Kinetic formation of iron oxide nanoparticles using un- and γ-irradiated singular molecular precursor of Tris(pentanedionato)iron(III) complex
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Iron oxide nanoparticles were synthesized from tris(pentanedionato)iron(III) singular molecular precursor through pyrolysis in static air. FT-IR, XRD, TGA, BET and SEM/EDS techniques were used to follow the reactions and identify the products. Single crystalline iron oxide sizes were found to be 18.23 ± 0.23, 16.42 ± 0.21, and 13.964 ± 0.11 nm using Scherrer equation, and the BET surface area were measured to be 110.909, 121.352, and 153.9911 m2g−1 for the corresponding iron oxides obtained through pyrolysis of un-irradiated and γ irradiated tris(2,4 pentanedionato)iron(III) with 100 and 300 kGy, respectively. Nonisothermal kinetics formation of iron oxide nanoparticles over the thermal decomposition of un-irradiated and γ-irradiated tris(pentanedionato)iron(III) molecular precursor with 100 and 300 kGy total γ-ray doses were investigated under air flow with a heating rate of 5, 10, 15, 20, and 25 °C/min, from 25 °C to 500 °C. Kinetic parameters were attained through model-fitting and model-free approaches, and artificial isokinetic relationship (IKR) for multi-step processes. The decomposition for both un-irradiated and γ-irradiated tris(pentanedionato)iron(III) molecular precursor with 100 and 300 kGy total γ-ray doses proceed over one major step with two-dimensional diffusion (bi-dimensional particle shape) Valensi equation (D2).
Keywords
Iron Oxide Nanoparticles
Tris(pentanedionato)iron(III)
Singular Molecular Precursor
Kinetic Formation
1 Introduction
Magnetite, iron (II,III) oxide, is one of the well characterized naturally existence inorganic compound with the formula Fe3O4. It has a cubic inverse spinel structure contain a cubic close packed array of oxide ions and the Fe2+ ions fill half of the octahedral sites and the Fe3+ divided evenly and fill the tetrahedral and the remaining other half octahedral sites.(Cornell & Schwertmann, 2003; Pereira et al., 2012; Wu et al., 2021) Due its structure and related chemical, electrical, and magnetic properties besides to its low toxicity, cost effective, stability it has enormous number technological applications in deferent fields including but not limited in medicine, data storage device, catalysis, and environmental remediation.(Ajinkya et al., 2020; Al-Anazi, 2022; Israel et al., 2020; Teja & Koh, 2009; Xu et al., 2012) Despite the various chemical approaches employed to Magnetite iron oxide, there remain challenges related to suboptimal quality, unclear morphology, prolonged synthesis times, and high-temperature requirements. To address these issues, a more favourable synthesis approach such as co-precipitation (Petcharoen & Sirivat, 2012; Valenzuela et al., 2009), solvothermal (Bastami et al., 2012; Bastami et al., 2014), hydrothermal (Kumar et al., 2020; Torres-Gómez et al., 2019) and others are sought. Among these techniques, the electrochemical method stands out as it allows for the rapid formation of iron oxide nanoparticles at ambient temperature. However, the disadvantages of sonoelectrochemical synthesis include limited control, leading to variations in product quality and consistency. operational costs, equipment complexity, limited scalability for industrial applications due to equipment constraints and energy requirements, and environmental impact such as noise pollution, the disposal of related waste materials can pose environmental concerns, and sonochemical reactions can sometimes lead to unwanted side reactions or byproducts, reducing the selectivity of the synthesis process (Dudchenko et al., 2022). The high-temperature pyrolysis method provides distinct advantages in iron oxide nanoparticle synthesis compared to the cost-effective sonoelectrochemical approach. Pyrolysis allows for precise control over particle size and crystallinity, ensuring consistent, high-quality results, while sonoelectrochemical methods may yield less predictable outcomes due to their limited control, making pyrolysis a preferred choice for precise and reliable nanoparticle production. It has been established that the iron oxide particles size, sizes distribution, shapes, and surface nature impact the magnetic properties of iron oxide nanoparticles.(Carvalho et al., 2013; Filippousi et al., 2014; Ibarra-Sánchez et al., 2013; Muro-Cruces et al., 2019; Ngoi et al., 2021; Roca et al., 2019) One widely used way to control physicochemical properties such as particles sizes and size distribution is to produce magnetic nanoparticles by a high-temperature decomposition treatment of singular inorganic molecular precursor.(Belaïd et al., 2018; Maity et al., 2009; Miguel-Sancho et al., 2012; Roca et al., 2019; Roca et al., 2006; Siddiqi et al., 2007; Toyos-Rodríguez et al., 2019) On the other effective used way, the gamma Co-60 ray irradiation is desirable for producing controlled size nanoparticles in large scale with clean process.(Choi et al., 2005; Flores-Rojas et al., 2020; Jurkin et al., 2016; Marić et al., 2020; Nguyen et al., 2019; Remita et al., 1996) Although there are many studies have investigated the role of γ-irradiation on the thermal decomposition of inorganic precursor, the kinetics of the decomposition of the un- and γ-irradiated singular molecular precursor of tris(pentanedionato)iron(III) complex to obtain iron oxide nanoparticles have been considered less. Thermogravimetry analysis technique (TGA) is very valuable method particularly for exploring the decomposition reactions mechanism and its kinetics.(Laye et al., 2007). The influence of gamma irradiation on the weight loss behavior observed in the Thermogravimetric Analysis (TGA) curve of an inorganic complex is a pivotal aspect, offering profound insights into the material's thermal stability and decomposition characteristics. TGA, a method tracking weight changes in response to controlled temperature variations, becomes a potent tool to discern alterations induced by gamma irradiation in the molecular structure and properties of the inorganic complex. Thus herein, the main objective of the research is to investigate the impact of γ-irradiation on the structure of the singular molecular precursor tris(pentanedionato)iron(III) complex and its subsequent influence on the structure and morphology of iron oxide nanoparticles. Iron oxide nanoparticles were synthesized through pyrolysis of both unirradiated and γ-irradiated singular molecular precursors at different temperatures. The kinetics of the thermal decomposition of unirradiated and γ-irradiated tris(pentanedionato) iron(III) samples were also assessed and compared. Both model-fitting and isoconversional methods were employed to examine the reliability of each model category in describing the pyrolysis kinetics of unirradiated and γ-irradiated tris(pentanedionato) iron(III) samples, aiming to produce iron oxide nanoparticles. We elucidate the decomposition reaction mechanism of the tris(pentanedionato)iron(III) complex and its kinetics using thermal kinetic analysis, which appears to proceed through a two-dimensional diffusion process (bi-dimensional particle shape) described by the Valensi equation (D2). This suggests that stable nuclei form at a slower rate than their subsequent growth. Our results highlight the significant influence of pyrolysis temperature and absorbed dose on controlling the structure and morphology of iron oxide nanoparticles.
2 Experimental
Powder of tris(2,4-pentanedionato)iron(III)-Fe(C5H7O2)3 > 98 % was obtained commercially from TCI and were used without any further purification. The irradiation process involved compressing tris(2,4-pentanedionato)iron(III) samples in glass vials under vacuum, followed by γ-irradiation at a dose rate of approximately 26 kGy/h with constant intensity, utilizing a Co-60 Gamma cell 220 (Nordion MDS, Ontario, Canada). The absorbed doses administered were 100 and 300 kGy. Calibration of the radiation source was performed using a Fricke ferrous sulfate dosimeter for comparison. Dose calculations involved adjustments to the photon mass attenuation and energy-absorption coefficient for both the sample and the dosimeter solution. These corrections were meticulously applied to ensure the accuracy of the computed doses (Spinks & Woods, 1990). Tris(2,4-pentanedionato)iron(III) samples were calcined at 180, 200, 220, 240, 260, and 280 °C for 6 h each. The un-calcined and calcined samples before and after irradiations were characterized through recording the FTIR spectra in the range of 4000–400 cm−1 as KBr pellets with of a concentration of 1 % using a Perkin–Elmer FT-IR spectrometer, and collecting XRD patterns at the diffraction angles range of 5° to 100° utilizing Bruker (model D8 ADVANCE) X-ray diffractometer with a nickel filter and Cu Kα radiation (λ = 1.5406 A° at 40 kV and 40 mA). The thermal decomposition analysis of tris(2,4-pentanedionato)iron(III)-samples before and after radiation were carried using Perkin–Elmer (dynamic) thermogravimetric techniques. The kinetic analysis was calculated from temperature measurements made during the actual period of a material decomposition employing model-fitting and model-free methods to produce consistent kinetic characteristics from nonisothermal experiments in which the instrument was programmed to heat the sample from 25 °C to 500 °C at a constant heating rate of 5, 10, 15, 20, and 25 °C/min under air flow. Surface area and pore size of samples were measured via automated gas sorption analyser (Quantachrome Instruments) using N2 adsorption/desorption isotherms. Samples were initially outgassed at 60 °C for 60 min under vacuum to a final pressure of 0.35 Pa and then the data obtained over the relative pressure range of (P/P°= 0.05–––0.99). The data were calculated based on Brunauer-Emmett-Teller (BET) equation, Langmuir models Barrett-Joyner-Halenda (BJH). Samples morphology and surface composition were acquired utilizing Scanning Electron Microscopy (SEM/EDX, JEOL JSM-6380LA) using different magnification powers and operating between 5 and 20 kV. Samples were displayed on a carbon tube with coating with gold before being examined. chemical elements of the sample were determined using Elemental Analysis (EDX) at 20 kV.
3 Results and discussion
3.1 Characterization of iron oxide nanoparticles and γ- irradiation role in its formation
The role of irradiation on the characteristic FTIR bands of tris(2,4-pentanedionato)iron(III) precursors were elucidated and shown in Fig. 1. As shown, the FTIR spectra of un-irradiated and γ-irradiated tris(2,4-pentanedionato)iron(III) with 100 and 300 kGy, respectively; in agreement with the literature (Slabzhennikov et al., 2003; Tayyari & Milani-nejad, 2000) displayed the characteristic bands assigned to various vibration modes of acetylacetonate functional groups with no disappearance or appearance of new bands as were recorded in the FTIR spectra of γ-irradiated tris(2,4-pentanedionato)iron(III) samples with 100 and 300 kGy total γ ray doses. However, a decrease in the intensity of most characteristic bands was recorded as results of γ-irradiation. The bands intensities designated to ν Fe-O bond in the range of 799 to 433 cm−1 were more affected by irradiation than any other bands in the spectrum. The decreased in the intensity of this band could be attributed to bond divided or split influenced by γ irradiation.(Aly & Elembaby, 2020; El-Boraey et al., 2022) IR bands for tris(2,4-pentanedionato)iron(III) and their assignments are listed in Table 1.
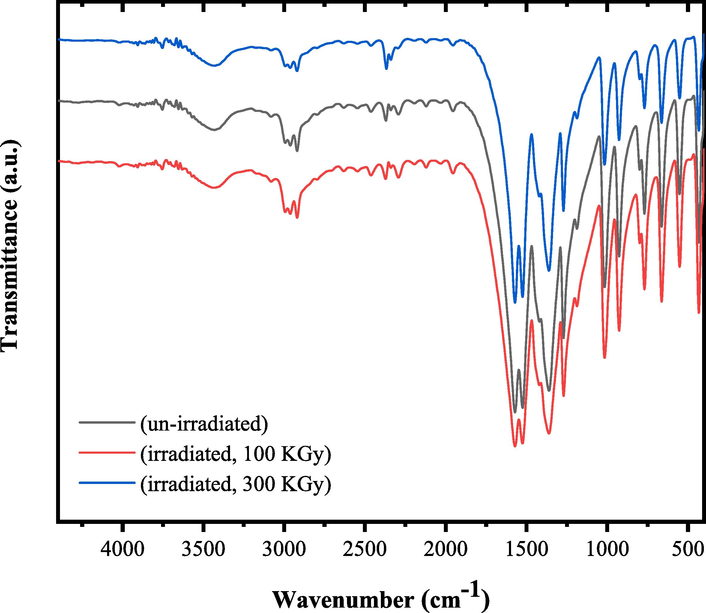
- FTIR spectra of un-irradiated and γ irradiated tris(2,4-pentanedionato)iron(III) with 100 and 300 KGy, respectively.
| Frequency (cm−1) | |||
|---|---|---|---|
| band assignment* | un-irradiated | γ- irradiated (100 kGy) | γ- irradiated (300 kGy) |
| ν(OH) | 3429 | 3429 | 3428 |
| νa(CH3) in plane | 3005 | 3005 | 3005 |
| νa(CH3) out of plane | 2961 | 2962 | 2962 |
| ν(OH) + νs(CH3) | 2919 | 2919 | 2920 |
| νa(C–C–C-O) + δ(OH) + ν(C-O) | 1571 | 1571 | 1571 |
| ν(C–C) + δ(OH) | 1525 | 1525 | 1525 |
| δa(CH3) in plane + δs(CH3) out of plane + νa(C–C–C-O) + δ(OH) + δ(CH) + ν(C-CH3) | 1426 | 1426 | 1426 |
| δs(CH3) | 1361 | 1361 | 1361 |
| νs(C–C–C-O) + ν(C-O) + δ(OH) + ν(C-CH3) | 1271 | 1271 | 1271 |
| δ(C–H) olefinic + π(CH3) + γ(OH) | 1188 | 1188 | 1188 |
| π(CH3) + ρ(CH3) + γ(OH) + τ(C–C–C–H) + τ(O-C–C–H) | 1017 | 1017 | 1017 |
| ρ(CH3) + γ(OH) + ν(C-O) + ν(Fe-O-C) | 927 | 927 | 927 |
| ρ(C–H) | 799 | 799 | 799 |
| γ(C–H) olefinic | 770 | 770 | 770 |
| Γ ring + ρ(CH3) + τ(C–C–C–H) + τ(O-C–C–H) | 664 | 664 | 664 |
| Δ ring + π(CH3) + ν(Fe-O) + γ(Fe-O-C) + δ(O-C–C) | 552 | 552 | 552 |
| ν(Fe-O) + δ(O-Fe-O) + Δ ring + δ(C-CH3) | 433 | 433 | 434 |
| *ν, stretching; δ, in plane bending; γ, out of plane bending; π, CH3 out of plane rocking; ρ, CH3 in plane rocking; Δ, in plane ring deformation; Γ, out of plane ring deformation; τ, torsion; s, symmetric; a, asymmetric. | |||
The role of irradiation on the XRD patterns of tris(2,4-pentanedionato)iron(III) precursors were also explored as shown in Fig. 2 which presented XRD patterns of un-irradiated and γ irradiated tris(2,4 pentanedionato)iron(III) with 100 and 300 kGy, respectively. The main peaks located at 10.99◦ (0 2 0), 13.27◦ (consisting of two superimposed peaks, 13.28◦ (0 0 2) and 13.26◦ (2 0 1)), 17.08◦ (0 2 2), 21.54◦ (3 2 1), 22.96◦ (2 0 3), 23.95◦ (2 3 2) and 25.43◦ (2 2 3) matching a reference primitive orthorhombic phase (ICCD Card No: 00–030-1763) (Dudek et al., 2020). XRD diffractograms proved that orthorhombic tris(2,4 pentanedionato)iron(III) was only dominated phase in un-irradiated and γ irradiated samples and thus maintains its structural integrity after exposure to the irradiation dose. However, tris(2,4 pentanedionato)iron(III) exposure to the 300 kGy irradiation dose not only resulting in broadening of the main peaks but also appears a loss of peak intensity in addition to the 020 peak 2θ position specifically shift to 10.70°. This then demonstrates that irradiation doses with 300 kGy doses cause decreasing the degree of crystallinity and more damage to tris(2,4 pentanedionato)iron(III) than 100 kGy doses.
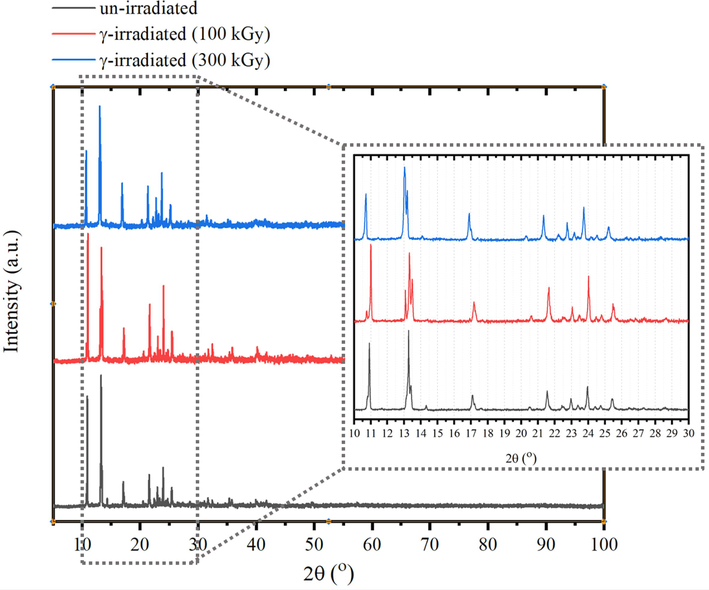
- XRD patterns (a) of un-irradiated and γ irradiated tris(2,4 pentanedionato)iron(III) with 100 and 300 kGy, respectively.
The irradiation effect on the morphology and surface elemental compositions of tris(2,4-pentanedionato)iron(III) precursors samples were examined as Fig. 3 displayed the effects of γ-ray on SEM images and EDS analysis of un-irradiated and γ irradiated tris(2,4 pentanedionato)iron(III) with 100 and 300 kGy samples. It can be clearly seen that the structure of inspected samples is ambiguous in nature, displaying a random size distribution for both un-irradiated and γ-irradiated samples containing of nearly spherical-shape nanoparticles that agglomerated together to form significant large grains that uniformly distributed throughout the matrix of the samples. The agglomeration of the grain crystallites increases with increasing the γ-irradiation with no discrete particles are noticeable in precursors samples. The EDS spectra revealed distinct signals at 0.25, 0.50, 0.57, and 6.40, corresponding to C, O, and Fe, respectively. Additionally, unassigned signals at 0.00 and 2.2 were identified, associated with C and Au. Tris(pentanedionato)iron(III) complexes exhibit minimal alterations when subjected to 100 and 300 kGy doses of gamma irradiation, as depicted in Fig. 4. Despite the application of high-energy photons inducing subtle changes evident in the resulting TGA curve, the complex remains remarkably stable. The intricate portrayal of the complex's thermal decomposition, leading to the formation of iron oxide and the subsequent release of byproducts, is clearly manifested in the TGA curve. The discernible impact of gamma irradiation on weight loss is characterized by slight shifts in the onset temperature of decomposition, fluctuations in the degradation rate, and modest alterations in the overall mass loss profile, reflecting the stability of the complex under the influence of gamma radiation. The TGA curves of un-irradiated tris(2,4 pentanedionato)iron(III) and γ-irradiated with 100 kGy displayed one major decomposition step with weight loss of 85.4 % in the range of 180 to 240 ℃ attributed to the thermolysis of pentanedionato moiety of the tris(2,4 pentanedionato)iron(III) phase resulting in the rapid formation of degradation products of gaseous residues and small iron oxide nuclei. The final weight of 14.6 % (calculated 21.8 %) is presumed to be the residue of the oxygen deficient iron oxide material. TGA curve demonstrates that γ-irradiated tris(2,4 pentanedionato)iron(III) with 100 kGy shows no apparent response to gamma irradiation doses and retains the thermolysis behaviour without any observed changes. Thermolysis of γ-irradiated tris(2,4 pentanedionato)iron(III) with 300 kGy rate dose exhibited similar behaviour with one major step with weight loss of 78.6 % in the range of 180 to 240 ℃ due to decomposition of pentanedionato moieties, and the final weight of 21.4 % (calculated 21.8 %) is presumed to be iron oxide products. The continuous weight loss on approaching 500 ℃ as consequence of decomposition of remaining carbonaceous species.
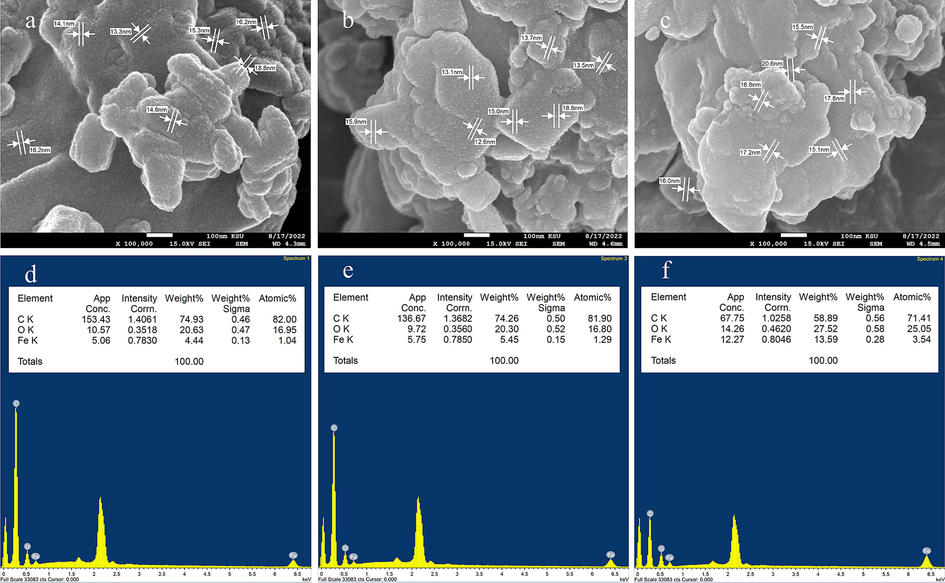
- SEM/EDS analysis of un-irradiated (a and d) and γ irradiated tris(2,4 pentanedionato)iron(III) with 100 (b and e) and 300 kGy (c and f), respectively.
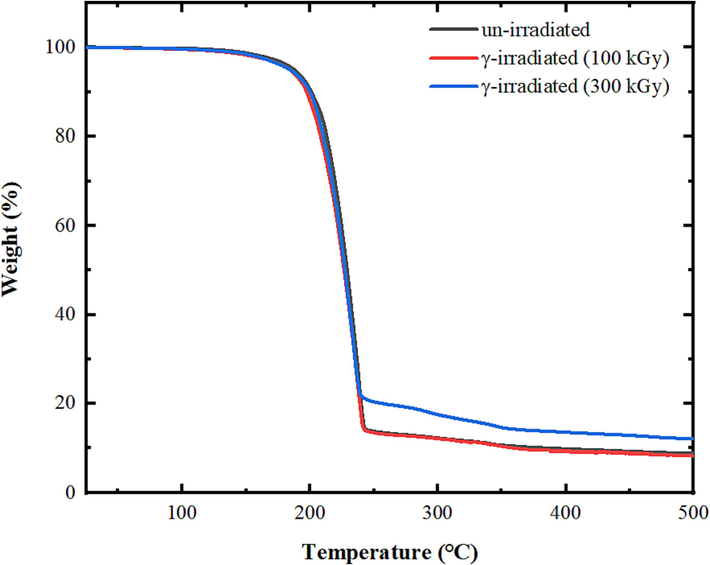
- Effects of γ-ray on the thermal decomposition behaviour of tris(pentanedionato)iron(III) samples.
The un-irradiated and γ irradiated tris(2,4 pentanedionato)iron(III) with 100 and 300 kGy, were calcined at selected temperature of 180, 200, 220, 240, 260, and 280 ℃ for 6 h each. Figs. 5 to 7 show the temperature-dependent FTIR spectra of un- and γ-irradiated calcined tris(2,4-pentanedionato)iron(III) with 100 and 300 kGy, respectively. The characteristic absorption bands of 2,4-pentanedionato gradually decrease with the increasing temperature and disappear when the temperature reaches 280 ℃, which indicates completion of the decomposition of 2,4-pentanedionato residues. The observed FTIR bands in the range of 800–––400 cm−1, attributed to Fe-O vibrations, exhibit an intriguing behavior of increasing intensity with rising temperature. These bands are linked to the vibrational modes associated with specific iron oxide phases, and their variations at different temperatures provide valuable insights into the thermal evolution of the formed iron oxide. At 240 °C, the presence of bands at 660, 614, 550, 446, and 428 cm−1 aligns with the characteristic vibrational modes of magnetite (Fe3O4). The increased intensity of these bands at higher temperatures may indicate enhanced crystallinity or phase transitions within the magnetite structure. Upon further heating to 260 °C, additional bands at 728, 692, 640, 560, 480, 444, and 428 cm−1 emerge. These bands are associated with vibrational modes characteristic of maghemite (γ-Fe2O3). The growing intensity of these bands suggests a temperature-induced transformation towards maghemite, possibly indicating the conversion from magnetite to maghemite. At 280 °C, the vibrational bands at 728, 697, 640, 560, 484, 444, and 424 cm−1 remain, affirming the persistence of maghemite characteristics. The variations in band intensity across temperatures imply dynamic phase changes and the influence of thermal treatment on the iron oxide system. However, it's important to note that the identification of phases based on FTIR bands is indicative, and definitive confirmation often requires complementary techniques such as X-ray diffraction (XRD). The observed changes underscore the sensitivity of FTIR spectroscopy to alterations in the crystalline structure and phase composition of iron oxides under different thermal conditions (Stoia et al., 2016; Waldron, 1955; Yadav et al., 2020). Moreover, the observed broad band at 3401 cm−1 and band at 1624 cm−1 were attributed to both symmetrical and unsymmetrical modes of O–H bonds of adsorbed water layer associated to the surface iron atoms. The temperature-dependent FTIR spectra of γ-irradiated calcined tris(2,4-pentanedionato)iron(III) with 100 and 300 kGy as seen in demonstrated similar behaviour with decreasing intensities of the iron oxide characteristic absorption bands for the calcined sample of tris(2,4-pentanedionato)iron(III) precursor with 300 kGy sample related to the calcined un-irradiated sample of tris(2,4-pentanedionato)iron(III) precursor.
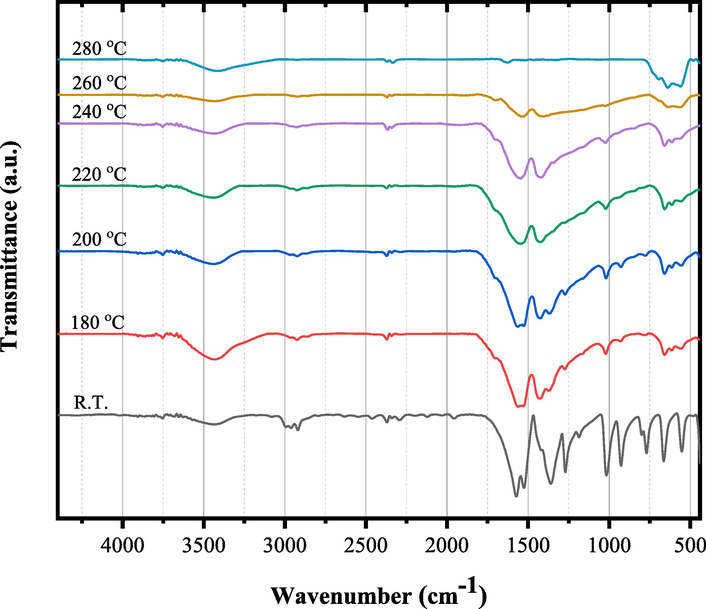
- FT-IR spectra of un-irradiated tris(2,4-pentanedionato)iron(III) calcined at different temperatures for 6 h.
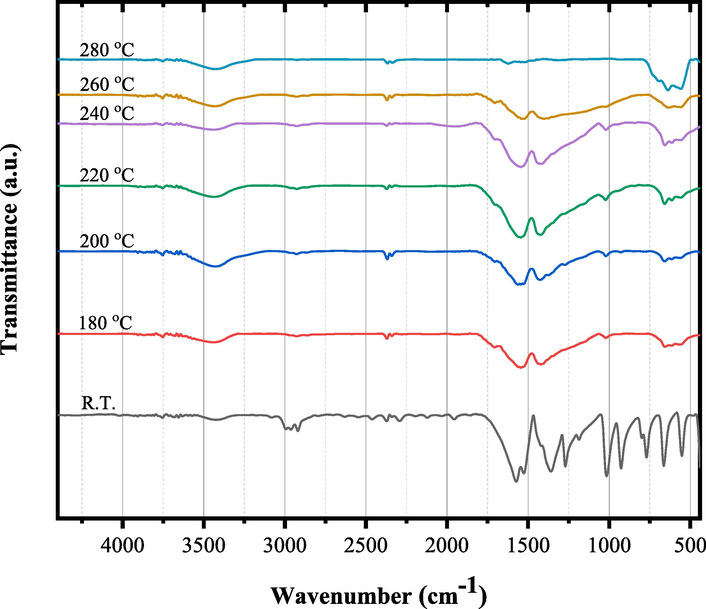
- FT-IR spectra of γ-irradiated tris(2,4-pentanedionato)iron(III) with 100 kGy radiation dose calcined at different temperatures for 6 h.
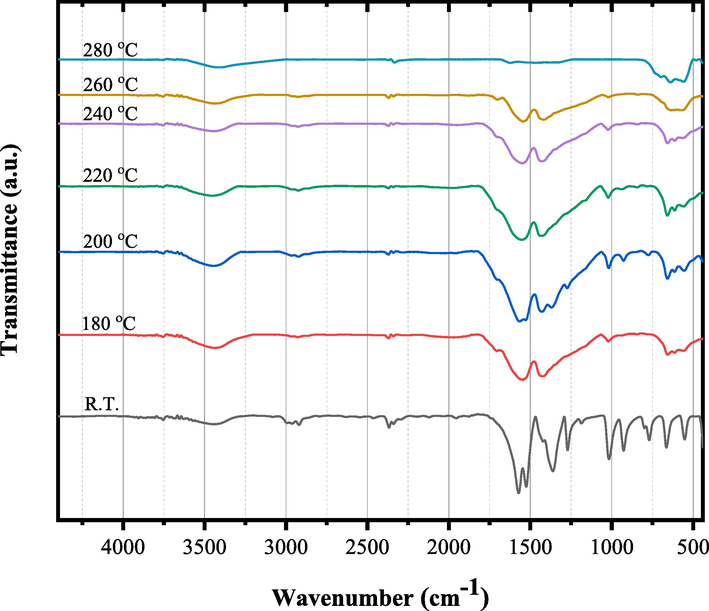
- FT-IR spectra of γ-irradiated tris(2,4-pentanedionato)iron(III) with 300 kGy radiation dose calcined at different temperatures for 6 h.
Figs. 8–10 show the temperature-dependent XRD patterns of un- and γ-irradiated calcined tris(2,4-pentanedionato)iron(III) with 100 and 300 kGy, respectively. Through calcination at 180, 200, 220, 240, 260, and 280 ℃ for 6 h each, an immediate structural loss of the corresponding tris(2,4-pentanedionato)iron(III) precursor was observed accompanied by the emission of carbonous gases from 2,4-pentanedionato moieties decomposition resulting in the formation of amorphous phases contain iron oxide. Moreover, the reflection signal located at 2θ of 36° in the diffractogram of calcined sample at 220 ℃ started to appear signalling the formation of iron oxide material phase. Upon increasing of calcination temperatures reaching 280 ℃ as shown in Fig. 11, All the reflections in the XRD patterns can be indexed to Magnetite iron oxide phase (ICCD Card No: 00–003-0863). The characteristic peaks at 30.4°, 35.8°, 43.5°, 53.9°, 57.4°, and 63.2° match to the (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), and (4 4 0) crystal faces of Fe3O4, respectively. The signals get well defined and narrowed indicating the effect of pyrolysis temperatures on the XRD patterns in alignment with the FTIR and TGA analyses conforming of nanocrystalline iron oxide phase developments. The peaks are found at 12.77° can be attributed to the amorphous phase of carbon or to deletion of some specific interlayered atoms leads to increased intensities.(Sun et al., 2017) The crystallite size of the prepared iron oxide samples was calculated by averaging the values obtained from the (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), and (4 4 0) diffraction peaks, utilizing the Debye-Scherrer equation (Eq. (1):
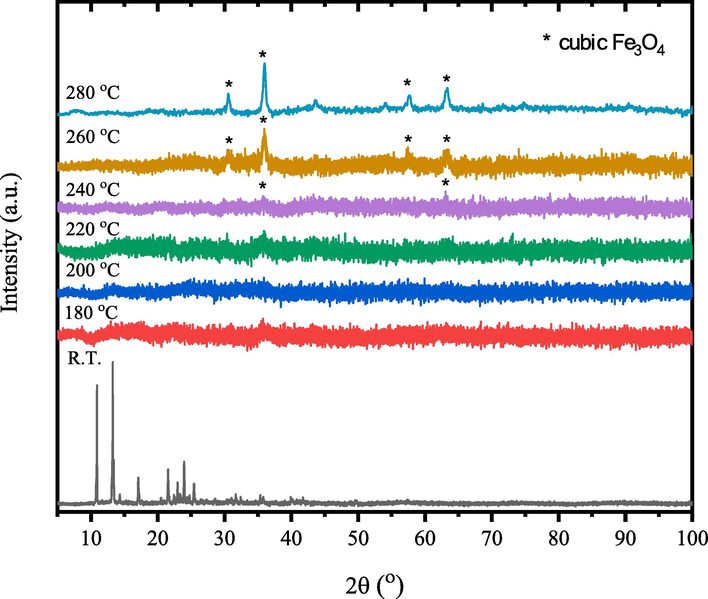
- Temperature-dependent XRD patterns of the un-irradiated tris(2,4-pentanedionato)iron(III).
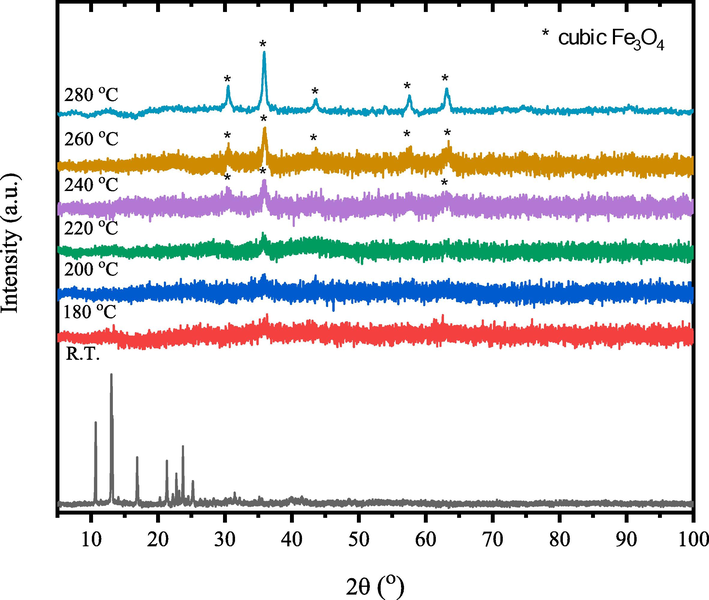
- XRD patterns of γ-irradiated tris(2,4-pentanedionato)iron(III) with 100 kGy radiation dose calcined products at different temperatures for 6 h.
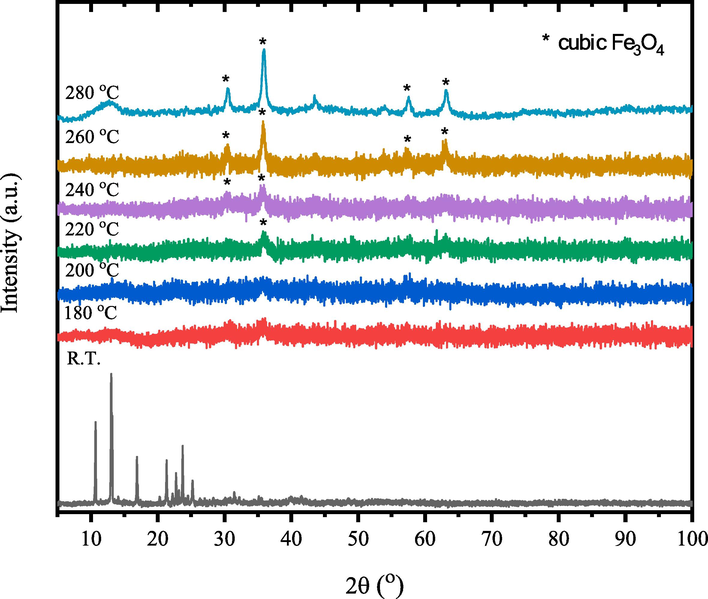
- XRD patterns of γ-irradiated tris(2,4-pentanedionato)iron(III) with 300 kGy radiation dose calcined products at different temperatures for 6 h.
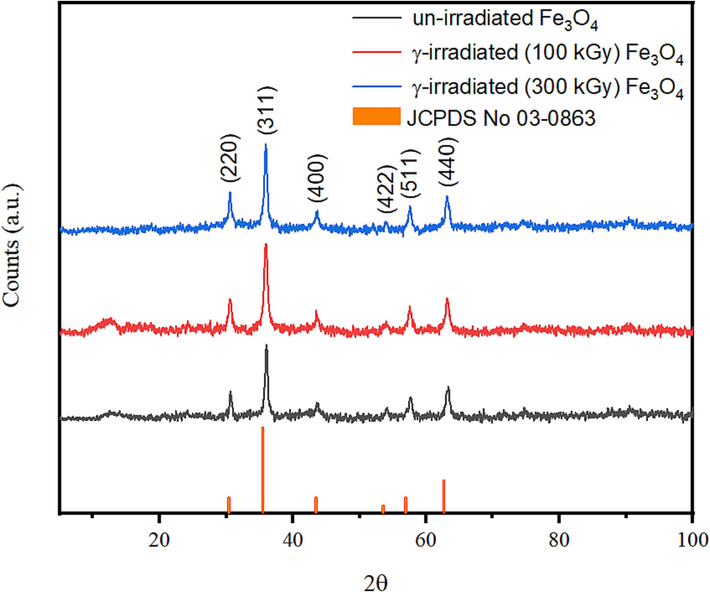
- XRD patterns of the solid residues obtained from pyrolysis of un-irradiated and γ-irradiated tris(2,4-pentanedionato)iron(III) precursors with 100 and 300 kGy, respectively.
Where, D is the diameter of crystalline size, λ is the wavelength of the incident beam, β is the full width half maxim ‘FWHM’, and θ is the Bragg’s angle. The crystallite size as Table 2 demonstrated represents the size of the individual crystalline domains within the nanoparticles. In the un-irradiated sample of iron oxide samples obtained from pyrolysis, the crystallite size is 18.23 ± 0.23 nm, which decreases to 16.42 ± 0.21 nm and 13.964 ± 0.11 nm for the 200 kGy and 300 kGy irradiated samples, respectively. The reduction in crystallite size with increased irradiation suggests a transformation in the crystal structure, potentially due to radiation-induced defects or phase changes. Fig. 12 clearly indicated that the as-prepared iron oxide materials were nearly spherical-shape nanoparticles with average size of 28.65 ± 4.08, 27.35 ± 6.14, and 22.99 ± 4.46 nm for samples obtained from pyrolysis of un-irradiated and γ-irradiated tris(2,4-pentanedionato)iron(III) precursors with 100 and 300 kGy, respectively (Table 2). The particles agglomerated together, and elements distributed uniformly all over the matrix of the samples. These results confirmed that the corresponding oxides kept the spherical morphology with a broader size distribution of the apparent original precursors. The agglomeration of the grain crystallites increases, and the particle size decreases with increasing the γ-irradiation. Pore size and BET surface area are vital indicators of the nanoparticles' structural characteristics (Figure S4 in SF Text). In the un-irradiated sample, the pore radius is 37.651 Å, while the BET surface area is 110.909 m2/g. With irradiation, the pore radius decreases to 23.152 Å (200 kGy) and 23.294 Å (300 kGy), and the BET surface area increases to 121.352 m2/g (200 kGy) and 153.991 m2/g (300 kGy). The reduction in pore radius and increase in BET surface area suggest enhanced porosity and surface reactivity, possibly attributable to the radiation-induced structural modifications. The dynamic changes in crystallite size, pore size, and BET surface area highlight the influence of irradiation on the physical properties of the nanoparticles. These alterations are pivotal for tailoring nanoparticles to potential specific applications, whether in catalysis, sensing, or drug delivery, and underscore the importance of a comprehensive understanding of their evolving characteristics under different irradiation conditions. Fig. 12 also demonstrate the EDS analysis of un-irradiated and γ irradiated of the solid residues obtained from pyrolysis of tris(2,4-pentanedionato)iron(III) precursors with 100 and 300 kGy, respectively. The un-irradiated sample showcases weight percentages of 33.43 % oxygen (O) and 66.57 % iron (Fe). The calculated Fe/O ratio approximates 2, indicating a molecular formula close to O2Fe1. This aligns with the expected composition of iron oxide, potentially magnetite, emphasizing the dominant role of iron in the molecular structure. Upon irradiation with 100 kGy, a shift in weight percentages to 30.87 % O and 69.13 % Fe alters the Fe/O ratio to approximately 2.24, suggesting a modified molecular formula of O1.9Fe1.2. The increased iron content implies potential phase transformations induced by irradiation, influencing the material's composition. At 300 kGy, weight percentages further change to 23.71 % O and 76.29 % Fe. The Fe/O ratio increases to about 3.21, indicating a shift towards O1.4 Fe1.3. This substantial alteration suggests a more pronounced impact of irradiation, potentially leading to the formation of iron-rich phases. The observed trends in molecular formulas and Fe/O ratios imply a dynamic response to radiation. The increasing Fe/O ratio suggests progressive iron enrichment, possibly indicating the formation of iron oxides with higher irradiation doses. Acknowledging the simplified nature of these estimations, further characterization techniques like Mössbauer spectroscopy are essential for precise phase identification. Overall, the EDS data illuminates the evolving molecular composition and Fe/O ratios, offering valuable insights into irradiation's impact on the material's structure and potential phase transformations.
| Iron oxide samples | Crystallite Size (nm) | Particle size(nm) | Surface area (m2/g) | Pore volume (cm3/g) | Pore radius(Å) |
|---|---|---|---|---|---|
| un-irradiated | 18.23 ± 0.23 | 28.65 ± 4.08 | 110.909 | 0.236 | 37.651 |
| irradiated (100 kGy) | 16.42 ± 0.21 | 27.35 ± 6.14 | 121.352 | 0.310 | 23.152 |
| irradiated (300 kGy) | 13.964 ± 0.11 | 22.99 ± 4.46 | 153.991 | 0.361 | 23.294 |
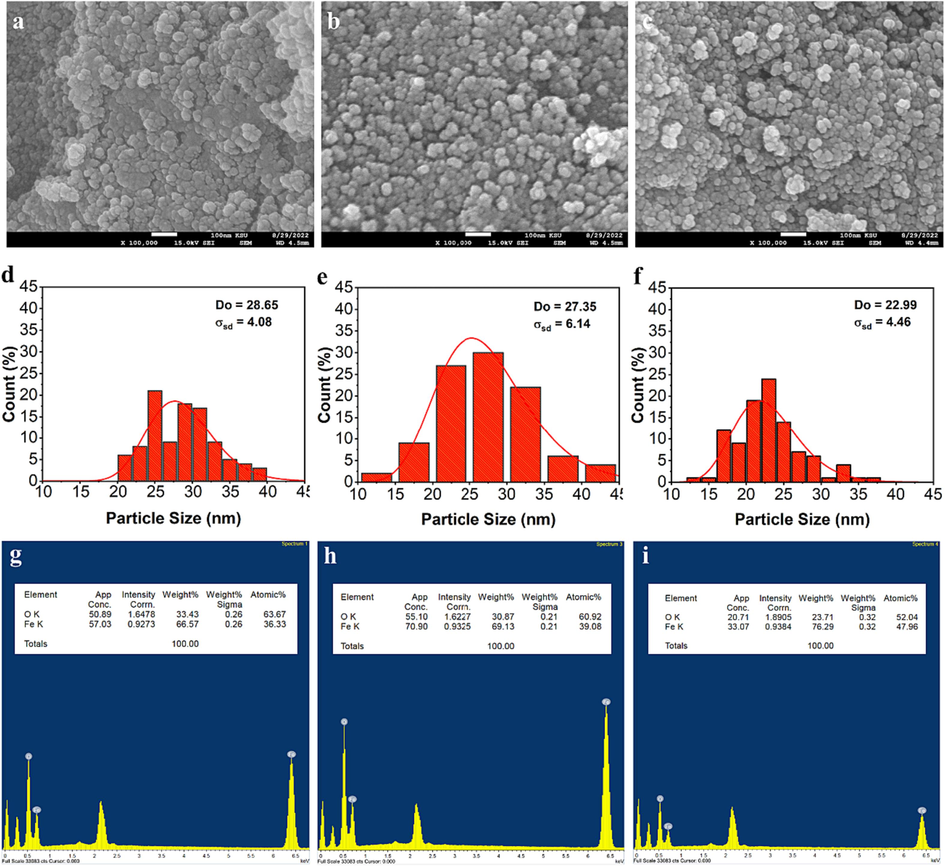
- SEM image, particle size distribution histogram, and EDS analysis of un-irradiated (a, d and g) and γ irradiated of the solid residues obtained from pyrolysis of tris(2,4-pentanedionato)iron(III) precursors with 100 (b, e and h) and 300 kGy (c, f and i), respectively.
3.2 Kinetic Studies
The solid-state decomposition reactions of tris(pentanedionato) iron (III) can be depicted through with a single-step kinetic equation according to Eq. (2).
Where
In this expression, A represents the pre-exponential factor (min−1),
Here,
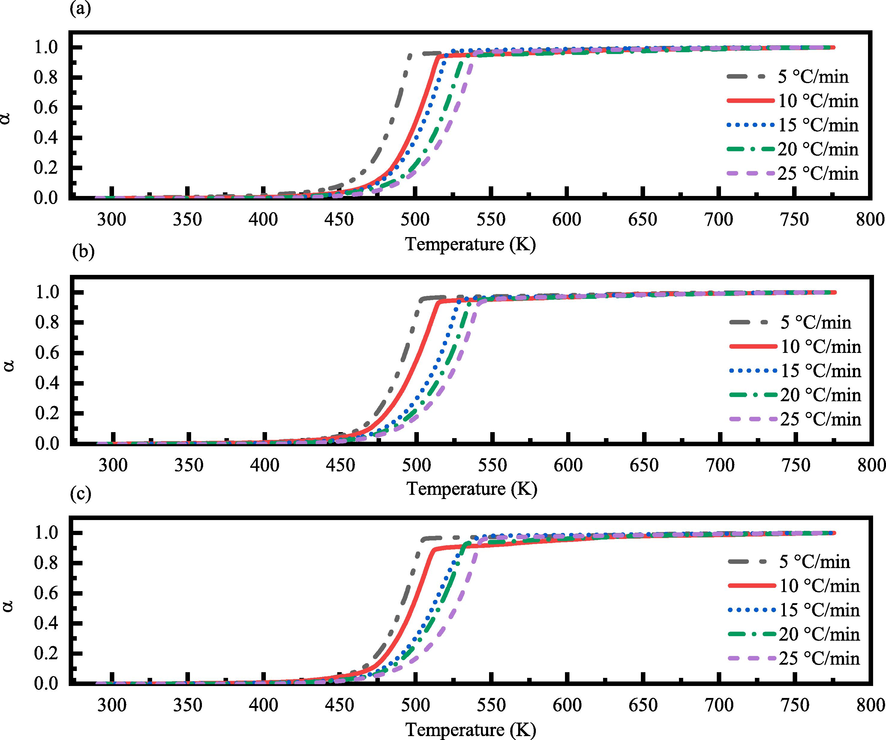
-
α-T (K) curves of the thermal decomposition for (a) un-irradiated, (b) γ-irradiated with 100 kGy radiation dose, and (c) γ-irradiated with 300 kGy radiation dose of tris(pentanedionato) iron (III) samples in static air at different heating rates.
Where
3.2.1 Model-fitting approaches
The solid-state reaction model equations, Table A in SF Text, employed into experimental data to obtain the kinetic parameters through model-fitting approaches using Coates and Redfern (CR) and Clark and Kennedy (CK) model displayed in Eqs. (6) and (7). (Coats & Redfern, 1964; Kennedy & Clark, 1997; Vyazovkin, 2000; Vyazovkin & Wight, 1999a)
Where
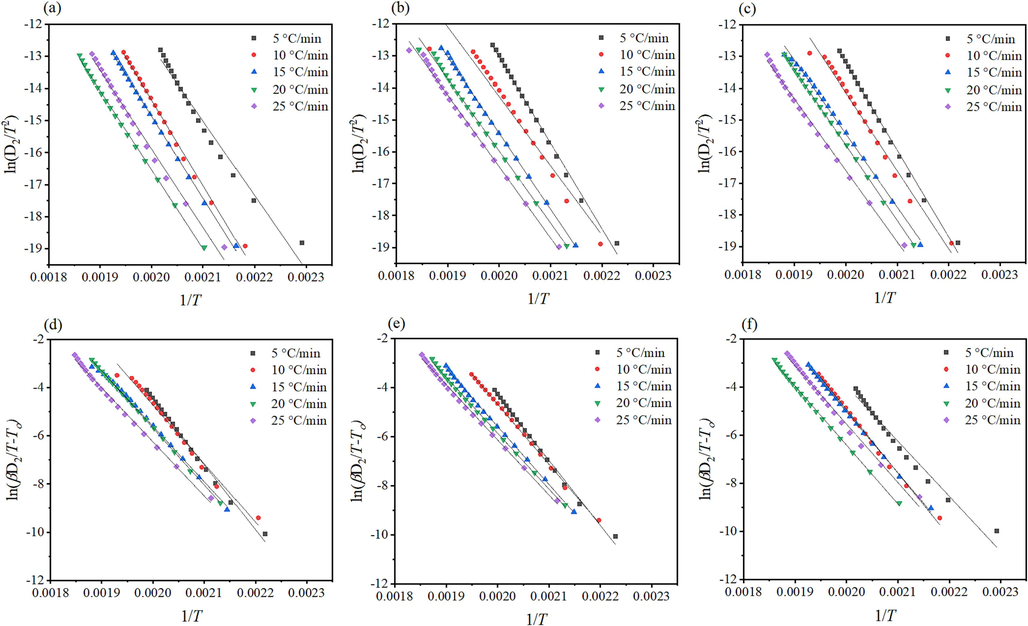
- Kinetic analysis of α/T (K) curves of the thermal decomposition of tris(pentanedionato) iron (III) samples according to CR (top) and CK (bottom) for (a and d) un-irradiated, (b and e) γ-irradiated with 100 kGy, and (c and f) γ-irradiated with 300 kGy radiation dose.
3.2.2 Isoconversional approaches
In nonisothermal experiments, the difficulty lies in distinguishing temperature dependence k(T) from the reaction model f(α), resulting in uncertainties in Arrhenius parameters that compensate for disparities between assumed and actual reaction models. Model-fitting provides a single, averaged activation energy, overlooking variations with temperature and conversion extent. Isoconversional methods address these challenges by determining activation energy without assuming the reaction model, thereby providing more consistent kinetic results across experiments. (Vyazovkin & Wight, 1999b) Isoconversional approaches were utilized to estimate the relation between activation energy (
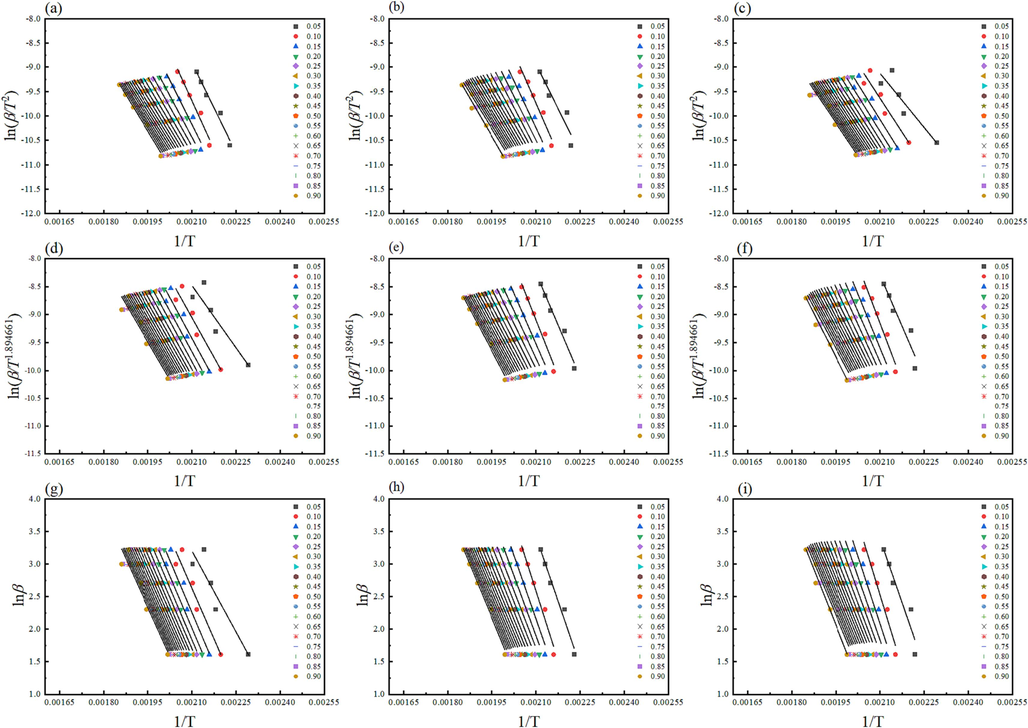
- Kinetic analysis of the thermal decomposition of tris(pentanedionato)iron (III) samples according to KAS (top), T (middle), and FWO (bottom) for (a, d, and g) un-irradiated, (b, e, and h) γ-irradiated with 100 kGy, and (c, f, and i) γ-irradiated with 300 kGy radiation dose.
Further, Vyazovkin (VYZ) isoconversional approximation with 5th degree Senum-Yang approximation (Eqs. (11) and (12) was used by Gorbachev (Eq. (13), Agrwal (Eq. (14), and Sivaubramanin-Cai (Eq. (15) integrations to determine the apparent activation energy (
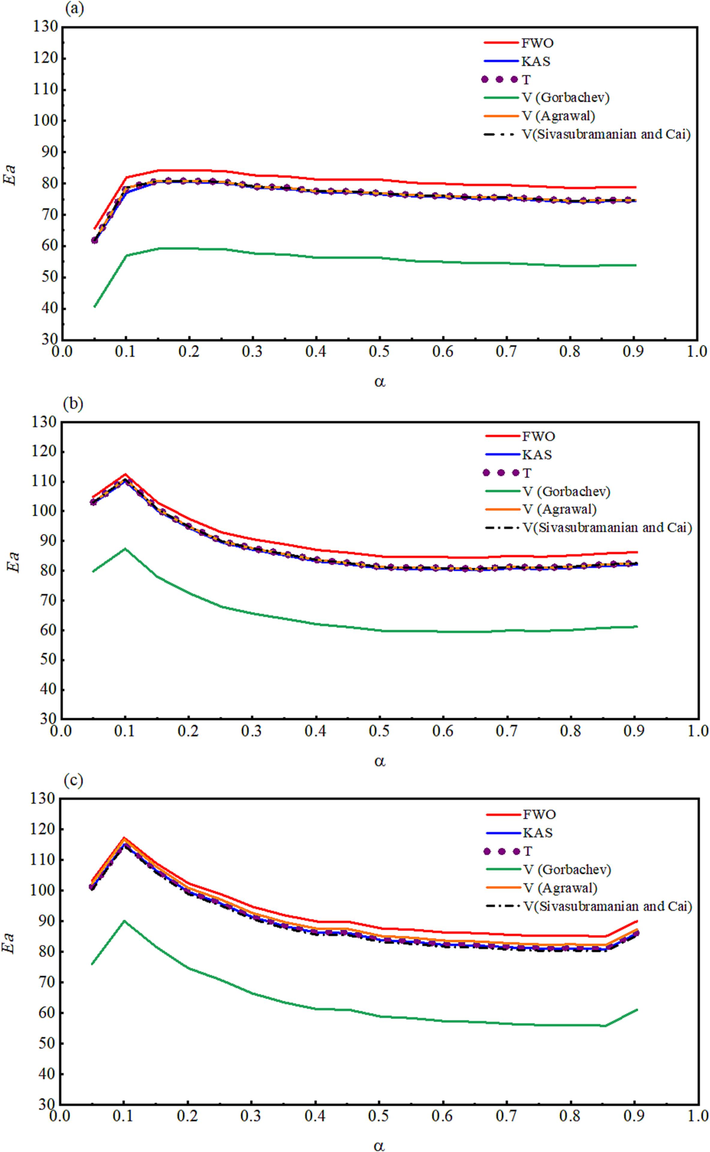
- The activation energy plotted as a function of the extent of the conversion for (a) un-irradiated, (b) γ-irradiated with 100 kGy, and (c) γ-irradiated with 300 kGy radiation doses.
3.2.3 Compensation effect
The validity of the compensation effect is related to the linearity of the relationship between lnA and
Where j represents one of the possible models fj(α) assumed to illustrate the reaction processes,
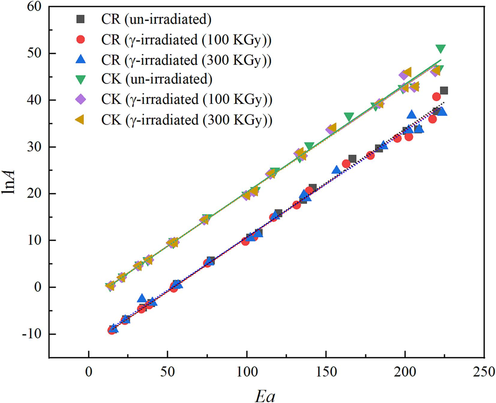
- The isokinetic relationships obtain by CR and CK methods for un-irradiated and γ- irradiated tris(pentanedionato)iron(III) samples.
| Method |
|
|
kiso (min−1) | Tiso (K) | r2 |
|---|---|---|---|---|---|
| CR (un-irradiated) | −12.4080 | 0.2315 | 4.09 × 10-6 | 519.5639 | 0.9966 |
| CR (γ-irradiated (100 kGy)) | −12.4020 | 0.2298 | 4.11 × 10-6 | 523.4075 | 0.9959 |
| CR (γ-irradiated (300 kGy)) | −11.9180 | 0.2265 | 6.67 × 10-6 | 531.0333 | 0.9957 |
| CK (un-irradiated) | −2.7136 | 0.2307 | 6.63 × 10-2 | 521.3656 | 0.9962 |
| CK (γ-irradiated (100 kGy)) | −2.5951 | 0.2287 | 7.46 × 10-2 | 525.9250 | 0.9965 |
| CK (γ-irradiated (300 kGy)) | −2.6055 | 0.2287 | 7.39 × 10-2 | 525.9250 | 0.9965 |
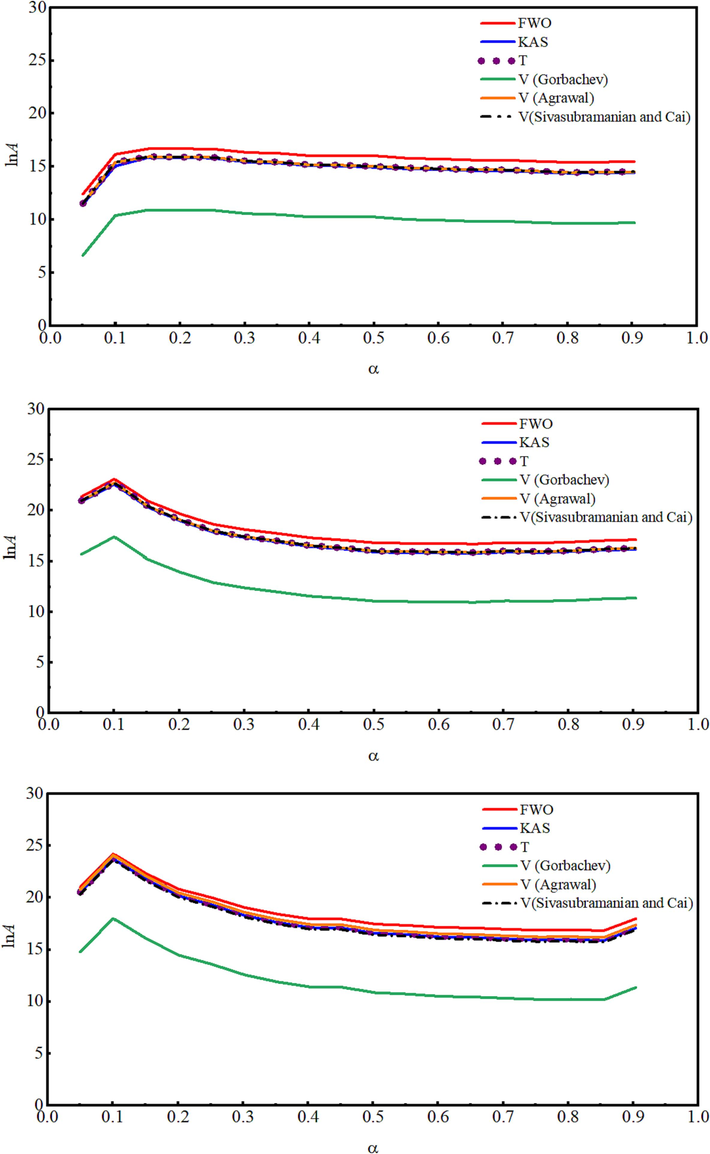
- The lnA dependence on the extent of the conversion for (a) un-irradiated, (b) γ-irradiated with 100 kGy, and (c) γ-irradiated with 300 kGy radiation doses.
4 Conclusions
In conclusion, the investigation into the impact of γ-irradiation on tris(2,4-pentanedionato)iron(III) complexes has provided a comprehensive understanding of structural, morphological, and kinetic changes. The FTIR spectra revealed a decrease in intensity, particularly in the ν Fe-O bond region, indicating the influence of γ-irradiation on bond integrity. XRD patterns demonstrated that while the orthorhombic phase of tris(2,4-pentanedionato)iron(III) remained dominant, higher irradiation doses led to broadening and intensity loss, indicating decreased crystallinity and more significant damage. SEM images and EDS analysis confirmed the formation of spherical nanoparticles with minimal alteration under irradiation. TGA curves displayed subtle shifts in decomposition parameters, emphasizing the stability of the complex under γ-irradiation. Calcination revealed temperature-dependent phase transitions, with FTIR and XRD analyses indicating the successfully synthesized of iron oxide from direct pyrolysis of tris(pentanedionato)iron(III) singular molecular precursor in static air. The crystallite size reduction with increased irradiation suggests structural modifications induced by radiation. EDS analysis demonstrated changing molecular compositions, emphasizing radiation's impact on material structure. Kinetic studies revealed a single-step decomposition reaction proceed through two-dimensional diffusion (bi-dimensional particle shape) Valensi equation (D2) as the stable nuclei would form slower than their subsequent growth rate, along with irradiation influencing activation energy and reaction rates. Isoconversional approaches highlighted enhanced porosity and surface reactivity. The compensation effect and lnAα relationship underscored the complex's dynamic response to irradiation. Overall, this study elucidates the intricate interplay of γ-irradiation on tris(2,4-pentanedionato)iron(III) complexes, providing valuable insights for tailoring these materials for various applications.
Acknowledgments
The author acknowledges the Researchers Supporting Project number (RSP2023R243), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Magnetic Iron Oxide Nanoparticle (IONP) Synthesis to Applications: Present and Future. Materials. 2020;13(20):4644.
- [Google Scholar]
- Iron-based magnetic nanomaterials in environmental and energy applications: a short review. Curr. Opin. Chem. Eng.. 2022;36:100794
- [CrossRef] [Google Scholar]
- Kinetic analysis of nonisothermal decomposition of (Mg5(CO3)4(OH)2·4H2O/5Cr2O3) crystalline mixture. Journal of King Saud University - Science. 2009;21(2):133-143.
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopic characterization and study the effect of gamma irradiation on VO2+, Mn2+, Zn2+, Ru3+, Pd2+, Ag+ and Hg2+ complexes and antibacterial activities. Arab. J. Chem.. 2020;13(2):4425-4447.
- [CrossRef] [Google Scholar]
- The mathematical origins of the kinetic compensation effect: 1. the effect of random experimental errors [10.1039/C1CP22666E] PCCP. 2012;14(1):318-326.
- [CrossRef] [Google Scholar]
- Role of polymeric surfactants on the growth of manganese ferrite nanoparticles. Chem. Eng. J.. 2012;210:157-165.
- [CrossRef] [Google Scholar]
- Influences of spinel type and polymeric surfactants on the size evolution of colloidal magnetic nanocrystals (MFe2O4, M= Fe. Mn). Frontiers of Chemical Science and Engineering. 2014;8(3):378-385.
- [CrossRef] [Google Scholar]
- Influence of experimental parameters on iron oxide nanoparticle properties synthesized by thermal decomposition: size and nuclear magnetic resonance studies. Nanotechnology. 2018;29(16):165603
- [CrossRef] [Google Scholar]
- On the Compensation Effect in Heterogeneous Catalysis. J. Phys. Chem. B. 2003;107(35):9325-9331.
- [CrossRef] [Google Scholar]
- Iron oxide nanoparticles: the Influence of synthesis method and size on composition and magnetic properties. J. Solid State Chem.. 2013;201:144-152.
- [CrossRef] [Google Scholar]
- Preparation of catalytically efficient precious metallic colloids by γ-irradiation and characterization. Colloids Surf A Physicochem Eng Asp. 2005;256(2):165-170.
- [CrossRef] [Google Scholar]
- 1964/01/01) Kinetic Parameters from Thermogravimetric Data. Nature. 1964;201(4914):68-69.
- [CrossRef] [Google Scholar]
- Cornell, R. M., & Schwertmann, U. 2003. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Wiley. https://books.google.com.sa/books?id=dlMuE3_klW4C.
- The Compensation Effect in Heterogeneous Catalysis. In: Frankenburg W.G., Komarewsky V.I., Rideal E.K., eds. Advances in Catalysis. Vol Vol. 7. Academic Press; 1955. p. :75-91.
- [CrossRef] [Google Scholar]
- Magnetite Nanoparticles: Synthesis and Applications in Optics and Nanophotonics. Materials. 2022;15(7):2601.
- [Google Scholar]
- Collation Efficiency of Poly(Vinyl Alcohol) and Alginate Membranes with Iron-Based Magnetic Organic/Inorganic Fillers in Pervaporative Dehydration of Ethanol. Materials. 2020;13(18):4152.
- [Google Scholar]
- Influence of high energy γ-irradiation on some binuclear transition metal complexes of pentadentate ligand: Spectral, thermal, modelling, X-ray diffraction, morphological and solid electrical conductivity. Appl. Radiat. Isot.. 2022;182:110121
- [CrossRef] [Google Scholar]
- Surfactant Effects on the Structural and Magnetic Properties of Iron Oxide Nanoparticles. J. Phys. Chem. C. 2014;118(29):16209-16217.
- [CrossRef] [Google Scholar]
- Gamma-irradiation applied in the synthesis of metallic and organic nanoparticles: A short review. Radiat. Phys. Chem.. 2020;169:107962
- [CrossRef] [Google Scholar]
- Nov-Dec). General Treatment of the Thermogravimetry of Polymers. J Res Natl Bur Stand A Phys Chem. 1966;70a(6):487-523.
- [CrossRef] [Google Scholar]
- Chapter 3 - Kinetic Background to Thermal Analysis and Calorimetry. In: Brown M.E., ed. Handbook of Thermal Analysis and Calorimetry. Vol Vol. 1. Elsevier Science B.V; 1998. p. :147-224.
- [CrossRef] [Google Scholar]
- Solid-state Decompositions — Stagnation or Progress? J. Therm. Anal. Calorim.. 2000;60(3):863-877.
- [CrossRef] [Google Scholar]
- Key Parameters for Scaling up the Synthesis of Magnetite Nanoparticles in Organic Media: Stirring Rate and Growth Kinetic. Ind. Eng. Chem. Res.. 2013;52(50):17841-17847.
- [CrossRef] [Google Scholar]
- Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. J. Control. Release. 2020;320:45-62.
- [CrossRef] [Google Scholar]
- Gamma-irradiation synthesis of iron oxide nanoparticles in the presence of PEO, PVP or CTAB. Radiat. Phys. Chem.. 2016;124:75-83.
- [CrossRef] [Google Scholar]
- A new method for the analysis of non-isothermal DSC and diffraction data. Thermochim Acta. 1997;307(1):27-35.
- [CrossRef] [Google Scholar]
- Role of isoconversional methods in varying activation energies of solid-state kinetics: II. Nonisothermal kinetic studies. Thermochim Acta. 2005;436(1):101-112.
- [CrossRef] [Google Scholar]
- Reaction Kinetics in Differential Thermal Analysis. Anal. Chem.. 1957;29(11):1702-1706.
- [CrossRef] [Google Scholar]
- Temperature selectivity for single phase hydrothermal synthesis of PEG-400 coated magnetite nanoparticles [10.1039/D0DT01318H] Dalton Trans.. 2020;49(25):8672-8683.
- [CrossRef] [Google Scholar]
- Laye, P. G., Warrington, S. B., Group, T. M., Heal, G. R., Price, D. M., Wilson, R., & Haines, P. 2007. Principles of Thermal Analysis and Calorimetry. Royal Society of Chemistry. https://books.google.com.sa/books?id=Rm0oDwAAQBAJ.
- Studies of magnetite nanoparticles synthesized by thermal decomposition of iron (III) acetylacetonate in tri(ethylene glycol) J. Magn. Magn. Mater.. 2009;321(19):3093-3098.
- [CrossRef] [Google Scholar]
- γ-irradiation generated ferrous ions affect the formation of magnetite and feroxyhyte. Radiat. Phys. Chem.. 2020;170:108648
- [CrossRef] [Google Scholar]
- Synthesis of Magnetic Nanocrystals by Thermal Decomposition in Glycol Media: Effect of Process Variables and Mechanistic Study. Ind. Eng. Chem. Res.. 2012;51(25):8348-8357.
- [CrossRef] [Google Scholar]
- Muro-Cruces, J., Roca, A. G., López-Ortega, A., Fantechi, E., del-Pozo-Bueno, D., Estradé, S., Peiró, F., Sepúlveda, B., Pineider, F., Sangregorio, C., & Nogues, J. 2019. Precise Size Control of the Growth of Fe3O4 Nanocubes over a Wide Size Range Using a Rationally Designed One-Pot Synthesis. ACS Nano, 13(7), 7716-7728. https://doi.org/10.1021/acsnano.9b01281.
- Morphological structure details, size distributions and magnetic properties of iron oxide nanoparticles. J. Ind. Eng. Chem.. 2021;95:37-50.
- [CrossRef] [Google Scholar]
- Nguyen, T. K. L., Nguyen, N. D., Dang, V. P., Phan, D. T., Tran, T. H., & Nguyen, Q. H. 2019. Synthesis of Platinum Nanoparticles by Gamma Co-60 Ray Irradiation Method Using Chitosan as Stabilizer. Advances in Materials Science and Engineering, 2019, 9624374. https://doi.org/10.1155/2019/9624374.
- Superparamagnetic MFe2O4 (M = Fe Co, Mn) Nanoparticles: Tuning the Particle Size and Magnetic Properties through a Novel One-Step Coprecipitation Route. Chem. Mater.. 2012;24(8):1496-1504.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B. 2012;177(5):421-427.
- [CrossRef] [Google Scholar]
- Chapter 8 - Controlled Rate Thermal Analysis and Related Techniques. In: Brown M.E., ed. Handbook of Thermal Analysis and Calorimetry. Vol Vol. 1. Elsevier Science B.V; 1998. p. :423-443.
- [CrossRef] [Google Scholar]
- Bimetallic AgPt and AuPt aggregates synthesized by radiolysis. Radiat. Phys. Chem.. 1996;47(2):275-279.
- [CrossRef] [Google Scholar]
- Synthesis of Monodispersed Magnetite Particles From Different Organometallic Precursors. IEEE Trans. Magn.. 2006;42(10):3025-3029.
- [CrossRef] [Google Scholar]
- Design strategies for shape-controlled magnetic iron oxide nanoparticles. Adv. Drug Deliv. Rev.. 2019;138:68-104.
- [CrossRef] [Google Scholar]
- Kinetic analysis of thermal decomposition for penicillin sodium salts: Model-fitting and model-free methods. Journal of Pharmaceutical and Biomedical Analysis. 2002;29(6):1031-1043.
- [CrossRef] [Google Scholar]
- Model-free kinetics analysis of waste PE sample. Thermochim Acta. 2006;451(1):27-33.
- [CrossRef] [Google Scholar]
- Model-free method for isothermal and non-isothermal decomposition kinetics analysis of PET sample. Thermochim Acta. 2006;444(1):46-52.
- [CrossRef] [Google Scholar]
- Learning about epoxy cure mechanisms from isoconversional analysis of DSC data. Thermochim Acta. 2002;388(1):289-298.
- [CrossRef] [Google Scholar]
- Numerical Data for Some Commonly Used Solid State Reaction Equations [https://doi.org/10.1111/j.1151-2916.1966.tb13289.x] J. Am. Ceram. Soc.. 1966;49(7):379-382.
- [CrossRef] [Google Scholar]
- Thermal stability, sublimation pressures and diffusion coefficients of some metal acetylacetonates. Surf. Coat. Technol.. 2007;201(22):9055-9059.
- [CrossRef] [Google Scholar]
- Normal Vibration Calculations for Iron Tris(acetylacetonate) Russ. J. Coord. Chem.. 2003;29(7):484-488.
- [CrossRef] [Google Scholar]
- Investigation of magnetite nanoparticles stability in air by thermal analysis and FTIR spectroscopy. J. Therm. Anal. Calorim.. 2016;125(3):1185-1198.
- [CrossRef] [Google Scholar]
- New complete assignment of X-ray powder diffraction patterns in graphitic carbon nitride using discrete Fourier transform and direct experimental evidence [10.1039/C7CP05242A] PCCP. 2017;19(38):26072-26084.
- [CrossRef] [Google Scholar]
- Analysis of dynamic kinetic data from solid-state reactions. J. Therm. Anal.. 1980;18(2):247-261.
- [CrossRef] [Google Scholar]
- Vibrational assignment of acetylacetone. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2000;56(14):2679-2691.
- [CrossRef] [Google Scholar]
- Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater.. 2009;55(1):22-45.
- [CrossRef] [Google Scholar]
- Torres-Gómez, N., Nava, O., Argueta-Figueroa, L., García-Contreras, R., Baeza-Barrera, A., & Vilchis-Nestor, A. R. 2019. Shape Tuning of Magnetite Nanoparticles Obtained by Hydrothermal Synthesis: Effect of Temperature. Journal of Nanomaterials, 2019, 7921273. https://doi.org/10.1155/2019/7921273.
- Toyos-Rodríguez, C., Calleja-García, J., Torres-Sánchez, L., López, A., Abu-Dief, A. M., Costa, A., Elbaile, L., Crespo, R. D., Garitaonandia, J. S., Lastra, E., García, J. A., & García-Alonso, F. J. 2019. A Simple and Reliable Synthesis of Superparamagnetic Magnetite Nanoparticles by Thermal Decomposition of Fe(acac)<sub>3</sub>. Journal of Nanomaterials, 2019, 2464010. https://doi.org/10.1155/2019/2464010.
- Influence of stirring velocity on the synthesis of magnetite nanoparticles (Fe3O4) by the co-precipitation method. J. Alloy. Compd.. 2009;488(1):227-231.
- [CrossRef] [Google Scholar]
- Chapter 2 - Thermodynamic Background to Thermal Analysis and Calorimetry. In: Brown M.E., ed. Handbook of Thermal Analysis and Calorimetry. Vol Vol. 1. Elsevier Science B.V; 1998. p. :75-145.
- [CrossRef] [Google Scholar]
- Kinetic concepts of thermally stimulated reactions in solids: A view from a historical perspective. Int. Rev. Phys. Chem.. 2000;19(1):45-60.
- [CrossRef] [Google Scholar]
- Isothermal and Nonisothermal Reaction Kinetics in Solids. In Search of Ways toward Consensus. Chem. A Eur. J.. 1997;101(44):8279-8284.
- [CrossRef] [Google Scholar]
- Kinetics of Thermal Decomposition of Cubic Ammonium Perchlorate. Chem. Mater.. 1999;11(11):3386-3393.
- [CrossRef] [Google Scholar]
- Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340–341:53-68.
- [CrossRef] [Google Scholar]
- 09/15/) Infrared Spectra of Ferrites. Physical Review. 1955;99(6):1727-1735.
- [CrossRef] [Google Scholar]
- Investigation of Commercial Iron Oxide Nanoparticles: Structural and Magnetic Property Characterization. ACS. Omega. 2021;6(9):6274-6283.
- [CrossRef] [Google Scholar]
- Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ.. 2012;424:1-10.
- [CrossRef] [Google Scholar]
- Yadav, B., Singh, R., Vishwakarma, A., & Kumar, N. 2020. Facile Synthesis of Substantially Magnetic Hollow Nanospheres of Maghemite (γ-Fe2O3) Originated from Magnetite (Fe3O4) via Solvothermal Method. Journal of Superconductivity and Novel Magnetism, 33. https://doi.org/10.1007/s10948-020-05481-7.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105531.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







