Translate this page into:
In situ synthesis and preconcentration of cetylpyridinium complexed hexaiodo platinum nanoparticles from spent automobile catalytic converter leachate using cloud point extraction
⁎Corresponding author at: National Centre for Compositional Characterisation of Materials (NCCCM), Bhabha Atomic Research Centre (BARC), ECIL PO, Hyderabad 500062, India. koramaganti@gmail.com (Aruna Jyothi Kora)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present study, a green chemistry based cloud point extraction (CPE) method has been developed for the in situ synthesis and preconcentration of cetylpyridinium complexed hexaiodo platinum nanoparticles (Pt-I NP) from the leachate of spent automobile catalytic converter using potassium iodide (KI) and assisted by a combination of cationic and non-ionic surfactants; cetylpyridinium bromide (CPB) and Triton X-114, respectively. The process parameters such as concentrations of hydrochloric acid, platinum, KI, sodium chloride, CPB, Triton X-114; incubation temperature and complexation time on CPE were optimized. The synthesized nanoparticles were characterized by UV–visible spectroscopy (UV–vis), transmission electron microscopy (TEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and zeta potential techniques. The formation of Pt-I NP was followed with UV–vis. The XRD pattern established the tetragonal crystal structure of the produced nanoparticles. The nanoparticles were spherical in shape and the particle size obtained with TEM was about 5.6 nm. Further, the preconcentrated nanoparticles were quantified by continuum source electro-thermal atomic absorption spectrometry (ET-AAS) and a preconcentration factor of 25 was obtained for a reaction volume of 25 mL. The accuracy of the developed method was confirmed by analyzing the certified reference materials such as CCRMP PTM-1a (copper-nickel sulphide matte) and CCRMP PTC-1a (copper-nickel sulphide concentrate). The current CPE protocol demonstrates advantages such as simultaneous synthesis and preconcentration; synthesis at micromolar concentration from metal scrap, higher nanoparticle recovery; biodegradability and biocompatibility of the employed surfactants and dual solubility of the synthesized Pt-I NP. Thus, the developed method can be applied for the separation, large scale synthesis and preconcentration of Pt-I NP from various environmental and industrial wastes, in a single pot.
Keywords
Cetylpyridinium complexed hexaiodo platinum nanoparticles (Pt-I NP)
Catalytic converter
Characterization
Cloud point extraction
ET-AAS
Solubility
1 Introduction
Platinum nanoparticles find their use in various fields such as catalyst in industrial processes (Hosseini et al., 2013; Nguyen Viet et al., 2010), fuel cells, gas sensor, chemiresistor sensor (Hosseini et al., 2013; Li et al., 2010), hydrogen storage, electrocatalyst (Nguyen Viet et al., 2010), mercury detection (Tan et al., 2009), anticancer agent (Bendale et al., 2012) etc. Earlier various chemical methods for the synthesis of platinum have been reported in literature. They include shape (Nguyen Viet et al., 2010) and size (Yamada et al., 2005) controlled syntheses for the production of platinum nanoparticles. These nanoparticles were produced by reducing platinum halides or anionic platinum chloride precursors with hydrogen (Lei et al., 2014) borohydride (Hosseini et al., 2013; Nazar et al., 2011), sodium citrate (Li et al., 2009), hydrazine (Soleimani et al., 2014), ethylene glycol (Nguyen Viet et al., 2010); sonochemical reduction (Mizukoshi et al., 2001; Nakanishi et al., 2005), hydrosilylation (Huang et al., 2004), atomic layer deposition (Christensen et al., 2009) etc. While the platinum oxide nanoparticles are produced under basic aqueous environment by hydrolytic decomposition of platinum halides, in the presence of stabilizers including surfactants, polymers and ligands (He et al., 2007).
Different protocols have been applied for separation and preconcentration of nanoparticles such as liquid-liquid extraction (Hartmann and Schuster, 2013), magnetic fields, size-exclusion chromatography, hydrodynamic chromatography, counter-current chromatography, field flow fractionation, density-gradient centrifugation, electrophoresis, selective precipitation, membrane filtration and cloud point extraction (CPE) (Kowalczyk et al., 2011; Liu et al., 2012). But the time consuming, labor intensive method of liquid–liquid extraction is limited by the requirement of higher volumes of toxic organic solvents and low enrichment factors (Hartmann and Schuster, 2013). Among which, CPE has gained much attention of the researchers. This is mainly due to the high extraction efficiency, adsorptive preconcentration and preconcentration factor; reduction in extractant loss, simple, low cost, easy handling and less environmentally hazardous, thus making it as a suitable method for separation and preconcentration of nanoparticles from a variety environmental and biological matrices (Liu et al., 2012; Nazar et al., 2011). The method CPE has been used explicitly employed for the preconcentration of various precious nanoparticles including gold, silver and palladium (Hartmann and Schuster, 2013; Kowalczyk et al., 2011; Liu et al., 2012; Nazar et al., 2011; Wu and Tseng, 2011). However, there was no report in literature about the utilization of CPE for platinum nanoparticles.
Generally using traditional synthetic methods, it is difficult to synthesize platinum iodide nanoparticles (Pt-I NP). In an earlier study, employing single-walled carbon nanotubes as a dual functional reaction vessel and template; Pt-I NP were produced (Stoppiello et al., 2017). In other reports, the additive, iodide ion mediated shape controlled synthesis of platinum nanocrystals was attempted in the presence of cetyltrimethylammonium bromide (CTAB) (Li et al., 2018) and polyacrylic acid (Yamada et al., 2005), as they bind strongly with platinum than any other halide ions. In the present study, a simple CPE method based on green chemistry was developed for the in situ synthesis and simultaneous preconcentration of Pt-I NP from spent automobile catalytic converter leachate samples using potassium iodide and a combination of cationic and non-ionic surfactants; cetylpyridinium bromide and Triton X-114, respectively. In previous reports, the spent catalytic converters samples were digested with aqua regia either by refluxing for 90 min (Yu et al., 2010) or in a closed microwave digestion at an elevated temperature and pressure of 220 °C and 800 psi for 15 min (Reddy et al., 2013). However, most of these reported hydrometallurgical protocols necessitate the presence of extremely aggressive conditions such as highly acidic environment and oxidizing agents including the application of aqua regia. But, the utilization of highly corrosive aqua regia makes the large scale recycling process less environment friendly due to the formation of nitrosyl chloride that decomposes into noxious nitric oxide and chlorine gases. The detrimental effects of nitric oxide on biota and environment are well known through the ozone layer depletion and acid rain production. The aqua regia extraction also produces hazardous nitrogen dioxide gas, a causative air pollutant of occupational exposure. Also, it leads to the production of large quantum of secondary chemical wastes (Jha et al., 2013a). Further, the replacement of aqua regia with cyanide extraction is left with limitations such as requirement of elevated temperature and pressure (200–250 °C and 10 bar), making the process very much energy intensive (Jha et al., 2013a; Patel and Dawson, 2015; Suoranta et al., 2015). This situation intrigued us to develop an eco-friendly platinum extraction method from the spent catalytic converter, as a potential alternative to both aqua regia and cyanide extraction. Hence, an attempt has been made by increasing the oxidizing power of HCl by the addition of hydrogen peroxide during extraction. Among the strong inorganic acids commonly used in the industries, HCl is less corrosive and least hazardous, which is stable upon storage at intermediate concentrations. The components of high oxidation potential; Cl2 (aq) and HClO produced during the closed leaching with HCl and H2O2 mixture aid in leaching of platinum from the catalytic converter. Most importantly, the produced byproduct during the dissolution of platinum is water, which is benign (Kizilaslan et al., 2009). The effect of different parameters such as concentrations of hydrochloric acid, platinum, potassium iodide, sodium chloride, cetylpyridinium bromide, Triton X-114; incubation temperature and complexation time on CPE was studied. The produced nanoparticles were characterized by UV–visible spectroscopy, transmission electron microscopy, X-ray diffraction, X-ray photoelectron spectroscopy and zeta potential techniques. Further, the preconcentrated nanoparticles were quantified by continuum source electro-thermal atomic absorption spectrometry (ET-AAS), a technique that permits determination in small sample volumes enriched with surfactants (Hartmann and Schuster, 2013).
According to the available information in literature, probably this is the first report where the CPE method is used for the simultaneous in situ synthesis and adsorptive preconcentration of Pt-I NP from the catalytic converter leachate matrix. In earlier reported methods, aqueous metallic solutions prepared from pure platinum salts such as platinum halides or anionic platinum chloride were used for the nanoparticle synthesis (Lee et al., 2008; Lei et al., 2014). Whereas, the current method utilizes the metal scrap i.e. spent catalytic converter as a matrix for the synthesis of Pt-I NP. Also, it is a one pot method wherein both synthesis and preconcentration occurs in the same reaction vessel, thus reducing the number of steps. Further, the synthesis was carried from leachate at a dilute metal precursor concentration of micromolar platinum (250 ng/mL), against the millimolar concentration used for most chemical methods (Lei et al., 2014) . The nanoparticles produced by chemical methods are very popular and the chemicals employed for production are highly reactive, generate unwanted by-products and cause potential environmental and biological risks (Raveendran et al., 2003; Vigneshwaran et al., 2006). Most importantly, Triton X-114, a non-ionic surfactant used in the current protocol is bestowed with virtues such as (i) solubility in aqueous and most polar organic solvent media, (ii) compatibility with other anionic, cationic and non-ionic surfactants, (iii) biodegradability and (iv) biocompatibility (Company; Glembin et al., 2014). While the other employed cationic surfactant, CPB is known to be biodegradable in the environment (Verschueren, 1983). Interestingly, the preconcentrated nanoparticles in surfactant rich phase (SRP) exhibited dual solubility, i.e. soluble both in aqueous and organic media. The aqueous solubility property of the produced nanoparticles can be used for various biological applications such as the synthesis of platinum based anti-cancer drugs. The organic miscibility phenomenon of the nanoparticles can be exploited for coating of Pt-I NP on automobile catalytic converters. Thus, the proposed CPE protocol overcomes the limitations posed by the other chemical methods by utilizing the platinum metal scrap for the Pt-I NP synthesis and the preconcentrated nanoparticles are readily available for applications. Thus, the method adopts the basic principles of green chemistry (Anastas and Warner, 1998).
2 Materials and methods
2.1 Materials
37% Hydrochloric acid (HCl), potassium iodide (KI), sodium chloride (NaCl), xylene, methanol, 30% hydrogen peroxide (H2O2) (E. Merck, Mumbai, India), chloroplatinic acid hexahydrate, cetylpyridinium bromide (CPB) and Triton X-114, (Sigma-Aldrich, Bengaluru, India) of analytical reagent grade were used. Pt (IV) stock solution (1 mg/mL) was prepared by dissolving the chloroplatinic acid hexahydrate in 10% (v/v) HCl. The working standards were prepared by subsequent dilutions of the stocks. All the solutions were prepared in ultrapure water obtained from a Milli-Q™ water purification system (Millipore, Bengaluru, India). 10% (w/v) stock solutions of non-ionic (Triton X-114) and cationic (CPB) surfactants were prepared in water and methanol, respectively. The spent automobile catalytic converters obtained from a local scrap recycler were used for the nanoparticle synthesis. The converters were sequentially cleaned with ultrapure water; methanol and xylene, respectively for removing the dust and carbonaceous hydrocarbons deposited on the surface and then air dried for 2 h. The cleaned catalytic converters were grounded in a planetary ball mill and sieved to get 90 µm sized powders. Accurately weighed 5 g of spent automobile catalytic converter powder samples were transferred into a closed reagent bottle and leached with 4 mL of HCl and H2O2 mixture (3:1 volume ratio) at 70 °C for 60 min. The leaching was repeated twice and after cooling the leachate was made to 20 mL with ultrapure water. The procedural blanks were also maintained in a similar manner.
2.2 CPE procedure for the synthesis and preconcentration of Pt-I NP
An aliquot of catalytic converter leachate sample containing platinum was taken in a polypropylene tube and 0.5 mL of HCl was added. To this solution, 0.2 mL of 2% (w/v) of KI, 1 mL of 30% (w/v) of NaCl, 0.5 mL of 10% (w/v) CPB and 2 mL of 10% (w/v) of Triton X-114 were added. The volume was made to 10 mL with ultrapure water and mixed well for 2 min. Then, the mixture was heated on a water bath at 70 °C for 40 min, which led to gravitational phase separation. Then, 1 mL of brownish green coloured SRP formed at the bottom of the tube was easily separated by decanting the bulk aqueous phase, after cooling the solution in a refrigerator for 10 min. The viscosity of the SRP containing Pt-I NP was reduced by dissolving it in 1 mL of methanol containing 20 µl of HNO3. The solution was made to 3 mL with water, characterized for Pt-I NP and analyzed for platinum content using continuum source ET-AAS. The process blanks were also prepared and analyzed in a similar way. A schematic flow chart showing the effect of different parameters on the synthesis and preconcentration of Pt-I NP from catalytic converter is given in Supplementary information (Sup. Fig. 1). Also, the presence of other metals in the leachate and their effect on the recovery of nanoparticles was studied.
2.3 Characterization and quantification of synthesized Pt-I NP
The UV–visible absorption spectra of the Pt-I NP solutions were noted using Analytik Jena AG, Specord 200 Plus UV–visible spectrophotometer (Jena, Germany), at a wavelength range of 250–600 nm. Before collecting the spectra, all the solutions were diluted 10 times. The X-ray diffraction analysis was conducted with a Rigaku, Ultima IV diffractometer (Tokyo, Japan) using monochromatic Cu Kα radiation (λ = 1.5406 Å) running at 40 kV and 30 mA. The intensity data for the nanoparticle solution deposited on a glass slide was collected over a 2θ range of 25-90° with a scan rate of 1°/min. The X-ray photoelectron spectroscopy analysis was carried out with VG ESCA LAB MK II spectrophotometer employing Al Kα as the incident X-ray source (1486.6 eV) of photoelectrons and a hemispherical analyzer (150 mm diameter) for data collection. The instrument was calibrated by measuring the Au 4f7/2 binding energy at 83.9 eV and the binding energy peak of C1s at 284.6 eV was used as an internal standard for correcting the in binding energy shift in peaks. The size and shape of the nanoparticles were obtained with FEI Tecnai 20 G2 S-Twin (Eindhoven, Netherlands) transmission electron microscope, operating at 200 kV. The samples for electron microscopy were prepared by depositing a drop of colloidal solution on a carbon coated copper grid and drying at room temperature. The zeta potential of the produced nanoparticles was assessed with a Malvern Zetasizer Nano ZS90 (Malvern, UK).
The concentration of platinum in the dissolved SRP containing Pt-I NP was determined by an Analytik Jena AG, Contra AA 700 continuum source ET-AAS (Jena, Germany). A transversely heated graphite tube, MPE 60 autosampler and xenon short arc lamp in hot spot mode operated at 300 W as a continuum radiation source were used. A high resolution double monochromator consisting of a prism and an echelle grating monochromator providing a spectral bandwidth per pixel of ca. 2 pm at 200 nm was used. A linear CCD array detector with a total of 588 pixels, of which 200 pixels were used for the determination of dispersed radiation. The platinum absorption was measured using the central pixel (CP) 1 ± pixels at a spectral interval of 6 pm. In all the stages, argon gas of 99.99% purity was used as a purging gas at flow rate of 250 mL/min, except during atomization stage. A spectral line at 265.9450 nm was used for platinum quantification. The standardized temperature programme for the platinum determination is shown in Table 1.
Step
Temperature (°C)
Ramp (°C/s)
Hold (s)
Drying 1
80
6
20
Drying 2
90
3
20
Drying 3
110
5
10
Pyrolysis
1100
300
10
Gas adaption
1200
0
5
Atomization
2200
1000
4
Cleaning
2400
500
3
3 Results and discussion
The conditions used in the current study on platinum leaching from catalytic converter (5 g of powdered converter in 8 mL of HCl + H2O2 at 70 °C for 2 h) were compared in terms of acid concentration, pulp density, leaching temperature and reaction time, with previous reports on the use of binary mixtures of HCl and H2O2. In an earlier study carried out on platinum recovery from scrap automotive catalytic converter, leaching was carried out at 70 °C for 2 h with a pulp density of 50 g/L using 10.9 M of HCl (Kizilaslan et al., 2009). In another study, leaching was attempted at 65 °C for 3 h with a pulp density of 500 g/L employing 11.6 M of HCl (Harjanto et al., 2006). While, in the present report the leaching was done at 70 °C for 2 h with a pulp density of 625 g/L using 9 M of HCl. Thus, the study achieved platinum leaching at higher pulp density with a lower concentration of acid at a comparable leaching temperature and reaction time. In this study, we have optimized the CPE parameters for the synthesis and preconcentration of Pt-I NP from the leachate of catalytic converter. The effect of each variable process parameter such as concentrations of HCl, KI, NaCl, CPB, Triton-X114, platinum; incubation temperature and complexation time were optimized. The concentration of other metals in the leachate and their interference on the preconcentration of nanoparticles is given in Supplementary information (Sup. Table 1).
3.1 CPE parameter optimization for quantitative synthesis and preconcentration of Pt-I NP
3.1.1 Effect of HCl concentration
It is known that the acid concentration plays a critical role in clouding, mixed micelle phase separation; dissolution of sample matrices and formation of stable and selective extractable species (Meeravali and Jiang, 2008). Hence, the concentration effect of HCl was studied in the range of 0.2–30% (v/v) at 250 ng/mL of platinum, 0.1% KI, 3% NaCl, 0.5% CPB and 1.5% Triton X-114 and the extraction recovery of Pt-I NP is shown in Fig. 1(a). The recovery of nanoparticles increased with an increase in HCl concentration up to 10% and remained more or less same with a further increase in acid concentration. Hence, 10% of HCl at which 100% recovery was noted was selected for further probing the effect of other parameters. The acid concentration optimized in the current study is comparable with previous study on preconcentration and separation of ultra trace levels of Pt from road dust samples by CPE (Meeravali and Jiang, 2008). This could be due to an increase in the concentration of charged species in the surrounding micro-environment of the mixed micelles at higher acidity (Meeravali et al., 2013). The optimized acid concentration of 1.2 M in the present report is comparable with 1 M HCl used in the previous cloud point extraction work carried out on microwave-assisted leaching of catalyst (Suoranta et al., 2015).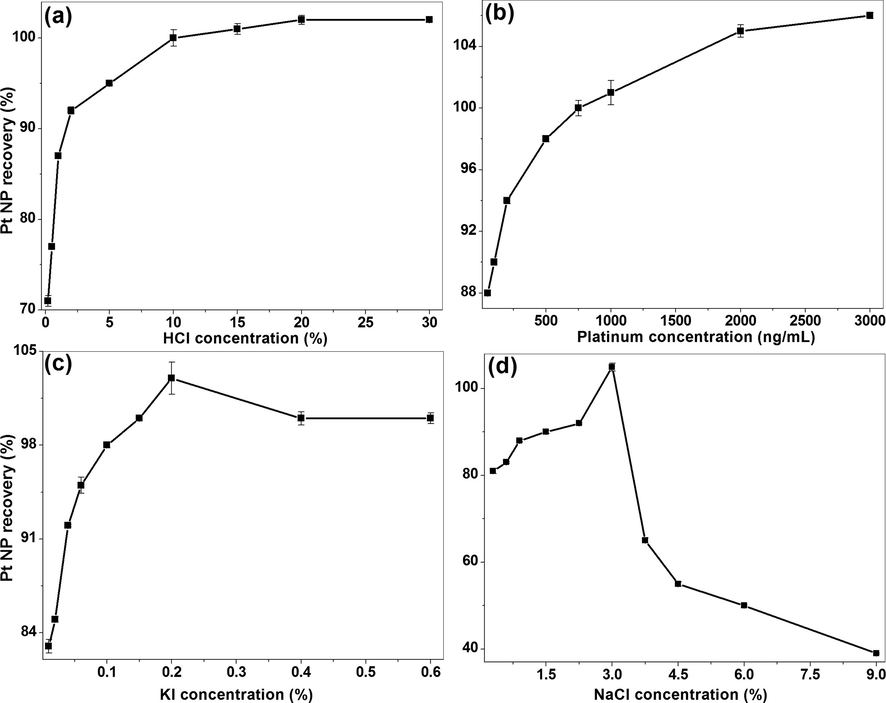
The effect of different concentrations of various parameters on Pt-I NP recovery (%), (a) HCl (Conditions: 250 ng/mL platinum, 0.1% KI, 3% NaCl, 0.5% CPB and 1.5% Triton X-114), (b) platinum (Conditions: 10% HCl, 0.1% KI, 3% NaCl, 0.5% CPB and 1.5% Triton X-114), (c) KI (Conditions: 250 ng/mL platinum, 10% HCl, 3% NaCl, 0.5% CPB and 1.5% Triton X-114) and (d) NaCl (Conditions: 250 ng/mL platinum, 10% HCl, 0.2% KI, 0.5% CPB and 1.5% Triton X-114).
3.1.2 Effect of platinum concentration
The effect of different concentrations of platinum on Pt-I NP recovery was studied in the range of at 50–3000 ng/mL at 10% HCl, 0.1% KI, 3% NaCl, 0.5% CPB and 1.5% Triton X-114. The Pt-I NP extraction efficiency increased linearly with an increase in metal concentration from 50 to 500 ng/mL and 100% recovery was obtained at 750 ng/mL and the trend was continued up to 3000 ng/mL. Thus, the complete Pt-I NP recovery was noted in the concentration range of 750–3000 ng/mL, suggesting the application of the current method for the extraction recovery of platinum over a broad range of metal concentrations [Fig. 1(b)].
3.1.3 Effect of KI concentration
As an alternative to aqua regia and cyanide extraction, the platinum group metals were recovered from the fuel cells using KI leaching (Patel and Dawson, 2015). This has led us to study the effect of KI on Pt-I NP synthesis. The extraction was carried out in the concentration range of 0.01–0.6% (w/v) at 250 ng/mL of platinum, 10% HCl, 3% NaCl, 0.5% CPB and 1.5% Triton X-114. The metal extraction efficiency increased with an increase in KI concentration and reached maximum at 0.2% and saturated beyond this concentration [Fig. 1(c)]. The KI is acting as a stabilizing agent for platinum by forming hexaiodo platinate complex and other reducing species. So it helps in the formation of charged Pt-I NP and in improving the stability. Hence, the optimization of KI is critical in improving the Pt-I NP synthesis.
3.1.4 Effect of NaCl concentration
The electrolyte, NaCl role as a salting out agent has been studied in the concentration range of 0.3–9% (w/v) at 250 ng/mL of platinum, 10% HCl, 0.2% KI, 0.5% CPB and 1.5% Triton X-114. The Pt-I NP extraction increased from 0.3 to 3% and beyond which the extraction efficiency was decreased. The optimum recovery (105%) was obtained at 3% of NaCl [Fig. 1(d)]. The enhanced extraction in the presence of NaCl is due to the surface charge neutralization of mixed micelles with sodium ions, demonstrated by drastic increase in the hydrophobic nature leading to a decrease in the clouding temperature (Meeravali and Kumar, 2012).
3.1.5 Effect of CPB concentration
The concentration of cationic surfactant CPB in the range of 0.2–1% (w/v) on Pt-I NP recovery was studied at 250 ng/mL of platinum, 10% HCl, 0.2% KI, 3% NaCl and 1.5% Triton X-114. The recovery increased from 0.2 to 0.75% and reached saturation at 1% with a recovery 104% [Fig. 2(a)]. Hence, an optimized concentration of 1% CPB was selected for successive studies. Beyond this concentration (1.5–3%), no phase separation was observed as it exceeds the critical micellar concentration of CPB. The results show that without CPB, the clouding system acts as a non-ionic surfactant based CPE due to the absence of electrostatic interacting sites in the non-ionic extracting micelles. It is important to note that the colour of the recovered Pt-I NP solution exhibits brownish green colour which is distinguishable from the yellow colour of the platinum iodo complex. It was noted that the intensity of SRP solution colour is directly proportional to the concentration of Pt-I NP present in the sample.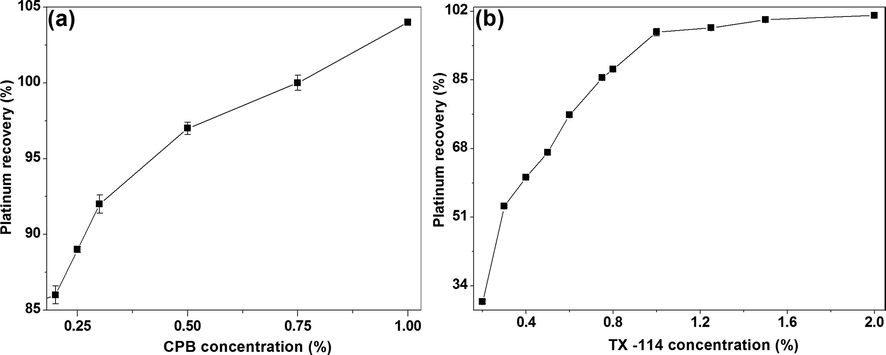
The effect of different concentrations of surfactants, (a) CPB (Conditions: 250 ng/mL platinum, 10% HCl, 0.2% KI, 3% NaCl and 1.5% Triton X-114) and (b) Triton X-114 (Conditions: 250 ng/mL platinum, 10% HCl, 0.2% KI, 3% NaCl and 1% CPB) on Pt-I NP recovery.
3.1.6 Effect of Triton X-114 concentration
The concentration of non-ionic surfactant is critical for the quantitative extraction of analytes in CPE process and it determines the preconcentration factor and phase volume ratio (Meeravali et al., 2010). Hence, the Triton X-114 micelles were utilized for preconcentrating the synthesized Pt-I NP from bulk aqueous phase into a small micelle rich phase in the presence of an electrolyte NaCl. The Pt-I NP recovery studies were carried out in the concentration range of 0.2–2% (w/v) Triton X-114 at 250 ng/mL of platinum, 10% HCl, 0.2% KI, 3% NaCl and 1% CPB. The extraction efficiency was linearly increased from 0.2 to 1.5% and reached saturation at 2% with 101% Pt-I NP recovery [Fig. 2(b)]. Hence, an optimal concentration of 2% Triton X-114 was chosen. It should be noted that in the absence of Triton X-114, only cationic micelle are present in the solution and not able to show clouding and phase separation phenomena due to the high repulsion between the charged head groups. The recovery increased from 0.2 to 0.75% and reached saturation at 1% with a recovery 104% [Fig. 2(a)]. Hence, an optimized concentration of 1% CPB was selected for successive studies. Beyond this concentration (1.5–3%), no phase separation was observed as it exceeds the critical micellar concentration of CPB.
3.1.7 Effect of incubation temperature and complexation time
The complexation of platinum metal and its efficient transfer into micellar aggregates depend upon incubation temperature and time (Meeravali et al., 2010). Hence, the effect of incubation temperature on Pt-I NP recovery was studied in the range of 30–80 °C for 30 min at 250 ng/mL platinum, 10% HCl, 0.2% KI, 3% NaCl, 1% % CPB and 2% Triton X-114. The optimum incubation temperature of the solution was found to be 70 °C [Fig. 3(a)]. It is known that the high temperature provides better dehydration of the micelles and also reduces the volume of the SRP (Meeravali and Jiang, 2008). Further, at this temperature and similar conditions the standardization of incubation time was carried out between 10 and 60 min. It was observed that the transfer of Pt-I NP is a slow process and requires a minimum complexation time of 40 min for its completion of reaction between analytes and solubilizing sites of micelle. Thus, an incubation time of 40 min was chosen for the present study [Fig. 3(b)]. It is evident from the observed zeta potential value of +28.5 mV for the CPB micellar sample solution, which was found to decrease to +12.3 mV in the presence of Triton X-114. Also, the hydrophobicity of the extracting species and the kinetics of their transfer into the micelle aggregates during phase separation are key factors in non-ionic surfactant based CPE methods (Meeravali et al., 2013).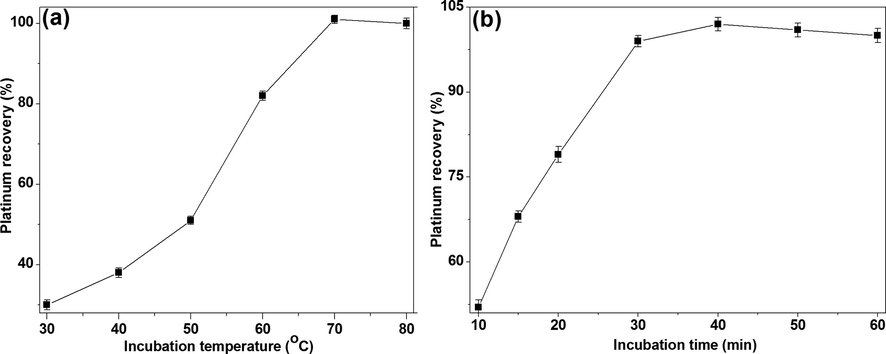
The effect of (a) incubation temperature and (b) complexation time on Pt-I NP recovery (%) at 250 ng/mL platinum, 10% HCl, 0.2% KI, 3% NaCl, 1% CPB and 2% Triton X-114.
3.1.8 Optimization of furnace pyrolysis temperature
The optimization of furnace temperature program is necessary for the determination of platinum using ETAAS. Hence, the effect of furnace pyrolysis temperature on platinum absorbance signal at 100 ng/mL was carried out in the range of 800–1900 °C at an atomization temperature of 2200 °C with an argon flow of 250 mL/min (Fig. 4). It was observed that the integrated absorbance values were increased with an increase in pyrolysis temperature from 800 to 1100 °C and a further increase caused a decrease in the absorbance signal. Thus, a pyrolysis temperature of 1100 °C was chosen for platinum determination. In general, the utilization of higher pyrolysis temperature reduces the background absorbance signal by removing the major components of SRP, thereby increasing the sensitivity of the analyte.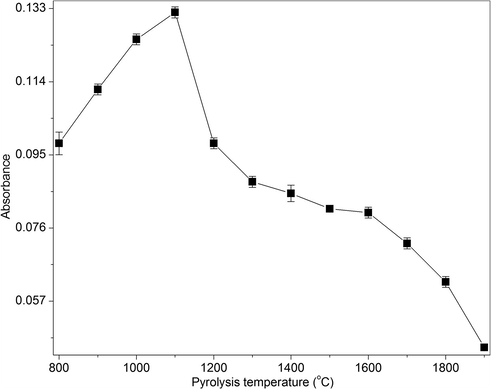
The effect of pyrolysis temperature on the absorbance of platinum at 100 ng/mL of platinum, 10% HCl, 0.2% KI, 3% NaCl, 1% CPB and 2% Triton X-114.
3.2 Characterization of Pt-I NP
3.2.1 UV–visible spectroscopy (UV–vis)
At optimized concentrations of 10% HCl, 0.2% KI, 3% NaCl, 1% CPB and 2% Triton X-114, the formation of triiodide ion and Pt-I NP was followed using UV–vis at 250 ng/mL of platinum (Fig. 5). The aqueous solution of catalytic converter leachate showed a discrete absorption peak at 256 nm, indicating the presence of Pt (IV) ions. In the solution containing HCl and KI, the formation of yellow coloured triiodide ion (I3−) can be clearly observed from the two characteristic peaks at 289 and 351 nm (Balcerzak and Kaczmarczyk, 2001). Under acidic conditions and in the presence of KI, the major, stable iodoplatinate anion produced is hexaiodoplatinate (PtI62−). It is due to the instantaneous formation of PtI62− at a lower potential of 0.40 V, in comparison with other species such as Pt4+ (1.15 V) and PtCl62− (0.657 V) (Jha et al., 2013b). The formation of PtI62− in the presence of iodide is accounted from the characteristic absorption peak at 495 nm (Balcerzak and Kaczmarczyk, 2001; Mizukoshi et al., 2001). The ease of transformation of platinum into iodide complex is routinely employed for the spectrophotometric determination platinum in the solutions of aqua regia digested catalysts (Balcerzak and Kaczmarczyk, 2001). On addition of CPB, the colour of the solution containing Pt, KI and NaCl changed to dark brown colour which could be due to the formation of platinum-iodide-cetylpyridinium ion ternary ion association complex (Kyzas and Bikiaris, 2014), resulting from the electrostatic interaction of anionic hexaiodoplatinate and cationic surfactant (Meeravali et al., 2014). The appearance of dark brown colour in the solution is confirming the formation of hexaiodo platinum nanoparticles (Pt-I NP) arising possibly from the charge transfer complex of hexaiodoplatinate with CPB. This is further reflected in terms of shift in the triiodide absorption maxima to 295 and 364 nm. Upon heating with Triton X-114, the preconcentrated, stabilized nanoparticles collected in the SRP were changed to dark green colour (Inset of Fig. 5). The solution showed peaks at absorption maxima at 277 and 367 nm attributed to shift in absorption maxima of triiodide. The mechanism involved in the formation of the Pt-I NPs from the spent automobile catalytic converter powder is shown in Fig. 6. It is known that the colour of the nanoparticles depends upon the particle size, shape, interparticle distance, surface charge, composition, concentration and surrounding environment. The colour of Pt-I NP synthesized at variable concentrations of platinum, showing different colours is given in Supplementary information (Sup. Fig.2). It is significant to note that the preconcentrated nanoparticles were soluble both in aqueous medium as well as organic solvent methanol.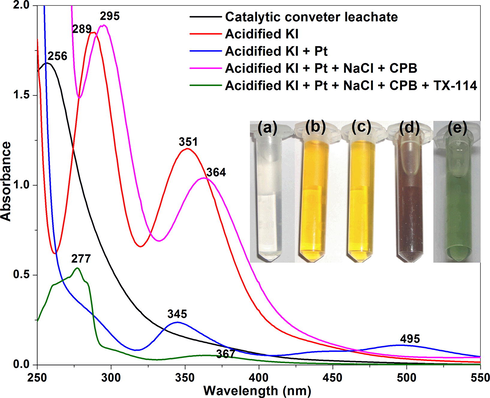
The UV–vis absorption spectra of Pt-I NP synthesized at optimized conditions of CPE (250 ng/mL platinum, 10% HCl, 0.2% KI, 3% NaCl, 1% CPB and 2% Triton X-114). Inset: Colour of the solution (a) catalytic converter leachate, (b) acidified KI, (c) acidified KI + Pt, (d) acidified KI + Pt + NaCl + CPB and (e) acidified KI + Pt + NaCl + CPB + Triton X-114.
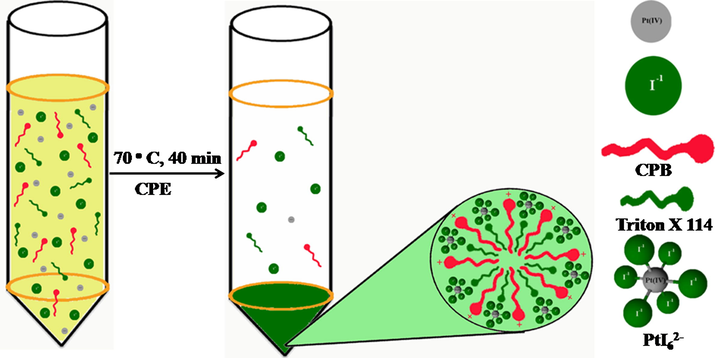
The mechanism involved in the formation of the Pt-I NPs from spent automobile catalytic converter leachate.
3.2.2 Transmission electron microscopy (TEM)
The TEM images of Pt-I NP synthesized at optimum conditions of 250 ng/mL of platinum, 10% HCl, 0.2% KI, 3% NaCl, 1% CPB and 2% Triton X-114 is shown in Fig. 7. The generated nanoparticles were spherically shaped and the size of the particles ranged from 4.0 to 7.4 nm. The average particle size obtained from the corresponding diameter distribution was about 5.6 ± 1.0 nm [Fig. 7(d)]. The selected-area electron diffraction (SAED) pattern showed in Fig. 7(e) exhibits concentric rings with intermittent spots, confirming the crystallinity of the formed nanoparticles.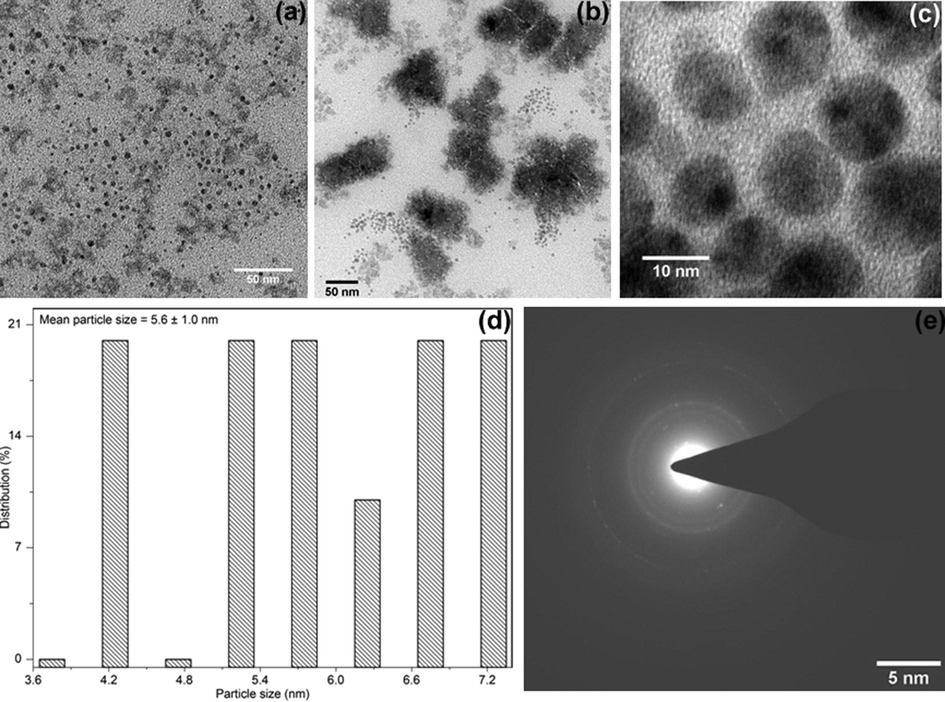
The TEM image of Pt-I NP synthesized at optimized conditions of CPE, at (a) 50 nm scale, (b) micellar aggregates, (c) 10 nm, (d) histogram showing the particle size distribution and (e) corresponding SAED pattern.
3.2.3 X-ray diffraction (XRD)
The difractogram of the preconcentrated Pt-I NP is shown in Fig. 8. There were four well-defined characteristic diffraction peaks at 27.6°, 32°, 45.9 and 53.1°, respectively, indexed to reflections from (2 0 2), (2 2 0), (2 2 4) and (4 2 0) planes of tetragonal crystal structure of dipotassium hexaiodoplatinate. These values are in agreement with standard values (JCPDS PDF card 01–076-2332). Thus, the XRD pattern further substantiates the highly crystalline nature of Pt-I NP observed from SAED pattern (Fig. 7). It is pertinent to note that the absence of metallic platinum peaks in difractogram at 39.76°, 46.24°, 67.45, 81.29° and 85.71° indicates that the platinum in nanoparticles is not reduced to elemental state but it is indeed in Pt (IV) state (JCPDS PDF card 004–0802). The presence of Pt (IV) is confirmed from the presence of characteristic absorption peak at 495 nm in UV–vis (Mizukoshi et al., 2001).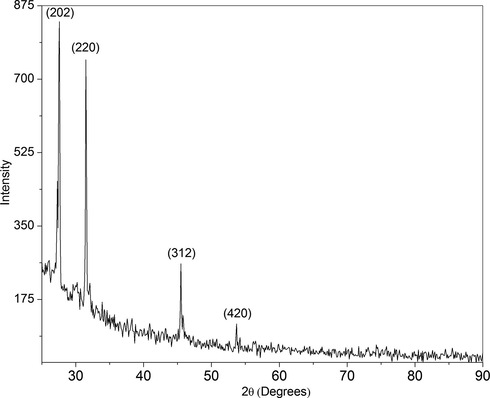
X-ray diffraction pattern of Pt-I NP showing the tetragonal crystal structure.
3.2.4 X-ray photoelectron spectroscopy (XPS)
The 4f spectrum for the Pt-I NP deconvoluted into two distinctive peaks is shown in Fig. 9(a). The binding energies of Pt 4f5/2 and 4f7/2 were found at 74.28 and 68.68 eV, respectively, corresponding to Pt (IV) state. Also, no 4f7/2 peaks at 71.1 and 72.82 eV indicating the absence of platinum in (II) and zero valent states (Dablemont et al., 2008; Fu et al., 2001; Li et al., 2015; Wu et al., 2014). Thus, the results further confirm the findings of XRD. The spin orbit 3d spectral doublets of metal iodide were found at 630.19 and 618.82 eV, corresponding to 3d3/2 and 3d5/2, respectively (Reiller et al., 2006) [Fig. 9(b)].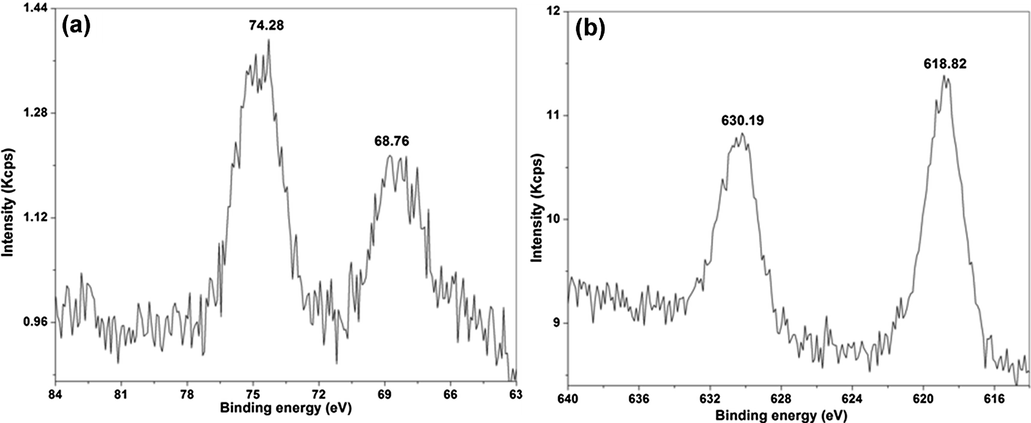
The XPS spectra of Pt-I NP showing the binding energy of (a) Pt 4f region and (b) I 3d region.
3.3 Analytical figures of merit
The preconcentration factor (PCF) is the ratio of Pt-I NP concentration in SRP to that of the initial aqueous phase. The phase volume ratio (PVR) is defined as the ratio between final volume of SRP to that of the aqueous phase. Both these factors depend upon the initial aqueous phase volume and added diluents volumes to volume of SRP after phase separation (Meeravali and Jiang, 2008). The PCF and PVR were calculated for the developed CPE method at optimized conditions, by varying the sample volumes from 10 to 100 mL. It was noted that with an increase in sample volume, there was a raise in the SRP volume, leading to larger volumes of required diluents for efficient dissolution. The PVR decreased with an increase in the sample volume and reached a plateau from 25 to 75 mL and further increased at 100 mL. The PCF for platinum increases from 5 to 25, when the sample volume is increased from 10 to 25 mL, remains saturated from 50 to 75 mL and decreased at 100 mL. Hence, the optimized sample volume is 25 mL and the PVR and PCF were found to be 0.04 and 25, respectively (Fig. 10). It is significant to note that around 94% recovery of Pt-I NP was obtained in the current method at 1.28 µM concentration of platinum (250 ng/mL). As there were no reports on the preconcentration of Pt-I NP by CPE, we have compared the recoveries with earlier CPE studies on gold and palladium nanoparticles. The recoveries were found to be 52% and 50% for gold and palladium nanoparticles, synthesized at millimolar concentration, respectively (Nazar et al., 2011). The accuracy of the proposed procedure was validated by analyzing the certified reference materials including CCRMP PTM-1a and CCRMP PTC-1a, after closed acid leaching. The obtained values are in good agreement with the certified values based on student t-test at 95% confidence level. The values obtained for various spent automobile catalytic converter leachate samples are also given (Table 2). In literature, various nanomaterials such as carbon nanotubes surface functionalized with amine (Zhang et al., 2015), chitosan (Dou et al., 2019); and SiO2 hybrid materials functionalized with amine and polyphenols (Huang et al., 2018) are reported for the adsorptive preconcentration of Cu metal ion.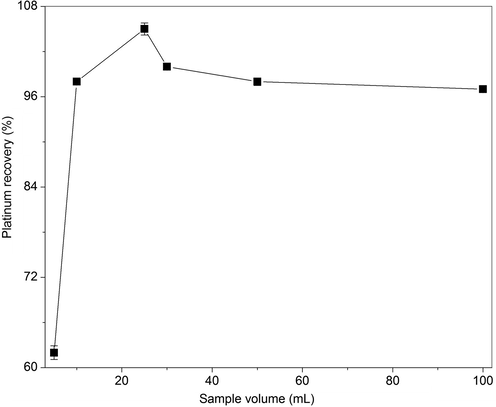
The effect of sample volume on the preconcentration factor (PCF) and phase volume ratio (PVR) for Pt-I NP by CPE method.
Type of matrix
Measured value (µg/g)
Certified value (µg/g)
PTM-1a
7.02 ± 1.3
7.29 ± 0.22
PTC-1a
2.8 ± 0.8
2.72 ± 0.11
Catalytic converter 1
656 ± 9.6
–
Catalytic converter 2
689 ± 3.1
–
Catalytic converter 3
782 ± 2.6
–
Catalytic converter 4
896 ± 6.1
–
Catalytic converter 5
1060 ± 7.2
–
Catalytic converter 6
1072 ± 8.3
–
4 Conclusions
A simple and novel cloud point procedure has been developed for the simultaneous synthesis and preconcentration of Pt-I NP from the catalytic converter leachate. In this single pot method, the formation and preconcentration of 5.6 nm sized Pt-I NP was noted at a diluted metal precursor concentration. Thus, the current study paves a way for the synthesis of Pt-I NP, which are inaccessible and difficult to produce in traditional methods (Stoppiello et al., 2017). Interestingly, the demonstrated dual solubility of the preconcentrated Pt-I NP makes them as ideal candidate for both chemical and biological applications. They could find a variety of technological applications such as anticancer drug synthesis, optoelectronics (Evans et al., 2018), solving unknown protein structures (Tanley et al., 2014), semiconductors (Pal, 2018) etc. Also, the biodegradability of the employed surfactants and the utilization of metal scrap in the present protocol follow the principles of green chemistry. The developed method can also be exploited for the synthesis and preconcentration of other platinum group metals from electronic waste etc. Our current objectives are oriented in this direction.
References
- Green Chemistry: Theory and Practice. New York: Oxford University Press; 1998. p. :30.
- Rapid derivative spectrophotometric method for the determination of platinum in Pt-Ru/C catalyst using iodide media. Anal. Sci.. 2001;17:1321-1324.
- [Google Scholar]
- Green synthesis, characterization and anticancer potential of platinum nanoparticles Bioplatin. J. Chin. Integr. Med.. 2012;10(6):681-689.
- [Google Scholar]
- Controlled growth of platinum nanoparticles on strontium titanate nanocubes by atomic layer deposition. Small. 2009;5(6):750-757.
- [Google Scholar]
- Company, T.D.C. Triton X-114 -Technical data sheet-Dow, vol. 2017, pp. Form No. 119-01884-1107.
- FTIR and XPS study of Pt nanoparticle functionalization and interaction with alumina. Langmuir. 2008;2008(24):5832-5841.
- [Google Scholar]
- Functionalization of carbon nanotubes with chitosan based on MALI multicomponent reaction for Cu2+ removal. Int. J. Biol. Macromol.. 2019;136:476-485.
- [Google Scholar]
- Hydrogen bonding controls the structural evolution in perovskite-related hybrid platinum(IV) Iodides. Inorg Chem. 2018;57(16):10375-10382.
- [Google Scholar]
- Surface Modification of small platinum nanoclusters with alkylamine and alkylthiol: an XPS study on the influence of organic ligands on the Pt 4f binding energies of small platinum nanoclusters. J. Colloid Interface Sci.. 2001;243(2):326-330.
- [Google Scholar]
- Micelle mediated extraction of fatty acids from microalgae cultures: Implementation for outdoor cultivation. Sep. Purif. Technol.. 2014;135:127-134.
- [Google Scholar]
- Leaching of Pt, Pd and Rh from automotive catalyst residue in various chloride based solutions. Mater. Trans.. 2006;47(1):129-135.
- [Google Scholar]
- Species selective preconcentration and quantification of gold nanoparticles using cloud point extraction and electrothermal atomic absorption spectrometry. Anal. Chim. Acta. 2013;761:27-33.
- [Google Scholar]
- Size control synthesis of polymer-stabilized water-soluble platinum oxide nanoparticles. J. Colloid Interface Sci.. 2007;308(1):105-111.
- [Google Scholar]
- A new application of functionalized platinum nanoparticles as chemiresistor coating. Measurement. 2013;46(9):3328-3332.
- [Google Scholar]
- Formation and characterization of water-soluble platinum nanoparticles using a unique approach based on the hydrosilylation reaction. Langmuir. 2004;20(12):5145-5148.
- [Google Scholar]
- Facile preparation of polyethylenimine-tannins coated SiO2 hybrid materials for Cu2+ removal. Appl. Surf. Sci.. 2018;427:535-544.
- [Google Scholar]
- Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: a review. Hydrometallurgy. 2013;133:23-32.
- [Google Scholar]
- Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: a review. Hydrometallurgy. 2013;133:23-32.
- [Google Scholar]
- Towards environmentally safe recovery of platinum from scrap automotive catalytic converters. Turk. J. Eng. Environ. Sci.. 2009;33:83-90.
- [Google Scholar]
- Nanoseparations: strategies for size and/or shape-selective purification of nanoparticles. Curr. Opin. Colloid Interface Sci.. 2011;16(2):135-148.
- [Google Scholar]
- Molecular imprinting for high-added value metals: an overview of recent environmental applications. Adv. Mater. Sci. Eng.. 2014;2014:8.
- [Google Scholar]
- Synthesis of heterogeneous catalysts with well shaped platinum particles to control reaction selectivity. Proc. Natl. Acad. Sci.. 2008;105(40):15241-15246.
- [Google Scholar]
- Morphology-controlled growth of Pt nanoparticles taking advantage of smaller molecule and inorganic salt. Acta Mater.. 2014;63:202-208.
- [Google Scholar]
- BSA-stabilized Pt nanozyme for peroxidase mimetics and its application on colorimetric detection of mercury(II) ions. Biosens. Bioelectron.. 2015;66:251-258.
- [Google Scholar]
- Electrochemical patterning of platinum nanoparticles for methanol oxidation on single-walled carbon nanotube films. Meeting Abstr.. 2010;MA2010-01(1):73.
- [Google Scholar]
- Synthesis of platinum nanocrystals within iodine ions mediated. J. Nanomater.. 2018;2018:10.
- [Google Scholar]
- Green synthesis of highly stable platinum nanoparticles stabilized by amino-terminated ionic liquid and its electrocatalysts for dioxygen reduction and methanol oxidation. Electrochem. Commun.. 2009;11(2):351-354.
- [Google Scholar]
- Methods for separation, identification, characterization and quantification of silver nanoparticles. TrAC Trends Anal. Chem.. 2012;33:95-106.
- [Google Scholar]
- Interference free ultra trace determination of Pt, Pd and Au in geological and environmental samples by inductively coupled plasma quadrupole mass spectrometry after a cloud point extraction. J. Anal. At. Spectrom.. 2008;23(6):854-860.
- [Google Scholar]
- An acid induced mixed-micelle mediated cloud point extraction for the separation and pre-concentration of platinum from road dust and determination by inductively coupled plasma mass spectrometry. Anal. Methods. 2010;2(8):1101-1105.
- [Google Scholar]
- Determination of Cd, Pb, Cu, Ni and Mn in effluents and natural waters by a novel salt induced mixed-micelle cloud point extraction using ETAAS. Anal. Methods. 2012;4(8):2435-2440.
- [Google Scholar]
- Microwave assisted aqua regia extraction of thallium from sediment and coal fly ash samples and interference free determination by continuum source ETAAS after cloud point extraction. Talanta. 2013;104:180-186.
- [Google Scholar]
- Sequential extraction of platinum, cisplatin and carboplatin from environmental samples and pre-concentration/separation using vesicular coacervative extraction and determination by continuum source ETAAS. Talanta. 2014;118:37-44.
- [Google Scholar]
- Preparation of platinum nanoparticles by sonochemical reduction of the Pt(IV) ions: role of surfactants. Ultrason. Sonochem.. 2001;8(1):1-6.
- [Google Scholar]
- Characterization of binary gold/platinum nanoparticles prepared by sonochemistry technique. Appl. Surf. Sci.. 2005;241(1–2):209-212.
- [Google Scholar]
- Separation and recycling of nanoparticles using cloud point extraction with non-ionic surfactant mixtures. J. Colloid Interface Sci.. 2011;363(2):490-496.
- [Google Scholar]
- The synthesis and characterization of platinum nanoparticles: a method of controlling the size and morphology. Nanotechnology. 2010;21(3):035605.
- [Google Scholar]
- Pal, S., 2018. Pyridine: A Useful Ligand in Transition Metal Complexes. https://www.intechopen.com/books/pyridine/pyridine-a-useful-ligand-in-transition-metal-complexes (Ed.) P.P. Pandey, IntechOpen.
- Recovery of platinum group metal value via potassium iodide leaching. Hydrometallurgy. 2015;157:219-225.
- [Google Scholar]
- Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc.. 2003;125(46):13940-13941.
- [Google Scholar]
- Phase transfer catalyst assisted directly suspended droplet microextraction of platinum from geological and spent automobile converter samples prior to HR-CS AAS determination. Anal. Methods. 2013;5(9):2343-2351.
- [Google Scholar]
- Iodination of humic acid samples from different origins. Radiochim. Acta. 2006;94:739-745.
- [Google Scholar]
- Effect of surfactant type on platinum nanoparticle size of composite Pt/α-Al2O3 catalysts synthesized by a microemulsion method. J. Colloid Interface Sci.. 2014;426:287-292.
- [Google Scholar]
- A one-pot-one-reactant synthesis of platinum compounds at the nanoscale. Nanoscale. 2017;9(38):14385-14394.
- [Google Scholar]
- Recovery of palladium, platinum, rhodium and ruthenium from catalyst materials using microwave-assisted leaching and cloud point extraction. Hydrometallurgy. 2015;154:56-62.
- [Google Scholar]
- Visual and colorimetric detection of Hg2+ by cloud point extraction with functionalized gold nanoparticles as a probe. Chem. Commun.. 2009;45:7030-7032.
- [Google Scholar]
- The binding of platinum hexahalides (Cl, Br and I) to hen egg-white lysozyme and the chemical transformation of the PtI6 octahedral complex to a PtI3 moiety bound to His15. Acta Crystallogr F Struct. Biol. Commun.. 2014;70(Pt 9):1132-1134.
- [Google Scholar]
- Handbook of environmental data on organic chemicals (second ed.). New York: Van Nostrand Reinhold Co.; 1983.
- A novel one-pot 'green' synthesis of stable silver nanoparticles using soluble starch. Carbohydr. Res.. 2006;341(12):2012-2018.
- [Google Scholar]
- Citrate-capped platinum nanoparticle as a smart probe for ultrasensitive mercury sensing. Anal. Chem.. 2014;86(21):10955-10960.
- [Google Scholar]
- Combined cloud point extraction and Tween 20-stabilized gold nanoparticles for colorimetric assay of silver nanoparticles in environmental water. Anal. Methods. 2011;3(12):2915-2920.
- [Google Scholar]
- Synthesis and size control of Pt nanocubes with high selectivity using the additive effect of NaI. Chem. Lett.. 2005;34(7):1050-1051.
- [Google Scholar]
- Development of cloud point extraction for simultaneous extraction and determination of platinum and palladium using Inductively Coupled Plasma Optical Emission Spectrometry in platinum-palladium spent catalysts. Anal. Lett.. 2010;43(6):972-982.
- [Google Scholar]
- Preparation of amine functionalized carbon nanotubes via a bioinspired strategy and their application in Cu2+ removal. Appl. Surf. Sci.. 2015;343:19-27.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.10.008.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







