Translate this page into:
Isolation of herbicidal compounds, quercetin and β-caryophyllene, from Digera muricata
⁎Corresponding author. muhammad.akbar@uog.edu.pk (Muhammad Akbar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Synthetic herbicides are available to control weeds but these herbicides have many concerns. Eco-friendly herbicides obtained from plants is a better alternative to synthetic ones. Despite that the herbicidal activity of Digera muricata extracts have been reported but there are no studies regarding the isolation and identification of herbicidal compounds from D. muricata. Herein, we are reporting the identification of two herbicidal compounds from chloroform extract obtained from D. muricata. The chloroform extract was initially tested on the germination and early growth of two weeds; Avena fatua and Melilotus indicus as well as wheat where a significant decline in % age germination and growth of both weeds was observed. Among the 8 different fractions obtained using different chromatographic techniques, fractions 2 and 7 were found to be phytotoxic to both test weeds. The herbicidal efficacy was tested at 200, 150, 100, 50, 25 μg/ml. These two fractions were further purified on Reversed Phase High Pressure Liquid Chromatography (RP-HPLC). Fraction 2 yielded 3 sub-fractions (2A, 2B & 2C), while fraction 7 yielded 2 sub-fractions (7A, 7B). Fraction 2B caused 43%, 53%, and 57% decline in seed germination, shoot dry weight, and root dry weight of A. fatua, while these values against M. indicus were 50%, 81% and 84%, respectively. Fraction 7A caused 25%, 36%, and 42% decline in seed germination, shoot dry weight, and root dry weight of A. fatua, while these values against M. indicus were 35%, 62% and 69%, respectively, at 200 μg/ml conc. Cyanazine caused 61% and 50% reduction in seed germination of A. fatua and M. indicus, respectively. The herbicidal effects of these two fractions were found nonsignificant against wheat. Fourier Transform Infrared Spectroscopy (FT-IR), elemental analysis (C,H) and Nuclear Magnetic Resonanace (NMR) analyses of these two fractions depicted the presence of quercetin (Fraction 2B) and β-caryophyllene (Fraction 7A). In post emergence bioassays, the isolated compounds caused significant decrease in the biomass of both weeds. Plasma membrane integrity assays revealed electrolyte leakage in treated leaf discs of both weeds. It was concluded that quercetin and β-caryophyllene isolated from D. muricata exhibited toxicity towards both test weeds without harming wheat.
Keywords
Digera muricata
Herbicidal
Phytotoxic
Quercetin
β-caryophyllene
1 Introduction
Weeds increase abiotic stresses like utilization of water, light, nutrients and unavailability of growth resources for crops (Soares et al., 2019; Silva et al., 2013). The roots of different plants excrete certain chemicals, produced during metabolism and are termed as allelochemicals (Broeckling et al., 2008). These allelochemicals are used by the plants for interactions with other plants and the environment (Aslani et al., 2014). These allelochemicals may interfere with the physiology of other plants and may enhance or retard the germination of the recipient ones (Majeed et al., 2017). The germination of Pirsabaq cultivar was totally failed due to aqueous extracts of Melilotus indicus (Siyar et al., 2017). Weeds e.g., Avena fatua, M. indicus and Polypogon hissaricus are the most dangerous weeds that decrease the wheat production in many regions of the world, including Pakistan (Siyar et al., 2019; Kong et al., 2019).
Despite the great success and widespread adoption of herbicides by farmers, the continued and heavy use of herbicides not only destroyed the ecosystem but also posed serious health risks and the evolution of herbicide resistant weeds (Iqbal et al., 2019). Owing to these negative effects of these weed killers, now there is demand of new groups of substances that are biodegradable, having plant based origin and may have the possibilities to be used as herbicides (Aryakia et al., 2015: Anwar et al., 2019). The allelochemicals disturb the growth of undesired plants by altering their cell structure, inhibit the cell multiplication, disruption in the plasma membrane or can create troubles in uptake of dissolved minerals and water. Utilization of these allelochemicals is environment-friendly and it helps to retard the applications of herbicides. So, allelopathy is an alternative method to control growth of undesired plants (Nawaz et al., 2020).
Plant-based natural herbicides can reduce the application of synthetic herbicides due to having the potential of producing those set of substances that are useful for weed killing mechanisms (Anwar et al., 2019; Ahmed et al., 2014). As an example, leaves aqueous extract of Jimson weed (Datura stramonium) at concentrations of 5, 15, 30, 40, 50 and 60% reduced the germination of sugar beet (Beta vulgaris) and A. retroflexus by 89% and 55%, at 5% concentration. However, at all other concentrations, 100% decline in germination was recorded (Yorulmaz et al., 2020). In another study, seed sprouting, seedling height, seedling vigor index and biomass of Parthenium hysterophorus were suppressed significantly due to the application of extracts of Datura metel, Mangifera indica, Azadirachta indica and Tagetes erectus at 50% and 75% concentration (Ramachandran et al., 2020). In another study, leaf extract of nerium (Nerium oleander), olives (Olea europaea) and castor oil (Ricinus communis) stopped the germination of purple nut sedge (Cyperus rotundus). Nerium extract caused 90% inhibitory effect at 5 and 10%, however, the 2.5% concentration showed the lowest inhibition effects on the germination of weeds, while in 2nd experiment, 10% nerium extract exhibited 100% inhibition (Al-Samarai et al., 2018). In another study, the isolated compounds viz., diterpenes, manool oxide and sesquiterpene hydrocarbons from Pinus nigra showed strong herbicidal efficacy against various weeds like Phalaris canariensis, Trifolium campestre and Sinapis arvensis (Amri et al., 2017).
There are few reports of phytotoxic activity of Digera muricata (Family Amaranthaceae). D. muricata aqueous extract decreased the germination and growth of Pennisetum glaucum. Major compounds detected by Gas Chromatography Mass Spectrometry (GC/MS) included quercetin, sinapic, ferluic acid, cystine, berbine and limonene from roots and shoots of D. muricata (Aziz & Shaukat, 2014). In another styudy, D. muricata extracts at 1, 3 and 5% concentrations significantly decreased the seed germination and seedling growth of Pennisetum typhoideum (Bindu & Jain, 2011). As the phytotoxic activity of D. muricata extracts have been reported by earlier workers so we hypothesized that D. muricata should possess herbicidal/phytotoxic compounds. To the best of our knowledge, previously there are no studies regarding the isolation and identification of herbicidal compounds from D. muricata, so herein we isolated the actual bioactive compounds from D. muricata and tested for their herbicidal activity. Identification of actual herbicidal compounds would act as structural analogues to synthesize of new herbicidal compounds (Natural herbicides) by herbicide companies to safeguard environment and human health.
2 Materials and methods
2.1 Collection of weeds
Two knotty weeds of wheat crop viz., Avena fatua and Melilotus indicus were chosen for study. The ripened/mature seeds of these weeds were collected in the month of May from wheat fields of District Gujrat, Pakistan at the end of the wheat growing season. These seeds were sun dried for 7 days and then packed in paper bags for further use.
2.2 Collection of test plant material
Test plant namely Digera muricata was harvested at flowering stage in the month of October, from the fields at Islam Nagar and Sabowal village, Gujrat, Punjab, Pakistan, to evaluate its herbicidal potential against the selected weeds namely M. indicus and A. fatua.
2.3 Preparation of extracts of test plant
For the preparation of plant extracts, procedure described by Akbar et al. (2020) was adopted with modifications. After collection, the plants were surface washed with water sprinkler to remove impurities like dust particles. Immediately after washing procedure, whole plants were air dried with the aid of fan to remove excessive moisture. These plants were cut with a fodder cutter into 2 cm pieces and the cut plant pieces were dried under shade to avoid leaching of metabolites by rainwater. The dried chopped plant material was ground to fine powder and incubated or soaked in autoclaved water @ 10 g: 100 ml (w/v) for 24 h at 50 °C in a water bath (H H-4). After the incubation period, the mixture was filtered through 4 layers of cheese cloth followed by filtration through Whatman filter paper no.1., using vacuum flask to get water extract of test plant. The water extract obtained in this way was regarded as original or 10% extract concentration. Extraction of metabolites was carried out using chloroform solvent in 1:1 ratio of 10% extract (400 ml) and chloroform (400 ml), in separating funnel (1L). The process was repeated thrice to maximize the extraction of compounds. These extracts were concentrated in a rotatry evaporator at 40 °C. Finally, the chloroform solvent was evaporated from the extract by continuous air flow on the extract contained in glass beaker.
2.4 Chromatographic procedures
Initailly, compounds present in chloroform extract of D. muricata were separated by Thin Layer Chromatography (TLC) for the optimization of maximum number of separated compounds and solvent system. The solvent system n-hexane and ethanol (1:3) was able to isolate 8 fractions. To get the fractions for bioassays, Preparative Thin Layer Chromatographic (PTLC) plates (20x20 cm) were used with the same solvent system. Fractions showing bioactivity (fractions 2 & 7) were further purified by Reversed Phase High Pressure Liquid Chromatography (RP-HPLC). Gradient elution was employed with solvent system dH2O and acetonitrile. Fraction 2 yielded 3 sub-fractions (2A, 2B & 2C), while fraction 7 yielded 2 sub-fractions (7A, 7B). The herbicidal activity was tested at 200, 150, 100, 50, 25 μg/ml.
2.5 Pre emergence in vitro bioassays using chloroform extracts and fractions
Seeds of Triticum aestivum, M. indicus and A. fatua were surface sterilized with 1% sodium hypochlorite for 10 min (Akbar & Javaid, 2013) and then placed in Petri dishes (9 cm diameter) having sterilized filter paper moistened with 2.5 ml of plant extract. Two concentrations of chloroform extract (2 mg/ml) and diluted with dH2O (1 mg/ml) were investigated. The control plant received the same amount of distilled water. Data regarding seed germination and early seedling growth were recorded after 14 days. Following parameters viz., seed germination, shoot dry weight, and root dry weight were noted to assess the herbicidal activity. The herbicidal activity of pure compounds was compared with broad-spectrum herbicide, cyanazine. Following treatments were investigated in in vitro assays. Each treatment was replicated thrice & there were 10 seeds per Petri plate.
Treatments in in vitro assays
Set 1- Melilotus indicus
T1 = Distilled water; T2 = 1 mg/ml chloroform extract; T3 = 2 mg/ml chloroform extract
Set 2- Avena fatua
T1 = Distilled water; T2 = 1 mg/ml chloroform extract; T3 = 2 mg/ml chloroform extract
Set 3- (1–8 Fractions) Melilotus indicus, Avena fatua
T1 = Distilled water; T2 = 25 μg/ml; T3 = 50 μg/ml; T4 = 100 μg/ml; T5 = 150 μg/ml; T6 = 200 μg/ml
Set 4- Subfractions/purified compounds/herbicide (Cyanazine) (2A, 2B & 2C) & (7A, 7B) (Melilotus indicus, Avena fatua & wheat); fraction concentrations as in set 3.
2.6 Spectroscopic analysis
Fractions showing herbicidal activity were analysed by spectroscopic methods to identify the active compounds.
The 1H NMR and 13C NMR spectra (Chloroform-d) were recorded using a Bruker 500 MHz spectrometer (Bruker, Freemont, CA, USA) and Fourier Transform Infrared Spectroscopy (FT-IR) analysis was carried out on Shimadzu FTIR–8400S spectrometer (Kyoto, Japan, υ, cm-1). Melting point was determined by using a Digimelt MPA 160, USA apparatus and are reported uncorrected. Elemental analysis (C, H) was carried out on a Flash 2000 series elemental analyzer with thermal conductivity detector (TCD) system (Thermo Flash 2000 elemental analyzer, Thermo Fisher Scientific Inc., Waltham, MA, USA) and results are with ± 0.3%.
2.7 Post emergence herbicidal activity
2.7.1 Pot experiments
Pot experiments were conducted with A. fatua, M. indicus and wheat. Seeds were surface sterilized and sown in 500 g of soil contained in pots. After emergence, seedlings were thinned to keep 3 uniform seedlings in each pot. Sprays with desired concentrations of purified compounds as well as commercial herbicide (Cyanazine) were done at 3–4 leaves stage for A. fatua and wheat, while M. indicus seedlings were sprayed at 6–7 leaves stage. All mixtures contained 2% Tween 20 and sprays were done with manual spray bottle. Fresh weight measurements of shoot were done after 14 days of compounds/herbicide applications.
Treatments in post emergence pot assays
Following treatments were investigated in post emergence pot assays for purified compounds and cyanazine against A. fatua, M. indicus and wheat. Each treatment was replicated 5 times.
T1 = Distilled water; T2 = 25 mM; T3 = 50 mM; T4 = 100 mM; T5 = 150 mM; T6 = 200 mM T7 = 150 mM; T8 = 200 mM.
2.7.2 Plant plasma membrane integrity assays
For membrane integrity assays (an indicator of cellular damage), five leaves discs of 10 mm diameter were cut from plants after 2 h of application of the mixtures. These cut discs were transferred into 10 ml of dH2O and shaken on a shaker for 3 h at 90 rpm. The mixtures were then transferred into test tubes for the measurements of electrolyte concentrations with a conductivity meter. Five replications were done for membrane integrity assays (Bainard et al., 2006).
2.8 Experimental design
Completely Randomized Design (CRD) was followed in in vitro and pot assays.
2.9 Data analysis
Data were analyzed by analysis of variance (ANOVA) followed by Tukey’s test to delimit treatment means using computer software Minitab 19.
3 Results and discussion
3.1 Herbicidal activity of chloroform extract of Digera muricata on the germination and growth of Petri plate grown Avena fatua and Melilotus indicus
The effect of chloroform extracts of D. muricata regarding germination of seeds and early seedling growth of two weeds viz., A. fatua and M. indicus was found significant. The chloroform extract showed reduction in germination of A. fatua and M. indicus by 46 and 62% at 1 mg/l concentration, respectively, whereas when the concentration was increased to 2 mg/l, there was 75 and 81% reduction in germination of seeds (Table 1). The chloroform extract of D. muricata showed reduction in shoot dry weight of A. fatua by 32% at 1 mg/l concentration, whereas when the concentration was increased to 2 mg/l, 73% reduction in shoot dry weight was observed. The dry weight of root was reduced to 35 and 60%, under the application of 1 mg/l and 2 mg/l concentrations of chloroform extract of D. muricata, respectively. Data show means ± Standard error of 3 replications. Different letters show significance between the treatments at P ≤ 0.05, as determined by ANOVA & Tukey’s Test.
Treatments
Avena fatua
Melilotus indicus
T1 = dH2O
93 ± 3.3a
87 ± 3.3a
T2 = 1 mg/ml chloroform extract
50 ± 5.7b
33 ± 3.3b
T3 = 2 mg/ml chloroform extract
23 ± 3.3c
17 ± 3.3c
The susceptibility of M. indicus towards the chloroform extracts of D. muricata, was also found significant. The chloroform extract showed a reduced shoot dry weight by 60% at 1 mg/l concentration, whereas when the concentration was increased to 2 mg/l, 95% reduction in shoot dry weight was observed. The dry weight of root was reduced to 63 and 95%, under the application of 1 mg/l and 2 mg/l concentrations of chloroform extract of D. muricata, respectively (Figs. 1 & 2). In an investigation, the ethanol extract of Sapindus mukorossi was phytotoxic to A. fatua and A. retroflexus. Five phytotoxic compounds from S. mukorossi extract were identified. Of these five compounds, d-pinitol and oleanolic acid hindered the development of both test weeds (Ma et al., 2018). Two flavonoids; pectolaring and scutellarein 4′‐methyl ether and two sesquiterpene lactones, elemanolide 11(13)‐dehydromelitensin β‐hydroxyisobutyrate and acanthiolide, isolated from leaves extract of Onopordum acanthium, showed phytotoxic activity against etiolated wheat coleoptiles. Elemanolide 11(13)‐dehydromelitensin β‐hydroxyisobutyrate arrested the development of wheat (Watanabe et al., 2014).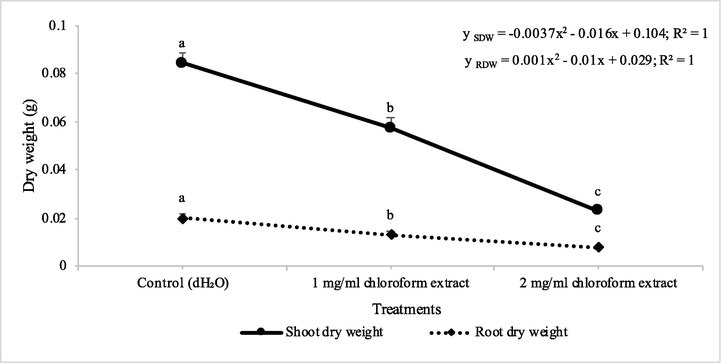
Herbicidal activity of different concentrations of chloroform extract of Digera muricata on shoot and root dry weight of Petri plate grown Avena fatua. ANOVA and Tukey's range test were done on Minitab 19 with 3 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show significance.
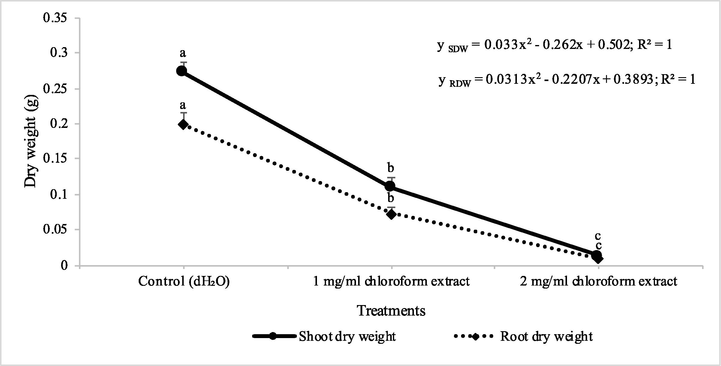
Herbicidal activity of different concentrations of chloroform extract of Digera muricata on shoot and root dry weight of Petri plate grown Melilotus indicus. ANOVA and Tukey's range test were done on Minitab 19 with 3 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show significance.
3.2 Herbicidal activity of fraction 2 & 7 from chloroform extract of Digera muricata on the germination and growth of Petri plate grown Avena fatua and Melilotus indicus
Fraction 2 from chloroform extract of D. muricata decreased the shoot dry biomass of A. fatua by 7, 18, 26, 38 and 45% at concentrations of 25, 50, 100, 150 and 200 μg/ml, respectively, while root dry biomass of A. fatua was inhibited by 11, 24, 27, 29 and 36%, respectively. On the other hand, fraction 7 from chloroform extract of D. muricata decreased the shoot and root dry biomass of A. fatua by 7, 16, 19, 20% and 24% and 5, 9, 12, 23 and 30%, at concentration of 25, 50, 100, 150 and 200 μg/ml, respectively (Figs. 3 & 4). Germination of seeds of A. fatua was also negatively affected by the applications of different employed concentrations of fraction 2 and fraction 7. There was 7, 11, 21, 25 and 36% decrease in the germination of seeds of A. fatua by the application of different concentrations of fraction 2, while these values for fraction 7 were 7, 11, 14, 14 and 18%, respectively.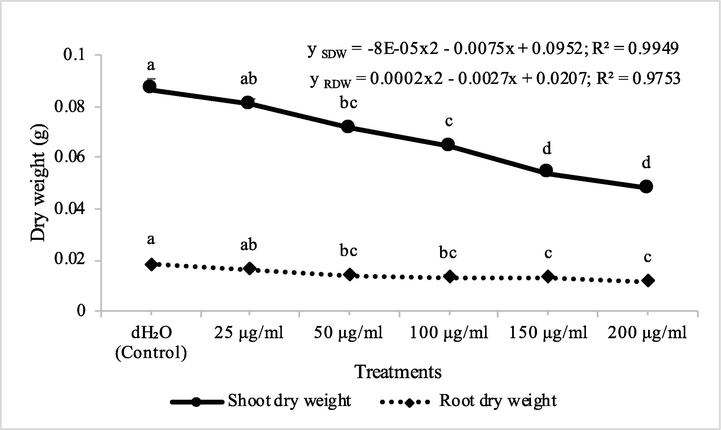
Herbicidal activity of different concentrations of fraction 2 from chloroform extract of Digera muricata on shoot and root dry weight of Petri plate grown Avena fatua. ANOVA and Tukey's range test were done on Minitab 19 with 3 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show significance.
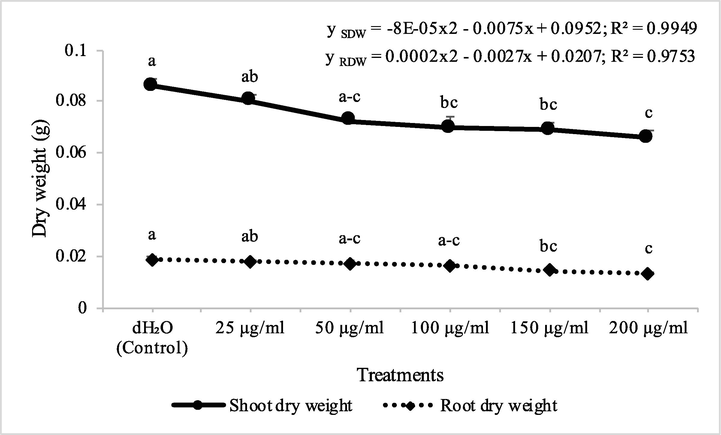
Herbicidal activity of different concentrations of fraction 7 from chloroform extract of Digera muricata on shoot and root dry weight of Petri plate grown Avena fatua. ANOVA and Tukey's range test were done on Minitab 19 with 3 replicates at P ≤ 0.05. Y error bars represent standard rrror of means and different letters show significance.
Fraction 2 decreased the shoot dry biomass of M. indicus by 8, 22, 38, 54 and 62% at concentrations of 25, 50, 100, 150 and 200 μg/ml, respectively, while these concentations of fraction 2 decreased the root dry biomass of A. fatua by 10, 15, 20, 39 and 59%, respectively. On the other hand, fraction 7 decreased the shoot dry biomass of M. indicus by 6, 23, 30, 36 and 50% at fraction 7 concentrations of 25, 50, 100, 150 and 200 μg/ml, respectively, while root drybiomass of M. indicus was declined to 5, 16, 24, 34 and 48%, respectively (Figs. 5 & 6). There was 8, 12, 12, 23 and 35% decrease in the germination of seeds of M. indicus by the application of different concentrations of fraction 2, while these values for fraction 7 were 4, 8, 8, 15 and 23%, respectively (Table 2). Data show means ± standard error of 3 replications. Different letters show significance between the treatments at P ≤ 0.05, as determined by ANOVA & Tukey’s range test.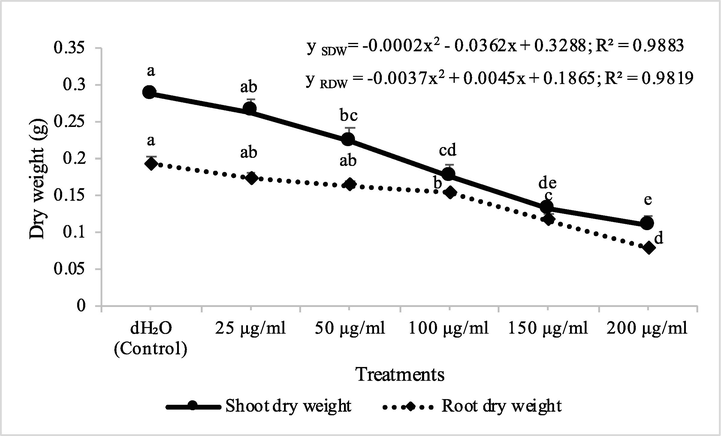
Herbicidal activity of different concentrations of fraction 2 from chloroform extract of Digera muricata on shoot and root dry weight of Petri plate grown Melilotus indicus. ANOVA and Tukey's range test were done on Minitab 19 with 3 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show significance.
Herbicidal activity of different concentrations of fraction 7 from chloroform extract of Digera muricata on shoot and root dry weight of Petri plate grown Melilotus indicus. ANOVA and Tukey's range test were done on Minitab 19 with 3 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show Ssignificance.
Treatments
Avena fatua
Melilotus indicus
Fraction 2
Fraction 7
Fraction 2
Fraction 7
T1 = dH2O
93 ± 3.3a
93 ± 3.3a
87 ± 3.3a
87 ± 3.3a
T2 = 25 μg/ml
87 ± 3.3b
87 ± 3.3b
80 ± 5.8b
83 ± 6.7b
T3 = 50 μg/ml
83 ± 3.3c
83 ± 3.3c
77 ± 3.3bc
80 ± 5.8bc
T4 = 100 μg/ml
73 ± 3.3d
80 ± 5.8 cd
77 ± 5.8bc
80 ± 5.7bc
T4 = 150 μg/ml
70 ± 5.7e
80 ± 5.7 cd
67 ± 5.7d
73 ± 3.3c
T6 = 200 μg/ml
60 ± 5.7f
77 ± 3.3d
57 ± 3.3e
67 ± 3.3 cd
3.3 Herbicidal activity of fraction 2B & 7A from chloroform extract of Digera muricata on the germination and growth of Petri plate grown Avena fatua and Melilotus indicus
Fraction 2B from chloroform extract of D. muricata decreased the shoot and root dry biomass of A. fatua by 9, 28, 30, 49 and 53% and 13, 26, 40, 51 and 57% at concentrations of 25, 50, 100, 150 and 200 μg/ml concentration, respectively. On the other hand, fraction 7A decreased the shoot and root dry biomass of A. fatua by 9, 18, 21, 31 and 36% and 7, 17, 20, 29 and 42% at concentrations of 25, 50, 100, 150 and 200 μg/ml, respectively (Figs. 7 & 8). Germination of seeds of A. fatua was reduced by the applications of different employed concentrations of fraction 2B and fraction 7A. There was 11, 14, 21, 32 and 43% decrease in the germination of seeds of A. fatua by the application of different concentrations of fraction 2B, while these values for fraction 7A were 14, 18, 21, 25 and 25%, respectively (Table 2). Cynara cardunculus extracts showed herbicidal/allelopathic potency against weeds that included Trifolium incarnatum, Silybum marianum and Phalaris minor. From the aerial parts of C. cardunculus, syringic acid, p-coumaric acid, myricitrin, quercetin and naringenin were detected (Kaab et al., 2020).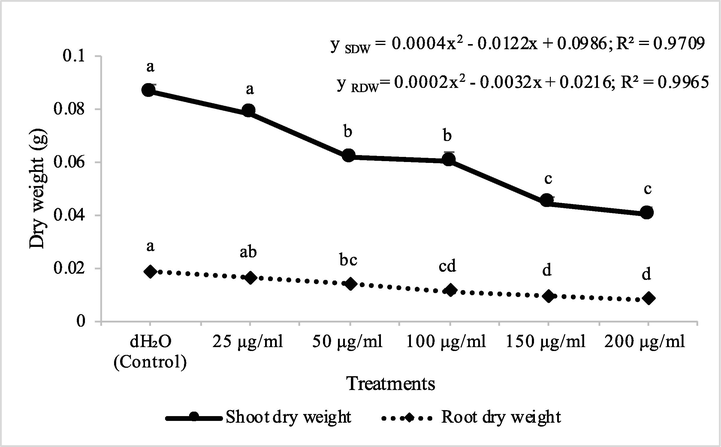
Herbicidal activity of different concentrations of fraction 2B from chloroform extract of Digera muricata on (A) shoot dry weight and root dry weight of Petri plate grown Avena fatua. ANOVA and Tukey's range test were done on Minitab 19 with 3 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show significance.
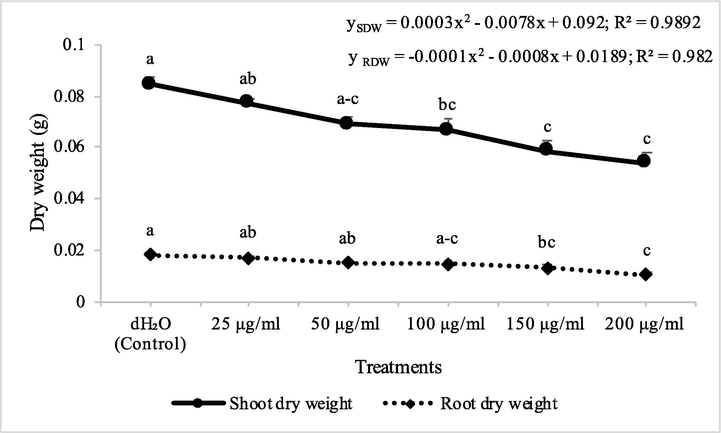
Herbicidal activity of different concentrations of fraction 7A from chloroform extract of Digera muricata on shoot and root dry weight of Petri plate grown Avena fatua. ANOVA and Tukey's range test were done on Minitab 19 with 3 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show significance.
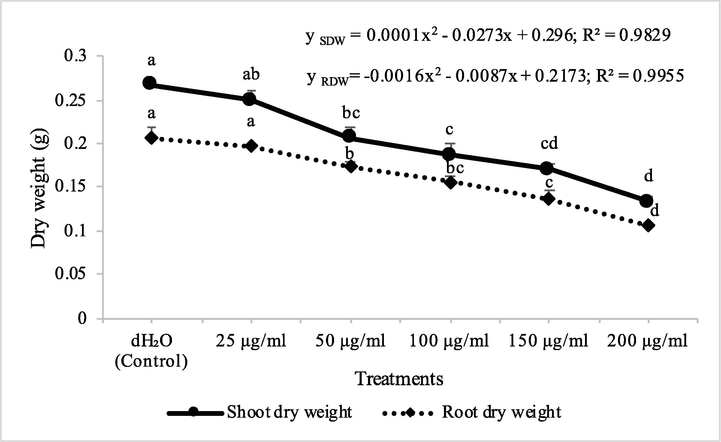
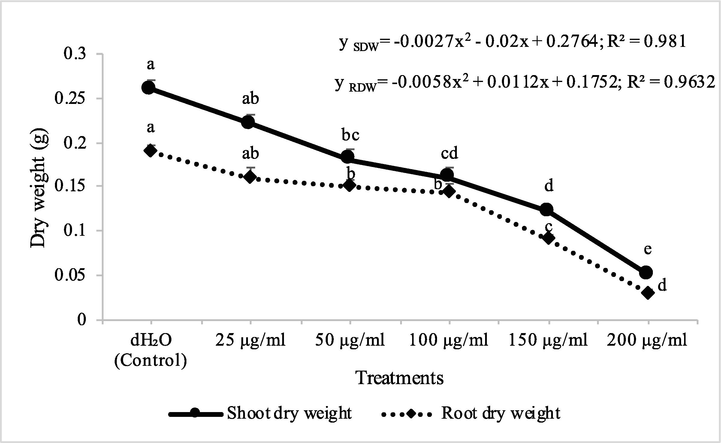
Fraction 2B from chloroform extract of D. muricata decreased the shoot dry biomass of M. indicus by 15, 31, 38, 53 and 81% at 25, 50, 100, 150 and 200 μg/ml concentration, respectively, while these concentations of fraction 2B decreased the root dry biomass of M. indicus by 16, 21, 25, 53 and 84%, respectively. On the other hand, fraction 7A from chloroform extract of D. muricata decreased the shoot dry biomass of M. indicus by 11, 31, 37, 52 and 62% at fraction 7A concentration of 25, 50, 100, 150 and 200 μg/ml, respectively, while these concentations of fraction 7A from chloroform extract of D. muricata decreased the root dry biomass of M. indicus by 17, 21, 27, 44 and 62%, respectively (Figs. 9 & 10). Germination of seeds of M. indicus was also decreased by the application of different employed concentrations of fraction 2B and fraction 7A. There was 27, 31, 38, 50 and 50% decrease in the germination of seeds of M. indicus by the application of different concentrations of fraction 2B, while these values for fraction 7A were 8, 15, 23, 35 and 35%, respectively. The effect of synthetic herbicide, cyanazine was comparable with fraction 2B. This herbicide decreased the seed germination of A. fatua by 25, 32, 36, 43 and 61%, while, these values for M. indicus were 19, 23, 31, 46 and 50% at concentrations of 25, 50, 100, 150 and 200 μg/ml, respectively (Table 3). Data show means ± standard error of 3 replications. Different letters show significance between the treatments at P ≤ 0.05, as determined by ANOVA & Tukey’s range test.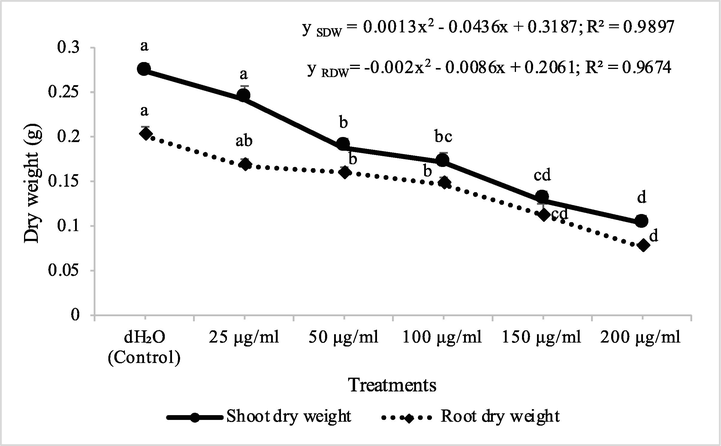
Herbicidal activity of different concentrations of fraction 7A from chloroform extract of Digera muricata on shoot and root dry weight of Petri plate grown Melilotus indicus. ANOVA and Tukey's range test were done on Minitab 19 with 3 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show significance.
Herbicidal activity of different concentrations of fraction 2B from chloroform extract of Digera muricata on shoot and root dry weight of Petri plate grown Melilotus indicus. ANOVA and Tukey's range test were done on Minitab 19 with 3 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show significance.
Treatments
Avena fatua
Melilotus indicus
Fraction 2B
Fraction 7A
Herbicide
Fraction 2B
Fraction 7A
Herbicide
T1 = dH2O
93 ± 3.3a
93 ± 3.3a
93 ± 3.3a
87 ± 3.3a
87 ± 3.3a
87 ± 3.3a
T2 = 25 μg/ml
83 ± 3.3b
80 ± 5.8b
70 ± 5.8b
63 ± 3.3b
80 ± 0b
70 ± 5.8b
T3 = 50 μg/ml
80 ± 5.8bc
77 ± 3.3bc
63 ± 5.7bc
60 ± 5.8bc
73 ± 3.3c
67 ± 3.3bc
T4 = 100 μg/ml
73 ± 3.3c
73 ± 3.3c
60 ± 5.7c
53 ± 5.7c
67 ± 3.3d
60 ± 5.7d
T4 = 150 μg/ml
63 ± 3.3d
70 ± 5.8c
53 ± 3.3c
43 ± 3.3d
57 ± 3.3e
47 ± 3.3e
T6 = 200 μg/ml
53 ± 3.3e
70 ± 5.8c
37 ± 3.3d
43 ± 3.3d
57 ± 3.3e
43 ± 3.3e
The synthetic herbicide, cyanazine, used as reference compound, decreased the shoot dry biomass of A. fatua by 24, 39, 41, 56 and 65%, while root dry biomass of A. fatua was inhibited to 24, 46, 50, 61 and 68% at herbicide concentrations of 25, 50, 100, 150 and 200 μg/ml, respectively. On the other hand, cyanazine decreased the shoot dry biomass of M. indicus by 24, 34, 54, 65 and 83%, while, root dry biomass of M. indicus was inhibited to 16, 21, 25, 53 and 84% at herbicide concentrations of 25, 50, 100, 150 and 200 μg/ml, respectively (Figs. 11 & 12).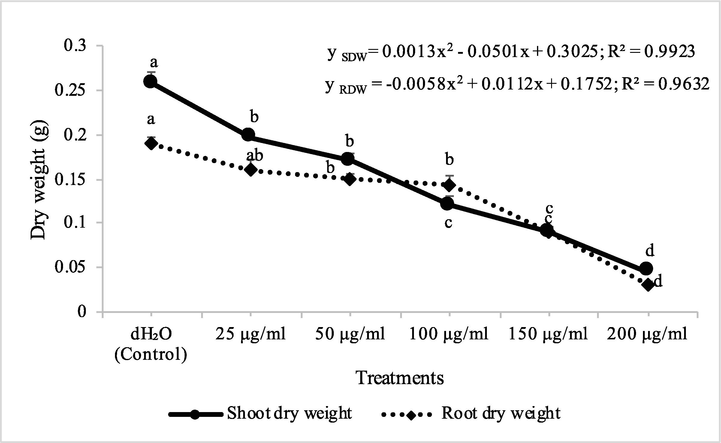
Herbicidal activity of different concentrations of cyanazine herbicide on shoot and root dry weight of Petri plate grown Melilotus indicus. ANOVA and Tukey's range test were done on Minitab 19 with 3 replicates at P ≤ 0.05. Y Error bars represent standard error of means and different letters show significance.
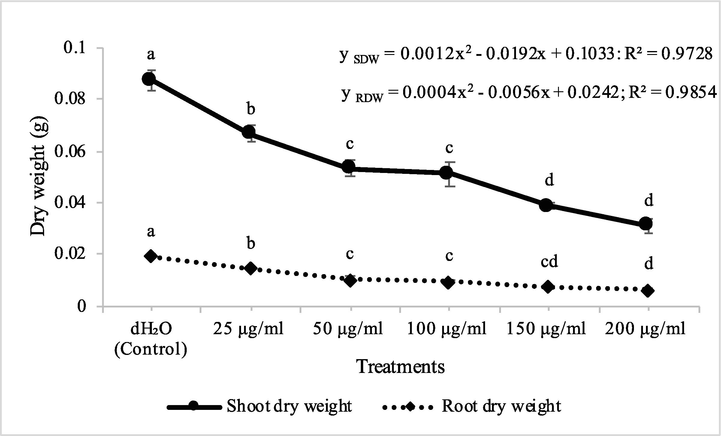
Herbicidal activity of different concentrations of cyanazine herbicide on shoot and root dry weight of Petri plate grown Avena fatua. ANOVA and Tukey's range test were done on Minitab 19 with 3 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show significance.
Parthenium hysterophorus is also known for phytotoxic behaviour against seedling growth of lettuce and some other plant seedlings (Shi et al., 2020). A novel kaurene-type compound, wedelienone was identified from aqueous methanol extract of Wedelia chinensis. Phytotoxic property of wedelienone was monitored by measuring inhibited growth of cress and timothy (Phleum pratense) at concentrations greater than 10–30 μM (Das et al., 2020). Brassicaceae seed meals (BSM) also showed herbicidal activity on the growth of wild oat, ryegrass, lettuce and pigweed. Among different BSM amendments, white mustard seed meals at 2000 kg ha−1 significantly inhibited the growth of tested weeds (Handiseni et al., 2011). Metabolites of Aureobasidium pullulans are known to possess herbicidal activity against Galium aparine, Chenopodium album, Malva crispa, Polygonum lapathifolium and A. fatua. G. aparine and M. crispa exhibited significant decline in fresh weight. Metabolites of A. pullulans exhibited little to non-toxic herbicidal effects on wheat, Vicia faba, Hordeum vulgare, Brassica napus and Pisum sativum (Guo et al., 2020). In the present study, the effect of extracts and fractions as well as synthetic herbicide against wheat was in general, non significant, so these data were not presented in this article.
3.4 Spectroscopic analysis
Spectroscopic analysis of active fractions revealed the presence of quercetin (Fraction 2B) and β-caryophyllene (Fraction 7A).
Quercetin
Solid; Mp: 316 °C; FT-IR (cm−1): 3485 (OH), 1580 (C⚌O), 1480 (C⚌C), 1220 (C—O): 1H NMR (500 MHz, Chloroform-d) δ 9.69 (s, 1H, H-11), 9.12 (s, 1H, H-20), 8.95 (s, 1H, H-21), 7.59 (dd, J = 7.5, 1.6 Hz, 1H, H-15), 7.32 (d, J = 1.5 Hz, 1H, H-19), 6.83 (d, J = 7.5 Hz, 1H, H-16), 6.29 (d, J = 1.4 Hz, 1H, H-7), 6.15 (d, J = 1.4 Hz, 1H, H-9). 13C NMR (125 MHz, Chloroform-d) δ 175.81 (C-3), 164.57 (C-8), 161.40 (C-10), 157.65 (C-1), 147.69 (C-17), 146.80 (C-5), 145.09 (C-18), 136.19 (C-4), 121.89 (C-14), 119.94 (C-15)), 115.60 (C-16), 115.17 (C-19), 103.23 (C-2), 99.54 (C-9), 94.34 (C-7). Chemical Formula: C15H10O7; Molecular Weight: 302.24; Elemental Analysis: Calculated (C, H) 59.61, 3.34; Found; (C, H) 59.63, 3.36. The physical and spectroscopic data agreed with the literature for quercetin (Fernández-Aparicio et al., 2021; Grande, et al., 1985).
β-caryophyllene
Solid; FT-IR (cm−1): 3395 (OH), 2904 (sp2 CH), 1485 (C⚌C): 1H NMR (500 MHz, Chloroform-d) δ 5.26 (dddd, J = 7.1, 4.8, 2.1, 0.9 Hz, 1H, H-4), 4.84 (ddq, J = 4.0, 2.0, 1.1 Hz, 2H, H-15), 2.44 (qt, J = 7.0, 1.1 Hz, 1H, H-16), 2.03 – 1.88 (m, 6H, H-1,8,9), 1.73 – 1.60 (m, 3H, H-12,17), 1.57 (s, 3H, H-10), 1.52 – 1.38 (m, 2H, H-2), 1.00 (d, J = 1.6 Hz, 3H,H-14), 0.95 (d, J = 1.5 Hz, 3H, H-13). 13C NMR (125 MHz, Chloroform-d) δ 154.47 (C-7), 135.45 (C-5), 124.52 (C-4), 111.39 (C-15), 54.15 (C-3), 47.37 (C-6), 39.79 (C-12), 39.20 (C-1), 34.53 (C-8), 33.33 (C-11), 28.26 (C-9), 27.50 (C-2), 26.37 (C-13,14), 16.56 (C-10). Chemical Formula: C15H24; Molecular Weight: 204.36; Elemental Analysis: Calculated (C, H) 88.16, 11.84; Found; (C, H) 88.14, 11.82. The physical and spectroscopic data agreed with the literature for β-caryophyllene (Dahham et al., 2015). The structures of these two compounds are presented in Fig. 13.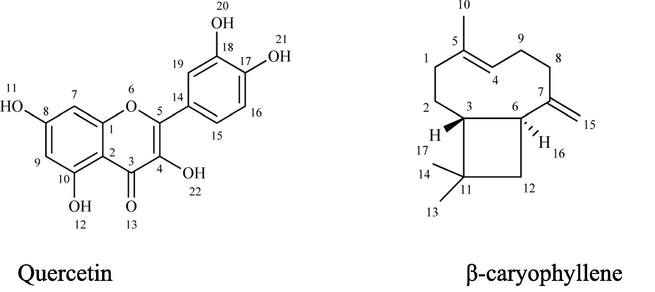
Structures of identified compounds; quercetin β-caryophyllene.
In an investigation, essential oil from Tagetes erecta exhibited herbicidal activity in terms of seed germination inhibition in weed, Echinichloa crus-galli. It’s mode of action was described as blockage of α-amylase activity. Gas Chromatography Mass Spectrometry (GC/MS) analysis depicted the presence of piperitone, piperitenone, ocimine, sesquiterpenoids mainly neophytadiene and caryophyllene (Laosinwattana et al., 2018). Berberine, a plant secondary metabolite revealed more herbicidal effects than traditional herbicide glyphosate (Zhang et al., 2018). Extracted oils (EOs) from vegetative parts of Copaifera species and isolated active compounds (sesquiterpene hydrocarbons, including germacrene D, β-caryophyllene, α-humulene, δ-elemene, and δ-cadinene) by GC/MS depicted the phytotoxic activity against Mimosa pudica and Senna obtusifolia. M. pudica was more sensitive to these phytotoxic compounds in comparison with S. obtusifolia (Gurgel et al., 2019). EOs of various plant species showed phytotoxic effects against germination and early growth of Cucumis sativus and Solanum lycopersicum. Among oils extracted from different plants, Oregano was the most injurious as compared to rosemary EO (Ibáñez et al., 2020). EOs of Vitex negundo, showed herbicidal effect against A. fatua and E. crus-galli. GC/MS analysis depicted that V. negundo EO was rich in β-caryophyllene (Issa et al., (2020). EOs of Piper dilatatum and Piper divaricatum also exhibited phytotoxic effects against Lolium perenne and Lactuca sativa. Among isolated compounds, e.g., apiol (79%), eugenol (37%), methyl eugenol (36%), δ-3-carene (35%) and limonene (27%) at higher concentrations (Jaramillo-Colorado et al., 2019). Extracts from vegetative parts of Inula graveolens also revealed the phytotoxic activity against the growth of different monocotyledonous plant spp. In in-vitro experiments, plant extracts showed strong inhibitory effect, reduced the emergence rate and the growth of tested plant spp. Two phytotoxic compounds named as 2,3,11b,13-tetrahydroaromaticin (THA) and ilicic acid were isolated from plant extracts of I. graveolens (Abu Irmaileh et al., 2015). The compound royleanumioside, was separated from the chloroform extract of Teucrium royleanum which showed moderate phytotoxic activity against lettuce seedlings (Ahmad et al., 2015). Similarly, phenolic compounds isolated from water and methanol extracts of Chrysanthemoides monilifera exhibited phytotoxicity against Isotoma axillaris (Al Harun et al., 2015). Needles, stems and cones of Pinus halepensis also possess phytotoxic activities against weeds. Compounds named as a-pinene and and (Z)-caryophyllene were detected as major chemical constituents in various parts of P. halepensis. These extracts significantly inhibited seed germination of test weeds (Amri et al., 2012). A compound α-phellandrene, terpinen-4-ol, trans-pinocarveol and pinocarvone isolated from EOs of Wedelia trilobata, were responsible for the herbicidal activity against shoot and root growths of weedy rice and lettuce (Azizan et al., 2020).
Allelopathic interactions play very important role in the agro-ecosystems. The literature abounds with examples of plant extracts or extracts of other organisms showing allelopathic interactions in terms of modeling the growth of certain crops/plants. However, there are less examples showing actual compounds exhibiting phytotoxicity/herbicidal activity. Flavonoids are widely distributed in different plant parts. e.g., quercetin and kaempferol have already been described as allelopathic molecules. Moreover, the derivatives of quercetin like quercetin 3-methyl ether and its 4′-O-glucoside and 7-O-glucoside completely inhibited the growth of Arabidopsis thaliana at 100 ppm (Parvez et al., 2004). Quercetin, isolated from Nerium oleander declined germination of seeds, root/shoot growth of Parthenium hysterophorus in a dose dependent manner (Rajyalakshmi et al., 2011). Quercetin is a stronger inhibitor of auxin polar transport (Hernandez et al., 2009; Lewis et al., 2011). Flavonoids, such as quercetin, have been shown to inhibit polar auxin transport and to enhance consequent localized auxin accumulation in planta. Quercetin isolated from root of Fagopyrum esculentum showed inhibitory effect on Phelipanche ramosa. Besides reduction of radicle growth, haustorium induction was observed at the tip of P. ramosa radicles treated with quercetin at 0.1–0.05 mM (Fernández-Aparicio et al., 2021). The quercetin, apigenin and daucosterol isolated from Shibataea chinensis Nakai were more phytotoxic to germination (IC50 = 7.26–7.95 ppm), root (IC50 = 2.97–6.33 ppm) and shoot elongation (IC50 = 4.89–7.74 ppm) of lettuce than glyphosate (IC50 values greater than 20 ppm). Dihydroquercetin isolated from Egyptian plant Jasonia montana also showed the herbicidal activity against Convolvulus arvensis and Calystegia inflata, Portulaca oleracea and Arabidopsis thaliana. Dihydroquercetin isolated from J. montana decreased C. arvensis fresh biomass by 89.8%, when compared with control (Balah, 2011). In another investigation, dihydroquercetin isolated from Centaurea maculosa was shown to be phytotoxic in terms of inhibition of seed germination and growth of various plants, while quercetin was not phytotoxic to these plants (Bais et al., 2003).
β-caryophyllene, a sesquiterpene, has been described to be having allelopathic activities. β-caryophyllene affected seed germination and seedling growth of Brassica campestris, Raphanus sativus, Lactuca sativa and Mikania micrantha. Phytotoxicity of β-caryophyllene against these plants was dose-dependent and varied with the receptor plants (Wang et al., 2010). In another investigation, β-caryophyllene inhibited seedling growth of B. campestris and R. sativus (Wang et al., 2009). β-caryophyllene is also reported from Artemisia lavandulaefolia, and inhibited the seedling growth of Achyranthes japonica (Kil et al. 2000). β-caryophyllene isolated from Senecio salignus inhibited root length of Physalis ixocarpa by 42 and 53% at 50 and 150 μg/mL respectively. However, β-caryophyllene inhibited root length of E. crus-galli by 30% at 150 μg/ml concentration. β-caryophyllene also inhibited the dry biomass of P. ixocarpa by 37.5% in comparison with control at 100 μg/ml concentration.
3.5 Herbicidal activity of quercetin, β-caryophyllene and cyanazine on the fresh biomass of pot grown Avena fatua and Melilotus indicus
The toxic effects of quercetin, β-caryophyllene and cyanazine on the fresh shoot biomass were found significant against A. fatua and M. indicus. In A. fatua, the effect of 25–50 mM each of quercetin, β-caryophyllene and cyanazine was nonsiginificant when compared to control as well as when compared amonst themselves. However, the higher concentrations(100–300 mM) of quercetin, β-caryophyllene and cyanazine were found to cause significant decline in the fresh biomass of A. fatua. A dose dependant response was recorded from 25 to 200 mM concentrations, however, further increased concentrations did not depict significant effects. The highest inhibition in the fresh biomass of A. fatua was observed by the application of cyanazine followed by quercetin and β-caryophyllene was the least effective when compared with either quercetin or cyanazine.
In M. indicus, the effect of 25–50 mM of quercetin was found non significant however, the higher concentrations were found having significant effect on the fresh biomass of M. indicus. In case of β-caryophyllene and cyanazine, significant effects were observed at 50 mM and higher concentrations and these effects were obviously dose dependent up to 250 mM concentrations. Beyond 250 mM, nonsignificant effects of quercetin, β-caryophyllene and cyanazine were recorded (Figs. 14 and 15). The effects of quercetin, β-caryophyllene and cyanazine against wheat were in general non significant, so data were not presented in this paper. Quercetin was more active as compared to β-caryophyllene but the cyanazine was even more effective when compared to quercetin. Application of phytotoxic compounds/essential oil is known to cause decrease in the biomass of test plants (Bainard et al., 2006; Ben Kaab et al., 2020).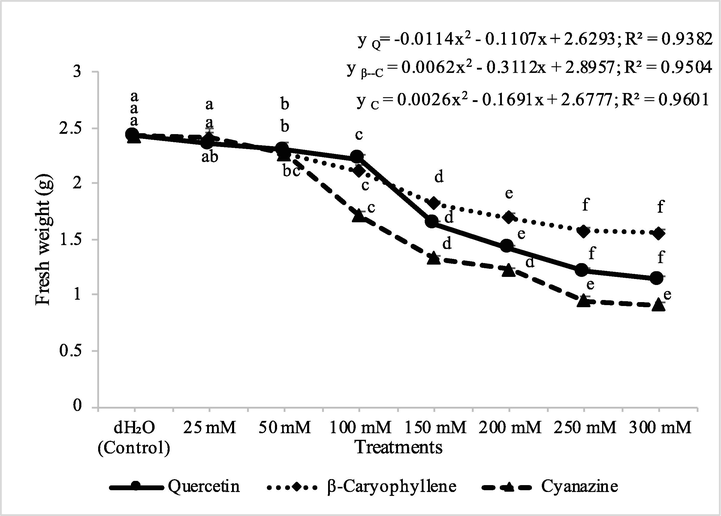
Herbicidal activity of different concentrations of quercetin, β-caryophyllene and cyanazine herbicide on shoot fresh weight of pot grown Avena fatua. ANOVA and Tukey's range test were done on Minitab 19 with 5 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show significance.
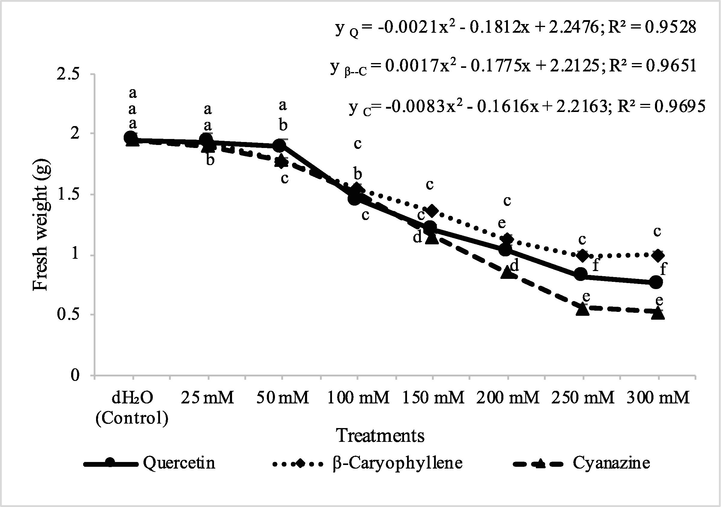
Herbicidal activity of different concentrations of quercetin, β-caryophyllene and cyanazine herbicide on shoot fresh weight of pot grown Melilotus indicus. ANOVA and Tukey's range test were done on Minitab 19 with 5 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show significance.
3.6 Effect on plant plasma membrane integrity
The application of quercetin, β-caryophyllene and cyanazine significantly increased electrolyte leakage in both test weeds viz., A. fatua and M. indicus, and therefore increased conductivity of solutions. The highest conductivity was observed by the application of, cyanazine against both the test weeds. Quercetin was more effective in the increase of conductivity as compared to β-caryophyllene. M. indicus was more sensitive to the application of quercetin, β-caryophyllene and cyanazine. The effect of all these compounds on disintegration of membrane was in general dose dependent. The application of quercetin, β-caryophyllene and cyanazine caused significant loss of membrane integrity and this was evident by the increased electrolyte leakage in both test weeds viz., A. fatua and M. indicus (Figs. 16 & 17). Natural compounds like clove oil and its main constituents namely eugenol, β-caryophyllene, and α-humulene are known to cause membrane damage in planta and therefore caused electrolyte leakage leading to cell death (Stokłosa et al., 2012). In another study, clove oil and eugenol caused leaf cell membrane damage and electrolyte leakage in broccoli, lambsquarters and redroot pigweed (Bainard et al., 2006). Along with membrane damage, flavonoids like quercetin inhibited ATP production (Stenlid 1970). Flavonoids also influence auxin transport (Peer and Murphy, 2007) and promote the allelopathic effects (Bais et al., 2006), such as kaempferol, quercetin and naringenin (Macías et al., 2007). Moreover, these compounds are considered potent allelochemicals disturbing the mitochondrial O2 regulation (Moreland and Novitski, 1988). Flavonoids destroy indoleacetic acid via IAA oxidase, resulting in the inhibition of ATP formation. Flavonoids may also interfere with the mode of actions of plant hormones such as auxins [Peer and Murphy 2007]. Quercetin can interact with components of both the ATP-generating and electron transport pathways. Interference with the phosphorylation pathway was evidenced by the higher sensitivity of the phosphorylation reaction than coupled whole-chain electron transport, inhibition of cyclic phosphorylation, inhibition of the light-activated Mg2+-ATPase, and inhibition of the heat-activated Ca2+-ATPase associated with CF1 in chloroplasts of Spinacea oleracea. (Moreland and Novitzky, 1988). Any modification of the plant plasma membrane structure by allelopathic substances disturb its function and integrity (Lins et al., 2019). In that regard, the phytotoxic effects observed in the present study may be linked to the fact that plant plasma membrane is one of the targets of the test compounds, due to their lipophilicity and small size, as shown for some essential oil compounds (Lebecque et al., 2018). Mode of action of β-caryophyllene was described as inhibition of photosystem II and induction of chlorosis on treated leaves (Sánchez-Muñoz et al., 2012). Hence, it may be argued that the phytotoxic effects observed during the present study may be attributed to multiple effects of bioactive compounds as discussed by earlier workers because natural compounds may have more than one target site in the plants. In the present investigation, wheat was found resistant to the application of isolated compounds as well as commercial herbicide, cyanazine. The resistance of wheat to applied compounds as well as cyanazine could be explained due to the fact that the weeds are more sensitive to these compounds than the crops (Grichi et al., 2016), hence, these molecules can act as lead compounds for developing natural herbicides.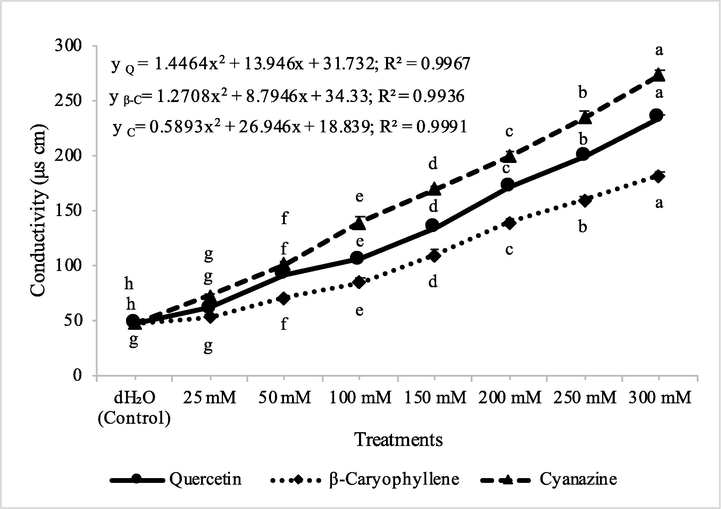
Conducivity measurements at different concentrations of quercetin, β-caryophyllene and cyanazine herbicide on Avena fatua. ANOVA and Tukey's range test were done on Minitab 19 with 5 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show significance.
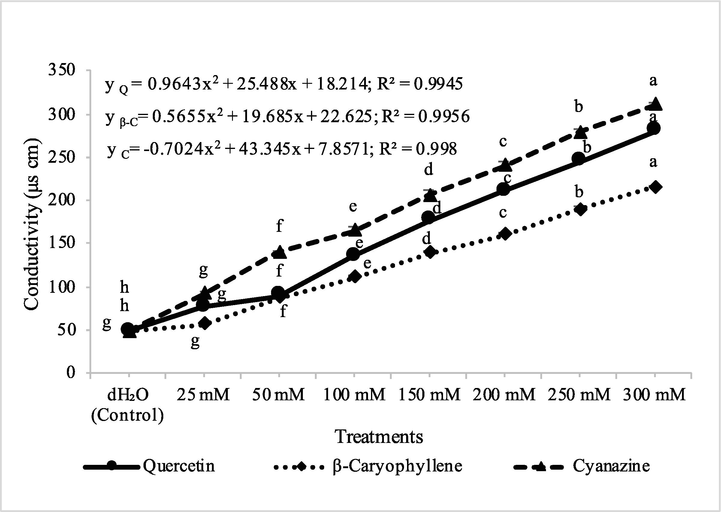
Conducivity measurements at different concentrations of quercetin, β-caryophyllene and cyanazine herbicide on Melilotus indicus. ANOVA and Tukey's range test were done on Minitab 19 with 5 replicates at P ≤ 0.05. Y error bars represent standard error of means and different letters show significance.
4 Conclusions and recommendations
The present study demostrated the herbicidal potential of Digera muricata against Avena fatua and Melilotus indicus. In vitro experiments depicted that chloroform extract of D. muricata as well as the purified compounds viz., quercetin and β-caryophyllene significantly decreased dry biomass of A. fatua and M. indicus. In post emergence bioassays, the isolated compounds significantly decreased the biomass of both weeds. Membranne integrity assays depicted the electrolyte leakage in the both weeds by the application of quercetin, β-caryophyllene and cyanazine. As there were no bad effects of isolated compounds of D. muricata against wheat, so, total synthesis of these compounds can be made and used to manage weeds in wheat.
Acknowledgements
One of the authors, Dr. Muhammad Akbar is highly indebted to Al-Balqa Applied University, Al-Salt, Jordan, for providing funding to present this manuscript as oral presentation in International Conference on Applied Chemistry and Biotechnology; ICACB-2022, held on May 10-12, 2022.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Selective phytotoxic activity of 2, 3, 11β, 13-tetrahydroaromaticin and ilicic acid isolated from Inula graveolens. Nat. Prod. Res.. 2015;29:893-898.
- [CrossRef] [Google Scholar]
- Royleanumioside–a new phytotoxic triterpenoid from Teucrium royleanum. J. Asian Nat. Prod. Res.. 2015;17:838-842.
- [CrossRef] [Google Scholar]
- The efficiency of allelochemicals in the seed powder of Eruca sativa in controlling weeds in Pisum sativum. Middle East J. Agric. Res.. 2014;3:757-762.
- [Google Scholar]
- Prospects of using fungal metabolites for the management of Rumex dentatus, a problematic weed of wheat. Int. J. Agric. Biol.. 2013;15:1277-1282.
- [Google Scholar]
- Antibacterial and antioxidant activities of slender amaranth weed. Planta Daninha. 2020;38
- [CrossRef] [Google Scholar]
- Identification and phytotoxicity assessment of phenolic compounds in Chrysanthemoides monilifera subsp. monilifera (Boneseed) PLos One.. 2015;10:e0139992.
- [Google Scholar]
- Reducing environmental pollution by chemical herbicides using natural plant derivatives allelopathy effect. Ann. Agric. Environ. Med.. 2018;25:449-452.
- [CrossRef] [Google Scholar]
- Chemical composition, phytotoxic and antifungal activities of Pinus pinea essential oil. J. Pest. Sci.. 2012;85:199-207.
- [CrossRef] [Google Scholar]
- Essential oils of Pinus nigra JF Arnold subsp. laricio Maire: chemical composition and study of their herbicidal potential. Arab. J. Chem.. 2017;10:S3877-S3882.
- [CrossRef] [Google Scholar]
- Comparative allelopathic activity of Rhazya stricta, Pinus roxburghii, Carica papaya and Lantana camara. Appl. Ecol. Environ. Res.. 2019;17:7175-7187.
- [CrossRef] [Google Scholar]
- Evaluating allelopathic effects of some plant species in tissue culture media as an accurate method for selection of tolerant plant and screening of bioherbicides. J. Agric. Sci. Technol.. 2015;17:1011-1023.
- [Google Scholar]
- Allelopathic effect of methanol extracts from Tinospora tuberculata on selected crops and rice weeds. Acta Agric. Scand. A Anim. Sci.. 2014;64:165-177.
- [CrossRef] [Google Scholar]
- Allelopathic potential of Digera muricata, a desert summer annual. Pak. J. Bot.. 2014;46:433-439.
- [Google Scholar]
- Discrimination and prediction of the chemical composition and the phytotoxic activity of Wedelia trilobata essential oil (EO) using metabolomics and chemometrics. Plant Biosyst.. 2020;156:1-16.
- [CrossRef] [Google Scholar]
- Phytotoxicity of clove oil and its primary constituent eugenol and the role of leaf epicuticular wax in the susceptibility to these essential oils. Weed Sci.. 2006;54(5):833-837.
- [CrossRef] [Google Scholar]
- Structure dependent phytotoxicity of catechins and oher flavonoids: Flavonoid conversions by cell-free protein extracts of Centaurea maculosa (Spotted Knapweed) roots. J. Agric. Food Chem.. 2003;51:897-901.
- [CrossRef] [Google Scholar]
- The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol.. 2006;57:233-266.
- [Google Scholar]
- Allelopathic characteristics of Jasonia montana for weeds control. Aust. J. Basic Appl. Sci.. 2011;5:98-106.
- [Google Scholar]
- Cynara cardunculus crude extract as a powerful natural herbicide and insight into the mode of action of its bioactive molecules. Biomolecules. 2020;10(2):209.
- [CrossRef] [Google Scholar]
- Allelopathic effect of Digera muricata L. mart on in vitro seed germination of Pennisetum typhoideum. Int. J. Plant Sci.. 2011;6:332-334.
- [Google Scholar]
- Root exudates regulate soil fungal community composition and diversity. Appl. Environ. Microbiol.. 2008;74:738-744.
- [CrossRef] [Google Scholar]
- The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules.. 2015;26;20(7):11808–29
- [CrossRef] [Google Scholar]
- A kaurene-type novel phytotoxic substance in Wedelia chinensis. Tetrahedron Lett.. 2020;61:151600
- [CrossRef] [Google Scholar]
- Allelopathic effect of quercetin, a flavonoid from Fagopyrum esculentum roots in the radicle growth of Phelipanche ramosa: quercetin natural and semisynthetic analogues were used for a structure-activity relationship investigation. Plants. 2021;10:543.
- [CrossRef] [Google Scholar]
- Phytotoxic effects of essential oil from Eucalyptus cinerea and its physiological mechanisms. J. New Sci.. 2016;15:1289-1296.
- [Google Scholar]
- Herbicidal activity of Aureobasidium pullulans PA-2 on weeds and optimization of its solid-state fermentation conditions. J. Integr. Agric.. 2020;19:173-182.
- [CrossRef] [Google Scholar]
- Chemical compositions and herbicidal (phytotoxic) activity of essential oils of three Copaifera species (Leguminosae-Caesalpinoideae) from Amazon-Brazil. Ind. Crops. Prod.. 2019;142:111850
- [CrossRef] [Google Scholar]
- Herbicidal activity of Brassicaceae seed meal on wild oat (Avena fatua), Italian ryegrass (Lolium multiflorum), redroot pigweed (Amaranthus retroflexus), and prickly lettuce (Lactuca serriola) Weed Technol.. 2011;25:127-134.
- [CrossRef] [Google Scholar]
- How relevant are flavonoids as antioxidants in plants? Trends Plant Sci.. 2009;14:125-132.
- [CrossRef] [Google Scholar]
- Phytotoxic effects of commercial essential oils on selected vegetable crops: cucumber and tomato. Sustain. Chem. Pharm.. 2020;15:100209
- [CrossRef] [Google Scholar]
- Weed control through allelopathic crop water extracts and S-metolachlor in cotton. Inf. Process. Agric.. 2019;7:165-172.
- [CrossRef] [Google Scholar]
- Appraisal of phytotoxic, cytotoxic and genotoxic potential of essential oil of a medicinal plant Vitex negundo. Ind. Crops. Prod.. 2020;145:112083
- [CrossRef] [Google Scholar]
- Volatile composition and biocidal (antifeedant and phytotoxic) activity of the essential oils of four Piperaceae species from choco-colombia. Ind. Crops. Prod.. 2019;138:111463
- [CrossRef] [Google Scholar]
- Screening of Tunisian plant extracts for herbicidal activity and formulation of a bioherbicide based on Cynara cardunculus. S. Afr. J. Bot.. 2020;128:67-76.
- [CrossRef] [Google Scholar]
- Allelopathic effects of Artemisia lavandulaefolia. Korean J. Ecol.. 2000;23:149-155.
- [Google Scholar]
- Allelochemicals and signaling chemicals in plants. Molecules.. 2019;24:2737.
- [CrossRef] [Google Scholar]
- Chemical composition and herbicidal action of essential oil from Tagetes erecta L. leaves. Ind. Crops. Prod.. 2018;126:129-134.
- [CrossRef] [Google Scholar]
- Interaction between the barley allelochemical compounds gramine and hordenine and artificial lipid bilayers mimicking the plant plasma membrane. Front. Plant. Sci.. 2018;1–13
- [CrossRef] [Google Scholar]
- Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development.. 2011;138:3485-3495.
- [CrossRef] [Google Scholar]
- Insights into the relationships between herbicide activities, molecular structure and membrane interaction of cinnamon and citronella essential oils components. Int. J. Mol. Sci.. 2019;20:4007.
- [Google Scholar]
- Potent herbicidal activity of Sapindus mukorossi Gaertn against Avena fatua L. and Amaranthus retroflexus L. Ind. Crops. Prod.. 2018;122:1-6.
- [CrossRef] [Google Scholar]
- Allelopathy – a natural alternative for weed control. Pest Manag. Sci.. 2007;63:327-348.
- [CrossRef] [Google Scholar]
- In vitro allelopathic effect of aqueous extracts of sugarcane on germination parameters of wheat. Acta Agric. Slov.. 2017;109:349-356.
- [CrossRef] [Google Scholar]
- Interference by flavone and flavonols with cloroplasts mediated electrons transport and phosphorylation. Phytochemistry. 1988;27:3359-3365.
- [Google Scholar]
- Ecological management of agricultural pests through allelopathy. Co-Evol. Sec. Met.. 2020;543–574
- [CrossRef] [Google Scholar]
- Effects of quercetin and its seven derivatives on the growth of Arabidopsis thaliana and Neurospora crassa. Biochem. Syst. Ecol.. 2004;32:631-635.
- [CrossRef] [Google Scholar]
- Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci.. 2007;12(12):556-563.
- [CrossRef] [Google Scholar]
- Inhibitory effects of Nerium oleander L. and its compounds, rutin and quercetin, on Parthenium hysterophorus L. J. Agric. Sci.. 2011;3:123-137.
- [CrossRef] [Google Scholar]
- Influence of different botanicals extract on the management of (Parthenium hysterophorus L.) for eco-friendly approach. J. Pharmacogn. Phytochem.. 2020;6:409-417.
- [Google Scholar]
- The sesquiterpenes β-caryophyllene and caryophyllene oxide isolated from Senecio salignus act as phytogrowth and photosynthesis inhibitors. Molecules. 2012;17(2):1437-1447.
- [CrossRef] [Google Scholar]
- The phytotoxic activity of Parthenium hysterophorus L. seedlings on a range of pasture species. Crop. Prot.. 2020;137:105211
- [CrossRef] [Google Scholar]
- Períodos de interferência de plantas daninhas na cultura do girassol. Bragantia.. 2013;72:255-261.
- [CrossRef] [Google Scholar]
- Allelopathic effects of some common weeds prevailing in wheat fields on growth characteristics of wheat (Triticum aestivum L.) PSM. Biol. Res.. 2017;2:124-127.
- [Google Scholar]
- Allelopathic effect of aqueous extracts of three weed species on the growth and leaf chlorophyll content of bread wheat. Acta Ecol. Sin.. 2019;39:63-68.
- [CrossRef] [Google Scholar]
- Effects of competition and water deficiency on sunflower and weed growth. Rev. Caatinga.. 2019;32:318-328.
- [CrossRef] [Google Scholar]
- Flavonoids as inhibitors of the formation of adenosine tri-phosphate in plant mitochondria. Phytochemistry. 1970;9:2251-2256.
- [Google Scholar]
- Phytotoxic activity of clove oil, its constituents, and its modification by light intensity in broccoli and common lambsquarters (Chenopodium album) Weed Sci.. 2012;60(4):607-611.
- [CrossRef] [Google Scholar]
- Cloning, expression and wounding induction of β-caryophyllene synthase gene from Mikania micrantha HBK and allelopathic potential of β-caryophyllene. Allelopathy J.. 2009;24(1):35-44.
- [Google Scholar]
- Responses of Mikania micrantha, an invasive weed to elevated CO2: induction of β-caryophyllene synthase, changes in emission capability and allelopathic potential of β-caryophyllene. J. Chem. Ecol.. 2010;36:1076-1082.
- [CrossRef] [Google Scholar]
- Phytotoxic potential of Onopordum acanthium L. (Asteraceae) Chem. Biodivers.. 2014;11:1247-1255.
- [CrossRef] [Google Scholar]
- Allelopathic effect of jimson weed (Datura stramonium L.) on seed germination. Türk Tarım. Doğa. Bilim. Derg.. 2020;7:793-797.
- [CrossRef] [Google Scholar]
- Cytotoxicity of the natural herbicidal chemical, berberine, on Nicotiana tabacum Bright yellow-2 cells. Pestic. Biochem. Phys.. 2018;152:131-137.
- [CrossRef] [Google Scholar]







