Translate this page into:
In vitro biological activities and preliminary phytochemical screening of different extracts from Achillea sintenisii Hub- Mor
⁎Corresponding author at: Faculty of Veterinary Medicine, Department of Pharmacology and Toxicology, Istanbul University- Cerrahpasa, 34500, Istanbul, Turkey. cerenis@iuc.edu.tr (Ceren Anlas),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study was designed to investigate in vitro biological activities and phytochemical composition of aqueous and ethanolic extracts from Achillea sintenisii Hub- Mor. (AS). To determine the chemical composition of AS extracts, phytochemical analyses were performed by using HPLC–ESI-Q-TOF-MS-MS. Afterwards, both extracts were investigated in terms of their effect on fibroblast proliferation, collagen synthesis, and hydrogen peroxide-induced damage. In addition to cell culture analysis, antibacterial, antioxidant, hyaluronidase inhibitory activities and total phenolic contents of the extracts were analyzed in cell-free systems. Our results demonstrated that the aqueous and ethanolic extracts did not show cytotoxic activity on fibroblasts, on the contrary, promoted fibroblast proliferation. Both AS extracts potently inhibited hyaluronidase activity and the inhibitory effect of ethanolic extract was comparable with the positive control, especially at high concentrations. The aqueous extract was the potent stimulator of collagen synthesis at 200 µg/mL concentration. Although the ethanolic extract showed antibacterial activity against all gram-positive bacteria, the aqueous extract was only effective against K. pneumoniae and B. subtilis. The ability of AS extracts, which have a rich phenolic compound content (≥50 mg GAE/g), to scavenge free radicals and protect fibroblasts against hydrogen peroxide-induced damage can be considered as a result of their antioxidant potential. Our findings scientifically support the widespread use of this plant, by demonstrating the pharmacological properties of the extracts.
Keywords
Achillea sintenisii
Fibroblast proliferation
Antioxidant activity
Antibacterial activity
Collagen synthesis
Hyaluronidase inhibitory activity
1 Introduction
In parallel with the growth of the world population, the increase in diversity of diseases and the inadequacy of conventional therapies have accelerated researchers on discovering novel therapeutic agents in the field of medicine (Menaa et al., 2013). In this scope, bioactive constituents isolated from plants have gained attention as one of the major sources to provide safe/ effective molecules to modern medicine and scientific research has focused more on natural remedies. On the other hand, people have turned to low-cost and easily accessible natural products especially in developing countries for the treatment of many ailments including cold, gastrointestinal disorders and skin diseases (Khan and Ahmad, 2019). Natural treatment options are used not only in developing countries as well as preferred in developed countries to avoid the undesirable effects associated with modern treatments (Bagde et al., 2010). In this context, plant-based extracts have been widely used for centuries and this tendency is continuously growing. The traditional use of herbal preparations is also encouraged by the World Health Organization (WHO) directives. According to WHO reports, approximately 80 % of the population uses herbal treatment options for primary health care in developing countries (WHO 2005). In addition, WHO reports point out that 25 % of modern medicines prescribed are of plant origin (WHO 2005; Khan and Ahmad 2019). Despite the widespread use of plants for medicinal purposes, only 15 % of the plant species in the world have been evaluated in terms of their pharmacological activity (Süntar 2020). Therefore, the lack of sufficient scientific evidence supporting the claimed efficacy is the crucial problem limiting their rational use in the health field.
The genus Achillea L. (Asteraceae), which is widespread in many parts of the world, mainly in Europe, Asia, North America and South Australia, has approximately more than 130 species (Paduch et al., 2008; Agar et al., 2015). Turkey is considered one of the most important diversity centers for the genus Achillea L. (Agar et al., 2015). According to the Turkish Plants Data Service, this genus is represented by 48 taxa in the Turkish flora and the rate of endemism is over 50 % (TUBİVES, 2018). Achillea sintenisii Hub- Mor. (AS), which is the subject of the present study, is one of these endemic species and is widely distributed in the Central Anatolia region of Turkey. The species of the genus Achillea are enormously rich in different types of secondary metabolites and accordingly have valuable biological properties, especially for dermatological applications (Strzępek-Gomólka et al., 2021). Scientific studies have shown that major constituents of Achillea species such as chlorogenic acid, apigenin-7-O-glucoside, and schaftoside stimulate collagen expression through different mechanisms (Dorjsembe et al., 2017). Chlorogenic acid has the ability to inhibit matrix metalloproteinase (MMP)- 9, which is one of the proteases associated with the inflammatory process and also plays an important role in the degradation of collagen (Jin et al., 2005; Benedek et al., 2007; Bor et al., 2022). Apigenin and luteolin isolated from Achillea species have been reported to suppress the expression of inflammatory mediators and contribute to the prevention of long-term inflammation, which is an important strategy for wound treatment (Dorjsembe et al., 2017). Also, A. millefolium extracts are known as powerful antioxidants in relation to the phenolic components they contain including luteolin-7-O-glucoside, chlorogenic acid, and rutin (Vitalini et al., 2011). It has also been suggested that isovitexin flavonoid isolated from some Achillea species, such as A. alpina L., has the potential to promote skin whitening associated with down-regulation of intracellular tyrosinase activity (Lee et al., 2019). In this context, various extracts prepared from this genus have been used as active ingredients in skin healing and conditioning products in the pharmaceutical industry (Strzępek-Gomólka et al., 2021). Besides their industrial use, many Achillea species, including Achillea sintenisii Hub- Mor., have been used in traditional medicine as a natural remedy for pain, inflammation, wounds, and bleeding (Strzępek-Gomólka et al., 2021; Sezik et al., 2001). In addition to the traditional use of Achillea species, the wound healing (Küpeli Akkol et al., 2011; Agar et al., 2015), antinociceptive (Küpeli et al., 2007), antimicrobial (Candan et al., 2003; Karaalp et al., 2009), antispasmodic (Karamenderes and Apaydın, 2003), anti-inflammatory (Gómez et al., 1999) and antioxidant (Konyalioglu and Karamenderes, 2005; Anlas et al., 2017) activities of several Achillea species have been confirmed by scientific reports. Previous studies on AS have demonstrated that the methanolic extract and essential oil from this species possess strong antimicrobial activity against various bacteria and yeast (Sökmen et al., 2003). However, to the best of our knowledge, there is not any comprehensive study concerning the biological activities of AS extracts.
In the present study, it was aimed to provide scientific support to the traditional use of AS extracts with pharmacological activity studies. It is known that the type of extraction solvent and technique may have a significant impact on the quantity and variety of isolated bioactive compounds and simultaneously on the biological activity of extract (Rafińska et al., 2019). In this context, we used two extracts (ethanol and water) obtained from AS by different extraction techniques and primarily performed the phytochemical screening of the extracts by using high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (HPLC–ESI-Q-TOF-MS-MS). Afterwards, the antibacterial, antioxidant and hyaluronidase inhibitory activities of the extracts were assessed in cell-free systems; as well as the effects on fibroblast proliferation, collagen synthesis, and protection against hydrogen peroxide (H2O2) induced damage were investigated in in vitro systems.
2 Materials and methods
2.1 Plant material
AS was collected from Sivas, Turkey (Between Sivas- Hafik, Topçuyeniköy- 1384 m) in June 2013 (geographic coordinates: 39°46′23.0″N 37°34′48.1″E). A voucher specimen is deposited in the herbarium of Cumhuriyet University, Faculty of Science, Department of Biology (CUFH-AA 5044). Before starting the extraction process, the collected plant samples were dried at room temperature (20–24◦C).
2.2 Extraction process
In the extraction process, water and ethanol (96 %) were used to isolate different bioactive constituents from the aerial parts of AS. The aqueous extract (ASA) was prepared by boiling 25 g of the powdered plant material in deionized water (500 mL) during 5 min (Albayrak et al., 2012). Then the extract was allowed to stand for 10 min at room temperature. To obtain the ethanolic extract (ASE), the same amount of plant material was macerated with ethanol for 10 consecutive days, at room temperature (Ustuner et al., 2019). All these extracts were concentrated at 40 °C under reduced pressure and dried by freeze-dryer. The extracts were stored at –20 °C until analysis. The extraction yields of ASA and ASE were 7.9 % and 3.2 % respectively.
2.3 Phytochemical screening (HPLC ESI-Q-TOF MS-MS analysis)
To identification of bioactive compounds in AS extracts, HPLC ESI-Q-TOF MS-MS analyses were performed using Agilent 1260 series coupled with Agilent 6550 iFunnel high-resolution mass spectrometry (Agilent Technologies, Inc., CA, USA). Jet stream electrospray ionization technique was used in negative ionization mode. Agilent TC C-18 (4.6 mm × 150 mm × 5 µm) analytical column was used for chromatographic separation. Data interpretation was carried out by using Agilent MassHunter software B.06.01 and Metlin Metabolite database. HPLC ESI-Q-TOF MS-MS conditions were summarized in Table 1. Deionized water (1 mL) and methanol–water mixture (1 mL) were used to dissolve ASA and ASE (10 mg), respectively. All the samples were filtered (0.45 μm) before the injection.
HPLC conditions
Column temperature
30 °C
Injection volume
5.0 µL
Analysis time
82 min
Mobile phase A- B
10.0 mM ammonium acetate in water- Acetonitrile
Flow rate
0.65 mL/min
Solvent composition timetable
5 % B (0 min)
5 % B (4 min)
10 % B (12 min)
10 % B (15 min)
20 % B (28 min)
40 % B (48 min)
Detection wavelength
260 nm
MS conditions
Ionization mode
Negative
Drying gas flow, N2
14.0 L/min
Drying gas temperature
200 °C
Nebuliser
40 psi
Sheat gas flow, N2
11.0 L/min
Sheath gas temperature
375 °C
Capillary voltage
1100 V
Nozzle voltage
2000 V
Mass range
20–1500 amu
Reference ion
966.000725
2.4 Total phenolic content (TPC) estimation
The content of phenolics in AS extracts were quantitatively determined using the Folin-Ciocalteu (FC) assay (Dominquez et al. 2005). Briefly, 10 µL of the methanolic solutions of the extracts (at a concentration of 2 mg/mL) were mixed with 150 µL of FC reagent diluted with water (1:4, v/v). After 3-min incubation, 50 µL of sodium carbonate solution was added and this mixture incubated for 2 h in the dark. Finally, the absorbance of each well was read at 725 nm. Gallic acid (0, 0.05, 0.1, 0.2 and 0.4 mg/mL) was used as the standard phenolic compound and the calibration curve of gallic acid was drawn to calculate the total phenolics. TPCs of AS extracts were expressed as milligram (mg) of gallic acid equivalent (GAE) per gram (g) of extract.
2.5 2,2-diphenyl-1 picrylhydrazyl (DPPH) scavenging assay
DPPH free radical scavenging ability of AS extracts was determined following the method described by Yang et al. (2009). Serial dilutions of AS extracts (25 to 200 μg/ mL, 200 μL) and ascorbic acid (positive control) were mixed with freshly prepared DPPH solution (0.15 mM, 50 μL). After 30-min incubation in the dark, the absorbance of each well was measured by a microplate reader (at 517 nm wavelength). The IC50 values, defined as the extract concentration needed to decrease the initial free radical by 50 %, were calculated using a log-dose inhibition curve.
2.6 Antibacterial activity (Agar dilution assay)
The agar dilution assay was carried out to determine the antibacterial activities of the extracts following the procedure described by the Clinical and Laboratory Standards Institute (CLSI, 2006). In this context, minimum inhibitory concentrations (MICs) of the extracts against both gram-positive (Bacillus subtilis, Staphylococcus aureus, Bacillus cereus, Staphylococcus epidermidis) and gram-negative bacteria (Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumonia) were detected. Gentamicin sulfate was used as the reference standard for antibacterial activity against both gram-positive and gram-negative bacteria, as it is a broad-spectrum antibiotic (Kimna et al., 2018). For antibacterial analysis, the extracts were dissolved at a concentration of 80 mg/mL and diluted with cation-adjusted Mueller Hinton broth to obtain final concentrations of 0.015625 to 8 mg/mL. Each inoculum (1 mL) was poured into petri dishes and mixed with 9 mL of Muller–Hinton agar (45–50 °C) by gentle circular rotation. Then, the mixture was kept cool at room temperature (25 ± 2 °C). A bacterial suspension (adjusted to about107 CFU/mL) was prepared and added to each well of the microplate. The replicator was placed into the microplate to soak the pins and transfer them onto the agar plate. The MIC values were determined as the lowest extract concentrations that prevent bacterial growth after 24 h of incubation at 37 °C.
2.7 Hyaluronidase inhibitory activity
The inhibitory effect of AS extracts on the hyaluronidase, an enzyme responsible for the degradation of hyaluronic acid, was measured by a turbidimetric method. The method is based on the remaining hyaluronic acid interacts with the albumin solution and creates turbidity (Kass and Seastone, 1944; Tolksdorf and McCready, 1949). In this experiment, tannic acid was used as a reference, and albumin was used as a blank. The samples were run in three parallel. First, 20 µL of samples at different concentrations (50, 100, and 200 µg/mL) were added to the wells on the ELISA microplate. Hyaluronic acid (0.4 mg/mL, 10 μL) was added to the samples and incubated at 37 °C for 4–5 min. Then, hyaluronidase enzyme (0.05 mg/mL, 10 μL) was added to the mixture and stored for 10 min. Finally, 180 µL of albumin solution was added to the microplate wells brought to room temperature and incubated for further 10 min. The absorbance was read in a microplate reader at 540 nm. The percentage inhibition was calculated according to this formula:
Percentage of hyaluronidase inhibition (%) = [(Sample absorbance- Control absorbance) / Control absorbance] x100.
2.8 Cell culture conditions
Cell culture analyses were carried out on fibroblast cell line (3 T3-Swiss albino mouse, ATCC-CCL-92). Briefly, the cells were nurtured in Dulbecco’s Modified Eagle’s Medium-F12 supplemented with penicillin/streptomycin solution (1 %) and fetal bovine serum (10 %). A humidified ambiance (37 °C, 5 % CO2) was set up for the growth of the cells. The culture medium was changed every 48 h until the cultures reached confluence. For the cell culture analysis, fibroblasts were detached from the culture flask using trypsin-EDTA solution (0.25 %). The stock solutions of plant extracts were prepared by dissolving the extracts in dimethyl sulfoxide (DMSO) and diluting with cell culture medium. The final DMSO concentration did not exceed 0.2 % throughout the study and the control cells were treated with the corresponding amount of DMSO.
2.8.1 Cell proliferation assay (MTT assay)
The effects of Achillea sintenisii Hub- Mor. extracts on fibroblast proliferation were determined by MTT (3- [4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) assay. For this purpose, mouse fibroblasts were seeded to the wells of a 96-well plate at a density of 1 × 104 cells per well and incubated for 24 h for cell attachment. Then, the medium was gently removed from the wells and the cells were further incubated with varying concentrations of (50, 100, and 200 µg/mL) AS extracts for 72 h. The MTT cell proliferation assay kit was used according to the manufacturer's instructions (Roche Applied Science). The absorbance of each well was read at 595 nm using the multimode microplate reader. The effects of AS extracts on fibroblast proliferation were assessed as the percent cell viability where untreated cells were considered as 100 % viable. The cell viability percentage was calculated as follows:
Percentage of cell viability (%) = (Absorbance of treated cells / Absorbance of control cells) × 100.
2.8.2 Hydroxyproline assay
To quantify the effect of Achillea sintenisii Hub- Mor. extracts on collagen production, hydroxyproline assay was carried out following to the method published by Jorge et al. (2008). 3 T3 mouse fibroblasts were seeded at a density of 1 × 103 cells per well, and the plates were incubated at 37 °C for 24 h. After incubation, the culture medium was discarded and cells were exposed to serial dilutions of extracts (50, 100, and 200 µg/mL) in fresh medium for 72 h. In the positive control group, fibroblasts were treated with ascorbic acid (25 µg/mL). The supernatant of each well was collected and hydrolyzed with 6 N hydrochloric acid (HCl) at 120 °C by using an autoclave. Chloramine-T solution was added to the hydrolyzed samples first, and after 20 min of the incubation period at room temperature, Ehrlich's reagent was added to the mixture. The absorbance of the samples was measured at 550 nm by a multimode microplate reader, following 15 min of incubation at 65 °C. To determine the hydroxyproline content in the samples, a standard curve was prepared by hydroxyproline standard in concentrations ranging from 0 to 10 μg/mL. The results were expressed as µg/mL of hydroxyproline.
2.8.3 Antioxidant activities of the extracts on mouse fibroblasts
The effect of AS extracts on the H2O2-induced oxidative damage was investigated by the method published by Annan and Dickson (2008). The cells were seeded in a 96-well plate (5 × 103 cells/well) and incubated at 37 °C overnight. Afterwards, the culture medium was discarded and two independent experiments were performed on the cells. In the first experiment, fibroblasts were pre-treated with the extracts at different concentrations (50, 100, and 200 µg/mL) for 24 h and subsequently incubated with H2O2 (10−4 M) for 3 h. In the second protocol, fibroblasts were treated with the same concentrations of AS extracts and H2O2 (10−4 M) simultaneously for 3 h. In both experiments, MTT assay was used to measure the protective effect of AS extracts against cellular damage induced by H2O2 in fibroblasts. As the positive control group, fibroblasts were treated with Catalase (250 IU/mL) in both experiments.
2.9 Statistical analysis
Results were presented as mean ± standard error of the mean (SEM) from three independent experiments. Data were analyzed using one-way (ANOVA) followed by Student’s t-test. (SPSS 15.0, SPSS Inc., Chicago, IL, USA). Differences were considered to be significant at P < 0.05.
3 Results and discussion
3.1 HPLC ESI-Q-TOF MS-MS analysis
In this study, the compounds were putatively identified by their mass spectrometric ions through comparison with the literature and library database search from the instrument software. NIST library matching percentage was higher than 99.99 % for identification of all compounds. The MS spectrum and HPLC chromatogram of ASA and ASE were shown in Fig. 1 and Fig. 2, respectively. Chlorogenic acid, isoschaftoside isomers, luteolin derivatives (glucoside and methyl ether glucuronide), vicenin 2, rutin, quercetin glucoside, isorhamnetin derivatives (glucoside and glucoside rhamnoside) and dicaffeoylquinic acid isomers were identified in ASA. Almost all the compounds found in ASA were also identified for ASE, except dicaffeoylquinic acid and its isomers. The difference between AS extracts can be clearly seen in Fig. 1 at mainly 39.65 min and minorly 40.79; 41.86 mins.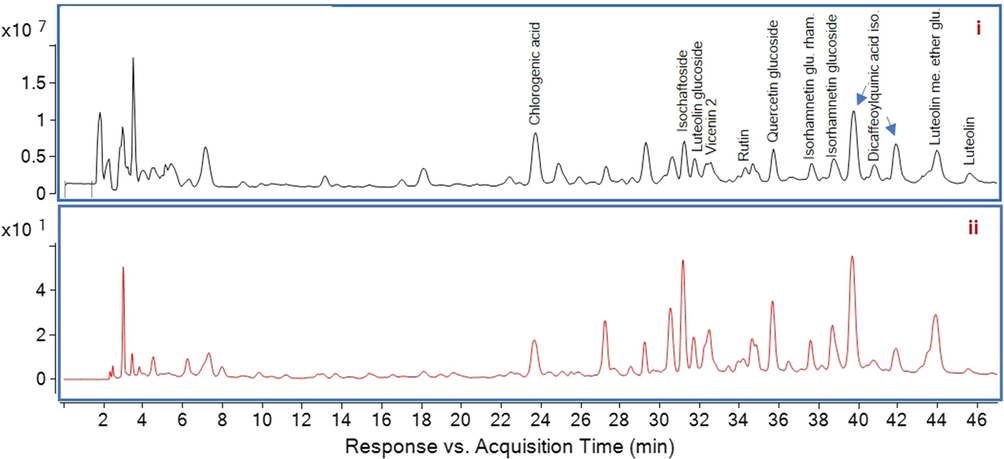
MS spectrum (i) and HPLC chromatogram (ii) of ASA. ASA, Aqueous extract; HPLC, high-performance liquid chromatography; MS, mass spectrometry.
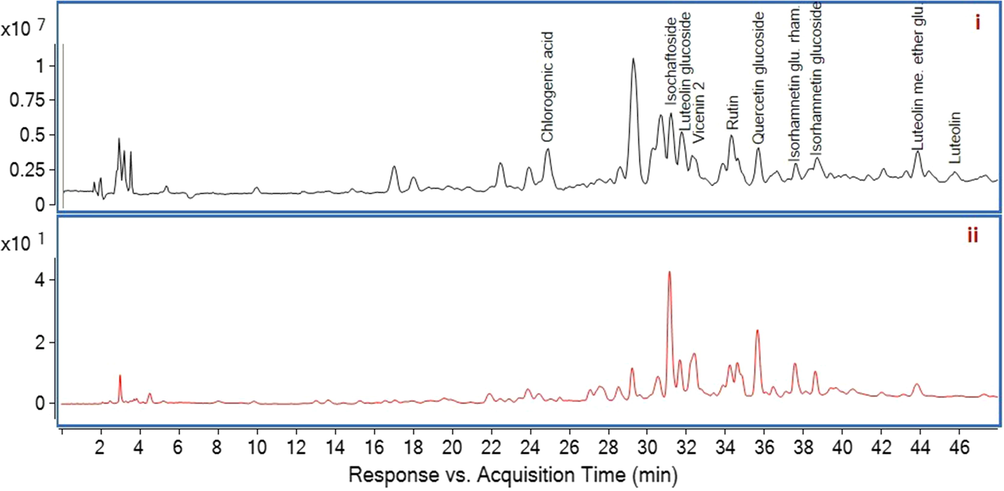
MS spectrum (i) and HPLC chromatogram (ii) of ASE. ASE, Ethanolic extract; HPLC, high-performance liquid chromatography; MS, mass spectrometry.
Separated compounds were identified by HPLC ESI-Q-TOF MS-MS in negative ionization mode. All identified compounds including retention time, molecular weight, relative abundances (%) and fragments were summarized in Table 2. Chlorogenic acid showed [MH]- value at m/z 353.0881 with the fragment ions at m/z 191 and 179 due to the loss of a caffeoyl group and a quinic group, respectively (Chen et al., 2012). Isoschaftoside showed [MH]- value at m/z 563.1407 with the fragment ion at m/z 311 and luteolin glucoside showed [MH]- value at m/z 447.0863 with the fragment ion at m/z 285. Both product ions prove the lack of a glucoside group (Plazonic et al., 2009). Vicenin 2 showed [MH]- value at m/z 593.1504. Rutin showed [MH]- value at m/z 609.1409 with the fragment ion at m/z 301 by losses of rutinose group (Tohma et al., 2016). Quercetin glucoside showed [MH]- value at m/z 463.0814 and gave fragment ion at m/z 301. Isorhamnetin glucoside rhamnoside and isorhamnetin glucoside showed [MH]- value at m/z 623.1641 and 477.0990, respectively, which are interpreted from the instrument database. Dicaffeoylquinic acid showed [MH]- value at m/z 515.1310 with fragment ions 353 (chlorogenic acid), 191, 179 (Bejaoui et al., 2003). The obtained spectra showed the [MH]- molecular ion at m/z 477.0966 gave fragment ions 299, 285, 199, 175 which was attributed to the luteolin methyl ether glucuronide by interpretation from the instrument database. Luteolin gave [MH]- value at m/z 285.0405 with fragment ions at m/z 199, 151 and 133 (Tohma et al., 2016). ASA: A. sintenisii Hub- Mor. aqueous extract, ASE: A. sintenisii Hub- Mor. ethanolic extract.
Compounds
tR(min)
[M−H]-
Fragment ions
Relative abundance for ASA (%)
Relative abundance for ASE (%)
Chlorogenic acid
23.67
353.0881
191, 179
6.53
2.19
Isochaftoside
31.16
563.1407
311
3.04
6.94
Luteolin glucoside
31.76
447.0863
285
1,58
5.31
Vicenin 2
32.57
593.1504
456, 474, 576
2.32
3.02
Rutin
34.64
609.1409
301
1.47
6.20
Quercetin glucoside
35.71
463.0814
301
2.50
2.59
Isorhamnetin glucoside rhamnoside
37.58
623.1641
477, 316
1.58
1.17
Isorhamnetin glucoside
38.78
477.0990
316
2.83
3.72
Dicaffeoylquinic acid isomers
39.65
515.1310
353, 191, 179
15.59
0.89
Luteolin methyl ether glucuronide
43.86
477.0966
299, 285, 199, 175
5.73
3.04
Luteolin
45.53
285.0405
199, 151, 133
1.19
1.27
The phenolic contents of water and water–ethanol extracts obtained from AS have been previously investigated and similar to our results, the presence of quercetin glucoside and luteolin glucoside in both extracts has been reported (Şabanoğlu et al., 2017). However, the presence of different phytocomponents including chlorogenic acid or dicaffeoylquinic acid, which are the main bioactive constituents of many Achillea species, in AS extracts were demonstrated for the first time in this study.
3.2 DPPH scavenging capacities and total phenolic content of A. sintenisii Hub- Mor. extracts
Free radicals are intermediates formed during cell metabolism and essential for critical cellular functions (such as leukocyte adhesion, signal transduction, platelet aggregation, etc.) under normal conditions (Bruce and Buehler, 2012). However, their excessive production can cause oxidative stress, which can damage to cellular reactions and lead to various chronic diseases (Zhang et al., 2020). For example, excessive amounts of free radicals generated on the wound surface in response to skin injury may also delay the healing process by destroying lipids, proteins, and essential components of extracellular matrix (Ustuner et al., 2019). It was suggested that treatment options which act to modulate free radical formation can be a beneficial approach in the management of degenerative disorders (Henriques et al., 2006). In this context, subsequent scientific studies have reported that plants with high antioxidant capacity may down-regulate degenerative processes and effectively reduce the incidence of many disorders related with oxidative stress (Sultana et al., 2009). The oxidation reducing properties of plant-derived antioxidants may occur by interrupting the free radical chain reaction or by directly scavenging reactive oxygen species. In this direction, we investigated the antioxidant activities of AS extracts by determining their DPPH free radical scavenging abilities.
The percentage of DPPH radical scavenging activity ranged from 19.19 % to 50.67 % for ASE and from 16.9 % to 53.56 % for ASA. On the other hand, the standard compound, ascorbic acid, inhibited the DPPH radical more strongly up to 95.79 %. ASA and ASE demonstrated moderate and very similar activity against DPPH free radical with IC50 values of 183.13 ± 1.10 µg/mL and 184.31 ± 0.80 µg/mL, respectively (Table 3), however, this activity was not as strong as ascorbic acid (27.63 ± 1.12 µg/mL). In previous studies conducted on other Achillea species, high variation has been reported among DPPH radical scavenging capacities of the extracts due to various factors such as temperature or altitude difference in the region where the plants were collected and genetic differences (Gharibi et al., 2013). Our results demonstrated that the aqueous and ethanolic extract of AS has similar radical scavenging potential to the ethanolic extracts of A. wilhelmsii and A.cucullata with close IC50 values (118.90 µg/mL and 132.55 µg/mL, respectively) (Nickavar et al.,2006; Eruygur et al., 2019). Similar to the results of our study, it is noteworthy that the DPPH scavenging activities of A.wilhelmsii and A. cucullata extracts were not as pronounced as rutin and gallic acid, which are used as reference compounds for radical scavenging activity. These results can be interpreted as the fact that although the extracts belonging to Achillea species have DPPH free radical scavenging activity, these effects are not as strong as standard antioxidants. On the other hand, the reduction of DPPH radical is highly correlated with the presence of hydroxyl groups (phenolic or non-phenolic), which act as hydrogen donors to stabilize the free radicals (Mensor et al., 2001). The results of the phytochemical analysis in this study evidenced the presence of phenolic compounds (rutin, luteolin, quercetin, etc.) containing hydroxyl groups in AS extracts. In this context, the free radical scavenging capacities of AS extracts can be attributed to the presence of these compounds, which have the ability to stabilize free radicals. ASA: A. sintenisii Hub- Mor. aqueous extract, ASE: A. sintenisii Hub- Mor. ethanolic extract, GAE: Gallic acid equivalent, TPCs: Total phenolic contents.
Extracts
DPPH scavenging activity [IC50 (µg/mL)]
TPCs [mg GAE/g Extract]
ASA
183.13 ± 1.10
58.62 ± 0.95
ASE
184.31 ± 0.80
110.0 ± 0.84
In the present study, we also determined the amount of TPC. The TPCs of ASA and ASE were determined as 58.62 ± 0.95 mg GAE/g and 110 ± 0.84 mg GAE/g, respectively. According to Agourram et al. (2013), the TPC of plant extracts could be subdivided as follows: high content (≥50 mg GAE/g), medium content (50–20 mg GAE/g), and low content (<20 mg GAE/g). Considering this classification, it can be concluded that both AS extracts have a rich content of phenolic compounds that act as highly effective scavengers of various oxidizing molecules and free radicals (Montoro et al., 2005).
3.3 Antibacterial activities of A. Sintenisii Hub- Mor. Extracts
Antibacterial activities of AS extracts were investigated against four strains of gram-positive and three strains of gram-negative bacteria. The antibacterial activities of AS extracts expressed with MIC values are summarized in Table 4. ASE exhibited antibacterial activity against all of the tested gram-positive bacteria (B.subtilis, S.aureus, S.epidermidis, B.cereus) with MIC values ranging from 0.5 to 8 mg/mL, while ASA was only effective against K. pneumonia and B. subtilis with a MIC value of 4 mg/mL. B.subtilis was sensitive to both ASE and ASA, while P.aeruginosa and P.mirabilis were resistant to both AS extracts. Our findings are consistent with the results of the study conducted by Sökmen et al. (2003) who reported that the essential oil of this plant did not show any antibacterial activity against P. mirabilis and P. aeruginosa. On the other hand, our findings reveal that ASE has a better antibacterial profile against B.cereus and S.aureus with lower MIC values than those reported for the essential oil of the same plant (Sökmen et al., 2003). ASA: A. sintenisii Hub- Mor. aqueous extract, ASE: A. sintenisii Hub- Mor. ethanolic extract, NI: not inhibition, a: positive control.
Plant material
Minimum Inhibitory Concentrations (mg/mL)
B.subtilis
B.cereus
S.aureus
S.epidermidis
P.mirabilis
P. aeruginosa
K. pneumoniae
ASA
4
NI
NI
NI
NI
NI
4
ASE
2
4
8
0.5
NI
NI
NI
Gentamicin a
0.0005
0.0008
0.0005
0.0005
0.00025
0.00025
0.00025
According to the classification reported by Fabry et al. (1998), crude plant extracts should have MIC values < 8 mg/mL, to be considered as useful therapeutically. In this direction, we can conclude that especially ASE has sufficient antibacterial activity against many gram-positive bacteria. This activity can be attributed to the presence of bioactive molecules with previously demonstrated antibacterial potential, such as quercetin or chlorogenic acid (Yang et al., 2020; Lou et al., 2011). It is noteworthy that, although ASA contains these molecules, it showed no antibacterial activity against most gram-negative and gram-positive bacteria tested. This situation may be a result of the inefficient diffusability of aqueous extracts in the agar medium and the low ability of aqueous extracts to damage bacterial cell walls, as previously reported by Kaczorova et al. (2021).
3.4 Hyaluronidase inhibitory effects of A. sintenisii Hub- Mor. extracts
Hyaluronic acid, one of the main glycosaminoglycans found in the extracellular matrix of connective tissues, plays an essential role in many pathological and physiological processes including embryogenesis, angiogenesis, wound healing, cell differentiation/proliferation, and inflammation (Girish et al., 2009; Gonzalez- Rico et al., 2019). As natural component of extracellular matrix, hyaluronic acid is the focus of scientific research especially related with the skin-health (Li et al., 2020). Hyaluronidase-mediated degradation of hyaluronic acid can increase the permeability of connective tissue, impair the structural integrity of the extracellular matrix, as well as affect progression of cancer, spread of toxins or microbial pathogenesis (McCook et al., 2015; Girish and Kemparaju 2007; Li et al., 2020). Therefore, inhibition of hyaluronidase activity is critical in many pathological conditions including skin wounds, allergy or inflammation, and is considered as a promising strategy in reducing the progression of diseases which are associated with up-regulation of this enzyme (Li et al., 2020; Arunkumar et al., 2021). Hyaluronidase inhibitory activities of many plant species have been demonstrated by scientific reports (Kim et al., 1995; Liyanaarachchi et al., 2019). However, no study has been found in the literature on the hyaluronidase inhibitory activities of Achillea species, which are especially used in traditional medicine to treat skin inflammation and wounds. In the present study, it was thought that the investigation of hyaluronidase inhibitory activity could contribute to the elucidation of the mechanism of action of Achillea species on skin disorders and AS extracts were evaluated for their in vitro hyaluronidase inhibitory activities.
The inhibitory effects of AS extracts on hyaluronidase enzyme were presented in Table 5. Both of the extracts exhibited concentration-dependent and significant hyaluronidase inhibitory activity at all concentrations, compared with the control. The inhibitory activity of ASE was very close to tannic acid, especially at high concentrations. Previous studies have reported that identified flavonoid glycosides in AS extracts such as quercetin and luteolin glycosides can able to inhibit the hyaluronidase activity and lead to a more stable extracellular matrix (Kim et al., 2005; Gendrisch et al., 2021). Hence, it is thought that these phytochemicals contained in the extracts may be responsible for the hyaluronidase inhibitory activity. In particular, the inhibitory activity of ASE is very close to tannic acid, indicating that this extract may serve as a potent natural hyaluronidase inhibitor and play an important role in hyaluronidase-mediated pathological processes. ASA: A. sintenisii Hub- Mor. aqueous extract, ASE: A. sintenisii Hub- Mor. ethanolic extract.
Plant material
Concentrations
50 µg/mL
100 µg/mL
200 µg/mL
ASA
64.5 ± 2.73***
71.77 ± 1.71***
72.8 ± 1.08***
ASE
69.45 ± 1.83***
83.69 ± 2.06***
84.16 ± 2.25***
Tannic acid
79.94 ± 1.05***
82.18 ± 2.45***
85.95 ± 1.49***
3.5 Effect of A. sintenisii Hub- Mor. extracts on fibroblast proliferation
In the present study, the effects of AS extracts on the proliferation of fibroblasts were investigated by MTT assay, which is a useful method used for screening the cytotoxic or proliferative effects of plant extracts (Mektrirat et al., 2020). The viability of fibroblasts treated with various concentrations of AS extracts was presented in Fig. 3. As shown in the figure, the percentage of cell viability treated with ASA and ASE varied between 108.70 % and 115.73 % and 99.09 %- 111.4 %, respectively. ASA (at all concentrations) and ASE (at 100 µg/mL concentration) significantly stimulated fibroblast proliferation compared with the control group. The results of the MTT assay also contributed interpretation of the cytotoxic activity of AS extracts on fibroblasts. None of the extracts significantly reduced fibroblast viability compared with non-treated cells at the applied concentrations. Considering these results it can be concluded that AS extracts have a good safety profile on healthy cells by not adversely interfering with cell viability, as well as promote the healing process of skin by stimulating fibroblast proliferation.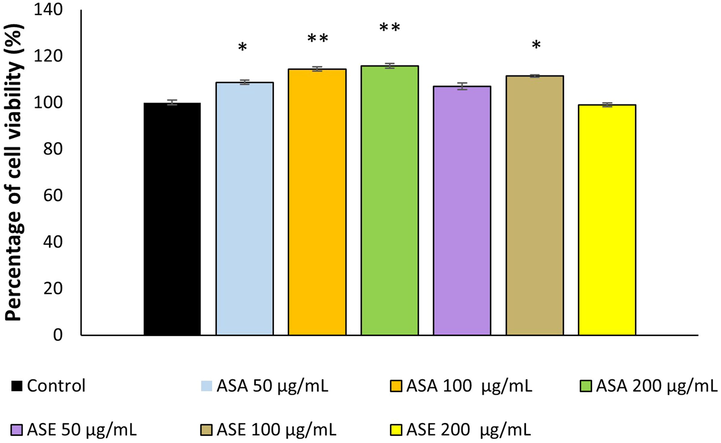
Percentage of fibroblast viability (%) following treatment with different extracts of A. sintenisii Hub-Mor., determined by MTT assay. ASA, Aqueous extract; ASE: Ethanolic extract. * P < 0.05, **P < 0.01 versus control cells.
The results of our study were consistent with previous reports on the toxicity potential of different Achillea species on healthy cells. In the study conducted by Bali et al. (2015), the cytotoxicity potential of Achillea teretifolia Willd was investigated on human gingival fibroblasts and was reported that the methanolic and aqueous extracts did not induce cytotoxicity up to concentrations of 0.45 mg/mL and 1.4 mg/mL, respectively. In another study, evaluating the proliferative effect of hydroalcoholic extract from Achillea millefolium leaves on skin fibroblasts, Ghobadian et al. (2015) reported that the extract can stimulate fibroblast proliferation at < 20.0 mg/mL concentrations. Similarly, Agar et al. (2015) reported that A. kotschyi Boiss. subsp. kotschyi exhibited potent proliferative activity on mouse fibroblasts at 2.5–20 µg/mL concentration. It has been previously proven that many phytochemicals of AS, such as Vicenin-2, quercetin glucoside, and rutin, that we have identified both in ASA and ASE can promote fibroblast proliferation (Dinda et al., 2016; Tan et al., 2020; Muhammad et al.,2013). It is thought that individual or synergistic effects of these phytoconstituents can be responsible for the proliferative activities of AS extracts. The only notable difference between the phytochemical contents of the extracts was the presence of dicaffeoylquinic acid isomers in ASA. The significant and pronounced proliferative activity of ASA at all tested concentrations is likely to be related to dicaffeoylquinic acid isomers, which have been reported to act on fibroblasts in the skin healing process (Chiangnoon et al., 2022).
3.6 Effect of A. sintenisii Hub- Mor. extracts on collagen synthesis
Collagens are the principal structural protein of all connective tissues and are also present in the interstitial tissue of virtually all parenchymal organs (Sandhu et al., 2012). Collagens provide support to many tissues including cartilage, tendon, bone, and ligaments and play a vital role in maintaining the structural integrity of these tissues (Rodriguez et al. 2018). Previous studies have demonstrated that extracts obtained from various Achillea species have the ability to stimulate collagen production in dermal fibroblasts (Agar et al., 2015). From this point of view, in our study, the effects of AS extracts on collagen production were evaluated by measuring hydroxyproline level, which is considered a marker for collagen biosynthesis (Anlas et al., 2019). The changes in hydroxyproline levels following treatment with ASA and ASE were demonstrated in Fig. 4. Treatment of cells with ASE resulted in an approximately 1.05 to 1.25-fold increase in hydroxyproline synthesis compared with untreated cells (P < 0.05). On the other hand, ASA significantly stimulated hydroxyproline synthesis (2.51-fold) compared with control only at the highest concentration (P < 0.01).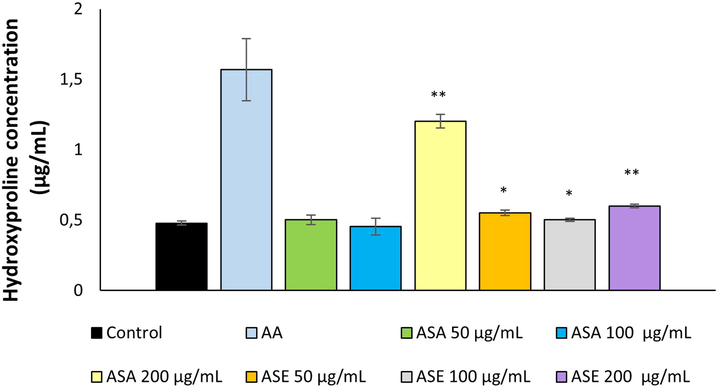
Hydroxyproline content in the groups treated with different extracts from A. Sintenisii Hub-Mor. AA, Ascorbic acid; ASA, Aqueous extract; ASE, Ethanolic extract. * P < 0.05, **P < 0.01 versus control cells.
To the best of our knowledge, there is no study revealing the effect of Achillea species on collagen production, by directly measuring the hydroxyproline level in fibroblasts. On the other hand, our findings are consistent with the results of studies demonstrating the effects of various Achillea species on collagen synthesis by different in vivo and in vitro methods. In the study conducted by Agar et al. (2015), the morphometric analysis revealed that methanolic extracts of Achillea kotschyi Boiss. subsp. kotschyi and Achillea coarctata Poir. significantly increase the number of collagen granules in mouse fibroblasts. Similarly, histological observations on wound healing properties of Achillea biebersteinii Afan. indicated that especially the n-hexane extract has stimulated the formation of collagen fibers (Küpeli Akkol et al., 2011). Considering our results, it can be thought that AS extracts may contribute to the treatment of pathologies characterized by collagen disorders by promoting collagen synthesis, especially at high concentrations. The effect of AS extracts on collagen synthesis can be attributed to the presence of phenolics present in the extracts (such as rutin), which have previously proven to be effective on collagen biosynthesis in dermal fibroblasts (Jampa et al., 2022; Stipcevic et al.,2006).
3.7 Protective effect of A. sintenisii Hub- Mor. extracts on H2O2– induced damage
Oxidative stress has a crucial role in the development and pathogenesis of many diseases, by causing profound changes in biological structures, such as cellular membranes, proteins, lipids, and nucleic acids (Luo et al., 2018). In recent years, interest in plant-derived antioxidants which have protective properties against oxidative stress-induced damage caused by free radicals has increased (Sharathchandra and Rajashekhar, 2013). In this context, it has been revealed that many plant species have high antioxidant capacity, especially in relation to their polyphenol and flavonoid contents (Konyalioglu and Karamenderes, 2005; Ustuner et al., 2019). Previous reports on different Achillea species have demonstrated that this plant displayed pronounced antioxidant activity by protecting the cells against oxidative damage induced by H2O2 (Varasteh-kojourian et al. 2016). Accordingly, in the present study, AS extracts were assessed for their ability to protect fibroblasts against H2O2-induced damage using two different treatment protocols.
As shown in Fig. 5 and Fig. 6, treatment with H2O2 alone, reduced the viability of fibroblasts to approximately 54 %. The viability of cells pre-treated and co-treated with AS extracts was 1.89 to 2.20-fold and 1.58 to 2.12-fold higher respectively, compared with the cells treated with H2O2 alone. In the pre-treatment protocol, ASA (at 200 µg/mL concentration) offered the highest protection against oxidative damage almost comparable with that of catalase (positive control). Both extracts exhibited significant protective activity against H2O2-induced damage in both co-treatment and pre-treatment protocols.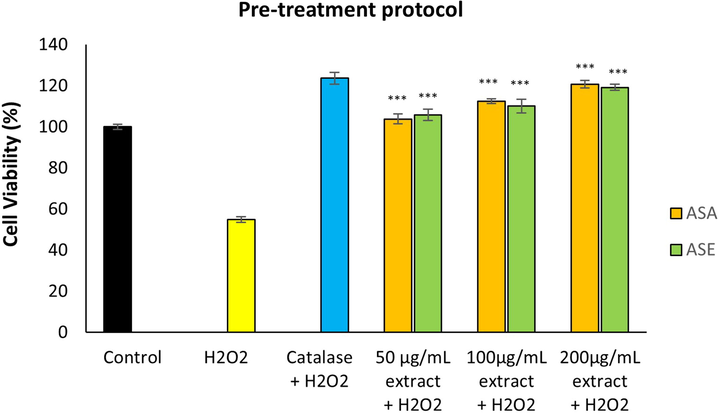
Effect of A. sintenisii Hub-Mor. extracts on hydrogen peroxide-induced damage in fibroblasts following pre-treatment protocol·H2O2, Hydrogen peroxide; ASA, Aqueous extract; ASE, Ethanolic extract. ***P < 0.001 versus cells exposed to only H2O2 without extract.
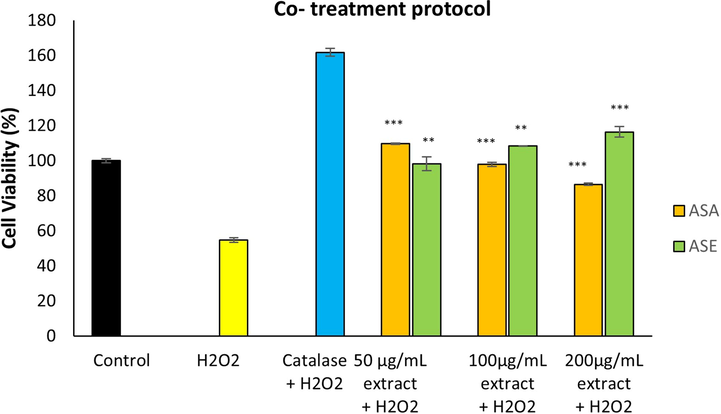
Effect of A. sintenisii Hub-Mor. extracts on hydrogen peroxide-induced damage in fibroblasts following co-treatment protocol·H2O2, Hydrogen peroxide; ASA, Aqueous extract; ASE, Ethanolic extract. **P < 0.01, ***P < 0.001 versus cells exposed to only H2O2 without extract.
Our findings are similar to the results of the study conducted by Varasteh-kojourian et al. (2016), who reported that methanolic extract of Achillea biebersteinii can inhibit H2O2– induced cytotoxicity in fibroblasts. Additionally, Konyalioglu and Karamenderes (2005) have reported that different Achillea species protected the antioxidant enzyme levels of erythrocytes and leukocytes against H2O2-induced oxidative damage, and this effect might be attributed to the rich phenolic contents of the extracts. Since AS extracts contain a wide variety of phytoconstituents with antioxidant properties (luteolin, quercetin, rutin, isorhamnetin, etc.), as demonstrated by our phytochemical analyses, it is thought that cytoprotective effects of the extracts may be a result of interactions of different antioxidants in the extracts (Gong et al.2020; Boots et al., 2008; Ozgen et al., 2016).
4 Conclusion
Our findings scientifically support the traditional use of this plant by demonstrating its ability to proliferate fibroblasts, promote collagen synthesis, protect cells against oxidative damage, as well as exhibit antibacterial, antioxidant, and hyaluronidase inhibitory activities. In addition to the therapeutic potential of AS extracts, they did not exhibit cytotoxic activity on fibroblasts, suggesting their safety profile. The present study also provides important data regarding the phytochemical composition of two different AS extracts. Summarizing the results of this study, it can be concluded that AS extracts may have therapeutic potential in many pathological conditions associated with oxidative stress, hyaluronidase inhibition, collagen synthesis, or inflammation, as a result of their valuable pharmacological activities. Also presented results suggest that AS extracts could be used in the development of new cosmetics as a result of their effects, especially on collagen synthesis, cell proliferation, and hyaluronidase inhibition. This is the first report in the literature investigating various biological activities of AS extracts. However, further detailed in vivo studies are needed to reveal their effects on the biological system.
Funding
This work was supported by the Department of Scientific Research Projects, Istanbul University-Cerrahpasa, Turkey [Grant No: 22886].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Comparative studies on phenolic composition, antioxidant, wound healing and cytotoxic activities of selected Achillea L. species growing in Turkey. Molecules. 2015;20:17976-18000.
- [CrossRef] [Google Scholar]
- Phenolic content, antioxidant potential, and antimicrobial activities of fruit and vegetable by-product extracts. Int. J. Food Prop.. 2013;16(5):1092-1104.
- [CrossRef] [Google Scholar]
- Antioxidant and antimicrobial activities of different extracts of some medicinal herbs consumed as tea and spices in Turkey. J. Food. Biochem.. 2012;36:547-554.
- [CrossRef] [Google Scholar]
- A comparative study on the antioxidant activities and phenolic contents of different extracts of Achillea nobilis subsp. sipylea and Alcea apterocarpa (Fenzl) Boiss, endemıc plants in Turkey. Fresenius Environ. Bull.. 2017;26(2):1423-1430.
- [Google Scholar]
- In vitro evaluation of the therapeutic potential of Anatolian kermes oak (Quercus coccifera L.) as an alternative wound healing agent. Ind. Crops Prod.. 2019;137:24-32.
- [CrossRef] [Google Scholar]
- Evaluation of wound healing actions of Hoslundia opposita Vahl, Anthocleista nobilis G. Don and Balanites aegyptiaca L. J. Sci. Technol.. 2008;28:26-35.
- [CrossRef] [Google Scholar]
- Brown seaweed as a source of anti-hyaluronidase compounds. S. Afr. J. Bot.. 2021;139:470-477.
- [CrossRef] [Google Scholar]
- Interaction of mycobiont: Piriformospora indica with medicinal plants and plants of economic importance. Afr. J. Biotechnol.. 2010;9(54):9214-9226.
- [Google Scholar]
- In vitro anti-oxidant, cytotoxic and pro-apoptotic effects of Achillea teretifolia Willd extracts on human prostate cancer cell lines. Phcog. Mag.. 2015;11(44):308-315.
- [CrossRef] [Google Scholar]
- Metabolic profile of the bioactive compounds of Ormenis africana Jord. and Fourr. (Asteraceae) an endemic species from Tunisia. Int. J. Adv. Res.. 2003;1:124-131.
- [Google Scholar]
- Achillea millefolium L. sl–Is the anti-inflammatory activity mediated by protease inhibition? J. Ethnopharmacol.. 2007;113(2):312-317.
- [CrossRef] [Google Scholar]
- Bor, E., Koca Caliskan, U., Anlas, C., Durbilmez, G. D., Bakirel, T., Ozdemir, N., 2022. Synthesis of Persea americana extract based hybrid nanoflowers as a new strategy to enhance hyaluronidase and gelatinase inhibitory activity and the evaluation of their toxicity potential. Inorg. Nano-Met. Chem. https://doi.org/10.1080/24701556.2022.2072342.
- Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol.. 2008;585:325-337.
- [CrossRef] [Google Scholar]
- The free radical theory of aging and antioxidant supplements: a systematic review. Evid. -Based Complement. Altern. Med.. 2012;17(3):218-220.
- [CrossRef] [Google Scholar]
- Antioxidant and antimicrobial activity of the essential oils and methanol extracts of Achillea millefolium susp. millefolium Afan. (Asteraceae) J. Ethnopharmacol.. 2003;87(2–3):215-220.
- [Google Scholar]
- Determination of phenolic acids and flavonoids in Taraxacum formosanum Kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci.. 2012;13:260-285.
- [Google Scholar]
- Phytochemical analysis, antioxidant, and wound healing activity of Pluchea indica L. (Less) branch extract nanoparticles. Molecules. 2022;27:635.
- [CrossRef] [Google Scholar]
- CLSI, 2006. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Sixteenth informational supplement. CLSI document M100-S16 [ISBN 1-56238-588-7]; Clinical and Laboratory Standards Institute: Wayne, PA, USA.
- The water fraction of Calendula officinalis hydroethanol extract stimulates in vitro and in vivo proliferation of dermal fibroblasts in wound healing. Phytother. Res.. 2016;30:1696-1707.
- [CrossRef] [Google Scholar]
- Antioxidant activities of extracts from Barkleyanthus salicifolius (Asteraceae) and Penstemon gentianoides (Scrophulariaceae) J. Agric. Food Chem.. 2005;53(15):5889-5895.
- [CrossRef] [Google Scholar]
- Achillea asiatica extract and its active compounds induce cutaneous wound healing. J. Ethnopharmacol.. 2017;206:306-314.
- [CrossRef] [Google Scholar]
- Screening the in vitro antioxidant, antimicrobial, anticholinesterase, antidiabetic activities of endemic Achillea cucullata (Asteraceae) ethanol extracts. S. Afr. J. Bot.. 2019;120:141-145.
- [CrossRef] [Google Scholar]
- Antibacterial activity of East African medicinal plants. J. Ethnopharmacol.. 1998;60:79-84.
- [CrossRef] [Google Scholar]
- Luteolin as a modulator of skin aging and inflammation. BioFactors. 2021;47:170-180.
- [CrossRef] [Google Scholar]
- Total phenolic content and antioxidant activity of three Iranian endemic Achillea species. Ind. Crops Prod.. 2013;50:154-158.
- [CrossRef] [Google Scholar]
- In vitro evaluation of Achillea Millefolium on the production and stimulation of human skin fibroblast cells (HFS-PI-16) Med. Arh.. 2015;69(4):212-217.
- [CrossRef] [Google Scholar]
- The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci.. 2007;80:1921-1943.
- [CrossRef] [Google Scholar]
- Hyaluronidase inhibitors: a biological and therapeutic perspective. Curr. Med. Chem.. 2009;16(18):2261-2288.
- [CrossRef] [Google Scholar]
- Study of the topical anti-inflammatory activity of Achillea ageratum on chronic and acute inflammation models. Z. Naturforsch. C. 1999;54(11):937-941.
- [CrossRef] [Google Scholar]
- Isorhamnetin: a review of pharmacological effects. Biomed. Pharmacother.. 2020;128
- [CrossRef] [Google Scholar]
- The role of hyaluronic acid and versican in the skin extracellular matrix. Biomecánica. 2019;27:35-49.
- [CrossRef] [Google Scholar]
- Free radical production and quenching in honeys with wound healing potential. J. Antimicrob. Chemother.. 2006;58(4):773-777.
- [CrossRef] [Google Scholar]
- Multiple bioactivities of Manihot esculenta leaves: UV filter, anti oxidation, anti-melanogenesis, collagen synthesis enhancement, and anti-adipogenesis. Molecules. 2022;27:1556.
- [CrossRef] [Google Scholar]
- A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sci.. 2005;77(22):2760-2769.
- [CrossRef] [Google Scholar]
- Evaluation of wound healing properties of Arrabidaea chica verlot extract. J. Ethnopharmacol.. 2008;118(3):361-366.
- [CrossRef] [Google Scholar]
- Influence of extraction solvent on the phenolic profile and bioactivity of two Achillea species. Molecules. 2021;26:1601.
- [CrossRef] [Google Scholar]
- Evaluation of antimicrobial properties of Achillea L. flower head extracts. Pharm. Biol.. 2009;47(1):86-91.
- [CrossRef] [Google Scholar]
- Karamenderes, C., Apaydın, S., 2003. Antispasmodic effect of Achillea nobilis L. subsp. sipylea (O. Schwarz) Bässler on the rat isolated duodenum. J. Ethnopharmacol. 84 (2-3), 175-179. https://doi.org/10.1016/s0378-8741(02)00296-9.
- The role of the mucoid polysaccharide (hyaluronic acid) in the virulence of group A hemolytic streptococci. J. Exp. Med.. 1944;79(3):319-330.
- [Google Scholar]
- Herbal medicine: current trends and future prospects. In: Khan M.S.A., Ahmad I., Chattopadhyay D., eds. New Look to Phytomedicine: Advancements in Herbal Products as Novel Drug Leads. Academic Press; 2019. p. :3-13.
- [Google Scholar]
- Identification and in vitro biological activities of flavonols in garlic leaf and shoot: inhibition of soybean lipoxygenase and hyaluronidase activities and scavenging of free radicals. J. Sci. Food Agric.. 2005;85:633-640.
- [CrossRef] [Google Scholar]
- Inhibitory effects of herbal medicines on hyaluronidase activity. Korean J. Pharmacogn.. 1995;26(3):265-272.
- [Google Scholar]
- Novel zein-based multilayer wound dressing membranes with controlled release of gentamicin. J. Biomed. Mater. Res. Part B. 2018;107B:2057-2070.
- [CrossRef] [Google Scholar]
- The protective effects of Achillea L. species native in Turkey against H2O2-induced oxidative damage in human erythrocytes and leucocytes. J. Ethnopharmacol.. 2005;102(2):221-227.
- [CrossRef] [Google Scholar]
- Evaluation of anti-inflammatory and antinociceptive activity of five Anatolian Achillea species. Turkish J. Pharm. Sci.. 2007;4(2):89-99.
- [Google Scholar]
- Evaluation of the wound healing potential of Achillea biebersteinii Afan. (Asteraceae) by in vivo excision and incision models. Evid.- Based Complement. Alternat. 2011:474026.
- [CrossRef] [Google Scholar]
- Antioxidant and antimelanogenic activities of compounds isolated from the aerial parts of Achillea alpina L. Chem. Biodivers.. 2019;16(7):e1900033.
- [CrossRef] [Google Scholar]
- In silico evaluation of antimicrobial, antihyaluronidase and bioavailability parameters of rosmarinic acid in Perilla frutescens leaf extracts. SN Appl. Sci.. 2020;2:1547.
- [CrossRef] [Google Scholar]
- Tyrosinase, elastase, hyaluronidase, inhibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind. Crops Prod.. 2019;111:597-605.
- [CrossRef] [Google Scholar]
- Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci.. 2011;76(6):398-403.
- [CrossRef] [Google Scholar]
- Protective effects of radish (Raphanus sativus L.) leaves extract against hydrogen peroxide-induced oxidative damage in human fetal lung fibroblast (MRC-5) cells. Biomed. Pharmacother.. 2018;103:406-414.
- [CrossRef] [Google Scholar]
- In vitro inhibition of hyaluronidase by sodium copper chlorophyllin complex and chlorophyllin analogs. Clin. Cosmet. Investig. Dermatol.. 2015;8:443-448.
- [CrossRef] [Google Scholar]
- Phytochemical and safety evaluations of volatile terpenoids from Zingiber cassumunar Roxb. on mature carp peripheral blood mononuclear cells and embryonic zebrafish. Molecules. 2020;25:613.
- [CrossRef] [Google Scholar]
- Anti-inflammatory benefits of pentacyclic triterpenes. In: Watson R.R., Preedy V.R., eds. Bioactive Food As Interventions For Arthritis And Related Inflammatory DiseAses. Academic Press; 2013. p. :413-430.
- [Google Scholar]
- Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res.. 2001;15:127-130.
- [CrossRef] [Google Scholar]
- Structure–antioxidant activity relationships of flavonoids isolated from different plant species. Food Chem.. 2005;9:349-355.
- [CrossRef] [Google Scholar]
- Muhammad, A.A., Pauzi, N.A.S., Arulselvan, P., Abas, F., Fakurazi, S., 2013. In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam. Biomed Res. Int. http://dx.doi.org/10.1155/2013/974580.
- Comparison of the free radical scavenging activity of six Iranian Achillea species. Pharm. Biol.. 2006;44(3):208-212.
- [CrossRef] [Google Scholar]
- Antioxidant activity of Quercetin: a mechanistic review. Turkish J. Agric. - Food Sci. Technol.. 2016;4(12):1134-1138.
- [Google Scholar]
- Essential oil composition and in vitro biological activity of Achillea millefolium L. extracts. J. Pre-Clin. Clin. Res.. 2008;2(1):49-58.
- [Google Scholar]
- Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules. 2009;14(7):2466-2490.
- [CrossRef] [Google Scholar]
- Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chem.. 2019;289:16-25.
- [CrossRef] [Google Scholar]
- Collagen: a review on its sources and potential cosmetic applications. J. Cosmet. Dermatol.. 2018;17:20-26.
- [CrossRef] [Google Scholar]
- Secondary metabolites of Achillea sintenisii HUB. MOR. FABAD J. Pharm. Sci.. 2017;42:191-197.
- [Google Scholar]
- Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J. Ethnopharmacol.. 2001;75:95-115.
- [CrossRef] [Google Scholar]
- Antioxidant activity in the four species of cyanobacteria isolated from a sulfur spring in the western ghats of Karnataka. Int. J. Pharm. Bio. Sci.. 2013;4(1):275-285.
- [Google Scholar]
- Antimicrobial activity of essential oil and methanol extracts of Achillea sintenisii Hub. Mor. (Asteraceae) Phytother. Res.. 2003;17(9):1005-1010.
- [CrossRef] [Google Scholar]
- Effect of different flavonoids on collagen synthesis in human fibroblasts. Plant Foods Hum. Nutr.. 2006;61:29-34.
- [CrossRef] [Google Scholar]
- Strzępek-Gomółka, M., Gaweł-Bęben, K., Kukula-Koch, W., 2021. Achillea species as sources of active phytochemicals for dermatological and cosmetic applications. Oxid. Med. Cell. Longev. https://doi.org/10.1155/2021/6643827.
- Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167-2180.
- [CrossRef] [Google Scholar]
- Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem. Rev.. 2020;19:1199-1209.
- [CrossRef] [Google Scholar]
- Tan, W.S., Arulselvan, P., Ng, S.F., Taib, C.N.M., Sarian, M.N. Fakurazi, S., 2020. Healing effect of vicenin-2 (VCN-2) on human dermal fibroblast (HDF) and development VCN-2 hdrocolloid film based on alginate as potential wound dressing. Biomed Res. Int. https://doi.org/10.1155/2020/4730858.
- RP-HPLC/MS/MS Analysis of the phenolic compounds, antioxidant and antimicrobial activities of Salvia L. species. Antioxidants. 2016;5(4):38.
- [CrossRef] [Google Scholar]
- TUBİVES, 2018. http://194.27.225.161/yasin/tubives/index.php?sayfa=1&tax_id=4996 (accessed 02.05.2022).
- In vitro evaluation of antioxidant, anti-inflammatory, antimicrobial and wound healing potential of Thymus sipyleus Boiss. Subsp. Rosulans (Borbas) Jalas. Molecules. 2019;24:3353.
- [CrossRef] [Google Scholar]
- Antioxidant, cytotoxic and DNA protective properties of Achillea eriophora DC. and Achillea biebersteinii Afan. extracts: a comparative study. Avicenna J. Phytomed.. 2016;7(2):157-168.
- [Google Scholar]
- Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim. Pol.. 2011;58(2):203-209.
- [Google Scholar]
- WHO, 2005. National policy on traditional medicine and regulation of herbal medicines: report of a WHO global survey. World Health Organization, Geneva. https://apps.who.int/iris/bitstream/handle/10665/43229/9241593237.pdf?sequence=1&isAllowed=y (accessed 12.05.2022).
- Yang, D., Wang, T., Long, M., Li, P., 2020. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxid. Med. Cell. Longev. https://doi.org/10.1155/2020/8825387.
- Radical scavenging activity of the essential oil of silver fir (Abies alba) J. Clin. Biochem. Nutr.. 2009;44(3):253-259.
- [CrossRef] [Google Scholar]
- Antioxidant, anti-inflammatory and cytotoxic activities of polyphenols extracted from Chroogomphus rutilus. Chem. Biodivers.. 2020;17(1)
- [CrossRef] [Google Scholar]







