In situ visual and content changes analysis of coumarins in Radix Angelicae dahuricae by LSCM combined with LC-MS technology
⁎Corresponding author. liqian1984@gsau.edu.cn (Qian Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this study, a simple, accurate and green localization method of coumarins in Radix Angelicae dahuricae was established with fresh Radix Angelicae dahuricae as research material to reveal the distribution and accumulation of coumarins, based on frozen section and fluorescence imaging technology. The best frozen section conditions were established by comparing the effects of different cryoprotectants on the quality of Radix Angelicae dahuricae's frozen sections, according to the loss of coumarins and the complexity of the operation process. The coumarin components in Radix Angelicae dahuricae at different stages were located and quantitatively analyzed, and coumarin components distribution positions and content changes were identified using fluorescence imaging combined with LC-MS technology. The results showed that 30 μm slice thickness with 15 % glycerin protectant treatment is the best condition for frozen section. Fluorescence imaging showed that coumarins in Radix Angelicae dahuricae were mainly distributed in secretory tissue, the content over different periods showed an “S” curve of growth and coumarins reached their highest content in early September. The distribution and accumulation of coumarins in roots were revealed, which provided a reference for the synthesis and metabolism mechanism of metabolites in medicinal plants and the quality evaluation of traditional Chinese medicine.
Keywords
Radix Angelicae dahuricae
LC-MS
LSCM
Coumarins
1 Introduction
Radix Angelicae dahuricae is the dry root of Angelica dahurica (Fisch, ex Hoffm.) Benth.et Hook.f. or Angelica dahurica (Fisch. ex Hoffm.) Benth.et Hook.f.var.formosana (Boiss.) Shan et Yuan (Pharmacopoeia of the People's Republic of China, 2020). Pharmacological effects of Radix Angelicae dahuricae extracts include antioxidation (Piao et al., 2004), anti-inflammatory (Mehnaz et al., 2012), skin whitening (Cho et al., 2006), antitumor (Kim et al., 2007) and anti-Alzheimer (Marumoto and Miyazawa, 2010) effects. It contains coumarins, volatile oils, polysaccharides and other chemical components (Shu et al., 2020). Coumarins, as one of the important active ingredients of Radix Angelicae dahuricae, have the effects of relieving acute allergic reaction, muscle relaxation, vasodilation and anticancer (Cheng et al., 2016; Chiou et al., 2001; García-Argáez et al., 2000; Li and Wu, 2017) after chronic inflammatory reaction. At present, the research on coumarins in Radix Angelicae dahuricae is mainly focused on extraction, separation and pharmacological efficacy, and the correlation between structural development and coumarins accumulation site is less studied. The author plans to use plant segmentation technology combined with fluorescence imaging technology to carry out relevant research on the accumulation rules of effective components in Radix Angelicae dahuricae.
Frozen sectioning is an experimental technique to quickly freeze plant tissues at low temperatures and slice them after reaching a certain hardness (Boughton and Thinagaran, 2018). Compared to other production techniques, it is easy to maintain the original life form, and has the advantages of being fast, simple and easy to operate. It is commonly used in histochemistry, immunolocalization, in situ hybridization and other studies (Hernandez and Costa, 2022). At present, frozen sectioning technology has matured frozen sectioning conditions in animal and human research. However, due to the high-water content and cell wall of plant cells, the hardness of plant tissues becomes larger and easy to break after freezing, making it difficult to cut sections with complete structure (Hasilo et al., 2014; Wang and Guo, 2021). Therefore, this study obtained the best production method of Radix Angelicae dahuricae by optimizing the frozen section conditions. Fluorescence imaging technology is to use computers to simulate the visual function of the human eye, obtain fluorescence images of plants without damaging the appearance of plants and affecting their growth (Fu et al., 2022; Hui et al., 2017), and conduct relative quantitative analysis of the content of effective components in plant tissues by using fluorescence intensity through image processing algorithms (Chen et al., 2022; Ji et al., 2022). This technology has been applied in Traditional Chinese Medicine (Loh et al., 2021; Kucherenko et al., 2010), biology (Chunyan and Qiangbin, 2019) and clinical medicine (Zhao et al., 2022). Laser scanning confocal microscope (LSCM) has attracted a lot of attention because of its advantages of high resolution and depth tomography. It has the characteristics of non-destructive real-time observation of living cells and biological tissue samples, so it has become a powerful tool for the detection of fluorescent materials in the biological and medical fields (Zeng et al., 2020). Compared to ordinary optical microscopes, LSCM has significant advantages in spatial resolution, noninvasive and non-invasive real-time optical segmentation, three-dimensional imaging, etc. (Jariya et al., 2014). Compared with other processing methods, fluorescence imaging technology uses the characteristics of coumarins autofluorescence, and does not require dyeing and heating treatment, reflecting the concept of green environmental protection. In addition, liquid chromatography mass spectrometry (LC-MS) is a widely used technical means in recent years. It is highly sensitive and selective, which can ensure accurate quantitative analysis of trace and multi-component in complex systems (Lianza et al., 2022). At present, there are increasing studies on simultaneous quantitative analysis and pharmacokinetic analysis of various furanocoumarins in Radix Angelicae dahuricae by LC-MS technology (Chen et al., 2015; Kang et al., 2008; Zhao et al., 2016). Therefore, the frozen section combined with LSCM technology based on the spontaneous fluorescence characteristics of compounds in plants can be used to quickly locate and analyze compounds. In addition, LC-MS technology can be used for quantitative analysis of compounds.
In this study, the best sectioning method for Radix Angelicae dahuricae was established by using frozen sectioning technology. Combined with fluorescence imaging and LC-MS technology, the localization and quantitative analysis of coumarins in Radix Angelicae dahuricae at different growth stages were carried out, and the best slicing method was established to reveal the distribution and accumulation of coumarins in the root. It provides an effective theoretical basis for the production, distribution and accumulation of chemical compounds in Radix Angelicae dahuricae, as well as a reference for the metabolic mechanism and quality evaluation of traditional Chinese medicine.
2 Materials and methods
2.1 Plant materials
Radix Angelicae dahuricae was collected from Maxia Town (Huating City, Gansu Province, China, 165°65′E, 35°22′N). The roots of Angelica dahurica CV. Yubaizhi, an umbrella plant, was identified by Professor Yuan Chen (Department of Chinese Herbal Medicine, Gansu Agricultural University, China). Fresh Radix Angelicae dahuricae in different growth periods (March, May, July, September and November) was used as the material.
2.2 Chemicals and instruments
Glycerin (lot: 20210506; Sinopharm Chemical Reagent, Beijing, China). OCT embedding agent (lot: 14020108926; Leica, Wetzlar, Germany). Imperatorin (lot: 18051502; purity ≥ 98.0 %), isoimperatorin (lot: 18062202; purity ≥ 98.0 %), xanthotoxin (lot: 21070206; purity = 99.88 %), 7-hydroxycoumarin (lot: 20052901; purity = 99.07 %) and bergapten (lot: 171205001; purity ≥ 98.0 %) were sourced from Chengdu Pufeide Biotechnology (Chengdu, China). Methanol (lot: 20191120; Sinopharm Chemical Reagent, Beijing, China) was chromatographic purity.
Laser scanning confocal microscope (model: LSCM 800; Carl Zeiss, Germany), forward inverted integrated fluorescence microscope (model: RVL100-G; ECHO, America), freezing microtome (model: CM1950; Leica, Germany), triple four pole liquid chromatography-mass spectrometer (model: 1290–6460; Agilent, America), fluorescence spectrophotometer (model: F97; Shanghai Lingguang Technology Co., ltd.).
2.3 Selection of the best frozen section method
2.3.1 Screening of slice thickness
Fresh Radix Angelicae dahuricae was washed and cut into small segments of ∼ 0.5 cm to obtain a sample. Samples were poured into 15 % glycerin and vacuumized until the sample sank to the bottom of the bottle. Then the glycerin solution on the surface was sucked dry. The sample was immersed in the pre-cooled sample holder with OCT embedding agent, frozen on the freezing table until OCT turned white, and then moved to the head of the slicer for slicing. They were cut into 20 µm, 25 µm, 30 µm, 35 µm, 40 µm thick pieces, integrity and stretch were observed under the bright-field of the forward inverted integrated fluorescence microscope.
2.3.2 Screening of cryoprotectants
In the frozen sectioning process of plant tissue, cryoprotectants play an important role in the stability of active components in plant tissue and the integrity of sectioning. Currently glycerin and sucrose are commonly used as cryoprotectants (Bromfield and Rosa, 2013). Protectants can combine with free water in plant tissue and reduce damage to ice crystals to cells. Therefore, Radix Angelicae dahuricae samples were placed in distilled water, sucrose solution with mass fractions of 5 %, 10 %, 15 %, 20 %, 25 % and glycerin solution with the same volume fraction, and vacuumized until the materials were sunk to the bottom of the bottle. The best cryoprotectant was obtained by comparing the quality of slices treated with different cryoprotectants.
2.3.3 Establishing the evaluation equation for the frozen section
Traditional evaluation methods cannot judge the change in overall slice quality caused by the small concentration change of cryoprotectant, so we (Chen et al., 2022) improved it in a previous study. The evaluation equation of frozen section of medicinal plant Angelica sinensis was established, y = λ1a + λ2b + λ3c + λ4d + λ5e + λ6f + λ7g(λ1 ∼ λ7 is the grade coefficient, indicating the importance of each item, λ1 + λ2 + λ3 + λ4 + λ5 + λ6 + λ7 = 20). A represents the integrity of the observed object (the integrity of the secretory cavity and catheter), b refers to the retention degree of the test content (the retention degree of coumarin), c refers to the integrity of the cork layer and cork inner layer, d refers to the difficulty of freezing the material, e refers to the slice stretch, f refers to the integrity of tissue and cells, g refers to the reduction of ice crystal damage. The slices of the same concentration were repeated for 3 times, and the image quality of the 3 repetitions was scored according to items a to g, with a full score of 5 points for each item. The final total score is calculated after the average of the total scores of three repetitions. The evaluation equation is y = 5a + 4b + 3d + 2 (c + e + f + g). The higher the score, the better the slice quality.
2.4 Localization of coumarins in Radix Angelicae dahuricae by fluorescence microscopy
2.4.1 Observation of coumarins autofluorescence
Coumarins can produce blue fluorescence under UV irradiation, and are widely used because of their high quantum yield and stability (Zhao et al., 2016). Therefore, imperatorin was selected for spontaneous fluorescence observation, which is the high content of coumarin in Radix Angelicae dahuricae. A small amount of imperatorin standard was placed on the concave glass slide, and 15 % glycerin was added to seal the slide. The slides were placed under the DAPI channel of the forward inverted integrated fluorescence microscope for observation.
2.4.2 Localization of coumarins in Radix Angelicae dahuricae root.
Samples obtained from frozen sections are fixed with 95 % ethanol for 30 min to remove coumarins, because coumarins are readily soluble in methanol, ethanol, chloroform and other organic solvents (Sun et al., 2020). After the film was sealed, it was observed under the DAPI channel of the forward inverted integrated fluorescence microscope.
2.5 Relative quantification of coumarins in Radix Angelicae dahuricae by LSCM
2.5.1 Determination of coumarins fluorescence parameters in Radix Angelicae dahuricae
In this study, imperatorin and isoimperatorin standards were selected to determine coumarin fluorescence parameters, as they were high-content compounds in Radix Angelicae dahuricae. Imperatorin and isoimperatorin weighed 0.2 mg, dissolved in a 10 mL volumetric flask of methanol, and then ultrasonically dissolved for 2 min to obtain the test solution, respectively. The test solution was placed in the cuvette and fed into the F97 fluorescence spectrophotometer. Combined with the excitation wavelength of LSCM, the fluorescence value of each substance was detected to determine the best excitation wavelength of Radix Angelicae dahuricae in LSCM.
2.5.2 Relative quantitative analysis of coumarins in Radix Angelicae dahuricae at different periods
According to the research of Qiang Lu (Lu, 2009), the growth and development period of Radix Angelicae dahuricae can be divided into four stages. Seedling stage: mid-September to early March. Leaf growth period: early March to early May. Root growth period: from early May to mid-July. Harvest period. mid-July to mid-September. Therefore, fresh Radix Angelicae dahuricae from five different growth periods in early March, early May, mid-July, early September and early November were selected as materials. Fluorescence intensity refers to the intensity of the light emitting fluorescence. The fluorescence intensity is proportional to the concentration of the fluorescent substance, so the average fluorescence intensity is used to represent the coumarin content. Tissue sections of Radix Angelicae dahuricae at different stages were prepared under selected optimum frozen section conditions, and then fluorescence imaging was observed and photographed under LSCM with the selected excitation wavelength. Fluorescence images of 10 different visual fields were obtained for each tissue section, and the average fluorescence intensity was recorded.
2.6 Quantitative analysis of coumarins in Radix Angelicae dahuricae at different periods by LC-MS
2.6.1 Preparation of the control solution
Standards for imperatorin, isoimperatorin, bergapten, xanthotoxin and 7-hydroxycoumarine (Fig. 1), all at 1 mg, were accurately weighed and dissolved in methanol solution, respectively. Then, a control solution (100 µg/mL) was prepared. They are taken in an appropriate amount, mixed and diluted to obtain a mixed control solution with a concentration of 10 µg/ mL.
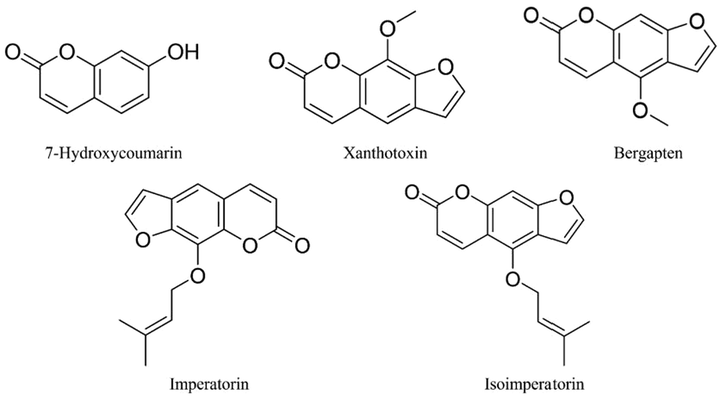
- Structures of coumarins of Radix Angelicae dahuricae.
2.6.2 Preparation of test solution
Radix Angelicae dahuricae in different periods was dried at 37 ℃ (Zhang, 2005) and then crushed and sieved through 40 mesh. The medicinal powder of Radix Angelicae dahuricae in different periods was weighed to 3 g, added 30 mL of methanol in a conical flask, soaked for 30 min, extracted in a 40 ℃ water bath by ultrasound (power: 360 W, frequency: 40KHz) for 30 min, cooled to room temperature, and then methanol was used to make up the lost weight. A certain amount of solution was taken into a centrifuge tube and centrifuged into a high-speed centrifuge (speed: 8000r/min, time: 10 min), and then the supernatant was taken for 0.22 µm microporous membrane filtration to obtain the test solution.
2.6.3 LC-MS conditions
The data were obtained by liquid chromatography mass spectrometry (Agilent 1290–6460, U.S.A.). Chromatographic column: Agilent Eclipse Plus-C18 (150 mm × 2.1 mm, 1.8-µm); mobile phase: 0.1 % formic acid (a)–methanol (b), gradient elution (0–0.5 min, 5 %-15 % B; 0.5–0.8 min, 15 %-28 % B; 0.8–1.0 min, 28 %–32 % B; 1.0–1.2 min, 32 %-38 % B; 1.2–1.5 min, 38 %-45 % B; 1.5–4.5 min, 45 %-65 % B; 4.5–5.5 min, 65 %-95 %B; 5.5–6.0 min, 95 %-5% B; 6.0–8.0 min, 95 %-5% B); Column temperature: 35 ℃; Flow rate: 0.3 mL/min; Injection volume: 10 µL.
Mass spectrometry conditions: the dry gas and atomizer are nitrogen; the temperature of dry gas is 295 ℃, the flow rate of dry gas is 11 L/min, the atomizer pressure is 15 psi, the electric spray ion source (ESI), the spray voltage is-4.0 KV, the positive and negative ion monitoring mode and multiple reaction scanning mode (MRM) are selected.
3 Results and discussion
3.1 Selection of the best method of frozen section
3.1.1 Investigation of optimum thickness of frozen sections
As shown in Fig. 2, when the slice thickness of Radix Angelicae dahuricae is less than 30 µm, the degree of tissue fragmentation and the difficulty of slicing increase. When the slice thickness exceeds 30 µm, the slice is too thick and the field of vision is dark, although the slice integrity and stretch are high, resulting in reduced cell tissue clarity. Therefore, 30 µm was chosen as the slice thickness of Radix Angelicae dahuricae.
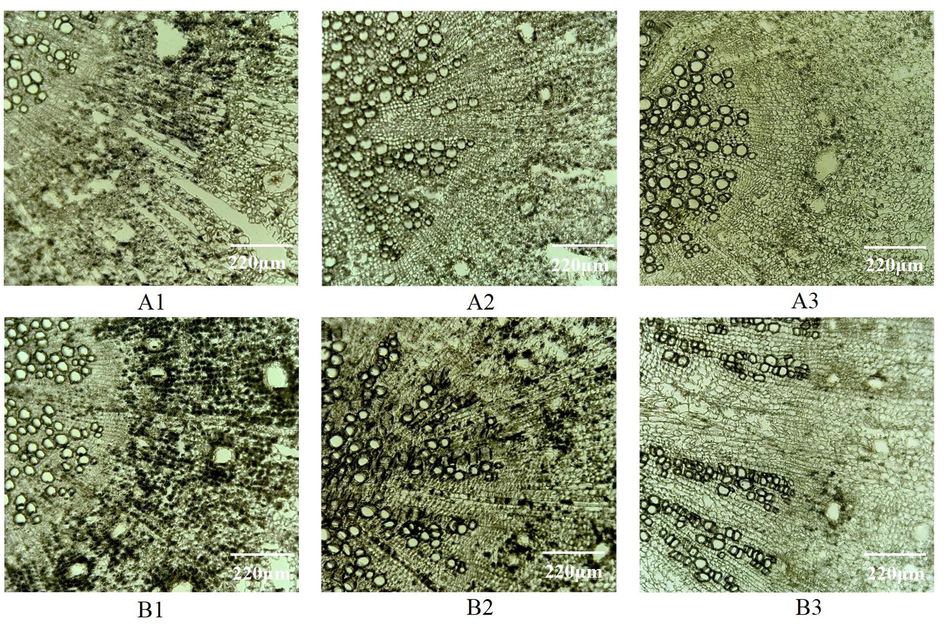
- Microscopic observation on frozen sections of Radix Angelicae dahuricae with different thickness Notes: A1:20 µm, A2:25 µm, A3:30 µm, B1:35 µm, B2:40 µm, B3:30 µm.
3.1.2 The best condition of frozen section
It could be seen from Table 1 and Fig. 3 that different concentrations of glycerin and sucrose as protectants have a significant impact on the integrity of frozen sections of Radix Angelicae dahuricae. When the concentration of glycerin and sucrose is less than 15 %, slice integrity gradually increases with the increase of concentration. When the concentration exceeds 15 %, it is difficult to freeze and slice the tissue, and the slices are difficult to transfer to the slide. However, when sucrose solution of the same concentration is used as a cryoprotectant, the integrity of the observed object is lower than that of glycerin solution. It could be concluded that soaking in sucrose for a long time will lead to softening of tissue cells in Radix Angelicae dahuricae, and the degree of chip fragmentation is serious through the slicing process. The integrity of no cryoprotectant and distilled water as protectants is poor, as shown in Fig. 3 (C2, C3). Therefore, when 15 % glycerin is selected as the most cryoprotectant for the frozen section of Radix Angelicae dahuricae, the section quality is the best and the integrity is high, as shown in Fig. 3(A2). The use of protectants plays an important role in the stability of active ingredients in plant tissues and the integrity of sections in the freezing process of plant tissues.
| Processing method | a | b | c | d | e | f | g | Total score1) |
|---|---|---|---|---|---|---|---|---|
| 5 % glycerin | 3 | 3 | 2 | 4 | 2 | 3 | 3 | 60.67d, e |
| 10 % glycerin | 3 | 3 | 3 | 4 | 3 | 3 | 4 | 68.00c, d |
| 15 % glycerin | 4 | 4 | 5 | 4 | 3 | 4 | 4 | 87.33a |
| 20 % glycerin | 4 | 4 | 4 | 3 | 2 | 4 | 4 | 75.33b, c |
| 25 % glycerin | 4 | 3 | 3 | 2 | 4 | 2 | 4 | 62.67d, e |
| 5 % sucrose | 2 | 1 | 2 | 4 | 1 | 2 | 2 | 39.00f |
| 10 % sucrose | 3 | 2 | 3 | 4 | 2 | 3 | 3 | 54.00e |
| 15 % sucrose | 3 | 2 | 2 | 3 | 3 | 1 | 4 | 55.00e |
| 20 % sucrose | 4 | 3 | 3 | 4 | 4 | 3 | 5 | 77.67b |
| 25 % sucrose | 4 | 2 | 2 | 4 | 5 | 3 | 3 | 68.33c, d |
| no cryoprotectant | 1 | 4 | 3 | 4 | 1 | 5 | 2 | 55.67e |
| distilled water | 1 | 3 | 2 | 4 | 3 | 4 | 4 | 55.67e |
Note: 1) different letters mean the difference of data in the same column is statistically significant (p < 0.05). The scores in the table are the average of three times.
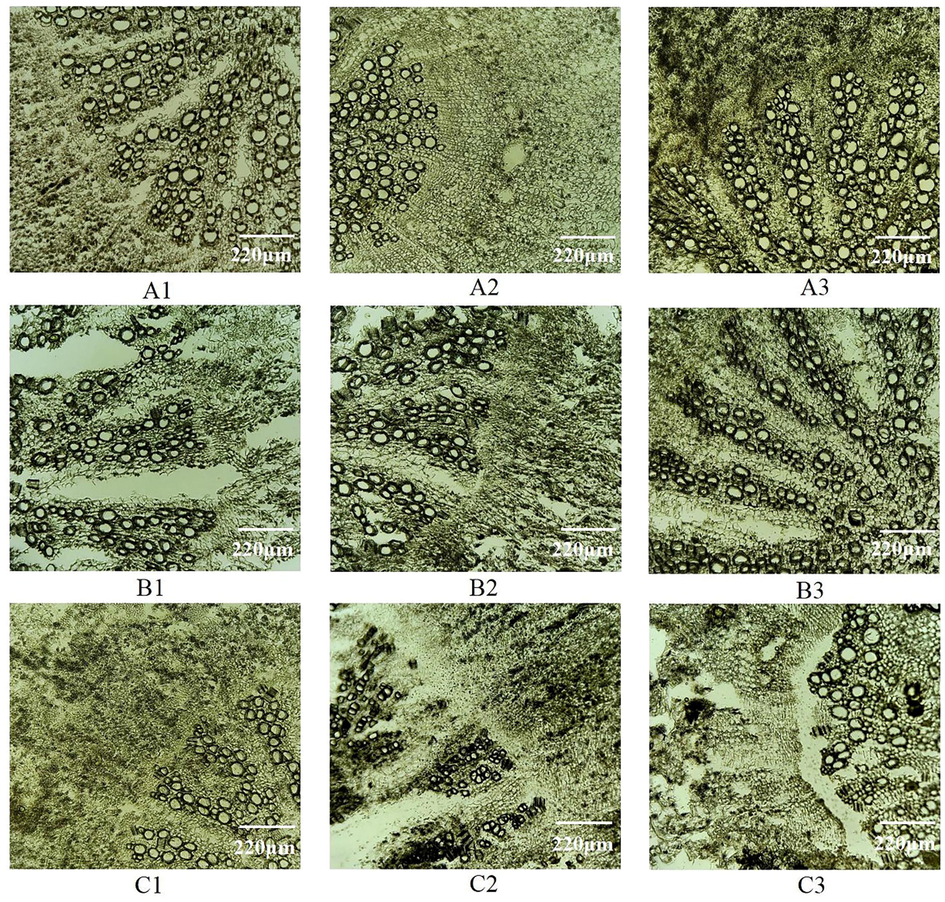
- Microscopic observation of frozen sections of Radix Angelicae dahuricae with different sectioning methods Notes: A1: 5% glycerin treatment, A2:15% glycerin treatment, A3:25%glycerin treatment, B1:5% sucrose treatment, B2:15% sucrose treatment, B3:20%sucrose treatment, C1:25% sucrose treatment, C2: distilled water treatment, C3: no cryoprotectant.
3.2 Distribution of coumarins in Radix Angelicae dahuricae
The frozen sections of Radix Angelicae dahuricae were observed under the DAPI channel of the forward inverted integrated fluorescence microscope. The cork layer, the cork inner layer secretory cavity, the phloem secretory tract and the xylem all emitted blue fluorescence, which was consistent with the fluorescence emitted by the imperatorin standard (Fig. 4A1), so it is difficult to determine the location of the coumarin. Therefore, the ethanol soluble coumarin property was used for the control test. After Radix Angelicae dahuricae slices were treated with ethanol control group, the blue fluorescence of the secretory cavity of the inner layer of cork and the phloem secretory tract disappeared, but the cork layer and xylem still had blue fluorescence (Fig. 4A2-4, C1-3). This shows that fluorescence in the secretory cavity of the inner layer of the cork and the phloem secretory tract was produced by coumarin. In Radix Angelicae dahuricae, coumarin is mainly distributed in secretory tissues, such as secretory cavities and secretory ducts (Fig. 4B1-4).
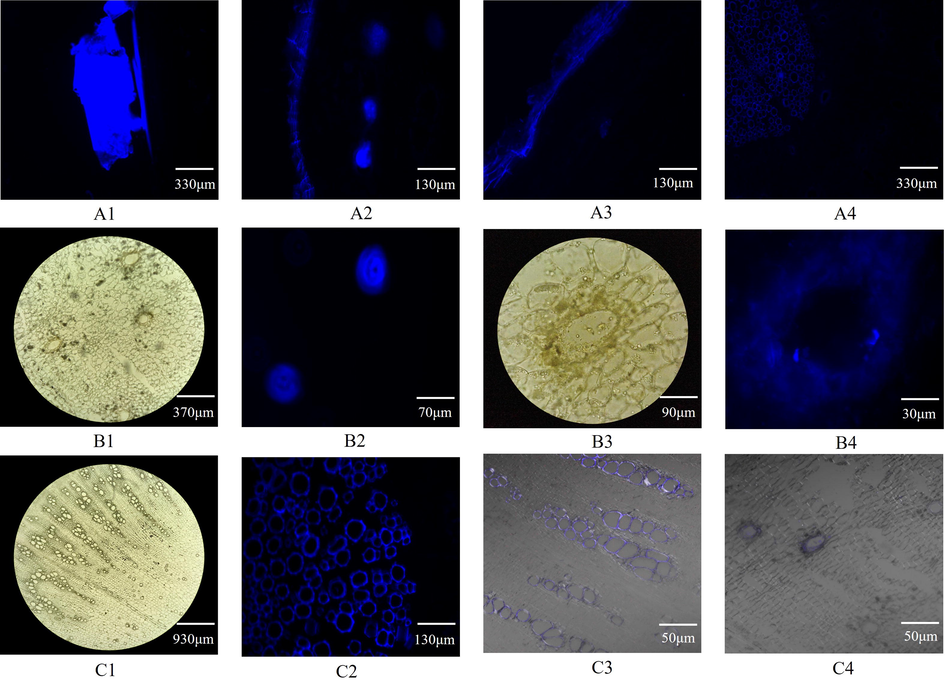
- Microstructure and coumarin localization in Radix Angelicae dahuricae Notes: A1 (imperatorin), A2 (cork layer, cork inner layer, phloem), A3 (cork layer, cork inner layer and phloem after ethanol treatment), A4 (cork inner layer, phloem and xylem after ethanol treatment), B2 (cork inner layer secretory cavity), B4 (secretory cavity), C2 (xylem) are the images observed in the DAPI channel of the forward inverted integrated fluorescence microscope. B1 (cork inner layer secretory cavity), B3 (secretory cavity), C1 (xylem) are the images observed in the bright field of the forward inverted integrated fluorescence microscope. C3 (xylem) and C4 (cork inner layer secretory cavity) are the images observed by superimposing the bright field on the LSCM AF405 channel.
3.3 Relative quantitative study of coumarins in Radix Angelicae dahuricae by LSCM
3.3.1 Determination of fluorescence parameters of coumarins in Radix Angelicae dahuricae
Combined with the excitation wavelength of LSCM, 405 nm, 488 nm, 568 nm and 647 nm were used as excitation wavelengths, respectively. The fluorescence value of each standard was detected, as shown in Table 2 and Fig. 5. Imperatorin and isoimperatorin both excited fluorescence at different excitation wavelengths. Both have the highest fluorescence value at 405 nm excitation wavelength and the lowest fluorescence value at 647 nm excitation wavelength. Therefore, 405 nm was selected as the excitation wavelength for the subsequent test.
| Excitation wavelength (nm) | Imperatorin | Isoimperatorin | ||
|---|---|---|---|---|
| Emission | Fluorescence | Emission | Fluorescence | |
| Wavelength (nm) | value | Wavelength (nm) | value | |
| 405 | 408 | 8479 | 409 | 9956 |
| 488 | 490 | 5400 | 491 | 8154 |
| 568 | 569 | 1935 | 571 | 3225 |
| 647 | 650 | 689 | 651 | 1380 |
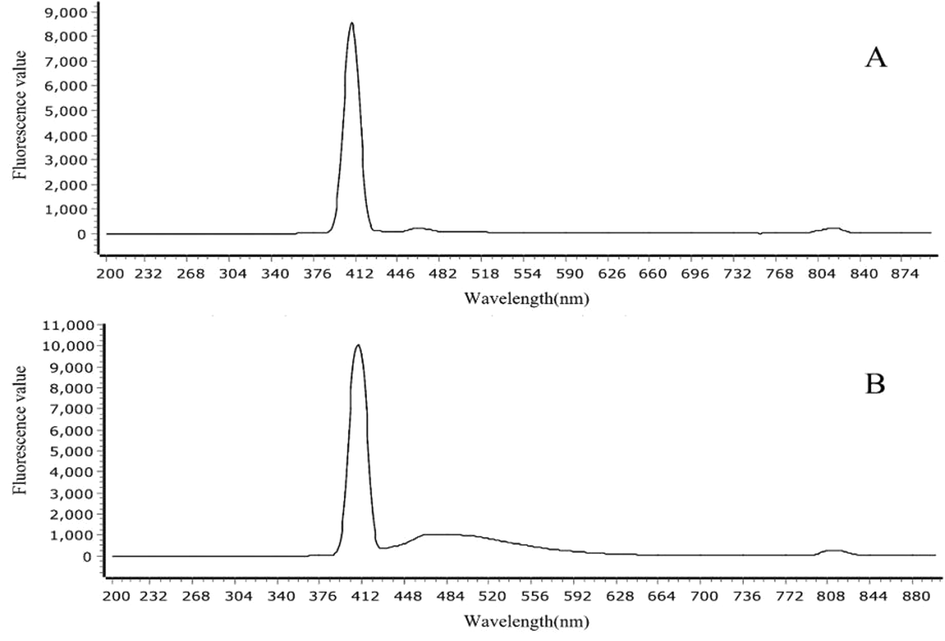
- Fluorescence spectrum of standard (excitation wavelength = 405) Notes: A: imperatorin; B: isoimperatorin.
3.3.2 Fluorescence imaging results of coumarins in Radix Angelicae dahuricae at different periods
The average fluorescence intensity of coumarin in Radix Angelicae dahuricae at different periods was analyzed using the coumarin fluorescence imaging method of coumarin combined with LSCM and ZEN 2 software, and it was found that the average fluorescence intensity at different periods was different (Table 3).
| Group | Month | ||||
|---|---|---|---|---|---|
| Early March | Early May | Mid-July | Early September | Early November | |
| 1 | 174.575 | 260.727 | 353.573 | 459.044 | 355.781 |
| 2 | 167.129 | 263.632 | 355.388 | 431.322 | 322.436 |
| 3 | 168.716 | 262.027 | 359.510 | 450.299 | 314.536 |
| 4 | 158.641 | 263.281 | 362.018 | 459.622 | 306.370 |
| 5 | 155.959 | 265.058 | 368.269 | 454.467 | 303.939 |
| 6 | 161.848 | 264.697 | 366.596 | 488.166 | 315.295 |
| 7 | 158.783 | 255.157 | 361.567 | 403.715 | 303.306 |
| 8 | 169.658 | 253.533 | 358.636 | 415.210 | 348.519 |
| 9 | 164.409 | 241.068 | 391.156 | 413.413 | 316.251 |
| 10 | 171.976 | 264.283 | 352.162 | 470.840 | 314.596 |
| Average value | 165.170 | 259.346 | 362.888 | 444.610 | 320.103 |
The average fluorescence intensity of coumarin in Radix Angelicae dahuricae showed a gradual increase from seedling stage to harvest stage. In early March, the average fluorescence intensity of coumarin in Radix Angelicae dahuricae at seedling stage was the lowest, only 165.170. In mid-July, the average fluorescence intensity of coumarin increased by 103.542, indicating that the root growth period is the key period for coumarin content accumulation. The average fluorescence intensity of coumarin in Radix Angelicae dahuricae during harvest time in early September was the highest, reaching 444.610. The nutrient growth period of Radix Angelicae dahuricae is from March to October, which is conducive to nutrient accumulation in the root. After November, Radix Angelicae dahuricae entered the reproductive growth period. In order to maintain life metabolism, the root system consumed some dry matter, so the average fluorescence intensity of coumarin began to decrease. In conclusion, the content of coumarin in Radix Angelicae dahuricae showed an “S” trend during the growth period (Fig. 6).
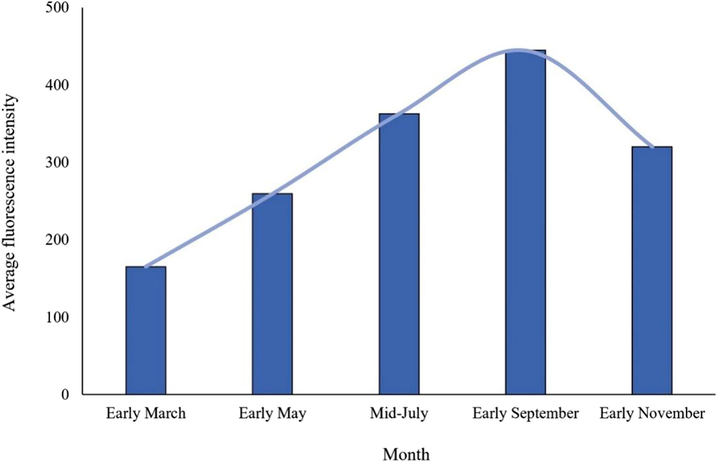
- Variation trend of coumarin average fluorescence intensity in Radix Angelicae dahuricae in different periods.
3.4 Quantitative study of coumarins in Radix Angelicae dahuricae at different periods by LC-MS
3.4.1 Methodological validation
The linear relationship, precision, repeatability, stability and sample recovery of the determination method were investigated. The correlation coefficient of the linear regression equation for imperatorin, isoimperatorin, bergapten, xanthotoxin and 7-hydroxycoumarine in their linear concentration ranges is 0.9985 ∼ 0.9996, and the linear range is 0.16 ∼ 6.00 μg/mL (Table 4). In the precision experiment, the RSD calculated by peak area is 0.59 %∼2.19 %. In the repeatability experiment, the RSD calculated by peak area is 0.49 %∼2.70 %. In the stability experiment, the test solution was injected and measured at 0, 2, 4, 8 and 12 h respectively, and the RSD of its peak area was 0.30 %∼2.45 %. The recovery rate of the sample is over 90.08 %, and the RSD is less than 3.94 %. Which met the requirements of quantitative analysis.
| Compound | Regression equation | Correlation coefficient | Linear range (µg/mL) |
|---|---|---|---|
| 7-Hydroxycoumarin | Y = 16.9390X-212.5958 | 0.9987 | 0.16 ∼ 6.00 |
| Xanthotoxin | Y = 66.6885X + 2.4322 | 0.9982 | 0.16 ∼ 6.00 |
| Bergapten | Y = 172.7384X + 2818.2264 | 0.9996 | 0.16 ∼ 6.00 |
| Imperatorin | Y = 457.7008X + 14140.3218 | 0.9996 | 0.16 ∼ 6.00 |
| Isoimperatorin | Y = 296.6266X-5347.3684 | 0.9985 | 0.16 ∼ 6.00 |
3.4.2 Sample content determination results
After fresh samples were harvested, they were dried at 37℃ to reduce volatilization of Radix Angelicae dahuricae during drying. As shown in Fig. 7 and Fig. 8, the coumarins content reached the highest in the early September, and increased most rapidly in the mid-July when the root was growing for a long time, indicating that this period is the accumulation period of coumarins content in Radix Angelicae dahuricae. The contents of imperatorin and isoimperatorin were high, which increased until the harvest period, and began to decrease during the reproductive growth period. The content of 7-hydroxycoumarin is less and tends to be stable. The content of bergapten lactone increased in the early stage of growth of Radix Angelicae dahuricae, and the content changed little in the late stage. Xanthotoxin content tended to be stable before reaching the harvest period, and began to decrease during the reproductive growth period. Total coumarin content in Radix Angelicae dahuricae in different periods increased in an “S” curve. In this work, the same batch of samples in the same period were analyzed both by LC-MS and LSCM. The average fluorescence intensity measured by LSCM represented the coumarin content. Therefore, the analysis results of total coumarin content change in different periods by LC-MS and LSCM were consistent, which verified the accuracy of the experiment.
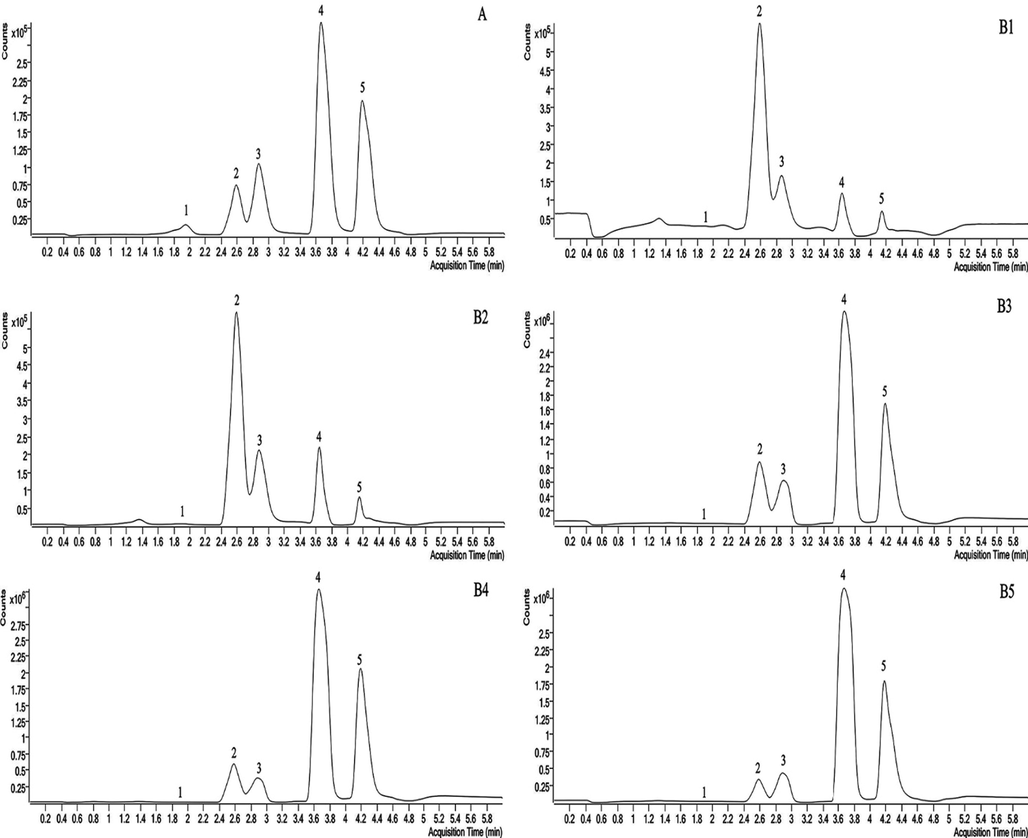
- LC-MS diagram of representative coumarins in Radix Angelicae dahuricae at different periods Notes: 1: 7-hydroxycoumarin, 2: xanthotoxin, 3: bergapten, 4: imperatorin, 5: isoimperatorin, A: mixed control solution, B1: early March, B2: early May, B3: mid-July, B4: early September, B5: early November.
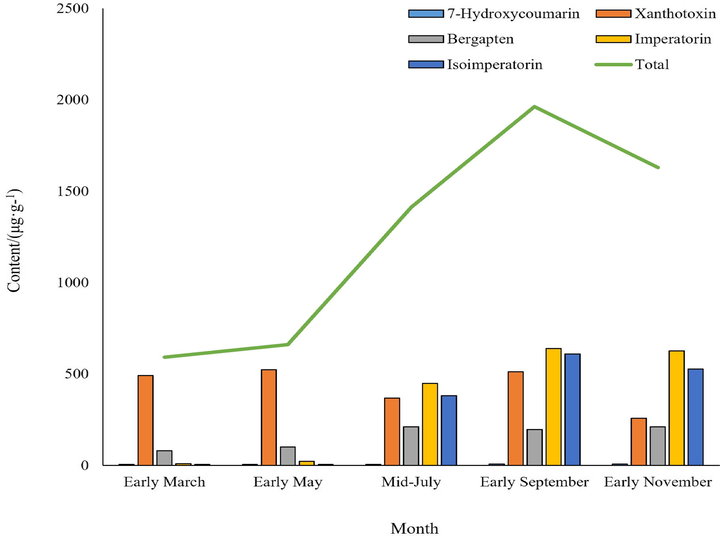
- Variation trend of coumarins content in Radix Angelicae dahuricae at different periods by LC-MS.
4 Conclusion
In this study, coumarins in Radix Angelicae dahuricae were visually analyzed in situ using frozen section and fluorescence imaging technology. The quality assessment method and the best conditions for the frozen section were established. Radix Angelicae dahuricae was treated with 15 % glycerin protectant, and the slice thickness was 30 µm is the best condition. The accumulation site of coumarins could be observed conveniently, quickly and accurately by using frozen section combined with fluorescence imaging technology, without dyeing and heating, reducing coumarins loss, avoiding the use of carcinogens such as dyes, and conforming to the development concept of green chemistry. This study showed that coumarins existed in secretory tissue. Coumarins in Radix Angelicae dahuricae at different periods were statistically analyzed by LSCM combined with LC-MS, and the law of coumarins accumulation and harvest time was obtained. The positioning and quantitative analysis of coumarins in Radix Angelicae dahuricae provides an effective theoretical basis for studying the production, distribution and accumulation of other active ingredients, and provides a certain reference for ensuring the quality of medicinal materials.
Funding
This work was supported by the National Natural Science Foundation of China (31860102), Youth Tutor Fund project of Gansu Agricultural University (GAU-QDFC-2020-2), FuXi Young Talents Introduction Projects of Gansu Agricultural University (GSAU-RCZX201704) and Outstanding Graduate Student Innovation Star Project in Gansu Province(2022CXZXS-021).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mass spectrometry imaging (msi) for plant metabolomics. Methods Mol. Biol.. 2018;1778:241-252.
- [Google Scholar]
- Rapid gelatin embedding procedure for frozen brain tissue sectioning. J. Histotechnol.. 2013;15(2):1301-1315.
- [Google Scholar]
- Separation and simultaneous quantification of nine furanocoumarins from radix angelicae dahuricae using liquid chromatography with tandem mass spectrometry for bioavailability determination in rats. J. Sep. Sci.. 2015;38(24):4216-4224.
- [Google Scholar]
- Location analysis of volatile oil in Angelica sinensis by frozen section and fluorescence imaging. Chin. J. Exp. Tradit. Med. Form. 2022;28(01):182-188.
- [Google Scholar]
- Synthesis and fluorescent study of 5-phenyl furocoumarin derivatives as vasodilatory agents. Bioorg. Med. Chem. Lett.. 2016;26(2):640-644.
- [Google Scholar]
- Vasorelaxing effect of coumarins from cnidium monnieri on rabbit corpus cavernosum. Planta Med.. 2001;67(3):282-284.
- [Google Scholar]
- New cosmetic agents for skin whitening from Angelica dahurica. J. cosmet. Sci.. 2006;57(1):11-21.
- [Google Scholar]
- Advanced NIR-II fluorescence imaging technology for in vivo precision tumor theranostics. Adv. Ther.. 2019;2(9):1900053.
- [Google Scholar]
- In vivo diagnostics of abiotic plant stress responses via in situ real-time fluorescence imaging. Plant Physiol.. 2022;190(1):196-201.
- [Google Scholar]
- Anti-inflammatory activity of coumarins from decatropis bicolor on tpa ear mice model. Planta Med.. 2000;66(3):279-281.
- [Google Scholar]
- Assessment of human islet grafts in frozen sections of cd-1 athymic nu/nu mouse liver for molecular analysis. Transpl. P. 2014;46(6):1956-1959.
- [Google Scholar]
- The fluorescence-activating and absorption-shifting tag (fast) enables live-cell fluorescence imaging of methanococcus maripaludis. J. Bacteriol.. 2022;2022:e0012022.
- [Google Scholar]
- Early identification of herbicide stress in soybean (Glycine max (L.) Merr.) using chlorophyll fluorescence imaging technology. Sensors-Basel. 2017;18(1):21.
- [Google Scholar]
- Characteristics of laser scanning confocal microscopes for surface texture measurements. Surf. Topogr- Metrol.. 2014;2(1):014003
- [Google Scholar]
- Fluorescence imaging technique was used to study the accumulation pattern of coumarin components in Angelicae Pubescentis Radix. World Chinese Medicine 2022:1-12.
- [Google Scholar]
- Chromatographic fingerprint analysis and characterization of furocoumarins in the roots of angelica dahurica by hplc/dad/esi-msn technique. J. Pharmaceut. Biomed.. 2008;47(4–5):778-785.
- [Google Scholar]
- Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines. Phytother. Res.. 2007;21(3):288-290.
- [Google Scholar]
- Paraffin-embedded and frozen sections of drosophila adult muscles. J. Visualized Exp.. 2010;46:e2438.
- [Google Scholar]
- Coumarins from the roots of Angelica dahurica cause anti-allergic inflammation. Exp. Ther. Med.. 2017;14(1):874-880.
- [Google Scholar]
- In vitro α-glucosidase inhibition by brazilian medicinal plant extracts characterised by ultra-high performance liquid chromatography coupled to mass spectrometry. J. Enzym. Inhib. Med. Ch.. 2022;37(1):554-562.
- [Google Scholar]
- Use of intraoperative frozen section in the surgical management of patients with nonmelanoma skin cancer. J. Skin Cancer. 2021;2021:4944570.
- [Google Scholar]
- Comparative study on the growth characteristics and yield and quality of Chuan Bai Zhi. Chengdu University of TCM; 2009.
- Beta-secretase inhibitory effects of furanocoumarins from the root of Angelica dahurica. Phytother. Res.. 2010;24(4):510-513.
- [Google Scholar]
- Antioxidant, anti-inflammatory and antiproliferative activity of Angelica dahurica roots. J. Food Biochem.. 2012;38:281-292.
- [Google Scholar]
- Antioxidative activity of furanocoumarins isolated from Angelicae dahuricae. J. Ethnopharmacol.. 2004;93(2–3):243-246.
- [Google Scholar]
- Isolation, structure elucidation, tyrosinase inhibitory, and antioxidant evaluation of the constituents from Angelica dahurica roots. J. Nat. Med.-Tokyo. 2020;74(2):456-462.
- [Google Scholar]
- Synthesis and application of coumarin fluorescence probes. Rsc Adv.. 2020;10(18):10826-10847.
- [Google Scholar]
- Applications of cryo-sectioning technology in higher plants. Plant Physiol. J.. 2021;57(05):1047-1054.
- [Google Scholar]
- Visual research filler network structure in polymer composites and its structure-activity relationship by fluorescent labeling and lscm. Polym. Test. 2020;90
- [Google Scholar]
- Studies on quality character formation and cultivation regulation and control of Angelica dahurica. China Agricultural University; 2005.
- Identification of filigree pattern increases the diagnostic accuracy of micropapillary pattern on frozen section for lung adenocarcinoma. Histopathology. 2022;81(1):119-127.
- [Google Scholar]
- Simultaneous determination and pharmacokinetics of sixteen angelicae dahurica coumarins in vivo by lc–esi-ms/ms following oral delivery in rats. Phytomedicine. 2016;23(10):1029-1036.
- [Google Scholar]







