Translate this page into:
Investigating the efficiency of the microwave heating process for the carbothermic synthesis of cobalt/nickel alloys
⁎Corresponding author. hbeiki@qiet.ac.ir (Hossein Beiki)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
High energy consumption and its associated pollution fare among the significant issues of metal production systems. Traditional furnaces currently used in the production of alloys. The microwave heating process is a novel approach with numerous advantages compared to the conventional heating processes. On the other hand, cobalt/nickel alloy, is an important alloy with extensive applications in jet engines, gas turbines, and petroleum refining. The present research addressed the efficiency of the microwave heating process for the carbothermic synthesis of cobalt/nickel alloys. Moreover, the morphology of the produced material was evaluated. The synthesis method was based on the reduction of cobalt/nickel oxides to cobalt/ nickel alloys. Two parameters of the reaction time and the composition of the granules containing cobalt/nickel oxides were investigated to assess the efficiency of the reduction process. The results showed a decrease in the oxygen content from 20 to about 10 wt% by prolonging the processing time from 10 to 20 min. XRD analysis also indicated no crystal structure related to nickel oxide in the samples processed for 20 min. The diameter of the crystals also increased by about 71 % by prolonging the reaction time from 10 to 20 min, suggesting the growth of the crystals and enhanced adhesion of the particles. The number of oxygen atoms was also decreased by about 50 % upon increasing the reaction time from 10 to 20 min, indicating a better reduction reaction. A rise in the nickel oxide content in the raw granule from 30 % to 60 %, led to a 3.8-fold enhancement in the number of oxygen atoms in the reduced product.
Keywords
Microwave heating
Carbothermic Synthesis
Cobalt/Nickel alloys
Reduction Reaction
1 Introduction
Our surrounding environment is deteriorating rapidly due to human beings’ destructive urban and industrial activities (Pazhoohan et al., 2019). One of these damaging activities is the significant consumption of fossil fuels for transportation, domestic and industrial means, and metal smelting industries, especially iron and copper smelting (Beiki and Soukhtanlou, 2020; Nourollahi, 2022). Fossil fuels usage produce gases harmful to the atmosphere, resulting Greenhouse effect and severe climate changes. Severe climate changes lead to droughts or unexpected rainfalls. In Iran, in the beginning ten days of 2019 spring (March 21 to 30), more than 42 people died due to unexpected rains and floods. Nowadays, a wide range of energy and fuel resources are needed to meet the needs of human societies. The environment has suffered irreparable damage, by human use of fossil fuels. To reduce these losses and destructions, it is recommended to use alternative green fuels and renewable sources of energy to save energy (Beiki et al., 2009; Amui Khorshidi et al., 2022; Ansarian and Beiki, 2022).
The use of microwave irradiation in the frequency range of 300 MHz to 300 GHz, in the metallurgical processes can offer advantages such as rapid process, material sensitivity, volumetric and uniform heating, facile power control, decreased activation energy, and lower CO2 emissions (compared to the conventional heating) (Chun, 2017; Borah, 2022). In the conventional methods of metal oxide reduction process, energy consumption is very high as a result, environmental pollution increases. In addition, the amount of reduction degree and metal oxide conversion in the conventional heating process are much lower than those in the microwave heating (Chen, 2017; Anwar, 2015; Oueslati, 2020). The idea of applying microwave heating, which is a kind of radio waves, to improve mineral and metallurgical processes was introduced about 40 years ago (Samouhos, 2012). Most published studies have reported that microwave heating provides an efficient extractive metallurgy process in terms of economy and product quality (Hao, 2023).
The effect of microwave irradiation on iron phase and gangues were investigated, revealing that microwave heating, even at lower temperatures (about 800 °C), can be accelerated the reduction process due to microwave heating advantages; it could be enhanced the densification of the metallic iron phase and separation of the iron and slag phase (Chun, 2017). Samouhos et al. (Samouhos, 2012) reported about 70 % the reduction degree for reduction process of nickeliferous laterite ore under 800 W and 2.45 GHz microwave heating after 480 s heating. They also observed that the mass of the laterite-lignite sample caused the reduction degree to be dropped to 25 % under the same conditions (Samouhos, 2012). By using microwave-assisted pyrolysis, the degradation of the Ni-Co-Al material was investigated, indicating that the microwave heating rate was 3.5 °C/s, which was nearly 20 times faster than the conventional heating rate (Diaz, 2018). The effect of sulfur on iron oxide reduction under microwave irradiation (1050 W and 2.45 GHz) was investigated, showing a gap between the reduced iron and its’ surrounding. The gap in the high-sulfur sample enhanced the H2-reduction reaction as compared with another phase (a pure magnetite sample), that was probably improved the gas accessibility to unreact parts (Amini, 2020). By using blast furnace dust and hazardous electric arc furnace dust, which were distributed in the core and shell of pellets, Ye et al. (Ye, 2021) produced direct reduction iron during microwave heating (1400 W, 2.45 GHz). They concluded that it was necessary to increase the C/O molar ratio in the composite pellets to achieve a high reduction degree (>90 %) such that by increasing the C/O molar ratio from 0.55 to 0.7, the iron oxide in samples was reduced completely (around 94 %) (Ye, 2021). For the purpose of investigating cobalt producing from the cobalt oxide reduction under the microwave heating on an experimental scale, Shojae and Khoshandam employed a new two-step fixed bed reactor (Shojae and Khoshandam, 2021). The first step was the pyrolysis reaction of glycerol as an indirect reducing agent, and the second step was the reduction reaction of cobalt oxide granules that were embedded in the bed of activated carbon (AC). Both steps were merged and occurred in the fixed bed reactor, led to reduce operating and fixed cost. Their results revealed that the conversion rate significantly increased with reducing agent amount and temperature. The conversion rate reached 100 % for 7 cc glycerol at 810 °C and 91.75 % at 900 °C after 4 min (Shojae and Khoshandam, 2021). Similarly, they studied iron/cobalt alloy production using glycerol as an indirect reducing agent under the microwave heating both experimentally and numerically. The authors showed that the conversion rate increased with amount of FeO in the sample. It was reported that the conversion rate of (FeO)0.8/(CoO)0.2 to Fe/Co alloy reached 92 % at 850 °C and 7 cc reducing agent (glycerol) (Shojae and Khoshandam, 2021). In the study of Mitra et al. (Mitar, 2021), microwave thermal synthesis of hematite (α-Fe2O3) and goethite (α-FeOOH) from 0.1 M FeCl3 solution was performed under 800 W microwave irradiation at 150 °C, 200 °C and 250 °C for 20 min. Their results demonstrated that the transformation of goethite to hematite rate was slowed down in 0.25 % cetyltrimethylammonium bromide as a cationic surfactant, at 200 °C. Still, at 250 °C and in other concentrations, cetyltrimethylammonium bromide had no effect on transformation rate (Mitar, 2021).
Cobalt/nickel alloys have vast applications in various industries such as petrochemical, oil and gas refinery, renewable energy production, water electrolysis, and so on (Beiki and Keramati, 2019; Feng, 2017; Oriňáková, 2006; Lupi et al., 2009). Recently, in the study by Maurya et al. (Maurya, 2023) observed that cobalt/nickel alloys on the copper substrate could be a suitable choice for hydrogen production by water splitting. Due to the many applications and good features of cobalt/nickel alloys in the industry, it is necessary to provide new production methods with high energy efficiency. According to the literature review, no work has addressed the microwave-assisted reduction of cobalt/nickel oxides composite. Therefore, this experimental study is aimed to fill this gap by using AC (as an excellent receptor of microwave energy) in the production of cobalt/nickel alloys from cobalt/nickel oxides composite nanoparticles. A new microwave-assisted alloy production procedure was evaluated for producing cobalt/nickel alloys by reduction of cobalt/nickel oxides. To this end, the reactor and process of synthesis were first briefly introduced and the quality of raw materials was verified by characterization. In the next step, the influence of two different parameters of cobalt/nickel oxides composition and the reaction time was investigated on the rate of the reduction reaction. Finally, the results were compared with previous data to make a conclusion. The novelty of this paper lies on the carbothermic reaction basis of this system which was evaluated for the first time to produce cobalt/nickel alloys. Previous studies have only addressed the carbothermic reduction of iron oxide under microwave heating (Chun, 2017). As the temperature-rising trend of AC under microwave heating and its effect on the reduction reaction of metal oxides were previously explored (Shojae and Khoshandam, 2021), only two important parameters of sample composition and the time of reaction were focused. The first parameter is very important as each metal oxides have a distinct kinetic mechanism in the carbothermic reduction reaction under microwave heating, thus, the content of each material can affect the efficiency of process (Ghanbarabadi and Khoshandam, 2018).
2 Material and methods
2.1 Materials
Merck cobalt oxide and nickel oxide nanoparticles (US Research Nanomaterials, USA Co.) with 99 % purity were purchased, and Merck AC with 95 % purity was also used. In addition, a 20-liter cylinder containing argon gas with a high purity of 97 % was used. In addition, polyvinyl alcohol (PVA) supplied by Wanwei Co. (China) was also used.
2.2 The experimental system
In this research, a microwave oven with 2.45 GHz frequency, 1200 W fixed power, 42 L working volume, and 45.8 × 30.8 × 54.4 cm3 dimensions were utilized for the synthesis of cobalt/nickel alloys. Fig. 1 shows a schematic of the utilized setup. As can be seen, this system consisted of a quartz reactor with a volume equal to 0.00942 m3, to perform the carbothermic reduction reaction of cobalt/nickel oxides to cobalt/nickel alloys under microwave heating. In addition, an argon cylinder was used as a carrier gas. The inlet flow of argon gas was adjusted by using a rotameter. The argon carrier gas flow was fixed to be 33.4 ml/min before and after each experiment for 30 min. The reactor was vertically placed in the microwave oven, and its upper part was covered with a Teflon cap. The melting temperature threshold of this cap was estimated to be nearly 300 °C. A k-type thermocouple was used to measure the temperature. Two holes were installed on the cap to enter the carrier gas and to drive out the gases resulting from the reaction along with the carrier gas. To provide the required heat for the reactions, an AC bed was used as a microwave absorber to convert microwaves into heat. The laboratory condenser was only used to cool the gaseous products from the reactor to prevent possible harm.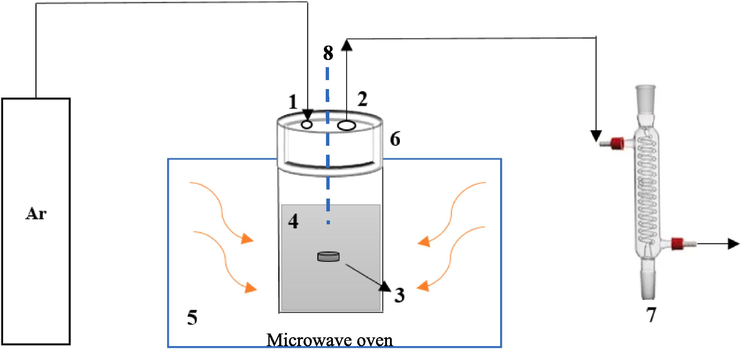
The setup used in this research. 1- Argon gas inlet, 2- gas outlet, 3- metal oxide granule, 4- AC bed, 5- microwave oven, 6- Teflon cap and 7- laboratory condenser, 8- Thermocouple.
2.3 The experimental procedure and employed characterizations
For investigating the efficiency of microwave heating at the carbothermic synthesis of cobalt/nickel alloys, two parameters of the time of reaction and the sample composition were concerned. Since, the effect of temperature elevation on the reaction under microwave heating was previously addressed and its direct impact on the reduction reaction was determined (Shojae and Khoshandam, 2021), the parameter of temperature was not considered. Therefore, three reaction timings of 10, 15, and 20 min were selected, and to evaluate the sample composition, different amounts of cobalt/nickel oxides were prepared as granules. Table 1 shows the prepared samples and their compositions.
Sample type
(CoO)x/(NiO)1-x, x=
Sample 1 (S1)
0.8
Sample 2 (S2)
0.6
Sample 3 (S3)
0.3
For each experiment, different amounts of cobalt/nickel metal oxides were prepared in the form of cylindrical granules, as can be seen in Fig. 2, and placed in the AC bed. In order to make granules, firstly, different amounts of metal oxides were appropriately mixed on a laboratory watch glass several times. Then, 5 % PVA dissolved in deionized water as a binder was sprayed on mixed powder to ensure the cohesion of particles after pressing tasks. To stabilize the granule particles, at each stage, the granules produced were compacted by a manual granulator device under 10 bar press. Finally, the obtained granules were calcined at 400 °C for 1 h in a box furnace, and the resulting material was washed twice with deionized water and dried in an oven (at 80 °C for 12 h). Fig. 2 depicts the produced granules before the heating operation.
A picture from the produced granule, a diameter of 1.5 cm and a thickness of 0.5 cm.
To characterize the reduced granules, the analysis of scanning electron microscopy, or SEM (TESCAN), field emission scanning electron microscopy, or FESEM (TESCAN, MIRA III), X-Ray diffraction analysis, or XRD (PWa730-PHILIPS, Cu-Ka source), and energy dispersive spectroscopy, or EDS (TESCAN, SAMX detector) were utilized.
3 Result and discussion
3.1 Characteristics of purchased materials
Fig. 3 shows the TEM image and XRD pattern of purchased nickel oxide powder. Based on the TEM image, it can be concluded that the particle size of nickel oxide is less than 20 nm, and the shape of its particles is quasi-spherical. Based on the XRD analysis, the purity of the nickel oxide was also confirmed, and no disturbing peaks were observed in the XRD pattern. Fig. 4 also shows the TEM image and XRD pattern of purchased cobalt oxide powder. As can be seen, based on the FESEM analysis, the size of the used cobalt oxide particles is around 50 nm, and its shape is spherical.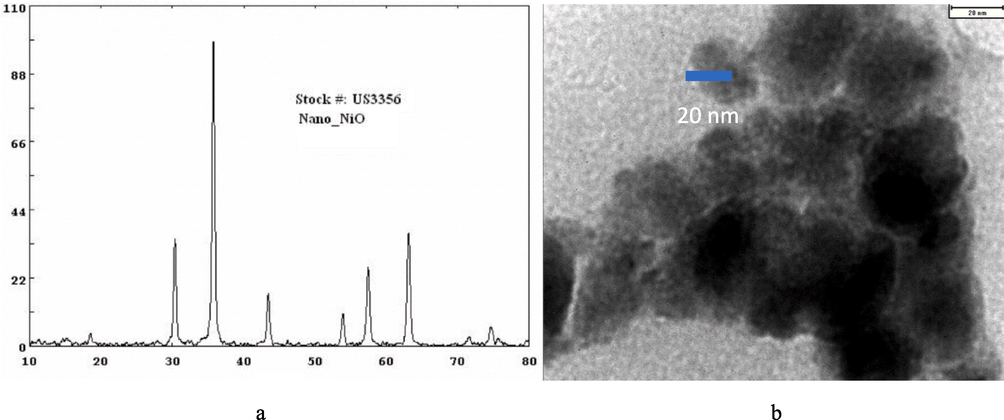
Analyzes related to purchased nickel oxide. a: XRD analysis and b: TEM analysis (received from US Research Nanomaterials company).
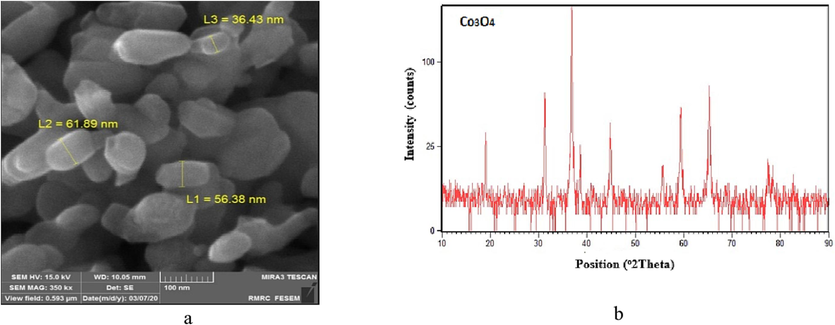
XRD and FESEM analyses related to cobalt oxide. a: TEM analysis and b: XRD pattern.
Scherer's equation is presented in Eq. (1) (Matin, 2010):
Based on Eq. (1), the mean crystal size of cobalt oxide powder was determined to be 32 nm. Fig. 5 shows the EDS analysis of pure cobalt oxide. As observed, cobalt oxide powder contains about 71 % by weight of cobalt and 29 % by weight of oxygen.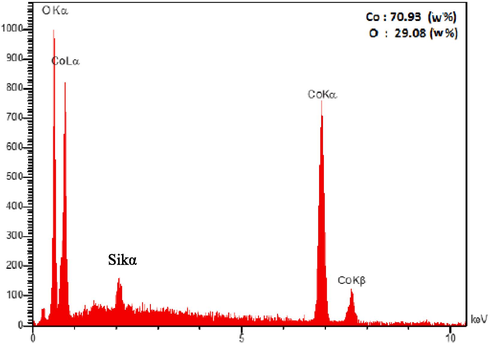
EDS analysis of pure cobalt oxide.
3.2 The effect of reaction time
For the experiments related to the reaction time, S1 sample was selected. This sample had 80 wt% cobalt oxide and was selected to compare with a related reference (Ghanbarabadi and Khoshandam, 2018).
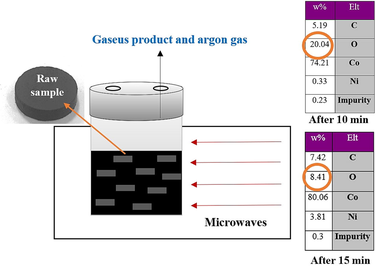
Fig. 6 demonstrates the results of analyzing the reduction of cobalt/nickel oxides granules to cobalt/nickel alloys for different reaction times in the S1 sample. As seen, nickel particles exhibited different morphologies at different reaction times. For the reaction times of 15 min and 20 min, the particles well grew which might increase the porosity of the sample. For the reaction time of 20 min, the sintering rate of the semi-spherical particles was much higher than those reacted for 10 min. In the case of 15 min reaction time, the nickel particles showed a rod-shaped form while spherical particles were achieved at the reaction time of 10 min. Based on Fig. 6 (200 nm magnification), the size of the particles in 20 min reaction time was greater than that of 10 min reaction time. Higher surface melting also occurred due to the prolonged the reaction time and more contact of granules with high-temperature AC bed particles with metal which in turn, enhanced the adhesion of the particles and increased the formation of large particles. Therefore, the reason for the increase in the size of the particles during 20 min reaction time is the surface melting and adhesion of particles. Noteworthy, microwave radiation affected the function of thermocouple in measuring the temperature trend which led to an error of ∼ 3 %.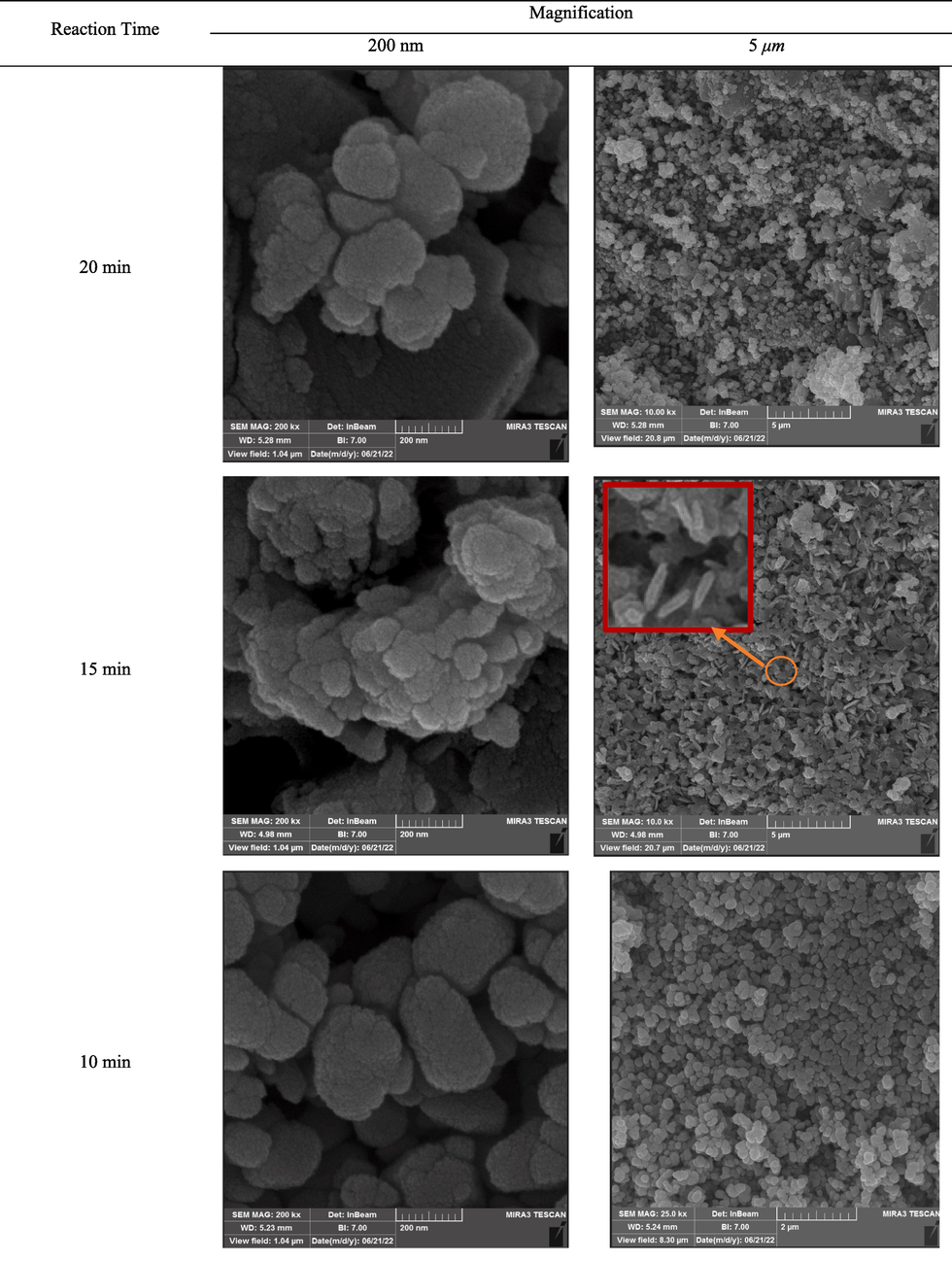
FESEM picture of cobalt/nickel alloy from the reduction reaction with different reaction times; the red marked rectangle shows the nod-shape nickel oxide.
Fig. 7 shows the EDS analyses related to the samples produced at reaction times of 20 min, 15 min and 10 min. Based on these patterns, any significant impurities were not detected in the produced materials. In addition, with an increase in the time of reduction reaction, the amount of oxygen in the molecular structure of the produced materials decreased significantly. As can be observed, the peak related to the oxygen element was determined to be significantly variable, indicating the percentage change of the oxygen weight fraction in the sample composition. The oxygen content of the product was reduced by prolonging the reaction time from 10 to 15 min and more. The reduction of oxygen content demonstrated the higher reduction rate of metal oxides to cobalt/nickel alloy.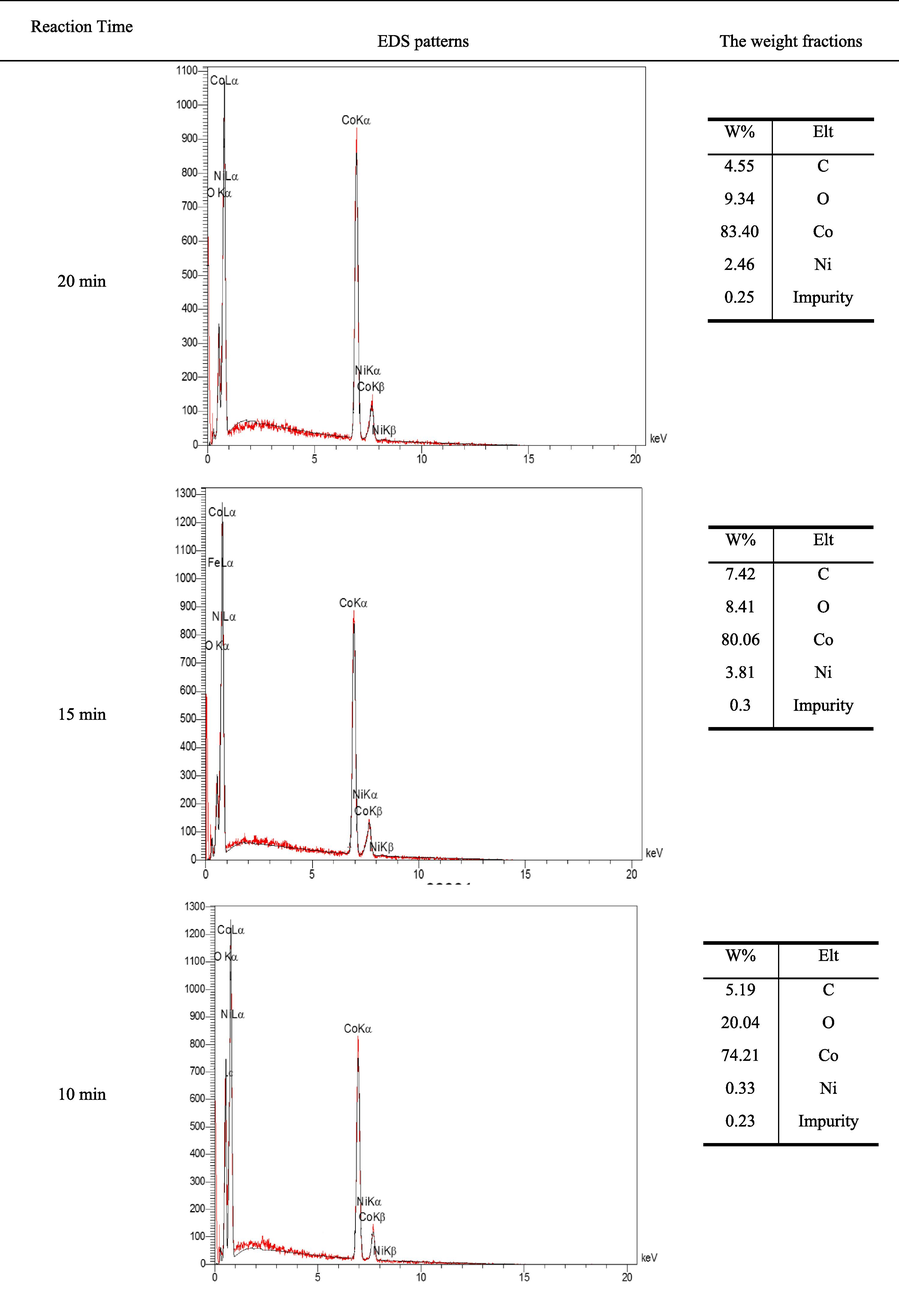
The EDS of produced alloys at different times of reduction reaction, for S1.
Fig. 8 shows the XRD analyses of the obtained products at different times of reduction reaction. As can be seen, the reaction time of 20 min caused the proper conversion of metal oxides to cobalt/nickel metals without any side peaks. For the reaction time of 15 min, the peak near 44° is related to the cobalt/nickel alloy. In XRD pattern, the peaks near the 52° and 78° positions are related to pure nickel and cobalt oxide. In the case of 10 min reaction time, only nickel oxide was converted to nickel. Based on Fig. 8, no crystalline structure of nickel oxide was found in the samples with reaction time of 20 min, reflecting that all nickel oxide particles were converted into nickel. However, considering peaks in all positions, the peaks related to cobalt oxide in the system were clearly visible, suggesting a far faster carbothermic reduction reaction of nickel oxide to nickel than the reduction reaction of cobalt oxide to cobalt in a constant reduction reaction time. Based on Scherer's Eq. (1), the size of crystals in cases of obtained materials at different reaction times was calculated and presented in Table 2.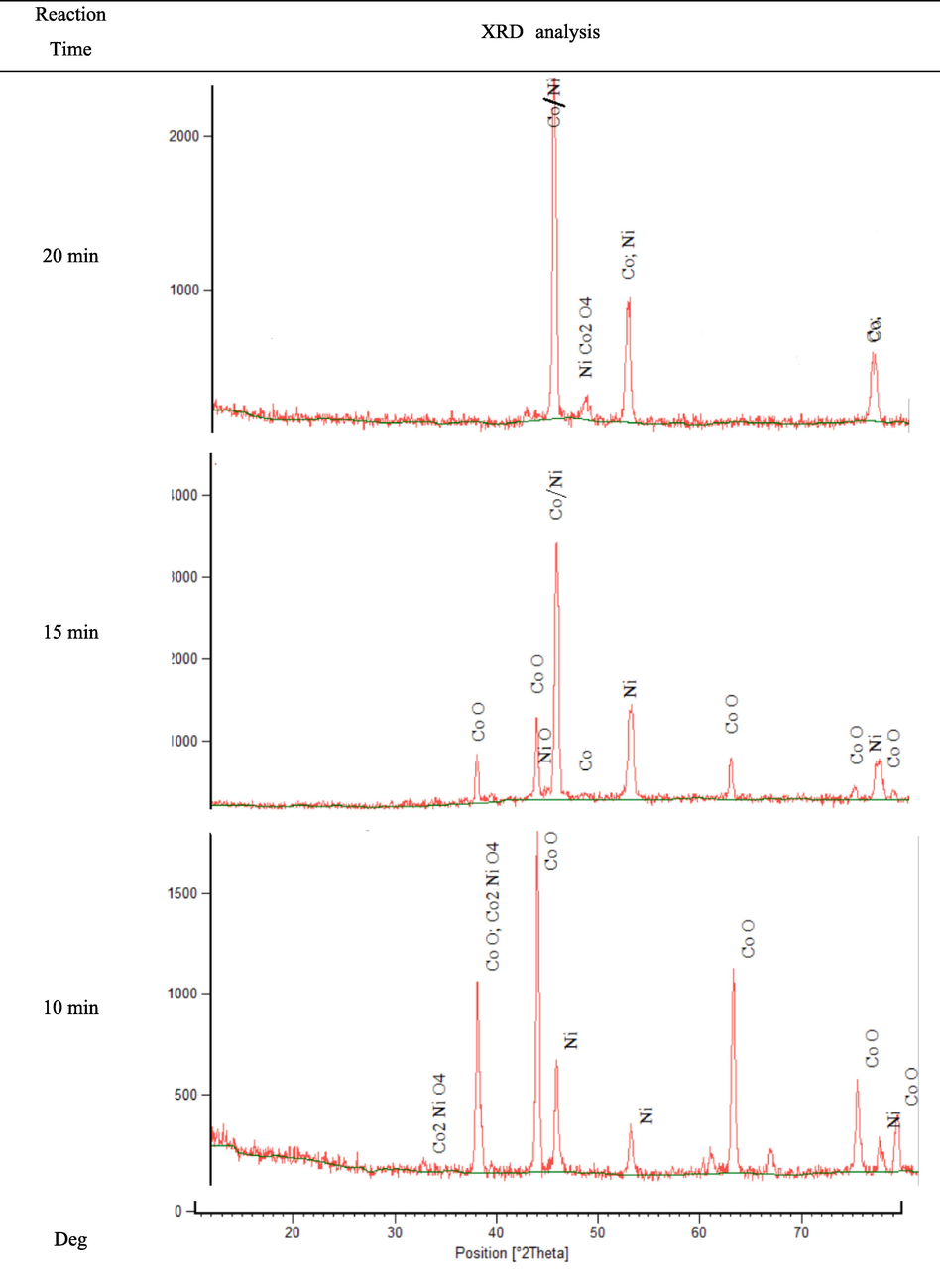
XRD patterns of reduced samples at different times of reduction reaction (For S1).
The time of reduction reaction
20 min
15 min
10 min
The crystalline size (nm)
48
47
29
As can be seen in Table 2, by increasing the time of reduction reaction from 10 min to 20 min, the crystalline size of obtained alloys was increased. During the reduction of metal oxides, in the first steps of the reaction, the amount of porosity between the particles decreased with an increase in the temperature of granules. In addition, with the increasing of the AC bed temperature and residence time of the granules in the AC bed, the sintering rate of the particles increased, and crystals with a larger diameter were formed. As a result, increasing the diameter of the pores between the crystals can be justified.
3.3 The effect of cobalt/nickel oxides composition
In the previous section, different reaction times were evaluated. As the reaction time of 15 min led to an acceptable reduction, it was selected for the rest experiments.
Fig. 9 shows the FESEM analyses related to the reduction of raw granules containing cobalt/nickel oxides with different compositions under 15 min microwave heating. As observed in Fig. 9 (magnification of 200 nm), the cohesion and agglomeration of the particles was more in the S1 compared to S3, leading to higher porosities on the surface of samples. The increase of these porosities facilitated the reaction of cobalt/nickel oxides with AC particles, and a purer alloy was produced considering S3. According to the FESEM images, it can be seen that the crystal growth rate of the particles in S1 is much better than S3, due to making a better cohesion of particles.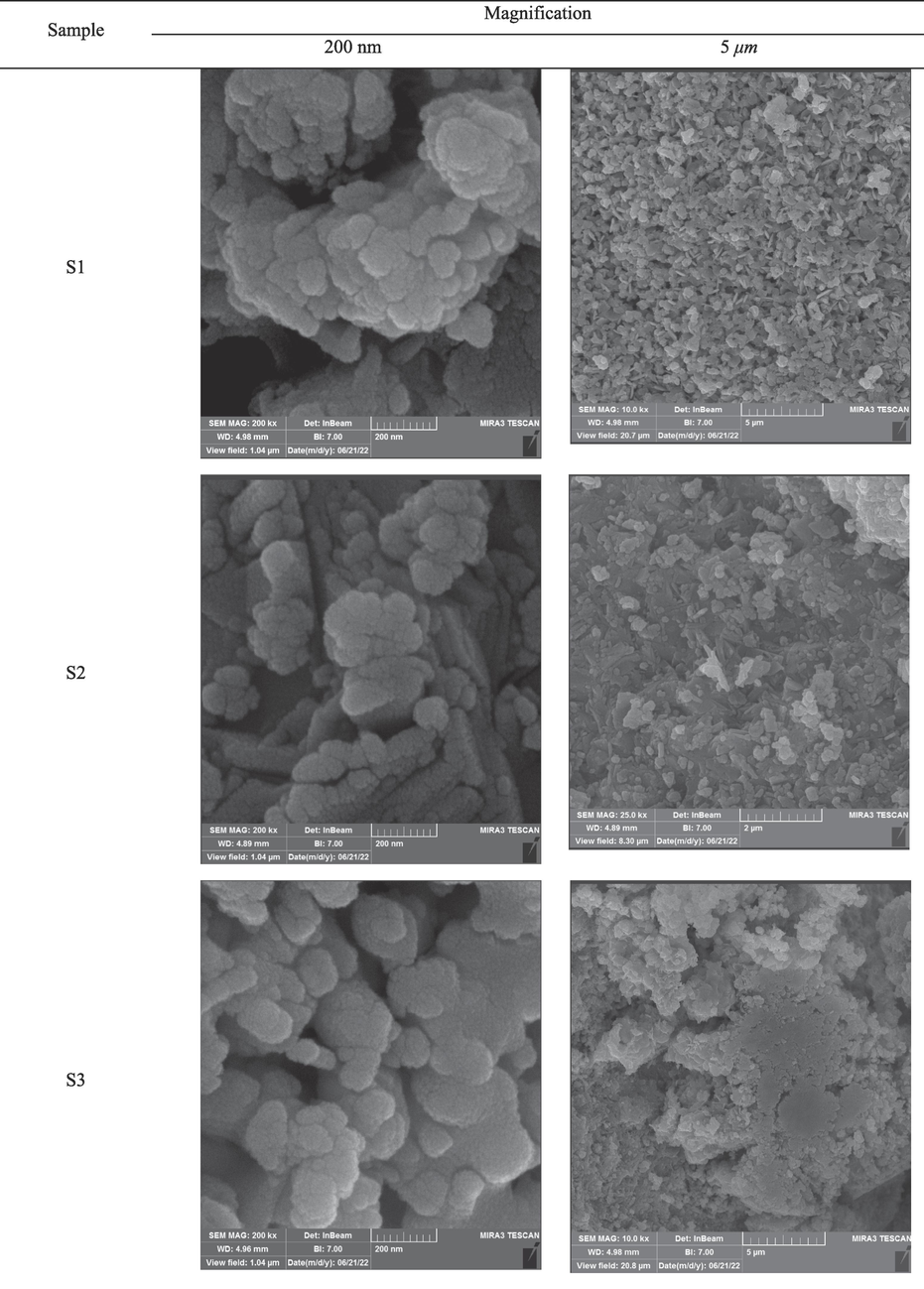
FESEM pictures of Ni/Co alloy obtained from the reduction reaction with different compositions of granule (at 15 min).
Fig. 10 shows the EDS analyzes related to the product of the reduction reaction of granules containing three different amounts of cobalt/nickel oxides. As can be seen, for S3 the weight percentage of oxygen atoms was much lower than S2. Based on Fig. 10, for S2, more amount of carbon particles was penetrated through the granule’s porosities, indicating more or bigger cracks compared to S1 and S3. However, the weight percentage of oxygen atoms of S2 and S3 was much higher than S1. Fig. 11 demonstrates the XRD analysis related to the products obtained from the reduction reaction of granules with different compositions of metal oxides. As can be seen, in cases of S1 and S2, almost all nickel oxide particles were converted to nickel metal, and there were not any significant peaks related to nickel oxide crystals. In addition, in the XRD pattern of S1, the peaks related to cobalt oxide were very weak and can be ignored compared to S3. The lower number of oxygen atoms in S1 is also proof of the better reduction reaction of S1 compared to S3. As can be seen, considering S2 or S3, the nickel oxide was reduced better than cobalt oxide due to thermodynamics reasons or higher reaction rate.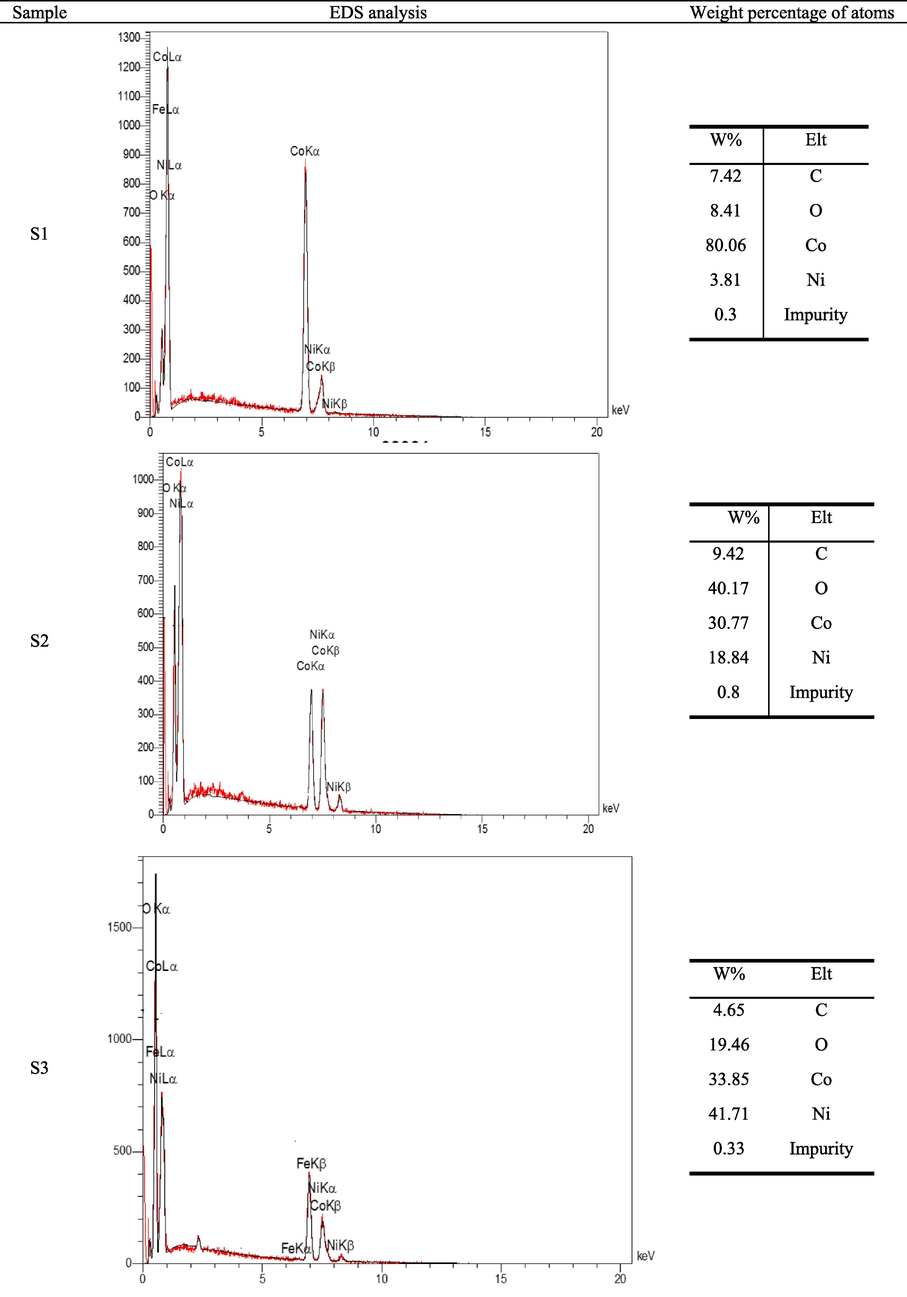
The EDS analysis of produced alloys with different compositions of granules (at 15 min).
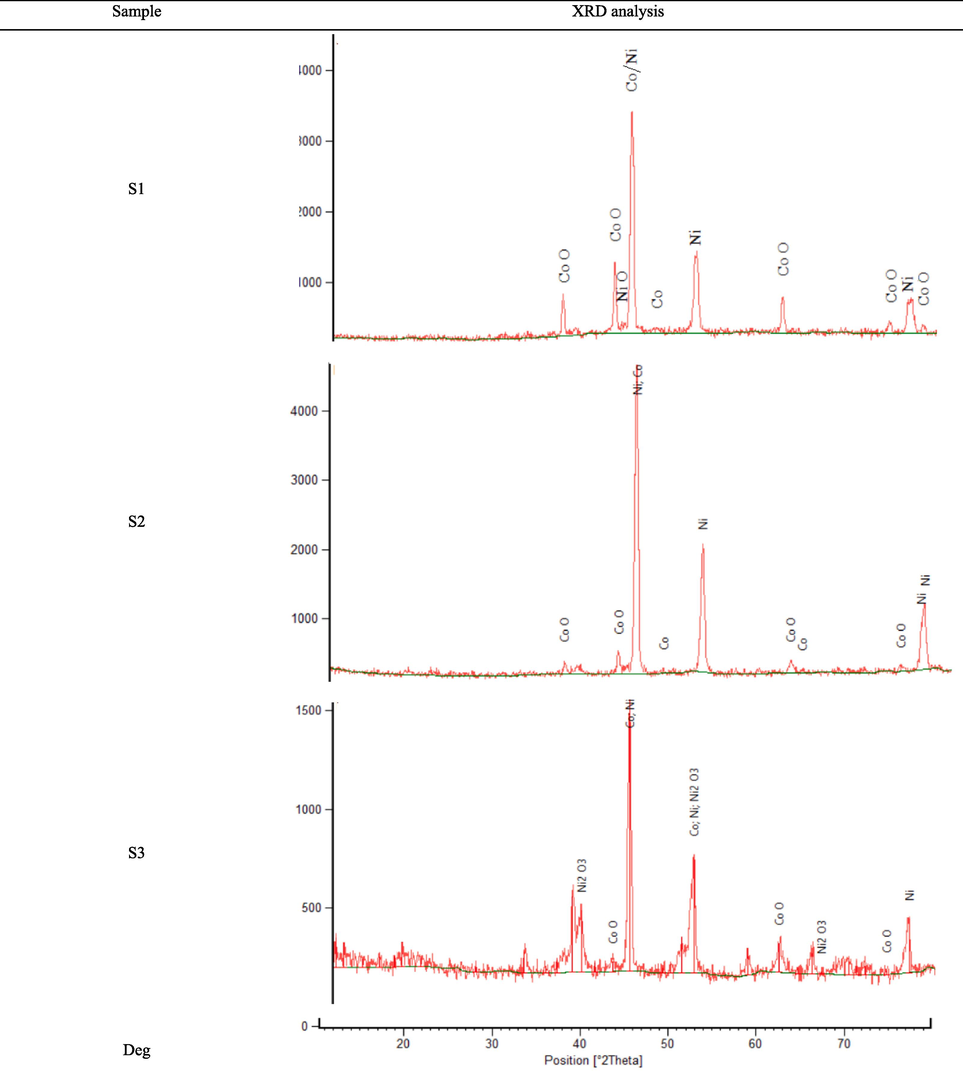
XRD analysis related to the products obtained from the reduction reaction of granules with different compositions of cobalt/nickel oxides.
The findings of this research are in contrast with the results of Ref. (Lebukhova and Karpovich, 2008) declaring the faster reduction reaction rate of nickel oxide to nickel compared to the reduction rate of cobalt oxide to cobalt at a constant temperature. In addition, the rate of reduction was considerably increased by using microwave heating in this work compared to the use of electrical furnaces due to the following reasons:
In the reduction reaction under microwave heating, the kinetics of chemical reactions are changed, and the conditions are entirely different compared to traditional heating methods.
The surface cracks forming on the particles during the reduction reaction by microwave heating can dramatically affect the final reduction reaction rate (Lebukhova and Karpovich, 2008; Singh, 2018). The surface cracks were determined to be different for each material, and cobalt oxide probably caused more surface cracks here. In Fig. 12, the SEM analysis related to S2 and S3 was presented. As can be seen, there were inevitable surface cracks on the samples, which are associated with the effect of the microwave heating method. As also mentioned by Chun Long (Chun, 2017), the surface crack on the material under a microwave can be due to applying of thermal stress caused by the heating selectivity of the microwave oven. Moreover, a rise in the reaction surface by these cracks provides larger spaces for gas diffusion, hence, promoting the reduction reaction.These surface cracks on produced alloys caused due to using the microwave heating process have different advantages and disadvantages. The main advantage of these cracks is an effective and faster reduction process at low temperatures, but these surface cracks can negatively affect the quality of synthesized material. However, it should be noted that these cracks usually occur at low temperatures, and by increasing the temperature of the process, materials can be obtained without surface cracks.
The SEM analysis of produced alloys with different compositions; some possible surface cracks were signed with red marks.
3.4 Future perspectives
The microwave heating process was determined to be very promising for the carbothermic synthesis of Co/Ni alloy. However, this paper was also faced with limitations and numerous gaps and challenges remained unanswered. In this regard, one can construct a semi-industrial microwave-based system and consider the economical evaluations to synthesize different alloys compared to traditional heating methods. Furthermore, the use of various microwave receptors can be very effective in the synthesis of alloys because they can provide higher temperatures compared to activated carbon. However, the catalytic activity of activated carbon helps the reduction process and the use of a different receptor has a side negative effect on its catalytic activity that should be considered when one uses another microwave receptor instead of activated carbon. Moreover, microwave heating also may be used to synthesize robust catalysts. because of its rapid temperature rising trend.
4 Conclusion
In this research, the efficiency of the microwave heating process was explored during the carbothermic synthesis of cobalt/nickel alloys. The morphology of the produced materials was also evaluated. The efficiency of the reduction process was also assessed, considering two parameters of the reaction time and the composition of the granules containing cobalt/nickel oxides. Three samples with different cobalt contents were prepared in the form of cylindrical pellets using the PVA bonder and hydraulic tableting. Accordingly, prolonging the reaction time from 10 to 20 min decremented the weight percentage of oxygen from 20 % to about 10 %. Furthermore, no crystal nickel oxide structures were detected after the 20 min. The size of the crystals was enhanced by increasing the reaction time from 10 to 20 min, suggesting the growth of the crystals and more agglomerated particles. The number of oxygen atoms was also decreased by about 50 % upon prolonging the reaction time from 10 to 20 min, indicating a better reduction reaction. A rise in the content of cobalt oxide from 30 % (S3) to 60 % (S2) improved the reduction reaction and the amount of oxygen atoms in the product. Regarding the decisive role of surface cracks in the reaction kinetics, future works can be focused on the underlying reasons of crack formation upon microwave heating to develop novel and more efficient processes.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of sulfur on hydrogen-reduction behavior of iron oxide during microwave heating. Miner. Eng.. 2020;148:106198
- [Google Scholar]
- Using a novel continuous bioreactor in enhancing the biogas production. Biomass Convers. Bioref. 2022
- [Google Scholar]
- Nanofluids application to promote CO2 absorption inside a bubble column: ANFIS and experimental study. Int. J. Environ. Sci. Technol. 2022:1-12.
- [Google Scholar]
- Microwave chemistry: Effect of ions on dielectric heating in microwave ovens. Arab. J. Chem.. 2015;8(1):100-104.
- [Google Scholar]
- Improvement of Methane Production from Sugar Beet Wastes Using TiO2 and Fe3O4 Nanoparticles and Chitosan Micropowder Additives. Appl. Biochem. Biotechnol.. 2019;189(1):13-25.
- [Google Scholar]
- Determination of optimum insulation thicknesses for salinity gradient solar pond’s bottom wall under different climate conditions. SN Appl. Sci.. 2020;2(7):1284.
- [Google Scholar]
- Pore network model for catalytic dehydration of methanol at particle level. AIChE J.. 2009;55(2):442-449.
- [Google Scholar]
- Recent advances in the microwave- and ultrasound-assisted green synthesis of coumarin-heterocycles. Arab. J. Chem.. 2022;15(3):103654
- [Google Scholar]
- Rapid thermal decomposition of manganese ore using microwave heating. J. Alloys Compd.. 2017;699:430-435.
- [Google Scholar]
- Influence of microwave heating on the microstructures of iron ore pellets with coal during reduction. Ironmak. Steelmak.. 2017;44(7):486-491.
- [Google Scholar]
- Degradation mechanism of nickel-cobalt-aluminum (NCA) cathode material from spent lithium-ion batteries in microwave-assisted pyrolysis. Metals. 2018;8(8):565.
- [Google Scholar]
- Single crystalline ultrathin nickel–cobalt alloy nanosheets array for direct hydrazine fuel cells. Adv. Sci.. 2017;4(3):1600179.
- [Google Scholar]
- Controlled synthesis of Nix-Co (1–x) bimetallic nanoparticles using the thermogravimetric method. Chem. Eng. Res. Des.. 2018;137:174-185.
- [Google Scholar]
- Typical growth of SiC fibers prepared by microwave heating with NiCl2 catalyst. J. Alloy. Compd. 2023169437
- [Google Scholar]
- Carbothermic reduction of copper, nickel, and cobalt oxides and molybdates. Inorg. Mater.. 2008;44(8):890-893.
- [Google Scholar]
- Nickel–cobalt electrodeposited alloys for hydrogen evolution in alkaline media. Int. J. Hydrogen Energy. 2009;34(5):2101-2106.
- [Google Scholar]
- Alkaline-and template-free hydrothermal synthesis of stable SnO2 nanoparticles and nanorods for CO and ethanol gas sensing. Sens. Actuators B: Chem.. 2010;151(1):140-145.
- [Google Scholar]
- Substrate dependent electrodeposition of Ni–Co alloy for efficient hydrogen evolution reaction. Electrocatalysis. 2023;14(1):68-77.
- [Google Scholar]
- Rapid microwave method for synthesis of iron oxide particles under specific conditions. Crystals. 2021;11(4):383.
- [Google Scholar]
- Investigating the simultaneous effect of effective parameters on the performance of salinity gradient solar ponds using Taguchi analysis: Determination of optimum thickness of the middle layer. Alex. Eng. J.. 2022;61(7):5607-5619.
- [Google Scholar]
- Recent developments in the electrodeposition of nickel and some nickel-based alloys. J. Appl. Electrochem.. 2006;36(9):957-972.
- [Google Scholar]
- Assessment of conventional and microwave heating effects on the variation of the bioactive compounds of Chétoui VOO using HPLC-DAD-ESI-TOF-MS. Arab. J. Chem.. 2020;13(1):954-965.
- [Google Scholar]
- Experimental investigation and adaptive neural fuzzy inference system prediction of copper recovery from flotation tailings by acid leaching in a batch agitated tank. Int. J. Miner. Metall. Mater.. 2019;26(5):538-546.
- [Google Scholar]
- A novel two-step fixed bed reactor for the reduction of cobalt oxide under microwave heating. Mater. Sci. Eng. B. 2021;267:115085
- [Google Scholar]
- The experimental and modeling study on a new process of iron/cobalt alloy production using indirect reducing agent of glycerol under microwave heating. Chem. Eng. Process.-Process Intensif. 2021108765
- [Google Scholar]
- Double layer microwave absorber based on Cu dispersed SiC composites. Adv. Powder Technol.. 2018;29(9):2019-2026.
- [Google Scholar]
- Toward environmentally friendly direct reduced iron production: A novel route of comprehensive utilization of blast furnace dust and electric arc furnace dust. Waste Manag.. 2021;135:389-396.
- [Google Scholar]







