Iron-catalyzed synthesis of N-heterocycles via intermolecular and intramolecular cyclization reactions: A review
⁎Corresponding authors at: Department of Chemistry, Government College University Faisalabad, 38000, Pakistan(Tahir Maqbool), Department of Physics, Zhejiang Normal University, Jinhua, Zhejiang 312004, China (Ghulam Abbas Ashraf). drtahirmaqbool@gcuf.edu.pk (Tahir Maqbool), ga_phy@yahoo.com (Ghulam Abbas Ashraf) drtahirmaqbool@gcuf.edu.pk (Ghulam Abbas Ashraf) ga_phy@yahoo.com (Ghulam Abbas Ashraf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Small N-heterocyclic molecules are important scaffolds in the pharmaceutical industry and most FDA-approved drugs are nitrogen-containing heterocycles. Chemists try to employ iron-based catalysts for organic transformations due to their abundance, economic, easily accessible and environment-friendly behaviour. N-heterocycles are synthesized by the cyclization reactions. This review covered the synthesis of N-heterocycles by employing iron-based catalysts via intermolecular or intramolecular cyclizations.
Keywords
Iron, catalyst
N-heterocycles
Intermolecular
Intramolecular
1 Introduction
Heterocycles of nitrogen-containing compounds are one of the most important structural components of medicines, and according to a study by the US Food and Drug Administration, 59% of unique small-molecule medications include a nitrogen heterocycle (Vitaku et al., 2014; Łowicki and Przybylski, 2022; Petri, 2020). In physiologically active compounds, both unsaturated and saturated N-heterocycles are common, and they are becoming more appealing substrates for the synthesis of novel medications (Fig. 1). Several indoles and azaindole compounds are effective cancer drugs (Pyta, 2022; Dong, 2016; Klich, 2016). Pemigatinib is an FDA-approved reference medicine (Han, 2020). Bioactive quinazolinone and quinazoline-based alkaloids include fenquizone and Camptothecin (CPT, 2). Quinazoline compounds are suspected inhibitors of epidermal growth factor (EGF) and have antiviral, antitubercular, and antibacterial properties and tyrosine kinase receptors (Cagir and Luotonin, 2003; Chan, 2009; Tsou, 2001; Wakeling, 1996; Kung, 1999; Jung, 2016; Liang et al., 2011; Przybylski, 2009; Domagalska, 2016). Lipitor, a pyrrole-based inhibitor, is a 'superb' medicine that is widely prescribed and improves the health of millions of people by decreasing cholesterol (Thompson, 2001). Saturated N-heterocycles, such as focalin and balaglitazone, are also medicinally important compounds that are used to treat ADHD (attention deficit hyperactivity disorder) and as antidiabetic agents, respectively (Pantaine, 2019; Skrzypczak and Przybylski, 2022). Piperidines, piperazines, and pyrrolidines are the most common saturated N-heterocycles in medicinal drugs (Vitaku et al., 2014; Skrzypczak and Przybylski, 2022; Pyta, 2014; Pyta, 2019; Surette et al., 2021). The formation of N-heterocycles has traditionally been a significant study field in synthetic organic chemistry. To approach the formation of N-heterocycles, traditionally named reactions have now been developed (Knölker and Reddy, 2002). Despite the value of traditional synthetic techniques, recent advances in synthetic chemistry have highlighted novel sustainable synthetic approaches that are focused on ecologically friendly alternatives to traditional methods (Daştan et al., 2012; Bilal, 2021; Kanwal, 2022; Ahmad, 2021). Indeed, drug development initiatives require easy and environmentally friendly access to a large range of N-heterocyclic molecules. Because N-heterocycles are found in both natural and synthetic molecules and have a wide range of applications and medical significance, their synthesis is an essential component of research in synthetic chemistry.
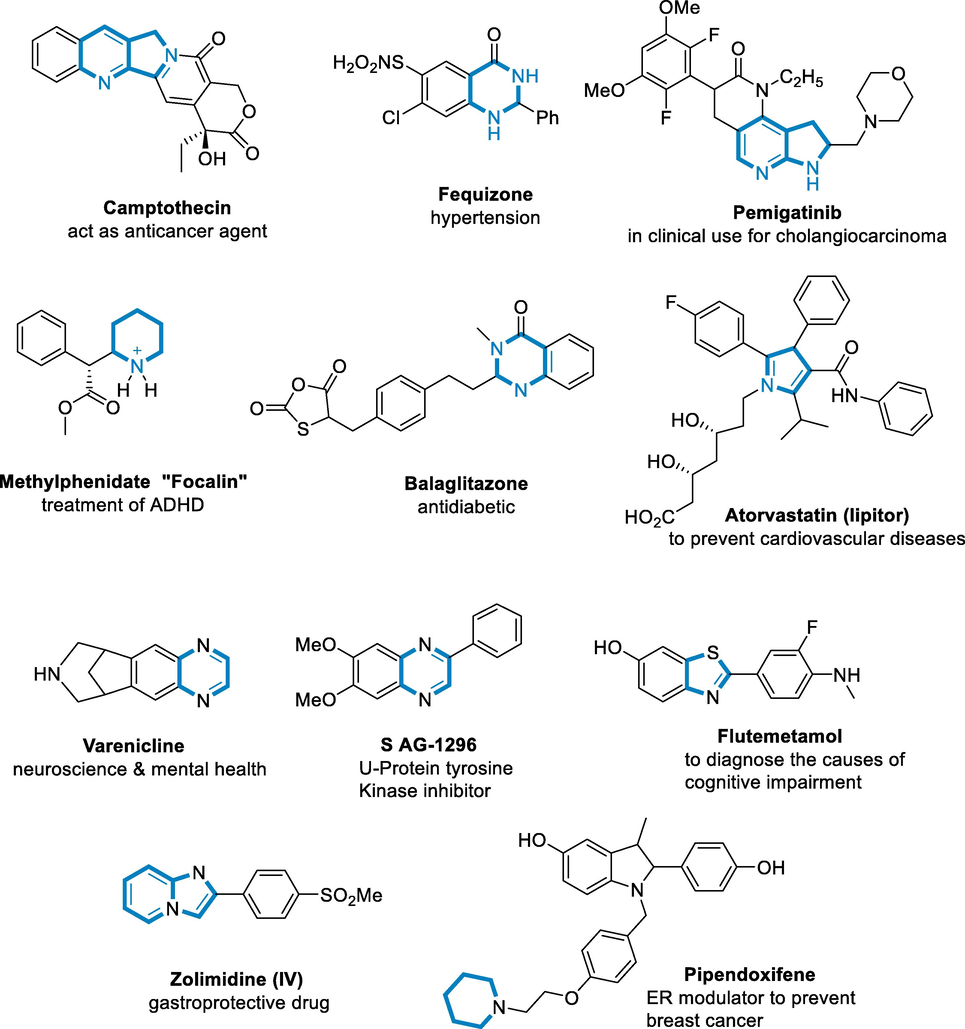
- Some representative bioactive examples of N-heterocycles.
The research of innovative techniques for heterocycle syntheses that use efficient and atom-saving pathways is a trend right now. Transition metal-catalyzed reactions are the most appealing technique among several novel synthetic transformations because they may directly produce multiple substituted molecules from widely accessible starting materials under moderate parameters (Bagley, 2003; McReynolds et al., 2004; Alonso et al., 2004; Deiters and Martin, 2004; Patil and Yamamoto, 2008; Yet, 2000). Transition metal-catalyzed coupling transformations and heteroannulation are useful and convenient tools in organic synthesis for the assembly of N-heterocycles (Bilal, 2021; Alberico et al., 2007; Dick and Sanford, 2006). C–N-bonds are a potentially effective way to introduce nitrogen into chemical scaffolds for the formation of nitrogen-containing molecules (Hili and Yudin, 2006; Bariwal and Van der Eycken, 2013). After Ullmann and Goldberg reported the Cu-mediated formation of the C–N bond reaction of an amine with aryl halides nucleophiles, Buchwald and Hartwig separately discovered Cu and Pd-catalyzed amination procedures, opening the door for the production of a range of useful N-heterocycles (Shin et al., 2015; Surry and Buchwald, 2011; Hartwig, 2008; Goldberg, 1906; Ullmann, 1903). Currently, first-row transition metals have seen a surge in applications in synthetic organic chemistry because of their increased availability, low toxicity, cheap cost, and amazing synthetic flexibility (Gandeepan, 2018; Sreedevi, 2019; Loup, 2019).
Iron is the frequent second metal on the planet, and it is commercially accessible in a range of salts and complexes. Iron salts have also grown popular due to their low cost and environmental friendliness. These concerns prompted scientists to focus their attention on iron-based catalysis for mild and environmentally friendly reactions (Bolm, 2004; Correa et al., 2008; Nakamura and Yoshikai, 2010; Plietker, 2008; Plietker, 2011). Iron catalysis has become even more important in recent years; particularly for large-scale applications in industries. Bolm et al. in 2004, reviewed the organic transformations of iron-catalyzed processes (Bolm, 2004). The same group 2008; described the progress of carbon-heteroatom and heteroatom-heteroatom bond formation processes by using iron-catalyst (Correa et al., 2008). In 2010; Nakamura et al. summarized their work on elevated Fe-catalyzed C-C bond formation processes (Nakamura and Yoshikai, 2010). In addition; Iron catalysis has been the subject of two monographs (Plietker, 2008).
Iron is a potent and necessary catalyst that plays a key role in biological and synthetic chemistry because iron easily accesses multiple oxidation states such as Fe0, FeII, FeIII, and FeIV. It shows a wide range of functional groups with Lewis acid interactions (Alonso et al., 2004; Han, 2014; Han et al., 2010). For most hydrocarbon oxidations using iron enzymes or synthetic catalysts, short-lived high-valent Fe-oxo intermediates (FeIV = O or FeV = O) are proposed as the active oxidants (Gelalcha, 2014). The Friedel–Crafts reaction, the Kumada cross-coupling reaction, the Nazarov reaction, and the Fenton reaction are only a few examples of iron-mediated transformations. Iron salts have recently been described as effective catalysts for additions, functionalization, and oxidative couplings, particularly C-H oxidative radical couplings. By using iron catalysis, N-heterocycle analogues have also been synthesized. According to a review of literature on iron-catalyzed synthesis of N-heterocycles, iron complexes elevate the activation of the unsaturated functionality by coordinating with the π-electrons (for C≡C, C = C) or heteroatoms (for C = NH, C = O) to enhance the attack of the nucleophile at the respective C-atoms.
This review focuses on iron-catalyzed chemical transformations via intra and intermolecular for the synthesis of nitrogen-containing compounds by C-H activation, oxidation, coupling, cyclization, etc. and includes the literature up to 2021.
2 Synthesis of N-heterocycles via intramolecular cyclization reactions
2.1 From carbonyl compounds
In important pharmaceutical and natural products, nitrogen-containing heterocycles are preferred scaffolds (Hazelard et al., 2017; Trowbridge et al., 2020; Ricci, 2008). The accessibility and ecofriendly nature of iron catalysts provide nitrene insertion which is the utmost intriguing way to C-H amination (Wang and Deng, 2018; Plietker and Röske, 2019; Liu, 2019; Liu, 2020).
Zhong et al., developed in situ intramolecular amidations of N-benzoyloxyureas by using iron-catalyst [Fe(OTf)2] and bipyridine without employing extrinsic oxidants. Using aliphatic C(sp3)-H amidation, a variety of cyclic ureas are produced in high yields. Cyclic urea (2) was synthesized by using N-benzoyloxylurea (1) as a substrate and10 mol% of FeCl2 as a catalyst in the presence of ligands such as bipyridine (L1), K2CO3 as a base, and acetonitrile at the temperature of 40 °C for 6 h. A variety of substituents like halogens at the para-position of the phenyl group and the electron-rich groups were well tolerated in good to high yields. A precursor having a nitro group containing an electron-withdrawing group afforded product in lesser yield (71%) (Scheme 1) (Zhong, 2021).
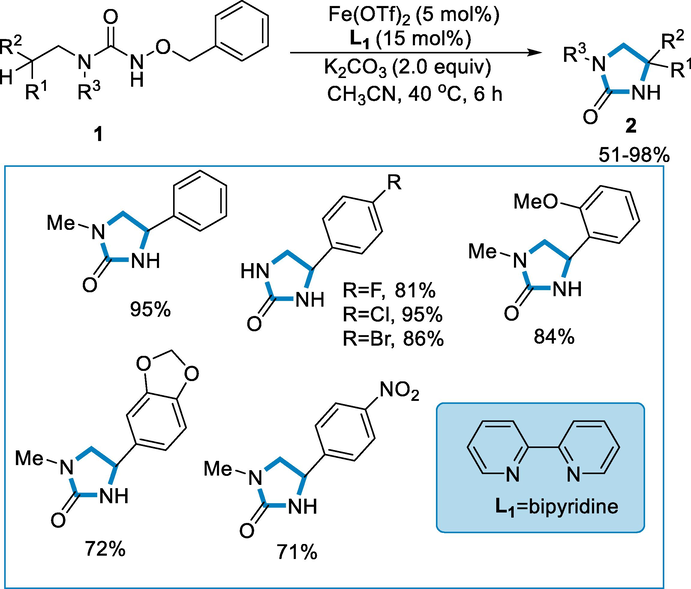
- Intramolecular amidation of N-benzoyloxyureas by using iron-catalyst.
Furthermore, numerous naturally produced alkaloids and biologically active compounds contain benzo[b]carbazoles, a form of polycyclic structure (Miller and McCarthy, 2012; May and Moody, 1984; Asche, 2005; Hande, 2008; Ramkumar and Nagarajan, 2014; Schmidt et al., 2012). Because of its chemiluminescent and optoelectronic capabilities, the coplanar structure of these molecules offers a wide range of applications in materials science (Wu, 2005; Levick, 2012; Levick, 2014; Bałczewski et al., 2012). Pericyclic reactions have historically been thought of as a cost-effective way to create bonds and are most common in tandem reactions (Sankararaman and Reactions-A, 2005; Qin, 2020; Arns and Barriault, 2007; Poulin et al., 2009). The exo-dig cyclization of alkynes by using iron-catalyst produces a reactive intermediate of vinylidene, which is then trapped in a pericyclic reaction to produce benzo[b]carbazoles from certain substrates in one step (Anderson, 2011; Nicolaou and Chen, 2009; Wang, 2011; Grigg, 2006).
The corresponding benzo[b]carbazoles (4) were synthesized by using aroyl substrate (3) catalyzed by 20 mol% Fe(OTf)2 at 130 °C temperature. The reaction went well for a wide range of 5H-benzo[b]carbazoles (4) by using aroyl moieties in R1 (electron-donating), R2 replaced with chloro, fluoro, methyl, and R3 with both electron-rich or poor groups in moderate yields (Scheme 2).
![Iron-catalyzed synthesis of benzo[b]carbazoles by using aroyl substrates.](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104095-fig4.png)
- Iron-catalyzed synthesis of benzo[b]carbazoles by using aroyl substrates.
The carbonyl group of the benzoyl moieties (3) has transformed into its enolic form (5), and alkyne has been coupled by Lewis acid, allowing for a 5-exo-dig cyclization and consequent proto demetallation, generating intermediate (6) of vinylidene. Intermediate (6) has transformed to its enolic form (7) and 6-π electrocyclization produced intermediate (8), whereas aromatization by aerial oxidation produced the hydroxy-5H-benzo[b]carbazole intermediate (3a). When the oxygen of sulfonyl intermediate (3a) interacted with Fe(OTf)2, the intermediate (9) was formed, and the five-membered intermediate (10) has been formed by the successive nucleophilic interaction of phenolic OH. Finally, by rearranging intermediate (10), the desired product (4) has been obtained, and the catalyst has been recycled for the next process (Scheme 3) (Boominathan, 2015).
![Mechanistic pathway for the synthesis of benzo[b]carbazoles.](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104095-fig5.png)
- Mechanistic pathway for the synthesis of benzo[b]carbazoles.
Loup and Coworkers reported indoles are synthesized by the reductive cyclization of ortho-vinylanilides which catalyze by a stable iron complex at room temperature (Andersson and Munslow, 2008; Trost and Ball, 2005; Gribble, 2010; Kaushik, 2013). The reaction yields through the vinyl group hydromagnesiation and trapped in situ produced benzyl carbanion by an intramolecular electrophile.
Herein, the corresponding indoles (12) were synthesized by using derivatives of benzamides (11) catalyzed by an iron catalyst such as Fe1 = [PrBIPFeCl2] (BIP = bis(min))pyridine) (10 mol%) and EtMgBr in Et2O, THF at rt. for 1 h. A wide variety of benzamides (11) consisting of electron-rich and withdrawal groups were synthesized 2-arylindoles (12) in affordable yield (Scheme 4).
- Synthesis of substituted indoles using an iron catalyst.
The following has the process of making an indole: Intermediate (17) has produced by hydromagnesiation of the selective iron catalyst. The in situ produced benzyl carbanion can attack an amide substrate, affording intermediates (18 or 19). Based on control testing, the above step appears a significant Grignard reaction that may not require an iron catalyst. Finally, after the water operation, the intermediate (20) has available, which can easily be shortened and refined to produce indole (12). However, the method that requires protonation (18) cannot be completely removed. Intermediates connected to both (20) and (18) have been previously proposed or found to be intermediate in multi-step indole synthesis (Scheme 5) (Lautens et al., 2021).
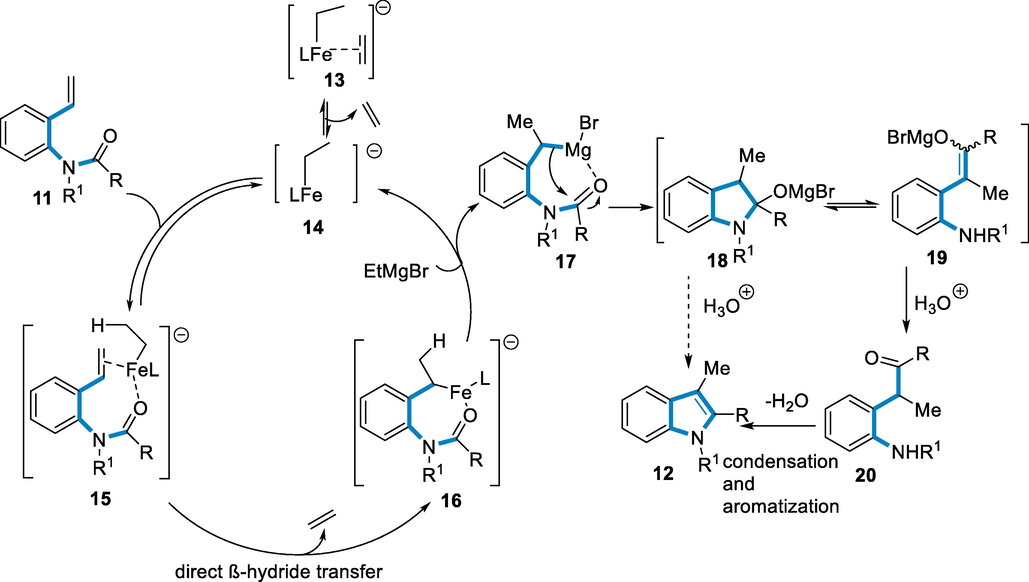
- Mechanistic pathway for the synthesis of substituted indoles.
Furthermore, Imidazolinone is a key heterocyclic structure found in a variety of bioactive compounds. A novel catalytic system made up of FeCl2 and β-discriminate ligand may convert α-azidyl amide intermediates to synthesis imidazolinone by the intramolecular C(sp3)-H amination (King et al., 2011; Iovan and Betley, 2016; Wilding et al., 2017; Huang and Che, 2015; Liu, 2013). Azides are a frequent supply of nitrogen for this purpose (Bräse, 2005; Intrieri, 2014; Shin et al., 2015).
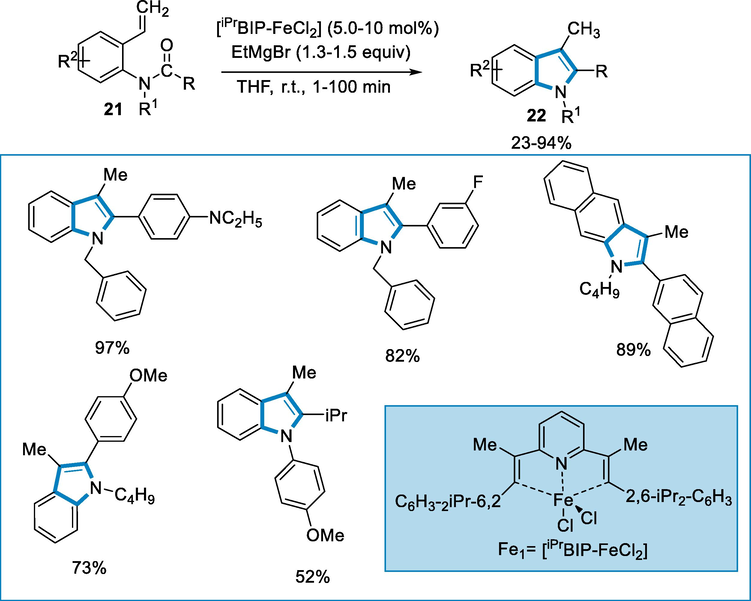
The 2-azido-N,N-diarylmethyl-2-methylpropanamides (21) react in presence of ligand β–diketiminate such as L2 = [N((4E,2Z)-4-(mesitylimino)pent-2-en-2-yl)-2,4,6-trimethylaniline] to produced imidazolinone (22) by using iron catalyst such as FeCl2 (20 mol%) at 100 °C for 12 h. The reaction went well for the series of 2-azido-N,N-diarylmethyl, 2-methyl propanamides but the benzyl ring substituent had a little consequence on the reaction (not affect the selectivity). The yields of desired compounds were good to fair (Scheme 6).
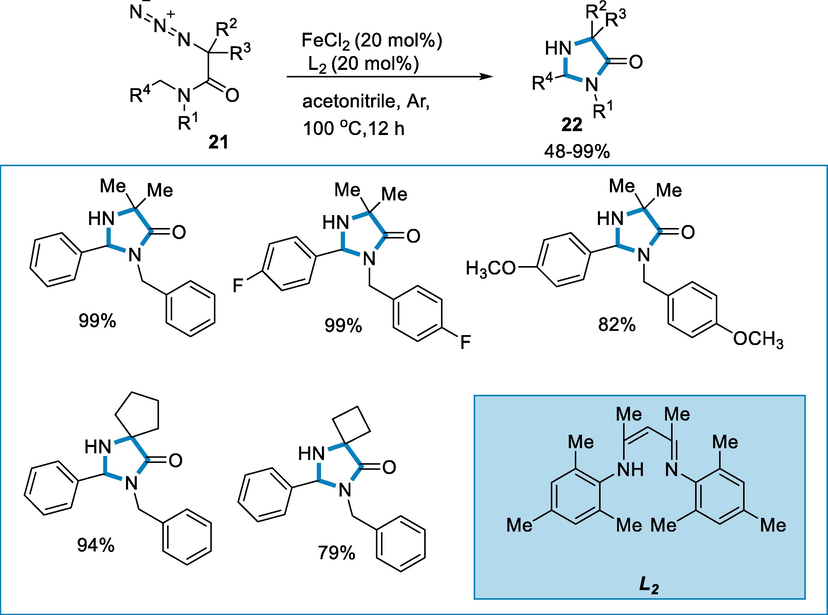
- Synthesis of imidazolinone by using FeCl2 as a catalyst.
There has been presented a probable mechanism: The in situ generated NacNacFeII complex (23) combines with the precursor to produce radical of ferric-iminyl complex (24), which has subsequently been subjected to intramolecular hydrogen atom transfer (HAT) to yield intermediary (26). A quick radical interaction converts the intermediate (26) to product imidazolinone (22). Apart from this route, the reactions might also continue through the reasonably stable ferric-imido intermediate (25) by direct C-H inclusion (path B). Because no precursors could be identified and defined, these two pathways could not be differentiated at this time (Scheme 7) (Zhao, 2019).
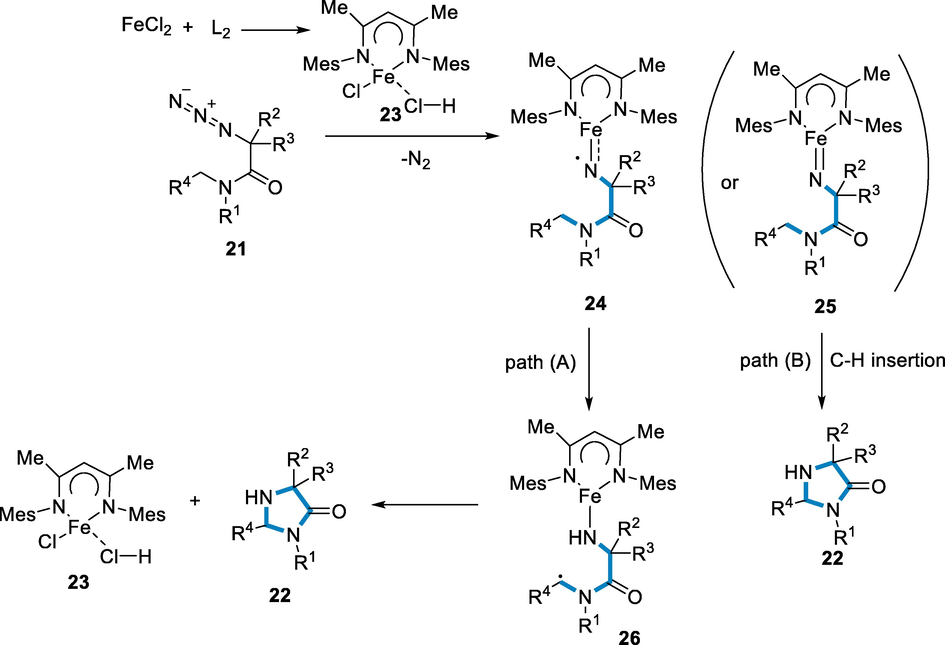
- Mechanistic pathway for the synthesis of imidazolinone.
Furthermore, the earth-abundant transition metal of the first row (iron) is non-toxic metal, which is attractive from an economic, environmental, and health perspective (Gandeepan, 2018; Bolm, 2009; Chirik and Morris, 2015; Beller, 2019). The simple transformation of nitrogen-containing benzoyloxyureas to 2-imidazolidones, which are significant scaffolds in bioactive chemicals, is catalyzed by ferrous chloride in conjunction with 1,10-o-phenanthroline (Patocka, 2020; Borsini, 2002). The C-H amination of propargylic, benzylic, allylic, and fully inactivated aliphatic C(sp3/sp2)-H bonds occur under mild circumstances such as accessible ligand without an inert environment or dry solvents by employing a simple iron salt (van Vliet and de Bruin, 2020; Shimbayashi, 2019).
The reaction conditions for the synthesis of 2-imidazolidones (28) were N-benzoyloxyureas (27) as a substrate by using the catalyst of iron such as FeCl2·4H2O (0.02 mmol), 1,10-o-phenanthroline, MeCN, and Na2CO3 at 50 °C for 14 h. The amination(C-H) of benzylic C(sp3)-H bonds, tolerates a wide range of nitrogen derivatives at the benzoyloxyureas, as well as various groups of alkyl, and also trimethylsilyl methyl. In the phenyl moiety at para-position, both substituents electron-poor and rich were effectively transformed. The C-H amination of propargylic, allylic and 2°/3° aliphatic C(sp3)-H bonds were effectively synthesized respective 2-imidazolidones (28) in fair to good yields (68–95%) (Scheme 8).
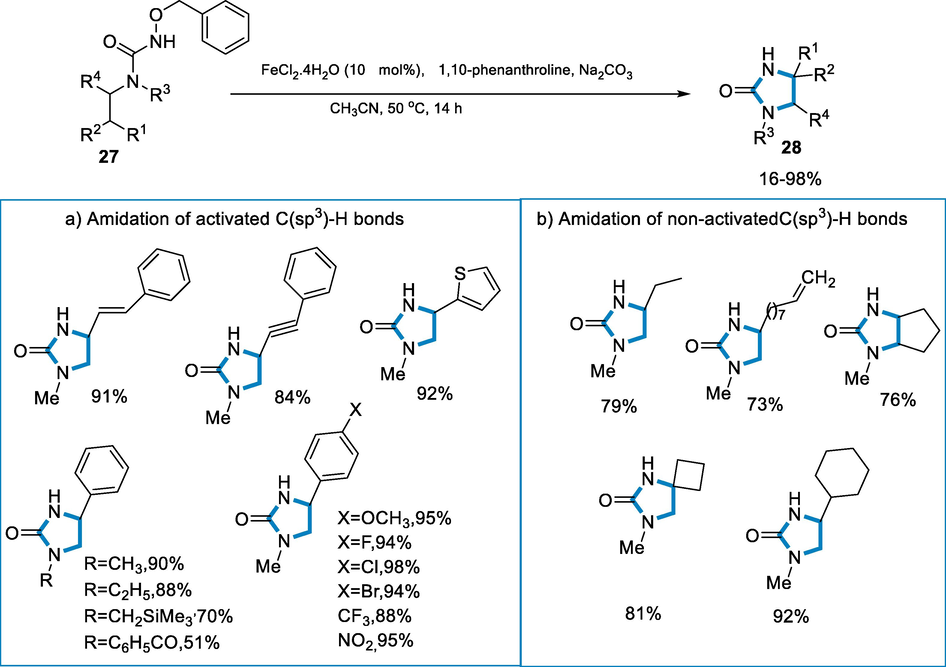
- Iron catalyzed synthesis of imidazolidin-2-ones.
The active catalyst of FeII attached to the 1,10-Phenanthroline ligands which correlate to the nitrogen-containing benzoyloxyurea, and an intermediate iron nitrenoid (29) has generated after deprotonation of the N-H group and then leaving group benzoate has released. In its triplet state, the intermediate iron nitrenoid proceeds through a 1,5-hydrogen atom transfer (HAT) to produce a radical intermediate (30), which has proceeded by a fast rebound of radical to form a newly C-N bond (31). The iron catalyst has been regenerated after the formation of imidazolidin-2-ones (28) for the further catalytic cycle (Scheme 9) (Jarrige, 2021).
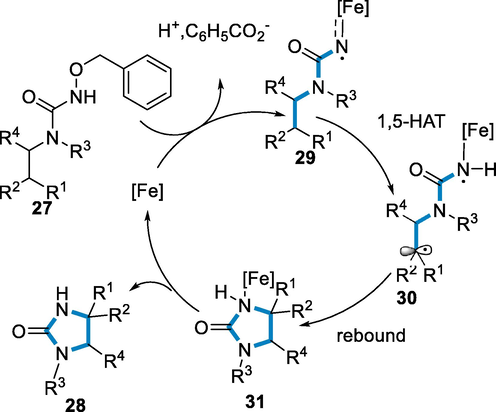
- Mechanistic pathway for the synthesis of imidazolidin-2-ones.
FeIII(TPP)Cl is a biocompatible, non-toxic, and inexpensive iron catalyst for the aromatic amidation of (sp2)C-H via cyclization of substrates such as N-Tosyloxyarylcarbamate intramolecularly under mild conditions (low catalyst loading, room temperature, external free oxidant, and water-soluble side product) to biologically important benzoxazolones (Liu et al., 2010; Liu, 2015; Singh et al., 2016; Prasanthi, 2018; Hennessy and Betley, 2013; Poupaert et al., 2005; Bach, 2015).
Benzoxazolones (33) were synthesized by using N-tosyloxyarylcarbamates (32) as substrate by using an iron catalyst such as FeIII(TPP)Cl (2 mol%) at the temperature of 25 °C for 6–12 h. N-tosyloxyarylcarbamates were well tolerated with diverse substrates such as aryl, alkyl, alkoxy, and halogen groups. The electronic effect was observed on 4-substituted-carbamate in which electron-poor substituents provided reduced yields whereas electron-donating substituents (Me, alkoxy) provide good to excellent yields (Scheme 10).
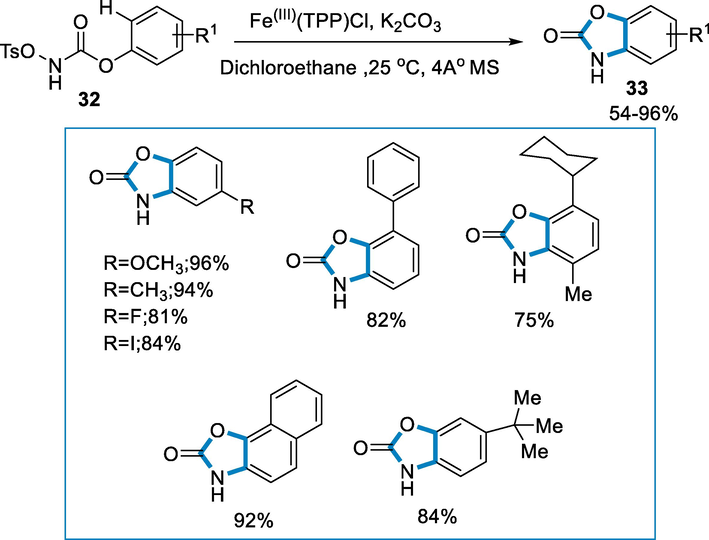
- Intramolecular C(sp2)–H amidation of N-tosyloxyaryl carbamates by using an iron(III) catalyst.
The N-Tosyloxy carbamate (32) initially coordinates with FeIII-porphyrin resulting in the intermediate of FeV–nitrenoid (34), then by stepwise electrophilic substitution undergoes nitrenoid insertion by aromatic C-H bond via an intermediate of arenium-ion (35) to give the corresponding benzoxazolones (33) (Scheme 11) (Prasanthi, 2018).
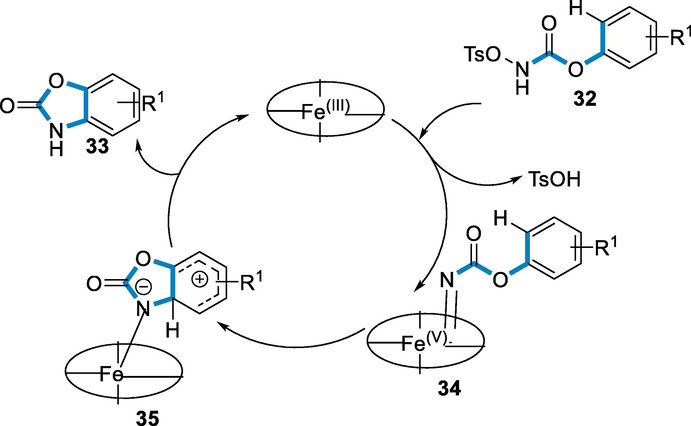
- Mechanistic pathway for the synthesis of Benzoxazolones.
2.2 From esters
Nitrogen containing heterocycles of sulfamate esters are frequent moieties in synthetic organic intermediates, agrochemicals and pharmaceutics (Hazelard et al., 2017; Roose, 2015; Córdova et al., 2007). Intramolecular C-H amination by using iron-catalyst of sulfamate-esters utilizing simple and inexpensive ligands. The reaction is accelerated by the addition of a second ligand(bipyridine) which also increase the yield (Zhang et al., 2014).
The aliphatic substrates of sulfamate ester (36) with the iron catalyst such as Fe(ClO4)2 (10 mol%) in the presence of ligand (L1) bipyridine in CH3CN, and C6H5I(OCO2CF3) at the temperature of 80 °C for the production of desired N-heterocycle products. The allylic C-H bonds and various benzylic derivatives including both electron-poor and rich substrates were aminated in excellent yield (54–78%). Secondary alcohol-derived sulfamate esters (37) were functionalized in high yield (Scheme 12) (Liu, 2019).
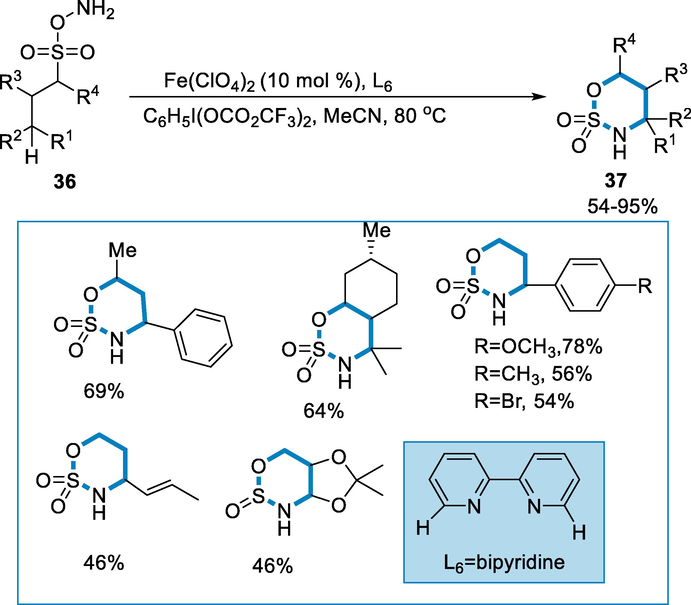
- Intramolecular C-H amination of sulfamate esters catalyzed by iron.
2.3 From sulfonamide
Sultams (cyclic sulfonamides) are the preferred scaffold of pharmaceutics and agrochemicals because of their extensive range of bioactivities. Direct synthesis of cyclic sulfonamides in the presence of ligand (amino pyridine) by using iron catalyst through intramolecular amidation of C-H(sp3). This method features an accessible catalyst and tolerates a wide range of substrates (Drews, 2000; Scozzafava, 2003; Scott and Njardarson, 2019; Feng, 2016; Inagaki, 2000; Donkor, 2000; Wells, 2001; Lad, 2017; Zhuang, 2003; Fejerman, 2012; Poutsiaka, 1961).
The reaction conditions for the synthesis of sultams (39) were 3-phenylpropane-1-sulfonamide (38) as a standard substrate by using a catalyst such as Fe(ClO4)2 (10 mol%) and ligand such as L3 = N-methyl-1,(pyridine-2-yl)-N-(pyridine-2-ylmethyl)methenamine at the temperature of 80 °C for 2 h. Substituents at the para-position of the aromatic ring, containing both electron-deficient and rich moieties yielded the required ɣ-sultams in moderate to fair (52–88%) yields. In meta and ortho-positions substituents of the aromatic ring were likewise tolerated, afforded good (61–89%) yields of the respective products (Scheme 13) (Zhong, 2019).
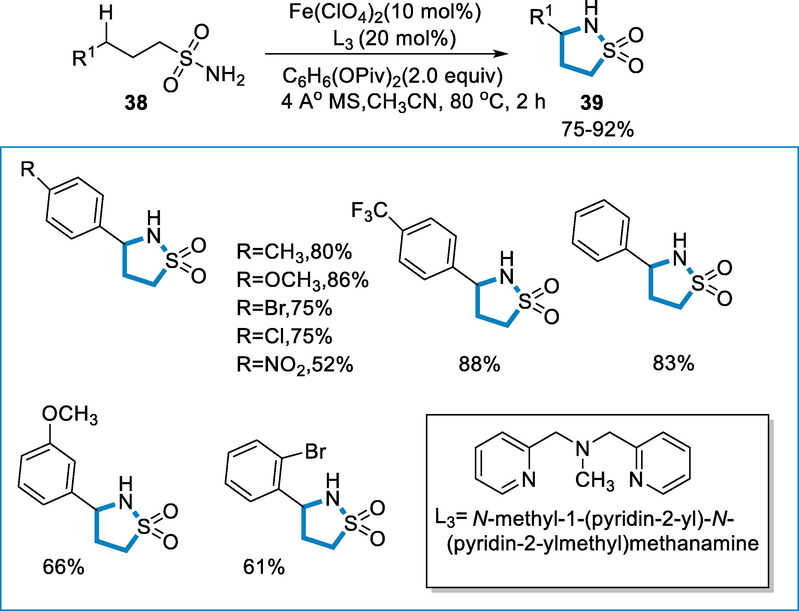
- Intramolecular C-H(sp3) amidation by using an iron catalyst.
2.4 From halides
5,10-Diaryl-5, 10-dihydrophenazines (DADHAPs) are important moieties of organic luminescent materials, have magnetic properties, and use as photoredox catalysts. The one-pot DADHPs synthesis from diphenylamines with new Fe-catalyzed amination of the C-F process was developed. The catalytic FeCl2 and 1,2-dibromoethane stoichiometric, homo-dimerization of magnesium diarylamides along with defluorinated cyclization intramolecularly (double-ortho C-F amination), yielding the corresponding DADHPs with perfect regio-control (Okamoto, 2003; Zhang, 2014; Lee, 2015; Theriot, 2016; Lim, 2017; Ramsey, 2017).
The synthesis of DADHP (41) by tandem intramolecular C − F amination using the amine substrate such as 2-fluoro-N-phenylaniline (40) in the presence of FeCl2 (5 mol%) as a catalyst at 100–120 °C for 12 h. The DADHP (41) synthesis was relaid on the improved process, which yielded DADHP derivatives in fair to good yields with perfect regio-selectivity. The reaction was well tolerated by various electron-withdrawing groups on the aromatic ring at different positions and also include phenazine core afforded good to excellent yield (61–91%). It is essential to know that the C-F (ortho) amination occurred regio-selectively when the fluoro group was present in the meta- and para-positions. (Scheme 14).
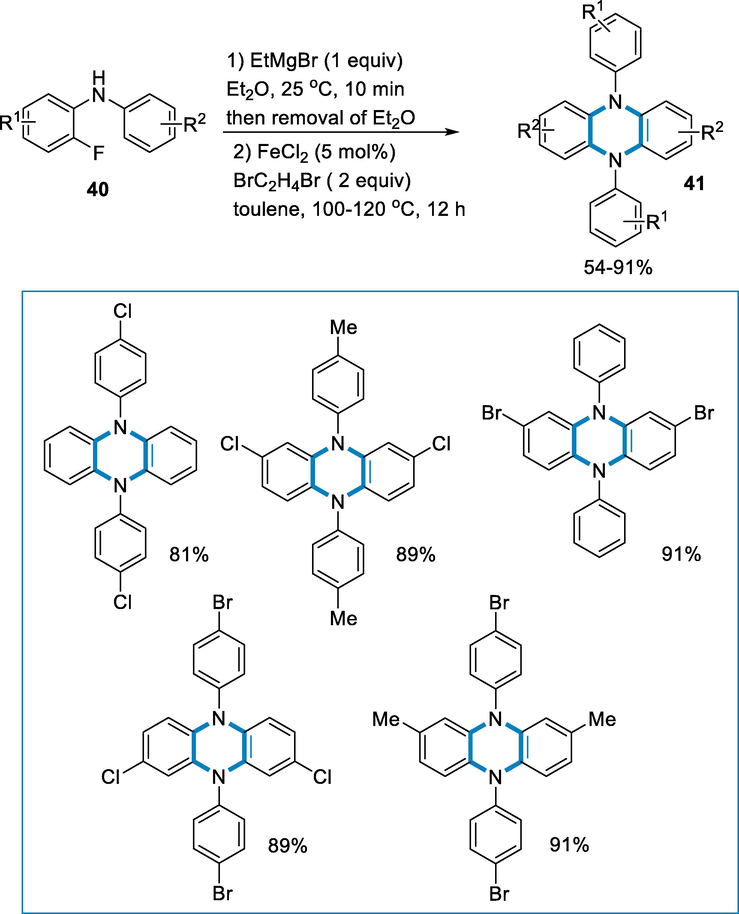
- Synthesis of DADHP by tandem intramolecular C − F amination.
The production of dinuclear tetra-amide iron complexes occurs before the creation of the C-N bond. The synthesis of magnesium amide dimer (42) was suggested by the substantial precipitation of MgBr2 in the solution of magnesium diarylamide with toluene. The cyclic four-membered iron diamide complex (43) has been formed via trans-metalation of FeCl2 with magnesium amide. The (C-F) amination occurs intermolecularly most likely through the route of SNAr to yield the iron amide complex containing the relevant o-phenylenediamine (45) which has been assisted by fluoro substituent coupling to the adjacent iron centre (44). The (C-F) amination of the benzene-1,2-diamineoccur intramolecular through the route of SNAr (46) yields the equivalent DADHP (41) in the second step. The fluoro groups improved the reactivity of SNAr reaction of the aryl ring when the substrate is 2,4-difluoro-N-phenylaniline was employed, allowing tandem C-F aminations without 1,2-dibromoethane and iron-catalyst, afforded in poor yield under the same reaction temperature and time. Although it deduces that it might oxidize the iron di-amide species to stimulate SNAr processes, the role of 1,2-dibromoethane has unknown (Scheme 15) (Aoki, 2019).
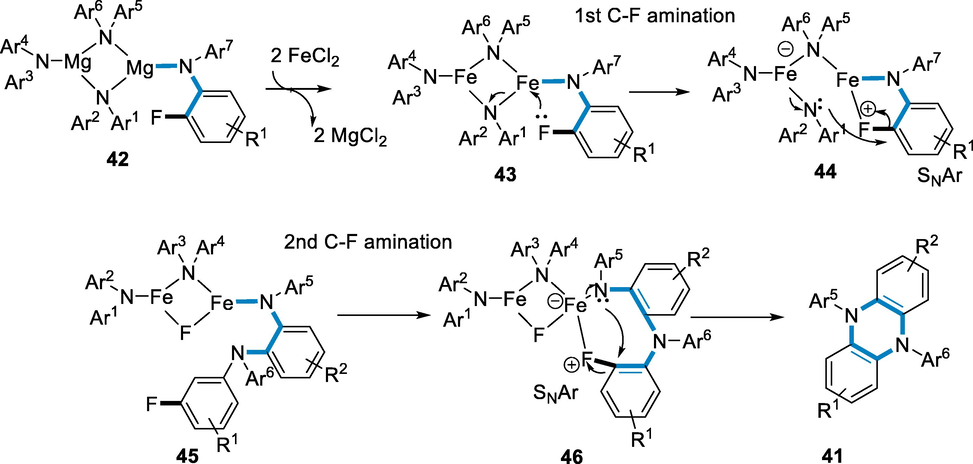
- Mechanistic pathway for the synthesis of DADHP.
2.5 From alkenes
Nitrogen-containing heterocycles of a six-membered ring may be adjacent in a variety of bioactive natural compounds, α-polyenyl-substituted piperidines being one of the most common. Micro-grewiapine A, Corydendramine A, and B and microcosamine A and B, are trisubstituted piperidines with the N- and polyenyl substituents differing only in nature (Vitaku et al., 2014; Pelletier and Joshi, 1991; O’Hagan, 2000).
The synthesis of cyclized product α-dienyl monosubstituted piperidines (48) was isolated in moderate yield from diene (47) as a substrate by using iron-catalyst such as FeCl3·6H2O (5 mol%) in CH3CN at room temperature for 17 h. Terminal diene was not restricted to cyclization, on C9-C10 double bond wide range of substituted dienes were tolerated (R1 = Me, R2 = H). When the CH3 group was replaced with a benzyl group, the yield in CH2Cl2 remained satisfactory (73%), but less in acetonitrile (58%). Functionalized α-dienyl-piperidines with an alkenyl tributylstannane or alkenyl iodide might be obtained by cyclizing the corresponding dienes. (Scheme 16) (Gonnard et al., 2017).
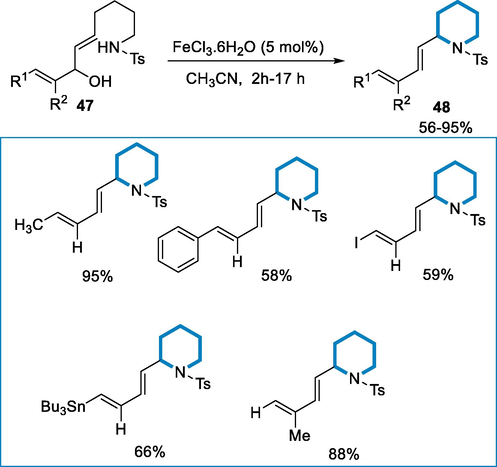
- Synthesis of α-dienyl-monosubstituted piperidines by using FeCl3·6H2O.
Piperidine derivatives are bioactive N-heterocycles synthesized by iron catalyst through heterocyclization in an environmentally friendly manner (Guerinot, 2010). The generalized reaction for the formation of piperidine derivatives (50) from aminoacetates (49) by using FeCl3·6H2O as a catalyst at room temperature for 30 min. The piperidine derivatives (50) were obtained in high amounts with great diastereoselectivity (dr = 97:3) in the preference of cis-isomer via cyclization of N-tosylamides with pentyl group having a linear side chain. For isopropyl substrates at C1, the outcome was good because only one diastereomer was produced in quantitative yield (dr > 99:1). The cyclization produced the cis-2,6-disubstituted piperidine (50) in a reasonable diastereomeric ratio (cis/trans;dr = 92:8) whenever a CH3 substituent with a low steric demand was introduced. A benzyl derivative was likewise tolerated, indicating that R1 group at C1 had no impact on the cyclization's result (Scheme 17) (Cornil, 2015).
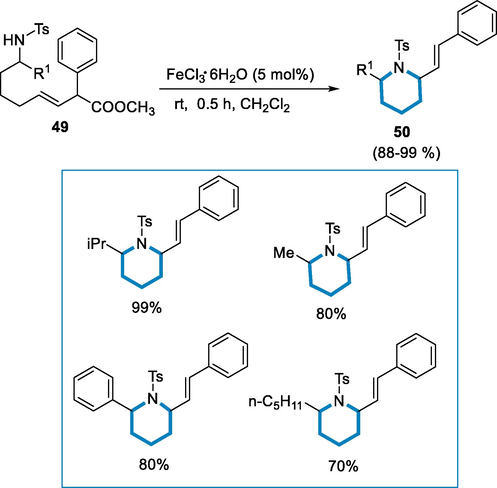
- Synthesis of 2,6-disubstituted-piperidine by using FeCl3·6H2O.
Azidoaryl(alkenes) is catalyzed by the iron complex through intramolecular C-H direct amination into the respective indoles by using mild reaction conditions and with moderate loading of catalyst. C-H direct activation is the difficult reaction in the catalysis of organometallic (Bauer and Knölker, 2015; Yang and Huang, 2015).
The reaction conditions were 2.5 mol% of TBA[Fe] and (azidoaryl)alkenes (51) as substrates in the presence of a solvent such as 1,2-dichloroethane for the synthesis of indole derivatives (52) at 100 °C for 1 h of microwave irradiation (200 W). Electron-donating and withdrawing (azidoaryl)alkenes (51) were reactive and modify into the desired indoles in high yields. Z- and E- (azidoaryl)alkenes configurations were uniformly reactive, and indole derivatives were isolated in good yields. Moreover, even tri-substituted olefins were reactive under these conditions (Scheme 18).
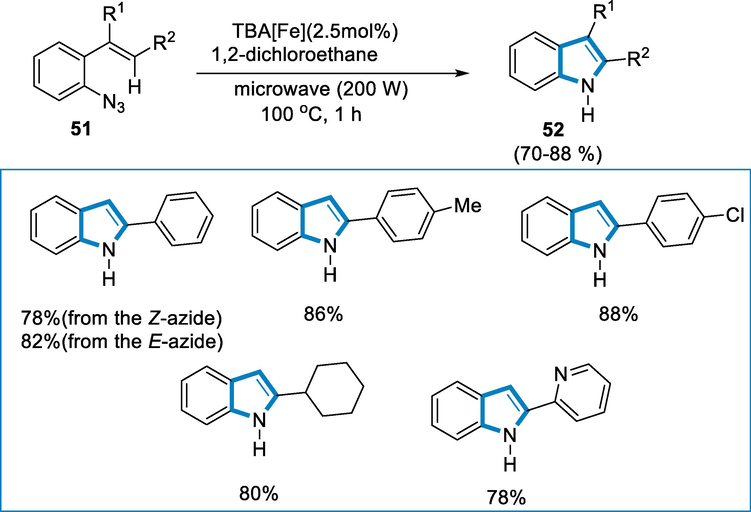
- Iron catalyzed synthesis of indole derivatives.
The aryl azide reacts with the[Fe(CO)3(NO)] anion (53) to form iron nitrene intermediate (55), with the release of N2, which generate a partial positive charge on the ortho C-atom, as shown by the intermediate (56). For the C-N bond-forming a reaction to give (57), charge transfer from metal to ligand occurs then by 1,5-hydrogen shift the transformation of indole (58) (Scheme 19) (Alt and Plietker, 2016).
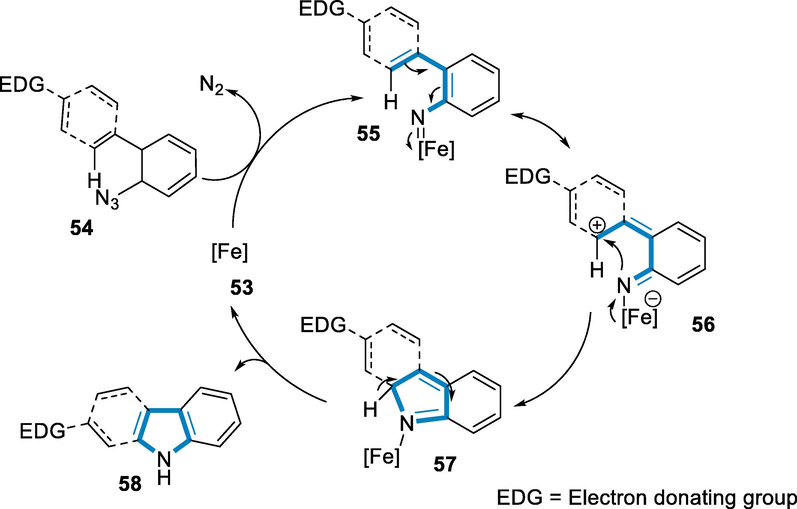
- Mechanistic pathway for the synthesis of indole derivatives.
Indoles and their derivatives have significant properties synthetically and biologically; as a result, they have attracted great attention toward synthetic approaches (Sundberg, 1996; Sharma et al., 2010; Andrea, 2018; Humphrey and Kuethe, 2006). Indoles are synthesized by intramolecular C-H iron-catalyzed amination and azidoacrylates as precursors (Liu et al., 2010; Bandini and Eichholzer, 2009).
The reaction conditions were azidoacrylate (59) as a substrate and Fe(OTf)2 as an iron-catalyst in the solvent THF at 80 °C temperature for 24 h for the synthesis of respective indoles (60). Azido acrylates (59) with electron-rich and poor groups (halo, benzyl, and trifluoromethyl groups) at the position of para reacted well to afford the respective indoles (60) in good yields. The catalysis was successful with substrates containing a variety of substituents, regardless of their position on the aryl ring. The presence of a methoxy substituent at ortho-position had no effect, while substrates containing para and meta disubstituted aromatic groups generated only one isomer in all circumstances and the yields were good (88–99% ) (Scheme 20) (Bonnamour and Bolm, 2011).
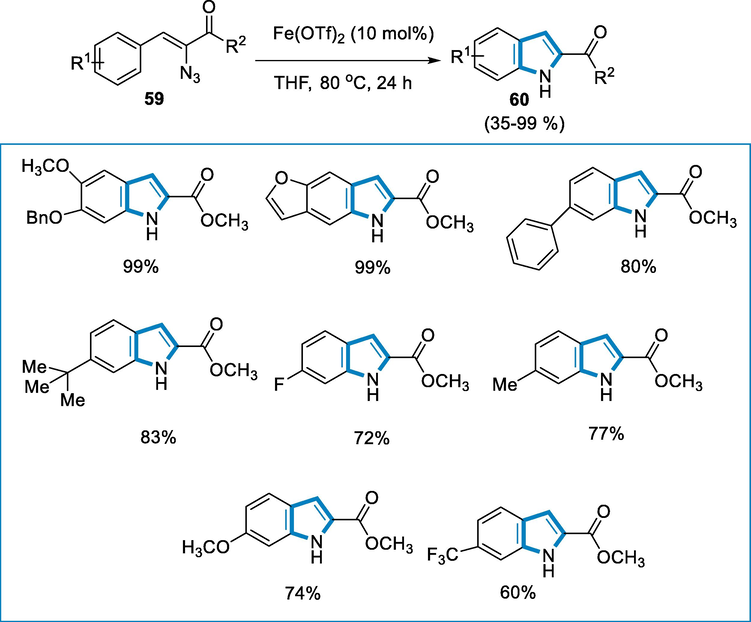
- Synthesis of indole derivatives by intramolecular C-H amination catalyzed by ferrous triflate.
Azaindoles are bioisosteres of indoles that have exceptional properties in the field of medicinal chemistry, drug discovery, and related fields of research (Smirnov, et al., 1997; Zhao and Wang, 2010). The azaindoles use as compared to the indoles is limited due to the dearth of appropriate synthetic methods. Roy and coworkers reported an iron-catalyzed amination for the formation of N-heterocycle in which tertrazoles have amide functionality with aliphatic bonds of C(sp3)-H (Das, 2020).
The reaction of tetrazole substrates (61) with the iron catalyst of 5.0 mol% Fe(TPP)Cl at the temperature of 80–130 °C for 12–24 h for the synthesis of respective azaindoles. The electron-rich and poor groups in the pyridine ring were well tolerated, and then rearrangement of reaction occurs to afford the complex N-heterocycles in high isolated yield (Scheme 21).
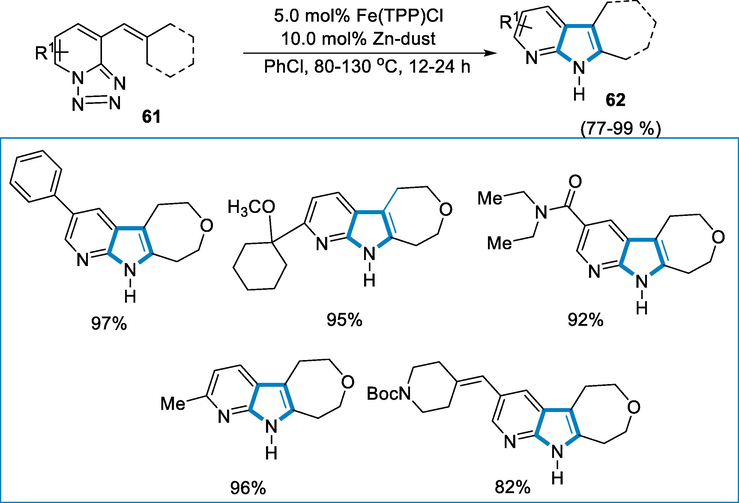
- Iron-catalyzed amination of tetrazole substrates.
The process has a typical radical activation mechanism in the first step Zn reduces FeIII(TPP)Cl by single-electron transfer for the formation of active catalytic species FeII(TPP) (63), which was confirmed by the red shift in the UV–visible spectra. In the next step, the N is lost to form α-FeIII-nitrene intermediate (a), when the substrate is coordinated to generate a metal-bound complex (64). The nitrene (a) then interacts with the pendant carbon double bond to form the benzylic pyridine radical (b). Following that, 1,2-migration happened to make intermediate (63), which after the shift of 1,3-H gave us the respective product (62) and allowed the catalytic cycle to regenerate (Scheme 22) (Roy, 2021).
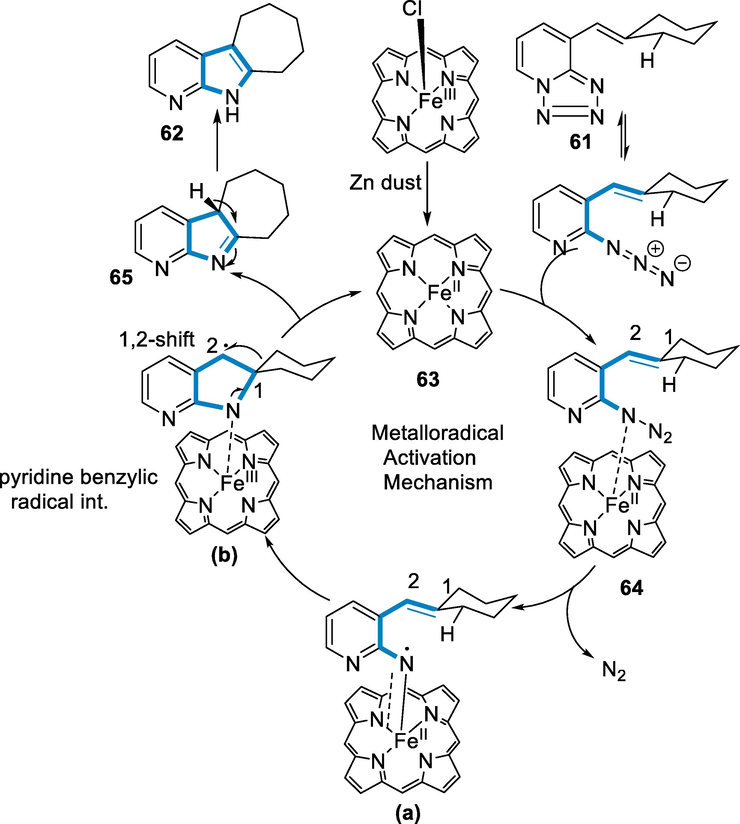
- Mechanistic pathway for the synthesis of azaindoles.
Alkaloids are naturally active pharmaceutical compounds that contain a nitrogen atom and are synthesized by nitrene insertion and transfer reactions. An iron catalyst such as [Fe(F20TPP)Cl] is an effective catalyst for the synthesis of alkaloids including indolines, tetrahydroquinolines, indoles, quinazolinones, and dihydroquinazolinones through intramolecular C–H(sp3and sp2) amination (Liu and Che, 2010).
Indoles (67) were synthesized by following reaction conditions such as aryl azide (66) as a substrate with an iron catalyst [Fe(F20TPP)Cl] (0.004 mmol) and 4A˚ molecular sieves in anhydrous ClCH2CH2Cl under nitrogen atmosphere. Indoles were produced in high yields from substituted methyl-α-azido-cinnamates, as well as ones with electron-deficient or rich groups. In the α-position of cinnamates, aryl nitrene was inserted and ortho-azido-cinnamates may also provide the respective indoles in fair to high yields by using iron catalysts such as [Fe(F20TPP)Cl] with complete azide consumption (Scheme 23).
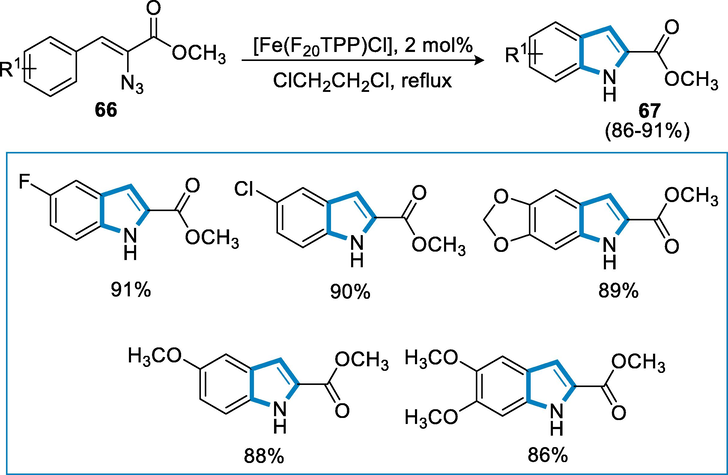
- Iron-catalyzed synthesis of indoles through C-H amination of azides.
The mechanism has been catalyzed by [Fe(F20TPP)Cl] of the amination of C(sp2)-H bonds that may contain iron-nitrene/imido complex has very similar to the mechanism proposed for dirhodium by Driver and co-workers. In the first step, Iron-nitrene/imido complex (69) has formed by the destruction of aromatic azide (66) catalysed by [Fe(F20TPP)Cl]. An intramolecular hydrogen atom abstraction mechanism might then produce a benzyl radical intermediate (70). The benzyl radical intermediate undergoes collapse for the generation of the C-N bond and collapse/rotation operations to provide a combination of trans- and cis-isomer (71) (trans: cis = 0.58:1) (Scheme 24) (Liu et al., 2010).
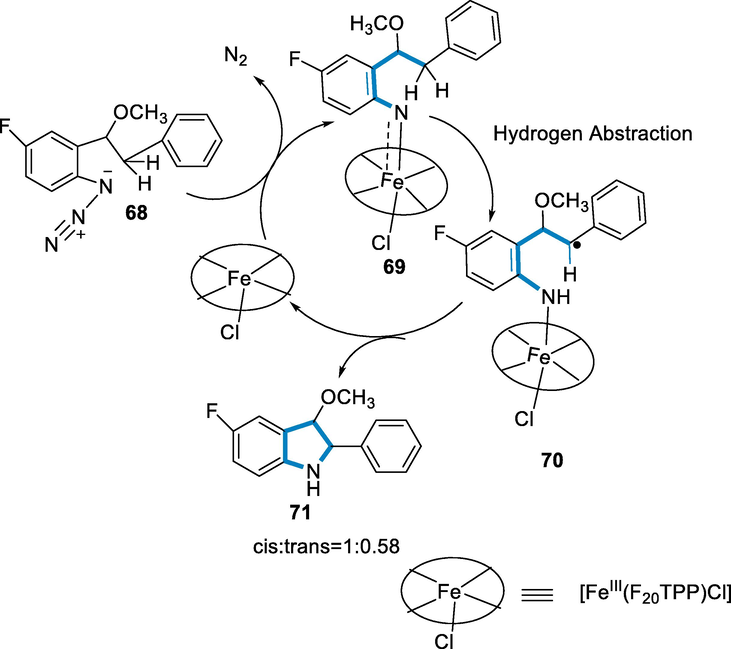
- Mechanistic pathway for the synthesis of indoles.
Indoles and secondary amines are preferential scaffolds of natural materials, medicines and dyes synthesized by ironII/(EtO)3SiH complex through the reductive alkylation via intramolecular addition of alkenes to N-H bonds of amine. The reaction is an effective method for constructing novel C-N bonds and manipulating (hetero)aromatic amines (Pirnot et al., 2016; Utsunomiya, 2003; Zhang et al., 2009; Nguyen et al., 2014; Shigehisa, 2014; Musacchio, 2017).
The reaction conditions for the synthesis of substituted indoles (73) were 0.5 mol% catalyst Fe2 = [Cp*Fe(1,2-Ph2PC6H4S)] loading to a relative substrate such as ortho-nitrostyrene (72) in the presence of (EtO)3SiH and THF at 60 °C for 6 h. Several derivatives of indole were synthesized in high yields and functional groups like OCH3, Br, F, CF3, and cyclic alkenes in the presence of nitro structures (six to eight-membered rings) were well tolerated under these conditions. The tricyclic fused indole derivatives were synthesized in high yield in 1,2-dimethoxy-ethane at 80 °C (Scheme 25) (Song, 2019).
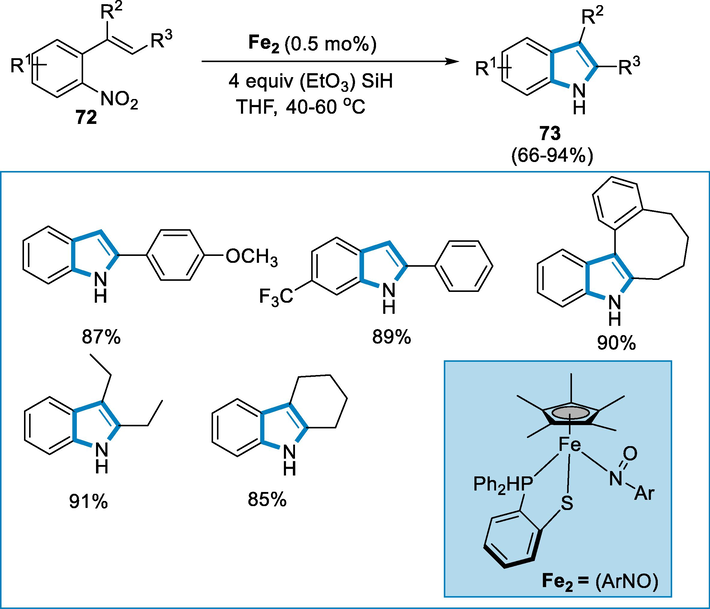
- Iron catalyzed synthesis of indole derivatives.
Pyrrolines are significant N-heterocyclic scaffolds in the area of bioactive chemicals and naturally present products, such as the biological and pharmacological uses of β-homoprolines. Pyrrolines are synthesized from γ,δ unsaturated oxime esters by the carbonylation of radicals and the intramolecular amination of alkenes (Vitaku et al., 2014; Fukuda, 2014; Lauder, 2017; Cordero, 2013). The method includes transition-iron catalyzed activation of oxime-esters (N-O) bond to form iminyl radicals, follows by intramolecular cyclization to generate radicals of new C-atom, which are then caught by C-O and converted into the desired products (Shimbayashi, 2018; Gu, 2019; Jiang, 2015; Jiang and Studer, 2017; Chen, 2015; Su, 2015).
The reaction conditions for the synthesis of pyrrolines (75) were oxime esters (74) as substrates and methanol with iron-catalyst such as Fe(acac)3 (5 mol%) in DCE as a solvent at 100 °C temperature for 20 h under CO (50 bar.). A wide range of aryl and heteroaryl oxime-esters (74) with both electron-deficient and rich substrates on the aryl ring in various places were tolerated to synthesized the respective products in excellent yields. Other substrates, such as thiophene and 2-naphthalene, as well as adamantine derivatives of O-benzoyl oximes, were also given desired products in good yield (Scheme 26) (Zhang, 2020).
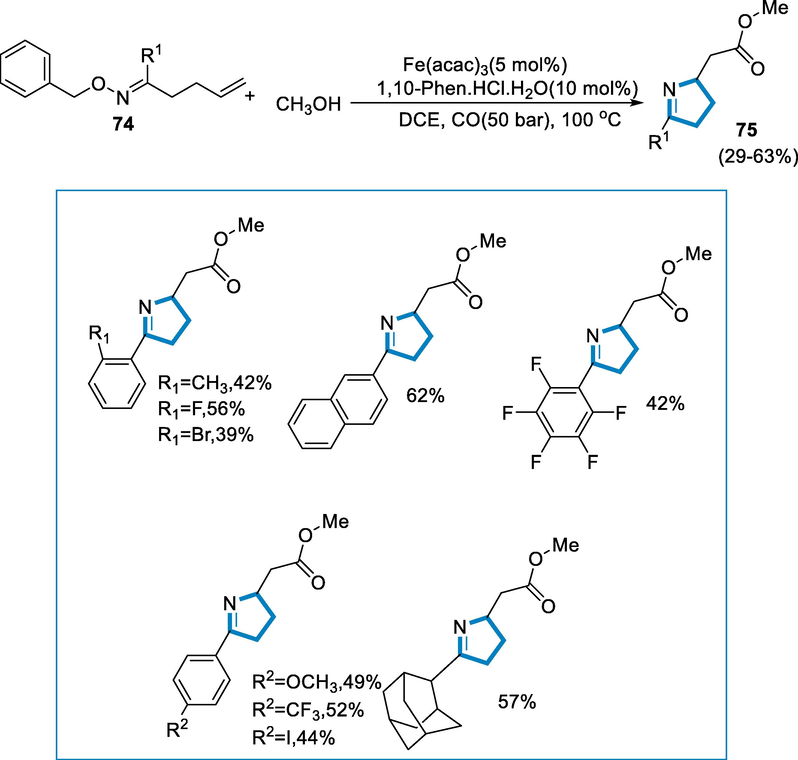
- Intramolecular amination of alkenes for the synthesis of pyrrolines.
2.6 Miscellaneous compounds
Indole moieties can be present in a wide range of bioactive natural compounds and pharmaceutics (Shvartsberg, 2009; Brancale and Silvestri, 2007). The 2,3-disubstituted indoles synthesis is developed by using a catalytic and general approach by employing aromatic of C-H amination through FeCl2 catalyzed opening ring of 2H-azirines. The procedure can also be used to make azaindoles (Prasanthi, 2018; Bach, 2015; Alt and Plietker, 2016).
The 2,3-disubstituted indoles (77) were formed by using 2H-azirine (76) as a substrate with the iron catalyst of 5 mol% FeCl2 at the temperature of 70 °C for 24 h in THF. The transformation is well tolerated in a variety of functional groups including amides, cyclopropyl, CF3, OTBS, halides, OPiv, and aryl substitution undergoes functionalization on the aromatic ring (Scheme 27).
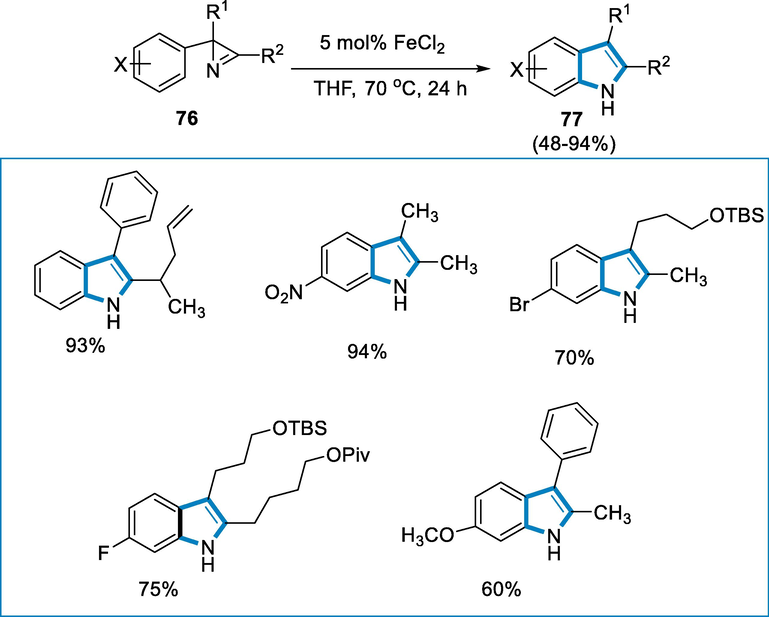
- Synthesis of 2,3-disubstituted indoles using FeCl2 as a catalyst.
Azirine Iron-complex (78) is formed by the immediate integration of FeII to the N-atom of imine 2H-azirine (76); subsequent dissociation of the C-N bond forms vinyl-imene iron-complex (79); and as a result, corresponding indole (77) are generated by a 5-centred 6-π-electrocyclization of (79) through intermediates (80). The rearrangement was most likely assisted by nitrene iron-complex (79) (Scheme 28) (Jana, 2010).
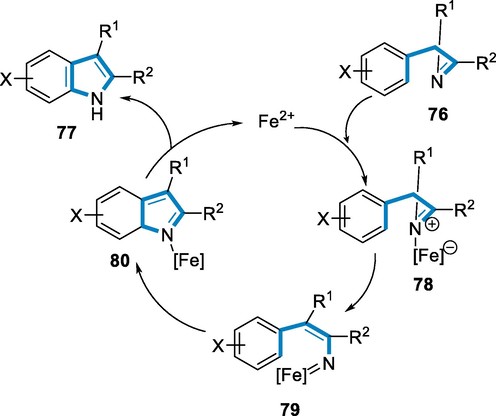
- Mechanistic pathway for the synthesis of 2,3-disubstituted indoles.
The intramolecular amination of unactivated C(sp3)-H bonds catalyzes by iron nucleophilic complex such as Bu4N[Fe(CO)3(NO)] (TBA[Fe]) through the activation of aryl azides to afford indoline derivatives. The amination of C(sp3)-H bonds catalyzed by two possible strategies are; C-H-aminations involving intense oxidants to create nitrenoid species or the “ oxidant-free ” procedure, which depends on azides as N-donor reagents (Shin et al., 2015; Yu and Shi, 2010; Louillat and Patureau, 2014; Jiao et al., 2016; Lee, 2017).
Treating o-tert-butyl aryl azide (81) with iron-complex Bu4N[Fe(CO)3(NO)] (TBA[Fe]) (2.0 mol-%) at 0.5 mmol scales in dry DMF and DCE using microwave irradiation for 1 h at 120 °C for the formation of indoline derivatives (82). Due to the instability of some of the products Boc2O was added subsequently. Electron-withdrawing groups show improved reactivity by changing the substitution pattern proximal to the reactive C-H bond and the substitution within the aryl azide substrates had some effect on the reaction conditions, although isolated yields were moderate to excellent irrespective of the substituent's electronic nature (Scheme 29).
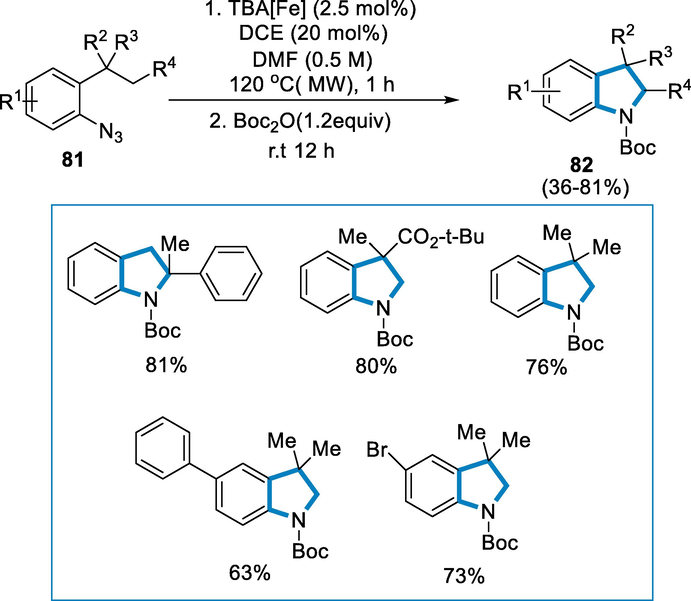
- Activation of aryl azides for the synthesis of substituted indoline.
The amination of C-H bond intramolecularly explained by a complementary proton transfer as the C-H bond activation and increase in the C-H acidity favoured a preferred form of 6-membered rings. The intramolecular amination of C(sp3)-H bonds in azido arylalkanes to the respective indoline derivatives has been catalyzed by the iron nucleophilic complex Bu4N[Fe(CO)3(NO)]. The catalyst may be made in a single step from low-cost Fe(CO)5 on a large scale, making the technique extremely appealing from a synthetic perspective (Scheme 30) (Alt et al., 2017).
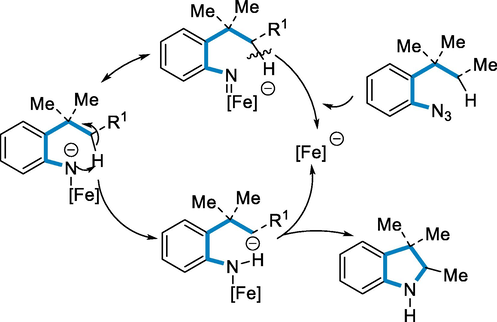
- Mechanistic pathway for the synthesis of substituted indoline.
Indoline substituents are preferred scaffolds of pharmaceuticals, natural products, and physiologically active compounds that can significantly alter a molecule's chemical, physical, and biological characteristics (Richter, 2017). Although indoline and 7-azaindoline derivatives are key elements for the development of physiologically active structures their preparation techniques differ (Chen et al., 2016). The formation of inert C(sp3)-H bonds in organic compounds is significant because it improves chemical synthesis efficiency (Dick and Sanford, 2006; Davies and Manning, 2008; Brückl, 2012; Abrams et al., 2018). Current functionalized C–H bond approaches usually need substrate pre-oxidation, directing group changes, and the use of strong oxidants, resulting in de-novo synthesis with limited generality (Lyons and Sanford, 2010; Labinger and Bercaw, 2002; Ryabov, 1990).
For the synthesis of azaindoline derivatives (84) substrate (83) was formed after the cross-coupling reaction of 8-Bromo-tetrazole and either in the presence of alkene or alkyne followed by the hydrogenation and the reaction conditions were 5.0 mol% Fe-cat. in the10.0 mol% Zn-dust and C6H6 at 120–150 °C temperature for 72 h. The azaindoline derivatives (84) were produced in high yields by intramolecular aminations of the substrates with strong bonds of C–H and also with different bond dissociation energies. A substrate with benzylic, inert alkyl, and homo-benzylic C–H linkages promote amination at the inert and strong C–H bonds, affording only one isomer (Scheme 31).
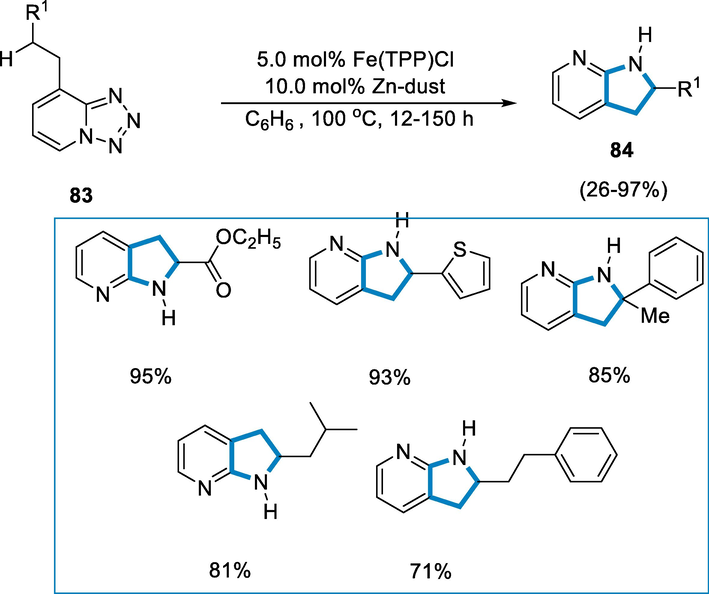
- Intramolecular amination of C-H bond for the synthesis of azaindoline derivatives.
The FeII(TPP) (85) active catalytic species are produced when FeIII(TPP)Cl has been reduced by Zn through a single-electron transfer. Tetrazole (83) then forms a metal-bound complex (86) with the catalyst, which generates the radical intermediate (87) when the N2 gas has been lost. The bond of C–H broke intramolecularly from the intermediate (87), which has the amination rate-limiting step, producing the intermediate (88). As a result, the intermediate (88) creates the aminated product (84) by the substitution of radical and catalytic cycle recovery (Scheme 32) (Das, 2020).
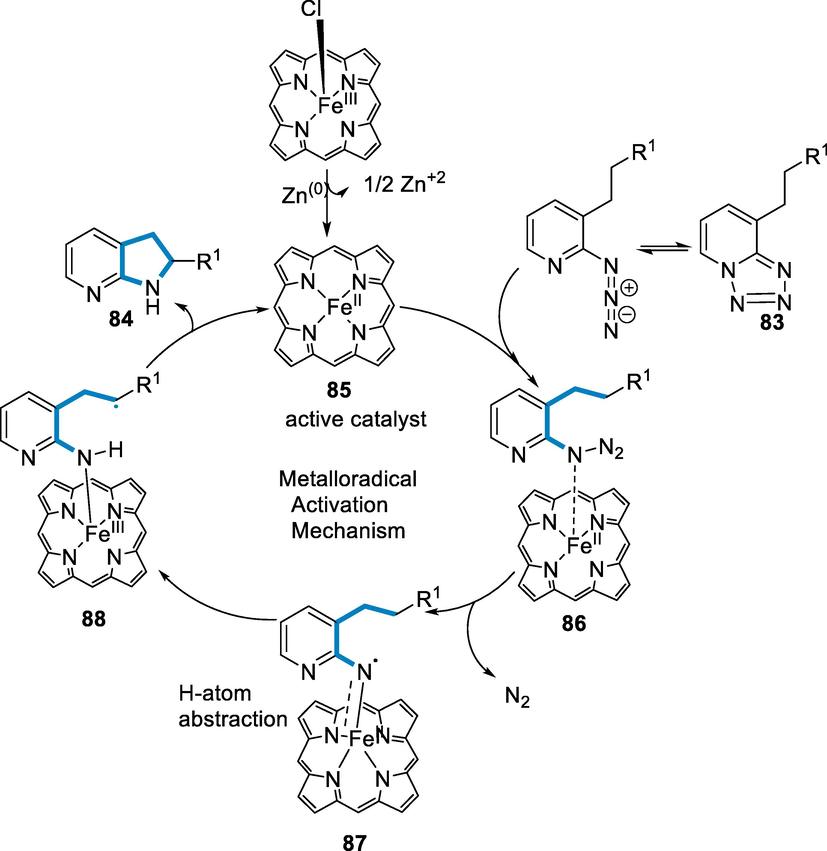
- Mechanistic pathway for the synthesis of azaindoline derivatives.
Cyclic amines such as N-heterocycles are significant elements in the formation of naturally occurring physiological active compounds, pharmacological moieties, and substances. In present chemical synthesis, the ability to manipulate traditionally non-reactive functional groupings is essential. The formation of an iron-dipyrrinato catalyst that enhances C–H aliphatic direct amination by using the reactivity of an iron-borne metal–ligand with multiple bonds. When organic azides are exposed to an iron-dipyrrinato catalyst, it generates products of cyclic amine with complex patterns in core substitution (Labinger and Bercaw, 2002; Zalatan and Du Bois, 2009).
The reaction conditions for the formation of pyrrolidines (90) were a wide range of aliphatic azide derivatives (89) in the presence of catalyst Fe3 (20 mol%) with Boc2O, benzene, and 65 °C temperature for 12 h. When substrates with allylic, tertiary or benzylic, C–H linkages were exposed to Fe3 (10 mol%), the appropriate Boc-protected pyrrolidine (90) was extracted in excellent yield. It was also feasible to catalytically functionalize a 2 °C–H bond, which afforded 1-Boc-2-ethyl pyrrolidine at a less yield. The poor yield was due to the generation of undesirable linear by-products that compete with the cyclization at 2 °C–H bond. Exposure of tertiary and benzylic alcohols to trimethylsilylazide and boron trifluoride diethyl etherate, was immediately transformed to the appropriate azide (Scheme 33).
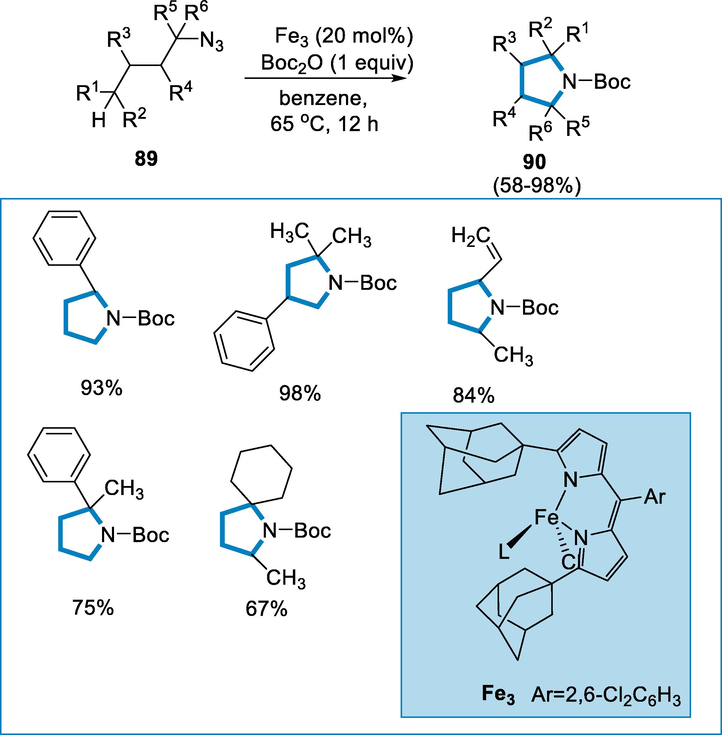
- Synthesis of pyrrolidines from aliphatic azides using an iron catalyst.
The three-step process of functionalization for the C–H bond involves (a) the alkyl azide (89) substrate oxidizing the iron-catalyst from FeII to FeIII, (b) (path A) for the generation of Fe-III amide and an alkyl radical hydrogen atom abstraction occur intramolecularly (c) (path A’) recombination of radical to synthesized the respective N-heterocycle (90). The carbon-hydrogen bond insertion directly by the intermediate of radical FeIII-imido couldn’t be eliminated (path B). Both processes involve the coordination of substrate carbon–hydrogen bond to the active iron-imido radical. The imido-radical has been most likely found in the plane formed by the dipyrrin ligand and the iron, with significant pyrrolide-adamantyl derivatives on each side. The substrate of carbon-hydrogen bond must reach the imido-radical next to the ligand of chloride to achieve this configuration. Functionalized C–H bond has fast when this orientation has been obtained. For benzylic substrates, the incoherent intramolecular kinetic isotope effect implies a sequential process (path A). When the functionalization of stronger substrate carbon-hydrogen bonds occurred, the mechanism of reaction may switch to a (path B) direct insertion pathway. The high spin Fe-imido radical for the synthesis method of inactivated and activated aliphatic substrates of carbon-hydrogen bond with the oxidative potency of the transiently generated. This iron-based cyclization of linear azides allows for quick entrance into complicated N-heterocyclic products (90) from easily available substrates, which hasn't been possible with azide photolysis or traditional Hoffmann-Löffler Freytag methods. The techniques discussed here may be used to create various saturated, cyclic compounds (Scheme 34) (Hennessy and Betley, 2013).
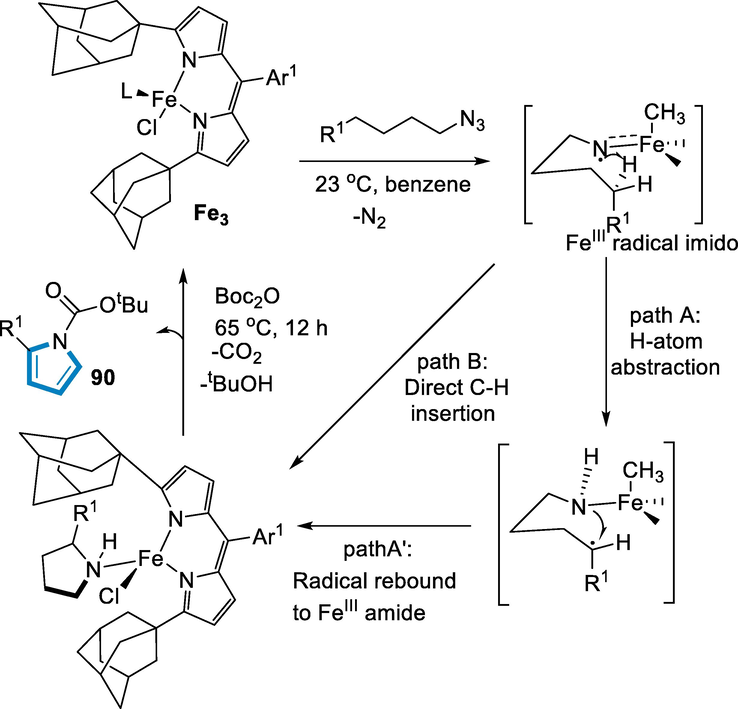
- Mechanistic pathway for the synthesis of pyrrolidines.
Tetrahydropyrrolizines are produced via the cycloaddition reaction by using an iron-catalyst of disubstituted alkenes with alkene-tethered oxime esters, which is a common structural motif in bicyclic alkaloids. The process is carried out through a series of cycloaddition reactions. Intra and Intermolecular cyclization is occurred respectively with a disubstituted-alkene in a regioselective manner, with the initial synthesis of an imine moiety playing a key role (Robertson and Stevens, 2014; Robertson and Stevens, 2017).
The reaction conditions for the formation of tetrahydro pyrrolizines (93) were oxime ester (91) with a variety of disubstituted-alkenes (92) in the presence of FeSO4·7H2O (0.03 mmol, 5 mol%) as a catalyst, and L4= (DTBPY) 4,4′-di-tert-butyl-2,2′-bipyridyl as a ligand with C6H6 at the 120 °C temperature for 12 h. The oxime-ester (91) reacted with several 1,2-disubstituted alkenes (92) containing both electron-rich and poor groups on the aromatic group at the β-position resulting in high yields of the appropriate product and arenes with deficient electrons also afforded better yield. Not only ketones but also nitrile might be useful reaction partners, resulting in high yields of tetrahydro pyrrolizines (93) (Scheme 35).
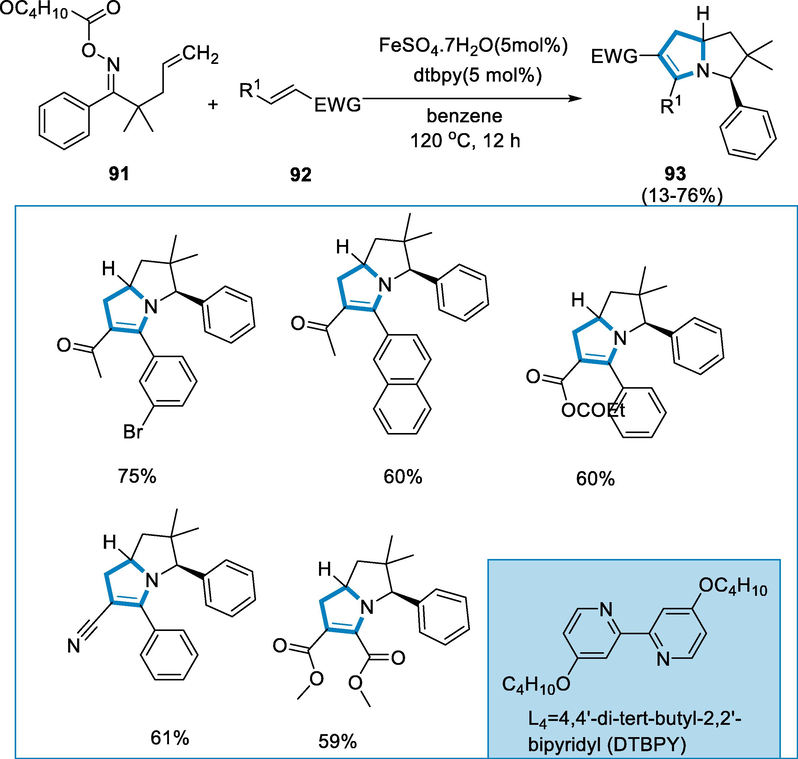
- Iron-catalyzed cycloaddition reaction for the synthesis of tetrahydropyrrolizines.
In a feasible process, iron cleaves the N-O bond of (91) to generate intermediate (95), which then undergoes 5-Exo cyclization to give (96). Intermediate (96) and the equivalent iron adduct (97) may be in equilibrium. Then, with alkene (92), a region-selective radical addition occurs, producing (98). Intermediate (98) has a single electron oxidized by Fe to generate the cation (99), which then is ionically cyclized to form bicyclic iminium (101). Alternately, cyclization of radical with an imine substituent to produce (100) before oxidation to get (101) has another possibility. The process with electron-poor alkenes reinforces this. In the catalytic system, both mechanisms compete. The ylide of azomethine intermediate (102), which may have a conventional resonance structure (103′), has then deprotonated by pivalate. Finally, at the carbanion at C(5) (104) protonated followed by deprotonation on the acyl-group at the α-position completes the process (Scheme 36) (Shimbayashi, 2018).
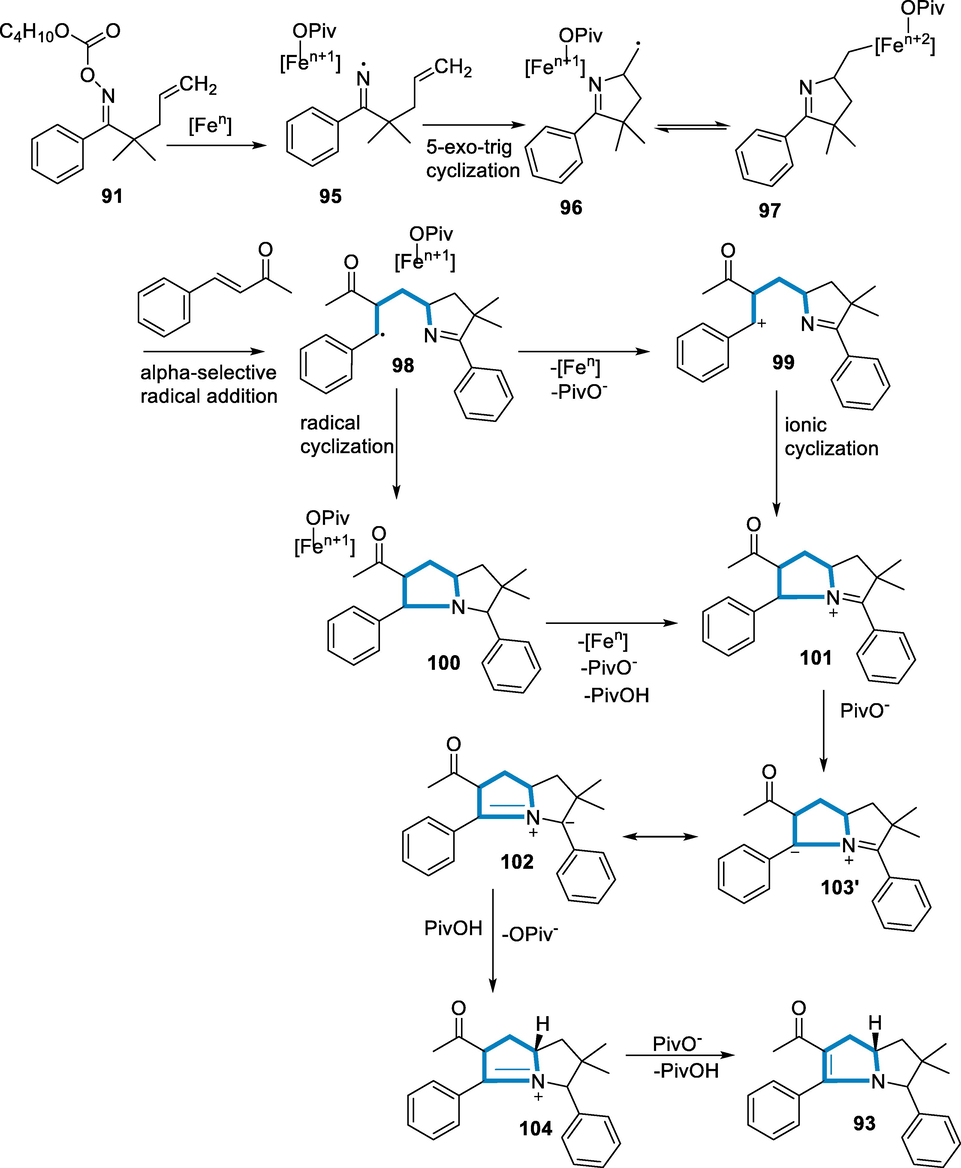
- Mechanistic pathway for the synthesis of tetrahydropyrrolizines.
Derivatives of Purine have various biological functions, and the core purine is employed as a key element to address a wide range of biological molecules. Derivatives of 1,3-bis(4-methoxybenzyl) pyrido[1,2-e] Purine-2,4(1H,3H)-diones were synthesized via direct amination using iron-catalyst to process on readily available 5-(pyridine-2-ylamino) pyrimidine-2,4(1H,3H)-diones and oxygen (O2) might be utilized as an oxidase in the reaction. The approach has, chemo-selectivity and the functional group affinity towards halo groups enable post functionalized annulated ring (Legraverend and Grierson, 2006; Federico and Spalluto, 2012; Baraldi, 2011; Hollinshead, 2012; Blanchard, 2012; Shi, 2012).
The reaction conditions for the derivatives of 1,3-bis(4-methoxybenzyl)-pyrido[1,2-e] purin-2,4(1H,3H)-diones (106) were 5-(pyridine-2-ylamino) pyrimidine-2,4(1H,3H)-diones (105) as a substrate with the catalyst (FeCl2·4H2O (15–40 mol%) in the DMSO under O2 atmosphere (balloon) at the temperature of 120 °C for 24–48 h. The corresponding derivatives of 1,3-bis(4-methoxybenzyl)-pyrido[1,2-e] purin-2,4(1H,3H)-diones (106) were produced in fair to moderate yields and has functional group compatible over a various functional groups (Br, Cl, COOMe, C2H5, CH3, Ph). However, there was no steric impact owing to substituents at the difficult C-3 position (Me, Et, Ph) (Scheme 37).
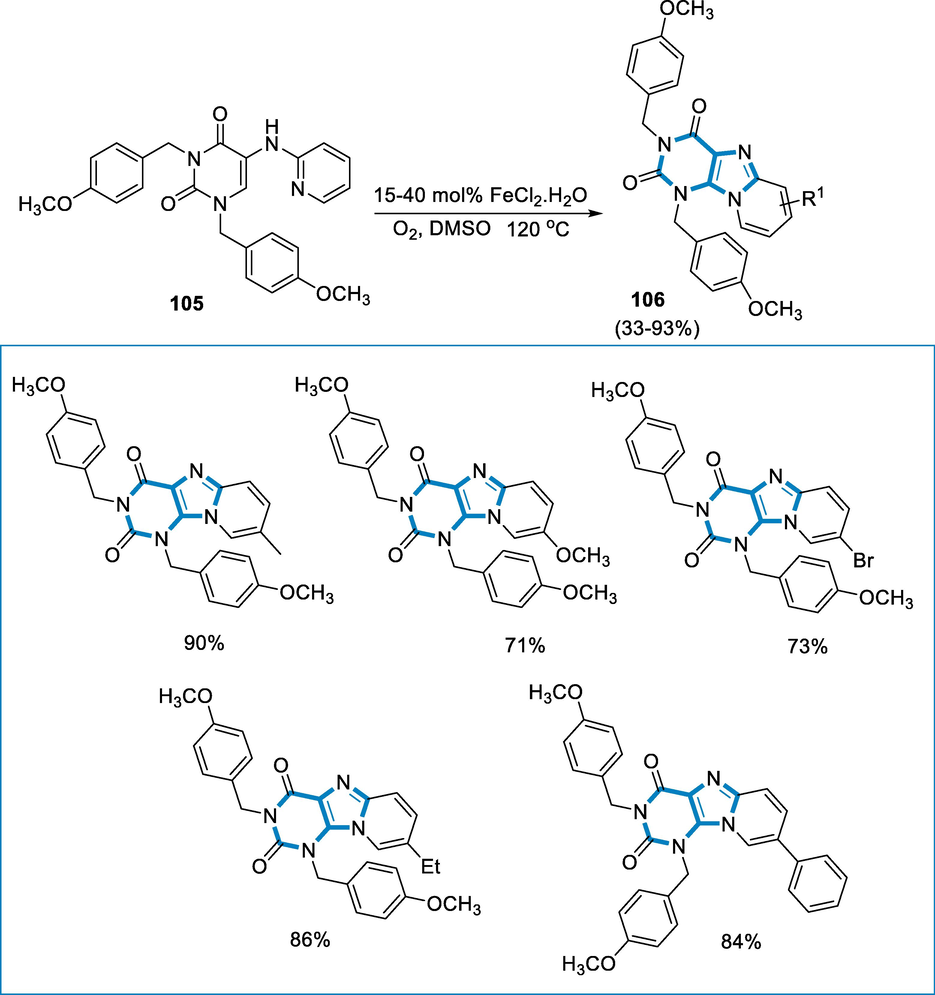
- Purine derivatives synthesized by using iron as a catalyst.
Because the intermediate radical (107) and cation (108) have become unstable by resonance in the ring of pyridine, in this case, a radical mechanism would also be consistent with the more difficult reaction seen with electron-withdrawing groups as substituents. The difficulty in the closure of the ring affected between C5- and C3-substituted moieties with a hetero-atom (OCH3 and Br) may have been related to hydrogen bonding intramolecularly that prevents radical production. As a result, the electronic effects of carbon-3 and 5 substituents should be comparable. Alternatively, in the instance of C3 substitution, a non-planar orientation that only has an inductive impact resulting in destabilization (steric-suppression of resonance) might explain these findings (Scheme 38) (Maes et al., 2013).
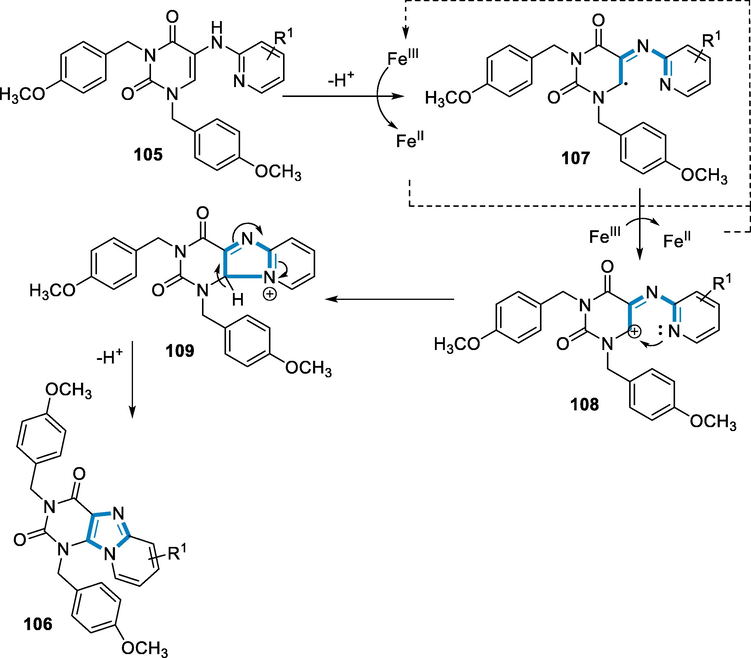
- Mechanistic pathway for the synthesis of purine derivatives.
N-heterocycles of benzothiazoles are important moieties in pharmaceutical industries. The formation of C-S bonds through oxidative direct intramolecular via functionalized carbon-hydrogen bond is an adorable method for the formation of benzothiazole. Pyridine had a critical role in the high yields and selectivities, according to preliminary mechanistic analyses (Li et al., 2007; Li et al., 2008; Wen, 2010; Li, 2009; Song, 2009; Yoshikai, 2009; Massari, 2010; Wang, 2012; Aiello, 2008).
N-aryl alkyl thioamides (110) employed as the substrate, under optimization conditions such as oxidant Na2S2O8 in DMSO with (10 mol%) the iron catalyst FeCl3 in the presence of pyridine gives the good yield of benzothiazoles (111). In good to high yields (71–92%) of N-aryl-tert-pentane thioamide with both electron-rich and poor substituents could accomplish C–S/C–H activation bond synthesized easily. When R was NHPh, a yield of 50% was achieved (Scheme 39).
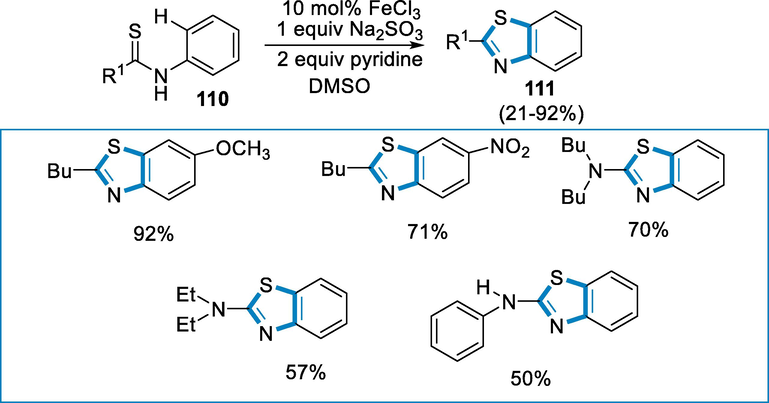
- Iron catalyzed synthesis of benzothiazole.
Through the process, I, FeIII oxidized the substrate N-phenyl benzothioamide (110) and subtraction of electron and proton to synthesize the intermediate of thionyl-radical, while FeIII has been reduced to FeII. Na2S2O8 reoxidized FeII species to regenerate FeIII. The product 2-phenyl benzothiazole was then obtained by the process of cyclization with the intermediate of thionyl-radical by the process of oxidation with Na2S2O8 (Scheme 40) (Wang, 2012).
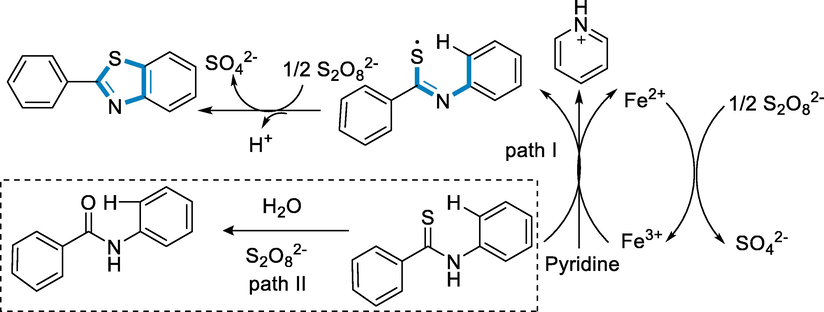
- Mechanistic pathway for the synthesis of benzothiazole.
[FeIII(TF4DMAP)Cl] may effectively catalyse alkyl-azides amination of the C(sp3)-H bond intramolecularly. The reactions have good regio- and chemo-selectivity with a variety of substrate scope and are also worthwhile for the complexes of late-stage functionalization which are natural/bioactive compounds with low catalyst loadings as 1 mol%. Iron complexes are more economical and biocompatible than other transition-metal catalysts, and they frequently have distinctive catalytic activity in nitrene-insertion processes of azides (Wang and Deng, 2018; Iovan and Betley, 2016; Hennessy and Betley, 2013; Liu and Che, 2010; Che et al., 2011; Che, 2011; Zhang and Deng, 2012; Iovan, 2017; Shing, 2018; Paradine and White, 2012).
Fe4 = [FeIII(TDCPP)-(IMe)2]I may effectively catalyze amination of carbon-hydrogen bond intramolecularly of a wide range of alkyl-azides (112). After all, the reaction needed a 10 mol% catalyst loading to provide good substrate conversion and product yields (113). By using 3 mol% [Fe(TF4DMAP)Cl], various alkyl azides (112) suffered C-H amination intramolecularly in 45–90% isolated yields (Scheme 41) (Du, 2019).
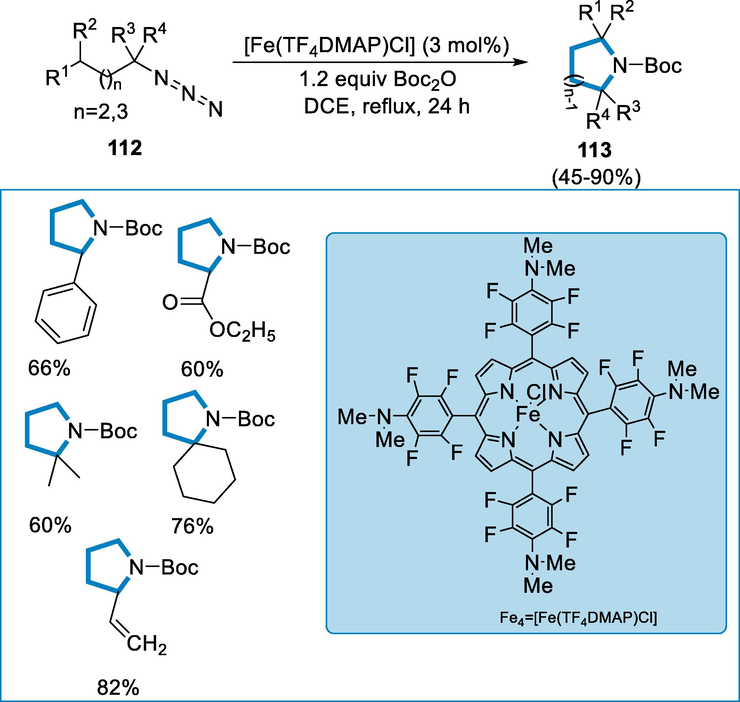
- Intramolecular C(sp3)-H amination of alkyl azides.
3 Intermolecular cyclization
3.1 From internal alkynes
Transition-iron-catalysis is one of the most efficient techniques for constructing pyridine cores via cycloaddition [2 + 2 + 2] process of alkynes and nitrile. In comparison to other late transition metals, iron is considered an affordable, innocuous, largely non-toxic, and ubiquitous metal. Iron catalysis may successfully stimulate a variety of reactions (Bolm, 2004; Vollhardt, 1984; B nnemann, H., , 1985; Varela and Saá, 2003; Chopade and Louie, 2006; Shaaban et al., 2011; Tanaka, 2012).
Diyne (120) and cyanamide (121) were substrates that react to give 2-aminopyridine (122) and the reaction conditions were FeI2 as a catalyst in the presence of Zn, THF, and dppp, at rt. for 24 h and use of naphthalene as internal standard. Dialkylcyanamides (121) reacted efficiently with malonate-diyne (120) to afford desired products (122) in fair to moderate yields (Scheme 42) (Wang, 2013).
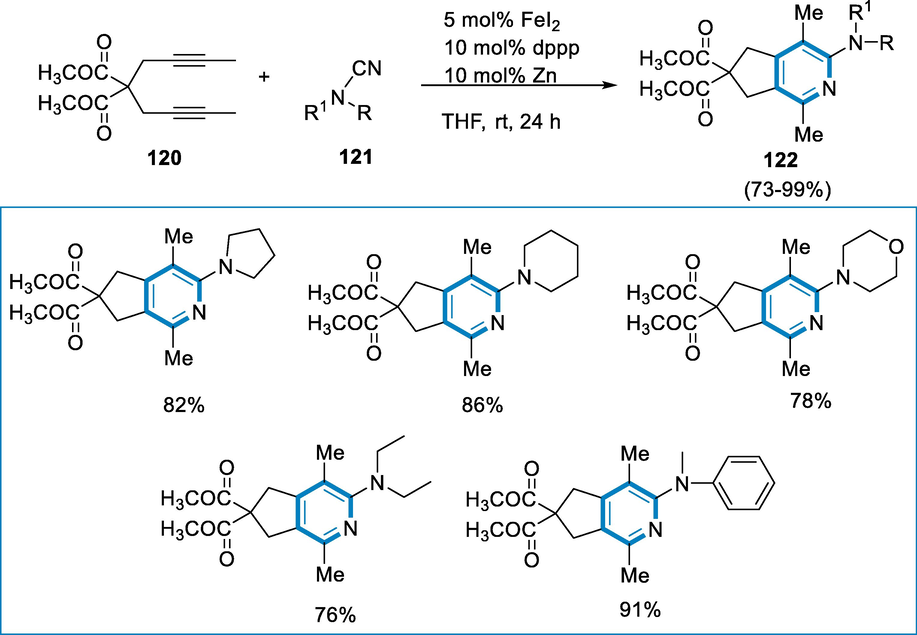
- Synthesis of 2-aminopyridine by using FeI2 as a catalyst.
Naturally, bioactive products, medicines, agrochemicals, and functional materials all include fused pyridines. They are also extremely valuable as bases, synthetic intermediates, and ligands, in chemical synthesis. As a result, much effort has gone into developing efficient ways for the formation of pyridine-fused scaffolds. Alkenes and alkyne-oximes as starting materials for an iron-catalyzed radical relay protocol provide a variety of structurally significant cyclopenta-fused pyridines, a useful substrate found in important compounds, in fair to good yields with good tolerance of functional groups. Prefatory biological research revealed that several of the pyridines have potent anti-inflammatory properties (Henry and De, , 2004; Hill, 2010).
Oxime (123) and N-benzyl maleimide (124) react under the reaction conditions such as Fe(acac)3 and PhCO2Na after stirring in 1,4-dioxane at 80 °C for 12 h, fused pyridines (125) are synthesized in excellent yields. The reaction tolerated both meta and para substituents well, however, the yield was reduced when an ortho methyl group was present. In the conversions, heterocycle-containing oximes showed excellent reactivity. Oxacyclopenta afforded fused-pyridine in good yield when the oxygen atom of oxime at the β-position interacted with N-benzyl maleimide (124) (Scheme 43) (Du, 2020).
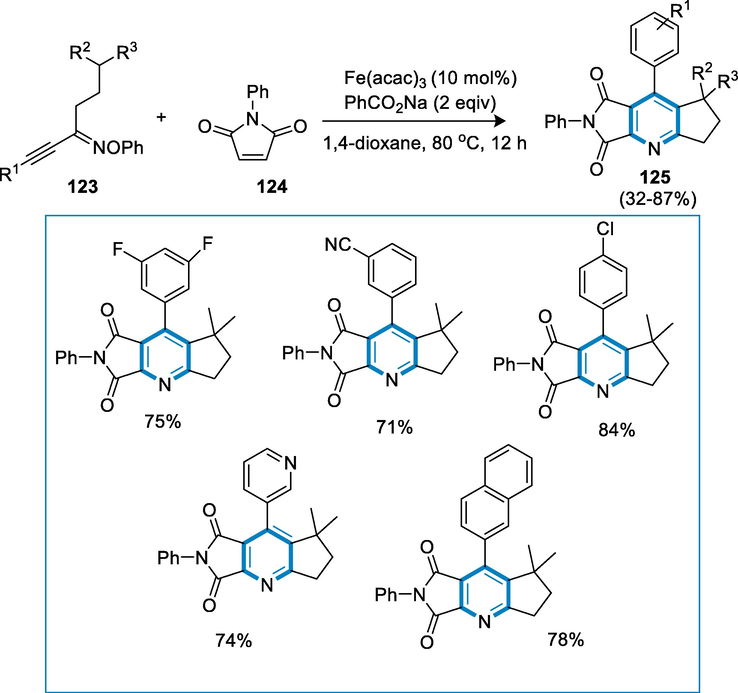
- Iron catalyzed synthesis of pyridine derivatives.
Iron is a desirable catalyst source because of its availability and inexpensive. The iron complexes promote a variety of catalytic reactions. The cycloaddition of alkyne and alkynes-nitriles is catalyzed by the Fe(OAc)2 with electron-rich groups and sterically hindered pyridyl-bisimine ligand. Several pyridine derivatives are produced in affordable yield.
The optimized reaction conditions for the synthesis of pyridine (128) were 10 mol% iron catalyst Fe(OAc)2, bisimine (L5), alkyne nitrile (126), and alkyne (127) in DMF, rather than DMA, at 85 °C. The Fe(OAc)2/L5-catalyzed cycloaddition of alkynes (127) and alkyne nitriles (126) produce moderate isolated yields of corresponding pyridine structures (128) (Scheme 44) (D’Souza et al., 2011).
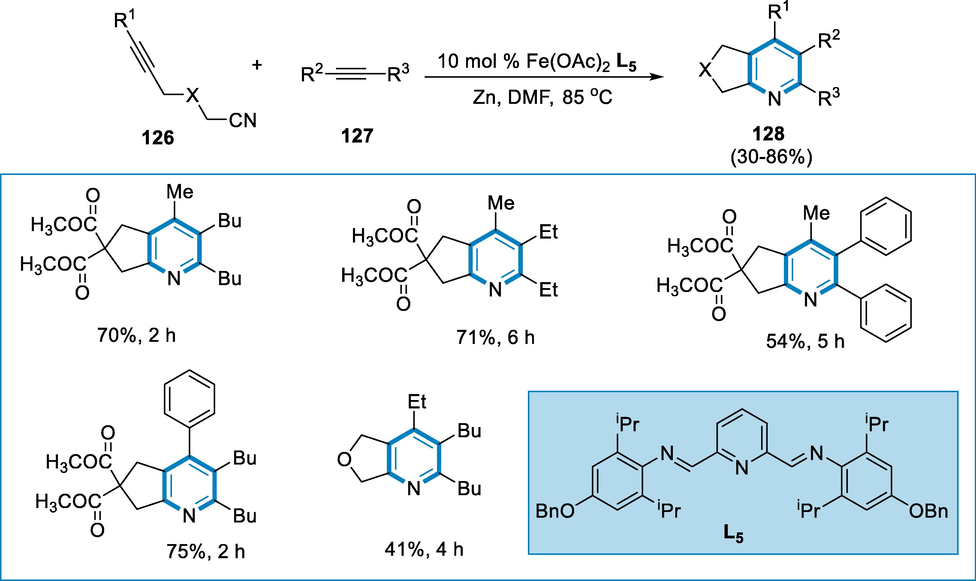
- Synthesis of substituted pyridine by using iron as catalyst.
The imidazo[1,2-α]-pyridine is an important nitrogen compound since it has so many biological functions (Joule et al., 2020). The imidazo[1,2-α]-pyridine structures are found in therapeutically prescribed medications such as zolpidem, zolimidine, alpidem, necopidem, olprinone, and saripidem. There has been established effective intermolecular amino oxygenation of 2-aminopyridines by using iron-catalyst with a variety of ynals. Due to its cost-effectiveness, non-toxicity, quick accessibility, stability, extraordinary reactivity, and environmental friendliness, iron salts are established to be a plausible replacement for other metal catalysts, and significant development has been achieved (Shi, 2012; Maes et al., 2013; Li et al., 2007; Li et al., 2008).
The synthesis of 3-aroylimidazo[1,2-α]-pyridines (130) from 2-pyridinyl amine (128) and benzyl propiolaldehyde (129) under the reaction conditions such as FeCl3 (5 mol%) as an iron-catalyst with toluene and air at 60 °C for 18 h. The 2-pyridinyl amine (128) substrates tolerate electron-deficient and rich groups on the pyridine ring which present the respective products in moderate to high yields. A nitro group-containing substrate could also provide an adequate yield. The conditions were tolerant to a variety of aromatic ring substituents. The substituent at the ortho position of the aromatic ring affected the yield (Scheme 45).
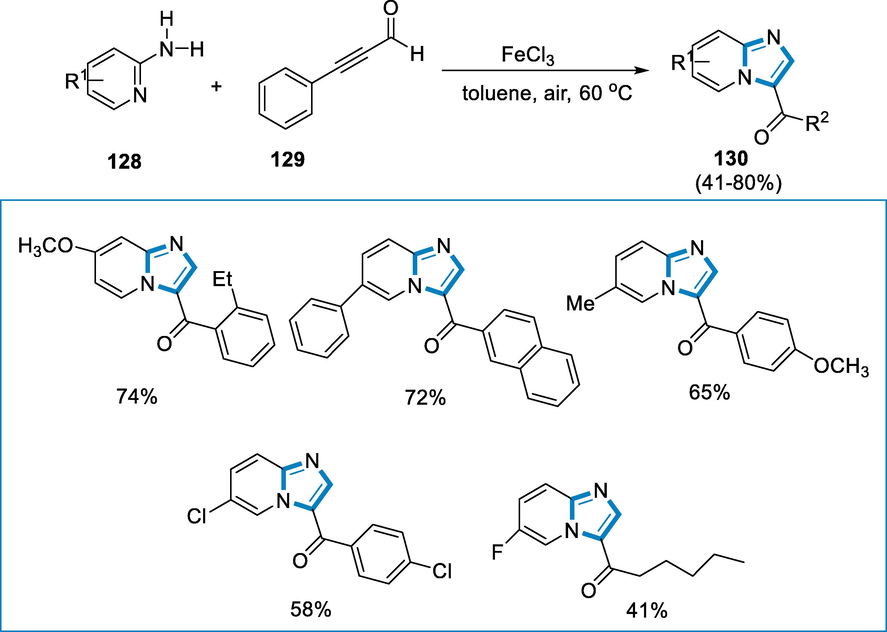
- Iron-catalyzed intermolecular amino oxygenation of 2-pyridinylamines.
Imine intermediate (131) was produced from an aldehyde by a condensation process with a 1° amine in the mechanism by using an iron-catalyst for the formation of 3-aroylimidazo[1,2-α]-pyridines (130) from 2-pyridinyl amines (128) and benzyl propiolaldehyde (129). The iron complex has then covalently linked to the ring of pyridine and the triple(sp) bond to form an intermediate (132). Following that, an intramolecular cyclization method has been used to make carbene intermediate (133). Finally, carbene oxidation with oxygen produced the corresponding product 3-aroylimidazo[1,2-α]-pyridine (130) (Scheme 46) (Bilal, 2021).
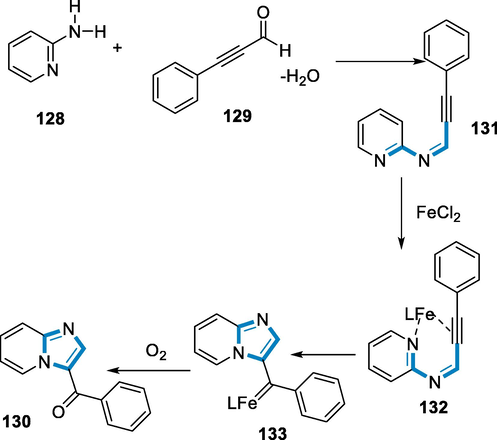
- Mechanistic pathway for the synthesis of pyridine derivatives.
2,3,4-trisubstituted and 2,3-disubstituted quinolines are effectively syntheses from substituted imines and electron-withdrawing alkynes in excellent yields using FeCl3 promoted carbon–carbon bond formation. Iron plays a catalytic role in this reaction which is numerous on Earth and chemically stable, is progressively appealing for its environmentally benign nature and potential efficiency equivalent to that of other transition metals in a variety of domains, including C-H activation, C-H oxidation, cycloaddition, and Fe-porphyrin chemistry (Bolm, 2004; Sun et al., 2011; Möller, 2010; Ohara, 2001; Norinder, 2008; Kadish et al., 2010).
The reaction conditions were imine (134) and alkyne (135) as substrates in the presence of FeCl3 (0.5 mmol), and dichloroethane as a solvent at the temperature of 80 °C with the blowing of air into the system for 5 h affording quinoline (136) in good yields. The amine moieties with electron-donating groups interacted efficiently with alkyne (135), producing excellent yields of quinolone (136). The addition of a halogen group to the amine or aldehyde substrate was particularly well tolerated by the reaction. Despite this, the reaction was severely suppressed by the nitro group. The fact that aliphatic aldimines generated from paraformaldehyde and aniline or other aliphatic aldehydes had decreased selectivity and produced indistinguishable compositions (Scheme 47).
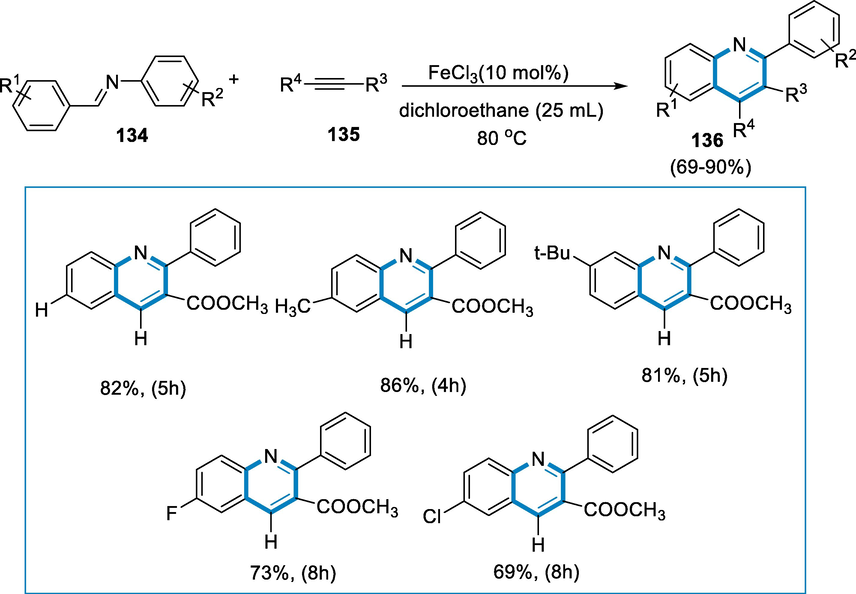
- Iron catalyzed synthesis of substituted quinolones.
The production of an alkenyl carbocation (137) by iron-catalyst FeCl3 as a Lewis acid alkynoate attacking which has been the initial step. Following that, an aromatic electrophilic substitution takes place between the alkenyl cations (137) and (134), resulting in the intermediate (138), which has a Friedel-Crafts reaction. Then, with the assistance of the chloride ion, (138) has activated by a proton to raise the carbon electro positivity of imine (136), which encourages the cyclization to form (140) with the release of FeCl3. The formation of the intermediate (138) and subsequent cyclization has a cooperative process. Following the oxidation of (140) with FeCl3, (136) and FeCl2 have been produced. For the completion of the catalytic cycle, oxidized FeCl2 has been produced by FeCl3. Furthermore, starting with aniline, benzaldehyde, and (135) with FeCl3, a standard three-component method for the transformation was investigated. The catalyst and (135) have been added to the mixture of in-situ after the condensation between aniline and benzaldehyde to get the corresponding quinoline (136) in excellent yields. This method has effective for assembling 2,3-substituted quinolones (136) from aromatic amines, aldehydes, and electron-poor alkynes in a systematic way (Scheme 48) (Li, 2014).
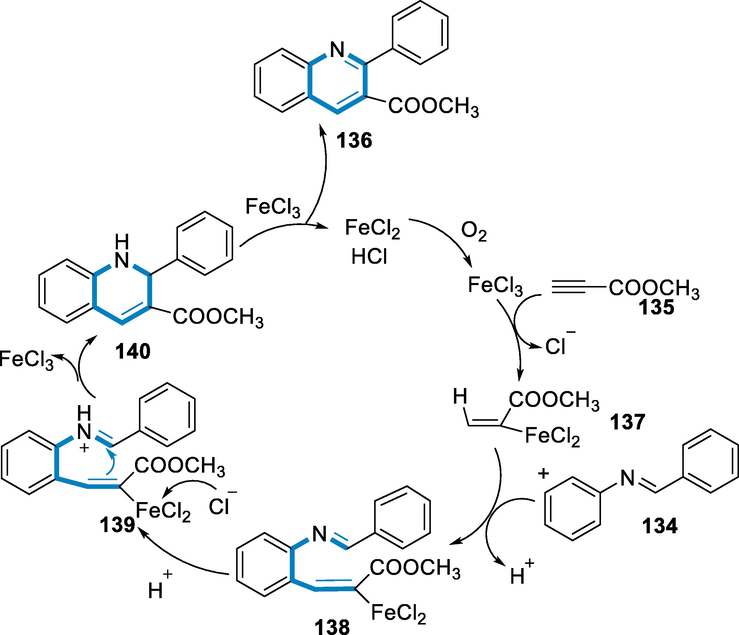
- Mechanistic pathway for the synthesis of substituted quinolones.
The quinoline nucleus is a common structural scaffold in a wide range of natural and synthetic substances with essential biological and pharmacological properties and as a result, effective synthetic methods for highly functionalized quinoline derivatives have been established. (Michael, 2004; Ito, 2004; Grougnet, 2005; Chen, 2004; Butenschön et al., 2001). FeCl3 catalyzed with no ligand or additive, organic transformations of terminal alkynes, primary amines, and aldehydes yielded derivatives of 2,4-disubstituted-quinoline in excellent yields with great atom economy (Cao, 2009). The process would probably go via tandem oxidation/cyclization/addition/ condensation/ reactions, with water only as a side product.
The reaction conditions were alkyne (140), aldehyde (141), and amine (142) with the iron-catalyst FeCl3 (16 mg, 0.01 mmol) in the solvent 1,2-dichloroethane (DCE) at 120 °C for 12 h under an air atmosphere for the formation of quinoline derivatives (143). The quinoline derivatives (143) were obtained in good yields from derivatives of benzaldehyde with both electron-rich and poor groups. The substrates for this simultaneous conversion were p-toluidine, p-anisidine, p-chloroaniline, and o-toluidine which afforded good yields of the appropriate products. Substituted phenylacetylenes, such as p-chloro phenylacetylene, p-methyl phenylacetylene, diphenylacetylene, and p-fluoro phenylacetylene, were also afforded to be appropriate moieties for this cyclization, and necessary products were produced in moderate yields. When aliphatic aldehyde (isobutyraldehyde) was used in the process, the desired product was afforded in less quantity (Scheme 49).
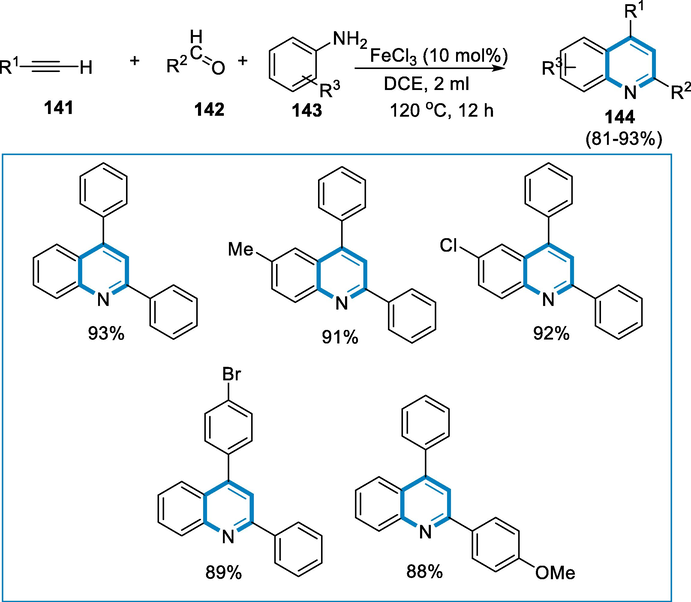
- Iron-catalyzed three-component reaction for the synthesis of quinoline derivatives.
A probable mechanism of the three-component iron-catalyzed one-pot sequential transformation of aldehyde, amine, and alkyne has been proposed: The FeCl3 catalyst presumably has one terminal-alkynyl group due to the un-reactivity of the FeIII alkynyl-complex. To create propargylamine, the synthesized alkynyl-complex was nucleophilically added to an imine generated in-situ from 1° amine and aldehyde. The nucleophilic intramolecular assault by the nitrogen-containing substituted benzyl ring linked to the nitrogen might be promoted by FeCl3 as Lewis acid activating the triple bond of propargylamine. The FeIII vinyl-complex was then decomposed to provide an intermediate of dihydroquinoline and recycled Fe-catalyst for future reactions. The produced dihydroquinoline might be further oxidized by O2 with atmospheric oxygen to yield the quinoline product (136) (Scheme 50) (Zhang et al., 2011).
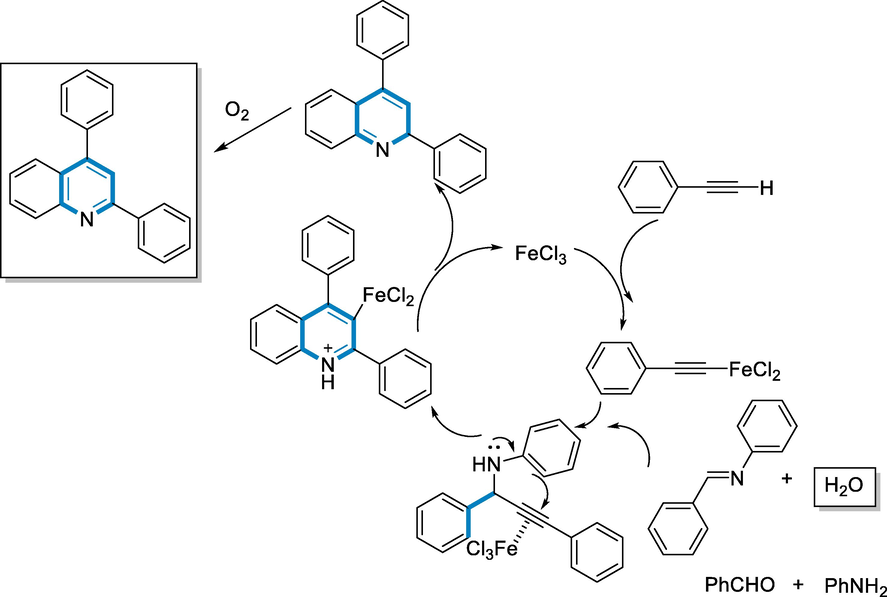
- Mechanistic pathway for synthesis of quinoline derivatives.
3.2 From terminal alkynes
Formation of quinoxalines utilizing a variety of terminal alkyne and O-phenylenediamine derivatives, in the presence of Fe3O4@Cu2O.Fe3O4@Cu2O-rGO nanocomposite demonstrates not only increased catalytic activity but also outstanding regenerate functionality. This is owing to its favourable features, such as high surface area, porous volume, distinctive semi-exposed structure, and increased mass transfer.
The reactions were synthesized in sealed tubes using o-phenylenediamines (141), aromatic alkynes (142), 1 mol% of Fe3O4@Cu2O-rGO, and DMAP in each solution at the temperature of 70℃ for 8 h to provide corresponding quinoxalines (143) product in isolated yields. The compounds were produced in good yields from 3,4-Diaminoisole dihydrochloride and 2,4-diamino toluene with an electron-rich group and by using 4-Bromo-1,2-diaminobenzene with an electron-withdrawing group. Fe3O4@Cu2O-rGO nanocomposite demonstrated greater product yields in all alkynes employed for evaluation, only one isomer was produced when OCH3, CH3, and Br groups were replaced in the substrates (Scheme 51) (Wang, 2015).
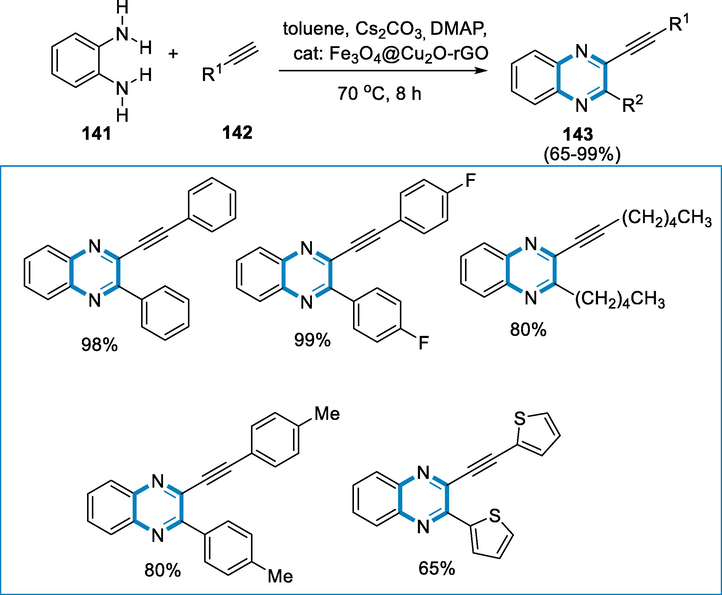
- Iron catalyzed synthesis of quinoxalines.
The antimicrobial, anti-inflammatory, antibacterial, and analgesic capabilities of the ring system of quinoline may be found in a wide range of medications. They also serve as valuable ligands and substrates in organic reactions. The formation of quinolone-3-carbonyl is described using an effective cascade Michael iron-catalyzed addition–cyclization of amino aromatic ortho-compounds such as O-aminoaryl ketones, O-amino benzyl alcohols, and O-amino aryl aldehydes with ynones. Using the catalyst of IronIIIchloride-hexahydrate in the air, the reactions undergo to generate quinolone-3-carbonyl derivatives without or with a substituent at the carbon-4 position in moderate to excellent yield (Michael, 2005; Roma, et al., 2000; Chen, 2001; Kleeman, et al., 2001; PB, V.B.N.A.P., Monga V. Jain R. Kaur S. Singh pp. Bioorg. , 2004; Nakatani et al., 2000; Nakatani et al., 2001; Nguyen, 1998; Shih, 2006).
The reactions undergo the following conditions for the formation of quinolone-3-carbonyl derivatives (146) were 2-amino benzaldehyde (144) with 1-phenylprop-2-yn-1-one (145) using 20 mol% of a catalyst such as FeCl3·6H2O in 1,4-dioxane at 80 °C in the presence of air for 4 h. The quinolone (146) was obtained in excellent yields by reacting ynone with an electron-rich (para-CH3) and (-p-OMe) aromatic group with 2-amino benzaldehyde (144) (Scheme 52) (Li, 2011).
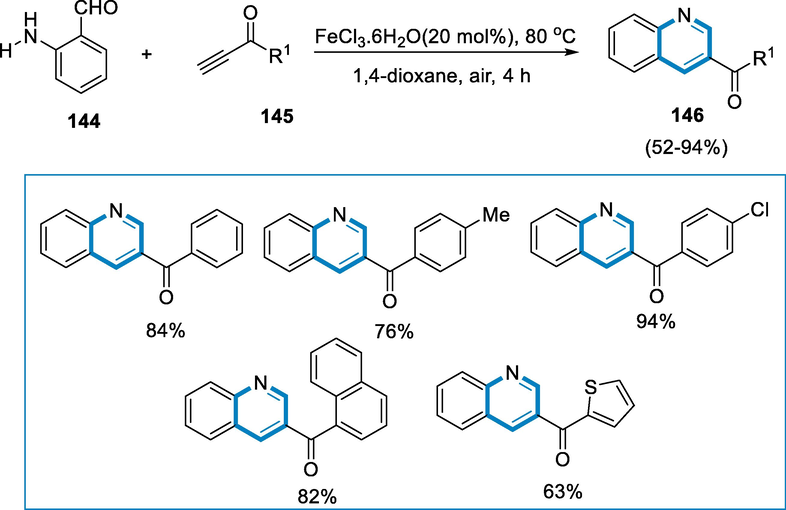
- Cascade Michael iron-catalyzed addition–cyclization of o-amino aromatic compounds.
Quinolines and their substituted structures are present in a vast variety of naturally occurring products and have a diverse range of biological functions. Many synthetic compounds with pharmacological characteristics use the quinoline skeleton as a helpful synthesis intermediate. Furthermore, these chemicals are very well ligands for making OLED phosphorescent complexes. The formation of quinoline derivatives from alkenes or alkynes and N-alkyl-anilines using oxidative iron-catalyzed coupling processes afforded a wide range of quinolones derivatives ranging from moderate to high yield (Balasubramanian and Keay, 1996; Musiol, 2010; Korivi and Cheng, 2006; Kim, 2005).
The reaction of N-alkyl aniline (147) with phenylacetylene (148) by FeCl3 iron salts (10 mol%) with the oxidant di-tert-butyl-peroxide in the solvent of ethylene dichloride at 80 °C which was carried out in a Schlenk reaction tube for the synthesis of a variety of quinolone (149). A variety of N-alkyl-anilines (147) and derivatives of ethyne (148) was evaluated under the conventional reactions conditions in a synthetic technique for substituted quinolones (149) employing oxidative coupling reactions, and afforded a range from good to high yield. The presence of bulky moieties at para-position had no steric influence on the transformation of N-alkyl aniline (147) with different alkyne derivatives, including electron-poor or rich moieties on the aromatic ring of the alkynes (Scheme 53) (Liu, 2012).
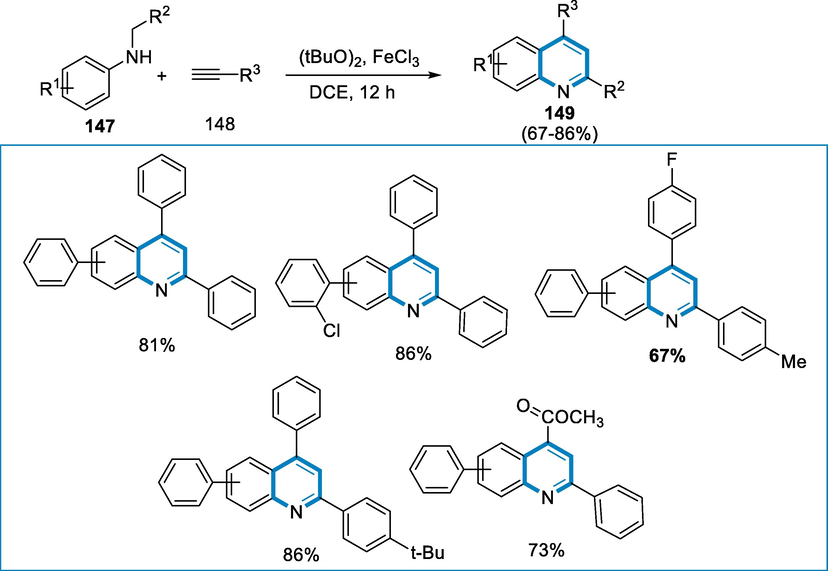
- Iron-catalyzed oxidative coupling for the synthesis of quinoline derivatives.
Quinolines and their derivatives are present in various physiologically active natural compounds, as well as being essential raw materials in the chemicals and medicinal industries (Sawada, 2004; Strekowski, l., 2003). The three-component coupling catalyzed by FeCl3 of amines, alkynes, and aldehydes yields quinolines simply and cost-effectively. From inexpensive and commonly accessible starting materials, a sequence of 2,4-disubstituted quinolines has been produced (Bolm, 2004; Saberi, 2013; Clark, 2014).
The reaction conditions were aldehyde (150), amine (151), alkyne (152) as a substrate, and FeCl3 (0.1 mmol) as a catalyst with toluene at 110 °C, under an air atmosphere the desired quinoline (153) was afforded in good yields. The aldehyde (150) substrate scope was first explored using phenylacetylene and aniline as model substrates. When the aryl aldehyde had an electron-rich or poor substituent, the reactions performed easily, affording fair to good yields of the quinolones (153). Both p-toluidine and p-methoxyaniline were preferred derivatives for this reaction, and the corresponding products were afforded moderate yields. The reaction conditions were also applicable to halogen-containing anilines, yielding the desirable quinolones (153) in good to high yield. The alkynes such as substituted phenylacetylenes, heteroaromatic alkynes, and aliphatic alkynes, were all excellent substrates for this conversion, with reasonable to good yield (Scheme 54).
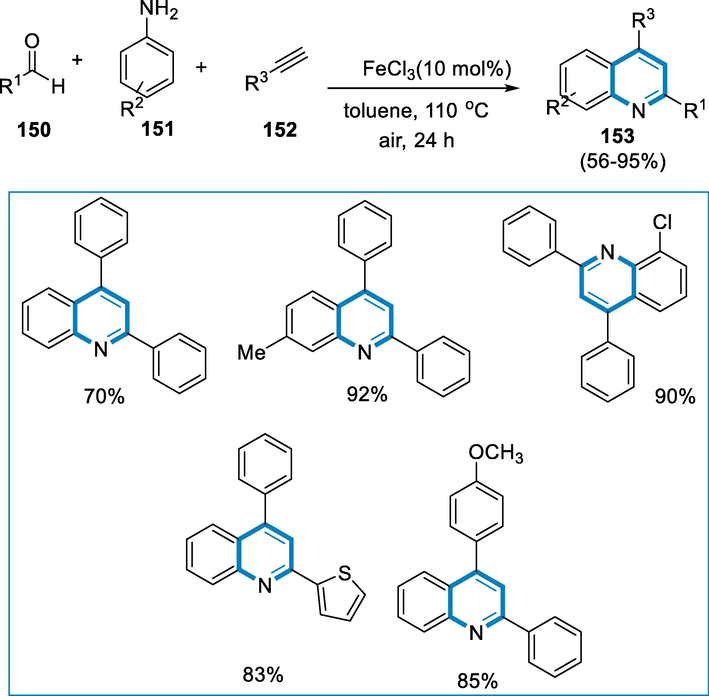
- Three-component coupling by FeCl3-catalyzed for the synthesis of quinolines.
Intermediate (154) has been created by coupling imine and alkyne to Fe(III) in situ and then adding alkyne to imine to make propargylamine intermediate (155), which then undergoes intramolecular hydroarylation of alkyne to form dihydroquinoline intermediate (156). The formation of the propargylamine intermediate (155) and the following hydroarylation reaction are a coordinated process in this condition since the propargylamine has been not detected in the reaction mixture. The quinoline product (153) has been obtained after the final oxidation of C by O2 in the air (Scheme 55) (Cao, 2009).
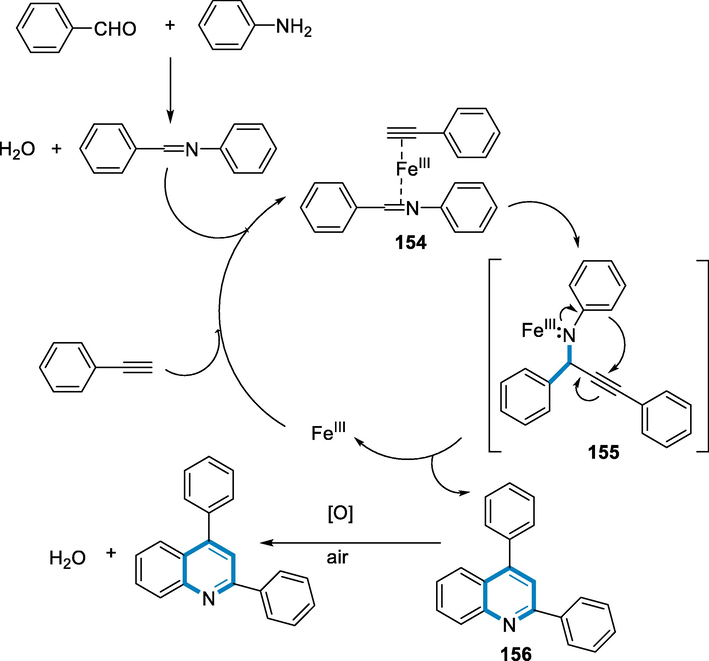
- Mechanistic pathway for the synthesis of quinolines.
Indoles are particularly desirable among nitrogen heterocycles since they are found in a wide range of physiologically active natural compounds, alkaloids, and medicines. Using ironII phthalocyanine as the catalytic precursor and CO as the reductant, 3-aryl indoles from their respective nitrosoarenes and alkynes are converted into the corresponding indoles in high yield with remarkable functional group tolerance. Other reductants, such as isopropanol, can also be used to substitute CO gas (Gupta et al., 2007; Pindur and Lemster, 2001; Yang et al., 2004; Gul and Hamann, 2005; Aygun and Pindur, 2003).
The reaction conditions for the synthesis of 3-aryl indoles (159) were alkynes (157) react with (158) nitrosobenzene, Fe(Pc) (0.02 mmol,11.4 mg) as a catalyst in the presence of DCE (2 mL), CO (30 bar) at 120 °C temperature for 12 h. The substrates scope of alkynes (157) for this reaction was tested and the desired indole products were produced in good yields. In comparison to terminal alkynes with electron-withdrawing and electron-donating substitutes were found to react efficiently, and the corresponding 3-aryl indole (159) was extracted in high yields. Furthermore, functional groups containing halogen groups also tolerated well to provide required indoles in afforded yield. These indole compounds with halogen functional groups are suitable for further modification via cross-coupling reactions. However, the reaction of diphenylacetylene with nitrosobenzene produced just a trace of the desired indole product, and when the pent-1-yne was utilized as the substrate, no indole product was identified (Scheme 56) (Yin et al., 2017).
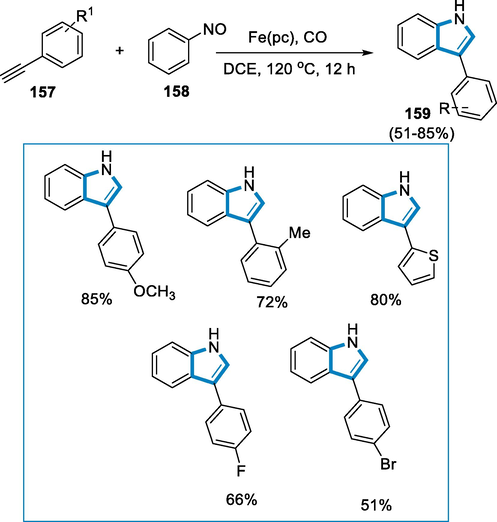
- Synthesis of 3-aryl indoles by using iron(II) as a catalyst.
3.3 From carbonyl compounds
A significant number of bioactive natural products and pharmacological molecules have benzoxazole moiety as a fundamental structural characteristic. The aerobic oxidation method is catalyzed by FeCl3 for the production of benzoxazoles, in which FeCl3 functions as an oxidizer and O2 acts as an oxidant. Chemists are increasingly interested in iron as green chemistry and cost-effective catalyst. This approach has been used to synthesize JTP-426467 and has proven to be successful on a variety of substrates (Correa et al., 2008; Viirre et al., 2008; Ten Brink et al., 2004; Beller, 2004; Kawashita, 2003; Chen, 2008).
The reaction conditions were 2-aminophenol (160) and aldehyde (161) as model substrates, FeCl3 (0.05 mmol) as a catalyst in the presence of toluene at the temperature of 110 °C, under 1 atm O2 atmosphere for the formation of benzoxazoles (162). The reactions proceeded easily to provide the respective benzoxazoles (162) with good to excellent yields whether the aromatic aldehyde has an electron-donating group or an electron-withdrawing group. The intended benzoxazole was subsequently achieved in 88% yield when a bulky 1-naphthaldehyde was used as substrate. 4-pyridine carboxaldehyde was also compatible with the reaction, affording a 67% yield of the corresponding product (Scheme 57).
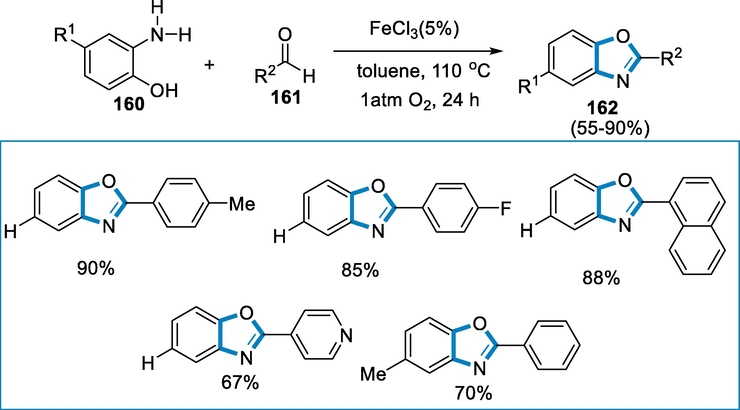
- Iron catalyzed aerobic oxidation for the synthesis of benzoxazoles.
JTP-426467, a selective antagonism for peroxisome proliferator-activated receptor, was produced using this process. From carboxylic acid (163), benzoyl chloride (164) was obtained, which was then converted into amide using 4-amino benzyl alcohol, followed by oxidation with Dess-Martin Perio-dinane to provide the related aldehyde (165). The predicted product JTP-426467 was obtained in 55% by reacting aldehyde (165) with 2-amino-4-methyl phenol under standard conditions for 48 h (Scheme 58) (Cao et al., 2010).
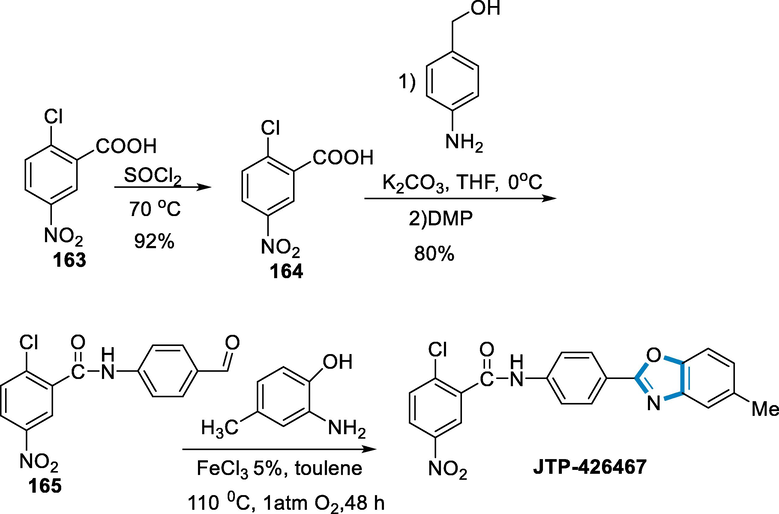
- Synthesis of JTP-426467.
Azolines are particularly important among them as 3-oxazolines are studied less than other chemicals in the sequence, most likely due to synthesis difficulties. By treating substituted vinyl azides with Togni's reagent and substoichiometric quantities of ferrous chloride, 2,2,2-trifluoroethyl-substituted 3-oxazolines, 3-thiazolines, and 5,6-dihydro-2H-1,3-oxazines are produced. 1,n-HAT reactions are performed by the newly produced imiyl radicals (Vitaku et al., 2014; Chen et al., 2014; Taylor, 2016; Akhtar, 2017; Reyes-Arellano et al., 2016; Capaldo and Ravelli, 2017; Nechab et al., 2014; Li, 2018; Chiba and Chen, 2014; Stateman et al., 2018; Protti et al., 2015; Chen, 2016; Jin, 2018).
The reaction conditions for the formation of 3-oxazolines (168) were 2-azidoallyl diarylmethyl ethers (166) and Togni’s trifluomethylating reagent (167) was activated by the addition of FeCl2 (20 mol%) in the presence of dry dichloromethane (DCM) at the room temperature for 30 min. 2-azidoallyl diarylmethyl ethers (167) with two identical 4-substituted aryl groups were used as the substrate. The resultant 3-oxazolines (168) were in excellent yield (ranging from 81% to 98%) with the 4-fluoro- and 4-methyl substituents. As a result, the substituents with electron-donating function appeared to obstruct the formation of the heterocycle, likely due to excessive radical or cation stabilization. It was found that using freshly made Togni's reagent was advantageous. The aromatic substituent's placement was crucial since 3-oxazolines (168) carrying trifluoromethyl were produced in only moderate and low yields. Furthermore, thioether interacted with 3-thiazoline in a 63% yield (Scheme 59) (Terhorst, 2020).
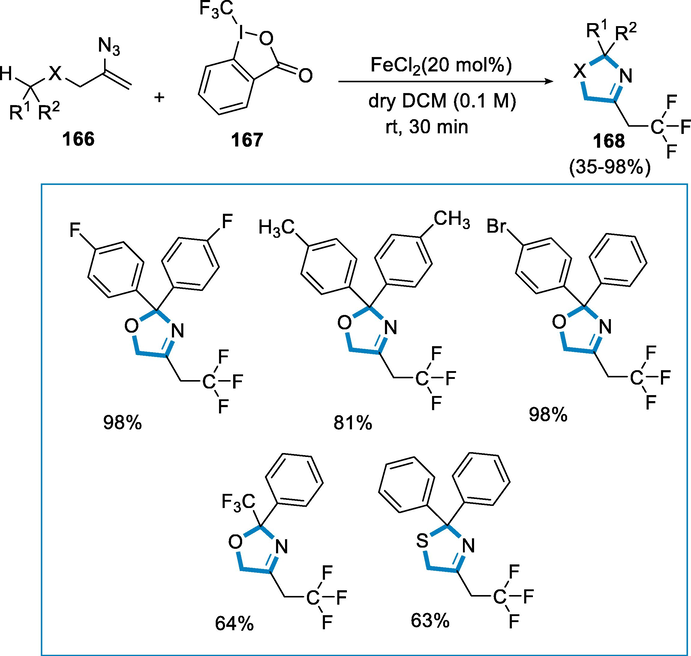
- Iron catalyzed synthesis of 3-oxazolines.
Quinazolinone compounds are a kind of heterocyclic chemical that may be found in a wide range of natural and synthesized materials. Anti-inflammatory, anti-tubercular, antimalarial, antifungal, antidiabetic, and anticancer properties are all present in these substances. Microwave-assisted iron-catalyzed cyclization with or without ligand in water or DMF was developed as a green, quick, and effective technique for manufacturing quinazolinone derivatives from substituted 2-halo benzoic acids and amidines (Ma, 1997; Griess, 1869; Koepfli et al., 1947; Witt and Bergman, 2003; Mhaske and Argade, 2006; Connolly, 2005; Ma et al., 2005).
The reaction conditions for the synthesis of quinazolinone derivatives (171) were 2-halo benzoic acid (169) with acetamidine hydrochloride (170) as a substrate in the presence of Fe2(acac)3 as a catalyst with 1,2-Dimethylethylenediamine (DMEDA), Caesium carbonate (Cs2CO3), and Dimethylformamide (DMF). With the majority of the substrates, moderate to high yields were achieved for the synthesis of quinazolinone derivatives (171) from different substituted 2-halo benzoic acids with acetamidine and benzimidamide. Aryl chlorides < aryl bromides < aryl iodides have the relative reactivity of substituted 2-halo benzoic acids. The reactivity of substituted 2-halo benzoic acids with electron-donating groups was however greater than that of other substituted 2-halo benzoic acids (Scheme 60) (Zhang, 2009).
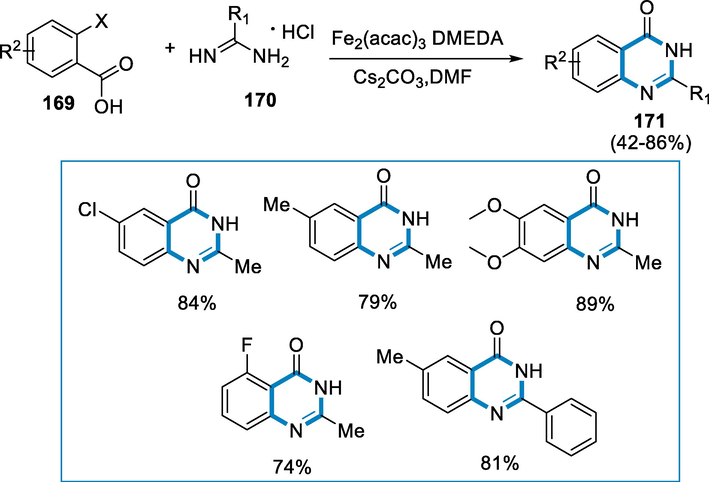
- Microwave-assisted iron-catalyzed cyclization for the synthesis of quinazolinone.
The reaction of aldehydes, anilines, and nitroalkanes with a catalytic quantity of Ferric chloride yielded a series of substituted quinolines. By using aza-Henry cyclization under ambient air, the reaction is a simple, efficient, one-pot, three-component domino method that obtained good yields of the products. This protocol's major features include the use of widely accessible chemicals as starting ingredients, an affordable metal catalyst, aerobic reaction conditions, tolerance of a wide variety of functional groups, and operational simplicity (Sun et al., 2011; Kim, 2005; Buchwald and Bolm, 2009; Sherry and Fürstner, 2008; Foley and Tilley, 1998; Kaminsky and Meltzer, 1968; Hosseinzadeh et al., 2017; Rossiter, 2005; Bizzarri, 2018; Bangcuyo, 2002; Tong, 2003; Nodes, 2009).
Synthesis of substituted quinolines (175) in presence of various nitroalkanes (174), the reaction conditions were anilines (172) and aldehydes (173) in the presence of 20 mol% FeCl3 at 90 °C for 6 h. The impact of aryl aldehyde substituents was first investigated. The intended products were achieved in moderate to high yields in the majority of situations. Benzaldehydes containing electron-donating substituents like CH3 and OCH3, as well as electron-withdrawing substituents like F, Cl, and Br, interacted successfully to provide the respective 2-aryl quinoline derivatives. The benzaldehyde moiety's strong electron-withdrawing group, CF3, efficiently produced the intended product without ease. The occurrence of both electron-withdrawing and electron-donating substituents in the same aniline moiety produced good yields of the quinoline derivatives (Scheme 61).
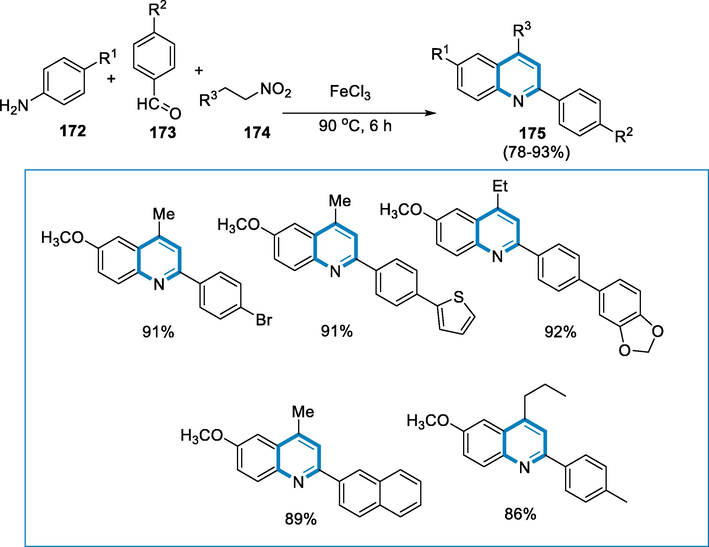
- One-pot, three-component synthesis of substituted quinolines.
The process starts with the synthesis of imine (176), which then reacts with the nitro alkane (174) to generate the aza-Henry adduct (177). The nitro group of the aza-Henry intermediate (177) has been rearranged to form another intermediate (178) with a gem hydroxyl group on nitrogen. The ortho cyclization of intermediate (178) and the elimination of HNO and H2O form a new carbon–carbon bond with nearby carbenes (179). Finally, oxidation transformed the rearrangement of carbene dihydroquinoline to the appropriate quinolone (175) (Scheme 62) (Mahato, 2019).
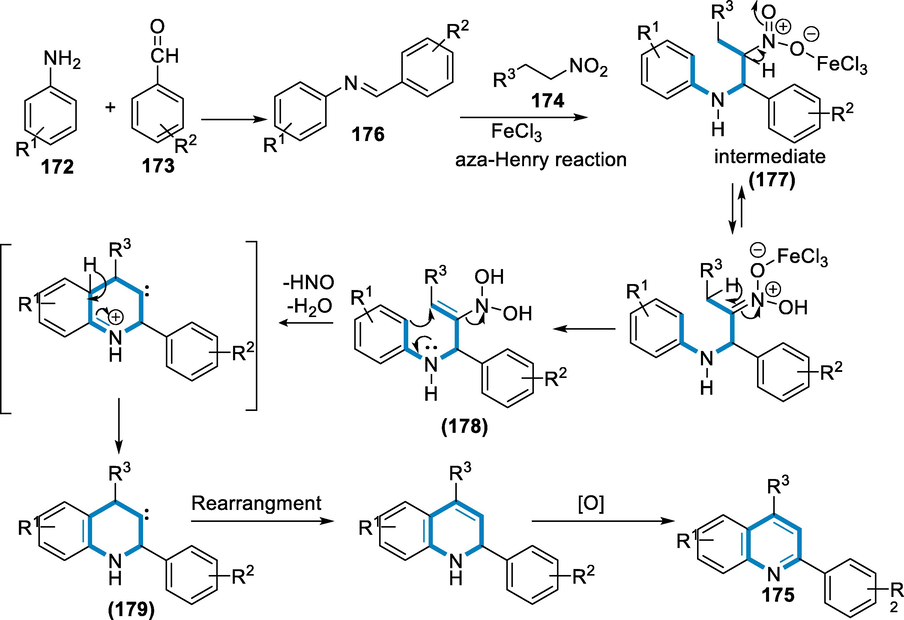
- Mechanistic pathway for the synthesis of substituted quinolines.
Substituted imidazoles have been reported to have impressive bioactivities, such as antimicrobial, anti-inflammatory, anti-parasitic, antidiarrheal, platelet aggregation inhibitors, antiseizure agents, and glucagon receptor antagonism activity. The Fe3O4@SiO2/bipyridinium nanocomposite (Fe3O4@SiO2/BiPy2 2Cl–) is used as a heterogeneous catalyst in the multi-component cyclo-condensation reaction of 1,2-dicarbonyl compounds, aldehydes, and N-containing compounds under solvent-free conditions to synthesis various multi-substituted imidazoles. Simple magnetic decantation can easily recover the catalyst, and it can be reused numerous times without reducing its catalytic activity (Uçucu et al., 2001; Chang, 2001).
The one-pot three-component condensation reaction of benzyl (180), benzaldehyde (181), and NH4OAc as N-source was studied as a model in the presence of 0.05 g Fe3O4@SiO2/BiPy2 2Cl– as a catalyst under solvent-free thermal conditions at 120 °C for the synthesis of multi-substituted imidazoles (182) and (183). Synthesis of a variety of 2,4,5-triaryl substituted imidazoles (182) and 1,2,4,5-tetrasubstituted imidazoles (183) with diverse steric and electronic characteristics proved the universality and synthetic extent of this coupling procedure. Aromatic aldehydes with electron-donating or electron-withdrawing substituents interacted effectively in all cases, affording the desired products in moderate to good yields in short reaction durations (Scheme 63) (Hosseini et al., 2021).
- One-pot three-component condensation reaction for the synthesis of substituted imidazoles.
Imidazoles, which have a five-member ring structure and are polar show a wide range of biologically important properties such as antagonists, biocides, plant herbicides, anti-inflammatory, anti-carcinogenic, antibacterial, muscle relaxant, and anti-tubercular activity (Shalini et al., 2010; Shaterian and Ranjbar, 2011). Fucoidan (FU), a fucose-rich sulfated polysaccharide derived from the brown algae Fucus vesiculosus, was utilized to prepare magnetic Fe3O4@FU in situ in this procedure. The catalytic activity of Fe3O4@FU was used to synthesize tri- and tetra-substituted imidazoles using three-component and four-component reactions, respectively, involving benzyl, aldehydes, NH4OAc, and amine under reflux in ethanol (Mei, 2008; Balalaie and Arabanian, 2000).
The reaction conditions were benzyl (184), aldehydes (185), ammonium acetate (186), and aniline187 as model substrates in the presence of EtOH as a solvent and Fe3O4@FU nanocomposite (12 mg) as a catalyst for the synthesis of imidazoles derivatives (188). In such a reaction, both electron-donating and electron-withdrawing substitutions were present in aromatic aldehydes and anilines. While the presence of electron-donating groups resulted in lower reaction yields and the presence of electron-withdrawing groups resulted in greater yields with lower reaction durations (Scheme 64).
- Iron catalyzed synthesis of imidazoles derivatives.
The suggested mechanism for the synthesis of imidazole has benzyl (184), aldehyde (185), ammonium acetate (186), and amine (187) in four one-pot processes. Fe3O4@FU has been used to activate aldehyde and 1,2-diketone, and then amine has been added to the aldehydes to generate an imine intermediate, which has been attacked by ammonia (released from ammonium acetate) to form the amine intermediate (189). The amine intermediate (189), interacted with the activated carbonyl groups of benzil to produce the intermediate (190). After dehydration, the imidazole derivative (188) has been produced, followed by a 1.5H-shift (Scheme 65) (Banazadeh et al., 2020).
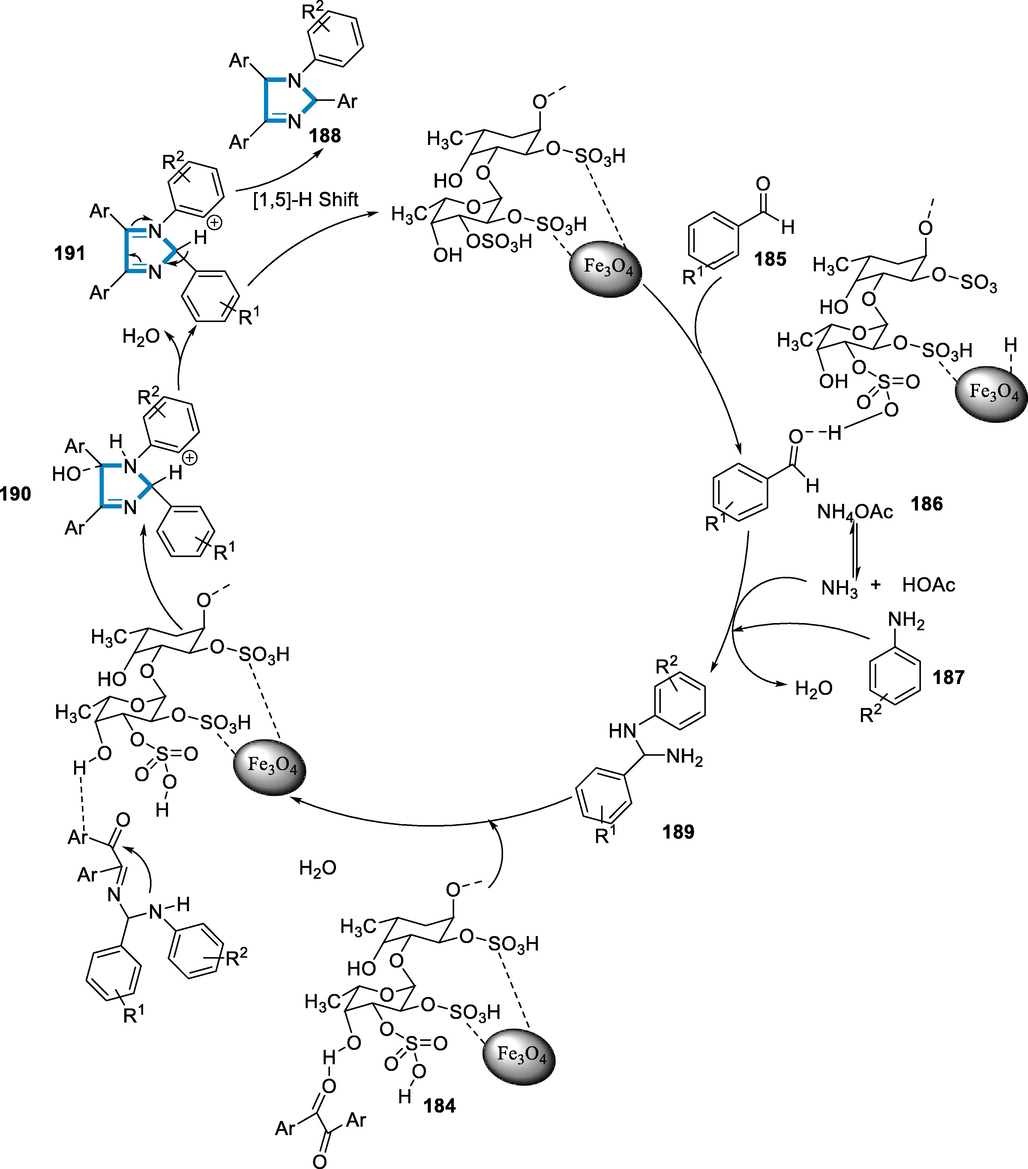
- Mechanistic pathway for the synthesis of imidazoles derivatives.
Ferric chloride is combined with an organic phosphonate ligand to produce an organic–metal nano-catalyst. Its catalytic activity was tested in the synthesis of heterocyclic compounds (2,4,5-trisubstituted imidazoles derivatives) under optimised conditions, and the product is produced with high efficiency and in a short time. For the production of trisubstituted imidazole derivatives, iron-phosphonate nanostructures are effective. For a variety of reasons, using iron compounds as catalysts in organic synthesis is desirable, it is the most widespread metal in the earth's crust, making it significantly less expensive than the most often utilized precious metals (Shaabani, 2009; Verma et al., 2013).
The reaction conditions were benzaldehyde (193), benzyl (192), and ammonium acetate (194) as model substrates in the presence of 0.05 g of Fe-DTPMP as a catalyst at the temperature of 100 °C for the synthesis of trisubstituted imidazoles (195). A diverse array of aromatic aldehydes interacted efficiently to produce excellent yields of 2,4,5-trisubstituted imidazoles (195). High yields of products were seen when electron-withdrawing substituents were present in the aromatic ring of the aldehydes, however, the effect was reversed when the electron-donating substituents were present. The shortest reaction time occurs with 4-N,N-dimethyl amino benzaldehyde, which can be attributed to the steric impact of the bulky group at the para position of benzaldehyde (Scheme 66).
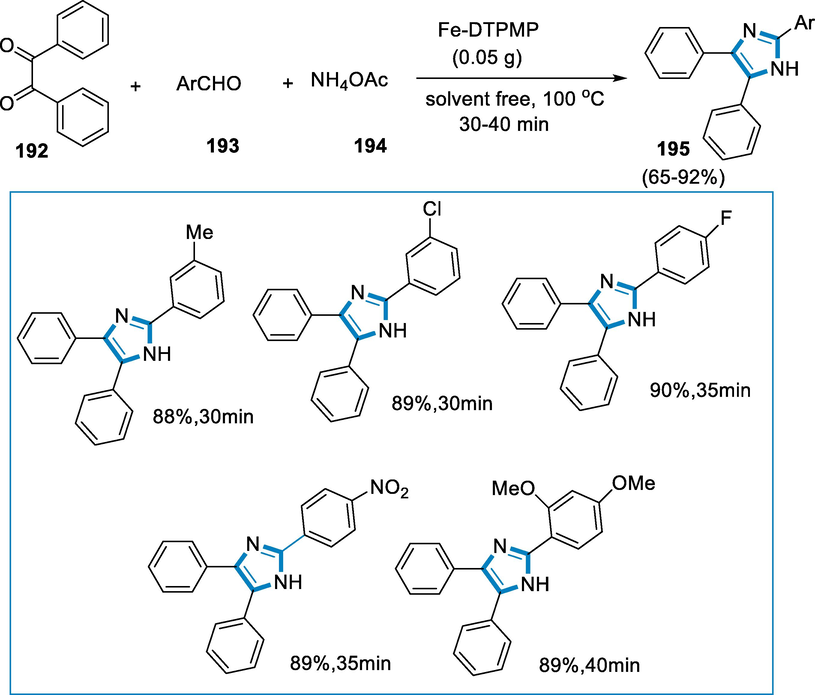
- Synthesis of trisubstituted imidazole derivatives by using an iron catalyst.
The Fe-centre of the catalyst coordinates to the carbonyl oxygen atom of the cyclic aldehyde (193), similar to Lewis acid sites, hence activating the carbonyl carbon atom in the production of 2,4,5-trisubstituted imidazoles (195). The diamine intermediate (196) has been produced by reacting activated aldehyde with two equivalents of ammonium acetate (194). In the presence of Fe-DTPMP, this intermediate condenses with activated benzyl (192) to create intermediate (197), which then undergoes a (Vitaku et al., 2014; Łowicki and Przybylski, 2022; Petri, 2020; Pyta, 2022; Dong, 2016)-H shift to provide the trisubstituted imidazoles (195). The catalyst's tertiary amine groups assist in the elimination of protons during the water removal stage. The synthesis of tri-substituted imidazole derivatives (195) has been catalyzed by the coordination of iron and tertiary amines in the catalyst (Scheme 67) (Arpanahi and Goodajdar, 2020).
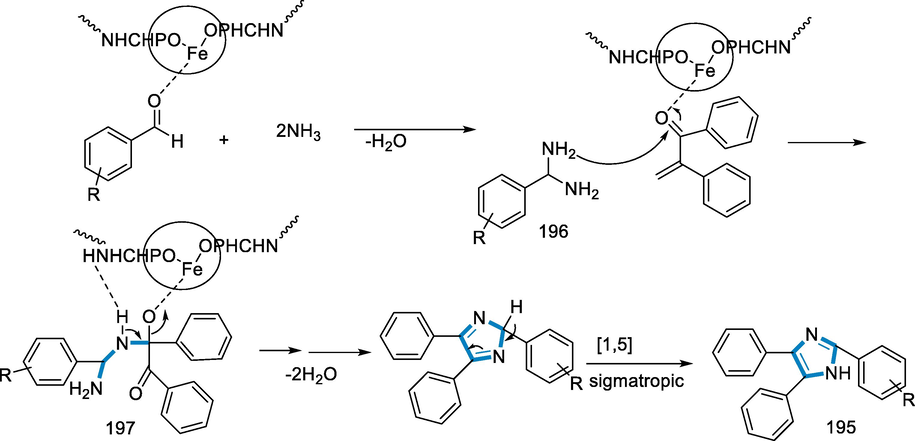
- Mechanistic pathway for the synthesis of trisubstituted imidazole derivatives.
Biocides (in particular, herbicides, fungicides, and growth regulators), powerful angiotensin-II receptor antagonists, glucagon receptor antagonists, and inhibitors of interleukin 1 and 5 lipoxygenases are all examples of substituted imidazoles (Lombardino and Wiseman, 1974; Chang and S. O’, Keefe, M. Pang, A. Rolando, WK Hagmann. , 2001). CuII immobilized on guanidinated epibromohydrin functionalized Fe2O3@TiO2 (Fe2O3@TiO2EGCuII) is used for the synthesis of 2,4,5trisubstituted and 1,2,4,5 tetrasubstituted imidazoles via condensation reactions of numerous aldehydes with benzil and ammonium acetate or ammonium acetate and amine. CuII nano-catalyst Fe2O3@TiO2EG is discovered to be an eco-friendly and highly effective nano-catalyst that could be readily handled, regenerated, and recycled numerous times without reducing its activity (Jahanshahi et al., 2016; Masjed, 2016; Jahanshahi and Akhlaghinia, 2017).
The reaction conditions for the synthesis of 1,2,4,5‐tetrasubstituted imidazoles (200) and 2,4,5‐trisubstituted imidazoles (201) were benzyl (198), aromatic aldehydes (199), ammonium acetate, and a selection of primary aromatic amines in the presence of γ‐Fe2O3@TiO2‐EG‐CuII as catalyst under the solvent‐free condition at 100 °C temperature. The efficient one-pot, multi-component cyclo condensation processes produced 2,4,5-trisubstituted imidazoles (201) in good yields from a wide range of aromatic aldehydes (199) bearing both electron-donating and electron-withdrawing substituents. Remarkably, both heteroaromatic and aliphatic aldehydes produced good yields of the respective imidazoles. Electron-withdrawing substituents on the aromatic ring of aldehydes and electron-donating substituents on the aromatic ring of primary amines might almost similarly help to accelerate the condensation processes (Scheme 68).
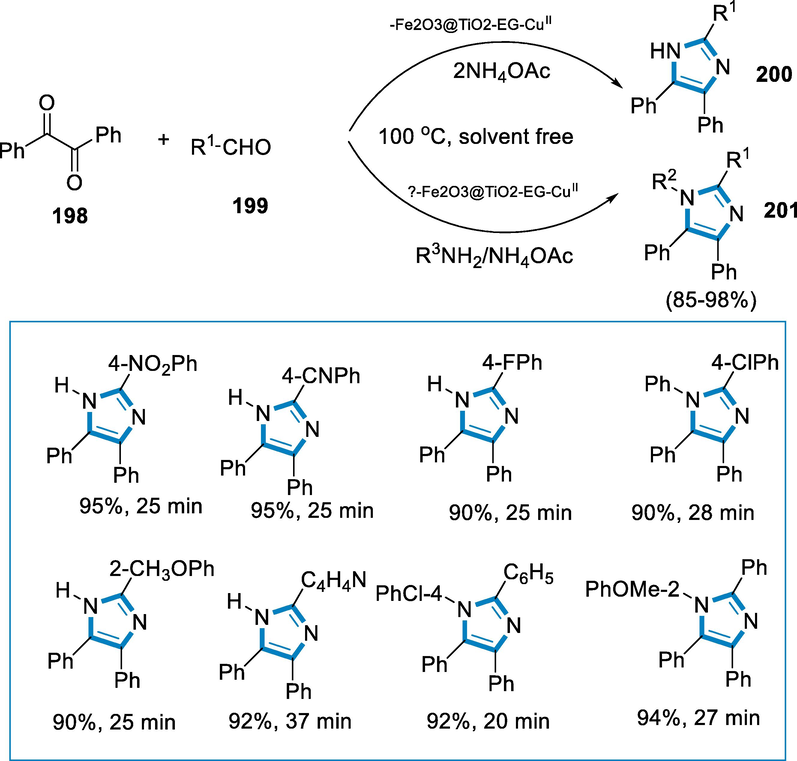
- One-pot, multi-component cyclo-condensation processes for the synthesis of 2,4,5-trisubstituted imidazoles.
The production of aryl aldimine (203) has been thought to be the consequence of the nucleophilic reaction of ammonia nitrogen (obtained from NH4OAc) on the activated carbonyl group of aldehyde. By enhancing the electrophilicity of the C-N on the aryl aldimine (203) toward the nucleophilic attack of ammonia or amine, the catalyst favours the production of intermediate (204). Between benzil and ammonia, there is no potential for imino ketone (202) to form. In the presence of Fe2O3@TiO2EG Cu(II), intermediate (204) compresses with benzil to form intermediate (205) or (206), which then rearranges to 2,4,5-trisubstituted imidazole (200) or 1,2,4,5-tetrasubstituted imidazole (201) via a (Vitaku et al., 2014; Dong, 2016) hydrogen shift or dehydration (Scheme 69) (Nejatianfar et al., 2018).
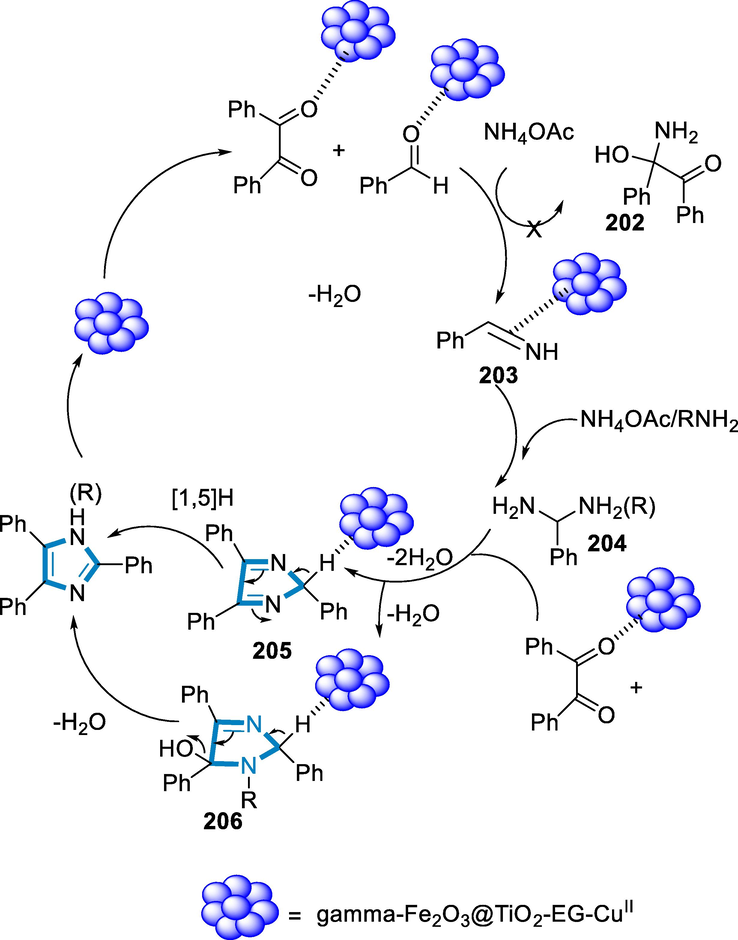
- Mechanistic pathway for the synthesis of 2,4,5-trisubstituted imidazoles.
An effective one-pot, three-component cascade method for the synthesis of imidazo[1,2-α]pyridines is demonstrated by utilizing a catalytic quantity of Ferric chloride. The reaction of readily accessible aldehydes with 2-aminopyridines in a combination of nitroalkane and DMF afforded a series of imidazo[1,2-α]pyridines. A sequential aza-Henry cyclization is thought to be involved in this conversion. Because of their relatively low cost, stability, quick affordability, inertness, and eco-friendly, an iron-based catalyst is demonstrated to induce a wide range of organic transformations, including cross-couplings, allylations, hydrogenations, and direct C-H bond functionalizations. In numerous medications, fused imidazoles are shown to be a significant structural scaffold. They also act as β-amyloid inhibitors, agonists of GABA and benzodiazepine receptors, and cardiotonic drugs (Sun et al., 2011; Hatakeyama, 2012; Lhassani, 1999; Humphries, 2006; Fookes, 2008).
The reaction conditions were 2-aminopyridine (207), substituted aldehyde (208) in presence of 20 mol % of FeCl3 in a mixture of nitromethane (209), and DMF at 110 °C in the air for the synthesis of a series of imidazo[1,2-α]pyridines (210). Under the optimum reaction conditions, the electron-rich and electron-deficient aldehydes interacted successfully with different 2-aminopyridines to produce the required products in good yields. Aldehydes with an electron-donating OCH3 group on the aromatic ring also performed well (Scheme 70).
![Synthesis of substituted imidazo[1,2-α]pyridines by using an iron catalyst.](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104095-fig72.png)
- Synthesis of substituted imidazo[1,2-α]pyridines by using an iron catalyst.
Imine (211) has been generated through the condensation between 2-aminopyridine (215) and aldehyde (216). The introduction of nitromethane (209) to the imine results in the production of the aza-Henry product (212). FeCl3 has been thought to have assisted these two steps by enhancing the electrophilicity of the aldehyde (216) and imine. Intermediate (212) tautomerizes to intermediate (213), which proceeds to intramolecular cyclization to yield intermediate (214). Following the removal of both water and nitroxyl, the final product (217) has been obtained (HNO). FeCl3 can also behave as a Lewis acid, making intramolecular cyclization easier (Scheme 71) (Santra, 2014).
![Mechanistic pathway for the synthesis of substituted imidazo[1,2-α]pyridines.](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104095-fig73.png)
- Mechanistic pathway for the synthesis of substituted imidazo[1,2-α]pyridines.
Naturally substituted imidazoles, as well as their synthesized derivatives, have a diverse set of biological functions, making them appealing to organic chemists. P38 MAP kinase inhibitors, anti-inflammatory drugs, bio-fertilizers, insecticides, pesticides, anti-angiogenesis agents, and therapeutic compounds are only a few of their uses (MaGee et al., 2013; Keivanloo, 2013). The Fe3O4–PEG–Cu catalyst is assessed for its catalytic activity in the production of highly substituted imidazoles. The current iron catalyst is very active and environment-friendly for the production of 2,4,5-trisubstituted imidazoles and 1,2,4,5-substituted imidazoles. The catalyst has magnetic characteristics, allowing them to be rapidly separated from the reaction liquid using a simple magnet.
The reaction conditions for the synthesis of 1,2,4,5‐tetrasubstituted imidazoles (220) and 2,4,5‐trisubstituted imidazoles (221) were benzyl (218), benzaldehyde (219), aniline, NH4OAc, 10 mol% catalyst Fe3O4– PEG–Cu at 110 °C temperature. Several aldehydes and amines were utilized as substrates for the synthesis of 1,2,4,5-tetrasubstituted imidazoles (220) and 2,4,5-trisubstituted imidazoles (221), with both electron-donating and electron-withdrawing substituents present on the aromatic aldehydes and primary amines, the reactions were similarly simple, affording high yields of the respective imidazoles (Scheme 72).
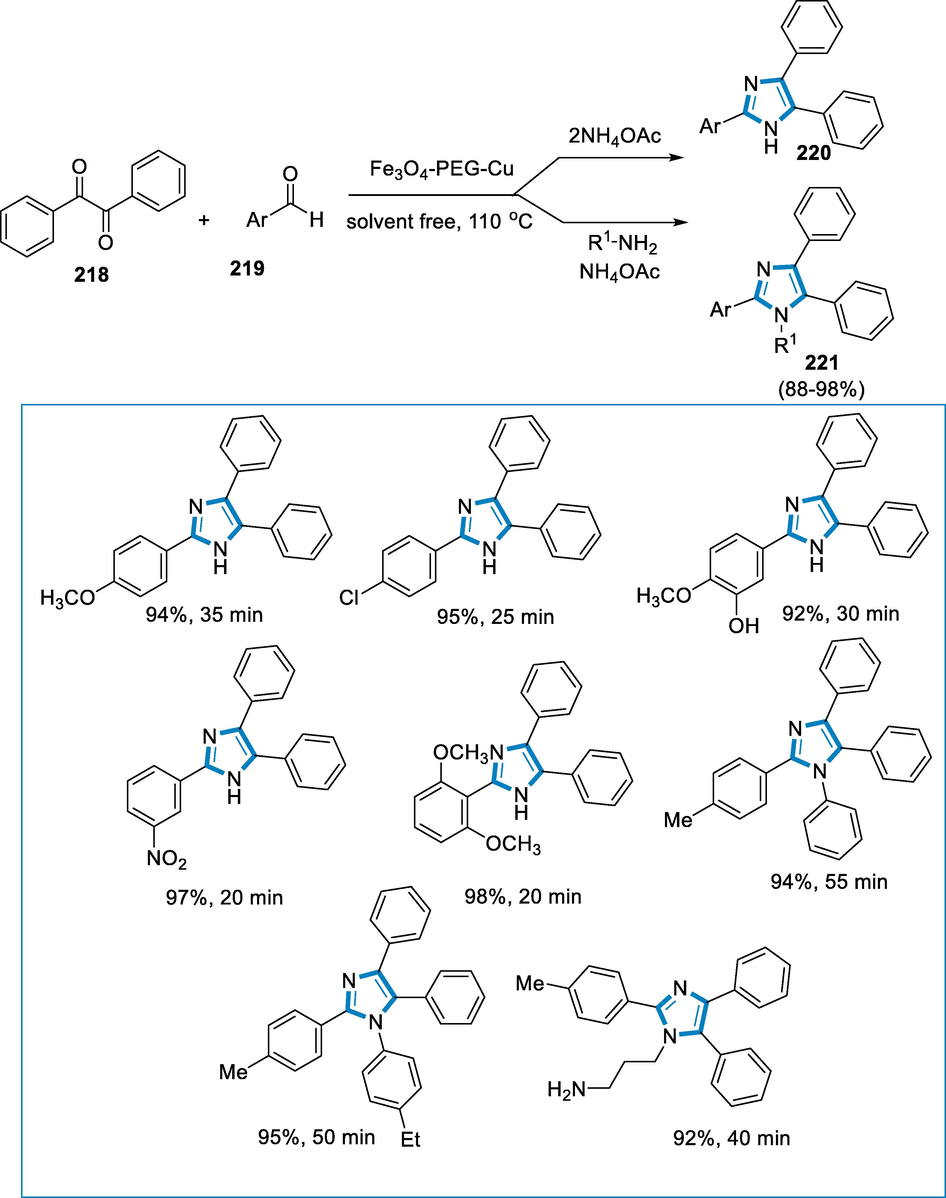
- Iron catalyzed synthesis of substituted imidazoles.
The Fe3O4–PEG–Cu catalyst enables the synthesis of highly substituted imidazoles, and the reaction proceeds through the diamine intermediate (222), which has been produced by the Cu on the surface of nanocomposites activating an aldehyde carbonyl group. The intended product has been obtained by condensing diamine with 1,2-diketone, dehydrating it, and then rearrangement through the imino intermediate (223) (Scheme 73) (Zarnegar and Safari, 2014).
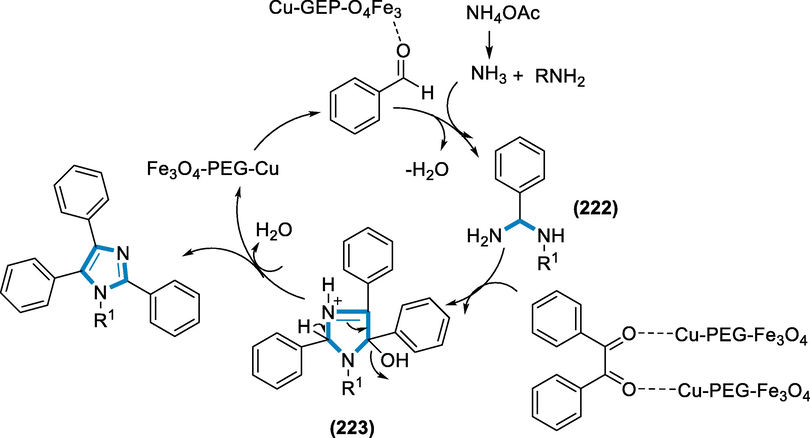
- Mechanistic pathway for the synthesis of substituted imidazoles.
1,2,3-triazoles comprise the fundamental structure in a significant number of natural products, physiologically and pharmaceutically active compounds, discovering an effective technique for their synthesis has attracted organic synthesis's interest. Because of their ecologically friendly, cost-effective, easy separation with an external magnet, and reusability qualities, magnetic nanoparticles are widely exploited in organic transformations. CuFe2O4 nanoparticles facilitate the production of N-2-substituted 1,2,3-triazoles (Hein and Fokin, 2010; Decréau et al., 2010; Jewett and Bertozzi, 2010; Hua and Flood, 2010; Mamidyala and Finn, 2010; Hänni and Leigh, 2010; Le Droumaguet et al., 2010; Yang, 2015; Gangaprasad, 2015; Gawande et al., 2013; Baig and Varma, 2013; Gawande, 2012; Gawande, 2013; Gawande, 2013).
The reaction conditions for the formation of 1,2,3-triazoles (225) were chalcone (224), NaN3, CuFe2O4 (1 equiv.) in the DMSO, at 80 °C temperature for 24 h, under air, then 2-NO2-C6H4F was added to the mixture, and the reaction continued for 5 h. The varied chalcones (224) with a variety of functional groups, such as electron-withdrawing groups afforded good yield as compared to the electron-donating groups. However, most reactions went well, affording moderate to high yields of N-2-aryl-substituted 1,2,3-triazoles (225). The substituent's type and orientation in the aromatic ring (R1 or R2) affected the yields of the corresponding products (Scheme 74).
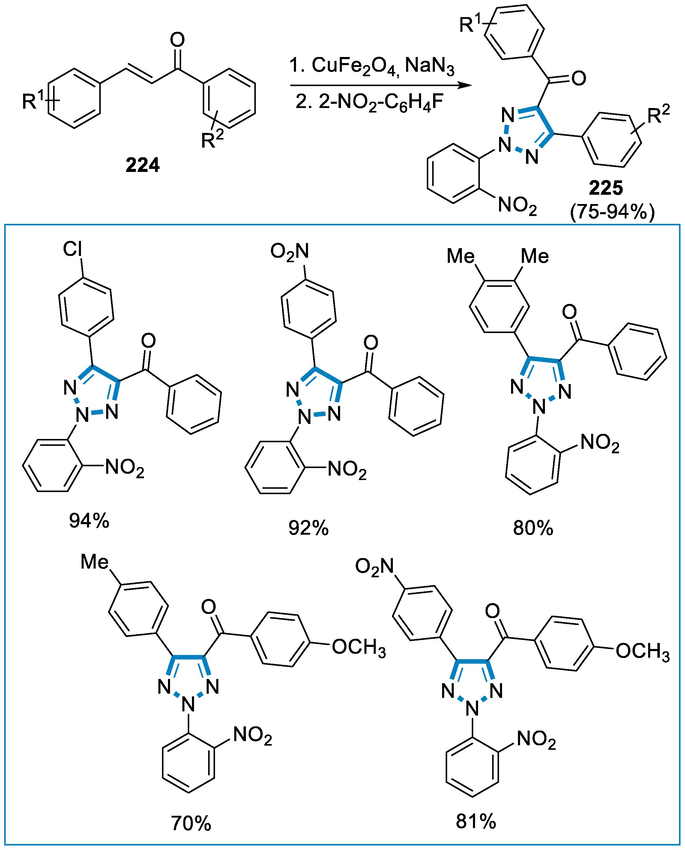
- Synthesis of 1,2,3-triazoles by using iron as a catalyst.
Although the mechanism for this response had not evident, a possible explanation has been hypothesized based on the literature and this experiment. The reaction of sodium azide with chalcones (224) in the presence of CuFe2O4 yielded an intermediate (226), which was subsequently nucleophilically reacted with an aromatic halide to get the desired product (227) (Scheme 75) (Dong et al., 2016).
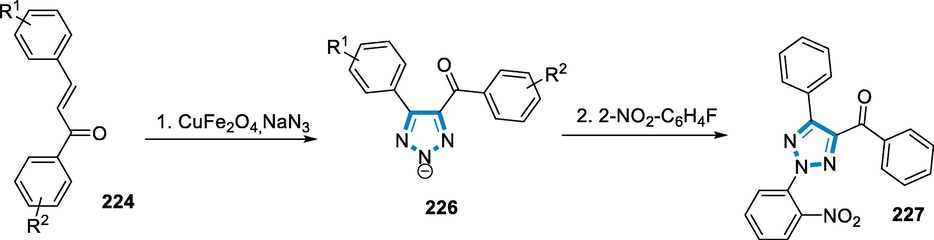
- Mechanistic pathway for the synthesis of 1,2,3-triazoles.
Quinazolinones are a prominent family of annulated six-membered nitrogen heterocycles found in natural compounds and synthesized medicines. The synthesis of quinazolinones occurs from methyl arenes and anthranilamides by cross-dehydrogenative coupling. The benzylic sp3 carbon C–H functionalization is accomplished under air by di-tert-Butyl peroxide, and the annulation process is completed by an amination–aerobic oxidation process catalyzed by iron. Iron is a transition metal that is affordable, nontoxic, and ecologically friendly (Bauer and Knölker, 2015; Mhaske and Argade, 2006; Hour, 2000; Henderson, 2006; Alagarsamy and Pathak, 2007).
The reaction conditions for the formation of quinazolinones (230) were 2-anthranilamide (228) with toluene (229) in the presence of iron catalyst FeCl3·6H2O and oxidant such as di-tert-Butyl peroxide(DTBP) with DMSO (dimethyl sulfoxide) as a solvent at the temperature of 110 °C for 40 h and air. 2-phenyl quinazolinones (230) containing N-substituents were effectively synthesized from the respective 2-amino N-substituted benzamides using a wide variety of anthranilamides (228), yields were good when less nucleophilic amides were replaced with an aryl group. Other heteroarene methyl-substituted amides, in addition to benzyl, produced the necessary compounds in good yields. Furthermore, there was no obvious variation in the substituents on the substrate's aromatic ring. The annulated products of iodide and bromide were similarly preserved which indicates that they are effective functional groups for subsequent processing (Scheme 76).
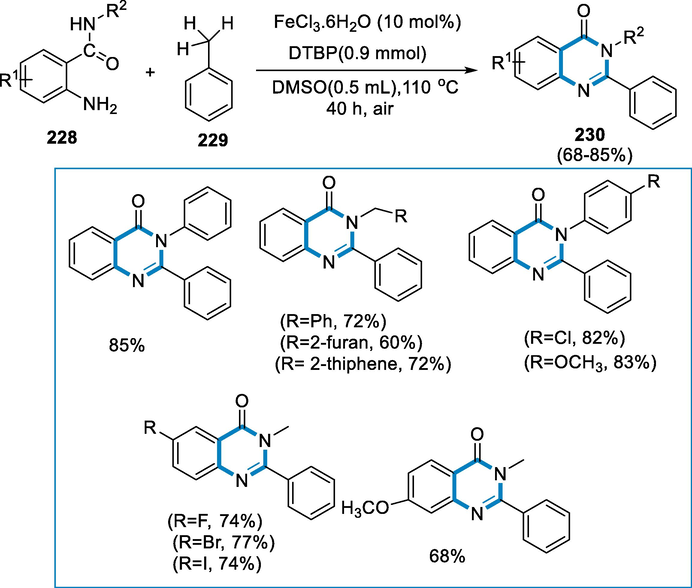
- Synthesis of quinazolinones by cross-dehydrogenative coupling using iron catalyst.
Initially, t-butoxy radicals have been produced by homolysis of di-tert-butyl peroxide (DTBP). The benzyl radical (229a) has been generated when the t-butoxy radical removes H• from the benzylic carbon of toluene. The t-butoxy benzyl ether (229)-OtBu was formed as an intermediate by combining these two radicals H• was extracted from (229)-OtBu by another t-butoxy radical, which then autolysis to generate benzaldehyde (231). The iron(III) salt may be implicated in the single-electron transfer oxidation of (229), particularly in the production of radical species. Following the production of benzaldehyde (231), it interacted with 2-anthranilamide (228), resulting in the synthesis of imine (232) with the support of the iron catalyst. The final product (233) has been obtained by annulating imine (232) by nucleophilic intramolecular amination followed by aerobic oxidation. Furthermore, direct amination of (228) by radical species (229a) or (229b) could not be eliminated. The synthesis of benzaldehyde (231) has been enhanced by exposure to air via a different mechanism. The combination of (229a) with O2, followed by the removal of H• from the solvent, produced benzyl hydrogen peroxide in the presence of oxygen gas (229 or DMSO). Then, under the reaction parameters, benzyl hydrogen peroxide might be transformed into benzaldehyde (231). The synthesis of aldehydes, as well as the total process, was enhanced by oxygen gas in this route (Scheme 77) (Jang, 2020).
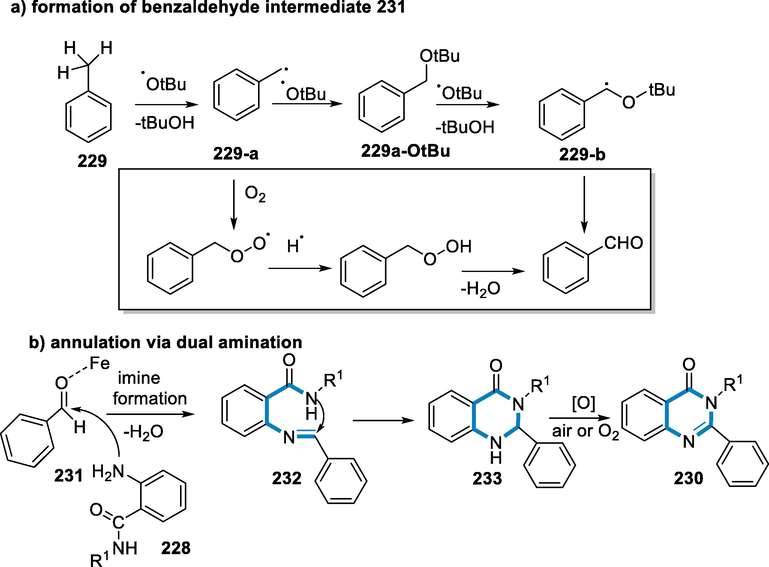
- Mechanistic pathway for the synthesis of quinazolinones.
Carboxylic acid derivatives and readily accessible o-substituted anilines synthesize 2-substituted quinazolin-4(3H)-ones from iron-catalyzed aerobic oxidative functionalization of the sp3 C-H interaction. The procedure uses a bio-friendly iron catalyst in conjunction with air as the sole oxidant or molecular oxygen to give broad and eco-friendly access to Nitrogen-heterocyclic compounds, which are abundant core units of many physiologically active pharmaceuticals and natural products (Bolm, 2004; Sun et al., 2011; Gonzalez-de-Castro et al., 2014; DeSimone, 2004; PD, L., Springthorpe B. , 2007; Wu, 2013; Wang et al., 2006; Balakumar, 2010; Chen, 2015; Yang, 2012; Nguyen et al., 2013; Jia and Li, 2014; Klein and Plietker, 2013; Majumdar, 2014).
o-Aminobenzamide (234) and aryl acetic acid (235) were the substrates for the synthesis of 2-substituted quinazolin-4(3H)-ones (236) in the presence of 10 mol% FeCl3 at 100 °C temperature in DMF (dimethyl formamide) as a solvent under dioxygen atmosphere. The quinazolin-4(3H)-ones (236) were easily formed by reacting O-aminobenzamide (234) with various aryl acetic acids (235) that include both electron-rich and electron-poor groups on the aryl rings. In the current catalytic system, important functional groups such as OCH3, NH2, OH, NO2, Br, and Cl all survived, and the desired products were generated in excellent yields (Scheme 78).
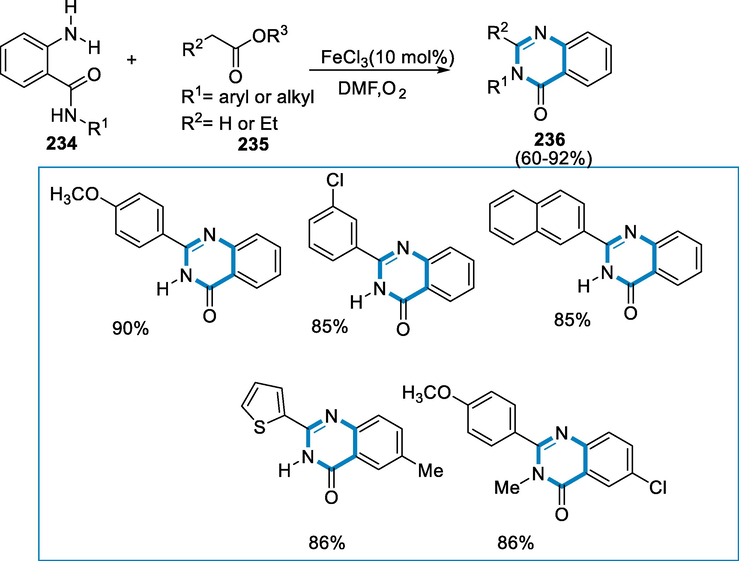
- Iron-catalyzed aerobic oxidative functionalization of the C(sp3)-H interaction for the synthesis of 2-substituted quinazolin-4(3H)-ones.
The C-H bonds have been oxidized to generate -hydroxycarboxylic acid through a radical route in the Fe/O2 system, followed by dehydrogenation to provide 2-oxo-2-carboxylic acid, according to a probable reaction pathway for the current iron-catalyzed aerobic oxidative functionalization of C(sp3)-H bonds. Under atmospheric oxygen, the resultant aldehyde is generated which has caught by o-substituted anilines, resulting in N-heterocyclic compounds (Scheme 79) (Chen, 2015).
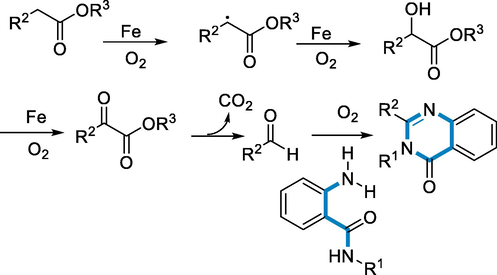
- Mechanistic pathway for the synthesis of 2-substituted quinazolin-4(3H)-ones.
Pyrido[4,3-d]pyrimidines show a variety of antimicrobial, antipyretic, antimicrobial, antiviral, anti-inflammatory, and antifungal characteristics are among the many biological actions of pyrimidines. A novel phenylpyrido[4,3-d] series is discovered, under solvent-free conditions. Pyrimidine-2-amine derivatives are synthesised by reacting (E-3,5-bis(benzylidene)-4-piperidones with guanidine carbonate in the presence of (Fe2O3-MCM-41-nPrNH2 as a magnetically recoverable nano-catalyst. Magnetic iron oxide nanoparticles are particularly appealing due to their unique features and prospective uses in a variety of fields, including magnetically assisted medication delivery, MRI contrast agents, hyperthermia, and biomolecule magnetic separation (Hurlbert and Valenti, 1968; Althuis et al., 1979; Althuis, 1980; Mo, 2009; Elslager, et al., 1972; Rosowsky et al., 1995; El-Subbagh, 2000).
The reaction conditions for efficient synthesis of Pyrido[4,3-d]pyrimidines and its derivatives (239) through the two-component reactions in the presence of magnetically recoverable catalyst [(Fe2O3-MCM-41- nPrNH2] between (E-3,5-bis(benzylidene)-4- piperidones (237) and guanidine carbonate (238) under solvent-free conditions. For the synthesis of phenylpyrido[4,3-d]pyrimidines (239), a range of aromatic aldehydes with both electron-rich and electron-deficient groups were used and the results showed that the reaction occurred well in all cases. It's worth noting that 3,5-dibenzylidene piperidin-4-one with electron-deficient groups reacted quickly, whereas those with electron-donating groups needed longer reaction periods. Electron-deficient groups on phenyl rings cause more electronic positive charge on the -atoms than electron-donating moieties (Scheme 80).
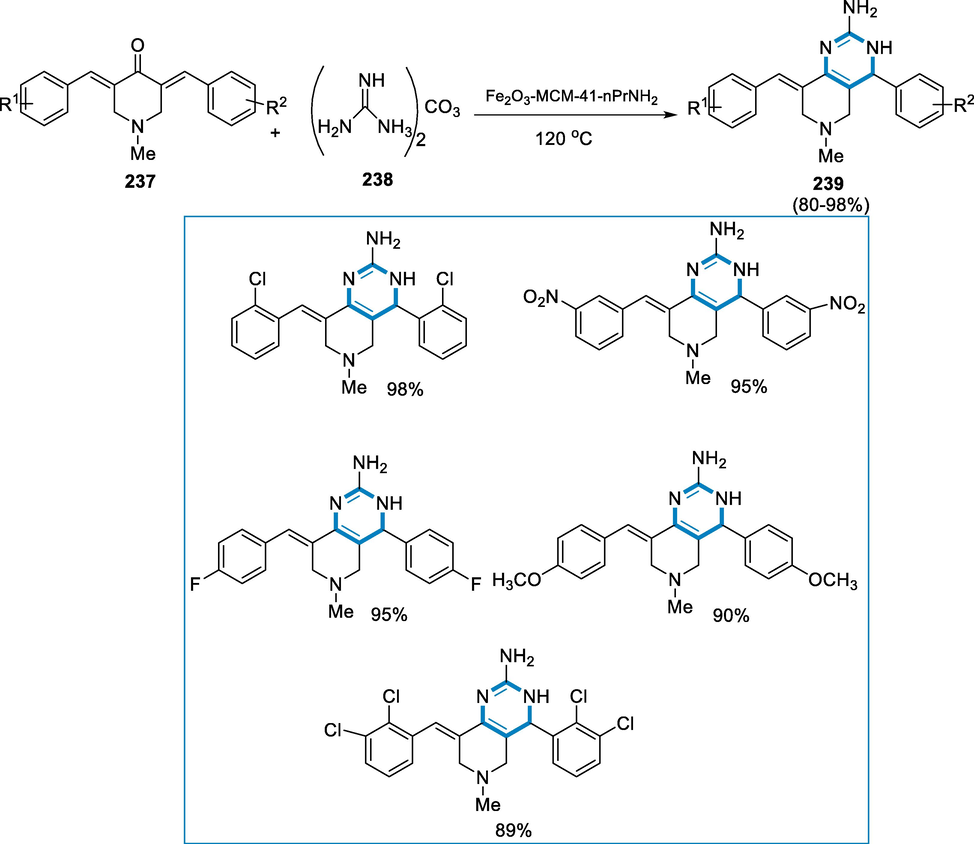
- Iron catalyzed synthesis of 2-aminopyrimidine derivatives.
The presented mechanism has been that the reactants easily transfer toward the nanocatalyst channels due to the two-dimensional pores of MCM-41, and they have been accompanied by the inherent hydrogen bonding of –OH and –NH2 groups, both of which have been capable of bonding with the carbonyl oxygen of the 3,5-dibenzylidene piperidin-4-one (237) moiety to increase its electrophilicity. It has been necessary to convert guanidine carbonate to a free base in the presence of a base (NH2 groups). The free base of guanidine carbonate (238) has been first added to 3,5-dibenzylidene piperidin-4-one (237) to produce intermediates (240), (241). Following that, the water has removed for the synthesis of the final product (239) (Scheme 81) (Rostamizadeh, 2013).
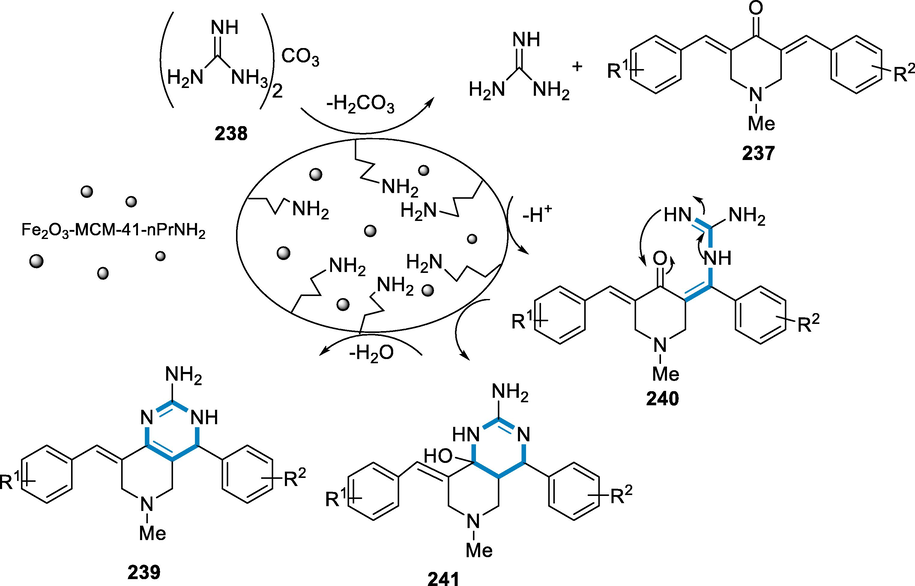
- Mechanistic pathway for the synthesis of 2-amino pyrimidine derivatives.
3.4 From nitriles
The azepine, which is present in a wide range of natural products and physiologically active compounds, has a broad substrate range and strongly functional group compatibility. A range of structurally unique and intriguing azepine derivatives are successfully synthesized in good to fair yield by combining iminyl radical-triggered 1,5-hydrogen atom transfer with (5 + 1) or (5 + 2) annulation procedures. This technique employs FeCl2 as a catalyst and easily accessible oximes. Many useful substances may be easily produced from annulation products (Renfroe et al., 1984; Tomasi, 2010; Dudognon, 2018; Pellissier, 2011; Pellissier, 2018).
Oximes (242) and acrylonitrile (243) were reacted in the presence of FeCl2 (10 mol%) as the catalyst, 1,4-dioxane as the solvent, and PivONa as the additive at 100 °C for 12 h to develop an effective catalytic system to realize the (5 + 2) annulation reaction for the synthesis of derivatives of azepine (244). FeCl2 exhibited the best catalytic reactivity toward azepine (244) synthesis. Substrate (242) oxime reacted well with acrylonitrile (243), affording the desired products, and a variety of functional groups such as OCH3, (CH3)3SiCl, ROR1, CN, CF3, OCF3, Cl, I, Br, F, CnH2n were well tolerated. Several heterocycle-derived oximes, such as those produced from quinoline, benzodioxole, thiophene, pyridine, benzothiazole, isoquinoline, furan, and benzofuran, were also favourable to this method. Ethyl/ethyl and phenyl/methyl were examples of substituents (R1 and R2) at the γ-position of the oxime moiety. Cyclic moieties were also used as substituents (Scheme 82).
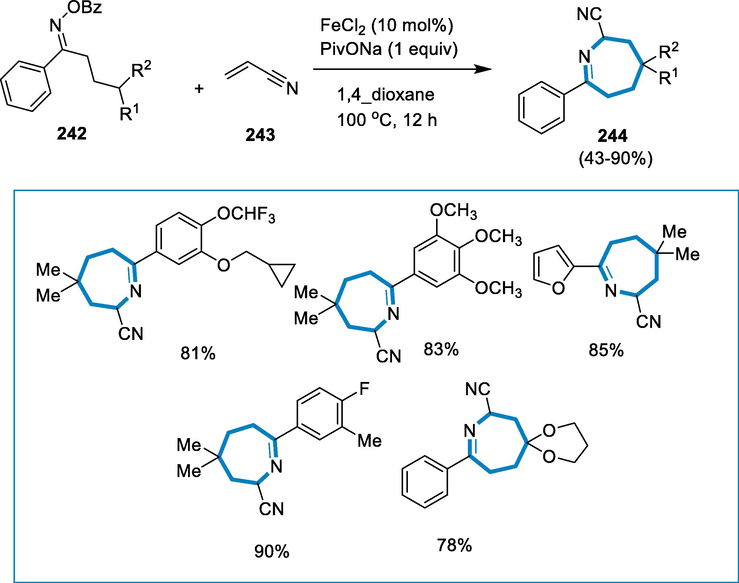
- (5 + 1) or (5 + 2) annulation procedures for the synthesis of azepine derivatives.
Transfer of single-electron to the oxime from FeII occurs in N-O bond breaking, generating a FeIII and an iminyl radical (245) in the cascades 1,5-HAT/(5 + 2) annulation process. The second undergoes 1,5- HAT, which causes the formation of an alkyl carbon radical (246). The synthesis of new carbon radical (247) by the addition of γ-carbon radical to the alkene. The carbon radical (247) would react with the intramolecular imine group to generate an intermediate (248), which could then be oxidized by FeIII to form an iminium ion (249) while the FeII catalyst has been regenerated. The azepine derivative (244) would be produced after deprotonation. FeIII salts can also cause the reaction, and one explanation for this could be because the FeIII salts were converted to FeII species first under the reaction circumstances (e.g., 1,4- dioxane) (Scheme 83) (Liang, 2020).
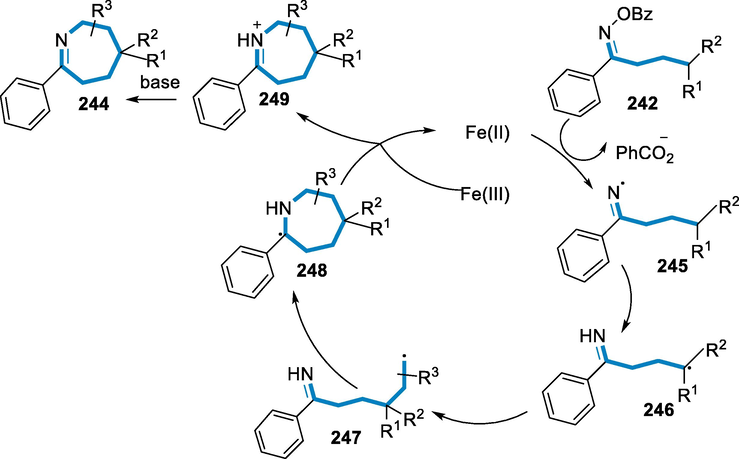
- Mechanistic pathway for the synthesis of azepine derivatives.
Benzothiadiazine-1,1-dioxide and quinazolinone derivatives have a wide range of biological and therapeutic properties. Benzothiadiazine-1,1-dioxide derivatives (such as diazoxide) are proven to diminish AMPA receptor desensitisation and enhance defective synaptic transmission of functions, making them beneficial for the treatment of early stages Alzheimer's disease (De Tullio, 2005; Boverie, 2005; De Tullio, 2003; Coghlan et al., 2001; Braghiroli, 2002; Nagarajan, 2001; Tedesco, 2006). The cascade coupling processes allow for the effective iron-catalyzed production of quinazolinone and benzothiadiazole-1,1-dioxide derivatives. There is no need for an external ligand or additive FeCl3 to be used as the catalyst and the starting substrates are widely accessible such as 2-halo benzenesulfonamides, 2-bromo benzoic acids, and amidine hydrochlorides (Tedesco, 2006; Ikawa, 2007).
The reaction conditions for the formation of 1,2,4-Benzothiadiazine-1,1-Dioxide (252) and quinazolinone derivatives (254) were substituted 2-halobenzene-sulfonamide (250), substituted 2-halo benzoic acid (253); respectively and amidine hydrochloride (251) were the substrates FeCl3 (0.1 mmol) as a catalyst with Cs2CO3 and DMF for 12 h at the temperature of 120 °C under nitrogen atmosphere. For all of the substrates evaluated, under the optimum coupling procedures afforded the required 1,2,4-benzo thiadiazine-1,1-dioxide (252) derivatives in moderate to high yields. The aryl iodide converted to the intended product better than the aryl bromides for substituted 2-halo benzenesulfonamides. There were no considerable reactivity differences amongst the amidines studied in this coupling process. The electronic impact of substituted groups in 2-bromo benzoic acids, including electron-donating, -neutral, and -withdrawing substituents, had no noticeable reactivity variation. Amidines, both aliphatic and aromatic, produced excellent yields (Scheme 84) (Yang, 2009).
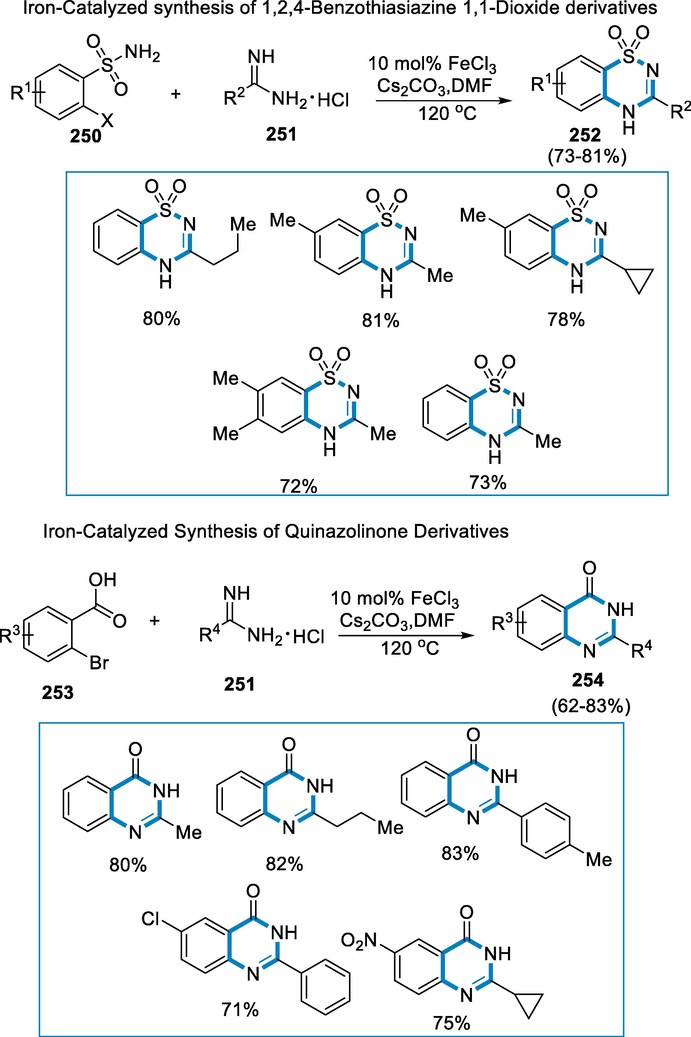
- Iron-catalyzed production of quinazolinone and benzothiadiazole-1,1-dioxide derivatives.
Natural compounds and medicines with exceptional biological activity have the 2-amino benzothiazole core as a preferred scaffold. Iron-catalyzed tandem reactions of 2-iodo anilines with isothiocyanates in water, leading to the synthesis of diverse 2-amino benzothiazoles. The transformation went efficiently in the presence of octadecyl trimethyl ammonium chloride as a phase-transfer catalyst (PTC), and the respective products are synthesized in moderate to high yields (Paget, 1969; Lours, 1970; Suter and Zutter, 1967; Shirke, 1990; Hays et al., 1994; Jimonet, 1999; Ding and Wu, 2007; Ding et al., 2009; Ding, 2007; Ding et al., 2008; Ding et al., 2007; Ding and Wu, 2008; Ding et al., 2009; Ding, 2009; Ding and Wu, 2008; Ding, 2010).
2-iodo aniline (255) and isothiocyanate (256) were reacted in the presence of a catalyst such as FeCl3 (5 mol%) with the L6 = 1,10-phenanthroline, PTC-4 (octadecyl trimethyl ammonium chloride), DABCO (1,4-diazabicyclo[2.2.2]octane), and H2O at the temperature of 80 °C for the synthesis of 2-amino benzothiazoles (257). The method's range and specificity were demonstrated by altering the 2- iodo benzenamine, which could be easily produced from 4-substituted benzenamines, and the reactions yielded the appropriate 2-amino benzothiazole (257) in good to high yields. The results showed that numerous functional groups on the phenyl ring of 2-iodo benzenamine, such as CF3, F, Cl, and CH3, were well tolerated (Scheme 85) (Ding, 2010).
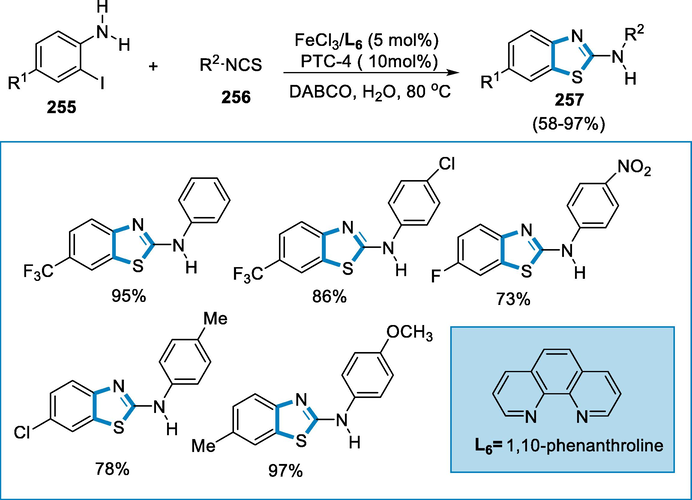
- Iron-catalyzed tandem reaction for the synthesis of 2-aminobenzothiazoles.
3.5 From alcohols
Pyrimidine's existence in a biological system as well as its widespread in synthetic chemistry, among the other nitrogen-containing compounds, show its importance. They can also be used in light-emitting devices as photoactive materials. A broad range of 2,4,6-trisubstituted pyrimidines is produced through dehydrogenative coupling of amidines with primary and secondary alcohols using a FeII-complex incorporating redox non-innocent 2-phenyl azo-(1,10-phenanthroline) ligand as a catalyst (Joule and Mills, 2012; Raw and Taylor, 2010; Park et al., 2017; Gao, 2017).
The pyrimidine derivatives (261) synthesis substrates were benzyl alcohol (259), 1-phenyl ethanol (258), and guanidine (260). In non-polar solvents such as toluene and xylene, the reaction proceeds well; however, polar solvents yield only traces or no product but t-BuOK was effective. The pyrimidine 261 was afforded at a good yield of 82% when the coupling of (258), (259), and (260) was carried out at 100 °C for 16 h in the presence of 0.5 equiv. of t-BuOK and 3.0 mol% of Fe5. With a wide range of electron-rich, -deficient, and heteroaromatic functionalities on both 1° and 2° alcohols, the reaction proceeds smoothly (Scheme 86).
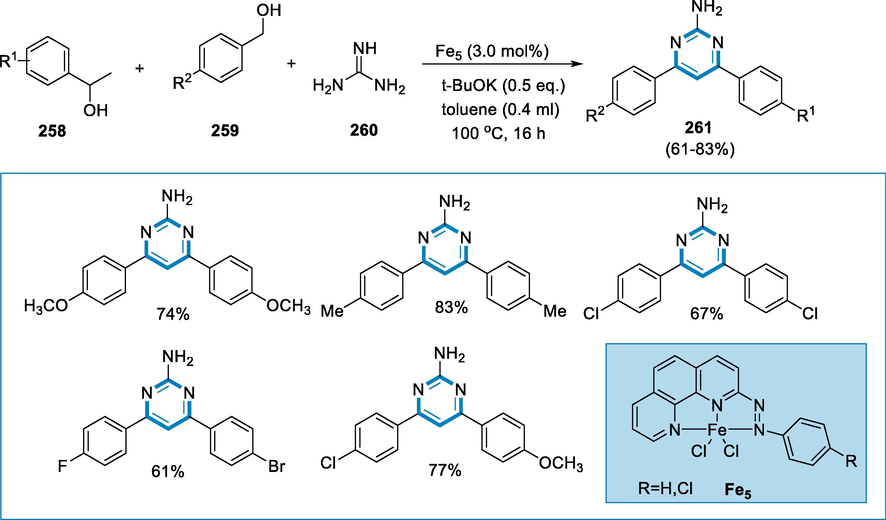
- 2,4,6-trisubstituted pyrimidines synthesis through the dehydrogenative coupling of amidines.
The process begins with the dehydrogenation of 1° and 2° alcohols, which have been catalyzed by Fe5. In a one-electron hydrogen atom transfer (HAT) reaction, the iron-stabilized azo-anion radical takes hydrogen atoms from the alcohol to generate a ketyl radical intermediate, which then undergoes quick one-electron oxidation to yield the corresponding carbonyls. The carbonyls generated in situ follow base-promoted condensation to form the α-β-unsaturated ketone, which has been converted to pyrimidines by base-mediated condensation with amidine and intramolecular cyclization (Scheme 87) (Mondal, 2020).
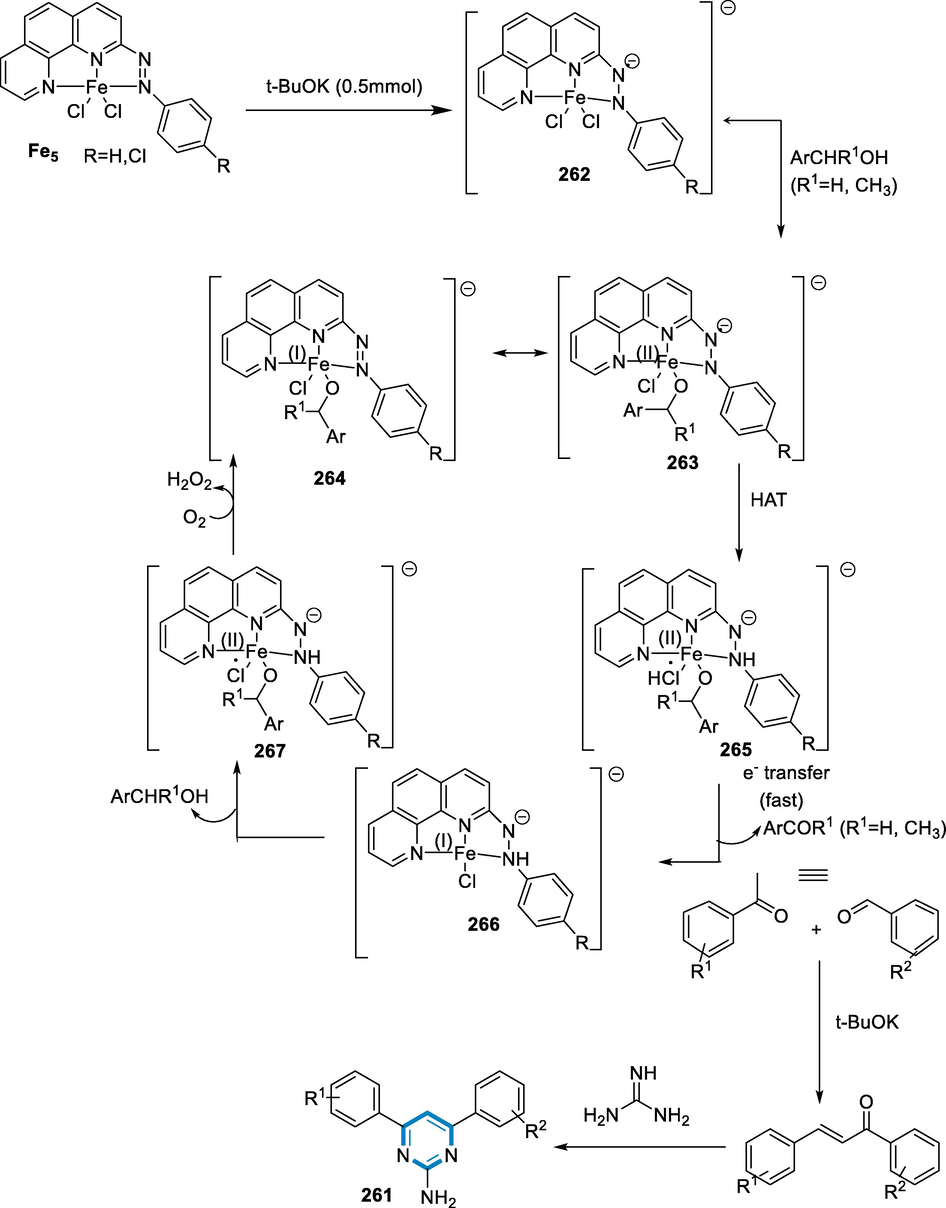
- Mechanistic pathway for the synthesis of 2,4,6-trisubstituted pyrimidines.
Quinoxaline is an N-heterocyclic chemical having benzene and pyrazine rings. Natural compounds and physiologically active compounds have quinoxaline structures in abundance. Quinoxaline moieties are found in a variety of commercial medications, including sulfaquinoxaline, quinacillin, clofazimine, and echinomycin. Transfer hydrogenative condensation of 2-nitro aniline with vicinal diols through the one-pot synthesis of quinoxalines by iron-catalyst. The Knolker complex such as tricarbonyl (h4-cyclopentadienone) iron, stimulated the reduction of nitroarenes and, oxidation of alcohols generating carbonyl and 1,2-diaminobenzene intermediates in situ. The iron complex is activated using trimethylamine N-oxide. For transfer hydrogenative condensation, a variety of unsymmetrical and symmetrical vicinal diols are being used, affording quinoxaline derivatives in excellent yield (Liu, 2020; Ajani, 2014; Parhi, 2013; Hajri, 2016; Zhao, 2019; Liang, 2020; Kothavale et al., 2019; Li, 2019).
The reaction conditions of transfer hydrogenative condensation for the quinoxalines,2-nitroaniline (268) were (0.4 mmol) and 1-phenylethane-1,2-diol (269), (0.8 mmol) react in the presence of an iron catalyst (Fe6) (2 mol%), Me3NO (4 mol%) and solvent (1 mL) in a sealed tube at 150 °C for 24 h to give the isolated yields of quinoxalines (270). Condensation proceeded by cyclization with 2-nitroaniline (268) generates 2-alkyl-substituted quinoxalines (270) in excellent yields from alkyl-substituted unsymmetrical vicinal diols (269). Electron-rich groups (CH3 and OCH3) with nitro aniline gave greater yields of quinoxalines than electron-withdrawing groups (Cl) with substrates. The hydrogenative condensation of symmetrical diols with 2-nitro aniline (268) by transfer hydrogenation. 2-nitro aniline (268) with electron-rich groups (CH3 and OCH3) gave high yields of the corresponding products. Although 2-nitro aniline with electron-deficient groups (Cl, Br, I, and CF3) are less efficient than the electron-donating groups (Scheme 88).
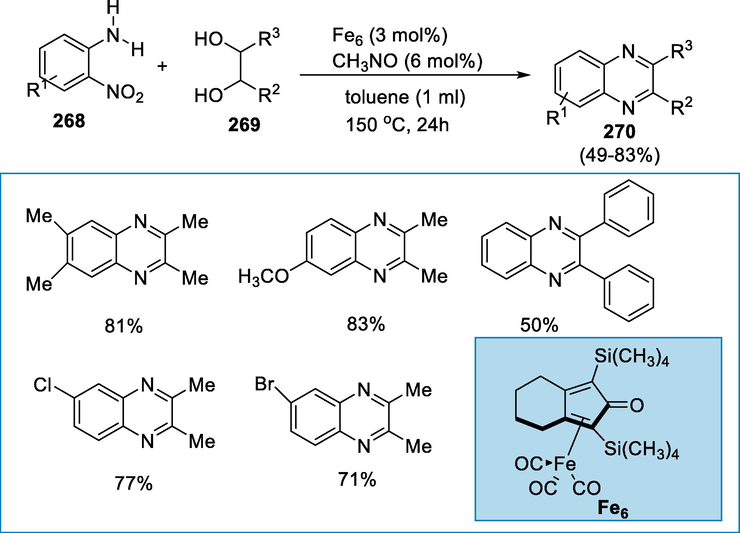
- One-pot synthesis of quinoxalines by iron-catalyst.
The plausible mechanism for the transfer hydrogenative condensation of 2-nitro aniline (268) with vicinal diols (269); Me3NO oxidizes a CO ligand on Knolker complex Fe6 and liberates CO2. The activated iron-catalyst has been produced, which mediates the oxidation of diols (269) to provide the intermediate of carbonyl and [Fe]–H2. The α-hydroxyimine intermediate (272 or 273) has been produced via condensation of carbonyl compounds with 2-nitro aniline (268). The hydrogenation of nitro groups and oxidation of the residual alcohol through an iron-mediated transfer hydrogenation mechanism can then yield α-keto amine intermediates (274) or (275). Finally, the α-keto amine intermediate (274 or 275) passes through intramolecular condensation and aromatization, affording the desired products (Scheme 89) (Putta, 2021).
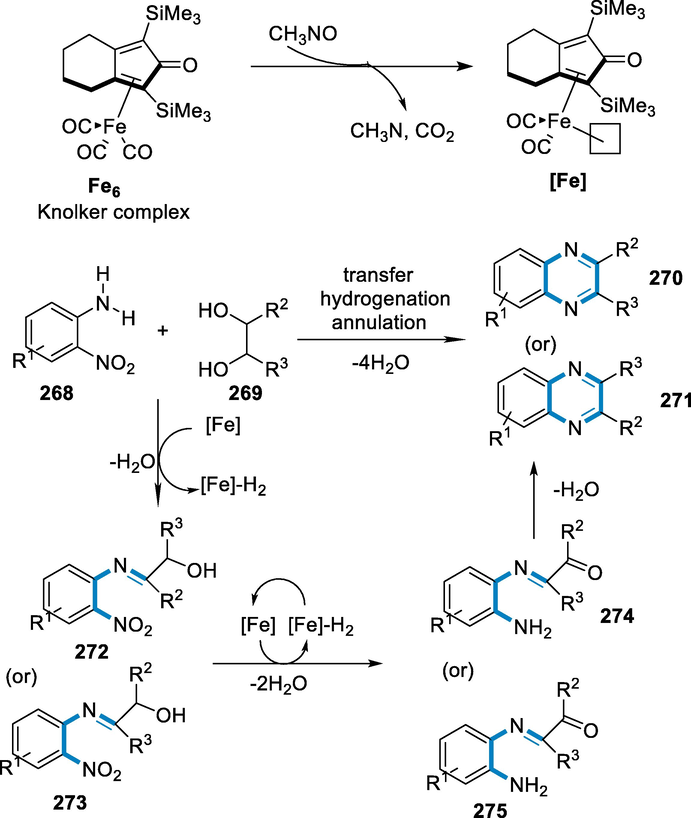
- Mechanistic pathway for the synthesis of quinoxalines.
Quinazolinone is a fundamental basis in natural compounds that have a wide variety of biochemical and pharmaceutical properties. The conversion of primary alcohols with o-amino benzamide into N-heterocycles, such as quinazolinone derivatives is effectively established using an iron-catalyzed one-step one-pot oxidative method. Both alkyl and aryl primary alcohols are reactive in the ecologically friendly methodology, which showed strong functional group compatibility.
The reaction conditions for the formation of quinazolinone (278) were various types of primary alcohols (276) and o-amino benzamide (277) as model substrates in the presence of catalyst FeCl3 (2 mol%), an oxidant in decane such as tert-butyl hydroperoxide(TBHP), DMSO, under an air atmosphere, at 60 °C temperature for 7 h. Numerous primary alcohols (276) were explored using the optimal conditions. Good to fair yields were obtained for several substituted benzyl alcohols with electron-donors and deficient groups. 2-furyl methanol, a heteroaryl substrate, yields the corresponding product in an excellent (76%) yield. More difficult alkyl primary alcohols, such as octanol, were used to illustrate the system's broad synthetic applicability, and the desired products were obtained with fair yields (Scheme 90) (Zhao, 2014).
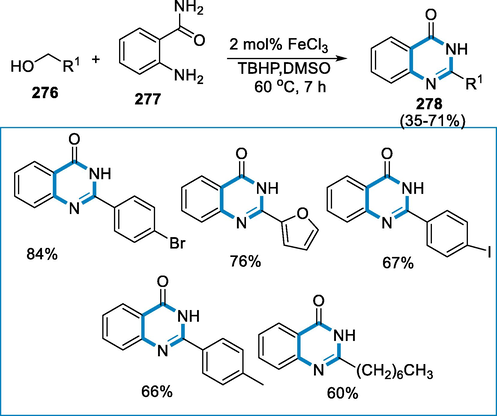
- Synthesis of quinazolinone derivatives using an iron-catalyzed one-pot oxidative method.
3.6 From amines
DBTD (3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides) is a key synthetic target in a wide range of physiologically active chemicals, psychoactive drugs, and artificial medicines. Using iron as the catalyst and air as the oxidant, a simple and one-pot synthesis of 3,4-dihydro-2H-1,2,4-benzothiadiazine1,1-dioxides by oxidative condensation of 2-amino benzenesulfonamide with benzylamines is generated. Under moderate conditions, this iron-catalyzed aerobic oxidative transformation has high efficiency, strong functional group tolerance, and broad substrate scope, and the use of air as the only oxidant makes this technique highly practical and cost-effective (Goffin, 2018; Impagnatiello, 1997; Bourasset, 2005; Phillips, 2002; Chen, 2010; Alukal and Lepor, 2016; Inagaki, 2013; Ravez, 2015).
The reaction conditions for the formation of 3,4-dihydro-2H-1,2,4- benzothiadiazine 1,1-dioxides (281) (DBTD) were 2-amino benzenesulfonamide (279) with a range of benzylamines (280) with catalyst FeBr2(10 mol%) and chlorobenzene at 100 °C temperature under air atmosphere. Evaluating 2-amino benzenesulfonamide (279) with a variety of benzylamines (280) revealed the substrate scope for the production of numerous DBTD derivatives (281). The condensation of benzylamines (280) with electron-rich and electron-deficient substituents on the aromatic rings was carried out easily, affording the required products in excellent quantities. Under the reaction circumstances, various types of functional groups such as F, Cl, CH3, OCH3, and R-O-CH2-O-R moieties were tolerated well. The reaction efficiency was unaffected by moving the OCH3 group from the para to the meta and ortho positions. The bulky substrate 1-(1-naphthyl)methanamine, in addition to benzylamines (280), was also compatible with this reaction, generating the desired product in 74% yield (Scheme 91).
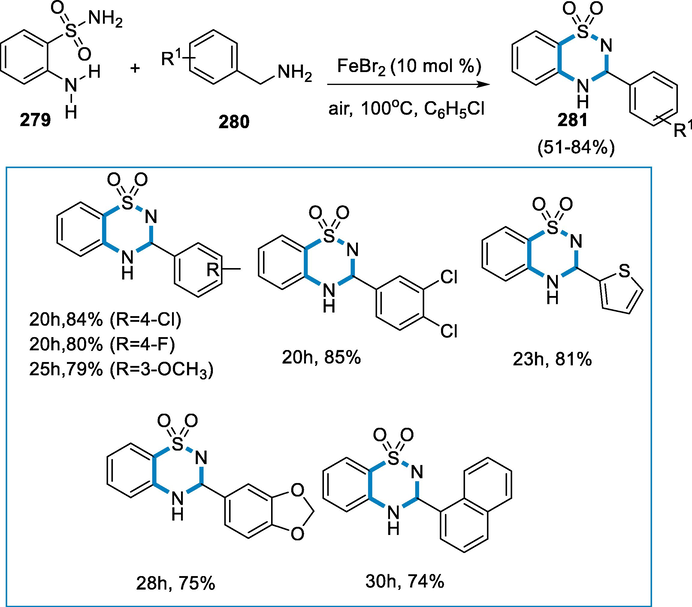
- One-pot synthesis of 3,4-dihydro-2H-1,2,4-benzothiadiazine1,1-dioxides by oxidative condensation.
The benzylamine (280) produces the homo-imine (282) by oxidative self-condensation, which has a probable mechanism for this oxidative process. The homo-imine (282) may then be readily transmitted using 2-amino benzenesulfonamide (279) to get amidoamine (283). At the same time, the aldehyde (284) produced in small amounts by the hydrolysis of imine (282) may condense with arylamines (280) and (279) to yield amidoamine (283). The required DBTD chemical (281) has been produced by intramolecular cyclization of aminoimine (283) obtained from (282), (284), and substrate (279) (Scheme 92) (Gopalaiah, 2019).
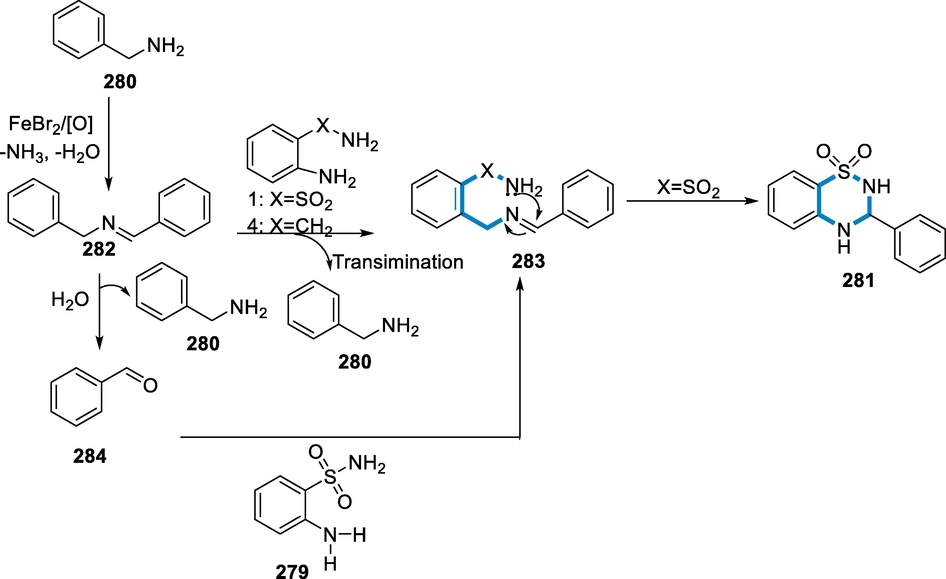
- Mechanistic pathway for the synthesis of 3,4-dihydro-2H-1,2,4-benzothiadiazine1,1-dioxides.
3.7 From alkenes
Aziridines are effective ligands for catalysis, pharmacophores, and flexible intermediates of bioactive materials and fine chemicals, hence aziridination processes are essential in the synthetic industry. Iron N,N′-dimethyl-N,N′-bis(2-pyridinylmethyl)cyclohexane-1,2-diamine-Fe(MCP)- and Fe(PDP)-type complexes catalyze an effective nitrogen-transfer process. The ligand's 2-pyridinylmethyl substitution does not affect reaction efficiency; however, modifications in the ligand are used to sterically protect the iron core which improves the reaction cycle. This aziridination reaction takes place under moderate circumstances and does not involve the addition of further olefin (Moragas, 2014; Ismail et al., 2009; Jarzyński, 2017; Ferioli, et al., 2005; Chen, 2013; Hu, 2004).
The alkene (285), PhINNs, as substrates for the synthesis of aziridines (286) in the presence of 5 mol% Fe7 complex and CH3CN at the temperature of 21 °C. Under the optimized conditions, limiting quantities of electronically varied styrenes react with moderate efficiency to generate racemic aziridines. The reaction tolerates a pendant acetate, nitrile, bromine atom, phenolic ether. Aziridination of isomerically pure cis-1-phenyl-1-propene proceeds with an erosion of the original olefin geometry (Scheme 93) (Shehata et al., 2018).
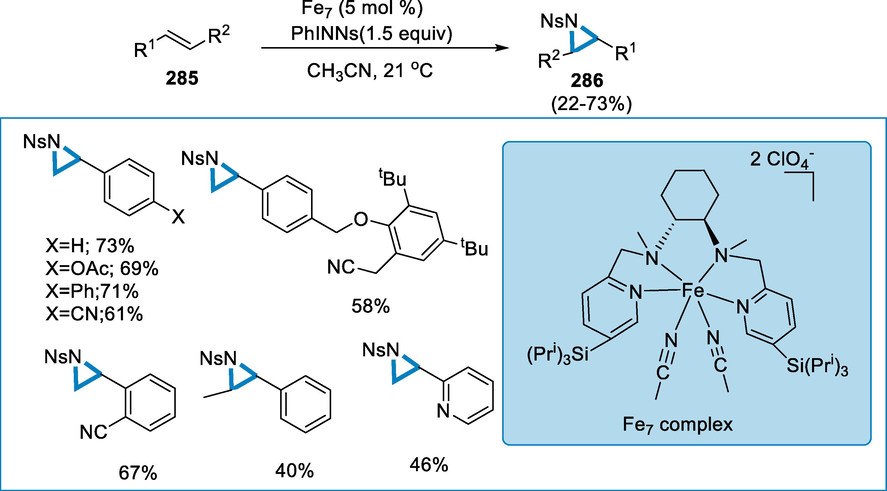
- Iron catalyzed synthesis of Aziridines.
Sulfone-containing oxindoles compounds have major functions in materials, chemical products, and medications. Sulfonyl substituted oxindoles, a new aerobic oxidative sulfonyl-carbocyclization of activated alkenes is developed. As a green oxidant, molecular oxygen is used, and it serves a critical role in beginning this transition, making the protocol relatively simple to follow. This sulfone integration process is ecologically friendly and practical since it uses easily accessible benzene sulfonic acids, an affordable iron salt catalyst, and air as the oxidant (Metzner and Thuillier, 2013; Renaud and Sibi, 2001).
The reaction conditions were N-methyl, N-aryl acrylamide (287) and benzenesulfinic acid by using 10% Fe(NO3)3·9H2O as a catalyst in MeCN at the temperature of 100 °C for 12 h in the presence of air for the synthesis of sulfone-containing oxindoles (288). Using the improved reaction conditions, the substrate scope of this aerobic sulfon-carbocyclization of alkenes was expanded to include different N-aryl acrylamides (287). N-aryl acrylamides (287) with both electron-rich and electron-deficient groups at the aromatic ring performed well and produced good to high yields of the respective products. In general, substrates that have an electron-deficient group can provide greater yields. Under these moderate reaction conditions, halo-substituents (F, Cl, Br, and I) were well tolerated with the N-Methyl-N-phenyl methacryl amides (287) which provide good yields (88–96%) of the respective halo-substituted oxindoles (Scheme 94).
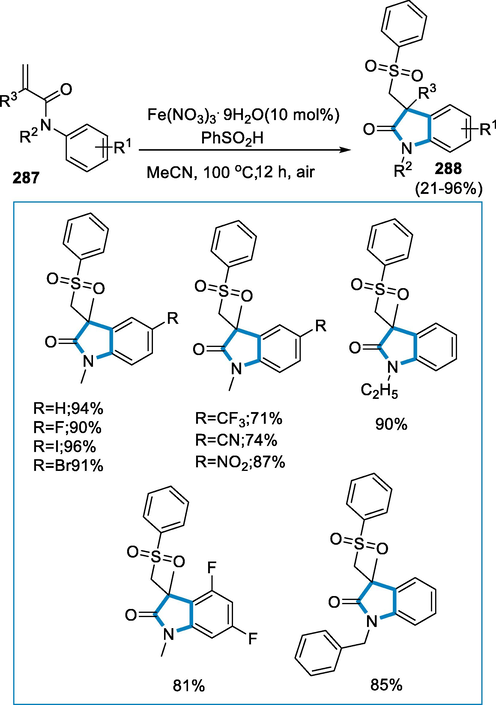
- Aerobic oxidative sulfonyl-carbocyclization of activated alkenes for the synthesis of Sulfonyl substituted oxindoles.
A plausible mechanism has been given; initially, O2 could readily begin with the sulfonyl radical. Following radical addition to activated alkene (287), a radical intermediate has been formed. The radical intermediate (289) undergoes intramolecular carbocyclization to produce the radical intermediate (290), which has to be oxidized by FeIII to produce the cationic intermediate (291) through the SET process. After a deprotonation process, intermediate (291) yields a terminal product (288). FeII might be easily oxidized to FeIII in the presence of O2 to fulfil the catalytic cycle (Scheme 95) (Shen, 2014).
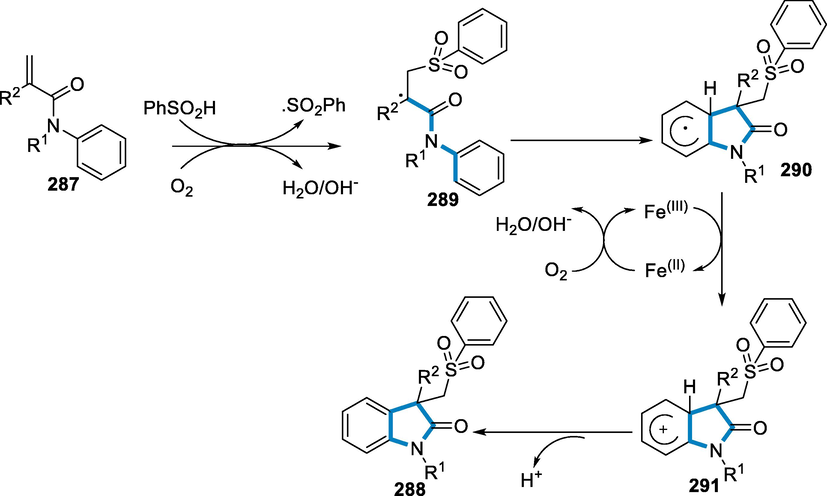
- Mechanistic pathway for the synthesis of Sulfonyl substituted oxindoles.
Aziridines are found in a variety of pharmacological and natural medicines with strong biological activity, and they are useful building blocks for a variety of N-heterocycles. The aziridination of olefins with equimolar quantities of iminoiodinane is possible due to a catalytic system based on ionic liquid, ferrous triflate, and quinaldic acid (Padwa and Woolhouse, 1984; Kasai and Kono, 1992; Reddy et al., 2005; Surendra et al., 2005; Evans et al., 1994; Fioravanti, 1985).
The reaction conditions were styrene derivatives (292) react with sulfonamides (293) in the presence of Fe(OTf)2 (5 mol%), quinaldic acid (294), and hydrophobic ethylmethylimidazolium bis[(trifluoromethyl)sulfonyl]- amide (emim BTA) (295) and CH3CN at 85 °C temperature for the synthesis of aziridines (296). The iron-catalyzed aziridination process worked well with styrene derivatives as a substrate and produced products with good to high yields. P-methyl styrene and p-nitrostyrene produced the greatest results. The corresponding aziridines (296) were obtained in acceptable yields from p-acetoxy styrene, p-cyanostyrene, and p-trifluoromethyl styrene (Scheme 96) (Mayer et al., 2008).
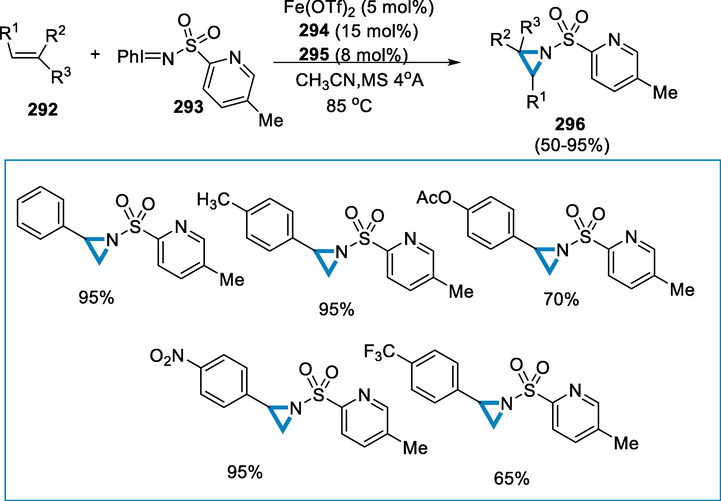
- Synthesis of aziridines by using iron as a catalyst.
Imidazoles and their derivatives are an important class of N-heterocycles chemicals which have a wide range of pharmaceutically active drugs and natural products. Multi-substituted imidazoles are important to core structures in synthetic chemistry because of their unique biological activity, including antimicrobial, anticancer, antibiotic, antivirus, and anti-inflammatory properties. Because of its low cost, simple accessibility, stability, non-toxicity, and eco-friendliness, iron catalysts are significant and efficient catalysts in numerous organic processes (Heeres and Antimycotic imidazoles. Part 4. , 1979; Hunkeler, 1981; Hamdouchi, 1999; Nakano, 2000; Pan, 2010).
The reaction conditions for the synthesis of imidazoles (299) were benzamidine (297) and 1-(2-nitro vinyl)-benzene (298) as a substrate in the presence of FeCl3(20 mmol%) catalyst and DMF at 90 °C for 4 h. The required compounds (299) were produced in good to high yields using a range of N-aryl benzamidines (297) as partners. High yields were found in benzamidines (297) with electron-rich substituents on R1 and R2. Electron-deficient substituted benzamidines, afforded low yields, with some (such as nitro) unable to produce the desired product. The yield was increased to 86 % when benzamidine contained two electron-rich groups (Scheme 97).
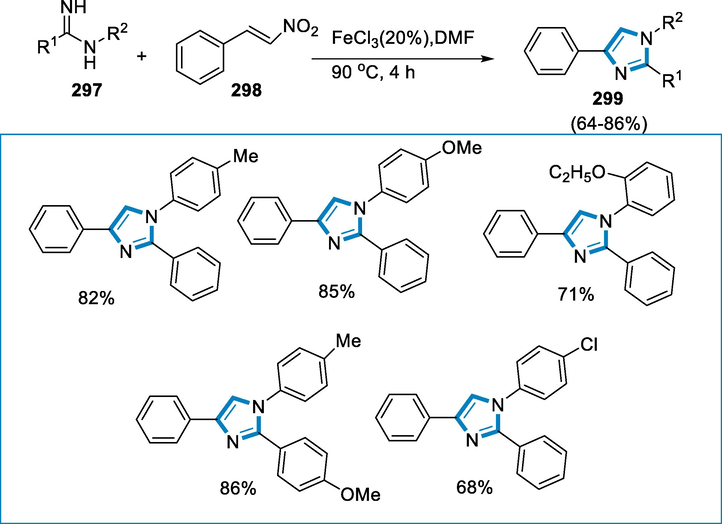
- Iron catalyzed synthesis of imidazoles.
The reaction's mechanism has been suggested as follows: Initially, the intermediate (302) was generated by adding N-p-tolyl benzamidine (300) to 1-(2-nitro vinyl)-benzene through Michael addition (298). The intermediate (303) was created from the (302) using FeCl3 as a Lewis acid and another intramolecular nucleophilic addition. After eliminating nitroxyl (HNO) and H2O, the end product (301) was produced from intermediate (303) (Scheme 98) (Liu et al., 2013).
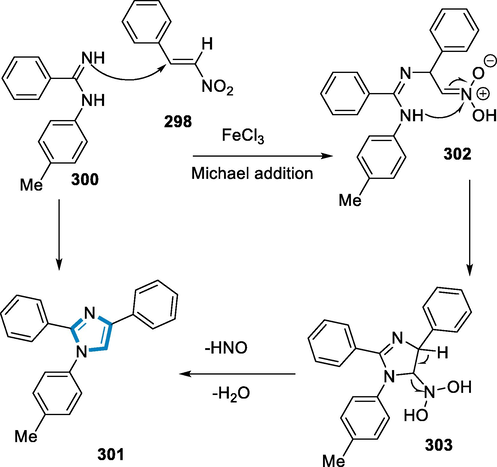
- Mechanistic pathway for the synthesis of imidazoles.
3.8 From miscellaneous compounds
A novel iron aziridination catalyst is developed from the tetraimidazolium substrate (Me,EtTCPh)(I)4, which is supported by a macrocyclic tetracarbene ligand. In a “C2 + N1″ addition reaction, this iron complex catalyzes the aziridination of electron-rich aryl azides and a broad variety of substituted aliphatic alkenes, including tetrasubstituted ones. Without a noticeable decrease in yield, the catalyst can be recovered and utilized up to three times more (Kasai and Kono, 1992; Liu et al., 2007; Colandrea et al., 2003; Sharma, 2009; Loncaric and Wulff, 2001).
Synthesis of aziridines (306) required the following reaction conditions; a 0.1 mol% catalyst loading of Fe8 (iron complex) with an excess of alkene (305), aryl azide (304), and without solvent. After 18 h at 90 °C, the reaction was complete (all of the organic azides had reacted), the reaction mixture was cooled to room temperature, and the catalyst was removed by filtration over Celite. The iron-catalyst effectively conducted aziridination with 1-decene and electron-deficient azides such as 1-azido-4-(trifluoromethyl)benzene. Both cis- and trans-substituted and disubstituted alkenes were found to be effective. With just 0.1% catalyst loading, the yield for 9-(p-tolyl)-9-azabicyclo[6.1.0]nonane was practically quantitative (97%). Because of the steric bulkiness of the propyl groups, the reaction with trans-4-octene was slow and generated less yield (Scheme 99).
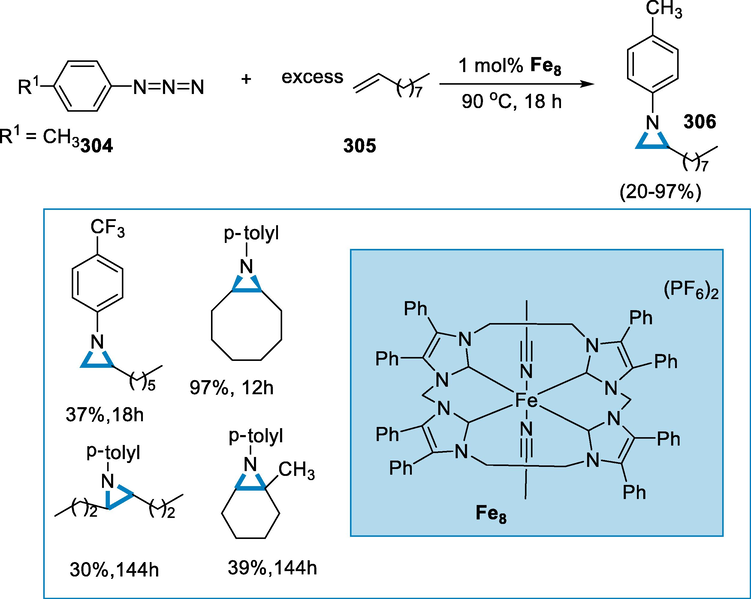
- C2 + N1″ addition reaction for the synthesis of aziridines by using iron complex.
A prospective intermediate in this reaction mechanism has an ironIV imide (308) in aziridination reactions with aryl azides (304). Iron imides in the 2+, 3+, and 4+ oxidation states are stabilized by three-fold-symmetric strong σ-donor ligands, however, these complexes do not react with alkenes to form aziridines (Scheme 100) (Cramer and Jenkins, 2011).
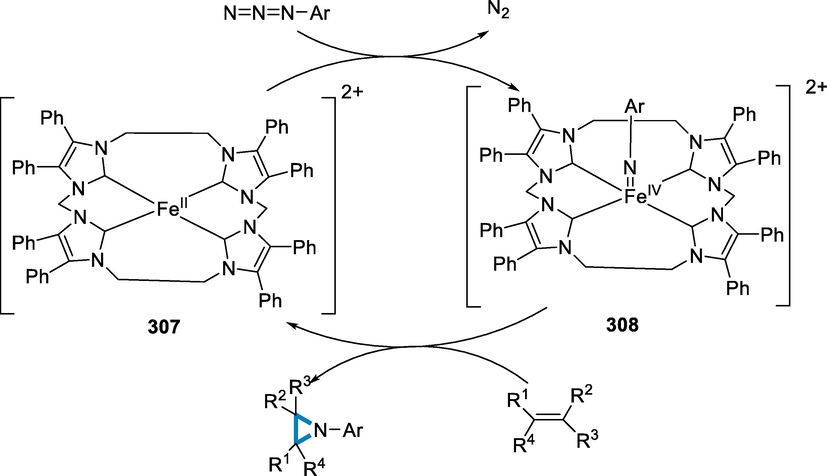
- Mechanistic pathway for the synthesis of aziridines.
Functionalized fullerenes, a family of high-performance nano-molecules, are finding numerous useful uses as supramolecular chemistry, adaptable building blocks in nanomaterials, pharmacology, and perovskite solar cells. For the synthesis of N-containing pyrrolidine- [2′,3′:1,2]fullerenes, an ironII-catalyzed redox-neutral radical cascade reaction of (Liu, 2019) fullerene with, γ,δ-unsaturated oxime esters is used. The transformation occurs through intramolecular cyclization and has a straight forward operation, functional group compatibility, a large substrate scope, and is also suitable for scale-up synthesis, allowing access to a variety of free pyrrolidino[2′,3′:1,2]fullerenes (Prato, 1997; Giacalone et al., 2009; Guldi, 2009; Imahori, 2007; Zieleniewska, 2018; Megiatto et al., 2020; Nakamura and Sawamura, 2001; Castro, 2017; Nierengarten, 2017; Li et al., 2012; Cui et al., 2017; Matsuo, 2012; Wang, 2015; Maroto, 2014; Li, 2016; Aghabali, 2016; Ueda, 2016; Zhou and Wang, 2016; Li et al., 2017; Hussain, 2019; Maroto, 2015; Giron, 2018; Maggini et al., 1993; Gan, 1998; Gan, 1996; Komori, 1996; Koeppe, 2005; Yang, 2016; Shi, 2016).
The reaction conditions for the formation of pyrrolidine- [2′,3′:1,2]fullerenes (310) were O-acyl oxime (309) in the presence of FeCl2·4H2O as a catalyst in a co-solvent of o-dichlorobenzene (ODCB) and CH3CN at various temperatures and time under a nitrogen atmosphere. The reaction conditions were used to test the redox-neutral cascade reaction of aryl and heteroaryl, γ,δ-unsaturated oxime esters tethered to a terminal olefinic unit. In good to high yields, all processes underwent cascade cycloaddition to produce desirable monosubstituted free pyrrolidino[2′,3′:1,2]fullerenes (310). The transition tolerated several functional groups that adorned the phenyl ring of oxime esters, such as OCH3, Br, I, and CN groups. When substrates were compared with electron-deficient groups and electron-rich groups, electron-rich groups had higher reactivity and yielded the desired product in a higher yield at a lower reaction temperature which demonstrates that the electronic effect of the substituent has a clear impact on the reaction (Scheme 101).
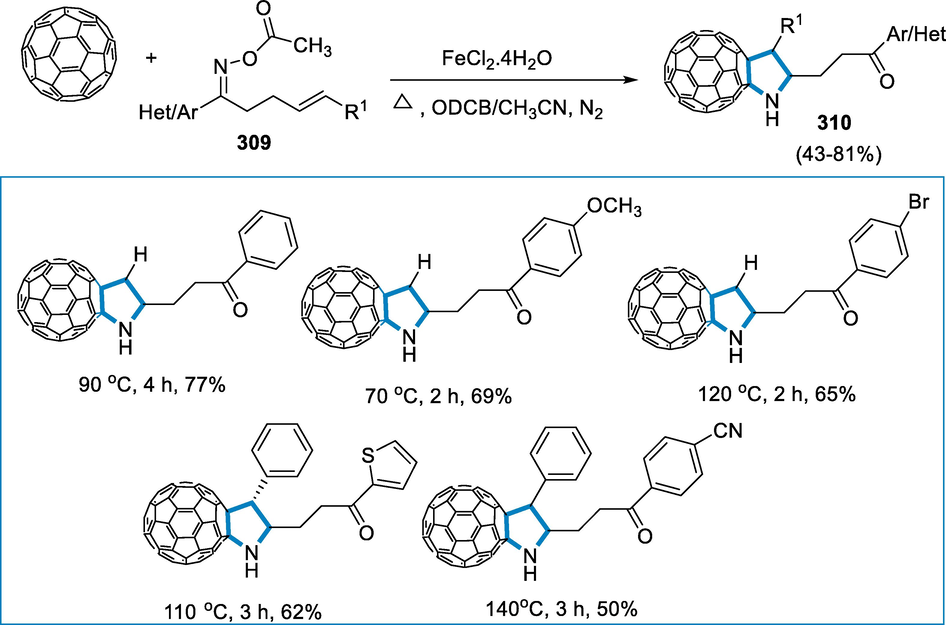
- IronII-catalyzed redox-neutral radical cascade reaction for the synthesis of functionalized fullerenes.
A plausible mechanism has been given; the iminium radical (311) has been formed by the oxidative addition of FeII to the NO bond of O-acyl oximes (309), which then undergoes intramolecular cyclization to give intermediate (312). The fullerene radical (313) has been formed when radical intermediate (312) has been added to C60. An intermolecular radical cyclization with an imine moiety occurs in route I, resulting in (314) intermediate. With the release of FeII, radical (314) has been oxidized by FeIII produced in situ to create cationic species (315). Following that, adding FeCl2·4H2O hydrate water and/or concurrent water to (315) produces the unstable intermediate (316), which then changes into the end product (310). Another, less desirable path-II cannot be entirely ruled out in comparison to pathway-I. In pathway-II, the fullerene radical (313) has been oxidized by FeIII to produce fullerene cation (317), which is then cyclized intermolecularly to produce iminium (318). After that, (318) goes through a similar hydrolysis procedure to get to (310). Furthermore, the process might occur in the presence of FeIII salts, implying that iron with a higher oxidation state could be produced, however, the specific mechanism of the FeIII-catalyzed reaction and the change in an oxidation state of FeIII remain unknown (Scheme 102) (Wu, 2020).
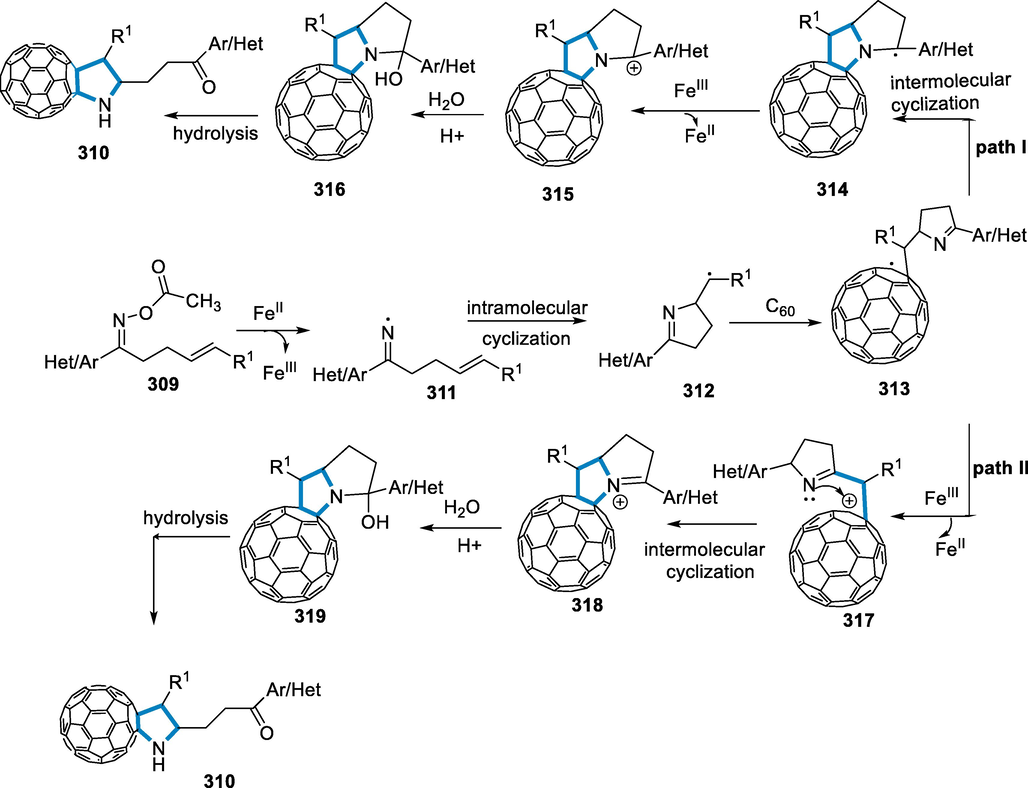
- Mechanistic pathway for the synthesis of functionalized fullerenes.
Bioactive natural items, medicines, and functional materials all include aza-heterocycles. (Vitaku et al., 2014; Łowicki and Przybylski, 2022; Pyta, 2022)-triazines are synthesized in the presence of iron catalyst by [3 + 3]-annulation of N-alkyl aziridines with bicyclic diaziridines. 1,3-dipolar cycloadditions are involving these two saturated building elements which provide a simple technique for delivering a synthetic basis for heterocycle synthesis, and the iron catalyst is the focus of recent sustainable cycloaddition reactions due to its low toxicity, environmental friendliness, and low cost (Vitaku et al., 2014; Bolm, 2004; Correa et al., 2008; Bauer and Knölker, 2015; Nakamura and Yamamoto, 2004; D'Souza and Mueller, 2007; Blunt, 2012; Shang et al., 2017; Enthaler et al., 2008; Fürstner, 2009; Kennedy, 2019).
The reaction conditions for the formation of (Vitaku et al., 2014; Łowicki and Przybylski, 2022; Pyta, 2022)-triazines (322) were a series of diaziridines (320) with 1-(4-chlorobenzyl)-2-(p-tolyl)aziridine (321) as a standard substrate with FeCl3 (10 mol%) as a catalyst in the presence of CH2Cl2 at room temperature for 5 min. Diaziridines (320) with both electron-donating and electron-withdrawing substituents at the 4-position of the aromatic rings, such as Br and OCH3 groups, were effectively involved in the reaction and produced high yields of cycloadducts. The reaction of the heteroaromatic diaziridines proceeded without a problem, yielding good results. In addition, polycyclic-aromatic diaziridines (320) with 2-fluorene and 3,3-disubstituted diaziridine functionality were shown to be accessible and yielded well. These findings imply that different substitution patterns in diaziridines (320) can also be responded to (Scheme 103).
![[3 + 3]-annulation of N-alkyl aziridines by iron catalyst.](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104095-fig105.png)
- [3 + 3]-annulation of N-alkyl aziridines by iron catalyst.
The chelation of unactivated aziridines (321) with FeCl3 can lead to the synthesis of (323), which can lead to stereospecific ring-opening utilizing azomethine imine intermediate (323) to provide 324, which indicates a probable process for the formation of (Vitaku et al., 2014; Łowicki and Przybylski, 2022; Pyta, 2022)-triazines (322). The latter can result in intramolecular cyclization, resulting in the delivery of the desired heterocycles (322). During the interactions of aziridine with the azomethine imine (323), the resulting diastereoselectivity has been predominantly regulated by steric considerations. As a result, the sterically less inhibited approach the (path-A) favoured the synthesis of the trans-selective cycloadduct. Path-B, which might lead to cis-selectivity, has unfavored due to steric hindrance (Scheme 104) (Sarkar, 2020).
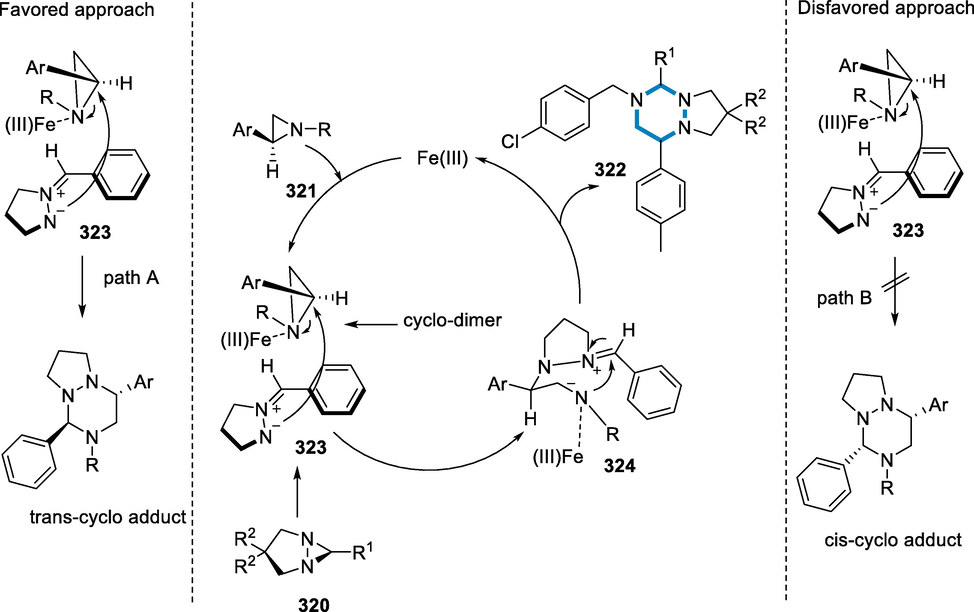
- Mechanistic pathway for the synthesis of (Vitaku et al., 2014; Łowicki and Przybylski, 2022; Pyta, 2022)-triazines.
4 Conclusion
Iron is one of the most abundant and non-toxic metals on earth due to which synthetic chemists are interested in using iron-based catalysts in organic synthesis. N-heterocycles are very active pharmacores and part of many FDA-approved drugs. Iron catalyzed synthesis of N-heterocycles is well explored by chemists. This review covered the synthesis of N-heterocycles such as indoles, indolines, pyridine, imidazoles, aziridines, benzoxazoles, quinazoline etc by inter- and intramolecular cyclization reactions via various iron complexes, salts and nano-particles. N-heterocylic fullurenes can also be synthesized by iron catalysts. Moreover, the mechanistic studies, substrate scope and the biological potential of the N-heterocycles are discussed. In future, chemists will discover new iron-based catalytic systems which should be greener, economic, industrious and sustainable. These catalytic systems will use to synthesize heterocycles by using abundant feedstocks. This review helps synthetic chemists and medicinal chemists to explore this valuable field for the synthesis of N-heterocyclic drugs.
Acknowledgement
This research was funded by china postdoctoral science foundation fund ZC304021910.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Recent applications of C-H functionalization in complex natural product synthesis. Chem. Soc. Rev.. 2018;47(23):8925-8967.
- [Google Scholar]
- Silver (I)-mediated modification, dimerization, and polymerization of an open-cage fullerene. J. Am. Chem. Soc.. 2016;138(50):16459-16465.
- [Google Scholar]
- Facile Synthesis of 5-Aryl-N-(pyrazin-2-yl) thiophene-2-carboxamides via Suzuki Cross-Coupling Reactions, Their Electronic and Nonlinear Optical Properties through DFT Calculations. Molecules. 2021;26(23):7309.
- [Google Scholar]
- Synthesis and biological properties of benzothiazole, benzoxazole, and chromen-4-one analogues of the potent antitumor agent 2-(3, 4-dimethoxyphenyl)-5-fluorobenzothiazole (PMX 610, NSC 721648) J. Med. Chem.. 2008;51(16):5135-5139.
- [Google Scholar]
- Present status of quinoxaline motifs: Excellent pathfinders in therapeutic medicine. Eur. J. Med. Chem.. 2014;85:688-715.
- [Google Scholar]
- Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med. Chem.. 2017;125:143-189.
- [Google Scholar]
- Synthesis and antihypertensive activity of novel 3-benzyl-2-substituted-3H-[1, 2, 4] triazolo [5, 1-b] quinazolin-9-ones. Bioorg. Med. Chem.. 2007;15(10):3457-3462.
- [Google Scholar]
- Aryl− aryl bond formation by transition-metal-catalyzed direct arylation. Chem. Rev.. 2007;107(1):174-238.
- [Google Scholar]
- Transition-metal-catalyzed addition of heteroatom− hydrogen bonds to alkynes. Chem. Rev.. 2004;104(6):3079-3160.
- [Google Scholar]
- Iron-Catalyzed Intramolecular C (sp2)− H Amination. Angew. Chem. Int. Ed.. 2016;55(4):1519-1522.
- [Google Scholar]
- Iron-Catalyzed Intramolecular Aminations of C (sp3)− H Bonds in Alkylaryl Azides. Angew. Chem. Int. Ed.. 2017;56(35):10582-10586.
- [Google Scholar]
- Structure-activity relationships in a series of novel 3, 4-dihydro-4-oxopyrimido [4, 5-b] quinoline-2-carboxylic acid antiallergy agents. J. Med. Chem.. 1980;23(3):262-269.
- [Google Scholar]
- Development of ethyl 3, 4-dihydro-4-oxopyrimido [4, 5-b] quinoline-2-carboxylate, a new prototype with oral antiallergy activity. J. Med. Chem.. 1979;22(1):44-48.
- [Google Scholar]
- Cascade polycyclisations in natural product synthesis. Org. Biomol. Chem.. 2011;9(11):3997-4006.
- [Google Scholar]
- Modern reduction methods. John Wiley & Sons; 2008.
- Book Review: Indole ring synthesis: From natural products to drug discovery. Curr. Org. Synth.. 2018;15(2):152-153.
- [Google Scholar]
- Ligand-Free Iron-Catalyzed C-F Amination of Diarylamines: A One-Pot Regioselective Synthesis of Diaryl Dihydrophenazines. Org. Lett.. 2019;21(2):461-464.
- [Google Scholar]
- Cascading pericyclic reactions: building complex carbon frameworks for natural product synthesis. Chem. Commun.. 2007;22:2211-2221.
- [Google Scholar]
- Iron–phosphonate nanomaterial: as a novel and efficient organic–inorganic hybrid catalyst for solvent-free synthesis of tri-substituted imidazole derivatives. J. Inorg. Organomet. Polym Mater.. 2020;30(7):2572-2581.
- [Google Scholar]
- Synthesis, antitumour activity and structure–activity relationships of 5H-benzo [b] carbazoles. Bioorg. Med. Chem.. 2005;13(3):819-837.
- [Google Scholar]
- Chemistry and biology of new marine alkaloids from the indole and annelated indole series. Curr. Med. Chem.. 2003;10(13):1113-1127.
- [Google Scholar]
- Organocobalt Compounds in the Synthesis of Pyridines? An Example of Structure? Effectivity Relationships in Homogeneous Catal sis. Angew. Chem., Int. Ed. Engl.. 1985;24(4):248-262.
- [Google Scholar]
- Benzoxazolone carboxamides as potent acid ceramidase inhibitors: synthesis and structure–activity relationship (SAR) studies. J. Med. Chem.. 2015;58(23):9258-9272.
- [Google Scholar]
- One-pot synthesis of pyridines or pyrimidines by tandem oxidation-heteroannulation of propargylic alcohols. Synlett. 2003;2003(10):1443-1446.
- [Google Scholar]
- Magnetically retrievable catalysts for organic synthesis. Chem. Commun.. 2013;49(8):752-770.
- [Google Scholar]
- Synthesis, anti-inflammatory evaluation and docking studies of some new fluorinated fused quinazolines. Eur. J. Med. Chem.. 2010;45(11):4904-4913.
- [Google Scholar]
- One-pot synthesis of tetrasubstituted imidazoles catalyzed by zeolite HY and silica gel under microwave irradiation. Green Chem.. 2000;2(6):274-276.
- [Google Scholar]
- In Comprehensive Heterocyclic Chemistry II; Katritzky, AR, Rees, CW, Scriven, EFV. Eds. Pergamon Press: Oxford. 1996;5:245.
- [Google Scholar]
- Nowe narzędzie do syntezy organicznych materiałów optoelektronicznych. Chemik. 2012;66(1):11-20.
- [Google Scholar]
- Synthesis, Characterization, and Catalytic Properties of Magnetic Fe3O4@ FU: A Heterogeneous Nanostructured Mesoporous Bio-Based Catalyst for the Synthesis of Imidazole Derivatives. Front. Chem.. 2020;8:1023.
- [Google Scholar]
- Catalytic functionalization of indoles in a new dimension. Angew. Chem. Int. Ed.. 2009;48(51):9608-9644.
- [Google Scholar]
- Quinoline-containing, conjugated poly (aryleneethynylene) s: Novel metal and H+-responsive materials. Macromolecules. 2002;35(5):1563-1568.
- [Google Scholar]
- New 2-heterocyclyl-imidazo [2, 1–i] purin-5-one derivatives as potent and selective human A3 adenosine receptor antagonists. J. Med. Chem.. 2011;54(14):5205-5220.
- [Google Scholar]
- C-N bond forming cross-coupling reactions: an overview. Chem. Soc. Rev.. 2013;42(24):9283-9303.
- [Google Scholar]
- The current status and future trends in oxidation chemistry. Adv. Synth. Catal.. 2004;346(2–3):107-108.
- [Google Scholar]
- Synthesis of Indoles via Intermolecular and Intramolecular Cyclization by Using Palladium-Based Catalysts. Catalysts. 2021;11(9):1018.
- [Google Scholar]
- Sustainable metal complexes for organic light-emitting diodes (OLEDs) Coord. Chem. Rev.. 2018;373:49-82.
- [Google Scholar]
- 2-Anilino-4-aryl-8H-purine derivatives as inhibitors of PDK1. Bioorg. Med. Chem. Lett.. 2012;22(8):2880-2884.
- [Google Scholar]
- Iron (II) Triflate as a Catalyst for the Synthesis of Indoles by Intramolecular C− H Amination. Org. Lett.. 2011;13(8):2012-2014.
- [Google Scholar]
- An Iron-Catalyzed Cascade Approach to Benzo [b] carbazole Synthesis Followed by 1, 4-Sulfonyl Migration. Chemistry–A. European Journal. 2015;21(8):3193-3197.
- [Google Scholar]
- Neuropharmacokinetics of a new α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (ampa) modulator, S18986 [(S)-2, 3-dihydro-[3, 4] cyclopentano-1, 2, 4-benzothiadiazine-1, 1-dioxide], in the rat. Drug Metab. Dispos.. 2005;33(8):1137-1143.
- [Google Scholar]
- Effect on K ATP Channel Activation Properties and Tissue Selectivity of the Nature of the Substituent in the 7-and the 3-Position of 4 H-1, 2, 4-Benzothiadiazine 1, 1-Dioxides. J. Med. Chem.. 2005;48(10):3492-3503.
- [Google Scholar]
- Synthesis of 3, 4-Dihydro-2 H-1, 2, 4-benzo-thiadiazine 1, 1-Dioxide Derivatives as Potential Allosteric Modulators of AMPA/Kainate Receptors. J. Med. Chem.. 2002;45(12):2355-2357.
- [Google Scholar]
- Indole, a core nucleus for potent inhibitors of tubulin polymerization. Med. Res. Rev.. 2007;27(2):209-238.
- [Google Scholar]
- Organic azides: an exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed.. 2005;44(33):5188-5240.
- [Google Scholar]
- Innate and guided C-H functionalization logic. Acc. Chem. Res.. 2012;45(6):826-839.
- [Google Scholar]
- On the role of metal contaminants in catalyses with FeCl3. Angew. Chem.. 2009;121(31):5694-5695.
- [Google Scholar]
- Angular methoxy-substituted furo-and pyranoquinolinones as blockers of the voltage-gated potassium channel Kv1. 3. J. Med. Chem.. 2001;44(8):1249-1256.
- [Google Scholar]
- A naturally occurring human DNA topoisomerase I poison. J. Am. Chem. Soc.. 2003;125(45):13628-13629.
- [Google Scholar]
- Iron (III)-Catalyzed and Air-Mediated Tandem Reaction of Aldehydes, Alkynes and Amines: An Efficient Approach to Substituted Quinolines. Chemistry–A. European Journal. 2009;15(26):6332-6334.
- [Google Scholar]
- Synthesis of substituted benzoxazoles by the iron (III)-catalyzed aerobic oxidation process. Science China Chemistry. 2010;53(1):130-134.
- [Google Scholar]
- Hydrogen atom transfer (HAT): a versatile strategy for substrate activation in photocatalyzed organic synthesis. Eur. J. Org. Chem.. 2017;2017(15):2056.
- [Google Scholar]
- Modulatory effects and action mechanisms of tryptanthrin on murine myeloid leukemia cells. Cell. Mol. Immunol.. 2009;6(5):335-342.
- [Google Scholar]
- Substituted imidazoles as glucagon receptor antagonists. Bioorg. Med. Chem. Lett.. 2001;11(18):2549-2553.
- [Google Scholar]
- Bioorg. Med. Chem. Lett. 2001;11(2549):10.1016.
- Selective functionalisation of saturated C-H bonds with metalloporphyrin catalysts. Chem. Soc. Rev.. 2011;40(4):1950-1975.
- [Google Scholar]
- Catalysis by Fe= X Complexes (X= NR, CR 2) In: Iron Catalysis. Springer; 2011. p. :111-138.
- [Google Scholar]
- Synthesis and antibacterial evaluation of certain quinolone derivatives. J. Med. Chem.. 2001;44(14):2374-2377.
- [Google Scholar]
- Synthesis and anti-inflammatory evaluation of 4-anilinofuro [2, 3-b] quinoline and 4-phenoxyfuro [2, 3-b] quinoline derivatives. Part 3. Bioorganic & medicinal chemistry. 2004;12(2):387-392.
- [Google Scholar]
- Efficient Aerobic Oxidative Synthesis of 2-Substituted Benzoxazoles, Benzothiazoles, and Benzimidazoles Catalyzed by 4-Methoxy-TEMPO. Angew. Chem. Int. Ed.. 2008;47(48):9330-9333.
- [Google Scholar]
- Acetic acid derivatives of 3, 4-dihydro-2 H-1, 2, 4-benzothiadiazine 1, 1-dioxide as a novel class of potent aldose reductase inhibitors. J. Med. Chem.. 2010;53(23):8330-8344.
- [Google Scholar]
- Application of chiral N-tert-butylsulfinyl vinyl aziridines in Rh (I) catalyzed 1, 4-addition of aryl boronic acids to cyclic enones. Chem. Commun.. 2013;49(57):6433-6435.
- [Google Scholar]
- Palladium (0)-Catalyzed Iminohalogenation of Alkenes: Synthesis of 2-Halomethyl Dihydropyrroles and Mechanistic Insights into the Alkyl Halide Bond Formation. Angew. Chem. Int. Ed.. 2015;54(10):3092-3096.
- [Google Scholar]
- Iron-catalyzed aerobic oxidative functionalization of sp 3 C-H bonds: a versatile strategy for the construction of N-heterocycles. Catal. Sci. Technol.. 2015;5(4):2197-2202.
- [Google Scholar]
- Intramolecular 1, 5-H transfer reaction of aryl iodides through visible-light photoredox catalysis: a concise method for the synthesis of natural product scaffolds. Chem. Commun.. 2016;52(38):6455-6458.
- [Google Scholar]
- Nitrogen heterocycle-containing materials for highly efficient phosphorescent OLEDs with low operating voltage. J. Mater. Chem. C. 2014;2(45):9565-9578.
- [Google Scholar]
- Rh (II)-Catalyzed formation of pyrrolo [2, 3-b] quinolines from azide-methylenecyclopropanes and isonitriles. Chem. Commun.. 2016;52(9):1967-1970.
- [Google Scholar]
- sp 3 C-H oxidation by remote H-radical shift with oxygen-and nitrogen-radicals: a recent update. Org. Biomol. Chem.. 2014;12(24):4051-4060.
- [Google Scholar]
- Getting down to earth: the renaissance of catalysis with abundant metals. Acc. Chem. Res.. 2015;48(9):2495.
- [Google Scholar]
- [2+ 2+ 2] cycloaddition reactions catalyzed by transition metal complexes. Adv. Synth. Catal.. 2006;348(16–17):2307-2327.
- [Google Scholar]
- Doctoring the bled: Medical auxiliaries and the administration of rural life in colonial Algeria, 1904-1954. Princeton University; 2014.
- Recent developments in the biology and medicinal chemistry of potassium channel modulators: update from a decade of progress. J. Med. Chem.. 2001;44(11):1627-1653.
- [Google Scholar]
- Synthesis of the mitomycin and FR900482 ring systems via dimethyldioxirane oxidation. Org. Lett.. 2003;5(6):785-787.
- [Google Scholar]
- Synthesis of quinazolinones and quinazolines. Tetrahedron. 2005;43(61):10153-10202.
- [Google Scholar]
- Synthesis of 4-hydroxy-β3-homoprolines and their insertion in α/β/α-tripeptides. Amino Acids. 2013;44(2):769-780.
- [Google Scholar]
- Direct catalytic asymmetric Mannich reactions and surroundings. Amino Group Chemistry: From Synthesis to the. Life Sci. 2007:185-205.
- [Google Scholar]
- Iron-and indium-catalyzed reactions toward nitrogen-and oxygen-containing saturated heterocycles. Acc. Chem. Res.. 2015;48(3):761-773.
- [Google Scholar]
- Iron-catalysed carbon–heteroatom and heteroatom–heteroatom bond forming processes. Chem. Soc. Rev.. 2008;37(6):1108-1117.
- [Google Scholar]
- Synthesis of aziridines from alkenes and aryl azides with a reusable macrocyclic tetracarbene iron catalyst. J. Am. Chem. Soc.. 2011;133(48):19342-19345.
- [Google Scholar]
- Fullerene derivatives for the applications as acceptor and cathode buffer layer materials for organic and perovskite solar cells. Adv. Energy Mater.. 2017;7(10):1601251.
- [Google Scholar]
- Iron-catalyzed cycloaddition of alkynenitriles and alkynes. Org. Lett.. 2011;13(11):2936-2939.
- [Google Scholar]
- Iron-catalyzed amination of strong aliphatic C (sp3)–H bonds. J. Am. Chem. Soc.. 2020;142(38):16211-16217.
- [Google Scholar]
- Environmentally benign synthesis of heterocyclic compounds by combined microwave-assisted heterogeneous catalytic approaches. Green Chem.. 2012;14(1):17-37.
- [Google Scholar]
- Catalytic C-H functionalization by metal carbenoid and nitrenoid insertion. Nature. 2008;451(7177):417-424.
- [Google Scholar]
- Toward tissue-selective pancreatic b-cells Katp channel openers belonging to 3-alkylamino-7-halo-4 H-1, 2, 4-benzothiadiazine 1, 1-dioxides. J. Med. Chem.. 2003;46(15):3342-3353.
- [Google Scholar]
- 3-Alkylamino-4 H-1, 2, 4-benzothiadiazine 1, 1-dioxides as atp-sensitive potassium channel openers: Effect of 6, 7-disubstitution on potency and tissue selectivity. J. Med. Chem.. 2005;48(15):4990-5000.
- [Google Scholar]
- Electrochemical applications. How click chemistry brought biomimetic models to the next level: electrocatalysis under controlled rate of electron transfer. Chem. Soc. Rev.. 2010;39(4):1291-1301.
- [Google Scholar]
- Synthesis of oxygen-and nitrogen-containing heterocycles by ring-closing metathesis. Chem. Rev.. 2004;104(5):2199-2238.
- [Google Scholar]
- Privileged structures: applications in drug discovery. Comb. Chem. High Throughput Screening. 2004;7(5):473-493.
- [Google Scholar]
- Transition metal catalyzed oxidative functionalization of carbon–hydrogen bonds. Tetrahedron. 2006;11(62):2439-2463.
- [Google Scholar]
- Selective Synthesis of 2, 3-Disubstituted-2 H-isoindol-1-ylphosphonate and 2, 3-Disubstituted-1, 2-dihydroiso-quinolin-1-ylphosphonate via Metal-Tuned Reaction of α-Amino (2-Alkynylphenyl) methylphosphonate. The Journal of organic chemistry. 2007;72(14):5439-5442.
- [Google Scholar]
- Highly efficient electrophilic cyclization of N′-(2-alkynylbenzylidene) hydrazides. Tetrahedron Lett.. 2009;50(3):340-342.
- [Google Scholar]
- Silica Gel-Promoted Tandem Addition− Cyclization Reactions of 2-Alkynylbenzenamines with Isothiocyanates. J. Comb. Chem.. 2010;12(3):370-373.
- [Google Scholar]
- Synthesis of 2-aminobenzothiazole via FeCl3-catalyzed tandem reaction of 2-iodoaniline with isothiocyanate in water. Green Chem.. 2010;12(9):1607-1610.
- [Google Scholar]
- An efficient route to 2, 3-disubstituted-1, 2-dihydroisoquinolin-1-ylphosphonates via CuI-catalyzed three-component reactions. Tetrahedron. 2007;63(49):12166-12171.
- [Google Scholar]
- Access to Functionalized Isoquinoline N-Oxides via Sequential Electrophilic Cyclization/Cross-Coupling Reactions. Adv. Synth. Catal.. 2008;350(11–12):1850-1854.
- [Google Scholar]
- AgOTf-catalyzed one-pot reaction of 2-alkynylbenzaldehyde, amine, and sodium borohydride. Tetrahedron Lett.. 2008;49(17):2752-2755.
- [Google Scholar]
- Tandem Electrophilic Cyclization−[3+ 2] Cycloaddition− Rearrangement Reactions of 2-Alkynylbenzaldoxime, DMAD, and Br 2. The Journal of organic chemistry. 2009;74(2):921-924.
- [Google Scholar]
- Tandem cyclization-[3+ 3] cycloaddition reactions of 2-alkynylbenzaldoxime: synthesis of fused 1, 2-dihydroisoquinolines. Tetrahedron Lett.. 2009;50(2):198-200.
- [Google Scholar]
- Lewis acid-and organocatalyst-cocatalyzed multicomponent reactions of 2-alkynylbenzaldehydes, amines, and ketones. Org. Lett.. 2007;9(24):4959-4962.
- [Google Scholar]
- A facile route to 2, 4-dihydro-1 H-benzo [d][1, 3] thiazines via silver-catalyzed tandem addition-cyclization reactions. J. Comb. Chem.. 2008;10(4):541-545.
- [Google Scholar]
- 16-membered macrolide lactone derivatives bearing a triazole-functionalized arm at the aglycone C13 position as antibacterial and anticancer agents. ChemMedChem. 2016;11(17):1886-1891.
- [Google Scholar]
- Novel natural product-and privileged scaffold-based tubulin inhibitors targeting the colchicine binding site. Molecules. 2016;21(10):1375.
- [Google Scholar]
- Synthesis of N-2-aryl-substituted 1, 2, 3-triazoles mediated by magnetic and recoverable CuFe 2 O 4 nanoparticles. Res. Chem. Intermed.. 2016;42(7):6231-6243.
- [Google Scholar]
- Drug discovery: a historical perspective. 2000;287:1960-1964.
- Multi-component syntheses of heterocycles by transition-metal catalysis. Chem. Soc. Rev.. 2007;36(7):1095-1108.
- [Google Scholar]
- An Effective [FeIII (TF4DMAP) Cl] Catalyst for C-H Bond Amination with Aryl and Alkyl Azides. Org. Lett.. 2019;21(4):895-899.
- [Google Scholar]
- Iron-Catalyzed Radical Relay Enabling the Modular Synthesis of Fused Pyridines from Alkyne-Tethered Oximes and Alkenes. Angew. Chem.. 2020;132(52):23963-23970.
- [Google Scholar]
- Organocatalytic Enantioselective Synthesis of Tetrahydropyridines. Eur. J. Org. Chem.. 2018;2018(20–21):2432-2442.
- [Google Scholar]
- Folate antagonists. 7. Antimalarial, antibacterial, and antimetabolite effects of 2, 4‐Diamino‐6‐(benzyl and pyridylmethyl)‐5, 6, 7, 8‐tetrahydropyrido [4, 3‐d] pyrimidines. J. Heterocycl. Chem.. 1972;9(5):1113-1121.
- [Google Scholar]
- Synthesis and biological evaluation of certain α, β-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J. Med. Chem.. 2000;43(15):2915-2921.
- [Google Scholar]
- Sustainable metal catalysis with iron: from rust to a rising star? Angew. Chem. Int. Ed.. 2008;47(18):3317-3321.
- [Google Scholar]
- Development of the copper-catalyzed olefin aziridination reaction. J. Am. Chem. Soc.. 1994;116(7):2742-2753.
- [Google Scholar]
- Therapeutic potential of A2 and A3 adenosine receptor: a review of novel patented ligands. Expert Opin. Ther. Pat.. 2012;22(4):369-390.
- [Google Scholar]
- Sulthiame add-on therapy in children with focal epilepsies associated with encephalopathy related to electrical status epilepticus during slow sleep (ESES) Epilepsia. 2012;53(7):1156-1161.
- [Google Scholar]
- Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr. Top. Med. Chem.. 2016;16(11):1200-1216.
- [Google Scholar]
- Steric Effects in Enantioselective Allylic Alkylation Catalysed by Cationic (η3‐Allyl) palladium Complexes Bearing Chiral Pyridine‐Aziridine Ligands. Wiley Online Library; 2005.
- N-Protected. alpha.-amino ketones from enamines and (ethoxycarbonyl) nitrene. J. Org. Chem.. 1985;50(25):5365-5368.
- [Google Scholar]
- Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol. Ther.. 1998;79(1):55-87.
- [Google Scholar]
- Synthesis and biological evaluation of substituted [18F] imidazo [1, 2-a] pyridines and [18F] pyrazolo [1, 5-a] pyrimidines for the study of the peripheral benzodiazepine receptor using positron emission tomography. J. Med. Chem.. 2008;51(13):3700-3712.
- [Google Scholar]
- Isolation and biosynthesis of preussin B, a pyrrolidine alkaloid from Simplicillium lanosoniveum. J. Nat. Prod.. 2014;77(4):813-817.
- [Google Scholar]
- From oblivion into the limelight: iron (domino) catalysis. Angew. Chem. Int. Ed.. 2009;48(8):1364-1367.
- [Google Scholar]
- Synthesis of fullerene amino acid derivatives by direct interaction of amino acid ester with C60. The Journal of Organic Chemistry. 1996;61(6):1954-1961.
- [Google Scholar]
- Synthesis of pyrrolidine ring-fused fullerene multicarboxylates by photoreaction. The Journal of Organic Chemistry. 1998;63(13):4240-4247.
- [Google Scholar]
- A new paradigm of copper oxide nanoparticles catalyzed reactions: Synthesis of 1, 2, 3-triazoles through oxidative azide-olefin cycloaddition. RSC Adv.. 2015;5(78):63473-63477.
- [Google Scholar]
- Synthesis of hierarchical graphdiyne-based architecture for efficient solar steam generation. Chem. Mater.. 2017;29(14):5777-5781.
- [Google Scholar]
- Regio-and Chemoselective Reduction of Nitroarenes and Carbonyl Compounds over Recyclable Magnetic Ferrite Nickel Nanoparticles (Fe3O4 Ni) by Using Glycerol as a Hydrogen Source. Chemistry–A. European Journal. 2012;18(40):12628-12632.
- [Google Scholar]
- Magnetically recyclable magnetite–ceria (Nanocat-Fe-Ce) nanocatalyst–applications in multicomponent reactions under benign conditions. Green Chem.. 2013;15(5):1226-1231.
- [Google Scholar]
- Magnetite-supported sulfonic acid: a retrievable nanocatalyst for the Ritter reaction and multicomponent reactions. Green Chem.. 2013;15(7):1895-1899.
- [Google Scholar]
- Nano-magnetite (Fe 3 O 4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev.. 2013;42(8):3371-3393.
- [Google Scholar]
- Biomimetic Iron-Catalyzed Asymmetric Epoxidations: Fundamental Concepts, Challenges and Opportunities. Adv. Synth. Catal.. 2014;356(2–3):261-299.
- [Google Scholar]
- Fullerene-Containing Polymers: An Overview. Fullerene Polymers: Synthesis, Properties and Applications 2009:1-14.
- [Google Scholar]
- Reversible Stereodivergent Cycloaddition of Racemic Helicenes to [60] Fullerene: A Chiral Resolution Strategy. Org. Lett.. 2018;20(7):1764-1767.
- [Google Scholar]
- 7-Phenoxy-substituted 3, 4-dihydro-2 H-1, 2, 4-benzothiadiazine 1, 1-dioxides as positive allosteric modulators of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) receptors with nanomolar potency. J. Med. Chem.. 2018;61(1):251-264.
- [Google Scholar]
- Ueber phenylirungen bei gegenwart von kupfer als katalysator. Ber. Dtsch. Chem. Ges.. 1906;39(2):1691-1692.
- [Google Scholar]
- Iron-Catalyzed Synthesis of α-Dienyl Five-and Six-Membered N-Heterocycles. Eur. J. Org. Chem.. 2017;2017(41):6160-6167.
- [Google Scholar]
- Dehydrogenative α-oxygenation of ethers with an iron catalyst. J. Am. Chem. Soc.. 2014;136(23):8350-8360.
- [Google Scholar]
- Straightforward Access to 3, 4-Dihydro-2H-1, 2, 4-benzothiadiazine 1, 1-dioxides and Quinazolines via Iron-Catalyzed Aerobic Oxidative Condensation of Amines. ChemistrySelect. 2019;4(18):5200-5205.
- [Google Scholar]
- Heterocyclic Scaffolds II:: Reactions and Applications of Indoles. Springer Science & Business Media; 2010.
- Ueber die einwirkung des cyans auf anthranilsäure. Ber. Dtsch. Chem. Ges.. 1869;2(1):415-418.
- [Google Scholar]
- Palladium catalysed bis-and tris-cyclisations furnishing fused cyclopropyl carbo/heterocycles. Tetrahedron. 2006;62(41):9523-9532.
- [Google Scholar]
- Koniamborine, the First Pyrano [3, 2-b] indole Alkaloid and Other Secondary Metabolites from Boronella k oniambiensis. J. Nat. Prod.. 2005;68(7):1083-1086.
- [Google Scholar]
- Iminyl radical-triggered intermolecular distal C (sp3)–H heteroarylation via 1, 5-hydrogen-atom transfer (HAT) cascade. Org. Lett.. 2019;21(4):917-920.
- [Google Scholar]
- FeCl3-catalyzed highly diastereoselective synthesis of substituted piperidines and tetrahydropyrans. Org. Lett.. 2010;12(8):1808-1811.
- [Google Scholar]
- Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci.. 2005;78(5):442-453.
- [Google Scholar]
- Bis and tris indole alkaloids from marine organisms: new leads for drug discovery. Curr. Med. Chem.. 2007;14(16):1789-1803.
- [Google Scholar]
- Synthesis and evaluation of in vitro antiproliferative activity of new ethyl 3-(arylethynyl) quinoxaline-2-carboxylate and pyrido [4, 3-b] quinoxalin-1 (2H)-one derivatives. Eur. J. Med. Chem.. 2016;124:959-966.
- [Google Scholar]
- 2-Amino-3-substituted-6-[(E)-1-phenyl-2-(N-methylcarbamoyl) vinyl] imidazo [1, 2-a] pyridines as a novel class of inhibitors of human rhinovirus: stereospecific synthesis and antiviral activity. J. Med. Chem.. 1999;42(1):50-59.
- [Google Scholar]
- The importance of indole and azaindole scaffold in the development of antitumor agents. Eur. J. Med. Chem.. 2020;203:112506
- [Google Scholar]
- Highly efficient Cu (I)-catalyzed synthesis of N-heterocycles through a cyclization-triggered addition of alkynes. J. Am. Chem. Soc.. 2010;132(3):916-917.
- [Google Scholar]
- Han, J., 2014. Synthesis of N-heterocycles through cyclization-triggered tandem additions to alkynes and the study of promoters in ionic reactions.
- The application of CuAAC ‘click’chemistry to catenane and rotaxane synthesis. Chem. Soc. Rev.. 2010;39(4):1240-1251.
- [Google Scholar]
- Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res.. 2008;41(11):1534-1544.
- [Google Scholar]
- Iron-catalyzed aromatic amination for nonsymmetrical triarylamine synthesis. J. Am. Chem. Soc.. 2012;134(50):20262-20265.
- [Google Scholar]
- J. Pharm. Sci. 1994;83:1425.
- Catalytic C-H amination at its limits: challenges and solutions. Org. Chem. Front.. 2017;4(12):2500-2521.
- [Google Scholar]
- Synthesis and antifungal activity of ketoconazole, a new potent orally active broad-spectrum antifungal agent. J. Med. Chem.. 1979;22(8):1003-1005.
- [Google Scholar]
- Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper (I) acetylides. Chem. Soc. Rev.. 2010;39(4):1302-1315.
- [Google Scholar]
- Targeting the α-folate receptor with cyclopenta [g] quinazoline-based inhibitors of thymidylate synthase. Bioorg. Med. Chem.. 2006;14(14):5020-5042.
- [Google Scholar]
- Complex N-heterocycle synthesis via iron-catalyzed, direct C-H bond amination. Science. 2013;340(6132):591-595.
- [Google Scholar]
- Nat. Biol: Chem; 2006.
- Recent strategies for the synthesis of pyridine derivatives. Chemistry–A. European Journal. 2010;16(40):12052-12062.
- [Google Scholar]
- Discovery and optimization of novel purines as potent and selective CB2 agonists. Bioorg. Med. Chem. Lett.. 2012;22(15):4962-4966.
- [Google Scholar]
- Fe3O4@ SiO2/Bipyridinium Nanocomposite as a Magnetic and Recyclable Heterogeneous Catalyst for the Synthesis of Highly Substituted Imidazoles Via Multi-Component Condensation Strategy. Polycyclic Aromat. Compd.. 2021;41(4):761-771.
- [Google Scholar]
- Pharmacological effects of a synthetic quinoline, a hybrid of tomoxiprole and naproxen, against acute pain and inflammation in mice: a behavioral and docking study. Iranian journal of basic medical sciences. 2017;20(4):446.
- [Google Scholar]
- 6-Alkylamino-and 2, 3-Dihydro-3 ‘-methoxy-2-phenyl-4-quinazolinones and related compounds: Their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J. Med. Chem.. 2000;43(23):4479-4487.
- [Google Scholar]
- Click chemistry generates privileged CH hydrogen-bonding triazoles: the latest addition to anion supramolecular chemistry. Chem. Soc. Rev.. 2010;39(4):1262-1271.
- [Google Scholar]
- Yungen Liu,[Guo-Qiang Chen,[Chun-Wai Tse,[Xianguo Guan,[Zheng-Jiang Xu,[b] Jie. Chem. Asian J. 2015;10:100-105.
- [Google Scholar]
- Practical methodologies for the synthesis of indoles. Chem. Rev.. 2006;106(7):2875-2911.
- [Google Scholar]
- 8-Fluoroimidazo [1, 2-a] pyridine: Synthesis, physicochemical properties and evaluation as a bioisosteric replacement for imidazo [1, 2-a] pyrimidine in an allosteric modulator ligand of the GABAA receptor. Bioorg. Med. Chem. Lett.. 2006;16(6):1518-1522.
- [Google Scholar]
- Condensed pyrimidine systems. XXIV. The condensation of 2, 4, 6-triaminopyrimidine with malondialdehyde derivatives. J. Med. Chem.. 1968;11(4):708-710.
- [Google Scholar]
- Palladium-catalyzed heteroannulation of indole-1-carboxamides with [60] fullerene and subsequent electrochemical transformations. Org. Lett.. 2019;21(21):8568-8571.
- [Google Scholar]
- Pd-catalyzed amidations of aryl chlorides using monodentate biaryl phosphine ligands: A kinetic, computational, and synthetic investigation. J. Am. Chem. Soc.. 2007;129(43):13001-13007.
- [Google Scholar]
- Creation of fullerene-based artificial photosynthetic systems. Bull. Chem. Soc. Jpn.. 2007;80(4):621-636.
- [Google Scholar]
- 7-Chloro-3-methyl-3, 4-dihydro-2H-1, 2, 4-benzothiadiazine S, S-dioxide: A partial modulator of AMPA receptor desensitization devoid of neurotoxicity. Proc. Natl. Acad. Sci.. 1997;94(13):7053-7058.
- [Google Scholar]
- Novel antiarthritic agents with 1, 2-isothiazolidine-1, 1-dioxide (γ-sultam) skeleton: cytokine suppressive dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase. J. Med. Chem.. 2000;43(10):2040-2048.
- [Google Scholar]
- Linagliptin provides effective, well-tolerated add-on therapy to pre-existing oral antidiabetic therapy over 1 year in Japanese patients with type 2 diabetes. Diabetes Obes. Metab.. 2013;15(9):833-843.
- [Google Scholar]
- Organic azides:“energetic reagents” for the intermolecular amination of C-H bonds. Chem. Commun.. 2014;50(78):11440-11453.
- [Google Scholar]
- Diastereoselective C− H bond amination for disubstituted pyrrolidines. Angew. Chem.. 2017;129(49):15805-15808.
- [Google Scholar]
- Characterization of iron-imido species relevant for N-group transfer chemistry. J. Am. Chem. Soc.. 2016;138(6):1983-1993.
- [Google Scholar]
- Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem.. 2009;44(9):3373-3387.
- [Google Scholar]
- Chemical Constituents of Glycosmis a rborea: Three New Carbazole Alkaloids and Their Biological Activity. J. Nat. Prod.. 2004;67(9):1488-1491.
- [Google Scholar]
- Sulfonated Honeycomb Coral (HC-SO3H): a new, green and highly efficient heterogeneous catalyst for the rapid one-pot pseudo-five component synthesis of 4, 4′-(aryl methylene) bis (3-methyl-1H-pyrazol-5-ol) s. Chem. Pap.. 2017;71(7):1351-1364.
- [Google Scholar]
- 6, 29210. CAS: Crossref; 2016.
- Fe (II)-Catalyzed Amination of Aromatic C− H Bonds via Ring Opening of 2 H-Azirines: Synthesis of 2, 3-Disubstituted Indoles. Org. Lett.. 2010;12(17):3736-3739.
- [Google Scholar]
- Synthesis of 2-aryl quinazolinones via iron-catalyzed cross-dehydrogenative coupling (CDC) between N-H and C–H bonds. Org. Biomol. Chem.. 2020;18(28):5435-5441.
- [Google Scholar]
- Efficient Amination of Activated and Non-Activated C (sp3)− H Bonds with a Simple Iron-Phenanthroline Catalyst. Angew. Chem. Int. Ed.. 2021;60(12):6314-6319.
- [Google Scholar]
- Highly enantioselective asymmetric reactions involving zinc ions promoted by chiral aziridine alcohols. Tetrahedron Asymmetry. 2017;28(12):1774-1779.
- [Google Scholar]
- Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev.. 2010;39(4):1272-1279.
- [Google Scholar]
- Iron-catalyzed/mediated oxidative transformation of C-H bonds. Org. Chem. Front.. 2014;1(2):194-214.
- [Google Scholar]
- Visible-Light-Promoted Iminyl-Radical Formation from Acyl Oximes: A Unified Approach to Pyridines, Quinolines, and Phenanthridines. Angew. Chem. Int. Ed.. 2015;54(13):4055-4059.
- [Google Scholar]
- Iminyl-Radicals by Oxidation of α-Imino-oxy Acids: Photoredox-Neutral Alkene Carboimination for the Synthesis of Pyrrolines. Angew. Chem.. 2017;129(40):12441-12444.
- [Google Scholar]
- Catalytic methods for aromatic C-H amination: an ideal strategy for nitrogen-based functional molecules. ACS Catal.. 2016;6(2):610-633.
- [Google Scholar]
- Riluzole series. Synthesis and in vivo “antiglutamate” activity of 6-substituted-2-benzothiazolamines and 3-substituted-2-imino-benzothiazolines. J. Med. Chem.. 1999;42(15):2828-2843.
- [Google Scholar]
- Nickel-catalyzed remote arylation of alkenyl aldehydes initiated by radical alkylation with tertiary α-carbonyl alkyl bromides. Org. Lett.. 2018;20(5):1435-1438.
- [Google Scholar]
- Heterocyclic chemistry at a glance. John Wiley & Sons; 2012.
- Heterocyclic chemistry. CRC Press; 2020.
- Development of potent chemical antituberculosis agents targeting Mycobacterium tuberculosis acetohydroxyacid synthase. Int. J. Antimicrob. Agents. 2016;48(3):247-258.
- [Google Scholar]
- The porphyrin handbook. World scientific. 2010;2011:1-20.
- Quinoline antibacterial agents. Oxolinic acid and related compounds. J. Med. Chem.. 1968;11(1):160-163.
- [Google Scholar]
- Synthesis of Functionalized Thiophene Based Pyrazole Amides via Various Catalytic Approaches: Structural Features through Computational Applications and Nonlinear Optical Properties. Molecules. 2022;27(2):360.
- [Google Scholar]
- Direct and practical synthesis of 2-arylbenzoxazoles promoted by activated carbon. Org. Lett.. 2003;5(20):3713-3715.
- [Google Scholar]
- Boehmite nanoparticles, an efficient green catalyst for the multi-component synthesis of highly substituted imidazoles. Appl. Catal. A. 2013;467:291-300.
- [Google Scholar]
- Regio-and diastereoselective iron-catalyzed [4+ 4]-cycloaddition of 1, 3-dienes. J. Am. Chem. Soc.. 2019;141(21):8557-8573.
- [Google Scholar]
- Efficient electrogenerated chemiluminescence from cyclometalated iridium (III) complexes. J. Am. Chem. Soc.. 2005;127(6):1614-1615.
- [Google Scholar]
- Catalytic C− H bond amination from high-spin iron imido complexes. J. Am. Chem. Soc.. 2011;133(13):4917-4923.
- [Google Scholar]
- Pharmaceutical substances, synthesis, patents, applications (4th edn Thieme). Stuttgart; 2001.
- Iron-catalysed carbon–carbon single bond activation. Org. Biomol. Chem.. 2013;11(8):1271-1279.
- [Google Scholar]
- Synthesis, antibacterial, and anticancer evaluation of novel spiramycin-like conjugates containing C (5) triazole arm. J. Med. Chem.. 2016;59(17):7963-7973.
- [Google Scholar]
- Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev.. 2002;102(11):4303-4428.
- [Google Scholar]
- An alkaloid with high antimalarial activity from Dichroa Febrifuga1. J. Am. Chem. Soc.. 1947;69(7):1837.
- [Google Scholar]
- Complexation of pyrrolidinofullerenes and zinc-phthalocyanine in a bilayer organic solar cell structure. Appl. Phys. Lett.. 2005;87(24):244102
- [Google Scholar]
- Unusual reactions of C60 with aldehydes in the presence of aqueous ammonia. Tetrahedron Lett.. 1996;37(23):4031-4034.
- [Google Scholar]
- Nickel-catalyzed cyclization of 2-iodoanilines with aroylalkynes: An efficient route for quinoline derivatives. The Journal of organic chemistry. 2006;71(18):7079-7082.
- [Google Scholar]
- Isomeric quinoxalinedicarbonitrile as color-managing acceptors of thermally activated delayed fluorescent emitters. ACS Appl. Mater. Interfaces. 2019;11(19):17583-17591.
- [Google Scholar]
- Structure− activity relationships of novel 2-substituted quinazoline antibacterial agents. J. Med. Chem.. 1999;42(22):4705-4713.
- [Google Scholar]
- Piperlongumine derived cyclic sulfonamides (sultams): Synthesis and in vitro exploration for therapeutic potential against HeLa cancer cell lines. Eur. J. Med. Chem.. 2017;126:870-878.
- [Google Scholar]
- Synthesis and reactivity of propargylamines in organic chemistry. Chem. Rev.. 2017;117(24):14091-14200.
- [Google Scholar]
- Iron-Catalyzed Reductive Cyclization by Hydromagnesiation: A Modular Strategy Towards N-Heterocycles. Angew. Chem. Int. Ed. 2021
- [Google Scholar]
- Controlled emission colors and singlet–triplet energy gaps of dihydrophenazine-based thermally activated delayed fluorescence emitters. J. Mater. Chem. C. 2015;3(10):2175-2181.
- [Google Scholar]
- Enhanced electrocatalytic activity via phase transitions in strongly correlated SrRuO 3 thin films. Energy Environ. Sci.. 2017;10(4):924-930.
- [Google Scholar]
- The purines: Potent and versatile small molecule inhibitors and modulators of key biological targets. Bioorg. Med. Chem.. 2006;14(12):3987-4006.
- [Google Scholar]
- Phase tag-assisted synthesis of benzo [b] carbazole end-capped oligothiophenes. Org. Lett.. 2012;14(22):5744-5747.
- [Google Scholar]
- A Sm (II)-Mediated cascade approach to dibenzoindolo [3, 2-b] carbazoles: Synthesis and evaluation. Org. Lett.. 2014;16(8):2292-2295.
- [Google Scholar]
- Synthesis and antiviral activity of imidazo [1, 2-a] pyridines. Eur. J. Med. Chem.. 1999;34(3):271-274.
- [Google Scholar]
- Cross Dehydrogenative Arylation (CDA) of a Benzylic C H Bond with Arenes by Iron Catalysis. Angew. Chem. Int. Ed.. 2009;48(21):3817-3820.
- [Google Scholar]
- Iron-catalyzed cascade reaction of ynone with o-aminoaryl compounds: a Michael addition–cyclization approach to 3-carbonyl quinolines. Tetrahedron Lett.. 2011;52(4):530-533.
- [Google Scholar]
- FeCl3-promoted formation of C-C bonds: synthesis of substituted quinolines from imines and electron-deficient alkynes. Tetrahedron. 2014;70(46):8971-8975.
- [Google Scholar]
- Fullerene-Based Macro-Heterocycle Prepared through Selective Incorporation of Three N and Two O Atoms into C60. Angew. Chem. Int. Ed.. 2016;55(47):14590-14594.
- [Google Scholar]
- Distal radical migration strategy: an emerging synthetic means. Chem. Soc. Rev.. 2018;47(3):654-667.
- [Google Scholar]
- Novel mitochondrial-targeted thiadiazolo [3, 4-g] quinoxaline dyes as efficient photosensitizers for ultra-low dose operable photodynamic therapy. Chem. Commun.. 2019;55(76):11390-11393.
- [Google Scholar]
- FeCl2-Catalyzed Selective C C Bond Formation by Oxidative Activation of a Benzylic C H Bond. Angew. Chem. Int. Ed.. 2007;46(34):6505-6507.
- [Google Scholar]
- Palladium-catalyzed synthesis of [60] fullerene-fused benzofurans via heteroannulation of phenols. Chem. Commun.. 2017;53(11):1852-1855.
- [Google Scholar]
- Functional fullerenes for organic photovoltaics. J. Mater. Chem.. 2012;22(10):4161-4177.
- [Google Scholar]
- Iron-Catalyzed C C Bond Formation by Direct Functionalization of C H Bonds Adjacent to Heteroatoms. Angew. Chem. Int. Ed.. 2008;47(39):7497-7500.
- [Google Scholar]
- Iron-Catalyzed, Iminyl Radical-Triggered Cascade 1, 5-Hydrogen Atom Transfer/(5+ 2) or (5+ 1) Annulation: Oxime as a Five-Atom Assembling Unit. Angew. Chem. Int. Ed.. 2020;59(43):19222-19228.
- [Google Scholar]
- Rational design of efficient orange-red to red thermally activated delayed fluorescence emitters for OLEDs with external quantum efficiency of up to 26.0% and reduced efficiency roll-off. J. Mater. Chem. C. 2020;8(5):1614-1622.
- [Google Scholar]
- Intramolecular charge transfer and ion pairing in N, N-diaryl dihydrophenazine photoredox catalysts for efficient organocatalyzed atom transfer radical polymerization. J. Am. Chem. Soc.. 2017;139(1):348-355.
- [Google Scholar]
- Synthesis of substituted quinolines by iron-catalyzed oxidative coupling reactions. Tetrahedron Lett.. 2012;53(49):6654-6656.
- [Google Scholar]
- Nonheme iron-mediated amination of C (sp3)–H bonds. Quinquepyridine-supported iron-imide/nitrene intermediates by experimental studies and DFT calculations. J. Am. Chem. Soc.. 2013;135(19):7194-7204.
- [Google Scholar]
- [Fe (F20TPP) Cl]-Catalyzed Amination with Arylamines and [Fe (F20TPP)(NAr)](PhI NAr) Intermediate Assessed by High-Resolution ESI-MS and DFT Calculations. Chemistry–An Asian Journal. 2015;10(1):100-105.
- [Google Scholar]
- Iron-catalyzed C-H amination and its application in organic synthesis. Tetrahedron. 2019;75(44):130607
- [Google Scholar]
- Iron-catalyzed intramolecular amination of aliphatic C-H bonds of sulfamate esters with high reactivity and chemoselectivity. Org. Lett.. 2019;21(8):2673-2678.
- [Google Scholar]
- Iron-and cobalt-catalyzed C (sp 3)–H bond functionalization reactions and their application in organic synthesis. Chem. Soc. Rev.. 2020;49(15):5310-5358.
- [Google Scholar]
- Synthesis and pesticidal activities of new quinoxalines. J. Agric. Food. Chem.. 2020;68(28):7324-7332.
- [Google Scholar]
- [FeIII (F20-tpp) Cl] Is an Effective Catalyst for Nitrene Transfer Reactions and Amination of Saturated Hydrocarbons with Sulfonyl and Aryl Azides as Nitrogen Source under Thermal and Microwave-Assisted Conditions. Chemistry–A. European Journal. 2010;16(34):10494-10501.
- [Google Scholar]
- Copper-catalyzed tethered aziridination of unsaturated N-tosyloxy carbamates. The Journal of organic chemistry. 2007;72(15):5587-5591.
- [Google Scholar]
- Iron (III)-catalyzed synthesis of multi-substituted imidazoles via [3+ 2] cycloaddition reaction of nitroolefins and N-aryl benzamidines. Tetrahedron. 2013;69(45):9417-9421.
- [Google Scholar]
- [Fe (F 20 TPP) Cl] catalyzed intramolecular C-N bond formation for alkaloid synthesis using aryl azides as nitrogen source. Chem. Commun.. 2010;46(37):6926-6928.
- [Google Scholar]
- Preparation and antiinflammatory activity of some nonacidic trisubstituted imidazoles. J. Med. Chem.. 1974;17(11):1182-1188.
- [Google Scholar]
- An efficient synthesis of (−)-Chloramphenicol via asymmetric catalytic aziridination: a comparison of catalysts prepared from triphenylborate and various linear and vaulted biaryls. Org. Lett.. 2001;3(23):3675-3678.
- [Google Scholar]
- Enantioselective C− H Activation with Earth-Abundant 3d Transition Metals. Angew. Chem. Int. Ed.. 2019;58(37):12803-12818.
- [Google Scholar]
- Cascade synthetic strategies opening access to medicinal-relevant aliphatic 3-and 4-membered N-heterocyclic scaffolds. Eur. J. Med. Chem. 2022114438
- [Google Scholar]
- Palladium-catalyzed ligand-directed C− H functionalization reactions. Chem. Rev.. 2010;110(2):1147-1169.
- [Google Scholar]
- Two new pyrroloquinazolinoquinoline alkaloids from Peganum nigellastrum. Heterocycles. 1997;46:541-546.
- [Google Scholar]
- Luotonin A: a lead toward anti-cancer agent development. Heterocycles. 2005;65(9):2203-2219.
- [Google Scholar]
- Synthesis of C8 N9 Annulated Purines by Iron-Catalyzed C H Amination. Chemistry–A. European Journal. 2013;19(28):9137-9141.
- [Google Scholar]
- Highly efficient and eco-friendly synthesis of 2-alkyl and 2-aryl-4, 5-diphenyl-1H-imidazoles under mild conditions. Tetrahedron Lett.. 2013;54(21):2591-2594.
- [Google Scholar]
- Addition of azomethine ylides to C60: synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc.. 1993;115(21):9798-9799.
- [Google Scholar]
- Facile synthesis of substituted quinolines by iron (iii)-catalyzed cascade reaction between anilines, aldehydes and nitroalkanes. Org. Biomol. Chem.. 2019;17(34):7907-7917.
- [Google Scholar]
- In situ click chemistry: probing the binding landscapes of biological molecules. Chem. Soc. Rev.. 2010;39(4):1252-1261.
- [Google Scholar]
- Chiral fullerenes from asymmetric catalysis. Acc. Chem. Res.. 2014;47(8):2660-2670.
- [Google Scholar]
- Enantiospecific cis–trans Isomerization in Chiral Fulleropyrrolidines: Hydrogen-Bonding Assistance in the Carbanion Stabilization in H2O@ C60. J. Am. Chem. Soc.. 2015;137(3):1190-1197.
- [Google Scholar]
- Direct synthesis of nitriles from aldehydes and hydroxylamine hydrochloride catalyzed by a HAP@ AEPH2-SO3H nanocatalyst. Aust. J. Chem.. 2016;70(1):33-43.
- [Google Scholar]
- A 1, 8-naphthyridone derivative targets the HIV-1 Tat-mediated transcription and potently inhibits the HIV-1 replication. J. Med. Chem.. 2010;53(2):641-648.
- [Google Scholar]
- Design concept for high-LUMO-level fullerene electron-acceptors for organic solar cells. Chem. Lett.. 2012;41(8):754-759.
- [Google Scholar]
- A concise synthesis of the antitumour alkaloid ellipticine. J. Chem. Soc., Chem. Commun.. 1984;14:926-927.
- [Google Scholar]
- Iron-catalysed aziridination reactions promoted by an ionic liquid. Chem. Commun.. 2008;45:5975-5977.
- [Google Scholar]
- Synthesis of phosphorus and sulfur heterocycles via ring-closing olefin metathesis. Chem. Rev.. 2004;104(5):2239-2258.
- [Google Scholar]
- Design, synthesis and photoinduced processes in molecular interlocked photosynthetic [60] fullerene systems. Chem. Soc. Rev.. 2020;49(1):8-20.
- [Google Scholar]
- (S)-Pyrrolidine sulfonamide catalyzed asymmetric direct aldol reactions of aryl methyl ketones with aryl aldehydes. Tetrahedron Lett.. 2008;49(17):2681-2684.
- [Google Scholar]
- Sulfur reagents in organic synthesis. Elsevier; 2013.
- The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron. 2006;62(42):9787-9826.
- [Google Scholar]
- Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep.. 2004;21(5):650-668.
- [Google Scholar]
- Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep.. 2005;22(5):627-646.
- [Google Scholar]
- RSC Adv.. 2012;2:8883. (c) H.-J. Knölker and KR Reddy. Chem. Rev, 2002. 102: p. 4303
- Synthesis and biological activity of 4-methylene-pyrido [4, 3-d] pyrimidines. J. Heterocycl. Chem.. 2009;46(4):579-583.
- [Google Scholar]
- Selective Iron-Catalyzed Oxidation of Phenols and Arenes with Hydrogen Peroxide: Synthesis of Vitamin E Intermediates and Vitamin K3. Chemistry–A. European Journal. 2010;16(34):10300-10303.
- [Google Scholar]
- Iron Catalyzed Synthesis of Pyrimidines Under Air. Adv. Synth. Catal.. 2020;362(3):594-600.
- [Google Scholar]
- Asymmetric synthesis of trisubstituted aziridines via aza-Darzens reaction of chiral sulfinimines. Org. Lett.. 2014;16(24):6290-6293.
- [Google Scholar]
- Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science. 2017;355(6326):727-730.
- [Google Scholar]
- Mechanism and impact of allosteric AMPA receptor modulation by the AmpakineTM CX546. Neuropharmacology. 2001;41(6):650-663.
- [Google Scholar]
- Nakamura, E., Sawamura, M., 2001. Pure and Applied Chem. 73, 355; e) Nakamura, E., Isobe, H., 2003. Acc. Chem. Res. 36: p. 807.
- Transition-metal-catalyzed reactions in heterocyclic synthesis. Chem. Rev.. 2004;104(5):2127-2198.
- [Google Scholar]
- Low-valent iron-catalyzed C− C bond formation− addition, substitution, and C− H bond activation. The Journal of Organic Chemistry. 2010;75(18):6061-6067.
- [Google Scholar]
- Synthesis and biological activities of novel antiallergic agents with 5-lipoxygenase inhibiting action. Bioorg. Med. Chem.. 2000;8(2):373-380.
- [Google Scholar]
- Recognition of a single guanine bulge by 2-acylamino-1, 8-naphthyridine. J. Am. Chem. Soc.. 2000;122(10):2172-2177.
- [Google Scholar]
- Improved selectivity for the binding of naphthyridine dimer to guanine–guanine mismatch. Bioorg. Med. Chem.. 2001;9(9):2381-2385.
- [Google Scholar]
- 1, n-Hydrogen-Atom Transfer (HAT) Reactions in Which n≠ 5: An Updated Inventory. Chemistry–A. European Journal. 2014;20(49):16034-16059.
- [Google Scholar]
- Cu (II) immobilized on guanidinated epibromohydrin-functionalized γ-Fe2O3@ TiO2 (γ-Fe2O3@ TiO2-EG-Cu (II)): A highly efficient magnetically separable heterogeneous nanocatalyst for one-pot synthesis of highly substituted imidazoles. Appl. Organomet. Chem.. 2018;32(2):e4095
- [Google Scholar]
- Synthesis of 13 H-benzo [6, 7]-and 13 H-benzo [4, 5] indolo [3, 2-c]-quinolines: a new series of potent specific ligands for triplex DNA. J. Am. Chem. Soc.. 1998;120(11):2501-2507.
- [Google Scholar]
- Selective autoxidation of benzylamines: application to the synthesis of some nitrogen heterocycles. Green Chem.. 2013;15(10):2713-2717.
- [Google Scholar]
- anti-Markovnikov Hydroamination of Alkenes Catalyzed by a Two-Component Organic Photoredox System: Direct Access to Phenethylamine Derivatives. Angew. Chem. Int. Ed.. 2014;53(24):6198-6201.
- [Google Scholar]
- The art of total synthesis through cascade reactions. Chem. Soc. Rev.. 2009;38(11):2993-3009.
- [Google Scholar]
- Fullerene hexa-adduct scaffolding for the construction of giant molecules. Chem. Commun.. 2017;53(87):11855-11868.
- [Google Scholar]
- Enantioselective intramolecular Michael addition of nitronates onto conjugated esters: access to cyclic γ-amino acids with up to three stereocenters. J. Am. Chem. Soc.. 2009;131(44):16016-16017.
- [Google Scholar]
- Iron-Catalyzed Direct Arylation through Directed C− H Bond Activation. J. Am. Chem. Soc.. 2008;130(18):5858-5859.
- [Google Scholar]
- Pyrrole, pyrrolidine, pyridine, piperidine and tropane alkaloids. Nat. Prod. Rep.. 2000;17(5):435-446.
- [Google Scholar]
- [2+ 2]-Cycloaddition reaction of styrene derivatives using an Fe (III) salt catalyst. Chem. Lett.. 2001;30(7):624-625.
- [Google Scholar]
- Facile synthesis of 5, 10-diaryl-5, 10-dihydrophenazines and application to EL devices. Org. Lett.. 2003;5(3):373-376.
- [Google Scholar]
- Aziridines, Azirines and Fused Ring derivatives In Comprehensive Heterocyclic Chemistry. Oxford: Pergamon; 1984.
- Heterocyclic substituted ureas. II. Immunosuppressive and antiviral activity of benzothiazolyl-and benzoxazolylureas. J. Med. Chem.. 1969;12(6):1016-1018.
- [Google Scholar]
- Iron-Catalyzed N-Alkylation of Azoles via Oxidation of C− H Bond Adjacent to an Oxygen Atom. Org. Lett.. 2010;12(9):1932-1935.
- [Google Scholar]
- Photoredox radical/polar crossover enables construction of saturated nitrogen heterocycles. Org. Lett.. 2019;21(7):2317-2321.
- [Google Scholar]
- Iron-catalyzed intramolecular allylic C-H amination. J. Am. Chem. Soc.. 2012;134(4):2036-2039.
- [Google Scholar]
- Antibacterial activity of quinoxalines, quinazolines, and 1, 5-naphthyridines. Bioorg. Med. Chem. Lett.. 2013;23(17):4968-4974.
- [Google Scholar]
- Pyrimidine-based twisted donor–acceptor delayed fluorescence molecules: a new universal platform for highly efficient blue electroluminescence. Chem. Sci.. 2017;8(2):953-960.
- [Google Scholar]
- Coinage metal-assisted synthesis of heterocycles. Chem. Rev.. 2008;108(8):3395-3442.
- [Google Scholar]
- Phenytoin–An anti-seizure drug: Overview of its chemistry, pharmacology and toxicology. Food Chem. Toxicol.. 2020;142:111393
- [Google Scholar]
- Med. Chem. 2004;12:4179.
- The influence of drug-like concepts on decision-making in medicinal chemistry. Nat Rev Drug Discov. 2007;6:881-890.
- [Google Scholar]
- Carbon-13 and proton NMR shift assignments and physical constants of norditerpenoid alkaloids. In: Alkaloids: Chemical and Biological Perspectives. Springer; 1991. p. :297-564.
- [Google Scholar]
- Recent developments in the [5+ 2] cycloaddition. Adv. Synth. Catal.. 2011;353(2–3):189-218.
- [Google Scholar]
- Recent developments in the [5+ 2] cycloaddition. Adv. Synth. Catal.. 2018;360(8):1551-1583.
- [Google Scholar]
- Bioactive pyrrole-based compounds with target selectivity. Eur. J. Med. Chem.. 2020;208:112783
- [Google Scholar]
- 5′-Alkyl-benzothiadiazides: a new subgroup of AMPA receptor modulators with improved affinity. Bioorg. Med. Chem.. 2002;10(5):1229-1248.
- [Google Scholar]
- Advances in marine natural products of the indole and annelated indole series: chemical and biological aspects. Curr. Med. Chem.. 2001;8(13):1681-1698.
- [Google Scholar]
- Copper hydride catalyzed hydroamination of alkenes and alkynes. Angew. Chem. Int. Ed.. 2016;55(1):48-57.
- [Google Scholar]
- Recent advances in Fe-catalyzed C-H aminations using azides as nitrene precursors. Catal. Sci. Technol.. 2019;9(16):4188-4197.
- [Google Scholar]
- Iron catalysis in organic chemistry: reactions and applications. John Wiley & Sons; 2008.
- Iron catalysis: fundamentals and applications. Springer Science & Business Media; 2011.
- Pericyclic domino reactions: concise approaches to natural carbocyclic frameworks. Chem. Soc. Rev.. 2009;38(11):3092-3101.
- [Google Scholar]
- 2 (3H)-benzoxazolone and bioisosters as “privileged scaffold” in the design of pharmacological probes. Curr. Med. Chem.. 2005;12(7):877-885.
- [Google Scholar]
- Effects of benzydroflumethiazide (Naturetin) on the renal excretion of calcium and magnesium by dogs. Toxicol. Appl. Pharmacol.. 1961;3(4):455-458.
- [Google Scholar]
- Iron-catalyzed arene C-H amidation using functionalized hydroxyl amines at room temperature. ACS Catal.. 2018;8(9):8369-8375.
- [Google Scholar]
- [60] Fullerene chemistry for materials science applications. J. Mater. Chem.. 1997;7(7):1097-1109.
- [Google Scholar]
- Photocatalytic C H Activation by Hydrogen-Atom Transfer in Synthesis. ChemCatChem. 2015;7(10):1516-1523.
- [Google Scholar]
- Antifungal activity of alkyl and heterocyclic aza-derivatives of gossypol as well as their complexes with NaClO4 against Fusarium oxysporum f. sp. lupini. Bioorg. Med. Chem. Lett.. 2009;19(7):1996-2000.
- [Google Scholar]
- Iron-catalyzed one-pot synthesis of quinoxalines: transfer hydrogenative condensation of 2-nitroanilines with vicinal diols. RSC Adv.. 2021;11(30):18225-18230.
- [Google Scholar]
- Structure and evaluation of antibacterial and antitubercular properties of new basic and heterocyclic 3-formylrifamycin SV derivatives obtained via ‘click chemistry’approach. Eur. J. Med. Chem.. 2014;84:651-676.
- [Google Scholar]
- Specific interactions between rifamycin antibiotics and water influencing ability to overcome natural cell barriers and the range of antibacterial potency. ACS Infect. Dis.. 2019;5(10):1754-1763.
- [Google Scholar]
- Regioselective approach to colchiceine tropolone ring functionalization at C (9) and C (10) yielding new anticancer hybrid derivatives containing heterocyclic structural motifs. J. Enzyme Inhib. Med. Chem.. 2022;37(1):597-605.
- [Google Scholar]
- Transition-Metal Catalysis of Triene 6π Electrocyclization: The π-Complexation Strategy Realized. Angew. Chem. Int. Ed.. 2020;59(41):17958-17965.
- [Google Scholar]
- Total synthesis of ellipticine quinones, olivacine, and calothrixin B. The Journal of organic chemistry. 2014;79(2):736-741.
- [Google Scholar]
- Photoinduced organocatalyzed atom transfer radical polymerization using continuous flow. Macromolecules. 2017;50(7):2668-2674.
- [Google Scholar]
- Expert Opin. Ther. Patents. 2015;25(7):1.
- Recent advances in the chemistry of 1, 2, 4-triazines. Adv. Heterocycl. Chem.. 2010;100:75-100.
- [Google Scholar]
- A mild and efficient synthesis of α-tosylamino ketones from aryl aziridines in the presence of β-cyclodextrin and NBS in water. Tetrahedron Lett.. 2005;46(8):1299-1301.
- [Google Scholar]
- Radicals in organic synthesis. Wiley-vch; 2001.
- Heterocyclic Compounds: Azepines. Part. 1984;1:671.
- Synthesis of azolines and imidazoles and their use in drug design. Med Chem (Los Angeles). 2016;6:561-570.
- [Google Scholar]
- Amino group chemistry: from synthesis to the life sciences. John Wiley & Sons; 2008.
- Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature. 2017;545(7654):299-304.
- [Google Scholar]
- 1, 8-Naphthyridines IV. 9-Substituted N, N-dialkyl-5-(alkylamino or cycloalkylamino)[1, 2, 4] triazolo [4, 3-a][1, 8] naphthyridine-6-carboxamides, new compounds with anti-aggressive and potent anti-inflammatory activities. Eur. J. Med. Chem.. 2000;35(11):1021-1035.
- [Google Scholar]
- Amines, Aliphatic, Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH; 2015.
- Synthesis and antifolate activity of 2, 4-diamino-5, 6, 7, 8-tetrahydropyrido [4, 3-d] pyrimidine analogues of trimetrexate and piritrexim. J. Heterocycl. Chem.. 1995;32(1):335-340.
- [Google Scholar]
- Synthesis and anthelmintic properties of arylquinolines with activity against drug-resistant nematodes. Bioorg. Med. Chem. Lett.. 2005;15(21):4806-4808.
- [Google Scholar]
- Catalytic activity of (Fe2O3)-MCM-41-nPrNH2 magnetically recoverable nanocatalyst for the synthesis of phenylpyrido [4, 3-d] pyrimidins. J. Nanosci. Nanotechnol.. 2013;13(7):4925-4933.
- [Google Scholar]
- Iron-Catalyzed Radical Activation Mechanism for Denitrogenative Rearrangement Over C (sp3)–H Amination. Angew. Chem. Int. Ed.. 2021;60(16):8772-8780.
- [Google Scholar]
- Mechanisms of intramolecular activation of carbon-hydrogen bonds in transition-metal complexes. Chem. Rev.. 1990;90(2):403-424.
- [Google Scholar]
- Preparation of carbon nanotube-supported α-Fe 2 O 3@ CuO nanocomposite: a highly efficient and magnetically separable catalyst in cross-coupling of aryl halides with phenols. Catal. Sci. Technol.. 2013;3(8):2025-2031.
- [Google Scholar]
- Pericyclic Reactions-A Textbook: Reactions, Applications and Theory. Wiley-VCH; 2005.
- Iron (III)-catalyzed three-component domino strategy for the synthesis of imidazo [1, 2-a] pyridines. Tetrahedron Lett.. 2014;55(37):5151-5155.
- [Google Scholar]
- Expedient iron-catalyzed stereospecific synthesis of triazines via cycloaddition of aziridines with diaziridines. Chem. Commun.. 2020;56(23):3381-3384.
- [Google Scholar]
- A new series of highly potent non-peptide bradykinin B2 receptor antagonists incorporating the 4-heteroarylquinoline framework. Improvement of aqueous solubility and new insights into species difference. J. Med. Chem.. 2004;47(7):1617-1630.
- [Google Scholar]
- Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem. Rev.. 2012;112(6):3193-3328.
- [Google Scholar]
- Analysis of US FDA-approved drugs containing sulfur atoms. Sulfur Chemistry 2019:1-34.
- [Google Scholar]
- Construction of fused heterocycles by metal-mediated [2+ 2+ 2] cyclotrimerization of alkynes and/or nitriles. Tetrahedron. 2011;67(34):6095-6130.
- [Google Scholar]
- Cellulose sulfuric acid: an efficient biopolymer-based catalyst for the synthesis of oxazolines, imidazolines and thiazolines under solvent-free conditions. Appl. Catal. A. 2009;358(2):146-149.
- [Google Scholar]
- Imidazole and its biological activities: A review. Der Chemica Sinica. 2010;1(3):36-47.
- [Google Scholar]
- Synthesis and QSAR modeling of 2-acetyl-2-ethoxycarbonyl-1-[4 (4′-arylazo)-phenyl]-N, N-dimethylaminophenyl aziridines as potential antibacterial agents. Eur. J. Med. Chem.. 2009;44(1):251-259.
- [Google Scholar]
- Biological importance of the indole nucleus in recent years: a comprehensive review. J. Heterocycl. Chem.. 2010;47(3):491-502.
- [Google Scholar]
- An environmental friendly approach for the synthesis of highly substituted imidazoles using Brønsted acidic ionic liquid, N-methyl-2-pyrrolidonium hydrogen sulfate, as reusable catalyst. J. Mol. Liq.. 2011;160(1):40-49.
- [Google Scholar]
- Iron (MCP) Complexes Catalyze Aziridination with Olefins As Limiting Reagents. The Journal of organic chemistry. 2018;83(9):5072-5081.
- [Google Scholar]
- Iron-catalyzed aerobic difunctionalization of alkenes: a highly efficient approach to construct oxindoles by C-S and C–C bond formation. Chem. Commun.. 2014;50(31):4115-4118.
- [Google Scholar]
- The promise and challenge of iron-catalyzed cross coupling. Acc. Chem. Res.. 2008;41(11):1500-1511.
- [Google Scholar]
- Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant. Chem. Soc. Rev.. 2012;41(8):3381-3430.
- [Google Scholar]
- Synthesis and Functionalization of Symmetrical 2, 5-Diaryl Fulleropyrrolidines: Ferric Perchlorate-Mediated One-Step Reaction of [60] Fullerene with Arylmethanamines. The Journal of organic chemistry. 2016;81(5):1769-1777.
- [Google Scholar]
- Catalytic hydroamination of unactivated olefins using a Co catalyst for complex molecule synthesis. J. Am. Chem. Soc.. 2014;136(39):13534-13537.
- [Google Scholar]
- Novel carbazole/fluorene hybrids: Host materials for blue phosphorescent OLEDs. Org. Lett.. 2006;8(13):2799-2802.
- [Google Scholar]
- Facile construction of tetrahydropyrrolizines by iron-catalyzed double cyclization of alkene-tethered oxime esters with 1, 2-disubstituted alkenes. Org. Lett.. 2018;20(10):3044-3048.
- [Google Scholar]
- Recent Progress on Cyclic Nitrenoid Precursors in Transition-Metal-Catalyzed Nitrene-Transfer Reactions. Chemistry–A. European Journal. 2019;25(13):3156-3180.
- [Google Scholar]
- Transition-metal-catalyzed C-N bond forming reactions using organic azides as the nitrogen source: a journey for the mild and versatile C–H amination. Acc. Chem. Res.. 2015;48(4):1040-1052.
- [Google Scholar]
- Acc. Chem. Res. 2015
- N-Heterocyclic Carbene Iron (III) Porphyrin-Catalyzed Intramolecular C (sp3)–H Amination of Alkyl Azides. Angew. Chem. Int. Ed.. 2018;57(37):11947-11951.
- [Google Scholar]
- Synthesis and antitubercular activity of some new 2-(substituted arylamino)-5, 6-disubstituted/6-substituted benzothiazoles. Indian Drugs. 1990;27(6):350.
- [Google Scholar]
- Synthesis of benz [f] indole-4, 9-diones via acetylenic derivatives of 1, 4-naphthoquinone. Tetrahedron Lett.. 2009;50(49):6769-6771.
- [Google Scholar]
- Rhodium (II)-Catalyzed Undirected and Selective C (sp2)–H Amination en Route to Benzoxazolones. ACS Catal.. 2016;6(10):6520-6524.
- [Google Scholar]
- Modifications, biological origin and antibacterial activity of naphthalenoid ansamycins. Nat. Prod. Rep. 2022
- [Google Scholar]
- Structural diversity and biological relevance of benzenoid and atypical ansamycins and their congeners. Nat. Prod. Rep. 2022
- [Google Scholar]
- Photophysics and biological applications of 7-azaindole and its analogs. ACS Publications; 1997. p. :2758-2769.
- Direct functionalization of benzylic C-Hs with vinyl acetates via Fe-catalysis. Chem. Commun.. 2009;40:6002-6004.
- [Google Scholar]
- Iron-catalyzed reductive coupling of nitroarenes with olefins: Intermediate of iron–nitroso complex. ACS Catal.. 2019;10(1):276-281.
- [Google Scholar]
- Recent Trends in Iron-Catalyzed Reactions towards the Synthesis of Nitrogen-Containing Heterocycles. Adv. Synth. Catal.. 2019;361(10):2236-2249.
- [Google Scholar]
- Remote C-H functionalization via selective hydrogen atom transfer. Synthesis. 2018;50(08):1569-1586.
- [Google Scholar]
- Copper-Catalyzed Cyclization and Azidation of γ, δ-Unsaturated Ketone O-Benzoyl Oximes. Adv. Synth. Catal.. 2015;357(1):64-70.
- [Google Scholar]
- Indoles Academic Press. San Diego 1996:113.
- A mild and efficient procedure for the oxidation of epoxides and aziridines using cerium (IV) ammonium nitrate and NBS. Tetrahedron Lett.. 2005;46(23):4111-4113.
- [Google Scholar]
- The enzymes of the rifamycin antibiotic resistome. Acc. Chem. Res.. 2021;54(9):2065-2075.
- [Google Scholar]
- Dialkylbiaryl phosphines in Pd-catalyzed amination: a user's guide. Chem. Sci.. 2011;2(1):27-50.
- [Google Scholar]
- Studien über Benzthiazole als eventuelle orale Antidiabetica. Helv. Chim. Acta. 1967;50(4):1084-1086.
- [Google Scholar]
- RHODIUM-CATALYZED< 2+ 2+ 2> CYCLOADDITION FOR THE SYNTHESIS OF SUBSTITUTED PYRIDINES, PYRIDONES. AND THIOPYRANIMINES. Heterocycles. 2012;85(5):1017-1043.
- [Google Scholar]
- Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem.. 2016;14(28):6611-6637.
- [Google Scholar]
- 3-(1, 1-Dioxo-2 H-(1, 2, 4)-benzothiadiazin-3-yl)-4-hydroxy-2 (1 H)-quinolinones, potent inhibitors of hepatitis C virus RNA-dependent RNA polymerase. J. Med. Chem.. 2006;49(3):971-983.
- [Google Scholar]
- The Baeyer− Villiger reaction: New developments toward greener procedures. Chem. Rev.. 2004;104(9):4105-4124.
- [Google Scholar]
- Syntheses of Trifluoroethylated N-Heterocycles from Vinyl Azides and Togni’s Reagent Involving 1, n-Hydrogen-Atom Transfer Reactions. Org. Lett.. 2020;22(12):4766-4770.
- [Google Scholar]
- Organocatalyzed atom transfer radical polymerization driven by visible light. Science. 2016;352(6289):1082-1086.
- [Google Scholar]
- Foundations for blockbuster drugs in federally sponsored research. FASEB J.. 2001;15(10):1671-1676.
- [Google Scholar]
- Targeting the polyamine transport system with benzazepine-and azepine-polyamine conjugates. J. Med. Chem.. 2010;53(21):7647-7663.
- [Google Scholar]
- “Turn-on” conjugated polymer fluorescent chemosensor for fluoride ion. Macromolecules. 2003;36(8):2584-2586.
- [Google Scholar]
- Addition of metalloid hydrides to alkynes: hydrometallation with Boron, Silicon, and Tin. Synthesis. 2005;2005(06):853-887.
- [Google Scholar]
- New strategies for the transition-metal catalyzed synthesis of aliphatic amines. Chem. Rev.. 2020;120(5):2613-2692.
- [Google Scholar]
- 6-Substituted-4-(3-bromophenylamino) quinazolines as putative irreversible inhibitors of the epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor (HER-2) tyrosine kinases with enhanced antitumor activity. J. Med. Chem.. 2001;44(17):2719-2734.
- [Google Scholar]
- Synthesis and analgesic activity of some 1-benzyl-2-substituted-4, 5-diphenyl-1H-imidazole derivatives. Il Farmaco. 2001;56(4):285-290.
- [Google Scholar]
- Regio-and stereo-selective intermolecular [2+ 2] cycloaddition of allenol esters with C 60 leading to alkylidenecyclobutane-annulated fullerenes. Chem. Commun.. 2016;52(89):13175-13178.
- [Google Scholar]
- Ueber eine neue Bildungsweise von Diphenylaminderivaten. Ber. Dtsch. Chem. Ges.. 1903;36(2):2382-2384.
- [Google Scholar]
- Rhodium-catalyzed anti-Markovnikov hydroamination of vinylarenes. J. Am. Chem. Soc.. 2003;125(19):5608-5609.
- [Google Scholar]
- Dioxazolones: stable substrates for the catalytic transfer of acyl nitrenes. ACS Catal.. 2020;10(8):4751-4769.
- [Google Scholar]
- Construction of pyridine rings by metal-mediated [2+ 2+ 2] cycloaddition. Chem. Rev.. 2003;103(9):3787-3802.
- [Google Scholar]
- Copper-catalyzed domino annulation approaches to the synthesis of benzoxazoles under microwave-accelerated and conventional thermal conditions. The Journal of organic chemistry. 2008;73(9):3452-3459.
- [Google Scholar]
- Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals: miniperspective. J. Med. Chem.. 2014;57(24):10257-10274.
- [Google Scholar]
- Cobalt-Mediated [2+ 2+ 2]-Cycloadditions: A Maturing Synthetic Strategy [New Synthetic Methods (43)] Angew. Chem., Int. Ed. Engl.. 1984;23(8):539-556.
- [Google Scholar]
- Specific inhibition of epidermal growth factor receptor tyrosine kinase by 4-anilinoquinazolines. Breast Cancer Res. Treat.. 1996;38(1):67-73.
- [Google Scholar]
- Iron-Mediated [3+ 2] or [3+ 3] Annulation of 2-(2-(Ethynyl) phenoxy)-1-arylethanones: Selective Synthesis of Indeno [1, 2-c] chromenes and 5 H-Naphtho [1, 2-c] chromenes. Org. Lett.. 2011;13(1):14-17.
- [Google Scholar]
- Fe-catalysed oxidative C-H functionalization/C–S bond formation. Chem. Commun.. 2012;48(1):76-78.
- [Google Scholar]
- Iron-catalyzed cycloaddition reaction of diynes and cyanamides at room temperature. The Journal of organic chemistry. 2013;78(7):3065-3072.
- [Google Scholar]
- Functionalization of [60] Fullerene via Palladium-Catalyzed C-H Bond Activation. In: CH Bond Activation and Catalytic Functionalization I. Springer; 2015. p. :119-136.
- [Google Scholar]
- Coordinated assembly of a new 3D mesoporous Fe 3 O 4@ Cu 2 O–graphene oxide framework as a highly efficient and reusable catalyst for the synthesis of quinoxalines. Chem. Commun.. 2015;51(24):5069-5072.
- [Google Scholar]
- Recent Advances in Iron-Catalyzed C—H Bond Amination via Iron Imido Intermediate. Chin. J. Chem .. 2018;36(12):1222-1240.
- [Google Scholar]
- Benign and efficient synthesis of 2-substituted 4 (3 H)-quinazolinones mediated by iron (III) chloride hexahydrate in refluxing water. Bull. Chem. Soc. Jpn.. 2006;79(9):1426-1430.
- [Google Scholar]
- 1, 2-benzothiazine 1, 1-dioxide P2–P3 peptide mimetic aldehyde calpain I inhibitors. J. Med. Chem.. 2001;44(21):3488-3503.
- [Google Scholar]
- Iron-Mediated Direct Suzuki− Miyaura Reaction: A New Method for the ortho-Arylation of Pyrrole and Pyridine. Org. Lett.. 2010;12(12):2694-2697.
- [Google Scholar]
- High-spin iron imido complexes competent for C-H bond amination. J. Am. Chem. Soc.. 2017;139(34):12043-12049.
- [Google Scholar]
- Recent developments in the field of quinazoline chemistry. Curr. Org. Chem.. 2003;7(7):659-677.
- [Google Scholar]
- Indolo [3, 2-b] carbazole-based thin-film transistors with high mobility and stability. J. Am. Chem. Soc.. 2005;127(2):614-618.
- [Google Scholar]
- Palladium-catalyzed carbonylative synthesis of quinazolinones from 2-aminobenzamide and aryl bromides. Chemistry–A. European Journal. 2013;19(38):12635-12638.
- [Google Scholar]
- Iron-Catalyzed Redox-Neutral Radical Cascade Reaction of [60] Fullerene with γ, δ-Unsaturated Oxime Esters: Preparation of Free (N–H) Pyrrolidino [2′, 3′: 1, 2] fullerenes. Org. Lett.. 2020;22(18):7327-7332.
- [Google Scholar]
- Environmentally friendly iron-catalyzed cascade synthesis of 1, 2, 4-benzothiadiazine 1, 1-dioxide and quinazolinone derivatives. J. Comb. Chem.. 2009;11(4):653-657.
- [Google Scholar]
- Copper-Catalyzed Domino Synthesis of Benzimidazo [2, 1-b] quin-azolin-12 (6H)-ones Using Cyanamide as a Building Block. Adv. Synth. Catal.. 2012;354(2–3):477-482.
- [Google Scholar]
- Regioselective synthesis of triazoles via base-promoted oxidative cycloaddition of chalcones with azides in aqueous solution. RSC Adv.. 2015;5(116):95833-95839.
- [Google Scholar]
- Reaction of C60 with Inactive Secondary Amines and Aldehydes and the Cu (OAc) 2-Promoted Regioselective Intramolecular C-H Functionalization of the Generated Fulleropyrrolidines. The Journal of organic chemistry. 2016;81(22):11201-11209.
- [Google Scholar]
- Progress in studies of novel marine bis (indole) alkaloids. Curr. Org. Chem.. 2004;8(17):1691-1720.
- [Google Scholar]
- Transition-metal-catalyzed direct addition of unactivated C-H bonds to polar unsaturated bonds. Chem. Rev.. 2015;115(9):3468-3517.
- [Google Scholar]
- Iron-Catalyzed Regioselective Synthesis of 3-Arylindoles. ChemistrySelect. 2017;2(23):6689-6692.
- [Google Scholar]
- Iron-Catalyzed Chemoselective ortho Arylation of Aryl Imines by Directed C H Bond Activation. Angew. Chem.. 2009;121(16):2969-2972.
- [Google Scholar]
- CH activation. Springer; 2010.
- Catalytic activity of Cu nanoparticles supported on Fe 3 O 4–polyethylene glycol nanocomposites for the synthesis of substituted imidazoles. New J. Chem.. 2014;38(9):4555-4565.
- [Google Scholar]
- Microwave-assisted synthesis of quinazolinone derivatives by efficient and rapid iron-catalyzed cyclization in water. Green Chem.. 2009;11(11):1881-1888.
- [Google Scholar]
- Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photonics. 2014;8(4):326-332.
- [Google Scholar]
- Iron-catalyzed carbonylative cyclization of γ, δ-unsaturated aromatic oxime esters to functionalized pyrrolines. Chem. Commun.. 2020;56(51):7045-7048.
- [Google Scholar]
- CH bond amination by iron-imido/nitrene species. Chin. Sci. Bull.. 2012;57(19):2352-2360.
- [Google Scholar]
- Intermolecular hydroamination of ethylene and 1-alkenes with cyclic ureas catalyzed by achiral and chiral gold (I) complexes. J. Am. Chem. Soc.. 2009;131(15):5372-5373.
- [Google Scholar]
- Iron-catalyzed tandem reactions of aldehydes, terminal alkynes, and primary amines as a strategy for the synthesis of quinoline derivatives. J. Heterocycl. Chem.. 2011;48(1):153-157.
- [Google Scholar]
- Three-coordinate cobalt (IV) and cobalt (V) imido complexes with N-heterocyclic carbene ligation: synthesis, structure, and their distinct reactivity in C-H bond amination. J. Am. Chem. Soc.. 2014;136(44):15525-15528.
- [Google Scholar]
- Iron-catalyzed oxidative synthesis of N-heterocycles from primary alcohols. RSC Adv.. 2014;4(13):6486-6489.
- [Google Scholar]
- Synthesis, characterization and charge storage properties of π-biindolo [2, 3-b] quinoxaline for solution-processing organic transistor memory. Dyes Pigm.. 2019;167:255-261.
- [Google Scholar]
- Iron-catalyzed intramolecular C-H amination of α-azidyl amides. Org. Lett.. 2019;21(6):1559-1563.
- [Google Scholar]
- Luminescence and reactivity of 7-azaindole derivatives and complexes. Chem. Soc. Rev.. 2010;39(8):3142-3156.
- [Google Scholar]
- Synthesis of Sultams and Cyclic N-Sulfonyl Ketimines via Iron-Catalyzed Intramolecular Aliphatic C-H Amidation. Org. Lett.. 2019;21(15):5808-5812.
- [Google Scholar]
- Iron-Catalyzed Intramolecular C—H Amidation of N-Benzoyloxyureas. Chin. J. Chem .. 2021;39(4):855-858.
- [Google Scholar]
- Synthesis of [60] Fullerene-Fused Spiroindanes by Palladium-Catalyzed Oxidative Annulation of [60] Fullerene with 2-Aryl Cyclic 1, 3-Dicarbonyl Compounds. Org. Lett.. 2016;18(11):2616-2619.
- [Google Scholar]
- Design and synthesis of 8-hydroxy-[1, 6] naphthyridines as novel inhibitors of HIV-1 integrase in vitro and in infected cells. J. Med. Chem.. 2003;46(4):453-456.
- [Google Scholar]
- Fullerenes–how 25 years of charge transfer chemistry have shaped our understanding of (interfacial) interactions. Chem. Soc. Rev.. 2018;47(3):702-714.
- [Google Scholar]







