Translate this page into:
Ion exchange pattern-based 18β-glycyrrhetinic acid containing pyridinium salts derivatives as novel antibacterial agents with low toxicity
⁎Corresponding authors. xiangzhou@gzu.edu.cn (Xiang Zhou), zhoux1534@163.com (Xiang Zhou), syang@gzu.edu.cn (Song Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
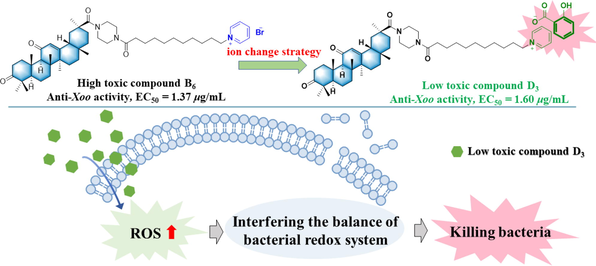
Abstract
Studies in the past few years have shown that simple salts, such as pyridinium salts and imidazole salts, have excellent antibacterial activity. However, their application in drugs and agrichemicals development has been limited due to their high toxicity. In this study, we found that synthesized target compounds with pyridinium salt unit A1 and B6 displayed excellent anti-Xac (Xanthomonas. axonopodis pv. citri) and Xoo (Xanthomonas oryzae pv. oryzae) activity, with EC50 values of 7.67 μg/mL and 1.37 μg/mL, respectively. On searching for a toxicity reduction strategy, an ion exchange method was adopted to obtain a new type of various organic acid-decorated pyridinium salt derivatives containing with glycyrrhetinic acid framework, and their structure was characterized by FTIR and 1H NMR. Furthermore, bioassays and toxicity tests were used to show that these organic acid-exchanged compounds not only exhibited lower toxicity to plants but also had excellent antibacterial activity towards virulent phytopathogenic bacteria, especially compound D3, which provided an EC50 of 1.60 μg/mL toward Xoo. Subsequently, the pot experiments determined that compound D3 exhibited suitable protective and curative activities for the management of rice bacterial leaf blight at 200 μg/mL, with control efficacies of 54.09% and 38.50%, respectively. In addition, studies evaluating potential antibacterial mechanisms suggested that apoptosis was responsible for the antibacterial behavior of the target compounds. Overall, this study proved that the ion exchange method was a feasible strategy to reduce the toxicity of pyridinium salts and was promising for use in the development of novel antimicrobial agents.
Keywords
Pyridinium salts
Phytotoxicity
Ion exchange strategy
Antibacterial activity
- Xoo
-
Xanthomonasoryzae pv. oryzae
- Xac
-
Xanthomonas axonopodis pv. citri
- BT
-
Bismerthiazol
- TC
-
Thiodiazolecopper
- GA
-
18β-glycyrrhetinicacid
- ROS
-
Reactive oxygen species
- OPO
-
essentialoiloforangepeel
Abbreviations
1 Introduction
Plant bacterial diseases refer to physiological lesions of cells and tissues of plants that occur subsequent to being infected with phytopathogenic bacteria. A phenomenon which has occurred in important grain and industrial crops, such as cereals, vegetables, and fruit trees, and are the second most severe plant disease only after fungal infection. In rice production, two common bacterial diseases, bacterial blight and bacterial leaf streak, have been frequently responsible for global outbreaks, seriously affecting an area of 330,000 to 660,000 hm2 each year, and usually resulting in a yield reduction of about 10% in production (Chen et al., 2021; Hu, 2022; Zhao et al., 2022). Similarly, citrus canker is a notorious bacterial disease in the citrus industry, which can severely affect the citrus tree causing the fruit to drop prematurely, with a significant impact on its economic value. However, this disease is highly contagious and has worried homeowners around the world, and some researchers reported that the field incidence rate could reach 15% in Hunan province, China (Luo et al., 2015; Feng, 2021; Zhang et al., 2021). Furthermore, bacterial wilt in the Solanaceae plant is also a devastating disease that can cause a loss of yield of 20% to 50%, and affects peppers, tomatoes, tobacco, and potatoes (Huang et al., 2017; He et al., 2021; Xu et al., 2021). After kiwifruit is infected with canker, local canker rots, causing the tree to weaken; the diseased plant rate is as high as 20% (Li et al., 2013). For all these plants, bacterial diseases have the characteristics of explosiveness, epidemic, destructiveness, and difficulty in control. Therefore, there will be severe damage when plants are infected with bacterial diseases, resulting in a severe reduction in production yields and quality. To date, chemical control has always been the most adopted and efficient measure in most cases of epidemics. However, because the long-term continuous use and abuse of a small variety of chemical agents, including cupric pesticide, streptomycin sulfate, and bismerthiazol (BT), not only leads to a series of environmental and ecological problems, such as increased resistance of phytopathogenic bacteria, excessive pesticide residues, and soil and water pollution, but also seriously affects the safety of agricultural products (Wang et al. 2022). Under the background of “China’s 14th Five-Year Plan,” green agricultural development has become the dominant direction of future agriculture. Thus, the development of efficient, green, and environmentally friendly agrochemicals has become a great challenge (Chen and Chen, 2022).
The chemical modification of active natural products has strongly motivated the discovery and development of new pesticides and medicines (Butler, 2005; Camp et al., 2012; Hüter, 2011; Joo, 2014; Marrone, 2019; Wu et al., 2019). 18β-glycyrrhetinic acid (GA), a pentacyclic triterpenoid, is an active ingredient extracted from the rhizomes of Glycyrrhiza uralensis Fisch and other plants (Song et al., 2022), which has attracted a great deal of attention due to its extensive applications in food additives, cosmetics, matrix materials and medicine (Jager et al., 2009; Xu et al., 2012; Zhang et al., 2013; Huang et al., 2018). In addition, GA is an ideal lead compound; it not only exhibits diverse bioactive activities including anti-inflammatory (Radwan et al., 2016), antiviral (Zígolo et al., 2018), antibacterial (Yang et al., 2020), and antitumor (Sun et al., 2019) activities, but also has a modifiable chemical structure, given a hydroxyl group at the 3-position, an unsaturated ketone at the 11- and 13-positions, and a carboxyl group at the 20-position (Gaware et al., 2011; Hussain et al., 2018). However, some undesirable physical and chemical properties of GA have severely limited its application prospects, such as high hydrophobicity, low bioavailability, poor water solubility, and permeability (Tian et al. 2012; Lei et al. 2016; Dai et al., 2018). As for the derivatives of pyridinium salts, a quaternary ammonium salt has been suggested, which exhibits excellent antibacterial (Zhao and Sun, 2006; Sundararaman et al., 2013), anticancer (Fahs et al., 2014), and other biological activities, and can also be used as a surfactant (Zhen et al., 2011) (Fig. 1). Many studies have verified that salt-based antibacterial agents based on pyridinium have excellent water solubility and are relatively less toxic to mammalian cells, but are highly toxic to microorganisms and some plants, and may be the main reason for their rarely reported antibacterial agent in agricultural production (Chen et al., 2019). More interestingly, through the use of rational molecule modification, some pyridinium salt derivatives not only have outstanding antibacterial competence but have also exhibit low toxicity to nontarget organisms. Therefore, further investigation is needed to identify new functions and applications of pyridinium salt derivatives.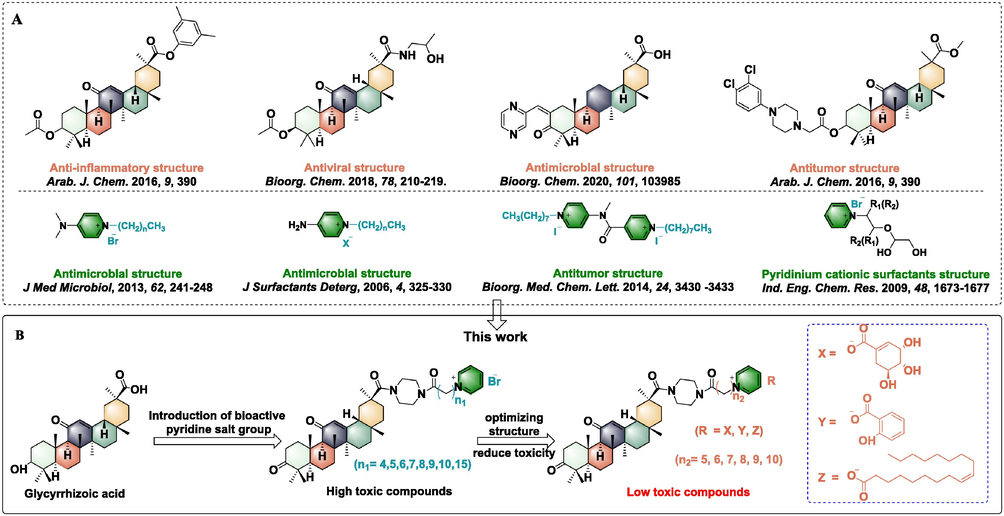
A) Reported bioactive molecules containing 18β-glycyrrhetinic acid or pyridine salt; B) The design strategy for target molecules.
Hence, to improve the water solubility and bioactivity of GA, a series of pyridinium salt-based GA derivatives were prepared, and their in vitro and in vivo antibacterial activities and primary antibacterial mechanisms were studied using a series of biochemical assays. Furthermore, an ion exchange method was employed to successfully reduce their phytotoxicity.
2 Materials and methods
2.1 Instruments and chemicals
The NMR spectra of the title compounds were obtained on a JEOL-ECX500 apparatus (Akishima, Japan) or a Bruker Biospin AG-400 apparatus (Bruker Optics, Germany) using TMS as the internal standard. HRMS spectra were obtained on a Q-Exactive Orbitrap MS apparatus (Thermo Fisher Scientific, USA). Infrared (IR) spectra were collected using a Fourier transform infrared spectrometer (Thermo Scientific Nicolet iS50, Thermo Fisher Scientific). A Nova Nano SEM 450 instrument captured scanning electron microscope (SEM) images. Fluorescent images were collected using an Olympus BX53 microscope (Olympus, Tokyo, Japan). Optical density was recorded on a CytationTM 5 multi-mode readers (BioTek Instruments, Inc. USA). 18β-Glycyrrhetinic acid (purity > 97%) was purchased from Energy Chemical of Saen Chemical Technology (Shanghai) Co., Ltd.
2.2 Experimental section
For in vitro antibacterial bioassays, apoptosis responses, ROS detection, fluorescent images, and enzyme activity assays were performed as described in our previous studies (Zhao et al., 2019, Wang et al., 2020; Xiang et al., 2020).
2.3 Toxicity assays of compound D3 in rice plant
The toxicity assay of compound D3 was conducted based on our reported method with slight modifications. Briefly, rice plants (Fengyouxiangzhan variety) grown at the maximum leaf stage were used in this experiment. Then, compounds B6 and D3 were prepared in sterile water at the final concentration of 200 μg/mL. Subsequently, 20 mL volumes of the solutions were evenly sprayed on rice leaves and grown in a greenhouse. Finally, the condition of the rice leaves was observed 14 days after spraying. Each experiment was performed in duplicate, and rice plants treated only with an equivalent amount of sterile water and grown under the same conditions were used as a blank control.
2.4 In vivo bioassay against rice bacterial blight
The in vivo antibacterial activities of compound D3 against rice bacterial leaf blight were performed based on our reported method with some modifications (Huang et al., 2021). Bismerthiazol (BT, 90% technical material) and thiodiazole copper (TC, 20% suspending agent) were used as positive controls, and rice plants (variety Fengyouxiangzhan) cultured in a greenhouse for 2 months were used. For protective assays, different adjuvants were added to a compound D3 solution at 200 μg/mL to obtain a mixture that included essential oil of orange peel (OPO) at a dose of 0.1% (v/v). Then 20 mL of the solution was evenly sprayed onto the rice leaves and the Xoo cell solution (OD595 = 0.6–0.8) was inoculated using the leaf clipping method at 24 h after spraying. To determine the curative activity, Xoo cells were first inoculated and then the mixture was sprayed 24 h after inoculation. All treatments were cultured in a climate chamber (95% RH) with 16 h lighting at 28 °C and 8 h dark at 25 °C. The results were observed 14 days after inoculation.
2.5 Dual AO/EB fluorescent staining
Acridine orange/ethidium bromide (AO/EB) staining was conducted using Kasibhatla et al.’s method with some modifications (Kasibhatla et al., 2006). Briefly, 2 mL of Xoo culture solution (OD595 = 0.6–0.8) was exposed to compound D3 at different concentrations at 28 °C for 12 h. Subsequently, the treated Xoo cells were collected by centrifugation (6000 × g, 5 min, 4 °C), washed with sterile water (1 mL × 2), then resuspended in sterile water (1 mL) and the supernatant was discarded. For compound D3 treatments or control were then mixed with 5 μL of the dye mixture of AO (10 μg/mL)/EB (100 μg/mL) in the ratio of 1: 1 and incubated at 28 °C for 20 min with Xoo cells. Subsequently, 1 mL of sterile water, was added, mixed well, resuspended, and the supernatant was discarded. Finally, all samples were observed and immediately captured using an Olympus BX53 microscope (Olympus, Tokyo, Japan).
2.6 Accumulation of reactive oxygen species triggered by target compound D3
The reactive oxygen species (ROS) content in D3-triggered pathogens (Xoo) was measured using an ROS assay kit (Beyotime Institute of Biotechnology, Haimen, China). Briefly, 1 mL of Xoo culture solution (OD595 = 0.6–0.8) was exposed to compound D3 at different concentrations at 28 °C for 12 h. Subsequently, Xoo cells (OD595 is 0.6–0.8) were collected by centrifugation (6000 × g, 5 min, 4 °C), washed with sterile water (1 mL × 2), and resuspended in sterile water (1 mL). Finally, 0.5 mL of the resuspended solution was mixed with 1 µL of the Beyotime dyeing solution in a dark environment for 20 min, and all samples were observed and captured with an Olympus BX53 microscope (Olympus, Tokyo, Japan).
3 Results and discussion
3.1 Synthesis and antibacterial activity of target compounds A1-A7 and B1-B7
The synthetic route and structure of pyridinium salt-decorated 18β-glycyrrhetinic derivatives possessing flexible alkyls are illustrated in Scheme 1. Briefly, the piperazine group was first introduced into the 30-position of carbon of GA via a classical condensation reaction to provide intermediate 1. Then, key intermediates 2–8 were prepared by a substitution reaction between acyl chloride containing different alkyl chain lengths and intermediate 1. Finally, target molecules (A1-A7) were obtained by a substitution reaction between intermediates 2–8 and pyridine at 53 °C. In addition, target compounds B1-B7 were synthesized using a route similar to (Scheme 2) to compounds A1-A7. In summary, intermediates 2–8 were oxidized to obtain intermediates 9–15, which were then substituted with pyridine at 53 °C to obtain the target compounds B1-B7. All molecular structures were confirmed by 1H NMR, 13C NMR, and HRMS (for detailed information see the supplementary data Figs. S1-S87). Bioassays of the target compounds against phytopathogenic bacteria were evaluated using the turbidimeter test. The results of the bioassay indicated that some of the synthesized compounds displayed remarkable antibacterial activity against phytopathogenic bacteria, including Xanthomonas oryzae pv. oryzae (Xoo) and X. axonopodis pv. citri (Xac) (Table 1). In detail, most target molecules (A1-A7 and B1-B7) showed vastly improved antibacterial activity of GA (EC50 > 100 μg/mL) against anti-Xoo and anti-Xac, with EC50 values ranging from 1.37 to 64.80 μg/mL, which suggested that the pyridinium salts moiety was a key pledge to improve antibacterial potency. The best antibacterial activity against Xac was compound A1 whose 3-hydroxyl group was not oxidized, with an EC50 value of 7.67 μg/mL. Next, the 3-position hydroxyl group of 18β-glycyrrhetinic acid was oxidized to a series of target compounds, with the growth of the flexible alkyl chain, the anti-Xoo EC50 value showed a trend of first increasing and then decreasing. Of these, compounds with chain lengths of 8, 9, and 10 (corresponding to compounds B4, B5, and B6) exhibited EC50 values of 3.38, 2.29 and 1.37 μg/mL, respectively. Furthermore, compounds with flexible alkyl chain lengths of 5, 6, and 7 (corresponding to compounds A1, A2, and A3) showed more significant inhibition of Xac growth, affording EC50 values of 7.67, 9.55 and 8.16 μg/mL, respectively. Further studies showed that this ability was increased against Xoo and decreased against Xac, while the 3-position hydroxyl group of compounds A1-A7 was oxidized to the carbonyl group (compounds B1-B7). Based on the bioassay results, we found that the pyridine salt moiety played an extremely important role in enhancing antibacterial activity.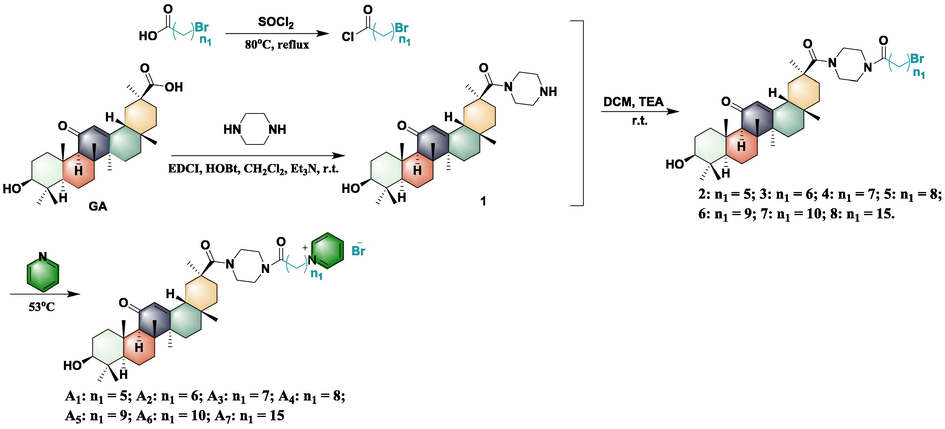
Synthesis of pyridine salt-decorated 18β-glycyrrhetinic acid derivatives A1-A7.
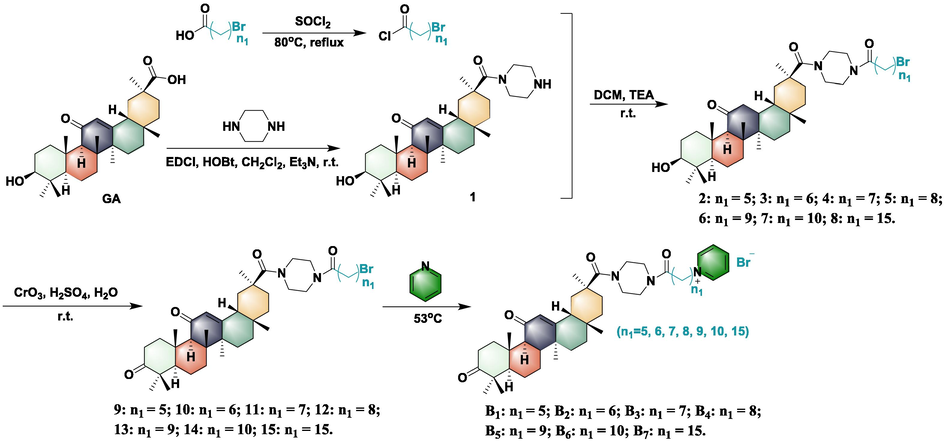
Synthesis of pyridine salt-decorated 18β-glycyrrhetinic acid derivatives B1-B7.
Compd.
Xoo
Xac
regression eq.
EC50(μg/mL)
regression eq.
EC50(μg/mL)
GA
–
>100
–
>100
1
y = 5.403x − 0.099
8.78 ± 0.69
y = 0.927x + 3.865
16.70 ± 1.27
2
–
>100
y = 1.263x + 3.203
26.40 ± 1.73
3
–
>100
–
>100
4
–
>100
y = 0.440x + 4.230
56.00 ± 5.85
5
–
>100
y = 1.271x + 3.341
20.20 ± 0.83
6
–
>100
–
>100
7
–
>100
–
>100
8
–
>100
–
>100
9
–
>100
–
>100
10
–
>100
–
>100
11
–
>100
–
>100
12
–
>100
–
>100
13
–
>100
–
>100
14
–
>100
–
>100
15
–
>100
–
>100
A1
y = 5.835x + 0.443
8.57 ± 1.05
y = 1.784x + 3.421
7.67 ± 0.32
A2
y = 2.214x + 3.406
5.24 ± 0.76
y = 1.659x + 3.374
9.55 ± 0.52
A3
y = 2.690x + 3.106
5.06 ± 0.47
y = 0.490x + 4.553
8.16 ± 0.74
A4
y = 2.898x + 3.410
3.54 ± 0.20
y = 1.459x + 3.521
10.30 ± 0.12
A5
y = 4.965x + 2.317
3.47 ± 0.28
y = 0.387x + 4.406
34.10 ± 3.41
A6
y = 12.62x − 5.340
6.60 ± 0.67
y = 1.002x + 3.756
17.40 ± 1.05
A7
–
>100
–
>100
B1
y = 3.743x + 1.345
9.47 ± 0.94
y = 0.945x + 3.986
11.80 ± 0.28
B2
y = 6.376x − 1.139
9.18 ± 0.98
y = 1.044x + 3.394
34.50 ± 2.17
B3
y = 8.796x − 2.497
7.12 ± 0.15
y = 2.354x + 2.694
9.53 ± 1.18
B4
y = 4.618x + 2.557
3.38 ± 0.10
y = 0.686x + 4.127
18.70 ± 2.31
B5
y = 8.480x + 1.950
2.29 ± 0.29
y = 0.614x + 4.134
25.60 ± 2.52
B6
y = 5.794x + 4.211
1.37 ± 0.03
y = 0.391x + 4.385
37.40 ± 2.60
B7
–
>100
–
>100
BT
y = 1.289x + 2.898
42.60 ± 3.11
–
>100
TC
y = 2.819x − 0.856
119.44 ± 2.54
y = 7.726x − 9.949
86.09 ± 2.87
3.2 Synthesis, antibacterial activity, and rice leaf toxicity test of further optimized compounds C1-C9 and D1-D9
Based on the bioassay results of the compounds in series A and B, molecule B6 with the highest activity (EC50 = 1.37 μg/mL to anti-Xoo) was chosen to perform the phytotoxicity test on the rice plant. The results showed (Fig. 2) that compound B6 exhibited marked toxicity for the rice plant and produced a large number of black spots on the rice leaves 14 days after spraying at 200 μg/mL. Meanwhile, we found that some quaternary ammonium compounds were very effective against both Gram-positive and Gram-negative bacteria, but they were also quite toxic (Schoenberg et al., 1974; Thorsteinsson et al., 2003). Bodor et al. (1980) proposed that the toxicity of quaternary ammonium surfactants is mainly related to various biological effects of the quaternary ammonium head and its metabolites (such as oxidative dealkylation). Thus, they designed and synthesized a new class of “soft antibacterial agents” containing ester moieties to prevent and reduce drug-related toxicity effects, which cleaved into non-toxic moieties by chemical or enzyme hydrolysis after exerting antibacterial effects. Encouraged by these findings, to reduce phytotoxicity, an ion exchange method was adopted to optimize the structure of six active compounds A1, A2, A3, B4, B5, and B6. In detail, the ion exchange first involved switching between the bromide anion and hydroxide anion in the methanol solution at room temperature to obtain the key intermediates 16–21. Then, salicylic acid, shikimic acid, and oleic acid were sequentially introduced in anhydrous methanol at 70 °C, and a class of novel pyridinium salt-decorated 18β-glycyrrhetinic derivatives (C1-C9 and D1-D9) were formed via two-step reactions (Schemes 3 and 4). Furthermore, in vitro bioassays (shown in Tables 2 and 3) indicated that compounds with different acids, i.e., compounds A1, A2, A3, B4, B5, and B6, could effectively enhance anti-Xac ability. In particular, compound D8 provided an EC50 = 2.07 μg/mL toward anti-Xac, which was significantly higher than that of the corresponding compound A1 (EC50 = 7.67 μg/mL). Meanwhile, as for anti-Xoo, the antibacterial activities of optimized compounds were basically maintained. Of these, compounds D3 and D9 achieved more significant effects, with EC50 values of 1.60 and 2.98 μg/mL, respectively. In addition, compounds with alkyl chain lengths of 10 were more dominant in anti-Xoo than other alkyl chain lengths. Furthermore, the phytotoxicity test of active compound D3 (EC50 = 1.60 μg/mL to anti-Xoo) was evaluated in rice plants. The results indicated (Fig. 2) that compound D3 did not produce any adverse impact on rice leaves 14 days after spraying at 200 μg/mL. Altogether the results suggested that the ion exchange method was a feasible strategy to reduce the phytotoxicity of pyridinium salts while effectively maintaining the antibacterial potency.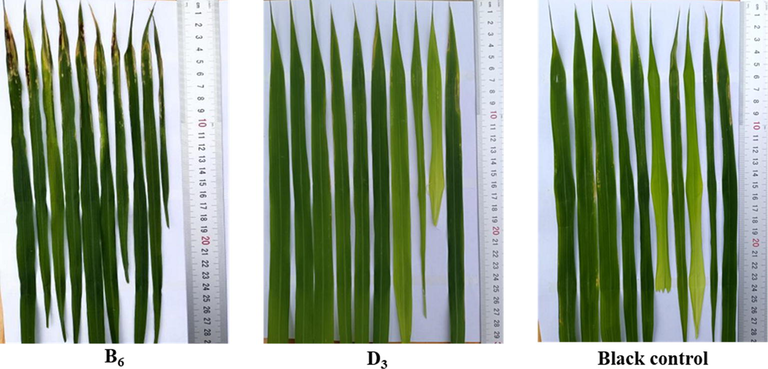
In vivo phytotoxicity test of B6, D3, and CK in rice under greenhouse conditions at 200 μg/mL.
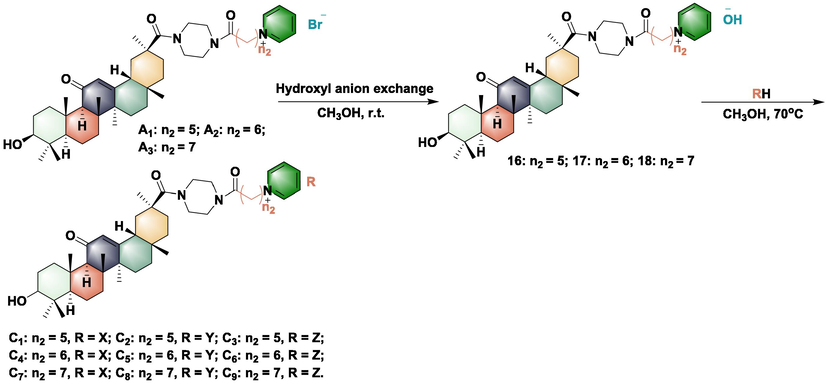
Synthesis of optimized compounds C1-C9.
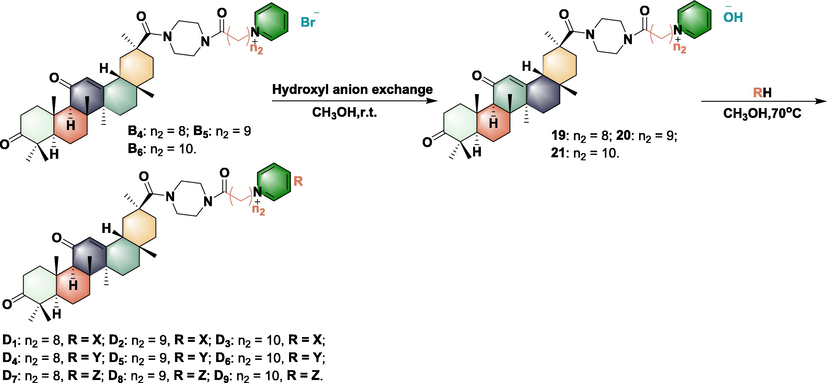
Synthesis of optimized compound D1-D9.
Compd.
Xoo
Xac
regression eq.
EC50 (μg/mL)
regression eq.
EC50 (μg/mL)
C1
y = 2.208x + 2.463
14.08 ± 1.50
y = 2.327x + 2.279
14.76 ± 0.53
C2
y = 3.254x + 0.799
19.53 ± 1.66
y = 1.676x + 3.604
6.80 ± 0.45
C3
y = 1.189x + 3.477
19.06 ± 2.17
y = 0.961x + 4.363
4.59 ± 0.70
C4
y = 1.014x + 3.901
12.11 ± 2.41
y = 1.964x + 3.354
6.88 ± 0.60
C5
y = 2.312x + 2.431
12.90 ± 0.70
y = 5.272x − 2.372
25.03 ± 1.66
C6
y = 1.816x + 2.614
20.59 ± 2.14
y = 1.143x + 3.792
11.40 ± 1.74
C7
y = 1.983x + 3.383
6.5 ± 1.16
y = 1.400x + 3.360
14.81 ± 0.75
C8
y = 1.400x + 3.360
6.17 ± 2.30
y = 1.735x + 3.147
11.69 ± 0.73
C9
y = 1.676x + 3.604
17.25 ± 0.03
y = 1.767x + 2.953
14.39 ± 0.83
BT
y = 1.289x + 2.898
42.60 ± 3.11
–
>100
TC
y = 2.819x − 0.856
119.44 ± 2.54
y = 7.726x − 9.949
86.09 ± 2.87
Compd.
Xoo
Xac
regression eq.
EC50 (μg/mL)
regression eq.
EC50 (μg/mL)
D1
y = 1.388x + 4.009
5.17 ± 0.54
y = 2.074x + 3.226
7.16 ± 0.67
D2
y = 2.075x + 4.156
4.18 ± 0.09
y = 3.194x + 1.774
10.23 ± 0.79
D3
y = 2.094x + 4.575
1.60 ± 0.34
y = 0.325x + 4.698
8.48 ± 0.07
D4
y = 1.717x + 3.810
4.93 ± 0.93
y = 1.703x + 1.936
3.82 ± 0.14
D5
y = 1.009x + 4.113
7.56 ± 0.75
y = 1.909x + 3.775
4.38 ± 0.61
D6
y = 8.912x − 0.498
4.14 ± 0.20
y = 2.253x + 2.733
10.15 ± 0.20
D7
y = 2.373x + 2.296
13.77 ± 0.63
y = 2.063x + 2.224
22.17 ± 1.29
D8
y = 1.781x + 3.048
12.46 ± 0.08
y = 1.658x + 4.475
2.07 ± 0.58
D9
y = 1.899x + 4.099
2.98 ± 0.39
y = 0.763x + 4.516
6.08 ± 0.19
BT
y = 1.289x + 2.898
42.60 ± 3.11
–
>100
TC
y = 2.819x − 0.856
119.44 ± 2.54
y = 7.726x − 9.949
86.09 ± 2.87
3.3 Structural characterization of compounds B6, D3, and D4
FTIR was used to monitor the functional groups of compounds B6 and D3. The FTIR spectra (Fig. 3) of compounds D3 and B6 presented peaks at 2922 and 2759 cm−1 which were attributed to the H-stretch vibration of the pyridine ring, and peaks at 1709 and 1610 cm−1 indicated the characteristic absorption of the carbonyl group at the 3-position and α, β-unsaturated ketones at the 11,12 position of the 18β-glycyrrhetinic acid skeleton. As shown in Fig. 3b, the spectrum of D3 showed the in-plane bending vibration absorption of the benzene ring C–H and the ortho-disubstituted characteristic absorption of the benzene ring skeleton, with peaks at 1459, 1378, and 757 cm−1. Furthermore, a weak absorption band appeared at 2005 cm−1 that was assigned to the overtone peak of the benzene ring of compound D3. Regarding compound D4, an absorption peak (Fig. 3a) appeared at 1546 cm−1, which was attributed to the characteristic absorption of the shikimic acid double bond. Altogether these FTIR data indicated that salicylic acid and shikimic acid were successfully introduced into compounds D3 and D4, respectively. Furthermore, the structure of these newly synthesized compounds (compound B6 and D3) was also confirmed by 1H NMR (as shown in Fig. 4). The chemical shifts of the protons around the pyridine nitrogen atoms shifted to a high field after the bromide ion was exchanged for other acid anions, which may be due to the electron cloud density being increased by hydrogen bonding or electrostatic attraction. The spectroscopy evidence indicated that the different acid anions were successfully introduced and that the acid anions were probably arranged around the pyridinium cations by a series of intermolecular interactions.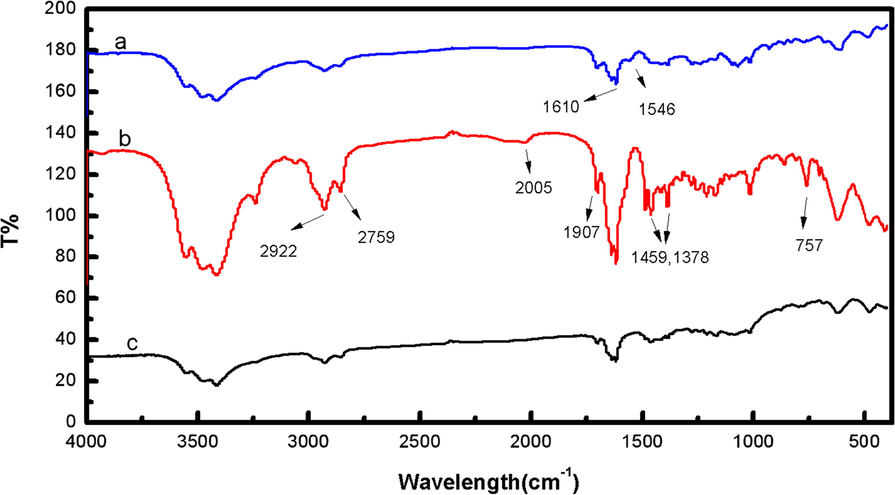
FTIR spectra of compounds (a) D4, (b) D3, and (c) B6.
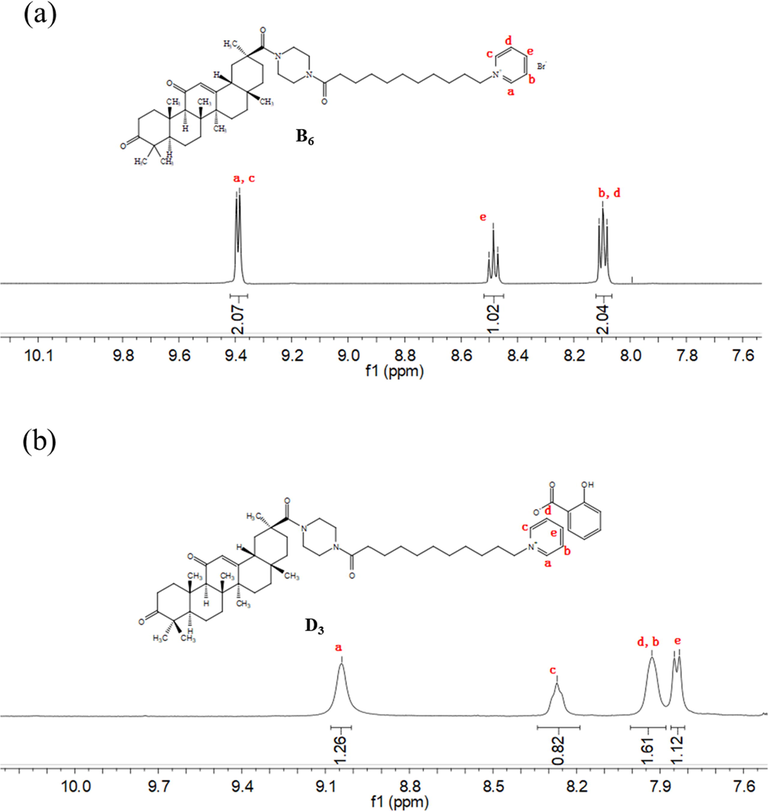
1H NMR confirmed the structures of compounds (a) B6 and (b) D3.
3.4 In vivo bioassay against rice bacterium blight
To validate the potential application of compound D3 in the management of plant bacterial diseases, an in vivo bioassay against rice bacterial blight was assessed using a pot experiment under greenhouse conditions. Meanwhile, 0.1% (v/v) orange peel essential oil (OPO) was added to improve the surface wettability of compound D3 in rice leaves, which aims to reduce losses during spraying and increase efficacy. As shown in Fig. 5 and Table 4, compound D3 could effectively control rice bacterial blight at 200 μg/mL with protective and curative activities of 54.09% and 38.50%, respectively, which were much better than commercial bactericides BT (44.04% and 37.00%) and TC (43.82% and 35.50%). Expectedly, the protective activity (54.62%) and curative activity (46.52%) of compound D3 were mildly enhanced after adding 0.1% OPO. Altogether, these findings suggested that 18-glycyrrhetinic derivatives coated with pyridinium salt-decorated 18β-glycyrrhetinic derivatives were powerful alternatives for protecting plants from bacterial infections, and the addition of OPO could effectively promote the potency.
Curative and protective activities of compounds D3, BT, and TC against rice bacterial blight under greenhouse conditions at 200 μg/mL.
Chemicals
Protect activity
Curative activity
Morbidity (%)
Disease index (%)
Control efficiencyb (%)
Morbidity (%)
Disease index (%)
Control efficiencyb (%)
D3
100
40.48
54.09B
100
54.67
38.50B
D3-OPO
100
40.00
54.62A
100
47.14
46.52A
TC
100
49.52
43.82D
100
57.33
35.50D
BT
100
49.33
44.04C
100
56.00
37.00C
CKa
100
88.15
100
88.89
3.5 Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis
Acridine orange (AO)/ethidium bromide (EB) double staining assay was used to observe the apoptosis of Xoo cells triggered by compound D3. AO is a fluorescent nucleic acid cationic dye that can stain live and dead cells, causing cells to emit bright green fluorescence. EB can only penetrate cells with damaged membranes, causing cells to emit orange fluoresce. Apoptotic cells displayed an orange fluorescence in a merged image when dyed using the AO/EB double staining method (Guo, 1998). Thus, a fluorescence microscope was used to observe the experimental results of AO/EB double staining in this study and the results showed that the Xoo cell treated with compound D3 emitted bright red fluorescence after staining with AO/EB, while the control group only emitted green fluorescence, indicating that compound D3 could cause certain damage to the cytomembrane of Xoo cells. Meanwhile, with increased concentration of compound D3, the intensity of red and orange fluorescence had obviously increased, but a visible decrease was observed in green fluorescence (Fig. 6), which indicated that the degree and amount of damaged and apoptotic cells increased evidently with increasing concentration of compound D3. These results suggested that our designed compound could induce the cell apoptosis of phytopathogenic bacteria.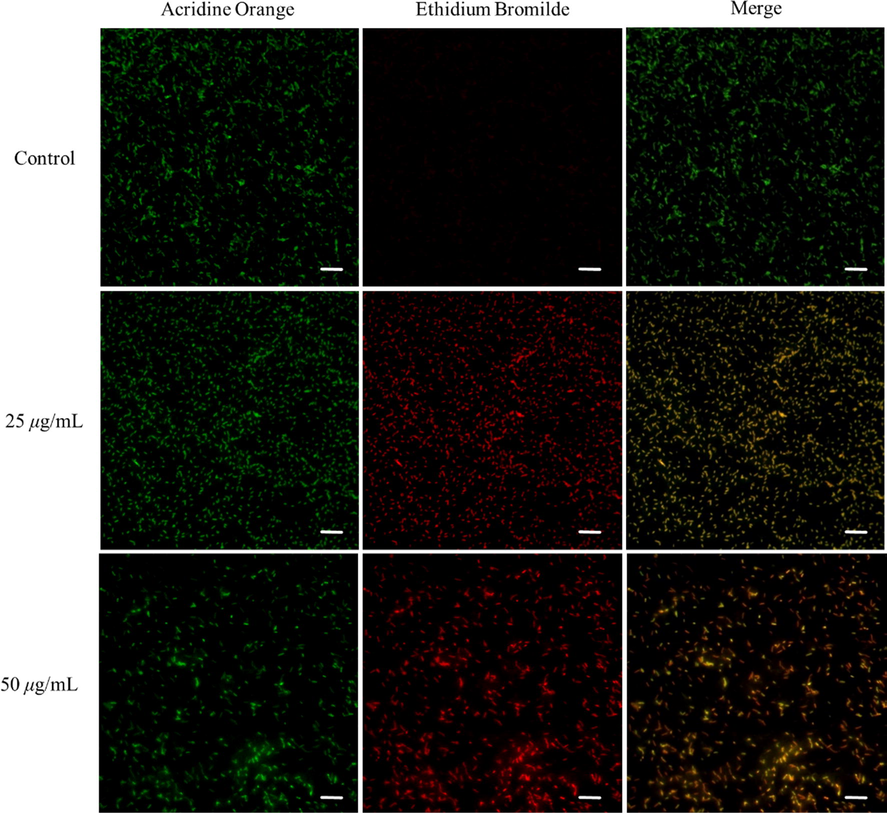
AO/EB dual fluorescence staining results for Xoo after incubation with D3 for 12 h. All the scale bars for are 10 μm.
3.6 Fluorescence imaging of dichlorofluorescein from ROS-induced Xoo pathogen
Excess ROS is a crucial factor in triggering the apoptosis (Mehraj et al., 2022), so the accumulation of ROS in Xoo pathogens was detected using the fluorescent probe DCFH-DA in this experiment. The results showed (Fig. 7) that ROS levels obviously increased in Xoo cells in a concentration-dependent manner when incubated with compound D3 for 12 h, which was higher than that of the control group (0 μg/mL). These findings suggested that our designed compounds could interfere with the balance of the redox system in phytopathogenic bacteria, resulting in massive accumulation of ROS, inducing an apoptotic response, and ultimately leading to bacterial death.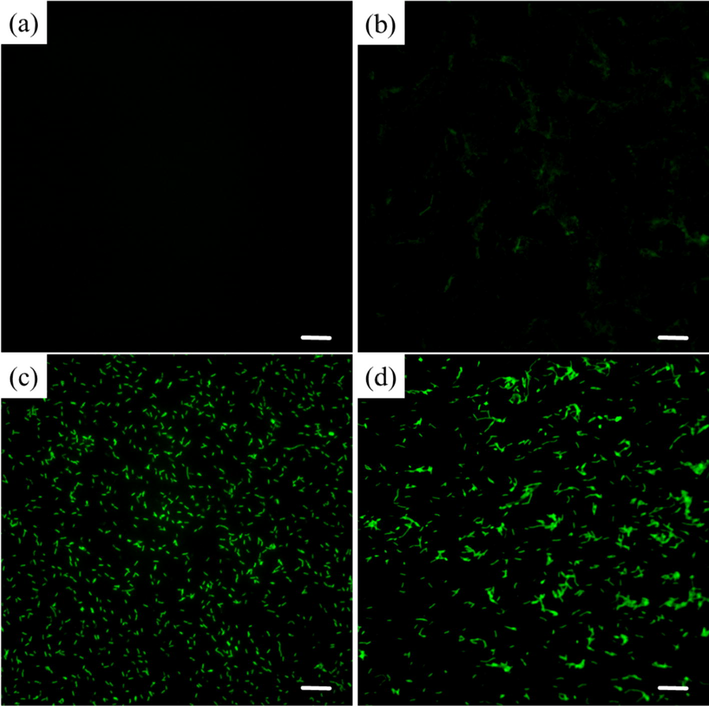
Fluorescence images of Xoo stained with the nonfluorescent oxidation-responsive dye DCFH-DA after treatment with elevated dosages of D3 (a) 0 μg/mL, (b) 12.5 μg/mL, (c) 25 μg/mL, and (d) 50 μg/mL. Scale bars for (a)–(d) are 10 μm.
3.7 SEM images of bacterial morphological changes
SEM was used to observe the morphological changes of Xoo pathogens treated with different concentrations of compound D3. The results (Fig. 8) showed that the morphology of the bacterial cell membrane was indeed affected by compound D3. Compared to the control group (Fig. 8a), the surface of Xoo cells showed a noticeable shrinkage and depression when treated with compound D3, and the phenomenon became more evident with elevated concentrations (Fig. 8b-d). Altogether, these findings showed that compound D3 had a considerable ability to affect physiological and biochemical processes, including altering the bacterial defense system, promoting ROS accumulation, and leading to morphological changes, which are consistent with the mechanisms of cell apoptosis.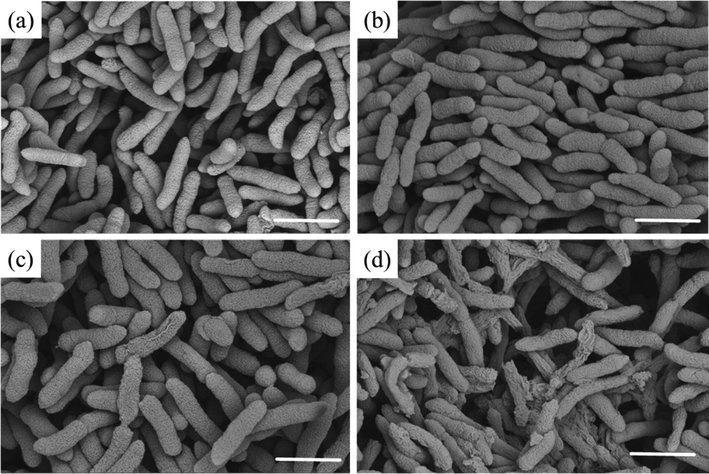
SEM images for Xoo after incubation with various concentrations of D3. (a) 0 μg/mL, (b) 6.25 μg/mL, (c) 12.5 μg/mL, and (d) 25 μg/mL. Scale bars for (a)–(d) are 10 μm.
4 Conclusion
In summary, a class of novel pyridinium salt-decorated 18β-glycyrrhetinic derivatives were designed, synthesized, and their structures were optimized. The results of the antibacterial bioassay showed that compounds D3 and D8 could efficiently attack two pernicious phytopathogenic bacteria, including Xoo and Xac, with EC50 values of 1.60 and 2.07 μg/mL, respectively. The pot experiment revealed that compound D3 had excellent curative and protective activities in vivo for the management of rice bacterial blight at 200 μg/mL, of 54.09% and 38.50%, respectively. The phytotoxicity test showed compound D3 had markedly lower phytotoxicity than B6, which suggested that the ion exchange method was a feasible means to reduce the adverse effects of pyridinium salt on the plant. Furthermore, AO/EB double staining assays indicated that these compounds could induce strong apoptotic effects on pathogens tested at lower drug concentrations. ROS detection studies showed that compound D3 could remarkably induce ROS production in bacteria, which in turn interfered with the bacterial redox system, caused irreversible damage, contraction of the bacterial cell membrane, and finally bacterial death. SEM observations showed that our designed compounds could cause bacteria cell shrinkage and depression, effects which were in accordance with the phenomenon of apoptosis. Overall, the findings suggest that these novel types of pyridinium salt-decorated 18β-glycyrrhetinic derivatives may act as a reference for the development of new bactericide alternatives and provide a rationale for overcoming the limitations in the application of pyridinium salts in agricultural and medical development.
CRediT authorship contribution statement
Jing-Jing He: Writing – original draft, Investigation, Data curation. Ting Li: Data curation. Hong-Wu Liu: Writing – review & editing. Lin-Li Yang: Data curation. Yi-Hong Yang: Data curation. Qing-Qing Tao: Data curation. Xiang Zhou: Resources, Funding acquisition, Writing – review & editing. Pei-Yi Wang: Methodology. Song Yang: Conceptualization, Funding acquisition, Project administration, Resources, Validation, Writing – review & editing.
Acknowledgments
We acknowledge the supports from National Natural Science Foundation of China (21877021, 32160661, 32202359), the Guizhou Provincial S&T Project (2018[4007]), the Guizhou Province [Qianjiaohe KY number (2020)004], Program of Introducing Talents of Discipline to Universities of China (D20023, 111 Program) and GZU (Guizhou University) Found for Newly Enrolled Talent (No. 202229).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Soft drugs. 1. Labile quaternary ammonium salts as soft antimicrobials. J. Med. Chem.. 1980;23(5):469-474.
- [CrossRef] [Google Scholar]
- Natural products to drugs: natural product derived compounds in 300 clinical trials. Nat. Prod. Rep.. 2005;22(2):162-195.
- [CrossRef] [Google Scholar]
- Drug-like properties: guiding principles for the design of natural product libraries. J. Nat. Prod.. 2012;75(1):72-81.
- [CrossRef] [Google Scholar]
- Occurrence conditions and influencing factors of bacterial diseases in rice. Mod. Agric. Sci. Technol.. 2021;120(21):116-117.
- [CrossRef] [Google Scholar]
- Plant bacterial diseases and their control measures. J. Zhejiang Agric. Sci.. 2022;63(08)
- [CrossRef] [Google Scholar]
- Construction of pyridinium/N-chloramine polysiloxane on cellulose for synergistic biocidal application. Cellulose.. 2019;26(8):5033-5049.
- [CrossRef] [Google Scholar]
- Enzymatic synthesis of novel glycyrrhizic acid glucosides using a promiscuous bacillus glycosyltransferase. Catalysts. 2018;8(12):615.
- [CrossRef] [Google Scholar]
- Development of a novel, multifunctional, membrane-interactive pyridinium salt with potent anticancer activity. Bioorg. Med. Chem. Lett.. 2014;24(15):3430-3433.
- [CrossRef] [Google Scholar]
- Feng, Q., 2021. Discussion on green control of citrus canker. Plant Prot. Sci. 41 (8), 96–98. https://1672-6820(2021)08-0096-03.
- Synthesis of new glycyrrhetinic acid derived ring A azepanone, 29-urea and 29-hydroxamic acid derivatives as selective 11beta-hydroxysteroid dehydrogenase 2 inhibitors. Bioorg. Med. Chem.. 2011;19(6):1866-1880.
- [CrossRef] [Google Scholar]
- Cell and molecular biology experiment operation guide. Ac. J. Sec. Mil. Med. Univ.. 1998;18(006):551.
- [Google Scholar]
- Research progress in biological control of tobacco bacterial wilt. Plant Doctor.. 2021;34(2):4-8.
- [CrossRef] [Google Scholar]
- Etiology and control measures of bacterial stripe disease in rice. Seed Sci. Technol.. 2022;40(05):97-99.
- [CrossRef] [Google Scholar]
- Optimization of 1,2,3-triazole-tailored carbazoles as prospective antibacterial alternatives with significant in vivo control efficiency and unique mode of action. J. Agric. Food. Chem.. 2021;69(16):615-4627.
- [CrossRef] [Google Scholar]
- Control effect of three copper preparations on pepper bacterial wilt. Modern Agrochem.. 2017;16(1)
- [CrossRef] [Google Scholar]
- Synthesis and cytotoxicity evaluation of pentacyclic triterpene-phenol nitrogen mustard conjugates. Chem. Nat. Compd.. 2018;54(1):106-111.
- [CrossRef] [Google Scholar]
- Therapeutic potential of glycyrrhetinic acids: a patent review (2010–2017) Expert Opin. Ther. Pat.. 2018;28(5):383-398.
- [CrossRef] [Google Scholar]
- Use of natural products in the crop protection industry. Phytochem. Rev.. 2011;10(2):185-194.
- [CrossRef] [Google Scholar]
- Pentacyclic triterpene distribution in various plants-rich sources for a new group of multi-potent plant extracts. Molecules. 2009;14(6):2016-2031.
- [CrossRef] [Google Scholar]
- Natural product-derived drugs for the treatment of inflammatory bowel Diseases. Intest. Res.. 2014;12(2):103-109.
- [CrossRef] [Google Scholar]
- Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. Cold Spring Harb. Protoc.. 2006;2006(3):799-803.
- [CrossRef] [Google Scholar]
- Enhanced oral bioavailability of glycyrrhetinic acid via nanocrystal formulation. Drug Deliv. Transl. Res.. 2016;6(5):519-525.
- [CrossRef] [Google Scholar]
- Research progress of kiwifruit bacterial ulcer disease. J. Huazhong Agric. Univ.. 2013;32(5):124-133.
- [CrossRef] [Google Scholar]
- Luo, K., Cai, H.L., Zhou, Z.C., Xiao, L.P., Xiao, Q.M., Li, X., Tang, Q.J., 2015. Research on the prevention and treatment of Tangerine Rhizoma disease. Mod. Agric. Sci. Technol. (8), 123–124. https://1007-5739(2015)08-0123-02.
- Pesticidal natural products-status and future potential. Pest. Manag. Sci.. 2019;75:2325-2340.
- [CrossRef] [Google Scholar]
- Adapalene and doxorubicin synergistically promote apoptosis of TNBC cells by hyperactivation of the ERK1/2 pathway through ROS induction. Front. Oncol.. 2022;12:938052
- [CrossRef] [Google Scholar]
- Synthesis and biological activity of new 18β-glycyrrhetinic acid derivatives. Arab. J. Chem.. 2016;9(3):390-399.
- [CrossRef] [Google Scholar]
- Palladium-catalyzed carboalkoxylation of aryl, benzyl, and vinylic halides. J. Org. Chem.. 1974;39(10):3318-3326.
- [CrossRef] [Google Scholar]
- Novel 18β-glycyrrhetinic acid amide derivatives show dual-acting capabilities for control of plant bacterial diseases through ROS-mediated antibacterial efficiency and activation of plant defense responses. J. Integr. Agr.. 2022;12(21)
- [CrossRef] [Google Scholar]
- Modification, antitumor activity, and targeted PPARγ study of 18β-glycyrrhetinic acid, an important active ingredient of licorice. J. Agric. Food. Chem.. 2019;67(34):9643-9651.
- [CrossRef] [Google Scholar]
- 1-Alkyl-(N, N-dimethylamino) pyridinium bromides: inhibitory effect on virulence factors of Candida albicans and on the growth of bacterial pathogens. J. Med. Microbiol.. 2013;62(2):241-248.
- [CrossRef] [Google Scholar]
- Self-assembly and liver targeting of sulfated chitosan nanoparticles functionalized with glycyrrhetinic acid. Nanomed-Nanotechnol.. 2012;8(6):870-879.
- [CrossRef] [Google Scholar]
- Novel piperazine-tailored ursolic acid hybrids as significant antibacterial agents targeting phytopathogens Xanthomonas oryzae pv. oryzae and X. axonopodis pv. citri probably directed by activation of apoptosis. Pest. Manag. Sci.. 2020;76(8):2746-2754.
- [CrossRef] [Google Scholar]
- Novel 1, 3, 4-Oxadiazole thioether and sulfone derivatives bearing a flexible N-heterocyclic moiety: synthesis, characterization, and anti-microorganism activity. Arab. J. Chem.. 2022;2(16):104479.
- [CrossRef] [Google Scholar]
- Natural products that target virulence factors in antibiotic resistant Staphylococcus aureus. J. Agric. Food Chem.. 2019;67(48):13195-13211.
- [CrossRef] [Google Scholar]
- Synthesis of novel 18β-glycyrrhetinic piperazine amides displaying significant in vitro and in vivo antibacterial activities against intractable plant bacterial diseases. Pest Manage. Sci.. 2020;76(9):2959-2971.
- [CrossRef] [Google Scholar]
- Research progress on green control technology of soil-borne diseases of pepper in protected areas. Anhui Agric. Sci. Bull.. 2021;27(23):121-123.
- [CrossRef] [Google Scholar]
- A new E-ring gamma-lactone pentacyclic triterpene from Lysimachia clethroides and its cytotoxic activities. Chem. Nat. Compd.. 2012;48(4):597-600.
- [CrossRef] [Google Scholar]
- Synthesis, anti-microbial and anti-inflammatory activities of 18β-glycyrrhetinic acid derivatives. Bioorg. Chem.. 2020;101
- [CrossRef] [Google Scholar]
- Occurrence and control status of main diseases and insects in citrus in Hunan Province. Hunan Agric. Sci.. 2021;12:61-64.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of novel pentacyclic triterpene derivatives as potential PPAR gamma agonists. Med. Chem.. 2013;9(1):118-125.
- [CrossRef] [Google Scholar]
- Research status and prospect of green pest control technology in rice. J. Huazhong Agric. Univ.. 2022;41(1):92-104.
- [CrossRef] [Google Scholar]
- Identification of racemic and chiral carbazole derivatives containing an isopropanolamine linker as prospective surrogates against plant pathogenic bacteria: in vitro and in vivo assays and quantitative proteomics. J. Agric. Food. Chem.. 2019;67(26):7512-7525.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of antimicrobial cationic surfactants: aminopyridinium salts. J. Surfactants Deterg.. 2006;9(4):325-330.
- [CrossRef] [Google Scholar]
- Progress of research on antimicrobial agents and their application to textiles. Text. Res. J.. 2011;32(11):153-161.
- [CrossRef] [Google Scholar]
- Chemoenzymatic synthesis of new derivatives of glycyrrhetinic acid with antiviral activity molecular docking study. Bioorg. Chem.. 2018;78:210-219.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supporting information including 1H NMR, 13C NMR and HRMS spectra associated. Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104771.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







