Translate this page into:
Inositol hexakisphosphate induces apoptosis, cell cycle arrest in non-Hodgkin’s Burkitt lymphoma cells and mediates anti-angiogenic, antitumor effects in T-cell lymphoma bearing Swiss albino mice
⁎Corresponding authors at: Department of Oncology, The Second People’s Hospital of Kunshan, Suzhou 215300, China (S. Yang). Nanosynthesis Unit, Nanome Consulting, Salem 636008, Tamil Nadu, India (J. Antony Jacob). joeantonyjacob@gmail.com (Joe Antony Jacob), joeantonyjacob@hotmail.com (Joe Antony Jacob), huanghuikk@163.com (Sufang Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cancer ranks top as one among the leading cause of non-infectious deaths in humans. Lymphoma occurs as an outcome of uncontrollable proliferation of immune cells. Dalton’s ascites lymphoma (DAL) is a well-established type of non-Hodgkin's T-cell lymphoma of murine models. Inositol hexakisphosphate (IP6) is a phosphate-storage antioxidant present in high volumes among cereals and legumes consumed as a human diet. Based on this background, in the present study, the cytotoxic effects of IP6 were studied in Raji cells, whereas, the antitumor and anti-angiogenic effects were tested in DAL-induced mice. IP6 possessed cytotoxic effects in vitro, induced apoptosis and cell cycle arrest at the G2/M phase in Raji cells. In vivo, eighteen swiss albino male mice were divided into three groups of six mice each. IP6 was effective in prevention of blood vessel formation. The tumor burden reduced considerably as evidenced by body weight and tumor volume. The hematological and biochemical parameters were revived to near-normal in the treatment groups. The antioxidant profile improved significantly after treatment. Reduction in lipid peroxidation and the levels of cellular metabolites indicate the potential of IP6 in the inhibition of DAL-induced intracellular oxidative stress. Altogether, IP6 possessed cytotoxic effects in vitro with anti-angiogenic and antitumor effects in vivo.
Keywords
Lymphoma
IP6
Cytotoxic
Anti-angiogenic
Antitumor
1 Introduction
Cancers are the second-most cause for deaths related to non-communicable diseases (Wu et al., 2020). In 2018, 9.6 million deaths were associated to cancer as demonstrated by the GLOBOCAN (Oh et al., 2020a). Lymphomas are cancers that arise due to uncontrolled proliferation of B and T lymphocytes classified by enlarged lymph nodes (Mugnaini and Ghosh, 2016, Matasar and Zelenetz, 2008). Deficiency of the immune system caused by viral infections is an established risk factor for lymphoma (Biggar et al., 2006). The components of microenvironment of tumors and a failure in immune surveillance can lead to the progression of blood-related malignancies such as lymphomas (Upadhyay et al., 2015, Álvaro et al., 2010). American Cancer Society evaluates the frequency of cancer prevalence, new cases, deaths and survival in the United States. The interrelated data derived from the North American Association of Central Cancer Registries indicated an expected 77,240 new cases and 20,140 deaths linked to non-Hodgkin’s T-cell lymphoma in United States for the year 2017 (Siegel et al., 2017). The same estimate in the United States predicts 80,470 new cases and 20,250 deaths for the year 2022 (Siegel et al., 2022). DAL is a type of non-Hodgkin's T-cell lymphoma of murine models transplanted for better understanding of treatment mechanisms and the discovery of novel drugs for neoplasms (Koiri et al., 2017, Kumari et al., 2017).
Although targeted immune system oriented chemotherapy and checkpoint inhibitors have revolutionized therapeutic approaches for lymphoma, the associated adverse effects remain a concern (Wang et al., 2020, Ayyappan and Maddocks, 2019). Identification of phytoconstituents for medical management and the elimination of diseases such as cancer has reached new heights because of the access to natural products in traditional and modern medicine (Yuan et al., 2016).
IP6 also known as phytic acid or phytate, is a phosphate-storage antioxidant found in high volumes among plants and lesser volumes in animal cells (Letcher et al., 2008, Graf et al., 1987). Humans have consumed cereals and legumes as a part of their diet from times unknown. Dietary phytic acids are present in high-fiber cereals and legumes, especially in oilseeds (ranging from 1 to 10 gm per 100 gm of dry weight). IP6 consumed as a dietary component assimilate into cells via several means including endocytosis. It is known to possess protective and therapeutic effects against cancer in murine models at a dose of 1–5 mM or in combination with 5-flourouracil (Schlemmer et al., 2009, Masunaga et al., 2019, Schröterová et al., 2010, Shamsuddin, 2002, Al-Fatlawi et al., 2014, Kaur et al., 2020). Intake of fibers rich in IP6 (1 gm per day) among western diets is usual. It is digested by microbial community of the gut through secretion of enzymes such as phytases and phosphatases. The intake of IP6 is inversely correlated to cancer as evidenced by human trials conducted at pilot scale. The mechanisms of action comprise cell cycle arrest, induction of apoptosis, inhibition of PI3K/Akt pathway, activation of Wnt/β-catenin pathway, antioxidant and anti-invasive properties (Bizzarri et al., 2016).
Based on the aforementioned background, we used IP6 as a therapeutic agent against Raji cells in vitro and DAL cells in Swiss albino male mice.
2 Materials and Method
2.1 Cell culture
Burkitt’s lymphoma cells Raji were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 50 IU/mL penicillin, and 50 mg/mL streptomycin in a humidified atmosphere of 5% CO2 at 37 °C.
2.2 Detection of apoptotic cell death by flow cytometry
All experiments were performed using cells after 24 hrs when the confluence of 2 × 106 cells/mL was reached in six-well plates. Later, varying concentrations of IP6 (1 μM, 3 μM and 5 μM) were loaded onto each well for 24, 48 and 72 hrs. After treatment, the medium was removed and the cells were washed with 1X PBS and trypsinated for 3–5 mins at 37 °C. Following this, the cells were centrifuged at 2000 rpm for 5 mins at 4 ℃, washed with 1X PBS and re-suspended in 100 μL/well of the 1X binding buffer. Annexin V-FITC (1 μL/well) and 1 μL of propidium iodide were added to stain the cells after which the fluorescence was analysed by flow cytometry (A00-1–1102, Beckman Coulter, USA).
2.3 Cell cycle analysis
After a confluence of 3 × 105 cells/mL was reached, varying concentrations of IP6 were added at varying time durations as mentioned earlier. The cells were washed with 1X PBS, trypsinated, centrifuged at 2000 rpm at 4 ℃ for 5 mins. After centrifugation, the pellet was re-suspended in 500 μL of 70% ice-cold ethanol and incubated for 2 hrs at 4 ℃. The cells were washed with PBS, re-suspended in 100 μL propidium iodide/RNaseA solution (cell cycle buffer). After an incubation of 30–60 mins, the cells were analysed by flow cytometry (A00-1–1102, Beckman Coulter, USA).
2.4 Institutional approval, animal maintenance and in vivo experiment
The animal study was approved by Institutional Animal Ethics Committee of Bharathidasan University and adhered to the CPCSEA regulations. Adult male Swiss albino mice (30–35 gm) were housed under controlled temperature and hygiene under sanitized conditions in polypropylene cages with sterile paddy husk in normal day and night (12-hour light: 12-hour dark) conditions. Standard laboratory diet (22% crude protein, 4.12% crude oil, 2.79% crude fiber, 7.81% ash and 1.34% sand silica) and water were used to feed the animals.
Eighteen mice were randomized into 3 groups of six animals each: normal, DAL induced and DAL induced mice treated with IP6 at a dose of 300 mg/kg. The animals injected with DAL cells, were kept as such for three days. Three days into the experimental period, the animals were administered with IP6 every day, intraperitoneally for a period of ten days. Thus, the total experimental period was 13 days. Body weight was measured and recorded from all animals every day from the start to the end of the experiment. The antitumor effects of IP6 were assessed after 10 days of treatment, following euthanasia, at the point considered to be the end of the experiment. Intraperitoneal ascites fluid was collected from the induced and treated groups at the end of the trial and the tumor volume was ascertained in tumor-induced and treated animals. The degree of blood vessel growth and formation was used to determine angiogenesis.
2.5 Trypan blue exclusion method for assessment of cytotoxicity in vitro
Sterile PBS was injected and DAL cells were collected into a Petri dish from the peritoneal region of tumor-bearing mice. The cells were incubated at 37 °C for 2 h, followed by aspiration performed to dislodge the non-adherent cells. The cell suspension was then treated with IP6 (0.5 and 1 mg/mL, which resembles 150 and 300 mg/kg BW in mice). Later, cell viability was determined using trypan blue staining and the treated cells were observed using a hemocytometer (Shrivastava and Ganesh, 2010, Sriram et al., 2010).
2.6 Analysis of in vivo parameters
Blood collected from the animals of each group was used to separate the serum by centrifuging at 2500 rpm for 15 min. The separated serum was used for biochemical, hematological and antioxidant assays. Assay of biochemical parameters include the analysis of serum glutamate oxalate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT) and alkaline phosphatase (ALP) levels. RBC, WBC and hemoglobin were the hematological parameters analysed. Serum levels of cellular metabolite lactate dehydrogenase (LDH), enzymatic antioxidant superoxide dismutase (SOD) and non-enzymatic antioxidant glutathione (GSH) were measured. Lipid peroxidation marker malondialdehyde (MDA) was measured using centrifugation of the supernatant collected from the liver homogenate at 12000 rpm for 30 min (Antony et al., 2013, Zhang et al., 2019).
2.7 Histopathology
Liver tissue was fixed in 10% formalin for further use. Paraffin sections of 5 µm thickness were prepared, stained with hematoxylin and eosin, observed under a microscope and photographed at 40X magnification (Ye et al., 2009).
2.8 Statistical Analysis
All data were expressed as mean ± SD. The statistical significance was assessed by one- way analysis of variance (ANOVA) using SPSS version 17 and the individual comparisons were obtained by Duncan’s multiple range test (DMRT). A value of p < 0.05 was considered to be statistically significant among the groups. Alike alphabets in lower-case indicate no significant change, however, unlike alphabets specify significant change between the groups (Zar, 1984).
3 Results and Discussion
3.1 IP6-induced apoptotic cell death in Raji Cells
Apoptosis is a normal mechanism for maintaining homeostasis in cells during development and aging. There are several stimuli that can lead to an apoptotic trigger. Although all these stimuli cannot lead to cell death, interaction with anticancer drugs can result in cell death through apoptosis in a p53 dependent mechanism (Elmore, 2007). The Annexin V-FITC/PI staining is an appropriate and subtle method to detect cellular apoptosis by detecting the stage of apoptosis (early or late) by the virtue of plasma or nuclear membranes (Rieger et al., 2011, Filograna et al., 2015).
In this study, the early apoptotic cells increased from 2.42% in control to 4.03% in cells treated with 1 µM IP6. This percentage increased considerably to 4.52% and 5.01% when treated with 3 µM and 5 µM IP6 after 24 hrs. The late apoptotic cells increased from 0.69% in control to 0.99% (1 µM), 1.03% (3 µM) and 1.11% (5 µM) in treated cells. After treatment of 72 hrs, the early apoptotic cells increased from 1.78% in control to 2.31% (1 µM), 2.88% (3 µM) and 3.03% (5 µM) in treated cells. The late apoptotic cells increased from 0.77% in control to 1.45% in 5 µM IP6 treated cells [Fig. 1]. These results indicate that apoptosis was effectively induced in Raji cells treated with IP6.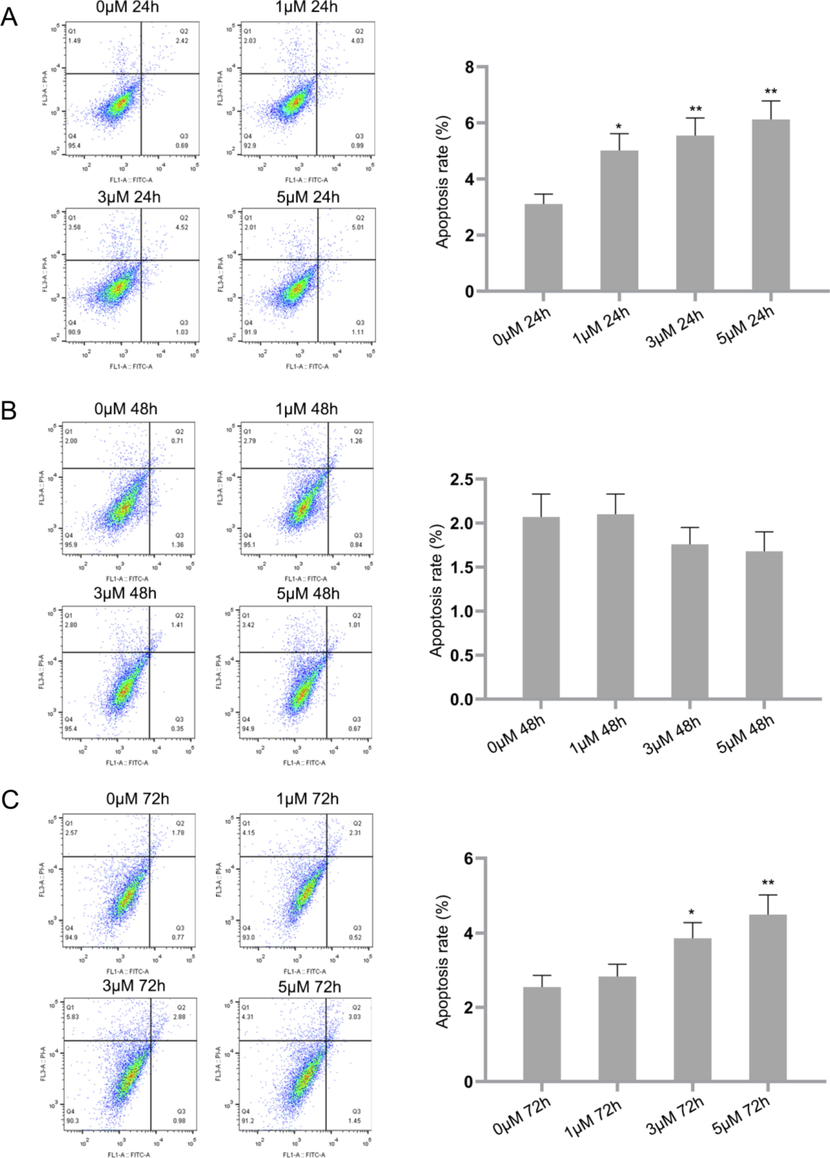
IP6 induces apoptosis in Raji cells as evidenced by Annexin V-FITC/PI staining using flow cytometry. P value of<0.05 (*P < 0.05, **P < 0.01, compared with the control group) are considered significant.
3.2 IP6-induced cell cycle arrest at G2/M phase
Cell cycle arrest and apoptosis occur as a result of the activation of p53 which may prevent tumor cell from developing (Chen, 2016). The cell cycle plays key roles in replication of a cell, cell death and its functioning (Ramadoss and Sivalingam, 2021). In the first phase called growth 1 phase or gap 1 phase (G1), the cells will produce new proteins and enlarge in size until it reaches a G1 checkpoint (Maira et al., 2007). The S phase of synthesis phase occurs between the G1 phase and G2 phase (Growth 2 or Gap 2 phase) where the DNA replication occurs for multiplying the genetic content (Fischer et al., 2018). The cells at G2 phase grow rapidly, synthesize proteins and prepares itself for mitotic phase (Li et al., 2015). Connecting to this, several cell types have modified the expressions of regulatory molecules such as cyclins and cyclin-dependent serine/threonine kinases (CDKs) to regulate the cell cycle. Novel anticancer agents are targeted towards these molecules for better management of diseases such as cancer. In this view, intervening or causing cell cycle arrest may be a promising approach for cancer therapy (Karthi et al., 2016, Guo et al., 2015). Analysis of cell cycle by flow cytometry can determine the phase of cell cycle in which the cells being studied are part of (Pozarowski and Darzynkiewicz, 2004).
Although a large population of cells were part of the G0/G1 phase, and the increase in percentage was time- and dose-dependent, the changes were not significant in comparison to the control. After 24 hrs of treatment with IP6, significant increase in occurrence of cells at G2/M phase was observed at doses of 3 µM and 5 µM in comparison to the control [Fig. 2A]. The percentage of cells at G2/M and S phases decreased with increasing doses of IP6 compared to the cells at G0/G1 phase. Yet, the increase in percentage of cells at G2/M phase was significant when compared to the percentage of control cells after 72 hrs [Fig. 2C]. This indicates that IP6 could induce cell cycle arrest at G2/M phase significantly in Raji cells after 72 hrs at all three doses tested.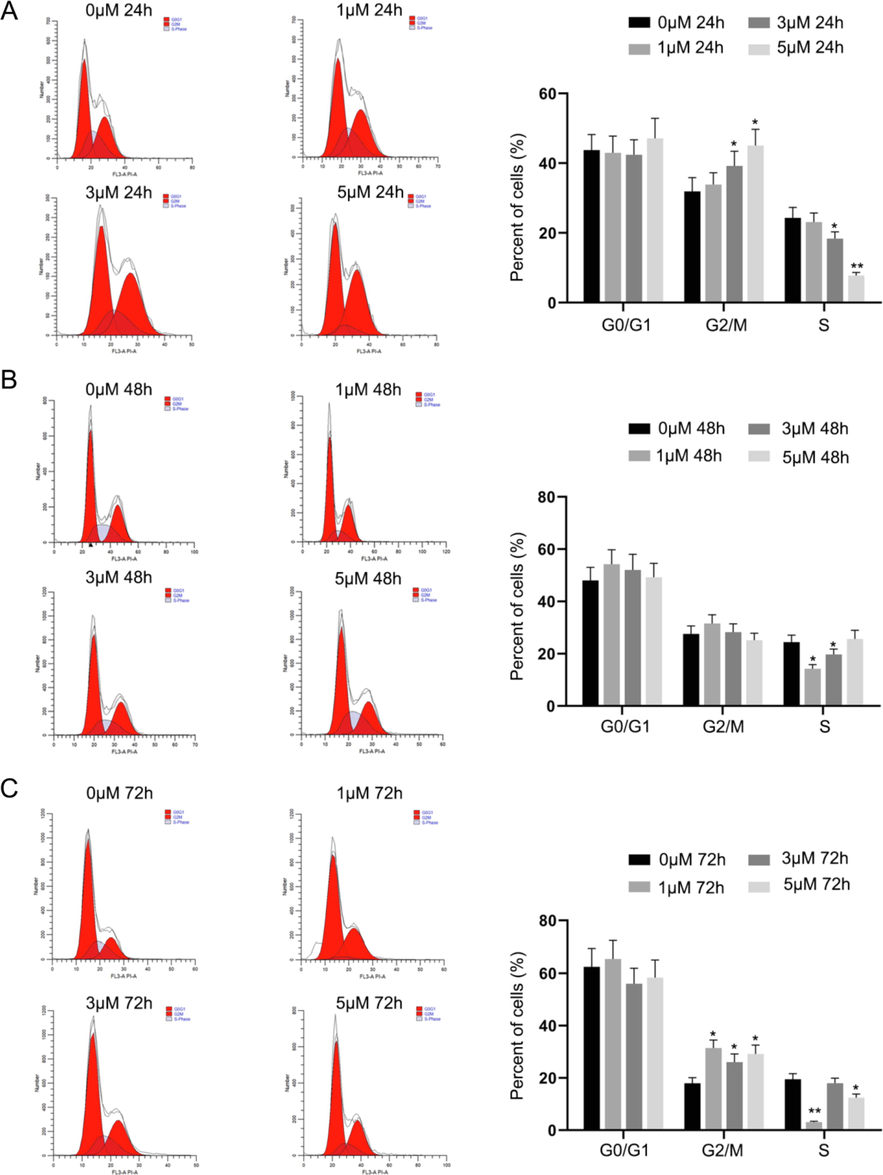
IP6 induced cell cycle arrest at G2/M phase in Raji cells as analysed by flow cytometry. P value of<0.05 (*P < 0.05, **P < 0.01, compared with the control group) are considered significant.
Drugs or compounds which can decrease the viability of neoplastic cells, induce cell cycle arrest and apoptosis in such cells are considered effective anticancer agents (Lee et al., 2020). Detection of apoptosis by Annexin V-FITC/PI assay and the cell cycle arrest at G2/M phase indicated that IP6 is an effective anti-neoplastic entity.
3.3 In vitro analysis of cell death by trypan blue staining
Thousands of ethnomedical plants are explored every year which are known to possess potent anticancer effects (Dai and Mumper, 2010). Cytotoxic agents isolated from these plants can both inhibit cell proliferation and result in cell lysis thereby forming the basis for treatment of malignancies (Sui et al., 2013, Seca and Pinto, 2018). Trypan blue staining is an exclusion technique in which dead cells are stained blue, whereas, the viable cells are not, because of their intact membranes. This happens as a consequence of apoptosis and loss of membrane integrity among dead cells (Khairunnisa and Karthik, 2014). According to the trypan blue staining, increased cell death was observed among DAL cells treated with 300 mg/kg of IP6. Therefore, 300 mg/kg of IP6 was used for in vivo experimentation [Fig. 3].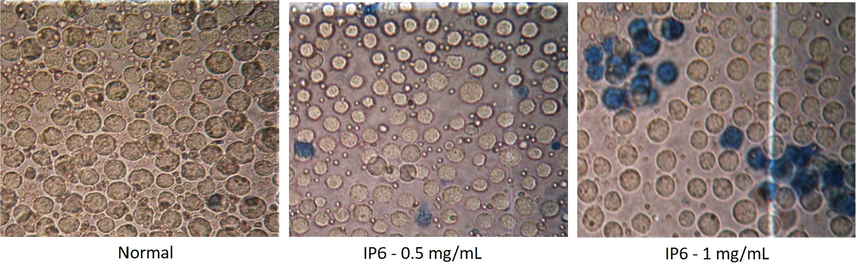
Cell viability assessment by trypan blue staining using various doses of IP6 at 40X magnification.
3.4 Tumor burden altered significantly after IP6 treatment
Changes were observed in body weights of treated and control animals. Body weights of IP6 treated mice decreased significantly in comparison to the DAL induced mice [Fig. 4A]. There was a reduction in tumor volume [Fig. 4B]. This reduction in body weight was linked to decrease in number of viable tumor cells and the volume of ascitic fluid, which is a necessity to achieve nutritional sustainability among tumor models. This is an indication of systemic or local cytotoxic effects (Dolai et al., 2012, Samudrala et al., 2015). A drop in tumor volume is a critical parameter for identification of an effective anticancer drug (Kathiriya et al., 2010).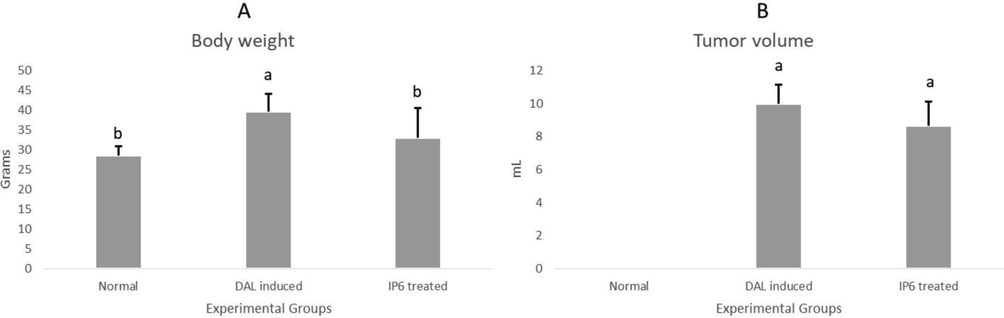
Tumor burden of the experimental animals before and after treatment with IP6 (a) changes in body weight (b) changes in tumor volume.
3.5 IP6 curbed angiogenesis in tumor-induced mice
The formation of new blood and lymphatic vessels, necessary for tumor metastasis, occur as a result of angiogenesis and lymphangiogenesis. This system supplies nutrients, oxygen, helps in removal of waste products and supports tumor cells to metastasize. When the vascular system does not give the cancer cells the provision of such supply, they die due to necrosis or apoptosis. Angiogenesis occurs in several stages among tumor cells that include membrane destruction and resultant hypoxia as the preliminary step. The next steps involve the migration, proliferation (once every 1000 days) and stabilization of endothelial cells that are activated by the influence of angiogenesis (Nishida et al., 2006, Yoo and Kwon, 2013, Rajabi and Mousa, 2017). Controlling or inhibiting angiogenesis is a key approach in cancer treatment (Lee et al., 2015). The identification of drugs or compounds with anti-angiogenic activity can help reduce the adverse effects of drugs intended to be used as antitumor medications (Abdallah et al., 2018). Post-dissection, the peritoneal region was observed for vessel growth, which indicated enlarged or increased growth of capillaries in DAL mice. The treatment with IP6 restricted the vessel growth in tumor induced mice. This indicates the anti-angiogenic effect of IP6 [Fig. 5].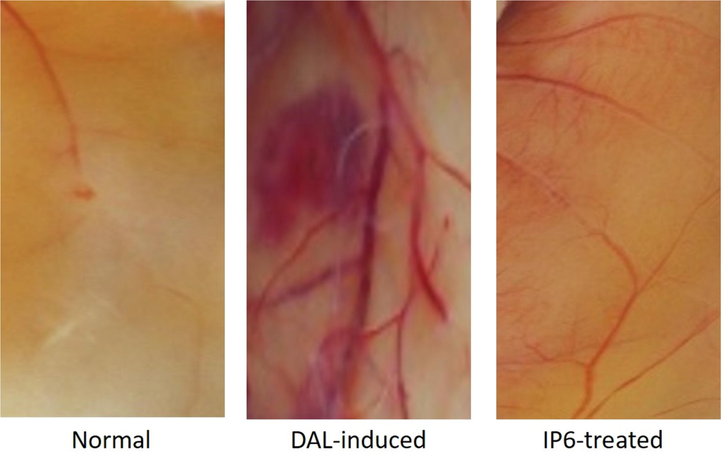
Anti-angiogenic effects as observed by changes in vessel growth of the peritoneal regions of normal, DAL-induced and IP6 treated mice.
3.6 Hematological changes were significant after IP6 treatment
Myelosuppression and anaemia, arise mutually as a result of reduction in RBC and percent hemoglobin due to deficiency of iron or other hemolytic conditions. These are major parameters to be addressed during cancer therapy and are prognostic factors for progression of lymphoma (Ghosh et al., 2011, Hasenclever et al., 1998, Knight et al., 2004). Leukocytosis is a determining factor for inflammation and is representative for oncogenesis and progression (Iida et al., 2012, Kleppe et al., 2018). Declined RBC and hemoglobin levels along with elevated WBC levels were observed in this study. This indicates that there was a significant therapeutic effect of IP6 on myelosuppression, anaemia and leukocytosis in DAL mice by creating a profile of typical hematology [Fig. 6].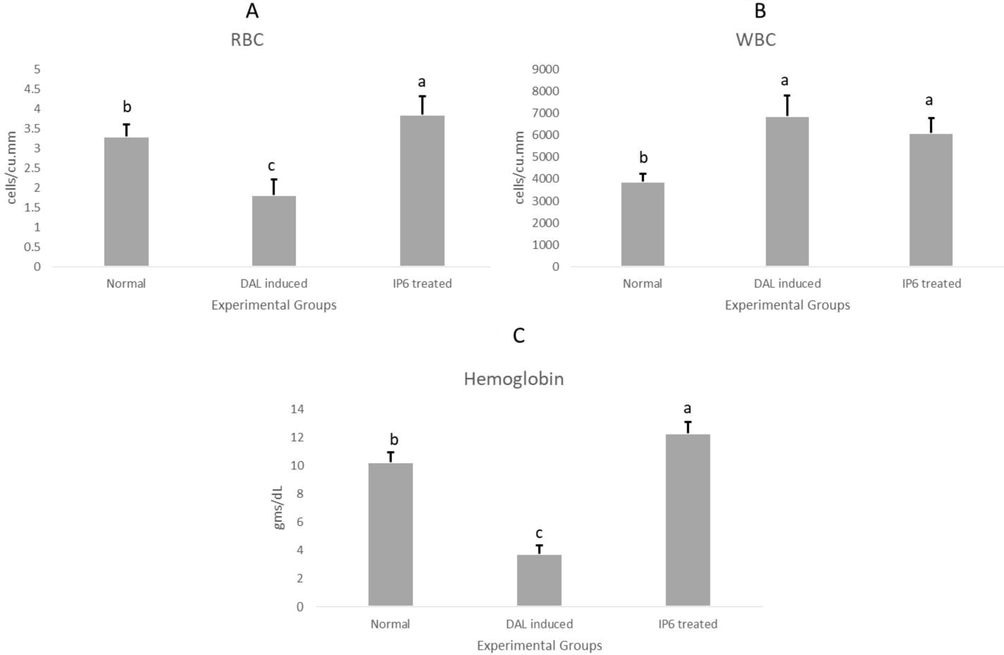
Changes in hematological parameters of the experimental animals before and after treatment with IP6 (a) RBC (b) WBC (c) Hemoglobin.
3.7 Biochemical parameters altered significantly after IP6 treatment
Although liver is far from tumor-affected peritoneum, it is affected by disorders such as cancer. Liver seems to be the primary site preferred for such metastatic changes (Seyfried and Huysentruyt, 2013). High levels of hepatic transaminases such as ALP, SGOT and SGPT are indicative of liver damage in such cases (Oh et al., 2020b, Zachariah et al., 2017, Haldar et al., 2010). The high levels of these transaminases in DAL mice reverted to near-normal in IP6 treated group [Table 1A].
Parameters
Normal mice
DAL-induced mice
IP6 treated mice
Biochemical parameters
1A. Liver function test
SGOT (IU/L)
258.92 ± 36.12 a
286.10 ± 16.26 a
243.72 ± 33.99 a
SGPT (IU/L)
51.24 ± 2.16b
60.66 ± 1.71 ab
75.84 ± 20.75 ab
ALP (IU/L)
77.90 ± 3.16b
91.78 ± 4.13 a
65.98 ± 17.41c
1B. Enzymatic antioxidant
SOD (IU/mg)
8.37 ± 0.34b
7.47 ± 0.19 a
7.76 ± 0.19b
1C. Non-enzymatic antioxidant
GSH (μmoles/gm)
5.82 ± 0.14b
5.07 ± 0.53b
13.57 ± 0.68 a
1D. Lipid peroxidation marker
MDA (nmoles/gm)
1.15 ± 0.11b
1.31 ± 0.07 a
1.08 ± 0.03b
1E. Cellular metabolite
LDH (IU/L)
1227.4 ± 118.22c
3722.2 ± 263.16 a
1638.78 ± 163.54b
Cancer cells can generate excessive levels of hydrogen peroxide and the resultant reactive oxygen species (ROS) which can cause mutations and cause injury to normal cells leading to oncogenesis (He et al., 2017, Han and Chen, 2013). Dietary antioxidants can convert these free radicals into wastes or by-products that are eventually released by the body (Esquivel-Chirino et al., 2013). SOD, is an enzymatic antioxidant which can catalyse the simultaneous oxidation and reduction of superoxide anion to hydrogen peroxide. This peroxide is later detoxified into oxygen and water by enzymes and other components of antioxidant system (Asakura and Kitahora, 2018). GSH, a non-enzymatic antioxidant can play dual roles in cancer. On the positive note, it can scavenge free radicals and aid as an adjuvant for enzymes involved in detoxification of carcinogens (Balendiran et al., 2004, Sznarkowska et al., 2017). Therefore, a failure in antioxidant defence can lead to redox imbalance that cancer cells relish on (Snezhkina et al., 2019). The failure in antioxidant defence observed among DAL mice was reformed to normalcy in IP6 treated group [Table 1B and 1C].
MDA is an end-product of oxidative degeneration which can result in the release of toxic substances such as hydroperoxides through lipid peroxidation (Helbock et al., 1993). Treatment with IP6 reduced the MDA levels significantly which signifies the reduction in lipid peroxidation of mice induced with lymphoma [Table 1D]. LDH is a standard diagnostic marker for violent forms of cancers such as lymphoma, which indicates the intracellular metabolism, mode of glycolysis and neoplastic conversion. It is generally augmented in cancer patients and protects tumor cells from hypoxia‐induced necrosis [Table 1E] (Forkasiewicz et al., 2020, Jurisic et al., 2015, Feng et al., 2018). Reduction in lipid peroxidation and an increase in antioxidant system that possess SOD and GSH shows the potential of IP6 in inhibition of DAL-induced intracellular oxidative stress that occurred as a result of damage induced by free radicals (Thavamani et al., 2014).
3.8 Significant histological changes after IP6 treatment
The cells of livers of normal mice showed well-defined cytoplasm, prominent nucleus and distinct central vein. Centrilobular hepatic necrosis, vacuolization, disintegrated cell membranes and disintegrated central vein with dilated sinusoids were the damages observed in the livers of DAL induced mice. The damages observed in architecture of livers of DAL mice were revived to normalcy in mice treated with IP6 [Fig. 7]. This elucidates the protective effects of IP6 on liver after tumor induction.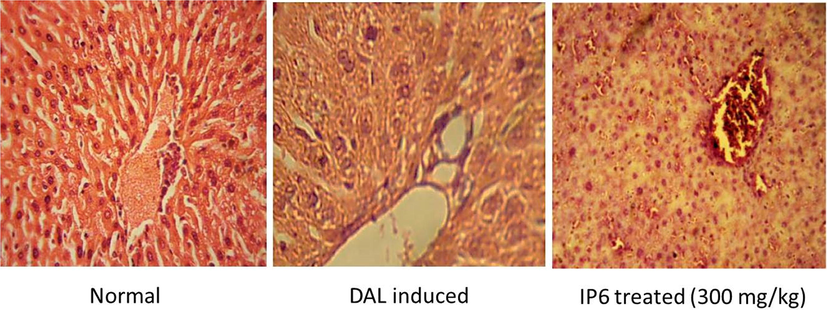
Histopathological examinations of livers of normal, DAL-induced and IP6 treated mice at 40X magnification.
4 Conclusions
Based on the above-mentioned facts and results discussed, we report the cytotoxic effects in vitro of IP6 in addition to anti-angiogenic and antitumor effects in DAL mice in vivo. This is a first-ever report according to the knowledge of the authors on antitumor effect of IP6 in lymphoma-induced mice model. According to the study, IP6 possessed cytotoxic effects in vitro, induced apoptosis and cell cycle arrest in Raji cells. In vivo, treatment with IP6 resulted in DAL-cell death as per trypan blue staining. Blood vessel growth was reduced in tumor induced mice after IP6 administration indicating anti-angiogenic effects. The physical observation and the analyses of hematological and biochemical parameters indicate the positive effects of IP6 in tumor mice. The antioxidant system was significantly revived. To conclude, IP6 induced apoptosis and cell cycle arrest in Raji cells in vitro. Treatment with IP6 reduced the tumor burden and its severity. In addition, IP6 possessed anti-angiogenic and antitumor properties in vivo. Hence, further studies are warranted on the mechanism of such effects to use IP6 for clinical applications in cancer management.
Acknowledgements
This work was supported by Kunshan Science and Technology Special Project (No. KS1905). We thank Nanome Consulting for their valuable contribution in preparation of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- Anti-angiogenic activity of Middle East medicinal plants of the Lamiaceae family. Mol. Med. Rep.. 2018;18:2441-2448.
- [Google Scholar]
- Anticarcinogenic activity of rice bran phytic acid against human breast cancer cell line (MCF-7) Asian J Pharm Clin Res. 2014;7:151-155.
- [Google Scholar]
- ÁLVARO, T., DE LA CRUZ-MERINO, L., HENAO-CARRASCO, F., VILLAR RODRÍGUEZ, J. L., VICENTE BAZ, D., CODES MANUEL DE VILLENA, M. & PROVENCIO, M. 2010. Tumor Microenvironment and Immune Effects of Antineoplastic Therapy in Lymphoproliferative Syndromes. J. Biomed. Biotechnol. 2010, 846872.
- In vivo antitumor activity of biosynthesized silver nanoparticles using Ficus religiosa as a nanofactory in DAL induced mice model. Colloids Surf., B. 2013;108:185-190.
- [Google Scholar]
- Antioxidants and polyphenols in inflammatory bowel disease: ulcerative colitis and Crohn disease. Polyphenols: Prevention and treatment of human disease. Elsevier; 2018.
- Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786-3791.
- [Google Scholar]
- Broad Spectrum Anticancer Activity of Myo-Inositol and Inositol Hexakisphosphate. Int. J. Endocrinol.. 2016;2016:5616807.
- [Google Scholar]
- CHEN, J. 2016. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harbor perspectives in medicine, 6, a026104–a026104.
- Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313-7352.
- [Google Scholar]
- Evaluation of antitumor activity and in vivo antioxidant status of Anthocephalus cadamba on Ehrlich ascites carcinoma treated mice. J. Ethnopharmacol.. 2012;142:865-870.
- [Google Scholar]
- Elmore, S. 2007. Apoptosis: a review of programmed cell death. Toxicologic Pathol. 35, 495–516.
- Inflammatory environmental, oxidative stress in tumoral progression. Inflamm. Environ. Oxidative Stress Tumoral Progress 2013:187-208.
- [Google Scholar]
- Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med.. 2018;7:6124-6136.
- [Google Scholar]
- Analysis of the catecholaminergic phenotype in human SH-SY5Y and BE (2)-M17 neuroblastoma cell lines upon differentiation. PLoS ONE. 2015;10:e0136769
- [Google Scholar]
- FISCHER, M., DANG, C. V. & DECAPRIO, J. A. 2018. Chapter 17 - Control of Cell Division. In: HOFFMAN, R., BENZ, E. J., SILBERSTEIN, L. E., HESLOP, H. E., WEITZ, J. I., ANASTASI, J., SALAMA, M. E. & ABUTALIB, S. A. (eds.) Hematology (Seventh Edition). Elsevier.
- The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell. Mol. Biol. Lett.. 2020;25:35.
- [Google Scholar]
- Evaluation of antitumor activity of stigmasterol, a constituent isolated from Bacopa monnieri Linn aerial parts against Ehrlich Ascites Carcinoma in mice. Oriental Pharm. Experim. Med.. 2011;11:41-49.
- [Google Scholar]
- Effects of karanjin on cell cycle arrest and apoptosis in human A549, HepG2 and HL-60 cancer cells. Biol. Res.. 2015;48:1-7.
- [Google Scholar]
- Antitumor activity of Sansevieria roxburghiana rhizome against Ehrlich ascites carcinoma in mice. Pharm. Biol.. 2010;48:1337-1343.
- [Google Scholar]
- Oxidative Stress Induces Mitochondrial DNA Damage and Cytotoxicity through Independent Mechanisms in Human Cancer Cells. Biomed Res. Int.. 2013;2013:825065
- [Google Scholar]
- A prognostic score for advanced Hodgkin's disease. N. Engl. J. Med.. 1998;339:1506-1514.
- [Google Scholar]
- Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem.. 2017;44:532-553.
- [Google Scholar]
- Toxic hydroperoxides in intravenous lipid emulsions used in preterm infants. Pediatrics. 1993;91:83-87.
- [Google Scholar]
- White blood cell count and risk of gastric cancer incidence in a general Japanese population: the Hisayama study. Am. J. Epidemiol.. 2012;175:504-510.
- [Google Scholar]
- The Actual Role of LDH as Tumor Marker, Biochemical and Clinical Aspects. Adv. Exp. Med. Biol.. 2015;867:115-124.
- [Google Scholar]
- Pelargonidin induces apoptosis and cell cycle arrest via a mitochondria mediated intrinsic apoptotic pathway in HT29 cells. RSC Adv.. 2016;6:45064-45076.
- [Google Scholar]
- KATHIRIYA, A., DAS, K., KUMAR, E. & MATHAI, K. 2010. Evaluation of antitumor and antioxidant activity of Oxalis corniculata Linn. against Ehrlich ascites carcinoma on mice.
- KAUR, V., GOYAL, A. K., GHOSH, G., CHANDRA SI, S. & RATH, G. 2020. Development and characterization of pellets for targeted delivery of 5-fluorouracil and phytic acid for treatment of colon cancer in Wistar rat. Heliyon, 6, e03125-e03125.
- Evaluation of in-vitro apoptosis induction, cytotoxic activity of Hymenodictyon excelsum (Roxb) Wall in Dalton’s lymphoma ascites (DLA) and Lung fibroblast-Mouse L929 cell lines. JAPS. 2014;4:11-17.
- [Google Scholar]
- KLEPPE, M., KOCHE, R., ZOU, L., VAN GALEN, P., HILL, C. E., DONG, L., DE GROOTE, S., PAPALEXI, E., HANASOGE SOMASUNDARA, A. V., CORDNER, K., KELLER, M., FARNOUD, N., MEDINA, J., MCGOVERN, E., REYES, J., ROBERTS, J., WITKIN, M., RAPAPORT, F., TERUYA-FELDSTEIN, J., QI, J., RAMPAL, R., BERNSTEIN, B. E., BRADNER, J. E. & LEVINE, R. L. 2018. Dual Targeting of Oncogenic Activation and Inflammatory Signaling Increases Therapeutic Efficacy in Myeloproliferative Neoplasms. Cancer Cell, 33, 29-43.e7.
- Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am. J. Med.. 2004;116:11-26.
- [Google Scholar]
- Dalton’s lymphoma as a murine model for understanding the progression and development of T-Cell lymphoma and its role in drug discovery. Int. J. Immunother. Cancer Res. 2017;3:001-006.
- [Google Scholar]
- Amelioration of Dalton’s lymphoma–induced angiogenesis by melatonin. Tumor Biol.. 2017;39 1010428317705758
- [Google Scholar]
- Anti-angiogenic effect of Nelumbo nucifera leaf extracts in human umbilical vein endothelial cells with antioxidant potential. PLoS ONE. 2015;10:e0118552
- [Google Scholar]
- Synthesis and evaluation of novel anticancer compounds derived from the natural product Brevilin A. ACS Omega. 2020;5:14586-14596.
- [Google Scholar]
- Do mammals make all their own inositol hexakisphosphate? Biochem. J.. 2008;416:263-270.
- [Google Scholar]
- LI, Y., BARBASH, O. & DIEHL, J. A. 2015. 11 - Regulation of the Cell Cycle. In: MENDELSOHN, J., GRAY, J. W., HOWLEY, P. M., ISRAEL, M. A. & THOMPSON, C. B. (eds.) The Molecular Basis of Cancer (Fourth Edition). Philadelphia: W.B. Saunders.
- MAIRA, M. S., PEARSON, M. A., FABBRO, D. & GARCÍA-ECHEVERRÍA, C. 2007. 7.01 - Cancer Biology. In: TAYLOR, J. B. & TRIGGLE, D. J. (eds.) Comprehensive Medicinal Chemistry II. Oxford: Elsevier.
- Anti-cancer activity of the cell membrane-permeable phytic acid prodrug. Bioorg. Chem.. 2019;92:103240
- [Google Scholar]
- Overview of lymphoma diagnosis and management. Radiol. Clin. North Am.. 2008;46(175–98):vii.
- [Google Scholar]
- Causes of death among cancer patients in the era of cancer survivorship in Korea: Attention to the suicide and cardiovascular mortality. Cancer Med.. 2020;9:1741-1752.
- [Google Scholar]
- Prognostic impact of the combination of serum transaminase and alkaline phosphatase determined in the emergency room in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. PLoS ONE. 2020;15:e0233286.
- [Google Scholar]
- Vanillin extracted from proso and barnyard millets induces cell cycle inhibition and apoptotic cell death in MCF-7 cell line. J. Cancer Res. Ther.. 2021;17:1425.
- [Google Scholar]
- Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J. Visualized Experim.. 2011;JoVE:2597.
- [Google Scholar]
- Evaluation of antitumor activity and antioxidant status of Alternanthera brasiliana against Ehrlich ascites carcinoma in Swiss albino mice. Pharmacognosy Res.. 2015;7:66-73.
- [Google Scholar]
- Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res.. 2009;53:S330-S375.
- [Google Scholar]
- Effect of phytic acid and inositol on the proliferation and apoptosis of cells derived from colorectal carcinoma. Oncol. Rep.. 2010;23:787-793.
- [Google Scholar]
- Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci.. 2018;19:263.
- [Google Scholar]
- SHAMSUDDIN, A. M. 2002. Anti‐cancer function of phytic acid. Int. J. Food Sci. Technol. 37, 769–782.
- Tumor inhibition and Cytotoxicity assay by aqueous extract of onion (Allium cepa) & Garlic (Allium sativum): an in-vitro analysis. Int. J. Phytomed.. 2010;2
- [Google Scholar]
- SIEGEL, R. L., MILLER, K. D., FUCHS, H. E. & JEMAL, A. 2022. Cancer statistics, 2022. CA: A Cancer Journal for Clinicians, 72, 7-33.
- SIEGEL, R. L., MILLER, K. D. & JEMAL, A. 2017. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians, 67, 7-30.
- ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longevity. 2019;2019:6175804.
- [Google Scholar]
- Antitumor activity of silver nanoparticles in Dalton's lymphoma ascites tumor model. Int. J. Nanomed.. 2010;5:753-762.
- [Google Scholar]
- Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis.. 2013;4:e838.
- [Google Scholar]
- Inhibition of cancer antioxidant defense by natural compounds. Oncotarget. 2017;8:15996-16016.
- [Google Scholar]
- Anticancer activity of cissampelos pareira against dalton's lymphoma ascites bearing mice. Pharmacognosy magazine. 2014;10:200-206.
- [Google Scholar]
- Advances in targeted therapy for malignant lymphoma. Signal Trans. Targeted Therapy. 2020;5:15.
- [Google Scholar]
- Effects of herbal and mushroom formulations used in Traditional Chinese Medicine on in vitro human cancer cell lines at the preclinical level: an empirical review of the cell killing mechanisms. Process Biochemistry 2020
- [Google Scholar]
- Hepatoprotective effects of Coptidis rhizoma aqueous extract on carbon tetrachloride-induced acute liver hepatotoxicity in rats. J. Ethnopharmacol.. 2009;124:130-136.
- [Google Scholar]
- Angiogenesis and Its Therapeutic Opportunities. Mediators Inflamm.. 2013;2013:127170
- [Google Scholar]
- The Traditional Medicine and Modern Medicine from Natural Products. Molecules (Basel, Switzerland). 2016;21:559.
- [Google Scholar]
- Hepatic transaminases as predictors of liver injury in abdominal trauma. Int. Surg. J.. 2017;5:181-186.
- [Google Scholar]
- ZAR, J. 1984. Biostatistical Analysis–2nd ed. Prentice‐Hall, Englewood‐Cliffs, New Jersey.
- Hepatoprotective effect of silver nanoparticles synthesized using aqueous leaf extract of Rhizophora apiculata. Int. J. Nanomed.. 2019;14:3517.
- [Google Scholar]







