Translate this page into:
Magnetic photocatalyst Bi3O4Cl/MnxZn1-xFe2O4: Facile synthesis and its photocatalytic activity
⁎Corresponding authors. cqwhl@sicnu.edu.cn (Hailong Wang), xulj@cqu.edu.cn (Longjun Xu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Herein, a Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalyst based on MnxZn1-xFe2O4 is fabricated by hydrothermal and roasting methods. The performances of the obtained photocatalysts were detected via XRD, SEM, EDS, TEM, UV–vis, PL and nitrogen absorption-desorption equipment, which indicated the successful combination of nanosheet Bi3O4Cl and granular MnxZn1-xFe2O4. The activity of the photocatalyst was investigated under solar light with rhodamine B (RhB) as a simulated pollutant, which achieved high catalytic and magnetic properties. In addition, 99.5 % degradation of RhB was displayed by MB-10(10 %MnxZn1-xFe2O4/Bi3O4Cl). The Bi3O4Cl/MnxZn1-xFe2O4 photocatalyst was regarded as having excellent kinetic properties for recycling and magnetism due to its low hysteresis loss illustrated in the hysteresis loop images. In addition, the average recovery of the samples was 91.3 %, which successfully verified the excellent stability of the composites in RhB degradation. This paper demonstrates that the doping of magnetic material onto Bi3O4Cl is a feasible approach to remove RhB. The recovery of the photocatalyst, which provides low cost and highly efficient photocatalysis, is guiding a brand-new strategy for the elimination of organic contaminants.
Keywords
Bi3O4Cl/MnxZn1-xFe2O4
Hydrothermal-roasting method
Photocatalytic activity
Magnetic recovery
1 Introduction
The development of society has led to an increased consumption of products to improve people’s quality of life (Hu et al., 2020). However, the use of a large number of environmentally toxic dyes is usually overshadowed by the pleasing consumption, which causes the retention of contamination in the environment, resulting in irreparable consequences (Ma et al., 2020; Ma et al., 2020; Jing et al., 2020; Niu et al., 2020; Frindy and Sillanpää, 2020). Thus, the elimination of toxic dyes is crucial. Physical absorption, ion exchange, chemical and biological oxidation, biodegradation, photoelectrocatalysis, membrane filtration, coagulation and adsorption have been utilized to remove dyes such as RhB throughout history (Zhang et al., 2020; Wang et al., 2021; Khalid et al., 2020; Ghorai et al., 2021; Luo et al., 2020; Bao et al., 2021; Cainglet et al., 2020; Zhao et al., 2023; Sohrabi and Ameri, 2015). However, there are still dyes remaining in the environment even after the processes listed, causing severe damage to Earth. Thus, the elimination of dyes has become a hot topic, and numerous studies have presented a practical solution.
Photocatalysts, which have been confirmed to decompose dyes to small molecules by direct light activation, are ideal candidates to remove aquatic pollutants due to their low cost, ease of control and environmental friendliness (Acharya et al., 2020; Li et al., 2020; Wang et al., 2020; Talreja et al., 2021; Behnood and Sodeifian, 2021; Bhatt et al., 2021; Ye et al., 2021; Cui et al., 2021; Sharma et al., 2020; Sun et al., 2018; Senthil et al., 2019). Titanium dioxide has been widely investigated due to its acute photocatalytic activity, photostability and nontoxicity (Nabi et al., 2021; Li et al., 2020; Ziental et al., 2020; Pan et al., 2020; Eddy et al., 2021). However, the photocatalytic reaction of titanium dioxide only occurs in the ultraviolet excitation band, and the recombination of holes and electrons results in low quantum efficiency, which leads to the weak activity of TiO2 (Li et al., 2021; Pant et al., 276 (2021); Li et al., 2021). Akyüz (Akyüz, 2021) successfully prepared a rGO-TiO2-CdO-ZnO-Ag composite photocatalyst under the sol–gel method, which displayed preferable photocatalytic activity than pristine TiO2 in condition of visible light irradiation. Zhao et al. (Zhao et al., 2021) first prepared numerous self-assembled WO3-decorated TiO2 nanocrystallites with the combination of strategic integration and membrane water filtration to identify the magnetic behaviour and photocatalytic activity. However, due to the difficulty of recovery, the environment would suffer great pollution by modified TiO2 photocatalysts. In addition, the minimization of cost leads to the recovery of the photocatalyst after utilization, and MnxZn1-xFe2O4 is prepared to enhance the photocatalytic activity and magnetic recovery rate (Cheng et al., 2021; Jiang et al., 2021). To meet the ideal outcome, a new photocatalyst has been investigated.
Wrapped by two chlorine atoms, the unique layered structure of Bi3O4Cl has attracted extensive interest. As a member of Silén, the superior photocatalytic efficiency Bi3O4Cl displayed in the inner electrostatic field perpendicular to the laminar layer, which promotes greater segregation of photogenerated electron-hole pairs. Xu (Xu et al., 2020) prepared Bi3O4Cl by calcining Bi2O3 and BiOCl under high temperature, which presented high photocatalytic degradation efficiency under visible light irradiation. Notably, Yuan (Yuan et al., 2022) successfully prepared the S-scheme Bi7O9I3/g-C3N4/Bi3O4Cl composite photocatalyst by an oil bath method, which exhibited better photocatalytic degradation efficiency than pure Bi3O4Cl. However, these studies exhibited tedious steps and high energy consumption in preparation. Moreover, for the sake of cost reduction, any photocatalyst should be recoverable, and the general application to add magnetic material in the preparation of composite photocatalyst. That is, the photocatalytic degradation efficiency could be improved by recycling magnetism in a magnetic composite photocatalyst.
In this work, Bi3O4Cl prepared by a hydrothermal-roasting method could be a rapid, simple and effective synthesis pathway. In addition, MnxZn1-xFe2O4, known as a typical soft magnetic material, exhibits an effective saturation magnetization, perfect permeability, low loss, high stability, and an ideal recycling efficiency (Cheng et al., 2021). Additionally, since it is used as a magnetic substance, the combination of Bi3O4Cl and MnxZn1-xFe2O4 could be an ideal candidate to meet the demands. Herein, Bi(NO3)3·5H2O and NaCl were utilized as raw materials to prepare Bi3O4Cl by a hydrothermal method, and MnxZn1-xFe2O4 was added into the combination. Thus, a Bi3O4Cl/MnxZn1-xFe2O4 magnetic composite photocatalyst was prepared, which presents an effective and simple method for the preparation of Bi3O4Cl, promoting the application and development of photocatalytic technology in controlling environmental pollution.
2 Experimental
2.1 Experimental materials
Chengdu Kelong Chemical Co., Ltd. (Chengdu, China) purchased Bi(NO3)3·5H2O, NaCl, NaOH, ZnSO4·7H2O, MnSO4·H2O, Fe2(SO4)3, and C2H6O2. It is noteworthy that all chemical reagents were of analytical grade and can be used directly without any purification.
2.2 Preparation of Bi3O4Cl/MnxZn1-xFe2O4
Herein, by the application of hydrothermal and roasting methods, bismuth oxychloride/manganese and zinc ferrite (Bi3O4Cl/MnxZn1-xFe2O4) composite photocatalysts were synthesized by preparing Bi(NO3)3·5H2O and NaCl as raw materials and preparing MnxZn1-xFe2O4 as an additive. It was synthesized by the following procedures presented in Fig. 1. First, a certain amount of ZnSO4·7H2O, MnSO4·H2O, and Fe2(SO4)3 mixed solution was dissolved in distilled water and stirred ultrasonically for 10 min. Then 2 mol/L sodium hydroxide solution was added dropwise. The pH of the solution was adjusted to 13 and stirred for 10 min. Then the mixed solution was placed in a high-pressure reactor at 200 °C for 5 h. After washing and drying, the MnxZn1-xFe2O4 magnetic matrix was obtained.Then, 20 mL of ethylene glycol, 0.97 g of Bi(NO3)3·5H2O were mixed into a beaker and sonicated for 10 min. MnxZn1-xFe2O4 accounting for 5 %∼25 % of the total mass were added into the levitated solution and mechanically stirred for 10 min. After that, 50 mL of NaCl (0.0134 mol/L) was added dropwise to the levitated solution. After stirred for 10 min again, the mixture was calcined at 160 °C for 12 h to obtain the intermediate product in the reaction at room temperature. The specimens were scrubbed with anhydrous ethanol and aqua destillata many times and desiccated at 75 °C for 12 h. The samples were ground into 100 mL ceramic crucible, continuously roasted at 500 °C for 2 h and ground to obtain Bi3O4Cl/MnxZn1-xFe2O4 at room temperature. In addition, 5 % ∼ 25 % MnxZn1-xFe2O4/Bi3O4Cl were named MB-5, MB-10, MB-15, MB-20, and MB-25, respectively.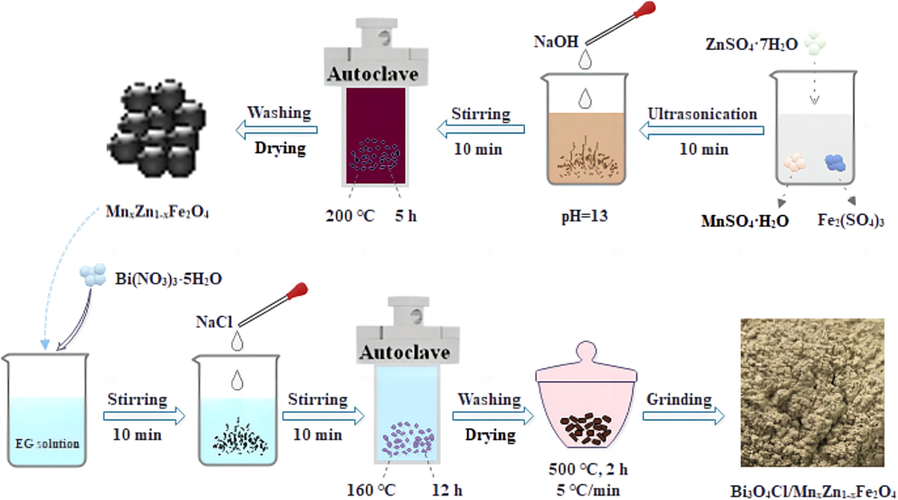
Preparation flow of Bi3O4Cl/MnxZn1-xFe2O4.
2.3 Characterization
The phase structure and crystallographic structure of the Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalyst were obtained by X-ray diffraction (Shimadzu XRD 6000, Japan) with monochromated high-intensity Cu Ka radiation. The surface appearance of the composites was characterized by SEM (S4800, Japan). Through TEM (Tecnai G2 F20, USA), the appearance and microstructure of the samples were further displayed. A specific surface and aperture analyser (Quadrasorb 2 MP, USA), which was applied to analyse the composites, exhibited the specific surface area (BET). In addition, UV–vis diffuse reflectance spectra (DRS) were acquired on a Shimadzu UV-2600 UV–Vis spectrophotometer (TU-1901, China). With excitation at a wavelength of 380 nm, photoluminescence (PL) spectra of the powder samples were recorded with a fluorescence spectrophotometer (F-4700, Japan). Furthermore, electrochemical impedance spectroscopy (EIS, CHI660E, CHI Shanghai, Inc.) were utilized to confirm the separation efficiency and interfacial charge transfer of the Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalyst.
2.4 Photocatalytic evaluation
RhB was applied as a simulated dye wastewater, which evaluated the degradation activity of the photocatalytic composite under irradiation with visible light. The simulated pollutant (10 mg/L) was prepared with the addition of 100 mg photocatalyst and placed into darkness for 30 min with stirring to achieve adsorption equilibrium. At ten-minute intervals, the absorbance was measured during the photodegradation process under a xenon lamp (CEL-HXF300-T3, China). The photodegradation process could be presented via a pseudofirst-order reaction under low RhB conditions, which follows the formula,
3 Results and discussion
3.1 Photocatalytic activity
Photocatalytic performance of the photocatalyst was measured under simulated solar light irradiation, as illustrated in Fig. 2(a). In 100 min, the degradation rates of MB-5, MB-10, MB-15, MB-20, and MB-25 for RhB were 99.6 %, 99.5 %, 98.9 %, 93.3 % and 78.5 %, respectively. The kinetic model analysis confirmed that the degradation of RhB by the Bi3O4Cl/MnxZn1-xFe2O4 composite magnetic photocatalyst met the first-order reaction kinetics model. MB-5 exhibited perfect photocatalytic degradation of RhB with 0.0195 min−1. MB-10 displayed weaker catalytic degradation but outperformed MB-5 in magnetic recovery, which was chosen as the best candidate to be test analysed.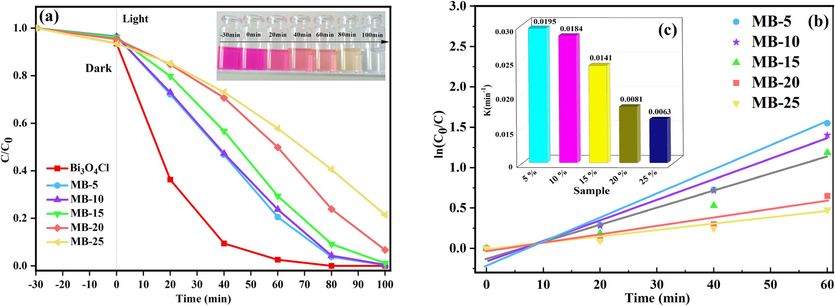
(a) Photocatalytic degradation; (b) kinetic linear simulation curves of RhB over Bi3O4Cl and Bi3O4Cl/MnxZn1-xFe2O4; (c) values of reaction rate constants.
3.2 Structure characteristics
The crystalline formation of the composites could be investigated via X-ray diffraction (XRD) patterns shown in Fig. 3. The different peaks of Bi3O4Cl and MnxZn1-xFe2O4 were indexed to monoclinic Bi3O4Cl (JCPDS No.: 36-0760) and cubic MnxZn1-xFe2O4 (JCPDS No.: 74-2399), respectively. The diffraction peaks at 2θ = 24.31°, 29.13°, 29.68°, 31.65°, 38.77°, 43.36° and 45.25° could be perfectly indexed to monoclinic Bi3O4Cl. By the Scherrer formula, the mean grain size of Bi3O4Cl was determined to be 29.9 nm. Compared with pristine Bi3O4Cl, the appearance of characteristic peaks associated with Bi3O4Cl in Bi3O4Cl/MnxZn1-xFe2O4 and the characteristic peaks at 2θ = 29.91°, 42.94° and 62.01° corresponding to MnxZn1-xFe2O4 could still be recognized, confirming the successful incorporation of MnxZn1-xFe2O4 onto Bi3O4Cl.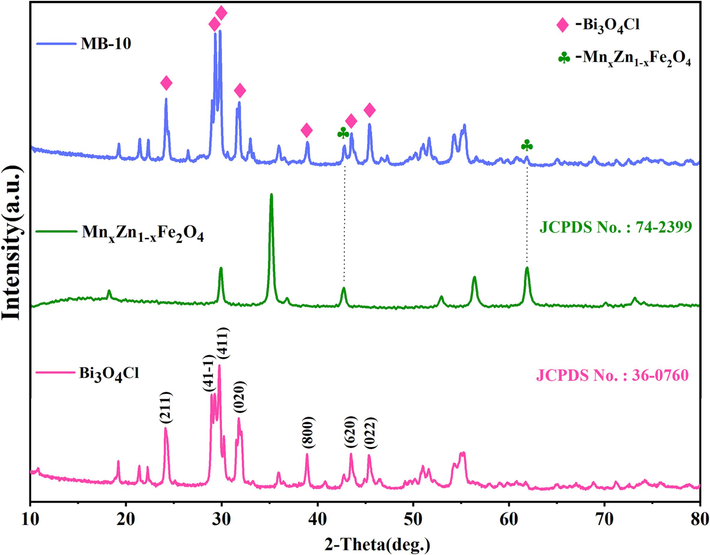
XRD patterns of Bi3O4Cl, MnxZn1-xFe2O4 and MB-10.
The surface morphologies of the samples are observed via SEM and EDS mapping, as presented in Fig. 4. Compared with granular MnxZn1-xFe2O4, Bi3O4Cl showed irregular nanosheets. SEM and EDS mapping of the results revealed the combination of Bi3O4Cl and MnxZn1-xFe2O4, corresponding to XRD. Only Bi, Cl, O, Mn, Zn and Fe were uniformly distributed in the composite. These results indicate that the Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalysts obtained were pure.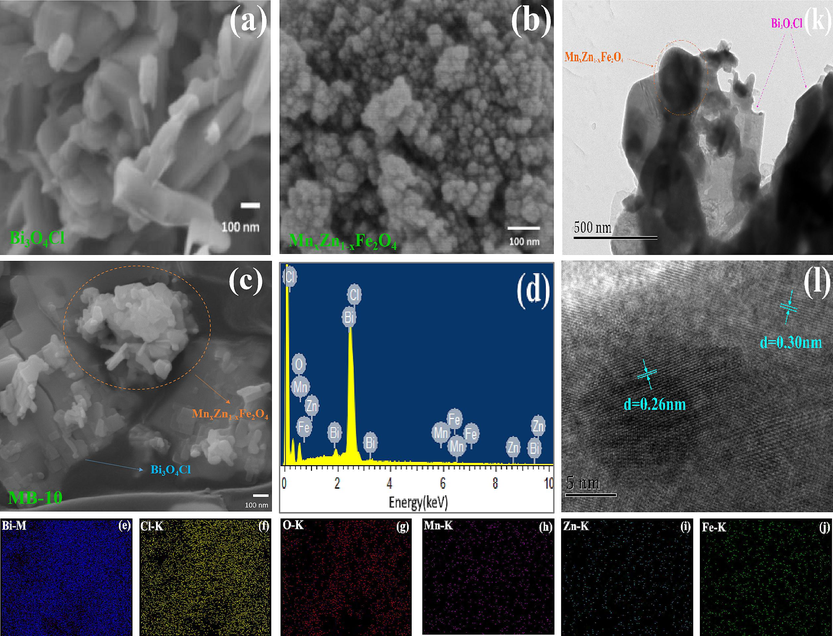
(a)-(c) SEM images of Bi3O4Cl, MnxZn1-xFe2O4 and MB-10; (d) EDS spectrum of MB-10; (e)-(j) EDS elemental mapping of Bi, Cl, O, Mn, Zn and Fe; (k) TEM image of MB-10; (l) HRTEM image of MB-10.
Transmission electron microscopy (TEM) is used to express the surface morphologies of Bi3O4Cl/MnxZn1-xFe2O4. The TEM images of the Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalyst investigated in Fig. 4(k) confirm the doping of MnxZn1-xFe2O4 onto Bi3O4Cl shown in SEM. The HRTEM exhibited in Fig. 4(l) shows a spacing of 0.26 nm and a crystal lattice fringe spacing of 0.30 nm, identified with (4 1 1) crystal Bi3O4Cl and (3 1 1) crystal MnxZn1-xFe2O4, respectively. This clearly exhibits the differences between each crystal plane and confirms the successful preparation of the Bi3O4Cl/MnxZn1-xFe2O4 compound.
The chemical composition of support surface of MB-10, which was surveyed by XPS survey spectra, proving the constitution and chemical shift of of Bi3O4Cl and MnxZn1-xFe2O4. According to Fig. 5(a), MB-10 exhibits all elements of Bi, Cl, Mn, O, Zn, C, and Fe, which are in consistent with the HRTEM and EDS outcomes. The spectrum of Cl 2P could be separated into two characteristic peaks at 199.2 eV (Cl 2P3/2) and 197.7 eV (Cl 2P1/2), indicating the existence of Cl– (Zhou et al., 2018). In the meantime, the characteristic peaks at 163.9 eV (Bi 4f5/2) and 158.6 eV (Bi 4f7/2) in the Bi 4f spectrum confirmed the existent form of Bi3+ (Li et al., 2018). The O 1s spectrum can fit the characteristic peak at 529.2 eV illustrated in Fig. 5(d). The major peaks at 651.5 eV, 709.2 eV, and 1017.5 eV in Mn 2p, Fe 2p and Zn 2p spectrum are attributed to MnxZn1-xFe2O4 presented in Fig. 5(e-g). Moreover, the peaks of O 1s, Bi 4f and Br 3d in MB-10 slightly shift to the weaker binding energy direction through the intense interaction between Bi3O4Cl and MnxZn1-xFe2O4 (compared to pure Bi3O4Cl). In summary, the interface between Bi3O4Cl and MnxZn1-xFe2O4 is formed by chemical interactions rather than simple physical mixing.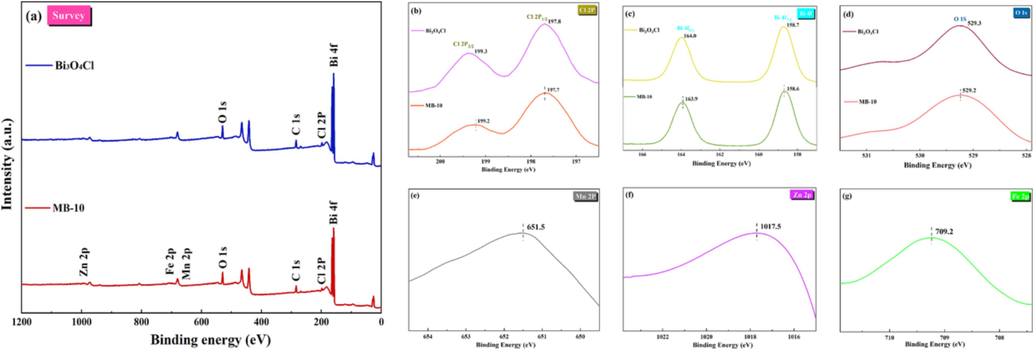
(a) X-ray photoelectron spectroscopy (XPS) survey spectra; (b–g) corresponding high-resolution XPS spectra of each element.
The aperture partition and Brunauer–Emmett–Teller surface area (SBET) of the Bi3O4Cl/MnxZn1-xFe2O4 magnetic composite photocatalyst were investigated by N2 adsorption and desorption isotherms, as exhibited in Fig. 6. The whole specimens were presented typical Type IV isotherms, indicating the presence of mesoporous within the production. At higher relative pressures P/P0, type H3 hysteresis loop was observed, suggesting the existence of slit-shaped macropores. Fig. 6 illustrates the pore size distribution of the Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalyst, and the most likely pore size distribution is 21.2 nm. The total pore volume of Bi3O4Cl/MnxZn1-xFe2O4 was 0.0203 cm3/g. The SBET values of Bi3O4Cl/MnxZn1-xFe2O4 and Bi3O4Cl were 7.27 and 5.82 m2/g, respectively. Typically, the superior specific surface area of the photocatalysts was essential to higher adsorption of RhB, since more active loci could be furnished.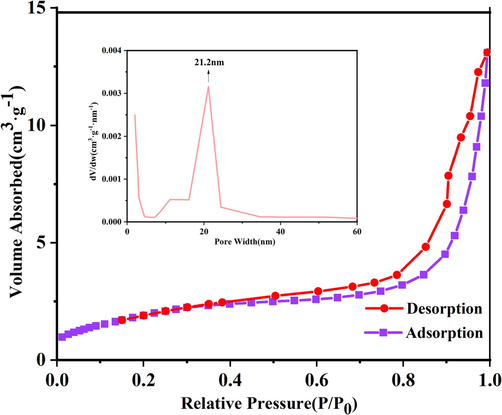
The N2 adsorption-desorption isotherms of MB-10 (Inset is pore diameter distribution curve).
3.3 Optical properties
Upon UV–vis diffuse reflection spectra, the photoresponsibility of the Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalyst and Bi3O4Cl are investigated in Fig. 7(a). UV and visible light could be absorbed by both. In comparison with pristine Bi3O4Cl, the Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalyst absorbed a smaller wavelength of 420 nm, which demonstrated a narrower response range for visible light of the Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalyst leading to weaker photocatalytic activity.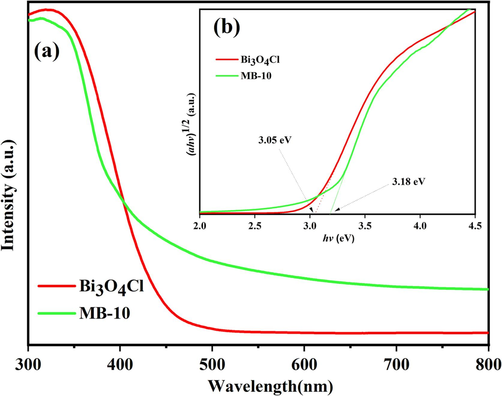
(a) UV–vis DRS of Bi3O4Cl and MB-10; (b) The (ahv)1/2-hv curve of Bi3O4Cl and MB-10.
The (ahv)1/2-hv images are obtained in Fig. 7(b) since Bi3O4Cl is an indirect band gap semiconductor (Lin et al., 2006). Band gap energy (Eg) is shown at the intersection point of the curve tangent extension line and the abscissa axis. The calculated results indicated that the Eg values of Bi3O4Cl and Bi3O4Cl/MnxZn1-xFe2O4 were 3.05 eV and 3.18 eV, respectively. Compared with Bi3O4Cl, Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalysts exhibited a higher band gap energy, which indicates that the greater difficulty in yielding electrons and holes than Bi3O4Cl was responsible for the weaker photocatalytic activity of Bi3O4Cl/MnxZn1-xFe2O4.
3.4 Photoelectrochemical properties
Typically, the weaker the PL intensity is, the less the compound probability of photogenerated h+ and e− is, which results in the longer life period of photogenerated carriers. Steady-state photoluminescence (PL) is applied to explore the migration efficiency and segregation of photoinduced electron-hole pairs investigated in Fig. 8. Under photoexcitation, the Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalyst showed a greater photocurrent response intensity than Bi3O4Cl, which indicates the higher photogenerated carrier recombination rate of the Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalyst than Bi3O4Cl. Furthermore, the greater efficiency of electron-hole separation after coupling decreases the lifetime of light-generated holes and electrons, which confirmed the slightly weaker photocatalytic activity of the Bi3O4Cl/MnxZn1-xFe2O4 composite magnetic photocatalyst compared with pure Bi3O4Cl.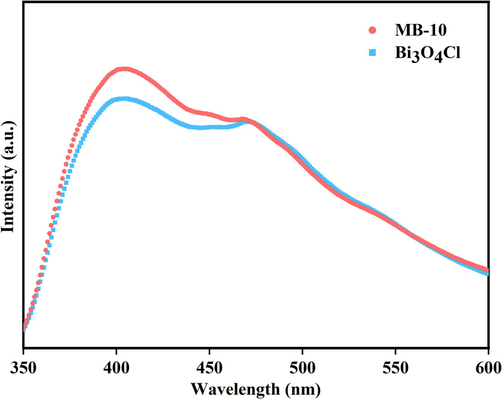
PL emission spectra of Bi3O4Cl和MB-10.
Fig. 9 shows the electrochemical impedance spectroscopy (EIS) analysis for Bi3O4Cl and the prepared Bi3O4Cl/MnxZn1-xFe2O4. Bi3O4Cl presents a smaller curvature radius than the Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalyst, which demonstrates a better interfacial charge transfer and separation efficiency and exhibits a greater conductivity.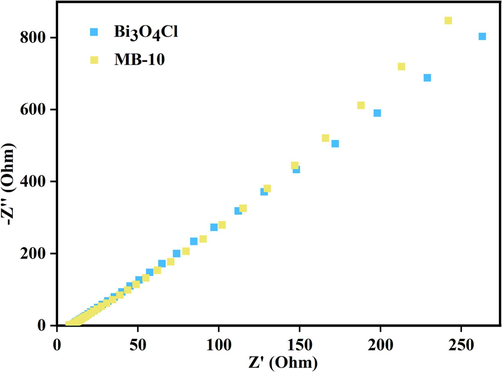
EIS of Bi3O4Cl and MB-10.
The TPR results of pristine Bi3O4Cl and Bi3O4Cl/MnxZn1-xFe2O4 are investigated in Fig. 10 by an electrochemical workstation. Irradiated by simulated sunlight and obtained energy, the electrons transformed, resulting to an instantaneous increase in the photocurrent density of the reaction system and reaching the peak. Notably, the lowest photocurrent density was observed in the absence of light. Furthermore, the transient photocurrent density of pristine Bi3O4Cl is 0.56 µA·cm−2, which was nearly 2 times that of Bi3O4Cl/MnxZn1-xFe2O4, proving the superior election mobility performance.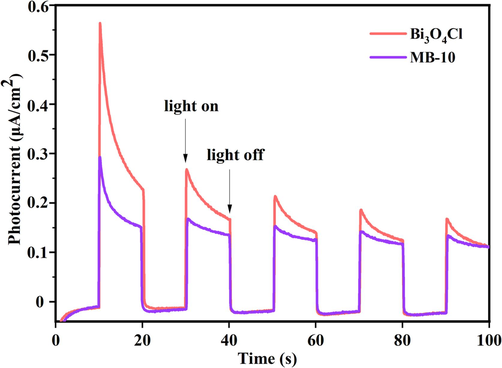
Transient photocurrent response (I-t) of Bi3O4Cl and MB-10.
3.5 Magnetic properties
Fig. 11 illustrates the hysteresis loop diagrams of MnxZn1-xFe2O4 and Bi3O4Cl/MnxZn1-xFe2O4 composite photocatalysts. MnxZn1-xFe2O4 exhibited a greater hysteresis loop and coercivity, which can foster magnetic induction intensity and a lower hysteresis loss. Herein, the coercivity (Hc) of MnxZn1-xFe2O4 was 42 G, and the residual magnetization (Mr) was 6.8 emu/g. Compared with the saturation magnetization (Ms) of 70 emu/g owing to MnxZn1-xFe2O4, Bi3O4Cl/MnxZn1-xFe2O4 displayed a weaker Ms of 6.42 emu/g. The Mr of Bi3O4Cl/MnxZn1-xFe2O4 retained 0.52 emu/g, which indicates a lower MnxZn1-xFe2O4, and the calculation process was responsible for the weaker residual magnetization (Mr). Given an external magnetic field, Bi3O4Cl exhibited no magnetism, while Bi3O4Cl/MnxZn1-xFe2O4 displayed an ideal magnetism sharply. Hence, Bi3O4Cl/MnxZn1-xFe2O4 could be regarded as a composite photocatalyst with excellent magnetic properties for recycling and magnetism.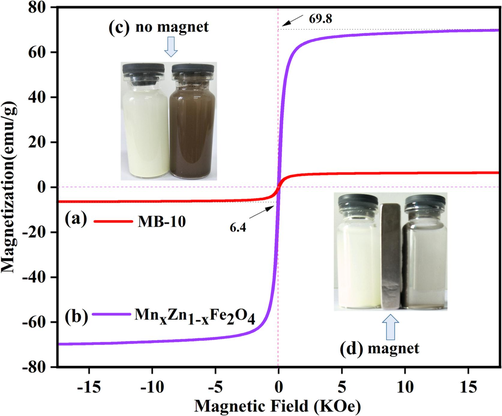
(a)-(b) The magnetic hysteresis loops; (c) The suspension of Bi3O4Cl and MB-10; (d) The suspension of Bi3O4Cland MB-10 under the magnetic field.
3.6 Stability and recycling ability
The stability of the composite is proven by circular photodecomposition of RhB solution under the same conditions as presented in Fig. 12. Given an accurate weighing, the recovery of each catalyst was 91.3 %. After 5 cycles, no distinct deterioration could be observed in the degradation curves, and the recovery of the catalyst was 82.2 %, which verifies the excellent stability of the composites in RhB degradation.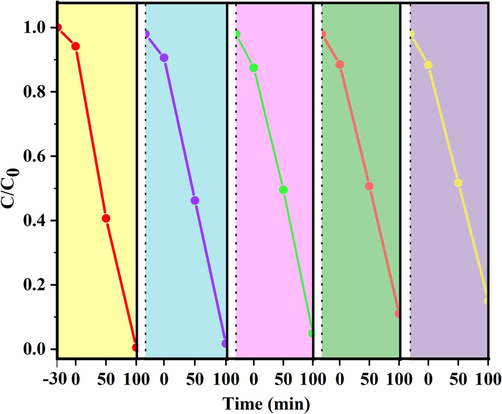
Degradation rate of RhB on MB-10 after being recycled.
3.7 Photocatalytic mechanism
Free radical capture experiments are prepared to reveal the main active substances by the samples investigated in Fig. 13. IPA (Isopropyl alcohol), BQ (benzoquinone) and AO (ammonium oxalate) were prepared in the experiment to detect hydroxyl radicals (·OH), photogenerated holes (h+) and superoxide anion rabical (
), respectively. Confirming their effects on catalytic degradation in the reaction system. After adding AO, IPA and BQ, the degradation rate of RhB retained 44.5 %, 32.6 % and 8.2 %, respectively. This indicates the importance of h+, ·OH and
.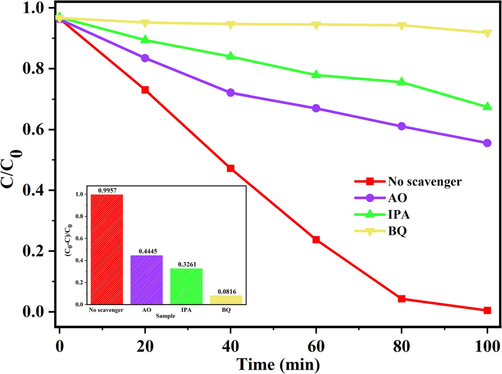
Effect of radical scavengers on RhB photocatalytic degradation of MB-10.
In addition, the band gap energies of the specimens could be initially evaluated under UV–vis DRS. The fitted Eg of Bi3O4Cl was estimated to be 3.05 eV. In addition, the EVB values of Bi3O4Cl and MnxZn1-xFe2O4 were 3.43 eV and 1.17 eV, respectively. However, with EVB higher than 2.3 eV, Bi3O4Cl fostered OH– oxidation to ·OH(OH–/·OH, 2.3 eV vs. NHE) by photogenerated holes (h+) (Li et al., 2017). MnxZn1-xFe2O4 with weaker EVB negatively contributed to the inability to oxidize OH– to ·OH. Furthermore, compared with −0.33 eV, the lower ECB of MnxZn1-xFe2O4 displayed perfect O2 reduction to
(O2/
, −0.33 eV vs. NHE) (Fu et al., 2012), while Bi3O4Cl with a higher ECB exhibited nothing on it. The Z-type heterojunction (Zhao et al., 2023; Zhao et al., 2023) Bi3O4Cl/MnxZn1-xFe2O4 is investigated in Fig. 14, which reveals the possible pathway to catalyse the mechanism. The intensive combination of e− on Bi3O4Cl and h+ on MnxZn1-xFe2O4 contributes to the Z-type heterojunction. The h+ on Bi3O4Cl had two pathways, and some of them degraded RhB directly. Others effectively promoted OH– oxidation to ·OH, which eliminated RhB later. Concurrently, the e− on MnxZn1-xFe2O4 reduced O2 to
, confirming the duration of RhB under
. The purpose and discussion of RhB degradation by the Bi3O4Cl/MnxZn1-xFe2O4 composite magnetic photocatalyst were exhibited under the reaction mechanism, which matches the results of free radical capture.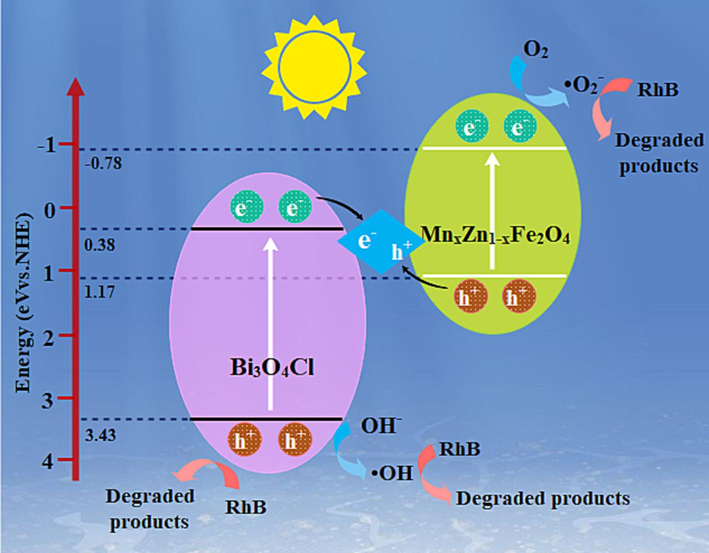
Photocatalytic mechanism of RhB dye degradation over Bi3O4Cl/MnxZn1-xFe2O4.
4 Conclusions
In summary, by the hydrothermal-roasting method, Bi(NO3)3·5H2O and NaCl were used as raw materials, MnxZn1-xFe2O4 was used as the magnetic substrate, and the Bi3O4Cl/MnxZn1-xFe2O4 composite magnetic photocatalyst was successfully fabricated. RhB was utilized as the target degradation to evaluate the performance of the photocatalysts, and the obtained Bi3O4Cl/MnxZn1-xFe2O4 photocatalyst exhibited an excellent photocatalytic behaviour, which could contribute to the doping of MnxZn1-xFe2O4 onto Bi3O4Cl. Since more active sites were provided, with a higher specific surface area, the Bi3O4Cl/MnxZn1-xFe2O4 photocatalyst was essential to the exhibition of higher adsorption of RhB. MB-10 exhibited fine magnetic recovery. In addition, an efficient preparation method is also proposed in this paper, which provides a simple and feasible approach for obtaining Bi3O4Cl/MnxZn1-xFe2O4 photocatalysts. Thus, an ideal potential application for the removal of environmental pollutants in the area of photocatalysis is available.
CRediT authorship contribution statement
Hailong Wang: Conceptualization, Resources, Writing – review & editing, Visualization, Supervision, Funding acquisition. Dan Yang: Writing – original draft, Visualization. Longjun Xu: Conceptualization, Resources, Writing – review & editing, Visualization, Supervision, Funding acquisition.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (NSFC, 51374259).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- MoS2-mesoporous LaFeO3 hybrid photocatalyst: Highly efficient visible-light driven photocatalyst. Int. J. Hydrogen. Energ.. 2020;45:11502-11511.
- [CrossRef] [Google Scholar]
- rGO-TiO2-CdO-ZnO-Ag photocatalyst for enhancing photocatalytic degradation of methylene blue. Opt. Mater.. 2021;116:111090
- [CrossRef] [Google Scholar]
- A ternary photocatalyst with double heterojunctionsfor efficient diesel oil degradation. ChemistrySelect. 2021;6:3117-3125.
- [CrossRef] [Google Scholar]
- Novel ZnCo2O4 embedded with S, N-CQDs as efficient visible-light photocatalyst. J. Photoch. Photobio. a. 2021;405:112971
- [CrossRef] [Google Scholar]
- New insights into the degradation of synthetic pollutants in contaminated environments. Chemosphere. 2021;268:128827
- [CrossRef] [Google Scholar]
- Organic polyelectrolytes as the sole precipitation agent in municipal wastewater treatment. J. Environ. Manage.. 2020;271:111002
- [CrossRef] [Google Scholar]
- A novel visible-light-driven ternary magnetic photocatalyst MnxZn1-xFe2O4/C/CdS: fabrication, characterization and application. Mater. Chem. Phys.. 2021;262:124308
- [CrossRef] [Google Scholar]
- Magnetically separable and recyclable ternary photocatalyst MnxZn1-xFe2O4/BiVO4/MnO2 with excellent photocatalytic activity. Vacuum. 2021;187:110133
- [CrossRef] [Google Scholar]
- Catalytic degradation of carbamazepine by a novel granular visible-light-driven photocatalyst activating the peroxydisulfate system. Chem. Eng. J.. 2021;421:127867
- [CrossRef] [Google Scholar]
- Photocatalytic phenol degradation by silica-modified titanium dioxide. Appl.-Sci.. 2021;11:9033.
- [CrossRef] [Google Scholar]
- Synthesis and application of novel α-Fe2O3/graphene for visible-light enhanced photocatalytic degradation of RhB. Mater. Des.. 2020;188:108461
- [CrossRef] [Google Scholar]
- V2O5 /Al2O3 composite photocatalyst: preparation, characterization, and the role of Al 2O3. Chem. Eng. J.. 2012;180:170-177.
- [CrossRef] [Google Scholar]
- Facile synthesis of CuCr2O4/BiOBr nanocomposite and its photocatalytic activity towards RhB and tetracycline hydrochloride degradation under household visible LED light irradiation. J. Alloy. Compd.. 2021;867:157947
- [CrossRef] [Google Scholar]
- Step-scheme NiO/BiOI heterojunction photocatalyst for rhodamine photodegradation. Appl. Surf. Sci.. 2020;511:145499
- [CrossRef] [Google Scholar]
- Carbon-coated MnxZn1-xFe2O4 as a magnetic substrate for Zn0.8Cd0.2S applied in photocatalytic rhodamine B. Opt. Mater.. 2021;112:110767
- [CrossRef] [Google Scholar]
- Synthesis and characterization of ZnO-TiO2/CoFe2O4 hollow photocatalyst: magnetic recovery and highly efficient photocatalytic performance. Funct. Mater. Lett.. 2020;13:2051027.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of RhB from an aqueous solution using Ag3PO4/N-TiO2 heterostructure. J. Mol. Liq.. 2020;313:113522
- [CrossRef] [Google Scholar]
- Ordered bicontinuous mesoporous polymeric semiconductor photocatalyst. ACS Nano. 2020;14:13652-13662.
- [CrossRef] [Google Scholar]
- In-situ photocalorimetry-fluorescence spectroscopy studies of RhB photocatalysis over Z-scheme g-C3N4@Ag@Ag3PO4 nanocomposites: a pseudo-zero-order rather than a first-order process. Appl. Catal. B-Environ.. 2017;217:591-602.
- [CrossRef] [Google Scholar]
- Blue light-powered hydroxynaphthoic acid-titanium dioxide photocatalysis for the selective aerobic oxidation of amines. J. Colloid Interface Sci.. 2021;602:534-543.
- [CrossRef] [Google Scholar]
- Vacancy-rich monolayer BiO2-x as a highly efficient UV, visible, and near-infrared responsive photocatalyst. Angew. Chem. Int. Ed.. 2018;57:491-495.
- [CrossRef] [Google Scholar]
- Photodegradation of benzothiazole ionic liquids catalyzed by titanium dioxide and silver-loaded titanium dioxide. Chinese J. Chem. Eng.. 2020;28:1397-1404.
- [CrossRef] [Google Scholar]
- Two-dimensional sulfur- and chlorine-codoped g-C3N4/CdSe-amine heterostructures nanocomposite with effective interfacial charge transfer and mechanism insight. Appl. Catal. B-Environ.. 2021;280:119452
- [CrossRef] [Google Scholar]
- Photocatalytic activity of a bi-based oxychloride Bi3O4Cl. J. Phys. Chem. B. 2006;110:24629-24634.
- [CrossRef] [Google Scholar]
- Study on the catalytic performance of LaMnO3 for the RhB degradation. J. Taiwan. Inst. Chem. E.. 2020;109:15-25.
- [CrossRef] [Google Scholar]
- Fabrication of graphene/Bi12O17Cl2 as an effective visible-light photocatalyst. Mater. Rws. Bull.. 2020;122:110690
- [CrossRef] [Google Scholar]
- 2D layered MoS2 loaded on Bi12O17Cl2 nanosheets: an effective visible-light photocatalyst. Ceram. Int.. 2020;46:7438-7445.
- [CrossRef] [Google Scholar]
- Green synthesis of spherical TiO2 nanoparticles using Citrus Limetta extract: excellent photocatalytic water decontamination agent for RhB dye. Inorg. Chem. Commun.. 2021;129:108618
- [CrossRef] [Google Scholar]
- Fe-doped UiO-66 combined with ZnIn2S4 for adsorption and degradation of RhB under visible light. Nano. 2020;15:1-11.
- [CrossRef] [Google Scholar]
- Manipulating spin polarization of titanium dioxide for efficient photocatalysis. Nat. Commun.. 2020;11:418.
- [CrossRef] [Google Scholar]
- Ag3PO4-TiO2-Carbon nanofiber Composite: an efficient Visible-light photocatalyst obtained from eelectrospinning and hydrothermal methods. Sep. Purif. Technol.. 2021;276:119400
- [CrossRef] [Google Scholar]
- Facile fabrication of a new BiFeWO6/α-AgVO3 composite with efficient visible-light photocatalytic activity for dye-degradation [J] Opt. Mater.. 2019;92:284-293.
- [CrossRef] [Google Scholar]
- Recent progress on heterostructures of photocatalysts for environmental remediation. Mater. Today: Proc.. 2020;32:584-593.
- [CrossRef] [Google Scholar]
- Adsorption equilibrium, kinetics, and thermodynamics assessment of the removal of the reactive red 141 dye using sesame waste [J] Desalin. Water Treat.. 2015;57(38):18087-18098.
- [CrossRef] [Google Scholar]
- A facile synthesis of visible-light driven rod-on-rod like α-FeOOH/α-AgVO3 nanocomposite as greatly enhanced photocatalyst for degradation of rhodamine B [J] Catalysts. 2018;8(9)
- [CrossRef] [Google Scholar]
- Bimetal (Fe/Zn) doped BiOI photocatalyst: an effective photodegradation of tetracycline and bacteria. Chemosphere. 2021;280:130803
- [CrossRef] [Google Scholar]
- Ag NPs decorated C-TiO2/Cd0.5Zn0.5S Z-scheme heterojunction for simultaneous RhB degradation and Cr(VI) reduction. Environ. Pollut.. 2021;286:117305
- [CrossRef] [Google Scholar]
- A spherical TiO2-Bi2WO6 composite photocatalyst for visible-light photocatalytic degradation of ethylene. Colloid Surf. a. 2020;602:125048
- [CrossRef] [Google Scholar]
- Synthesis of Bi3O4Cl nanosheets with oxygen vacancies: the effect of defect states on photocatalytic performance. Appl. Surf. Sci.. 2020;507:144806
- [CrossRef] [Google Scholar]
- Simultaneous removal of organic pollutants and heavy metals in wastewater by photoelectrocatalysis: a review. Chemosphere. 2021;273:128503
- [CrossRef] [Google Scholar]
- Fabrication of a dual S-scheme Bi7O9I3/g-C3N4/Bi3O4Cl heterojunction with enhanced visible-light-driven performance for phenol degradation. Chemosphere. 2022;287:132241
- [CrossRef] [Google Scholar]
- Carbon covered Ag and Bi nanoparticles uniformly dispersed in porous carbon matrix: synergistic effect for removal of RhB. Mater. Lett.. 2020;275:128099
- [CrossRef] [Google Scholar]
- Bi4O5Br2 nanoflower and CdWO4 nanorod heterojunctions for photocatalytic synthesis of ammonia [J] ACS Appl. Nano Mater.. 2023;6(17):15709-15720.
- [CrossRef] [Google Scholar]
- Fabrication of novel BiPO4/Bi4O5Br 2 heterojunctions for improving photoactivity in N2 fixation and dye degradation [J] Mater. Res. Bull.. 2023;2023(167):112377
- [CrossRef] [Google Scholar]
- One-step preparation of novel Bi-Bi2O3/CdWO4 Z-scheme heterojunctions with enhanced performance in photocatalytic NH3 synthesis [J] J. Alloy. Compd.. 2023;2023(968):171956
- [CrossRef] [Google Scholar]
- Mesoporous WO3/TiO2 spheres with tailored surface properties for concurrent solar photocatalysis and membrane filtration. Chemosphere. 2021;263:128344
- [CrossRef] [Google Scholar]
- In Situ grown AgI/Bi12O17Cl2 heterojunction photocatalysts for visible light degradation of sulfamethazine: efficiency, pathway, and mechanism. ACS Sustain. Chem. Eng.. 2018;6:4174-4184.
- [CrossRef] [Google Scholar]
- Titanium dioxide nanoparticles: prospects and applications in medicine. Nanomaterials-Basel. 2020;10:387.
- [CrossRef] [Google Scholar]







