Translate this page into:
In-vitro cytotoxicity of synthesized phthalide-fused indoles and indolines against HL-60 and HepG2 cells
⁎Corresponding author. aishah80@ukm.edu.my (Siti Aishah Hasbullah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Phthalide derivatives bearing indole or indoline moieties were successfully synthesized via eco-friendly method and were evaluated for their antiproliferative activity on HL-60 and HepG2 cell lines in vitro. At a final concentration of 100 μM, most of the compounds showed moderate potency on both the cell lines tested. Compound 3b bearing 5-chloro substituted indoline had the best potency against HL-60 and HepG2 cell lines with IC50 values of 45.4 and 57.7 μM, respectively. It was also found that replacement of a conjugated indoline to indole moiety gave better antiproliferative activity on HL-60 cells by almost two-fold. Morphological observation demonstrated numerous fragmented nuclei which are indicative of apoptosis. Molecular docking studies predicted non-covalent interactions and H-bonding of selected compounds with the P2 binding hot spot of the anti-apoptotic protein, Bcl-2, formed by Asp108, Phe109, Met112, Leu134, Arg143, Ala146 and Val153. Overall, our work highlights the potential of synthesized phthalide-fused indoles or indolines as antitumor agents.
Keywords
Phthalide
Indole
Indoline
Antiproliferative activity
Molecular docking
1 Introduction
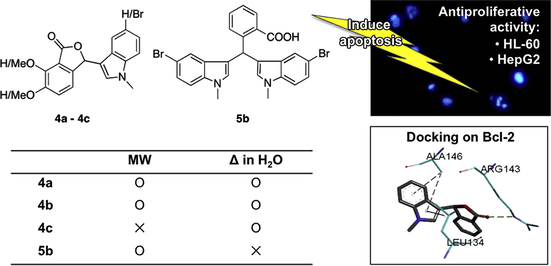
We have previously synthesized phthalide-fused indolines linked by an exocyclic double bond (Sheryn et al., 2018). In this study, the synthesized 3-substituted phthalides were linked to an indole at C3 position of the lactone ring and possess a similar framework as noscapine, a phthalideisoquinoline alkaloid, by replacing the isoquinoline unit with an indole moiety (Fig. 1). Noscapine is a prominent antitussive drug with remarkable cytotoxicity towards a variety of cancer cell types while having no significant toxicity to normal cells (Ke et al., 2000; Porcù et al., 2014; Rida et al., 2015). Indole containing products are found naturally through marine organisms, particularly from the marine sponges (Mollica et al., 2012). Compounds containing the indolic system have been reported to give high cytotoxicity and established promising antitumor properties against various human tumor cell lines (Haider et al., 2007; Ma et al., 2015; Sri Ramya et al., 2017). Similarly, natural occurring and synthetic bisindolylmethanes (BIMs), have recently attracted much attention for their anticancer potency (Chen et al., 2017; Hatti et al., 2015; Kamal et al., 2010).
The chemical structure of noscapine and its isoquinoline scaffold.
Microwave irradiation is becoming more prevalent in organic syntheses because of its advantages over the conventional method as well as environmental benefits. Microwave irradiation generally boosts chemical reactions by reducing reaction time and improved reaction yields (Penieres-Carrillo et al., 2017; Raunak et al., 2005; Solanki and Shekhawat, 2012; Srivastava et al., 2013). The conventional synthesis of 3-indolyl-substituted phthalide with the use of catalyst required a reaction time between 2 and 24 h, However, the reactions performed by microwave irradiation could be accelerated from 2 h to 5 min (Tang et al., 2012). In addition, a review displayed examples of specific reactions occurred by microwave irradiation which do not happen under conventional heating (Dallinger and Kappe, 2007).
In continuation of our interest in the syntheses of 3-substituted phthalide derivatives, herein we report synthesis utilizing eco-friendly method such as the use of microwave energy source for the activation of reactions, catalyst-free conditions and non-toxic solvent like water. All the derivatives were evaluated for their cytotoxic effect on human leukemia HL-60 and human hepatoma HepG2 cells.
2 Experimental
2.1 General methods
All chemicals and solvents were obtained from commercially available sources and used without further purification. All reactions were performed under conventional reflux and microwave irradiation methods. Microwave irradiation was performed using Monowave 50 (Anton Paar) employing sealed reaction vessels and an internal temperature probe and pressure sensor. MS spectra were obtained using the Waters UPLC-MS system (Aquity UPLC XevoQTof). 1H and 13C NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer at 400 MHz and 100 MHz, respectively using TMS as internal standard. IR spectra were recorded on a Perkin Elmer Spectrum 400 FT-IR/FT-FIR spectrometer equipped with a PIKE GladiATR. Column chromatography was performed on silica gel 60 (0.06–0.2 mm).
2.2 Cell culture
HepG2 cell line was kindly provided by the Division of Antioxidant Research (Life Science Research Center, Gifu University) and HL-60 cell line was obtained from DS Pharma Biomedical Co., Ltd. (Osaka, Japan). The HepG2 and HL-60 cell lines were cultured in Dulbecco’s modified eagle medium (DMEM) (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and RPMI 1640 media (Wako Pure Chemical Industries, Ltd., Osaka, Japan), respectively. The cultured media were supplemented with 10% heat inactivated fetal bovine serum (FBS), 1% antibiotics, penicillin-streptomycin (Gibco®, Life Technologies, Thermo Fisher Scientific Inc., MA, USA). Cells were maintained at 37 °C under a humidified atmosphere of 5% CO2.
2.3 In vitro CCK-8 assay
Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies Inc. (Kumamoto, Japan) and noscapine hydrochloride hydrate, purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) was used as the positive control. The in vitro cytotoxic activities of compounds were evaluated against two human cancer cell lines (HL-60 and HepG2). The cells (100 μL, 2 × 104 cells/mL) were seeded in 96-well plates. After 24 h incubation, test compounds dissolved in DMSO were added to the cell culture at a final DMSO concentration of less than 1%. After 48 h of subsequent incubation, CCK solution (10 μL) was added and the plates were further incubated for 2 h. Visible absorption was measured at 436 nm using a microplate reader (Emax precision microplate reader, Molecular Devices).
2.4 Morphological changes
For the morphological assessment of apoptosis, compound (100 μM) was added to cultured HL-60 cells (1 mL, 2 × 105 cells/mL) and incubated for 48 h. In addition, 10 μL of DMSO alone was added to another set of cells as the solvent control. The culture medium was stained with Hoechst 33342 solution (5 μL) and incubated for another 30 min. After centrifugation, the excess dye was removed and the collected cells were rinsed with PBS and then examined under a fluorescence microscope (Axiovert 40, Carl Zeiss).
2.5 Docking and modeling studies
The crystallographic coordinates of Bcl-2 co-crystallized with navitoclax (PDB ID: 4LVT (Souers et al., 2013)) was obtained from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (http://www.rcsb.org) and was used in performing the docking studies. The 3D structure of Bcl-2 was first cleaned and the inhibitor binding site was accounted for further analysis. All compounds were drawn using Chem3D software and their conformations were optimized using Gaussian09 software. The geometry optimization of compounds was carried out using DFT methods at the B3LYP/3-21G level. All docking simulations were performed using AutoDock version 4.2 for 100 iterations each compound. Grid maps were prepared with 50 × 62 × 52 points with a point spacing of 0.375 Å. The grid box was allocated at the center of the protein using x,y,z coordinates of 6.021, −3.611, −5.598, respectively. The typical parameters set by the AutoDock 4.2 package for Lamarckian genetic algorithm were population size of 150 individuals, maximum 2.5 × 106 of energy evaluations and maximum 27,000 of generations per iteration. The best ranking pose of compounds toward Bcl-2 was visualized with Discovery Studio version 4.5.
2.6 Synthesis of phthalide-fused indoles and indolines
The preparations of compounds 4a–c were preceded by microwave irradiation or reflux in water. The chemical structures of the synthesized compounds were confirmed by spectroscopic studies.
2.6.1 Conventional
A mixture of a phthalaldehydic acid derivative (1.1 mmol) and an indole derivative (1.0 mmol) was stirred in distilled water (5.0 mL) in reflux conditions for 24 h. The crude precipitate formed was filtered, washed with minimal amount of cold ethanol and acetone and finally dried in oven to give pure products (as monitored on TLC and spectral data) except for 4a, which formed red colored sticky product, was purified by column chromatography (EtOAc/petroleum ether 1:2).
2.6.2 Microwave irradiation
A mixture of a phthalaldehydic acid derivative (1.1 mmol) and an indole derivative (1.0 mmol) was mixed in EtOH (0.5 mL) in a reaction vessel. The mixture was then irradiated in Monowave 50 at 160 °C for 5 min and while stirring at 500 rpm. The reaction mixture was evaporated in vacuo and the product obtained was washed with a minimal amount of cold EtOH and purified by recrystallization to give pure products (as monitored on TLC and spectral data).
2.6.3 3-(1-Methylindol-3-yl)isobenzofuran-1(3H)-one (4a) (Noland and Johnson, 1960)
Compound 4a was synthesized from starting materials 2-carboxybenzaldehyde (1a) and 1-methylindole (2c). This product was purified by recrystallization from acetone-EtOH to give yellow solid; IR (ATR, υmax/cm−1): 1736 (C⚌O), 1553, 1475, 1464 (C⚌C), 1284 (C—N), 1065 (C—O), 921, 745; 1H NMR (400 MHz; CDCl3) δ (ppm): 3.77 (s, 3H, N-CH3), 6.79 (s, 1H, H-2), 7.05–7.09 (m, 2H, H-2′ and ArH-7′), 7.17 (dt, 1H, J = 8.0, 0.8 Hz, ArH-8′), 7.26 (ddd, 1H, J = 8.3, 7.1, 1.4 Hz, ArH-6′), 7.34 (dt, 1H, J = 8.0, 1.0 Hz, ArH-5′), 7.46 (dd, 1H, J = 7.4, 1.0 Hz, ArH-8), 7.62 (ddd, 1H, J = 7.6, 1.6, 0.8 Hz, ArH-6), 7.69 (td, 1H, J = 7.6, 1.2 Hz, ArH-7), 8.04 (dt, 1H, J = 7.6, 1.0 Hz, ArH-5); 13C NMR (101 MHz, CDCl3) δ (ppm): 33.0 (N-CH3), 77.7 (C-2), 109.6 (C-1′), 109.8 (Ar-CH), 119.1 (Ar-CH), 120.1 (Ar-CH), 122.5 (Ar-CH), 123.2 (Ar-CH), 125.6 (Ar-CH), 126.3 (Ar-C), 126.8 (Ar-C), 129.3 (C-2′), 129.3 (Ar-CH), 134.1 (Ar-CH), 137.5 (Ar-C), 149.3 (Ar-C), 170.7 (C⚌O); HRESITOFMS: m/z calcd for C17H13NO2Na [M+Na]+ 286.0844, found 286.0844.
2.6.4 3-(5-Bromo-1-methylindol-3-yl)isobenzofuran-1(3H)-one (4b)
Compound 4b was synthesized from starting materials 2-carboxybenzaldehyde (1a) and 5-bromo-1-methylindole (2d). This product was purified by recrystallization from acetone-EtOH gave colorless needles; IR (ATR, υmax/cm−1): 1750 (C⚌O), 1475, 1463 (C⚌C), 1293 (C—N), 1063 (C—O), 936, 783 (C-Br), 710; 1H NMR (400 MHz; DMSO‑d6) δ (ppm): 3.78 (3H, s, N-CH3), 7.02 (s, 1H, H-2), 7.12 (d, 1H, J = 1.6 Hz, ArH-5′), 7.30 (dd, 1H, J = 8.6, 1.8 Hz, ArH-7′), 7.47 (d, 1H, J = 8.8 Hz, ArH-8′), 7.50–7.53 (m, 2H, H-2′ and ArH-8), 7.70 (t, 1H, J = 7.4 Hz, ArH-6), 7.81 (td, 1H, J = 7.5, 0.9 Hz, ArH-7), 7.99 (d, 1H, J = 7.6 Hz, ArH-5); 13C NMR (101 MHz, DMSO‑d6) δ (ppm): 33.3 (N-CH3), 77.4 (C-2), 109.1 (C-1′), 112.8 (Ar-C), 113.1 (Ar-CH), 121.0 (Ar-CH), 123.9 (Ar-CH), 124.9 (Ar-CH), 125.5 (Ar-CH), 126.1 (Ar-C), 128.0 (Ar-CBr), 130.1 (Ar-CH), 132.2 (C-2′), 135.2 (Ar-CH), 136.3 (Ar-C), 149.6 (Ar-C), 170.4 (C⚌O); HRESITOFMS: m/z calcd for C17H12NO2NaBr [M+Na]+ 363.9949, found 363.9928.
2.6.5 6,7-Dimethoxy-3-(5-bromo-1-methylindol-3-yl)isobenzofuran-1(3H)-one (4c)
Compound 4c was synthesized from starting materials 6-formyl-2,3-dimethoxybenzoic acid (1b) and 5-bromo-1-methylindole (2d). White solid product was obtained; IR (ATR, υmax/cm−1): 1741 (C⚌O), 1500, 1475, 1425 (C⚌C), 1269 (C—N), 1046 (C—O), 939, 768 (C-Br), 706; 1H NMR (400 MHz; CDCl3) δ (ppm): 3.73 (s, 3H, N-CH3), 3.95 (s, 3H, O-CH3), 4.18 (s, 3H, O-CH3), 6.57 (s, 1H, H-2), 7.01 (s, 1H, H-2′), 7.03 (dd, 1H, J = 8.4, 0.8 Hz, ArH-8), 7.17 (d, 1H, J = 8.4 Hz, ArH-8′), 7.24 (d, 1H, J = 8.4 Hz, ArH-7), 7.31 (dd, 1H, J = 8.6, 1.8 Hz, ArH-7′), 7.39 (d, 1H, J = 1.2 Hz, ArH-5′); 13C NMR (101 MHz, CDCl3) δ (ppm): 33.2 (N-CH3), 56.9 (O-CH3), 62.6 (O-CH3), 75.7 (C-2), 110.0 (C-1′), 111.3 (Ar-CH), 113.5 (Ar-C), 117.6 (Ar-CH), 118.8 (Ar-C), 119.4 (Ar-CH), 121.7 (Ar-CH), 125.4 (Ar-CH), 128.0 (Ar-CBr), 130.0 (C-2′), 136.1 (Ar-C), 141.7 (Ar-C), 148.2 (Ar-COMe), 152.8 (Ar-COMe), 167.9 (C⚌O); HRESITOFMS: m/z calcd for C19H16NO4NaBr [M+Na]+ 424.0160, found 424.0151.
2.6.6 2-(Bis(5-bromo-1-methylindol-3-yl)methyl)benzoic acid (5b)
Compound 5b was synthesized from starting materials 2-carboxybenzaldehyde (1a) and 5-bromo-1-methylindole (2d). This product was purified by recrystallization from acetone-EtOAc to obtain off-white solid; IR (ATR, υmax/cm−1): 3061, 2935 (broad, O—H), 1676 (C⚌O), 1477 (C⚌C), 1265 (C—N), 1047 (C—O), 774 (C—Br), 722; 1H NMR (400 MHz; DMSO‑d6) δ (ppm): 3.69 (s, 6H, 2 × N-CH3), 6.81 (s, 2H, 2 × H-2′), 6.90 (s, 1H, H-7), 7.23 (dd, 2H, J = 8.8, 2.0 Hz, 2 × ArH-7′), 7.29–7.33 (m, 2H, ArH-3 and ArH-5), 7.37–7.45 (m, 5H, ArH-4, 2 × ArH-5′ and 2 × ArH-8′), 7.79 (dd, 1H, J = 7.8, 1.4 Hz, ArH-6); 13C NMR (101 MHz, DMSO‑d6) δ (ppm): 33.0 (2 × N-CH3), 34.1 (C-7), 111.7 (2 × Ar-C), 112.5 (2 × Ar-CH), 117.2 (2 × C-1′), 121.4 (2 × Ar-CH), 124.1 (2 × Ar-CH), 126.7 (Ar-CH), 129.0 (2 × C-2′), 129.8 (Ar-CH), 130.2 (Ar-CH), 130.3 (2 × Ar-CBr), 131.1 (Ar-C), 131.8 (Ar-CH), 136.1 (2 × Ar-C), 144.5 (Ar-C), 170.0 (C⚌O); HRESITOFMS: m/z calcd for C26H20N2O2NaBr2 [M+Na]+ 572.9789, found 572.9778.
3 Results and discussion
3.1 Reaction and mechanism
We have previously synthesized 3a–c by conventional method and microwave irradiation using a domestic microwave oven (Sheryn et al., 2018). Scheme 1 and Table 1 show the eco friendly synthesis of 3-substituted phthalide derivatives by microwave irradiation (160 °C, 5 min) or reflux in water (100–120 °C, 24 h). Compounds 4a–c were successfully synthesized using the conventional reflux method. The same reaction scheme was followed in an attempt to produce 4a–c using the Monowave 50 reactor. However, only 4a could be obtained in 54% yield. In a similar reaction, 5b was obtained as the main product in 28% reaction yield instead, with a trace of 4b (7% yield). 4b was produced with good yield (81%) when refluxed in water. Generally, 4a–c gave better yields by the conventional method, whereas microwave irradiation further activated the alkylation process to give bisindole 5b.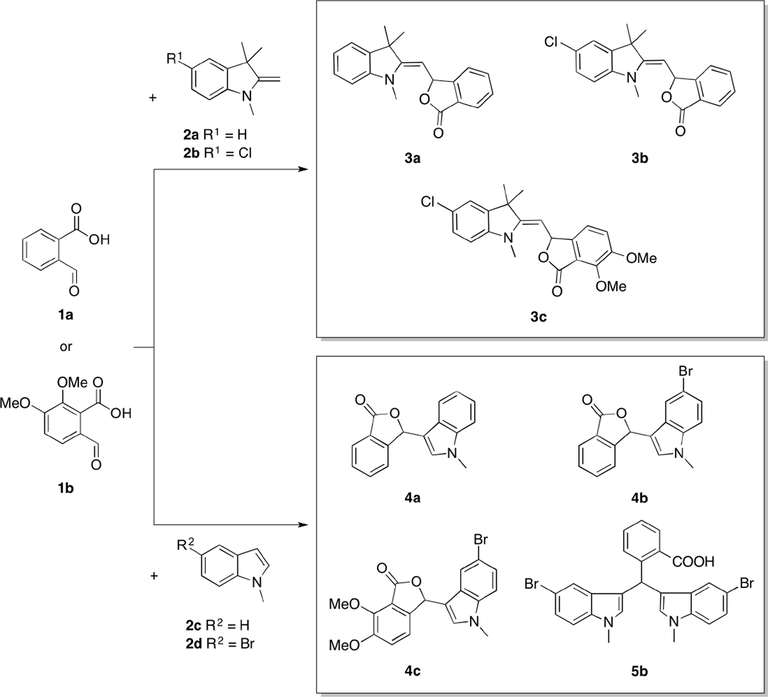
Synthesis of phthalide-fused indolines (3a–c), phthalide-fused indoles (4a–c) and a bisindole (5b) by microwave irradiation (160 °C, 5 min) or reflux in water (100–120 °C, 24 h).
Product
Microwave irradiation
Conventional Reflux
Time (min)
Yield (%)
Time (h)
Yield (%)
3aa
5
72
24
79
3ba
1
80
24
77
3ca
1
80
24
68
4a
5
54
24
38
4b
5
7
24
81
4c
5
NOb
24
36
5b
5
28
24
NOb
A plausible mechanism for the synthesis of phthalide-fused indole is proposed in Scheme 2. The reaction occurred via condensation of a Fischer base with aldehyde (Chunaev et al., 1982). The first step is initiated via nucleophilic attack of the Fischer base on the aldehyde group of a 2-formylbenzoic acid derivative to form an iminium ion intermediate. Next, a carbinol intermediate is formed by intramolecular proton transfer, followed by the release of the lone pair on amine nitrogen. Subsequent cyclization by condensation reaction gave a five-membered ring lactone. Another unit of indole could be fused when the furanone ring was fragmented upon protonation catalyzed by 2-formylbenzoic acid (Chen et al., 2017; Lin and Sun, 2008).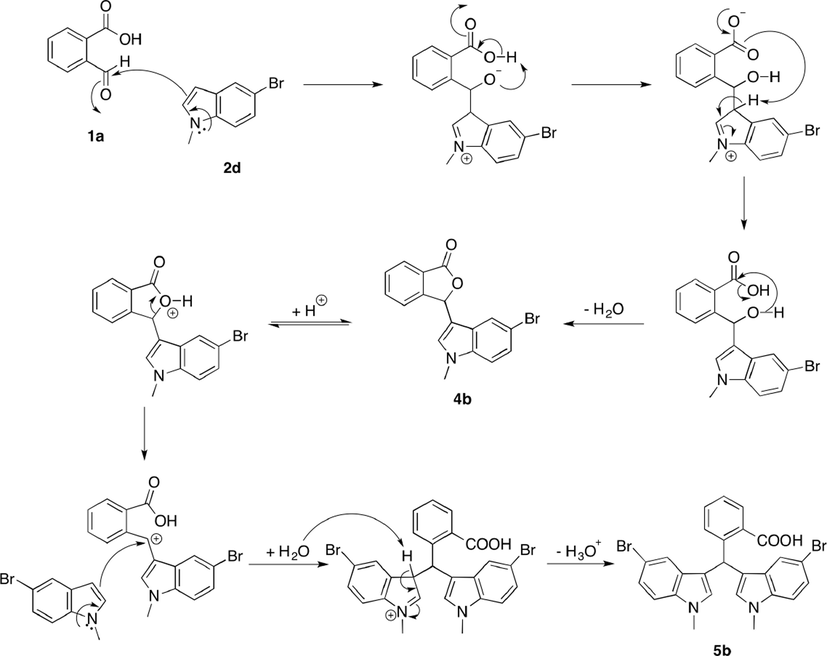
Plausible mechanism for the synthesis of 4b and 5b.
3.2 Spectroscopic studies
Spectral data (FT-IR, NMR and MS) are analyzed to elucidate the structure of synthesized compounds (Supplementary Material Figs. S1–S24). The FT-IR spectra of 4a–c exhibited a strong band at 1736–1750 cm−1, signifying stretching frequencies of the phthalide carbonyl esters. Whereas the FT-IR spectrum of 5b showed a strong band at 1676 cm−1 which is a typical stretching for carbonyl of benzoic acid and was observed together with a broad OH band. The C—O—C stretching frequencies were observed at 1046–1065 cm−1, indicating the formation of lactone ring (Mahmoodi and Salehpour, 2003).
Generally, the 1H NMR spectra of 4a–c showed the presence of methylamine protons (N-CH3) at 3.73–3.78 ppm, a chiral proton (CH-O) at 6.57–7.02 ppm, an olefinic proton of the enamine (C⚌CH-N) detected as a singlet at 7.01–7.50 ppm and aromatic protons resonated between 7.30 and 8.04 ppm. Two new singlets were detected in the spectrum of 4c at 3.95 and 4.18 ppm, with an integral of 3 protons each belong to the methoxy substituents. The positions of aromatic protons on both the phthalide and indole moieties were determined through COSY analysis. The aromatic protons on phthalide moiety (7.46–8.04 ppm) were found to be slightly more deshielded as compared to aromatic protons on the indolic system (7.05–7.47 ppm) due to electron withdrawing effect caused by the carbonyl group. These values are similar with the range of the previously reported individual entities (Chitra et al., 2017; Zhang et al., 2010).
The 13C NMR spectra showed methylamine carbon (N—CH3) at 33.0–33.3 ppm, chiral carbon (CH—O or C-2) at 75.7–77.7 ppm, olefinic carbons overlapped with aromatic carbons between 109.1 and 152.8 ppm and a most downfield quaternary peak of the carbonyl carbon at 167.9–170.7 ppm. Two new methoxy carbon (O—CH3) peaks were observed for 4c at 56.9 ppm and 62.6 ppm. By comparing the spectra of 4b and 4c, presence of the dimethoxy substituents in 4c exhibited a slight upfield shifts on the chiral carbon (C-2) and carbonyl carbon (C⚌O) by 1.7 ppm and 2.5 ppm, respectively. Analysis of 1D and 2D NMR data led us to the assignment of aromatic carbons of compounds 4a–c. Comparison of the 13C NMR spectra indicated chemical shift differences to the substituted patterns on aromatic rings (Fig. 2). By comparing the spectra of 4a and 4b, small downfield and upfield shifts were observed for ortho carbons (C-5′ and C-7′) and carbons on meta position (C-4′ and C-8′), respectively, due to the electron withdrawing effect of bromine atom. In addition to that, C-6′ was shifted to downfield by 5.5 ppm when bromine was directly attached to it. On the other hand, the aromatic carbons (C-3, C-4, C-7 and C-8) on the dimethoxy phthalide substituent (4c) showed upfield shifts due to the shielding effect of the electron donating methoxy groups, whereas C-5 and C-6 were shifted to downfield at 148.2 ppm and 152.8 ppm as an electronegative oxygen atom was attached to them.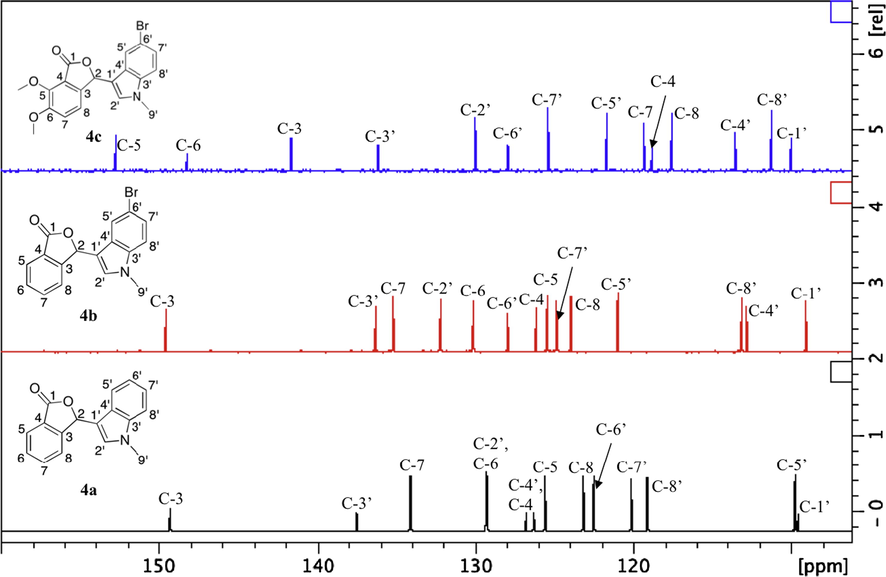
The 13C NMR spectra of 4a–c at aromatic region.
The 1H and 13C NMR spectra of 5b showed that the two indole molecules have chemically equivalent protons and carbons of the same multiplicity. A total of 17 carbon peaks was observed in 13C NMR of 5b, nine (9) of which are of higher intensity were assigned as carbon signals of the bisindole molecules.
Results for mass analysis were found to be in good agreement with the calculated values.
3.3 In vitro cytotoxicity
The antiproliferative activities of all the synthesized compounds were assessed against HL-60 and HepG2 cell lines using the CCK-8 assay (Kakumu et al., 2014). The cell viability results are summarized in Fig. 3 and Table 2, with noscapine hydrochloride (Nos) as the positive control. Dose-dependant cytotoxic effect was observed for all compounds on both cells except for 3a and 3c in HL-60 and HepG2 cells, respectively. At a final concentration of 100 μM, all compounds possessed cytotoxic potency and inhibited cell proliferation of the two cell lines. Moreover, phthalide bearing 5-chloro substituted indoline, 3b, was the most potent compound at 100 μM with cell viability of 11.7 and 24.0% for HL-60 and HepG2 cells, respectively. However, compound 3c was inactive and failed to inhibit the proliferation of HepG2 cells even at 100 μM.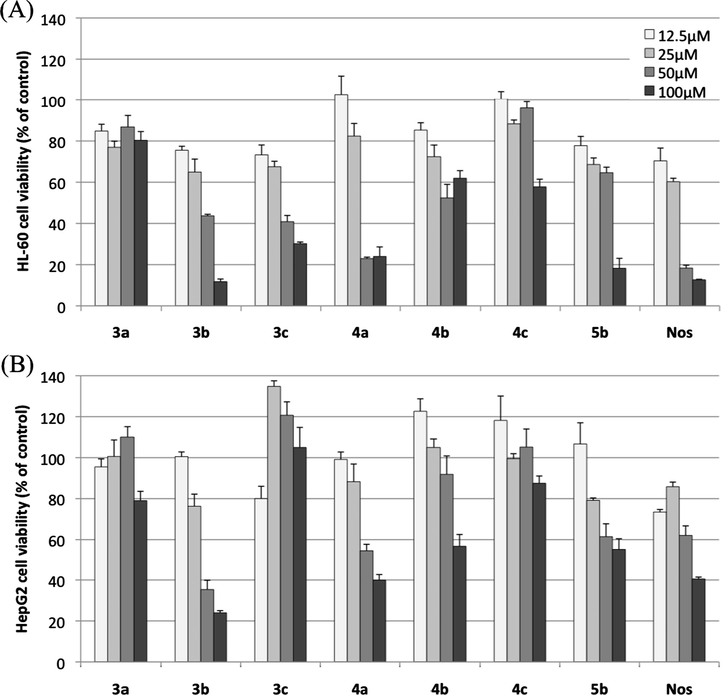
Antiproliferative effects of phthalide-fused indoles and indolines on (A) HL-60 cells and (B) HepG2 cells (means ± SEMs, n = 3). Results are presented as percent viability of treated cells compared to that of untreated control.
Compound
HL-60
HepG2
Cell viability (% of control)
IC50 (μM)
Cell viability (% of control)
IC50 (μM)
3a
80.3 ± 4.3
>100
79.0 ± 4.5
>100
3b
11.7 ± 1.3
45.4
24.0 ± 1.1
57.7
3c
30.2 ± 0.8
52.8
105.0 ± 9.8
>100
4a
24.0 ± 4.6
55.9
39.9 ± 2.7
76.9
4b
61.9 ± 3.7
>100
56.7 ± 5.8
>100
4c
57.7 ± 3.7
>100
87.5 ± 3.6
>100
5b
18.2 ± 4.8
57.8
54.9 ± 5.5
97.0
Noscapine
12.6 ± 0.3
32.5
40.5 ± 0.9
80.6
The drug concentration (μM) giving a 50% reduction in cellular viability (IC50) was calculated for each compound. The non-substituted indoline, 3a did not show inhibition more than 50% compared to 3b. This signifies the importance of halide substitution for inhibition activity to take place and may be associated with the hydrophobic effect of the Cl group, which improved cellular uptake of compound. Results for compounds 3a and 4a show that the replacement of conjugated indoline to indole moiety resulted in better activity on the two cell lines tested. On the other hand, the non-substituted indole on compound 4a possessed greater activity than those with 5-bromo substituents (4b–c). Overall, the dimethoxy substitution on the phthalide moiety did not show any significant effect on the antiproliferative activity. Bisindole 5b showed better cytotoxicity against HL-60 than its monomeric counterpart, 4b. This finding was supported by a literature which revealed that bis- and tris-indole substituted compounds displayed stronger cytotoxicity than mono-indole substituted compounds (Pingaew et al., 2013).
Nuclear morphology studies were carried out to validate apoptosis induction of three selected compounds with the best cytotoxic activity. The microscopic images of HL-60 cells stained with Hoechst 33342 are shown in Fig. 4. The compounds 3b, 4a or 5b-treated cancer cells (B, C, and D) showed different cell shapes from the untreated control group (A). The observed alterations caused by chromatin condensation, membrane blebbing and nuclear fragmentation are morphological features typical of apoptosis (Rello et al., 2005). These results suggested that the synthesized compounds were able to induce apoptosis in HL-60 cells.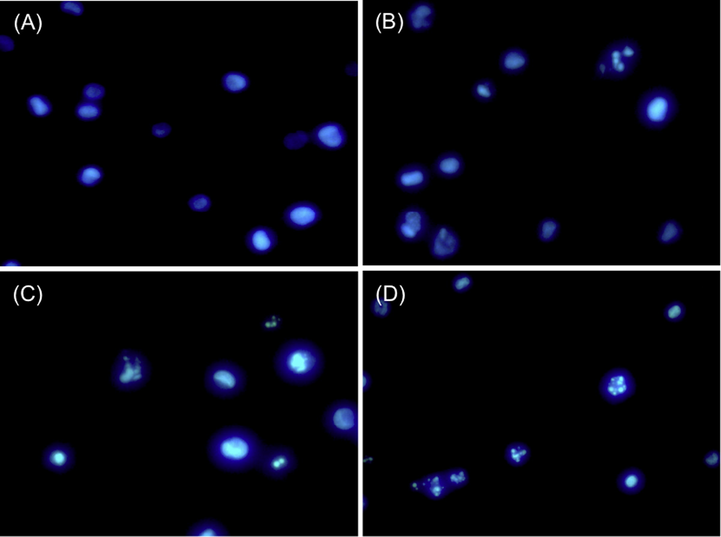
Morphological changes of HL-60 cells induced by synthesized compounds at a final concentration of 100 μM for 48 h. The cells were stained in Hoechst 33342 and viewed under a fluorescence microscope. (A) Controlled HL-60 cells (DMSO alone); (B) 3b in HL-60; (C) 4a in HL-60; (D) 5b in HL-60.
3.4 Docking and modeling studies
Many human tumors are formed due to an overexpression of the Bcl-2 family of anti-apoptotic proteins, which also promotes the resistance of tumor cells to conventional chemotherapeutic drugs (Ashkenazi et al., 2017; Chaabane et al., 2013). The molecular docking simulation methods were carried out according to a previous report to explore their possible binding modes and interactions with this receptor and their potential in suppressing the anti-apoptotic proteins, which inhibit apoptosis in cancer cells (Gyebi et al., 2017). The top three synthesized compounds that showed the best potency were docked into the navitoclax (an inhibitor) binding hot spots of Bcl-2. The co-crystallized ligand navitoclax was re-docked as validation of the docking protocol and yielded RMSD of 1.126 Å (Supplementary Material Fig. S25). All the docked compounds showed good fitting with the receptor and achieved best configuration with binding free energies (ΔG) of −16.61, −16.68, −16.36 and −15.10 kcal/mol for compounds 3b, 4a, 5b and noscapine, respectively. Compound 4a gave the best docking score although 3b and noscapine was the most potent drugs, experimentally.
Generally, the docked compounds were incorporated at the P2 hot spot of the protein formed by Asp108, Phe109, Met112, Leu134, Arg143, Ala146 and Val153 (Fig. 5). Among these amino acid residues, Ala146 and Leu134 formed π-alkyl interaction with compounds 3b and 4a via the aromatic indole scaffold (Supplementary Material Fig. S26). On the contrary, the bisindole moieties on compound 5b formed π-anion interactions with Asp108. A conventional H-bond was observed between Arg143 and carbonyl (C⚌O) on the phthalide scaffold of 4a. Both the indole and phthalide scaffolds could attribute to the binding affinity of Bcl-2, hence, blocking the signal pathway of Bcl-2 for triggering apoptosis in tumor cells.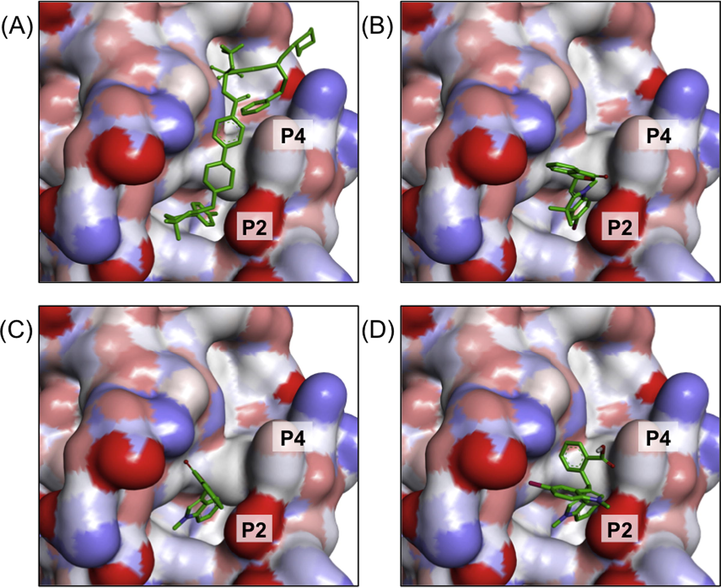
3D molecular surface map of Bcl-2 showing the docked poses of (A) navitoclax, (B) 3b, (C) 4a and (D) 5b into P2 and P4 binding hot spots.
4 Conclusions
The 3-substituted phthalide derivatives were successfully synthesized via the eco-friendly methods using the microwave irradiation or by reflux with water as a solvent. The synthesized phthalide-fused indoles and indolines have moderate cytotoxicity against the tested HL-60 and HepG2 cell lines. Further alteration of the structure or incorporation of varying substituents could improve the antitumor potential of these compounds and aid in the discovery of new cytotoxic agents.
Acknowledgment
This work was supported by Universiti Kebangsaan Malaysia (DIP-2015-015 and FRGS/1/2018/STG01/UKM/02/14) and Japan Student Services Organization Scholarship (JASSO). We appreciate great supports from Prof. Kaori Tanaka (Division of Anaerobe Research, Life Science Research Center, Gifu University) on the bioassays work. We are also grateful to the members of the Koketsu laboratory for their endless assistance.
References
- From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discovery. 2017;16:273.
- [CrossRef] [Google Scholar]
- Autophagy, apoptosis, mitoptosis and necrosis: interdependence between those pathways and effects on cancer. Archivum Immunologiae et Therapiae Experimentalis.. 2013;61:43-58.
- [CrossRef] [Google Scholar]
- Synthesis of biologically active bis(indolyl)methane derivatives by bisindole alkylation of tetrahydroisoquinolines with visible-light induced ring-opening fragmentation. Asian J. Org. Chem.. 2017;6:426-431.
- [CrossRef] [Google Scholar]
- Indole-3-acetic acid/diol based pH-sensitive biological macromolecule for antibacterial, antifungal and antioxidant applications. Int. J. Biol. Macromol.. 2017;95:363-375.
- [CrossRef] [Google Scholar]
- Reaction of the Fischer base with 8-hydroxy-1-naphthaldehydes. Investigation of the reaction products by 13C NMR spectroscopy. Chem. Heterocycl. Compd.. 1982;18:1164-1169. %@ 0009-3122
- [CrossRef] [Google Scholar]
- Microwave-assisted synthesis in water as solvent. Chem. Rev.. 2007;107:2563-2591.
- [CrossRef] [Google Scholar]
- Iloneoside: a cytotoxic ditigloylated pregnane glycoside from the leaves of Gongronema latifolium Benth. Nat. Prod. Res.. 2017;1–5
- [CrossRef] [Google Scholar]
- Synthesis and in-vitro antitumor activity of 1-[3-(indol-1-yl) prop-1-yn-1-yl] phthalazines and related compounds. Molecules. 2007;12:1900-1909.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of 1, 3, 4-oxadiazole-linked bisindole derivatives as anticancer agents. Monatshefte für Chemie-Chemical Monthly.. 2015;146:1699-1705.
- [CrossRef] [Google Scholar]
- Phytochemical analysis and antileukemic activity of polyphenolic constituents of Toona sinensis. Bioorg. Med. Chem. Lett.. 2014;24:4286-4290.
- [CrossRef] [Google Scholar]
- Synthesis of 3,3-diindolyl oxyindoles efficiently catalysed by FeCl3 and their in vitro evaluation for anticancer activity. Bioorg. Med. Chem. Lett.. 2010;20:5229-5231.
- [CrossRef] [Google Scholar]
- Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses. Cancer Immunol. Immunother.. 2000;49:217-225.
- [CrossRef] [Google Scholar]
- Highly efficient synthesis of 3-indolyl-substituted phthalides via Friedel-Crafts reactions in water. Tetrahedron Lett.. 2008;49:5343-5346.
- [CrossRef] [Google Scholar]
- Design, synthesis, biological evaluation and preliminary mechanism study of novel benzothiazole derivatives bearing indole-based moiety as potent antitumor agents. Eur. J. Med. Chem.. 2015;96:173-186.
- [CrossRef] [Google Scholar]
- Direct synthesis of γ-substituted phthalides via ortho-aryl benzoic acid mediated benzyl radical cyclization. J. Heterocycl. Chem.. 2003;40:875-878. https://doi.org/doi:10.1002/jhet.5570400520
- [Google Scholar]
- Synthesis and bioactivity of secondary metabolites from marine sponges containing dibrominated indolic systems. Molecules. 2012;17:6083.
- [CrossRef] [Google Scholar]
- 3-(Indolyl)-Phthalides and (2-Carboxybenzyl)-Indoles. J. Am. Chem. Soc.. 1960;82:5143-5147.
- [CrossRef] [Google Scholar]
- Synthesis of novel benzimidazole-diindolylmethane hybrid compounds within the green chemistry context. Organic Chem.. 2017;210–221
- [CrossRef] [Google Scholar]
- Synthesis and structure–activity relationship of mono-indole-, bis-indole-, and tris-indole-based sulfonamides as potential anticancer agents. Mol. Diversity. 2013;17:595-604.
- [CrossRef] [Google Scholar]
- Novel 9′-substituted-noscapines: synthesis with Suzuki cross-coupling, structure elucidation and biological evaluation. Eur. J. Med. Chem.. 2014;84:476-490.
- [CrossRef] [Google Scholar]
- Microwave mediated synthesis of spiro-(indoline-isoxazolidines): mechanistic study and biological activity evaluation. Tetrahedron. 2005;61:5687-5697.
- [CrossRef] [Google Scholar]
- Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments. Apoptosis. 2005;10:201-208.
- [CrossRef] [Google Scholar]
- The noscapine chronicle: a pharmaco-historic biography of the opiate alkaloid family and its clinical applications. Med. Res. Rev.. 2015;35:1072-1096.
- [CrossRef] [Google Scholar]
- Solvent-free microwave accelerated synthesis and structural characterization of phthalide-fused indolines. Heterocycles. 2018;96:839-849.
- [CrossRef] [Google Scholar]
- Eco-friendly synthesis and potent antifungal activity of 2-substituted coumaran-3-ones. Nus. Biosci.. 2012;4:101-104.
- [Google Scholar]
- ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med.. 2013;19:202.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of curcumin inspired indole analogues as tubulin polymerization inhibitors. Eur. J. Med. Chem.. 2017;127:100-114.
- [CrossRef] [Google Scholar]
- Microwave assisted synthesis and photochemical reactions of 3-(amine dithiocarbamyl) phthalides. Der Pharma Chemica.. 2013;5:137-144.
- [Google Scholar]
- TsOH· H2O-catalyzed friedel-crafts of indoles of 3-hydroxyisobenzofuran-1 (3H)-one with indoles: highly synthesis of 3-indolyl-substituted phthalides. Modern Res. Catal.. 2012;1:11.
- [CrossRef] [Google Scholar]
- Synthesis of chiral 3-substituted phthalides by a sequential organocatalytic enantioselective Aldol-Lactonization reaction. Three-step synthesis of (S)-(−)-3-butylphthalide. J. Organic Chem.. 2010;75:368-374.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.02.002.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







