Translate this page into:
Investigation on the g-C3N4 encapsulated ZnO nanorods heterojunction coupled with GO for effective photocatalytic activity under visible light irradiation
⁎Corresponding author. krmurthin@yahoo.co.in (K. Ramamurthi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present work reports on a novel ternary nanocomposite of ZnO rods encapsulated by graphitic like carbon nitride (g-C3N4) and coupled with graphene oxide (GO) prepared by ultrasonication assisted hydrothermal method which provides enhanced photocatalytic activity and stability. Field emission scanning electron microscopy analysis showed that the surface morphology of ZnO, g-C3N4 (prepared by heating method) and the GO contains nanorods structures, sheet like structures and sheets with porous structures respectively. Formation of rod like structures of ZnO and thin sheet like structures of g-C3N4 were observed from transmission electron microscopy analysis. Transmission electron microscopy analysis of g-C3N4 (6 wt%)/ZnO nanocomposites showed that ZnO nanorods are encapsulated by the thin sheets of g-C3N4 and g-C3N4(6 wt%)/ZnO/GO (30 mg) ternary nanocomposites contains porous structures of GO. The optical band gap of ZnO nanoparticles was shifted from 3.08 eV to 2.85 eV for g-C3N4(6 wt%)/ZnO/GO(30 mg) ternary nanocomposites. Under visible light irradiation the ZnO nanorods and g-C3N4 (6 wt%)/ZnO showed photodegradation efficiency of ∼21% and 90% respectively for 120 min whereas g-C3N4 (6 wt%)/ZnO/GO(30 mg) showed about 99% photodegradation efficiency in a time period of 14 min. The recycle process carried out for g-C3N4(6 wt%)/ZnO/GO(30 mg) composites up to five cycles showed 91.5% of photodegradation in the fifth cycle for a time period of 14 min. Total Organic Carbon (TOC) analysis shows removal of carbon content 83% in 28 min. The Gas Chromatography-Mass Spectroscopy analysis shows the intermediate products of 1,2 benzenedicarboxylic acid and phthalic acid during the RhB dye photodegradation process. The radical trapping experiment reveals that the photo-induced holes (h+) are one of the main reactive species involved in the degradation of the RhB.
Keywords
g-C3N4/ZnO/GO ternary nanocomposite
Ultrasonication
Hydrothermal
Optical band gap
Raman analysis
Photocatalysts
1 Introduction
Rapid developments in the various industries during the last few decades, specifically the growth and expansion in the organic chemical, petrochemical and other such industries, have released waste water containing organic contaminants such as pesticides, herbicides and dyes which mix directly and pollute the clean water. The commonly used dyes in textile industries are toxic and carcinogenic in nature which affect the ground water system and cause severe health issues. It is very difficult to degrade RhB because of its complex structure (Neppolian et al., 2002; Gogate et al., 2004). Hence developing various strategies to treat the contaminants and protect the water from the contamination have been the subject matter of active research. Various processes such as adsorption, biological, chemical oxidation, photocatalytic and incineration techniques have been used to treat the waste water (Khin et al., 2012). Among the various methods photocatalytic method utilizes clean energy source (natural sunlight) for the purpose of environmental remediation through pollutant degradation and has been widely used (Tong et al, 2012). However, treatment of polluted water continues to be a great challenge. Nanoparticles are tiny materials having size in the range from 1 to 100 nm. Large surface area of the nanoparticles exhibit unique physical and chemical properties. Most importantly the optical properties depend on the size, which imparts different colors due to absorption in the visible region. Reactivity, toughness and other properties of nanoparticles are also dependent on their unique size, shape and structure. Due to these characteristics, they are suitable candidates for various commercial and domestic applications, which include catalysis, imaging, chemical and biological sensing, energy-based research, and environmental remediation (Khan et al., 2019). In this concern nanomaterials show excellent oxidation and reduction behavior due to their large reactive surface area and high reactivity. The nanostructured materials are used in various applications such as photocatalyst (Li et al., 2017), energy storage (Guo et al., 2016). In addition, the mobility of nanomaterials in solution is relatively high and hence the whole solution volume can be quickly scanned with small amounts of nanomaterials due to their nanometer size (Sanchez et al., 2011), which enhances the degradation level of organic pollutants present in the contaminated water. Further, semiconducting metal oxide nanoparticles based photocatalysts are proved to be the promising candidates for environmental remediation (Kochuveedu et al., 2017). Zinc oxide (ZnO) nanoparticles, one of the multifunctional semiconducting metal oxides, possessing wide direct band gap of 3.37 eV and large exciton binding energy of 60 meV, are proved to be most suitable for photocatalytic application (Jung et al., 2008).
The ZnO nanoparticles were synthesized by different methods such as solution (Uthirakumar and Hong, 2009) sol-gel (Zak et al., 2011), hydrothermal (Faisal et al., 2012) and precipitation methods (Chen et al., 2008). Further tuning the nanoparticles morphology, size and their dimension acquired considerable importance due to their ability in enhancing their physical and chemical properties for effective environmental applications (Xia et al., 2003; Zhang et al., 2002; Alivisatos, 1996; Sheikholeslami et al., 2018; Elhag et al., 2015; Khun et al., 2015). In these aspects thus the ZnO nanoparticles possessing various morphologies such as flower (Ameen et al., 2013), timber (Jia et al., 2012), nanosheets (Li et al., 2010), spheres (Das et al., 2013), hierarchical (Motaung et al., 2014), nanorods (Shouli et al., 2010), porous hollow spheres (Wang et al., 2010), mesoporous (Xu et al., 2010) and dandelion-like (Fan et al., 2014) structures are potential in enhancing their surface area and hence the photocatalytic activity. Therefore researchers have been interested in synthesizing ZnO nanoparticles with 1D nanostructures such as nanowires (Lu et al., 2006), nanoneedles (Wu et al., 2006), nanotubes (Wang et al., 2008) and nanorods (Baruah and Dutta, 2009; Moussa et al., 2016) as they offer large surface area eventhough the ZnO nanoparticles show less photo-energy conversion efficiency in the UV region (only 3–5% of the solar spectrum) due to their low electron transfer rate and rapid electron-hole pair recombination rate (Zhou et al., 2014). Recent reports show that heterojunctions formed with ZnO nanocomposites reduce the electron-hole pair recombination rate and enhance the photocatalytic efficiency (Chen et al., 2014; Pawar et al., 2016). Hence efforts have been taken to tune the band gap of ZnO nanostructures through synthesizing ZnO based nanocomposites, so as to utilize the visible part of sun light also in enhancing the efficiency of photodegradation. Thus, synthesizing various photocatalysts and tuning the band gap through heterojunction is gained importance in the area of photocatalytic application.
Recently, stable and metal free organic graphitic carbon nitride (g-C3N4) semiconductor is reported as one of the active visible light photocatalytic materials (Wen et al., 2016; Martha et al., 2013; Patnaik et al., 2016). The g-C3N4 possessing π-conjugated electronic structure with the band gap of about 2.7 eV (Zhang et al., 2013), shows superior thermal, chemical, mechanical, electrical and optical properties (Huang et al., 2013). Further the g-C3N4/ZnO nanocomposites, synthesized with various concentration of g-C3N4 and ZnO are proved to be the potential candidates to enhance the efficiency of photocatalytic activity under visible light irradiation. The ZnO/g-C3N4 nanocomposites synthesized by microwave method showed photocatalytic degradation of ∼98% in a time period of 80 min against Rhodamine B (RhB) dye under visible light (Osman et al., 2017). The g-C3N4/ZnO composite photocatalysts synthesized with various weight percent of ZnO by a simple calcination process increased the photodegradation efficiency of methyl orange about to 3 times under visible light irradiation and p-nitrophenol to about 6 times as compared with that of pure g-C3N4 (Sun et al., 2012). The ZnO/g-C3N4 nanocomposite heterostructures prepared by reflux method exhibited 99% of photocatalytic activity in a time period of 140 min against methylene blue under the illumination of visible light as compared to that of pure g-C3N4 (Fageria et al., 2015). Thus the previous reports evidently show that the g-C3N4/ZnO binary nanocomposites acquired relatively high photocatalytic activity under visible light irradiation and enhance the photocatalytic efficiency against organic pollutants. Graphene and its derivatives are 2D one-atom-thick monolayers of sp2-bonded carbon, which exhibit excellent electronic properties, mechanical behavior and ultrahigh surface area with high-performance in photocatalytic application (Dato et al., 2008). The ZnO/g-C3N4 binary and ZnO/g-C3N4/GO (graphene oxide) ternary nanocomposites synthesized by co-precipitation method enhanced the photodegradation efficiency against methylene blue (MB) to 99% (90 min) and 99.5% (15 min) respectively under Xenon light irradiation (Wan-Kuen Jo and Selvam, 2015). Thus literature reports clearly reveal the possibilities of enhancing photocatalytic activity using binary and ternary nanocomposites. Hence motivated by previous studies carried out to enhance the photocatalytic activity of nanocomposites by forming the heterojunction against the RhB dye degradation, we report in the present work for the first time, the formation of heterojunction due to the encapsulation of ZnO nanorods by g-C3N4 coupled with porous structure of GO prepared by ultrasonication assisted hydrothermal method for effective degradation of RhB dye under visible light irradiation. Ultrasonication treatment provides unusual reaction condition in precursor solution due to the formation of compressive acoustic waves which create bubbles that cannot be realized by other methods (Bang and Suslick, 2010; Suslick, 1990). Further the prepared binary and ternary nanocomposites were characterized to analyze their structural, optical and morphological properties and photocatalytic activity against RhB under visible light irradiation.
2 Materials, methods and characterization
2.1 Materials
Analytical grade (AR) zinc acetate dihydrate ((ZnCH3COO)2·2H2O) and sodium hydroxide (NaOH), polyethylene glycol 400 (PEG 400), graphite powder, potassium permanganate (KMnO4), concentrated hydrochloric acid (HCl), concentrated sulfuric acid (H2SO4), hydrogen peroxide (H2O2) and phosphoric acid (H3PO4) were purchased from Sisco Research Laboratories Pvt. Ltd. - India and used. Melamine powder (AR) was purchased from Sigma-Aldrich. In all the experiment ethanol was used as solvent.
2.2 Synthesis process
Synthesis process adopted to prepare GO, g-C3N4, ZnO, g-C3N4/ZnO and g-C3N4/ZnO/GO nanocomposites is presented in Fig. 1.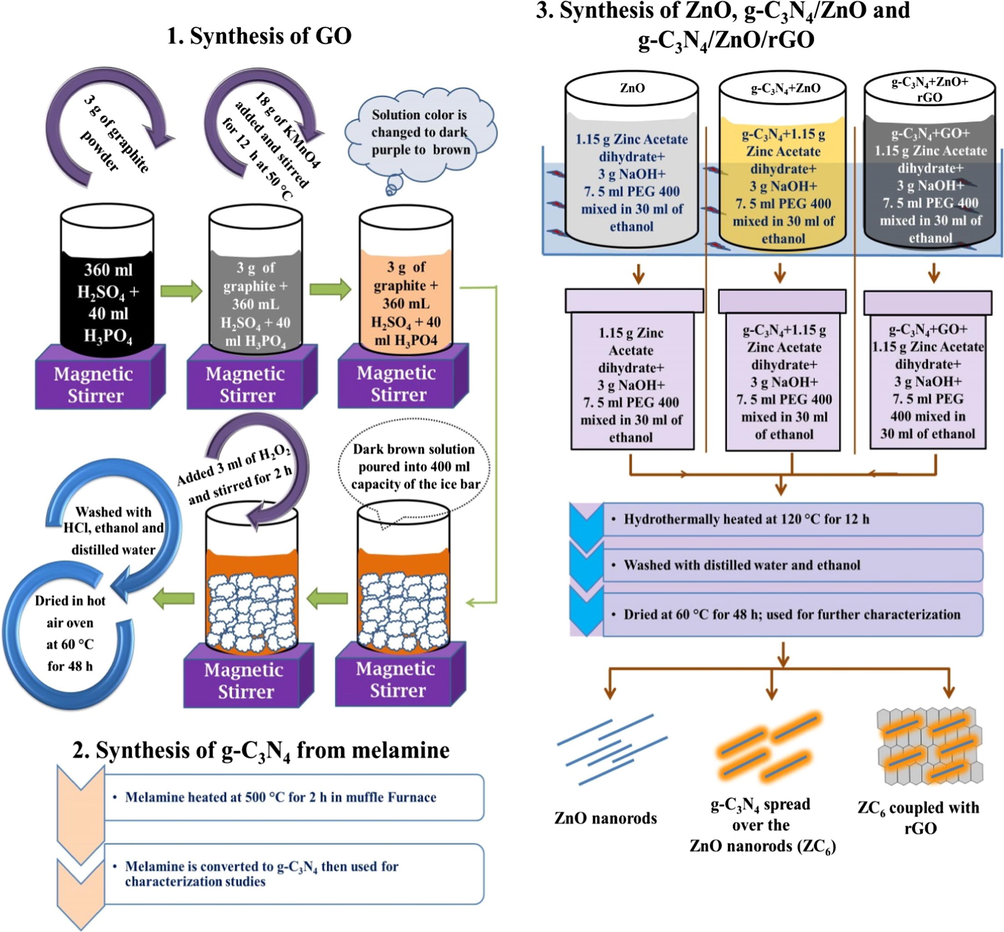
Synthesis method of GO, g-C3N4, ZnO, g-C3N4/ZnO and g-C3N4/ZnO/GO nanocomposites.
2.2.1 Synthesis of graphene oxide
The GO was synthesized following the improved method (Marcano et al., 2010). In a typical synthesis process 360 ml of concentrated H2SO4 and concentrated 40 ml of H3PO4 were mixed properly and 3 g of graphite powder was added slowly to the solution by continuously stirring it for 3 h. Subsequently 18 g of KMnO4 was ground properly and slowly added to that solution. Further the solution was continuously stirred for 12 h at 50 °C using a hot plate magnetic stirrer. During this reaction process the solution color was changed to dark purple and to green and then to dark brown. The dark brown solution was poured into a beaker of 400 ml capacity kept with ice bar and 3 ml of H2O2 was added to this solution and stirred for 2 h. Then the residue particles were washed with HCl for three times and further washed with ethanol and distilled water for several times. Finally the particles were dried at 60 °C in hot air oven for 48 h which yielded GO.
2.2.2 Synthesis of graphitic carbon nitride
Graphitic carbon nitride was synthesized by heating the melamine. 2 g of melamine powder was taken in silica crucible and kept in a muffle furnace. Then muffle furnace temperature was increased in step of 3 °C/min up to 500 °C and maintained at 500 °C for 2 h (Liao et al., 2012). The yellow color product of g-C3N4 was collected and ground into powder in an agate mortar and then used for further characterization studies.
2.2.3 Synthesis of ZnO nanorods
ZnO nanorods were prepared by ultrasonication assisted hydrothermal method (Fan et al., 2016). In a typical synthesis process 1.15 g of zinc acetate dihydrate was dissolved in 30 ml of ethanol; 3 g of NaOH and 7.5 ml of PEG 400 were added to the solution and ultrasonicated for 30 min. Further the solution was transferred to a Teflon lined stainless steel autoclave (50 ml capacity) and the autoclave was heated at 120 °C for 12 h in a muffle furnace and then naturally allowed to reach the room temperature. The product of white ZnO particles was collected, washed with distilled water and ethanol several times. Then the ZnO particles were dried at 60 °C for 48 h and used for further studies.
2.2.4 Synthesis of g-C3N4/ZnO and g-C3N4/ZnO/GO nanocomposites
In the synthesis of g-C3N4/ZnO binary nanocomposites, 0.023 g (2 wt%) of g-C3N4 was dispersed in 30 ml ethanol and the solution was ultrasonicated for 30 min. Then 1.15 g of zinc acetate dihydrate, 3 g of NaOH and 7.5 ml of PEG 400 were added to the solution and ultrasonicated for 30 min. Then the solution was transferred to a Teflon lined stainless steel autoclave and heated at 120 °C for 12 h in a muffle furnace and allowed to cool to room temperature naturally. Final product of g-C3N4/ZnO nanocomposites were washed with distilled water and ethanol for several times and dried at 60 °C for 48 h and the sample is mentioned as ZC2 then used for characterization studies. The same process was followed to prepare g-C3N4/ZnO binary nanocomposites using 0.046 g (4 wt%), 0.069 g(6 wt%) and 0.092 g (8 wt%) of g-C3N4 and the samples are mentioned as ZC4, ZC6 and ZC8 respectively. The ZC6 binary nanocomposites showed enhanced degradation efficiency. The ZC6/GO(x) ternary nanocomposites were prepared varying the amount of GO (x = 10, 20, 30 and 40 mg) as follows: In the synthesis process 6 wt% of g-C3N4 was added to the ultrasonicated solution containing 1.15 g of zinc acetate dihydrate/3g of NaOH/7.5 ml of PEG 400 and then ultrasonicated for 30 min. Subsequently, different amount of GO (x = 10, 20, 30 and 40 mg) was added in separate experiment and ultrasonicated for 30 min. Then the solution was transferred to a Teflon lined stainless steel autoclave and heated at 120 °C for 12 h and the collected particles were washed with distilled water and ethanol for several times and dried at 60 °C for 48 h and the ternary nanocomposites are named as ZC6G10, ZC6G20, ZC6G30 and ZC6G40 respectively for 10, 20, 30 and 40 mg of GO added in the synthesis process.
2.3 Characterization
Structural properties of the synthesized nanoparticles/nanocomposites were analyzed by X-ray diffraction technique (PANalytical’s X’Pert Pro with Cu Kα radiation). Surface morphology and elemental composition of the synthesized samples were studied by Field Emission Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy (FEI Quanta FEG200). Optical band gap of the synthesized nanoparticles was evaluated by Diffuse Reflectance Spectral analyses (Shimadzu, UV-2600 spectrophotometer). Size and structure of the nanoparticles were obtained by transmission electron microscope and high resolution transmission electron microscope (Philips FEI, TECNAI, G2 20s-TWIN with an accelerating voltage of 200 kV). Structure and crystallinity of the nanoparticles were analyzed by Raman Spectroscopy (Renishaw (UK) In Via Raman microscope; incident light wavelength is 633 nm from He-Ne Laser). Photoluminescence of the samples was obtained from Model S4 PIONEER BRUKER spectrometer (excitation wavelength of the sample about λ = 360 nm). The rate of mineralization (carbon removal) was also analyzed using Total Organic Carbon (TOC) analysis (Shimadzu, TOC-L, Japan).
2.3.1 Photocatalytic activity tests
Photocatalytic activity of the prepared samples was analyzed by immersion type photoreactor made up of double layer quartz tube container and the photocatalytic dye degradation was tested under visible light irradiation (150 W tungsten lamp at λ > 400 nm; intensity ∼23,000 lx). During the photocatalytic analysis 5 ppm of RhB dye was prepared in 100 ml distilled water. The absorption spectrum recorded using UV–visible spectrometer showed the maximum absorption intensity at 554 nm. Further, 0.1 g of the prepared catalyst was added to the dye solution and the solution was continuously stirred for 30 min under dark conditions to analyze the adsorption and desorption equilibrium between the catalyst and RhB dye. Then the solution was continuously stirred for 2 h under the visible light irradiation (λ > 400 nm) and at every 30 min interval UV–visible absorption spectrum was recorded to analyze the degradation efficiency of the catalyst. Photocatalytic degradation percentage (%D) was calculated from the formula %D = ((Co − C/Co) × 100 where, Co is the initial concentration of the dye solution and C is the concentration of the dye solution measured at different time interval during the photocatalytic reaction.
3 Results and discussion
3.1 XRD analysis
The X-ray diffraction (XRD) pattern of the bare ZnO, g-C3N4, GO, ZC6 and ZC6G30 nanocomposites are shown in Fig. 2. Bare ZnO nanoparticles show sharp XRD peaks (Fig. 2) at 2θ = 31.82°, 34.46°, 36.30°, 47.58°, 56.63°, 62.87°, 66.40°, 67.97° and 97.11° due to diffraction from (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2) and (2 0 1) planes respectively. These peaks agree well with the hexagonal wurtzite structure of ZnO (JCPDS card no. 89-0510). The XRD peaks of the g-C3N4 observed at 2θ = 13.07° and 27.74° are indexed to (1 0 0) and (0 0 2) planes (Yua et al., 2015). The XRD peak observed at 2θ = 10.27° is due to (0 0 1) plane of GO and the interplanner spacing obtained from the Bragg’s equation (Cullity, 1978) is 0.86 nm. The XRD results of ZC6 nanocomposites show slightly diminished the XRD peak intensity than that of ZnO and the XRD peak intensity of ZC6G30 is slightly diminished than that of ZC6 (Fig. 2) and no characteristic peak is identified related to g-C3N4 and GO in the synthesized (binary and ternary) nanocomposites. This may be due to low level addition of g-C3N4 and GO in the ZnO nanoparticles which resulted in the absence of the long range order stacking arrangement (Wang et al., 2017a, 2017b). The XRD peak intensity of (1 0 0) plane of ZnO nanoparticles is slightly higher than that of (0 0 2) plane. But the ZC6 and ZC6G30 nanocomposites show that the XRD intensity of (0 0 2) plane of ZnO is higher than that of the (1 0 0) plane. Thus the order of more dominant XRD peak intensity in ZnO is (1 0 1) > (1 0 0) > (0 0 2); however ZC6 and ZC6G30 nanocomposites changed the order of the intensity of the XRD peaks as (1 0 1) > (0 0 2) > (1 0 0). Previous reports suggest that enhancement in the (0 0 2) polar facets of ZnO nanoparticles provide high surface area and favors to improve the photocatalytic activity (Zeng et al., 2009; Wang et al., 2011a, 2011b). In the present work, the XRD analysis reveals that the ZC6 binary and ZC6G30 ternary nanocomposite possess relatively enhancement in (0 0 2) polar plane which can be expected to enhance the dye degradation efficiency of the nanocomposites under the visible light irradiation.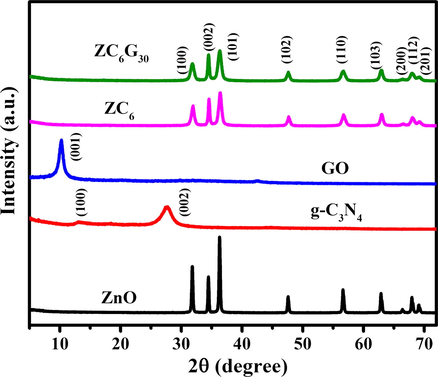
XRD pattern of ZnO, g-C3N4, GO, ZC6 binary and ZC6G30 ternary nanocomposite.
3.2 Field emission scanning electron microscopy analysis
Surface morphology examined by Field Emission Scanning Electron Microscopy (FESEM) analysis, reveals the formation of ZnO nanorods with different length and width along with nearly the regular and elongated hexagonal structures (Fig. 3a). The average length and width of the ZnO nanorods is ∼500 nm and ∼100 nm respectively. Morphology of the g-C3N4 shows thick sheet like structures (Fig. 3b). GO acquired (Fig. 3c) stacking arrangement of thin layer sheets. The binary nanocomposite of ZC6 shows formation of g-C3N4 over ZnO nanorods (Fig. 3d). The ZC6G30 ternary nanocomposites exhibit densely arranged sheet over the ZnO nanorod (Fig. 3e). Thus the addition of g-C3N4 and GO over the ZnO nanorods exhibits effective change in the binary and ternary morphology nanocomposites which are expected to play an important role for photocatalytic activity.
FESEM images of (a) ZnO nanorod; (b) g-C3N4; (c) GO; (d) ZC6 binary nanocomposite (e) ZC6G30 ternary nanocomposite.
3.3 Transmission electron microscopy analysis
Structure and size of the synthesized ZnO, g-C3N4, ZC6 and ZC6G30 nanocomposites were analyzed by Transmission Electron Microscopy (TEM) and the results are given in Fig. 4. Bare ZnO nanoparticles show rods like structures (Fig. 4a); average length and width of the nanorods is ∼400 nm and ∼50 nm respectively. High Resolution TEM (HRTEM) analyses show (Fig. 4b) that the lattice fringes spacing value is 0.24 nm and this value matches well with the d value (0.24 nm) obtained from Bragg’s equation from the XRD peak of (1 0 1) plane of the present work. Selected Area Electron Diffraction (SAED) pattern given in the inset of the Fig. 4b confirms the polycrystalline nature of ZnO. The Energy Dispersive X-ray Analysis (EDS) of the ZnO nanorods confirms the presence of Zn and O (Fig. 4c). TEM analysis of bare g-C3N4 shows sheet like structures (Fig. 4d) and its the magnified image reveals stacking arrangement of thin layer sheets (Fig. 4e). The SAED pattern shows the polycrystalline nature of g-C3N4 (inset of Fig. 4e). Fig. 4f shows the EDS spectrum of g-C3N4 which confirms the presence of C and N related to g-C3N4.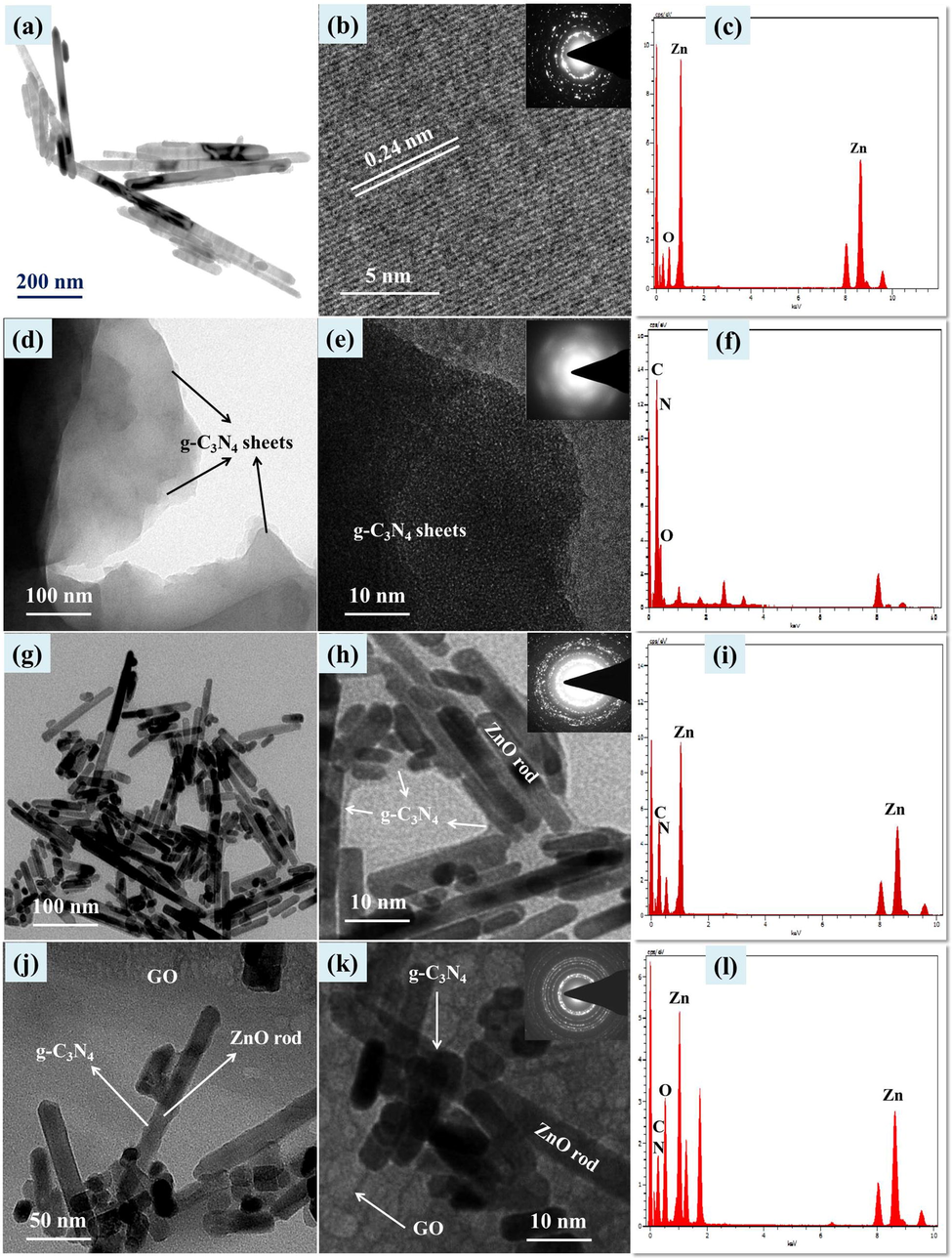
ZnO nanorod images: (a) TEM, (b) HRTEM (Inset SAED) and (c) EDS spectrum. g-C3N4 images: (d, e) TEM, (Inset SAED) and (f) EDS spectrum; g-C3N4(6 wt%)/ZnO images of (g, h) TEM, (Inset SAED) and (i) EDS spectrum; g-C3N4(6 wt%)/ZnO/GO(30 mg) images of (j) TEM, (k) HRTEM (Inset SAED) and (l) EDS spectrum.
The TEM image of ZC6 binary nanocomposite is presented in Fig. 4g which reveals that the formation of ZnO nanorods are encapsulated by thin layer sheets of g-C3N4. Further, HRTEM image confirms that the ZnO nanorods are encapsulated by the formation of thin sheets of g-C3N4 (Fig. 4h) and the SAED of the binary nanocomposites of ZC6 (inset Fig. 4h) shows polycrystalline nature. The EDS pattern of the ZC6 binary nanocomposite (Fig. 4i) shows the presence of Zn, O, C and N. The TEM image of ZC6G30 ternary nanocomposite (Fig. 4j) reveals that the ZnO nanorods are encapsulated by thin layers of g-C3N4 and coupled with porous structures of GO. The ZnO nanorods encapsulated by thin layer structures of g-C3N4 and coupled with GO are shown in Fig. 4k. The ternary ZC6G30 nanocomposite shows (Fig. 4k) the formation of sheet like structure of g-C3N4 and the sheet like porous structure of GO. The SAED pattern confirm (inset of Fig. 4k) that the ZC6G30 ternary nanocomposite is polycrystalline nature. The EDS spectrum of the ZC6G30 ternary nanocomposite shown in Fig. 4l confirms the presence of Zn, O, C and N. Thus the addition of g-C3N4(6 wt%) is converted into thin layers under the ultrasonication process which encapsulate ZnO nanorods whereas the ZC6 is coupled with the porous structure of GO and then form ternary nanocomposite. Element distribution in the ZnO, ZC6 and ZC6G3 nanocomposite was analyzed by FESEM mapping scanning method and the images are given in Fig. 5. The ZnO nanorods show distribution of Zn and O (Fig. 5a1-a3). Fig. 5b1-b5 and c1-c5 shows distribution of Zn, O, C and N elements in binary and ternary nanocomposite respectively. The images show uniform distribution of Zn, O, C and N across the nanorods.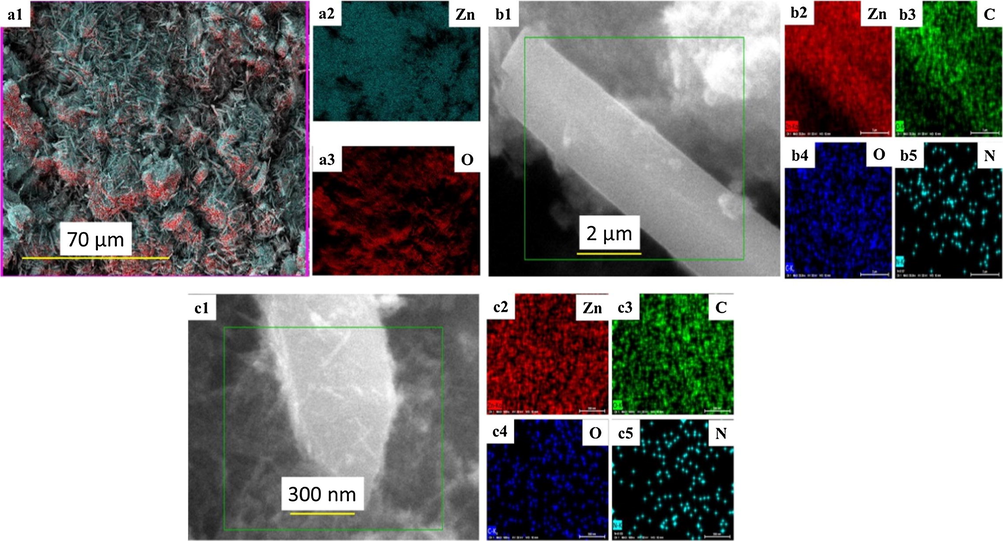
Elemental mapping of ZnO (a1-a3); ZC6 binary (b1-b5) and ZC6G30 ternary nanocomposite (c1-c5).
3.4 XPS analysis
X-ray photoelectron spectrum (XPS) was used to investigate the electronic environment and valence states of elements of ZC6G30 sample. The XPS spectra of the ZC6G30 ternary nanocomposite are presented in Fig. 6. The XPS survey spectrum of ZC6G30 is given in Fig. 6a which confirms the presence of carbon, nitrogen, oxygen and zinc. The Zn 2p spectrum (Fig. 6b) exhibits peak at 1022.4 eV and 1045.5 eV, which are attributed to the binding energy values of Zn 2p3/2 and Zn 2p1/2 respectively. The present values compare well with the XPS results provided by Shifu et al., 2009. The O 1s spectrum is given in Fig. 6c. The main peak centered at 531.8 eV is attributed to O2− ions of ZnO wurtzite structure. The shoulder peak observed at 532.6 eV is attributed to the O2− ions in the oxygen deficient regions within the ZnO matrix (Deng et al., 2008). The N 1s spectrum presented in Fig. 6d shows the peak at 399.8 eV and 400. 8 eV which are assigned to C—N⚌C and N—(C)3 respectively (Xiang et al., 2011). The deconvoluted C 1s spectrum (Fig. 6e) shows three peaks at about 285.9 eV, 286.4 eV and 289.8 eV. The peak centered at 285.9 eV is assigned to pure graphitic sites in the carbon nitride matrix, whereas the peak at 286.4 eV is attributed to the sp2-hybridized carbon atoms bonded to N in an aromatic ring. The peak at 289.8 eV can be assigned to the sp2-hybridized carbon in the aromatic ring attached to the NH2 group (Deng et al., 2012). Moreover, the appearance of N 1s and C 1s peaks indicate the formation of heterojunctions in binary and ternary nanocomposite.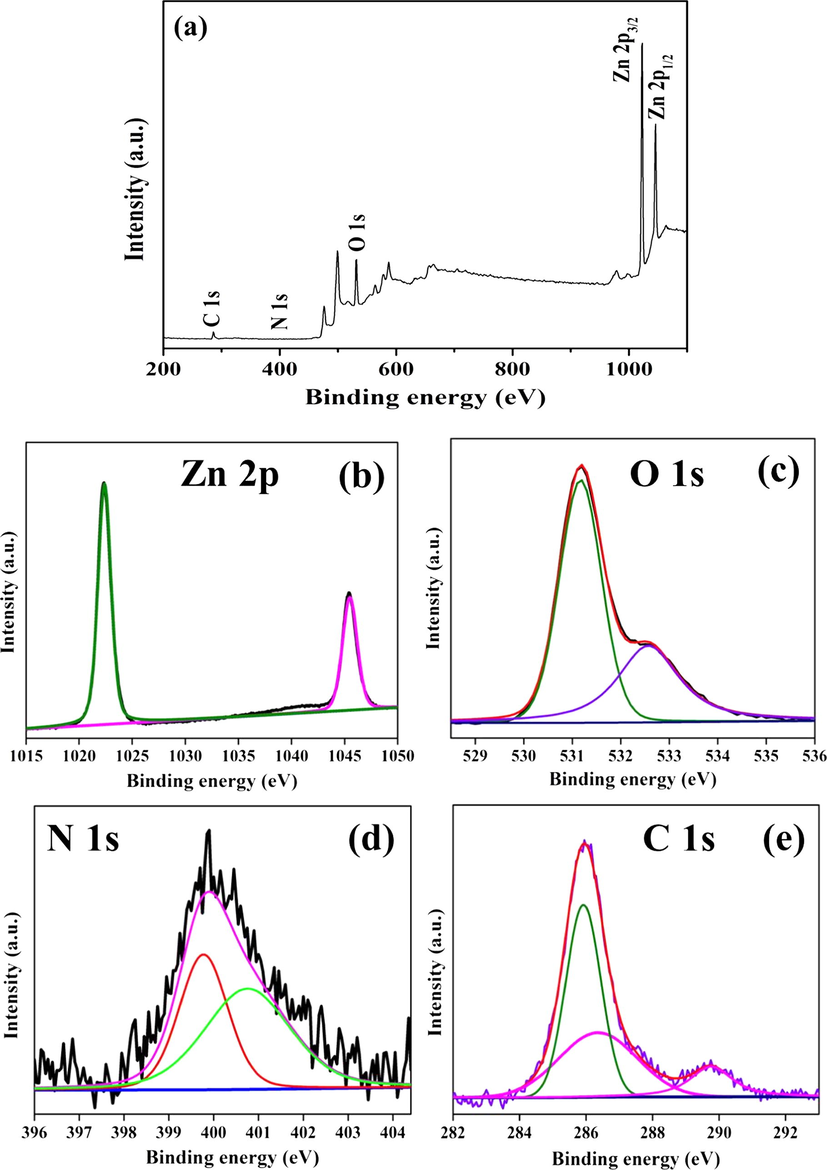
XPS analysis for (a) survey spectrum; (b) Zn 2p; (c) O 1s; (d) N 1s and (e) C 1s of the ZC6G30 ternary nanocomposite.
3.5 Raman and Fourier transform infrared spectral analysis
Structure, crystallinity and defects of the synthesized nanoparticles can be analyzed by Raman Spectroscopy. The Raman spectra of ZnO, g-C3N4, GO, ZC6 binary and ZC6G30 ternary nanocomposites recorded are shown in Fig. 7. The Raman vibrational frequencies along with the frequency assignment of ZnO, g-C3N4, GO, and ZC6 and ZC6G30 are summarized in Table 1. The Raman spectra of ZC6 binary and ZC6G30 ternary nanocomposites are shown in Fig. 7d. The intensity of ZnO stretching vibration observed at 439 cm−1 in ZC6 binary nanocomposite is decreased when compared with that of ZnO and a less intense peak observed at 775 cm−1 is due to the g-C3N4. Further, in the ZC6G30 ternary nanocomposite the intensity of ZnO stretching vibrational mode observed at 439 cm−1 is diminished when compared with that of ZC6 and vibrational mode observed at 775 cm−1 is related to g-C3N4. The vibrational peaks observed at 1353 cm−1 and 1579 cm−1 in ZC6G30 are due to D and G band of GO respectively (Ramachandran et al., 2015) Thus the Raman spectral analyses reveal that the crystallite arrangement in ZnO decreases in binary ZC6 and ternary ZC6G30 nanocomposites than that of ZnO which match well with the XRD results.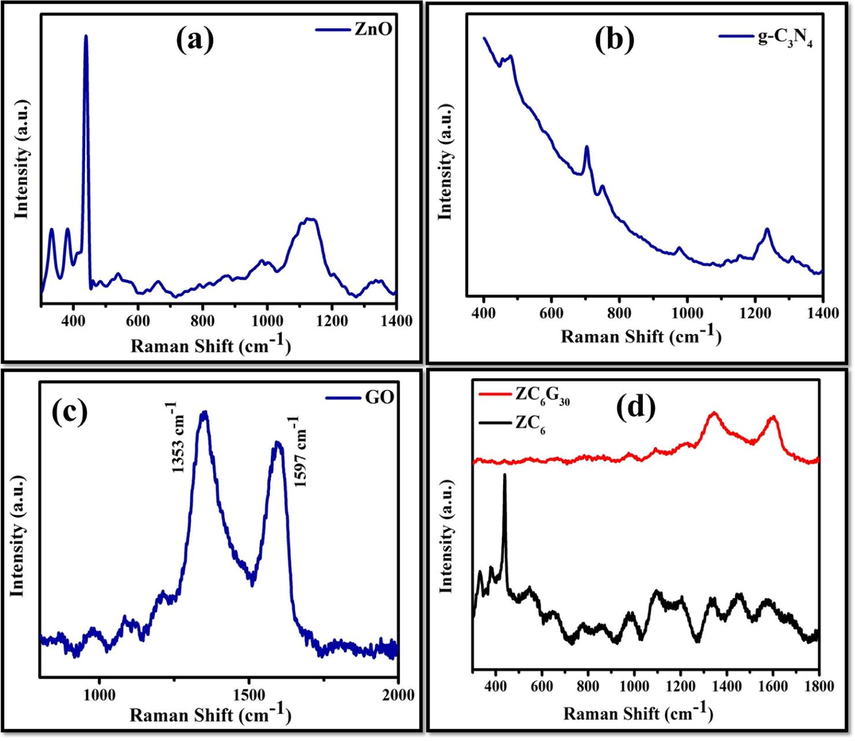
Raman spectral analysis of (a) ZnO; (b) g-C3N4; (c) GO; (d) ZC6 binary and ZC6G30 ternary nanocomposite.
Sample
Peaks (cm−1)
Tentative frequency assigned
Reference
ZnO
439
Raman active E2 mode of hexagonal ZnO
Pudukudy et al. (2014)
331
3E2H-E2L modes of ZnO
Xingfu et al. (2008)
382
A1 transverse optical (TO) mode
Xingfu et al. (2008)
663
A1(LO)+E2(low) modes
Kuriakose et al. (2014)
412
E1 (TO) mode
Scepanovic et al. (2010)
g-C3N4
455, 478, 703, 752, 1235, 1307
Related to graphitic carbon nitrite
Xiang et al. (2011), Hu et al. (2015)
GO
1353
Defects in the graphitic structure (D-band)
Ramachandran et al. (2015)
1579
sp2 hybridized carbon atoms in graphitic structure (G-band)
Ramachandran et al. (2015)
ZC6
439
Raman active E2 mode of hexagonal ZnO
Pudukudy et al. (2014)
775
Related to graphitic carbon nitrite
Xiang et al. (2011), Hu et al. (2015)
ZC6G30
439
Raman active E2 mode of hexagonal ZnO
Pudukudy et al. (2014)
775
Related to graphitic carbon nitrite
Xiang et al. (2011); Hu et al. (2015)
1353
Defects in the graphitic structure (D-band)
Ramachandran et al. (2015)
1579
sp2 hybridized carbon atoms in graphitic structure (G-band)
Ramachandran et al. (2015)
Fourier transform infrared (FTIR) spectra of ZnO, ZC6 binary and ZC6G30 ternary nanocomposites were recorded in the range of 400–4000 cm−1 and are shown in Fig. 8. The frequency observed at 559 cm−1 corresponds to Zn-O bending vibrations (Wan-Kuan Jo and Selvam, 2015). In the ZC6 binary ZC6G30 ternary nanocomposites the peak observed at ∼805 cm−1 is assigned to the s-triazine ring of g-C3N4 which indicates the intense interaction between ZnO and g-C3N4 (Liu et al., 2011; Wan et al., 2015). The several bands observed in the range of 650–1200 cm−1 are attributed to g-C3N4 (Liu et al., 2008). The band appeared at 1240 cm−1 in ZC6G30 ternary nanocomposite is assigned to aromatic C-N stretching (Liu et al., 2008; Zhu et al., 2014). The bands at 1432 cm−1, 1561 cm−1 and 1630 cm−1 are assigned to typical stretching vibrational modes of heptazine-derived repeating units (Li et al., 2014). The broad band obtained around 3000–3500 cm−1 is assigned to the hydroxyl group of H2O as well as to the —NH moieties (Wang et al., 2012; Zhu et al., 2014). Thus in the present investigation the FTIR spectral analysis confirms the formation of binary and ternary nanocomposites.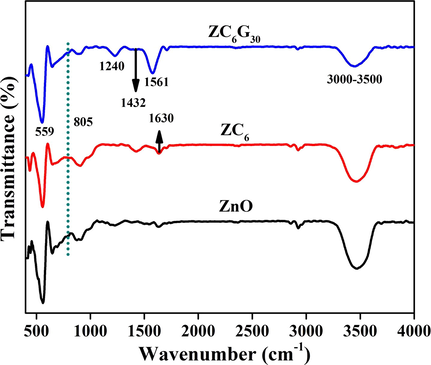
FTIR spectral analysis of ZnO, ZC6 binary and ZC6G30 ternary nanocomposite.
3.6 DRS spectral analysis
UV–Visible diffuse reflectance spectra recorded for the ZnO nanoparticles, g-C3N4, ZC6 and ZC6G30 nanocomposites are shown in Fig. 9a. The ZnO nanorods and g-C3N4 show absorption edge at 400 nm and 450 nm respectively. Further, ZC6 shows a shift in the absorption edge towards the higher wavelength side to 415 nm and the absorption intensity is also slightly increased when compare with that of ZnO nanorods. Thus shift in the absorption edge towards visible side implies that the formation of tight chemically bonded interface between g-C3N4 and ZnO phases (Wang et al., 2017a, 2017b). The DRS spectral analysis of ZC6G30 ternary nanocomposites shows a slight shift in the absorption towards the visible range to 425 nm and absorption intensity is also increased.
Absorption spectra of (a) ZnO, g-C3N4, ZC6 binary and ZC6G30 ternary nanocomposite; (b) hν vs (αhν)2 plot of ZnO, g-C3N4, ZC6 binary and ZC6G30 ternary nanocomposite.
The direct optical band gap of the bare ZnO nanorods, g-C3N4, ZC6 and ZC6G30 nanocomposites was calculated from the equation αhν = B (hν − Eg)1/2 (Tauc and Grigovici, 1974). The direct optical band gap is calculated by plotting the value of (αhν)2 (Y-axis) as a function of hν (X-axis) and extrapolating the straight line portion to (αhν)2 = 0 (Fig. 9b). The ZnO nanorods acquire the band gap of about 3.08 eV and the g-C3N4 shows the band gap about 2.60 eV. The binary ZC6 and ternary ZC6G30 nanocomposites acquired the band gap 2.9 eV and 2.85 eV respectively. In the present work DRS spectral analysis results reveal that ZC6G30 ternary nanocomposite shows enhanced absorption intensity and the band gap shifted to visible region. Thus the ZC6G30 ternary nanocomposite can be expected to yield better degradation efficiency under the visible light irradiation.
3.7 Photoluminescence spectroscopy
The charge separation capacity of the electron-hole pair is evaluated by the Photoluminescence (PL) spectral analysis. The room temperature PL emission spectra of the ZnO, g-C3N4, ZC6 and ZC6G30 are presented in Fig. 10. ZnO nanorods show emission peak at 399 nm due to excitonic band-to-band radiative emission and the trap-state emission observed at 460 nm is originating from oxygen and zinc intrinsic defects (Ahsanulhaq et al., 2007). The intensity of the PL emission peak at 399 nm is decreased in the ZC6 binary nanocomposite and slightly shifted towards higher wavelength side which confirms the shift in the band gap towards the visible region. However, the PL intensity in ZC6 decreases due to decrease in the charge recombination rate which indicates the effective charge carrier separation capacity. The PL emission spectra of ZC6G30 ternary nanocomposite shows quenching in the PL emission intensity at 399 nm than that of ZC6 binary nanocomposite. This evidently shows that ZC6G30 ternary nanocomposite effectively conducts the photo-excited electrons to form superoxide radicals and hence reduces the charge recombination rate due to effective charge carrier separation capacity and enhances the photocatalytic efficiency.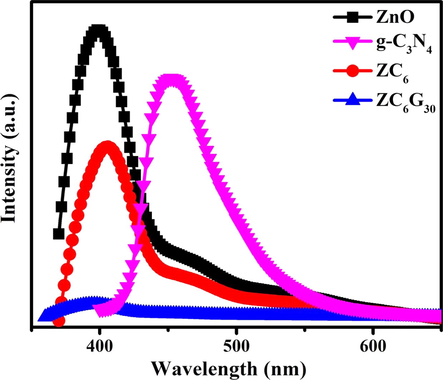
PL spectral analysis of ZnO, g-C3N4, ZC6 binary and ZC6G30 ternary nanocomposite.
4 Photocatalytic activity
4.1 Photocatalytic activity of bare ZnO, g-C3N4, binary and ternary nanocomposite
Photocatalytic activity of ZnO and g-C3N4 and binary nanocomposite was tested against RhB dye under visible light irradiation for a time period of 120 min and the results are shown in Fig. 11a. The synthesized ZnO nanorods show the absorption edge at 400 nm and the corresponding bad gap is about 3.08 eV. Pawar et al., 2016, also reported that the prepared ZnO nanoparticles showed a direct band gap of 3.07 eV. In the present work bare ZnO (Eg ∼ 3.08 eV) and g-C3N4 (Eg ∼ 2.65 eV) show ∼21% and 51% of the RhB dye degradation respectively. The ZC2 and ZC4 binary nanocomposites show ∼68% and 85% of RhB dye degradation. Further, ZC6 binary nanocomposite shows relatively enhanced RhB dye degradation of 90%. However the photocatalytic degradation is suppressed to 81% in ZC8. Thus the results show that among the prepared binary nanocomposites, ZC6 containing 6 wt% of g-C3N4 shows relatively effective RhB dye degradation of ∼90% under visible light irradiation in a time period of 120 min; thus showing an effective suppression in charge recombination rate due to the formation of heterojunction between g-C3N4 and ZnO.
(Co/C) versus time plot of (a) ZnO, g-C3N4 and different wt.% of g-C3N4 in g-C3N4/ZnO binary nanocomposites under visible light irradiation; (b) different amount of GO in g-C3N4/ZnO/GO ternary nanocomposites under visible light irradiation and Inset figure shows absorption spectra of RhB dye during the degradation.
Further the photocatalytic activities of ternary nanocomposites was tested against RhB dye for a total time period of 28 min under visible light irradiation and the photodegradation efficiency was estimated at every 7 min interval. Fig. 11b shows that the ternary nanocomposite of ZC6G10 and ZC6G20 possesses 80% and 88% of RhB dye degradation respectively. The ZC6G30 ternary nanocomposite shows relatively enhanced photodegradation efficiency to ∼99%. Further, ZC6G40 ternary nanocomposite shows decreased degradation efficiency of 90% against RhB dye. Thus in the present work the ZC6G30 ternary nanocomposite shows remarkable photodegradation efficiency to 99% against RhB dye in a time period of 14 min. The absorption ability of the ternary nanocomposite ZC6G30 is provided in the inset of Fig. 11b.
Adsorption property of the ZC6G30 ternary nanocomposite and self-degradation efficiency of the RhB dye was evaluated to confirm the degradation efficiency of the ternary nanocomposite. The ZC6G30 ternary nanocomposite shows 24% of the RhB dye adsorption capacity for a time period of 28 min (Fig. 12a). Further RhB dye shows about 6% of self-degradation for 28 min. Hence it is confirmed in the present work that the degradation of RhB dye is effectively enhanced under the visible light irradiation due to in the presence of ZC6G30 ternary nanocomposite.
(a) Adsorption and self-degradation analysis; (b) A series of trapping experiments for the degradation of RhB; (c) recycle process of ZC6G30 ternary nanocomposites against RhB dye for five cycles; (d) TOC analysis of ZC6G30 ternary nanocomposite.
In general, during the photocatalytic activity the reactive species, hydroxyl radicals (•OH), superoxide anions (O2•) and holes (h+) degrade the organic pollutants (Zhou et al., 2014). In order to understand the active species involved in the photocatalytic degradation of RhB in the presence of g-C3N4/ZnO/rGO photocatalyst, radicals trapping experiments were carried out and the results are presented in Fig. 12b. In the presence of •OH radicals scavenger (benzoic acid (BA) 0.5 mM) the degradation of RhB dye is only about 86% for a time period of 28 min under the visible light irradiation. These results reveal that the photocatalytic degradation of RhB dye is not only mediated through •OH radicals reaction. Hence the radical trapping experiment was performed under N2 atmosphere by continuously purging high purity N2 gas throughout the reaction process under ambient condition. This eliminates the dissolved oxygen content from the reaction solution and thereby prevents the formation of O2−•. The study shows about 76% degradation of RhB dye in 28 min under the visible light irradiation.
Thus the experimental results clearly show that the RhB is not only degraded by the •OH and O2−• radicals. Hence, in order to analyze the exact reactive species involved in RhB degradation, 10 mM of disodium ethylenediaminetetraacetate (EDTA) was added, as an effective h+ scavenger in the RhB solution. It is observed that the rate of RhB degradation is about 9%. Thus the present work shows that the photo-induced holes (h+) are one of the main reactive species involved in the degradation of the RhB under the visible light irradiation. These results compare well with the previous reports (Zhou et al., 2014; Wang et al., 2011a, 2011b).
The stability of the nanocomposite was analyzed by the recycle process up to five cycles and the results are shown in Fig. 12c. In the first cycle, ZC6G30 ternary nanocomposite shows 99% of photocatalytic degradation efficiency in a time period of 14 min. Then the ternary nanocomposites were separated by centrifugation and then used for the next cycles. In the fifth cycle ZC6G30 ternary nanocomposite shows 91.5% of degradation in a time period of 14 min. Thus the results evidently show that the synthesized ternary nanocomposite can be reused.
Furthermore, the Total Organic Carbon (TOC) analysis was used to confirm the removal of carbon content from RhB dye solution during the photodegradation process. The results of TOC analysis carried out for a total period of 28 min at 7 min time interval are shown in Fig. 12d. Initially, removal of carbon content in RhB dye is zero. Increase in the degradation time to 7 min, 14 min, 21 min and 28 min shows the removal of carbon content of about 58%, 78%, 81% and 83% respectively. In the present investigation, 83% of carbon content is removed in 28 min which confirms that ZC6G30 ternary nanocomposite possesses effective degradation efficiency under the visible light irradiation.
In order to confirm the degradation efficiency of the ZC6G30 ternary nanocomposites the colorless aqua solution of 4-cholorophenol (4-CP) (2.5 × 10−4 M, 100 ml) was tested under the visible light irradiation up to 28 min and the absorbance results are presented in Fig. 13. Initial solution of the 4-CP shows the maximum absorption peak at 224 nm which decreases with increasing irradiation time up to 28 min. The ZC6G30 ternary nanocomposite shows 85% of the degradation efficiency against 4-CP in a time period of 28 min. Thus, the present investigation reveals that the ZC6G30 ternary nanocomposite possesses relatively enhanced degradation efficiency against 4-CP under the visible light irradiation.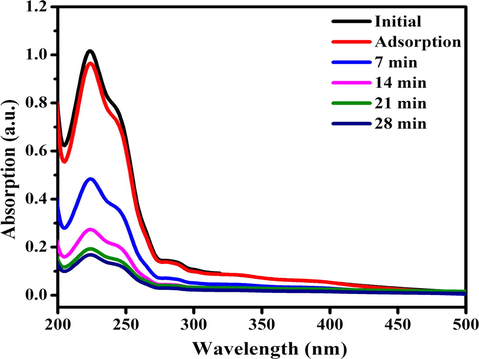
Absorbance spectra of 4-CP after photodegradation using ZC6G30 ternary nanocomposite.
4.2 Gas chromatography-mass spectroscopy analysis
The Gas Chromatography-Mass Spectroscopy (GC-MS) analysis was carried out for ZC6G30 ternary composite to detect the intermediates in RhB degradation process during the photocatalytic activity (GC-MS System with 60 m capillary column for the separation of VOCs). Previous reports reveal that the degradation of RhB dye may be divided into two processes: destruction of the conjugated structure and N-demethylation (Chen et al., 2002). Destruction of conjugated structure of RhB dye shows decrement in the absorption at 554 nm. Whereas if N-demethylation happens it shows a shift in the absorption peak towards the blue region (Bao et al., 2004). In addition the intensity of the absorbance at 259 nm and 354 nm is decreased due to benzene ring cracking. However, the absorbance at 554 nm decreases much faster than the other peaks (inset in Fig. 11b), thus revealing the conjugated structure of RhB dye is prior in degrade.
The GC-MS results obtained for the ZC6G30 sample through photodegradation process against RhB after visible light irradiation for a period of 7 min and 28 min are shown in Fig. 14(a–d). Further the results are summarized in Table 2. The predominant peak (A1) observed in GC (Fig. 14a, c) and in MS (Fig. 14b, d) at a retention time of 16.49 min identified is due to 1,2 benzenedicarboxylic acid (GC-MS library). Similarly the peak A2 observed in GC (Fig. 14a, c) and in MS (Fig. 14b, d) at a retention time of 19.52 min is due to phthalic acid (GC-MS library). The other unknown peaks are marked with the symbol X1-X5.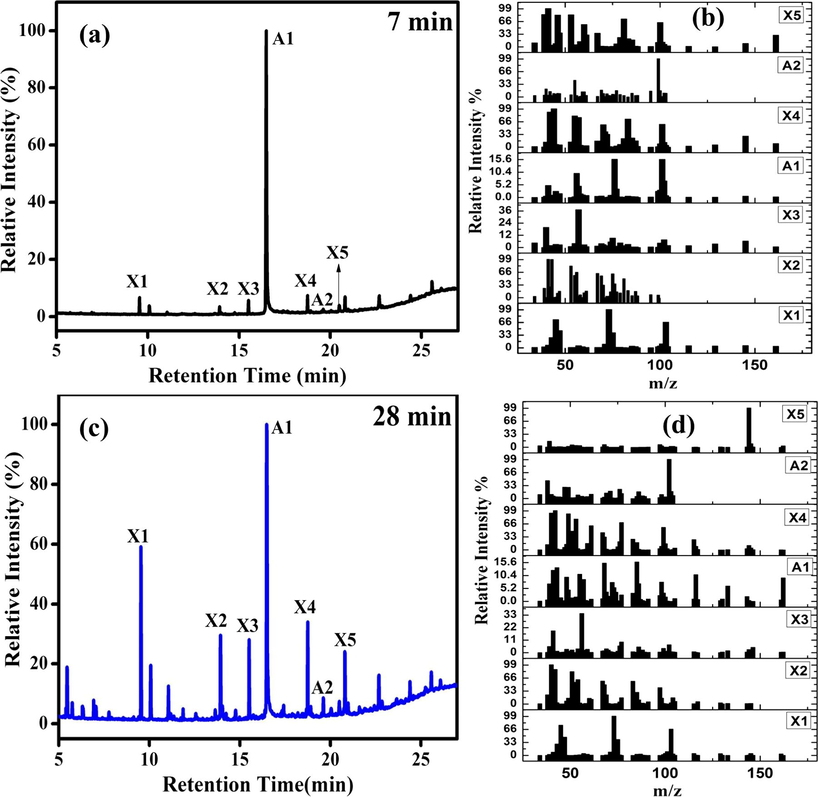
(a) GC of ZC6G30 ternary nanocomposite photodegradation of RhB dye after the light irradiation of 7 min; (b) mass spectra of the species observed in MS; (c) GC from the ZC6G30 ternary nanocomposite photodegradation of RhB dye after the light irradiation of 14 min and (d) mass spectra of the species observed in MS.
Gas chromatography peaks of ZC6G30
Retention time of peaks
T = 7 and T = 28
9.55 min, 10.08 min, 13.93 min, 15. 51 min, 16.49 min, 18.75 min, 19.52 min, 20.49 min, 20.80 min, 22.68 min, 24.4 min and 25.58 min
Thus it is confirmed in the present work, that the RhB dye is converted into 1,2 benzenedicarboxylic acid and phthalic acid during the photodegradation process. The results agree with the previous reports (Dai et al., 2017; Jing-yi et al., 2007; Cui et al., 2015). In the process RhB dye degrades into the intermediate products of 1,2 benzenedicarboxylic acid and phthalic acid and finally mineralize into CO2 and H2O.
4.3 Possible way of the charge transfer mechanism in binary (g-C3N4/ZnO) and ternary g-C3N4(6 wt%)/ZnO/GO nanocomposites under visible light irradiation
The photocatalytic activity of g-C3N4/ZnO heterojunctions can be explained in terms of the alignment of the valence band (VB) and the conduction band (CB) positions in the ZnO and g-C3N4. The g-C3N4 and ZnO band alignment is verified by potential energy level calculations, EVB = χ − Ec + 0.5Eg (Lin et al., 2007), where EVB is VB edge potential, χ is the electronegativity of the semiconductor, Ec is the energy of free electrons in hydrogen scale (∼4.5 eV), and Eg is the band gap energy of the semiconductor. Further, the edge potential for CB (ECB) can be calculated from the relation, ECB = EVB − Eg. The value of χ for ZnO is 5.79 eV, the band gap (Eg) of the as-synthesized ZnO material is 3.08 eV and calculated EVB and ECB of ZnO is +2.83 eV and −0.25 eV respectively. The calculated values of ECB and EVB of g-C3N4 is -1.04 eV and +1.56 eV respectively. These values compare well with the previous reports (Fageria et al., 2015).
Photocatalytic activity depends on the light harvesting, charge excitation and charge separation properties of the catalysts (Wen et al., 2017). The possible way of charge transfer mechanism in binary and ternary nanocomposite under the visible light irradiation is shown in Fig. 15. The ZnO nanorods (Eg ∼ 3.08 eV) and g-C3N4 (Eg ∼ 2.65 eV) show that the photodegradation efficiency of 21% and 51% respectively against RhB dye under the visible light irradiation for 120 min. However g-C3N4 (Eg = 2.65 eV) possesses high amount of photo-excited electrons than that of ZnO as its band gap lies in the visible region. Under the visible light irradiation g-C3N4 present in the g-C3N4/ZnO binary nanocomposite absorbs the visible light and hence the electrons are excited from valence band (VB) of g-C3N4 to its conduction band (CB). Further, these excited electrons migrate to the CB of ZnO because the CB edge potential of g-C3N4 −1.04 eV is more negative (Yu et al., 2015; Yan et al., 2010) than that of the ZnO −0.25 eV (Fu et al., 2008). Meanwhile the holes in the VB of ZnO are transferred to the VB of g-C3N4. Thus the ZC6 binary nanocomposite shows diminished electron-hole pair recombination rate, thereby enhancing the charge transfer capacity than that of ZnO and g-C3N4 as has also been observed from the PL spectral analysis.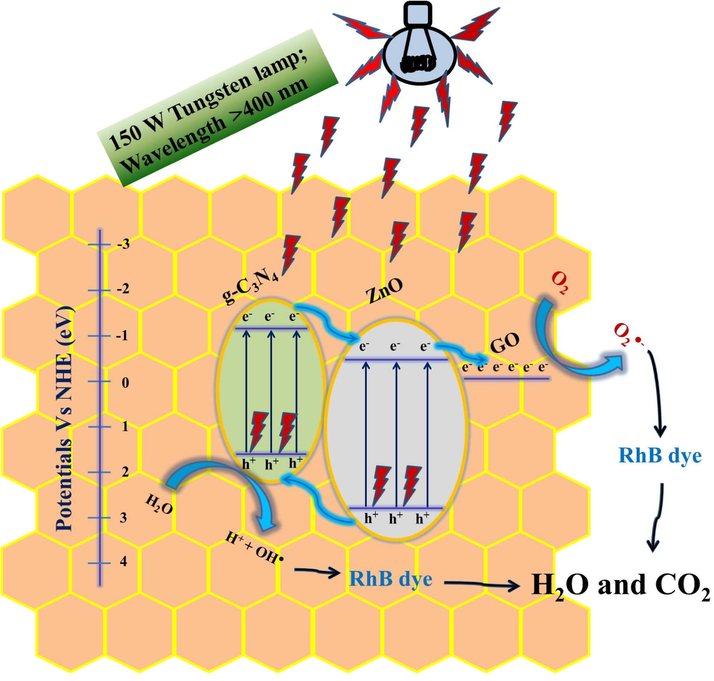
Working mechanism and charge transfer pathway in g-C3N4(6 wt%)/ZnO/GO(30 mg) of nanocomposite under visible light irradiation.
Hence, the 6 wt% of g-C3N4 in the binary catalysts (ZC6) seems to be the optimal one as it shows relatively maximum absorption in visible light and less PL intensity due to diminish in the electron-hole pair recombination rate. However, the excess amount of g-C3N4 above the optimal value of 6 wt% in the binary nanocomposites acts as electron-hole pair recombination center and decreases the photocatalytic activity (Yuan et al., 2016). Under the visible light irradiation ZC6G30 ternary nanocomposite excites the electrons from VB of g-C3N4 to its CB and the electrons migrate to the CB of ZnO and then migrate to the GO, as the work function of GO (-0.08 eV vs normal hydrogen electrode) is lower than that of the ZnO (Dai et al., 2015). Thus, in the ZC6G30 ternary nanocomposite GO acts as an electron acceptor which effectively suppresses the charge recombination rate (Fig. 7) and enhances the dye degradation efficiency (Fig. 8b). Thus the ZC6G30 ternary nanocomposite is the optimum level catalyst and shows maximum degradation efficiency. Above this optimum level of GO (30 mg) in ternary nanocomposites the degradation efficiency is decreased because the excess amount of GO present in the nanocomposites reduces the intensity of visible light necessary for the generation of electrons which subsequently reduces the electron transfer between CB of g-C3N4 and CB of ZnO.
In the present investigation ZC6 binary nanocomposite shows 90% of degradation efficiency in 120 min under visible light irradiation. The ternary nanocomposite of ZC6G30 (ZnO/g-C3N4 (6 wt%)/GO (30 mg)) shows 99% of photocatalytic activity under visible light irradiation (150 W; Tungsten lamp) for 14 min. Wan-Kuen Jo and Selvam, 2015, synthesized binary ZnO/g-C3N4(10–70 wt%) and ternary ZnO/g-C3N4(50 wt%)/GO(0.5–5 wt%) nanocomposites employing co-precipitation method and reported that the photocatalytic degradation efficiency of ZnO/g-C3N4(50 wt%) against methyl blue is 99% in 90 min and ZnO/g-C3N4(50 wt%)/GO(10 wt%) is 99.5% in 15 min under Xenon light irradiation (500 W). Thus the improved photocatalytic activity observed in the present work may be attributed to rod like structures of ZnO encapsulated by g-C3N4 and coupled with porous GO layered structure.
The photocatalytic degradation results clearly reveal that the ternary nanocomposite degradation efficiency is respectively ∼5 times and ∼2 times higher than that of ZnO and g-C3N4 in a time period of 14 min. Whereas, the recycle process confirms better stability of ZC6G30 for five cycles. Thus the ZC6G30 ternary nanocomposite prepared in the present work seems to be more effective in enhancing the photodegradation efficiency. This may be due to formation of g-C3N4 thin sheets under ultrasonication encapsulation of the ZnO nanorods coupled with GO sheets. Thus ternary nanocomposite prepared is capable to enhance the light absorption ability through the heterojunction formation which suppresses the photo-excited charge recombination rate during photocatalytic reaction against RhB dye.
5 Conclusions
In summary ZnO, binary and ternary nanocomposites synthesized via ultrasonication assisted hydrothermal method are subjected for measuring the photodegradation efficiency. The ZC6 binary and ZC6G30 ternary nanocomposite transformed the ZnO dominant XRD plane from (1 0 0) to (0 0 2). The TEM analysis shows the ZnO nanorods are encapsulated by g-C3N4 and then coupled with GO in ZC6G30 ternary nanocomposite. The binary nanocomposite ZC6 showed RhB dye degradation of about 90% under the visible light irradiation in a time period of 120 min. Ternary nanocomposite ZC6G30 showed RhB dye degradation of about 99% under visible light irradiation in a time period of 14 min; due to the light absorbing property of g-C3N4 in the visible range and reducing the electron hole-pair recombination rate due to the migration of electrons to the CB band of ZnO, in addition to the electron conducting property of GO. The recycle process carried out up to five cycles also confirmed the better stability of the ZC6G30 ternary nanocomposite against RhB due to the formation of ZnO-g-C3N4 heterojunction coupled with GO. TOC analysis confirms the degradation of RhB dye is due to nanocomposite. The intermediate products observed in the photocatalytic process by the GC–MS experiment are 1,2 benzenedicarboxylic acid and phthalic acid.
Acknowledgements
One of the authors (N. K) thanks SRM Institute of Science and Technology, Chennai for the award of SRM Institute of Science and Technology Junior Research Fellowship to carry out the research work. The authors thank Prof. B. Neppolian, Research Institute, SRM Institute of Science and Technology for his support during the progress of this work and Prof. D. John Thiruvadigal, Dean of Science and Dr. Preferential Kala, Head, Department of Physics and Nanotechnology, SRM Institute of Science and Technology, for extending facilities created under DST FIST-SR/FST/PSI-155/2010. The authors thank Dr. C. Gopalakrishnan and Dr. Helen Annal Therese, Nano Research Center, SRM Institute of Science and Technology, for extending the NRC facilities to record XRD pattern and FESEM images.
References
- Growth of aligned ZnO nanorods and nanopencils on ZnO/Si in aqueous solution: growth mechanism and structural and optical properties. Nanotechnology. 2007;18 115603(1-7)
- [Google Scholar]
- Nanoparticles: properties, applications and toxicities. Arabian J. Chem.. 2019;12(7):908-931.
- [CrossRef] [Google Scholar]
- Mineralization of rhodamine 6G dye over rose flower-like ZnO nanomaterials. Mater. Lett.. 2013;113:20-24.
- [Google Scholar]
- Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater.. 2010;22:1039-1059.
- [Google Scholar]
- Synthesis and characterization of nano-sized ZnO powders by direct precipitation method. Chem. Eng. J.. 2008;144:509-551.
- [Google Scholar]
- Synthesis and characterization of the ZnO/mpg-C3N4 heterojunction photocatalyst with enhanced visible light photoactivity. Dalton Trans.. 2014;43:13105-13114.
- [Google Scholar]
- Elements of X-ray diffraction (second ed.). Reading: Addison-Wesley; 1978.
- A high efficient graphitic- C3N4/BiOI/graphene oxide ternary nanocomposite heterostructured photocatalyst with graphene oxide as electron transport buffer material. Dalton Trans.. 2015;44:7903-7910.
- [Google Scholar]
- Morphology control of ZnO with citrate: a time and concentration dependent mechanistic insight. CrystEngComm. 2013;15:6349-6358.
- [Google Scholar]
- Substrate-free gas-phase synthesis of graphene sheets. Nano Lett.. 2008;8:2012-2016.
- [Google Scholar]
- Graphitic-carbon nitride support for the synthesis of shape-dependent ZnO and their application in visible light photocatalyst. RSC Adv.. 2015;5:80397-80409.
- [Google Scholar]
- Fabrication of ZnO nanoparticles based sensitive methanol sensor and efficient photocatalyst. Appl. Surf. Sci.. 2012;258:7515-7522.
- [Google Scholar]
- Polydopamine nanotubes as an effective fluorescent quencher for highly sensitive and selective detection of biomolecules assisted with exonuclease III amplification. Anal. Chem.. 2016;88:9158-9165.
- [Google Scholar]
- Synthesis and gas sensing performance of dandelion-like ZnO with hierarchical porous structure. Ind. Eng. Chem. Res.. 2014;53:12737-12743.
- [Google Scholar]
- Photocorrosion inhibition and enhancement of photocatalytic activity for ZnO via hybridization with C60. Environ. Sci. Technol.. 2008;42:8064-8069.
- [Google Scholar]
- Synthesis of MoS2/g-C3N4 nanocomposites with enhanced visible-light photocatalytic activity for the removal of nitric oxide (NO) Opt. Express. 2016;24:10205-10212.
- [Google Scholar]
- Facile synthesis of highly active g-C3N4 for efficient hydrogen production under visible light. J. Mater. Chem. A. 2013;1:7816-7824.
- [Google Scholar]
- An overview on structural, textural and morphological modulations of g- C3N4 towards photocatalytic hydrogen production. RSC Adv.. 2016;6:46929-46951.
- [Google Scholar]
- Non-noble metal-transition metal oxide materials for electrochemical energy storage. Energy Storage Mater.. 2016;15:171-201.
- [Google Scholar]
- Facile synthesis and shape evolution of well-defined phosphotungstic acid potassium nanocrystals as a highly efficient visible-light-driven photocatalyst. Nanoscale. 2017;9:216-222.
- [Google Scholar]
- Preparation, characterization and photocatalytic activity of N-containing ZnO powder. Chem. Eng. J.. 2009;148:263-269.
- [Google Scholar]
- A facile method to fabricate ZnO hollow spheres and their photocatalytic property. J. Phys. Chem. B. 2008;112:16-22.
- [Google Scholar]
- Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites. J. Phys. Chem. C. 2011;115:7355-7363.
- [Google Scholar]
- Synthesis and CO2 capture properties of mesoporous carbon nitride materials. Chem. Eng. J.. 2012;203:63-70.
- [Google Scholar]
- Enhancement of photocatalytic activity of Bi2WO6 hybridized with graphite-like C3N4. J. Mater. Chem.. 2012;22:11568-11573.
- [Google Scholar]
- Synthesis and characterization of microporous carbon nitride. Micro. Meso. Mater.. 2008;110:216-222.
- [Google Scholar]
- Carbon-doped ZnO hybridized homogeneously with graphitic carbon nitride nanocomposites for photocatalysis. J. Phys. Chem. C. 2014;118:10963-10971.
- [Google Scholar]
- Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem.. 2011;21:14398-14401.
- [Google Scholar]
- Synergistic effect of efficient adsorption g-C3N4/ZnO composite for photocatalytic property. J. Phys. Chem. Sol.. 2014;75:441-446.
- [Google Scholar]
- Heat transfer improvement and pressure drop during condensation of refrigerant-based nanofluid; an experimental procedure. Int. J. Heat Mass Transf.. 2018;122:643-650.
- [Google Scholar]
- Simultaneous nanostructure and heterojunction engineering of graphite carbon nitride via in situ Ag doping for enhanced photo electrochemical activity. Appl. Catal. B: Environ.. 2015;163:611-622.
- [Google Scholar]
- Synthesis and characterization of CeO2/g-C3N4 composites with enhanced visible-light photocatalytic activity. RSC Adv.. 2013;3:22269-22279.
- [Google Scholar]
- Photocatalytic activities of heterojunction semiconductors Bi2O3/BaTiO3: strategy for the design of efficient combined photocatalysts. J. Phys. Chem. C. 2007;111:18288-18293.
- [Google Scholar]
- Facet enhanced photocatalytic effect with uniform single-crystalline zinc oxide nanodisks. Chem. Phys. Lett.. 2009;472:90-95.
- [Google Scholar]
- Polar interface-induced improvement in high photocatalytic hydrogen evolution over ZnO-CdS heterostructures. Energy Environ. Sci.. 2011;4:3976-3979.
- [Google Scholar]
- Effect of transition metal ions on the TiO2-assisted photodegradation of dyes under visible irradiation: a probe for the interfacial electron transfer process and reaction mechanism. J. Phys. Chem. B. 2002;106:318-324.
- [Google Scholar]
- Highly efficient liquid-phase photooxidation of an azo dye methyl orange over novel nanostructured porous titanate-based fiber of self-supported radially aligned H2Ti8O17·1.5H2O nanorods. Environ. Sci. Technol. 2004;38:2729-2736.
- [Google Scholar]
- Detection of intermediates in the TiO2-assisted photodegradation of Rhodamine B under visible light irradiation. J. Environ. Sci.. 2007;19:892-896.
- [Google Scholar]
- Electrochemical oxidation of Rhodamine B: optimization and degradation mechanism. Int. J. Electrochem. Sci.. 2017;12:4265-4276.
- [Google Scholar]
- Photodegradation of Rhodamine B over Ag modified ferroelectric BaTiO3 under simulated solar, light: pathways and mechanism. RSC Adv.. 2015;5:30372-30379.
- [Google Scholar]
- Solar/UV-induced photocatalytic degradation of three commercial textile dyes. J. Hazard. Mater.. 2002;89:303-317.
- [Google Scholar]
- Destruction of Rhodamine B using novel sonochemical reactor with capacity of 7.5 l. Sep. Purif. Technol.. 2004;34:13-24.
- [Google Scholar]
- Habit-modifying additives and their morphological consequences on photoluminescence and glucose sensing properties of ZnO nanostructures, grown via aqueous chemical synthesis. Vacuum. 2015;116:21-26.
- [Google Scholar]
- Supramolecules-assisted ZnO nanostructures growth and their UV photodetector application. Solid State Sci.. 2015;41:14-18.
- [Google Scholar]
- Preparation, characterization and photocatalytic activity of porous zinc oxide superstructure. Mat. Sci. Semicon. Proc.. 2012;15:270-276.
- [Google Scholar]
- Fabrication of composite photocatalyst g-C3N4–ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans.. 2012;41:6756-6763.
- [Google Scholar]
- Sonochemical preparation of shape-selective ZnO nanostructures. Cryst. Growth Des.. 2008;8:265-269.
- [Google Scholar]
- A review on nanomaterials for environmental remediation. Energy Environ. Sci.. 2012;5:8075-8109.
- [Google Scholar]
- Visible light active photocatalysis on block copolymer induced strings of ZnO nanoparticles doped with carbon. J. Mater Chem. A.. 2017;1:898-905.
- [Google Scholar]
- Enhanced photocatalytic activity of Co doped ZnO nanodisks and nanorods prepared by a facile wet chemical method. Phys. Chem. Chem. Phys.. 2014;16:12741-12749.
- [Google Scholar]
- Multilayered ZnO nanosheets with 3D porous architectures: synthesis and gas sensing application. J. Phys. Chem. C. 2010;114:14684-14691.
- [Google Scholar]
- Graphene oxide modified g-C3N4 hybrid with enhanced photocatalytic capability under visible light irradiation. J. Mater. Chem.. 2012;22:2721-2726.
- [Google Scholar]
- Hydrothermal growth of large-scale micropatterned arrays of ultralong ZnO nanowires and nanobelts on zinc substrate. Chem. Commun. 2006:3551-3553.
- [Google Scholar]
- Appl. Mater. Interfaces. 2014;6:8981-8995.
- ZnO rods/reduced graphene oxide composites prepared via a solvothermal reaction for efficient sunlight-driven photocatalysis. Appl. Catal. B: Environ.. 2016;185:11-21.
- [Google Scholar]
- Efficient photocatalytic degradation of Rhodamine B dye using ZnO/graphitic C3N4 nanocomposites synthesized by microwave. Environ. Chem. Lett.. 2017;15:435-441.
- [Google Scholar]
- Integration of ZnO with g-C3N4 structures in core-shell approach via sintering process for rapid detoxification of water under visible irradiation. Curr. Appl. Phys.. 2016;16:101-108.
- [Google Scholar]
- Synthesis, characterization and photocatalytic activity of annealing dependent quasi spherical and capsule like ZnO nanostructures. Appl. Surf. Sci.. 2014;319:221-229.
- [Google Scholar]
- Solvothermal synthesis of Zinc sulfide decorated Graphene (ZnS/G) nanocomposites for novel Super capacitor electrodes. Electrochim. Acta. 2015;178:647-657.
- [Google Scholar]
- Ecotoxicity of, and remediation with, engineered inorganic nanoparticles in the environment Trends in the environment. Anal. Chem.. 2011;30:507-516.
- [Google Scholar]
- Raman study of structural disorder in ZnO nanopowders. J. Raman Spectrosc.. 2010;41:914-921.
- [Google Scholar]
- Different morphologies of ZnO nanorods and their sensing property. Sensor Actuat. B.. 2010;146:129-137.
- [Google Scholar]
- Liquid Semiconductors. New York: Plenum press; 1974. p. :159-220.
- Nano-photocatalytic materials: possibilities and challenges. Adv. Mater.. 2012;24:229-251.
- [Google Scholar]
- Effect of annealing temperature and pH on morphology and optical property of highly dispersible ZnO nanoparticles. Mater. Charact.. 2009;60:1305-1310.
- [Google Scholar]
- Controllable preferential-etching synthesis and photocatalytic activity of porous ZnO nanotubes. J. Phys. Chem. C. 2008;112:11738-11743.
- [Google Scholar]
- Enhancement of photocurrent and photocatalytic activity of ZnO hybridized with graphite-like C3N4. Energy Environ Sci.. 2011;4:2922-2929.
- [Google Scholar]
- Core-shell g-C3N4@ZnO composite as photoanodes with double synergistic effects for enhanced visible-light photocatalytic activities. Appl. Catal. B: Environ.. 2017;217:169-180.
- [Google Scholar]
- In-situ growth of g-C3N4 layer on ZnO nanoparticles with enhanced photocatalytic performances under visible light irradiation. Mater. Lett.. 2017;188:347-350.
- [Google Scholar]
- Template-free fabrication of porous zinc oxide hollow spheres and their enhanced photocatalytic performance. J. Porous Mat.. 2010;17:79-84.
- [Google Scholar]
- Enhanced visible light-driven photocatalytic performance of ZnO-g-C3N4 coupled with graphene oxide as a novel ternary nanocomposite. J. Hazard. Mater.. 2015;299:462-470.
- [Google Scholar]
- Room-temperature fabrication of highly oriented ZnO nanoneedle arrays by anodization of zinc foil. Nanotechnology. 2006;17:4936-4940.
- [Google Scholar]
- One-dimensional nanostructures: synthesis, characterization, and applications. Adv. Mater.. 2003;15:353-389.
- [Google Scholar]
- Microspheric organization of multilayered ZnO nanosheets with hierarchically porous structures. J. Phys. Chem. C.. 2008;112:11722-11728.
- [Google Scholar]
- Precursor template synthesis of three-dimensional mesoporous ZnO hierarchical structures and their photocatalytic properties. CrystEngComm. 2010;12:2166-2172.
- [Google Scholar]
- Organic-inorganic composite photocatalyst of g-C3N4 and TaON with improved visible light photocatalytic activities. Dalton Trans.. 2010;39:1488-1491.
- [Google Scholar]
- Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: a direct Z-scheme mechanism. J. Mater. Chem. A. 2015;3:19936-19947.
- [Google Scholar]
- Facile synthesis of g-C3N4 nanosheets/ZnO nanocomposites with enhanced photocatalytic activity in reduction of aqueous chromium (VI) under visible light. Nanomaterials. 2016;6:173-184.
- [Google Scholar]
- Effects of annealing temperature on some structural and optical properties of ZnO nanoparticles prepared by a modified sol-gel combustion method. Cerm. Int.. 2011;37:393-398.
- [Google Scholar]
- Control of ZnO morphology via a simple solution route. Chem. Mater.. 2002;14:4172-4177.
- [Google Scholar]
- Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Chem. Soc.. 2013;135:18-21.
- [Google Scholar]
- Preparation of visible light-driven g-C3N4@ZnO hybrid photocatalyst via mechanochemistry. Phys. Chem. Chem. Phys.. 2014;16:17627-17633.
- [Google Scholar]







