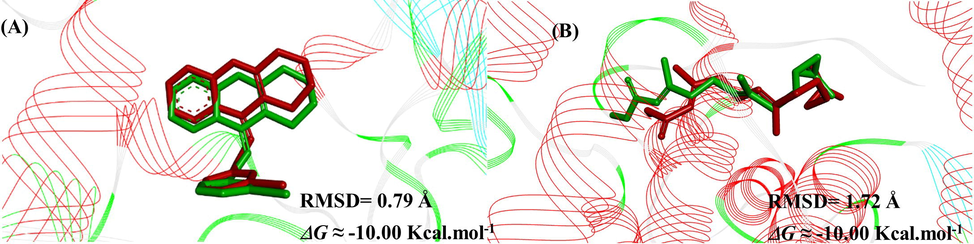
Superpositions of the crystallographic poses (in green) with the docked ones (in red) of the compounds (A) I40 and (C) JHIII, and respective RMSD and ΔG values.
Interactions between I40 and AChE observed in silico reproduced the interactions described in vitro, as π-π planar interaction with Trp 83 on β-sheet, π-π with the Tyr 370 on α-helix, π-π interaction with Tyr 71 on α-helix, hydrogen bond with Hys 480, and hydrophobic interactions with Tyr 370, among others (Harel et al., 2000).
The interactions between JHIII and juvenile hormone observed in silico reproduced the hydrophobic interactions described in vitro and validate the theoretical model of molecular docking, such as Tyr 33, Leu 37, Val 51, Trp 53, Tyr 64, Val 65, Val 68, and Tyr 129 in the α-helix; Leu 74 and Phe 144 on the β-sheet (Kim et al., 2017).
The binding affinities of terpenes found in the essential oils of each morphotype of A. triplinervis (Table 2) complexed with acetylcholinesterase and juvenile hormone receptors were predicted and compared with the binding affinity of inhibitors I40, JHIII, and pyriproxyfen to select secondary metabolites with an affinity for binding equal to or greater than positive controls. Five compounds showed potential inhibition of acetylcholinesterase receptors: aciphyllene, α-muurulene, and γ-gurjunene from morphotype A essential oil, and valencene and α-cedrene epoxide from morphotype B essential oil, as shown in Fig. 2.
Chemical Structure
Name
MAEO (%)
MBEO (%)
Reference
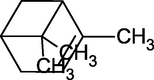
α-Pinene
0.93
1.25
Rodrigues et al. (2021)
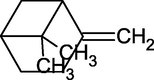
β-Pinene
2.16
2.26
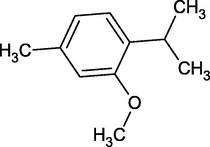
Thymol Methyl Ether
0.68
–
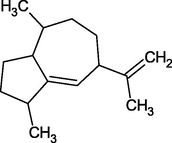
α-Gurjunene
0.98
0.41
β-Caryophyllene
45.93
–
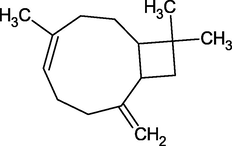
Thymohydroquinone Dimethyl Ether
32.93
84.53
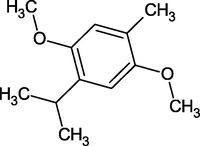
α-Humulene
1.60
1.11
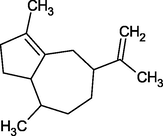
Aciphyllene
0.53
–
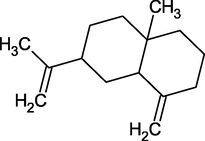
β-Selinene
0.88
0.41
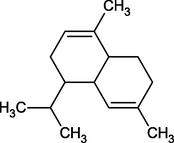
α-Muurulene
0.45
–
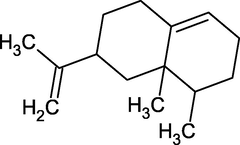
Valencene
–
0.53
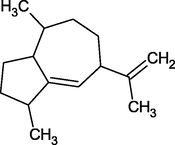
γ-Gurjenene
0.64
–
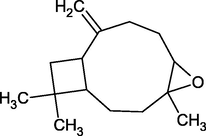
Caryophyllene Oxide
0.76
0.67
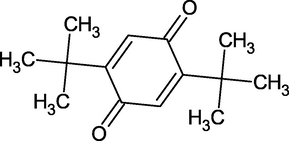
2,5-Di-Tert-Buthyl-1,4-Benzenoquinone
4.22
1.82
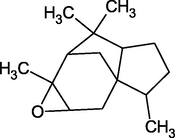
α-Cedrene Epoxide
–
0.93
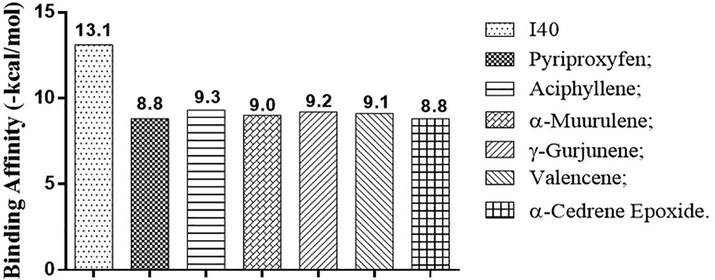
Results of binding affinity of the compounds os A. triplinervis morphotypes with the acetylcholinesterase (Drosophila Melanogaster) receptor (PDB ID 1QON).
Aciphyllene was the most promising metabolite that showed a binding affinity with the acetylcholine complex of −9.3 Kcal.mol−1, proceeded by γ-gurjunene, and valencene, which together have strong interaction in docking with the amino acid residues Tyr 71, Trp 83 and Tyr 370 that represent the acetylcholinesterase catalytic site and were similar to the in vitro experimental results described in the literature (Costa et al., 2019). These interactions reproduced the molecular ones observed in the positive controls and indicate inhibition of acetylcholinesterase receptors, as shown in Fig. 3: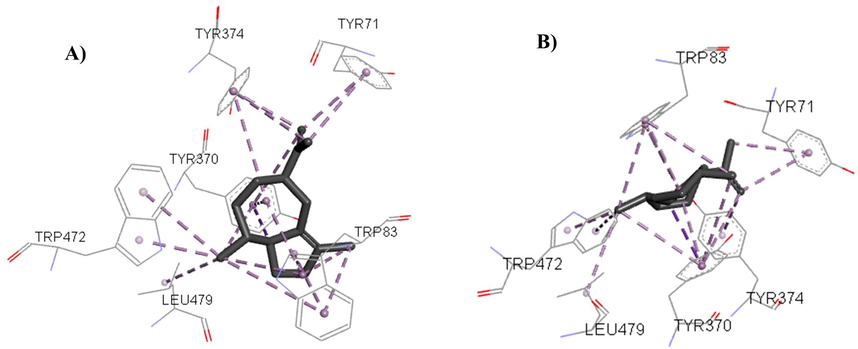
Interactions between the Drosophila Melanogaster acetylcholinesterase active site and the compounds (A) Aciphyllene and (B) γ-Gurjunene.
Four compounds showed a potential binding affinity for the inhibition of A. aegypti juvenile hormone receptors, among them α-muurulene, α-cedrene epoxide, and γ-gurjunene found only in morphotype A, and β-selinene in morphotype A and morphotype B, as shown in Fig. 4: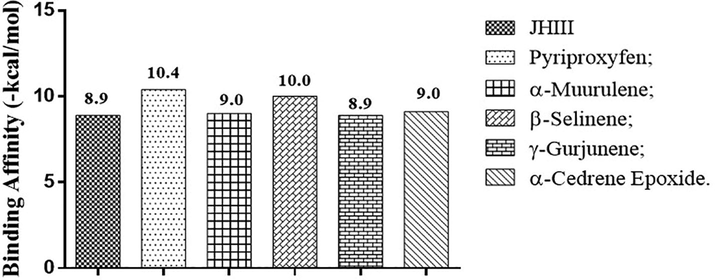
Results of binding affinity of the compounds with the A. aegypti juvenile hormone receptor (PDB ID 5V13).
Hydrophobic interactions described in the scientific literature for in vitro study were similar to those observed in docking for α-muurulene, β-selinene, and α-cedrene epoxide including those of Tyr-64, Trp-53, Val-65, Val-68, and Tyr-33 (Kim et al., 2017) and indicate the inhibition of A. aegypti juvenile hormone receptors, as shown in Fig. 5: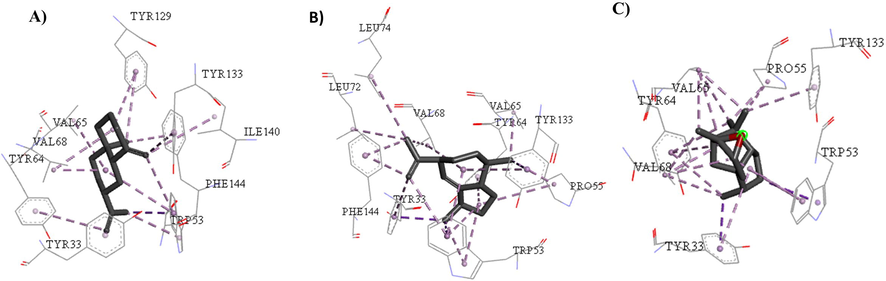
Interactions between the active site of A. aegypti juvenile hormone with (A) α-muurulene, (B) β-selinene and (C) α-cedrene epoxide.
The major compounds Thymohydroquinone Dimethyl Ether and β-caryophyllene did not predict significant binding affinity to inhibit acetylcholine and juvenile hormone receptors when compared to positive controls. However, molecular docking predicted that minor compounds present in morphotype A essential oil (α-muurulene, α-cedrene epoxide γ-gurjunene, and β-selinene) have greater potential to bind to acetylcholinesterase enzyme and juvenile hormone receptors when compared to morphotype B essential oil (β-selinene) through the individual or synergistic action of secondary metabolites.
The Centers for Disease Control and Prevention (CDC) bottle bioassay was used to assess whether nano-emulsions can help control the A. aegypti vector. The bioassay assesses the time and dose at which nano-emulsions arrive at the target site and cause mortality (Aïzoun et al., 2014).
The nano-emulsions used here were prepared according to our previous studies and demonstrated kinetic stability and monomodal distribution. Morphotype A essential oil nano-emulsion (MAEON) had a particle diameter equal to 101.400 ± 0.971 nm (PdI = 0.124 ± 0.009 and ZP = -19.300 ± 0.787 mV). Morphotype B essential oil nano-emulsion (MBEON) showed particle size 104.567 ± 0.416 nm (PdI = 0.168 ± 0,016 and ZP = -27.700 ± 1.307 mV).
The results showed that 100% of A. aegypti was susceptible to MAEON at 150 µg.mL−1 within 15 min of exposure. At the lowest concentration evaluated, 50 µg.mL−1, there was no mortality at the same time of exposure. There was no statistically significant difference between the concentration of MAEON at 150 μg.mL−1 and the positive control (p-value < 0.05, F = 32.602). MAEON showed a diagnostic dose of 150 µg.mL−1 in the diagnostic time of 15 min, as shown in Fig. 6.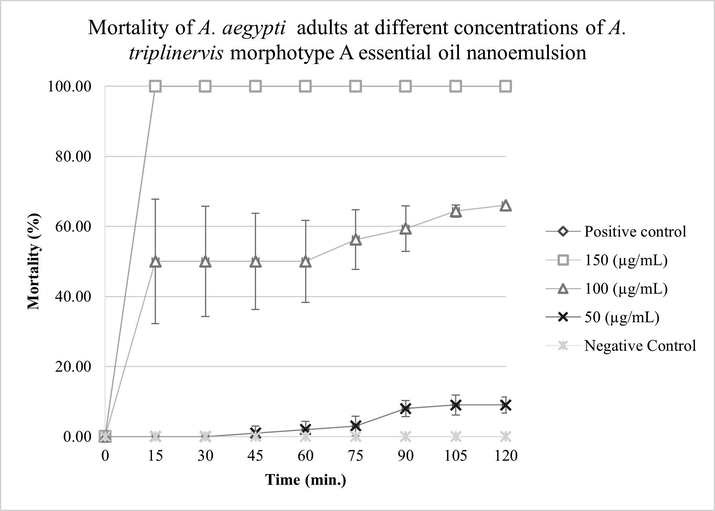
Mortality of A. aegypti adults as a function of time in different concentrations do A. triplinervis morphotype A essential oil nanoemulsion (MAEON). Malathion at 50 μg.mL−1 was used as a positive control.
For MBEON, the results indicate that 100% of A. aegypti were susceptible to 150 µg.mL−1 in 45 min of exposure. In the same exposure period, the lowest concentration (50 µg.mL−1) showed a mortality of approximately 0%. There was no statistically significant difference between the concentration of MBEON at 150 μg.mL−1 and the positive control (p-value < 0.05, F = 32.172). MBEON presented a diagnostic dose of 150 µg.mL−1 in a diagnostic time of 45 min, as shown in Fig. 7.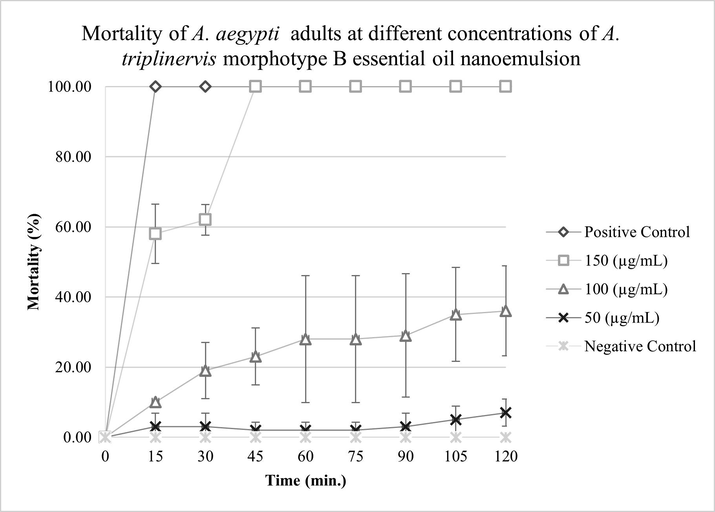
Mortality of A. aegypti adults as a function of time in different concentrations do A. triplinervis morphotype B essential oil nanoemulsion (MBEON). Malathion at 50 μg.mL−1 was used as a positive control.
MAEON and MBEON acted effectively in the control of A. aegypti at the same diagnostic dose, but at different diagnostic times and, according to the World Health Organization (World Health Organization, 2016), it is possible to conclude that A. aegypti adults were susceptible to A. triplinervis nano-emulsion.
The mosquito has a genetic preference to enter homes, blood feed on humans, and reproduce in relatively clean water in urban environments (Crawford et al., 2017). Due to the anthropophilic behaviour of A. aegypti, the acute dermal toxicity of A. triplinervis nano-emulsions was evaluated in non-target mammals.
During the assessment of acute toxicity, no mortality was observed in Swiss albino mice (Mus musculus) treated with MAEON and MBEON. Hippocratic screening indicated that animals treated with MAEON show symptoms of irritability, whereas animals treated with MBEON showed signs of irritability, itching, and corneal reflex, as shown in Table 3.
Groups
Gender
Number of animals
Number of Death
Symptoms
Control
Males
3
0
–
Female
3
0
–
MAEON
Males
3
0
Irritability
Female
3
0
Irritability
MBEON
Male
3
0
Irritability, itching
Female
3
0
Corneal reflex
The physiological parameters did not indicate significant differences in water consumption and food intake in the MAEON and MBEON groups compared to the control group. The animals showed weight gain in the MAEON and MBEON groups and differed from the control group, as shown in Table 4:
Parameter
Control
MAEON
MBEON
Male
Female
Male
Female
Male
Female
Water (mL)
13.71 ± 4.61
11.47 ± 3.19
12.80 ± 4.96
11.86 ± 4.83
11.04 ± 4.19
12.30 ± 4.12
Food (g)
6.67 ± 1.17
5.76 ± 0.66
6.80 ± 0.88
7.81 ± 1.96
6.20 ± 0.77
6.94 ± 1.18
Weight (g)
31.81 ± 1.82
24.25 ± 2.10
30.30 ± 2.69
25.84 ± 1.69
29.72 ± 2.97
25.85 ± 1.44
The evaluation of the relative weight of the organs showed that the livers of the group treated with MAEON increased significantly (*p < 0.05) when compared to the control group. Kidneys, Hearts, and lungs did not show changes in relative weight. The relative weights of the livers, kidneys, Hearts, and lungs of male and female mice treated with MAEON and MBEON are shown in Table 5.
Organs (%)
Control
MAEON
MBEON
Male
Female
Male
Female
Male
Female
Liver
5.99 ± 0.59
6.76 ± 0.81
7.52 ± 0.72
6.93 ± 0.86
7.54 ± 0.16*
6.73 ± 0.37
Kidneys
0.91 ± 0.05
0.93 ± 0.15
0.98 ± 0.04
0.93 ± 0.21
0.95 ± 0.05
0.80 ± 0.06
Heart
0.48 ± 0.01
0.72 ± 0.11
0.51 ± 0.31
0.55 ± 0.06
0.56 ± 0.02
0.64 ± 0.10
Lungs
0.65 ± 0.15
0.82 ± 0.18
0.87 ± 0.24
0.80 ± 0.16
0.65 ± 0.08
0.81 ± 0.24
In the histopathological evaluation of the dermis, acantholysis, infiltrated neutrophils, thickening of the corneal layer, formation of crusts, and presence of inflammatory cells were not found after the fourteenth day of treatment. Lungs, kidneys, livers, and dermis showed normal appearances. However, the presence of inflammatory cells was identified in the liver of animals treated with MAEON and MBEON. No histopathological changes were observed in the control group, as shown in Fig. 8: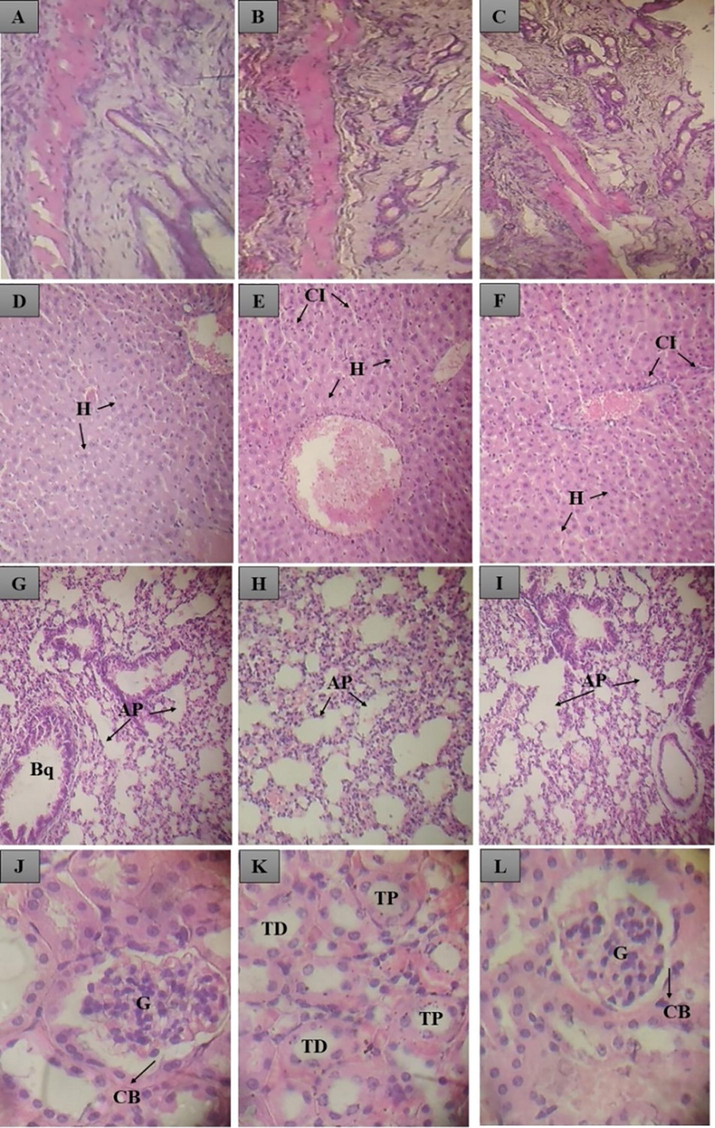
Histological section of Mus musculus treated cutaneously with the control (Tween 80 + 20), MAEON and MBEON. In A, B and C there is a normal region of the dermis of the animal treated with the control, MAEON and MBEON, respectively. In D, the normal liver of an animal treated with the control; in E and F, the liver of an animal treated with MAEON and MBEON, where normal hepatocytes (H) and the presence of inflammatory cells (CI) are observed. Em G, H e I, the lung of a normal animal treated with the control, MAEON and MBEON, where pulmonary alveoli (AP) and bronchioles (Bq) are observed. In J, K and L, normal kidney of an animal treated with the control, MAEON and MBEON, where the glomerulus (G), the Bowmam capsule (CB), distal tubule (TD), proximal tubule (TB) are observed.
A virtual prediction was performed using the ProTox server to investigate which compounds present in essential oils could be associated with the reported inflammation in the mouse liver. ProTox uses the two-dimensional similarity method to relate the chemical structure to the results available in the scientific literature database to predict the interactions of toxicophoric groups, determine the LD50 in rodents, and suggest a mechanism for the development of toxicity (Drwal et al., 2014). The analysis did not predict the hepatotoxicity associated with essential oil constituents of A. triplinervis morphotypes, as can be seen in the following Table 6:
Compounds
LD50 Toxica
Toxicity classb
Target
Prediction
Probability
α-pinene
3700
V
Hepatotoxicy
Inactive
0.86
β-pinene
4700
V
Hepatotoxicy
Inactive
0.80
Thymol methyl ether
880
IV
Hepatotoxicy
Inactive
0.74
γ-gurjunene
5000
V
Hepatotoxicy
Inactive
0.78
β-caryophyllene
500
V
Hepatotoxicy
Inactive
0.80
Thymohydroquinone dimethyl ether
880
IV
Hepatotoxicy
Inactive
0.72
α-humulene
4400
V
Hepatotoxicy
Inactive
0.83
Aciphyllene
4690
V
Hepatotoxicy
Inactive
0.73
β-selinene
5000
V
Hepatotoxicy
Inactive
0.79
α-muurulene
4400
V
Hepatotoxicy
Inactive
0.83
Valencene
5000
V
Hepatotoxicy
Inactive
0.76
γ-gurjunene
5000
V
Hepatotoxicy
Inactive
0.73
Caryophyllene oxide
5000
V
Hepatotoxicy
Inactive
0.80
2,5-di-tert-butyl-1,4-benzenoquinone
2400
V
Hepatotoxicy
Inactive
0.65
α-cedrene epoxide
5000
V
Hepatotoxicy
Inactive
0.83
Another in silico toxicity prediction was made using the Deductive Estimation of Risk from Existing Knowledge (DEREK) 10.0.2 program (Ridings et al., 1996). We have considered DEREK alerts of toxicity involving the human species and also classified them as plausible in mammals. DEREK program qualitatively predicts the toxicity of the compounds through a specialist system that focuses attention on the toxic action of chemical compounds.
In addition to toxicity, DEREK software can be used to identify aspects related to skin sensitization, irritation, and neurotoxicity (Ramos et al., 2020). Table 7 shows the results of toxicity predictions by the identification of toxicophore groups of A. triplinervis essential oils.
Compounds
Toxicity Prediction Alert (Lhasa Prediction)
Toxicophoric Group
Toxicity alert
Caryophyllene oxide
Irritation (of the eye) in human, mouse and rat
Epoxide
PLAUSIBLE
Irritation (of the skin) in human, mouse and rat
Skin sensitisation in human, mouse and rat
2,5-Di-tert-buthyl-1,4-benzoquinone
Skin sensitisation in human, mouse and rat
Quinone
PLAUSIBLE
α-cedrene epoxide
Irritation (of the eye) in human, mouse and rat
Epoxide
PLAUSIBLE
Skin irritation and sensitization were the toxicological parameters found in DEREK analyses. Therefore, these compounds were selected to evaluate the prediction of the biotransformation metabolism of xenobiotics in the liver that can cause toxicity through the MetaTox web server. Metabolism can increase the toxicity of a compound, in which case it is referred to as an activation reaction. More frequently, metabolism decreases the toxicity of a compound through a detoxification reaction.
A new freely available web-application MetaTox (https://www.way2drug.com/mg) for the prediction of xenobiotic metabolism and calculation of metabolites toxicity based on the structural formula of chemicals has been developed. The calculation of probability for generated metabolites is based on the analyses of “structure-biotransformation reactions” and “structure-modified atoms” relationships, using a Bayesian approach. Prediction of LD50 values is performed by GUSAR software for the parent compound and each of the generated metabolites using QSAR models created for acute rat toxicity with the intravenous type of administration (Rudik et al., 2016).
The results showed 20 xenobiotics metabolites from caryophyllene oxide, 19 from α-cedrene epoxide, and 4 from 2,5-di-tert-buthyl-1,4-benzenoquinone. The only xenobiotics metabolite that showed a probability of promoting a hepatotoxic effect was that derived from biotransformation 2,5-di-tert-buthyl-1,4-benzenoquinone through C-oxidation by cytochrome oxidase enzymes (Croom, 2012) and presented LD50 = 868.49 mg.kg−1, as shown in Fig. 9.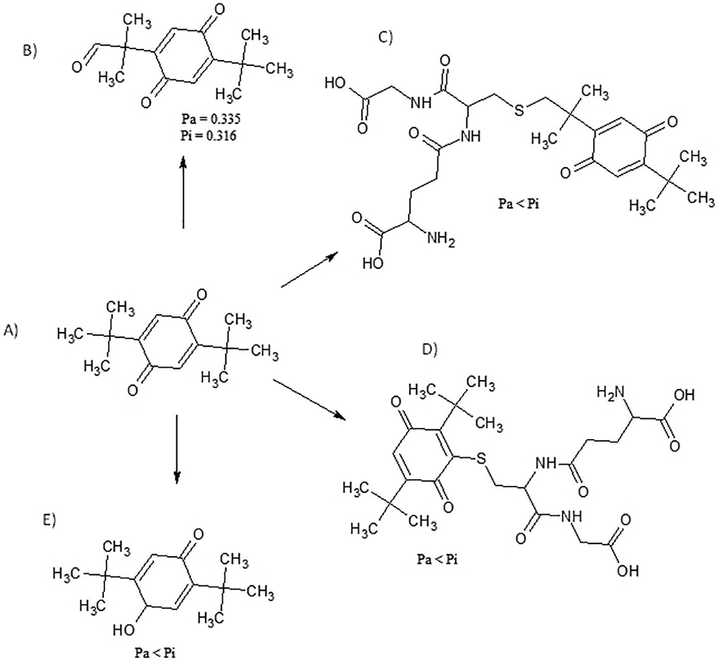
Biotransformation of (A) 2,5-di-tert-buthyl-1,4-benzenoquinone through (B) C-oxidation, (C) Glutathionation, (D) Glutathionation, and (E) Hydrogenation. Pa and Pi represent the probability of xenobiotic metabolites being active (Pa) or inactive (Pi) for hepatotoxicity.
4 Discussion
This study predicted that acyphyllene, α-muurulene, γ-gurjunene, valencene, and α-cedrene epoxide terpenes have the potential to inhibit D. Melanogaster acetylcholinesterase receptors through the catalytic site. These data are in accordance with studies available in the literature that demonstrate the inhibitory action of terpenes on the acetylcholine enzyme through docking molecular (Khanikor, 2013; Silva et al., 2014).
The molecular docking carried predicted the action of the essential oils of A. triplinervis morphotypes on D. melanogaster acetylcholinesterase receptors through complexation with the enzyme's catalytic site. As the literature reports that different enzymes belonging to the cholinesterase family share the same catalytic mechanism (Moralev et al., 2001), it is possible to infer that the essential oils of A. triplinervis morphotypes can also inhibit the acetylcholinesterase enzyme of A. aegypti.
The insecticidal activity of several essential oils has demonstrated a correlation with inhibition of the enzyme acetylcholinesterase (Seo et al., 2015). Perilla frutescens essential oil showed insecticidal activity against Drosophila suzukii (Park et al., 2016), Citrus sinensis essential oil showed insecticidal activity against Tribolium confusum, Callosobruchus maculatus, and Sitophilus oryzae (Oboh et al., 2017), and Ocimum tenuiflorum essential oil showed insecticidal activity against Sitophilus orzyae (Bhavya et al., 2018). All of these examples showed high inhibition of the AChE enzyme.
Another important strategy for controlling A. aegypti is focused on the morphogenesis and reproduction of insects by the regulation of juvenile hormones for the development of new insecticides (Cusson et al., 2013; Moralev et al., 2001). Our results showed that α-muurulene, β-selinene, γ-gurjunene, and α-cedrene epoxide have the potential to act as regulators of the juvenile hormone. The prediction in silico of this study is in agreement with experimental tests that demonstrated that terpenes from essential oils can block egg maturation in female mosquitos and disrupt the expression of the Juvenile Hormone-induced gene (Chokechaijaroenporn et al., 1994; Lee et al., 2018).
This study reported for the first time in the literature the evaluation of the adulticidal activity of nano-emulsion in A. aegypti using CDC bottle bioassay and reported exclusively the nanoinsecticidal potential of formulations based on A. triplinervis. Thus, it is possible to conclude that the essential oils of A. triplinervis morphotypes can be used in the chemical control of A. aegypti adults through the inhibitory properties of acetylcholinesterase enzyme and juvenile hormone receptors.
Studies regarding the adulticidal activity in A. aegypti have shown that Lippia origanoides essential oil (Carvacrol 50.6%) killed 100% at 300 µg.mL−1 in 60 min of exposure (Castillo et al., 2017; Chokechaijaroenporn et al., 1994). In another study with Melaleuca quinquenervia essential oil (28.7% 1,8-cienole and 25.2% viridiflorol) killed 100% of A. aegypti at 40,000 µg.mL−1 in 30 min.
From the point of view of nanoformulations, nanoparticles based on Heliotropium indicum presented mortality of 98.2% of A. aegypti at 250 µg.mL−1 in 24 h of exposure (Castillo et al., 2017; Veerakumar et al., 2014). In another study, nanoparticles based on Olea europaea showed mortality of 73.3% in D. melanogaster at 200 µg.mL−1 in 168 h of exposure (Araj et al., 2015; Veerakumar et al., 2014).
Comparing these results above with the dose and diagnostic time found for MAEON and MBEON, the nanoformulations based on A. triplinervis morphotypes essential oils can be used in the chemical control of A. aegypti and, as shown in the molecular docking simulations, with possible insecticidal action through inhibition of the catalytic site in enzyme acetylcholinesterase receptors and juvenile hormone receptors.
Aggregates of small lipophilic molecules or colloidal structures can influence the structure-ligand binding, producing non-specific mechanisms of action or the induction of conformational changes that affect the protein and can represent false-positive results. Thus, it is suggested the future perspectives for this study to evaluate the influence of the critical concentration of nano-emulsion colloidal aggregates on AChE and juvenile hormone receptors through molecular dynamics techniques (Ghattas et al., 2020; Vázquez et al., 2020).
Plant secondary metabolites have advantages as biopesticides because they are easily available, inexpensive, biodegradable, don’t leave chemical residues, and offer greater security to non-target species (Gupta et al., 2019). When secondary metabolites with insecticidal action are stabilized within a nanotechnological system, nanoinsecticides are more efficient due to the greater surface area, solubility, mobility, and low toxicity without the presence of organic solvents than the conventional use of many commercial pesticides. (Durán et al., 2016).
Nanoinsecticides can come into contact with non-target mammals through inhalation, the digestive tract, or skin absorption. The permeability of MAEON and MBEON over the skin can occur through pinocytosis by the transcellular mechanism and provoke its deleterious effects (Rai et al., 2018).
Corneal reflex was one of the toxicological effects found in the hippocratic screening and indicates an action on the central nervous system of nano-emulsions. This result is in line with what is reported in the literature, which describes the sedative, anxiolytic and antidepressant effects of A. triplinervis extracts (Melo et al., 2013).
Nano-emulsions caused subtle toxicological effects on the liver of mice that could be seen through the presence of inflammatory cells. Different results of this study showed a hepatoprotective effect of methanolic extract of the species (Bose et al., 2007).
The initial in silico prediction did not indicate a hepatotoxic effect of the chemical constituents of essential oils, indicating action resulting from the synergistic effect of essential oil compounds or the action of their biotransformation in the liver. However, the analysis of xenobiotic metabolism showed hepatotoxic action of the biotransformation of 2,5-di-tert-butyl-1,4-benzenoquinone. This biotransformation may have occurred through the action of the cytochrome P450 enzyme and is extremely selective in the oxidation of substrates as the first defense mechanism (Kliewer et al., 2002). However, it was not possible to state that nano-emulsions containing essential oil from A. triplinervis morphotypes have a hepatotoxic effect. Further studies are needed.
The itching was an effect described in the hypocratic screening and may be related to irritation and sensitivity caused by the oxide and quinone groups of Caryophyllene oxide, 2,5-Di-tert-buthyl-1,4-benzenoquinone, and α-cedrene epoxide in the skin predicted by the analyses of DEREK. The presence of a skin sensitization structural alert within a molecule indicates the molecule has the potential to cause skin sensitization. Whether the molecule is a skin sensitizer will also depend upon its percutaneous absorption. Generally, small lipophilic molecules are more readily absorbed into the skin and are therefore more likely to cause sensitization (Basketter and Scholes, 1992).
Thus, it is possible to conclude that nano-emulsions have low acute dermal toxicity and indicated that the LD50 is higher than 2000 mg.Kg−1 and can be used in the chemical control of A. aegypti adults.
5 Conclusions
Docking showed inhibition of the catalytic site of acetylcholinesterase enzyme receptors by acyphyllene, α-muurulene, γ-gurjunene, valencene, and α-cedrene epoxide, and inhibition of juvenile hormone receptors by α-muurulene, β-selinene, γ-gurjunene, and α-cedrene epoxide and indicated the insecticidal potential of A. triplinervis morphotypes in silica model.
The evaluation of adulticidal activity showed that the diagnostic dose of 150 µg.mL−1 in the diagnostic time of 15 min for MAEON, and MBEON was the same diagnostic dose in 45 min of exposure. These results indicate the susceptibility of A. aegypti against A. triplinervis nano-emulsions. Nano-emulsions have low acute dermal toxicity and the LD50 is higher than 2000 mg.Kg−1.
Acknowledgments
The Amapá Research Support Foundation (FAPEAP). To the Research Program for SUS – PPSUS – Ministry of Health. The Coordination for the Improvement of Higher Education Personnel (CAPES)/Ministry of Education (MEC). To the Pharmaceutical Research laboratory – UNIFAP under the responsibility of Dr. José Carlos Tavares Carvalho. To the Laboratory of Toxicology and Pharmaceutical Chemistry – UNIFAP under the responsibility of Dr. Mayara Amoras Teles Fujishima. The Dean of Research and Graduate Studies – PROPESPG.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Centre for Disease Control and Prevention (CDC) bottle bioassay: A real complementary method to World Health Organization (WHO) susceptibility test for the determination of insecticide susceptibility in malaria vectors. J. Parasitol. Vector Biol.. 2014;6:42-47.
- [Google Scholar]
- Juvenile hormone analog enhances Zika virus infection in Aedes aegypti. Sci. Rep.. 2021;11:21062.
- [CrossRef] [Google Scholar]
- Toxicity of Nanoparticles against Drosophila melanogaster (Diptera: Drosophilidae) J. Nanomaterials. 2015;2015
- [CrossRef] [Google Scholar]
- Validation of Eupatorium triplinerve Vahl Leaves, a Skin Care Herb from East Kalimantan, Using a Melanin Biosynthesis Assay. J. Acupuncture Meridian Studies. 2012;5
- [CrossRef] [Google Scholar]
- Comparison of the local lymph node assay with the guinea-pig maximization test for the detection of a range of contact allergens. Food Chem. Toxicol.. 1992;30
- [CrossRef] [Google Scholar]
- Ocimum tenuiflorum oil, a potential insecticide against rice weevil with anti-acetylcholinesterase activity. Ind. Crops Prod.. 2018;126
- [CrossRef] [Google Scholar]
- Hepatoprotective and Antioxidant Effects of Eupatorium ayapana against Carbon Tetrachloride nduced Hepatotoxicity in Rats. J. Pharmacol. Therapeut. 2007
- [Google Scholar]
- Brandão, L.B., Santos, L.L., Martins, R.L., Rodrigues, A.B.L., Rabelo, E. de M., Galardo, A.K.R., Almeida, S.S.M. da S. de, 2021. Larvicidal Evaluation against Aedes aegypti and Antioxidant and Cytotoxic Potential of the Essential Oil of Tridax procumbens L. Leaves. The Scientific World Journal 2021. https://doi.org/10.1155/2021/2172919.
- Brogdon, W.G., Chan, A., 2013. Diretriz para Avaliar a Resistência a Inseticida em Vetores Usando o Bioensaio com Garrafa do CDC, 1st ed.
- The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr.. 2013;109
- [CrossRef] [Google Scholar]
- Insecticidal and Repellent Activity of Several Plant-Derived Essential Oils Against Aedes aegypti. J Am Mosq Control Assoc. 2017;33
- [CrossRef] [Google Scholar]
- Chaves, R. do S.B., Martins, R.L., Rodrigues, A.B.L., Rabelo, É. de M., Farias, A.L.F., Brandão, L.B., Santos, L.L., Galardo, A.K.R., de Almeida, S.S.M. da S., 2020. Evaluation of larvicidal potential against larvae of Aedes aegypti (Linnaeus, 1762) and of the antimicrobial activity of essential oil obtained from the leaves of Origanum majorana L. PLOS ONE 15. https://doi.org/10.1371/journal.pone.0235740.
- Mosquito repellent activities of ocimum volatile oils. Phytomedicine. 1994;1
- [CrossRef] [Google Scholar]
- Principais Mosquitos de Importância Sanitária no Brasil (1st ed.). Rio de Janeiro: Editora Fiocruz; 1994.
- Costa, G., Ferreira, E.F.B., da S. Ramos, R., B. da Silva, L., M. F. de Sá, E., K. P. da Silva, A., M. Lobato, C., N. P. Souto, R., T. de P. da Silva, C.H., B. Federico, L., M. C. Rosa, J., B. R. dos Santos, C., 2019a. Hierarchical Virtual Screening of Potential Insectides Inhibitors of Acetylcholinesterase and Juvenile Hormone from Temephos. Pharmaceuticals 12. https://doi.org/10.3390/ph12020061.
- Population genomics reveals that an anthropophilic population of Aedes aegypti mosquitoes in West Africa recently gave rise to American and Asian populations of this major disease vector. BMC Biol.. 2017;15
- [CrossRef] [Google Scholar]
- Metabolism of Xenobiotics of Human Environments. In: Hodgson E., ed. Progress in Molecular Biology and Translational Science. Oxford: Academic Press; 2012.
- [CrossRef] [Google Scholar]
- Cusson, M., Sen, S.E., Shinoda, T., 2013. Juvenile Hormone Biosynthetic Enzymes as Targets for Insecticide Discovery, in: Ishaaya, I., Palli, S., Horowitz, A.R. (Eds.), Advanced Technologies for Managing Insect Pests. Springer Netherlands, Dordrecht. https://doi.org/10.1007/978-94-007-4497-4_3.
- ProTox: a web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res.. 2014;42
- [CrossRef] [Google Scholar]
- Nanobiotechnology Solutions against Aedes aegypti. J. Braz. Chem. Soc. 2016
- [CrossRef] [Google Scholar]
- Essential oil of Ayapana triplinervis from Reunion Island: A good natural source of thymohydroquinone dimethyl ether. Biochem. Syst. Ecol.. 2008;36
- [CrossRef] [Google Scholar]
- Ghattas, M.A., al Rawashdeh, S., Atatreh, N., Bryce, R.A., 2020. How Do Small Molecule Aggregates Inhibit Enzyme Activity? A Molecular Dynamics Study. Journal of Chemical Information and Modeling 60, 3901–3909. https://doi.org/10.1021/acs.jcim.0c00540.
- Long-term stability of food-grade nano-emulsions from high methoxyl pectin containing essential oils. Food Hydrocolloids. 2016;52
- [CrossRef] [Google Scholar]
- Gupta, R.C., Doss, R.B., Srivastava, A., Lall, R., Sinha, A., 2019. Nutraceuticals for Control of Ticks, Fleas, and Other Ectoparasites, in: Gupta, R., Srivastava, A., Lall, R. (Eds.), Nutraceuticals in Veterinary Medicine. Springer International Publishing, Cham. https://doi.org/10.1007/978-3-030-04624-8_43.
- Morphology and phylogenetic interrelationships of the Asteraceae, Calyceraceae, Campanulaceae, Goodeniaceae, and related families (Asterales) Am. J. Bot.. 1995;82
- [CrossRef] [Google Scholar]
- Haddi, K., Tomé, H.V.V., Du, Y., Valbon, W.R., Nomura, Y., Martins, G.F., Dong, K., Oliveira, E.E., 2017. Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti: a potential challenge for mosquito control. Sci. Rep. 7. https://doi.org/10.1038/srep46549.
- Three-dimensional structures of Drosophila melanogaster acetylcholinesterase and of its complexes with two potent inhibitors. Protein Sci.. 2000;9
- [CrossRef] [Google Scholar]
- Comparative mode of action of some terpene compounds against octopamine receptor and acetyl cholinesterase of mosquito and human system by the help of homology modeling and Docking studies. J. Appl. Pharm. Sci. 2013
- [CrossRef] [Google Scholar]
- A mosquito hemolymph odorant-binding protein family member specifically binds juvenile hormone. J. Biol. Chem.. 2017;292
- [CrossRef] [Google Scholar]
- The Nuclear Pregnane X Receptor: A Key Regulator of Xenobiotic Metabolism. Endocr. Rev.. 2002;23
- [CrossRef] [Google Scholar]
- Plant-derived compounds regulate formation of the insect juvenile hormone receptor complex. Pestic. Biochem. Physiol.. 2018;150
- [CrossRef] [Google Scholar]
- Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crops Prod.. 2010;31
- [CrossRef] [Google Scholar]
- Melo, A.S., Monteiro, M.C., da Silva, J.B., de Oliveira, F.R., Vieira, J.L.F., de Andrade, M.A., Baetas, A.C., Sakai, J.T., Ferreira, F.A., Cunha Sousa, P.J. da, Maia, C. do S.F., 2013. Antinociceptive, neurobehavioral and antioxidant effects of Eupatorium triplinerve Vahl on rats. J. Ethnopharmacol. 147. https://doi.org/10.1016/j.jep.2013.03.002.
- Moralev, S.N., Rozengart, E.V., Suvorov, A.A., 2001. The “Catalytic Machines” of Cholinesterases of Different Animals Have the Same Structure. Doklady Biochem. Biophys. 381. https://doi.org/10.1023/A:1013351127011.
- Chemical composition, insecticidal activity and persistence of three Asteraceae essential oils and their nano-emulsions against Callosobruchus maculatus (F.) J. Stored Prod. Res.. 2015;61
- [CrossRef] [Google Scholar]
- Nery, M.I.S., Potiguara, R.C.V., Kikuchi, T.Y.S., Garcia, T.B., Lins, A.L.F.A., 2014. Morfoanatomia do eixo vegetativo aéreo de Ayapana triplinervis (Vahl) R.M. King & H. Rob. (Asteraceae). Revista Brasileira de Plantas Medicinais 16. https://doi.org/10.1590/S1516-05722014000100009.
- Insecticidal activity of essential oil from orange peels (Citrus sinensis) against Tribolium confusum, Callosobruchus maculatus and Sitophilus oryzae and its inhibitory effects on acetylcholinesterase and Na+/K+-ATPase activities. Phytoparasitica. 2017;45
- [CrossRef] [Google Scholar]
- Insecticidal and acetylcholinesterase inhibitory activities of Lamiaceae plant essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae) Ind. Crops Prod.. 2016;89
- [CrossRef] [Google Scholar]
- Nano-emulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release. 2018;270
- [CrossRef] [Google Scholar]
- Ramos, R.S., Macêdo, W.J.C., Costa, J.S., da Silva, C.H.T. de P., Rosa, J.M.C., da Cruz, J.N., de Oliveira, M.S., de Aguiar Andrade, E.H., e Silva, R.B.L., Souto, R.N.P., Santos, C.B.R., 2020a. Potential inhibitors of the enzyme acetylcholinesterase and juvenile hormone with insecticidal activity: study of the binding mode via docking and molecular dynamics simulations. J. Biomol. Struct. Dyn. 38. https://doi.org/10.1080/07391102.2019.1688192.
- Computer prediction of possible toxic action from chemical structure: an update on the DEREK system. Toxicology. 1996;106
- [CrossRef] [Google Scholar]
- Rodrigues, A.B., Martins, R.L., Rabelo, É. de M., Tomazi, R., Santos, L.L., Brandão, L.B., Faustino, C.G., Ferreira Farias, A.L., Santos, C.B.R., Castro Cantuária, P., Galardo, A.K.R., Almeida, S.S.M. da S., 2021. Development of nano-emulsions based on Ayapana triplinervis essential oil for the control of Aedes aegypti larvae. PLOS ONE 16, e0254225. https://doi.org/10.1371/journal.pone.0254225.
- Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat. Commun.. 2017;8
- [CrossRef] [Google Scholar]
- Rudik, A. v., Dmitriev, A. v., Lagunin, A.A., Filimonov, D.A., Poroikov, V.V., 2016. Prediction of reacting atoms for the major biotransformation reactions of organic xenobiotics. Journal of Cheminformatics 8. https://doi.org/10.1186/s13321-016-0183-x.
- Rudik, A. v., Bezhentsev, V.M., Dmitriev, A.V., Druzhilovskiy, D.S., Lagunin, A.A., Filimonov, D.A., Poroikov, V.V., 2017. MetaTox: Web Application for Predicting Structure and Toxicity of Xenobiotics’ Metabolites. Journal of Chemical Information and Modeling 57. https://doi.org/10.1021/acs.jcim.6b00662.
- Oil from the fruits of Pterodon emarginatus Vog.: A traditional anti-inflammatory. Study combining in vivo and in silico. J. Ethnopharmacol.. 2018;222
- [CrossRef] [Google Scholar]
- GC–MS analysis of phytocomponents in the methanolic extract of Eupatorium triplinerve. Asian Pacific J. Tropical Biomed.. 2012;2
- [CrossRef] [Google Scholar]
- Larvicidal and Acetylcholinesterase Inhibitory Activities of Apiaceae Plant Essential Oils and Their Constituents against Aedes albopictus and Formulation Development. J. Agric. Food. Chem.. 2015;63
- [CrossRef] [Google Scholar]
- Acetylcholinesterase Inhibitory Activity and Molecular Docking Study of 1-Nitro-2-Phenylethane, the Main Constituent of Aniba canelilla Essential Oil. Chem. Biol. Drug Des.. 2014;84
- [CrossRef] [Google Scholar]
- Merging Ligand-Based and Structure-Based Methods in Drug Discovery: An Overview of Combined Virtual Screening Approaches. Molecules. 2020;25:4723-4750.
- [CrossRef] [Google Scholar]
- Evaluation of plant-mediated synthesized silver nanoparticles against vector mosquitoes. Parasitol. Res.. 2014;113
- [CrossRef] [Google Scholar]
- Test procedures for insecticide resistance monitoring in malaria vector mosquitoes (2nd ed.). Switzerland: WHO Press; 2016.
- The preparation, characterization, antimicrobial stability and in vitro release evaluation of fish gelatin films incorporated with cinnamon essential oil nanoliposomes. Food Hydrocolloids. 2015;43
- [CrossRef] [Google Scholar]







