Translate this page into:
In vitro antioxidant actions of sulfur-containing amino acids
⁎Corresponding author. leech@konkuk.ac.kr (Chi-Ho Lee)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Sulfur-containing amino acids such as methionine, cysteine, and taurine are present in animals and plants with biological functions. The aim of this study was to determine the antioxidant activities of representative sulfur-containing amino acids using various in vitro antioxidant assays including radical scavenger activities against DPPH•, ABTS•, and superoxide radicals, ferric reducing antioxidant power, hydrogen peroxide scavenging activity, and metal chelating activities. Of the three sulfur-containing amino acids, cysteine had the highest DPPH•, ABTS•, and O2− radical and H2O2 scavenging activities, FRAP, and metal chelating activities except for Fe2+ chelating. However, methionine and taurine failed to show DPPH• or ABTS• radical scavenging activity. Based on the results of the present study, sulfur-containing amino acids with excellent antioxidant abilities might be useful for the food processing industry as antioxidant additives to extend shelf-life of food or food products and offer beneficial pharmacological effect against cell damage caused by oxidative stress.
Keywords
Sulfur containing amino acid
Antioxidant
Cysteine
Radical scavenging activity
Oxidative stress
1 Introduction
Lipid oxidation is one of the main reasons behind food decay. Many antioxidants have been researched for the purpose of inhibiting lipid oxidation. Lipid oxidation is largely induced by three different routes: enzymatic oxidation, free-radical-mediated oxidation, and non-radical oxidation (Niki et al., 2005). The process of lipid oxidation related to free-radical chain mechanism is basically divided into three steps. In the initiation step, free radical is generated by the removal of unstable hydrogen atom bound with unsaturated fatty acids. In the step of propagation, hydroperoxides are produced by the initial reaction products with oxygen. In the step of termination, chemical reactions are terminated due to the formation of chemically stable form (Gray, 1978). Free radicals and reaction products can cause many biological problems such as cell injury and human diseases. Different antioxidants and antioxidant enzymes play important roles in scavenging free radicals (Fang et al., 2005).
Sulfur is involved in numerous biological and chemical reactions. Organic sulfur compounds are produced in plants and microorganisms. These organic sulfur compounds are then oxidized to sulfate which is then excreted through the urinary system in animals (Parcell, 2002). Sulfur can be combined with several chemical forms such as sulfur-containing amino acids (methionine, cysteine, homocysteine, and tauric acid) and glutathione (Brosnan and Brosnan, 2006). Sulfur containing amino acids (methionine and cysteine) are regarded as essential amino acids (Konashi et al., 2000). Song et al. (2013) have demonstrated that sulfur containing amino acids of serum in pigs are increased by feeding diet containing high sulfur content. Moreover, cysteine plays an important role in forming disulfide bond linkages between proteins such as insulin (Piste, 2013). Antioxidant effect of sulfur has been studied by some scientists to understand the mechanism of sulfur antioxidant (Atmaca, 2004; Battin and Brumaghim, 2009). Since sulfur amino acids can remove reactive oxygen species (ROS), sulfur amino acids can be used to reduce cell damage induced by oxidative stress (Moskovitz, 2005).
In previous studies, the antioxidant effects of sulfur containing amino acids have been theoretically explained. This study is aimed to investigate the effect of antioxidant capacity following as the different analytical measurements for finding antioxidant effects depends on sulfur-containing amino acids in vitro. Different sulfur containing amino acids may possess different antioxidant pathways depending on chemical forms (Parcell, 2002). Sulfur-containing amino acids with excellent antioxidant abilities might be useful for the food processing industry as antioxidant additives to extend shelf-life of food or food products. They might also offer beneficial pharmacological effect against cell damage caused by oxidative stress. Therefore, the objective of this study was to determine the antioxidant activities of sulfur-containing amino acids (Cys, Tau, and Met).
2 Material and methods
2.1 Chemicals and experimental design
Processed sulfur (97.9% inorganic sulfur) was purchased from Jungmin co., Ltd (Korea). l-Methionine, l-Cysteine and taurine were obtained from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany). All other chemicals in analytical grade were purchased from Sigma-Aldrich (Fig. 1). Experimental design of this study is shown in Fig. 1.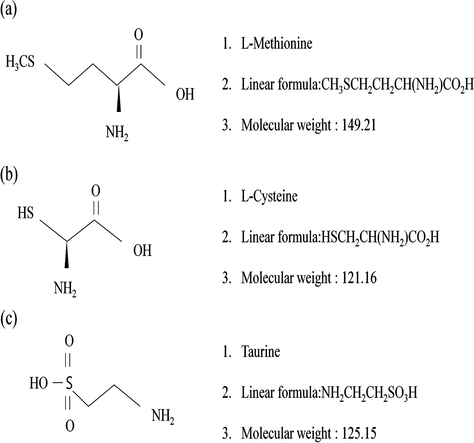
Structures of sulfur-containing amino acids: methionine, cysteine and taurine.
2.2 Total antioxidant ability
2.2.1 Ferric reducing antioxidant power (FRAP) assay
Ferric reducing antioxidant power (FRAP) assay was conducted using published method of Oyaizu (1986) with slight modifications. Briefly, different concentrations (1000, 2000, and 4000 ppm) of each amino acid were prepared in distilled water as stock solutions. One mL of the stock solution was mixed with 2.5 mL of sodium phosphate buffer (0.2 M at pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was placed at 50 °C for 20 min. After the incubation, 2.5 mL of 10% trichloroacetic acid was added to the mixture. The upper layer of the mixture was taken. It was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% FeCl3. The absorbance value of the solution was measured at wavelength of 700 nm using a spectrophotometer.
2.3 Metal chelating activity
2.3.1 Ferrous ions (Fe2+) chelating activity
The ferrous ion-chelating ability of sulfur compound was measured using the method of Dinis et al. (1994). Briefly, 0.4 mL of the stock solution of each sulfur amino acid (20 µg/mL) was mixed with 0.2 mL of 2 mM FeCl2. Then 0.4 mL of ferrozine (5 mM) was added to the mixture to initiate the reaction. The reacted mixture was shaken for 10 min at room temperature. The absorbance of the mixture was measured at wavelength of 562 nm. The ferrous ion-chelating activity was calculated using the following equation: where Acontrol 562 nm was the absorbance of the control reaction containing distilled water instead of sample and Asample 562 nm was the absorbance of the sample in the presence of sulfur-containing amino acid.
2.3.2 Copper (Cu2+) and zinc (Zn2+) chelating activity
Copper (Cu2+) and zinc (Zn2+) chelation activities was determined by the method of Asakura et al. (1990), the sulfur-containing amino acids were diluted into 20 µg/mL in distilled water. CuSO4 and ZnCl2 metal solutions were prepared at concentrations of 0.24 mM and 0.8 mM in the same buffer. One mL of the sample solution was mixed with 1 mL of metal solution followed by the addition of 0.1 mL of 1 mM tetramethylmurexide solution. Absorbance value was then recorded at A282/A530 for Cu2+ and A462/A530 for Zn2+ to obtain bound metal (%) using the following equation:
In order to calculate the percentage of metal chelating activity, standard curves of copper and zinc were prepared. The range of Cu2+ was from 0.025 to 0.125 mM/L. The range for Zn2+ was from 0.2 to 1.0 mM/L.
2.4 Hydrogen peroxide (H2O2) scavenging activity
Hydrogen peroxide scavenging ability was measured according to the method of Ruch et al. (1989). First, 43 mM of H2O2 stock solution was prepared in phosphate buffer (0.1 M at pH 7.4). Sulfur compound (1000 ppm) in 3.4 mL phosphate buffer was mixed with 0.6 mL of 43 mM of H2O2 in phosphate buffer solution. The absorbance value of the mixture was recorded at wavelength of 230 nm. Phosphate buffer without hydrogen peroxide was used as blank solution. The concentration of hydrogen peroxide (mM) was determined by using a standard curve (r2: 0.9865). H2O2 scavenging activity (%) of sulfur compounds was calculated using the following equation: where Acontrol 230 nm was the absorbance of control reaction containing distilled water instead of sample and Asample 230 nm was the absorbance of sample in the presence of sulfur-containing amino acid.
2.5 Radical scavenging activity
2.5.1 DPPH free radical scavenging activity
DPPH free radical scavenging activity assay was carried out using the method of Blois (1958) with slight modifications. Briefly, 0.5 mL of 0.1 mM DPPH in ethanol was added to 1.5 mL of sulfur compound at different concentrations in ethanol. This mixture was reacted in a dark room for 30 min. The absorbance values of samples were recorded at wavelength of 517 nm. A standard curve was prepared using different concentrations of DPPH (r2: 0.9985). The DPPH scavenging effect (%) was calculated using the following equation according to Gülcin et al. (2010). where Acontrol 517nm was the absorbance value of the control reaction containing distilled water instead of sample and Asample 517nm was the absorbance value of the sample in the presence of sulfur-containing amino acid.
2.5.2 ABTS radical cation scavenging activity
The ABTS radical scavenging activity was analyzed using the method of Re et al. (1999). Briefly, 2 mM of ABTS in ethanol was reacted with 2.45 mM potassium persulfate (K2S2O8) to generate ABTS+ in a dark room for 4 h. The ABTS+ solution was diluted to obtain an absorbance of 0.75 at wavelength of 734 nm by using 0.1 M sodium phosphate buffer (pH 7.4). One mL of diluted ABTS+ solution was then reacted with 3 mL of sulfur compound in distilled water for 30 min. ABTS+ scavenging effect (%) was calculated using the following equation: where Acontrol 734nm was the absorbance of the control reaction containing distilled water instead of the sample and Asample 734nm was the absorbance value of the sample in the presence of sulfur-containing amino acid.
2.5.3 Superoxide anion radical scavenging activity
Superoxide anion radical scavenging activity was measured using the method of Li et al. (2006). Briefly, 0.2 mL of sulfur compound stock solution was mixed with 0.4 mL of 150 µM of nitro blue tetrazolium (NBT) in phosphate-buffered solution (100 mM, pH 7.4), 0.4 mL of 460 µM β-nicotinamide adenine dinucleotide (β-NADH), and 0.05 mL of 60 μM phenazine methosulphate (PMS). After mixing well, the mixture was then placed at room temperature. After standing for 5 min, the absorbance value of the mixture was recorded at wavelength of 560 nm. Superoxide anion radical scavenging activity (%) was calculated using the following formula: where Acontrol 560nm was the absorbance value of the control reaction containing distilled water instead of the sample and Asample 560nm was the absorbance value of the sample in the presence of sulfur-containing amino acid.
2.6 Statistical analysis
All experiments were conducted in triplicates and all data were analyzed with SPSS 19.0. Results are expressed as means ± standard error of the means. One-way analysis of variance (ANOVA) was performed using ANOVA procedures. Significant differences between means were determined by Duncan’s Multiple Range tests. A p value of <.05 was regarded as statistically significant.
3 Results and discussions
3.1 Radical scavenging activity
3.1.1 DPPH and ABTS radical scavenging assays
DPPH and ABTS free radical scavenging activities of cysteine were linearly increased with increasing concentrations. However, Met and Tau did not possess DPPH or ABTS free radical scavenging activity (Fig. 2). Kavlentis (1988) has reported that cysteine can inhibit the formation of DPPH complex whereas methionine does not affect the DPPH experiment. Ripoll et al. (2012) have shown the taurine does not have ABTS radical scavenging ability. The addition of taurine is unable to decrease ROS production or inhibit lipid peroxidation in glutathione-depleted cell culture (Heidari et al., 2014). In the present study, ascorbic acid has more powerful DPPH radical scavenging activity than cysteine at the same concentrations (50–400 µg/mL). At 400 µg/mL, cysteine and ascorbic acid showed 84.42% and 94.94% of DPPH radical scavenging activity, respectively. However, the ABTS radical scavenging activity of cysteine was significantly higher than that of ascorbic acid at concentrations from 10 to 100 µg/mL. For instance, cysteine and ascorbic acid at 100 µg/mL had 96.77% and 91.78% ABTS radical scavenging activities, respectively. In general, ascorbic acid is considered as a representative antioxidant that can remove water-soluble free radical. Ascorbic acid is converted into ascorbate radical when electron of ascorbate radical supplies lipid radical to terminate the oxidative chain reaction (Nimse and Pal, 2015). In general, DPPH assay is used for ethanol-soluble free radicals, whereas ABTS assay is used to measure water-soluble free radicals. Kaviarasan et al. (2007) have found that ABTS assay is more sensitive than DPPH assay since DPPH radical is only involved in hydrogen (H+) transfer [DPPH• to DPPH-H] while ABTS radical is involved in the electron transfer pathway [ABTS• to ABTS]. According to the radical scavenging activity of cysteine, the presence of proteins containing disulfide bonds (Cys-Cys) might potentially increase the antioxidant ability of food.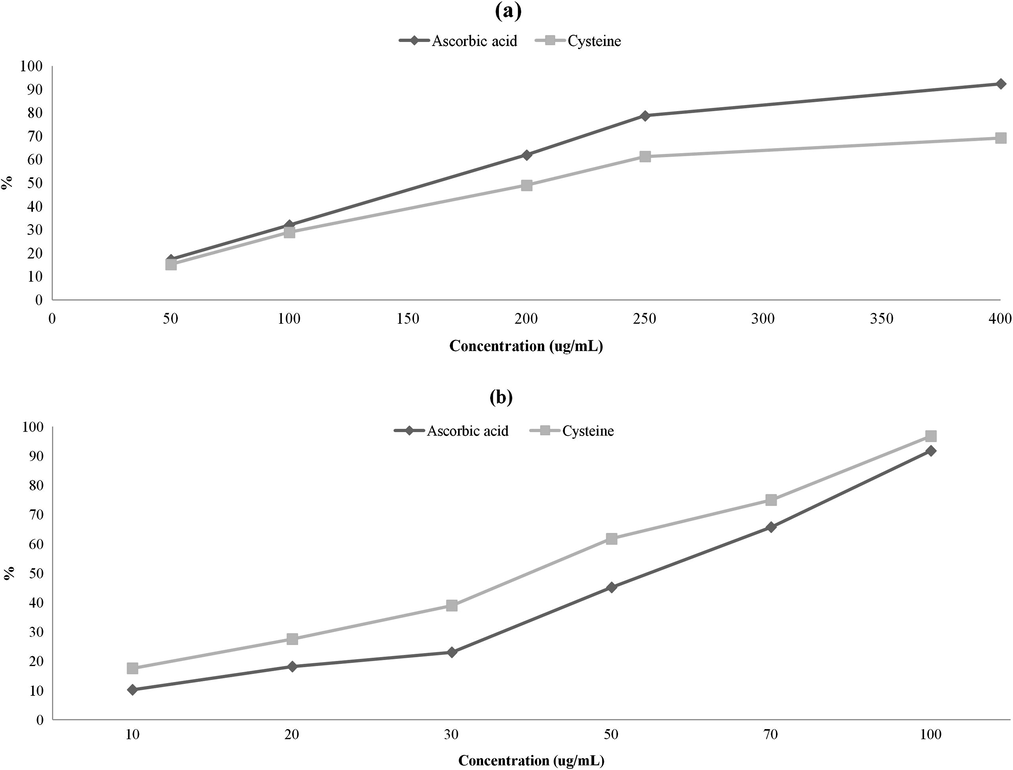
Radical scavenging activities of sulfur amino acids. (a) DPPH radical scavenging activity and (b) ABTS radical scavenging activity of different concentrations of sulfur-containing amino acids (cysteine, methionine and taurine) and ascorbic acid (reference antioxidant). Radical scavenging activities of methionine and taurine was not detected.
3.1.2 Superoxide anions radical scavenging activity
The principle of superoxide anion radical scavenging activity assay has been explained by Gülcin et al. (2010). The addition of PMS will induce the reduction of NBT with the generation of superoxide radicals (O2−) in PMS-NADH system. Blue formazan at 560 nm is then formed through the NBT reduction. Therefore, inhibition of blue formazan production can be expressed as a decrease in absorbance at 560 nm due to O2− scavenging. At the same concentration (1000 ppm), cysteine, taurine, methionine, and BHT exhibited 31.32, 22.27, 17.8, and 11.29% superoxide anion radical scavenging activities, respectively (Fig. 3). Cysteine showed the highest (p < .001) superoxide radical scavenging activity. The excellent superoxide radical scavenging activity of cysteine has been reported by Hussain et al. (1996). Likewise, methionine also possessed higher (p < .001) superoxide radical scavenging activity than BHT. It has been reported that methionine can be easily oxidized to methionine sulfoxide by some oxidants such as oxygen, ozone, hydrogen peroxide, and superoxide (Levine et al., 2000). In a previous study, the superoxide radical scavenging activity (the reduction of NBT to form the blue formazan at 560 nm) of taurine has been observed at concentrations from 30 mM to 60 mM (Oliveira et al., 2010). Kim and Cha (2009) have found that taurine can reduce oxidative damage by altering superoxides into taurine chloramines.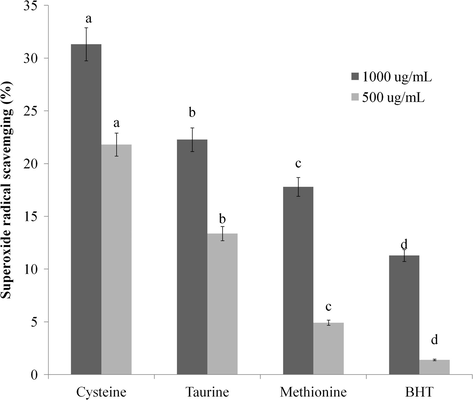
Effect of sulfur-containing amino acids on superoxide anion radical scavenging (%). Different letters within the same concentration indicate significant differences (p < .05).
3.2 Reducing power
FRAP results of cysteine and ascorbic acid are shown in Fig. 4. Methionine and taurine did not show any FRAP. FRAP measures the degree of residual Fe2+ generated from the reduction of Fe3+. Although cysteine showed some FRAP, its reducing ability was lower (p < .05) than ascorbic acid. At concentration of 1000 ppm, the absorbance values of ascorbic acid and cysteine after reducing Fe3+ were 2.837 and 2.689, respectively. According to Giles et al. (2003), the thiol group (SH) of cysteine in proteins can modulate the redox reactions such as the formation of cysteine radical (S•), cystine (S-S), cysteine sulfeninc acid (S-OH), and so on. Mancuso et al. (2010) have explained that the FRAP of human blood is increased when it is supplemented with cysteine-donor. Antioxidant systems are mostly associated with the inhibition of ROS production, metal chelating, or the removal of pro-oxidants by antioxidant enzyme activity, although the redox reaction is continuously occurring. Benzie and Strain (1996) have indicated that non-enzymatic antioxidants such as ascorbic acid and α-tocopherol have FRAP. Therefore, the reducing power of cysteine might be caused by the presence of thiol group.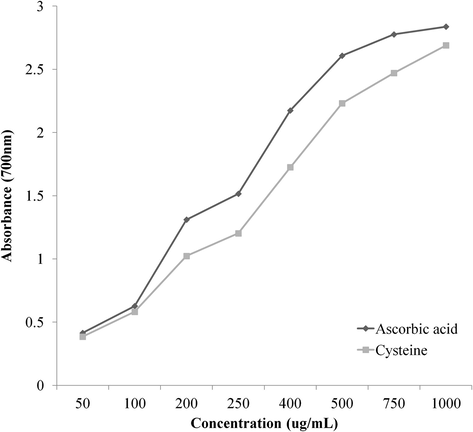
Total ferric reducing power (FRAP) of different concentrations of sulfur-containing amino acids (cysteine, methionine and taurine) and ascorbic acid (reference antioxidant).
3.3 Hydrogen peroxide scavenging activity
H2O2 scavenging activities of sulfur-containing amino acids (cysteine, methionine, and taurine) are shown in Table 1. At the same concentration (1000 ppm), BHT showed significantly higher H2O2 scavenging activity (79.81%) than sulfur-containing amino acids (Cys, 12.62%; Met, 20.66%; Tau, 52.01%). Although Met and Tau did not show radical scavenging activities against DPPH or ABTS, they showed higher (p < .001) H2O2 scavenging activity than Cys. Different superscript letters in the same row indicate significant differences during aging time, p < .05.
Cysteine
Methionine
Taurine
BHT
P-value
H2O2 scavenging activity (%)
12.62 ± 0.68d
20.66 ± 1.36c
52.01 ± 0.57b
79.81 ± 0.10a
<.001
Hydrogen peroxide is not directly toxic to human body. However, hydrogen peroxide continuously contributes as substrates to form ROS species through Fenton (Winterbourn, 1995) and Haber-Weiss reactions (Haber and Weiss, 1934): Fenton reaction: Fe2+ + H2O2 → Fe3+ + OH− + OH• Haber-Weiss reaction: O2•− + H2O2 → O2 + OH− + OH•
Therefore, decomposition of H2O2 is very essential in the antioxidant defense system to protect cell or food against oxidative stress. According to Halliwell and Rycker (1978), the thiol group in the cysteine residue can act as substrate for peroxidase to produce H2O2. During the scavenging of H2O2 using cysteamine generated by the degradation of cysteine, the loss of thiol group has been detected by Aruoma et al. (1988). This result might prove that the hydrogen peroxide scavenging activity of cysteine is negatively associated with the presence of thiol group. In the case of methionine, hydrogen peroxide (H2O2) is decomposed due to its reaction with methionine. Therefore, methionine is converted into methionine sulfoxide which is then reconverted to the methionine throughout the oxidation of NADPH (Atmaca 2004; Brosnan and Brosnan, 2006).
3.4 Metal chelating activity
The three sulfur-containing amino acids failed to show Fe2+ chelating ability. However, EDTA standard at 40 ppm showed high (87.17%) Fe2+ chelating ability (Table 2). In terms of Fenton reaction, inhibition on DNA damage is not affected when Fe2+ and hydrogen peroxide are added into cell (Battin and Brumaghim, 2008). On the other hand, the three sulfur-containing amino acids expressed copper (Cu2+) and zinc (Zn2+) chelating abilities in this study. The metal chelating abilities of all samples were decreased (p < .001) with increasing concentrations of metals. Relatively, cysteine showed higher chelating effects compared to other samples included with EDTA at same concentrations (p < .001). Regarding the chelating activity for 0.25 mM Cu2+, the chelating activity of cysteine was 81.87%. For other samples, their results were: EDTA, 56.26%; taurine, 27.32%; methionine, 46.91%; and ascorbic acid, 64.31%. Furia (1972) has reported that each sample can express different metal affinities (for example, cysteine: Cu2+, 19.2; Zn2+, 9.8; EDTA: Cu2+, 18.8; Zn2+, 16.5). Furia (1972) has reported that each sample can express different metal affinities (for example, cysteine: Cu2+, 19.2; Zn2+, 9.8; EDTA: Cu2+, 18.8; Zn2+, 16.5). Lower cysteine concentration is needed to show the same reduction for DNA damage caused by copper compared to methionine concentration (Battin and Brumaghim, 2008). Furthermore, the thiol group involved in cysteine structure has high heavy metal affinity (Piste, 2013). Therefore, the metal chelating ability of these sulfur-containing amino acids might be affected by several factors such as the number of binding-sites of chelator and metal affinity. Although zinc and copper are regarded as essential minerals in human body, toxic effects exist when the absorption of minerals is in excess (Cal et al., 2005). In particular, copper is regarded as a pro-antioxidant that produces free radical formation (Gaetke and Chow, 2003). Nandi et al. (2005) found that cysteine and methionine are considered as a metal chelator and reduction of oxidative stress in rats is proved by the cysteine and methionine supplementations. As shown in Fig. 1, cysteine only has a thiol group among sulfur containing amino acids. Thiol group is involved in oxidation-reduction reaction. It has antioxidant abilities such as metal chelating ability and free radical scavenging ability (Deneke, 2001). In addition, thiol groups present in protein and enzymes have strong metal affinity (Flora et al., 2008). The metal chelation activity of sulfur-containing amino acids could contribute to the excretion of toxic metal from the body (Flora and Pachauri, 2010). ND, Not detected. Different superscript letters in the same column indicate significant differences during aging time, p < .05.
Fe2+
Cu2+
Zn2+
0.25 mM
0.8 mM
0.25 mM
0.8 mM
EDTAA
87.17 ± 0.26
56.26 ± 2.50c
55.12 ± 0.86c
78.29 ± 0.14b
78.30 ± 0.99a
Taurine
ND
27.32 ± 1.52e
1.87 ± 1.38e
63.41 ± 0.24e
45.69 ± 0.22c
Methionine
ND
46.91 ± 1.04d
22.11 ± 1.79d
67.89 ± 2.34d
45.53 ± 0.16c
Ascorbic acid
ND
64.31 ± 0.92b
53.58 ± 1.17b
72.20 ± 1.94c
51.87 ± 0.22b
Cysteine
ND
81.87 ± 1.44a
73.25 ± 2.85a
88.29 ± 0.65a
76.50 ± 0.89a
P-value
–
P < .001
P < .001
P < .001
P < .001
4 Conclusion
The results of this study revealed that sulfur-containing amino acids had valuable antioxidant activities based on in vitro assays, including DPPH, ABTS, superoxide radical scavenging activities, FRAP, hydrogen peroxide scavenging, and metal chelating activities. Cysteine showed DPPH and ABTS radical scavenging similar to standard antioxidant (ascorbic acid). However, methionine and taurine failed to show DPPH• or ABTS• radical scavenging activity. Superoxide radical and hydrogen peroxide scavenging effects of the three sulfur-containing amino acids were detected. Ferrous chelating activity was not observed for the three amino acids. However, they showed chelating activities against other metals (copper and zinc). Based on our results, addition or supplementation with sulfur-containing amino acids to food products or human body might be useful for inhibiting oxidative stress or diminishing cell damage. However, in terms of oxidative stability, further investigation is needed to determine the antioxidant effects of food added with sulfur-containing amino acids as additives both in vitro and in vivo.
Acknowledgement
This study was supported by Konkuk University in 2016.
References
- Characterization of zinc chelating compounds in instant coffee. Agric. Biol. Chem.. 1990;54:855-862.
- [Google Scholar]
- Antioxidant effects of sulfur-containing amino acids. Yonsei. Med. J.. 2004;45(5):776-788.
- [Google Scholar]
- The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem. J.. 1988;256:251-255.
- [Google Scholar]
- Metal specificity in DNA damage prevention by sulfur antioxidants. J. Inorg. Biochem.. 2008;102:2036-2042.
- [Google Scholar]
- Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem. Biophys.. 2009;55(1):1-23.
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant power”: the FRAP assay. Anal. Biochem.. 1996;239:70-76.
- [Google Scholar]
- Antioxidant determinations by the use of a stable free radical. NLM. 1958;26:1199-1200.
- [Google Scholar]
- Essentiality, toxicology and chelation therapy of zinc and copper. Curr. Med. Chem.. 2005;12(23):2753-2763.
- [Google Scholar]
- Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys.. 1994;315:161-169.
- [Google Scholar]
- Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res.. 2008;128:501-523.
- [Google Scholar]
- Chelation in metal intoxication. Int. J. Environ. Res. Public Health. 2010;7:2745-2788.
- [Google Scholar]
- Furia, T.E., 1972. Sequestrants in food. In: Furia, T.E. (Ed.), CRC Handbook of Food Additives. Plenum Press, NY (Chapter 6).
- Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189:147-163.
- [Google Scholar]
- Metal and redox modulation of cysteine protein function. Chem. Biol.. 2003;10:677-693.
- [Google Scholar]
- Measurement of lipid oxidation: a review. J. Am. Oil Chem. Soc.. 1978;55(6):539-546.
- [Google Scholar]
- Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem.. 2010;3:43-53.
- [Google Scholar]
- The catalytic decomposition of hydrogen peroxide by iron salts. Proc. Roy. Soc. Lond. A Math. Phys. Sci.. 1934;147:332-351.
- [Google Scholar]
- Superoxide and peroxidase-catalysed reactions. Oxidation of dihydroxyfumarate, NADA and Dithiothreitol by horseradish peroxidase. Photochem. Photobiol.. 1978;28:757-763.
- [Google Scholar]
- Amodiaquine-induced toxicity in isolated rat hepatocytes and the cytoprotective effects of taurine and/or N-acetyl cysteine. Res. Pharm. Sci.. 2014;9(2):97-105.
- [Google Scholar]
- Role of metallothionein and other antioxidants in scavenging superoxide radicals and their possible role in neuroprotection. Neurochem. Int.. 1996;29(2):145-152.
- [Google Scholar]
- In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum graecum) seeds. Food Chem.. 2007;103:31-37.
- [Google Scholar]
- Selective spectrophotometric determination of cysteine in the presence of cystine, methionine, and the remaining amino acids using the palladium(II)-DPPH complex. Microchem. J.. 1988;37:129-131.
- [Google Scholar]
- Production of reactive oxygen and nitrogen species in phagocytes is regulated by taurine chloramines. Adv. Exp. Med. Biol.. 2009;643:463-472.
- [Google Scholar]
- Effects of dietary essential amino acid deficiencies on immunological variables in broiler chickens. Br. J. Nutr.. 2000;83:449-456.
- [Google Scholar]
- Oxidation of methionine in proteins: roles in antioxidant defense and cellular regulation. IUBMB Life.. 2000;50:301-307.
- [Google Scholar]
- Anti-oxidation and anti-microorganism activities of purification polysaccharide from Lygodium japonicum in vitro. Carbohydr. Polym.. 2006;66:1-9.
- [Google Scholar]
- Oxidative stress biomarkers in mitochondrial myopathies, basally and after cysteine donor supplementation. J. Neurol.. 2010;257:774-781.
- [Google Scholar]
- Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim. Biophys. Acta. 2005;1703(2):213-219.
- [Google Scholar]
- Effect of cysteine, methionine, ascorbic acid and thiamine on arsenic-induced oxidative stress and biochemical alterations in rats. Toxicology. 2005;211:26-35.
- [Google Scholar]
- Lipid peroxidation: Mechanism, inhibition, and biological effects. Biochem. Biophys. Res. Commun.. 2005;338:668-676.
- [Google Scholar]
- Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv.. 2015;5:27986-28006.
- [Google Scholar]
- Scavenging and antioxidant potential of physiological taurine concentrations against different reactive oxygen/nitrogen species. Pharmacol. Rep.. 2010;62(1):185-193.
- [Google Scholar]
- Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr.. 1986;44:307-315.
- [Google Scholar]
- Sulfur in human nutrition and applications in medicine. Altern. Med. Rev.. 2002;7:22-44.
- [Google Scholar]
- Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med.. 1999;26:1231-1237.
- [Google Scholar]
- Evaluation of natural substances’ protective effects against oxidative stress in a newly developed canine endothelial cell-based assay and in cell-free radical scavenging assays. Int. J. Appl. Res. Vet. M.. 2012;10(2):113-124.
- [Google Scholar]
- High sulfur content in corn dried distillers grains with solubles protects against oxidized lipids by increasing sulfur-containing antioxidants in nursery pigs. J. Anim. Sci.. 2013;91(6):2715-2728.
- [Google Scholar]
- Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol. Lett.. 1995;8283:969-974.
- [Google Scholar]







