Translate this page into:
Lycopodium japonicum: A comprehensive review on its phytochemicals and biological activities
⁎Corresponding author. ygchen48@126.com (Yegao Chen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Lycopodium japonicum Thunb (Lycopodiaceae) is a common and abundant plant widely distributed in China, Japan and countries of Southern Asia and used in traditional Chinese medicine for the treatment of sprains, strains and myasthenia. This review focuses on the phytochemicals and biological actions, with the objective of stimulating further studies on the plant. 132 chemical compounds have been identified and isolated from this plant, and the most important are alkaloids and serratane triterpenoids. The isolated compounds of L. japonicum were shown to possess acetylcholinesterase inhibitory, cytotoxic, anti-inflammatory, anti-HIV-1 and α-glucosidase inhibitory activities. Further studies should be carried out on this plant in order to disclose many more active principles and mechanisms of active components.
Keywords
Lycopodium japonicum
Phytochemicals
Alkaloids
Serratane triterpenoids
Bioactivity
1 Introduction
Lycopodium alkaloids are compounds isolated from plants of Lycopodiaceae and Huperziaceae, with diverse structures including many unusual skeletons of interest from biogenetic and biological points of view, and challenging targets for total synthesis (Hartrampf et al., 2017; Pinto et al., 2017; Saborit et al., 2016; Wang et al., 2017). Alkaloids are known to possess important bioactivities including acetylcholinesterase inhibitory activity (Benamar et al, 2016, 2017; Les et al, 2017). Since huperzine A, a potent, reversible and selective acetylcholinesterase inhibitor and a promising drug for the treatment of symptoms of Alzheimer’s disease was discovered from Huperzia serrata (Thunb. Ex Murray) Trev. (Huperziaceae), numerous efforts on the isolation of new potent alkaloids from H. serrata and related plants have been carried out by many research groups, which led to the isolation of a series of plant constituents, especially Lycopodium alkaloids with diverse structures (Hirasawa et al., 2018; Li et al., 2017; Nakayama et al., 2019; Tang et al., 2016). Lycopodium japonicum Thunb (Lycopodiaceae) is a common and abundant plant widely distributed in China, Japan and countries of Southern Asia and used in traditional Chinese medicine for the treatment of sprains, strains and myasthenia (Zhang & Zhang, 2004). In the last decade, there has been a dramatic progress in the chemical constituents and these compounds show potent bioactivities, such as acetylcholinesterase inhibitory, cytotoxic and anti-inflammatory activities. However, so far, no comprehensive review has been published. In the present review, we summarize systematically the research advances on the chemical constituents and their biological activities of L. japonicum reported in the literature, with the aim of providing a basis for further research of natural product drug discovery.
2 Chemical constituents
To date, 132 compounds have been isolated and identified from the club moss of L. japonicum, including 83 alkaloids (1–83), 36 triterpenoids (87–122), two diterpenoids (85, 86), one sesquiterpenoid (84), two sterols (123, 124), four flavans (125–128), two diaryl propanes (129, 130), one anthraquinone (1 3 1) and one phthalate (1 3 2). As it can be seen, alkaloids and serratane-type triterpenoids are the dominant chemical constituents in the plant L. japonicum. Their structures, names, and references are summarized in Tables 1–4 and Figs. 1–7.
No.
Name
Ref.
1
lycopodine
He et al. (2009)
2
lycodoline
Sun et al. (2008)
3
clavolonine
Li et al. (2012)
4
8β-acetoxy-12β-hydroxylycopodine
Li et al. (2012)
5
8β-hydroxylycodoline
Wang et al. (2013a)
6
12β-hydroxyacetylfawcettiine = acetyllycofawcine
Li et al. (2012)
7
acetylfawcettiine
Li et al. (2012)
8
lycofawcine
Wang et al. (2013a)
9
fawcettine
Zhu et al. (2019)
10
α-lofoline
Li et al. (2012)
11
deacetylfawcettiine
Wang et al. (2013a)
12
11α-O-acetyllycopodine
Liu and Wang (2012)
13
lycoposerramine M
Li et al. (2012)
14
8β-acetoxy-11α-hydroxylycopodine
Li et al. (2012)
15
8β-hydroxy-11α-acetoxylycopodine
Wang et al. (2013a)
16
11α-hydroxyacetylfawcettine
Wang et al. (2013a)
17
lycoclavine
He et al. (2014)
18
lycoposerramine L
Liu and Wang (2012)
19
6α-hydroxylycopodine
He et al. (2014)
20
6-epi-8β-acetoxylycoclavine
He et al. (2014)
21
serratezomine C
He et al. (2014)
22
6α,8β-dihydroxylycopodine
Wang et al. (2013a)
23
4α,8β-dihydroxylycopodine
Wang et al. (2013a)
24
4α,8β,12β-trihydroxylycopodine
Wang et al. (2013a)
25
lycoposerramine G
Wang et al. (2013a)
26
12-epilycodoline
Ge et al. (2016)
27
11β-hydroxy-12-epilycodoline
Shi & He, 2012
28
4α-hydroxyanhydrolycodoline
He et al. (2014)
29
4α,6α-dihydroxyanhydrolycodoline
He et al. (2014)
30
8β-hydroxylycoposerramine K
Wang et al. (2013a)
31
anhydrolycodoline
Wang et al. (2013a)
32
lycoposerramine K
He et al. (2014)
33
gnidioidine
Niu et al. (2015)
34
lucidioline
Sun et al. (2008)
35
diacetyllycofoline
Zhu et al. (2019)
36
flabelline
Niu et al. (2015)
37
12-deoxyhuperzine O
He et al. (2014)
38
8β-hydroxyhuperzine E
Wang et al. (2013a)
39
huperzine E
He et al. (2014)
40
fawcettine N-oxide
Zhu et al. (2019)
41
lycoposerramine F = miyoshianine A
Sun et al. (2008)
42
miyoshianine C
Sun et al. (2008)
43
acetylfawcettine N-oxide
Zhu et al. (2019)
44
12β-hydroxy-acetylfawcettiine N-oxide
Yang et al. (2016)
45
Lycoposerramine M N-oxide
Niu et al. (2015)
46
diphaladine A
He et al. (2014)
47
12-epilycodoline N-oxide
He et al. (2014)
48
lycoposerramine G nitrate
He et al. (2014)
No.
Name
Ref.
49
lycodine
Wang et al. (2013a)
50
N-methyllycodine
Wu et al. (2015)
51
hydroxypropyllycodine
Niu et al. (2015)
52
N-methylhydroxypropyllycodine
Wu et al. (2015)
53
α-obscurine
Sun et al. (2008)
54
des-N-methyl-α-obscurine
Li et al. (2012)
55
des-N-methyl-β-obscurine
Niu et al. (2015)
56
β-obscurine
Wu et al. (2015)
57
huperzinine
Wu et al. (2015)
No.
Name
Ref.
Fawcettimine
58
fawcettimine
He et al. (2009)
59
lycopoclavamine A
Li et al. (2012)
60
fawcettidine
Niu et al. (2015)
61
phlegmariurine B
Zhu et al. (2019)
62
lycojapodine A
He et al. (2009)
63
(15R)-14,15-dihydroepilobscurinol
Wang et al. (2013b)
64
obscurinine
Li et al. (2012)
65
isoobscurinine
Zhu et al. (2019)
66
6-hydroxyl-6,7-dehydro-8-deoxy-13-dehydroserratinine
Wang et al. (2013b)
67
8-deoxy-13-dehydroserratinine
Wang et al. (2013b)
68
15-epi-6-hydroxy-6,7-dehydro-8-deoxy-13-dehydroserratinine
Zhu et al. (2019)
69
lycoflexine
He et al. (2009)
70
17α-methyllycoflexine
Yang et al. (2018)
71
6-hydroxyl-6,7-dehydrolycoflexine
Wang et al. (2013b)
72
14,15-dehydrolycoflexine
Wang et al. (2013b)
73
lycojaponicumin E = palcernine A
Wang et al. (2012a), Yang et al. (2018)
74
lycoflexine N-oxide
Niu et al. (2015)
75
palhinine A
Wang et al. (2013b)
76
palhinine D
Wang et al. (2013a), Wang et al. (2016)
77
lycojaponicumin D
Wang et al. (2012a)
78
lycojaponicumin A
Wang et al. (2012b)
79
lycojaponicumin B
Wang et al. (2012b)
80
lycojaponicumin C
Wang et al. (2012b)
81
isopalhinine A
Yang et al. (2018)
82
lycopladine H
Yang et al. (2018)
Phlegmarine
83
lycocernuine
Yang et al. (2018)
No.
Name
Ref.
Serratene
87
serrat-14-en-3β-yl-acetate
Wang et al. (2014)
88
serrat-14-ene-3β,21β-diol
Shi et al. (2012)
89
21β-hydroxyserrat-14-en-3β-yl-acetate
Wang et al. (2014)
90
serratenediol
Shi et al. (2012)
91
3β-hydroxyserrat-14-en-21β-yl-formate
Wang et al. (2014)
92
21β-hydroxyserrat-14-en-3β-yl-formate
Wang et al. (2014)
93
diepiserratenediol
Ge et al. (2016)
94
serrate-14-en-3,21-dione
Wang et al. (2014)
95
3-epilycoclavanol
Yan et al. (2005a)
96
lycernuic acid A
Zhang et al. (2014)
97
lycojaponicuminol D
Zhang et al. (2014)
98
lycojaponicuminol E
Zhang et al. (2014)
99
phlegmaric acid
Zhang et al. (2014)
100
lycoclavanol
Yan et al. (2005a)
101
lycojaponicuminol A
Zhang et al. (2014)
102
japonicumin A
Li et al. (2006)
103
lycoclaninol
Yan et al. (2005a)
104
lycojaponicuminol F
Zhang et al. (2014)
105
16-oxo-3α-hydroxyserrat-14-en-21β-ol
Li et al. (2015)
106
3β, 21α-dihydroxyserrat-14-en-16-one
Yang et al. (2014)
107
lycernuic ketone C
Sun et al. (2017)
108
16-oxo-3α-hydroxyserrat-14-en-21α-ol
Shi et al. (2012)
109
3α,21α-dihydroxy-16-oxoserrat-14-en-24-yl p-coumarate
Sun et al. (2017)
110
japonicumin B
Li et al. (2006)
111
tohogenol
Sun et al. (2017)
112
japonicumin C
Li et al. (2006)
113
lycopodiin A
Yan et al. (2005a)
Onocerin
114
α-onocerin
Shi et al. (2012)
115
α-onoceradienedione
Wang et al. (2014)
116
26-nor-8-oxo-α-onocerin
Zhang et al. (2014)
117
lycojaponicuminol B
Zhang et al. (2014)
118
(3β,8β,14α,21α)-26,27-dinoronocerane-3,8,14,21-tetrol
Yan et al. (2005a)
119
(3β,8β,14α,21β)-26,27-dinoronocerane-3,8,14,21-tetrol
Yan et al. (2005a)
120
(3α,8β,14α,21β)-26,27-dinoronococerane-3,8,14,21-tetrol
Zhang et al. (2014)
121
lycojaponicuminol C
Zhang et al. (2014)
Lupane
122
betulin
Li et al. (2015)
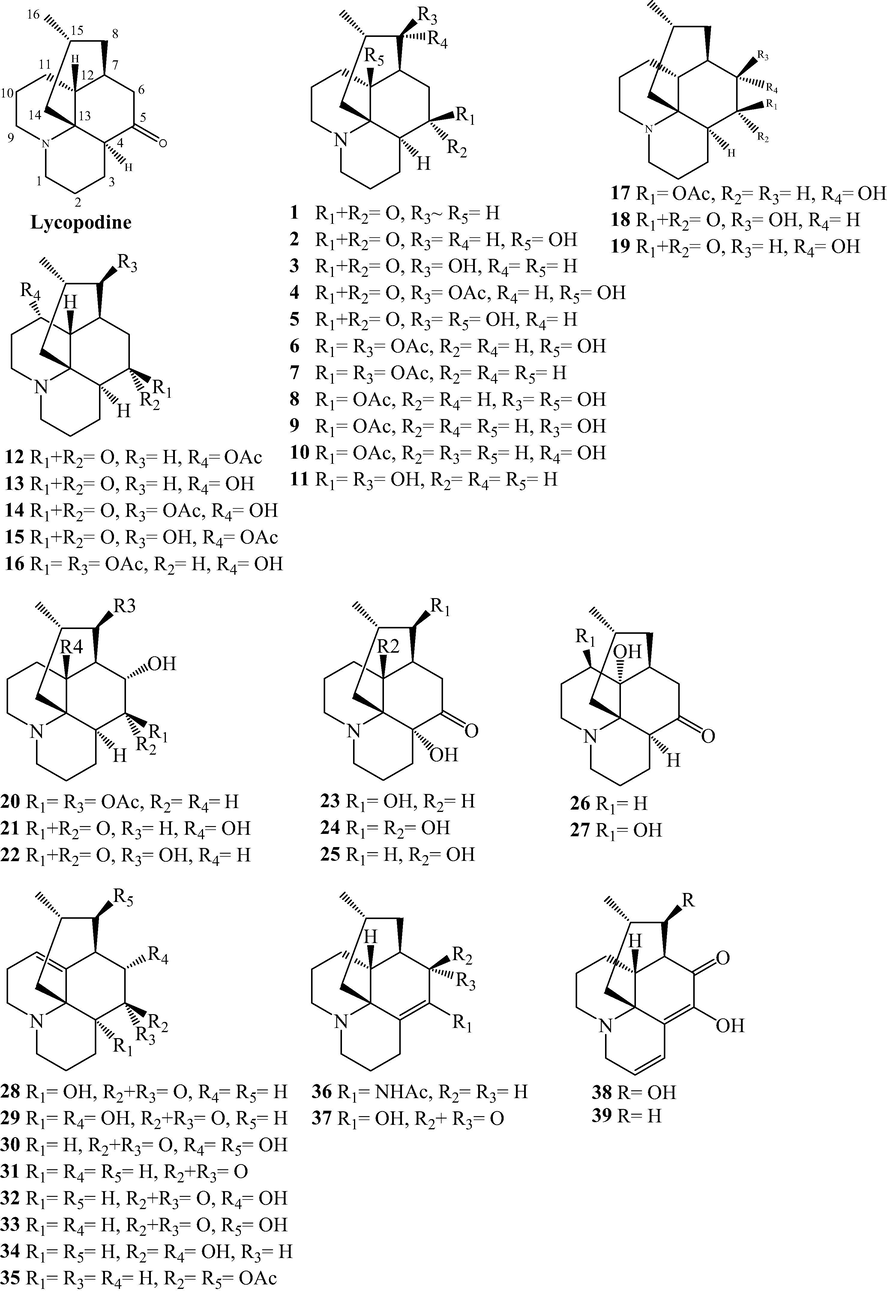
Lycopodine alkaloids from L. japonicum.
Lycopodine alkaloids from L. japonicum.
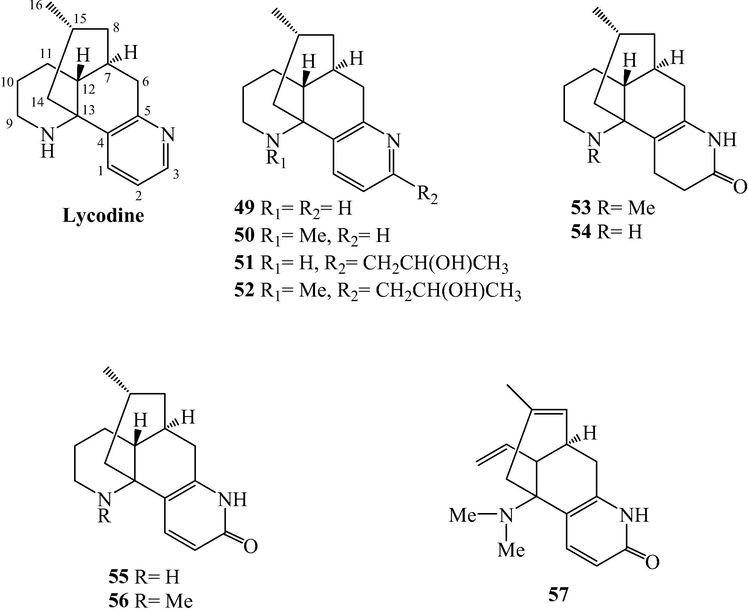
Lycodine alkaloids from L. japonicum.
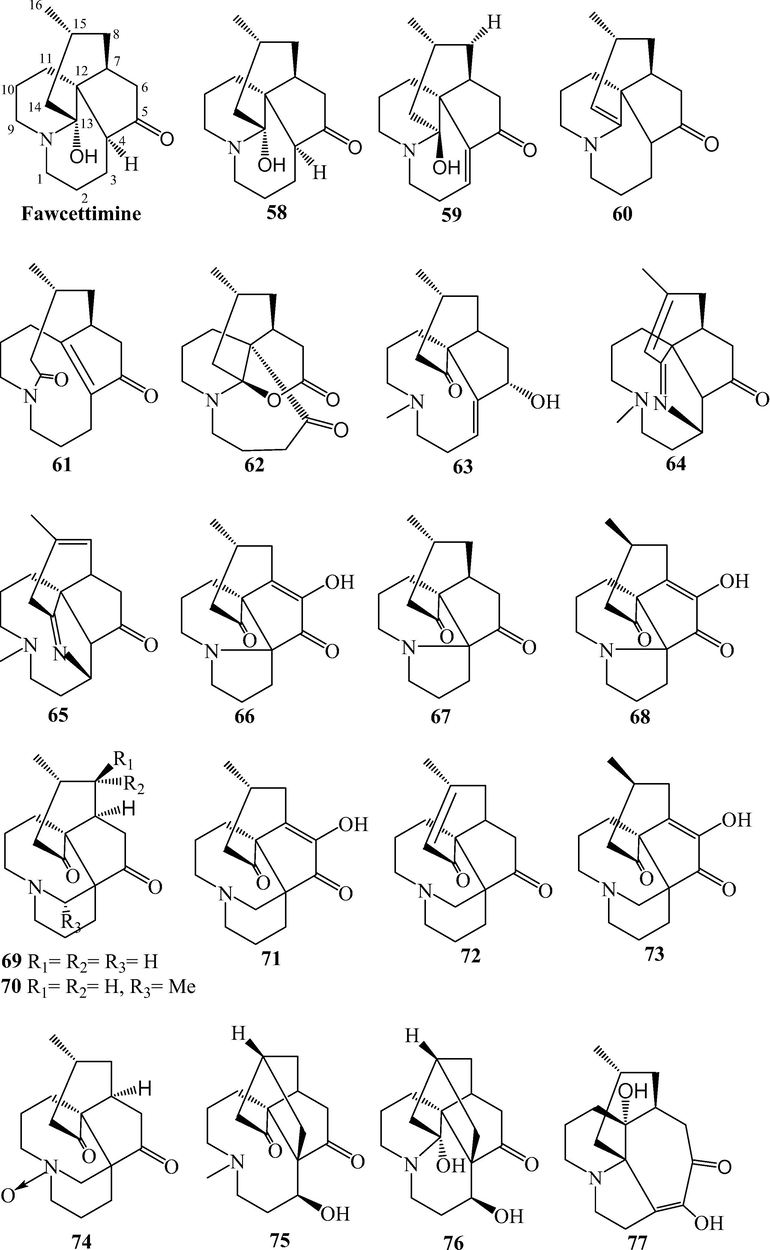
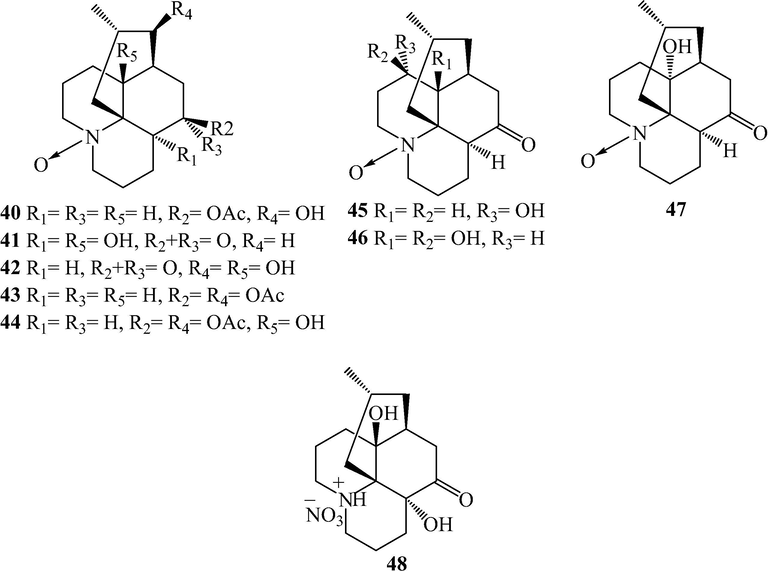
Fawcettimine and phlegmarine alkaloids from L. japonicum.
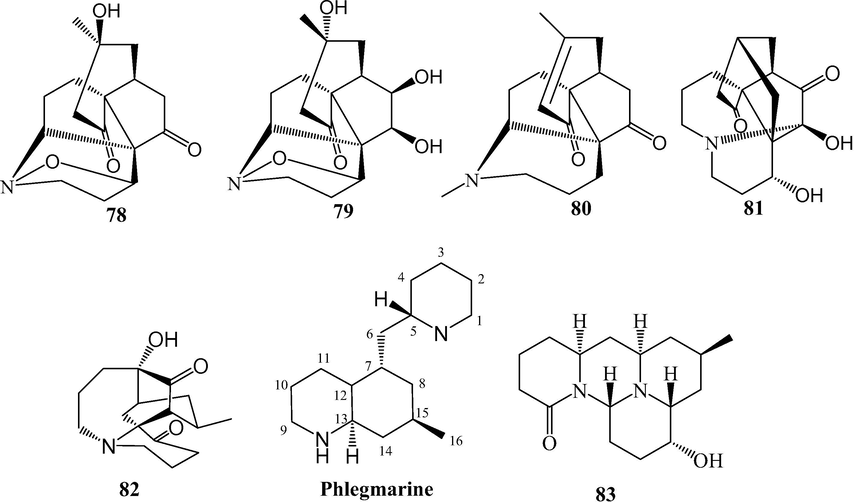
Fawcettimine and phlegmarine alkaloids from L. japonicum.
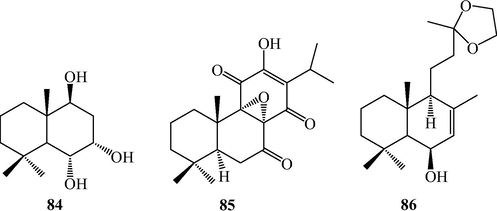
Sesquiterpenoid and diterpenoids from L. japonicum.
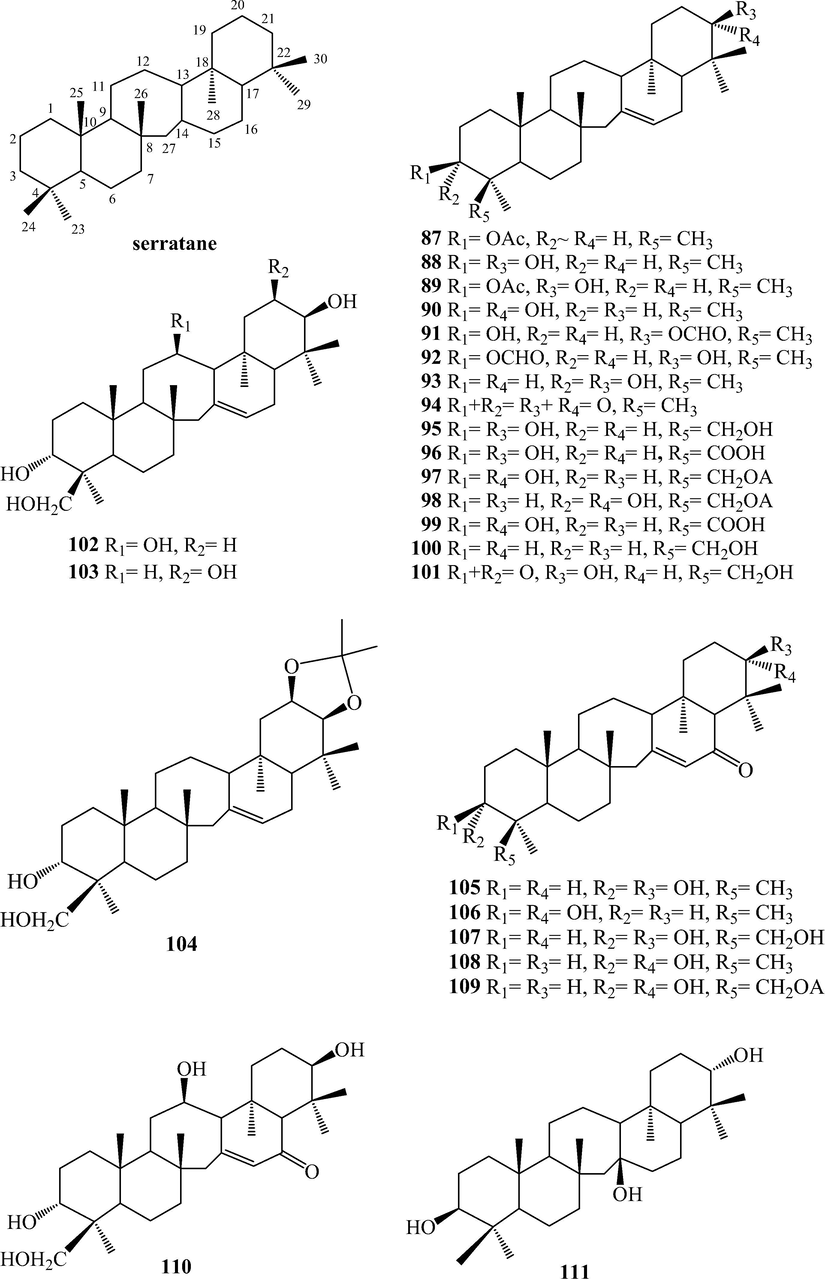
Triterpenoids from L. japonicum.
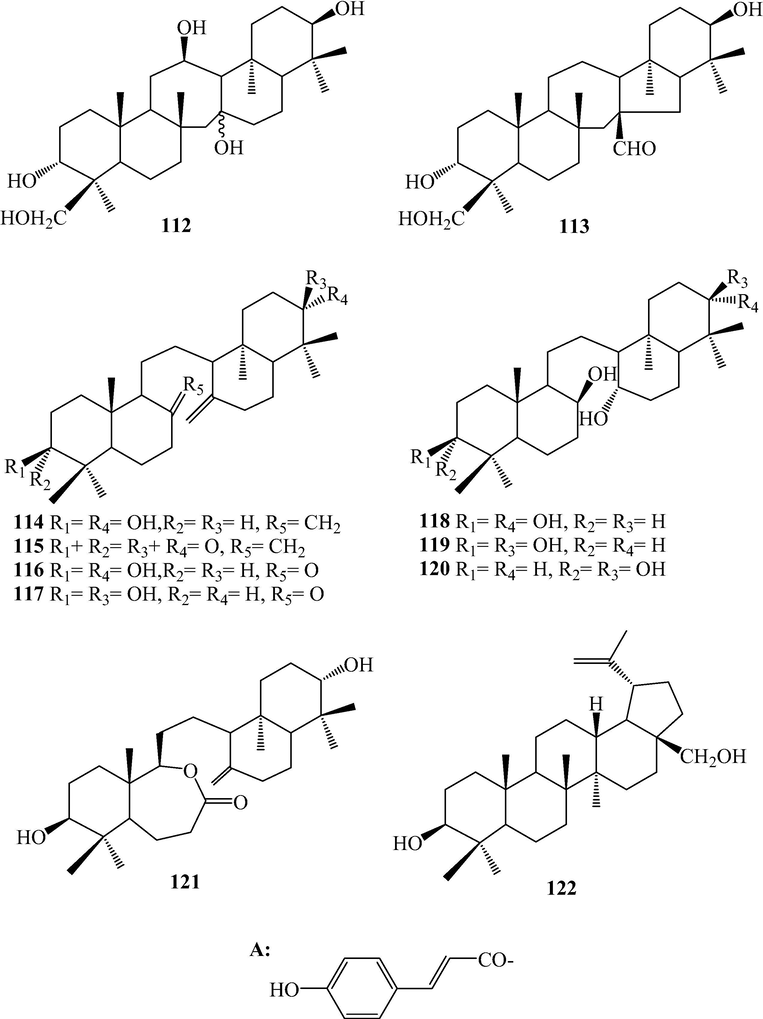
Triterpenoids from L. japonicum.
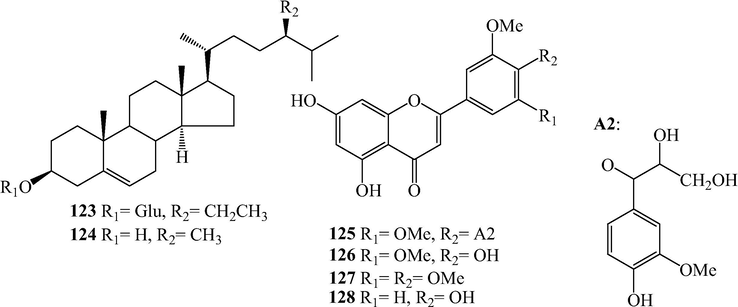
Sterols and flavones from L. japonicum.
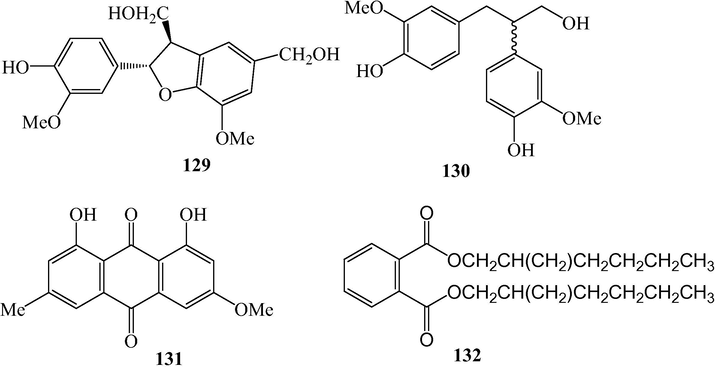
Diaryl propanes and other compounds from L. japonicum.
2.1 Alkaloids
Alkaloids are naturally occurring compounds containing basic nitrogen atoms. Alkaloids 1–83 have been isolated from L. japonicum. Alkaloids 1–48 were Lycopodine alkaloids. This class is characterized by four connected six-membered rings, with positions C-4, C-5, C-6, C-8, C-11 and C-12 usually being oxidized to hydroxyl and carbonyl groups or esterified by acetic acid, and the C⚌C bond may existed at C-2(3), C-4(5), and C-11(12). Flabelline (36) is an alkaloid with the lycopodine skeleton carrying a second nitrogen atom (Niu et al., 2015). In addition, the nitrogen could be oxygenated as N-oxide, such as in 40–47 (He et al., 2014; Niu et al., 2015; Sun et al., 2008; Yang et al., 2016; Zhu et al., 2019). Lycoposerramine G nitrate (48) was an artifact (He et al., 2014), which was produced during the isolation as verified by the TLC (Al2O3). Alkaloids 49–57 were lycodine alkaloids, which has four rings in general, with two nitrogen atom in pyridine or pyridone rings. Hydroxypropyllycodine (51) and N-methylhydroxypropyllycodine (52) are possessing a 19-carbon skeleton (Niu et al., 2015; Wu et al., 2015). Huperzinine (57), like huperzine A is the product of N-C-9 bond cleavage and elimination of C-9, giving a C15N2 skeleton with three rings (Wu et al., 2015). Alkaloids 58–82 belong to fawcettimine class, which could be regarded as the products of C-4(13) to C-4(12) bond migration from lycopodine group precursors, with C-13 usually being oxidized to hydroxyl group or forming C⚌C bond with C-14. Phlegmariurine B (61) was formed by further cleaving the C-12(13) bond (Zhu et al., 2019), whereas lycojapodine A (62) had a six-membered lactone ring between C-5 and C-13 with a novel 6/6/6/7 tetracyclic ring system, formed by the cleavage of C-4(5) bond, and bonded between C-4 and C-12 (He et al., 2009). Alkaloids 63–75 all possess the cleavage of N-C-13 bond. In obscurinine (64) and isoobscurinine (65), an extra ring is formed by a nitrogen atom bridging between C-3 and C-13 (Li et al., 2012; Zhu et al., 2019). whereras 6-hydroxyl-6,7-dehydro-8-deoxy-13-dehydroserratinine (66), 8-deoxy-13-dehydroserratinine (67) and 15-epi-6-hydroxy-6,7-dehydro-8-deoxy-13-dehydroserratinine (68) formed an additional pyrole ring between N and C-4 (Wang et al., 2013b; Zhu et al., 2019). Alkaloids 69–74 could be the products of N-methylation followed by C–C bond formation between the N-methyl and C-4 (He et al., 2009; Niu et al., 2015; Wang et al., 2012a; 2013a, 2013b; Yang et al., 2018). In palhinines A (75) and D (76), C-16 were fused to a new ring through a C-16(4) linkage (Wang et al., 2013b; 2016). Lycojaponicumin D (77) possesses an unprecedented 5/7/6/6 tetracyclic skeleton formed by an unusual C-3(13) linkage (Wang et al., 2012a). Lycojaponicumins A-C (78–80) represent a unique heterocyclic skeleton formed by the new linkage C-4(9) (Wang et al., 2012b). Notably, lycojaponicumins A and B are the first examples of natural products possessing a 5/5/5/5/6 pentacyclic ring system with a 1-aza-7-oxabicyclo[2.2.1]heptane moiety. Isopalhinine A (81) with C-4(16) and N-C-5 linkage, possesses a sterically congested architecture built with a tricyclo[4.3.1.03,7]decane (isotwistane) moiety and a 1-azabicyclo[4.3.1]decane moiety (Yang et al., 2018). Lycopladine H (82) is an unprecedented C16N-type Lycopodium alkaloid possessing a novel fused-tetracyclic ring system consisting of an azocane ring (C-9(14), C-5, and N-1) fused to a [2,2,2]-bicyclooctane ring and a 3-piperidone ring (Yang et al., 2018). Lycocernuine (83) is likely that it is formed by a 4 + 2 cycloaddition reaction with a derivative of phlegmarine that has undergone opening of ring D via cleavage of the C7(12) bond (Yang et al., 2018).
2.2 Terpenoids
2.2.1 Sesquiterpenoid
Japonicumin D (84), a unique and new C13 dinor-sesquiterpene was isolated from L. japonicum (Li et al., 2006).
2.2.2 Diterpenoids
The abietane-type diterpene, 8α,9α-epoxy-7-oxoroyleanon (85) and the labdane-type diterpene, (-)-13,13-ethylenedioxy-15,16-dinorlabd-7-en-6β-ol (86) were isolated from L. japonicum (Li et al., 2015; Wu et al., 2006).
2.2.3 Triterpenoids
Thirty-six triterpenoids, 87–122, were isolated from L. japonicum. Triterpenoids, 87–113, belong to serratane-type, in which positions C-3 and C-21 were usually being oxidized to hydroxyl groups, ketone or further esterified by acetic and formic acids and the C⚌C bond may existed at C-14(15). Sometimes, C-12, 20, and 24 also could be oxidized to hydroxyl groups, and C-24 could be oxidized further to carboxylic acid or esterified by p-hydroxycinnamic acid. Besides, C⚌C bond at C-14(15) may be hydrated to hydroxyl group and C-16 may be oxidized to ketone. Lycojaponicuminol F (104) bearing the isopropylidene acetal has not been reported in nature, and this functionality was suggested to arise from the acetone used for the extract separation by chromatography on silica gel (Zhang et al., 2014). In fact, lycoclaninol (103) is the precursor of 104 which was also isolated from this plant (Yan et al., 2005a). Lycopodiin A (113) was a 16(15 → 14) abeoserratan-15-al (Yan et al., 2005a). Triterpenoids 114–121 belong to onocerin type. Compounds 116–121 were noronocerins, and 121 was the first example of onoceranoid triterpene bearing a seven-member ring isolated from Lycopodiaceae plants (Zhang et al., 2014). Betulin (122) was a lupane triterpeoid.
2.3 Sterols
Daucosterol (123) and (24S)-24-methyl cholesterol (124) were isolated from L. japonicum (Li et al., 2015; Shi & He, 2012).
2.4 Flavones
A Flavones lycopodone (125) along with tricin (126), tricetin 3′,4′,5′-OMe (127) and 5,7,4′-trihydroxy-3′-methoxyflavone (128) were isolated (Yan et al., 2005b).
2.5 Diaryl propanes
Tomentosanan B (129) and (-)-1-(4′-hydroxy-3′-methoxyphenyl)-2-(4′’-hydroxy-3′’-methoxyphenyl)propan-3-ol (130) were isolated from L. japonicum (Li et al., 2015).
2.6 Others
Physcion (131), an anthraquinone and di-(2-ethylhexyl) phthalate (132) were isolated from L. japonicum (Cai et al., 1991; Li et al., 2015). Compound 132 is much likely a contaminator released from plastic containers (Bianco et al., 2014; Venditti, 2018).
3 Biological activities
3.1 Acetylcholinesterase inhibitory activity
Lycojapodine A (62) inhibited acetycholinesterase with an IC50 value of 90.3 μM (He et al., 2009), which was comparable to that of huperzine A, a potent, reversible and selective acetylcholinesterase inhibitor, and approved as the drug for treatment of Alzheimer’s disease in China, and marketed in USA as a dietary supplement (Ma & Gang, 2004). Lycoclavanol (100) and α-onocerin (114) showed acetylcholinesterase inhibition activity (20.0% and 39.0%, resp.) at 0.6 mg/mL concentration, with galanthamine as the standard (63.6%) (Yan et al., 2005a).
3.2 Cytotoxic activity
3-Epilycoclavanol (95) and lycopodiin A (113) showed moderate activity against human tumor A549 or K562 cells with IC50 of 10–100 μg/ml (Yan et al., 2005a). 95, lycernuic acid A (96), lycojaponicuminol F (104), 26-nor-8-oxo-α-onocerin (116), and lycojaponicuminol B (117) exhibited moderate activities against A549, hepatocellular carcinoma HepG2 and breast cancer MCF-7 with IC50 values of 2.28–11.81 μg/mL (Zhang et al., 2014). Lycopodone (125) and tricin (126) indicated moderate activity against human tumor K562 cells with IC50 of 10–100, and 11.68 μg/ml (Yan et al., 2005b).
3.3 Anti-inflammatory activity
Biological testing in vitro showed that ten alkaloids lycopodine (1), 8β-hydroxylycodoline (5), acetyllycofawcine (6), α-lofoline (10), deacetylfawcettiine (11), 11β-hydroxy-12-epilycodoline (27), lycojaponicumin D (77), and lycojaponicumins A-C (78–80) inhibited lipopolysaccharide (LPS)-induced pro-inflammatory factors in BV2 macrophages with IC50 of 4.23–64.97 μM (curcumin was used as the positive control, IC50 3.12 μM) (Wang et al., 2012a, 2012b; 2013a). Miyoshianine C (42) and lycoflexine N-oxide (74) exhibit the potent inhibition of NO release from LPS-induced RAW264.7 cells, with IC50 of 31.82 and 40.69 μM resp. (Niu et al., 2015).
3.4 Anti-HIV-1 activity
Lycojapodine A (62) was tested using the MTT method, showing an EC50 value of 85 μg/mL against HIV-1 (He et al., 2009).
3.5 α-Glucosidase inhibitory activity
Lycodine (49) showed weak α-glucosidase inhibitory activity, with inhibition of 30.56% at 100 μM (Yang et al., 2018), with acarbose as the positive control.
4 Conclusion
The chemical studies on L. japonicum have revealed that the typical constituents of this plant are mainly alkaloids and serratane-type triterpenoids. Other types of compounds such as onocerin type triterpenids, diterpenoids, flavones, and diaryl propanes are also important components. The biological research on the plant constituents showed that some components exhibit bioactivities, especially acetylcholinesterase inhibitory, cytotoxic and anti-inflammatory activities, which supported the use of L. japonicum in traditional medicines or revealed the new activities on modern pharmacological levels. Biological testing on anti-inflammatory activity could help fellow researchers to find more active compounds or active core framework and mechanisms of active components.
Declarations of interest
None.
Acknowledgement
The work was supported by a grant (21162045) from National Natural Science Foundation of China.
References
- Pyrrolizidine alkaloids from Solenanthus lanatus DC. with acetylcholinesterase inhibitory activity. Nat. Prod. Res.. 2016;30(22):2567-2574.
- [Google Scholar]
- Acetylcholinesterase inhibitory activity of pyrrolizidine alkaloids from Echium confusum Coincy. Nat. Prod. Res.. 2017;31(11):1277-1285.
- [Google Scholar]
- A new problem. Contamination of botanicals by phthalates. Rapid detection tests. Nat. Prod. Res.. 2014;28(2):134-137.
- [Google Scholar]
- The isolation and identification of the anthraquinones from Lycopodium japonicum L. and Lycopodium obscurum L. Acta Acad. Med. Shanghai. 1991;18(5):383-385.
- [Google Scholar]
- Alkaloids and triterpenoids from Lycopodium japonicum. J. Hainan Normal Univ. (Nat. Sci.). 2016;29(3):277-281.
- [Google Scholar]
- A conia-ene-type cyclization under basic conditions enables an efficient synthesis of (-)-lycoposerramine R. Angew. Chem. Int. Edit.. 2017;56(3):893-896.
- [Google Scholar]
- Lycojapodine A, a novel alkaloid from Lycopodium japonicum. Org. Lett.. 2009;11(6):1397-1400.
- [Google Scholar]
- Lycopodine-type alkaloids from Lycopodium japonicum. Nat. Prod. Bioprosp.. 2014;4(4):213-219.
- [Google Scholar]
- Hupercumines A and B, Lycopodium alkaloids from Huperzia cunninghamioides, inhibiting acetylcholinesterase. Org. Lett.. 2018;20(5):1384-1387.
- [Google Scholar]
- Everlasting flower (Helichrysum stoechas Moench) as a potential source of bioactive molecules with antiproliferative, antioxidant, antidiabetic and neuroprotective properties. Ind. Crop. Prod.. 2017;108:295-302.
- [Google Scholar]
- Chemical constituents from whole herb of Lycopodium japonicum. Chin. Tradit. Herb. Drugs. 2015;46(1):33-37.
- [Google Scholar]
- Neolignans and serratane triterpenoids with inhibitory effects on xanthine oxidase from Palhinhaea cernua. Fitoterapia. 2017;119:45-50.
- [Google Scholar]
- New alkaloids from Lycopodium japonicum. Chem. Pharm. Bull.. 2012;60(11):1448-1452.
- [Google Scholar]
- Japonicumins A-D: Four new compounds from Lycopodium japonicum. Helv. Chim. Acta. 2006;89(7):1467-1473.
- [Google Scholar]
- Study on chemical constituents of Lycopodium alkaloids. China J. Chin. Mater. Med.. 2012;37(4):475-477.
- [Google Scholar]
- Phlenumdines D and E, new Lycopodium alkaloids from Phlegmariurus nummulariifolius, and their regulatory effects on macrophage differentiation during tumor development. Phytochem. Lett.. 2019;29:98-103.
- [Google Scholar]
- Lycopodium alkaloids from Lycopodium japonicum. Chin. Tradit. Herb. Drugs. 2015;46(9):1269-1276.
- [Google Scholar]
- Access to enantiopure 5-, 7-, and 5,7-substituted cis-decahydroquinolines: enantioselective synthesis of (-)-cermizine B. Org. Lett.. 2017;19(7):1714-1717.
- [Google Scholar]
- Synthesis of (±)-serralongamine A and the revised structure of huperzine N. J. Org. Chem.. 2016;81(6):2629-2634.
- [Google Scholar]
- Serrantane triterpenoid from Lycopodium japonicum. Chin. J. Exper. Tradit. Med. Form.. 2012;18(9):90-92.
- [Google Scholar]
- A new serratene triterpenoid from Lycopodium japonicum. J. Asian Nat. Prod. Res.. 2017;19(3):299-303.
- [Google Scholar]
- A new alkaloid from Lycopodium japonicum Thunb. Helv. Chim. Acta. 2008;91(11):2107-2109.
- [Google Scholar]
- Annotinolides A-C, three Lycopodane-derived 8,5-lactones with polycyclic skeletons from Lycopodium annotinum. Org. Lett.. 2016;18(17):4376-4379.
- [Google Scholar]
- Venditti, A. 2018. What is and what should never be: artifacts, improbable phytochemicals, contaminants and natural products. Nat. Prod. Res. https://doi.org/10.1080/14786419.2018.1543674
- Total synthesis of Lycopodium alkaloids palhinine A and palhinine D. J. Am. Chem. Soc.. 2017;139(12):4282-4285.
- [Google Scholar]
- Lycojaponicumins D and E: Two new alkaloids from Lycopodium japonicum. Org. Lett.. 2012;22(14):5688-5691.
- [Google Scholar]
- Lycojaponicumins A-C, three alkaloids with an un precedented skeleton from Lycopodium japonicum. Org. Lett.. 2012;14(10):2614-2617.
- [Google Scholar]
- Five new fawcettimine-related alkaloids from Lycopodium japonicum Thunb. Fitoterapia. 2013;91(10):74-81.
- [Google Scholar]
- Nine new lycopodine-type alkaloids from Lycopodium japonicum Thunb. Tetrahedron. 2013;69(30):6234-6240.
- [Google Scholar]
- Two new triterpenoids from Lycopodium japonicum Thunb. Chin. J. Chem.. 2014;32(10):1007-1010.
- [Google Scholar]
- Corrigendum to “Five new fawcettimine-related alkaloids from Lycopodium japoniucm Thunb”. Fitoterapia. 2016;114:194.
- [Google Scholar]
- Isolation of a new lycodine alkaloid from Lycopodium japonicum. Nat. Prod. Res.. 2015;29(8):735-738.
- [Google Scholar]
- Three new triterpenoids from Lycopodium japonicum Thunb. Helv. Chim. Acta. 2005;88(2):240-244.
- [Google Scholar]
- Serratene triterpenoids in Lycopodium japonicum from Hubei province. Chin. Tradit. Herb. Drugs. 2014;45(24):3524-3527.
- [Google Scholar]
- A new Lycopodine-type alkaloid from Lycopodium japonicum. Nat. Prod. Res.. 2016;30(19):2220-2224.
- [Google Scholar]
- A new fawcettimine-related alkaloid from Lycopodium japonicum. Chem. Nat. Compd.. 2018;54(4):729-731.
- [Google Scholar]
- Lycojaponicuminol A-F: cytotoxic serratene triterpenoids from Lycopodium japonicum. Fitoterapia. 2014;96(11):95-102.
- [Google Scholar]
- Flora of China (Vol. 6). Science Press, Beijing; 2004. p. :33-34.
- Three new Lycopodium alkaloids from Lycopodium japonicum. J Asian Nat Prod Res.. 2019;21(1):17-24.
- [Google Scholar]







