Translate this page into:
Influence of ionic composition in aqueous solution on wettability of rock surface-experiment and economics evaluation
⁎Corresponding authors. malihua2004@126.com (Lihua Ma), yanhuizhe@163.com (Huizhe Yan), Yonghuiwang0510@163.com (Yonghui Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this study, the influence of different ionic composition in aqueous solution on the minerals surface wettability was studied. The differences effect of monovalent ion and divalent ions onto the wettability alteration were studied. The anions were Cl- and SO42-. The SO42- could make the minerals surface more hydrophilic. Besides, the influence of NaCl, MgCl2, CaCl2, Na2SO4, K2SO4 and MgSO4 on the mineral wettability alteration were studied. The results indicated that divalent ions showed significant impact on the minerals wettability alteration, compared with monovalent ion. The reasons were due to the fact that divalent ions showed higher ions adsorption than monovalent ion, and divalent ions have higher effect on compressing the electric double layer. The static contact angle and dynamic contact angle were measured. Different heavy oils were studied, including heavy oil with 100 ppm, heavy oil, heavy oil without resins, heavy oil without asphaltenes. The results showed that the asphaltenes would make it difficult for the heavy oils to liberate from minerals, thus decreasing the oil drops contact angle. Then the resins would decrease the heavy oil contact angles. CaCl2/MgCl2 and K2SO4 have synergistic effect on the change of the minerals surface wettability. Atomic force microscope (AFM) measurement indicated that the ions would effectively decrease the interaction force on the surface of heavy oil-minerals, which was beneficial to the heavy oil liberation. The roughness measurement indicated that the different ions would effectively increase the minerals surface wettability.
Keywords
Minerals
Wettability
Monovalent ion
Divalent ions
Dynamics contact angles
1 Introduction
Wettability was the basic property of the surface, which influenced the interface interactions, which was widely used in the emulsions formation (Hou et al., 2022; Jia et al., 2022), oil–water separation (Li et al., 2022; Sun et al., 2022; Qiu et al., 2018), enhanced oil recovery (Zhao et al., 2019), ect. Many researchers focused on the salinity effect on minerals surface wettability, and the detailed research progress was shown as follows. Besides, molecular dynamic simulation was used to study the molecule movement during the enhanced oil recovery process, and the salinity effect on oil recovery were studied (Badizad et al., 2020, Badizad et al., 2020, Badizad et al., 2020, Koleini et al., 2020, Badizad et al., 2021, Koleini et al., 2021, Sun et al., 2021). In recent years, many researchers focused on the salinity effect on rocks surface wettability (Liu et al., 2007, Gandomkar and Rahimpour 2017, Boumedjane et al., 2019, Tetteh et al., 2020, Kasha et al., 2021). Different salinities have different effect on the wettability alteration (Mugele et al., 2015, Alroudhan et al., 2016).
The formation brine would be helpful to the oil recovery. Altering the injected brine would alter the corresponding operation fees. Besides, the salinity alteration would be helpful to the oil recovery increase during the enhanced oil recovery process. Besides, due to the fact that the LSW methods was ion-engineered brine, and the brine composition would influence the heavy oil recovery.
Meriem Boumedjane et al. studied the ions Mg2+/SO42− and Ca2+/SO42− effect on the oil-wet calcite surfaces wettability alteration (Boumedjane et al., 2019). The results indicated that the determining ions (PDs) would alter the surface wettability, and the surface would be more hydrophilic. When the Mg2+ amount was equal to the SO42-, the surface wettability would show the highest. Asghar Gandomkar et al. (Boumedjane et al., 2019) studied the monovalent and divalent ions on oil/low salinity brine/limestone wettability alteration. The results indicated that the divalent ions (Ca2+, Mg2+, SO42-) had the similar wettability alteration on the limestone surface. Besides, the asphaltenes acid crude oil had positive effect on the wettability alteration for carbonate rocks. Mg2+/Ca2+ have synergistic effect with SO42- on the wettability alteration.
In addition, many researchers used the DFT and MD simulation of oil-rock system to study the wettability alteration mechanism (Li et al., 2021). Huifang Li et al. (Li et al., 2020) used the molecular dynamics and first-principles simulation methods to study the interactions between deionized water and brines, and the results indicated that two distinct water adsorption layers are formed by hydrogen bonding, and different ions played different role in the water-calcite (1 0 1 4) interaction. In addition, other researches indicated that the functionalization is no prerequisite of structural ordering and a distinct mass density profile perpendicular to the interface (Li et al., 2021). The corresponding rock surface wettability would influence the oil recovery, and the molecular dynamics simulation would be helpful to study the oil recovery mechanism (Singh et al., 2019).
Therefore, the main purpose of this study was to: (i) to study the monovalent and divalent ions effect on original rock surface wettability; (ii) to study the monovalent and divalent ions effect on the oil-wet rock surface wettability; (iii) to study the oil-solid interaction force and zeta potential of different monovalent and divalent ions.
2 Materials and discussion
2.1 Materials
NaCl, KCl, MgCl2, MgSO4, Na2SO4, CaCl2, purity > 99 %, were purchased from Aladdin Cop, Shanghai. The lipophilic carbonate asphalt rocks were obtained from Buton, Indonesia. The SNL 10 AFM probe employed was obtained from Brucker, Karlsruhe, Germany. Limestones were procured from Tianjin Liufang Co. ltd., Tianjin, China. The heavy oil was extracted from the Indonesian oil sands, Buton, Indonesia.
2.2 Contact angles measurement
In this study, the contact angles were used to evaluate the wettability of the minerals surface. The static contact angle and dynamic contact angle were measured.
2.3 Oil–solid interaction force measurement
The oil–solid interaction force was determined employing AFM measurements (Brucker, Karlsruhe, Germany). This determination is critical, as the oil-solid separation becomes more challenging when the oil–solid interaction forces increase (Liu et al., 2005, Xiang et al., 2019). During the measurements, the repulsive force would increase when the probe is close to the solid surface; however, when the probe was significantly removed from the solid surface, the force became an attractive force (Ren et al., 2009, Hogshead et al., 2011). This adhesive force was subsequently employed to evaluate the interaction force between the heavy oils and the minerals. The results indicated that the liberation of the heavy oil from the mineral surface became straightforward when less adhesive forces were present (Liu et al., 2003).
Bitumen was obtained from carbonate asphalt rocks through Soxhlet extraction. The bitumen (0.5 g) was subsequently dispersed into a 100 mL toluene solution to produce a 0.5 wt% bitumen–toluene solution, which was further diluted to 0.01 wt%. Next, the SiO2 microspheres were washed with acetone, ethanol, and distilled water, and the SiO2 microspheres were hydrophilic. Then, the SiO2 microspheres were placed into the vacuum desiccator, and there was 0.5 mL octyl trimethoxy silane in the bottom of the vacuum desiccator. In order to create the vacuum environment, the pump was used to vacuumize for 3 min. The then the SiO2 microspheres were in toe vacuum desiccator for 24 h for vapor deposition. The octyl trimethoxy silane would graft onto the hydrophilic SiO2 surface, and the SiO2 surface became hydrophobic (see Fig. 1).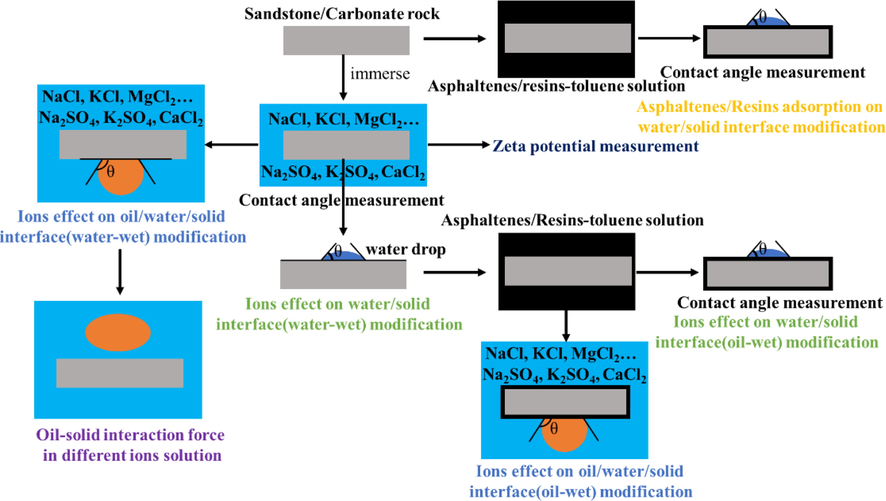
The study outline.
The hydrophobic SiO2 microspheres produced were subsequently washed with toluene and dried under nitrogen before they were added to the previously prepared 0.01 wt% bitumen–toluene solution for a period of 4 h. Finally, the solution was transferred drop-wise onto a glass slide, at which point the hydrophobic SiO2 microspheres were fixed onto the glass slide. The calcite surface could then be cleaned with acetone, ethanol, and distilled water, before a double-sided adhesive was attached to the calcite surface.
The interaction forces between heavy oil and CaCO3 surface in different saline solutions were measured using AFM (Bruker, Multimode 8) at room conditions. The speed of the probe was 2 μm/s, and the interaction forces were measured in 100 different points in order to obtain the average value, and the average value was the interaction force.
2.4 Zeta potential measurement
The charges associated with bitumen and CaCO3 in different saline water were investigated through an evaluation of the zeta potential (Liu et al., 2002, Ding et al., 2006). Here, the bitumen was dispersed in different ionic liquids employing the ultrasonic method. First, 0.5 g of bitumen was placed into 200 mL of saline solution, which was subsequently sonicated for 30 min. Then the bitumen zeta potentials would be measured. Next, 0.5 g of CaCO3 would be dispersed into 200 mL saline solution and sonicated for 30 min. All measurements were carried out three times, the average value was the zeta potential value.
2.5 Roughness measurement
In order to explore the different ions effect onto the limestone surface roughness, the roughness measurement was conducted. The detailed experiment procedures were as follows. Firstly, the limestones were treated by different aqueous solutions (with different ions), and then the limestones were naturally dried. Then the AFM measurement (Brucker, Karlsruhe, Germany) was used measure the roughness of the limestone.
3 Results and Discussion.
3.1 Monovalent anion (Cl-) effect on minerals wettability
3.1.1 Static contact angles measurement
Fig. 2 showed the different NaCl, KCl, CaCl2, MgCl2 effect on the limestone surface wettability alteration. As was shown in Fig. 2, when the salinity increased, the contact angle would decrease. However, when the salinity increased to 10000 ppm, the contact angle remained stable. In other words, the contact angle would not decrease when the salinity was higher than 10000 ppm. When NaCl, KCl, CaCl2 and MgCl2 concentrations were the same, the MgCl2 could make the contact angle show the lowest value, and the NaCl could make the contact angle show the highest value. The reason was due to the fact that divalent ions have more effect onto the minerals surface and compress the electric double layer (Lee et al., 2015, Adapa and Malani 2018).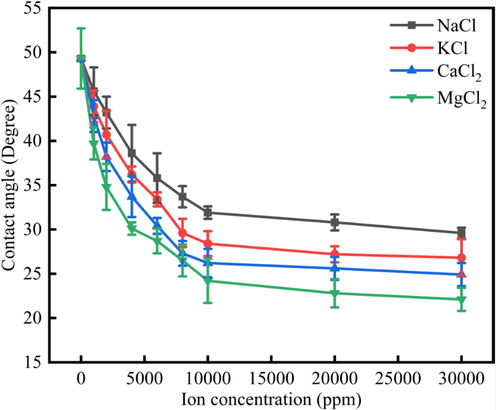
The contact angle of water drops onto the limestone surface under different ions concentration.
3.1.2 Dynamic contact angles measurement
Fig. 3 showed the dynamics contact angle of different systems in different ions and different heavy oils contents. The equilibration time for different systems were different. When the heavy oils were original heavy oil + 100 ppm asphaltenes, the equilibrium time was 72 h, which was higher than other heavy oil systems. When the heavy oils were original heavy oil, heavy oil without resins, heavy oil without asphaltenes, the equilibrium time was 48 h, 24 h, 24 h, respectively. The results showed that the lower asphaltenes content would help decrease the equilibrium time, which was due to the fact that asphaltenes would enhance the heavy oil-solid interaction force (Qao et al., 2018, Hou et al., 2021). Besides, when the heavy oil was the same, the contact angle followed the rule that θNaCl > θKCl > θCaCl2 > θMgCl2. The results indicated that the divalent ions would make the contact angle lower, and the divalent ions have higher effect on the heavy oil liberation.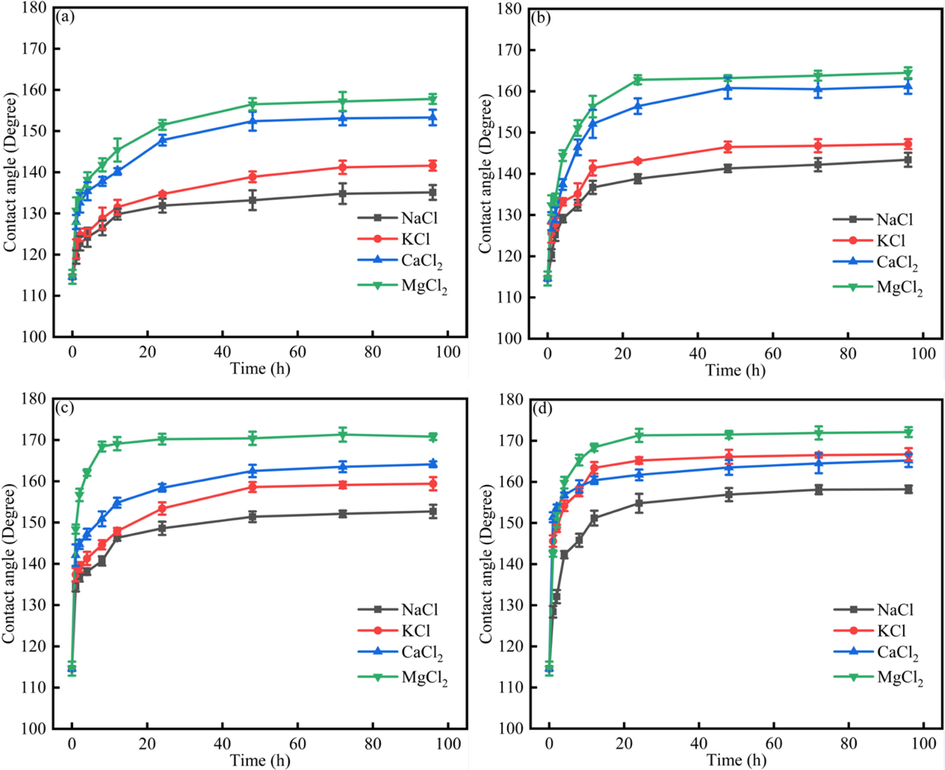
The dynamics contact angle of different systems under NaCl, KCl, CaCl2, MgCl2 solution (a) original heavy oil + 100 ppm asphaltenes; (b)original heavy oil; (c) heavy oil without resins; (d)heavy oil without asphaltenes.
Fig. 4 showed the contact angles of different oil drops onto limestone rock surface under different ions concentration (96 h). Fig. 4 showed that when the heavy oil was the same, the contact angle followed the rule that θMgCl2 > θCaCl2 > θKCl > θNaCl. When the ions were the same, the different heavy oil showed different contact angles, and when the heavy oil was without asphaltenes, the contact angle showed the highest value. And the heavy oil with 100 ppm asphaltenes would make the contact angle show the lowest value. For instance, when different heavy oil was dispersed into the NaCl solution, when the heavy oils were original heavy oil + 100 ppm asphaltenes, original heavy oil, heavy oil without resins and heavy oil without asphaltenes, the contact angles would be 135.1°, 143.4°,152.7°, 158.2°, respectively. For KCl, the contact angles would be 141.6°, 147.2°, 159.4°, 166.7°, respectively. For CaCl2, the contact angles would be 153.3°, 161.2°, 164.1°, 165.2°, respectively. For MgCl2, the contact angles would be 157.8°, 164.5°, 170.8°, 172.1°, respectively. The results indicated that different heavy oils would cause the different contact angles.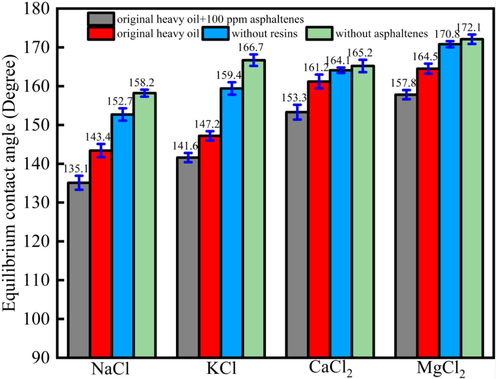
The contact angle of oil drops onto the limestone surface under different ions concentration (96 h).
3.2 Monovalent anion (SO42-) effect on minerals wettability
3.2.1 Static contact angles measurement
Fig. 5 showed the contact angle of water drops onto the limestone surface under different ions concentration (SO42-). For instance, when the different ions were treated the limestone surface, the contact angles followed the rule that θNa2SO4 > θK2SO4 > θMgSO4. The contact angles decreased with the increase of ions concentration, which was due to the fact that the Na2SO4, K2SO4 and MgSO4 would adsorb onto the limestone surface, and compress electric double layer, and then the limestone surface wettability alteration.
The contact angle of water drops onto the limestone surface under different ions concentration (SO42-).
3.2.2 Dynamic contact angles measurement
Fig. 6 showed the dynamics contact angle of different systems under Na2SO4, K2SO4, MgSO4 solution. Fig. 6 indicated that the contact angles would increase after the time continued. When the time was the same, the contact angle followed the rule that θMgSO4 > θK2SO4 > θNa2SO4. Besides, different heavy oil phases showed different effect on the contact angle alteration. When the asphaltenes content decreased, the heavy oil liberation would be enhanced, which indicated that the contact angles would decrease.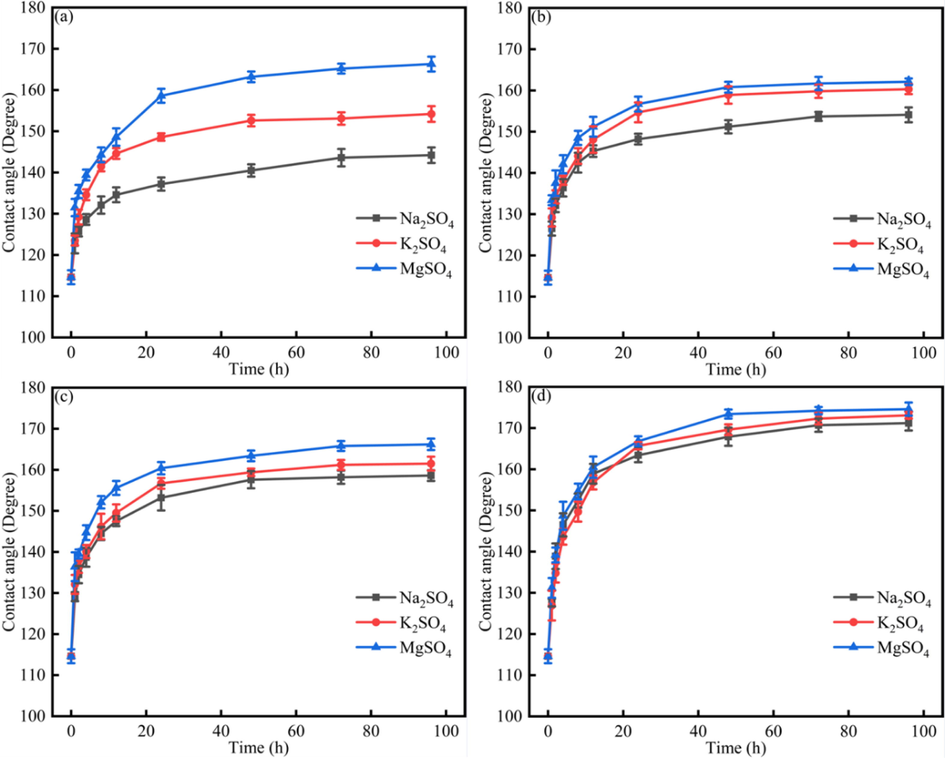
The dynamics contact angle of different systems under Na2SO4, K2SO4, MgSO4 solution (a) original heavy oil + 100 ppm asphaltenes; (b)original heavy oil; (c) without resins; (d)without asphaltenes.
Fig. 7 showed the contact angle of oil drops onto the limestone surface under different ions concentration (SO42-). The results showed that different sulfate ions showed different effect on the contact angles alteration. For Na2SO4, the contact angles for original heavy oil + 100 ppm asphaltenes, original heavy oil, heavy oil without resins, heavy oil without asphaltenes were 144.2°, 154.1°, 158.6°, 171.2°, respectively. For K2SO4, the contact angles were 154.2°, 160.3°, 161.5°, 173.1°, respectively. For MgSO4, the contact angles were 166.3°, 162.1°, 166.2°, 174.6°, respectively.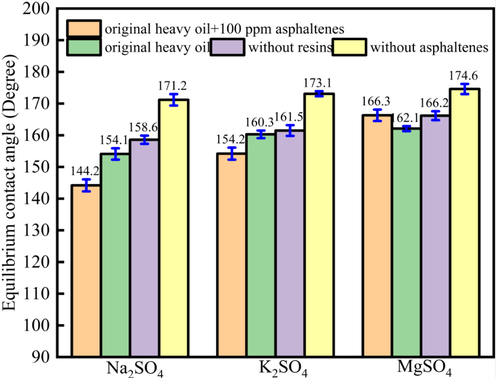
The contact angle of oil drops onto the limestone surface under different ions concentration (SO42-).
3.3 The synergistic effect between Ca2+/Mg2+ and SO42-
Fig. 8 showed the synergistic effect water drops of CaCl2, MgCl2 and K2SO4 solution. Fig. 8 indicated that the contact angles would decrease when the ions concentration increased, and when the ions concentration reached to 10000 ppm, the contact angles would become stable. The contact angles would decrease when the ions concentration increased because the ions adsorption would increase and the ions would compress the electric double layer, which would be beneficial to the contact angles decrease. After the contact angles reached equilibrium, when the ions concentrations were the same, the contact angles followed the rule that θCaCl2 > θK2SO4 > θMgCl2 > θ2CaCl2+K2SO4 > θ2MgCl2+K2SO4 > θ5CaCl2+K2SO4 > θ5MgCl2+K2SO4. All the results indicated that the CaCl2/MgCl2 has synergistic effect with the K2SO4 on the limestone wettability alteration.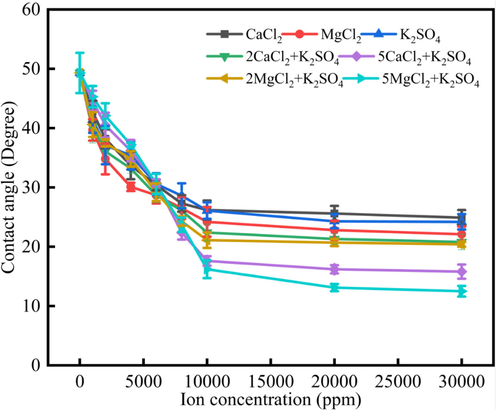
The synergistic effect water drops of CaCl2, MgCl2 and K2SO4 solution.
Fig. 9. The dynamics contact angle of different systems under different ions and different heavy oil phase. Fig. 9 showed that for different heavy oils, there exist the synergistic effect between CaCl2/MgCl2 and K2SO4, in other words, the 2CaCl2 and K2SO4 would make the contact angles higher than other aqueous phases.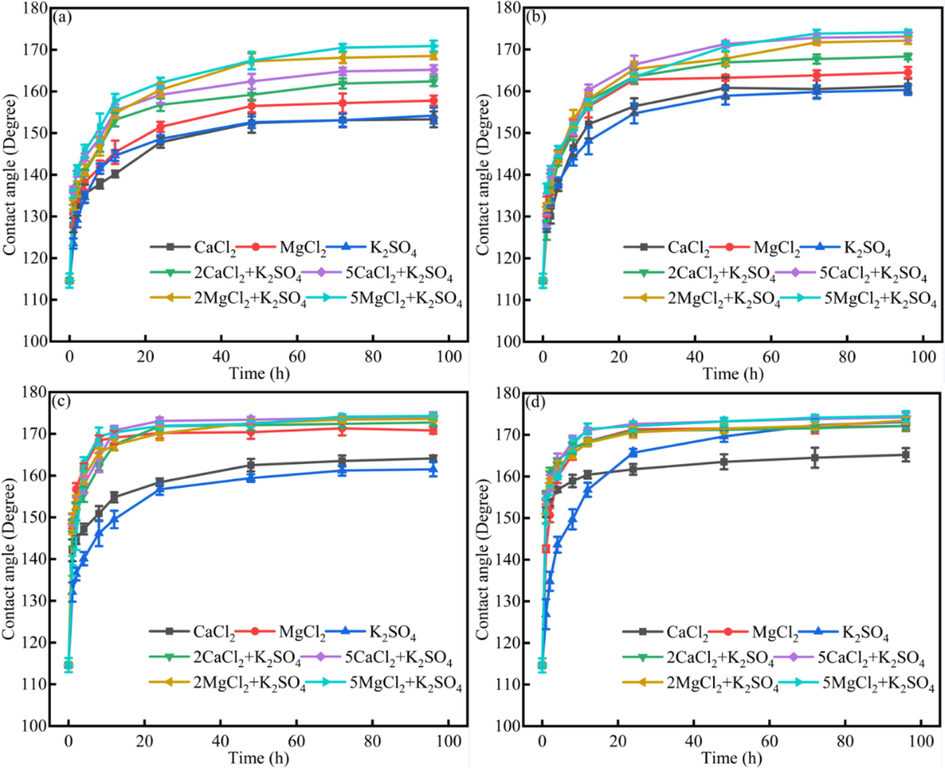
The dynamics contact angle of different systems under Na2SO4, K2SO4, MgSO4 solution (a) original heavy oil + 100 ppm asphaltenes; (b)original heavy oil; (c) without resins; (d)without asphaltenes.
Fig. 10 showed the synergistic effect oil drops of CaCl2, MgCl2 and K2SO4 solution. Fig. 10 indicated that there was synergistic effect on the contact angles alteration for the CaCl2/MgCl2 with the K2SO4. For instance, when the heavy oils were original heavy oil + 100 ppm asphaltenes, original heavy oil, heavy oil without resins, heavy oil without asphaltenes, the CaCl2 was used as the water phase, the contact angles would be 153.3°, 161.2°, 164.1°, 165.2°, respectively. When K2SO4 was used as the water phase, the contact angles would be 154.2°, 160.3°, 161.5°, 173.1°, respectively. When the 2CaCl2 was combined with the K2SO4, the contact angles would be 162.4°, 168.3°, 172.7°, 172.1°, respectively. The results indicated that the CaCl2 and K2SO4 have synergistic effect on the contact angles alteration.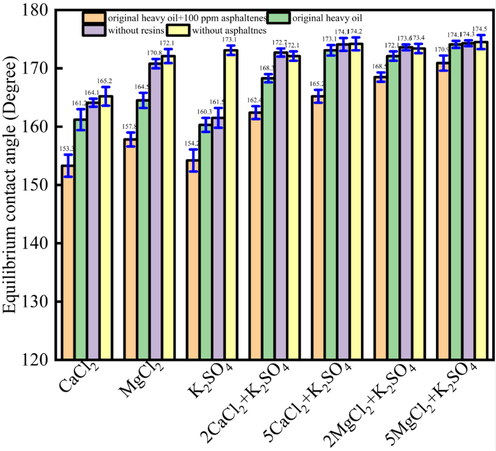
The synergistic effect oil drops of CaCl2, MgCl2 and K2SO4 solution.
Fig. 11 The contact angle of water drop onto the limestone surface under different ions concentration.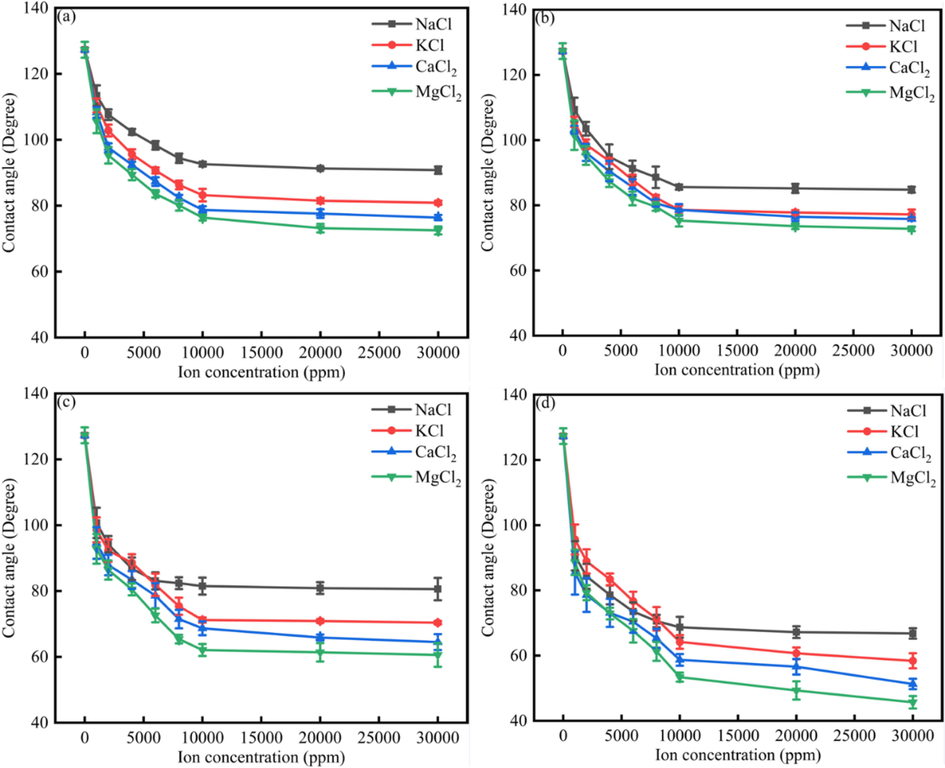
The contact angle of water drop onto the limestone surface under different ions concentration.
3.4 Oil-solid interaction force
Fig. 12 indicated that the interaction force between heavy oil and minerals surface in different aqueous solution showed different value. When the heavy oil and mineral surface were in the deionized water, the interaction force showed the highest value (5.27 mN/m). When the heavy oil and minerals surface were dipped into the salt solution, the heavy oil interaction force would decrease. When the anions were Cl-, the interaction force between heavy oil and minerals surface was higher than that for SO42-. For instance, the NaCl, KCl, CaCl2 and MgCl2 could decrease the oil-solid interaction force to 4.55 mN/m, 4.21 mN/m, 3.62 mN/m, 3.37 mN/m, respectively. The Na2SO4, K2SO4 and MgSO4 could decrease the contact angle to 4.12 mN/m, 3.89 mN/m, 2.75 mN/m, respectively. Besides, the AFM results indicated that when chloride salt and sulfate were combined together, the oil-solid interaction force would decrease further. When 5 MgCl2 was combined with K2SO4, the interaction force between heavy oil and minerals surface was 0.85 mN/m, which was the lowest value for different other salinity. The reason was due to the fact that different ions could make the interaction force decrease.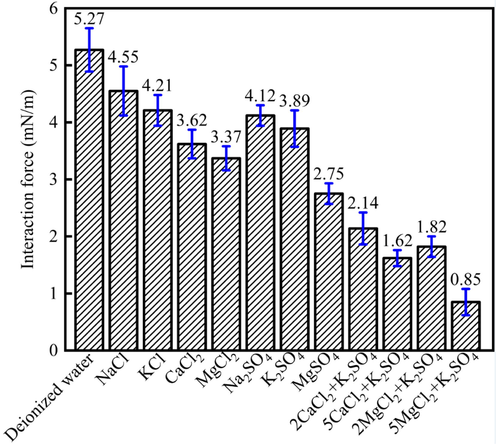
The interaction force between heavy oil and minerals surface in different aqueous solution.
3.5 Roughness analysis
Fig. 13 showed the roughness alteration of the limestone surface under different saline ions. Fig. 13 showed that different salinities would make the limestone surface showed different roughness, which was due to the fact that different ions would cause different effect on the roughness alteration. The limestone roughness was 8.2 nm when there were no salt ions. However, when the salt ions increase, the roughness would increase. For instance, when NaCl, KCl, CaCl2 and MgCl2 were treated the limestone surface, the limestone surface roughness was 15.4 nm, 20.7 nm, 32.1 nm, 36.4 nm, respectively. When the Na2SO4, K2SO4 and MgSO4 were treated the limestone surface, the limestone surface roughness was 24.5 nm, 29.8 nm, 36.6 nm, respectively. The results showed that when the cations were the same, the SO42- could make the limestone surface roughness increase. Besides, when different ions combined, the roughness would increase. Fig. 13 indicated that when the ions were combined together, the roughness would increase, the reason was due to the fact. Besides, higher roughness would make the minerals surface more hydrophilic, and the contact angle would decrease (Faibish et al., 2002, Gao et al., 2015).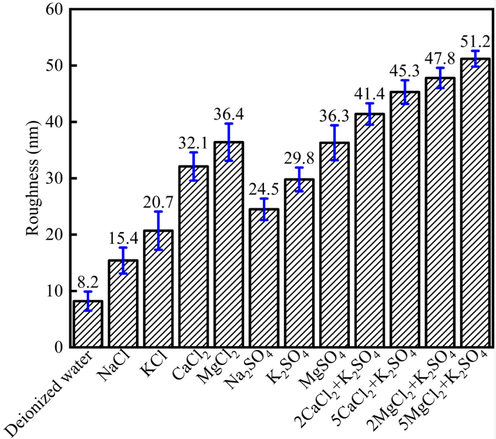
The roughness alteration of the limestone surface under different saline ions.
3.6 Economics evaluation
Fig. 14 showed the price (Yuan/ton) of different chemicals. The results showed that the heavy oil price was 4000 yuan/ton. But the ions concentration was ppm level, so it was economical feasible. In addition, the MgCl2 and Na2SO4 prices were low (450 yuan/ton, 500 yuan/ton), but the MgCl2 and Na2SO4 showed high effect on the wettability alteration, so these salts showed potential application effect.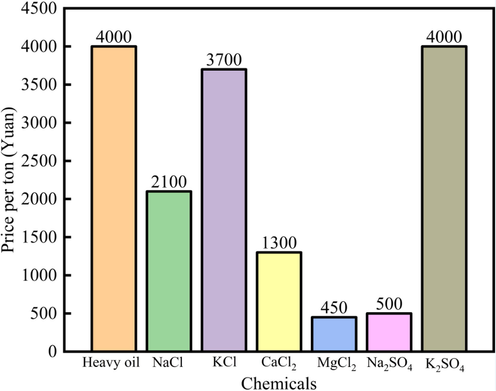
The price (Yuan/ton) of different chemicals.
4 Conclusions
In this study, the different ions effect on minerals surface wettability alteration effect were studied, and the detailed conclusions were as follows.
The ions would enhance the minerals surface hydrophilicity, and the minerals surfaces would be enhanced.
The divalent ions have higher effect onto the minerals surface than monovalent ion, which was due to the fact that divalent ions have higher effect on adsorption to the minerals surface and compress the electric double layer.
The dynamic contact angles measurement indicated that the asphaltenes would make the heavy oil contact angles lower, and then was the resins.
CaCl2/MgCl2 and Na2SO4/K2SO4 have synergistic effect on the minerals wettability alteration.
The AFM measurement indicated that the ions would decrease the heavy oil-solid interaction force. The roughness measurement indicated that the ions could increase the minerals surface roughness.
5 Fund
This work was supported by the Hebei Social Science Development Research Project under Grant 20220202093, and the Humanities and Social Science Research Project of Higher Education in Hebei Province under Grant SQ201027.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Role of hydration energy and co-ions association on monovalent and divalent cations adsorption at mica-aqueous interface. Sci. Rep.. 2018;8. 8(1):12198.
- [CrossRef] [Google Scholar]
- Zeta potential of intact natural limestone: impact of potential-determining ions Ca2+, Mg2+ and SO42-.Colloid Surf. A-Physicochem. Eng. Asp.. 2016;493:83-98.
- [CrossRef] [Google Scholar]
- A deep look into the dynamics of saltwater imbibition in a calcite nanochannel: temperature impacts capillarity regimes. Langmuir. 2020;36:9035-9046.
- [CrossRef] [Google Scholar]
- Ion-specific interactions at calcite-brine interfaces: a nano-scale study of the surface charge development and preferential binding of polar hydrocarbons. PCCP. 2020;22:27999-28011.
- [CrossRef] [Google Scholar]
- How do ions contribute to brine-hydrophobic hydrocarbon interfaces? An in silico study. J. Colloid Interface Sci.. 2020;575:337-346.
- [CrossRef] [Google Scholar]
- Atomistic insight into the behavior of ions at an oil-bearing hydrated calcite surface: implication to ion-engineered waterflooding. energy fuels.. 2021;35:13039-13054.
- [CrossRef] [Google Scholar]
- Experimental investigation of the concomitant effect of potential determining ions Mg2+/SO42- and Ca2+ /SO42- on the wettability alteration of oil-wet calcite surfaces. J. Pet. Sci. Eng.. 2019;179:574-585.
- [CrossRef] [Google Scholar]
- Effect of illite clay and divalent cations on bitumen recovery. Can. J. Chem. Eng.. 2006;84:643-650.
- [Google Scholar]
- Contact angle study on polymer-grafted silicon wafers. J. Colloid Interface Sci.. 2002;256:341-350.
- [CrossRef] [Google Scholar]
- The impact of monovalent and divalent ions on wettability alteration in oil/low salinity brine/limestone systems. J. Mol. Liq.. 2017;248:1003-1013.
- [CrossRef] [Google Scholar]
- Facile transformation of superhydrophobicity to hydrophilicity by silica/poly(epsilon-caprolactone) composite film. Appl. Surf. Sci.. 2015;359:209-214.
- [CrossRef] [Google Scholar]
- Studies of bitumen-silica and oil-silica interactions in ionic liquids. Energy Fuels.. 2011;25:293-299.
- [CrossRef] [Google Scholar]
- Surfactants enhanced heavy oil-solid separation from carbonate asphalt rocks-experiment and molecular dynamic simulation. Nanomaterials. 2021;11(7):1835.
- [CrossRef] [Google Scholar]
- A review on the application of nanofluids in enhanced oil recovery. Front. Chem. Sci. Eng.. 2022;16:1165-1197.
- [CrossRef] [Google Scholar]
- The construction of pseudo-Janus silica/surfactant assembly and their application to stabilize pickering emulsions and enhance oil recovery. Front. Chem. Sci. Eng.. 2022;16(7):1101-1113.
- [CrossRef] [Google Scholar]
- Interplay between Ca2+, Mg2+, and SO42- ions and their influence on the zeta-potential of limestone during controlled-salinity waterflooding. Energy Fuels.. 2021;35:12982-12992.
- [CrossRef] [Google Scholar]
- The impact of salinity on the interfacial structuring of an aromatic acid at the calcite/brine interface: an atomistic view on low salinity effect. J. Phys. Chem. B. 2020;124:224-233.
- [CrossRef] [Google Scholar]
- Atomistic insight into salinity dependent preferential binding of polar aromatics to calcite/brine interface: implications to low salinity waterflooding. Sci. Rep.. 2021;11:11967.
- [CrossRef] [Google Scholar]
- Influence of divalent cations adsorption on the performance of electromembrane process. Desalin. Water Treat.. 2015;53:2760-2766.
- [CrossRef] [Google Scholar]
- Facile generation of highly durable thiol-functionalized polyhedral oligomeric silsesquioxane based superhydrophobic melamine foam. Front. Chem. Sci. Eng.. 2022;16:1247-1258.
- [CrossRef] [Google Scholar]
- Mechanism of wettability alteration of the calcite 1014 surface. PCCP. 2020;22:15365-15372.
- [CrossRef] [Google Scholar]
- Modeling of n-alkanes on calcite/dolomite by molecular dynamics simulations and first-principles calculations. Advanced Theory and Simulations.. 2021;4:2100226.
- [CrossRef] [Google Scholar]
- Wettability alteration by magnesium ion binding in heavy oil/brine/chemical/sand systems - nalysis of electrostatic forces. J. Pet. Sci. Eng.. 2007;59:147-156.
- [CrossRef] [Google Scholar]
- Studies on bitumen-silica interaction in aqueous solutions by atomic force microscopy. Langmuir. 2003;19:3911-3920.
- [CrossRef] [Google Scholar]
- Interaction forces in bitumen extraction from oil sands. J. Colloid Interface Sci.. 2005;287:507-520.
- [CrossRef] [Google Scholar]
- Bitumen-clay interactions in aqueous media studied by zeta potential distribution measurement. J. Colloid Interface Sci.. 2002;252:409-418.
- [CrossRef] [Google Scholar]
- Ion adsorption-induced wetting transition in oil-water-mineral systems. Sci. Rep.. 2015;5(1):10519.
- [CrossRef] [Google Scholar]
- Interactions of asphaltene subfractions in organic media of varying aromaticity. Energy Fuels. 2018;32:10478-10485.
- [CrossRef] [Google Scholar]
- Superhydrophobic, mechanically flexible and recyclable reduced graphene oxide wrapped sponge for highly efficient oil/water separation. Front. Chem. Sci. Eng.. 2018;12:390-399.
- [CrossRef] [Google Scholar]
- Effect of weathering on surface characteristics of solids and bitumen from oil sands. Energy Fuels.. 2009;23:334-341.
- [CrossRef] [Google Scholar]
- Recent insights from computational materials chemistry into interfaces relevant to enhanced oil recovery. Advanced Theory and Simulations.. 2019;2(4):1-6.
- [CrossRef] [Google Scholar]
- Preparation and properties of a silver particle-coated and 1-dodecanethiol-modified superhydrophobic melamine sponge for oil/water separation. Front. Chem. Sci. Eng.. 2022;16(8):1237-1246.
- [CrossRef] [Google Scholar]
- Molecular physics in ion-bridging effect for wettability alteration of rock surfaces. Chem. Phys. Lett.. 2021;763:138201
- [CrossRef] [Google Scholar]
- Surface reactivity analysis of the crude oil-brine-limestone interface for a comprehensive understanding of the low-salinity waterflooding mechanism. Energy Fuels.. 2020;34:2739-2756.
- [CrossRef] [Google Scholar]
- Study of the role of sodium citrate in bitumen liberation. Energy Fuels.. 2019;33:8271-8278.
- [CrossRef] [Google Scholar]
- Pore-scale simulation of water/oil displacement in a water-wet channel. Front. Chem. Sci. Eng.. 2019;13:803-814.
- [CrossRef] [Google Scholar]







