Translate this page into:
In vitro wound healing evaluation, antioxidant and chemical profiling of Baeckea frutescens leaves ethanolic extract
⁎Corresponding author at: Integrative Pharmacogenomics Institute (iPROMISE), Universiti Teknologi MARA Selangor, Puncak Alam Campus, 42300 Bandar Puncak Alam, Selangor, Malaysia. hasseri2945@uitm.edu.my (Hasseri Halim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Occurrence of the wound or chronic wound, which results to disability, amputation and diminish quality of life, are leading to increased healthcare expenditure around the globe. Despite effective conventional wound healing medicines, the exploration of alternative medicines are continuing process as researchers seek for the approach to reduce the cost of wound healing management. In present study, wound healing properties of ethanolic extract of Baeckea frutescens leaves were determined by evaluating their cytotoxicity, proliferation and migration rate on two types of cells, keratinocytes (HaCaT) and fibroblasts (BJ). Furthermore, the antioxidant properties of this plant were determined by DPPH scavenging, ferric reducing antioxidant power (FRAP) and total phenolics content (TPC) assays. The phytochemistry of the extract was evaluated by phytochemicals screening and liquid chromatography mass spectrometry (LCMS) analysis. Results of this study indicated Baeckea frutescens extract increased the rate of proliferation and migration on both HaCat and BJ cells within their nontoxic doses. The extract also possessed a very good antioxidant property as demonstrated with high DPPH radical scavenging, FRAP and TPC values, comparable to that of Green tea extract, a widely known antioxidant. The phytochemistry analyses and LCMS exhibited the presence saponins, flavonoids, tannins and steroids in Baeckea frutescens extract possibly responsible to their antioxidant and wound healing properties.
Keywords
Baeckea frutescens
Antioxidant
Proliferation
Migration
Wound healing
Phytochemistry
1 Introduction
Skin is the largest and important organ that serves as a protective barrier against external stimuli and harmful agents such as microorganisms. A wound is a disruption to the skin either intentionally or unintentionally that changes the structural integrity of the skin. Wound healing is a complex biological and molecular process involving four overlapping phases; haemostasis, inflammation, proliferation and maturation phases. Haemostasis phase begins as the clotted blood, platelet and cross-linked fibrin formed a mesh to stop the bleeding. The temporary matrix is formed whereas cytokines and the growth factor are released to react and accelerate the healing process. The inflammatory phase takes place as the neutrophils and macrophages are recruited into the wounded site to clean the wounded area from debris and pathogen through phagocytosis and released of proinflammatory cytokines and growth factors (Shukla et al., 2019; Lucas et al., 2010; Koh & DiPietro, 2011). The proliferative stage is characterized by the restoration of damaged tissue by proliferation and migration of fibroblast, keratinocytes and endothelial cells. During this stage, abundant extracellular matrices are secreted, supporting cells migration that is essential for the repairing process, whereas new granulation tissue and blood vessel are formed to improve oxygenation at the wounded area. Collagen and fibronectin are further synthesized to complete the healing process (Landén et al., 2016). During the last stage, the maturation phase eventually takes place. Myofibroblasts, excess newly formed vessels and the extracellular matrix are removed by apoptosis, thus mediating the transition of granulation tissue into scar (Yamaguchi & Yoshikawa, 2001).
Baeckea frutescens, or locally known as Cucur atap in Malaysia is a shrub plant from the family of Myrtaceae. The plant grows on the top of mountain, Klang Gates Quartz Ridge and sandy coasts in Malaysia (Navanesan et al., 2015). In Malaysia, the essential oil of B. frutescens is traditionally used for massaging during postpartum confinement following childbirth and rheumatism (Navanesan et al., 2015). In Vietnam, the plant is traditionally used to treat headache, and rheumatism, whereas the leaves and flowers are used to ease menstrual disorder and indigestion problem (Bich et al., 2004). Other than being used for massaging aching muscles and as bath or tonic, the essential oil has also been used as an aromatherapy and inhaled to achieve focus and clear state of mind (Setzer et al., 2004). Additionally, Chinese folk medicine reported the use of B. frutescens plant to treat snake bite (Cheung and Li, 1980). Previous studies on the phytochemicals reported flavonoids including flavanone, flavonol, (Quang et al., 2008), flavonol glycoside (Lu et al., 2008), chromones, chromanones (Tsui and Brown, 1996), phloroglucinols (Fujimoto et al., 1996), tasmanones (Tam et al., 2004), pinoquercetin (Zhong et al., 1997) and quercetin (Lu et al., 2008) from B. frutescens.
Chronic wounds occur when the wound repair process is delayed or prolonged due to factors such as trauma, diabetes, infection, vascular disease or radiation (Järbrink et al., 2016). Apparently, chronic wound increases morbidity and healthcare costs especially among elderly and diabetic patients. Approximately 2% of all hospitalized patients have chronic wounds and older adult patients are at higher risks due to the impact of aging on the healing process (Powers et al., 2016). Even though the use of modern drug to treat wound healing proved to be effective, increased cost in wound healing management regime had prompted many researchers to find alternative candidates especially from plants with traditional used reputations. In view of the various therapeutic effects and traditional uses of B. frutescens, particularly regarding its ability to alleviate rheumatism and snake bites, it was proposed that this plant might also possess several other medicinal properties like wound healing ability. Therefore, this study was performed to evaluate the in vitro wound healing properties, antioxidant activity and analyze the chemical profile of B. frutescens ethanolic leaves extract. Present study showed that B. frutescens is a promising wound healing agent and this study served as a platform for future study to investigate the molecular mechanism of wound healing properties of this plant.
2 Material and methods
2.1 Chemicals and reagents
2,2-diphenyl-1-picrylhydrazyl (DPPH), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), acetate buffer, allantoin, dimethyl sulphoxide (DMSO), Folin-Ciocalteu reagent, gallic acid, hydrochloric acid (HCl), iron (III) chloride (FeCl3), iron (II) sulfate (FeSO4), TPTZ, phosphate buffer saline (PBS) tablet and sodium carbonate were purchased from Sigma-Aldrich, St. Louis, USA. Absolute ethanol and ethanol (95%) and were purchased from Merck, Malaysia. Dulbecco’s Modified Eagle’s Medium (DMEM), trypsin and penicillin-streptomycin were purchased from Nacalai Tesque, Japan. Green tea (Carmellia sinensis) extract was purchased from ChromaDex, USA.
2.2 Collection of plant materials and extract preparation
The Baeckea frutescens was collected at Setiu, Terengganu, Malaysia. The sample was authenticated by botanist, Ms. Tan Ai Lee at FRIM, Malaysia and a herbarium specimen with number (SBID: 044/20) was deposited at Faculty of Pharmacy, Universiti Teknologi MARA Selangor. This plant was cleaned prior to air dried for a week before the leaves were separated from its branches. The leaves were grinded to form a fine powder. The powder of leaf part of B. frutescens was macerated in 95% of ethanolic solvent (1:10) for 48 h and filtered using Whatman filter paper. The maceration and filtration process were performed in room temperature. The process was repeated twice, and the extracts filtered were pooled. The solvent was removed using rotary evaporator. The Baeckea frutescens leaves extract (BFLE) was collected and stored in −20 °C for further use. The BFLE was dissolved in 100% dimethyl sulfoxide (DMSO). The stock solution was then diluted with ultrapure water for phytochemical screenings and LCMS analysis, diluted with absolute ethanol in DPPH radical scavenging assay, diluted with distilled water in FRAP and TPC assays and diluted with DMEM for in vitro wound healing assays. Final concentration of DMSO in all cell culture assays did not exceed 0.5%, which was considered non-toxic to the cells (Galvao et al., 2014).
2.3 In vitro wound healing assays
2.3.1 Cell culture and maintenance
Immortalized human keratinocyte (HaCaT) and human dermal fibroblast (BJ) cells were purchased from ATCC and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Both cells were maintained and incubated in chamber in humidified condition (95% humidity) with 5% CO2 level at 37 °C. Cells were subcultured or seeded for experiments when it reached 80-95% confluency and the media was replaced for at least every 3 days.
2.3.2 Cells viability assay
Cells viability of HaCaT and BJ against BFLE were performed using MTT assay according to method described by Muniandy et al., (2018) with slight modifications. HaCaT and BJ cells were seeded in 96-well plate at 1 × 104 and 6 × 103 cells/well, respectively and were allowed to grow for 24. The cells were then treated with different concentrations of BFLE (0–1000 µg/ml) and were incubated for another 24 h. Following incubation period, the old medium was discarded and treated cells were rinsed with PBS. Complete medium containing 0.5% MTT was added to the cells and incubated for 2 h. Afterward, the complete medium containing 0.5% MTT was discarded and the DMSO was added to dilute the purple formazan crystal formed by mitochondria of living cells. The diluted formazan solution producing purple color was read spectrophotometrically at 570 nm. The experiment was performed in triplicate.
2.3.3 Cell proliferation assay
Cell proliferation assay was performed similar to cells viability assay except the exposure period of BFLE to HaCat and BJ was extended to 48 and 72 h, in addition to 24 h of exposure period. HaCaT and BJ cells were seeded in 96-well plate at 5 × 103 cells/well. The cells were allowed to grow with different concentrations of BFLE and incubated for another 24, 48 or 72 h. The proliferation assay requires less cells density compared to viability assay due to the longer incubation period which may cause over confluence of the cells in the plate. The concentrations applied in this assay were non-toxic concentrations to HaCaT and BJ cells as obtained from the result of cells viability assay, which were at 0–100 and 0–200 µg/ml, to HaCaT and BJ, respectively. The steps afterward was addition of MTT solution was similar to cells viability assay.
2.3.4 Scratching assay
Cells scratching assay was performed on HaCaT and BJ cells according to method described by Governa et al., (2019) with some modifications. HaCaT and BJ cells were seeded at 1 × 106 cells/well and 2 × 105 cells/well, respectively on 6-well plate. The cells were allowed to grow in CO2 chamber until it reached confluency. The differences on seeding density of both cell lines were due to their different sizes and different incubation time between both cells. After reaching confluency, cells were scrapped horizontally by using the 200 µl micropipette tips at three different areas on each well. The cells were then washed with PBS twice to remove the cells debris. Subsequently, the cells were treated with or without BFLE at different concentrations (25, 20, 10 and 5 µg/ml) or allantoin at 10 µg/ml (positive control). The photograph of cells was immediately taken under phase contrast microscopy at 10x magnification and denoted as 0 h. The cells were then incubated in CO2 chamber and the second and third set of images of cells were taken again at 6 and 12 h or 16 and 24 h, for HaCaT and BJ, respectively. The differences in incubation time between HaCaT and BJ were due to their different doubling time. HaCaT has more rapid doubling time compared to BJ thus require shorter incubation period. The images of cells were analysed by ImageJ software and the migration rate was calculated by comparing wound area of images at 6, 12 16 and 24 h to that of image at 0 h. The experiment was performed in triplicate of three independent experiments.
2.4 Antioxidant properties
2.4.1 DPPH radical scasvenging
DPPH radical scavenging activity of BFLE was determined according to method described by Blois (1958) with minor modifications. Briefly, 50 µl of BFLE (200, 100, 50, 25 and 12.5 µg/ml final concentration) or Green tea extract (same concentrations, used as positive control) was mixed with 50 µl DPPH solution (200 µM final concentration, diluted with ethanol) and 150 µl ethanol (absolute, AR grade) in 96-well microplate. The mixture was shaken for 15 s at 300 rpm and was left to stand for 30 min in dark at room temperature. The absorbance of the mixed solutions was measured spectrophotometrically at 520 nm. The experiment was performed by three independent experiments in triplicate. The DPPH radical scavenging was calculated using the following formula: % of DPPH scavenging effect = 100 − [(Asample-Ablank)/Acontrol × 100]
2.4.2 Ferric reducing antioxidant power (FRAP) value
The FRAP assay was performed according to the method of Benzie and Strain (1996) with some modifications. FRAP reagent was prepared by mixing acetate buffer (0.3 M, pH 3.6), a solution of 10 M TPTZ in 40 M HCl and 20 mM FeCl3 in 10:1:1 ratio. The assay was carried out in 96-well plate and was initiated when 200 µl of FRAP reagent was mixed with 20 µl of BFLE, Green tea extract or FeSO4 standard (500–2500 µmol/L) and the mixture were left to stand at room temperature for 4 min. Afterward, the plate was shaken at 300 rpm for 30 s and absorbance was measured spectrophotometrically at 593 nm. Aqueous solution of known Fe (II) concentration was used for calibration. A standard curve of FeSO4 was plotted at 500–2500 µmol/L. Result was expressed as mM FeSO4 equivalent per mg of extract. All tests were conducted in triplicate.
2.5 Total phenolic content (TPC)
Total phenolic content of BFLE was evaluated using Folin-Ciocalteu reagent according to the method of Singleton and Rossi (1965), with modifications into high-throughput microplate system. Briefly, distilled water 0.1 mL and 0.1 mL diluted Folin–Ciocalteu reagents (1:10 in distilled water) were added to 50 μl of BFLE or Green tea (positive control). The samples were left to stand for 5 min before 0.1 mL 7.5% sodium carbonate (w/v) was added. After 2 h, the absorbance was measured at 765 nm wavelength using a spectrophotometer. The calibration curve of gallic acid (GA) was used for the estimation of sample activity capacity.
2.6 Phytochemical screenings
BFLE was screened for the presence of secondary metabolites, including alkaloids, saponins, flavonoids, tannins, steroids, terpenoids and phenolics content according to procedures described by Zeng et al., (2016).
2.6.1 Alkaloids content
The test for alkaloids was carried out on 10 mg of BFLE mixed with 5 mL of ammonia and 2.5 mL of chloroform. After filtration, the supernatant was shaken with drops of 0.5 M sulfuric acid. The appearance of a creamy precipitate indicated the presence of alkaloids and was categorized as mild, moderate and high concentration based on colour scale.
2.6.2 Saponins content
The test for saponins was carried out by adding 10 mg of BFLE with 10 mL of ethanol. The solution was then sonicated for 30 min and filtered. After filtration, the supernatant was added with 5 mL of distilled water and vigorously shaken for 30 s. The appearance of a foam indicated the presence of saponins and was categorized as mild, moderate and high concentration based on foam formation scale.
2.6.3 Tannins content
A sample prepared with 5 mg of BFLE was dissolved in 10 mL of 70% ethanol. The sample was then diluted with sterile distilled water at a ratio of 1: 2 (v/v).Three drops of 10% (w/v) ferric chloride solution was then added. The appearance of a blue to black precipitate indicated the presence of tannins and was categorized as mild, moderate and high concentration based on colour scale.
2.6.4 Flavonoids content
A sample containing 5 mg of the BFLE was dissolved in 5 mL of absolute ethanol and treated with a few drops of concentrated HCl and 0.2 g of magnesium ribbon. The appearance of a pink-red color indicated the presence of flavonoids and was categorized as mild, moderate and high concentration based on colour scale.
2.6.5 Steroids and terpenoids content
Steroids and terpenoids were detected using the Liebermann-Burchard reaction. A solution containing 5 mg of BFLE dissolved in chloroform was filtered. The filtrate (2 mL) was added to 2 mL of acetic anhydride and 50% concentrated sulfuric acid. A blue-green ring indicated the presence of steroids while a red color indicated the presence of terpenoids and was categorized as mild, moderate and high concentration based on colour scale.
2.7 LCMS analysis
LCMS analysis was performed on LTQ Orbitrap Discovery LCMS system. The HPLC column was Luna Omega 3 µm Polar C18. The mobile phases were 0.1 % formic acid (A) and acetonitrile (B). The HPLC conditions were as followed: at 0 min, 95:5 (A:B), linear gradient to 50:50 (A:B) at 10, 5:95 (A:B) at 15 min and 95:5 at 20 min and hold on for 5 min. The flow rate was 0.2 µl/min with 10 µl injection volume. Mass spectra in the m/z range 50–1000 were obtained by negative ion (ESI) modes. The mass spectrometry conditions were as follows: sheath gas flow rate 18 arbitrary units, auxiliary gas flow rate 2 arbitrary units, spray voltage 4.40 kV, capillary temperature 285 °C, capillary voltage 18 V, tube lens 100 V. Data acquisition was performed using LTQ Tune Plus and data was analysed using Thermo Xcalibur Roadmap software.
2.8 Statistical analyses
All data were presented as mean ± standard deviation (SD). The differences between groups were analysed using one-way analysis of variance with post hoc Dunnett’s Multiple Comparison Test using Graphpad Prism 7 software. P value < 0.05 was considered as significantly different.
3 Results
3.1 Phytochemistry analysis
Phytochemistry analysis of BFLE detected the presence of saponins, condensed tannins, flavonoids and steroids (Table 1). Condensed tannins and flavonoids were present at moderate concentrations while saponins and steroids were detected at low concentration. Alkaloids and triterpenoids presence were not detected. ++ Moderate concentration, + low concentration, – not detected.
Phytochemistry components
Remarks
Alkaloids
–
Saponins
+
Condensed Tannins
++
Flavonoids
++
Triterpenoids
–
Steroids
+
3.2 LCMS/MS analyses
LCMS/MS results of BFLE are as depicted in Fig. 1 and Table 2. In summary, a total of 6 compounds were tentatively identified from LCMS/MS data and their identifications were confirmed from the data obtain from literatures and online databases (Table 2). Myricetin was tentatively identified (Lu et al., 2008) from deprotonated molecular ions [M−H]- at m/z 317.03 Pinoquercetin was tentatively identified from the deprotonated molecular ions [M−H]- at m/z 315.05. Next, quercetin-3-O-alpha-L-rhamnoside (Lu et al., 2008), 5,7-dihydroxy-6,8-dimethylflavanone (Nisa et al., 2016) and 6-Methylquercetin 7-O-β-D-glucopyranoside (Zhou et al., 2018) were tentatively identified from deprotonated molecular ions [M−H]- at m/z 447.20, 283.09 and 477.21, respectively.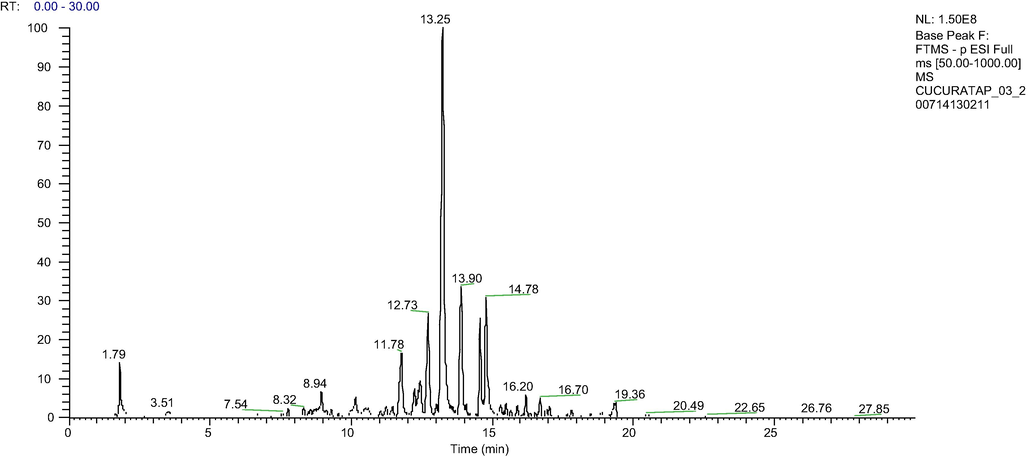
Total ion chromatogram of BFLE analyzed by HPLC-LTQ-Orbitrap MS System.
Retention time, min
Molecular ion (M-H)-
Molecular weight
Tentative identification
8.94
463.08
C21H19O12-
Myricetin 3-O-alpha-L-rhamnoside
10.15
317.03
C15H10O8
Myricetin
12.73
315.05
C16H12O7
Pinoquercetin
16.20
447.20
C21H20O11
Quercetin-3-O-alpha-L-rhamnoside
16.67
283.09
C17H16O4
5,7-dihydroxy-6,8-dimethylflavanone
16.70
477.21
C28H32O17
6-Methylquercetin 7-O-β-D-glucopyranoside
3.3 Antioxidant evaluations
Antioxidant properties of BFLE was determined by DPPH radical scavenging, total phenolic content (TPC) and ferric reducing antioxidant power (FRAP) assays. Result of DPPH scavenging shows a very good antioxidant property of BFLE with the extract exhibited the dose dependent effect on DPPH scavenging effect. Graph of dose–response curve plotted and the median inhibitory concentration (IC50) value of DPPH scavenging effect of BFLE obtained as 22.87 µg/ml. Green tea, which was used as positive control shows almost similar IC50 value of 16.69 µg/ml. Even though IC50 of Green tea was slightly lower than that of BFLE, the values are not significantly different between the two groups.
TPC of BFLE was obtained from the linear regression of gallic acid standard curve (data not shown) and the gallic acid equivalent (GAE) per gram extract was obtained from the equation obtained from that curve (y = 0.0625x − 0.0224, r2 = 0.9998). Results of TPC value of BFLE indicates that it has TPC value of 193.22 ± 10.02 mg GAE/g extract, higher than that of Green tea, with TPC value of 186.02 ± 11.79 mg GAE/g extract. However, the TPC values between the two groups were not significantly different.
Ferric reducing antioxidant power (FRAP) value was obtained from the linear regression of ferrous sulphate standard curve (data not show) and the FRAP value of was obtained from that curve (y = 0.6438x + 0.1042, r2 = 0.9999). The results dictate that the FRAP value of BFLE was 2.42 ± 0.15 mmoldm−3/g extract while FRAP value of Green tea is 3.19 ± 0.02 mmoldm−3/g extract (see Fig. 2).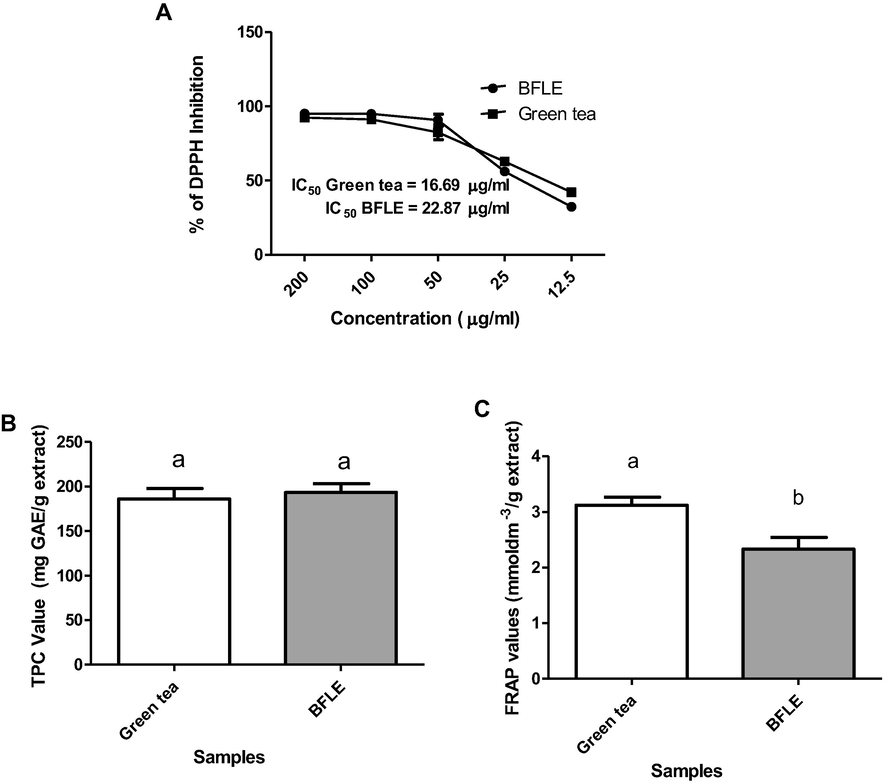
A) Percentage of DPPH inhibition on different concentrations of BFLE and Green tea extract and their IC50 values, TPC (B) and FRAP (C) values of BFLE and Green tea extract. TPC value were expressed as mg GAE per gram of extracts ± SD while FRAP value were expressed as mmoldm−3/g extract ± SD. The bars with different letters are significantly different from each other at P < 0.05.
3.4 Cells viability
Viability of HaCat and BJ cells against BFLE were tested with concentrations of 0–1000 µg/ml for 24 h treatment period. The results indicated that BFLE was toxic against both cells in dose-dependent manner but the level of toxicity were different between both cells. There is no significant difference between treatment of BFLE on HaCat at 15.63, 31.25 and 62.5 µg/ml compared to VC but cell viability was reduced as the concentration increased (dose dependent). In the meantime, treatment of BFLE on BJ at concentration of 31.25, 62.5 and 125 µg/ml significantly increased cell viability compared to that of VC (p < 0.05). However, treatment of BFLE at 15 and 250 µg/ml on BJ did not significantly alter cell viability while BJ cells viability were greatly reduced at concentration of 500 and 1000 µg/ml (not dose dependent) (see Fig. 3).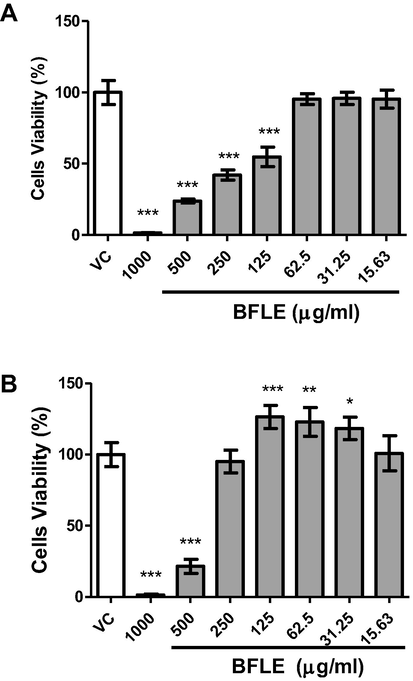
Viability of A) HaCaT and B) BJ cells against BFLE. Results were expressed as mean of percentage of cells viability ± SD. VC; vehicle control (untreated cells).* P < 0.05, ** P < 0.01, *** P < 0.001.
3.5 Cells proliferation
Cell proliferation assay was conducted on HaCaT and BJ cells at different range of concentrations, 0–100 and 0–200 µg/ml, respectively for 24, 48 and 72 h incubation periods. Results of proliferation show proliferative effects of BFLE on BJ at 25 and 12.5 µg/ml at 24 h compared to VC but no proliferative effect was observed on HaCaT. At 48 h, the proliferative effect of BFLE was observed on HaCaT at 6.25 and 3.13 µg/ml but there is no significant proliferative effect was observed on BJ cells. Both cells exhibited no proliferative effect at 72 h (see Fig. 4).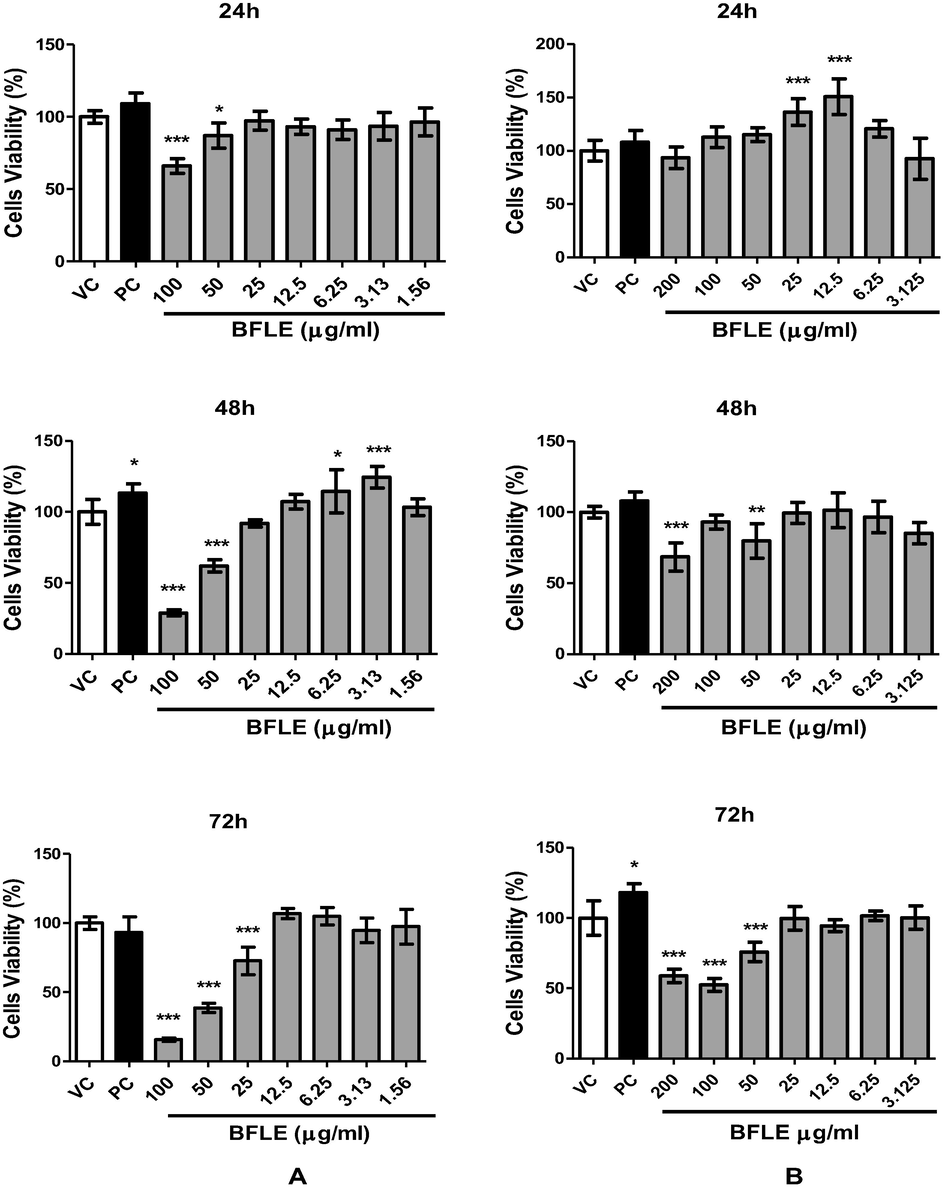
Proliferation of A) HaCaT and B) BJ against BFLE at 24, 48 and 72 h. Results were expressed as mean of percentage of cells viability ± SD. VC; vehicle control (untreated cells), PC; positive control (10 μg/ml allantoin).* P < 0.05, ** P < 0.01, *** P < 0.001 vs VC.
3.6 Cells migration
The migration rate of keratinocyte (HaCaT) and fibroblast (BJ) cells was measured by comparing the size of gaps on each well which mimics the wound and the image of the gaps was captured at different intervals of 0, 6, 12, 16 and 24 h after the scratch. The migration rate at 6, 12, 16 and 24 h was obtained by subtracting the size of the gap at 0 h to the size of gap at 6, 12, 16 and 24 h, respectively. The result indicates that BFLE at 25 and 20 µg/ml shows significantly high migration rate on HaCaT cell at 6 h, while at 12 h, it shows significantly higher migration rate compared to untreated control on all concentration tested. On BJ cells, the effect of BFLE on its migration rate was evident 10, 5 and 2.5 µg/ml at 16 h. At 24 h, only BFLE at 10 µg/ml showed significant effect of migration rate on BJ. Allantoin, the positive control used in migration study, significantly increase the migration rate of both HaCaT and BJ cells at 6, 12, 16 and 24 h. The concentrations used for migration assay were chosen based on the proliferation assay, which demonstrated that BFLE proliferation was better at 25 µg/ml and lower concentrations while it demonstrated sign of toxicity at concentration above 25 µg/ml when incubated for 72 h (see Figs. 5 and 6).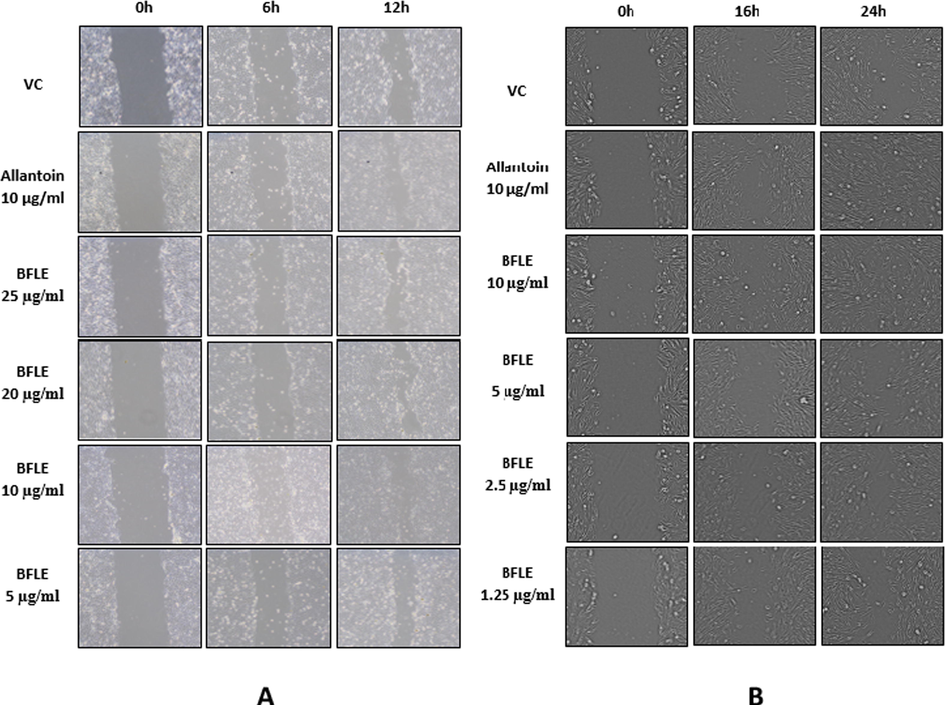
Migration of A) HaCaT against BFLE at 0, 6 and 12 h and B) BJ against BFLE at 0, 16 and 24 h. Data were presented as representative of image from three independent experiment taken from respective samples and time intervals.
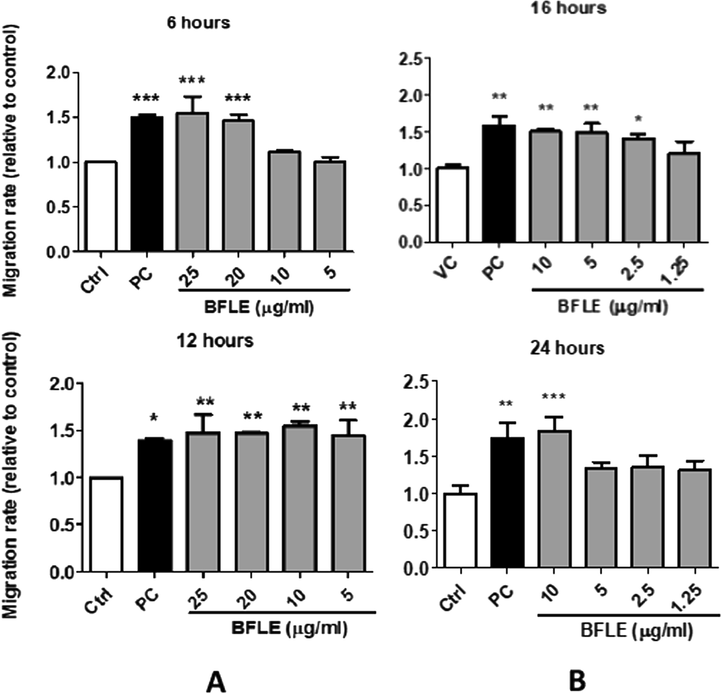
Effect of BFLE on relative migration rate of A) HaCaT cells at 6 and 12 h and B) BJ cells at 16 and 24 h. Data were expressed as mean of migration rate of HaCaT and BJ cells relative to untreated control. PC; positive control (10 μg/ml allantoin) * p < 0.05, ** p < 0.01 and p < 0.001 vs control.
4 Discussions
Wound healing occurs as cellular response towards injury and involves the activation of fibroblast, keratinocyte, endothelial cells and macrophages. Proliferation and migration of keratinocyte and fibroblast cells are involved, at least as a part of complex wound healing processes (Stamm et al., 2016). During injury, as the barrier disrupt, neutrophils, monocyte and macrophages recruited to the wound site and the abundant cellular matrices produced release several cytokines and growth factors, which in turn activated the keratinocyte and fibroblast cells (Pastar et al., 2014). Activated keratinocyte and fibroblast cells will migrate, proliferate, and produce several other inflammatory cytokines to promote the wound healing process.
Result of present study demonstrates that ethanolic extract of Baeckea frutescens leaves (BFLE) has the ability to increase the proliferation and migration of both keratinocyte (HaCaT) and fibroblast (BJ) cells but the pattern of the treatment effects are varied and are dose- and time-dependent. Proliferation of keratinocyte increased by 14 and 24% at concentration of 6.25 and 3.125 µg/ml, respectively after 48 h of treatment and proliferation of fibroblast was increased by 36 and 51% at concentration of 25 and 12.5 µg/ml, respectively after 24 h of treatment. Proliferation of both keratinocyte and fibroblast were not significantly increased by BFLE at 72 h across all concentrations and periods tested. BFLE also showed toxicity effect on HaCaT and BJ when treated at longer incubation period at higher concentrations. The results were similar with previous study by Governa et al., (2019), which implied that extract with higher concentrations and prolonged exposure were not imperatively produce more desirable effect. This is postulated to have caused by the effect of an elaborate balance between the anti-inflammatory and immune-stimulatory effect of polyphenols in the extract, which in turn reduce the production of pro-inflammatory cytokines and interact with cells proliferation, thus reducing its wound healing property (Chiocchio et al., 2019).
The morphological changes of both keratinocyte and fibroblast cells following treatment of BFLE at different concentrations (0, 5, 10, 20 and 25 µg/ml) observed by visual examination of images of scratch assay revealed that BFLE increased the migration of both cells compared to untreated cells. The relative migration rate of BFLE on HaCaT and BJ, measured as differences between wound area at 0, 6, 12 h, on HaCaT and 0, 12 and 24 h, on BJ, demonstrating wound healing properties of BFLE. The effect of BFLE on cells migration was shown to be more potent than that of allantoin, which is known to have the proliferative and migration effect (Forero-Doria et al., 2020). It is well established that both fibroblast and keratinocytes are two types of cells that are majorly involved in proliferative phase of wound healing and increasing their migration rate is very important to accelerate the process (Qiang et al., 2020). The migratory effect BFLE on both BJ (fibroblast) and HaCaT (keratinocyte) showed their great response on speeding the proliferative phase of wound healing and toward the closing of wound gaps during wound contraction.
Beside of its proliferative and migration effect on keratinocyte and fibroblast cells, this study also revealed the antioxidative properties of BFLE. Results of DPPH radical scavenging and FRAP assay determination have well demonstrated the antioxidative property of BFLE. BFLE showed remarkable DPPH radical scavenging effects, with IC50 value of 22.87 µg/ml and was not significantly different to IC50 value of Green tea with IC50 value of 16.69 µg/ml. TPC value of BFLE was also not significantly different to Green tea while the FRAP value of BFLE showed slightly lower value than that of Green tea (p < 0.001). Total phenolics content is known to be related to antioxidative properties and predominant in plant which mainly composed of phenolics acid, flavonoids, tannins, curcuminoids, coumarins, lignins and quinones. (Kaska; Ismail et al., 2017). Several studies have demonstrated the antioxidative properties of plant extracts were attributed to their phenolics content (Kaska et al., 2018; Ismail et al., 2017; Othman et al., 2014). Green tea, which was used as positive control in this study is a well known antioxidant and possesses health benefits (Rishi Raj Shrivastava et al., 2018). The similar antioxidant properties between BFLE and Green tea indicate that BFLE is a very good antioxidant thus may possess same health benefit of Green tea. Several studies have proved that antioxidant and wound-healing properties could be co-existed in certain plants (Süntar et al., 2012). For instance, study by Goorani et al, (2019) demonstrated an aqueous extract of Falcaria vulgaris, which has high antioxidant activity, showed a very good wound healing properties on cutaneous wound of Wistar rats. Similarly, recent study by Ahmad et al., (2021) indicated Marantodes pumilum has both antioxidant and wound healing effects on excisionally induced wound of rats.
It is well known that biological effects of natural products or medicinal plants are owing to their chemical properties. Result of phytochemical screening showed that BFLE has a moderate concentration of condensed tannins and flavonoids while steroids and saponins were detected at low concentration. Tannins and flavonoids have long been associated with plethora of biological properties and health benefits including antioxidants, anti-inflammatory, anticancer, cholesterol-lowering effect, arthritis and wound healing effect (Bondonno et al., 2019; Rex et al., 2018; Dabeek & Marra, 2019; Shao and Bao, 2019; Beninger & Hosfield, 2003). Recent study by Ambreen & Mirza (2020) has specifically demonstrated the wound healing property of tannins isolated from leaf callus culture of Achyranthes aspera and Ocimum basilicum. Further investigation by LCMS/MS has tentatively identified that flavonoids of BFLE were myricetin, myricetin 3-O-alpha-L-rhamnoside, quercetin-3-O-alpha-L-rhamnoside, 6-methylquercetin 7-O-β-D-glucopyranoside, pinoquercetin and 5,7-dihydroxy-6,8-dimethylflavanone based on their m/z masses. Myricetin and its derivative, myricetin-3-O-β-rhamnoside, were found to have in vitro healing properties by promoting both proliferation and migration of fibroblast cells and accelerating angiogenic process (Moghadam et al., 2017). Similarly, in vivo study performed by (Elshamy et al., 2020), demonstrated Myricetin from Tecomaria capensis v. aurea can stimulate wound contraction. In addition to that, Myricetin was also reported to suppress the ROS-induced oxidative stress (Wang et al., 2010). Inhibition of ROS has been implicated as indirectly promote migration and proliferation of cells (Yahaya et al., 2018). These findings suggest that Myricetin might the compound that contributed to wound healing properties of BFLE. However further investigation need to be performed to support this hypothesis.
5 Conclusions
The increased the rate of migration and proliferation on both keratinocyte and fibroblast cells indicated the wound healing properties of BFLE. The wound healing properties of BFLE may be related to their high antioxidant properties. The presence of saponins, flavonoids, tannins and steroids in the phytochemistry analysis and the presence of those compounds detected by LCMS analysis suggested the contribution of the compounds in the antioxidant and wound healing properties of BFLE.
Acknowledgement
This work was supported by FRGS-RACER grant by the Malaysian Ministry of Higher Education, project code RACER/1/2019/STG04/UITM//2 with project ID 16833, and UiTM grant (600-IRMI 5/3LESTARI (008/2019). We would like to thank Dr. Mary Khoo Gaik Hong for providing cell culture and microscopic viewing facilities as well as Mrs. Juliza Mohamed, Mrs. Rohana Sahdan, Miss Evana Kamaruddin, Miss Nurul Syamimi Suhaimi, Miss Asmiraizzlin Mad Kamal and Miss Siti Rajihawanie Adnan for facilitating in many aspects of this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of wound-healing and antioxidant effects of Marantodes pumilum (Blume) Kuntze in an excision wound model. Molecules.. 2021;26(1):228.
- [CrossRef] [Google Scholar]
- Evaluation of anti-inflammatory and wound healing potential of tannins isolated from leaf callus cultures of Achyranthes aspera and Ocimum basilicum. Pak. J. Pharm. Sci.. 2020;33
- [Google Scholar]
- Antioxidant activity of extracts, condensed tannin fractions, and pure flavonoids from Phaseolus vulgaris L. seed coat color genotypes. J. Agric. Food Chem.. 2003;51(27):7879-7883.
- [CrossRef] [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 1996;239(1):70-76.
- [Google Scholar]
- The medicinal plants and animals in Vietnam. Vol 1. Sci. Technol. Publ. House 2004:960-963.
- [Google Scholar]
- Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199-1200.
- [CrossRef] [Google Scholar]
- Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat. Commun.. 2019;10(1):1-10.
- [CrossRef] [Google Scholar]
- Chinese medicinal herbs of Hong Kong. Vol Vol 1. Hong Kong: Commercial Press; 1980.
- Wound healing and in vitro antiradical activity of five Sedum species grown within two sites of community importance in Emilia-Romagna (Italy) Plant Biosyst – Int. J. Dealing Aspects Plant Biol.. 2019;153(4):610-615.
- [CrossRef] [Google Scholar]
- Topical wound healing activity of myricetin isolated from Tecomaria capensis v. aurea. Molecules. 2020;25(21):4870.
- [CrossRef] [Google Scholar]
- Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients.. 2019;11:2288.
- [CrossRef] [Google Scholar]
- Supramolecular hydrogels based on cellulose for sustained release of therapeutic substances with antimicrobial and wound healing properties. Carbohydr. Polym.. 2020;242:116383
- [CrossRef] [Google Scholar]
- Phloroglucinols from Baeckea frutescens. Phytochemistry.. 1996;41(3):923-925.
- [CrossRef] [Google Scholar]
- Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J.. 2014;28(3):1317-1330.
- [CrossRef] [Google Scholar]
- Assessment of antioxidant and cutaneous wound healing effects of Falcaria vulgaris aqueous extract in Wistar male rats. Comparative Clin. Pathol.. 2019;28(2):435-445.
- [Google Scholar]
- Evaluation of the in vitro wound-healing activity of calabrian honeys. Antioxidants (Basel, Switzerland).. 2019;8(2):36.
- [CrossRef] [Google Scholar]
- Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo. J. Tradit. Complement. Med.. 2017;7(4):452-465.
- [CrossRef] [Google Scholar]
- Prevalence and incidence of chronic wounds and related complications: a protocol for a systematic review. Syst. Rev.. 2016;5(1):1-6.
- [Google Scholar]
- Evaluation of antioxidant properties, phenolic compounds, anthelmintic, and cytotoxic activities of various extracts isolated from Nepeta cadmea: an endemic plant for Turkey. J. Food Sci.. 2018;83(6):1552-1559.
- [CrossRef] [Google Scholar]
- Inflammation and wound healing: the role of the macrophage. Expert Rev. Mol. Med.. 2011;13:e23
- [CrossRef] [Google Scholar]
- Transition from inflammation to proliferation: a critical step during wound healing. Cell. Mol. Life Sci.: CMLS.. 2016;73(20):3861-3885.
- [CrossRef] [Google Scholar]
- A new flavonol glycoside from Baeckea Frutescens L. Yao Xue Xue Bao.. 2008;43(10):1032-1035. Chinese PMID: 19127867
- [Google Scholar]
- Differential roles of macrophages in diverse phases of skin repair. J. Immunol.. 2010;184(7):3964-3977. Epub 2010 Feb 22 PMID: 20176743
- [CrossRef] [Google Scholar]
- Wound healing potential of chlorogenic acid and Myricetin-3-O-β-Rhamnoside isolated from Parrotia persica. Molecules. 2017;22(9):1501.
- [Google Scholar]
- In vitro wound healing potential of stem extract of Alternanthera sessilis. Evid. Based Complement. Altern. Med.. 2018;2018
- [CrossRef] [Google Scholar]
- Evaluation of selected biological capacities of Baeckea frutescens. BMC Complementary and Alternative Medicine. 2015;15(1):1-8.
- [CrossRef] [Google Scholar]
- New acylphloroglucinol derivatives from the leaves of Baeckea frutescens. Phytochem. Lett.. 2016;15:42-45.
- [CrossRef] [Google Scholar]
- Phenolics, flavonoids content and antioxidant activities of 4 Malaysian herbal plants. Int. Food. Res.. 2014;21(2):18.
- [CrossRef] [Google Scholar]
- Epithelialization in wound healing: a comprehensive review. Adv. Wound Care (New Rochelle). 2014;3(7):445-464.
- [CrossRef] [Google Scholar]
- Wound healing and treating wounds: chronic wound care and management. J. Am. Acad. Dermatol.. 2016;74(4):607-625.
- [CrossRef] [Google Scholar]
- Keratinocyte autophagy enables the activation of keratinocytes and fibroblasts and facilitates wound healing. Autophagy 2020:1-16.
- [CrossRef] [Google Scholar]
- New flavonoids from Baeckea frutescens and their antioxidant activity. Natural Product Communications. 2008;3(5):755-758.
- [CrossRef] [Google Scholar]
- Phytochemicals as a potential source for anti-microbial, antioxidant and wound healing-a review. MOJ Biorg. Org. Chem.. 2018;2(2):61-70.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of Artemisia douglasiana leaf essential oil. Fitoterapia. 2004;75(2):192-200.
- [CrossRef] [Google Scholar]
- Rice Phenolics and Other Natural Products. In: Rice. AACC International Press; 2019. p. :221-271.
- [Google Scholar]
- Pharmacological control of inflammation in wound healing. J. Tissue Viability.. 2019;28(4):218-222. Epub 2019 Sep 14 PMID: 31542301
- [CrossRef] [Google Scholar]
- Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. American Journal of Enology and Viticulture. 1965;16:144-158.
- [Google Scholar]
- In vitro wound healing assays–state of the art. BioNano Mater.. 2016;17:79-87.
- [CrossRef] [Google Scholar]
- Wound healing and antioxidant properties: do they coexist in plants? Free Radicals Antioxidants. 2012;2(2):1-7.
- [CrossRef] [Google Scholar]
- Baeckea frutescens leaf oil from Vietnam: composition and chemical variability. Flavour and Fragrance Journal. 2004;19(3):217-220.
- [Google Scholar]
- Chromones and chromanones from Baeckea frutescens. Phytochemistry. 1996;43(4):871-876.
- [CrossRef] [Google Scholar]
- Myricetin suppresses oxidative stress-induced cell damage via both direct and indirect antioxidant action. Environ. Toxicol. Pharmacol.. 2010;29(1):12-18.
- [Google Scholar]
- Effect of ethnomedicinal extracts used for wound healing on cellular migration and intracellular reactive oxygen species release in SC-1 fibroblasts. South African J. Botany. 2018;118:11-17.
- [Google Scholar]
- Cutaneous wound healing: an update. J. Dermatol.. 2001;28(10):521-534. PMID: 11732719
- [CrossRef] [Google Scholar]
- Zeng, Q., Xie, H., Song, H., Nie, F., Wang, J., Chen, D., Wang, F., 2016. In vivo wound healing activity of Abrus cantoniensis extract. Evid. Based. Complement. Alternat. Med. 10.1155/2016/6568528.
- Three flavonol glycosides from leaves of Myrsine seguinii. Phytochemistry. 1997;46(5):943-946.
- [CrossRef] [Google Scholar]
- New flavonoids and methylchromone isolated from the aerial parts of Baeckea frutescens and their inhibitory activities against cyclooxygenases-1 and -2. Chin J Nat Med.. 2018;16(8):615-620. PMID: 30197127
- [CrossRef] [Google Scholar]







