Translate this page into:
Investigating the role of Cinnamomum verum in zebrafish swim bladder development and anti-cancer activity in human lung cancer cell lines
⁎Corresponding author. fmuhammad@ksu.edu.sa (Muhammad Farooq Khan),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Coughs and allergies are often treated at home with cinnamon (Cinnamomum verum). COVID-19 patients were also benefited by the herb. Aspirating C. verum powder caused pulmonary toxicity, hypercapnia, and respiratory failure in humans and animals. The toxicity of C. verum during fetal lung development is generally unknown. C. verum's effects on lung development were studied in zebrafish. Zebrafish lack lungs but have a swim bladder that functions like mammalian lungs. This study examined C. verum's role in embryonic lung formation using zebrafish swim bladder development. C. verum bark was extracted in methanol, chloroform, ethyl acetate, and hexane. Zebrafish embryos received serial dilutions of these extracts. Methanol extracts of C. verum were not harmful, while hexane, chloroform, and ethyl acetate extracts prevented swim bladder formation and caused neurotoxicity in zebrafish embryos. Organogenesis and lung tumorigenesis share biology. The anti-cancer effect of C. verum against lung cancer is unclear, hence an invitro cell viability study was performed utilizing three human lung cancer cell lines. All extracts decreased lung cancer cell viability, but hexane extract was most effective, inhibiting growth at IC 50 concentrations below 50 µg/ml. The LD50 dose of hexane extract in zebrafish embryonic toxicity exceeds 100 µg/ml, indicating more activity in cancer than normal cells. The extracts also exhibited significant level of ameliorative activity against CuSO4 induced oxidative stress in live zebrafish larvae. Hexane, ethyl acetate, and chloroform fractions have high cinnamaldehyde levels according to GC–MS analyses. Thus, cinnamaldehyde may be the key element in C. verum's lung toxicity and anticancer properties. This study suggested that C. verum crude extract or pure cinnamaldehyde could treat lung cancer. The dose in pregnant women must be carefully monitored to avoid teratogenic effects on fetus lung development.
Keywords
C. verum
Zebrafish swim bladder
Cinnamaldehyde
Oxidative stress
Lung cancer
1 Introduction
Several medicinal plants and herbs, including C. verum, are used in folk medicine against lung inflammation (Yakhchali et al., 2021). C. verum bark powder is routinely used as a home remedy to suppress cough, allergic reactions, and other respiratory disorders (Ranasinghe et al., 2013). C. verum was reported as an effective remedy for human lung diseases, and recently it was shown to have beneficial effects against COVID-19 (Barati et al., 2020, Yakhchali et al., 2021). The essential oils and other C. verum components possess antibacterial, antifungal, antioxidant, and antidiabetic properties. (Chao et al., 2005, Tung et al., 2010, Lee et al., 2018).
Very recently the pulmonary toxicity of C. verum in human have been reported. One study has reported the hypercapnia, lower airway obstruction, and respiratory failure of a pediatric patient who aspirated C. verum powdered. (Peir et al., 2022). In 2013 the American Association of Poison Control Centers (AAPCC) issued a warning for possible lung poisoning by C. verum after they found alarming signs such as burning, inflammation in the mouth, nose, and throat and fibrosis of lungs in experimental animals (Grant-Alfieri et al., 2013). The toxicity studies conducted in adult animals have shown that the use of C. verum is safe (Yun et al., 2018, Abdeen et al., 2019). However, the safety profile, of C. verum on embryonic development and specifically on embryonic lung formation, has yet to be discovered.
Swim bladder development in zebrafish (Danio rerio) has been suggested as a model of lung injury (Lee et al., 2019). Researchers have used zebrafish swim bladders to study human mucosal and fungus infections (Gratacap et al., 2013, Voelz et al., 2015). The swim bladder elastogenesis study provided the option of using this model as an in vivo injury-repair model for lung disease (Perrin et al., 1999). Zebrafish belong to the teleost family of fish, which do not have lungs, instead, they have an organ known as swim bladder. The swim bladder is an internal gas-filled organ that helps control buoyancy in fish. The swim bladders in fish are very much similar in structure and function to mammalian lungs (Daniels et al., 2004). The lungs of higher vertebrates develop similarly to the swim bladders of fish. Both arise as outgrowths from the esophagus, with the glottis occupying the same position (Daniels et al., 2004). Zebrafish embryos were used in this study as an in-vivo lung development model to investigate the toxicity of C. verum on the embryonic lung development.
It is known that organogenesis (organ formation) during embryonic development and postnatal carcinogenesis (tumour development) are similar biological events. Moreover, common molecular signatures such as Sonic Hedgehog are conserved between lung cancer and lung development in many species (Powers and Mu 2008, Kugler et al., 2015). We hypothesize if C. verum induced lung injury, it may negatively regulate the lung cancer cell proliferation and could be used as an anti-cancer agent against lung cancer. Even though the anticancer activity of C. verum has been reported in many cancers (Sadeghi et al., 2019, Caserta et al., 2023). We have not come across any study, reporting the anti-cancer action of C. verum in human lung cancer or in lung cancer cell lines. Hence in this study, the anticancer activity of C. verum was investigated in three types of human lung cancer cell lines, namely; A549, H-1650 and, H-1975 and a primary non-tumor HUVEC cell was used to evaluate the cytotoxicity of C. verum on normal cells.
The anti-inflammatory activity of cinnamon has been reported by in-vitro assays (Abeysekera et al., 2022), however, we have not come across any study, reporting the anti-inflammatory activity of C. verum in experimental animals. The zebrafish copper-induced inflammation model has been used previously to investigate the anti-inflammatory action of ethanol extract of Clerodendrum Cyrtophyllum Turcz (Nguyen et al., 2020). In this study, the CuSO4-induced oxidative stress in zebrafish embryo was used to evaluate the in vivo anti-inflammatory activity of C. verum extracts.
The lung toxicity in children and adult animals due to C. verum aspiration or ingestion is well known, but whether C. verum affects lung formation during fetus development in pregnant women or animals is largely unknown. It is therefore, this this study was designed to investigate the role of C. verum on lung organogenesis by using zebrafish swim bladder as in-vivo lung developmental model. Additionally, the anti-cancer activity of C. verum against lung cancer was studied in three different types of human cancer cell lines. Moreover, Zebrafish embryos were also used to examine the in vivo anti-inflammatory effect of C. verum. Lastly, the chemical characterization was done to identify major phytochemicals present in various polar fractions of C. verum to identify the principal ingredient for its biological activity.
2 Material and methods
The solvents used in this study were HPLC Plus grade and were purchased from Sigma Aldrich. Methanol (646377), chloroform (650498), hexane (34859), and ethyl acetate (650528). Cell culture reagents; DMEM (Dulbecco's Modified Eagle Medium) ThermoFisher cat # 11965092, Penicillin-Streptomycin (10,000 U/mL) ThermoFisher cat # 15140122, Gibco Fetal Bovine serum Thermo Fisher cat # 10270–106, Trypsin-EDTA solution Sigma Aldrich (cat # T4174). The cell culture plates, dishes, and flask from Corning (Corning USA).
2.1 Plant material and extraction
C. verum bark was obtained from Sorrah Saudi Arabia (https://www.sorrah.sa). Sixty grams of the powder was used for each solvent extraction. The extracts were prepared by the Soxhlet extraction method described previously (Farooq Khan et al., 2021).
2.2 Zebrafish
The wild-type zebrafish were obtained from the zebrafish international resource centre in Oregon, USA, and are kept in the animal facility bioproducts research chair, college of Science, Department of Zoology Saudi Arabia. The animals are fed twice daily with the Zeigler zebrafish diet (Zeigler Bros, Inc. Gardners, PA 17324 USA). The zebrafish is maintained by following the local and international regulations regarding the use and care of laboratory animals.
2.2.1 Ethical consideration
Zebrafish embryos are obtained by natural pair-wise breeding of Adult zebrafish. The embryos are screened, and synchronous-stage embryos are used for extract screening. Zebrafish embryos and larvae less than five days post fertilization (dpf) are used in this study, which has been exempted to take the approval from institutional review board as stated in (Strahle et al., 2012, Lackmann et al., 2018).
2.3 Zebrafish embryos treatment
2.3.1 In vivo toxicity assays
The embryos were obtained by natural pair-wise breeding of the fish. The synchronous stage embryos at the shield stage were exposed to serial dilution (1, 5, 15, 50, 150, and 500 µg/ml) of C. verum extracts in the embryo medium. The development toxicity of zebrafish embryos was recorded the next day by screening the embryos under a Leica stereo microscope. % lethality, tail detachment, hatching, abnormal organ development and developmental staging criteria were used to record the response of the embryos toward C. verum extracts treatment.
2.3.2 Oxidative stress assay
The zebrafish larvae at 3dpf were exposed to CuSO4 (10 µM) by following the method described by (Singh et al., 2022). Briefly, the zebrafish larvae (3dpf) were exposed to CuSO4 (10 µM) for one hour and then co-treated with serial dilution of each extract separately overnight. The CuSO4 (10 µM) only treated embryos served as positive control and un treated embryos served as negative control. The % survival of CuSO4 and C. verum extracts co-treated was compared with CuSO4 alone treated larvae.
2.4 In vitro anti-cancer activity
Three types of human lung cancer cell lines were used to evaluate the anti-cancer potential of C. verum extracts on lung cancer. The cell lines are obtained from American type cell collection Human lung cancer cell line A549 (ATCC cat # CCL-185), H-1650 (ATCC cat # CRL-5883), H-1975 (ATCC cat # CRL-5908) and one type of primary cell line HUVEC (ATCC cat # PCS-100–013). Cells were grown in DMEM (Dulbecco's Modified Eagle Medium) in a humified incubator at 37 ℃ with 5% CO2. In vitro cytotoxicity assays were carried out as described previously (Alqahtani et al., 2022). Briefly Cells were seeded at 2 × 104 per well of 6 well cell culture plates, and incubated with serial dilution (0, 0.5, 1.0, 3.0, 9.0, 27.0, 100.0 μg/mL) of extracts for 24 h. Control cells were treated with solvent (methanol 0.5% v/v). Three replicates were done for all experiments. DMEM with 10% FBS and 1% penicillin–streptomycin was used to grow the cells. The anticancer activity of C. verum extracts was assessed in vitro by MTT assay. To each well, 10 μL of 5 mg/mL MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) solution (made in PBS) was added and incubated for an additional 4 h. Finally, acidified isopropanol (0.01 N HCl) was added to dissolved MTT (formazan product). The dose–response curve was used to compute IC 50 values using Probit analysis. Cell viability was measured by using following formula.
2.4.1 Gas chromatography mass spectrophotometry (GC–MS)
The extract was derivatized using Bis (trimethylsilyl)trifluoroacetamide since it contains polar and non-polar analytes. After adding a 1:1 extract-BSTFA ratio, 1 µL of the extract was injected into GC–MS QP2010 GC–MS Shimadzu (Kyoto, Japan). 250 °C GC injection port temperature was used for all samples. Split vent opened in 1.0 min. Ion source and GC–MS interface temperatures were 200 and 220 °C, respectively. The oven temperature was programmed as follows: 40 °C for 1 min, 100 °C at 10 °C/min for 2 min, 165 °C at 10 °C/min for 0 min, 190 °C at 6 °C/min for 3 min, 220 °C at 3 °C/min for 3 min, and 240 °C at 2 °C/min for 1 min. To ensure target compound retention periods for qualitative analysis, scan mode data capture was used. Similarity searches and mass spectrum data from the NIST MS Library was used to identify the compounds present in crude extracts.
2.5 Statistical analysis
All the experiments repeated for at least three times and data are represented as the mean of triplicates ± standard deviation (SD). IC50 for cell lines and LD50 for zebrafish embryo were calculated using Probit analysis (Mekapogu 2017). Using a 2-tailed Student's t-test (GraphPad Prism v6), statistical significance was determined. The following P-values were considered significant: **P < 0.01; ***P < 0.001; ****P < 0.0001.
3 Results and discussion
3.1 Percent yield of different solvent extract
The cinnamon bark weighing 28.4 g was grinded to powder. Around 7 g of powder was used to prepare four types of extracts in solvents of methanol, chloroform, ethyl acetate and hexane. 500 ml of solvent was used for each extract. The percent yield for each solvent fraction is shown in Table 1. The maximum yield was from the methanol fraction (35%) while the least yield was from the chloroform fraction (6%).
Solvent
Quantity of C.verum powder used (grams)
Yield of extract
(grams)
% yield
Methanol
7.1
2.46
34.64
Hexane
7.1
0.95
13.42
Chloroform
7.1
0.43
6.15
Ethyl acetate
7.1
1.00
14.08
3.2 In vivo toxicity of C. verum extracts in zebrafish embryos
Zebrafish embryos were treated with serial dilution of each extract. The response of the embryos varied depending on the extract’s nature and the dose used. As shown in Fig. 1 and Table 2, the methanol extract (LD 50 ≥ 1 mg/ml) was safest for the embryos as compared with chloroform, hexane, and ethyl acetate extracts. The chloroform extract was the most toxic, with LD 50 values of just 2.42 µg/ml. In this study, the chloroform and hexane extract of C. verum showed more toxicity as compared to methanol, and ethyl acetate extracts. The hexane and chloroform extracts induced lethality in zebrafish treated embryos at concentrations of more than at 150 µg/ml. All the extracts were safe and did not induce any noticeable phenotype when used ≤ 50 µg/ml. *LD 50 is the average of three replicates ± standard deviation.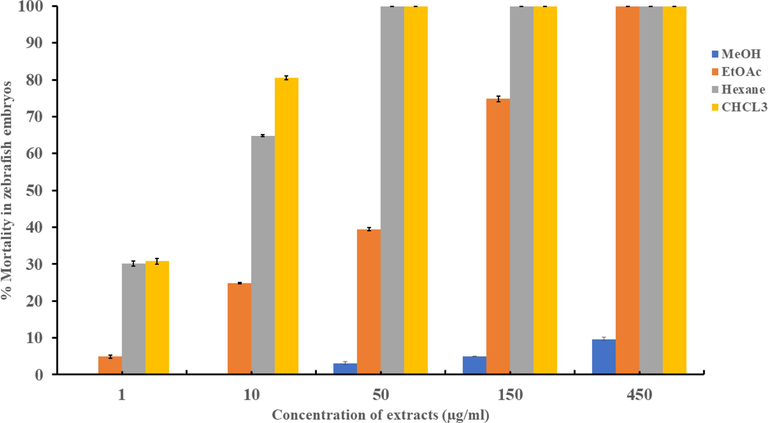
The dose–response of zebrafish embryos towards different extracts of C. verum. The data presented is the mean of three replicates, and the error bars represent the standard deviation.
Extract
LD 50 (µg/ml)*
1
Ethyl acetate
149.7 ± 0.35
2
Hexane
113.771 ± 0,57
3
Chloroform
112.42 ± 1.03
4
Methanol
850 ± 0.55
3.3 Hexane, ethyl acetate, and chloroform extracts of C. Verum induced craniofacial cartilage and notochord abnormities in zebrafish embryos
To investigate whether C. verum extracts induced any developmental toxicity (teratogenicity) in zebrafish embryos, the embryos were treated with sublethal a dose of extracts by which the treated embryos survived up to 3 days of treatment. To see the comparative teratogenic effects of different extracts, the zebrafish embryos were treated with an equal amount (100 µg/ml) of extracts. Each of the extracts was dissolved in methanol to prepare the final working solution, and hence the methanol (0.5% V/V) was used as mock control. As shown in Fig. 2A, no teratogenic effect was observed in mock treated embryos. The notochord in control embryos was straight and connected (NC pointed by black arrow). Similarly, no deformities were noted in craniofacial cartilage in mock-treated embryos (small black arrows under the mouth). The embryos which were treated with methanol extract of C. verum did not show any observable embryonic abnormalities (Fig. 2C). The highest level of embryonic abnormalities were observed in zebrafish embryos treated with C. verum chloroform extract. As shown in Fig. 2B, comparing to control embryos, these embryos are developmentally retarded and also cardiac edema is quite prominent in these embryos (Fig. 2B white arrow). The posterior trunk did not develop in 100% (n = 150) of chloroform treated embryos resulting in shortened bodies. The hexane and ethyl acetate extracts showed similar types of embryonic abnormalities as observed by chloroform extracts. Fig. 2D and E show the zebrafish embryos treated with hexane and ethyl acetate extract respectively. The craniofacial cartilage did not form in treated embryos and they showed an undulated notochord (black arrow NC).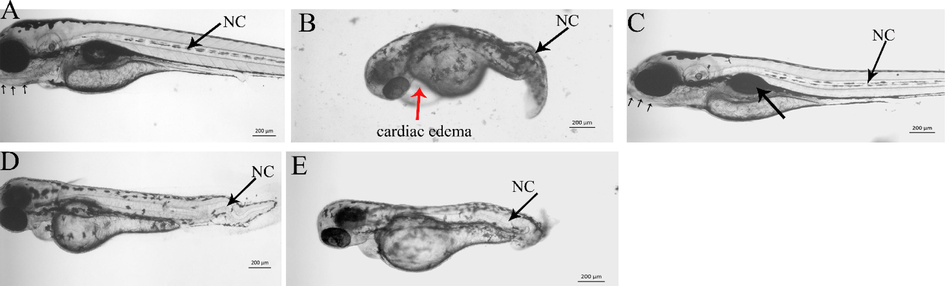
Craniofacial cartilage and notochord defects induced by C. verum extracts in zebrafish larvae. The photomicrograph is the representative image of the live zebrafish larvae snapped at four dpf. A) Control. Small arrows under the mouth indicate the normal development of craniofacial cartilage and the black arrows indicate the notochord (NC). B) Zebrafish embryos treated with 1 µg/ml of chloroform extract. The treated embryos showed severe developmental abrnomalities, smaller as compared to control larvae at the same stage.The posterior trunk did not develop as well. The embryos had severe cardiac edema (red arrow). C) zebrafish embryos treated with 500 µg/ml of methanol extract. The methanol extract did not induce any obvious defects, and the larvae's development and size were the same as control larvae at 4dpf. D) Zebrafish embryos treated with hexane extract. Moreover, craniofacial cartilage was also absent in these embryos. The embryos were smaller size as compared to the control. E). A clear undulation of notochord can easily be visualized in zebrafish embryos treated with ethyl acetate extract. Moreover, the craniofacial cartilage did not form in these larvae. F). All images are taken by keeping the larvae anterior to the left under the same magnification. The scale bar is shown at the right lower side of each image.
To evaluate whether the toxic effects were due to the extracts or solvents, the zebrafish embryos were treated with relevant solvents (as mock control for each solvent) which were used for the extraction. The concentration of the solvent was kept equal to the final highest working concentrations of the solvent (50 µL in 5 ml). As shown in Fig. 3, we have not detected any lethality and embryonic abnormalities in solvent alone treated embryos, which means that the teratogenic effects which were observed in zebrafish embryos were specific to the C. verum.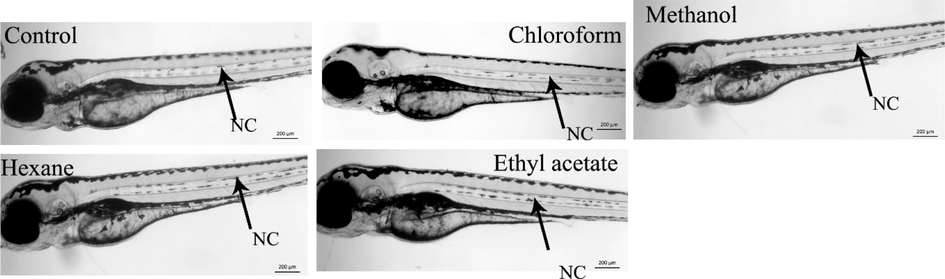
Solvent toxicity profiling in zebrafish embryos. Representative live images of zebrafish larvae at 3 days post fertilization. Control (no treatment), chloroform, methanol, hexane and ethyl acetate treated with 1% (V/V) solvents alone. The embryos were treated at shield stage (6 h post fertilization) and images were recorded at 3 days post fertilization.
C. verum has been extensively studied to investigate its various level of biological activities in many in-vitro and in-vivo systems. However, its safety profile on embryonic development is largely not known. Many studies have reported that C. verum did not induce toxicity when tested in adult experimental animals (mostly rodents). One study has reported normal body weight in rats after ingestion of C. verum by a 13-week repeat-dose oral toxicity assay. The same study has shown that C. verum extract was not mutagenic or clastogenic by in-vitro mammalian cell, and in vivo, bone marrow micronucleus assays (Yun et al., 2018). The aqueous extract of C. verum was used to treat female Sprague Dawley rats, and the authors suggested that the C. verum extract is safe when used below 0.5 g/kg dose (Abdeen et al., 2019, Hussain et al., 2019). Similarly, the ameliorative activity of C. verum essential oil has been demonstrated in rats against carbon tetrachloride-induced hepatoxicity (Bellassoued et al., 2019).
The chloroform extract of hexane in this study induced severe teratogenic phenotype at sublethal dose (100 µg/ml) in zebrafish embryos. Developmental delay was one of the most noticeable effects of chloroform extracts of C. verum at this concentration.
3.4 C. verum extracts perturbed the formation of the swim bladder in treated zebrafish embryos
Zebrafish embryos were treated with an equal concentration (100 µg/ml) of different extracts. The extracts were added at the shield stage (6 hpf), and the experiment lasted for four days, and swim bladder development was evaluated at 4dpf. The Mock (0.05% methanol V/V) treated embryos served as control. The black arrow in Fig. 4A, shows a fully developed and inflated swim bladder (SB) in control larvae. Similarly, methanol extract of C. verum did not cause toxicity and swim bladder formation in the zebrafish larvae. As the methanol extract did not cause swim bladder formation defects at 100 µg/ml, the concentration of methanol extract was increased up to 1000 µg/ml to see whether it could affect swim bladder development at higher concentration but it was well tolerated by zebrafish larvae and did not cause any obvious developmental defects in 10 times more concentration than other solvents extract. Zebrafish larvae treated with C. verum ethyl acetate extract (100 µg/ml) exhibited an inflated but small swim bladder (back arrow Fig. 4C). As shown in Fig. 4D, the swim bladder did not form in zebrafish embryos treated with C. verum hexane extract (100 µg/ml) at 4dpf, (absence of swim bladder is indicated by a white asterisk.) Similarly, the swim bladder did not form in zebrafish embryos treated with chloroform C. verum extract (100 (Fig. 4E).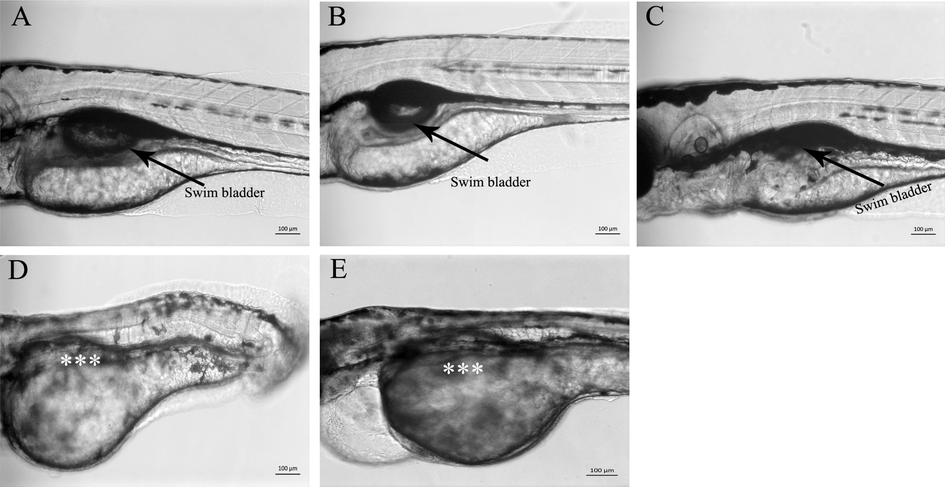
C. verum extracts blocked the formation and growth of swim bladder in zebrafish embryos. Representative images of live zebrafish larvae at 4dpf. Zebrafish embryos were treated with sub-lethal doses of C. verum extracts starting from the shield stage (6 hpf), and swim bladder development in control and treated larvae were recorded at 4dpf. A) Mock (0.5% methanol) treated control larvae with fully developed and inflated swim bladder (black arrow) B) Zebrafish larvae treated with methanol extract of C. verum (500 µg/ml). The methanol extract also did not affect the formation and growth of the swim bladder. C) Zebrafish larvae treated with C. verum ethyl acetate extract (10 µg/ml) show un-inflated and small-size swim bladder (back arrow) D). Hexane extract (5 µg/ml) treated larvae show the absence of swim bladder at 4dpf (white asterisk). E) Chloroform extract (2 µg/ml) treated larvae shows absence of swim bladder (white asterisk). All images were taken under the same magnification. The scale bar is shown at the bottom right corner.
Teleost (bony) fish do not have lungs, but they have a swim bladder, an organ that functions like the lungs. The swim bladders of fish develop similarly to the lungs of higher vertebrates. The anatomical structure, morphological development, and transcriptional patterns of the zebrafish swim bladder, which functions as a variable buoyancy device, are similar to those of the lung (Zheng et al., 2011, Cass et al., 2013). Both arise as outgrowths from the esophagus, with the glottis occupying the same position (M. 2023). We have not come across any report in which the effect of C. verum on embryonic lung development was studied in any animal model. Hence this is the first report demonstrating the role of C. verum on lung formation using zebrafish.
3.5 Ani-inflammatory activity of C. Verum extracts in CuSO4 induced oxidative stress in zebrafish embryos
The ameliorative function of C. verum against CuSO4 induced toxicity in zebrafish larvae was used as in-vivo model to measure the anti-inflammatory activity of extracts. Zebrafish larvae (3dpf) were exposed to 10 µM of CuSO4 for one hour and then co-treated with C. verum extracts with LD50 concentration (Table 1) for 12 to 15 h. Untreated larvae were negative control, and CuSO4 alone treated larvae as a positive control. The protective effect of C. verum extracts is shown in Fig. 5. Exposure to CuSO4 at a concentration of 10 µM induced 96% mortality in zebrafish larvae in 24 h. In contrast, no mortality was found in the negative control. The methanol extract (500 µg/ml) showed the weakest level of anti-inflammatory activity against CuSO4-induced oxidative stress and only 5% of zebrafish larvae survived by co-administration of methanol extract. Twenty one percent (21%) of zebrafish larvae survived, which were treated with 10 µM of CuSO4 and 110 µg/ml chloroform extract. Ethyl acetate (150 µg/ml) and hexane extract (110 µg/ml) showed strong protective activity against CuSO4-induced toxicity with 61 and 80% larvae survival. The protective action of ethyl acetate and hexane against CuSO4-induced toxicity was statistically significant, with a p-value of 0.05 compared with CuSO4 alone treated larvae.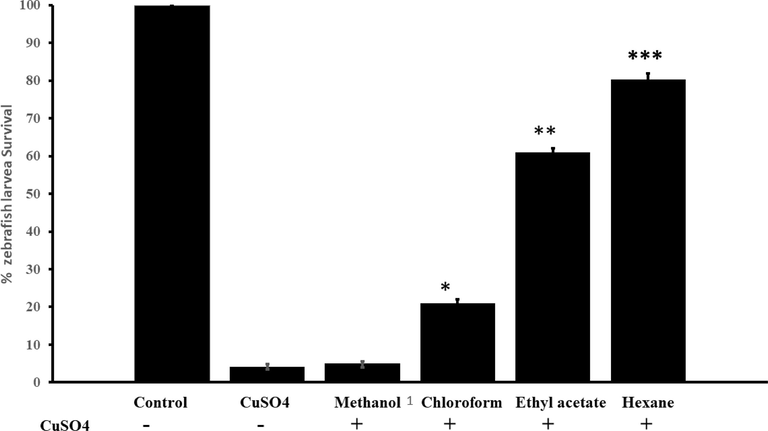
Organic extract of C. verum exhibited the highest anti-inflammatory activity in zebrafish larvae. Zebrafish larvae were exposed to 10 µM of CuSO4 for one hour and then co-exposed to C. verum extracts equal concentration (1 µg/ml) for 24 h. As indicated by bar graph, only 4% of larvae survived after 24 h treatment with CuSO4 alone. 100% larval survival was observed in negative control larvae (not exposed to CuSO4 or C. verum extracts). Methanol extract of C. verum showed the weakest anti-inflammatory activity, and only 2% of larvae survived, which were treated with CuSO4 and methanol extract. Hexane extract of C. verum showed strong ameliorated activity against CuSO4-induced toxicity with an 80% survival rate. The ethyl acetate showed moderate anti-inflammatory activity against CuSO4-induced toxicity. The data presented are the average of three independent experiments. *: indicates degree of statistical significance (*** p-value 0.0001, ** p value 0.001, * p value 0,01) between the CuSO4 alone and the larvae treated with CuSO4 and C. verum extracts. Error bars represent the ± standard deviation between three replicates.
Many in vitro and in vivo studies have documented C verum extracts or their components, especially cinnamaldehyde, as an anti-inflammatory agent (Abeysekera et al., 2022, Chen et al., 2022). Gunawardena et al. also reported that the highest level of anti-inflammatory activity was present in the organic fractions than the methanol and water extract of C. zeylanicum and C. cassia (Gunawardena et al., 2015). However, there is no report in which the anti-inflammatory activity of C. verum has been checked in live animals. Zebrafish possess an excellent in vivo model of inflammation as it shares several inflammatory genetic signatures with mammals (Zanandrea et al., 2020). Copper adversely affects organisms' development, physiology, reproduction, and survival and has immunosuppressive effects on certain fish species (Schink et al., 2018, Hong et al., 2020). Ethanol extract of leaves of C. cyrtophyllum showed protective activity against CuSO4 toxicity in 3dpf zebrafish larvae, and researchers proved that the protective activity was due to the high content of flavonoids in ethanol extract of leaves of C. cyrtophyllum which resulted in a high level of anti-oxidant (IC50 of 16.45 µg/mL) and anti-inflammatory. The co-administration of ethanol extract of leaves of C. cyrtophyllum and CuSO4 downregulated inflammatory response genes in zebrafish larvae (Nguyen et al., 2020). Similarly, the protective effect of acacetin against CuSO4-induced toxicity was also attributed to the anti-inflammatory activity of acacetin. The co-administration of acacetin resulted in upregulation of anti-oxidant genes and suppressing pro-inflammatory response genes in CuSO4-exposed zebrafish larvae (Singh et al., 2022).
3.6 The anticancer activity of C. Verum against human lung cancer cell lines
As presented in the previous section, most of the C. verum extracts affected the swim bladder formation in zebrafish embryos. We next tested whether these extracts could induce cytotoxicity in human lung carcinoma cell lines? Three types of human lung carcinoma cell lines, namely A549, H-1650, and, H-1975, and a primary cell (non-cancer) human umbilical vein endothelial cell (HUVEC), were selected for such investigation.
The 50% inhibitory concentration (IC50) of the extract against lung cancer cell and primary cell lines are presented in Table 3. The hexane extract of C. verum was most active against A549, H-1650, and H-1975 with IC50 values of 44.30 ± 0.58, 42.12 ± 057, 30.33 ± 0.57 µg/ml respectively. The methanol extract was not active in all three types of lung cancer cell line and HUVEC. The cytotoxicity C. verum extract in human lung cancer cell lines indicates that the co– related with the in vivo toxicity profile of these extracts in zebrafish embryos. The hexane extract showed maximum level of cytotoxicity with minim concentration as compared to chloroform, ethyl acetate and methanol. Its LD50 values in zebrafish embryos and IC50 concentration in HUVEC cell were more than almost double as compared its IC50 values in cancer cell lines, which shows that hexane extract showed higher toxicity towards cancer cell the normal cell. NA: Not active, Data are presented as the mean of three replication ± standard deviation.
Extract
Cytotoxicity IC50 (µg/ml)
HUVEC
A549
H-1650
H-1975
Methanol
NA*
NA
NA
NA
Chloroform
143.43 ± 0.58
116.72 ± 0.57
134.10 ± 0.58
145.71 ± 0.57
Ethyl acetate
107.69 ± 1.00
114.09 ± 1.52
114.92 ± 1.00
129.62 ± 0.58
Hexane
75.85 ± 0.57
44.30 ± 0.58
42.12 ± 057
30.33 ± 0.57
The anti-cancer activity of cinnamon against human cancer cell lines and xenograft cancer models has been reported previously. Cinnamomum cassia essential oil (CEO) infused chitosan nanoparticles suppressed the growth of 4T1 breast cancer cells in a mice xenograft model via inhibiting the expression of the Ki-67 protein (Xu et al., 2023). Cinnamaldehyde, the main ingredient has been suggested for the treatment of breast Cancer in women (Liu et al., 2020). The aqueous extract of cinnamon has been shown to inhibit the proliferation of bladder cancer 5637 cells by inhibiting glycolysis and induction of apoptosis (Aminzadeh et al., 2022). Proteasome inhibition by aromatic monophenols (procyanidin-B2), from cinnamon induced the death of prostate cancer cells by autophagy-dependent apoptosis (Gopalakrishnan and Ismail 2021). The anti-proliferative function of the cinnamon extract was also reported in three hematological cancer cell lines (Schoene et al., 2005).
3.7 Chemical composition of C. verum in different solvent fractions
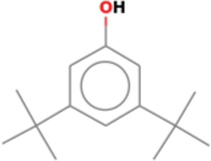
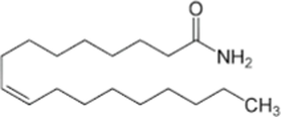
The biological activity of any crude extract is due to the presence of one or many phytochemicals in the crude extract. So, it is important to know what kind of phytochemicals are present in the crude extract. Gas chromatography coupled with mass spectrophotometry (GC–MS) is any easy technique to identify the different active compounds especially the volatile compounds in crude extract. The four solvent fractions (methanol, chloroform, ethyl acetate and hexane) of C. verum were analyzed by GC–MS and results are presented in Tables 4–7. We detected only two peaks in the methanol fraction and the identified compounds are Phenol, 3,5-bis(1,1-dimethylethyl), and 9-octadecenamide. Both of these compounds have been reported previously from the C. verum bark. The Phenol, 3,5-bis(1,1-dimethylethyl) from the bark of specie Cinnamomum Zeylanicum (Hameed et al., 2016), and 9-octadecenamide was identified in small quantity from the bark of C. cambodianum and C. caryophyllus (Son et al., 2014).
No
Retention Time
Compound in %
Compound Name
Formula
Structure
1
15.82
9.74
Phenol, 3,5-bis(1,1-dimethylethyl)
C14H22O
2
29.697
90.26
9-octadecenamide
C18H35NO
No
Retention Time
Compound in %
Compound Name
Formula
Structure
1
10.767
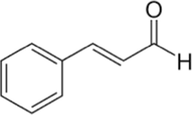
9.10
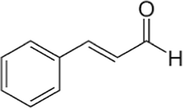
Cinnamaldehyde
C9H8O
2
13.535
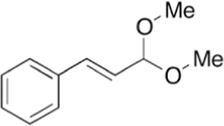
69.31
Cinnamaldehyde dimethyl acetal
C11H14O2

3
14.290
4.33
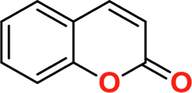
2H-1-Benzopyran-2-one
C10H9NO2
4
14.4870
1.55
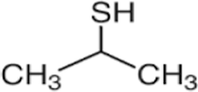
2-Thiapropane
C3H8S
5
16.618
3.50
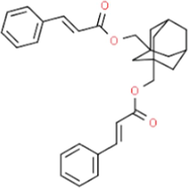
1,3-Bis(cinnamoyloxymethyl)adamantane
C30H32O
6
18.082
12.2
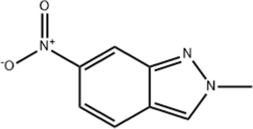
2-METHYL-6-NITRO-2H-INDAZOLE
C8H7N3O2
No
Retention Time
Compound in %
Compound Name
Formula
Structure
1
10.743
11.6
Cinnamaldehyde
C9H8O
2
13.059
8.50
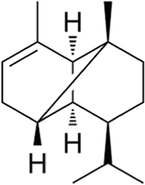
Copaene
C15H24
3
13.535
64.50
Cinnamaldehyde dimethyl acetal
C11H14O2
4
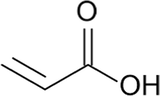
14.833
2.20
2-Propenoic acid
C3H4O2
5
15.144
3.23
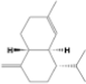
1,2,3,4,4a,5,6,8a-octahydro-7-methyl-4-methylene-1-(1-methylethyl)-, (1.alpha.,4a.beta.,8a.alpha.)-
C15H24
6
15.619
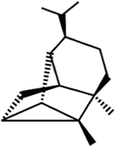
3.01
(+)-Cyclosativene
C15H24
7
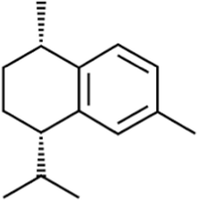
16.06
6.6
1 S-CIS-CALAMENENE
C15H22
8
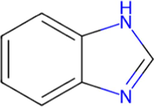
18.094
4.73
1H-Benzimidazole
C7H6N2
No
Retention Time
Compound in %
Compound Name
Formula
Structure
1
10.767
4.819
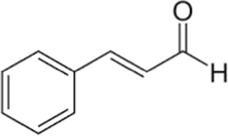
Cinnamaldehyde
C9H8O
2
13.528
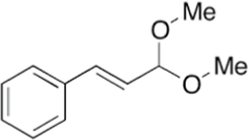
78.734
Cinnamaldehyde dimethyl acetal
C11H14O2
3
16.174
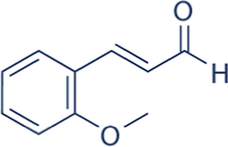
1.755
ORTHO METHOXY CINNAMIC ALDEHYDE
C10H10O2
4
18.094
14.692
2-METHYL-6-NITRO-2H-INDAZOLE
C8H7N3O2
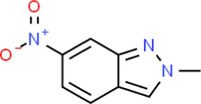
The Cinnamaldehyde was found in abundance in chloroform, hexane and ethyl acetate fractions. These fractions have Cinnamaldehyde mostly in cinnamaldehyde dimethyl acetal form. Cinnamaldehyde converted to Cinnamaldehyde dimethyl while using methanol for GC–MS which is a common phenomenon also has been reported previously (He et al., 2021). That mean that cinnamaldehyde dimethyl acetal is actually Cinnamaldehyde itself and is present from 60 to 80% in these fractions.
Several studies have reported cinnamaldehyde as the major ingredient (present as 65.00 to 80.00% in bark) and most of the biological properties of C. verum are attributed towards cinnamaldehyde (Singh et al., 2007, Rao and Gan 2014, Lu et al., 2022). We have observed the craniofacial cartilage and notochord deformities in zebrafish embryos exposed to 100 µg/ml of hexane, ethyl acetate and chloroform crude extract of C. verum. Surprisingly such type of deformities in zebrafish larvae were observed when zebrafish embryos were treated with pure cinnamaldehyde ((Bhattacharya et al., 2021)., which means that these toxicities are mainly due to cinnamaldehyde, and thus care should be taken by pregnant mothers, while using C. verum as whole or pure cinnamaldehyde. The neurotoxicity in zebrafish embryos upon exposure to cinnamaldehyde is also reported. The treated embryos had damaged ventricular structures, abnormal eyes, shortened body length, undulated trunk, and pericardial edema in zebrafish (Chang et al., 2022). We also have observed similar kind of neurotoxicity in treated embryos by hexane extracts. We have performed an online program (http://www.swisstargetprediction.ch/) to identify the protein targets in humans using the chemical structure of Cinnamaldehyde. The results have been shown as Supplementary Table S1. The Swiss protein target prediction shows the high probability of “Transient receptor potential cation channel subfamily A member 1 (TRPA1)” being a specific target of cinnamaldehyde. TRPA1 channel plays a vital role in regulating brain development and the physiological function of astrocytes (Shigetomi et al., 2011). Hence, it could be postulated that cinnamaldehyde present in C. verum could have blocked the activity of TRPA1 which resulted the neurotoxicity in treated zebrafish larvae. One study also reported the inhibition of angiogenesis blood vessels in transgenic zebrafish line (flk1:GFP) embryos by high doses of C. verum extract (Bansode et al., 2013). We have not observed any defects in angiogenesis blood vessels by exposure of C. verum extracts to TG (fli; EGFP) zebrafish embryos (data not shown). This could be due to the nature of the solvent used, as we have not used the DMSO, or the angiogenesis defects could be due to secondary affect due to the high dose of C. verum. The neuro and cardiovascular toxicity with high dose of C. verum in zebrafish embryos has been reported by other studies, but we have not found any study which reported the effect of C. verum on lung toxicity in embryos. This study is unique in the sense that the effect of C verum on zebrafish lung (swim bladder) development was being reported for the first time an also its anticancer potential against lung cancer.
4 Conclusion
Cinnamon (C. verum) is a popular home remedy for cough and allergies, and has been reported to have beneficial effects against COVID-19. However, the toxicity of C. verum during fetus lung development is not well understood. Zebrafish were used as an in vivo animal model to investigate the role of C. verum in embryonic lung development. C. verum extracts of variable polarity were Mreapted using methanol, chloroform, ethyl acetate, and hexane solvents. Methanol extracts did not show any toxicity towards zebrafish. However, hexane, chloroform, and ethyl acetate extracts blocked the formation of swim bladder and induced neurotoxicity in treated zebrafish embryos.. The invitro cell viability data from this study shows that hexane, chlorforom and ethyl acetate fractions inhibited the cell viability of three lung cancer cell lines. The GC–MS analysis showed the abundance of cinnamaldehyde compound in hexane, ethyl acetate, and chloroform fractions, suggesting that cinnamaldehyde could be the main ingredient responsible for lung toxicity and anticancer activity of C. verum. The hexane, chloroform, and ethyl acetate extract of C. verum also showed significant level of ameliorative activity against CuSO4-inducedd oxidative stress representing strong anti-anti-inflammatory action of these extracts.
Funding
This research was financially supported by Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia through (IFKSURC-1–0501).
6 Institutional review board statement
Zebrafish embryos and larvae that are used in this study are younger than five days post fertilization (dpf), which does not need approval from the institutional review board (IRB) as stated [19, 20].
Data availability statement
All data reported in this study has been included in the manuscript. Original images can be obtained from the corresponding author by reasonable request at any time.
Acknowledgments
The authors extend their appreciation to Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, for funding this research. (IFKSURC-1-0501)
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Protective effect of cinnamon against acetaminophen-mediated cellular damage and apoptosis in renal tissue. Environ. Sci. Pollut. R.. 2019;26:240-249.
- [CrossRef] [Google Scholar]
- Anti-inflammatory, cytotoxicity and antilipidemic properties: novel bioactivities of true cinnamon (Cinnamomum zeylanicum Blume) leaf. BMC Complement Med Ther.. 2022;22:259.
- [CrossRef] [Google Scholar]
- Cytotoxicity of newly synthesized quinazoline-sulfonamide derivatives in human leukemia cell lines and their effect on hematopoiesis in zebrafish embryos. Int. J. Mol. Sci.. 2022;23
- [CrossRef] [Google Scholar]
- Antitumor activities of aqueous cinnamon extract on 5637 cell line of bladder cancer through glycolytic pathway. Int. J. Inflam.. 2022;2022:3855368.
- [CrossRef] [Google Scholar]
- Cinnamon extract inhibits angiogenesis in zebrafish and human endothelial cells by suppressing VEGFR1, VEGFR2, and PKC-mediated MAP kinase. Food Sci. Nutr.. 2013;1:74-82.
- [CrossRef] [Google Scholar]
- Potential drugs and remedies for the treatment of COVID-19: a critical review. Biol Proced Online.. 2020;22:15.
- [CrossRef] [Google Scholar]
- Protective effect of essential oil of Cinnamomum verum bark on hepatic and renal toxicity induced by carbon tetrachloride in rats. Appl. Physiol. Nutr. Me.. 2019;44:606-618.
- [CrossRef] [Google Scholar]
- E-cigarette vaping liquids and the flavoring chemical cinnamaldehyde perturb bone, cartilage and vascular development in zebrafish embryos. Aquat. Toxicol.. 2021;240:105995
- [CrossRef] [Google Scholar]
- The anti-cancer effect of cinnamon aqueous extract: a focus on hematological malignancies. Life. 2023;13:1176.
- [Google Scholar]
- Expression of a lung developmental cassette in the adult and developing zebrafish swimbladder. Evol. Dev.. 2013;15:119-132.
- [CrossRef] [Google Scholar]
- Cinnamaldehyde causes developmental neurotoxicity in zebrafish via the oxidative stress pathway that is rescued by astaxanthin. Food Funct.. 2022;13:13028-13039.
- [CrossRef] [Google Scholar]
- Study on the antiinflammatory activity of essential oil from leaves of Cinnamomum osmophloeum. J. Agric. Food Chem.. 2005;53:7274-7278.
- [CrossRef] [Google Scholar]
- Cinnamic Aldehyde, the main monomer component of Cinnamon, exhibits anti-inflammatory property in OA synovial fibroblasts via TLR4/MyD88 pathway. J. Cell Mol. Med.. 2022;26:913-924.
- [CrossRef] [Google Scholar]
- The origin and evolution of the surfactant system in fish: insights into the evolution of lungs and swim bladders. Physiol. Biochem. Zool.. 2004;77:732-749.
- [CrossRef] [Google Scholar]
- Investigating the anticancer activity and characterization of bioactive constituents of Moricandia sinaica (Boiss.) Boiss through in vitro and in silico approaches in triple-negative breast cancer cell line. Appl. Sci.. 2021;11:1244.
- [Google Scholar]
- Aromatic monophenols from cinnamon bark act as proteasome inhibitors by upregulating ER stress, suppressing FoxM1 expression, and inducing apoptosis in prostate cancer cells. Phytother. Res.. 2021;35:5781-5794.
- [CrossRef] [Google Scholar]
- Ingesting and aspirating dry cinnamon by children and adolescents: the “cinnamon challenge”. Pediatrics. 2013;131:833-835.
- [CrossRef] [Google Scholar]
- Mucosal candidiasis elicits NF-kappa B activation, proinflammatory gene expression and localized neutrophilia in zebrafish. Dis. Model. Mech.. 2013;6:1260-1270.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts - identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. Food Funct.. 2015;6:910-919.
- [CrossRef] [Google Scholar]
- Evaluation of antifungal and antibacterial activity and analysis of bioactive phytochemical compounds of Cinnamomum Zeylanicum (Cinnamon Bark) using gas chromatography-mass spectrometry. Orient. J. Chem.. 2016;32:1769-1788.
- [CrossRef] [Google Scholar]
- Formation of cinnamaldehyde dimethyl acetal in methanol during analysis. J. Essent. Oil Res.. 2021;33:308-313.
- [CrossRef] [Google Scholar]
- Progress in the Research of the Toxicity Effect Mechanisms of Heavy Metals on Freshwater Organisms and Their Water Quality Criteria in China. J. Chem.. 2020;2020:9010348.
- [CrossRef] [Google Scholar]
- Protective effects of Cinnamomum zeylanicum L. (Darchini) in acetaminophen-induced oxidative stress, hepatotoxicity and nephrotoxicity in mouse model. Biomed. Pharmacother.. 2019;109:2285-2292.
- [CrossRef] [Google Scholar]
- Sonic hedgehog signaling in the lung. From development to disease. Am. J. Respir. Cell Mol. Biol.. 2015;52:1-13.
- [CrossRef] [Google Scholar]
- Novel procedures for whole organism detection and quantification of fluorescence as a measurement for oxidative stress in zebrafish (Danio rerio) larvae. Chemosphere. 2018;197:200-209.
- [CrossRef] [Google Scholar]
- A novel zebrafish model to emulate lung injury by folate deficiency-induced swim bladder defectiveness and protease/antiprotease expression imbalance. Sci. Rep.. 2019;9:12633.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effect of cinnamaldehyde and linalool from the leaf essential oil of Cinnamomum osmophloeum Kanehira in endotoxin-induced mice. J. Food Drug Anal.. 2018;26:211-220.
- [CrossRef] [Google Scholar]
- Targets and mechanism used by cinnamaldehyde, the main active ingredient in cinnamon, in the treatment of breast cancer. Front. Pharmacol.. 2020;11:582719
- [CrossRef] [Google Scholar]
- The therapeutic roles of cinnamaldehyde against cardiovascular diseases. Oxid. Med. Cell. Longev.. 2022;2022:9177108.
- [CrossRef] [Google Scholar]
- Mekapogu, A.R. 2017, 17 Ju;y 2017. Calculating LD50/LC50 using Probit Analysis in EXCEL Retrieved 12/08/2022, 2022, from https://probitanalysis.wordpress.com/author/alpharajm/.
- Anti-inflammatory and antioxidant properties of the ethanol extract of Clerodendrum Cyrtophyllum Turcz in copper sulfate-induced inflammation in zebrafish. Antioxidants (basel).. 2020;9
- [CrossRef] [Google Scholar]
- Case study: Cinnamon aspiration in a toddler causing severe ARDS requiring surfactant and extracorporeal membrane oxygenation. Pediatr Pulm.. 2022;57:325-329.
- [CrossRef] [Google Scholar]
- The zebrafish swimbladder: A simple model for lung elastin injury and repair. Connect Tissue Res.. 1999;40:105.
- [CrossRef] [Google Scholar]
- Genetic similarities between organogenesis and tumorigenesis of the lung. Cell Cycle. 2008;7:200-204.
- [CrossRef] [Google Scholar]
- Medicinal properties of 'true' cinnamon (Cinnamomum zeylanicum): a systematic review. Bmc Complem. Altern. M.. 2013;13:275
- [CrossRef] [Google Scholar]
- Cinnamon: a multifaceted medicinal plant. Evid. Based Complement. Alternat. Med.. 2014;2014:642942
- [CrossRef] [Google Scholar]
- Anti-cancer effects of cinnamon: insights into its apoptosis effects. Eur. J. Med. Chem.. 2019;178:131-140.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effects of cinnamon extract and identification of active compounds influencing the TLR2 and TLR4 signaling pathways. Food Funct.. 2018;9:5950-5964.
- [CrossRef] [Google Scholar]
- Water-soluble polymeric polyphenols from cinnamon inhibit proliferation and alter cell cycle distribution patterns of hematologic tumor cell lines. Cancer Lett.. 2005;230:134-140.
- [CrossRef] [Google Scholar]
- TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci.. 2011;15:70-80.
- [CrossRef] [Google Scholar]
- Copper sulfate induced toxicological impact on in-vivo zebrafish larval model protected due to acacetin via anti-inflammatory and glutathione redox mechanism. Comp. Biochem. Physiol. C: Toxicol. Pharmacol.. 2022;262:109463
- [CrossRef] [Google Scholar]
- A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol.. 2007;45:1650-1661.
- [CrossRef] [Google Scholar]
- Study on Cinnamomum oils: compositional pattern of seven species grown in Vietnam. J. Oleo Sci.. 2014;63:1035-1043.
- [CrossRef] [Google Scholar]
- Zebrafish embryos as an alternative to animal experiments–a commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol.. 2012;33:128-132.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activities of essential oils and their constituents from different provenances of indigenous cinnamon (Cinnamomum osmophloeum) leaves. Pharm. Biol.. 2010;48:1130-1136.
- [CrossRef] [Google Scholar]
- A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides. Dis. Model. Mech.. 2015;8:1375-1388.
- [CrossRef] [Google Scholar]
- Cinnamon cassia oil chitosan nanoparticles: Physicochemical properties and anti-breast cancer activity. Int. J. Biol. Macromol.. 2023;224:1065-1078.
- [CrossRef] [Google Scholar]
- Cinnamon and its possible impact on COVID-19: The viewpoint of traditional and conventional medicine. Biomed. Pharmacother.. 2021;143:112221
- [CrossRef] [Google Scholar]
- In vitro and in vivo safety studies of cinnamon extract (Cinnamomum cassia) on general and genetic toxicology. Regul. Toxicol. Pharm.. 2018;95:115-123.
- [CrossRef] [Google Scholar]
- Zebrafish as a model for inflammation and drug discovery. Drug Discov. Today. 2020;25:2201-2211.
- [CrossRef] [Google Scholar]
- Comparative transcriptome analyses indicate molecular homology of zebrafish swimbladder and mammalian lung. Plos One.. 2011;6:e24019
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105361.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







