Translate this page into:
Iron(III) oxide-hydroxide modification on Pterocarpus macrocarpus sawdust beads for direct red 28 dye removal
⁎Corresponding author at: Department of Environmental Science, Khon Kaen University, Khon Kaen 40002, Thailand. pornprai@kku.ac.th (Pornsawai Praipipat)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The releasing of wastewater contaminated with direct red 28 (DR28) dye into receiving water is a concern because it is a polyaromatic structure with difficult biodegradation. It results in being persistent in the environment and being toxic to the ecosystem from accumulating through a food chain. Thus, it needs to eliminate DR28 dye from contaminated wastewater. Sawdust beads (SDB) and sawdust mixed with iron(III) oxide-hydroxide beads (SDFB) are prepared and characterized for DR28 dye removals. DR28 dye removal efficiencies are examined with various affecting factors by the batch tests and adsorption–desorption tests, and their adsorption patterns, rates, and mechanisms are determined by isotherms and kinetics models. Moreover, the thermodynamic studies are examined for the temperature effect. SDFB demonstrated a higher surface area and smaller pore size than SDB. SDB and SDFB were uneven shapes and heterogeneous fibrillar structures. Carbon, oxygen, calcium, chloride, sodium, O—H, C—H, C⚌C, —COOH, and C—O were found in SDB and SDFB. The points of zero charge of SDB and SDFB were 4.65 and 6.11. The conditions of 3.5 g, 50 ℃, pH 3, 60 mg/L and 2.5 g, 40 ℃, pH 3, 60 mg/L illustrated the highest DR28 dye removals of more than 82 % for SDB and SDFB, respectively. Thus, the material modification by iron(III) oxide-hydroxide improved the DR28 dye removal efficiency of sawdust material. Moreover, they could be reusability more than three times with DR28 dye removal of more than 63 %. SDB and SDFB corresponded to Langmuir and Freundlich models, respectively, and a pseudo-second-order was a good model to describe their adsorption mechanism. Both materials did not favor DR28 dye adsorptions with increasing temperature. Therefore, SDFB was a potential material for removing DR28 dye removal in the industrial wastewater.
Keywords
Industrial waste
Iron(III) oxide-hydroxide
Adsorption
Anionic dye
Reusability
1 Introduction
Increasing dye uses in the industrial production processes from pigment, dye, textile, leather, plastic, food, pharmaceutical, paint, and paper factories result in a high risk of dye-contaminated wastewater and affecting the environment because they are polyaromatic structures, toxicity to the ecosystem, bioaccumulation through the food web, and difficult biodegradation (Islam et al., 2023). Several anionic dyes of reactive, acid, azo, and direct are used in the above industries with different purposes. Since direct dyes are easy to use with long-lasting dyes and are cheaper price than other dye types, they are popularly used for dying lignin cellulose, and cotton, especially direct red 28 (DR28) dye. It is commonly used by several manufacturers of textile, printing, and paper (Benkhaya et al., 2020). Since the structure of DR28 dye includes two azo groups which are tetrazotized benzidine and naphthionic acid, it is difficult to degrade and be persistent in the environment for a long time (Siddiqui et al., 2023). In addition, the release of wastewater contaminated DR28 dye into water bodies blocks the photosynthesis of aquatic organisms and decreases dissolved oxygen in water affecting the water quality and aquatic life (Tkaczyk et al., 2020). Moreover, its toxicity also causes human carcinogens and anaphylactic shock (Oladoye et al., 2022). Therefore, the wastewater contaminated with DR28 dye needs to be treated before being released to the water bodies to protect the aquatic life and environment.
Several chemical or physiochemical methods such as coagulation-flocculation, precipitation, adsorption, chemical oxidation, electrochemistry, photochemical, reverse osmosis, and membrane filtration used to eliminate wastewater-contaminated dyes. Among those methods, adsorption is a good option because it offers an effective method, reasonable cost, adsorbent reusability, and various availability of adsorbent choices (Dutta et al., 2021). Commercial adsorbents, biosorbents, nanosorbents, and waste adsorbents are used to remove various dyes shown in Table 1. For commercial adsorbents, activated carbon, and chitosan have been used for acid yellow 23 and methylene blue (MB) dye adsorptions (Ahmad and Ansari, 2021; Khader et al., 2021). Pinus roxburghii leaves, Dodonaea viscosa bark, and apple leaves activated carbon are used to adsorb acid orange 74, methyl red, and basic blue 47 dyes as biosorbents (Abdel-Aziz et al., 2021; Gul et al., 2022; Rehman et al., 2019). For nanosorbents, MB dye is adsorbed by wheat straw modified with magnetic nanoparticles (Ebrahimian Pirbazari et al., 2014). For food waste adsorbents, lemon peel, chicken and duck eggshells, coffee waste modified with polyethylenimine, and almond shell are used to remove eosin, reactive blue 4 (RB4), reactive black 5, and crystal violet dyes (Bukhari et al., 2022; Loulidi et al., 2020; Praipipat et al., 2022b; Wong et al., 2020). Basic red 2 and RB4 dyes are adsorbed by agriculture waste adsorbents of sugarcane bagasse (Farahani et al., 2015; Praipipat et al., 2023h). For industrial waste adsorbents, sugarcane bagasse fly ash and sawdust are used for adsorbing DR28, RB4, MB, methyl violet (MV), and crystal violet dyes (Ahamad and Nasar, 2023; Esmaeili and Foroutan, 2019; Guechi et al., 2021; Muneer et al., 2021; Praipipat et al., 2023h, 2023d, 2023b). For DR28 dye adsorption, various adsorbent types above have been used to eliminate it. Zeolite A and chitosan are used as commercial adsorbents (Ahmad and Ansari, 2021; Khalaf et al., 2021), and calcinated kaolin is used as a biosorbent (Findik, 2023). The cotton stalks biochar modified with zinc oxide nanocomposite and carbon nanotube mixed metal oxides nanocomposites are used as nanosorbents (Iqbal et al., 2021; Yang et al., 2015). Moreover, banana peel, cabbage, coffee, rice husk, sugarcane bagasse, sugarcane bagasse fly ash, pine bark, and sawdust have been used for removing DR28 dye in many previous studies, and the details are demonstrated in Table 1. Among the adsorbents mentioned above, the waste adsorbents are interesting because only uses of waste adsorbents could reduce waste volumes for waste management but also they can improve wastewater quality by adsorbing several dyes. Especially, sawdust is a good selection because it has a high adsorption capacity among other adsorbents in Table 1. It consists of cellulose, hemicellulose, and lignin, and then it can adsorb dyes in wastewater. However, the modification of sawdust needs more investigations to deal with the high dye concentrations in real wastewater.
Materials
Dyes
Types
qm (mg/g)
References
Commercials
Activated carbon
Acid yellow 23
Anionic
10.20
(Khader et al., 2021)
Zeolite A
Direct red 28
Anionic
21.11
(Khalaf et al., 2021)
Chitosan
Direct red 28
Anionic
104.60
(Ahmad and Ansari, 2021)
Chitosan
Methylene blue
Cationic
99.01
(Ahmad and Ansari, 2021)
Biosorbents
Calcined kaolin
Direct red 28
Anionic
5.39
(Findik, 2023)
Pinus roxburghii leaves
Acid orange 74
Anionic
7.52
(Rehman et al., 2019)
Dodonaea viscosa bark
Methyl red
Anionic
2.54
(Gul et al., 2022)
Apple leaves activated carbon
Basic blue 47
Cationic
2.59
(Abdel-Aziz et al., 2021)
Nanosorbents
Cotton stalks biochar modified with zinc oxide nanocomposite
Direct red 28
Anionic
555.60
(Iqbal et al., 2021)
Carbon nanotube mixed metal oxides nanocomposites
Direct red 28
Anionic
1250.00
(Yang et al., 2015)
Wheat straw modified with magnetic nanoparticles
Methylene blue
Cationic
1374.60
(Ebrahimian Pirbazari et al., 2014)
Waste adsorbents
Food wastes
Banana peel
Direct red 28
Anionic
1.72
(Mondal and Kar, 2018)
Cabbage
Direct red 28
Anionic
2.31
(Wekoye et al., 2020)
Lemon peel
Eosin
Anionic
8.24
(Bukhari et al., 2022)
Chicken eggshell beads
Reactive blue 4
Anionic
24.10
(Praipipat et al., 2022b)
Duck eggshell beads
Reactive blue 4
Anionic
12.63
(Praipipat et al., 2022b)
Coffee waste modified with polyethylenimine
Reactive black 5
Anionic
77.52
(Wong et al., 2020)
Coffee waste modified with polyethylenimine
Direct red 28
Anionic
34.36
(Wong et al., 2020)
Almond shell
Crystal violet
Cationic
12.20
(Loulidi et al., 2020)
Agriculture wastes
Rice husk char
Direct red 28
Anionic
1.28
(Malik et al., 2020)
Rice husk
Direct red 28
Anionic
1.58
(Malik et al., 2020)
Rice husk char modified potassium hydroxide
Direct red 28
Anionic
2.04
(Malik et al., 2020)
Sugarcane bagasse
Basic red 2
Cationic
58.85
(Farahani et al., 2015)
Bagasse beads
Reactive blue 4
Anionic
2.77
(Praipipat et al., 2023h)
Sugarcane bagasse beads
Direct red 28
Anionic
3.24
(Patabandige et al., 2019)
Rice husk modified with phosphoric acid
Reactive black 5
Anionic
2.60
(Değermenci et al., 2019)
Corn silk
Reactive blue 19
Anionic
60.60
(Değermenci et al., 2019)
Corn silk
Reactive red 218
Anionic
51.60
(Farahani et al., 2015)
Industrial wastes
Bagasse fly ash beads
Reactive blue 4
Anionic
2.72
(Praipipat et al., 2023h)
Sawdust beads
Reactive blue 4
Anionic
7.61
(Praipipat et al., 2023b)
Pine bark
Direct red 28
Anionic
3.92
(Litefti et al., 2019)
Fly ash
Direct red 28
Anionic
22.12
(Harja et al., 2022)
Sugarcane bagasse fly ash beads
Direct red 28
Anionic
3.36
(Praipipat et al., 2023d)
Cedrus deodara sawdust
Direct red 28
Anionic
182.50
(Muneer et al., 2021)
Palm sawdust
Methylene blue
Cationic
53.95
(Esmaeili and Foroutan, 2019)
Eucalyptus sawdust
Methylene blue
Cationic
53.48
(Esmaeili and Foroutan, 2019)
Sour lemon sawdust
Methylene blue
Cationic
52.36
(Esmaeili and Foroutan, 2019)
Okoume sawdust
Methyl violet 2B
Cationic
102.04
(Guechi et al., 2021)
Azadirachta indica sawdust
Crystal violet
Cationic
270.27
(Ahamad and Nasar, 2023)
Since increasing the adsorbent capacity is needed to deal with a high dye concentration in wastewater, metal oxide is popularly used to modify adsorbent. It increases the specific surface area of adsorbent resulting in capable high dye removal. Iron(II or III) oxide, magnesium oxide (MgO), aluminum oxide (Al2O3), zinc oxide (ZnO), iron(III) oxide-hydroxide, and titanium dioxide (TiO2) are used in previous studies to encourage dye removal efficiency shown in Table 2. Iron(II or III) oxide is used to modify clay and wheat bran sawdust to remove alizarin red s, MB, and MV dyes (Fu et al., 2011; Pooladi et al., 2021). Lemon peel, chicken or duck eggshells, bagasse or bagasse fly ash, chitosan, activated carbon oak wood, and sawdust are modified by ZnO to adsorb RB4, DR28, malachite green, methyl violet 2B, and MB dyes (Foroutan et al., 2022; Muinde et al., 2020; Ngamsurach et al., 2022; Oyewo et al., 2020; Praipipat et al., 2022a, 2022b, 2023d). TiO2, Al2O3, and MgO are used for modifying bagasse or bagasse fly ash for RB4 and DR28 dye adsorptions (Praipipat et al., 2023h, 2023d). Since many previous studies affirmed the high capability of iron(III) oxide-hydroxide modified with various waste adsorbents for dye adsorptions shown in Table 2, it is a good choice of metal oxide for the raw material modification to improve its ability to remove target pollutants. Although iron(III) oxide-hydroxide has been applied for modifying raw materials of lemon peels, eggshells, bagasse, bagasse fly ash, and sawdust to adsorb RB4 dye, DR28 dye removal by those adsorbents has not yet been investigated. Therefore, this study is the first attempt to use sawdust modified iron(III) oxide-hydroxide to eliminate DR28 dye.
Modifications/Raw materials
Dyes
Types
qm (mg/g)
References
Zinc oxide (ZnO)
Lemon peel
Reactive blue 4
Anionic
2.59
(Praipipat et al., 2022a)
Chicken eggshell
Reactive blue 4
Anionic
20.41
(Praipipat et al., 2022b)
Duck eggshell
Reactive blue 4
Anionic
19.23
(Praipipat et al., 2022b)
Bagasse
Reactive blue 4
Anionic
3.18
(Ngamsurach et al., 2022)
Bagasse fly ash
Reactive blue 4
Anionic
6.78
(Praipipat et al., 2022b)
Sugarcane bagasse fly ash
Direct red 28
Anionic
3.90
(Praipipat et al., 2023d)
Chitosan
Malachite green
Cationic
11.00
(Muinde et al., 2020)
Activated carbon oak wood
Methyl violet 2B
Cationic
37.05
(Foroutan et al., 2022)
Sawdust
Methylene blue
Cationic
64.93
(Oyewo et al., 2020)
Titanium dioxide (TiO2)
Bagasse
Reactive blue 4
Anionic
5.55
(Praipipat et al., 2023h)
Bagasse fly ash
Reactive blue 4
Anionic
6.48
(Praipipat et al., 2023h)
Sugarcane bagasse fly ash
Direct red 28
Anionic
4.00
(Praipipat et al., 2023d)
Aluminum oxide (Al2O3)
Bagasse
Reactive blue 4
Anionic
3.41
(Praipipat et al., 2023h)
Bagasse fly ash
Reactive blue 4
Anionic
5.11
(Praipipat et al., 2023h)
Sugarcane bagasse fly ash
Direct red 28
Anionic
5.46
(Praipipat et al., 2023d)
Magnesium oxide (MgO)
Bagasse
Reactive blue 4
Anionic
5.55
(Praipipat et al., 2023h)
Bagasse fly ash
Reactive blue 4
Anionic
6.48
(Praipipat et al., 2023h)
Sugarcane bagasse fly ash
Direct red 28
Anionic
5.56
(Praipipat et al., 2023d)
Iron(II or III) oxide (Fe3O4)
Clay
Alizarin red s
Anionic
32.70
(Fu et al., 2011)
Wheat bran sawdust
Methylene blue
Cationic
51.28
(Pooladi et al., 2021)
Wheat bran sawdust
Methyl violet
Cationic
46.08
(Pooladi et al., 2021)
Iron(III) oxide-hydroxide
Lemon peel
Reactive blue 4
Anionic
3.23
(Praipipat et al., 2022a)
Chicken eggshell
Reactive blue 4
Anionic
30.49
(Praipipat et al., 2022b)
Duck eggshell
Reactive blue 4
Anionic
25.97
(Praipipat et al., 2022b)
Bagasse
Reactive blue 4
Anionic
3.77
(Praipipat et al., 2022b)
Bagasse fly ash
Reactive blue 4
Anionic
10.28
(Praipipat et al., 2022b)
Sawdust
Reactive blue 4
Anionic
10.31
(Praipipat et al., 2023b)
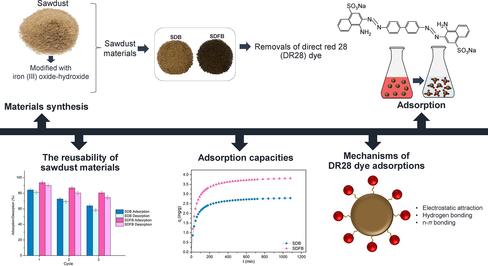
The current study aimed to synthesize sawdust beads (SDB) and sawdust mixed with iron(III) oxide-hydroxide beads (SDFB) to adsorb DR28 dye with various material characterizations on the physiochemical properties, surface structure, chemical elements, and chemical functional groups including the point of zero charge. DR28 dye removal efficiencies are examined through batch tests, adsorption–desorption tests, adsorption isotherms, adsorption kinetics, and thermodynamic studies.
2 Materials and methods
2.1 Raw material
Sawdust (Pterocarpus indicus) was collected from a sawmill in Udon Thani province, Thailand.
2.2 Chemicals
All chemicals were analytical grades (AR) used without purification which were ferric chloride hexahydrate (FeCl3·6H2O) (LOBA, India), sodium hydroxide (NaOH) (RCI Labscan, Thailand), sodium alginate (NaC6H7O6) (Merck, Germany), and calcium chloride dehydrate (CaCl2·2H2O) (RCI Labscan, Thailand), and DR28 dye (C32H22N6O6S2Na2) (Merk, Germany). For a pH adjustment, 0.1 M NaOH and 0.1 M hydrochloric acid (HCl) (RCI Labscan, Thailand) were used.
2.3 Sawdust material synthesis
The synthesis methods of SDB and SDFB explained by the schematic flow diagram are demonstrated in Fig. 1a and b which referred to the study of Praipipat et al. (Praipipat et al., 2023b).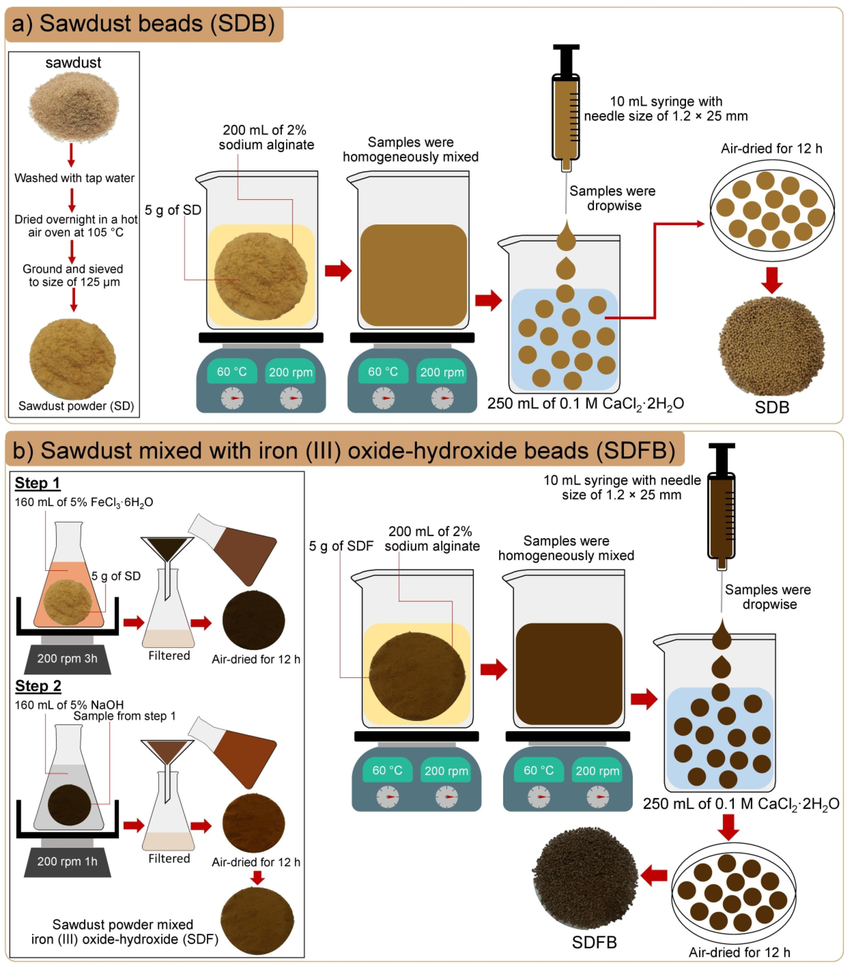
The synthesis methods of (a) SDB and (b) SDFB in the schematic flow diagram.
2.4 Material characterizations
Brunauer-Emmett-Teller (BET) (Bel, Bel Sorp mini X, Japan) is used to determine the physiochemical properties of SDB and SDFB, and their surface morphologies and chemical elements are explored by Field Emission Scanning Electron Microscopy and Focus Ion Beam (FESEM-FIB) with Energy Dispersive X-Ray Spectrometer (EDX) (FEI, Helios NanoLab G3 CX, USA). The chemical functional groups are identified by Fourier Transform Infrared Spectroscopy (FT-IR) (Bruker, TENSOR27, Hong Kong).
2.5 The point of zero charge
The surface charge of the adsorbent is necessary to investigate and predict how the adsorption occurs when the anionic pollutant might be adsorbed by a positively charged adsorbent. On the opposite, the cationic pollutant might be adsorbed by a negatively charged adsorbent. Thus, the points of zero charge (pHpzc) of SDB and SDFB for removing DR28 dye are required to explore this curiosity above which this study used the method of Praipipat et al. (Praipipat et al., 2023f). Firstly, 0.1 M NaCl solutions were prepared in pH 1–12, and 50 mL of each pH solution was contained in a 250 mL Erlenmeyer flask. Then, 0.1 g of SDB or SDFB were added and shaken by an orbital shaker (GFL, 3020, Germany) at 150 rpm for 24 h. The pHpzc was calculated from the plotting graph of ΔpH (pHfinal – pHinitial) versus pHinitial which a pH value was measured by a pH meter (Mettler Toledo, SevenGo with InLab 413/IP67, Switzerland).
2.6 Batch tests
Batch tests are investigated to explore the effects of dosage from 2 to 4 g, temperature from 30 to 70 ℃, pH 1–11, and concentration from 40 to 80 mg/L with a DR28 dye concentration of 60 mg/L, 200 mL of sample volume, 12 h, and 150 rpm of shaking speed for DR28 dye removals by SDB and SDFB. The lowest value of each affecting factor offering the highest DR28 dye removal efficiency is selected for the optimum condition (Praipipat et al., 2023b). The results were confirmed with the triplicate experiments and the average result was reported. The dye concentration was measured by UV–VIS Spectrophotometer (Hitachi, UH5300, Japan), and Equation (1) was used to calculate dye removal efficiency in the percentage (%).
2.7 The adsorption–desorption tests
The reusability of materials is a necessary investigation before applying them in the industry to evaluate the cost-effectiveness of the material in use. This study used the method of Praipipat et al. (Praipipat et al., 2023b) for investigating adsorption–desorption tests of SDB and SDFB for removing DR28 dye in three cycles. After DR28 dye adsorption, the saturated SDB or SDFB was added to 250 mL of Erlenmeyer flask containing 200 mL of 0.01 M NaOH solution, then it was shaken by an orbital shaker at 150 rpm for 6 h at room temperature. After that, they were filtrated and rinsed with deionized water, and they were air-dried. They were used for another adsorption cycle. Equation (2) is used to calculate the percentage of desorption efficiency.
2.8 Adsorption isotherms
Four isotherm models of Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich in the linear and nonlinear are applied to identify the adsorption patterns of SDB and SDFB following Eqs. (3)–(10), and the good fit model is chosen with the highest R2 value. The details are displayed in Table 3.
Models
Full equations
Plotting graph
Eq
References
Linear
Langmuir
(3)
(Langmuir, 1918)
Freundlich
(4)
(Freundlich, 1906)
Temkin
(5)
(Temkin and Pyzhev, 1940)
Dubinin-Radushkevich
(6)
(Dubinin and Radushkevich, 1947)
Nonlinear
Langmuir
(7)
(Langmuir, 1918)
Freundlich
(8)
(Freundlich, 1906)
Temkin
(9)
(Temkin and Pyzhev, 1940)
Dubinin-Radushkevich
(10)
(Dubinin and Radushkevich, 1947)
For an isotherm lab, 3.5 g of SDB or 2.5 g of SDFB were added to 250 mL Erlenmeyer flasks with different DR28 dye concentrations from 40 to 80 mg/L, and used 200 mL of sample volume, 150 rpm of shaking speed, pH 3, 50 °C, and 12 h.
2.9 Adsorption kinetics
Four kinetic models of pseudo-first-order kinetic, pseudo-second-order kinetic, elovich, and intraparticle diffusion in the linear and nonlinear are used to investigate the adsorption mechanism of SDB and SDFB following Eqs. (11)–(18), and the model obtaining the highest R2 is selected similar to the criteria of a good fit model on the adsorption isotherm. The details are displayed in Table 4.
Models
Full equations
Plotting graph
Eq
References
Linear
Pseudo-first-order
(11)
(Lagergren, 1898)
Pseudo-second-order
(12)
(Ho and McKay, 1999)
Elovich
(13)
(Elovich and Larinov, 1962)
Intraparticle diffusion
(14)
(Weber and Morris, 1963)
Nonlinear
Pseudo-first-order
(15)
(Lagergren, 1898)
Pseudo-second-order
(16)
(Ho and McKay, 1999)
Elovich
(17)
(Elovich and Larinov, 1962)
Intraparticle diffusion
(18)
(Weber and Morris, 1963)
For a kinetic lab, 17.5 g of SDB or 12.5 g of SDFB were added to 1000 mL of breaker with a DR28 dye concentration of 60 mg/L, 1000 mL of sample volume, 150 rpm of shaking speed, pH 3, and 18 h with the ambient temperature for studying DR28 dye adsorptions.
2.10 Thermodynamic studies
Thermodynamic study is generally used for investigating how much the temperature affects the adsorption process by the adsorbent which the different temperatures from 303.15 to 343.15 K referred from the previous study (Praipipat et al., 2023b) were applied to explore the temperature effect on DR28 dye removals of SDB and SDFB using Eqs. (19)–(21) to determine their thermodynamic parameters (MacQueen, 1967).
For the thermodynamic lab, 3.5 g of SDB or 2.5 g of SDFB were used with different temperatures from 303.15 to 343.15 K with a DR28 dye concentration of 60 mg/L, 200 mL of sample volume, 150 rpm of shaking speed, 12 h, and pH 3.
3 Result and discussion
3.1 The physical characteristics
The physical characteristics of SDB and SDFB are shown in Fig. 2a and b. They were spherical with a brown color corresponding to the color of sawdust, whereas SDB was a brighter brown color bead than SDFB. Thus, the modification by iron(III) oxide-hydroxide affected the material color to be a darker brown color in SDFB.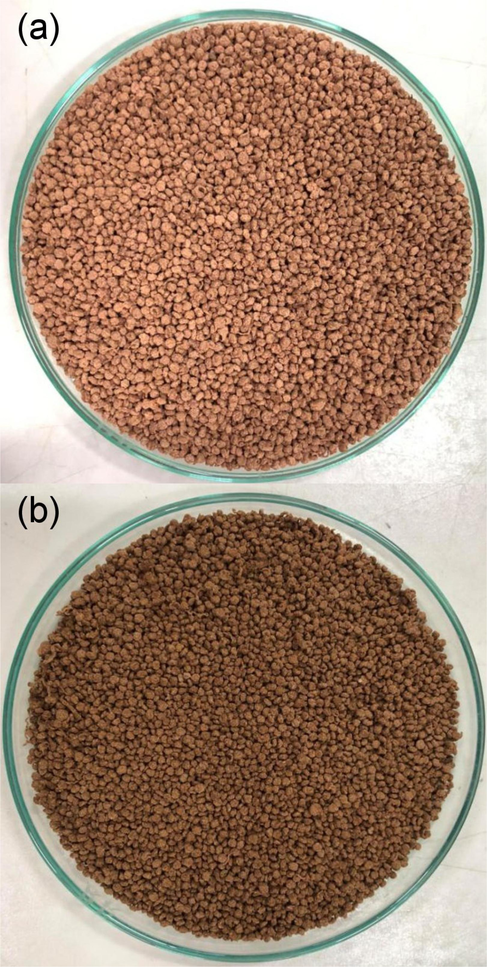
The physical characteristics of (a) SDB and (b) SDFB.
3.2 Material characterizations
3.2.1 Brunauer-Emmett-Teller (BET)
BET analysis is used for investigating the physicochemical properties of SDB and SDFB by Barrett-Joyner-Halenda (BJH) method shown in Table 5. The specific surface area, pore volume, and pore size of SDB were 1.138 m2/g, 0.301 cm3/g, and 4.634 nm, and the specific surface area, pore volume, and pore size of SDFB were 12.540 m2/g, 2.714 cm3/g, and 4.306 nm which they were closely values with another previous study from sawdust materials (Praipipat et al., 2023b). From these results, SDFB had a higher specific surface area with a smaller pore size than SDB which was a good characteristic of an adsorbent for a high dye removal. Thus, the iron(III) oxide-hydroxide modification highly supported the DR28 dye removal similar found in other previous studies that used iron(III) oxide-hydroxide to increase the material capacity (Praipipat et al., 2023g, 2023a, 2023e, 2023c).
SDB
SDFB
BET specific surface area (m2/g)
1.138
12.540
Pore volume (cm3/g)
0.301
2.714
Pore diameter size (nm)
4.634
4.306
3.2.2 Field Emission Scanning Electron Microscopy and Focus Ion Beam (FESEM-FIB) with Energy Dispersive X-Ray Spectrometer (EDX)
The surface structures of SDB and SDFB by FESEM-FIB at 1500X magnification with 100 µm are examined in Fig. 3a and b. The surface area of SDB and SDFB were uneven shapes and heterogeneous fibrillar structures corresponding to a wood structure similar to those observed in a previous study (Praipipat et al., 2023b).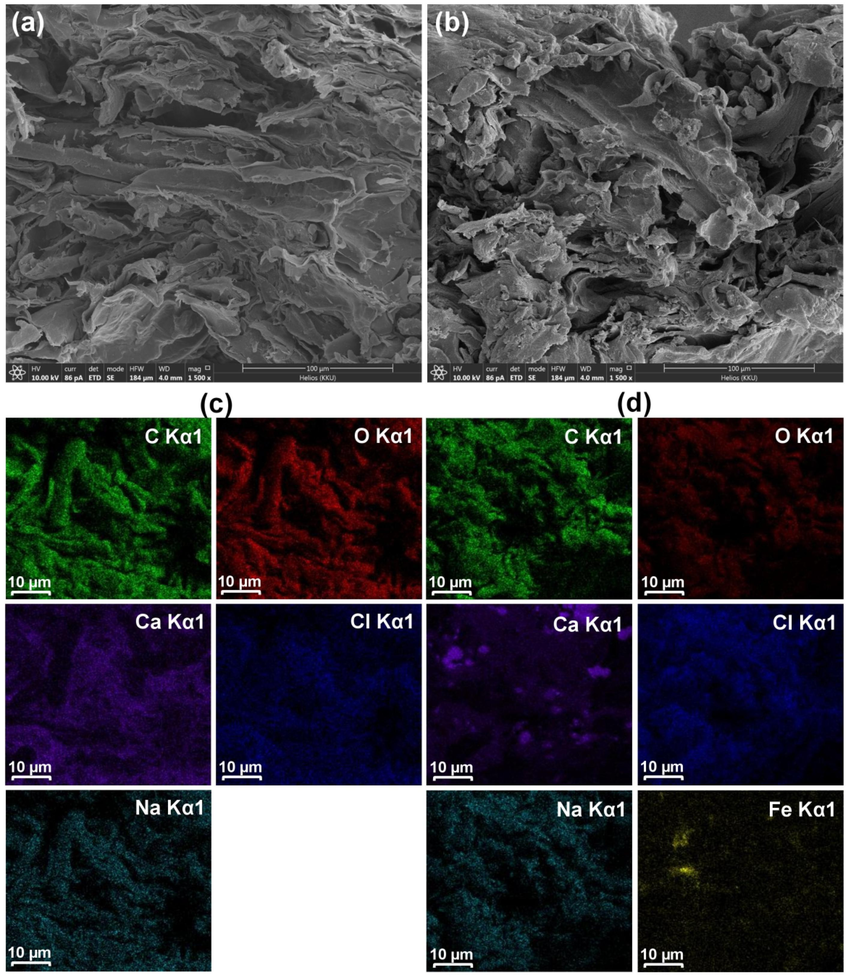
The surface morphologies of (a) SDB, (b) SDFB, and the elemental distributions of (c) SDB and (d) SDFB.
The chemical elements of SDB and SDFB are illustrated in Table 6 consisting of carbon (C), oxygen (O), calcium (Ca), chloride (Cl), and sodium (Na), while iron (Fe) was found in SDFB from modifying by iron(III) oxide-hydroxide corresponded to a previous study (Praipipat et al., 2023b). Moreover, their elemental mapping is demonstrated in Fig. 3c and d to verify the five main chemical elements of C, O, Ca, Cl, and Na detected in both materials and found they spread over their surfaces. Furthermore, SDFB also found the Fe distribution on its surface confirmed the addition of iron(III) oxide-hydroxide into SDFB.
Materials
Chemical elements (%wt)
C
O
Ca
Cl
Na
Fe
SDB
45.9
35.6
16.4
1.5
0.6
–
SDFB
36.7
32.3
15.6
1.3
0.5
13.6
3.2.3 Fourier Transform Infrared Spectroscopy (FT-IR)
The chemical functional groups of SDB and SDFB are determined by FT-IR, and their main chemical functional groups shown in FT-IR spectra are illustrated in Fig. 4a and b. In addition, the specific wavenumber of each chemical functional group in each material is illustrated in Table 7.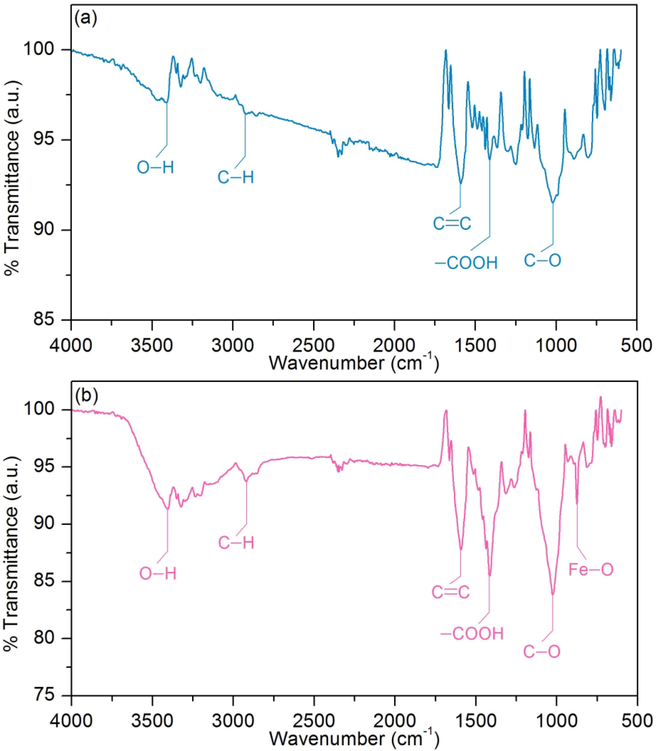
FT-IR spectra of (a) SDB and (b) SDFB.
Wavenumber (cm−1)
Assignments
Functional groups
References
SDB
SDFB
3413.76
3405.10
O—H
Hydroxyl, alcohol, and phenolic groups of lignin, cellulose, pectin, and fiber
(Rahman et al., 2021)
2935.55
2931.69
C—H
Methyl group (–CH2) in cellulose and hemicellulose
(Essabir et al., 2016)
1664.51
1664.18
C⚌C
Aromatic rings of lignin
(Tejada-Tovar et al., 2021)
1417.63
1419.56
—COOH
Carboxyl group of sodium alginate
(Zhong et al., 2020)
1028.02
1029.95
C—O
Carboxylic acids of lignin and hemicellulose
(Rahman et al., 2021)
–
813.93
Fe—O
Metal-oxygen
(Praipipat et al., 2023b)
3.2.4 The point of zero charge
The points of zero charge (pHpzc) of SDB and SDFB were 4.85 and 6.11, respectively demonstrated in Fig. 5a and b which iron(III) oxide-hydroxide could modify the sawdust material by increasing pHpzc similarly observed in many studies (Ngamsurach et al., 2022; Praipipat et al., 2022b, 2023f, 2023a). Therefore, the pH of the sample solution (pHsolution) < pHpzc should be the appropriate pH for DR28 dye adsorptions of SDB and SDFB corresponding to the occurrence of anionic dye adsorption and the previous study reported (Praipipat et al., 2023d).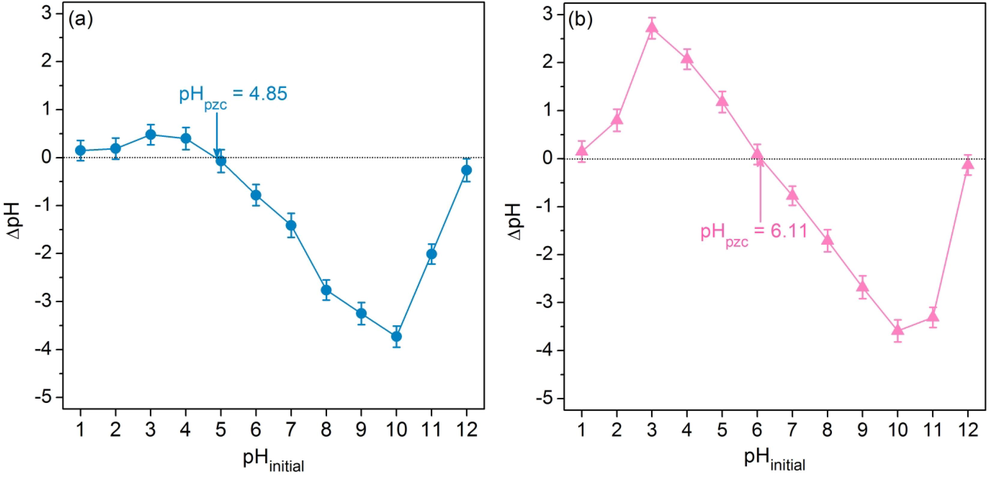
The point of zero charges of (a) SDB and (b) SDFB.
3.3 Batch tests
The batch tests of SDB and SDFB for DR28 dye adsorptions are shown in Fig. 6a–d which demonstrated the optimum dose, temperature, pH, and concentration of SDB and SDFB were 3.5 g, 50 ℃, pH 3, 60 mg/L for 82.93 % and 2.5 g, 40 ℃, pH 3, 60 mg/L for 91.64 %, respectively. SDFB illustrated a higher DR28 dye removal than SDB by spending less dosage and temperature than SDB obtaining a higher DR28 dye removal than SDB. Therefore, the modification by iron(III) oxide-hydroxide in sawdust material resulted in increased DR28 dye removal efficiency.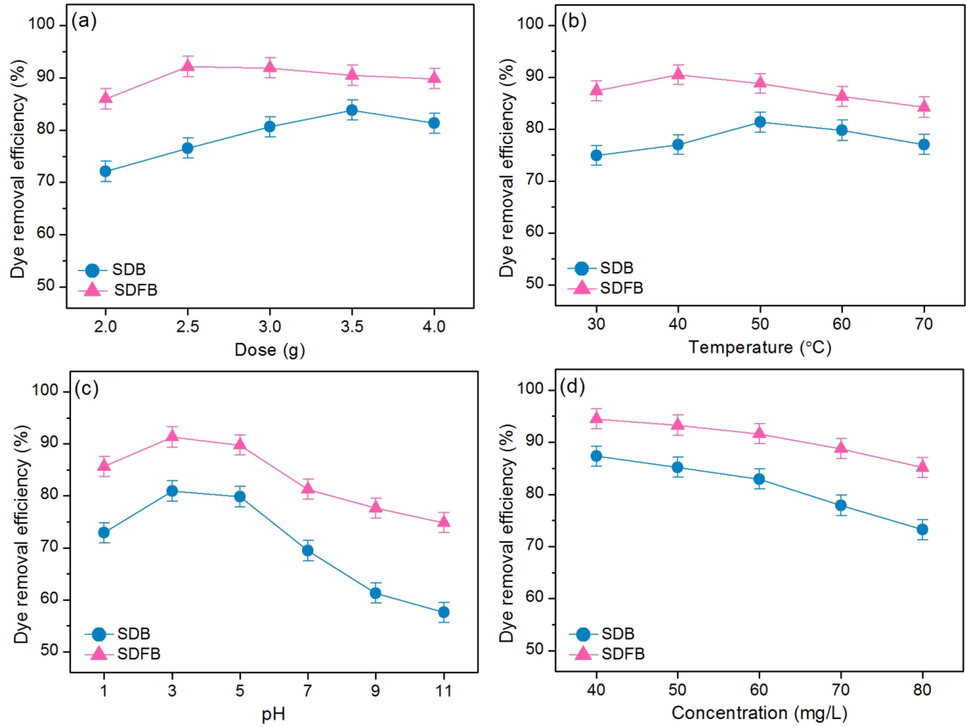
Batch tests for DR28 dye removals on the effects of (a) dose, (b) temperature, (c) pH, and (d) concentration of SDB and SDFB.
3.4 The adsorption–desorption tests
The adsorption–desorption tests of SDB and SDFB for removing DR28 dye in three cycles are reported in Fig. 7a and b. In three cycles, SDB had the percentages of adsorption–desorption in ranges of 63.87-–84.23 % and 58.30–81.22 % decreasing by 22.36 % and 22.92 %. While SDFB had the percentages of adsorption–desorption in ranges of 80.49–93.61 % and 74.48–90.11 % decreasing by 13.12 % and 15.63 %. As a result, SDFB had a higher DR28 dye adsorption than SDF corresponding to batch test results. Therefore, SDB and SDFB could reuse more than three cycles with high DR28 dye adsorptions of more than 63 %. For the evaluation of cost-effectiveness, not only SDB and SDFB could reuse more than three cycles with high DR28 dye adsorptions but also the use of sawdust from a sawmill could reuse waste and increase the value of waste by reusing it as a dye absorbent. Moreover, the material synthesis costs of SDB and SDFB were suitable costs of approximately 25 USD per kg. In addition, since SDB and SDFB are beaded materials, they are easy to separate from treated wastewater and reduce the operating cost of wastewater treatment. Therefore, they are potential materials for the feasibility of industrial applications for removing DR28 dye in wastewater, especially SDFB.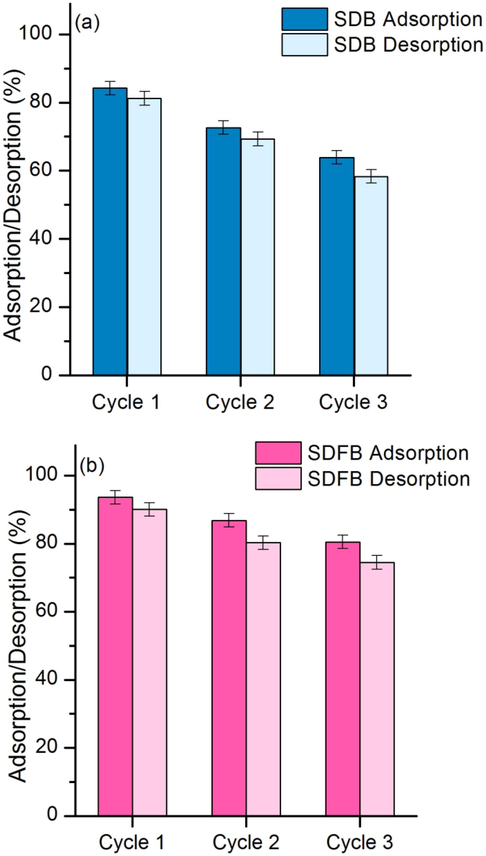
The desorption tests of (a) SDB and (b) SDFB.
3.5 Adsorption isotherms
The adsorption isotherms of SDB and SDFB for eliminating DR28 dye are shown in Fig. 8a–j and Table 8 which good fit models of SDB and SDFB were Langmuir and Freundlich, respectively. Therefore, SDB was the physical adsorption, while SDFB was the physiochemical adsorption. In addition, the linear and nonlinear plotting graphs are recommended to protect against data mistranslation similarly supported by previous studies (Ngamsurach et al., 2022; Praipipat et al., 2023b).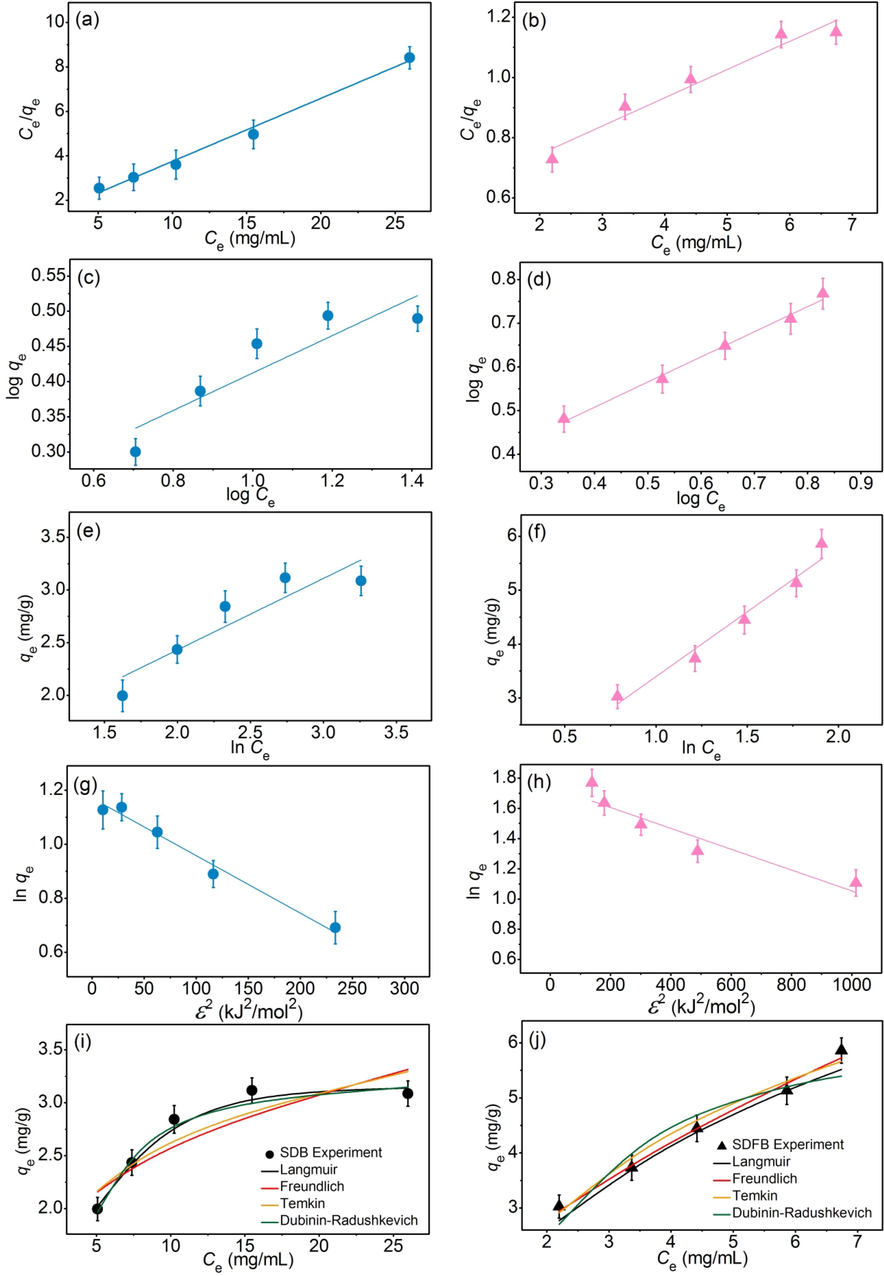
Graphs of (a and b) linear Langmuir, (c and d) linear Freundlich, (e and f) linear Temkin, (g and h) linear Dubinin- Radushkevich, and (i and j) nonlinear adsorption isotherms of SDB and SDFB for DR28 dye adsorptions.
Regression methods
Isotherm models
Parameters
SDB
SDFB
Linear
Langmuir
qm (mg/g)
3.534
10.593
KL (L/mg)
0.321
0.170
R2
0.991
0.957
Freundlich
1/n
0.269
0.580
KF (mg/g)(L/mg)1/n
1.399
1.884
R2
0.823
0.993
Temkin
bT (J/mol)
3896.547
1094.188
AT (L/g)
4.573
1.455
R2
0.845
0.969
Dubinin-Radushkevich
qm (mg/g)
3.208
5.800
KDR (mol2/J2)
0.002
0.001
E (kJ/mol)
15.430
26.726
R2
0.982
0.898
Nonlinear
Langmuir
qm (mg/g)
3.581
10.617
KL (L/mg)
0.388
0.160
R2
0.993
0.963
R2adj
0.991
0.950
RMSE
0.044
0.249
Freundlich
1/n
0.262
0.595
KF (mg/g)(L/mg)1/n
1.410
1.840
R2
0.819
0.990
R2adj
0.759
0.987
RMSE
0.253
0.126
Temkin
bT (J/mol)
3755.320
1026.477
AT (L/g)
4.572
1.488
R2
0.848
0.966
R2adj
0.798
0.955
RMSE
0.217
0.238
Dubinin-Radushkevich
qm (mg/g)
3.216
6.020
KDR (mol2/J2)
0.002
0.001
E (kJ/mol)
15.399
25.138
R2
0.979
0.891
R2adj
0.972
0.854
RMSE
0.080
0.427
In comparison, the maximum adsorption capacities of several waste adsorbents with or without modifications for DR28 dye removals are demonstrated and compared with this study in Table 9. SDFB examined the highest DR28 dye removal than other studies, whereas SDB showed a higher DR28 dye removal than waste adsorbents of rice husk, banana peel, cabbage, and sugarcane bagasse fly ash. Therefore, SDFB was a potential material for DR28 dye adsorption in industrial applications.
Materials
Conditions
qm (mg/g)
References
Rice husk char
0.5 g, 20 min, 30 °C, pH 4, concentration 20–100 mg/L, 15 mL
1.28
(Malik et al., 2020)
Rice husk
0.5 g, 20 min, 30 °C, pH 4, concentration 20–100 mg/L, 15 mL
1.58
(Malik et al., 2020)
Rice husk char modified potassium hydroxide
0.5 g, 20 min, 30 °C, pH 6, concentration 20–100 mg/L, 15 mL
2.04
(Malik et al., 2020)
Banana peel
1.5 g, 1.5 h, 40 °C, pH 10, concentration 20–40 mg/L, 80 mL
1.72
(Mondal and Kar, 2018)
Cabbage
2.0 g, 3 h, 25 °C, pH 8, concentration 4.88–48.76 mg/L, 50 mL
2.31
(Wekoye et al., 2020)
Pine bark
10 g/L, 25 °C, pH 6, concentration 5–100 mg/L, 100 mL
3.92
(Litefti et al., 2019)
Calcined kaolin
1.2 g, 2 h, 20 °C, concentration 20–50 mg/L, 200 mL
5.39
(Findik, 2023)
Sugarcane bagasse fly ash beads
2.5 g, 15 h, 30 °C, pH 3, concentration 30–90 mg/L, 100 mL
3.36
(Praipipat et al., 2023d)
Sugarcane bagasse fly ash beads doped with zinc oxide
2.5 g, 12 h, 30 °C, pH 3, concentration 30–90 mg/L, 100 mL
3.90
(Praipipat et al., 2023d)
Sugarcane bagasse fly ash beads doped with titanium dioxide
2.0 g, 15 h, 30 °C, pH 3, concentration 30–90 mg/L, 100 mL
4.01
(Praipipat et al., 2023d)
Sugarcane bagasse fly ash beads doped with aluminum oxide
1.5 g, 15 h, 30 °C, pH 3, concentration 30–90 mg/L, 100 mL
5.46
(Praipipat et al., 2023d)
Sugarcane bagasse fly ash beads doped with magnesium oxide
1.5 g, 12 h, 30 °C, pH 3, concentration 30–90 mg/L, 100 mL
5.56
(Praipipat et al., 2023d)
SDB
3.5 g, 12 h, 50 °C, pH 3, concentration 40–80 mg/L, 200 mL
3.53
This study
SDFB
2.5 g, 12 h, 50 °C, pH 3, concentration 40–80 mg/L, 200 mL
10.59
This study
3.6 Adsorption kinetics
The adsorption kinetics of SDB and SDFB for adsorbing DR28 dye are examined in Fig. 9a–j and Table 10. SDB and SDFB corresponded to the pseudo-second-order model which meant their adsorption mechanism was the chemisorption process that corresponded to many studies (Ngamsurach et al., 2022; Praipipat et al., 2022b, 2023b). In addition, the linear and nonlinear kinetic models corresponded to each other similarly to the adsorption isotherm results, so they were correct data translations in this study.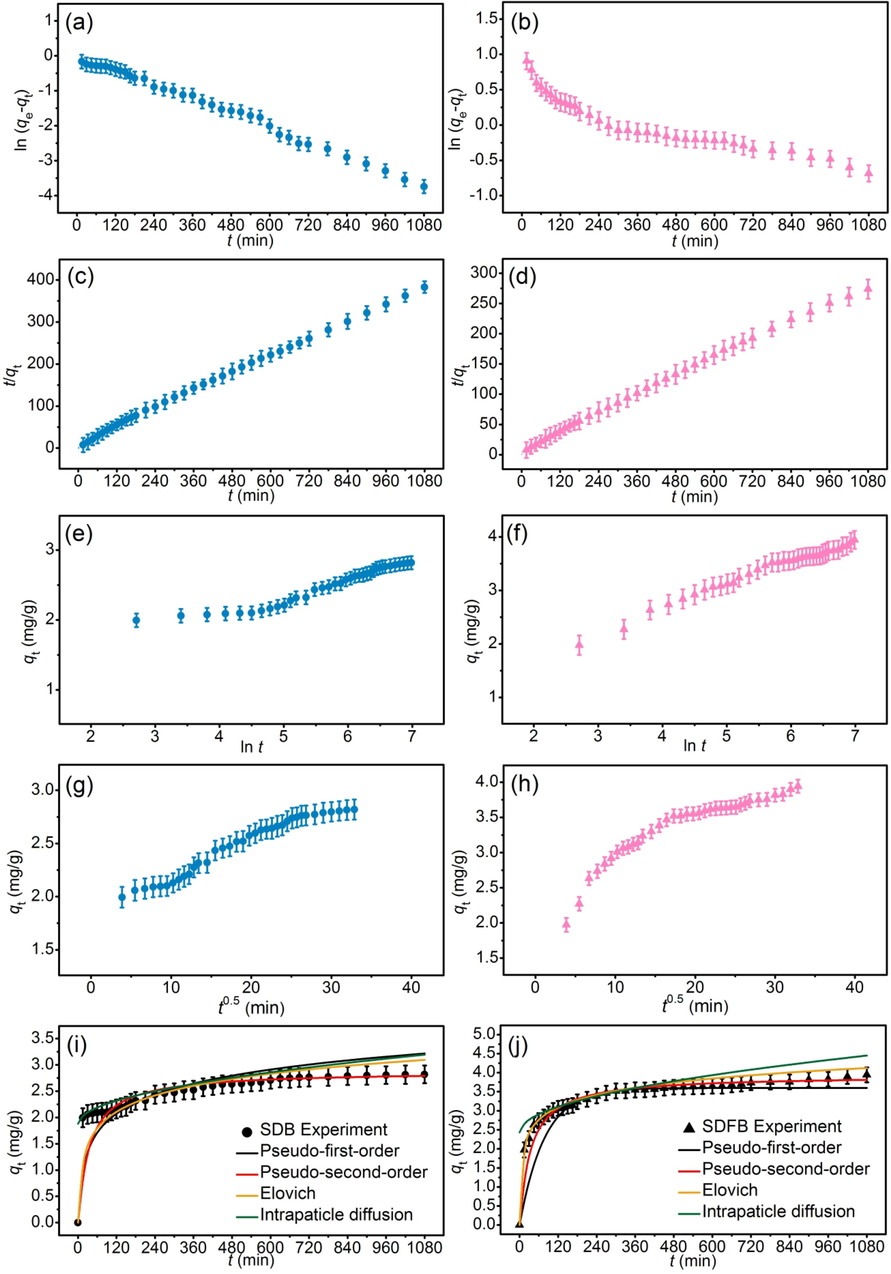
Graphs of (a and b) linear pseudo-first-order, (c and d) linear pseudo-second-order, (e and f) linear elovich model (g and h) linear intraparticle diffusion, and (i and j) nonlinear kinetic models of SDB and SDFB for DR28 dye adsorptions.
Regression methods
Kinetic models
Parameters
SDB
SDFB
Linear
Pseudo-first-order
qe (mg/g)
1.117
1.617
k1 (min−1)
0.003
0.001
R2
0.968
0.869
Pseudo-second-order
qe (mg/g)
2.881
3.931
k2 (g/mg·min)
0.010
0.008
R2
0.998
0.998
Elovich
α (mg/g·min)
5.096
14.999
β (g/mg)
3.015
2.362
R2
0.904
0.981
Intraparticle diffusion
ki (mg/g·min0.5)
0.033
0.052
Ci
1.862
2.396
R2
0.963
0.858
Nonlinear
Pseudo-first-order
qe (mg/g)
1.550
1.812
k1 (min−1)
0.004
0.002
R2
0.965
0.871
R2adj
0.964
0.867
RMSE
0.391
0.408
Pseudo-second-order
qe (mg/g)
2.895
3.927
k2 (g/mg·min)
0.013
0.009
R2
0.996
0.939
R2adj
0.995
0.938
RMSE
0.231
0.179
Elovich
α (mg/g·min)
5.148
15.270
β (g/mg)
3.451
2.470
R2
0.906
0.985
R2adj
0.903
0.984
RMSE
0.228
0.143
Intraparticle diffusion
ki (mg/g·min0.5)
0.036
0.061
Ci
1.879
2.432
R2
0.966
0.863
R2adj
0.965
0.859
RMSE
0.361
0.503
3.7 Thermodynamic studies
The thermodynamic studies of SDB and SDFB for DR28 dye adsorptions are illustrated in Table 11. In addition, their thermodynamic plots for DR28 dye adsorptions used to determine the values of ΔH° and ΔS° from the slope and intercept of their linear equations plotted by ln Kc versus 1/T are demonstrated in Fig. 10a and b. If the slope is a positive value, ΔH° is a negative value meaning the exothermic process. In addition, if the intercept is a negative value, ΔS° is a negative value meaning the decreasing of randomness during the adsorption process. Since their ΔG° values were negative which were favorable adsorption processes of a spontaneous nature. For ΔH° and ΔS°, both SDB and SDFB were also negative which were exothermic in nature (Praipipat et al., 2022b) and decreased the randomness during the adsorption process (Wong et al., 2019). Thus, DR28 dye adsorptions of both materials were not favorable when the temperature increased.
Materials
ΔG° (kJ/mol)
ΔH° (kJ/mol)
ΔS°
(J/mol K)
303.15 K
313.15 K
323.15 K
333.15 K
343.15 K
SDB
−1.23
−0.97
−0.75
−0.45
−0.21
−8.96
−25.49
SDFB
−3.98
−3.47
−3.10
−2.55
−2.17
−17.79
−45.61
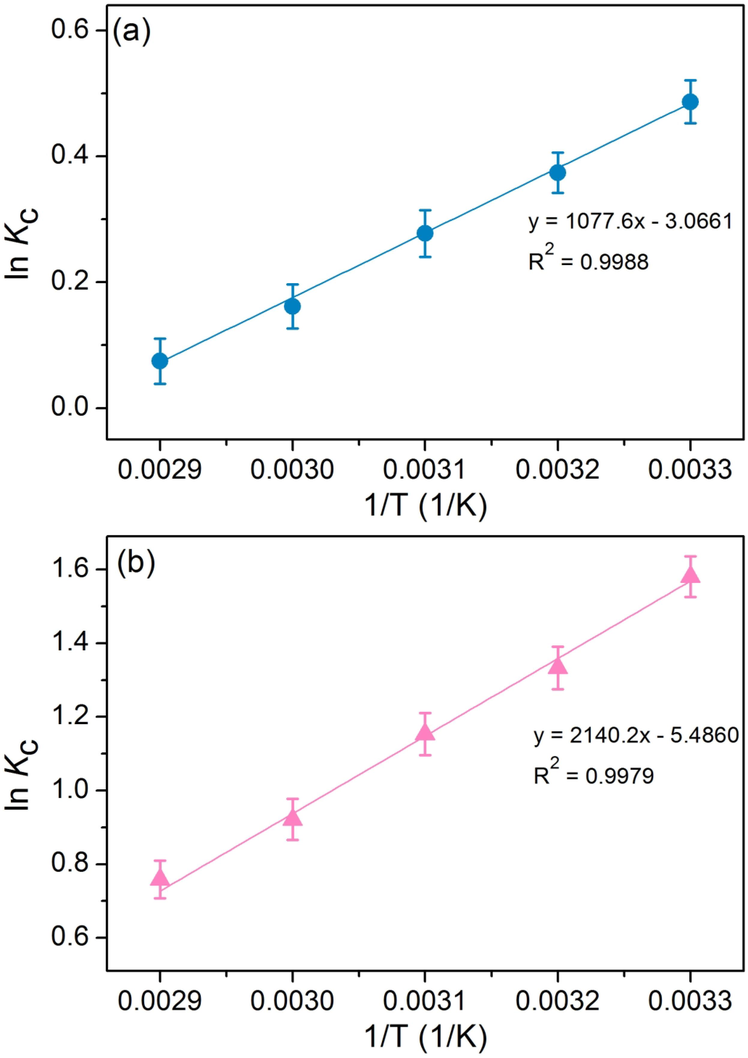
Thermodynamic plots of (a) SDB and (b) SDFB for DR28 dye adsorptions.
4 The possible mechanisms of SDB and SDFB for DR28 dye adsorptions
The possible mechanisms of SDB and SDFB for adsorbing DR28 dye could be explained by modifying ideas from the previous studies of Ngamsurach et al. (Ngamsurach et al., 2022) and Praipipat et al. (Praipipat et al., 2023b) which the electrostatic attraction, hydrogen bonding interaction, and n-π bonding interaction were main mechanisms of DR28 dye adsorptions. In addition, their main chemical functional groups of hydroxyl (–OH) and iron(III) oxide-hydroxide (–Fe(OH)3) occurring from sharing the electron with –OH had an important role in their adsorption mechanisms clearly described in Fig. 11a and b. For the electrostatic attraction, the positively charged of –OH on SDB and SDFB surfaces interacted with the negatively charged of sulfonate groups (–SO3-) of DR28 dye at an acidic solution which its pH < pHpzc of SDB and SDFB illustrated in Fig. 5a and b. For the hydrogen bonding interaction, the hydrogen ions in –OH caught up with the nitrogen ions in the DR28 dye. Finally, the oxygen atom in –OH interacted with the aromatic ring in the DR28 dye demonstrating the n-π bonding interaction.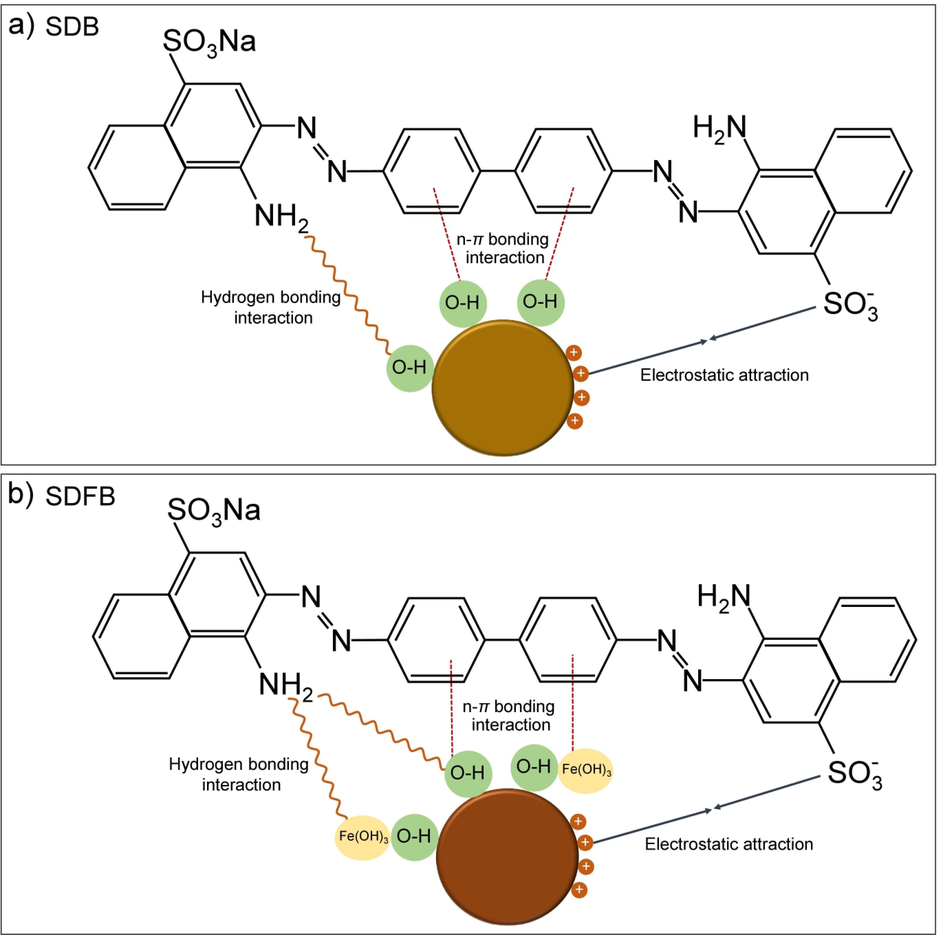
Possible mechanisms of DR28 dye adsorptions on (a) SDB and (b) SDFB.
5 Conclusion
Pterocarpus macrocarpus sawdust was used for synthesizing sawdust beads (SDB) and sawdust mixed with iron(III) oxide-hydroxide beads (SDFB) for DR28 dye adsorptions. Iron(III) oxide-hydroxide increased sawdust material efficiency by increasing the specific surface area to be more available DR28 dye adsorptions, and the batch test results also confirmed a higher DR28 dye removal of SDFB of 91.64 % than SDB of 82.93 % which SDFB also spent material dose and temperature less than SDB. Moreover, they also reused more than three cycles with high DR28 dye adsorptions of more than 63 %. Their DR28 dye adsorptions were an exothermic process that did not favor adsorption with increasing temperature. Three possible mechanisms of electrostatic attraction, hydrogen bonding interaction, and n-π bonding interaction could be good explanations for their DR28 dye adsorptions. Therefore, they are potential sawdust materials for removing DR28 dye contaminated in wastewater, especially SDFB.
For future works, the column experiments need to be studied before being applied in industry, and the competing ions might be necessary to explore their DR28 dye removal efficiencies of SDB and SDFB in case of real wastewater to confirm specific DR28 dye removals by them.
CRediT authorship contribution statement
Pornsawai Praipipat: Supervision, Conceptualization, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. Pimploy Ngamsurach: Investigation, Visualization, Writing – original draft. Piyaporn Khamkhae: Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- DFT and experimental study on adsorption of dyes on activated carbon prepared from apple leaves. Carbon Lett.. 2021;31:863-878.
- [CrossRef] [Google Scholar]
- Utilization of Azadirachta indica sawdust as a potential adsorbent for the removal of crystal violet dye. Sustain. Chem.. 2023;4:110-126.
- [CrossRef] [Google Scholar]
- Comparative study for adsorption of congo red and methylene blue dye on chitosan modified hybrid nanocomposite. Process Biochem.. 2021;108:90-102.
- [CrossRef] [Google Scholar]
- Classifications, properties, recent synthesis and applications of azo dyes. Heliyon. 2020;6:e03271.
- [Google Scholar]
- Removal of Eosin dye from simulated media onto lemon peel-based low cost biosorbent. Arab. J. Chem.. 2022;15:103873
- [CrossRef] [Google Scholar]
- Adsorption of reactive dyes on lignocellulosic waste; characterization, equilibrium, kinetic and thermodynamic studies. J. Clean. Prod.. 2019;225:1220-1229.
- [CrossRef] [Google Scholar]
- The equation of the characteristic curve of activated charcoal. Proc. USSR Acad. Sci.. 1947;55:327-329.
- [Google Scholar]
- Recent advances on the removal of dyes from wastewater using various adsorbents: a critical review. Mater. Adv.. 2021;2:4497-4531.
- [CrossRef] [Google Scholar]
- Fe3O4-wheat straw: preparation, characterization and its application for methylene blue adsorption. Water Resour. Ind.. 2014;7–8:23-37.
- [CrossRef] [Google Scholar]
- Theory of adsorption from solutions of non electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form,(II) verification of the equation of adsorption isotherm from solutions. Izv. Akad. Nauk. SSSR Otd. Khim. Nauk. 1962;2:209-216.
- [Google Scholar]
- Adsorptive behavior of methylene blue onto sawdust of sour lemon, date palm, and eucalyptus as agricultural wastes. J. Dispers. Sci. Technol.. 2019;40:990-999.
- [CrossRef] [Google Scholar]
- Mechanical and thermal properties of hybrid composites: oil-palm fiber/clay reinforced high density polyethylene. Mech. Mater.. 2016;98:36-43.
- [CrossRef] [Google Scholar]
- Adsorption of safranin O from aqueous phase using sugarcane bagasse. Int. J. Ecol. Sci. Environ. Eng.. 2015;2:17-29.
- [Google Scholar]
- Adsorption of diazo dye direct red-28 and tetra-azo dye direct black-22 using calcined kaolin in aqueous solutions. Bull. Chem. Soc. Ethiop.. 2023;37:593-610.
- [Google Scholar]
- Impact of ZnO and Fe3O4 magnetic nanoscale on the methyl violet 2B removal efficiency of the activated carbon oak wood. Chemosphere. 2022;286:131632
- [CrossRef] [Google Scholar]
- Effective adsorption of anionic dye, alizarin red S, from aqueous solutions on activated clay modified by iron oxide. Ind. Eng. Chem. Res.. 2011;50:9712-9717.
- [CrossRef] [Google Scholar]
- Biosorption of methyl violet 2B by chemically treated Okoume sawdust: kinetic, isotherm and thermodynamic studies. Alger. J. Eng. Res.. 2021;4:34-41.
- [Google Scholar]
- Efficient removal of methyl red dye by using bark of hopbush. Water. 2022;14:2831.
- [CrossRef] [Google Scholar]
- Recent advances in removal of Congo Red dye by adsorption using an industrial waste. Sci. Rep.. 2022;12:6087.
- [CrossRef] [Google Scholar]
- Pseudo-second order model for sorption processes. Process Biochem.. 1999;34:451-465.
- [CrossRef] [Google Scholar]
- Effective sequestration of Congo red dye with ZnO/cotton stalks biochar nanocomposite: MODELING, reusability and stability. J. Saudi Chem. Soc.. 2021;25:101176
- [CrossRef] [Google Scholar]
- Impact of textile dyes on health and ecosystem: a review of structure, causes, and potential solutions. Environ. Sci. Pollut. Res. 2023
- [CrossRef] [Google Scholar]
- Comparative performance between rice husk and granular activated carbon for the removal of azo tartrazine dye from aqueous solution. Desalin. Water Treat.. 2021;229:372-383.
- [CrossRef] [Google Scholar]
- Optimization of Congo red dye adsorption from wastewater by a modified commercial zeolite catalyst using response surface modeling approach. Water Sci. Technol.. 2021;83:1369-1383.
- [CrossRef] [Google Scholar]
- About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakademiens Handl.. 1898;24:1-39.
- [Google Scholar]
- The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc.. 1918;40:1361-1403.
- [Google Scholar]
- Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep.. 2019;9:16530.
- [CrossRef] [Google Scholar]
- Adsorption of crystal violet onto an agricultural waste residue: kinetics, isotherm, thermodynamics, and mechanism of adsorption. Sci. World J. 20205873521
- [CrossRef] [Google Scholar]
- Some observations concerning the van’t hoff equation. J. Chem. Educ.. 1967;44:755-756.
- [CrossRef] [Google Scholar]
- A comparative study of the adsorption of congo red dye on rice husk, rice husk char and chemically modified rice husk char from aqueous media. Bull. Chem. Soc. Ethiop.. 2020;34:41-54.
- [Google Scholar]
- Potentiality of banana peel for removal of Congo red dye from aqueous solution: isotherm, kinetics and thermodynamics studies. Appl. Water Sci.. 2018;8:157.
- [CrossRef] [Google Scholar]
- Adsorption of malachite green dye from aqueous solutions using mesoporous chitosan–zinc oxide composite material. Environ. Chem. Ecotoxicol.. 2020;2:115-125.
- [CrossRef] [Google Scholar]
- A brief study of adsorption of Congo red dye over sawdust of Cedrus deodara. Desalin. Water Treat.. 2021;235:272-282.
- [CrossRef] [Google Scholar]
- Modified beaded materials from recycled wastes of bagasse and bagasse fly ash with iron (III) oxide-hydroxide and zinc oxide for the removal of reactive blue 4 dye in aqueous solution. ACS Omega. 2022;7:34839-34857.
- [CrossRef] [Google Scholar]
- Toxicity and decontamination strategies of Congo red dye. Groundw. Sustain. Dev.. 2022;19:100844
- [CrossRef] [Google Scholar]
- Sawdust-based cellulose nanocrystals incorporated with ZnO nanoparticles as efficient adsorption media in the removal of methylene blue dye. ACS Omega. 2020;5:18798-18807.
- [CrossRef] [Google Scholar]
- H3PO4-activated sawdust and rice husk as effective decolorizers for textile wastewater containing Reactive Black 5. Int. J. Environ. Sci. Technol.. 2019;16:8375-8388.
- [CrossRef] [Google Scholar]
- Synthesis of wheat bran sawdust/Fe3O4 composite for the removal of methylene blue and methyl violet. Environ. Monit. Assess.. 2021;193:276.
- [CrossRef] [Google Scholar]
- Comparative reactive blue 4 dye removal by lemon peel bead doping with iron (III) oxide-hydroxide and zinc oxide. ACS Omega. 2022;7:41744-41758.
- [CrossRef] [Google Scholar]
- Chicken and duck eggshell beads modified with iron (III) oxide-hydroxide and zinc oxide for reactive blue 4 dye removal. Arab. J. Chem.. 2022;15:104291
- [CrossRef] [Google Scholar]
- Powdered and beaded zeolite A from recycled coal fly ash with modified iron (III) oxide-hydroxide for lead adsorptions. Environ. Nanotechnol, Monit. Manag.. 2023;20:100812
- [CrossRef] [Google Scholar]
- Powdered and beaded sawdust materials modified iron (III) oxide-hydroxide for adsorption of lead (II) ion and reactive blue 4 dye. Sci. Rep.. 2023;13:531.
- [CrossRef] [Google Scholar]
- The synthesis, characterizations, and lead adsorption studies of chicken eggshell powder and chicken eggshell powder-doped iron (III) oxide-hydroxide. Arab. J. Chem.. 2023;16:104640
- [CrossRef] [Google Scholar]
- Synthesis and characterization of metal oxide dopped beaded sugarcane bagasse fly ash for direct red 28 dye removal. J. Ind. Eng. Chem.. 2023;25:495-514.
- [CrossRef] [Google Scholar]
- Zeolite A powder and beads from sugarcane bagasse fly ash modified with iron(III) oxide-hydroxide for lead adsorption. Sci. Rep.. 2023;13:1873.
- [CrossRef] [Google Scholar]
- Modification of sugarcane bagasse with iron(III) oxide-hydroxide to improve its adsorption property for removing lead(II) ions. Sci. Rep.. 2023;13:1467.
- [CrossRef] [Google Scholar]
- Influence of duck eggshell powder modifications by the calcination process or addition of iron (III) oxide-hydroxide on lead removal efficiency. Sci. Rep.. 2023;13:12100.
- [CrossRef] [Google Scholar]
- Reactive blue 4 adsorption efficiencies on bagasse and bagasse fly ash beads modified with titanium dioxide (TiO2), magnesium oxide (MgO), and aluminum oxide (Al2O3) Ind. Crop. Prod.. 2023;191:115928
- [CrossRef] [Google Scholar]
- Activated Ailanthus altissima sawdust as adsorbent for removal of acid yellow 29 from wastewater: Kinetics approach. Water. 2021;13:2136.
- [CrossRef] [Google Scholar]
- Brilliant green and acid orange 74 dyes removal from water by Pinus roxburghii leaves in naturally benign way: an application of green chemistry. J. Chem.. 2019;2019:3573704
- [CrossRef] [Google Scholar]
- Investigation of congo red toxicity towards different living organisms: a review. Processes. 2023;11:807.
- [CrossRef] [Google Scholar]
- Potential use of residual sawdust of Eucalyptus Globulus Labill in Pb (II) adsorption: Modelling of the kinetics and equilibrium. Appl. Sci.. 2021;11:3125.
- [CrossRef] [Google Scholar]
- Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim. URSS. 1940;12:327-356.
- [Google Scholar]
- Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: a review. Sci. Total Environ.. 2020;717:137222
- [CrossRef] [Google Scholar]
- Kinetic and equilibrium studies of Congo red dye adsorption on cabbage waste powder. Environ. Chem. Ecotoxicol.. 2020;2:24-31.
- [CrossRef] [Google Scholar]
- Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL) J. Clean. Prod.. 2019;206:394-406.
- [CrossRef] [Google Scholar]
- Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci. Rep.. 2020;10:2928.
- [CrossRef] [Google Scholar]
- Enhanced adsorption of Congo red dye by functionalized carbon nanotube/mixed metal oxides nanocomposites derived from layered double hydroxide precursor. Chem. Eng. J.. 2015;275:315-321.
- [CrossRef] [Google Scholar]
- The role of sodium alginate in the flotation separation of apatite and dolomite. Powder Technol.. 2020;373:620-626.
- [CrossRef] [Google Scholar]







