Translate this page into:
Interaction of canadine with thymidylate synthase mediated its promising anticancer activity on MCF-7 human breast cancer cells
* Corresponding authors: E-mail addresses: qianlima-111@163.com (Y. Wang), baizhoulan@outlook.com (Z. Bai)
-
Received: ,
Accepted: ,
Abstract
In this study, the cytotoxic effects of canadine (xanthopuccine or tetrahydroberberine), a benzylisoquinoline alkaloid with a molecular formula of C20H21NO4, on the proliferation of Michigan Cancer Foundation-7 (MCF-7) human breast cancer cells and MCF-10 human normal mammary epithelial cells were assessed through 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Quantitative real-time PCR (qPCR), Quantitative real-time PCR ROS, Catalase (CAT), Superoxide dismutase (SOD), Glutathione peroxidase (GPx) and glutathione (GSH) content assays. The expression and activity of thymidylate synthase (TS) in canadine-treated MCF-7 cells were also explored using quantitative polymerase chain reaction (qPCR) and enzyme-linked immunosorbent assay (ELISA). Furthermore, the interaction of canadine with TS was investigated through spectroscopy and molecular docking. The results indicated that the IC50 concentrations of canadine were 17.50 μM in MCF-7 cells and >40 μM in MCF-10 cells. Colony-forming rates decreased to 95.23%, 80.95%, 71.42%, 47.61%, 47.69%, and 38.09% following the addition of canadine at the concentrations of 1, 5, 10, 20, 30, and 40 µM, respectively. It was also found that canadine induced cell-cycle arrest through the upregulation of p53 and p21 mRNA and apoptosis via the upregulation of the Bcl-2-associated X protein/ B-cell lymphoma 2 (BAX/BCL-2) ratio. Additionally, the data revealed that canadine-induced oxidative stress-mediated apoptosis through the upregulation of reactive oxygen species (ROS), downregulation of enzymatic and non-enzymatic mediators, Matrix Metalloproteinases (MMP) collapse, and cytochrome c release, all of which were modulated by the co-treatment of the cells with n-Acetylcysteine (NAC), a potential antioxidant. Moreover, canadine was found to downregulate Thymidylate synthase (TS) expression and activity, which was further evaluated by the Thymidylate synthase, small interfering RNA (TS siRNA) assay. The theoretical data indicated that there is a potential interaction (-6.80 kcal/mol) between canadine and the CYS195 residue in the active site of the TS, mediated by conventional hydrogen bonding, alkyl, and π-alkyl forces. Fluorescence spectroscopy measurements demonstrated TS’s interaction with canadine, leading to the formation of a static complex governed by hydrophobic forces. Calculations for thermodynamic and binding parameters showed that logKa, ΔH°, ΔS°, and ΔG° values were 5.11 ± 0.21, 66.46 ± 3.61 kJ/mol, 324.43 ± 16.73 J/mol K, and -29.24 ± 1.38, respectively. Spectroscopy measurements indicated substantial secondary and tertiary conformational alterations of the TS upon binding with canadine. These data may provide a new perspective on canadine as a potential anticancer molecule.
Keywords
Apoptosis
Breast cancer
Canadine
Spectroscopy
Thermodynamic
Thymidylate synthase
1. Introduction
Breast cancer is the most common neoplasm found in women worldwide, accounting for most cancer-related deaths among them. Unlike Western nations, China lacks a complete understanding of breast cancer, despite 5-year prevalence statistics indicating that the country accounts for 11% of cases globally, with a rapid increase in incidence over the last few years [1].
Despite utilizing various conventional strategies for managing breast cancer [2,3], addressing heterogeneity and cancer recurrence has presented unwanted challenges in inhibiting breast cancer proliferation and invasion [4].
Naturally based products derived from plants have been a source for developing several anticancer agents due to their significant structural diversity and functionality [5]. Indeed, plant-derived compounds have been used to develop potential anticancer drugs to mitigate the proliferation of cancer cells through the inhibition of histone deacetylases, microtubule disruption, apoptosis induction, metabolic re-modulation, histone and DNA modification, as well as anti-angiogenesis and anti-metastasis effects [6,5].
Benzylisoquinoline alkaloids have been widely used in the development of potential anticancer agents. For example, Sung et al. showed that noscapine, a benzylisoquinoline alkaloid, is able to increase the sensitization of leukemic cells to classical chemotherapeutic drugs and cytokines through regulation of the NF-κB signaling pathway [7]. Inoue et al. reported that benzylisoquinoline alkaloids, berberine, and coptisine, could serve as anticancer agents and modulate drug-resistant topoisomerase I mutants [8]. Kazemi Noureini et al. also proposed that benzylisoquinoline alkaloids might result in telomere shortening in michigan cancer foundation-7 (MCF-7) breast cancer cells [9]. Ren et al. also indicated that benzylisoquinoline alkaloids prohibit cell activation mediated by blocking TGF-β1/Smads and ERK1/2–related pathways [10].
Canadine (xanthopuccine or tetrahydroberberine) (C20H21NO4), is found in numerous plants of the Papaveraceae family and is a benzylisoquinoline alkaloid belonging to the protoberberine subgroup based on structural similarity [11]. Recently, Ma et al. displayed that canadine might inhibit the growth and invasion of cervical cancer associated with inhibiting MAGEA3 [12].
Thymidylate synthase (TS, a 70 kDa homodimeric enzyme) is known as a potential enzyme in the catalysis of a reaction that leads to the synthesis of 2′- desossithymidine-5′-monophosphate (dTMP), an important precursor for DNA replication. As a result, this enzyme has been shown to serve as a key target in the advancement of cancer chemotherapy [13-15]. The active conformation of the TS has been shown to be similar to the structure presented in all non-human Thymidylate synthase (TSs), with the side chain of CYS195 located inside the enzyme’s active site, where this amino acid residue plays an important role in catalytic activity. In the inactive form of the enzyme, the 181-197 loop in the monomer twists, leading to a relocation of the catalytic C195 amino acid residue towards the interface between the two monomers [16]. Based on these findings, any structural changes or ligand interactions with CYS195 in the active site may result in TS inactivation, which could be a potential cancer therapy strategy.
Consequently, the goal of the present investigation is to explore the anticancer mechanism of canadine on MCF-7 breast cancer cells by targeting TS and examining its expression, activity, and structural changes.
2. Materials and Methods
2.1 Cell and reagent
Human thymidylate synthase protein expression was conducted based on a previous study [17]. Canadine (Cat. No.: HY-N0925, Purity: 99.65%) was purchased from MedChemExpress LLC (NJ 08852, USA) and dissolved in Dimethyl sulfoxide (DMSO). The human breast cancer cell line, MCF-7, was purchased from Genechem. Co. Ltd. (Shanghai, China). The human normal mammary epithelial cell line, MCF-10A, was purchased from the Shanghai Institute of Cell Biology (Shanghai, China). Fetal bovine serum (FBS), Dulbecco’s Modified Eagle Medium (DMEM), DMEM/Nutrient mixture F12 (DMEM/F12), Rhodamine-123 (Rh-123), and penicillin-streptomycin were obtained from Gibco, USA. 1-Anilino-8-naphthalene sulfonate (ANS) anion, insulin, NAC (N-acetylcysteine), dichloro-dihydro-fluorescein diacetate (DCFH-DA), epidermal growth factor, and hydrocortisone were purchased from Sigma-Aldrich.
2.2. Cell culture
MCF-7 cells were cultured in DMEM medium with 1% penicillin/streptomycin, 1% l-glutamine, and 10% FBS. MCF-10 cells were cultured in DMEM-F12 medium containing hydrocortisone (0.05 mg/mL), insulin (0.01 mg/mL), and epidermal growth factor (0.02 mg/mL). Both cell lines were then incubated at 37°C with 5% CO2.
2.3. Cell viability assay
Cell viability was assessed using an 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di- phenytetrazoliumromide (MTT) assay. Briefly, cells (5 × 103 cells/well) seeded in 96-well plates and incubated overnight were treated with fresh medium supplemented with canadine at the indicated concentrations of 1 to 40 µM. After 24 hrs, MTT solution was added, the cells were incubated at 37°C for 4 hrs, and then MTT was replaced with DMSO. After 15 mins, the absorbance of the samples was read at 570 nm using a microplate reader (Bio-Tek).
2.4. Colony formation assay
The colony formation assay was done based on the method reported previously [18]. Briefly, cells (500 cells/well) seeded overnight were exposed to canadine at the indicated concentrations of 1 to 40 µM for 14 days. Then, the culture medium was removed, the plates were washed twice with PBS, and stained with crystal violet for 15 mins. The number of colonies were counted, and plating efficiency (%) was determined.
2.5. Cell cycle analysis
The quantification of cell-cycle arrest was determined by flow cytometry using propidium iodide (PI) staining [19]. Briefly, 24 hrs after canadine treatment, cells (1 × 106) were harvested, washed twice with PBS, resuspended in 70% ethanol, and fixed (overnight at 4°C). The cells were then washed with PBS and incubated with RNase A and PI at 37°C for 30 mins in the dark. The analysis was conducted using a flow cytometer (BD Biosciences, San Diego, CA, USA).
2.6. Detection of mitochondrial membrane potential (MMP)
The MMP dye, Rh-123, was used to quantify the decrease in MMP in MCF-7 cells treated with canadine, as described previously [20]. Briefly, cells seeded and treated with canadine for 24 hrs were washed twice with PBS and stained with 1 µM Rh-123 for 30 mins at ambient temperature in the dark. The fluorescence intensity of the samples was then measured with excitation and emission wavelengths of 488 and 530 nm, respectively, on a fluorescence microplate spectrometer (LS55, PerkinElmer, USA)
2.7. Measurement of reactive oxygen species (ROS) generation
Following treatment with canadine for 24 hrs, cells were stained with 2’,7’-Dichlorodihydrofluorescein diacetate (DCFH-DA) at a concentration of 10 μM and incubated for 30 mins in the dark. The fluorescence intensity of the cells was read at an emission/excitation wavelength of 485/535 nm using a fluorescence microplate spectrometer (LS55, PerkinElmer, USA).
2.8. Detection of oxidative stress markers
MCF-7 cells (1 x 106) seeded in T-25 flasks and allowed to attach for 24 hrs were then treated with canadine at a final concentration of 17.5 μM (IC50 concentration) for 24 hrs. The cells were then washed twice with PBS, detached, lysed with a sonicator, and centrifuged (10,000 rpm, 10 mins, 4°C). The supernatants were removed, and the protein concertation was determined using a Bradford assay kit (Sigma). Finally, a fixed amount of protein was used for superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and total glutathione (GSH) assays using relevant Cayman Chemicals kits (Ann Arbor, MI, USA).
2.9. TS enzyme activity assay
The TS enzyme activity assay was performed with a human TS enzyme-linked immunosorben assay (ELISA) Kit (Lanpai, Shanghai, China) based on the manufacturer’s instructions using the culture supernatant. The TS enzyme activity was reported relative to the negative untreated sample.
2.10. Transient transfection
The cells were transfected with small interfering RNA (siRNA) against TS using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions and a previous study [21].
2.11. Real-time quantitative PCR (RTqPCR)
Following treatment with canadine for 24 hrs, total RNA was extracted from MCF-7 cells using TRIzol reagent (Invitrogen), and reverse transcription was carried out with a two-step reverse-transcription reaction kit (Takara, Dalian, China). The RT-qPCR was conducted using an Applied Biosystems 7500 Real-time PCR system with a SYBR Green I Premix Ex Taq kit (Takara) in a final volume of 25 μL. The primers were obtained from Sangon Biotech (Shanghai, China), and their sequences were reported previously [22-24]. The thermal cycling conditions were done based on the previous report [22]. The level of gene expression was quantified relative to the level of GAPDH mRNA using the – ΔΔCT method.
2.12. ELISA analysis
Cells were washed with PBS, lysed with lysis-M buffer (Roche, IN, USA), and centrifuged (20,800 ×g for 10 mins at 4°C), and the supernatant was collected. Protein concentration determination was performed using the Bradford assay. The levels of proteins were quantified by ELISA according to the instructions provided by the manufacturer for the Human Cytochrome C ELISA Kit (ab221832) and the Human Thymidylate Synthase ELISA Kit (ab288923).
2.13. Molecular docking study
The 3D conformer of canadine was obtained from PubChem (CID: 34458). The 3D crystal structure of the TS protein was retrieved from the Protein Data Bank (PDB ID: 6QXG). Charge and hydrogen atom additions, along with water molecule removal, were performed on the protein structure. The AutoDock software was used for the blind molecular docking process. To ensure the accuracy of molecular docking, the exhaustiveness and energy range were set at 8 and 3, respectively. Default values were used for the rest of the parameters. The grid size was fixed at 60 x 50 x 60 Å along x, y, and z axes with 1 Å grid spacing.
2.14. Intrinsic and ANS fluorescence spectroscopy measurements
The fluorescence spectroscopy measurements were done using a fixed TS concentration (1 µM) after interaction with various concentrations of canadine (1–16 µM) on an LS50B Spectrometer (Perkin-Elmer). Excitation was set at 295 nm to explore the effect of canadine on the tryptophan microenvironment [25]. The emission wavelengths were read from 300 nm to 450 nm at five temperatures (ranging from 295 to 315 K). The Ex/Em slit widths were fixed at 10 nm in all measurements. The fluorescence spectroscopy measurements were performed 2 mins after ligand titration at a physiological pH of 7.4. The 1-Anilino-8-Naphthalene Sulfonate (ANS) fluorescence intensity was measured to study the structural changes of the TS. For this purpose, a fixed TS concentration (1 µM) was first combined with 50 µM ANS. Then, the solution was titrated with various concentrations of canadine (1–16 µM). In addition, the TS or TS-canadine complex (with a canadine concentration of 16 µM and ANS concentration of 50 µM) were heated for 15 mins at 60°C and then the ANS fluorescence spectra were recorded. Excitation was set at 360 nm, and the emission wavelengths were read from 400 nm to 600 nm at room temperature.
The fluorescence spectra were corrected against the tris-HCl buffer for background correction. Fluorescence intensity correction was also performed to exclude the inner filter effect based on a previous study [26].
2.15. UV-visible spectroscopy measurements
The UV-visible spectroscopy measurements were performed using a fixed TS concentration (1 µM) after interaction with different concentrations of canadine (1-16 µM) on a UV-visible Spectrophotometer (Hitachi U-3310, Japan). The TS or TS-canadine complex ( with a canadine concentration of 16 µM) were heated for 15 mins at 60°C, and then the UV-visible spectroscopy measurements were performed. The wavelengths were read from 250 nm to 320 nm at room temperature. The spectra were recorded 2 mins after ligand titration at a physiological pH of 7.4. The UV absorbance of canadine was utilized to normalize the UV-visible intensities of all spectra.
2.16. Circular dichroism (CD) spectroscopy measurements
The CD spectroscopy measurements were conducted with a fixed TS concentration (1 µM) after interaction with different concentrations of canadine (1-16 µM) using a J-815 spectropolarimeter (Jasco, Japan). The wavelengths were read from 250 nm to 190 nm at room temperature. The CD spectra were recorded 2 mins after canadine titration at a physiological pH of 7.4. The CD spectrum of canadine was used to normalize all spectra. The secondary structure fraction of the TS was determined using K2D2 software [27].
2.17. Statistical analysis
Data were reported as the average ± SD of three experiments. Data analysis was performed using an independent sample t-test via SPSS. A p-value < 0.05 was considered significant.
3. Results and Discussion
3.1. Canadine shows antiproliferative activity
We assessed the cytotoxic activity of canadine in two cell lines, including MCF-7 cancer cells and MCF-10 normal mammary epithelial cells. Results indicated that canadine had a potential antiproliferative activity in MCF-7 cancer cells (Figure 1a), while it showed no significant toxicity against MCF-10 normal mammary epithelial cells after 24 hrs. It was determined that the viability values of MCF-7 cells were reduced to 98.46%, 76.19%, 59.50%, 48.24%, 21.81%, and 18.42% following incubation with canadine at the concentrations of 1, 5, 10, 20, 30, and 40 µM, respectively, compared to untreated cells, considered as 100%. Meanwhile, the viability values of MCF-10 were reduced to 98.50%, 94.11%, 89.86%, 85.57%, 75.49%, and 67.92% after treatment with canadine at the concentrations of 1, 5, 10, 20, 30, and 40 µM, respectively, compared to untreated cells, considered as 100%.
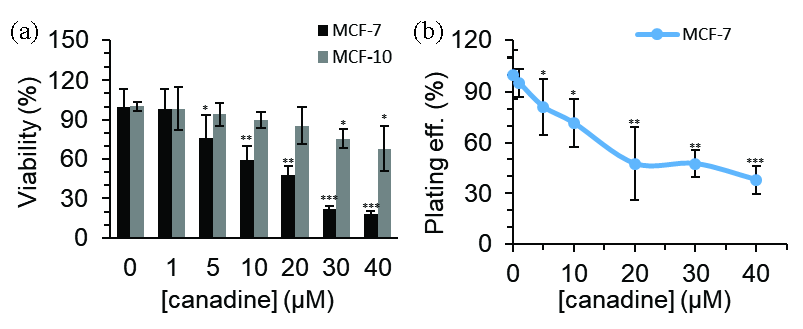
- MTT assay for the evaluation of the toxicity of canadine in (a) MCF-7 breast cancer cells and MCF-10 normal mammary epithelial cells after 24 hrs. (b) Colony formation assay for the evaluation of the antiproliferative effect of canadine in MCF-7 breast cancer cells. *P<0.05, **P<0.01, ***P<0.001.
The IC50 concentrations of canadine were determined to be approximately 17.50 μM in MCF-7 cells and >40 μM in MCF-10 cells. This indicates that MCF-7 cancer cells are more sensitive to canadine-induced cytotoxicity than MCF-10 normal mammary epithelial cells. Indeed, these data suggest that canadine is selective for MCF-7 breast cancer cells and less cytotoxic to non-cancerous cells. Breast cancer is one of the most prevalent types of cancer in the Chinese population; as a result, further investigations are required on this cell line.
We further explored the capability of canadine in inhibiting the clonogenic growth of MCF-7 cancer cells. As shown in Figure 1(b), canadine effectively inhibited the colony formation/growth of MCF-7 cancer cells. The colony-forming rates dropped to 95.23%, 80.95%, 71.42%, 47.61%, 47.69%, and 38.09% in the presence of canadine at the indicated concentrations of 1, 5, 10, 20, 30, and 40 µM, respectively, compared to untreated cells, considered as 100%. Together, these data showed that canadine has potential antiproliferative activity.
3.2. Canadine triggers cell cycle arrest and apoptosis
To further explore the cellular mechanism, the IC50 concentration (17.5 µM) of canadine from the MTT assay was selected. It is evident that the normal cell cycle has four stages, G1, S, G2, and M, and the advancement of anticancer agents has been carried out in such a way as to target specific stages of the cell cycle to inhibit the progression of cancer cells. Canadine (17.5 µM) was used for 24 hrs to assess cell cycle arrest in MCF-7 cells. The distribution of MCF-7 cells along the sub-G0-G1, G0-G1, S, and G2/M phases of the cycle has been shown in Figure 2(a). The results revealed that canadine triggered cell cycle arrest at the G2/M phase. It was quantified that the distribution of untreated MCF-7 cells in the G2/M phase was about 24.76%, which increased to 36.70% after treatment with 17.5 µM canadine for 24 hrs.
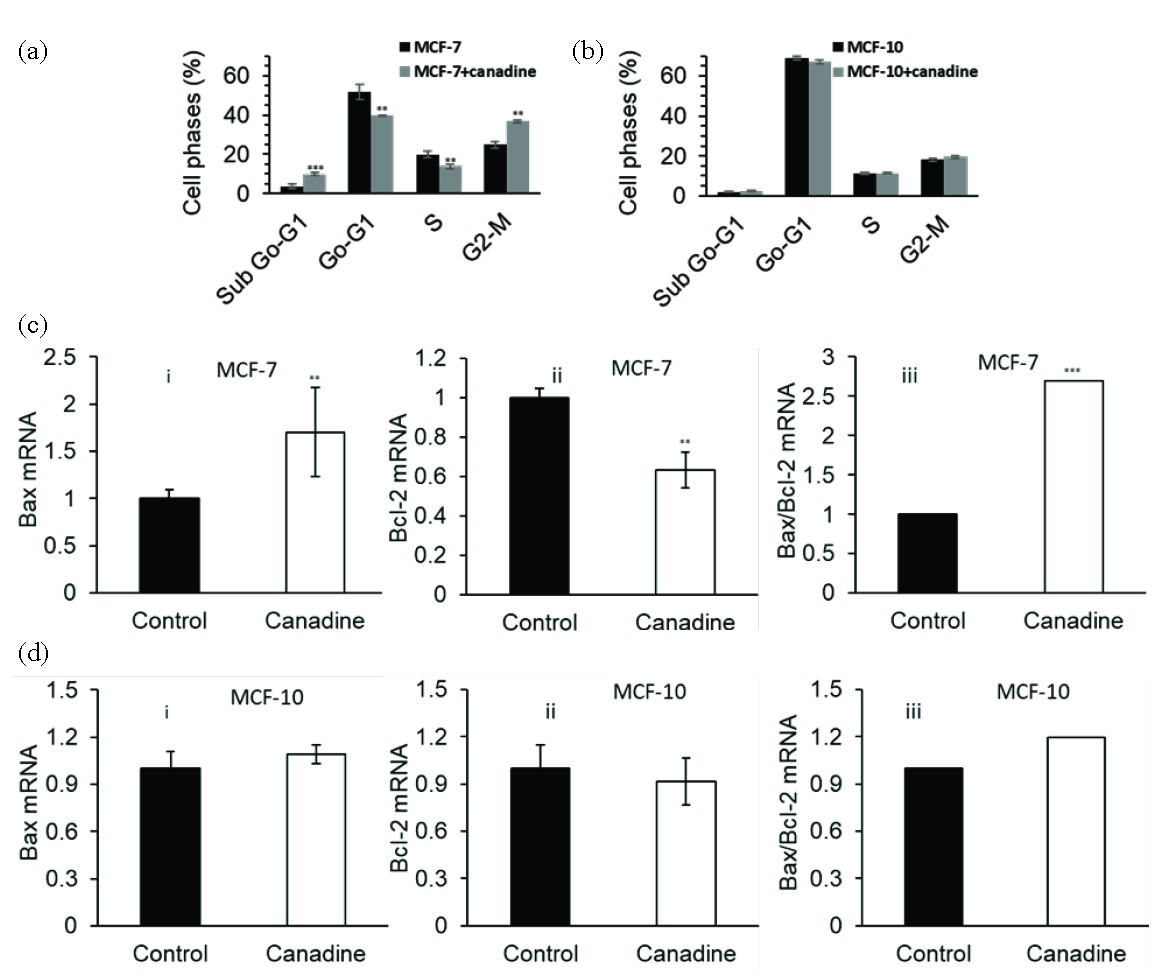
- Cell cycle assay for the evaluation of the toxicity of 17.5 µM canadine in (a) MCF-7 breast cancer cells and (b) MCF-10 normal mammary epithelial cells after 24 hrs. qPCR assay for the evaluation of the effect of 17.5 µM canadine on (c) (i) BAX mRNA expression, (ii) BCL-2 mRNA expression, (iii) BAX/BCL-2 mRNA ratio in MCF-7 breast cancer cells and on (d) (i) BAX mRNA expression, (ii) BCL-2 mRNA expression, (iii) BAX/BCL-2 mRNA ratio in MCF-10 normal mammary epithelial cells after 24 hrs. **P<0.01, ***P<0.001.
However, we found that 17.5 µM canadine after 24 hrs did not induce a significant cell cycle arrest in MCF-10 cells (Figure 2b). Additionally, canadine exhibited a 9.83% accumulation of MCF-7 cells in the sub-G0-G1 phase, whereas in control MCF-7 (untreated cells), only 3.70% were detected. These findings clearly indicate that canadine remarkably arrests the cell cycle at the G2/M phase and induces apoptosis.
Apoptosis is a natural signaling pathway essential for an organism’s growth cycle and homeostasis. Since this process can result in the elimination of undesirable cancer cells, it is considered a potential target for cancer therapy. To understand apoptosis induction triggered by canadine in MCF-7 cells, a qPCR assay was utilized. In cells, BAX upregulation and BCL-2 downregulation play a key role in the induction of apoptosis due to impaired mitochondrial membrane permeability. After 24 hrs of treatment with 17.5 µM canadine, the BAX and BCL-2 expressions at the mRNA level changed as BAX levels increased (Figure 2c(i)) and BCL-2 levels decreased significantly (Figure 2c(ii)) compared with control cells. Additionally, it was shown that 17.5 µM canadine resulted in a significant increase in the upregulation of the BAX/BCL-2 ratio at the mRNA level in MCF-7 cells after 24 hrs compared with control cells (Figure 2c(iii)). However, the incubation of MCF-10 cells with 17.5 µM canadine for 24 hrs did not induce any significant changes in the regulation of BAX mRNA (Figure 2d(i)), BCL-2 mRNA (Figure 2d(ii)), and the BAX/BCL-2 ratio (Figure 2d(iii)).
We further assessed the expression of effector mRNAs that might trigger cell cycle arrest and apoptosis induction in canadine-treated cancer cells. As shown in Figure 3, canadine activated p53 mRNA (Figure 3a) and stimulated p21 mRNA expression (Figure 3b) in MCF-7 cancer cells, while it did not induce any changes in the regulation of p53 mRNA (Figure 3c) and p21 mRNA (Figure 3d) in MCF-10 cells. P21, as a key cell cycle inhibitor, could trigger cell cycle arrest following the incubation of MCF-7 cells with canadine.

- qPCR assay for the evaluation of the effect of 17.5 µM canadine on (a) p53 mRNA expression and (b) p21 mRNA expression in MCF-7 breast cancer cells. qPCR assay for the evaluation of the effect of 17.5 µM canadine on (c) p53 mRNA expression and (d) p21 mRNA expression in MCF-10 normal mammary epithelial cells after 24 hrs. ***P<0.001.
We may, therefore, conclude that p53-targeted apoptotic mRNA. BAX was upregulated in canadine-treated cells, while BCL-2 mRNA expression as an anti-apoptotic marker was downregulated.
3.3. Canadine triggers oxidative stress-mediated apoptosis
Cell growth inhibition and apoptosis in MCF-7 cells could be associated with oxidative stress induced by canadine. Therefore, we performed ROS generation, SOD, CAT, GPx activity, as well as GSH content assays.
Therefore, we assessed the levels of ROS in cultured cells treated with either vehicle or 17.5 µM canadine for 24 hrs. The results showed that canadine significantly elevated ROS levels in MCF-7 cells compared with vehicle controls after 24 hrs (Figure 4a). The results indicated canadine-induced ROS generation in MCF-7 cells, and co-treatment with N-Acetyl-L-Cysteine (NAC) (0.5 mM), a potential antioxidant agent, could mitigate these excessive ROS. This data suggested that canadine could induce oxidative stress in MCF-7 cells. Excessive ROS generation and inhibition of cell growth could stem from the decreased SOD, CAT, and GPX activity, as well as reduced Glutathione (GSH) content. In fact, the expression and activity of these enzymatic and non-enzymatic molecules are key processes required for the modulation of accumulated ROS in the cells. Because co-treatment of the cells with NAC mitigates ROS levels, further studies were conducted to explore whether NAC co-treatment protects the MCF-7 cells from canadine-stimulated oxidative stress by rescuing the SOD, CAT, GPX activity, as well as GSH content.
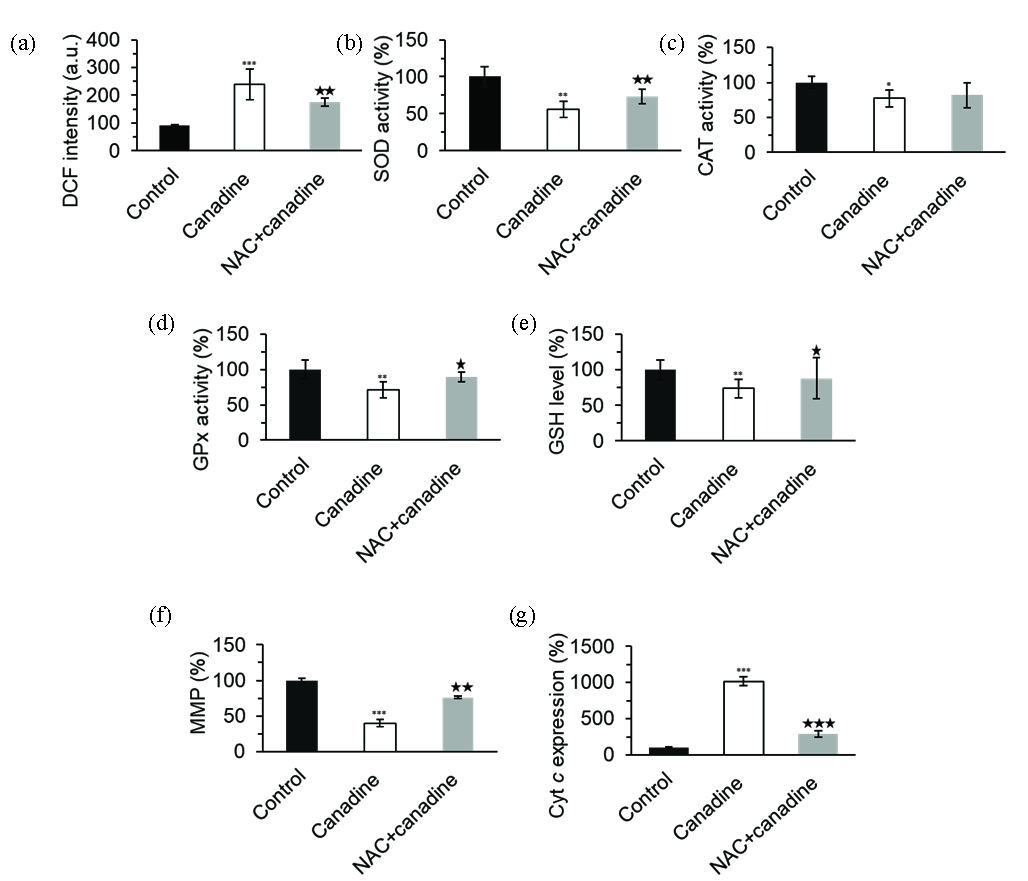
- The evaluation of the effect of 17.5 µM canadine or canadine + NAC on (a) ROS production, (b) SOD activity, (c) CAT activity, (d) GPx activity, (e) GSH level, (f) MMP collapse, and (g) Cytc expression in MCF-7 breast cancer cells after 24 h. *P<0.05, **P<0.01, ***P<0.001. *P<0.05, **P<0.01, ***P<0.001 relative to canadine-treated group. CAT: Catalase, SOD: Superoxide Dismutase, GPx: Glutathione Peroxidase, GSH: Glutathione.
Compared with vehicle controls, canadine (17.5 μM) significantly inhibited/mitigated the SOD (Figure 4b), CAT (Figure 4c), GPx (Figure 4d) activity, as well as GSH content (Figure 4e). The addition of NAC (0.5 mM) to cell culture media significantly recovered SOD/GPx activity and GSH content relative to the canadine-treated samples (Figure 4b-e).
We also investigated whether canadine-induced apoptosis was mediated by oxidative stress in MCF-7 cells. For this reason, we studied the effect of canadine alone or in combination with NAC on MMP loss and subsequent apoptosis. In fact, apoptosis-inducing agents can disrupt MMP and, as a result, stimulate the release of apoptotic mediators from mitochondria, such as cytochrome c. We, therefore, examined the impact of canadine on MMP loss and the release of cytochrome c. As shown in Figure 4(f), canadine resulted in a significant reduction in MMP, and co-treatment of MCF-7 cells with NAC for 24 hrs significantly recovered the MMP collapse induced by canadine.
Additionally, the pro-apoptotic marker BAX could modulate the mitochondrial pathway by stimulating cytochrome c release from the mitochondria. In parallel with BAX/BCL-2 overexpression and MMP collapse, as illustrated in Figure 4(g), cytochrome c release was detected following exposure of MCF-7 cells to canadine (17.5 μM) for 24 hrs. However, co-treatment of the cells with NAC (0. 5 mM) could mitigate the MMP collapse (Figure 4f) and cytochrome c release (Figure 4g) induced by canadine. Taken together, these data indicate that MMP collapse and cytochrome c release following canadine-induced apoptosis might be mediated by oxidative stress, and NAC co-treatment could alleviate these cytotoxic effects.
3.4. Canadine downregulated the expression and activity of the TS
The outcomes revealed that canadine significantly reduced the TS mRNA expression (Figure 5a), TS protein expression (Figure 5b), and TS activity (Figure 5c). We then assessed whether the downregulation of the TS by canadine provides any regulatory effect on the anticancer effects of canadine in MCF-7 cancer cells. Therefore, TS expression was silenced by transiently transfecting the MCF-7 cells with TS siRNA, and the cell proliferation and apoptosis assays (survivin gene expression) were conducted.

- The evaluation of the effect of 17.5 µM canadine or TS SiRNA or TS SiRNA+ canadine on (a) TS mRNA expression, (b) TS protein expression, (c) TS activity, (d) surviving MRNA expression, and (e) viability in MCF-7 breast cancer cells. The letters show the statistical significance when P< 0.05.
The data showed that the silencing of the TS further reduced survivin expression (Figure 5d) as an anti-apoptotic marker and enhanced the cytotoxicity (Figure 5e) of canadine in MCF-7 cells, revealing that canadine induced apoptosis and anticancer effects, at least, through the downregulation of the TS.
3.5. Canadine strongly binds TS
Without a substrate, the binding site of the TS adopts an open conformation, allowing for potential interaction with the dUMP substrate. The formation of a ternary TS-dUMP-mTHF complex leads to a minor structural change, causing displacement of the dUMP and the thiolation of CYS195. In the binary TS-dUMP complex, the phosphate group of dUMP interacts with four Arg residues, namely ARG50, ARG215, ARG175, and ARG176 [28]. Additionally, the residues ASN183 and ARG185 interact with dUMP, while other residues (175-176, 215-218, and 256-258) participate in nucleotide interactions. The catalytic site of TS, composed of residues 181-197, shows high flexibility and various conformations [28]. Therefore, in this study, we aimed to investigate the interaction of canadine with TS. As shown in Figure 6a(i, ii), canadine has a binding affinity for TS proximal to the active site. The binding affinity values between canadine and TS were calculated to be in the range of -6.80 kcal/mol to -5.36 kcal/mol for 10 runs. The conformation with the lowest binding energy of -6.80 kcal/mol was then recommended as the best conformer (Figure 6a).

- (a) (i, ii) The TS-canadine docked complex with two different illustrations and (b) the amino acid residues in the binding pocket.
The amino acid residues contributing to the interaction of canadine and TS are shown in Figure 6(b), and the resultant data have been summarized in Table 1.
| Interactions | Distance (Å) | Type |
|---|---|---|
| Ligand:O - CYS195:HN | 2.27419 | Conventional hydrogen bond |
| Ligand:H - GLU87:OE2 | 2.66142 | Carbon hydrogen bond |
| Ligand:H - PRO193:O | 2.69673 | Carbon hydrogen bond |
| Ligand - MET311 | 5.24007 | Pi-Sulfur |
| Ligand - TRP109 | 4.78103 | Pi-Pi stacked |
| Ligand - ILE108 | 4.86869 | Alkyl |
| Ligand - CYS195 | 4.61218 | Alkyl |
| Ligand - LEU192 | 4.83403 | Alkyl |
| Ligand:C - ILE108 | 4.29882 | Alkyl |
| Ligand:C - LEU221 | 4.33148 | Alkyl |
| Ligand:C - MET311 | 4.30316 | Alkyl |
| Ligand - TRP109 | 4.88467 | Pi-Alkyl |
| Ligand:C - PHE225 | 4.08201 | Pi-Alkyl |
| Ligand - CYS195 | 5.11456 | Pi-Alkyl |
| Ligand - ILE108 | 5.03855 | Pi-Alkyl |
| Ligand - LEU221 | 5.42349 | Pi-Alkyl |
Different hydrogen bonding, stacking, alkyl, and π-sulfur forces are involved in the interaction of canadine and TS. Interestingly, it was detected that LEU192 and CYS195 in the active site of the TS potentially interact with canadine. In fact, CYS195 was able to contribute to the formation of conventional hydrogen, alkyl, and π-alkyl bonds with canadine. As a catalytic residue, CYS195 in TS plays a key role in interaction with dUMP, the TS substrate. The results of this study indicate that this ligand could inhibit the CYS195 thiolation and subsequent inhibition of the TS activity [16].
3.6. Fluorescence spectroscopy measurements
The emission peak at 338 nm (Figure 7a) is a typical feature of tryptophan fluorescence, as already reported for other proteins [29,30].

- (a) The fluorescence spectroscopy study of the TS-canadine complex, (b)Stern-Volmer plots of the TS-canadine complex, (c) Modified Stern-Volmer plots of the TS-canadine complex, (d) van’t Hoff plot of the TS-canadine complex.
Canadine was shown to reduce the fluorescence intensity of TS at 295 K. Additionally, there was a significant shift to larger wavelengths (redshift) in the maximum emission wavelength from 338 to 344, suggesting that the interaction of canadine and TS leads to perturbation in the protein conformation [31]. In other words, following the interaction of canadine and TS, the induced structural changes lead to Trp movement residues from a nonpolar to a polar microenvironment [31].
3.7. Determination of the quenching mechanism
To determine the type of quenching mechanism occurring during the interaction of canadine and TS, the Stern-Volmer (SV) Eq. (1) was employed as follows:
where F0, F, kq, KSV, and τ0 represent TS fluorescence intensity, TS-canadine fluorescence intensity, the bimolecular quenching constant, the Stern–Volmer constant, and the lifetime of the fluorophore, 10-8 ns, respectively [32].
The reduction in TS fluorescence intensity may result from various molecular interactions, particularly the formation of the ground state TS-canadine complex or dynamic collisions. The latter is closely associated with the diffusion phenomenon, which accelerates with a rise in temperature. However, in static quenching, the breakdown of the TS-canadine complexes may occur following an increase in temperature, leading to a decrease in the values of the quenching constants [33]. Fluorescence spectroscopy was then performed at five different temperatures ranging from 295 to 315 K to explore the quenching mechanism associated with the interaction of the TS and canadine based on the recorded fluorescence quenching measurements. The linearity of the SV plots for the TS-canadine system at the five different temperatures is clearly observable in Figure 7(b). The calculated KSV and kq values exhibit an inverse correlation with temperature rise for the TS-canadine system (Table 2), suggesting that the quenching mechanism is static [34]. Additionally, kq is recognized as an important parameter in distinguishing whether the observed quenching mechanism results from collisional or dynamic quenching. For a static quenching mechanism, the kq value should exceed 2 × 1010 M−1 s−1.
| T (K) | KSV × 104 (L mol−1) | kq × 1012 (L mol−1 S−1) | R2 |
|---|---|---|---|
| 295 | 16.15 ± 0.69 | 16.15 ± 0.69 | 0.9375 |
| 300 | 14.13 ± 0.58 | 14.13 ± 0.58 | 0.9369 |
| 305 | 12.09 ± 0.51 | 12.09 ± 0.51 | 0.9585 |
| 310 | 9.40 ± 0.44 | 9.40 ± 0.44 | 0.9199 |
| 315 | 8.47 ± 0.39 | 8.47 ± 0.39 | 0.9787 |
As tabulated in Table 2, the calculated kq values at all five temperatures were significantly greater than 2 × 1010 M−1 s−1, reconfirming the occurrence of the static quenching mechanism in the formation of the TS-canadine system [33].
3.8. Binding parameter calculations
For a static protein-ligand complex, the binding parameters can be determined using the modified Stern-Volmer Eq. (2) [32]:
where, Ka is the binding constant and, n is the number of binding sites.
The graphical plots were generated to display the relationship between the log[(F0-F)/F] and log [canadine] (Figure 7c). The intercepts and slopes of these plots were then used to calculate the Ka and n values for the TS-canadine system across five different temperatures.
Table 3 shows an increase in binding parameters as the temperature rises in the TS-canadine system, which implies that the temperature favors the binding of the TS and canadine, and the interaction strengthens at higher temperatures [32]. This data clearly shows that there is a strong affinity between TS and canadine. Furthermore, the n values were close to one, suggesting that there is only one binding site on TS for canadine. This finding was already supported by molecular docking analysis in this study, which indicated that the active site of the TS is the most preferable site for the TS-canadine interactions.
| T (K) | logKa | n | R2 |
|---|---|---|---|
| 295 | 5.11 ± 0.21 | 1.00 ± 0.06 | 0.9279 |
| 300 | 5.27 ± 0.22 | 1.04 ± 0.06 | 0.9717 |
| 305 | 5.51 ± 0.25 | 1.10 ± 0.06 | 0.9806 |
| 310 | 5.65 ± 0.27 | 1.15 ± 0.07 | 0.9765 |
| 315 | 5.83 ± 0.27 | 1.19 ± 0.08 | 0.9936 |
3.9. Thermodynamic parameters
The thermodynamic parameters, including standard free energy changes (∆G°), standard entropy changes (∆S°), and standard enthalpy changes (∆H°), for the TS-canadine system were calculated using the Van’t Hoff and Gibbs Eqs. (3) and (4) [35]:
A plot (Figure 7d) of the lnKb against the reciprocal of temperature (1/T) exhibits strong linearity, revealing that thermodynamic parameters can be determined with high accuracy for the TS-canadine system. Subsequently, the calculated thermodynamic parameters have been summarized in Table 4.
| T (K) | ΔH° (kJ/mol) | ΔS° (J/mol K) | ΔG° (kJ/mol) |
|---|---|---|---|
| 295 | 66.46 ± 3.61 | 324.43 ± 16.73 | -29.24 ± 1.38 |
| 300 | -30.86 ± 1.39 | ||
| 305 | -32.49 ± 1.54 | ||
| 310 | -34.11 ± 1.63 | ||
| 315 | -35.73 ± 1.87 |
The negative values of ΔG° indicate that the interaction is spontaneous for the TS-canadine system [35]. Moreover, ∆H° and ∆S° values for the TS-canadine system were -66.46 ± 3.61 kJ/mol and 324.43 ± 16.73 J/mol K, respectively (Table 4). The presence of positive signs for ∆H° and ∆S° values suggests that the binding reaction between TS and canadine is endothermic and is driven mainly by entropy. The favorable entropy in this reaction displays that the hydrophobic forces are the dominant interactions in the formation of the TS-canadine system [36].
3.10. ANS fluorescence spectroscopy measurements
ANS, as an anion probe, typically binds non-covalently to the exposed hydrophobic moieties of proteins [37], which could be simply influenced by protein structural changes. Based on this fact, it was found that the ANS fluorescence intensity of the TS increases following interaction with canadine in a concentration-dependent manner (Figure 8a), suggesting that the surface hydrophobicity of the TS-complex is higher than that of free TS.
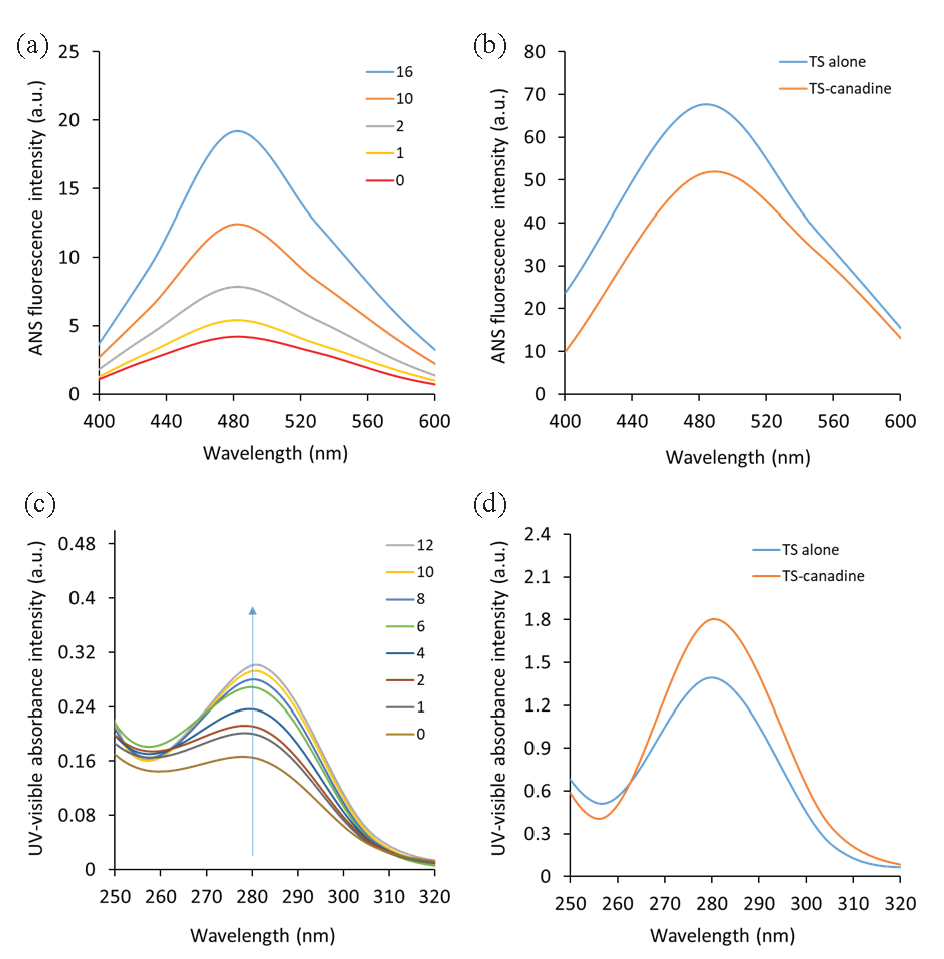
- (a) The ANS fluorescence spectroscopy study of the TS-canadine complex, (b) the ANS fluorescence spectroscopy study of the TS-canadine complex upon exposure to 60°C. (c) The UV-visible spectroscopy study of the TS-canadine complex, (d) the UV-visible spectroscopy study of the TS-canadine complex upon exposure to 60°C.
As anticipated, ANS is able to preferentially interact with denatured TS in the presence of canadine to reveal the conformational changes (unfolding or denaturation) of TS with canadine. The exposure of the TS’s hydrophobic parts following temperature elevation to 60°C was also assessed by ANS fluorescence spectroscopy.
ANS spectra of the TS or TS-canadine at 60°C showed that TS is not well-folded and that hydrophobic patches are exposed at 60°C (Figure 8b). Additionally, ANS fluorescence spectroscopy measurements showed an increased ANS fluorescence intensity for the TS-canadine at 60°C relative to TS, indicating TS unfolding in the presence of canadine.
3.11. UV–visible spectrophotometric measurements
UV–visible absorption spectroscopy is an important and sensitive technique widely used for probing the structural changes and the formation of static complexes [38,32]. UV-visible spectral changes, such as hypochromic or hyperchromic effects, as well as the red or blue shifts, can reveal the dominant interaction type between ligands and proteins [39,40]. Dynamic interactions arise exclusively from the interaction of ligands with aromatic residues in excited states, leaving the UV-visible absorption spectra unaffected. However, the formation of a ground-state complex causes changes in the absorption spectrum of the aromatic residues [32]. The UV-visible peak of the TS shows a maximum absorption peak of nearly 280 nm (Figure 8c), which is attributable to the aromatic amino acid residues, particularly Trp and Tyr [38]. Figure 8(c) depicts the absorption spectra of the TS following interaction with various concentrations of canadine. The detected increase in absorbance at 280 nm, combined with a red-shift primarily derived from Trp and Tyr residues, indicates the formation of a static TS-canadine complex, as well as structural changes in the protein near the Trp residue(s) [32]. This data is consistent with fluorescence quenching data, which showed the formation of a static complex as well as structural changes in TS upon interaction with canadine. This data aligns well with fluorescence spectroscopy measurements, revealing the conformational changes of the protein. Furthermore, the UV-visible spectra of TS or TS-canadine at 60°C revealed that this protein experienced significant unfolding, as evidenced by a considerable increase in UV-visible absorbance around 280 nm (Figure 8d). An additional increase in UV-visible absorbance around 280 nm for TS-canadine at 60°C compared with TS was also observed, exhibiting TS denaturation in the presence of canadine [41]. The conformational alteration of TS induced by canadine could be a possible basis for the further increase in UV-visible absorbance of the TS-canadine complex relative to TS around 280 nm [42,43].
3.12. Secondary structure measurement by CD
CD spectra of the TS and TS-canadine were recorded in the far-UV region. The CD spectrum of the TS showed two minima centered at 208 and 222 nm (Figure 9a), revealing the predominance of the α-helix fraction in the TS structure [44]. The determination of the secondary structure fractions, conducted by K2D2 software, showed that TS contains α-helix and random coil contents of 57.38 ± 2.19% and 28.44 ± 1.08%, respectively (Figure 9b), which is in good agreement with a previous study [44].
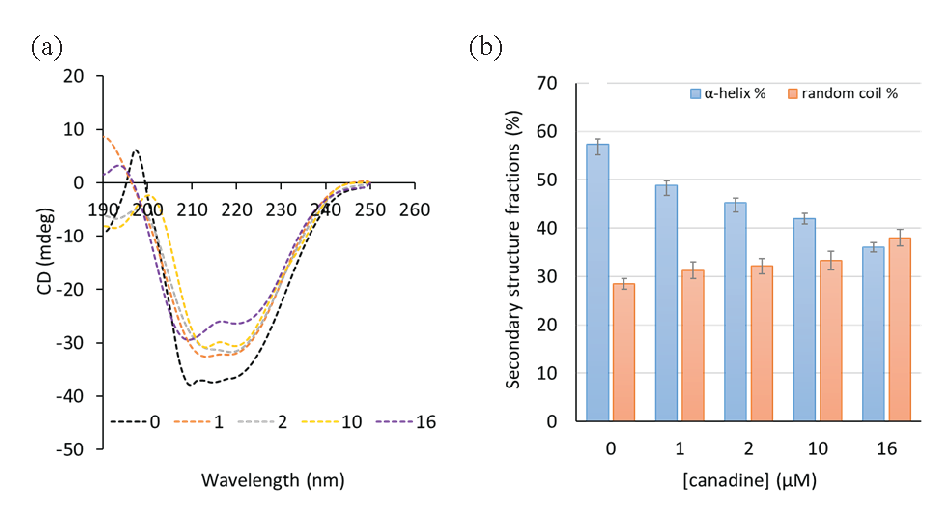
- The CD spectroscopy study of (a) the TS-canadine complex and (b) the secondary structure fractions.
It was observed that after the addition of canadine, the two minima significantly reduced relative to TS in a concentration-dependent manner (Figure 9a), revealing substantial structural changes in TS in the presence of canadine, which fully supports other spectroscopic data. Indeed, it was estimated that after the addition of 1, 2, 10, and 16 µM canadine, the α-helix contents were reduced to 48.83 ± 2.02%, 45.18 ± 1.88%, 42.01 ± 1.15%, and 35.98 ± 0 .89%, while the random coil contents increased to 31.28 ± 1.68%, 32.14 ± 1.62%, 33.28 ± 1.98%, and 37.98 ± 1.72%, respectively (Figure 9b).
3.13. Discussion
In this study, the anticancer effects of canadine as a benzylisoquinoline alkaloid on MCF-7 human breast cancer cells were explored using various cellular and molecular assays. Additionally, MCF-10 human normal mammary epithelial cells were utilized as a noncancerous cell line model. The mRNA/protein expression and activity of the TS in canadine-treated MCF-7 cells were investigated, further analyzed by molecular docking studies. It was shown that the IC50 concentrations of canadine were 17.50 μM and >40 μM in MCF-7 and MCF-10 cells, respectively. Colony-forming rates in MCF-7 cells were also demonstrated to be mitigated by the addition of canadine at the indicated concentrations. Inoue et al. also showed that benzylisoquinoline alkaloid-based derivatives, such as berberine and coptisine, exhibit potential inhibition of colony formation in different cell lines, especially MCF-7 cells [8]. Additionally, it has been revealed that phaeanthine, as a bisbenzylisoquinoline alkaloid, had a potential inhibitory effect on the proliferation and colony formation capacity of cervical cancer cells [45].
Moreover, canadine was shown to trigger cell cycle arrest and apoptosis mediated by the upregulation of p53, P21, and the BAX/BCL-2 ratio. Furthermore, the data disclosed canadine triggered oxidative stress-mediated apoptosis through MMP collapse and cytochrome c release. This mechanism was downregulated with the co-treatment of the MCF-7 cells with NAC. In line with these results, Kazemi Noureini et al. showed cell cycle arrest in MCF-7 cells through telomere shortening by benzylisoquinoline alkaloid chelidonine [9]. It has been reported that short telomeres could modulate intracellular signaling pathways that stimulate cell cycle arrest and apoptosis [9,46]. Moreover, Koutova et al. reported bersavine, as a bisbenzylisoquinoline alkaloid, to stimulate anticancer effects through ROS-mediated apoptosis in human leukemic cells [47]. Based on previous reports, bioactive compounds can lead to DNA damage and apoptosis mediated by the generation of ROS and MMP collapse in different cancer cells [48,49]. The outcomes of MMP and cytochrome c indicate that canadine was effective at the used concentration (17.5 μM). In this regard, Yuan et al. reviewed that MMP damage was detected along with a rise in cytochrome c release after exposure of breast cancer cells to natural products [50].
Moreover, it was noted that canadine decreased the expression of TS. Cabibi et al. reported that TS in primary and metastatic breast cancer nodes shows different expression levels [51] that are heavily associated with poor responses to chemotherapy in patients suffering from advanced breast tumors [52]. Therefore, TS, along with other DNA‐synthesizing enzymes such as thymidine kinase and thymidylate kinase, are known as potential targets in breast cancer chemotherapy [53,13,54].
The molecular docking analysis proved a high binding affinity (–6.80 kcal/mol) between canadine and TS. By comparing the theoretical analysis and experimental data on TS activity inhibition in the presence of canadine, we can discuss that the CYS195 residue, as a main residue in the active site of the TS, establishes several interactions with canadine through alkyl and π-alkyl forces. These forces then might inhibit the thiolation of CYS195, which is a crucial factor in active site closure and opening, as well as co-factor interaction, and, therefore, block the TS activity [28].
The fluorescence quenching study revealed the occurrence of a static quenching mechanism in the formation of the TS-canadine system derived from kq and KSV values [33]. The binding assay clearly showed a strong interaction between TS and canadine, which could indicate a possible interaction between these two molecules in vivo, though further research is needed in future studies. Furthermore, the n values of 1 were supported by molecular docking analysis, which indicated that canadine preferably binds to the active site of TS. Additionally, thermodynamic data were consistent with molecular docking analysis, which showed that the presence of hydrophobic residues and their corresponding forces are greater than those of other amino acid residues and their subsequent forces.
Moreover, the probable structural changes of the TS in the presence of canadine could be the reason for the further increase in ANS fluorescence intensity and UV-visible absorbance of the TS-canadine complex relative to TS [55]. Lastly, CD studies showed that canadine had a substantial effect on the secondary structure of the TS through a reduction of α-helix content and an increase of random coil fraction.
4. Conclusions
In conclusion, canadine showed a selective anticancer effect against MCF-7 breast cancer cells. Canadine was shown to induce cell cycle arrest at the G2/M phase, mediated by the upregulation of p53 and P21 mRNA. Canadine-induced oxidative stress-mediated apoptosis was evidenced by MMP collapse and cytochrome c release. It was shown that canadine potentially interacted with TS and could decrease the expression and activity of TS. Also, it was revealed that canadine could bind to TS, mediated by hydrogen bonds, and induce substantial effects on the secondary and tertiary structures of TS, assessed by several spectroscopic methods. In conclusion, this paper may hold outstanding prospects for the scientific discovery of a prospective anticancer molecule derived from canadine, which requires further in vitro and in vivo research.
CRediT authorship contribution statement
Yuchen Wang, Zhoulan Bai: Conceptualization, Methodology, Writing – Original Draft. Chaolin Zhang, Xiang Liu: Data Curation, Formal Analysis, Visualization. Hui Yu, Jiale He, Qilun Liu: Resources, Supervision, Writing – Review & Editing. Zhenning Tang, Zhoulan Bai: Project Administration, Funding Acquisition. All authors have read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no competing interest.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast cancer research and treatment. 2016;159:395-406. https://doi.org/10.1007/s10549-016-3947-0
- [Google Scholar]
- Breast cancer treatment. American Family Physician. 2021;104:171-8. https://doi.org/10.51866/mfpv16n2
- [Google Scholar]
- Combination of focused ultrasound, immunotherapy, and chemotherapy: new perspectives in breast cancer therapy. Journal of Ultrasound in Medicine. 2023;42:559-573. https://doi.org/10.1002/jum.16053
- [Google Scholar]
- Challenges for triple negative breast cancer treatment: Defeating heterogeneity and cancer stemness. Cancers. 2022;14:4280. https://doi.org/10.3390/cancers14174280
- [Google Scholar]
- Anticancer properties and mechanisms of botanical derivatives. Phytomedicine Plus. 2022;3:100396. https://doi.org/10.1016/j.phyplu.2022.100396
- [Google Scholar]
- Overview of major classes of plant-derived anticancer drugs. International Journal of Biomedical Science: IJBS. 2009;5:1. https://doi.org/10.59566/ijbs.2009.5070
- [Google Scholar]
- Noscapine, a benzylisoquinoline alkaloid, sensitizes leukemic cells to chemotherapeutic agents and cytokines by modulating the NF-κB signaling pathway. Cancer research. 2010;70:3259-3268. https://doi.org/10.1158/0008-5472.can-09-4230
- [Google Scholar]
- The benzylisoquinoline alkaloids, berberine and coptisine, act against camptothecin-resistant topoisomerase I mutants. Scientific Reports. 2021;11:7718. https://doi.org/10.1038/s41598-021-87344-2
- [Google Scholar]
- Telomere shortening in breast cancer cells (MCF7) under treatment with low doses of the benzylisoquinoline alkaloid chelidonine. PloS one. 2018;13:e0204901. https://doi.org/10.1371/journal.pone.0204901
- [Google Scholar]
- Benzylisoquinoline alkaloids inhibit lung fibroblast activation mainly via inhibiting TGF-β1/Smads and ERK1/2 pathway proteins. Heliyon. 2023;9:e16849. https://doi.org/10.1016/j.heliyon.2023.e16849
- [Google Scholar]
- In vitro and in silico acetylcholinesterase inhibitory activity of thalictricavine and canadine and their predicted penetration across the blood-brain barrier. Molecules. 2019;24:1340. https://doi.org/10.3390/molecules24071340
- [Google Scholar]
- Canadine inhibits epithelial mesenchymal transformation of HPV-negative cervical cancer. Tissue Barriers. 2023;12:2256641. https://doi.org/10.1080/21688370.2023.2256641
- [Google Scholar]
- Thymidylate synthase: a critical target for cancer chemotherapy. Clinical Colorectal Cancer. 2002;1:220-229. https://doi.org/10.3816/CCC.2002.n.003
- [Google Scholar]
- Targeting Thymidylate Synthase Enhances the Chemosensitivity of Triple-Negative Breast Cancer Towards 5-FU-Based Combinatorial Therapy. Frontiers in Oncology. 2021;11:656804. https://doi.org/10.3389/fonc.2021.656804
- [Google Scholar]
- Synthesis and biological evaluation of novel uracil derivatives as thymidylate synthase inhibitors. Applied Biochemistry and Biotechnology 2023:1-20. https://doi.org/10.1007/s12010-023-04367-3
- [Google Scholar]
- Intrinsic fluorescence of the active and the inactive functional forms of human thymidylate synthase. ChemBioChem. 2021;22:1800-1810. https://doi.org/10.1002/cbic.202000722
- [Google Scholar]
- Cooperative inhibition of human thymidylate synthase by mixtures of active site binding and allosteric inhibitors. Biochemistry. 2007;46:2823-2830. https://doi.org/10.1021/bi061309j
- [Google Scholar]
- Curcumin nicotinate selectively induces cancer cell apoptosis and cycle arrest through a P53-mediated mechanism. Molecules. 2019;24:4179. https://doi.org/10.3390/molecules24224179
- [Google Scholar]
- Xanthatin induces glioma cell apoptosis and inhibits tumor growth via activating endoplasmic reticulum stress-dependent CHOP pathway. Acta Pharmacologica Sinica. 2020;41:404-414. https://doi.org/10.1038/s41401-019-0318-5
- [Google Scholar]
- Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. Journal of Cellular Biochemistry. 2010;111:1426-1436. https://doi.org/10.1002/jcb.22869
- [Google Scholar]
- Gossypol sensitizes the antitumor activity of 5-FU through down-regulation of thymidylate synthase in human colon carcinoma cells. Cancer Chemotherapy and Pharmacology. 2015;76:575-586. https://doi.org/10.1007/s00280-015-2749-0
- [Google Scholar]
- Histone deacetylase inhibitor enhances sensitivity of non‐small‐cell lung cancer cells to 5‐FU/S‐1 via down‐regulation of thymidylate synthase expression and up‐regulation of p21waf1/cip1 expression. Cancer science. 2010;101:1424-1430. https://doi.org/10.1111/j.1349-7006.2010.01559.x
- [Google Scholar]
- Gene-silencing effects of anti-survivin siRNA delivered by RGDV-functionalized nanodiamond carrier in the breast carcinoma cell line MCF-7. International Journal of Nanomedicine 2016:5771-5787. https://doi.org/10.2147/IJN.S117611
- [Google Scholar]
- Melatonin promotes ATO-induced apoptosis in MCF-7 cells: Proposing novel therapeutic potential for breast cancer. Biomedicine & Pharmacotherapy. 2016;83:456-465. https://doi.org/10.1016/j.biopha.2016.07.004
- [Google Scholar]
- Quenching of thymidylate synthetase fluorescence by substrate analogs. Biochemical and Biophysical Research Communications. 1975;64:648-655. https://doi.org/10.1016/0006-291x(75)90370-8
- [Google Scholar]
- Evaluating the biomolecular interaction between delamanid/formulations and human serum albumin by fluorescence, CD spectroscopy and SPR: Effects on protein conformation, kinetic and thermodynamic parameters. Colloids and Surfaces B: Biointerfaces. 2024;239:113964. https://doi.org/10.1016/j.colsurfb.2024.113964
- [Google Scholar]
- K2D2: estimation of protein secondary structure from circular dichroism spectra. BMC Structural Biology. 2008;8:1-5. https://doi.org/10.1186/1472-6807-8-25
- [Google Scholar]
- Ensemble-based virtual screening of African natural products to target human thymidylate synthase. Journal of Molecular Graphics and Modelling. 2023;125:108568. https://doi.org/10.1016/j.jmgm.2023.108568
- [Google Scholar]
- Investigation of interaction between atazanvir sulphate and bovine serum albumin by using fluorescence spectroscopy. Indian Journal of Chemistry-Section A (IJCA). 2021;55:820-3. https://doi.org/10.56042/ijca.v55i7.11320
- [Google Scholar]
- Triplet state spectroscopy reveals involvement of the buried tryptophan residue 310 in Glyceraldehyde-3-phosphate dehydrogenase (GAPD) in the interaction with acrylamide. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2024;307:123622. https://doi.org/10.1016/j.saa.2023.123622
- [Google Scholar]
- Applications of fluorescence spectroscopy in protein conformational changes and intermolecular contacts. BBA Advances. 2023;3:100091. https://doi.org/10.1016/j.bbadva.2023.100091
- [Google Scholar]
- The Effect of Caffeine on the interaction between Bovine Serum Albumin and Losartan potassium by Spectroscopic Methods. Frontiers in Scientific Research and Technology 2024
- [Google Scholar]
- Credible parameter estimation of binding and quenching in protein–ligand systems via intrinsic fluorescence and dynamic simulations. Food Hydrocolloids. 2025;159:110608. https://doi.org/10.1016/j.foodhyd.2024.110608
- [Google Scholar]
- Binding constants and in silico analysis of albumin interaction with phenolic acids and flavonoids. Bulletin of Chemists and Technologists of Bosnia and Herzegovina. 2024;62 https://doi.org/10.35666/2232-7266.2024.62.03
- [Google Scholar]
- Conformational dynamics of trypsin in the presence of caffeic acid: a spectroscopic and computational investigation. Journal of Biomolecular Structure and Dynamics. 2024;42:3108-3117. https://doi.org/10.1080/07391102.2023.2212077
- [Google Scholar]
- Insights on the in-vitro binding interaction between donepezil and bovine serum albumin. BMC Chemistry. 2023;17:31. https://doi.org/10.1186/s13065-023-00944-z
- [Google Scholar]
- Conformational changes to zein and its binding interactions with sodium caseinate during the pH-driven self-assembly using multi-spectroscopic and hydrostatic methods. Food Hydrocolloids. 2023;136:108225. https://doi.org/10.1016/j.foodhyd.2022.108225
- [Google Scholar]
- Understanding the molecular interaction of BSA protein with antibiotic sulfa molecule (s) for novel drug development. Journal of Molecular Structure. 2023;1287:135697. https://doi.org/10.1016/j.molstruc.2023.135697
- [Google Scholar]
- Interaction of two commercial azobenzene food dyes, amaranth and new coccine, with human serum albumin: Biophysical characterization. ACS Food Science & Technology. 2023;3:955-968. https://doi.org/10.1021/acsfoodscitech.3c00125
- [Google Scholar]
- Biophysical and molecular modeling evidences for the binding of sulfa molecules with hemoglobin. Journal of Biomolecular Structure and Dynamics. 2023;41:3779-3790. https://doi.org/10.1080/07391102.2022.2057358
- [Google Scholar]
- Quercetin-induced inactivation and conformational alterations of alpha-2-macroglobulin: multi-spectroscopic and calorimetric study. Journal of Biomolecular Structure and Dynamics. 2020;38:4107-4118. https://doi.org/10.1080/07391102.2019.1671232
- [Google Scholar]
- Kolaflavanone of kolaviron selectively binds to subdomain 1B of human serum albumin: spectroscopic and molecular docking evidences. Computational Toxicology. 2020;13:100118. https://doi.org/10.1016/j.comtox.2020.100118
- [Google Scholar]
- Elucidating the Binding Interaction of a Highly Efficient Phytochemical-Sinapic Acid with Human Serum Albumin: A Spectroscopic and Calorimetric Approach. Journal of Macromolecular Science, Part B 2024:1-16. https://doi.org/10.1080/00222348.2024.2401234
- [Google Scholar]
- Human thymidylate synthase with loop 181–197 stabilized in an inactive conformation: ligand interactions, phosphorylation, and inhibition profiles. Protein Science. 2011;20:87-94. https://doi.org/10.1002/pro.539
- [Google Scholar]
- Exploration of Phaeanthine: A Bisbenzylisoquinoline Alkaloid Induces Anticancer Effect in Cervical Cancer Cells Involving Mitochondria-Mediated Apoptosis. ACS Omega. 2023;8:14799-14813. https://doi.org/10.1021/acsomega.3c01023
- [Google Scholar]
- Revisiting telomere shortening in cancer. Cells. 2019;8:107. https://doi.org/10.3390/cells8020107
- [Google Scholar]
- Bersavine: a novel bisbenzylisoquinoline alkaloid with cytotoxic, antiproliferative and apoptosis-inducing effects on human leukemic cells. Molecules. 2020;25:964. https://doi.org/10.3390/molecules25040964
- [Google Scholar]
- Hydroxysafflor yellow B induces apoptosis via mitochondrial pathway in human gastric cancer cells. Journal of Pharmacy and Pharmacology. 2022;74:1320-9. https://doi.org/10.1093/jpp/rgac044
- [Google Scholar]
- Betulinic acid inhibits the proliferation of human laryngeal carcinoma cells through reactive oxygen species-mediate mitochondrial apoptotic pathway. Toxicology in Vitro. 2023;95:105756. https://doi.org/10.1016/j.tiv.2023.105756
- [Google Scholar]
- Promoting apoptosis, a promising way to treat breast cancer with natural products: A comprehensive review. Frontiers in Pharmacology. 2022;12:801662. https://doi.org/10.3389/fphar.2021.801662
- [Google Scholar]
- Different expression of thymidylate synthase in primary tumour and metastatic nodes in breast cancer patients. Anticancer Research. 2007;27:2227-2230. https://doi.org/10.1080/15257770600894527
- [Google Scholar]
- Thymidylate synthase predicts poor response to pemetrexed chemotherapy in patients with advanced breast cancer. Oncology Letters. 2018;16:3274-3280. https://doi.org/10.3892/ol.2018.8973
- [Google Scholar]
- DNA‐synthesizing enzymes in breast cancer (thymidine kinase, thymidylate synthase and thymidylate kinase): Association with flow cytometric S‐phase fraction and relative prognostic importance in node‐negative premenopausal patients. International Journal of Cancer. 2001;95:56-61. DOI: 10.1002/1097-0215(20010120)95:1<56::aid-ijc1010>3.0.co;2-3
- [Google Scholar]
- Cellular and molecular basis of therapeutic approaches to breast cancer. Cellular Signalling. 2023;101:110492. https://doi.org/10.1016/j.cellsig.2022.110492
- [Google Scholar]
- The interaction of allicin with bovine serum albumin and its influence on the structure of protein. Process Biochemistry. 2022;112:139-144. https://doi.org/10.1016/j.procbio.2021.11.026
- [Google Scholar]







