Translate this page into:
Investigation of catalytic pyrolysis of spirulina for bio-oil production
⁎Corresponding authors. hallaj@ut.ac.ir (Ahmad Hallajisani), m_samipoor@iau-tnb.ac.ir (Mohammad Samipoorgiri)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The energy crisis, pollution, and environmental effects caused by the use of fossil fuels have made mankind use renewable energy; bio-oil is one of the types of renewable energy. A pyrolysis reactor with a diameter of 7.8 cm, a height of 23 m, a capacity of 1 L, and a reformer reactor with a diameter of 5.4 cm, a height of 20 cm, and a capacity of 0.45 L were used. The effect of using cobalt catalyst based on zeolite and its comparison with direct pyrolysis of microalgae Spirulina sp. was investigated. The optimum conditions for the highest bio-oil production efficiencies were temperature 468 ℃, carrier gas discharge rate equal to 0.31 , and heating rate equal to 14.4 ℃ . As shown by the results, adding the catalyst increased high heat value and bio-oil energy yield. The bio-oil from catalytic pyrolysis for Spirulina sp. demonstrates a higher heating value equal to 34.43 and these figures for direct pyrolysis were 27.22 . The bio-oil obtained from Spirulina sp. pyrolysis demonstrates the highest heat value (HHV), high cetane number, and low iodine number.
Keywords
Catalytic
Pyrolysis
Spirulina biomass
Bio-oil
HZSM-5
Zeolite
1 Introduction
A clean and renewable energy source obtained by burning different biofuels such as biodiesel, biogas, bioethanol, and bio-oil is bioenergy. These fuels are obtained from different bio sources like oil seeds, agricultural wastes, animal fat, microorganisms, aquatic plants (e.g. algae, ferns), urban wastes, sludges from wastewater treatment sites, and organic wastes (e.g. plastics) (Jalilian et al., 2020; Sun et al., 2022). Among the biofuels, bio-oil (products of thermal cracking of biomasses through a process called pyrolysis) does not need downstream processes, which makes it a promising choice (Ayub et al., 2022; Pourkarimi et al., 2020). Along with a variety of parameters like the type of biomass, heating rate, temperature, and rest time that affect the process of pyrolysis of biomass, using catalyzers (given the type and volume of catalyst) can be a key qualitative and quantitative factor in producing biofuel (Miao et al., 2004). The pyrolysis process can be catalytic or direct pyrolysis (Grierson et al., 2013; Miao et al., 2004). The bio-oil of catalytic pyrolysis has a better carbon content and lower oxygen, which increases its heat value and quality (Pourkarimi et al., 2019a).
The recommended mechanism for catalytic pyrolysis is a combination of free radicals and carbonium ion mechanisms (based on Bronsted-Lowry acid-based theory) (Sekar et al., 2021). The catalysts used in the process mostly contain metal compounds like metal oxides. In many cases, catalysts are comprised of two sections (base and booster), which improve the process yield. One of the main compounds used in the pyrolysis process, which is used as the base, is zeolite (Gökdai et al., 2010). Zeolite has a 3D, latticed, and pentagon molecular structure that is formed by alumina and silica at different ratios. Studies have listed a wide range of usage of zeolite to convert different biomasses such as cellulose, lignin, and algae biomasses into bio-oil through the pyrolysis process (Perego and Bosetti, 2011).
Spirulina microalgae are rich in protein and low in fat and are therefore a good feed for the pyrolysis process and the production of valuable aromatic substances such as solvents (Ayub et al., 2022; Naqvi et al., 2021). Low-lipid microalgae strains such as spirulina can easily adapt to the culture medium and grow faster than high-lipid strains in wastewater (Prabakaran et al., 2022). Spirulina is one of the most famous green–blue algal strains grown on a large scale. This strain is used as a dietary supplement due to its gamma linoleic acid and carotenes (Hou et al., 2022). The organic phase of chlorella pyrolysis bio-oil is of better quality than lignocellulosic biomass pyrolysis bio-oil due to its lower oxygen content, higher calorific value, lower acidity and density (Qian et al., 2021). The rapid pyrolysis of chlorella with zeolite catalyst with the weight ratio of 1:9, declared the efficiency of aromatic hydrocarbons to be 25 %, and the monoaromatic aromatics (such as BTX) in aromatic products were higher (Behera et al., 2022). Chaiwong et al. (Chaiwong et al., 2013) studied the slow pyrolysis of spirulina in the temperature range of 450–600 ℃ and reported the highest bio-oil yields at 550 ℃. Chagas et al. (Chagas et al., 2016) investigated the catalytic pyrolysis of bio-oil from spirulina pyrolysis with the help of several zeolite catalysts, the results of their research showed that the HZSM-5 catalyst with the weight ratio ( / = 23) due to its high acidity, had the highest conversion of biomass into bio-oil. In another similar study, Anand et al. (Anand et al., 2016) examined the rapid pyrolysis of spirulina and concluded that rapid pyrolysis produces some valuable chemicals and solvents. Plevin et al. (Plevin et al., 2022) examined the production of bio-oil using cellulose and lignin biomasses and zeolite catalysts; they reported that the catalyst deoxygenates the biomasses in the first phase and then facilitates hydrocarbons conversion of higher heat value. Pan et al. (Pan et al., 2010) examined the role of catalyst ratio to feed and temperature effect for Nannochloropsis sp. microalgae catalytic pyrolysis using HZSM-5 catalyst (Si/Al = 25), the ratio of feed to catalyst was equal to 1/20 % (w/w) and the operating temperature was 300–500 ℃; eventually, the top bio-oil yield was realized with a ratio of catalyst to feed equal to 20 %(w/w) and operation temperature equal to 400 ℃, which was about 25 %; moreover, oxygen production was decreased by the catalyst from 30.1 % to 19.5 % and increased value of heat from 24.6 to 32.7 (Mohammed et al., 2017). Mohamed et al. (Mohamed et al., 2013) conducted a study on using different ratios of alumina-based cobalt to feed (5–25 %) to covert fruit tree wastes into bio-oil using catalytic pyrolysis, they demonstrated that increasing the ratio of cobalt in the catalyst increased the yield of bio-oil and decreased coke, the highest bio-oil yield was equal to 41.89 % with alumina-based cobalt ratio of 20 %. Ma et al. (Ma et al., 2020) studied using zeolite catalyst ZSM-5 with a ratio to feed in the 2–10 % range for bio-oil production from Ulva microalgae at 400 ℃, they showed that the top bio-oil yield was 41.3 % with catalyst/feed ratio of 10 %. Chen et al. (Chen et al., 2021) examined bio-oil production using sawdust and corn waste pyrolysis at an operational temperature of 500 ℃, they used different HZSM-5/feed ratios (1:1, 1:5, and 1:10), and the top bio-oil yield was 6 % using sawdust with catalyst/feed ratio of 1:10, the highest level of aromatic compound production was equal to 27.3 %. Pourkarimi et al. (Pourkarimi et al., 2022) examined the production of bio-oil using two biomasses namely Azolla ferns and Ulva macroalgae, using two catalysts including HZSM-5 zeolite and zeolite-based molybdenum catalyst with catalyst/feed ratio (1:5 and 1:10); they found that the utmost energy efficiency and the lowest amount of pollutants for both Ulva bio-oil and Azolla were determined using /HZSM-5 with the catalyst to feed ratio 1:10, the uttermost bio-oil production yield of Azolla and Ulva were obtained using HZSM-5 catalyst and with catalyst/feed ratio equal 1:10.
In previous studies, the cost of making catalysts was high, which affects the use of this process on an industrial scale, so achieving an optimal process using an inexpensive catalyst was followed in this research as an innovative method to extract Bio-diesel. The aim of this study was to slow the pyrolysis of spirulina microalgae in both direct and catalytic pyrolysis modes. The effect of three factors of temperature, heating rate, and volumetric flow of carrier gas on bio-oil efficiency is investigated. Also, the effect of adding zeolite catalyst on the final quality of bio-oil is also examined.
2 Material and methodology
2.1 Preparation of biomass
Spirulina microalgae were purchased from the Iranian Genetic Resources Research Center. This strain was cultured in Zarouk culture medium in several stages to prepare a sufficient amount of dry biomass for testing. Spirulina microalgae, after being separated from the culture medium, were first washed twice with deionized distilled water and then dried in the sun for 48
and then in an oven at 55 ℃, the biomasses were ground to obtain a homogenous powder (particle size: 500–1000
). The results of elemental and approximate analysis along with the thermal value are given in Table 1.
Ultimate Analysis (Wt.%)
Biochemical composition (Wt.%)
Proximate Analysis (Wt.%)
HHV (
)
C
H
N
S
O
Protein
Carbohydrate
Lipid
Moisture
Volatiles
Fixed carbon
Ash
47.45
7.14
10.64
1.22
33.55
73.2
16.75
1.1
5.4
80.9
6.05
7.65
22.42
2.2 Preparation of catalysts
The zeolite base used in the study was HZSM-5 zeolite procured from Iran Zeolite with a Si/Al ratio of 40. According to the producer, the specific surface of zeolite was 370 , with pore size equal to 5.5 and crystallinity equal to 98 %. Cobalt (II) chloride hexahydrate ≥ 97 % was purchased from Sigma-Aldrich. Cobalt metal catalyst was prepared using a moist incubation method (Pourkarimi et al., 2020). To this end, adequate amounts of the cobalt (II) chloride hexahydrate was mixed with the HZSM-5 base solution (Okoye-Chine et al., 2019), and then the sample was stirred on a magnetic plate (40 , 25 ℃, and 4 ) to make sure that the active metal is adsorbed to the base. Afterward, the samples were dried in an oven (80 ℃, 18 ) and then calcinated in a furnace with a heating rate of 3℃ at 400 ℃. The catalyst powder was formed as a tablet using a hydraulic press device (60 ) and then grounded with a mesh number of 60 and catalyst powder particle sizes 250–420 µm were selected for reactor experiments.
2.3 Catalyst analysis
Using X-ray (XRD, PHILIPS PW1730, Netherlands), the structural information of the catalysts was obtained. X-ray light source was CU/Ka with wavelength of 1.54056 with 2θ values range from 10° to 90°, which was used to detect crystalline structure of Co/HZSM-5 catalyst. The surface morphology of the catalysts was studied by scanning electron microscope imaging (SEM, TESCAN MIRA III, Czech Republic). The functional groups of the catalyst were investigated and compared using the Fourier Transform Infrared Spectrometer (FTIR, THERMO AVATAR, USA). The specific surface of the catalysts, total volume of pores in the mass unit, and mean diameter of pores were obtained by Brunauer-Emmett-Teller theory (BET, BELSORP MINI II, Japan).
2.4 Experiment device
The experiment device was comprised of three major sections reformer (Sekar et al., 2021), pyrolysis reactor (Ayub et al., 2022), and condenser (Perego and Bosetti, 2011), which is illustrated in Fig. 1. Stainless steel 316 was used to build the reformer and pyrolysis reactor with electrical heat coat (Kumar et al., 2022). The system is comprised of three main parts, including pyrolysis reactor (4), reformer (10), and condenser (11). A pyrolysis reactor with a diameter of 7.8 cm, a height of 23 m, a capacity of 1
, and a reformer reactor with a diameter of 5.4 cm, a height of 20 cm, and a capacity of 0.45 L were used. The reformer section used a quartz cylinder (1 cm in diameter and 20 cm in height) as the catalyst container so that for each experiment, the catalyst would be placed inside and stabilized between the input and output (connected to the condenser).
was used as the condenser gas at 140 atm pressure, which was converted into 1 atm using a regulator (2). As a cooling system, water and ice circulation was used as the condenser to cool down and alter the pyrolysis vapors phase (Mohamed et al., 2013). Direct pyrolysis experiments were done using the same setting without the catalyst container of the reformer section (Kabir and Hameed, 2017).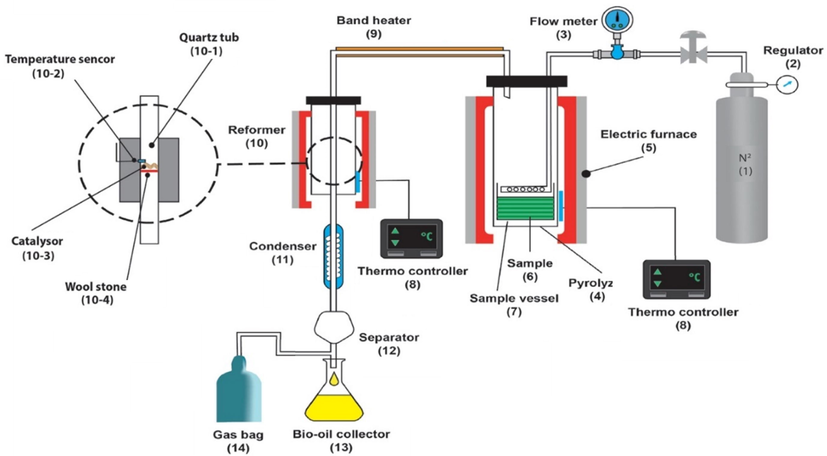
The pyrolysis device schematic.
2.5 Methodology
Similar to the method of Wu et al. (Wu et al., 2021): for each experiment, a 14 g dried biomass sample was placed in the reactor. Before beginning the pyrolysis experiments, nitrogen gas was passed through the system with a flow rate of 100 for 30 min to eliminate oxygen. The samples were heated from room temperature to different targets (425–625 ℃, 50 ℃) according to the designed heating rates (10–20 ℃ ) and nitrogen gas flow rates of 0.2–0.8 . The reactor was kept at the desired temperature for 30 min. At the end of each run, the system was turned off and allowed to cool to room temperature and the solid fraction (bio-char) was collected from the reactor. The liquid phase (bio-oil) generated from the cooling down of condensable gases was collected in a falcon (13), weighed, and stored for further analysis. The non-condensable gases were stored in a gasbag. All experiments were performed in duplicates and the average values are reported.
The optimum conditions for the highest bio-oil production efficiencies were temperature 468 ℃, carrier gas discharge rate equal to 0.31
, and heating rate equal to 14.4 ℃
. The reformer reactor was empty, but in catalytic pyrolysis, which is performed under optimal direct pyrolysis conditions, the catalyst is placed in the reformer reactor. The
/HZSM-5 catalyst with a loading ratio of 5 % (catalyst/feed ratio 1:10) was used. To this end, the catalyst container was prepared beforehand so that a metal mesh with a diameter of 1 cm would be fitted to the longitudinal center of the reformer cylinder and then an asbestos layer was used as coverage. Having bio-oil and biochar weighed, bio-oil, biochar, and biogas yield were obtained using Eqs. (1)–(3) (Biswas et al., 2017):
2.6 Analysis of the obtained bio-oil
To assess the quality of the bio-oil sample by detecting the compounds, the sample analysis was done using GC–MS 5975C (Agilent Technologies- American company). The qualitative parameters of the obtained fuel using CG-Mass and Biodiesel Analyzer (v.2.2), including iodine number, density, cetane number, and viscosity were determined. To perform elemental analysis, Flash EA1112 was used (Thermo Finnigan- American company) and the results yielded the highest heat value (HHV) using Eq. (4) (Pourkarimi et al., 2019b):
Where H, C, O, and S refer to hydrogen, carbon, oxygen, and sulfur weight percentage, which were measured using elemental analysis. Using Eq. (5), energy recovery for the two biomass type were examined (Song et al., 2014):
Where and are weight of biomass and bio-oil.
3 Result and discussion
3.1 Characterization
Fig. 2 illustrates XRD results for the catalyst used in the study. The pattern for HZSM-5 zeolite has a pointy and sharp peak at 2θ equal to 23.9 ° and two smaller peaks of the same size at 25.7 ° and 46 °. These peaks indicate the single-phase crystalline structure of the solid. As to
/HZSM-5 catalyst, it appears that the process of the metal deposit does not have any effect on the morphology and size of nanoparticles (Xiu and Shahbazi, 2012). As to Co catalyst based on zeolite base, adding the metal nanoparticles to the zeolite did not have a notable effect on the number and form of metallic catalyst peak compared to HZSM-5 catalyst. This can be due to the small size of metal nanoparticles and the low volume of loading on zeolite.
XRD analysis of the catalysts.
The SEM analysis results for cobalt catalyst based on zeolite are shown in Fig. 3. Clearly, HZSM-5 catalyst has particles with polyhedron and smooth surface smaller than 5 µm. In addition, as shown for metal catalysts based on zeolite, they have a structure similar to HZSM-5 (Hao et al., 2021). Apparently, the process of metal deposition does not have a notable effect on the morphology and size of the nanoparticles (Sahoo et al., 2022).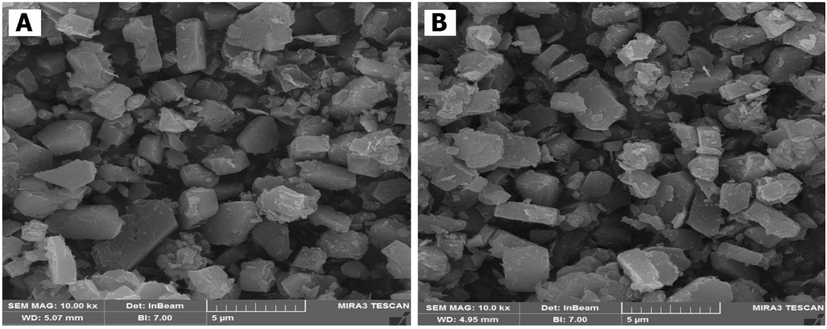
SEM catalyst analysis of HZSM-5 (a) Co/HZSM-5 (b).
According to Fig. 4 the FTIR spectrum of HZSM-5 is featured with two peaks at 454
and 548
which are because of
molecule vibration in zeolite (Behera et al., 2022). In addition, three peaks are observed at 796, 1089, and 1223
, which represent strain vibration of
structure (Hou et al., 2022). The next peak is at 1640
, which refer to vibration bond of water molecules. The last peak is at 3426
, which refers to
bond strain bond caused by the adsorbed water (Shen and Zhang, 2003). The FTIR spectrum for metal catalyst based on zeolite (Co/HZSM-5) is similar to HZSM-5. Therefore, adding extra compounds such as
, does not cause notable changes in HZSM-5 so that zeolite keeps its molecular structure (Sahoo et al., 2022).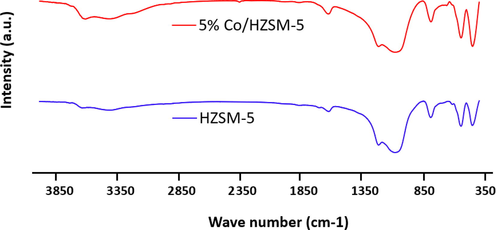
FTIR analysis of the catalysts.
The BET analysis including specific surface (
), total pores volume (
), and mean diameter of pores (
) is presented in Table 2. The specific surface and total pores volume of the Co/HZSM-5 catalyst is lower than that of the HZSM-5 catalyst, while the mean diameter of pores of the Co/HZSM-5 catalyst is greater than that of the HZSM-5 catalyst. The reason for this difference is metal nanoparticle residue on the base surface that might block smaller micro-pores on the surface. Moreover, the smaller zeolite pore blockage increases the mean pore size (Ren et al., 2018). The ICP analysis were conducted to determine metal nanoparticle volume on HZSM-5 in a more accurate way compared to the theoretical value (5 %) (Table 3). Taking into account the findings, the actual value of cobalt is acceptably near the expected values (Qian et al., 2021).
Catalysts
APD (nm)
ICP-OES
HZSM-5
329.197
0.059761
3.9659
–
Co/HZSM-5
280.279
0.02492
13.114
4.94
Catalysts
Bio-oil %
Bio-char %
Bio-gas %
Co/HZSM-5(1:10)
34.05
23.28
42.67
Direct Pyrolysis
48.51
32.25
19.24
3.2 Quantitative assessment of the pyrolysis products
Catalyst pyrolysis experiments and direct pyrolysis of spirulina are listed in Table 3. The highest yield of bio-oil for spirulina was 48.51 %, from direct pyrolysis process. The primary comparison between bio-oil yield from direct pyrolysis and catalyst pyrolysis indicates that bio-oil yield decreased by 14.46 % after adding the catalyst. With the catalyst, the performance of catalytic cracking reactions was improved and with an increase in the light products (gasses) yield, bio-oil and biochar yield decreased. Advantage of spirulina microalgae as a feedstock for biochar is its fast growth rate ranging from 10 to 27 day; which directly impacts the rate at which biochar can sequester carbon (Sekar et al., 2021). Miao et al. (Miao and Wu, 2004) studied fast pyrolysis of cultivated microalgae species. They obtained oil yields of 17.5 % and 23.7 % from autotropic microalgae like Chlorella protothecoides and Chlorella aeruginosa, respectively, whereas the yield of bio-oil was 57.9 % from heterotrophic Chlorella protothecoides. Thangalazhy- Gopakumar et al. (Thangalazhy-Gopakumar et al., 2012) reported 25 wt% (carbon yield) of aromatics from chlorella species via catalytic fast pyrolysis using HZSM-5 catalyst at high catalyst:algae mass ratio (9:1). Importantly, the aro- matic fraction contained more monoaromatics like BTX (benzene, toluene, xylene), and the aromatic yield was significantly higher than that derived from red oak (Thangalazhy-Gopakumar et al., 2012). In another study, Rizzo et al. (Rizzo et al., 2013) reported that the organic phase of bio-oil from pyrolysis of chlorella was superior to that from lignocellulosic biomass in terms of low oxygen content, high heating value, low acidity and low density.
3.3 Elemental analysis
The outcomes of bio-oil elemental analysis from direct pyrolysis and catalytic pyrolysis experiment, maximum heat value, and calculated energy recovery for spirulina is indicated in Table 3. As shown in Table 4, the HHV is 34.43
and the highest energy yield (48.37 %) belongs to bio-oil with catalytic pyrolysis. The reason for this can be carbon/oxygen ratio based on elemental analysis; so that the maximum carbon content (60.5 %) and the lowest oxygen content (14.32 %) was observed in bio-oil sample obtained through catalytic pyrolysis. The carbon content increase and oxygen content decrease can increase HHV. This shows that while production yield of bio-oil decreases with catalyst, there is a considerable growth in HHV and energy yield of the fuel. Among the advantages of catalytic pyrolysis, the H/C ratio increase and O/C ratio decrease in bio-oil yield can increase heat value and energy yield of the fuel. Vankrolen introduced a diagram to compare optimum performance of different fuels based on H/C and O/C ratio in fuels (Ben et al., 2019). Fig. 5 illustrates Vankrolen’s diagram. The areas marked on the diagram indicate the optimum H/C and O/C ratios for different types of fuels for which the HHV and energy yield are obtained. The optimum range determined for H/C pyrolysis oil is in 1.3–1.6 range and for O/C it is in 0.2–0.4 range. The H/C ratio for bio-oil is not in the optimum range of Vankrolen diagram; while in the case of O/C ratio, the direct pyrolysis O/C ratio is in 0.43 range, which is beyond the optimum range in Vankrolen’s diagram. In the case of bio-oil generated through catalytic pyrolysis, this range is 0.2–0.3, which is the optimum range. Therefore, the bio-oil generated through catalytic pyrolysis of bio-oil has an optimum performance in terms of Vankrolen’s diagram, while the bio-oil of direct pyrolysis does not have an optimum performance because of O/C ratio.
Catalysts
C
H
N
O
S
(H/C)*10
O/C
ER (%)
Co/HZSM-5 (1:10)
60.5
11.58
13.5
14.32
0.1
1.91
0.24
34.43
48.37
Direct Pyrolysis
54.5
9.1
12.5
23.7
0.1
1.67
0.43
27.22
36.8
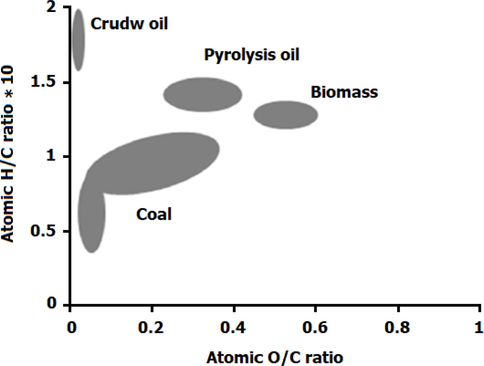
Vankrolen diagram.
3.4 Combinational analysis
The qualitative characteristics of biomass samples obtained from direct pyrolysis and catalytic pyrolysis can be studied through GC-Mass analysis. The GC-Mass analysis results for the samples are given in Table 5. Given the results of GC-mass analysis, the key compound groups in bio-oil samples include aromatic compounds, nitrogenated compounds, oxygenated compounds, Alkanes/Alkenes, and phenolic compounds. Using the catalyst, alkene/alkane content was increased. While the higher content of alkane/alkene can increase heat value of fuel, to some extent, presence of more efficient compounds like aromatics and oxygenated compounds overshadow their effect. Aromatic compound content increased due to using catalysts, which increased the HHV in comparison to the bio-oil collected from direct pyrolysis. It is notable that using catalysts can cause more complicated reactions and increase aromatic products yield. As to oxygenated compounds, catalytic pyrolysis decreased oxygenate compound ratio in a notable way with a positive impact on heat value of the fuel and burning quality. The protein content is decomposed by catalytic pyrolysis. The decomposed hydrogen compounds are converted into gas products, so that nitrogenated compounds content is lower in bio-oil obtained through catalytic pyrolysis compared to direct pyrolysis (Li et al., 2021). Eventually, using the catalyst decreased phenolic compounds. The increase in aromatic compounds due to the use of catalyst increases heat value of fuel, while it also increases the pollution caused by the burning the fuel when the increase in heat value is more than 25 % (Sun et al., 2014). In the case of the catalyst used in this study, aromatic content in bio-oil was 15.56 %. On the other hand, phenolic compound decreased notably due to the use of catalyst, which indicates a decrease in pollution load of bio-oil obtained from catalytic pyrolysis compared to the bio-oil obtained through direct pyrolysis.
Row
Component
Formula
Co/HZSM-5 (1:10)
Direct Pyrolysis
1
Phenol
C6H6O
0.75
1.25
2
4-ethyl-phenol
C8H10O
0.22
0.24
3
2-methyl-phenol
C7H8O
0.32
0.45
4
2–4-dimethyl-phenol
C8H10O
0.63
0.8
5
4-methyl-phenol
C7H8O
0.17
0.36
6
Toluene
C7H8
4.9
4.31
7
Styrene
C8H8
1.25
1.1
8
Ethyl benzene
C8H10
2.2
1.87
9
2-Methyl-naphtalen
C11H10
2.24
1.95
10
Indole
C8H7N
2.1
2.4
11
1H-indole 3-methyl
C9H9N
1.28
1.35
12
Hexadecanenitril
C16H31N
10.2
10.7
13
Hexadecaneamid
C16H33NO
9.4
15.5
14
Pyrazine
C4H4N2
0.75
0.8
15
Octadecaneamid
C18H37NO
2.98
3.2
16
Heptadecane
C17H36
6.2
3.1
17
d-Limonene
C10H16
1.4
0.7
18
Tetramethyl hexadecane
C20H40
6.55
4.4
19
Tetrahydro trimethylnaphtalene
C13H18
3.1
1.7
20
Furanmethanol
C5H6O2
0.45
0.7
21
3-methyl-butanal
C5H10O
0.5
0.75
Some of the qualitative specifications of the produced fuels including iodine number, cetane number, cinematic viscosity, density, and cloud point were determined using GC-Mass analysis results in Biodiesel Analyzer v.2.2. Table 6 indicates the qualitative parameters of the produced bio-oil and the standard values (EN14214-2012) (Pourkarimi et al., 2019b). With the catalyst, cetane number of bio-oil increased by 12.9 % and iodine number decreased by 9.1 %, which improved performance quality of the fuels. In addition, density and viscosity of the biofuels did not change notably after using the catalyst. However, the cloud point decreased to some extent, which makes the fuels suitable for lower temperatures. Using the catalyst had a positive impact on the quality of fuel (cetane and iodine numbers in particular) and the application range.
Quality parameter
Direct Pyrolysis
Co/HZSM-5 1:10
Standard EN14214
Cetan Number (CN)
60.3
68.1
51<
Iodine Value (IV) g/100 g oil
132
120
120>
Density (g/cm3)
0.94
0.85
0.86–0.9
Kinematic Viscosity (mm2/s)
4.6
4.3
3.5–5
Cloud Point (oC)
−4.8
−4.5
–
4 Conclusion
The effect of using cobalt catalyst based on zeolite was examined and compared with direct pyrolysis. To this end, catalytic pyrolysis of spirulina was performed in optimum operational condition and the results were compared with direct pyrolysis. Bio-oil production yields for direct pyrolysis was 48.51 % and for catalytic pyrolysis was 34.05 %. The optimum heat values for catalytic pyrolysis were 34.43 and for direct pyrolysis these values were 27.22 . This can be explained by an increase of carbon content along with a decline in oxygen content in the bio-oil samples obtained through catalytic pyrolysis. In addition, the bio-oil generated by catalytic pyrolysis was at optimum range defined for pyrolysis oil based on Vankrolen diagram. This indicates a higher qualitative performance in comparison with the bio-oil generated through direct pyrolysis. Qualitative parameters including cetane number and iodine number of the bio-oil obtained from catalytic pyrolysis were higher and lower compared to direct pyrolysis. This indicates that bio-oil has a higher quality through catalytic pyrolysis. However, catalyst caused no significant influence on density and viscosity. In short, using the catalyst increases maximum heat value and energy performance of the bio-oil, while it decreases the yield. Therefore, using catalytic pyrolysis is a potential way to produce bio-oil.
In the future, to increase the efficiency of this process, extensive research can be done on the genetic engineering of various microalgae, including spirulina. In addition, in the next researches, different catalysts can be studied in this process. Also, industrial scale process design for biodiesel production is particularly important.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Catalytic fast pyrolysis of Arthrospira platensis (spirulina) algae using zeolites. J. Anal. Appl. Pyrolysis. 2016;118:298-307.
- [CrossRef] [Google Scholar]
- Sustainable valorization of algae biomass via thermochemical processing route: an overview. Bioresour. Technol.. 2022;344:126399
- [CrossRef] [Google Scholar]
- Hydrothermal processing of microalgal biomass: Circular bio-economy perspectives for addressing food-water-energy nexus. Bioresour. Technol.. 2022;359:127443
- [CrossRef] [Google Scholar]
- A comprehensive characterization of pyrolysis oil from softwood barks. Polymers (Basel).. 2019;11:1387.
- [CrossRef] [Google Scholar]
- Pyrolysis of azolla, sargassum tenerrimum and water hyacinth for production of bio-oil. Bioresour. Technol.. 2017;242:139-145.
- [CrossRef] [Google Scholar]
- Catalytic pyrolysis-GC/MS of Spirulina: Evaluation of a highly proteinaceous biomass source for production of fuels and chemicals. Fuel. 2016;179:124-134.
- [CrossRef] [Google Scholar]
- Study of bio-oil and bio-char production from algae by slow pyrolysis. Biomass and Bioenergy. 2013;56:600-606.
- [CrossRef] [Google Scholar]
- Catalytic level identification of ZSM-5 on biomass pyrolysis and aromatic hydrocarbon formation. Chemosphere. 2021;271:129510
- [CrossRef] [Google Scholar]
- Comparison of the catalytic efficiency of synthesized nano tin oxide particles and various catalysts for the pyrolysis of hazelnut shell. Biomass and Bioenergy. 2010;34:402-410.
- [CrossRef] [Google Scholar]
- Life cycle assessment of a microalgae biomass cultivation, bio-oil extraction and pyrolysis processing regime. Algal Res.. 2013;2:299-311.
- [CrossRef] [Google Scholar]
- Catalytic co-pyrolysis of rice straw and ulva prolifera macroalgae: Effects of process parameter on bio-oil up-gradation. Renew. Energy. 2021;164:460-471.
- [CrossRef] [Google Scholar]
- Catalytic co-pyrolysis of oil sludge and biomass over ZSM-5 for production of aromatic platform chemicals. Chemosphere. 2022;291:132912
- [CrossRef] [Google Scholar]
- Macro and Micro Algae in Pollution Control and Biofuel Production – A Review. ChemBioEng Rev.. 2020;7:18-33.
- [CrossRef] [Google Scholar]
- Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew. Sustain. Energy Rev.. 2017;70:945-967.
- [CrossRef] [Google Scholar]
- Co-pyrolysis of de-oiled microalgal biomass residue and waste tires: deeper insights from thermal kinetics, behaviors, drivers, bio-oils, bio-chars, and in-situ evolved gases analyses. Chem. Eng. J.. 2022;446:137160
- [CrossRef] [Google Scholar]
- Applications of calcium oxide–based catalysts in biomass pyrolysis/gasification – A review. J. Clean. Prod.. 2021;291
- [CrossRef] [Google Scholar]
- Non-catalytic and catalytic pyrolysis of Ulva prolifera macroalgae for production of quality bio-oil. J. Energy Inst.. 2020;93:303-311.
- [CrossRef] [Google Scholar]
- High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J. Biotechnol.. 2004;110:85-93.
- [CrossRef] [Google Scholar]
- Fast pyrolysis of microalgae to produce renewable fuels. J. Anal. Appl. Pyrolysis. 2004;71:855-863.
- [CrossRef] [Google Scholar]
- The effects of holding time and the sweeping nitrogen gas flowrates on the pyrolysis of EFB using a fixed bed reactor. Procedia Eng., Elsevier Ltd 2013:185-191.
- [CrossRef] [Google Scholar]
- Valorization of Napier grass via intermediate pyrolysis: optimization using response surface methodology and pyrolysis products characterization. J. Clean. Prod.. 2017;142:1848-1866.
- [CrossRef] [Google Scholar]
- Recent developments on sewage sludge pyrolysis and its kinetics: resources recovery, thermogravimetric platforms, and innovative prospects. Comput. Chem. Eng.. 2021;150:107325
- [CrossRef] [Google Scholar]
- A critical review of the impact of water on cobalt-based catalysts in Fischer-Tropsch synthesis. Fuel Process. Technol.. 2019;192:105-129.
- [CrossRef] [Google Scholar]
- The direct pyrolysis and catalytic pyrolysis of Nannochloropsis sp. residue for renewable bio-oils. Bioresour. Technol.. 2010;101:4593-4599.
- [CrossRef] [Google Scholar]
- Biomass to fuels: The role of zeolite and mesoporous materials. Microporous Mesoporous Mater.. 2011;144:28-39.
- [CrossRef] [Google Scholar]
- Choices in land representation materially affect modeled biofuel carbon intensity estimates. J. Clean. Prod.. 2022;349:131477
- [CrossRef] [Google Scholar]
- Biofuel production through micro- and macroalgae pyrolysis – A review of pyrolysis methods and process parameters. J. Anal. Appl. Pyrolysis. 2019;142:104599
- [CrossRef] [Google Scholar]
- Biofuel production through micro-and macroalgae pyrolysis–a review of pyrolysis methods and process parameters. J. Anal. Appl. Pyrolysis. 2019;142:104599
- [Google Scholar]
- Factors affecting production of beta-carotene from Dunaliella salina microalgae. Biocatal. Agric. Biotechnol.. 2020;29:101771
- [CrossRef] [Google Scholar]
- Investigation of catalytic pyrolysis of Azolla filiculoides and Ulva fasciata for bio-oil production. Biochem. Eng. J.. 2022;178:108278
- [CrossRef] [Google Scholar]
- A state-of-the-art review on the environmental benefits and prospects of Azolla in biofuel, bioremediation and biofertilizer applications. Ind. Crops Prod.. 2022;183:114942
- [CrossRef] [Google Scholar]
- Enhanced production of renewable aromatic hydrocarbons for jet-fuel from softwood biomass and plastic waste using hierarchical ZSM-5 modified with lignin-assisted re-assembly. Energy Convers. Manag.. 2021;236:114020
- [CrossRef] [Google Scholar]
- Enhancement of aromatic products from catalytic fast pyrolysis of lignite over hierarchical HZSM-5 by piperidine-assisted desilication. ACS Sustain. Chem. Eng.. 2018;6:1792-1802.
- [CrossRef] [Google Scholar]
- Characterization of microalga Chlorella as a fuel and its thermogravimetric behavior. Appl. Energy. 2013;102:24-31.
- [CrossRef] [Google Scholar]
- Inspecting the bioenergy potential of noxious Vachellia nilotica weed via pyrolysis: thermo-kinetic study, neural network modeling and response surface optimization. Renew. Energy. 2022;185:386-402.
- [CrossRef] [Google Scholar]
- A review on the pyrolysis of algal biomass for biochar and bio-oil – Bottlenecks and scope. Fuel. 2021;283:119190
- [CrossRef] [Google Scholar]
- An experimental study of oil recovery from sewage sludge by low-temperature pyrolysis in a fluidised-bed☆. Fuel. 2003;82:465-472.
- [Google Scholar]
- Thermal cracking of Enteromorpha prolifera with solvents to bio-oil. Energy Convers. Manag.. 2014;77:7-12.
- [Google Scholar]
- Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J.. 2014;240:574-578.
- [Google Scholar]
- A state-of-the-art review on algae pyrolysis for bioenergy and biochar production. Bioresour. Technol.. 2022;346
- [CrossRef] [Google Scholar]
- Catalytic pyrolysis of green algae for hydrocarbon production using H +ZSM-5 catalyst. Bioresour. Technol.. 2012;118:150-157.
- [CrossRef] [Google Scholar]
- Pyrolysis of aquatic fern and macroalgae biomass into bio-oil: Comparison and optimization of operational parameters using response surface methodology. J. Energy Inst.. 2021;97:194-202.
- [CrossRef] [Google Scholar]
- Bio-oil production and upgrading research: a review. Renew. Sustain. Energy Rev.. 2012;16:4406-4414.
- [CrossRef] [Google Scholar]







