Translate this page into:
Ionic liquids enhanced oil recovery from oily sludge-experiment and mechanism
⁎Corresponding authors at: School of Chemical Engineering and Technology, Tianjin University, Tianjin, China (J. Hou). houjinjian@tju.edu.cn (Jinjian Hou)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The oily sludge would cause environment pollution, and would cause the heavy oil waste. Therefore, it was vital for us to find novel methods to obtain heavy oil from the oily sludges. In this study, the [C12mim][PF6] and [C12mim][Br] ionic liquids(ILs) were used to enhance the oil recovery. The toluene could obtain the highest oil recovery, and both the two ILs could increase the oil recovery. Toluene could obtain the highest oil recovery (89.4 wt%), and n-octane could obtain the lowest oil recovery (76.8 wt%). [C12mim] [PF6] could efficiently increase the heavy oil recovery to 91.2 wt%(by toluene). The [C12mim][Br] could increase the heavy oil recovery further. Both the [C12mim] [PF6] and the [C12mim][Br] ionic liquids could increase the heavy ois C/H ratio, decrease heavy oil viscosity and increase the sands hydrophilicity. The [C12mim][Br] ionic liquids showed better effect. In addition, the ionic liquids could increase the solvents recovery, and the ionic liquids recovery were high. Therefore, the ionic liquids enhanced oil recovery could be recycled to ten times. The two ionic liquids could effectively decrease the heavy oil interaction force, and when the ionic liquids increased to 200 ppm, the force remained stable. In the end, the ionic liquids enhancing solvent extraction mechanism was put forward.
Keywords
Oily sludge
Ionic liquids
Solvent extraction
Interface modification
1 Introduction
In recent years, environmental pollution has become a great concerned problem in all fields. In the field of the petrochemical industry, the environmental pollution caused by oily sludge attracts great attention of many researchers (Bhattacharya et al., 2021, Lu et al., 2021, Deng et al., 2022, Liu et al., 2022, Zhao et al., 2022a,b). The oily sludges were comprised of 30–50 % crude oil, 10–20 % solid and 30–50 % water. It’s an oily solid waste which is usually produced in the process of petroleum exploitation, petroleum refining, crude oil and refined oil transportation, etc (Zhao et al., 2022a,b). Due to the wide variety and complex nature of oily sludge, the corresponding treatment technology and equipment also show a diversification trend, such as landfill (Aguelmous et al., 2020), incineration (Gong et al., 2021), biological treatment (Saborimanesh 2021), pyrolysis (Ran et al., 2022), solvent extraction (Zhao et al., 2020, Hou et al., 2021, Song et al., 2022), etc. Compared with other methods about treating oil sands or oily sludges, solvent extraction method has obvious advantages. On the one hand, the solvent extraction process is simple, fast and has no energy consumption. On the other hand, the solvent extraction can treat most kinds of oily sludges and recover most petroleum hydrocarbons from oily sludges.
In the process of solvent extraction, a variety of organic solvents are used to extract oily sludge. Mohit et al. (2020) investigated the effectiveness of solvent extraction using MEK, xylene and solvent blends, which was composed of MEK and xylene, for oil recovery from oily sludge, respectively. Compared with MEK, xylene has a more significant performance in hydrocarbon recovery. Meanwhile, a higher oil recovery was achieved using solvent blend. The application of solvent extraction on oily sludge treatment is desirable, but there are also obvious disadvantage, such as unsatisfactory extraction efficiency, large volume of solvent consumption, and long extraction duration.
To further enhance solvent extraction process, ILs-solvent extraction method was proposed to apply on the heavy oil recovery of oily sludge (Zhu et al., 2020, Hu et al., 2021). Because ILs has conspicuous thermal and chemical stability, easy recovery property, it is considered as a “green and eco-friendly solvent”, more and more researchers put the attention on the application of ILs to recover heavy oil from oily sludges or oil sands. Tian et al. (2019) investigated the effectiveness of [Emim][BF4] on reinforcing oil recovery from crude oily sludge combining a solvent. Compared to cyclohexane extraction, [Emim][BF4]-cyclohexane extraction achieved a higher TPH recovery under the condition of shorter extraction time and lower solvent/sludge ratio. Zhu et al. (2020) explored the application of [Bmmim][PF6] in oil recovery from oily sludge by the solvent extraction method and analyzed the kinetic models of the solvent extraction and IL-solvent extraction. The results show that the [Bmmim][PF6] has a remarkable effect on the recovery of resins and asphaltenes. The extraction kinetics study indicate that the IL-enhanced extraction process fit the second-order pseudo-kinetic model, while the solvent extraction process fit the first-order pseudo-kinetic model. Although there are researchers have studied the application of several ILs in oily sludge extraction, due to the different physical and chemical properties of different ILs and oily sludge, the application of different ILs in assisting solvent extraction need to be further explored. Meanwhile, most of the studies on solvent extraction assisted by ILs do not involve the oil-solid interaction force, and mainly focus on macroscopic solvent extraction experiments (Sun et al., 2021). Besides, the experimental material of IL-solvent extraction is mainly oil sands, few researchers have explored the role and mechanism of ILs on the extraction of oily sludge, so more efforts are needed to study the mechanism of ILs on solvent extraction of oily sludge.
The purpose of this study mainly includes three parts. (i) The solvents and solvents-ILs ([C12mim] [PF6], [C12mim] [Br]) were used to extract heavy oil from oily sludges, the heavy oil element, viscosity and SARA analysis were conducted. (ii) The solvent recovery, ionic liquids recovery and the oil recovery recovery after recycle. (iii) The mechanism of ILs enhanced oil recovery was investigated.
2 2.Materials and methods
2.1 Materials
Toluene, n-octane, xylene, cyclohexane, n-decane, [C12mim][Br], [C12mim][PF6] were analytically pure from Aladdin Cop, Shanghai, China. The oily sludge samples were from the Shanghai, China. The [C12mim][Br] and [C12mim][PF6] ionic liquids structure were shown in Fig. 1.![The chemical structures of ILs: (a) [C12mim] [PF6]; (b) [C12mim] [Br].](/content/184/2022/15/11/img/10.1016_j.arabjc.2022.104210-fig1.png)
The chemical structures of ILs: (a) [C12mim] [PF6]; (b) [C12mim] [Br].
2.2 Solvent extraction and ILs-solvent extraction.
2.2.1 Solvent extraction
The oily sludge was put into a drying oven with the temperature set at 100℃ and dried for 24 h to remove the water in the oily sludge. 5 g dehydrated sludge sample was added into a flask, then 30 mL solvent was put into the same flask, the mixture was thoroughly mixed and placed in a water bath with a stirring rate of 700 r/min. After reaction for 60 min, the mixture was quickly transferred to a centrifuge for solid–liquid separation. The mixture was divided into solid phase and liquid phase, the upper layer is the solvent-oil mixture, and the lower layer is the residual solids. The solvent was recycled from the heavy oil by spinning method and used to the solvent extraction of next oil sludge sample. Then the heavy oil mass was measured, and the oil recovery was calculated.
2.2.2 ILs-solvent extraction
5 g dehydrated sludge sample was added into a flask, then 30 mL solvent and 10 mL 200 ppm ILs were added into the same flask, and then the experiment followed the 2.2.1 section. After centrifugation, the mixture was divided into three phases. The uppermost liquid phase is the mixture of solvent and oil, the middle liquid is the ILs, and the solid phase below is residual solids. The solvent and ILs were recovered. The oil recovery was calculated.
2.2.3 Recycle use
After the first extraction process, the recovered solvents and recovered ionic liquids would be used to extract oily sludges again, which was the second oil recovery. The process was repeated for ten times.
2.3 Element analysis measurement
The heavy oil element was calculated by the Elemental Analyzer, the C, H and other elements were calculated. Then the C/H ratio was calculated.
2.4 Contact angle measurement
The wettability of the sands after ILs-solvents extraction was evaluated by the water drops contact angle measurement (Sharifigaliuk et al., 2022). The detailed experiment procedures were as follows. Firstly, the residual solids after extraction were dried. Secondly, these drying solids were pressed into circular sheets with uniform thickness by a powder tablet press. Finally, the water drop contact angle of residual solids was tested, which was shown in Fig. 2.
The contact angles diagrammatic sketch.
2.5 Viscosity measurement
The viscosity of heavy oil from solvents-ILs was measured. The temperature control device of the rheometer was turned on and the temperature was set at 25℃. When the temperature is stable, an appropriate amount of heavy oil after extraction was poured into the measuring cylinder, then the cylinder was fixed, the rotor was installed and the relevant parameters were set. After the above operation, the viscosity of heavy oil could be measured.
2.6 SARA measurement
Because the composition of heavy oil is complex and the relative molecular weight is large, it is difficult to obtain its exact chemical composition by traditional chemical analysis methods (Wang et al., 2020). In order to facilitate research and engineering application, heavy oils are usually divided into four components (SARA) by chromatography according to the polarity of different components and their solubility in different organic solvents (Volkov et al., 2021). It has great significance to explore the composition and structure of heavy oil components for the separation and resource utilization of heavy oil. During the experiment, the ASTM D-4124 was used as the SARA analysis method (Wang et al., 2022).
1 g bitumen was placed in a beaker, 40 mL n-heptane was added, then the sample was sonicated for 4 h under the temperature of 50 °C. The mixture was composed of solution (saturates, aromatics, resins) and insoluble solids (asphaltenes), and the asphaltenes could be separated by centrifugal separation method from other components. Next, a neutral alumina column was prepared and the separated solution (saturates, aromatics, resins) was added in the top of the neutral alumina column. The saturates, aromatics, resin were eluted with n-heptane, toluene, methanol/toluene (v:v = 1:1), respectively.
2.7 Oil-solid interaction force measurement
The interaction force between oil and solid is an important factor affecting the solvent extraction of oil sludge. When the interaction force between oil and solid decreases, the separation of heavy oil from solid surface becomes easier (Shi et al., 2020). During the experiment, the oil-solid force interaction can be measured by AFM (atomic force measurement). In the process of AFM measurement, when the probe closes to the solid surface, the interaction force is repulsive and the repulsive force increases gradually with the shortening of the distance between probe and solid; when the probe leaves the solid surface, the interaction force changes to the attraction force and the attraction force increases with the increase of the distance. Previous studies have used this oil-solid interaction force to evaluate the difficulty level of separation of heavy oil and solid (Hou et al., 2022a, Hou et al., 2022b). The less attractive the oil-solid interaction force is, the easier it is to release heavy oil from the solid surface.
The bitumen used in the experiment was obtained by Soxhlet extraction. To obtain 1 wt% toluene-bitumen solution, the bitumen (1 g) was dispersed into 100 mL toluene. The SiO2 microspheres (hydrophilic) were washed with acetone, ethanol and distilled water, then the SiO2 microspheres were put in a vacuum desiccator (0.5 mL octyl trimethoxy silane was placed at the bottom of the vacuum desiccator) and dried under vacuum for 24 h. After vacuum drying, octyl trimethoxylsilane was grafted onto the hydrophilic SiO2 surface, which changed from hydrophilic to hydrophobic.
The hydrophobic SiO2 microspheres were subsequently washed with deionized water and dried under nitrogen, then the hydrophobic SiO2 microspheres were immersed for 4 h in 1 wt% bitumen-toluene solution. After immersion, the solution is transferred drop by drop onto the glass slide, and the microspheres will be fixed on the glass slide. The silicon slice was washed with acetone, ethanol and distilled water, then it was dried with nitrogen. AFM was used to measure the force curves between bitumen and silicon slice in different ILs ([C12mim][PF6], [C12mim][Br]) at different concentrations. The approach speed of the probe was set to 2 μm/s, and the repeating points with regular interval was 100 under each condition.
3 3.Results and discussion
3.1 ILs concentration optimization
As shown in Fig. 3, with the increase of ILs concentration, the oil recovery increases gradually. And when the concentration of [C12mim] [Br] reached about 200 ppm, oil recovery increased slightly. Therefore, 200 ppm [C12mim] [Br] was chosen as the ILs concentration in the ILs-solvent extraction.![The oil recovery alteration versus the [C12mim][Br] alteration.](/content/184/2022/15/11/img/10.1016_j.arabjc.2022.104210-fig3.png)
The oil recovery alteration versus the [C12mim][Br] alteration.
3.2 Heavy oil analysis and solid wettability analysis
The heavy oil recovery by different solvent extraction (toluene, n-octane, xylene, cyclohexane and n-decane) was shown in Fig. 4(a). The results show that n-octane, n-decane, cyclohexane and toluene have different oil recovery, and the oil recovery gradually increases depending on the above sequence. The highest oil recovery and the lowest oil recovery are obtained by toluene extraction and n-octane extraction respectively, and the values are 89.4 wt% and 76.8 wt% respectively. Different solvents showed different affinity with the heavy oil components, therefore the oil recovery was different for different solvents. The effect of IL([C12mim] [PF6], [C12mim] [Br]) on oil recovery was also shown in Fig. 4(a). Compared to pure solvent extraction, [C12mim] [PF6]-solvent extraction and [C12mim] [Br]-solvent extraction all show better oil recovery, and the oil recovery of [C12mim] [Br]-solvent extraction obtains the highest value under the same other experimental conditions.![(a) The oil recovery; (b) C/H ratio; (c) viscosity of heavy oil extracted from the solvents alone, solvents-[C12mim] [PF6] and solvents-[C12mim][Br] systems. (d) The water drop contact angles of the surface of the sand.](/content/184/2022/15/11/img/10.1016_j.arabjc.2022.104210-fig4.png)
(a) The oil recovery; (b) C/H ratio; (c) viscosity of heavy oil extracted from the solvents alone, solvents-[C12mim] [PF6] and solvents-[C12mim][Br] systems. (d) The water drop contact angles of the surface of the sand.
Fig. 4(b) shows the C/H ratio of oil extracted from oily sludge by pure solvent extraction or IL-solvent extraction. The C/H ratio of oil extracted by toluene is highest and the C/H ratio of oil extracted by n-octane is lowest, which represents that toluene could play a greater role in the extraction of oil with higher C/H ratio components, while n-octane could play a greater role in the extraction of oil with lower C/H ratio components. When the IL is used to reinforce solvent extraction, the component with higher C/H ratio will be extracted easily. Compared with [C12mim] [PF6], the effect of [C12mim] [Br] on the extraction of heavy oil with higher C/H ratio is more obvious.
Fig. 4(c) shows that the viscosities of the oil extracted by toluene, n-octane, xylene, cyclohexane, n-decane are 127.6 Pa·S, 165.2 Pa·S, 138.5 Pa·S, 143.4 Pa·S and 152.3 Pa·S, respectively. Compared with other solvents, toluene exhibits the greatest advantage in reducing the viscosity of oil. Depending on the research of Hou et al., the decrease in viscosity of oil is conducive to its separation from the solid surface, which represents that toluene shows the best performance in oil recovery, while n-octane shows the worst performance in oil recovery. The oil viscosity extracted by [C12mim][PF6]-toluene, [C12mim][PF6]-n-octane, [C12mim][PF6]-xylene, [C12mim] [PF6]-cyclohexane, [C12mim] [PF6]-n-decane are 89.2 Pa.S, 145.8 Pa.S, 104.6 Pa.S, 114.8 Pa.S, 123.1 Pa.S, respectively. Compared to pure solvent, [C12mim][PF6]-solvent exhibits a great decrease in the viscosity of the oil. And the [C12mim][Br]-solvent extraction shows the lower viscosity of oil compared with [C12mim][PF6]-solvent. The ILs ([C12mim] [PF6] or [C12mim][Br]) could obviously decrease the oil viscosity, the reason is that ILs ([C12mim] [PF6] or [C12mim][Br]) could dissolve part of oil, which is beneficial to enhance the oil recovery.
The contact angle of the oil sludge extracted by toluene, n-octane, xylene, cyclohexane, n-decane respectively was tested and the results were shown in Fig. 4(d). When [C12mim][PF6] or [C12mim][Br] was used to enhance the heavy oil recovery, the contact angles would decrease a lot, which represents that the wettability of the surface of the sand would become more hydrophilic. For instance, when [C12mim] [PF6] was used to reinforce the n-octane extraction, the contact angle decreased from 62.3° to 47.6°.
3.3 Sara analysis
Fig. 5 showed the saturates, aromatics, resins and asphaltenes content of heavy oil extracted from the solvents alone and [C12mim][PF6]/[C12mim][Br]-solvent systems. As was shown in Fig. 5, the ILs could effectively decrease the saturates/aromatics content, and increase the resins/asphaltenes content. For saturates, the toluene, n-octane, xylene, cyclohexane and n-decane could obtain the 23.5 wt%, 26.5 wt%, 24.1 wt%, 24.6 wt%, 25.2 wt% saturates content, respectively. When [C12mim][Br] was added to the solvent extraction system, the saturates content of toluene, n-octane, xylene, cyclohexane and n-decane decreases to 22.7 wt%, 25.2 wt%, 23.3 wt%, 23.6 wt%, 24.1 wt%, respectively. Combining the results of the C/H ratio, the existence of ILs will make the component with higher C/H ratio increase, and the resins and asphaltenes have higher C/H ration compared with saturates/aromatics. When the ILs was added to the solvent extraction system, the contents of the above four components will appear the change of Fig. 5.![(a) Saturates; (b) Aromatics; (c) Resins; (d) Asphaltenes content of heavy oil extracted from the solvents alone, solvents-[C12mim] [PF6]/C12mim][Br] systems.](/content/184/2022/15/11/img/10.1016_j.arabjc.2022.104210-fig5.png)
(a) Saturates; (b) Aromatics; (c) Resins; (d) Asphaltenes content of heavy oil extracted from the solvents alone, solvents-[C12mim] [PF6]/C12mim][Br] systems.
3.4 Element analysis
To further explored the contents of carbon element and hydrogen element in oil after extraction, element analysis experiments were designed, the results were shown in Table 1. The content of carbon element is more than 80 wt% and the content of hydrogen element is less than 11 wt%. In the solvent extraction experiments, the carbon content of oil which was extracted by toluene is highest and the hydrogen content is lowest. When the ILs was used to reinforce the solvent extraction, the carbon content increased and the hydrogen content decreases. Compared with [C12mim][PF6], the changes of carbon and hydrogen contents which was influenced by [C12mim][Br] is more obvious. The analysis of carbon and hydrogen contents gives us a deeper understanding of the respective changes of carbon and hydrogen when the C/H ratio changes. Meanwhile, the results of element analysis support the conclusion of SARA analysis from the other aspect, the driving effect of ILs on the detachment of the component with higher C/H ratio (such as resins and asphaltenes) from the solid surface is stronger than that of the component with lower C/H ratio from solid surface.
Solvent
Ionic Liquid
C (wt%)
H (wt%)
Other elements (wt%)
Without ILs
83.14
9.28
7.58
Toluene
[C12mim][PF6]
83.76
9.18
7.06
[C12mim][Br]
84.27
8.91
6.82
Without ILs
81.87
10.96
7.17
N-octane
[C12mim][PF6]
82.38
9.75
7.87
[C12mim][Br]
82.74
9.34
7.92
Without ILs
82.59
9.45
7.96
Xylene
[C12mim][PF6]
83.46
9.26
7.28
[C12mim][Br]
84.03
9.12
6.85
Without ILs
82.21
9.81
7.98
Cyclohexane
[C12mim][PF6]
82.61
9.33
8.06
[C12mim][Br]
83.88
9.16
6.96
Without ILs
82.04
9.96
8
N-decane
[C12mim][PF6]
82.56
9.47
7.97
[C12mim][Br]
82.96
9.29
7.75
3.5 Organic solvents recovery
Fig. 6 showed the organic solvents recovery of solvents alone, [C12mim][PF6]- solvent and [C12mim][Br]-solvent extraction system. Comparing with the IL-solvent extraction systems, the organic solvent recovery was low under the solvent extraction systems, which was due to that the ILs would alter the solid surface wettability and decrease the oil-solid interaction force, which decreased the adsorption of solvent on the solid surface and then increased the organic solvents recovery.![The organic solvents recovery of solvents alone, solvents-[C12mim][PF6], solvents-[C12mim][Br] extraction system.](/content/184/2022/15/11/img/10.1016_j.arabjc.2022.104210-fig6.png)
The organic solvents recovery of solvents alone, solvents-[C12mim][PF6], solvents-[C12mim][Br] extraction system.
3.6 ILs recovery
Fig. 7 showed the different ILs recovery of different extraction system. For different ILs-solvent extraction system, the ILs recovery was different. When the organic solvents were the same, the [C12mim] [Br] recovery was higher than [C12mim][PF6] recovery. For instance, the [C12mim][Br], [C12mim][PF6] recoveries were 97.2 wt%, 95.4 wt% for different ILs-solvent extraction system.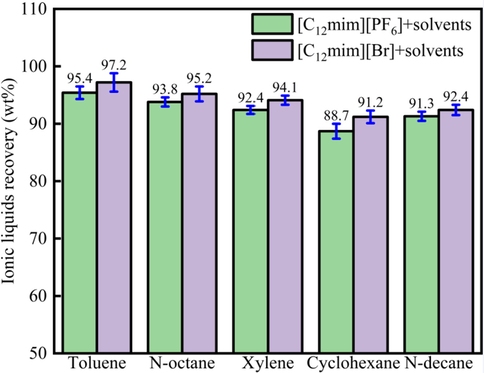
ILs recovery of different extraction system.
3.7 Interaction forces analysis
Fig. 8 showed the oil-solid interaction forces in different ILs([C12mim][PF6], [C12mim][Br]) concentrations. The results indicated that the ILs would decrease the interaction force, and the interaction force would decrease when the ILs concentration increased. The reasons for ILs decreasing oil-solid interaction force was as follows. On the one hand, the ILs could make oil-solid interface modification, and then the wettability of heavy oil would alter. On the other hand, the ILs would alter the surface charges of oil and sands, and then the electrostatic interactions between oil and sands would be altered.![The oil-solid interaction forces in different [C12mim][PF6], [C12mim][Br] ILs concentrations.](/content/184/2022/15/11/img/10.1016_j.arabjc.2022.104210-fig8.png)
The oil-solid interaction forces in different [C12mim][PF6], [C12mim][Br] ILs concentrations.
3.8 Recycle use
Fig. 9 showed the cyclic oil recovery alteration with different recycle times. The results indicated that the oil recovery decreased with the increase of recycle use time of solvent and ILs. As shown in Fig. 7, the oil recovery of [C12mim][Br]-toluene extraction is the highest under the same recycle use time. When the [C12mim][Br] and toluene were used in the 10 times, the oil recovery decreased about 10 wt%. On the contrary, the [C12mim][PF6]-n-octane extraction produced the lowest oil recovery, and when the [C12mim][PF6] and n-octane were used in the last recycle, there were about 12 wt% decline of oil recovery. Before and after IL-solvent extraction, the chemical properties of solvent and ILs did not change, and the reduction of oil recovery was due to solvent loss during extraction. The solvent loss included solvent adsorption, Operating loss, etc. On the whole, the combination of [C12mim][Br] and toluene produces the best results in the IL-solvent extraction, it’s also the optimal combination in the oil recovery.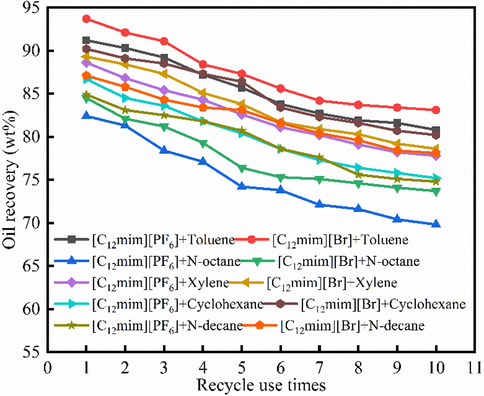
The cyclic oil recovery alteration with different recycle times.
3.9 The mechanism of solvent extraction and IL-solvent extraction
The solvent extraction mechanism and IL-solvent extraction mechanism are shown in Fig. 10. For the process of solvent extraction, heavy oil could directly dissolve in solvent. Especially under the action of external forces such as stirring, the electrostatic layer between the heavy oil and the solid surface is more likely to be destroyed, and the heavy oil can be separated from the solid surface faster (Lin et al., 2017). When the ILs is used to reinforce solvent extraction, anions are adsorbed on the solid surface, and cations tend to combine with anions and distribute around anions, which forms a positive layer on the solid surface; On the heavy oil surface, part of cations are adsorbed on the heavy oil surface, the alkyl chains of other cations are integrated into the heavy oil, which causes the positively charged head groups distribute on the heavy oil surface, forming a positively charged layer on the heavy oil surface, as shown in Fig. 10 (b). The adsorption of ILs on solids and heavy oil surfaces makes the solid surface and heavy oil surface contain same charge. Meanwhile, the cationic alkyl chains on the solid surface and heavy oil surface are distributed on the outermost side, which further hinders the combination of heavy oil and solid, and promotes the detachment of heavy oil from the solid surface.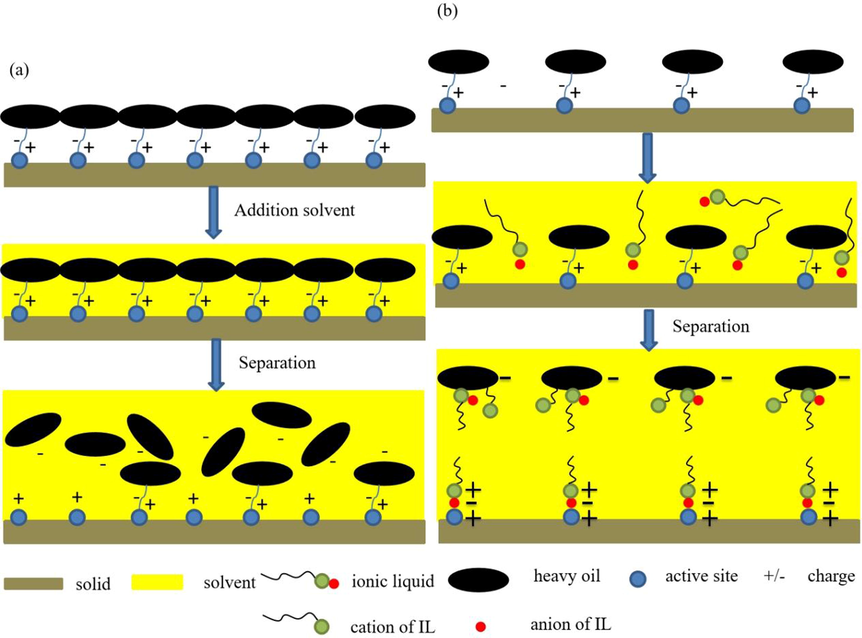
Solvent extraction and ILs-solvent extraction mechanism: (a) solvent extraction mechanism; (b) ILs-solvent extraction mechanism.
4 Conclusions
In this study, the [C12mim] [PF6] and [C12mim] [Br] was used to enhance the oil recovery from oily sludge, and the mechanism was explored, the conclusions were as follows:
In the pure solvent extraction system, five solvents were chosen to explore the effect of each solvent on heavy oil recovery from oil sludge. The toluene showed the best performance in heavy oil recovery and heavy oil recovery rate could reach 89.4 wt%. On the contrary, n-octane has the worst performance in heavy oil recovery, and its heavy oil recovery rate could only reach 76.8 wt%. Organic solvent extraction is mainly to recover heavy oil by dissolving heavy oil.
For the ILs-solvent extraction, [C12mim] [PF6] and [C12mim] [Br] were used to reinforce solvent extraction, respectively. The heavy oil recovery of [C12mim] [Br]-solvent extraction is higher than the heavy oil recovery of [C12mim] [PF6]-solvent extraction under the condition using the same organic solvent, and the combination of toluene and [C12mim] [Br] could make the heavy oil recovery reach 93.7 wt%, which is the maximum value of heavy oil recovery. The exist of ILs decrease heavy oil viscosity, increase heavy oil C/H ratio, and change the nature of the solid interface which makes the solid interface more hydrophilic, all these changes brought about by ILs make it easier for heavy oil to detach from solid surfaces, thus increasing heavy oil recovery.
Compared with pure solvent extraction, the solvent recovery in the system of ILs-solvent extraction is higher. Because the modification of solid surface by ILs reduces the loss of organic solvent adsorbed on solid surface. Meanwhile, [C12mim][Br] makes the solid more hydrophilic comparing with [C12mim] [PF6], which results in less loss of ILs. The solvent recovery and [C12mim] [PF6]/[C12mim] [Br] recovery was high after 10 recycle use.
Fund
This research was funded by Hebei Natural Science Funds for Young Scholar (B2021106003), Science and Technology Project of Hebei Education Department (BJ2021097) and 973 National Basic Research Program of China (2015CB251403).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Landfilling and composting efficiency to reduce genotoxic effect of petroleum sludge. Environ. Technol. Innovat.. 2020;20
- [CrossRef] [Google Scholar]
- Assessing pollution removal efficiencies of some selected parameters by applying different remediation techniques for petroleum oily sludge. Environ. Challenges. 2021;5
- [CrossRef] [Google Scholar]
- Investigating properties and intermolecular interactions of sludge bio-oil modified asphalt. J. Mol. Liq.. 2022;360
- [CrossRef] [Google Scholar]
- Study on migration characteristics of heavy metals during the oil sludge incineration with CaO additive. Chem. Eng. Res. Des.. 2021;166:55-66.
- [CrossRef] [Google Scholar]
- Surfactants Enhanced Heavy Oil-Solid Separation from Carbonate Asphalt Rocks-Experiment and Molecular Dynamic Simulation. Nanomaterials. 2021;11
- [CrossRef] [Google Scholar]
- A review on the application of nanofluids in enhanced oil recovery. Front. Chem. Sci. Eng.. 2022;16:1165-1197.
- [CrossRef] [Google Scholar]
- Ionic-liquid-enhanced solvent extraction mechanism: A novel concept. J. Environ. Chem. Eng.. 2022;10
- [CrossRef] [Google Scholar]
- Evaluation and Prediction on the Effect of Ionic Properties of Solvent Extraction Performance of Oily Sludge Using Machine Learning. Molecules. 2021;26
- [CrossRef] [Google Scholar]
- Recent Advances in Nonaqueous Extraction of Bitumen from Mineable Oil Sands: A Review. Org. Process Res. Dev.. 2017;21:492-510.
- [CrossRef] [Google Scholar]
- Hydrothermal carbonization of petrochemical sludge: The fate of hydrochar and oil components. J. Environ. Chem. Eng.. 2022;10
- [CrossRef] [Google Scholar]
- Oil recovery from polymer-containing oil sludge in oilfield by thermochemical cleaning treatment. Colloids Surf. A. 2021;611
- [CrossRef] [Google Scholar]
- Optimization of influential parameters of hydrocarbon recovery from waste oily sludge by solvent extraction using solvent blend. Environ. Monit. Assess.. 2020;192:407.
- [CrossRef] [Google Scholar]
- Rheological properties of asphalt mortar with silane coupling agent modified oil sludge pyrolysis residue. Constr. Build. Mater.. 2022;329
- [CrossRef] [Google Scholar]
- Toward sustainable remediation of oil sands fine Tailings-A review. J. Environ. Manage.. 2021;288:112418.
- [CrossRef] [Google Scholar]
- Comparative analysis of conventional methods for the evaluation of wettability in shales. J. Petrol. Sci. Eng.. 2022;208
- [CrossRef] [Google Scholar]
- Probing the interaction mechanism between oil droplets with asphaltenes and solid surfaces using AFM. J. Colloid Interface Sci.. 2020;558:173-181.
- [CrossRef] [Google Scholar]
- Kinetics of CO2 gas bubbling for the separation of residual solvent from waste solids: Effects of bubble size. J. Environ. Chem. Eng.. 2022;10
- [CrossRef] [Google Scholar]
- High-efficiency Extraction of Bitumen from Oil Sands Using Mixture of Ionic Liquid Emim BF4 and Dichloromethane. China Petrol. Process. Petrochem. Technol.. 2021;23:132-138.
- [Google Scholar]
- Ionic Liquid-Enhanced Solvent Extraction for Oil Recovery from Oily Sludge. Energy Fuels. 2019;33:3429-3438.
- [CrossRef] [Google Scholar]
- Low-field NMR-relaxometry as fast and simple technique for in-situ determination of SARA-composition of crude oils. J. Petrol. Sci. Eng.. 2021;196
- [CrossRef] [Google Scholar]
- Effects of SARA fractions on pyrolysis behavior and kinetics of heavy crude oil. Pet. Sci. Technol.. 2020;38:945-954.
- [CrossRef] [Google Scholar]
- Investigation on the oxidation thermal effects and kinetics of multivariate mixtures of heavy oil SARA fractions. Pet. Sci. Technol.. 2022;40:2049-2063.
- [CrossRef] [Google Scholar]
- Study on the oil-sludge separation by thermochemical method in rotating packed bed. Chem. Eng. Process. – Process Intensification. 2022;174
- [CrossRef] [Google Scholar]
- Co-pyrolysis of oil sludge with hydrogen-rich plastics in a vertical stirring reactor: Kinetic analysis, emissions, and products. Front. Environ. Sci. Eng.. 2022;16
- [CrossRef] [Google Scholar]
- Insight into essential channel effect of pore structures and hydrogen bonds on the solvent extraction of oily sludge. J. Hazard. Mater.. 2020;389
- [CrossRef] [Google Scholar]
- Application of hydrophobic ionic liquid [Bmmim][PF6] in solvent extraction for oily sludge. Chin. J. Chem. Eng.. 2020;28:2294-2300.
- [CrossRef] [Google Scholar]







