Translate this page into:
Levan polysaccharide from Erwinia herbicola protects osteoblast cells against lipopolysaccharide-triggered inflammation and oxidative stress through regulation of ChemR23 for prevention of osteoporosis
⁎Corresponding author at: No.1, Donggang West Road, Chengguan District, Lanzhou 730000, Gansu Province, China. ldyyjzwwj@163.com (Wenji Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Osteoblast cell injury is a type of degenerative disorder characterized by osteolysis. Levan polysaccharide is an active component of Erwinia herbicola, which shows potential anti-inflammatory and antioxidant properties. However, the protective effects of levan on lipopolysaccharide (LPS)-induced inflammatory and oxidative stress injury in osteoblast cells as an in vitro model of osteolysis remain largely unknown. The present study aimed to explore the functions of levan in LPS-triggered inflammation and oxidative stress in osteoblast cells (MC3T3-E1). The protective effects of levan on LPS-induced inflammatory and oxidative stress responses in MC3T3-E1 cells were assessed by quantification of superoxide dismutase (SOD) and catalase (CAT) activity as well as enzyme-linked immunosorbent assay (ELISA) for determination of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). Also, the key signaling pathway of ChemR23 was determined by qPCR analysis. Results showed that levan significantly alleviated LPS-induced deactivation of SOD and CAT activity. Levan also downregulated the expression of IL-6, TNF-α, and ChemR23 at mRNA level. These findings indicated that levan may protect MC3T3-E1 cells against LPS-triggered oxidative stress, and inflammation via regulation of ChemR23. This data may provide a potential basis for further clinical investigation of levan in the prevention and treatment of LPS-triggered bone resorption and osteolysis.
Keywords
Levan polysaccharide
Osteoblast cells
Inflammation
Oxidative stress
Signaling
1 Introduction
Pathogenic stimuli can irreversibly damage vital molecules such as nucleic acids, proteins, lipids, and lipoproteins and lead to cell dysfunction by induction of oxidative stress, inflammation, and apoptosis (Rani et al., 2016; Redlich and Smolen, 2012; Mollazadeh et al., 2015; Wauquier et al., 2009). It has been indicated that several mechanisms are involved in the deleterious effects of oxidative stress and inflammation on metabolic bone disease. For example, it has been indicated that production of reactive oxygen species (ROS) increases the induction of apoptosis and autophagy in osteoblast (MC3T3-E1) cells (Liu et al., 2018). Also, it has been shown that high glucose and corresponding oxidative stress and inflammation triggers autophagy of MC3T3-E1 cells (Wang et al., 2016). Furthermore, it has been suggested that induced apoptosis in osteoblast cells can be regulated by mitigating mitochondrial dysfunction and ROS-mediated oxidative stress and inflammation (Sun et al., 2019).
Antioxidants are able to protect biological systems against ROS-induced cell injury. Some microbial strains and medicinal plants contain high amounts of antioxidants that can be effective in human health (Swain and Rautray, 2021). Polysaccharides with microbial and plant origin are important and useful compounds with potential biomedical properties (Song et al., 2020; Rjeibi et al., 2020). For example, it has been shown that Lycium barbarum polysaccharide can show protective effects against induced oxidative stress and inflammation in PC12 cells (Gao et al., 2015) and osteoblast cells (MC3T3-E1) by regulating miR-200b-3p/Chrdl1/PPARγ signaling pathway (Jing et al., 2020). Furthermore, it has been indicated that polysaccharides from different sources can show protective effects against oxidative stress-induced cell mortality (Alencar et al., 2019; Olasehinde et al., 2020; Jayawardena et al., 2020).
Levan [a (2 → 6)-β-D-fructan] is produced by a wide range of bacterial strains and plant species which shows several potential physicochemical characteristics including high hydrophilicity, promising adhesiveness, and outstanding biocompatibility (González-Garcinuño et al., 2018). It has been also employed as a drug carrier in the form of nano-formulated structure to promote drug bioavailability (De Siqueira et al., 2020). Levan has been also reported to show antiviral (Gamal et al., 2020), antitumor (Queiroz et al., 2017), and antioxidant functions (Bouallegue et al., 2020).
On the other hand, the chemerin/CMKLR1 signaling pathway has been reported to play a key role in the adipogenesis and osteoblastogenesis of bone marrow stem cells (Muruganandan et al., 2010). It has been also reported that Resolvin E1 and chemokine-like receptor 1 can regulate bone preservation (Gao et al., 2013) under inflammatory conditions (El Kholy et al., 2018).
Although the biological activity of levan against some diseases have been reported, the exact mechanism by which levan induces its protective effects against expression of inflammatory mediators and oxidative stress through ChemR23 has not been fully explored. Therefore, the main objective and novelty of this study was to examine the protective effects of levan polysaccharide against lipopolysaccharide (LPS)-induced inflammation as a model and delineate its mechanism of action.
2 Materials and methods
2.1 Materials
Levan from Erwinia herbicola, MTT [3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide] and lipopolysaccharide (LPS) were obtained from Sigma-Aldrich (Shanghai, China). All cellular culture materials were purchased from Gibco Co. (Shanghai, China).
2.2 Methods
2.2.1 Cell culture and treatment
The osteoblast-like cells, MC3T3-E1, obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China), were cultured in the complete medium of α-MEM containing 10% FBS in a humidified incubator with 5% CO2 at 37 °C and 1% antibiotics. After reaching 70–0% confluency, the cells were cultured in differentiation medium (α-MEM) supplemented with 10 β-glycerol phosphate (mmol/L), L-ascorbic acid (50 mg/L), and dexamethasone (10 mmol/L) either alone or with different concentrations of LPS (0, 2, 5, 10, 20 and 50 μg/mL) or levan (0, 10, 50, 100, 500 and 1000 μg/mL) or left untreated (control) as the negative control for 72 h.
2.2.2 Cell viability assay
The viability of MC3T3-E1 cells was examined by the MTT assay. Briefly, after treating the cells with LPS or levan (72 h), followed by removal of the culture median, MTT was added and the cells were incubated for another 4 h. The absorbance was then read at 570 nm after adding DMSO for 5 min employing a Microplate Reader (Bio-Rad, USA).
2.2.3 Superoxide dismutase (SOD) and catalase (CAT) activity assays
After treatment and homogenization of the MC3T3-E1 cells, a fixed protein concentration was collected and the relative caspase-3, caspase-9, SOD, and CAT assays were carried out by relevant assay kits (Abcam, Shanghai, China) using a Microplate Reader (Bio-Rad, USA).
2.2.4 Enzyme-linked immunosorbent assay (ELISA) for determination of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α)
After treatment and homogenization of the MC3T3-E1 cells, a fixed protein concentration was collected and the relative quantities of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were determined by ELISA kit (Abcam, Shanghai, China).
2.2.5 Quantitative polymerase chain reaction (qPCR)
After treatment of the cells, the total RNA was extracted (TRIzol, Invitrogen), followed by doing reverse transcription (Transcriptor First Strand cDNA Synthesis Kit, Roche, USA). qPCR was then done with a One Step SYBR® master mix Kit (TaKaRa). The relative expression was then reported by using the 2−ΔΔCt method. The primers sequences are summarized in Table 1.
Primer
Forward
Reverse
ChemR23
5′-ATGGAGTACGACGCTTACAACG-3′
5′-GGTGGCGATGACAATCACCA-3′
TNF-α
5′-CGAGTCTGGGCAGGTCTACTTT-3′
5′-AAGCTGTAGGCCCCAGTGAGTT-3′
IL-6
5′-TGATGGATGCTTCCAAACTG-3′
5′-GAGCATTGGAAGTTGGGGTA5′-
β-actin
5′-TCCTCCTGAGCGCAAGTAC-3′
5′-CCTGCTTGCTGATCCACATCT-3′
2.3 Statistical analysis
The data were presented as the mean ± standard deviation (SD) of five experiments and P-values were calculated with one-way analysis of variance (ANOVA) using SPSS software.
3 Results
3.1 MTT assay
The alterations in the cell viability on treatment with LPS and levan in MC3T3-E1 cells at various concentrations at 72 h are shown in Fig. 1a and Fig. 1b, respectively. The cell viability was enhanced after treatment with LPS concentrations from 0 to 10 µg/mL, revealing a peak at 10 µg/mL (Fig. 1a). The effect of LPS on the cell viability was reduced at concentrations over 10 µg/mL (Fig. 1a). As the maximum value of the cell viability for LPS against MC3T3-E1 cells was detected at 10 µg/mL, this concentration was chosen to be the potential LPS concentration for MC3T3-E1 cells (Fig. 1a). It was also found that the cell viability increased as the levan concentration was increased, demonstrating a peak and reducing after 50 µg/mL at 72 h (Fig. 1b). In fact, the enhancement in the cell viability mitigated at levan concentrations higher than 50 μg/mL at 72 h, a levan concentration of 50 μg/mL was chosen for studying its protective effects against LPS-triggered inflammation and oxidative stress in MC3T3-E1 cells.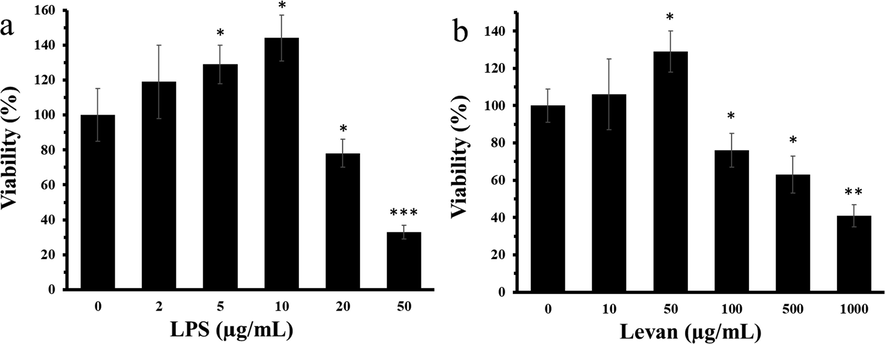
(a) The exploration of viability of MC3T3-E1 cells with increasing concentrations of lipopolysaccharide (LPS) for 72 h. (b) The exploration of viability of cells with increasing concentrations of levan for 72 h. Data are as the mean ± SD, (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001 relative to control (0) group.
3.2 SOD and CAT activity assay
The SOD and CAT activity was assessed to explore the oxidative stress stimulated by LPS and the antioxidant properties of levan. The data indicated that levan can significantly increase the SOD (Fig. 2a) and CAT (Fig. 2b) activity in MC3T3-E1 cells (P < 0.05) at 72 h. Afterwards it was observed that incubation of cells with LPS resulted in significant reduction of both SOD (Fig. 2a) and CAT (Fig. 2b) activity in MC3T3-E1 cells (***P < 0.001), whereas treatment of the cells with levan resulted in the significant recovery of SOD and CAT activity at 72 h.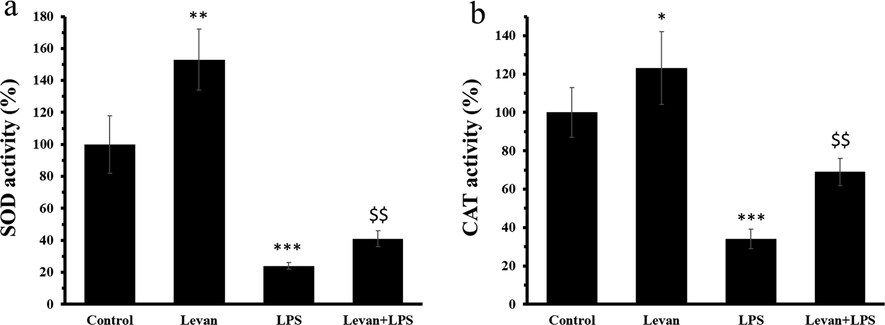
(a) The investigation of SOD activity in MC3T3-E1 cells. (b) The investigation of CAT activity in MC3T3-E1 cells. The cells were incubated either with levan (50 µg/mL) for 72 h or LPS (10 µg/mL) for 72 h or treated with levan and LPS for 72 h. Data are as the mean ± SD, (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001 relative to control group, $$P < 0.01 relative to LPS-treated group.
3.3 IL-6 and TNF-α protein level assay
The protein levels of IL-6 and TNF-α were assessed to examine the inflammatory response triggered by LPS and the anti-inflammatory effects of levan. The results displayed that incubation of cells with LPS caused a profound enhancement in protein levels of IL-6 (Fig. 3a) and TNF-α (Fig. 3b) in MC3T3-E1 cells (***P < 0.001), whereas treatment of the cells with levan caused the significant reduction of IL-6 and TNF-α at protein level at 72 h.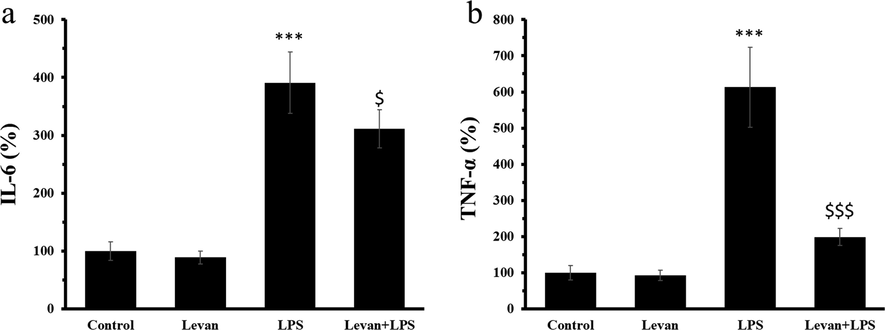
(a) The protein level of IL-6 in MC3T3-E1 cells. (b) The protein level of TNF-α in MC3T3-E1 cells. The cells were incubated either with levan (50 µg/mL) for 72 h or LPS (10 µg/mL) for 72 h or treated with levan and LPS for 72 h. Data are as the mean ± SD, (n = 5). ***P < 0.001 relative to control group, $P < 0.05, $$$P < 0.001 relative to LPS-treated group.
3.4 qPCR assay
The mRNA expression levels of IL-16, TNF-α, and ChemR23 were assessed in MC3T3-E1 cells at 72 h. It was seen that incubation of cells with LPS for 72 h led to a significant increase in the mRNA expression levels of IL-16 (Fig. 4a), TNF-α (Fig. 4b), and ChemR23 (Fig. 4c), whereas the treatment of the cells with levan mitigated the LPS-induced upregulation of these genes at 72 h.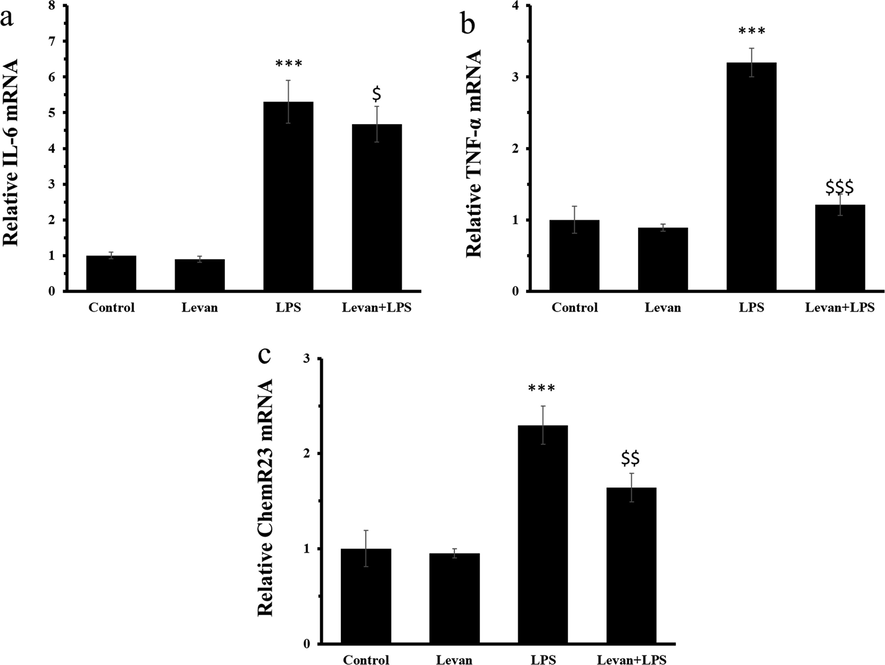
(a) The mRNA expression of IL-6 mRNA. (b) The mRNA expression level of TNF-α. (c) The mRNA expression level of ChemR23. The cells were incubated either with levan (50 µg/mL) for 72 h or LPS (10 µg/mL) for 72 h or treated with levan and LPS for 72 h. Data are as the mean ± SD, (n = 5). ***P < 0.001 relative to control group, $P < 0.05, $$P < 0.01, ###P < 0.001 relative to LPS-treated group.
4 Discussion
In the present study, we revealed that levan mitigates LPS-stimulated inflammation and oxidative stress in osteoblast cells, as evidenced by decrease in the levels of pro-inflammatory cytokines and increase in the SOD and CAT activity. We also found that the ChemR23 expression at mRNA was enhanced after incubation of osteoblast cells with LPS which was mitigated by levan, suggesting that levan might trigger a protective impact by interacting with this receptor. In summary, these data indicated that levan can be a potential polysaccharide that exerts protective effects against LPS-induced osteoblast cells injury by preventing inflammatory feedback and increasing the rate of SOD and CAT in osteoblast cells.
It has been reported that inflammation and oxidative stress result in bone loss (Braun and Schett, 2012). LPS has been used as a potential agent to induce oxidative stress and inflammation in osteoblast cells in vitro (Guo et al., 2014; Guo et al., 2015)
Levan has been shown to have potential antioxidant and anti-inflammatory activities (Abdel-Fattah et al., 2012; Srikanth et al., 2015).
Therefore, it was highly recommended to reveal the functional activities of levan in osteoblast cell injury.
Inflammatory cytokines lead to the inflammatory response against different stimuli. The levels of pro-inflammatory cytokines have been reported to be increased in the osteoblast cells in response to LPS (Guo et al., 2014; He et al., 2018). The inflammatory cytokines are believed to trigger bone resorption. Additionally, the neutralization of the cytokines resulted in the inhibition of the inflammatory feedback (Zhang et al., 2020). In the present study, incubation of osteoblast cells with LPS resulted in an increased mRNA and protein levels of IL-6 and TNF-α accompanied by a significant reduction in SOD and CAT activity, which were regulated by levan treatment. Therefore, levan may protect osteoblast cells from inflammatory injury by suppressing oxidative stress.
In fact, osteoblast cell inflammation is a crucial factor during osteolysis, and prevention of inflammation can play a key role in the bone resorption (Guo et al., 2018). We found that levan treatment protected osteoblast cells against inflammation through mitigation of the expression of pro-inflammatory mediators. Indeed, we used ELISA and qPCR analyses to explore LPS-induced inflammation in osteoblast cells. Our data showed that the LPS-induced expression of inflammatory cytokines was decreased by levan treatment. These data indicated that levan significantly protects osteoblast cells against LPS-induced bone destruction and osteolysis.
In addition, the overexpression of the ChemR23 by LPS was inhibited in the presence of levan, which contributed to the protective influence of levan against osteoblast cell injury. It has been well-documented that osteoblast cells express ChemR23 receptor (Zhao et al., 2021; Muruganandan et al., 2010; Gao et al., 2013; El Kholy et al., 2018).
It has been also shown that some bioactive materials, including levan in the form of organic thin films can regulate the osteoblast cell signaling response which can be extended to study potential bioactive interfaces for tissue engineering (Axente et al., 2014).
Our data that levan can decrease the ChemR23 at mRNA level, assessed under inflammatory condition is, to the best of our knowledge, a novel outcome.
5 Conclusion
In conclusion, our results showed that levan protects the osteoblast cells from inflammation and oxidative stress induced by LPS. The mechanism was probably associated with the inhibition of ChemR23 and TNF-α/IL-6 inflammatory signaling pathways and modulation of oxidative stress. These outcomes suggested that levan might be a promising lipid mediator for the treatment of osteolysis. This study was the beginning of the process to determine the efficacy of levan to treat osteoporosis and in the future studies, the potential application of levan in vivo and to treat people with osteoporosis can be further explored in detail.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci.. 2016;1(148):183-193.
- [Google Scholar]
- Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Discovery. 2012;11(3):234-250.
- [Google Scholar]
- Role of apoptosis in pathogenesis and treatment of bone-related diseases. J. Orthop. Surg. Res.. 2015;10(1):1-7.
- [Google Scholar]
- Oxidative stress in bone remodelling and disease. Trends Mol. Med.. 2009;15(10):468-477.
- [Google Scholar]
- Dexamethasone-induced production of reactive oxygen species promotes apoptosis via endoplasmic reticulum stress and autophagy in MC3T3-E1 cells. Int. J. Mol. Med.. 2018;41(4):2028-2036.
- [Google Scholar]
- High glucose induces autophagy of MC3T3-E1 cells via ROS-AKT-mTOR axis. Mol. Cell. Endocrinol.. 2016;5(429):62-72.
- [Google Scholar]
- Selenium-containing protein from selenium-enriched Spirulina platensis attenuates cisplatin-induced apoptosis in MC3T3-E1 mouse preosteoblast by inhibiting mitochondrial dysfunction and ROS-mediated oxidative damage. Front. Physiol.. 2019;9(9):1907.
- [Google Scholar]
- Estimation of trace elements, antioxidants, and antibacterial agents of regularly consumed Indian medicinal plants. Biol. Trace Elem. Res.. 2021;199(3):1185-1193.
- [Google Scholar]
- Antioxidant, anti-inflammatory and renoprotective effects of acidic-hydrolytic polysaccharides by spent mushroom compost (Lentinula edodes) on LPS-induced kidney injury. Int. J. Biol. Macromol.. 2020;15(151):1267-1276.
- [Google Scholar]
- Novel antioxidant, anti-α-amylase, anti-inflammatory and antinociceptive water-soluble polysaccharides from the aerial part of Nitraria retusa. Foods.. 2020;9(1):28.
- [Google Scholar]
- Protective effects of Lycium barbarum polysaccharide on 6-OHDA-induced apoptosis in PC12 cells through the ROS-NO pathway. Molecules. 2015;20(1):293-308.
- [Google Scholar]
- Lycium barbarum polysaccharide (LBP) inhibits palmitic acid (PA)-induced MC3T3-E1 cell apoptosis by regulating miR-200b-3p/Chrdl1/PPARγ. Food Nutrition Res.. 2020;64
- [Google Scholar]
- A novel antioxidant sulfated polysaccharide from the algae Gracilaria caudata: In vitro and in vivo activities. Food Hydrocolloids. 2019;1(90):28-34.
- [Google Scholar]
- Sulfated polysaccharides of some seaweeds exhibit neuroprotection via mitigation of oxidative stress, cholinergic dysfunction and inhibition of Zn–induced neuronal damage in HT-22 cells. BMC Complementary Med. Therap.. 2020;20(1):1.
- [Google Scholar]
- Antioxidant Potential of Sulfated Polysaccharides from Padina boryana; Protective Effect against Oxidative Stress in In Vitro and In Vivo Zebrafish Model. Mar. Drugs. 2020;18(4):212.
- [Google Scholar]
- Levan and levansucrases: polymer, enzyme, micro-organisms and biomedical applications. Biocatal. Biotransform.. 2018;36(3):233-244.
- [Google Scholar]
- Levan-based nanostructured systems: An overview. Int. J. Pharm.. 2020;30(580):119242
- [Google Scholar]
- Evaluation of the antivirus activity of Enterococcus faecalis Esawy levan and its sulfated form. Biocatal. Agri. Biotechnol.. 2020;1(28):101735
- [Google Scholar]
- Levan promotes antiproliferative and pro-apoptotic effects in MCF-7 breast cancer cells mediated by oxidative stress. Int. J. Biol. Macromol.. 2017;1(102):565-570.
- [Google Scholar]
- Levan from a new isolated Bacillus subtilis AF17: Purification, structural analysis and antioxidant activities. Int. J. Biol. Macromol.. 2020;1(144):316-324.
- [Google Scholar]
- Role of chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J. Bone Miner. Res.. 2010;25(2):222-234.
- [Google Scholar]
- Resolvin E1 and chemokine-like receptor 1 mediate bone preservation. J. Immunol.. 2013;190(2):689-694.
- [Google Scholar]
- Resolvin E1 promotes bone preservation under inflammatory conditions. Front. Immunol.. 2018;12(9):1300.
- [Google Scholar]
- Pathways for bone loss in inflammatory disease. Current Osteoporosis Rep.. 2012;10(2):101-108.
- [Google Scholar]
- Lipopolysaccharide (LPS) induces the apoptosis and inhibits osteoblast differentiation through JNK pathway in MC3T3-E1 cells. Inflammation.. 2014;37(2):621-631.
- [Google Scholar]
- SP600125 reduces lipopolysaccharide-induced apoptosis and restores the early-stage differentiation of osteoblasts inhibited by LPS through the MAPK pathway in MC3T3-E1 cells. Int. J. Mol. Med.. 2015;35(5):1427-1434.
- [Google Scholar]
- Antitumor and antioxidant activities of levan and its derivative from the isolate Bacillus subtilis NRC1aza. Carbohydr. Polym.. 2012;89(2):314-322.
- [Google Scholar]
- Antioxidant and anti-inflammatory levan produced from Acetobacter xylinum NCIM2526 and its statistical optimization. Carbohydr. Polym.. 2015;5(123):8-16.
- [Google Scholar]
- Monotropein attenuates ovariectomy and LPS-induced bone loss in mice and decreases inflammatory impairment on osteoblast through blocking activation of NF-κB pathway. Chem. Biol. Interact.. 2018;1(291):128-136.
- [Google Scholar]
- MMP9 protects against LPS-induced inflammation in osteoblasts. Innate Immunity.. 2020;26(4):259-269.
- [Google Scholar]
- N-acetyl cysteine inhibits lipopolysaccharide-mediated induction of interleukin-6 synthesis in MC3T3-E1 cells through the NF-kB signaling pathway. Arch. Oral Biol.. 2018;1(93):149-154.
- [Google Scholar]
- Chemerin/ChemR23 signaling mediates the effects of ultra-high molecular weight polyethylene wear particles on the balance between osteoblast and osteoclast differentiation. Ann. Transl. Med.. 2021;9(14)
- [Google Scholar]
- Combinatorial MAPLE gradient thin film assemblies signalling to human osteoblasts. Biofabrication.. 2014;6(3):035010
- [Google Scholar]







