Translate this page into:
Investigating the binding measurements of human α-acid glycoprotein with chlorambucil and dacarbazine in the presence of imidazolium based -ionic liquid by affinity capillary electrophoresis
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Human alpha (α1)-acid glycoprotein (AGP) is an acute phase protein whose plasma concentration increases several-folds in the presence of various diseases. The variability in AGP plasma concentration is expected to have a huge impact on the drug binding equilibrium. Therefore, a precise measurement of AGP-drug binding is of great demand for drug development. In the current study, an ionic liquid-based aqueous two-phase system combined with affinity capillary electrophoresis (ILATPS/ACE) was utilised in order to improve the accuracy of AGP-drug binding analysis through the measurements of electrophoretic mobilities. The utilisation of ILATPS has shown to have a positive impact on the stability of AGP activity solution during the storage for an extended period of time. In addition, the effect of various alkyl chains (C2-C10) of imidazolium-based ILs with concentrations ranging between 10.00 and 1000.0 μmol L−1 on the AGP binding with the anti-cancer drugs chlorambucil (CHL) and dacarbazine (DAC) was examined by the system developed (ILATPS/ACE). A 100.00 μmol L−1 1-ethyl-3-methylimidazolium chloride (EMImCl) prepared in the physiological buffer conditions containing AGP (5.00–100.00 µmol L−1) has provided an accurate apparent binding constant of 1.99 ± 0.11 and 6.95 ± 0.14 L mmol−1 with CHL and DAC respectively. Apart from the ACE analysis, EMImCl/phosphate buffer solution was found to be a distinguished system that could lengthen the stability of AGP activity for a period of time reaching 90 days during the solution storage at 4.00 °C. This effect is thought to be due to the easy conversion of one-phase EMImCl/phosphate buffer/AGP at the ambient lab temperature into the two-phase solution at refrigerator temperature, 4.00 °C, and vice versa. Therefore, the ILATP/ACE system could be used to enhance the accuracy for other AGP-drug bindings with a fast, easy to use, and cost-effective analysis.
Keywords
Affinity capillary electrophoresis
AGP
Apparent binding constants
Ionic liquids
- AC
-
acetanilide
- ACE
-
Affinity capillary electrophoresis
- AGP
-
human alpha (α1)-acid glycoprotein
- BMImBr
-
1-Butyl-3-methylimidazolium bromide
- BMImCl
-
1-butyl-3-methylimidazolium chloride
- BMImBF4
-
1-butyl-3-methylimidazolium tetrafluoroborate
- CHL
-
chlorambucil
- DAC
-
dacarbazine
- DMImCl
-
1-decyl-3-methylimidazolium chloride
- EMImCl
-
1-ethyl-3-methylimidazolium chloride
- HPAC
-
high performance affinity chromatography
- HMImCl
-
1-hexyl-3-methylimidazolium chloride
- IL
-
ionic liquid
- ILATPS
-
ionic liquid aqueous two phase system
- PRO
-
Propranolol
- EOF
-
Electroosmotic flow
Abbreviations
1 Introduction
The binding of prospective small molecule drugs to plasma is considered to be crucial in the field of drug discovery so as to comprehend their behavior in the human body (Arumugam et al., 2020; Šolínová et al., 2019). The human serum albumin (HSA) and human alpha (α1)-acid glycoprotein (AGP, also known as AAG or orosomucoid) are thought to be the most significant plasma proteins involving in drug binding. Though AGP is reported to have a moderately lower concentration compared with HSA in serum, AGP is a vital protein that has been involved in the binding as well as transport of various drugs (Lancioni et al., 2019; Eléonore et al., 2020; Olabi et al., 2018; Huang and Ung, 2013). Additionally, it possesses some unique drug binding properties, including, that there is one limiting binding site in every AGP combined with various acidic and basic drugs, based on the isoelectric point of AGP, pI (Albani et al., 1984; Schley and Mueller-Oerlinghausen, 1983). Furthermore, AGP is considered to be an acute phase protein, whose plasma concentration level raises in response to a variety of illnesses; for instance, cancer, inflammation or following trauma (burns, surgery, etc). Moreover, AGP serum concentration that is stable in the physiological conditions tends to go up several folds during the acute-phase reactions. The changes in AGP plasma concentration as well as its influence on the drug binding could lead to various changes including alterations in the drug’s mode of action, disposition, distribution, and elimination. Thus, accurate comprehension of this distinctive AGP interaction might afford an excellent benefit for drug discovery and development (Abd El-Hady and Albishri, 2018).
Affinity capillary electrophoresis (ACE) is considered to be one of the appropriate techniques utilised for the AGP-drug binding analysis owing to its high efficiency, fast analysis, and appropriateness for investigating high and week affinity interactions (El Deeb et al., 2013). In ACE, the AGP solution is freshly prepared in a phosphate buffer (67.00 mmol L−1, pH 7.45) and used as an additive background electrolyte (BGE) (El Deeb et al., 2014, 2016). When the voltage is applied, the alteration of the migration times of the injected drugs owing to their interactions with AGP is investigated and employed for calculating the binding values (Abd El-Hady and Albishri, 2018; Phillips, 2008).
One of the challenges faced during the analysis of ACE is the preservation of AGP activity against joule heating as well as its immobilization inside the fused silica capillary. This phenomenon is considered to hugely disturb the precision measurements of AGP-drug bindings (Hess et al., 1964; Tanaka and Terabe, 2001; Abd El-Hady and Albishri, 2012; Nehme et al., 2009; Albertsson, 1956). However, this challenge can be overcome via the appropriate choice of BGE components or coating of the inner surface of the fused capillary. Recently, the ILATPS/ACE (Gutowski et al., 2003; Breadmore, 2011) containing AGP was utilised to improve the activity and stability of AGP ultimately leading to enhance the accuracy of the AGP/drug binding constant determination (Abd El-Hady and Albishri, 2018). The ILATPS/ACE system consisting of unique BGE components including AGP (used as an additive in BGE) was prepared in a solution of short-chain (C2 - C10) imidazolium-based ionic liquids (ILs) (chaotropic salts) mixed with kosmotropic inorganic salts at a physiological pH. The BGE prepared was utilised to maintain the activity of AGP during the ACE analysis via two main forces of interaction namely the electrostatic and/or π-π between the IL and the negatively charged aromatic moieties present in the AGP ions (pI = 2.7) (Abd El-Hady and Albishri, 2018; Ruiz-Angel et al., 2007). These interactions caused no denaturation of the AGP molecule nor did they meddle with the binding of AGP with drugs (Abd El-Hady and Albishri, 2018). On the contrary, long chains of imidazolium ILs has shown to influence the AGP’s activity by enhancing the processes of unfolding (Baker and Heller, 2009). Additionally, ILATPS combined with ACE analysis has led to some specific features including enriched aqueous media, low denaturing properties of imidazolium ILs, and relatively low viscosity. The suitability of water-soluble imidazolium ILs with the buffer (phosphate) is considered to be a significant element in order to prevent the precipitation of salts that are formed by an anion exchange interaction (Breadmore, 2011).
Furthermore, aside from the ACE analysis, imidazolium ILs/phosphate solution has been found to be a distinguished system to enhance the stability of AGP activity during its storage for an extended period of time. This phenomenon includes the formation of a mono-phasic AGP-ILATPS solution at the room temperature and the ease of its conversion to a bi-phasic solution through decreasing the temperature to 4.00 °C. This conversion might be owing to the water layer being transferred to the salt-rich phase from the IL-rich phase through a drop in the temperature. This, in turn, leads the concentration of IL to increase and the salt-rich phase to become more diluted (Li et al., 2010; Bridges et al., 2007; Tursen et al., 2011). The salting-out induced ions are due to entropic effects, whereas the salting-in induced ions are owing to the lipophilic moieties of the IL.
The aim of this work was a continuation of the work conducted in our group (Abd El-Hady and Albishri, 2018), the AGP-ILATPS system was investigated with other drugs, chlorambucil (CHL) and dacarbazine (DAC) in order to further prove the impact of this system to accurately measure the drug’s binding with the human protein. Additionally, different short-chain imidazolium ILs were used in order to enhance the accuracy of AGP binding with the anti-cancer drugs, CHL and DAC by the ILATPS/ACE system. The effect of the concentration of ILs, IL-anions’ types, the chain length of ILs, and the concentration of AGP on the ACE analysis were also inspected. To support the accuracy of the binding values obtained by the ILATPS/ACE system, high performance affinity chromatography (HPAC) was employed. In addition, the stability of the stored AGP/ILATPS solution for an extended period of time at room temperature conditions was also investigated.
2 Experimental
2.1 Regents and materials
AGP (≥ 99% agarose gel electrophoresis, 41 kDa), KH2PO4, hydrochloric acid, sodium nitrate, acetanilide (AC), dipotassium hydrogen phosphate dihydrate, and sodium hydroxide were all supplied by Sigma-Aldrich, Germany. Fluka (Steinheim, Germany) was the supplier for DAC, CHL, and Propranolol (PRO). Aldrich (Steinheim, Germany) was the provider of 1-ethyl-3-methylimidazolium tetrafluoroborate (BMImBF4), 1-decyl-3-methylimidazolium chloride (DMImCl), 1-ethyl-3-methylimidazolium chloride (EMImCl), 1-ethyl-3-methylimidazolium bromide (EMImBr), 1-butyl-3-methylimidazolium chloride (BMImCl), and 1-hexyl-3-methylimidazolium chloride (HMImCl). AMilli-Rx apparatus (Millipore, Milford, MA, USA) was utilised in order to purify the water used throughout this work.
2.2 Instrumentations
The HPLC system utilised in this work was a PerkinElmer series 200 LC binary solvent delivery (San Diego, Canada) with a series 200 vacuum degasser. The injector used was a Rheodyne injection valve (model 7725) with series 200 UV/V which is a variable wavelength detector. The AGP column (2.1 × 100 mm, particle size 5 μm) was supplied by Sigma-Aldrich (Germany). The data attained was gathered via a Total ChromTM Chromatography data handling System. A software was used with the PerkinElmer 600 series Link Interface to governor the HPLC system. The temperature set throughout this work was maintained at 37.00 °C via the use of a column oven (Model 200, PerkinElmer, Canada). For the mobile phase filtration, a pump supplied by the Scientific Technical Supplies (Model D-6072 Dreieich, West Germany) was utilised.
An Agilent 7100 System (Waldbronn, Germany) with PDA as a detector was used for the ACE work. A bare fused-silica capillary (Agilent, Moers, Germany) with total length 64.55 cm, effective length 56.00 cm and an internal diameter (ID) 50.00 µm was used. Chemstation software was used in order to acquire the electropherograms obtained for this work.
In order to weigh the material used in this work, a Mettler PM 400 analytical balance (Switzerland) was utilised. A Jenway 3510 pH meter (UK) was employed in order to adjust the pH values for the solutions used.
2.3 Preparation of solutions
The stock solution of the phosphate buffer at a physiological pH 7.45 was made through mixing 80.25 mL K2HPO4 (67.00 mmol L−1) with 19.80 mL KH2PO4 (67.00 mmol L−1). The stock solutions of DAC, CHL, and AC were prepared by dissolving 6.25 mg, 6.24 mg, 37.50 mg in 25.00 mL of the phosphate buffer at the physiological (pH 7.45) respectively. Prior to the dilution step, 1.00 mL of AC with a concentration of 300.00 μg mL−1 was added to the solutions used for the analysis. As to the working solutions, they were made through the stock solutions in which an appropriate dilution of the stock solutions was made in 5.00 mL of the buffer (phosphate) at the physiological (pH 7.45). The solution AGP was made through dissolving the proper amount of AGP in a solution containing 67.00 mmol L−1of KH2PO4 (pH 7.45) and 100.00 μmol L−1 of EMImCl solutions. The solutions prepared were under the lab conditions, 25 ± 2 °C, and filtered via syringe filters (0.22 μm) prior to the ACE analysis.
2.4 Operating conditions
2.4.1 ACE measurements
As practiced, the new uncoated capillary was conditioned by running 1.00 mol L−1 of NaOH for 40.00 min and then deionized H2O for 10.00 min. Prior to the ACE analysis, 0.10 mol L−1 HCl for 10.00 min was run through the capillary for rinsing, followed by the deionized water for 3.00 min. Furthermore, between runs, HCl solution (0.10 mol L−1) was run for 1.00 min through the capillary followed by the deionized water for 2.00 min and then running buffer for 3.00 min. When the ACE analysis was complete at the end of the day, 0.10 mol L−1 HCl for 10.00 min was run through the capillary followed by the deionized water for 15.00 min. The pressure applied was 940.00 mbar, when the washing steps were conducted. The injection mode the pressure used were hydrodynamical and 25.00 mbar for 10.00 s, respectively. Moreover, all the injections were conducted at the inlet end of the capillary and the voltage used for ACE analysis was 20.00 kV with a positive polarity giving electric field strength (E) of 310.00 V/cm. The capillary’s temperature was set at 25.00 °C, which is equal to 37.00 °C inside capillary during the analysis, as reported (Yang and Hage, 1997), and the detection wavelength was 254.00 nm with a bandwidth of 4.00 nm for all the analytes investigated.
The effective mobility of each investigated analytes under the optimal experimental conditions of ILATPS/ACE system was calculated by subtracting the apparent effective mobility (µCHL = 3.01 – 1.82 × 10−4 cm2/V.s and µDAC = 2.62 – 1.77 × 10−4 cm2/V.s) from the EOF mobility (µeof = 5.19 × 10−4 cm2/V.s) giving values ranging from −2.18 to −3.37 cm2/V.s for CHL drug and from −2.57 to −3.42 cm2/V.s for DAC drug in the absence and the presence of 100.00 µmol L−1 AGP in the running buffer respectively. These values indicated that the anion forms of analytes are the predominant structures under the current experimental conditions.
In order to omit any possibility for the change in BGE viscosity due to the addition of AGP as mentioned in the previous report (Abd El-Hady et al., 2010) or the change in the net charge of the inner capillary surface due to the presence of IL as reported (Abd El-Hady and Albishri, 2018), the mobility ratio (R) values of the drugs were used in the current study and were calculated from the equation (2.1) below (Abd El-Hady and Albishri, 2018):
teof The tm of the marker (AC)
tdrug The tm of the drug investigated.
In order to improve the precision of calculated apparent binding constants, a non-linear regression equation (2.2) below was used (Abd El-Hady et al., 2010, 2015):
c(L) The concentration of the protein AGP ranging between 5.00 and 100.00 µmol L−1
Rf: The mobility ratio of the drug measured when AGP is absent
Ri: The mobility ratio of the drug measured when AGP is present
Rc: The R of the drug measured at a concentration of 125.00 µmol L−1for AGP, saturated level.
2.4.2 HPAC measurements
A 100% (v/v) 67.00 mmol L−1of K2HPO4 (pH 7.45) was employed as the mobile phase condition. In addition, the column temperature and flow rate were 37.00 °C and 0.50 mL min−1, respectively. Sodium nitrate, neutral compound, was utilised as a non-retained probe in order to determine the void time, ultimately used for a compound’s retention criterion. For conditioning the column to attain a stable baseline and a reproducible retention time, the column was conditioned with the mobile phase composition for 10.00 min under a flow rate of 0.10 mL min−1. In order to maintain the column, as recommended by the manufacturer, at the end of each day, 15% of 2-propanol in water for 15.00 mins was running through the column under a flow rate of 0.10 mL min−1. The injection volume was 10.00 ul and the wavelength chosen was 254.00 nm. Prior to the analysis, a 0.20 μm nylon filter was utilised to filter the analytes investigated followed by degassing for 15.00 min.
3 Results and discussion
3.1 Study of AGP binding with CHL and DAC by ILATP/ACE
To check the validity of our established ILATPS/ACE system, the well-known PRO-AGP binding model was used (Abd El-Hady and Albishri, 2018). In this study, seven concentrations of AGP ranging between 5.00 and 100.00 µmol L−1 were dissolved in a BGE containing 100.00 μmol L−1 EMImCl and 67.00 mmol L−1 K2HPO4 (pH 7.45). The mobility of PRO was normalised against the measured value for AC (300.00 µg mL−1) which was utilised as an electroosmotic flow marker (EOF) for calculating the mobility ratio, cancelling out its effects due to the viscosity change in the BGE following the addition of AGP. The non-linear regression approach was utilised to calculate the apparent binding constants between PRO and AGP (Abd El-Hady and Albishri, 2018). The ACE apparent binding constant calculated was 2.90 × 105 L mol−1 which does not differ significantly (P > 0.05) from the reference value (3.00 × 105 L mol−1) (Albani and Riva, 1989; Abd El-Hady and Albishri, 2018) indicating the suitability of the system developed.
Several rinsing procedures were utilised so as to avoid any changes in the migration time and peak areas of the analytes investigated which ultimately would have a negative impact on the reproducibility of the values obtained. The procedures included methanol, sodium hydroxide and hydrochloric acid to avoid any likelihood of EMImCl IL and AGP adsorption on the inner surface of capillary during the ACE analysis. The relative standard deviation (RSDs) obtained for the migration times and peak areas of the analytes investigated were ≥3 (n = 5) in the case of methanol and sodium hydroxide indicating the negative impact of these solutions (methanol and sodium hydroxide). Whereas when HCl solution was used, no changes were observed illustrating the suitability of this solution (HCl) for rinsing. Hence, the use of 0.10 mol L−1 HCl for 1 min followed by the deionised water for 2.00 min and then the BGE for 3.00 min was used to enhance the reproducibility of the migration times and peak areas yielding good precision values (RSDs < 0.9%, n = 5). This improvement of analysis was thought to be owing to the ability of HCl to eradicate any adsorbed molecules of EMImCl and/or AGP that might have existed on the interior surface of the capillary.
The developed ILATPS/ACE system was also employed to determine the apparent binding constants of two significant anti-cancerous drugs (CHL and DAC) in relation to the AGP protein. The effect of various types of alkyl chain imidazolium ILs combined with their concentrations ranging between 10.00 and 1000.00 μmol L−1, below their critical micelle concentrations (CMC), (Abd El-Hady et al., 2014) on CHL-AGP and DAC-AGP bindings were investigated as shown in Tables 1 and 2. As shown in the tables, the results related to the performance of studying ILs revealed that the utilisation of EMImCl up to 100.00 μmol L−1 demonstrated no significant deviation for the CHL-AGP and DAC-AGP apparent binding constants with the ILATPS/ACE in comparison with those measured by the HPAC. However, at 500.00 μmol L−1of EMImCl, a change with a significant differentiation (P < 0.05) was observed and this differentiation was increased as the concentration increased reaching 1000.00 μmol L−1, as illustrated in Tables 1 and 2. This variance might due to the water-miscible EMImCl IL acting almost like the chaotropic agents, in which, high concentrations are needed to denature the AGP to a complete unfolded state. Yet, in contradiction of the compounds investigated, high concentrations of EMImCl might lead to the AGP’s oligomerisation (Baker and Heller, 2009). Moreover, it was observed that utilising a longer alkyl chain CnMImCl (C2–C10) combined with their higher concentrations up to 1000.00 μmol L−1 greatly changed the apparent binding constants of the CHL-AGP and DAC-AGP (P < 0.05) in comparison with those binding values by HPAC, as reported in Tables 1 and 2. The reason for these changes might have been due to the increase of ILs lipophilicity from BMImCl to DMImCl, which as a result, un-preferably promoted their hydrophobic interactions with AGP. That is to say, IL cations with longer alkyl side chains affected the stability of AGP structure due to the increase in IL hydrophobic surface area contact with AGP molecule, and subsequently, has led to the AGP apparent binding constants deviation (Silva et al., 2014). In order to support this explanation; several techniques including fluorescence, UV–Vis, and FT-IR spectroscopy have been utilised and it has been concluded that the lipophilic force could alter the protein’s structure (Huang et al., 2013; Yan et al., 2012). In addition, the ILs anions effect on the CHL-AGP and DAC-AGP binding analysis were also examined. It was seen that the best results for the apparent binding constants were achieved in the presence of chloride and bromide anions unlike for tetrafluoroborate anion as indicated in Tables 1 and 2. The reason for this result could be attributed to the difference in size and electronegativity of the anions investigated. Therefore, the ionic liquid EMImCl is the optimum choice for studying the apparent binding constants of AGP with the drugs investigated. Fig. 1(A) and (B) illustrate the binding electropherograms between CHL and AGP in the absence and presence of EMImCl respectively. Fig. 2(A) and (B) demonstrate the binding electropherograms between DAC and AGP in the absence and presence of EMImCl respectively. Additionally, the traces “a” displays the CHL and AGP’s migration times when the AGP and EMImCl were absent, as seen in Fig. 1A and 2A. In the same wavelength, the traces “a” displays the CHL and AGP’s migration times when the AGP and EMImCl were present as seen in Fig. 1B and 2B. It was evidently seen that both CHL and DAC had a slower migration time than observed with the AC marker under the normal CZE mode, as shown in traces “a” in Fig. 1A and 2A. This behavior might owe to the dissociation of the COOH group forming a negatively charged CHL possessing a pKa of 5.80 as well as the phenomenon of tautomerisation of the amide group in DAC possessing a pKa of 4.45 under the physiological pH 7.45 (Abd El-Hady et al., 2015). Different letters (a,b,c) in the same column indicate that they are significant different (P < 0.05). Values are mean ± SD (n = 7). Different letters (a,b,c) in the same column indicate that they are significant different (P < 0.05).
Type of IL
Concentrations (µmol L−1) of IL
Binding constant (L mmol−1) by HPAC
10.00
100.00
500.00
1000.00
Apparent binding constants (L mmol−1) by ILATPS/ACE
EMImCl
1.99 ± 0.11a
1.99 ± 0.14 a
1.93 ± 0.15b
1.87 ± 0.17c
1.99 ± 0.10 a
BMImCl
1.94 ± 0.12b
1.92 ± 0.13b
1.86 ± 0.16c
1.80 ± 0.19 d
HMImCl
1.91 ± 0.09b
1.90 ± 0.11b
1.77 ± 0.16 cd
1.62 ± 0.32 e
DMImCl
1.81 ± 0.17c
1.72 ± 0.11 cd
1.60 ± 0.17 e
1.51 ± 0.21f
EMImBr
1.98 ± 0.17b
1.97 ± 0.05b
1.90 ± 0.09c
1.84 ± 0.06 cd
EMImBF4
1.82 ± 0.13c
1.77 ± 0.17 cd
1.68 ± 0.11 e
1.63 ± 0.13 e
Type of IL
Concentrations (µmol L−1) of IL
Binding constant (L mmol−1) by HPAC
10.00
100.00
500.00
1000.00
Apparent binding constants (L mmol−1) by ILATPS/ACE
EMImCl
6.95 ± 0.14 a
6.94 ± 0.11 a
6.89 ± 0.12b
6.80 ± 0.15c
6.94 ± 0.15 a
BMImCl
6.90 ± 0.10b
6.87 ± 0.11b
6.79 ± 0.16c
6.73 ± 0.15c
HMImCl
6.86 ± 0.12b
6.76 ± 0.11c
6.67 ± 0.10 cd
6.55 ± 0.12 e
DMImCl
6.81 ± 0.17b
6.73 ± 0.10c
6.61 ± 0.14 cd
6.51 ± 0.11 e
EMImBr
6.94 ± 0.17b
6.92 ± 0.12b
6.88 ± 0.11c
6.78 ± 0.06c
EMImBF4
6.81 ± 0.13b
6.72 ± 0.15c
6.64 ± 0.11 cd
6.59 ± 0.12 e
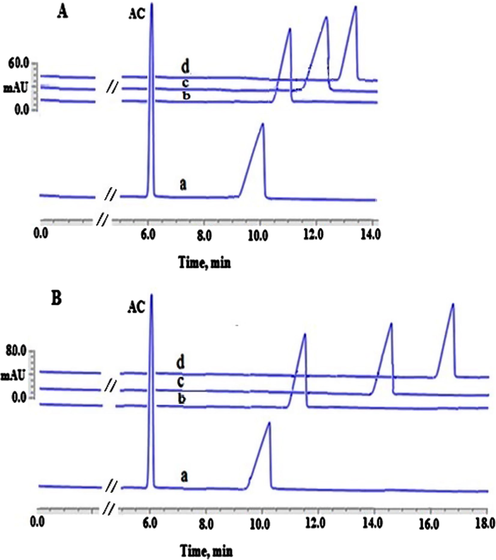
Illustration of the apparent binding between CHL and AGP absence (A) and presence of EMImCl (100 μmol L−1) (B). Experimental conditions: 100.00 µmol L−1 DAC; AGP concentrations (0) (a), (25.00 µmol L−1) (b), (50.00 µmol L−1) (c) and (100 µmol L−1) (d), phosphate buffer (67 mmol L−1) at pH 7.45, applied voltage 20.00 kV with a positive polarity, capillary’s temperature 25.00 °C, detection wavelength 254.00 nm, injection pressure 25.00 mbar for injection time 10.00 s.
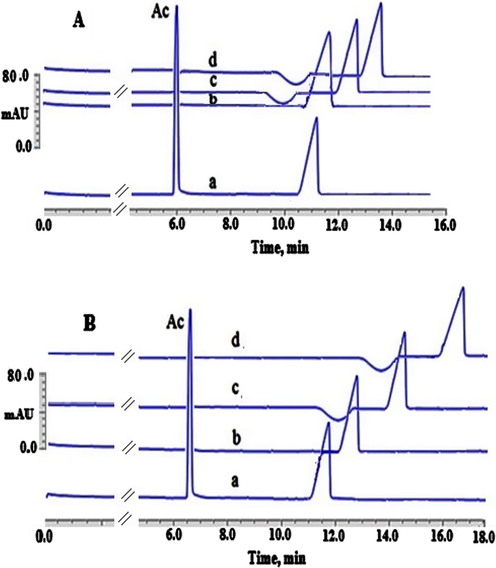
Illustration of the binding between DAC and AGP when EMImCl was absent (A) and when EMImCl was present (100.00 μmol L−1) (B). Experimental conditions: 100.00 µmol L−1 DAC; AGP concentrations (0) (a), (25.00 µmol L−1) (b), (50.00 µmol L−1) (c) and (100 µmol L−1) (d), phosphate buffer (67.00 mmol L−1) at pH 7.45, applied voltage 20.00 kV with a positive polarity, capillary’s temperature 25.00 °C, detection wavelength 254.00 nm, injection pressure 25.00 mbar for injection time 10.00 s.
The mobility ratios for CHL and DAC without the addition of EMImCl were 0.55 and 0.52, respectively. The same mobility ratios of CHL and DAC were attained when EMImCl (100.00 μmol L−1) was present. Thus, the interactions between the investigated drugs or AGP and EMImCl IL are thought to be very minimal. This is because there was no shift observed in the mobility ratios for both drugs when the IL was added. Additionally, an insignificant shift in the AC’s migration time (EOF marker) was observed when the IL or AGP was present in comparison with their absence. This finding might be referred to the change of BGE’s viscosity after the addition of IL or AGP. Yet, the effects of the mobility ratio in the apparent binding calculations was cancelled out. It was also seen that the investigated analytes’ migration times were clearly changed after adding various concentrations of AGP ranging from 25.00 µmol L−1 trace “b” to 100.00 µmol L−1 trace “d” in the presence of 100.00 μmol L−1 EMImCl (B) in comparison with their absences (A). These observations facilitated, consequently, the use of ACE for the calculation the apparent binding constant of CHL-AGP and DAC-AGP, as demonstrated in Tables 1 and 2. In addition, the HPAC technique was utilised as a verification approach in order to validate and increase the reliability and level of the confidence of the calculated apparent binding constants of CHL-AGP and DAC-AGP attained by the proposed ILATPS-ACE system. In HPAC, employing a AGP immobilized stationary phase is commonly practiced without the need for any stabilising agents such as ILs (Hage et al., 2011, 2009; Li et al., 2016). In the HPAC experiments, a model called zonal elution was utilised to gain information about the drug-protein binding equilibria. This was conducted through the injection of a 10.00 µL of 100.00 µmol L−1 CHL or 100.00 µmol L−1 DAC (Hage et al., 2011). The model was based on the interaction of the drugs investigated with the AGP immobilised stationary phase in the presence of 100% (v/v) K2HPO4 (67.00 mmol L−1) at pH 7.45 as the mobile phase (Hage et al., 2011). In order to examine the extent of this interaction between the drugs investigated and the AGP immobilised stationary phase, the plots of 1/k on Y-axis versus [drug] on X-axis was constructed (Hage et al., 2011, 2009; Li et al., 2016). As demonstrated in Tables 1 and 2; the calculated apparent binding constants by HPAC were found to be very close to those obtained by the ILATP-ACE when EMImCl (100.00 μmol L−1) was present without any substantial variance (P > 0.05) (Tables 1 and 2). Hence, the HPAC values attained were in line with the ILATP/ACE analysis indicating that EMImCl has indeed improved the stability of AGP in ACE analysis without any negative impact on the binding sites of AGP with CHL and DAC drugs. In addition, this alternative technique (HPAC) has, consequently, indeed increased the confidence and reliability of the results obtained and proposed by the ILATPS-ACE system for calculating the apparent binding values of CHL-AGP and DAC-AGP.
3.2 Stability of AGP during storage of BGE
In order to examine the stability of the AGP solution (25.00 µmol L−1) at the ambient conditions for a period of 30 days, 100.00 and 200.00 µmol L−1 of EMImCl were prepared. Table 3 demonstrates the mobility ratios of CHL and DAC during the investigated storage period as well as compares the results obtained in the absence of EMImCl. The comparison was made between the preserved protein solution containing the IL versus freshly prepared protein solution in the absence of IL. It was observed that the mobility ratios were quite constant in the presence of 100.00 µmol L−1 EMImCl compared with those in the absence of IL up to 30 days storage time under the ambient temperature. Moreover, the use of 100.00 µmol L−1 IL might decrease the cost of AGP’s consumption material as well as the time required for the ACE analysis without the need for a daily preparation of AGP solution as commonly practiced. However, a high concentration of EMImCl (200.00 µmol L−1) has failed to reserve the constant values of mobility ratios for the period studied. These observations demonstrated the vital impact of the concentration of EMImCl on the reservation of the AGP’s structure in the solutions. Furthermore, storing the AGP solution prepared in EMImCl/phosphate solution at 4.00 °C refrigeration temperature has indeed increased the AGP stability up to 90 days (data not shown). At 4.00 °C, a two-phases of IL-rich phase were created as well as a negatively charged AGP bound strongly to the positively charged EMIm in the IL-rich phase. This has led to, in turn, the π–π bonding with the imidazolium ring resulting in maintaining the AGP’s activity (Abd El-Hady and Albishri, 2018). Furthermore, the physiological pH 7.45 has also prevented the conformational changes of AGP and helped to maintain it. This behavior of EMImCl-ATPS involving its phase diagrams in K2HPO4 at different temperatures was previously investigated (Zafarani-Moattar and Hamzehzadeh, 2007). In general, ILATPS is based on a phase-splitting promoter (usually an inorganic salt) in order to facilitate the formation of two immiscible aqueous phases from a homogeneous aqueous solution of a hydrophilic ionic liquid. Therefore, an ionic liquid-rich and an inorganic salt-rich phase could be obtained, both of which often have water as the main component. Furthermore, the same occurred in the EMImCl-ATPS system, where the use of K2HPO4 salt-induced EMIm cation salted out and formed the upper IL-rich phase and K2HPO4-rich lower phase. Meanwhile, the water structure induces the chloride anions to increase the dielectric constant of the aqueous phase. This leads to more promoting of the low dielectric EMIm cations focusing in the upper phase concurs with more focusing of K2HPO4 salt in the lower phase. This helps to preserve the charge balance. The K+ ions are concentrated in the lower phase to balance Cl− anions (Zafarani-Moattar and Hamzehzadeh, 2007). With the addition of AGP, it promptly distributed into the upper IL-rich phase possessing the strongly bounded cationic EMIm via electrostatic attractions and π–π bonding. In addition, the impact of temperature on the separation of the bi-phasic system was also observed. On one hand, when the temperature is less than 4.00 °C, the formation of bi-phasic system is promoted; on the other hand, higher temperatures accelerate the movement of the IL and salt through the biphasic boundary resulting in one-phase’s formation. In the literature, the effect of temperature on the separation of the bi-phasic system was attributed to the ions’ hydration strength. The cause of this unusual phenomenon might be seen in a minor reduction of the Gibbs free energy of hydration for the IL ions with the reduction of the temperature (Du et al., 2007). Thus, the ions solubility of the IL goes down and therefore, the separation of the bi-phasic system occurs. After that, the formed bi-phasic system could be swiftly switched to a one-phase via shaking for a period of 3.00 min at the ambient temperature prior to the CE’s analysis (Zafarani-Moattar and Hamzehzadeh, 2007).
Time
Mobility ratio
CHL (100.00 µmol L−1)
DAC (100.00 µmol L−1)
Without EMImCl
100.00 µmol L−1 EMImCl
200.00 µmol L−1 EMImCl
Without EMImCl
100.00 µmol L−1 EMImCl
200.00 µmol L−1 EMImCl
1st day
0.553
0.553
0.550
0.539
0.539
0.534
5th day
0.553
0.553
0.550
0.539
0.538
0.533
10th day
0.552
0.552
0.549
0.538
0.538
0.533
15th day
0.552
0.552
0.548
0.537
0.537
0.531
20th day
0.551
0.551
0.546
0.537
0.537
0.531
25th day
0.551
0.551
0.544
0.537
0.536
0.530
30th day
0.551
0.551
0.544
0.536
0.536
0.530
4 Concluding remarks
In the current work, it was successfully investigated that the ILATPS/ACE system could be utilised to improve the activity and stability of AGP resulting in the enhancement of the accuracy of apparent binding measurement with the drugs investigated. The ILATPS/ACE system was found to be very sensitive to the type of IL anions, the concentration of ILs, as well as to the type of IL cations. The successful application of the accurate calculation of CHL-AGP and DAC-AGP apparent binding constants was attained through 100.00 μmol L−1 EMImCl prepared in 67.00 mmol L−1of phosphate buffer (pH 7.45). Furthermore, the EMImCl-ATPS approach developed was found to maintain the activity of AGP in the BGE under the lab environment, 25 ± 2 °C, for a period of 30 days. The main forces responsible for the stability of AGP activity were π–π and electrostatic interactions between EMIm cation and the AGP anions under the physiological pH 7.45. Moreover, at 4.00 °C, the ILATPS system has led to lengthening the stability of AGP activity for a period of time reaching 90 days. The proposed ILATPS system could be more looked at through an extensive investigation with various proteins, predominantly the proteins that are negatively charged at the physiological pH. Additionally, the ILATPS/ACE system could be applied for the binding analysis of proteins with a variety of acidic or basic drugs.
Acknowledgements
The author would like to thank Prof Hassan Albishri and Prof Deia Abd El-Hady for their valuable guide during this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- J. Chromatogr. B. 2012;911:180-185.
- Electrophoresis. 2015;36:3080-3087.
- M. Methods. 2018;146:120-125.
- J. Pharm. Biomed. Anal.. 2010;52:232-241.
- Electrophoresis. 2014;35:1956-1964.
- Br. J. Clin. Pharmacol.. 1984;18:244-246.
- Br. J. Clin. Pharmac.. 1989;27:229-234.
- Nature. 1956;177:771-774.
- Colloids Surf., A. 2020;601:124954-124964.
- Chem. Eng. J.. 2009;147:6-12.
- J. Chromatogr. A. 2011;1218:1347-1352.
- Green Chem.. 2007;9:177-183.
- Chem.-A Euro. J.. 2007;13:2130-2137.
- Trends Anal. Chem.. 2013;48:112-131.
- Electrophoresis. 2014;35:170-189.
- Electrophoresis. 2016;37:1591-1608.
- J. Chromatogr. A. 2020;1623:461209-461217.
- J. Am. Chem. Soc.. 2003;125:6632-6633.
- J. Sep. Sci.. 2009;32:835-853.
- Curr. Drug. Metab.. 2011;12:313-328.
- Science. 1964;143:1176-1177.
- Curr. Drug Metab.. 2013;14:226-238.
- Spectrochim. Acta. 2013;104:377-382.
- Talanta. 2019;146:448-454.
- Trends Anal. Chem.. 2010;29:1336-1346.
- Adv. Protein Chem. Struct. Biol.. 2016;102:1-39.
- Electrophoresis. 2009;30:1888-1898.
- Metheds. 2018;146:76-92.
- Electrophoresis. 2008;29:3257.
- J. Chromatogr. A. 2007;1151:65-73.
- Pharmacopsychiatry. 1983;16:82-85.
- Phys. Chem. Chem. Phys.. 2014;16:23394-23403.
- Anal. Chim. Acta. 2019;1052:170-178.
- J. Biochem. Biophys. Methods. 2001;48:103-116.
- Microchim. Acta. 2011;174:63-71.
- J. Luminesc.. 2012;132:622-628.
- J. Chromatogr. A. 1997;766:15-25.
- J. Chem. Eng. Data. 2007;52:1686-1692.







